Abstract
While current effective therapies are available for the symptomatic control of Parkinsońs disease (PD), treatments to halt the progressive neurodegeneration still do not exist. Loss of dopamine neurons in the substantia nigra pars compacta and dopamine terminals in the striatum drive the motor features of PD. Multiple lines of research point to several pathways which may contribute to the dopaminergic neurodegeneration. These pathways include extensive axonal arborization, mitochondrial dysfunction, dopamine’s biochemical properties, abnormal protein accumulation of α-synuclein, defective autophagy and lysosomal degradation, and synaptic impairment. Thus, understanding the essential features and mechanisms of dopaminergic neuronal vulnerability is a major scientific challenge and highlights an outstanding need for fostering effective therapies against neurodegeneration in PD. This article, which arose from the Movement Disorders 2018 Conference, discusses and reviews the possible mechanisms underlying neuronal vulnerability and potential therapeutic approaches in PD.
Introduction
The cardinal features of Parkinson disease (PD) were elegantly described some 200 years ago by James Parkinson and Jean-Martin Charcot. The second half of the 20th century produced remarkable advances in defining neurodegeneration of the nigrostriatal dopaminergic projection as the primary basis for PD, and major therapeutic developments such as levodopa and deep brain stimulation (1). Thus, clinical management of PD has improved substantially, diagnosis of genetic forms is readily available, patient quality of life continues to improve, and life expectancy is approaching that of the normal population. Furthermore, the clinical spectrum has expanded enormously with the recognition of a plethora of non-motor manifestations, particularly hyposmia, sleep disturbances, autonomic dysfunction, constipation, depression, and cognitive impairment. However, despite these major advances, there is still no treatment to stop progression of the disease, minimal therapeutic strategies exist to alleviate the nonmotor symptoms, and the underlying causes of PD are still unclear. This has produced a new clinical situation and phenotype of patients in whom the long-term evolution of neurodegeneration in PD interacts with aging and comorbidity. Accordingly, efforts to understand the mechanisms underlying neuronal loss and to halt disease progression are currently considered one of the most important unresolved issues in PD. This is particularly relevant regarding Lewy pathology (2), as major efforts are now underway to develop treatments that target abnormal aggregation of α-synuclein (α-syn) and other proteins. It is well known that α-syn aggregates are present in selected regions in the central and peripheral nervous system, suggesting that some neurons may be more susceptible to forming pathological inclusions. Examples of regions that harbor Lewy pathology include the olfactory bulb, retina, salivary gland, enteric nervous system, sympathetic ganglia, nerves of the cardiac conduction system, dorsal motor nucleus of the vagus, spinal cord, brainstem (including the substantia nigra pars compacta [SNc] and locus coeruleus), hypothalamus, hippocampus, and cortex (3–9). Why some neuron populations are more susceptible to forming Lewy pathology and the impact of these inclusions on neuronal function is thus an important area of current research (10, 11) .
Consequently, an outstanding question that remains to be answered is what makes particular subpopulations of neurons especially vulnerable in PD. An approximate 70% loss of dopamine (DA) neurons in the SNc defines PD, and contributes to the majority of motor features (12). Pigmented neurons are particularly susceptible, especially in the ventral tier of the SNc as assessed in post-mortem brains and recently, with in vivo neuromelanin-sensitve MRI (13). Although less well characterized, there is evidence for neuronal loss in other brain regions (14). A recent thorough review assessed all the published papers in which neuron counts in particular brain regions of PD patients were performed (14). Studies using unbiased, precise stereological counts showed loss of neurons, in addition to the SNc, in the pedunculopontine nucleus, dorsal motor nucleus of the vagus, the amygdala, and the cortex. However, the extent of cell loss in these brain areas (10–40%) was not as robust as in the SNc (70%), nor did it typically occur early in the disease process. There is a substantial decrease in glutamatergic neurons (up to 70%) in the parafascicular and centromedian nuclei of the thalamus, which project to the striatum (15), but the origin and significance of this early cell loss has yet to be clarified. Interestingly, three studies showed an up to 100% increase in DA neurons in the olfactory bulb, which suggests that not all dopaminergic cells are prone to neurodegeneration in the PD brain (16). Although loss of neurons in the locus coeruleus and raphe nucleus have also been reported, studies employing stereological cell counts of these important brain regions in PD are lacking.
In addition, treating disease progression and development of non-motor symptoms in PD and preventing progression from the brainstem to other brain areas is another critical unmet need in the field of PD. Neuronal loss in other non-dopaminergic brain regions conceivably contributes to some of the non-motor features of PD and thus, understanding the mechanisms that contribute to their neuronal dysfunction will be important but has not been elucidated. In addition, while synaptic loss has been appreciated for several years now in Alzheimer disease, it is only recently becoming clear that synaptic loss before neuronal death also occurs in PD.
In this article we focus on ascertaining and discussing mechanisms of neuronal vulnerability of the SNc dopaminergic striatal projection neurons, as this is better known and the primary target of probable therapeutic advances.
Role of axonal arborization
Neurons that are particularly vulnerable in PD including dopaminergic nigrostriatal neurons, cholinergic neurons of the pedunculopontine nucleus, and noradrenergic neurons of the locus coeruleus have long axons that are unmyelinated in the region of arborization. Nigrostriatal neurons exhibit a large synaptic tree (i.e. 1 axon has 2–6 × 105 synapses) which is likely to be required to sustain tonic DA release in the striatum (17, 18). Thus, extensive axonal arborization has been postulated as a key vulnerability factor in PD. The fundamental implication of this hypothesis is that such a highly branched axonal domain imposes extraordinary metabolic costs on these cells and places them close to their maximum capacity to manage elevated energetic demands, oxidant stress, protein delivery, and proteasomal systems (19–21). In the presence of the risk factors associated with PD, the subsets of neurons that express these characteristics will therefore begin to experience cellular dysfunction before other neuronal populations and ultimately lead to degeneration.
In a paper published in 2006, Parent and Parent raised the hypothesis that the highly collateralized nature of DA neurons, which they studied using single-neuron juxtacellular labelling (22–25), may underscore their risk to neurodegenerative, metabolic, or neurotoxic insults (26). Based on the increased vulnerability of DA neurons of the ventral tier of the SNc (projecting mostly to sensory-motor territories of the dorsal striatum), they further hypothesized that these neurons may have more extensive axonal arborization and be most energetically compromised compared to other DA neurons with a smaller axonal domain, such as those of the ventral tegmental area (VTA). This was subsequently expanded by Bolam and Pisaddaki (27) who estimated, based on the density of dopaminergic axon terminals in the caudate and putamen and the known number of DA neurons, that human SNc DA neurons may have over 1.5 million axon terminals apiece. Based on work in other systems (28), it is likely that a majority of these contain active mitochondria and thus locally generate ATP and reactive oxygen species.
1). Axonal arborization and neuronal susceptibility: Can it be validated?
Although simple and heuristically attractive, this hypothesis is not straightforward to test. We are limited in both our ability to measure multiple variables (the different physiological characteristics believed to underpin selective vulnerability) and to do this in experimental paradigms that are faithful and pertinent to the human pathological processes we are trying to understand.
First, comparative data are needed from a range of “vulnerable” and less vulnerable neurons in terms of their axonal complexity, number of axon terminals, and global size. Although tracing studies using juxtacellular labelling of single neurons or small population of neurons with dextran dyes or genetically-encoded virally-delivered reporter proteins have separately described the axonal arborization of subsets of SNc (Prensa and Parent, 2001; Matsuda et al., 2009) and VTA (29, 30) DA neurons, no direct comparative studies had been published until recently. Using an in vitro mouse DA neuron culture system, the axonal arborizations of SNc, VTA, and olfactory bulb DA neurons were compared (31). In this reduced system of DA neurons growing on supportive astrocytes, SNc DA neurons were found to have a two-fold larger axonal arborization compared to VTA DA neurons, and olfactory bulb DA neurons have a much smaller axonal arborization. Furthermore, it was recently shown using a virally-encoded tracer in the intact mouse brain that SNc DA neurons have a three-fold larger axonal arborization length compared to VTA DA neurons (14) Moving forward, similar and thorough comparative data need to be acquired for other potentially vulnerable neurons in PD for which only partial data presently exist (32–35).
Second, to further validate this hypothesis of a causative relationship between axonal arbor size and vulnerability, it is essential to obtain comparative data on the bioenergetic properties of highly branched neurons relative to neurons with smaller axonal arborizaton. Prior work has established that synaptic transmission is a key determinant of cellular energy use (36, 37), such that neurons with multiple active axon terminals along a highly branched axonal arborization would be predicted to have large energy requirements. Furthermore, it has been postulated that the energetic cost of action potential propagation may not increase in a purely linear fashion, but as a function of axonal arbor complexity(38).
ii). Impact of extensive axonal arborization on mitochondrial oxidative phosphorylation
Due to an absence of techniques, the measurement of mitochondrial oxidative phosphorylation (OXPHOS) in neuronal subpopulations in complex brain tissue is extremely challenging. However, data from in vitro studies support the hyperbranching hypothesis, including work that evaluated rates of oxygen consumption to estimate mitochondrial OXPHOS in FACS-purified primary mouse DA neurons (Pacelli et al., 2015) (31). These data revealed that the most arborized SNc DA neurons were also the ones with most active mitochondria compared to VTA DA neurons. Moreover, SNc DA neurons also demonstrated the highest rates of basal superoxide production, which supports the notion that elevated OXPHOS might be the origin of chronically elevated oxidant stress in these neurons(31). Interestingly, this study showed that the basal rate of OXPHOS in SNc DA neurons is relatively close to the maximal rate that can be sustained by the mitochondrial network of these cells, a situation that is likely to place them at risk in situations of cellular stress and perhaps lead to gradually impaired proteostasis and accumulation of pathological protein aggregates. The same study also showed that reducing the axonal arbor size of SNc DA neurons by exposing the neurons to the axonal guidance factor semaphorin 7A led to a subsequent decrease in OXPHOS and superoxide production, and in turn, an increase in resilience. Additional studies in support of the role of arborization and high energy consumption as a key vulnerability factor include two recent studies which showed that in DA neurons, the overexpression of SIRT3, a mitochondrial deacetylase well established to promote cellular resilience, leads to reduced basal rates of OXPHOS (39), increased antioxidant defense (40), and increased resilience. Compatible with the theory that elevated basal bioenergetics and oxidant stress in SNc DA neurons are key determinants of their vulnerability in response to cellular stressors of mitochondrial origin known to lead to PD, a recent study showed that deletion of the mitochondrial E3 ubiquitin ligase parkin leads to preferential loss of the most arborized SNc DA neurons (Giguère et al., 2018b). This suggests that mitochondrial dysfunction induced by autosomal recessive mutations in multiple mitochondrial-associated genes including Parkin, but also the mitochondrially-localized kinase PINK1, and DJ-1 all lead to forms of cellular stress that have more impact on SNc DA neurons compared to less arborized, more resilient neuronal populations.
iii). Therapeutic Outlook: Mitochondrial function and axonal arborization
It can be envisaged that different stressors involved in PD could preferentially drive the highly branched populations of dopaminergic SNc neurons beyond physiological capacity to cope with the associated high degree of mitochondrial oxidative phosphorylation. However, there has been no specific therapeutic approach based on this concept. Studies with antioxidants, MAO-B-inhibitors, creatinine, vitamins, or coenzyme Q10, to name a few, to reduce oxidative stress or enhance the supply of energy-rich substances have been carried out in de novo PD and in more advanced stages of PD without any net symptomatic or disease modifying benefit. Thus, other mechanisms must be investigated, such as toxicity caused by modifications to DA or impaired proteostasis pathways in neurons with long axons and extensive arborizations (41).
Role of biochemical phenotypes: catecholaminergic neurons and oxidized DA
Dopamine is oxidized, producing reactive oxygen species that contribute to neuronal toxicity. Rapid oxidation of DA into toxic quinones in dopaminergic neurons has also been proposed to play a contributing role in their degeneration in PD. However, this has not been systematically examined in human neurons until recently, thus allowing for an analysis of the role of oxidized DA in both familial and sporadic PD patient-derived neurons. Importantly, as the majority of PD mouse models demonstrate negligible nigral degeneration or behavioral phenotypes (42), patient-derived neurons may provide additional insight into PD pathogenesis. Multiple lines of evidence now implicate a key role for DA oxidation in driving selective vulnerability in patient neurons (Figure 1).
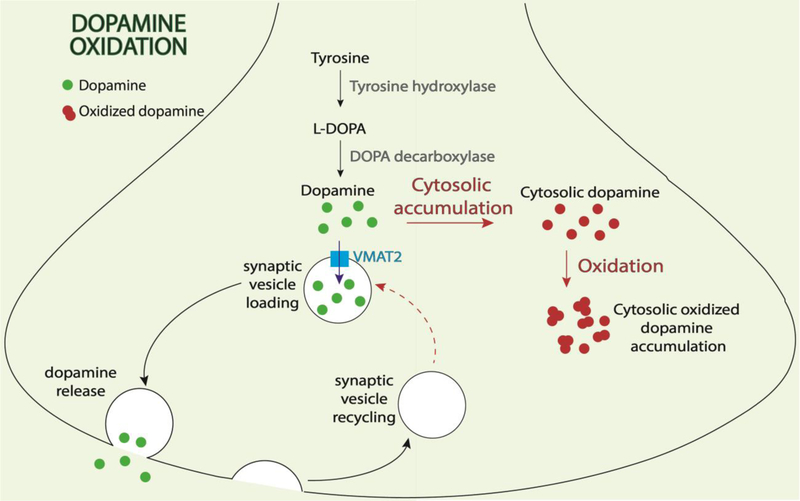
Figure 1. Role of oxidized dopamine in dopaminergic neuronal selective vulnerability.
Dopamine is normally generated from tyrosine and L-DOPA. It subsequently becomes incorporated into synaptic vesicles via VMAT2 and is finally released upon synaptic vesicle exocytosis. In PD dopaminergic patient neurons: 1) increased oxidation of cytosolic dopamine leads to generation of oxidized dopamine which can contribute to downstream mitochondrial and lysosomal dysfunction, increased α-synuclein oligomerization, and decreased lysosomal GBA1 enzyme activity; and 2) defective synaptic vesicle endocytosis contributes to inefficient synaptic vesicle recycling and increased cytosolic dopamine available for oxidation.
Using DA storage vesicles isolated from the striatum of PD patients and controls, it has been shown that the uptake of DA by VMAT2 is reduced in PD. Cytosolic DA is responsible for generation of damaging reactive oxygen species that contribute to degeneration of DA neurons (43). The first line of evidence comes from recent measurements of oxidized DA species in human neurons using near infrared fluorescence (nIRF) (44), which measures the fluorescent properties of o-quinones in both cells and tissues. Oxidized DA levels dramatically increase in a time-dependent manner in human iPSC (induced pluripotent stem cell)-derived dopaminergic neurons from both idiopathic patients and familial PD patients harboring Parkin, PINK1, DJ-1 loss of function mutations, or wild-type α-syn triplication mutations, as compared to control neurons (45). In contrast to human patient neurons, PD mouse models such as DJ-1 KO mice show negligible amounts of oxidized DA (45), consistent with the lack of nigral degeneration or behavioral phenotypes in the majority of PD mouse models (42). One factor potentially contributing to this species-specific difference is elevated levels of DA in control human neurons compared to mouse neurons (45). Supporting this, increasing DA synthesis in wild-type or DJ-1 KO mice with levodopa, the synthetic precursor of DA, is sufficient to induce oxidized DA accumulation in the SNc but not in other brain regions (45).
The second line of evidence arises from oxidized DA’s ability to mediate a pathogenic cascade involving mitochondrial and lysosomal dysfunction in neurons derived from PD. Elevated mitochondrial oxidant stress in human dopaminergic neurons drives a DA-dependent sequence of events that lead to lysosomal dysfunction and α-syn accumulation (45). Consistent with the species-specific oxidized DA increase found in human PD neurons, this toxic cascade was only observed in mouse dopaminergic neurons upon treatment with levodopa to induce oxidized DA accumulation. Oxidized DA’s ability to disrupt lysosomal dysfunction was further exacerbated by the fact that DA quinones directly modify cysteine residues in the catalytic site of the lysosomal enzyme GCase (GBA1) (45), the most common genetic risk factor for PD (46), resulting in decreased GCase enzymatic activity.
Moreover, oxidized DA also modifies α-syn to promote its aggregation (47–50). In particular, increased levels of cytosolic DA are toxic to DA neurons. Furthermore, deletion of α-syn protects DA neurons from L-DOPA-induced neurotoxicity, which support a toxic interaction between cytosolic DA and α-syn (51). Aggregation of α-syn caused by cytosolic, oxidized DA can be ameliorated in patient neurons by treatment with α-methyl-p-tyrosine (AMPT), which competitively inhibits tyrosine hydroxylase and prevents DA synthesis (45). Thus, as increased α-syn levels result in decreased GCase activity (52, 53), oxidized DA may further accelerate this bidirectional relationship in dopaminergic neurons leading to their selective vulnerability.
In addition, DA oxidation also contributes to mitochondrial dysfunction (45), resulting in a vicious cycle between DA and mitochondrial oxidation which may further contribute to lysosomal dysfunction in patient neurons. Surprisingly, early treatment with mitochondrial antioxidants in long-term midbrain cultures is sufficient to prevent these downstream toxic phenotypes (45), which suggests that early therapeutic interventions targeting oxidative stress may be viable for treating the pathogenesis of PD.
The third line of evidence stems from oxidized DA’s relationship with synaptic dysfunction. Recently, loss-of-function mutations in auxilin (DNAJC6) and synaptojanin-1 (SYNJ-1), which regulate clathrin-dependent synaptic vesicle endocytosis, have been associated with juvenile and early-onset atypical parkinsonism (54–58), further highlighting the role of synaptic dysfunction in this disease. At the nerve terminal, cytosolic DA must be rapidly packaged into newly formed synaptic vesicles to prevent its oxidization. Consequently, inefficient synaptic vesicle endocytosis and defective DA importing into vesicles may accelerate cytosolic oxidized DA accumulation and its downstream toxicity as discussed above (59–64). Indeed, loss of endogenous auxilin in human dopaminergic neurons is sufficient to induce oxidized DA accumulation, further supporting an important link between DA metabolism and synaptic function (65).
Role of selective vulnerability to α-syn
i). Local and global factors that influence vulnerability to Lewy pathology
In synucleinopathies, Lewy bodies (LBs) and Lewy Neurites (LNs) are distributed across multiple regions within the central and peripheral nervous systems with pathology present in both motor and non-motor systems (3, 8). Depending on disease stage, affected neurons can encompass several neurotransmitter systems, including catecholaminergic, glutamatergic, serotonergic, and cholinergic neurons (3, 66, 67). Lewy pathology is typically present in a subset of neurons within an individual neuroanatomical region, and a considerable proportion of neurons that share these same neurotransmitters do not feature α-syn inclusions over the course of a disease which can last up to decades. These observations further underline the contrasting susceptibilities to α-syn pathology between subpopulations of neurons, suggesting that cell-autonomous factors may further influence such selective vulnerability.
Large-scale genetic studies have identified variations in the α-syn gene (SNCA) as a major contributor to the risk of developing PD (68). Likewise, duplication and triplication of the SNCA gene encoding α-syn leads to early onset PD in which the age of onset inversely correlates with gene dosage (69). In addition to pointing towards a central, and potentially causative, role in PD, these data further imply that the propensity to develop Lewy pathology is dependent on the concentration of α-syn expressed within neurons. This hypothesis has largely been demonstrated in transgenic and viral overexpression PD models. For example, the accumulation of misfolded and post-translationally modified (e.g., Ser129 hyperphosphorylation) α-syn in animal and cell models, as well as their consequent phenotypes, are dependent on the number of alleles or genomes expressed (69–75).
A growing body of evidence also suggests that cells are capable of acquiring pathological α-syn from the extracellular environment which then catalyzes misfolding of the host cell’s α-syn pool into Lewy-like inclusions (76, 77). It follows that this prion-like mechanism, which relies on self-templating of a pathological seed, would proceed more rapidly when higher concentrations of α-syn are present in neurons. Consistent with this logic, the levels of α-syn pathology elicited when neurons are exposed to misfolded α-syn, either derived from synucleinopathy brains or in the form of recombinant α-syn fibrils, correlate strongly with the level of endogenous α-syn of the treated animal or cultured neurons (10, 78).
A similar relationship between α-syn expression and propensity to form Lewy pathology may also be acting at the regional and cellular levels. While a comprehensive survey of the physiological levels of α-syn has yet to be performed in CNS and PNS regions or individual neurons, the distribution pattern of Lewy pathology observed in postmortem synucleinopathy brains offers a number of clues regarding the relationship between the two. For example, in contrast to dopaminergic and noradrenergic neurons in the SNc and LC, respectively, inhibitory neurons are relatively spared from LBs in synucleinopathies (79). In agreement with this, levels of α-syn appear to be lower in GABAergic neurons and interneurons compared to other cells (79–81), whereas catecholaminergic neurons exhibit relatively high α-syn expression levels in both rodents and humans (80). On the other hand, glutamatergic neurons display a wide range of expression levels across different subpopulations. Correlating with their higher α-syn expression levels compared to other neurons containing this neurotransmitter, areas such as cortical layer 5, hippocampus, amygdala, and olfactory bulb are prominent hotspots for pathology, albeit at different stages of disease (67, 82, 83).
Interestingly, intracellular α-syn levels appear to vary considerably, even among glutamatergic excitatory neurons. Bulk RNAseq data suggests that α-syn expression across excitatory hippocampal neurons can differ by several fold and corresponds closely to subtypes belonging to different hippocampal subfields (84). Neurons deriving from the Cornu Ammonis (CA) bearing the master transcription factors Math2 (NEX-1/NeuroD6) or CTIP2 (Bcl11b) show the highest levels as confirmed by immunocytochemistry in cultured neurons (10). In contrast, glutamatergic neurons in the dentate gyrus (DG) containing another marker, Prox1, express markedly lower α-syn concentrations than CA neurons. Upon exposure to recombinant α-syn fibrils, CA neurons develop Lewy-like pathology much more rapidly and extensively than DG neurons, resulting in accelerated cell loss in the former and indicating that α-syn expression correlates tightly with both pathologic burden and degeneration. This pattern also coincides with the observation that CA subfields are more prone to pathology in in PDD and DLB (3, 85–87). In agreement with this, antisense oligonucleotide-mediated knockdown of α-syn reduces both the accumulation of pathology and cell death (88, 89).
Importantly, low α-syn expression levels alone do not confer total immunity to fibril-induced pathology, but rather slow down this process as evidenced by the observation that α-syn inclusions also emerge in DG neurons after targeted injection of fibrils into the hippocampus (10, 90, 91). While this response is muted compared to that seen in neighboring CA neurons, α-syn pathology nonetheless continues to amplify after the process has peaked in the CA, eventually also leading to neuron loss. A similar phenomenon is also observed following fibril injections into the mouse dorsal striatum where inclusions develop within GABAergic medium spiny projection neurons after a months-long delay. During this time pathology has already been established in surrounding afferent (e.g., nigrostriatal, amygdala, and corticostriatal) neurons (91, 92). These observations align with reports that inhibitory neurons and interneurons are also affected by Lewy pathology in PD and DLB, but to a lesser extent.
The increasing availability of single-cell analysis methods should provide additional insights into other cell-autonomous factors that contribute to relative vulnerability in synucleinopathies (93, 94). The most obvious candidates are processes that regulate α-syn levels through production and degradation. Consistent with this, several PD and DLB risk genes have functions that align with one or more of these above categories, for example α-syn expression (SNCA), proteastasis or lysosomal function (DJ-1, GBA, and ATP13A2) and intracellular trafficking (VSP35). Given the emerging realization that α-syn pathology can be transmitted between neurons at or within the vicinity of synapses (78), factors that modulate α-syn trafficking, internalization, or secretion would also be expected to influence vulnerability to developing α-syn pathology. Interestingly, both in vitro and in vivo studies indicate that the trafficking and cell-to-cell transmission of internalized α-syn species does not require α-syn expression in intermediary neurons (95, 96). Thus, overlaying the information gathered at the cellular level with the expanding number of brain connectivity datasets (94), which may be indicate the probable site of α-syn transfer or exposure, will provide a basis for understanding how pathology develops in a temporal-spatial manner.
In the meantime, available clinical-pathological and experimental data point towards two key concepts regarding selective vulnerability in individual neurons. First, while α-syn expression is necessary to support the development of Lewy pathology, intracellular α-syn concentration (and hence its modulators) appear to influence the rate at which it progresses. Furthermore, it is possible that α-syn pathology is present in neuronal populations at levels below the threshold for detection that may or may not emerge depending on disease duration. Second, selective vulnerability cannot be accounted for by α-syn expression levels alone and that these other cell-autonomous and non-autonomous risk factors intersect/interact within individual neurons.
ii). Therapeutic Outlook: Focus on α-syn
There are two dominant concepts currently in clinical trials related to α-syn. One relates to passive immunotherapy in two ongoing clinical Phase II trials by means of intravenous infusion of α-syn antibodies (97). One such antibody was recently demonstrated to be safe and tolerable in a Phase I clinical trial, reported by Prothena Corporation and Roche (PASADENA study) (98). Biogen has initiated another Phase I clinical trial (SPARK study) showing safety and tolerability of another antibody identified in a B-cell screen from healthy individuals (99, 100). AffiRis is also utilizing an active immunotherapy approach by stimulating an immune response to α-syn peptides (101).
The second approach to reducing alpha-synuclein aggregation relies on small molecules which have the property to block aggregation of alpha-synuclein monomers to oligomers. Two compounds are currently under investigation: the compound NPT200–11/UCB0599, from the company UCB, has passed Phase I testing in healthy volunteers, and Phase II in de novo idiopathic PD patients is currently under way. The second compound, ANLE138b (102) also blocks aggregation of alpha-synuclein as well as of tau- and ß-amyloid monomers. Preclinical testing of this compound has been completed, and it is ready to enter Phase I in healthy volunteers and de novo PD patients. Proclara Biosciences also has a compound, NPT088, in a Phase 1b clinical trial for Alzheimer disease, which may also be effective in preventing formation of amyloid aggregates of α-syn. In contrast to the antibodies against α-syn, these compounds are taken orally, easily pass the blood brain barrier, and accumulate in brain tissue in comparison to blood.
In any case, these trials will serve as valid examples for setting the methodology (i.e. minimal requirements) for future trials with putative disease-modifying molecules. This involves defining primary endpoints (motor symptoms) and secondary endpoints, such as non-motor symptoms. Methods to determine target engagement and ability of the compound to reduce pathologic α-syn are necessary Such methods may include: α-synuclein real time quaking-induced conversion [RT-QuIC) (103)] to quantify amounts of α-syn into fibrils in the cerebrospinal fluid, skin biopsy for phospho-alpha-synuclein aggregates in dermal fibers (104), and ultimately PET scans with Fluoro-Desoxy-Glucose (105) or even α-syn aggregate-selective ligands that are currently being developed. Importantly, finding a reliable recruitment assessment approach is necessary, including distinguishing between parkinsonism patients with or without Lewy pathology (106).
Role of proteostasis pathways in neuronal vulnerability
i). Autophagic and lysosomal degradative pathways
Dysfunction of the lysosomal/autophagic pathway has also been strongly implicated both genetically and functionally in PD (107). Indeed, mutations in the lysosomal hydrolase β-glucocerebrosidase (GCase) encoded by GBA1 represent the most common PD risk factor (108), while variants in the LIMP-2 gene (SCARB2) which traffics GCase to lysosomes (109) also associate with increased PD risk (110). This suggests that proper lysosomal trafficking of GCase is an important contributor to neuronal vulnerability in PD. Interestingly, sporadic PD patients also have reduced wild-type GCase activity and accumulation of GCase substrates in blood and postmortem brains (111–113). Thus, the level of GCase activity may be a potential biomarker for PD, and improving lysosomal GCase activity may be an efficacious therapeutic disease target. Of note, as increased α-syn levels and loss of GCase activity are bidirectionally regulated (53), decreased lysosomal GCase activity may be a common mechanism and potential target additionally relevant to other synucleinopathies besides PD, such as Lewy body disease (114, 115). Interestingly, as GBA1 mutations lead to loss of lysosomal ceramide production, increasing ceramide levels via acid ceramidase inhibition is also sufficient to prevent α-syn toxicity in PD patient-derived dopaminergic neurons (116), pointing to lysosomal ceramide levels as a contributing factor in dopaminergic neurodegeneration.
In addition, other endolysosomal genes have also been linked to familial PD, including autosomal dominant mutations in the retromer complex member VPS35 (117–119) and autosomal recessive mutations in the lysosomal cation channel PARK9/ATP13A2 (120). More recently, lysosomal genes CTSB, GALC, TMEM175, and ATP6V0A1 have also been identified as PD risk factors (68). Consistent with a role for lysosomes in PD neurodegeneration, lysosomal proteolysis (45) and lysosomal activity of multiple enzymes including GCase, cathepsin B, β-galactosidase, and hexosaminidase are significantly decreased in PD patient-derived dopaminergic neurons (45, 53, 121).
Damaged organelles and protein aggregates can also be delivered to lysosomes via autophagy, a dynamic process involving formation of autophagosomes which engulf cargo and subsequently fuse with lysosomes for their degradation (41). Multiple steps in the autophagic pathway have also been implicated in PD pathogenesis, including dysfunction in autophagosome formation (122), autophagosome axonal transport (123), and subsequent autophagosome/lysosomal fusion leading to autophagic cargo accumulation (124). Moreover, defects in chaperone-mediated autophagy (CMA), which involves direct translocation of substrates into the lysosome via the lysosomal receptor LAMP2A have also been implicated (125), and DA-modified α-syn is able to block CMA to potentially contribute to selective dopaminergic vulnerability in PD (48). Finally, autophagic cargo such as damaged mitochondria can also be selectively targeted to autophagosomes via mitophagy which involves functional PINK1 and Parkin (126), whose loss of function mutations lead to familial PD (127, 128).
ii). Functional crosstalk between mitochondria and lysosomes
Mitochondrial dysfunction has also long been implicated in PD neuronal vulnerability, with autosomal recessive mutations in mitochondrial-associated genes PINK1 (128), Parkin (127), and DJ-1 (129) resulting in early onset PD. Interestingly, inhibiting mitochondrial fission leads to decreased mitochondrial mass in axon terminals and results in selective degeneration of dopaminergic neurons in the SNc, but not the ventral tegmental area (VTA) which is less affected in PD (130). Thus, properly regulated mitochondrial dynamics may be particularly critical for dopaminergic neuronal viability and homeostasis. Of note, mitochondrial dysfunction may further converge with lysosomal dysfunction to drive PD pathogenesis. Recently, functional convergence of mitochondrial and lysosomal dysfunction in PD was elucidated in PD patient-derived dopaminergic neurons, whereby increased mitochondrial oxidant stress led to lysosomal dysfunction in PD patient-derived dopaminergic neurons and resulted in a pathogenic cascade mediated by DA oxidation (45), as discussed above.
In addition, dynamic inter-organelle contact sites were recently identified between mitochondria and lysosomes (131), demonstrating that these two organelles physically interact with one another independently of mitophagy or lysosomal engulfment of mitochondria to mediate their functional crosstalk. Mitochondria-lysosome contact sites allowed for bidirectional regulation of network dynamics of both organelles (131) whereby lysosomal contacts marked sites of mitochondrial fission allowing regulation of mitochondrial dynamics by lysosomes. Conversely, mitochondrial contacts regulated lysosomal RAB7 GTP state which is a master regulator of lysosomal dynamics (131). Thus, mitochondria–lysosome contact sites may contribute to the dysfunction observed in both organelles in PD, and misregulation of these contacts may exacerbate defective mitochondrial dynamics critical for nigral dopaminergic neuron survival (130).
The Synapse
i). Early Synaptic Defects
Evidence from imaging studies, immunohistochemistry of postmortem brains, and animal models indicate that synapse loss and axonal degeneration occur first in the disease process, possibly years before loss of neurons. It is therefore possible that therapeutic targets could be developed that prevent or rescue synaptic and axonal defects first, before major intractable neuronal death occurs.
ii). Loss of DA terminals precedes loss of soma in the SNc in PD patients
A study by Kordower et al. examined the magnitude of degeneration of nigrostriatal pathway at different stages of disease ranging from 1 to 27 years after diagnosis (132). This was the first study of its kind in that it analyzed the time course of DA terminal and neuronal loss. In years 1–3 after diagnosis there was a 35–75% loss of tyrosine hydroxylase and DA transporter labeled terminals in the putamen. After years 4 and beyond, DA terminal markers were essentially abolished in the putamen. In the SNc, there was a highly variable 50–90% loss of tyrosine hydroxylase-positive neurons at early time points after diagnosis with very little loss at later time points. Thus, remarkably, dopaminergic terminals and fibers are almost completely lost in PD while DA neuron soma are relatively less affected. These data are important because they suggest that the focus of therapies should shift to preserving dopaminergic terminals in the striatum. In addition, these data demonstrate that any trophic or neuro-restorative therapies should be carefully timed to the early stages of PD, when DA terminals still exist. Indeed, the clinical trials of adeno-associated viral administration of neurturin (CERE-10) to enhance DA neuron survival failed to improve motor phenotypes (133, 134). This is likely because the AAV was injected into the striatum at a time point when the DA terminals were absent, and thus the neurturin ligand could not induce neuronal survival signaling cascades.
Early detection of nigrostriatal terminal loss in people living with PD could help predict patients who might respond best to neuroprotective therapies. A study that included 78 subjects with idiopathic PD and 35 controls performed PET using ligands to estimate the densities of the vesicular monoamine transporter type 2 (VMAT), DA transporter, and the uptake of L-DOPA (135). At the initiation of motor features, there was a 58.9% loss of VMAT binding, 62.7% decline in DAT binding, and a 40% decline in L-DOPA uptake, compared with control subjects (136). The study also showed that there was a threshold for VMAT and DAT loss in the striatum before the appearance of motor signs; clinically noticeable manifestations developed only when there was >51% loss of VMAT ligand and >56% of loss DAT ligand. Thus, these data suggest a window of DA terminal loss before motor defects appear and a time period in which neuroprotective therapies may be most effective.
iii). Loss of DA terminals precede loss of soma in the SNc in rodent models of PD
Animal models of PD support findings in human brains that axonal abnormalities precede neuron death. In mice with AAV-mediated α-syn overexpression in the SNc, as early as 3 weeks after expression of AAV-α-syn there is a significant loss of DAT and VMAT position terminals, a significant reduction in DA reuptake rates, and evoked DA release in the striatum (137, 138). Dopamine neurons in the SNc only begin to show significant loss 5 weeks after AAV-α-syn injection. Titration of α-syn levels to induce loss of DA terminals in the striatum without neuronal loss in the nigra produces progressive motor impairments, suggesting that striatal terminal loss and not overt neuronal death is the major contributor to motor defects.
Pathologic α-syn causes axonal transport defects (123, 139), suggesting this may be a mechanism contributing to axonopathy. In rodent models, AAV-induced expression of A53T- or A30P-mutant forms of α-syn cause a reduction in the microtubule motor proteins kinesin and dynein in the striatum (139, 140). In contrast, levels of kinesin and dynein increase in the SNc, suggesting impaired transport from the soma to terminals. In primary neurons, exposure to fibrils causes formation of α-syn inclusions that biochemically and morphologically resemble LNs (141). These inclusions in the axon selectively block transport of endosomes and autophagosomes but do not impair transport of mitochondria or other organelles (123). Retrograde transport of endosomes is critical for delivery of signaling molecules to the soma where they activate transcription factors important for survival and neurite outgrowth. Impaired delivery of these signaling endosomes could thus further contribute to axonal degeneration.
Defects at the presynaptic terminal could put DA neurons preferentially at risk compared to other neuronal subtypes. First, damaged presynaptic proteins must travel retrogradely along the axon for degradation in lysosomes located in the cell body. The extensive arborization of DA neurons in the striatum, [one neuron is estimated to give rise to 245,000 release sites (27)] may reduce the efficiency by which damaged synaptic proteins are cleared from the cell. In addition, mitochondria are particularly abundant in the presynaptic terminal, and thus this compartment may be particularly sensitive to mitochondrial damage caused by oxidized DA at the presynaptic terminal (discussed above). Mitochondrial oxidative damage may also disrupt the function of autophagosomes located in the presynaptic terminal (142) as well as other organelles via membrane contact sites (143). Impaired autophagosomes may result in further buildup of damaged, toxic proteins in the DA neuron. Finally, α-synuclein is enriched in the presynaptic terminal (144) where oxidized DA enhances pathologic aggregation of the protein (145). Thus, DA terminals may be more susceptible to oxidized DA-induced α-synuclein aggregation and consequent dysfunction at the presynaptic terminal.
iv). Therapeutic Outlook- Axonal/Synaptic restoration
With respect to the “axonal dying back” hypothesis, the possibility of providing trophic support to axons at risk or in the process of degenerating by means of delivering trophic factors, either by focal intracranial application, by viral vector expression, by orally administered small molecules, or more recently by focal opening of the blood brain barrier (146), has emerged as a therapeutic principle. Although it does not address the underlying causes of PD, it offers a restorative component in PD therapy. Promoting trophic factors may be fundamentally important, as trophic factors are involved in the guidance and maintenance of arborization of the nigrostriatal neurons. This area reached clinical trials about 15 years ago with variable results and expectations ranging from high hopes to the idea of abandoning this approach (134). Recently, two publications reported that bilateral intraputaminal administration of glial cell line derived trophic factor (GDNF) resulted in a reduction in OFF time after 40- weeks. Also, F-L-Dopa PET imaging revealed a significant difference in tracer uptake in favor of the GDNF-based therapeutics. However, the study formally failed to meet its primary clinical endpoint in intermediate stage PD compared to placebo (133).
Of note, the preclinical studies that showed trophic factor protection of DA neurons were based on toxin models of PD and did not account for Lewy pathology. In the AAV-α-model, when α-syn is overexpressed in the SNc, GDNF does not protect against DA neuron loss. Aggregates of α-syn impair axonal transport of neurotrophic receptors to the soma where signaling cascades important to neuronal survival are activated (123). Interestingly, overexpression of Nurr1, a transcription factor activated by GDNF involved in DA synthesis, is protective in synucleinopathy models (147). Thus, treatments that directly activate Nurr1 in the nucleus may be a promising target.
Genes implicated in PD that play a role at the presynaptic terminal
i). α-Synuclein and pre-Synaptic dysfunction.
Dominantly inherited mutations in SCNA/PARK4, which encodes α-syn, the major component in LBs, was the first identified genetic cause for PD. Normally, the majority of α-syn concentrates at presynaptic terminals where it associates with synaptic vesicles (144, 148). Anatomical localization in the rodent brain shows differential α-syn expression in neuronal sub-types; α-syn is expressed in DA neurons of the SNc and VTA, and excitatory glutamatergic neurons throughout the brain, while expression in inhibitory neurons is brain-region dependent (80). Studies utilizing mice in which the α-syn gene has been knocked out, show that α-syn plays a role in DA release in the striatum and plays a role in tethering synaptic vesicles to the active zone (49, 144, 149). In addition, α-syn normally interacts with VAMP2 at the presynaptic terminal, which plays a critical role in synaptic vesicle endo/exocytosis (150).
In disease, pathological staging studies of PD brains from early to late disease show that axonal LNs form first before somal LBs (151). In dementia with LBs, another synucleinopathy with many features similar to PD, cortical presynaptic aggregates of α-syn are highly abundant (152). These presynaptic aggregates correspond to reduced dendritic spine density, suggesting that they impact synaptic function. Dendritic spine density is also reduced in rodent models of α-syn aggregation (153). Indeed, fibril-induced formation of α-syn aggregates causes major reduction in mushroom spine density at a timepoint preceding neuronal death (11). Exposure of neurons from α-syn knockout mice to fibrils does not affect spine density, demonstrating that corruption of endogenous α-syn causes synaptic structural defects. Thus, there is accumulating evidence that reduced spine density and perturbations in synapse function are pathologic features of synucleinopathies, including PD.
Early formation of fibril-induced α-syn aggregates increases the frequency of miniature excitatory postsynaptic currents (mEPSCs) and presynaptic docked vesicles (11). Enhanced frequency of mEPSCs reflects increased number or release probability of presynaptic vesicles. Thus, paradoxically, although dendritic spine density is reduced, presynaptic function is initially enhanced at these early time points. This phenotype is similar to what has been described in α-syn knockout mice, which show enhanced DA transmission and increased tethering of synaptic vesicles at the active zone (49). Thus, these findings suggest that initial formation of α-syn aggregates promotes a loss of function phenotype.
In addition, a DA/α-syn interaction also seems to play an important detrimental role in early synaptic dysfunction leading to neurodegeneration. High levels of DA cause oligomerization of α-syn (145). In addition, mutation of the DA-interacting site in α-syn prevents neurotoxicity. Thus, future studies to understand the role of oxidized DA, α-syn aggregation and a high burden on mitochondrial function at the presynaptic terminal will be critical for understanding nigrostriatal DA terminal degeneration.
ii). Dominantly inherited mutations in Leucine rich-repeat kinase 2 may also impact presynaptic function.
Mutations in leucine rich-repeat kinase 2 (LRRK2) are the most common cause of familial PD. Most PD-associated LRRK2 mutations increase its kinase activity. Consequently, LRRK2 kinase inhibitors are currently in clinical development for treatment of PD. LRRK2 contains multiple domains in addition to its kinase domain, including a Ras of Complex (Roc) GTPase and a C-terminus of Roc (COR) domain. LRRK2 also contains protein scaffolding domains including N-terminal armadillo, ankyrin, and leucine rich repeat domains and a C-terminal WD40 domain. LRRK2 has been implicated in membrane dynamics in multiple organelles including the trans-Golgi network, endosomes, lysosomes, and autophagosomes. LRRK2 has also been implicated in synaptic vesicle recycling (154). Mutant LRRK2 disrupts synaptic transmission in vivo (155–159). In cultured neurons, expression of the LRRK2 G2019S gain-of-function mutant and treatment with LRRK2 inhibitors both impair synaptic vesicle endocytosis and alter synaptic vesicle pool dynamics (160–163), indicating that LRRK2 kinase activity regulates synaptic function. Expression of PD-associated mutations in LRRK2 cause an initial increase in dopaminergic and glutamatergic activity in the striatum, followed by a decline (164, 165). LRRK2 phosphorylates endophilin (gene name SH3GL2) which is recruited to synaptic vesicles in the process of endocytosis from the plasma membrane and is responsible for vesicle uncoating before the synaptic vesicles undergo another round of membrane docking and release (166, 167). Recently, a select group of Rab GTPases were demonstrated to be bona fide substrates of LRRK2 activity (168). One of these includes Rab3A which also plays a critical role in synaptic vesicle dynamics whose expression is protective in α-syn models of DA neuron toxicity (169).
It is important to note that neither α-syn nor LRRK2 are necessary for presynaptic function, as mice in which either gene has been knocked out are still viable and have no gross neuroanatomical dysfunction. However, perturbations in the function of α-syn or LRRK2 over decades, along with the cumulative effects of aging, oxidative stress, and endosome/lysosomal dysfunction. are likely to compromise terminal function leading to loss of release sites, dying back of axons, and eventual neuron death.
The majority of current experiments and data derive from studies of nigro-striatal projections. In the future, it will be interesting and important clinically and therapeutically to determine if early synaptic dysfunction occurs in the cortex, hippocampus, and other brain regions which may contribute to cognitive impairment and non-motor symptoms in PD. Furthermore, given the particularly high concentration of α-syn at corticostriatal presynaptic terminals (80, 81), it will be interesting to determine how the effects of aggregated a-synuclein on activity of these excitatory neurons contributes to degeneration of SNc-striatal terminals.
iii). Other implicated genes that play a role in synaptic vesicle endo/exocytosis
In addition to the dominantly inherited SNCA and LRRK2 genes, a number of other genes have been linked to PD that are known to act primarily at the synapse; autosomal recessive mutations in PRKN, DNAJC6, and SYNJ1 lead to juvenile onset, atypical forms of PD, and variation at the locus is a PD risk factor (170) (Figure 2). Thus, the synapse is implicated as a key cellular site of disease onset in PD, which makes understanding the etiology of synaptic pathology key to early intervention (171). EndoA is a synaptic protein that facilitates synaptic vesicle recycling following endocytosis (172). Phosphorylation of EndoA by LRRK (the singular isoform for hLRRK½ in Drosophila) modulates synaptic vesicle cycling at the Drosophila NMJ (166). Mice deficient in different combinations of the three mammalian isoforms of EndoA show defects in synaptic transmission and varying degrees of neurodegeneration (173). It is thought that the main function of EndoA at the synapse is to recruit synj1, an inositol phosphatase essential to uncoating and thus recycling of endocytosed clathrin-coated vesicles (173). Auxilin, along with the heat shock protein, Hsc70, subsequently uncoats clathrin-coated vesicles, thus recycling them for another round of fusion and release of neurotransmitters. Knock-outs of synj1, auxilin, or EndoA all show accumulation of clathrin-coated vesicles in presynaptic terminals (173–175). LRRK2 has also recently been shown to phosphorylate auxilin in its clathrin-binding domain, and mutant LRRK2 is able to disrupt auxilin-clathrin interactions in patient-derived dopaminergic neurons contributing to synaptic vesicle defects and accumulation of oxidized DA (65). In addition, as homozygous mutations in synj1 have also been linked to early onset, atypical PD, mouse models carrying this mutation also demonstrate defective synaptic vesicle endocytosis as well as a surprising increase in both auxilin and parkin levels (176), further suggesting that multiple PD genes may converge on defective synaptic vesicle endocytosis (177).
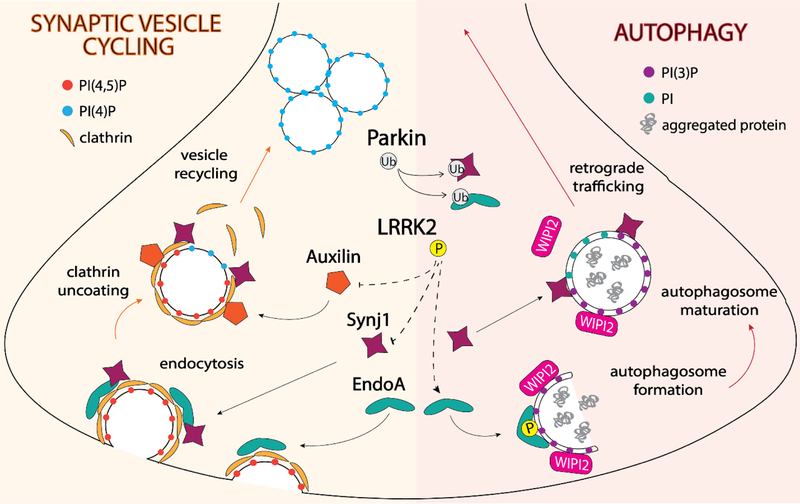
Figure 2. Parkinson’s disease genes function at the intersection of synaptic vesicle endo/exocytosis and autophagy at the synapse.
EndophilinA1 (EndoA) binds endocytosed vesicles and is responsible for recruiting the 5’-phosphatase synaptojanin1 (SynJ1), which together with auxilin, removes the clathrin coat so that vesicles can be recycled. Phosphorylation of auxilin and Synj1 by LRRK2 disrupts interactions with clathrin and EndoA, respectively. LRRK2 phosphorylation promotes the association of EndoA with highly-curved autophagosome membranes, where it recruits PI(3)P-binding proteins involved in autophagosome formation, such as WIPI2. SynJ1 converts PI(3)P to PI on the autophagosome membrane, which allows for dissociation of WIPI2 and maturation of autophagosomes, which are then trafficked back to the cell body for degradation. Parkin is also thought to regulate EndoA and SynJ1 activity through ubiquitination, although the functional consequence is not yet clear.
iv). Synaptic vesicle trafficking and autophagy.
The largely overlapping roles of LRRK2, EndoA, synj1, and auxilin in synaptic vesicle endocytosis implicates this process as a driver of neuronal toxicity in PD. However, PD cannot be entirely explained by defects in synaptic transmission, as loss of only a select set of proteins essential to synaptic function cause neurodegeneration while others do not (178). Interestingly, LRRK2, EndoA, synj1, and auxilin also all play integral roles at the intersection of synaptic vesicle trafficking and autophagy and may be necessary in maintaining proteostasis at the synapse (178–180).
Recently, autophagy has been shown to be highly active at the distal end of the axon where it transports cargo back to the cell body for degradation via the lysosome (142, 181). Aggregated α-syn impairs macroautophagy (124) as does knock-down of α-syn (182), which indicates α-syn may play a direct role in this process. A number of synaptic proteins, including α-syn and LRRK2 contain a KFERQ motif that targets them for degradation through CMA and microautophagy (125, 183). In addition, phosphorylation of EndoA by LRRK induces formation of highly curved membranes that promote autophagosome formation and recruit proteins necessary for autophagosome maturation (184). Moreover, knock-out mice of different EndoA isoforms exhibit dysregulated gene expression of autophagy-specific genes (185). Finally, synj1 has also recently been shown to play a similar role to EndoA in autophagosome formation and maturation at the Drosophila NMJ as well as in PD patient-derived neurons and zebrafish photoreceptors (186, 187). As parkin has also been shown to ubiquitinate both EndoA and synj1, and is elevated in both Endophilin knockout and synj1 mutant mice, these steps in synaptic vesicle trafficking and autophagy may be further regulated by parkin activity (188, 189); however, the precise functional consequence in patient neurons remains unclear.
Together, these recent studies identify the interplay of multiple PD-associated proteins at the synapse and further demonstrate that synaptic vesicle trafficking and autophagy are closely intertwined pathways. Given that multiple PD-associated genes play essential roles in both processes, mechanisms that drive neuronal toxicity in PD may lie at the intersection of these two pathways. Thus, determining which proteins regulate these pathways and what contributes to their age-related decline will be key to identifying novel treatments for early intervention in PD.
v). Therapeutic Outlook: Targeting gene defects
GBA:
Several clinical trials are underway in patients with GBA‐mutation related PD. The first trial tests the chaperone ambroxol (NCT02941822). This compound passes the blood brain barrier and increases GCase activity in brain of healthy non‐human primates. In addition, it reduces the stress of the endoplasmic reticulum due to accumulation of misfolded GCase. The second compound is the glucosylceramide synthetase inhibitor GZ/SAR402671 (NCT02906020), which reduces glycolysation of ceramide and thus increases the level of ceramide (see above – the beneficial role of increased ceramide in α‐syn toxicity). In addition, other angles for targeting GBA include activators which increase GCase enzyme activity (LTI-291) as well as increasing GCase levels using an AAV-based gene therapy approach (PR001). Due to the inverse relationship between the level of α‐syn and GCase activity as discussed above, these trials may also be of relevance to idiopathic PD.
LRRK2:
PD-linked mutations in LRRK2 account for 1% of all PD cases and 4% of familial PD cases. The majority of PD-associated mutations in LRRK2 increase the kinase activity of this protein. Thus, therapeutics that inhibit LRRK2 kinase activity are also in clinical trials for treatment of PD. For the Denali compound DNL201 (NCT03710707), safety and toxicity Phase I studies in healthy volunteers have successfully been completed, and the planning and implementation of a Phase Ib trial in PD patients with and without LRRK2 mutations (idiopathic PD) is underway. As with all clinical trials, biomarkers will be critical to determine LRRK2 kinase inhibitor engagement. Measurements of LRRK2 auto-phosphorylation in cerebrospinal fluid and urine will thus be critical to help guide assessment of LRRK2 inhibitor target engagement.
Conclusions
Aging is the single most important factor determining the risk of developing PD, similar to many other neurodegenerative diseases. There are also important gene/environmental interactions that increase susceptibility to PD. In addition, there are a growing number of factors that reduce susceptibility to PD (such as coffee drinking in some individuals). Importantly, nigrostriatal degeneration which is the driver of the motor features in PD has some specific characteristics that apply to the majority of patients at onset. These include: a) highly asymmetrical and focal onset of motor cardinal features; b) striatal DA loss that is relatively restricted to the caudal (motor) putamen contralateral to the clinically symptomatic side; c) Loss of dopaminergic cells in the SNc relatively limited to the ventro-lateral tier; d) DA putaminal loss exceeds by some 40% cell loss in SNc; and e) DA loss in the striatum and cell loss in the SNc progresses relatively orderly following a caudo-rostral and latero-medial pattern respectively (13).
These features imply that regardless of whether the propagation of toxic species in PD follows an orderly caudo-rostral pattern, the SNc lateral projection to the caudal putamen must have intrinsic anatomo-functional characteristic that make it especially vulnerable. Importantly, PD is not spontaneously present in any other species, and humans exhibit a number of specific motor, behavioral, and cognitive properties and capacities that are probably related to our ability to undergo age-related neurodegeneration (190).
Arguably, the greatest importance for deciphering the mechanisms and factors mediating neurodegeneration lies in the possibility of developing future treatments. Indeed, PD treatments are already evolving from a purely symptomatic fashion to a more disease-modifying approach. Thus, two passive immunotherapy trials with alpha-synuclein antibodies and two trials with compounds targeting PD-associated mutations in GBA will be completed in the next two to three years. Soon after, the results from two trials with a small molecule of the class of α‐syn aggregation blockers and trials with LRRK2-inhibitors will also be available. These studies will provide a wealth of insight into how to conduct disease-modifying trials in de novo sporadic PD, GBA-PD, LRRK2-PD, and AR-PD and in the intermediate time frame in prodromal PD patients.
We believe that the experimental scientific advances summarized in this article and in Figure 3 will continue to guide further clinical therapeutic developments with the aim to slow down or prevent the progression or even motor manifestations of PD. However, this may occur in a fashion similar to the oncology field, involving progressive recognition of etiopathogenic mechanisms and development of treatment combinations. Thus, we anticipate that this will involve PD patients being treated early in the disease process with a combination of both symptomatic and neuroprotective treatments, hopefully in the not too distant future.
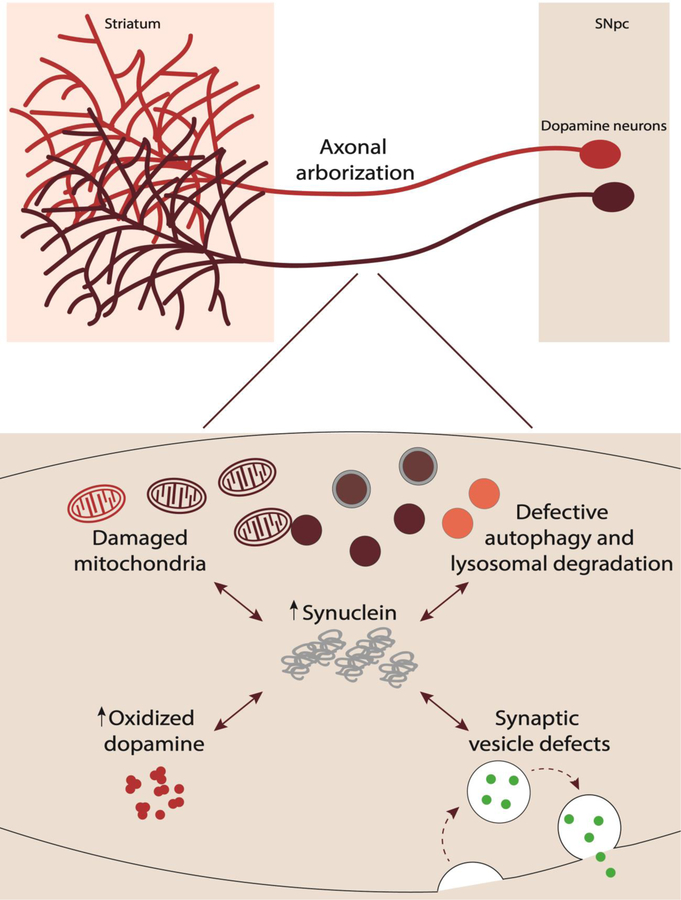
Figure 3. Contributors to neuronal vulnerability in Parkinson’s disease.
Dopaminergic neurons in the SNpc which project to the striatum degenerate in PD. Multiple factors contribute to this neuronal vulnerability including: 1) extensive axonal arborization; 2) mitochondrial dysfunction due to damaged mitochondria: 3) dopamine’s biochemical properties including increased oxidized dopamine: 4) abnormal protein accumulation of α-synuclein: 5) defective autophagy and lysosomal degradation: and 6) synaptic vesicle defects.
Acknowledgments
Financial Disclosures in Previous 12 Months:
Y.W. NIH: K99NS109252
K.L. NIH/NINDS: R01NS088322
S.B.N. Parkinson Canada graduate fellowship
L.-E.T. Canadian Institutes of Health Research, the Brain Canada and Krembli Foundations, and Parkinson Canada.
Z.Y. NIH/NINDS: P50NS0947331 and R01NS060123
D.K. NIH/NIA: R01NS076054 and NIH/NINDS: R01NS096240
W.O. Charitable Hertie Foundation, ParkinsonFonds Deutschland
J.O. Fundación BBVA; Obra Social Fundación “La Caixa” ); Plan Nacional: Ministerio de Economía y Competitividad: SAF2015–67239-P Ciberned
L.V.-D. NIH/NINDS: R01NS102257, P50 NS108675, Michael J. Fox foundation
References
- 1.Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 2017;32(9):1264–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature 1997;388(6645):839–40. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 4.den HJW, Bethlem J. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry 1960;23:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 1988;76(3):217–21. [DOI] [PubMed] [Google Scholar]
- 6.Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, et al. Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology 1999;52(6):1269–71. [DOI] [PubMed] [Google Scholar]
- 7.Stoessl AJ. Salivary gland biopsy for diagnosis of Parkinson’s disease? Lancet Neurol 2016;15(7):654–6. [DOI] [PubMed] [Google Scholar]
- 8.Adler CH, Beach TG. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord 2016;31(8):1114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 2009;15 Suppl 3:S1–5. [DOI] [PubMed] [Google Scholar]
- 10.Luna E, Decker SC, Riddle DM, Caputo A, Zhang B, Cole T, et al. Differential α-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta Neuropathologica 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froula JM, Henderson BW, Gonzalez JC, Vaden JH, McLean JW, Wu Y, et al. alpha-Synuclein fibril-induced paradoxical structural and functional defects in hippocampal neurons. Acta Neuropathol Commun 2018;6(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol 1990;27(4):373–85. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Bastida A, Lao-Kaim NP, Roussakis AA, Searle GE, Xing Y, Gunn RN, et al. Relationship between neuromelanin and dopamine terminals within the Parkinson’s nigrostriatal system. Brain 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giguere N, Burke Nanni S, Trudeau LE. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front Neurol 2018;9:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Ann Neurol 2000;47(3):345–52. [PubMed] [Google Scholar]
- 16.Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord 2004;19(6):687–92. [DOI] [PubMed] [Google Scholar]
- 17.Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci 2000;23(10 Suppl):S48–56. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci 2009;29(2):444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolam JP, Pissadaki EK. Living on the edge with too many mouths to feed: why dopamine neurons die. Movement disorders: official journal of the Movement Disorder Society 2012;27(12):1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misgeld T, Schwarz TL. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 2017;96(3):651–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao J, Bulgari D, Deitcher DL, Levitan ES. Limited distal organelles and synaptic function in extensive monoaminergic innervation. Journal of Cell Science 2017;130(15):2520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauthier J, Parent M, Lévesque M, Parent A. The axonal arborization of single nigrostriatal neurons in rats. Brain Res 1999;834:228–32. [DOI] [PubMed] [Google Scholar]
- 23.Parent A, Sato F, Wu Y, Gauthier J, Lévesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci 2000;23:S20–7. [DOI] [PubMed] [Google Scholar]
- 24.Parent M, Lévesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol 2001;439:162–75. [DOI] [PubMed] [Google Scholar]
- 25.Prensa L, Parent A. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 2001;21(18):7247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parent M, Parent A. Relationship between axonal collateralization and neuronal degeneration in basal ganglia. Journal of neural transmission. Supplementum 2006(70):85–8. [DOI] [PubMed] [Google Scholar]
- 27.Bolam JP, Pissadaki EK. Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord 2012;27(12):1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer TD, Dash PK, Liu J, Waxham MN. Morphology of mitochondria in spatially restricted axons revealed by cryo-electron tomography. PLoS biology 2018;16(9):e2006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aransay A, Rodríguez-López C, García-Amado M, Clascá F, Prensa L. Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Frontiers in Neuroanatomy 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-López C, Clascá F, Prensa L. The Mesoaccumbens Pathway: A Retrograde Labeling and Single-Cell Axon Tracing Analysis in the Mouse. Frontiers in Neuroanatomy 2017;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacelli C, Giguère N, Bourque M-J, Lévesque M, Slack RS, Trudeau L-É. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Current biology: CB 2015;25(18):2349–60. [DOI] [PubMed] [Google Scholar]
- 32.Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. The Journal of Comparative Neurology 1982;207(3):239–54. [DOI] [PubMed] [Google Scholar]
- 33.Baker KG, Halliday GM, Hornung JP, Geffen LB, Cotton RG, Törk I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience 1991;42(3):757–75. [DOI] [PubMed] [Google Scholar]
- 34.Vertes RP, Crane AM. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. The Journal of Comparative Neurology 1997;378(3):411–24. [DOI] [PubMed] [Google Scholar]
- 35.Ros H, Magill PJ, Moss J, Bolam JP, Mena-Segovia J. Distinct types of non-cholinergic pedunculopontine neurons are differentially modulated during global brain states. Neuroscience 2010;170(1):78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron 2012;75(5):762–77. [DOI] [PubMed] [Google Scholar]
- 37.Sobieski C, Fitzpatrick MJ, Mennerick SJ. Differential Presynaptic ATP Supply for Basal and High-Demand Transmission. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 2017;37(7):1888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pissadaki EK, Bolam JP. The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson’s disease. Frontiers in computational neuroscience 2013;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gleave JA, Arathoon LR, Trinh D, Lizal KE, Giguère N, Barber JHM, et al. Sirtuin 3 rescues neurons through the stabilisation of mitochondrial biogenetics in the virally-expressing mutant α-synuclein rat model of parkinsonism. Neurobiology of Disease 2017;106:133–46. [DOI] [PubMed] [Google Scholar]
- 40.Shi H, Deng H-X, Gius D, Schumacker PT, Surmeier DJ, Ma Y-C. Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Human Molecular Genetics 2017;26(10):1915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong YC, Holzbaur EL. Autophagosome dynamics in neurodegeneration at a glance. J Cell Sci 2015;128(7):1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron 2010;66(5):646–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pifl C, Rajput A, Reither H, Blesa J, Cavada C, Obeso JA, et al. Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J Neurosci 2014;34(24):8210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzulli JR, Burbulla LF, Krainc D, Ischiropoulos H. Detection of Free and Protein-Bound ortho-Quinones by Near-Infrared Fluorescence. Anal Chem 2016;88(4):2399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017;357(6357):1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 2009;361(17):1651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 2001;294(5545):1346–9. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest 2008;118(2):777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 2000;25(1):239–52. [DOI] [PubMed] [Google Scholar]
- 50.Wong YC, Krainc D. alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 2017;23(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009;62(2):218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong YC, Krainc D. Lysosomal trafficking defects link Parkinson’s disease with Gaucher’s disease. Mov Disord 2016;31(11):1610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 2011;146(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, et al. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One 2012;7(5):e36458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koroglu C, Baysal L, Cetinkaya M, Karasoy H, Tolun A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat Disord 2013;19(3):320–4. [DOI] [PubMed] [Google Scholar]
- 56.Olgiati S, Quadri M, Fang M, Rood JP, Saute JA, Chien HF, et al. DNAJC6 Mutations Associated With Early-Onset Parkinson’s Disease. Ann Neurol 2016;79(2):244–56. [DOI] [PubMed] [Google Scholar]
- 57.Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, et al. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum Mutat 2013;34(9):1208–15. [DOI] [PubMed] [Google Scholar]
- 58.Olgiati S, De Rosa A, Quadri M, Criscuolo C, Breedveld GJ, Picillo M, et al. PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family. Neurogenetics 2014;15(3):183–8. [DOI] [PubMed] [Google Scholar]
- 59.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 1978;14(4):633–43. [PubMed] [Google Scholar]
- 60.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A 1996;93(5):1956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci U S A 2014;111(27):9977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunn AR, Stout KA, Ozawa M, Lohr KM, Hoffman CA, Bernstein AI, et al. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson disease. Proc Natl Acad Sci U S A 2017;114(11):E2253–E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci 2007;27(30):8138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res 2007;1185:18–32. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen M, Krainc D. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proc Natl Acad Sci U S A 2018;115(21):5576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halliday GM, Del Tredici K, Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. Journal of Neural Transmission. Supplementum 2006(70):99–103. [DOI] [PubMed] [Google Scholar]
- 67.the Arizona Parkinson’s Disease C, Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathologica 2009;117(6):613–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 2017;49(10):1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science (New York, N.Y.) 2003;302(5646):841. [DOI] [PubMed] [Google Scholar]
- 70.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VMY. Neuronal α-Synucleinopathy with Severe Movement Disorder in Mice Expressing A53T Human α-Synuclein. Neuron 2002;34(4):521–33. [DOI] [PubMed] [Google Scholar]
- 71.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science (New York, N.Y.) 2000;287(5456):1265–9. [DOI] [PubMed] [Google Scholar]
- 72.Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, et al. Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proceedings of the National Academy of Sciences 2002;99(13):8968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, et al. Parkinson-Like Neurodegeneration Induced by Targeted Overexpression of α-Synuclein in the Nigrostriatal System. Journal of Neuroscience 2002;22(7):2780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America 2002;99(16):10813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wakamatsu M, Ishii A, Ukai Y, Sakagami J, Iwata S, Ono M, et al. Accumulation of phosphorylated alpha-synuclein in dopaminergic neurons of transgenic mice that express human alpha-synuclein. Journal of neuroscience research 2007;85(8):1819–25. [DOI] [PubMed] [Google Scholar]
- 76.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A 2009;106(47):20051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011;72(1):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological -Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012;338(6109):949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernstein H-G, Johnson M, Perry RH, LeBeau FEN, Dobrowolny H, Bogerts B, et al. Partial loss of parvalbumin-containing hippocampal interneurons in dementia with Lewy bodies. Neuropathology: Official Journal of the Japanese Society of Neuropathology 2011;31(1):1–10. [DOI] [PubMed] [Google Scholar]
- 80.Taguchi K, Watanabe Y, Tsujimura A, Tanaka M. Brain region-dependent differential expression of alpha-synuclein: α-Synuclein Differential Expression. Journal of Comparative Neurology 2016;524(6):1236–58. [DOI] [PubMed] [Google Scholar]
- 81.Taguchi K, Watanabe Y, Tsujimura A, Tatebe H, Miyata S, Tokuda T, et al. Differential Expression of Alpha-Synuclein in Hippocampal Neurons. PLoS ONE 2014;9(2):e89327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toledo JB, Gopal P, Raible K, Irwin DJ, Brettschneider J, Sedor S, et al. Pathological α-synuclein distribution in subjects with coincident Alzheimer’s and Lewy body pathology. Acta Neuropathologica 2016;131(3):393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marui W, Iseki E, Nakai T, Miura S, Kato M, Uéda K, et al. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. Journal of the Neurological Sciences 2002;195(2):153–9. [DOI] [PubMed] [Google Scholar]
- 84.Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N. Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. eLife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adamowicz DH, Roy S, Salmon DP, Galasko DR, Hansen LA, Masliah E, et al. Hippocampal α-Synuclein in Dementia with Lewy Bodies Contributes to Memory Impairment and Is Consistent with Spread of Pathology. The Journal of Neuroscience 2017;37(7):1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain: A Journal of Neurology 2014;137(Pt 9):2493–508. [DOI] [PubMed] [Google Scholar]
- 87.Pereira JB, Junqué C, Bartrés-Faz D, Ramírez-Ruiz B, Marti M- J, Tolosa E. Regional vulnerability of hippocampal subfields and memory deficits in Parkinson’s disease: Hippocampal Subfields in PD. Hippocampus 2013;23(8):720–8. [DOI] [PubMed] [Google Scholar]
- 88.Luna E, Decker SC, Riddle DM, Caputo A, Zhang B, Cole T, et al. Differential alpha-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta Neuropathol 2018;135(6):855–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volpicelli-Daley LA, Abdelmotilib H, Liu Z, Stoyka L, Daher JP, Milnerwood AJ, et al. G2019S-LRRK2 Expression Augments alpha-Synuclein Sequestration into Inclusions in Neurons. J Neurosci 2016;36(28):7415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, et al. Prion-like spreading of pathological α-synuclein in brain. Brain: A Journal of Neurology 2013;136(Pt 4):1128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nouraei N, Mason DM, Miner KM, Carcella MA, Bhatia TN, Dumm BK, et al. Critical appraisal of pathology transmission in the α-synuclein fibril model of Lewy body disorders. Experimental Neurology 2018;299(Pt A):172–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okuzumi A, Kurosawa M, Hatano T, Takanashi M, Nojiri S, Fukuhara T, et al. Rapid dissemination of alpha-synuclein seeds through neural circuits in an in-vivo prion-like seeding experiment. Acta Neuropathologica Communications 2018;6(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ecker JR, Geschwind DH, Kriegstein AR, Ngai J, Osten P, Polioudakis D, et al. The BRAIN Initiative Cell Census Consortium: Lessons Learned toward Generating a Comprehensive Brain Cell Atlas. Neuron 2017;96(3):542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding S-L, Royall JJ, Sunkin SM, Ng L, Facer BAC, Lesnar P, et al. Comprehensive cellular-resolution atlas of the adult human brain. The Journal of Comparative Neurology 2016;524(16):3127–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Annals of Neurology 2012;72(4):517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Helwig M, Klinkenberg M, Rusconi R, Musgrove RE, Majbour NK, El-Agnaf OMA, et al. Brain propagation of transduced α-synuclein involves non-fibrillar protein species and is enhanced in α-synuclein null mice. Brain 2016;139(3):856–70. [DOI] [PubMed] [Google Scholar]
- 97.Volpicelli-Daley L, Brundin P. Prion-like propagation of pathology in Parkinson disease. Handb Clin Neurol 2018;153:321–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schenk DB, Koller M, Ness DK, Griffith SG, Grundman M, Zago W, et al. First-in-human assessment of PRX002, an anti-alpha-synuclein monoclonal antibody, in healthy volunteers. Mov Disord 2017;32(2):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neff F, Wei X, Nolker C, Bacher M, Du Y, Dodel R. Immunotherapy and naturally occurring autoantibodies in neurodegenerative disorders. Autoimmun Rev 2008;7(6):501–7. [DOI] [PubMed] [Google Scholar]
- 100.Papachroni KK, Ninkina N, Papapanagiotou A, Hadjigeorgiou GM, Xiromerisiou G, Papadimitriou A, et al. Autoantibodies to alpha-synuclein in inherited Parkinson’s disease. J Neurochem 2007;101(3):749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, et al. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener 2015;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wagner J, Ryazanov S, Leonov A, Levin J, Shi S, Schmidt F, et al. Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol 2013;125(6):795–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 2016;3(10):812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim JY, Illigens BM, McCormick MP, Wang N, Gibbons CH. Alpha-Synuclein in Skin Nerve Fibers as a Biomarker for Alpha-Synucleinopathies. J Clin Neurol 2019;15(2):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meles SK, Renken RJ, Janzen A, Vadasz D, Pagani M, Arnaldi D, et al. The Metabolic Pattern of Idiopathic REM Sleep Behavior Disorder Reflects Early-Stage Parkinson Disease. J Nucl Med 2018;59(9):1437–44. [DOI] [PubMed] [Google Scholar]
- 106.Doppler K, Volkmann J, Sommer C. Skin biopsies in the differential diagnosis of parkinsonism: are we ready for simplified protocols? Brain 2016;139(Pt 1):e5. [DOI] [PubMed] [Google Scholar]
- 107.Blandini F, Cilia R, Cerri S, Pezzoli G, Schapira AHV, Mullin S, et al. Glucocerebrosidase mutations and synucleinopathies: Toward a model of precision medicine. Mov Disord 2019;34(1):9–21. [DOI] [PubMed] [Google Scholar]
- 108.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 2014;46(9):989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin X, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 2007;131(4):770–83. [DOI] [PubMed] [Google Scholar]
- 110.Hopfner F, Schulte EC, Mollenhauer B, Bereznai B, Knauf F, Lichtner P, et al. The role of SCARB2 as susceptibility factor in Parkinson’s disease. Mov Disord 2013;28(4):538–40. [DOI] [PubMed] [Google Scholar]
- 111.Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol 2012;72(3):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alcalay RN, Levy OA, Waters CC, Fahn S, Ford B, Kuo SH, et al. Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain 2015;138(Pt 9):2648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rocha EM, Smith GA, Park E, Cao H, Brown E, Hallett P, et al. Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann Clin Transl Neurol 2015;2(4):433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goker-Alpan O, Giasson BI, Eblan MJ, Nguyen J, Hurtig HI, Lee VM, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology 2006;67(5):908–10. [DOI] [PubMed] [Google Scholar]
- 115.Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol 2013;70(6):727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim MJ, Jeon S, Burbulla LF, Krainc D. Acid ceramidase inhibition ameliorates alpha-synuclein accumulation upon loss of GBA1 function. Human Molecular Genetics 2018;27(11):1972–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 2011;89(1):168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet 2011;89(1):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen X, Kordich JK, Williams ET, Levine N, Cole-Strauss A, Marshall L, et al. Parkinson’s disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A 2019;116(12):5765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 2006;38(10):1184–91. [DOI] [PubMed] [Google Scholar]
- 121.Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A 2016;113(7):1931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, et al. alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol 2010;190(6):1023–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM. Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell 2014;25(25):4010–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM. Lewy body-like alpha-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem 2013;288(21):15194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004;305(5688):1292–5. [DOI] [PubMed] [Google Scholar]
- 126.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015;85(2):257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998;392(6676):605–8. [DOI] [PubMed] [Google Scholar]
- 128.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 2004;304(5674):1158–60. [DOI] [PubMed] [Google Scholar]
- 129.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003;299(5604):256–9. [DOI] [PubMed] [Google Scholar]
- 130.Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko TS, et al. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci 2014;34(43):14304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wong YC, Ysselstein D, Krainc D. Mitochondria–lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 2018;554(7692):382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013;136(Pt 8):2419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marks WJ Jr., Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol 2010;9(12):1164–72. [DOI] [PubMed] [Google Scholar]
- 134.Olanow CW, Bartus RT, Volpicelli-Daley LA, Kordower JH. Trophic factors for Parkinson’s disease: To live or let die. Mov Disord 2015;30(13):1715–24. [DOI] [PubMed] [Google Scholar]
- 135.Asante EA, Smidak M, Grimshaw A, Houghton R, Tomlinson A, Jeelani A, et al. A naturally occurring variant of the human prion protein completely prevents prion disease. Nature 2015;522(7557):478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson’s disease. Brain 2011;134(Pt 11):3290–8. [DOI] [PubMed] [Google Scholar]
- 137.Lundblad M, Decressac M, Mattsson B, Bjorklund A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci U S A 2012;109(9):3213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis 2012;45(3):939–53. [DOI] [PubMed] [Google Scholar]
- 139.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci 2009;29(11):3365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain 2012;135(Pt 7):2058–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011;72(1):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maday S, Holzbaur EL. Compartment-Specific Regulation of Autophagy in Primary Neurons. J Neurosci 2016;36(22):5933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wong YC, Kim S, Peng W, Krainc D. Regulation and Function of Mitochondria-Lysosome Membrane Contact Sites in Cellular Homeostasis. Trends Cell Biol 2019;29(6):500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vargas KJ, Schrod N, Davis T, Fernandez-Busnadiego R, Taguchi YV, Laugks U, et al. Synucleins Have Multiple Effects on Presynaptic Architecture. Cell Rep 2017;18(1):161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mor DE, Daniels MJ, Ischiropoulos H. The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov Disord 2019;34(2):167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karakatsani ME, Blesa J, Konofagou EE. Blood Brain Barrier Opening with Focused Ultrasound in Two Experimental Models of Parkinson’s Disease. Movement Disorders: Official Journal of the Movement Disorder Society In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A. alpha-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med 2012;4(163):163ra56. [DOI] [PubMed] [Google Scholar]
- 148.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 1988;8(8):2804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scott D, Roy S. alpha-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci 2012;32(30):10129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burre J, Sharma M, Sudhof TC. alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A 2014;111(40):E4274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 152.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 2007;27(6):1405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blumenstock S, Rodrigues EF, Peters F, Blazquez-Llorca L, Schmidt F, Giese A, et al. Seeding and transgenic overexpression of alpha-synuclein triggers dendritic spine pathology in the neocortex. EMBO Mol Med 2017;9(5):716–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Roosen DA, Cookson MR. LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol Neurodegener 2016;11(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Islam MS, Nolte H, Jacob W, Ziegler AB, Putz S, Grosjean Y, et al. Human R1441C LRRK2 regulates the synaptic vesicle proteome and phosphoproteome in a Drosophila model of Parkinson’s disease. Hum Mol Genet 2016;25(24):5365–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li XT, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD, et al. Enhanced Striatal Dopamine Transmission and Motor Performance with LRRK2 Overexpression in Mice Is Eliminated by Familial Parkinson’s Disease Mutation G2019S. Journal of Neuroscience 2010;30(5):1788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu GX, Sgobio C, Gu XL, Sun LX, Lin X, Yu J, et al. Selective expression of Parkinson’s disease-related Leucine-rich repeat kinase 2 G2019S missense mutation in midbrain dopaminergic neurons impairs dopamine release and dopaminergic gene expression. Human Molecular Genetics 2015;24(18):5299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chou JS, Chen CY, Chen YL, Weng YH, Yeh TH, Lu CS, et al. (G2019S) LRRK2 causes early-phase dysfunction of SNpc dopaminergic neurons and impairment of corticostriatal long-term depression in the PD transgenic mouse. Neurobiol Dis 2014;68:190–9. [DOI] [PubMed] [Google Scholar]
- 159.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, et al. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A 2005;102(46):16842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arranz AM, Delbroek L, Van Kolen K, Guimaraes MR, Mandemakers W, Daneels G, et al. LRRK2 functions in synaptic vesicle endocytosis through a kinase-dependent mechanism. Journal of Cell Science 2015;128(3):541–52. [DOI] [PubMed] [Google Scholar]
- 161.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res 2008;314(10):2055–65. [DOI] [PubMed] [Google Scholar]
- 162.Belluzzi E, Gonnelli A, Cirnaru MD, Marte A, Plotegher N, Russo I, et al. LRRK2 phosphorylates pre-synaptic N-ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Molecular Neurodegeneration 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pan PY, Li X, Wang J, Powell J, Wang Q, Zhang Y, et al. Parkinson’s disease associated LRRK2 hyperactive kinase mutant disrupts synaptic vesicle trafficking in ventral midbrain neurons. J Neurosci 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matikainen-Ankney BA, Kezunovic N, Mesias RE, Tian Y, Williams FM, Huntley GW, et al. Altered Development of Synapse Structure and Function in Striatum Caused by Parkinson’s Disease-Linked LRRK2-G2019S Mutation. J Neurosci 2016;36(27):7128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Volta M, Beccano-Kelly DA, Paschall SA, Cataldi S, MacIsaac SE, Kuhlmann N, et al. Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K, et al. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 2012;75(6):1008–21. [DOI] [PubMed] [Google Scholar]
- 167.Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 2011;72(4):587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A 2008;105(1):145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bandres-Ciga S, Saez-Atienzar S, Bonet-Ponce L, Billingsley K, Vitale D, Blauwendraat C, et al. The endocytic membrane trafficking pathway plays a major role in the risk of Parkinson’s disease. Mov Disord 2019;34(4):460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 2010;67(6):715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, et al. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron 1999;24(1):143–54. [DOI] [PubMed] [Google Scholar]
- 173.Milosevic I, Giovedi S, Lou XL, Raimondi A, Collesi C, Shen HY, et al. Recruitment of Endophilin to Clathrin-Coated Pit Necks Is Required for Efficient Vesicle Uncoating after Fission. Neuron 2011;72(4):587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 1999;99(2):179–88. [DOI] [PubMed] [Google Scholar]
- 175.Yim YI, Sun T, Wu LG, Raimondi A, De Camilli P, Eisenberg E, et al. Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc Natl Acad Sci U S A 2010;107(9):4412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cao M, Wu Y, Ashrafi G, McCartney AJ, Wheeler H, Bushong EA, et al. Parkinson Sac Domain Mutation in Synaptojanin 1 Impairs Clathrin Uncoating at Synapses and Triggers Dystrophic Changes in Dopaminergic Axons. Neuron 2017;93(4):882–96 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D. Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Soukup SF, Vanhauwaert R, Verstreken P. Parkinson’s disease: convergence on synaptic homeostasis. EMBO J 2018;37(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sheehan P, Yue Z. Deregulation of autophagy and vesicle trafficking in Parkinson’s disease. Neurosci Lett 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pan PY, Zhu Y, Shen Y, Yue Z. Crosstalk between presynaptic trafficking and autophagy in Parkinson’s disease. Neurobiol Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol 2012;196(4):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yu WH, Dorado B, Figueroa HY, Wang LL, Planel E, Cookson MR, et al. Metabolic Activity Determines Efficacy of Macroautophagic Clearance of Pathological Oligomeric alpha-Synuclein. American Journal of Pathology 2009;175(2):736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 2013;16(4):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Soukup SF, Kuenen S, Vanhauwaert R, Manetsberger J, Hernandez-Diaz S, Swerts J, et al. A LRRK2-Dependent EndophilinA Phosphoswitch Is Critical for Macroautophagy at Presynaptic Terminals. Neuron 2016;92(4):829–44. [DOI] [PubMed] [Google Scholar]
- 185.Murdoch JD, Rostosky CM, Gowrisankaran S, Arora AS, Soukup SF, Vidal R, et al. Endophilin-A Deficiency Induces the Foxo3a-Fbxo32 Network in the Brain and Causes Dysregulation of Autophagy and the Ubiquitin-Proteasome System. Cell Reports 2016;17(4):1071–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vanhauwaert R, Kuenen S, Masius R, Bademosi A, Manetsberger J, Schoovaerts N, et al. The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. Embo Journal 2017;36(10):1392–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.George AA, Hayden S, Stanton GR, Brockerhoff SE. Arf6 and the 5 ‘ phosphatase of synaptojanin 1 regulate autophagy in cone photoreceptors. Bioessays 2016;38:S119–S35. [DOI] [PubMed] [Google Scholar]
- 188.Cao M, Milosevic I, Giovedi S, De Camilli P. Upregulation of Parkin in Endophilin Mutant Mice. Journal of Neuroscience 2014;34(49):16544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Trempe JF, Chen CXQ, Grenier K, Camacho EM, Kozlov G, McPherson PS, et al. SH3 Domains from a Subset of BAR Proteins Define a Ubl-Binding Domain and Implicate Parkin in Synaptic Ubiquitination. Molecular Cell 2009;36(6):1034–47. [DOI] [PubMed] [Google Scholar]
- 190.Hernandez LF, Obeso I, Costa RM, Redgrave P, Obeso JA. Dopaminergic Vulnerability in Parkinson Disease: The Cost of Humans’ Habitual Performance. Trends Neurosci 2019. [DOI] [PubMed] [Google Scholar]