Abstract
Background and Objectives:
Rapid Onset Dystonia Parkinsonism (RDP) is caused by ATP1A3 mutations. It has been characterized by rapid onset bulbar dysfunction, limb dystonia, bradykinesia and a rostrocaudal spatial gradient of expression, usually after a physiologic trigger. We reexamined whether these features were in fact characteristic.
Methods:
We characterized phenotypic variation within a cohort of 50 ATP1A3 mutation-positive subjects (carriers) and 44 mutation-negative family members (noncarriers). Potential subjects were gathered through referral for clinical suspicion of RDP or AHC. Inclusion criteria were having a ATP1A3 mutation or being a family member of such an individual.
Results:
We found RDP is underdiagnosed if only “characteristic” patients are tested. Rapid onset and bulbar predominance were not universally present in carriers. Among those with at least mild symptoms of dystonia, rostrocaudal severity gradient was rare (7%). Symptoms began focally but progressed to be generalized (51%) or multifocal (49%). Arm (41%) onset was most common. Arms and voice were typically most severely affected (48% and 44% respectively). Triggers preceded onset in 77% of subjects. Rapid onset, dystonia, parkinsonism, bulbar symptoms, headaches, seizures, frontal impairment, and a history of mood disorder and a history of psychosis were more common in carriers. Approximately half of proband mutations occurred de novo (56%).
Conclusions:
Our findings suggest that patients should not be excluded from ATP1A3 testing because of slow onset, limb onset, absent family history, or onset in middle adulthood. RDP should be strongly considered in the differential for any bulbar dystonia.
Keywords: RDP, Rapid-Onset Dystonia-Parkinsonism, ATP1A3, DYT12, Dystonia
Introduction:
Rapid-Onset Dystonia-Parkinsonism (RDP) was identified a quarter century ago when physicians noticed that stress-induced abrupt onset dystonia clustered in some families1. The phenotype was clinically defined as the onset over hours to days of bulbar dysfunction, limb dystonia, bradykinesia, and postural instability after a physiologic stimulus. Running, stress, and alcohol consumption were identified as common triggers2. Symptoms were observed to be levodopa-unresponsive and display a rostrocaudal spatial gradient of expression2, 3. Also known as Dystonia 12 (DYT12), RDP’s genetic basis was later identified as mutations in the ATP1A3 gene4. ATP1A3 encodes the α3 catalytic subunit of the Na+/K+-ATPase responsible for active maintenance of transmembrane ionic gradients4 and is expressed diffusely in neurons throughout the brain, including in the basal ganglia and cerebellum5, 6.
Identifying ATP1A3 as the underlying cause of RDP allowed the identification of atypical presentations, including gradual onset dystonia7–9, akinetic-rigid hemiparkinsonism8, and dystonia parkinsonism with diphasic symptom onset2. ATP1A3 mutations also underlie phenotypically distinct conditions including Alternating Hemiplegia of Childhood (AHC)10, 11, Cerebellar ataxia, Areflexia, Pes cavus, Optic atrophy and Sensorineural hearing loss syndrome (CAPOS)12, and Relapsing Encephalopathy with Cerebellar Ataxia (RECA), also known as Fever-Induced Paroxysmal Weakness and Encephalopathy (FIPWE)13, 14. The recognition of a single genetic cause has led to the possibility that these syndromes could be considered points along a single ATP1A3 disorder spectrum. A known genetic basis also allows us the opportunity to reassess whether ‘characteristic’ features in RDP are accurately considered to be typical. To address this, we characterize here phenotypic variations of RDP within a large cohort with (carriers, n=50) and without (noncarriers, n=44) ATP1A3 mutations.
Methods:
All participants signed an informed consent form in accordance with the Declaration of Helsinki and approved by the Wake Forest School of Medicine Institutional Review Board. Based on the current consensus on RDP 15 we adopted minimal criteria to define our cohort. Inclusion criteria were having a ATP1A3 mutation or being family to someone who did. Families were excluded from this analysis if movement abnormality onset occurred before 18 months of age in all affected carriers in the family.
Clinical:
This cohort was drawn from our database of individuals with ATP1A3 mutations and their family members. The proband of a family was defined as the individual who came to physician attention first in the family. A buccal or blood sample was obtained from subjects and DNA was extracted using the Puregene procedure (Qiagen systems, Minneapolis MN). The ATP1A3 gene was Sanger-sequenced using PCR primers and conditions previously described2. All carriers discovered were recruited to the database. Family members of carriers were also recruited and tested for ATP1A3 gene mutations and included whether mutation-positive or negative. Subjects were examined at a tertiary center for pediatric and adult movement disorders. Data were accrued from October 2007 through July 2017.
All cohort members underwent a structured interview (consisting of videotaped history-taking session and a subject questionnaire), neuropsychological testing, and a neurologic exam. The structured interview included items addressing family history, rapidity of symptom onset (rapid onset defined as plateauing within 30 days), affected body regions, associated triggers, medication response, seizures, and headaches. In assessing triggers the interview specifically enquired regarding physical activity, infection, heat/fever, psychological stress, and alcohol consumption2. The interview also allowed subjects to specify triggers using an open text field. The subject questionnaire was completed by the subject directly when possible, or by a family member when not. The neurological exam included a modified Unified Parkinson Disease Rating Scale Motor subsection (differing primarily in that scores were recorded solely for the more affected limb), and Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS)16. A standard UPDRS-3 subscale2, 17 and International Cooperative Ataxia Rating Scale (ICARS)18 were added to examinations conducted in 2014 and after. Neuropsychological and cognitive testing included the Trailmaking Part B19, Controlled Oral Word Association (COWA, linguistic and semantic verbal fluency)20, Hamilton Anxiety and Depression scales (HAM-A and HAM-D)21, 22, the Yale/Brown Obsessive Compulsive Symptom scale (YBOCS)23, the Composite International Diagnostic Interview (CIDI-Auto version)24, and the Structured Clinical Interview for DSM-IV (SCID)25. Subjects under 18 were administered the Structured Clinical Interview for DSM-IV Childhood Diagnoses (KID-SCID)26 instead of the SCID. Discrepancies in diagnoses generated by these instruments were adjudicated by a psychiatrist blinded to mutation status. If a subject had more than one SCID or KID-SCID and a subject received a psychiatric diagnosis at any administration, they were considered to have that diagnosis for the purposes of our prevalence analyses. Examinations were performed in person or via video by physicians who were unaware of carrier status but knew that RDP was a diagnostic possibility. Levodopa responsiveness was determined by structured interview.
Analyses:
Our analyses characterized symptoms typical of RDP and the degree of symptom variation within ATP1A3 mutation-positive individuals, as represented in our database through July 2017. To characterize these features, we compared individuals with ATP1A3 mutations (n=50) to family members without (n=44). We selected some features for comparison that have been considered to be typical of RDP15 and some that are described in other ATP1A3 phenotypes15, 27. This yielded 13 points of comparison: age of onset; rapidity of onset (rapid, non-rapid, or asymptomatic); dystonic symptoms; a gradient of dystonic severity (face > arm > leg); Parkinsonism (absent, possible/probable, absent); tremor (any, action and rest, action, none); bulbar symptoms; headaches; seizure history; frontal function (Trailmaking Part B, COWA linguistic verbal fluency, COWA semantic verbal fluency; all yielding T-scores: mean = 50, standard deviation = 10); psychiatric symptoms (HAM-A, HAM-D, YBOCS, and the SCID/KID-SCID); reflexes; and cerebellar symptoms. We also compared these groups demographically. We examined whether they had triggers that correlated with symptom onset or recrudescence. To reduce subject-based observer bias, we considered only events within one day of symptom onset for analysis as triggers. Data was supplemented from the videotaped history if missing.
Subjects without ATP1A3 mutations in our cohort sometimes reported symptoms and some subjects with ATP1A3 mutations did not. To separate subjects with overt disease from non-manifesting carriers we excluded carriers from our analyses if they had no more than slight symptoms of dystonia present, decreasing our n for carriers from 50 to 44. Symptoms were considered no more than slight if none of the nine regional BFMDRS severity items was scored as mild, moderate, or severe, and the BFMDRS total score was ≤ 7.5. For reference, a subject would have a total score of 7.5 if each of the nine dystonia severity items were rated as ‘slight,’ and each of the nine corresponding provoking items had the smallest possible positive rating. Data summaries were reported as means ± standard deviations, or counts and percentages. P values for between-group comparisons were based on chi-squared, Fisher’s exact and t tests. P ≤ 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 for Microsoft Windows (SAS Institute Inc., Cary, NC, USA).
Results:
Genetic characteristics:
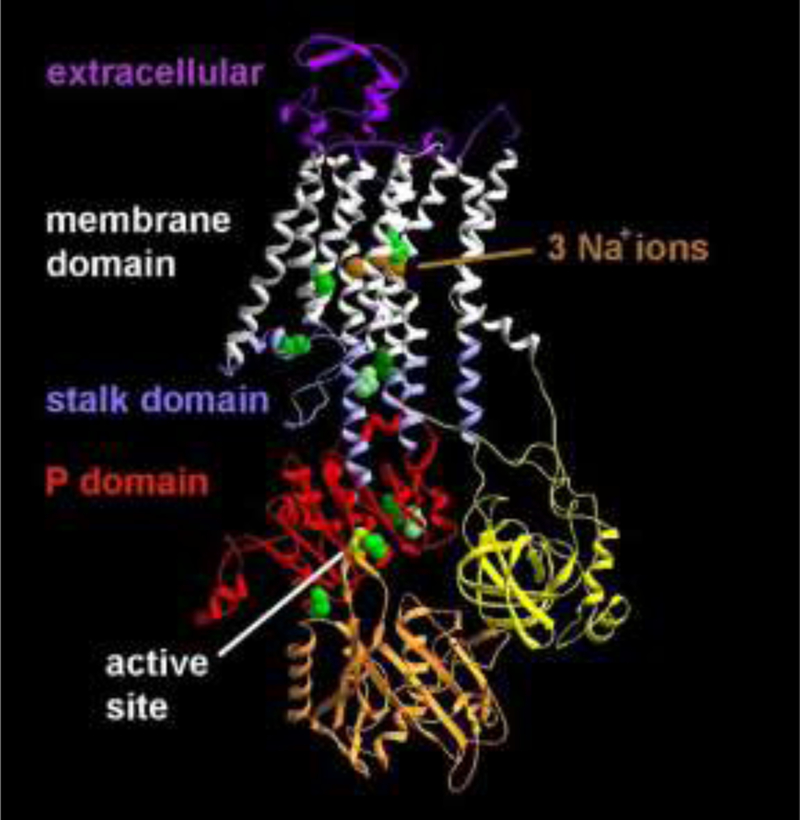
Our study cohort included 19 probands and 31 family members with ATP1A3 mutations, and 44 family members without mutations. No family had more than one proband. Probands were drawn from 19 families with no known relatives in common. Mutation characteristics are shown in Table 1, with locations in the protein crystal structure given in Figure 1. There were 10 distinct mutations associated with RDP in our cohort, and they occurred in several different domains of the protein (Figure 1). The most common mutation in our database was p.Thr613Met27, with five distinct families and 16 individuals with mutations out of 29 total family members. Other mutations that occurred in more than one family were p.Glu277Lys (three families, three individuals, eight total members), p.Asp923Asn (three families, three individuals, seven total members) and p.Asp801Tyr (two families, six total members). Another six mutations were found in single cases/families. De novo mutations represented more than half (56%) of all proband cases whose parental genetic status could be determined. We confirmed that the probands’ parents were ATP1A3 mutation-free in nine families, mutation-positive in seven families, but could not establish the probands’ parental genetic status in three (Table 1).
Table 1:
Mutation characteristics of subjects with ATP1A3 mutations with distribution of the RDP mutations in the structure listed in order of nucleotide change. Also given are the protein domains affected by each mutation and numbers of families in our cohort expressing that mutation. Numbers of carriers per family are also shown, and their genetic status.
| Mutation | Frequency | |||||
|---|---|---|---|---|---|---|
| nucleotide | amino acid | Domain | families with mut | carriers per family | total members in family | status |
| c.0829 G > A | p.Glu277Lys | stalk | 3 | 1 | 4 | unknown |
| 1 | 3 | de novo | ||||
| 1 | 1 | de novo | ||||
| c.1096 G > C | p.Asp366His | P domain, active site | 1 | 1 | 2 | de novo* |
| c.1108 A > G | p.Thr370Ala | P domain | 1 | 2 | 2 | familial* |
| c.1838 C > T | p.Thr613Met | P domain | 5 | 7 | 13 | familial |
| 5 | 5 | familial | ||||
| 2 | 5 | familial | ||||
| 1 | 1 | de novo | ||||
| 1 | 5 | de novo | ||||
| c.2134 T > C | p.Ser712Pro | P domain | 1 | 1 | 1 | de novo* |
| c.2140 G > A | p.Ala714Thr | P domain | 1 | 3 | 3 | familial |
| c.2273 T > G | p.Ile758Ser | stalk | 1 | 14 | 34 | familial |
| c.2401 G > T | p.Asp801Tyr | membrane | 2 | 5 | 5 | familial |
| 1 | 1 | unknown | ||||
| c.2767 G > A | p.Asp923Asn | membrane | 3 | 1 | 3 | unknown |
| 1 | 3 | de novo | ||||
| 1 | 1 | de novo | ||||
| c.2788 C > T | p.Arg930Trp | stalk | 1 | 1 | 2 | de novo |
denotes mutations reported here for the first time.
In some cases parental DNA was obtained to establish de novo mutations, but the parents were not study participants. Mut = ATP1A3 mutations.
Figure 1: Protein structure of the ATP1A3 Na,K-ATPase.
The sodium-bound form of the ATP1A3 Na,K-ATPase is shown in ribbon view, with the backbone locations of mutated amino acids in green and yellow. Three mutations were in the stalk domain. Two mutations were in the membrane domain not far from the ions, and five were in the P (phosphorylation) domain, including the aspartate (yellow) in the active site that is phosphorylated and dephosphorylated during each cycle of ion transport.
Analyses within mutation-positive subjects:
Our exclusion of carriers with no more than slight dystonia led to the exclusion of 6 non/mildly manifesting carriers from the analysis – 5 subjects who did not report symptoms and one subject with a low total BFMDRS score – for a remainder of 44 mutation-positive subjects included in analyses. Symptom onset was typically rapid, though a significant number of carriers reported onset over more than 30 days (20.5%, 9/44). Motor symptoms usually started in adolescence or early adulthood. Age of onset ranged from 3 to 59 years old (mean 22.8, SD±11.9), with 25% with onset from 3–16 years of age, 25% from 16–19.5, 25% from 19.5–27, and 25% from 27–59. There was typically a significant delay between symptom onset and diagnosis/enrollment with a mean age at initial study exam of 43 years. Subjects commonly reported triggers within a day of symptom onset. Thirty-one carriers provided information on the presence or absence of triggers. Seventy-seven percent had at least one of the stressors queried (physical activity, infection, heat/fever, psychological stress, and alcohol consumption) present. Of these, 16% reported a single trigger while 61% reported two or more. Other trigger types reported by carriers were sleep deprivation (n=2), childbirth (n=2), and drinking quinine-containing tonic water (n=1) or a “spiked” drink (n=1).
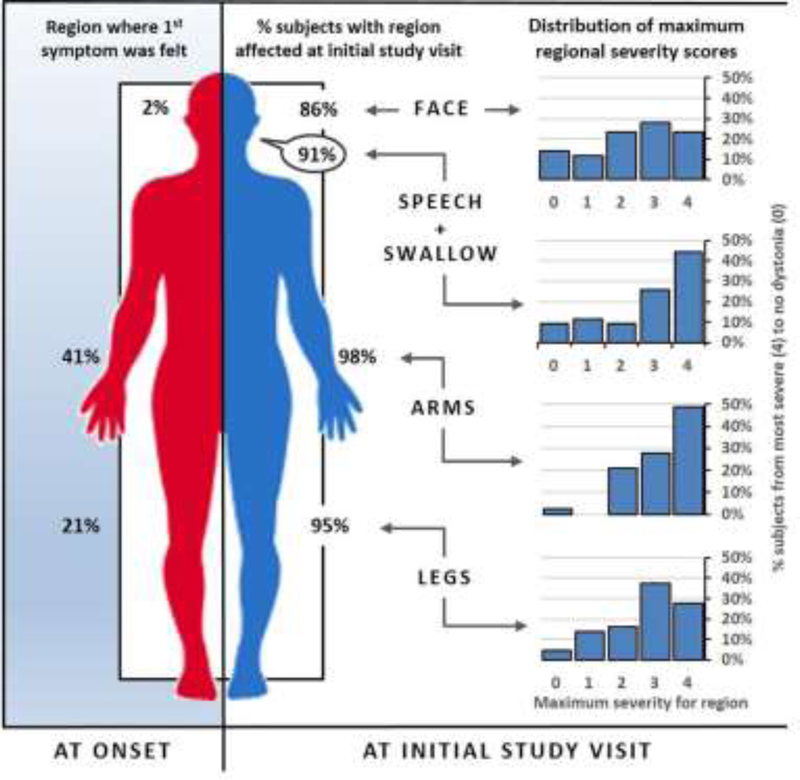
Fewer body regions were affected in our subjects at onset (mean age 23 years) than at time of study exam (mean age 43 years). Arms were the first body part affected in 41%, legs in 21%, and face in 2%. At study examination, arms were affected in 98%, legs in 95%, and face in 86% (Figure 2). Arms and voice were most severely affected (48% and 44% respectively) with some patients reporting multiple areas as equally affected. Dystonia severity varied considerably (Figure 2). Moderate to severe dystonia was more often seen in limbs (arms 77%, legs 65%), or speech and swallowing (70%), than the face (51%). A strict rostrocaudal spatial gradient of dystonia severity was present in only 7% of carriers (face/speech dystonia > arm dystonia > leg dystonia on the BFMDRS). Dystonia was generalized (51%) or multifocal (49%). No subjects displayed isolated segmental or focal dystonia at time of study exam. Parkinsonism was typical, with 77% of subjects displaying definite and 21% possible/probable parkinsonism. Parkinsonism and dystonia were strongly correlated (Spearman R=0.786 with a p=0.012) in the 9 patients with a full UPDRS3. Carriers uniformly lacked features characteristic of other parkinsonian and dystonic syndromes, such as pill-rolling tremor or diurnal fluctuation. Symptoms were not levodopa responsive in this cohort. Some patients reported subjective improvement with anticholinergics, benzodiazepines, botulinum toxin injections, and/or muscle relaxants. We did not perform off-medication evaluations in order to quantify degree of benefit.
Figure 2: Subjects with ATP1A3 mutations show progression to diffuse involvement without a rostrocaudal gradient of severity.
Predominance of dystonia by body region at onset (Left) and at initial study visit in symptomatic carriers (Right). Dystonia at initial study visit is subdivided into variation in carrier severity scores at a given region, and the percentage of carriers with moderate dystonia or greater at a given region. Carriers showed diffuse involvement without a clear rostrocaudal gradient of dystonia severity at the initial study visit. Symptoms typically began focally in arms or mouth, but were diffuse by initial study visit. All regions were affected in at least 85% of carriers but were not equally affected. Subjects most often displayed significant (moderate to severe) dystonia in their arms (77%) or speech or swallowing (70%). Fewer subjects scored in the moderate to severe range with respect to their legs (65%) or face (51%). Dystonia severity was derived from BFMDRS scores. Further detail on gradient calculation methodology is given in Supplemental Methods.
Thirty-one percent of carriers reported a history of seizure (n=12/39). With the exception of this history of seizure, we did not observe symptoms characteristic of the AHC phenotype of ATP1A3 in our cohort. All but one of these subjects reported that episodes occurred less than once a month, the lowest frequency that could be selected on our questionnaire. The carrier with more frequent seizures reported that they were occurring 5–6 times a week at time of study visit. Age at seizure onset was variable across carriers (mean 21.9 years ± 18.4). We also tested for features of other ATP1A3 phenotypes (CAPOS and RECA/FIPWE): cerebellar symptoms, areflexia, encephalopathy, or sudden weakness. There were no areflexic carriers. Ten carriers had formal cerebellar testing using the ICARS. Seven showed some cerebellar dysfunction while none of the 4 noncarriers displayed cerebellar abnormalities, but this difference between groups did not reach statistical significance (p=0.070). When cerebellar dysfunction occurred, subjects displayed decomposition or dysmetria of finger-to-nose (n=5), impaired heel-to-shin (n=4), and gaze nystagmus (n=2). Before instituting the ICARS, we had not detected cerebellar abnormalities on routine neurological exam. No carriers reported encephalopathy or sudden weakness. Headaches were relatively common, and occurred in 68% of carriers.
Non-Reporting/minimally symptomatic carriers:
Not all carriers reported motor symptoms. In our cohort of 50 carriers, 5 subjects did not. Those who did not self-report symptoms were mostly free of symptoms on assessment, whether by history, motor examination, or nonmotor testing. Two reported headaches. None displayed dystonia on formal examination (BFMDRS=0 in five). The small number of non-reporting carriers prevented further subgroup analyses. One subject did not meet our cutoff for a ‘symptomatic’ dystonia score. There were no clear demographic differentiators between non-reporting/minimally symptomatic carriers and other carriers. Age and gender were similar between these groups. There was no clustering within a mutation group or a protein domain.
Analyses between mutation positive and negative subjects:
Demographic variables were similar between the mutation-positive and mutation-negative subjects (61 male vs. 39% female, p=0.055). Age at exam did not significantly differ between the two groups, with a mean age of 43 years in the carriers vs. 37 years in non-carriers (p=0.341).
Dystonia, parkinsonism, bulbar symptoms, headaches, seizures, total reflex scores, impaired cognitive function, and a history of mood disorder and a history of psychosis were significantly more common in carriers than noncarriers (Tables 2 & 3). A history of seizure was significantly more common (31% of carriers vs. 7% of noncarriers, p = 0.009). As previously reported28, carriers were also significantly more likely to perform poorly on measures of cognitive function independently of motor dysfunction. Mean cognitive scores in the carriers were in the Mild Impairment range (Table 3) based on the tests’ normative values29, while noncarriers’ mean scores placed in the Average range on these measures. There was no significant difference between groups with respect to the psychiatric test results in our cohort; carriers did not score significantly higher on measures of anxiety, depression, or obsessive-compulsive disorder when examined. However carriers were significantly more likely than their noncarrier relatives to report a history of any mood disorder (50% vs 22%, p= 0.036) and a history of any psychotic disorder (22% vs 0%, p= 0.011), but were not significantly more likely to report a history of substance abuse or anxiety disorder (Table 2). Reflex scores were compared as the total across all joints, with each joint scored zero to four with higher scores indicating brisker reflexes (Table 2). Carriers had mildly brisker total reflex scores on average (22.7 with SD±7.0 vs. 18.8 with SD ±4.0, p=0.004, 38/44 and 39/44 assessed). There were no areflexic subjects within either cohort.
Table 2:
Statistical analysis of key motor features in those with and without ATP1A3 mutations. Percentages are given with respect to total assessed subjects. Where there is missing data due to patient-specific issues, information is given in Supplementary Data Table 1. Standard deviations are given in parentheses (SD) where appropriate. BMFDRS = Burke Fahn Marsden Dystonia Rating Scale, UPDRS = Unified Parkinson’s Disease Rating Scale motor subscale, Y-BOCS = Yale Brown Obsessive Compulsive Symptom Scale.
| Characteristics | Carriers N=44 |
Noncarriers N=44 |
p-Value | |
|---|---|---|---|---|
| Rapidity of onset | Rapid onset | 35 (79.5%) | 0 (0.0%) | 0.014 |
| Non-rapid onset | 9 (20.5%) | 3 (6.8%)† | ||
| No onset | 0 (0.0%) | 41 (93.2%) | ||
| F > A > L Gradient | Yes | 3 (7.0%) | 0 (0.0%) | 0.241 |
| No | 40 (93.0%) | 43 (100.0%) | ||
| Bulbar Symptoms | Yes | 41 (95.3%) | 2 (5.0%) | < 0.001 |
| No | 2 (4.7%) | 38 (95.0%) | ||
| Headaches | Yes | 26 (68.4%) | 17 (39.5%) | 0.031 |
| No | 12 (31.6%) | 26 (60.5%) | ||
| Seizures | Yes | 12 (30.7%) | 3 (7.1%) | 0.017 |
| No | 27 (69.2%) | 38 (90.5%) | ||
| I don’t know | 0 (0.0%) | 1 (2.4%) | ||
| Parkinsonism | Absent | 1 (2.3%) | 40 (97.6%) | < 0.0001 |
| Possible/Probable | 9 (20.9%) | 1 (2.4%) | ||
| Definite | 33 (76.7%) | 0 (0%) | ||
| Tremor | Any | 13 (29.5%) | 6 (14.0%) | 0.118 |
| Action & rest tremor | 3 (6.8%) | 0 (0.0%) | 0.103 | |
| Action only | 10 (22.7%) | 6 (14.0%) | 0.118 | |
| Rest only | 0 (0.0%) | 0 (0.0%) | ||
| None | 31 (70.5%) | 37 (86.0%) | ||
| BFMDRS | 53.2 (29.4) | 0.2 (0.6) | < 0.001 | |
| UPDRS3 | 42.6 (18.6) | 4.5 (0.6) | 0.002 | |
| Cerebellar Dysfunction | Yes | 7 (70.0%) | 0 (0.0%) | 0.070 |
| No | 3 (30.0%) | 4 (100.0%) | ||
| Reflexes | 22.7 (7.0) | 18.8 (4.0) | 0.004* | |
patient-reported, suspected to be functional.
reflex scores are given as a sum of limb reflex scores (bilateral biceps, triceps, brachioradialis, patellar, Achilles tendons with each scored 0–4, total range 0–40).
Further detail on calculation methodology is given in Supplemental Methods.
Table 3:
Statistical analysis of key nonmotor features in those with and without ATP1A3 mutations. Where there is missing data due to patient-specific issues, information is given in Supplementary Data Table 2. Numeric values are counts (column percentages) and means (SD). SCID = Structured Clinical Interview for DSM-IV, Y-BOCS = Yale Brown Obsessive Compulsive Symptom Scale. Of the psychiatric symptom scales, all except the SCID yield raw scores with higher values representing greater current symptom burden. The SCID yields lifetime diagnoses of psychiatric illness.
| Characteristics | Carriers N=44 |
Noncarriers N=44 |
p-Value | |
|---|---|---|---|---|
| Cognitive Function | Trailmaking B | 35.8 (10.3) | 51.2 (12.0) | < 0.001 |
| Linguistic Fluency | 33.9 (8.5) | 46.9 (9.2) | < 0.001 | |
| Semantic Fluency | 37.1 (13.1) | 51.4 (9.6) | < 0.001 | |
| Psychiatric symptoms | SCID Mood Disorder | 16 (50%) | 7 (22%) | 0.036 |
| SCID Anxiety Disorder | 13 (42%) | 12 (38%) | 0.080 | |
| SCID Psychosis | 7 (22%) | 0 (0%) | 0.011 | |
| SCID Substance Abuse | 11 (34%) | 8 (26%) | 0.590 | |
| Hamilton Anxiety | 7.2 (7.3) | 5.2 (8.7) | 0.240 | |
| Hamilton Depression | 10.5 (9.2) | 6.3 (7.7) | 0.051 | |
| YBOCS | 3.2 (6.3) | 0.9 (3.1) | 0.385 |
Some noncarriers did believe themselves to be experiencing motor symptoms (6.8%) but all reported non-rapid onset. They did not display significant dystonia (BMFDRS range 0–2). They rarely displayed parkinsonism (77% of carriers vs. 0% of noncarriers with definite parkinsonism, and 21% vs 2% with possible/probable parkinsonism, p < 0.001). Noncarriers rarely reported bulbar symptoms (5% of noncarriers vs. 95% of carriers, p < 0.001). Some noncarriers did not report symptoms but had mild motor signs on exam.
Discussion:
The classical clinical phenotype in RDP has been defined as an abrupt onset of dystonia with features of parkinsonism over a few minutes to 30 days, a clear rostrocaudal (face>arm>leg) gradient and prominent bulbar findings2. In-depth investigation of the largest-available cohort allowed us to show that not all of these classical features are truly characteristic of RDP, and that even ‘characteristic’ features may be absent in patients with ATP1A3 mutations. There is significant phenotypic variation in ATP1A3 mutation carriers even within the established RDP phenotype, particularly at disease onset. We hope this close analysis of a large cohort of carriers and their noncarrier family members will provide diagnostic guidance to clinicians encountering symptomatic patients. Appropriately categorizing subjects will be crucial for grouping them into meaningful categories for future treatment possibilities.
Revising prior diagnostic criteria for RDP
Based our analyses we recommend modifying the diagnostic criteria for RDP. The presence of a rostrocaudal gradient is infrequent (7%) and should not be required for diagnosis. Bulbar symptoms are likely to be present, but in our cohort were neither first nor most severe. In our sample subjects’ arms were most commonly first (41%) and most severely (48%) affected. Rapid onset should still be considered strongly suggestive and was present in 80% of carriers. The presence of bulbar symptoms remain a key feature of the phenotype. Speech and swallowing was impaired in 91% of carriers and moderate to severe in 70%. Most subjects with RDP report triggers, dystonia, and parkinsonism. The cognitive dysfunction we have previously reported continues to be evidenced, and this difference has persisted after controlling for psychomotor speed and severity of depressive symptoms 28. A history of mood disorders or psychotic episodes remains more common in carriers, though our clinical assessments of psychiatric symptoms did not, in contrast to our findings in a smaller RDP cohort30. Per our current analyses a history of seizures is more likely in carriers, and is helpful clue to the diagnosis when present.
Though the term RDP accurately captures the phenotype of many ATP1A3 mutation positive individuals, names which emphasize the genetic basis of the disorder (DYT-ATP1A3 or DYT12) or revised phenotype (Rapid-onset bulbar-predominant dystonia) may better encompass the condition. Given the degree of phenotypic variation, particularly at onset, we propose simple diagnostic criteria for RDP: 1) the presence of dystonia, 2) the absence of motor symptoms prior to 18 months of age and 3) the presence of a mutation in the ATP1A3 gene. Rapid onset, bulbar symptoms, and executive dysfunction are suggestive without being determinative.
Some carriers do not report symptoms while some noncarriers do
We have previously noted that some carriers do not report symptoms30,28. In this cohort this was the case in 10% of carriers (n=5). The absence of dystonia in these individuals was confirmed on examination. These subjectively asymptomatic subjects did not arise from a single kindred, and did not appear only in large families. Though we previously referred to these individuals as non-motor manifesting carriers it is not certain that they are objectively asymptomatic. The single subject enrolled after we implemented a revised and more extensive testing battery (i.e. including the ICARS and standard UPDRS-3) did display mild parkinsonism (UPDRS=15) and cerebellar symptoms (grade 1 impairment of heel to shin testing). Subjectively asymptomatic carriers may represent subjects who were vulnerable to developing symptoms but avoided triggers, or who had differential modification of gene expression or protein processing. Further study will be required to clarify this point. We also found it interesting that three of the mutation negative family members reported a movement disorder. It is possible that functional movement disorders are more common in family members of carriers, who would be expected to be more familiar with dystonic symptoms.
How similar is RDP to other ATP1A3 diseases?
Deviations from the classical RDP phenotype have previously been reported, if infrequently in adults. ATP1A3 mutations present differently when symptom onset occurs prior to 18 months of age (i.e. AHC)15, and so we chose age of symptom onset as our sole exclusion criterion for subjects with an ATP1A3 mutation (though others have reported subjects with p.Asp923Asn mutations with symptoms prior to 18 months of age, we did not observe this in our cohort)31, 32. The defining feature of AHC is the presence of bouts of plegia or dystonia with return towards baseline function between episodes. We did not see bouts of plegia in our cohort, suggesting motor symptom onset prior to 18 months is a valid dividing line between AHC and RDP phenotypes. Comparisons of seizures in RDP and in AHC will require further investigation. It is more difficult to assess the degree of representation of recently reported adult ATP1A3 phenotypes (CAPOS and RECA/FIPWE) within our cohort. Both have a prominent cerebellar component. In our cohort, ICARS-assessed carriers did not show a statistically greater level of cerebellar dysfunction.
An expanded phenotype for RDP
One of the earliest indicators for a diagnosis of RDP was recognition of a rostrocaudal gradient of symptoms. The quantitative studies within this cohort made it possible to investigate this in depth. It became clear that the word “gradient” is imprecise. It can refer to progression, severity, or prevalence. A rostrocaudal gradient of progression is not a characteristic of RDP. Symptoms can progress in any affected body part, and 62% in this cohort presented first with limb dystonia. Clinical assessments tend to encompass both severity and prevalence. Only a small minority of carriers displayed a rostrocaudal gradient of severity (7%). We did not find a rostrocaudal gradient of symptom prevalence either: more carriers reported moderate to severe dystonia in their arms (77%) than speech or swallowing (70%), legs (65%) or face (51%). Future studies of RDP may provide more information, but we recommend researchers distinguish between whether progression, severity, or symptom prevalence is being documented in any gradient observed.
This evolution of phenotype is a natural consequence of RDP’s transition from a set of symptoms to a disease anchored in ATP1A3 mutations. First reports were limited to families in whom linkage analyses could reveal the underlying genetic mutation. Identification of an underlying genetic cause has allowed individuals with less characteristic phenotypes to be checked for the condition. Our current analysis also takes advantage of more detailed data collection than was previously possible. Our analyses show that RDP is neither always associated with rapid onset nor with parkinsonism. Moreover, parkinsonism is not always an independent entity. It was strongly correlated with dystonia, suggesting that in many patients some of the UPDRS score was capturing bradykinesia and rigidity due to dystonia.
Accurate characterization will improve our ability to identify successful treatment modalities. Current treatments are limited in their success. No carrier in our cohort found levodopa to be effective. Most patients in our cohort received some combination of anticholinergics, benzodiazepines, botulinum toxin injections, or muscle relaxants. Benefit ranged from none to moderate. Though none of our subjects received Deep Brain Stimulation (DBS), RDP patients at other institutions have received DBS without significant improvement33–35.
Study limitations
Some analyses were limited due to small samples. Subjects were ascertained via referral rather than as a population-based sample. All probands were sufficiently characteristic of the accepted definition of RDP to warrant evaluation. We addressed this issue by repeating our analyses after excluding probands from the analysis. Most correlations were unaffected, but with probands excluded, seizure frequency was no longer significantly different between groups. Sample size and in-group phenotypic variability also precluded an analysis of genotype-phenotype variation across mutation loci. Based on the phenotypic range we have observed, we expect that animal models will be more likely to settle this issue than additional database accrual. Since there was typically a significant delay from onset to diagnosis, symptoms at onset and over the disease course were gathered via patient history. Historical detail can only be as accurate as the subject’s memory. Similarly, we left it to the patient to determine what might constitute a ‘seizure’ for the purposes of our questionnaire, and so can not differentiate between true epileptic events and paroxysmal non-epileptic events. Another issue is that portions of the structured clinical interview were form-based and filled out by the subjects/caregivers so that some analyses had missing data. Lastly, only a small minority of databased subjects have had multiple visits, precluding a prospective evaluation of variation over time. We continue to accrue subjects for this database, and hope to have sufficient power to ameliorate these limitations and answer additional questions in the future. In particular, we hope to test potential predictors of phenotypic severity and define the brain circuitry responsible. We are curious to see how trigger type affects symptom expression. Future analyses may also assess whether motor and cognitive symptoms covary, potentially addressing whether these deficits reflect shared brain circuits.
When should clinicians consider RDP?
Our analyses have significant implications for the characterization and differential diagnosis of dystonia. RDP’s apparent rarity may have been partially driven by testing for ATP1A3 primarily in those patients with more severe and ‘classic’ symptoms of the disease. Our results suggest strict criteria are counterproductive. Moreover scale-based assessments and preset questionnaires improve the detection of milder phenotypes compared to a routine history and exam. Symptoms in carriers can be mild and may not be spontaneously reported. Many subjects noted their dystonia as rapid only when asked to select between given time-frames. Some subjects with bradykinesia may fail to self-report as parkinsonian, leaving those symptoms undiagnosed. RDP should be strongly considered in the differential for any dystonia, particularly with onset over days to a few months, and when bulbar symptoms are present.
Supplementary Material
Acknowledgements:
We thank Karen Klein, MA, ELS (Research Support Core, WFUHS) for her editorial contributions to this manuscript.
Funding: Supported by NINDS (R01NS058949) and the CTSI Translational Imaging Program of Wake Forest School of Medicine.
• Dr. Haq has salary support from NINDS R01NS058949 (this project), NINDS 1U24NS107197–01, and NIAA: P30 AG049638. He has performed research for Allergan, Boston Scientific, Great Lakes Neurotechnology, Pfizer, and has consulted for Boston Scientific and Medtronics. His potential conflicts of interest are managed by Wake Forest School of Medicine.
• Dr. Snively receives salary support from NINDS R01NS058949
• Dr. Sweadner receives salary support from NINDS R01NS058949
• Ms. Suerken receives salary support from NINDS R01NS058949
• Mr. Cook receives salary support from NINDS R01NS05849
• Dr. Ozelius receives salary support from NINDS R01NS058949
• Ms. Miller receives salary support from NINDS R01NS058949
• Dr. McCall performs research for Merck and MECTA and consults for Sage Therapeutics and Anthem insurance company. He receives royalties from Wolters Kluwer Publishing.
• Dr. Whitlow has salary support from NINDS R01NS058949 (this project), NINDS R01NS091602, NINDS R01NS082453, NIMH 1R01MH116675, NIA R01AG058829, NIA R01AG055122, NIA R01AG054491, NIA R01AG040282, NIA P30AG049638, NCI P01CA207206, NIAAA P50AA026117, NCATS UL1TR001420, as well as the NCAA-DoD Alliance: Concussion Assessment, Research and Education (CARE) Consortium.
Footnotes
Disclosures:
• Dr. Brashear has salary support from NINDS R01NS058949 (this project), performs research for Revance and consults for Revance and Ipsen. Her conflicts of interest are being managed by Wake Forest School of Medicine.
References
- 1.Dobyns WB, Ozelius LJ, Kramer PL, et al. Rapid-onset dystonia-parkinsonism. Neurology 1993;43(12):2596–2602. [DOI] [PubMed] [Google Scholar]
- 2.Brashear A, Dobyns WB, de Carvalho Aguiar P, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain 2007;130(Pt 3):828–835. [DOI] [PubMed] [Google Scholar]
- 3.Pittock SJ, Joyce C, O’Keane V, et al. Rapid-onset dystonia-parkinsonism: a clinical and genetic analysis of a new kindred. Neurology 2000;55(7):991–995. [DOI] [PubMed] [Google Scholar]
- 4.de Carvalho Aguiar P, Sweadner KJ, Penniston JT, et al. Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 2004;43(2):169–175. [DOI] [PubMed] [Google Scholar]
- 5.Bottger P, Tracz Z, Heuck A, Nissen P, Romero-Ramos M, Lykke-Hartmann K. Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J Comp Neurol 2011;519(2):376–404. [DOI] [PubMed] [Google Scholar]
- 6.McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci 1991;11(2):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brashear A, Farlow MR, Butler IJ, Kasarskis EJ, Dobyns WB. Variable phenotype of rapid-onset dystonia-parkinsonism. Mov Disord 1996;11(2):151–156. [DOI] [PubMed] [Google Scholar]
- 8.Kamphuis DJ, Koelman H, Lees AJ, Tijssen MA. Sporadic rapid-onset dystonia-parkinsonism presenting as Parkinson’s disease. Mov Disord 2006;21(1):118–119. [DOI] [PubMed] [Google Scholar]
- 9.McKeon A, Ozelius LJ, Hardiman O, Greenway MJ, Pittock SJ. Heterogeneity of presentation and outcome in the Irish rapid-onset dystonia-parkinsonism kindred. Mov Disord 2007;22(9):1325–1327. [DOI] [PubMed] [Google Scholar]
- 10.Heinzen EL, Swoboda KJ, Hitomi Y, et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. NatGenet 2012;44(9):1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosewich H, Thiele H, Ohlenbusch A, et al. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol 2012;11(9):764–773. [DOI] [PubMed] [Google Scholar]
- 12.Demos MK, van Karnebeek CD, Ross CJ, et al. A novel recurrent mutation in ATP1A3 causes CAPOS syndrome. Orphanet J Rare Dis 2014;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano ST, Silver K, Young R, et al. Fever-Induced Paroxysmal Weakness and Encephalopathy, a New Phenotype of ATP1A3 Mutation. Pediatr Neurol 2017;73:101–105. [DOI] [PubMed] [Google Scholar]
- 14.Dard R, Mignot C, Durr A, et al. Relapsing encephalopathy with cerebellar ataxia related to an ATP1A3 mutation. Dev Med Child Neurol 2015;57(12):1183–1186. [DOI] [PubMed] [Google Scholar]
- 15.Rosewich H, Sweney MT, DeBrosse S, et al. Research conference summary from the 2014 International Task Force on ATP1A3-Related Disorders. Neurol Genet 2017;3(2):e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Rating scales for dystonia: a multicenter assessment. Mov Disord 2003;18(3):303–312. [DOI] [PubMed] [Google Scholar]
- 17.Goetz CG, Stebbins GT. Assuring interrater reliability for the UPDRS motor section: utility of the UPDRS teaching tape. Mov Disord 2004;19(12):1453–1456. [DOI] [PubMed] [Google Scholar]
- 18.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 1997;145(2):205–211. [DOI] [PubMed] [Google Scholar]
- 19.Washington DC- Adjutant General’s Office WD. Army Individual Test Battery. Manual of directions and scoring1944
- 20.Spreen O, Strauss E. A Compendium of Neuropsychological Tests New York: Oxford University Press, 1998. [Google Scholar]
- 21.Haskett R The role of antidepressant medication during ECT:new findings from OPT-ECT. J ECT 2007;23:56–56. [Google Scholar]
- 22.Guy W 048 HAMA Hamilton Anxiety Scale, ECDEU Assessment Manual1976 1976.
- 23.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. ArchGenPsychiatry 1989;46(11):1006–1011. [DOI] [PubMed] [Google Scholar]
- 24.Wittchen HU. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res 1994;28(1):57–84. [DOI] [PubMed] [Google Scholar]
- 25.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders- Patient Edition (SCID-I/P, 1½002 revsion) 2002.
- 26.Hien D, Matzner F, First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-child edition (version 1.0): Columbia University, New York, 1994. [Google Scholar]
- 27.Sweney MT, Newcomb TM, Swoboda KJ. The expanding spectrum of neurological phenotypes in children with ATP1A3 mutations, Alternating Hemiplegia of Childhood, Rapid-onset Dystonia-Parkinsonism, CAPOS and beyond. Pediatr Neurol 2015;52(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook JF, Hill DF, Snively BM, et al. Cognitive impairment in rapid-onset dystonia-parkinsonism. Mov Disord 2014. [DOI] [PMC free article] [PubMed]
- 29.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults: Psychological Assessment Resources, Inc., 2004. [Google Scholar]
- 30.Brashear A, Cook JF, Hill DF, et al. Psychiatric disorders in rapid-onset dystonia-parkinsonism. Neurology 2012;79(11):1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panagiotakaki E, De Grandis E, Stagnaro M, et al. Clinical profile of patients with ATP1A3 mutations in Alternating Hemiplegia of Childhood—a study of 155 patients. Orphanet Journal of Rare Diseases 2015;10(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roubergue A, Roze E, Vuillaumier-Barrot S, et al. The multiple faces of the ATP1A3-related dystonic movement disorder. Mov Disord 2013;28(10):1457–1459. [DOI] [PubMed] [Google Scholar]
- 33.Brücke C, Horn A, Huppke P, Kupsch A, Schneider G-H, Kühn AA. Failure of Pallidal Deep Brain Stimulation in a Case of Rapid-Onset Dystonia Parkinsonism (DYT12). Movement disorders clinical practice 2015;2(1):76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamm C, Fogel W, Wachter T, et al. Novel ATP1A3 mutation in a sporadic RDP patient with minimal benefit from deep brain stimulation. Neurology 2008;70(16 Pt 2):1501–1503. [DOI] [PubMed] [Google Scholar]
- 35.Deutschlander A, Asmus F, Gasser T, Steude U, Botzel K. Sporadic rapid-onset dystonia-parkinsonism syndrome: failure of bilateral pallidal stimulation. Mov Disord 2005;20(2):254–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.