Abstract
Focused ultrasound (FUS)-mediated blood-brain barrier (BBB) opening is currently being investigated in clinical trials. Here, we describe a portable clinical system with a therapeutic transducer suitable for humans, which eliminates the need for in-line MRI guidance. A neuronavigation-guided 0.25-MHz single-element FUS transducer was developed for noninvasive clinical BBB opening. Numerical simulations and experiments were performed to determine the characteristics of the FUS beam within a human skull. We also validated the feasibility of BBB opening obtained with this system in two non-human primates (NHPs) using FDA-approved treatment parameters. Ultrasound propagation through the human skull fragment caused 44.4 ± 1.3 % pressure attenuation at a normal incidence angle, while the focal size decreased by 3.3 ± 1.4 % and 3.9 ± 1.8 % along the lateral and axial dimension, respectively. Measured lateral and axial shifts were 0.5 ± 0.4 mm and 2.1 ± 1.1 mm, while simulated shifts were 0.1 ± 0.2 mm and 6.1 ± 2.4 mm, respectively. A 1.5-MHz passive cavitation detector transcranially detected cavitation signals of Definity microbubbles flowing through a vessel-mimicking phantom. T1-weighted MRI confirmed a 153 ± 5.5 mm3 BBB opening in two NHPs at MI of 0.4, using Definity microbubbles at the FDA-approved dose for imaging applications, without edema or hemorrhage. In conclusion, we developed a portable system for non-invasive BBB opening in humans, which can be achieved at clinically relevant ultrasound exposures without the need for in-line MRI guidance. The proposed FUS system may accelerate the adoption of non-invasive FUS-mediated therapies due to its low cost and portability.
Keywords: blood-brain barrier, focused ultrasound, clinical system, drug delivery
INTRODUCTION
Focused ultrasound (FUS) serves as a non-invasive and non-ionizing therapeutic modality, with applications in lithotripsy (Miller and Thomas 1996), tumor ablation (Xia et al. 2012), neuromodulation (Kamimura et al. 2016; Tyler et al. 2018) and essential tremor treatment (Elias et al. 2016; Lipsman et al. 2013). Microbubbles are routinely used as contrast agents in ultrasound imaging (Cosgrove and Harvey 2009) and as stress-mediators in ultrasound therapy (Coussios and Roy 2008) to deliver drugs into cells (Fan et al. 2012; Shamout et al. 2015), tumors (Arvanitis et al. 2018; Graham et al. 2014; Sun et al. 2017) or tissues (Kotopoulis et al. 2013). FUS in conjunction with systemically circulating microbubbles can perform targeted, non-invasive and reversible blood-brain barrier (BBB) opening (Hynynen et al. 2001; Konofagou 2012). FUS-mediated BBB opening has been successfully and safely tested for over 15 years in a variety of animal models, from rodents (Choi et al. 2007; Sheikov et al. 2008) to non-human primates (NHPs) (Arvanitis et al. 2012; Marquet et al. 2011; Wu et al. 2018). The success of these pre-clinical studies has paved the way towards clinical implementation of this technology.
The first published clinical study regarding the application of ultrasound and microbubbles to increase BBB permeability was by Carpentier et al. (Carpentier et al. 2016). The authors used an implantable 11.5-mm unfocused single-element 1.05-MHz transducer called SonoCloud© (Goldwirt et al. 2016; Horodyckid et al. 2017), which was fixed in the skull bone and connected to an external power supply via a transdermal needle. Glioblastoma multiforme (GBM) patients were enrolled in this study and were exposed to repeated monthly FUS treatments before receiving systemic chemotherapy with carboplatin. The results showed that BBB was disrupted at acoustic pressures of up to 1.1 MPa (i.e., mechanical index - MI: 1.07) without detectable adverse effects on MRI or clinical examination. Results from a larger cohort of 19 subjects showed an increase in median progression-free survival for patients with clear BBB disruption (Idbaih et al. 2019). The same group has developed a quantification method for FUS treatment assessment (Asquier et al. 2019) and is conducting a clinical trial with Alzheimer’s disease (AD) patients (). Additionally, GBM patients are currently treated with a new generation of SonoCloud© which enlarges the treatment volume by nine times (SC9; ); this device has recently received FDA approval for a phase 1/2a trial in the USA.
Another non-invasive approach involves the generation of FUS through a 1024-element 0.22-MHz hemispherical array embedded within the MRI bore. Real-time acoustic emission monitoring is used to determine the pressure levels during the FUS treatment (O’Reilly and Hynynen 2012). The first study showing BBB opening in 5 AD patients using the MR-guided ExAblate system developed by Insightec© (Insightec Inc., Tirat Carmel, Israel) was published by Lipsman et al. (Lipsman et al. 2018). AD patients received FUS treatments aimed at the dorsolateral prefrontal cortex. BBB opening was fully reversible, with no contrast enhancement detected 1 day after treatment. The same group recently published results from a trial with a cohort of 5 GBM patients, who have been treated with FUS in combination with temozolomide or doxorubicin (Mainprize et al. 2019). BBB opening was observed in all patients and an increase of the delivered chemotherapy was measured in the two patients for whom data was available. Currently, there are multiple clinical trials using the ExAblate Neuro system throughout the world, for targeted BBB opening in patients with GBM (, , , ), AD (, , ), Her2-positive breast cancer brain metastases (), amyotrophic lateral sclerosis (), and Parkinson’s Disease (PD) dementia (). Among the advantages of multi-element arrays are the ability to correct for skull-induced aberrations based on CT scans of the treated subject (Aubry et al. 2003; Clement and Hynynen 2002), simultaneous treatment monitoring via passive cavitation mapping (Jones et al. 2013), standing wave reduction, and flexibility in the positioning of the focal volume through electronic steering.
An alternative approach is to employ neuronavigation systems instead of MRI for FUS guidance. Neuronavigation-assisted BBB opening using a 0.4-MHz single-element transducer was proposed by Wei et al. (Wei et al. 2013). It was shown that precision of this technique was comparable to stereotactic procedures in a swine model, with targeting error of 2.3 ± 0.9 mm. BBB opening was observed above a derated pressure threshold of 0.43 MPa at 0.4 MHz (i.e., MI of 0.68), using constant infusion of 0.3 ml/kg/min SonoVue microbubbles. Clinical trials with this system, called NaviFUS©, are currently in progress in Taiwan, recruiting GBM () and drug resistant epilepsy () patients.
Recently, our group achieved BBB opening through neuronavigation targeting in a NHP model with simultaneous real-time passive cavitation detection (PCD) and passive acoustic mapping (PAM) (Wu et al. 2018). In this study, the average precision between planned and achieved targeting was 3.1 mm, compared to 4.3 mm of the frame-based stereotaxis. The BBB opening threshold was 350 kPa at 0.5 MHz (MI: 0.5), using 4–5 μm monodisperse microbubbles at a dose of 2.5 × 108 microbubbles/kg. We showed that 2D PAM can be used to predict and verify the BBB opening location. The total FUS procedure duration was less than 30 min, which is similar to current ultrasound imaging or radiation therapy duration.
Here, we sought to establish the clinical relevance of the previously described approach and develop a neuronavigation-guided focused ultrasound (NgFUS) system which is suitable for use in humans. Our objectives were to: 1) perform BBB opening at a lower frequency of 0.25 MHz, which would allow for lower attenuation/distortion of the ultrasound beam and would thus be more suitable for humans, 2) determine the transducer characteristics (e.g., radius of curvature and aperture size) which are required to expand the treatment envelope and enable coverage of the human brain using a single-element FUS transducer, 3) confirm the ability of low-frequency PCD transducers to detect cavitation signals through the human skull, 4) investigate whether BBB opening with real-time PCD monitoring is possible in a NHP model using Definity microbubbles at the FDA-approved dose for ultrasound imaging applications and clinically-relevant ultrasound parameters.
MATERIALS AND METHODS
Numerical simulations
Numerical simulations of ultrasound propagation through the human skull were performed in 2D using the k-Wave acoustics toolbox (Treeby et al. 2012; Treeby and Cox 2010), to test different transducer characteristics. We first investigated the trade-off between the focal depth and aperture size, i.e. the f-number, within the human skull. Our aim was to determine the center frequency, outer diameter, and radius of curvature, in order to be able to target both cortical and subcortical regions of the human brain, thus enlarging the treatment envelope. We tested three different transducer configurations (Table 1), based on commercially available low-frequency models (transducer 1: Sonic Concepts H-149 and transducer 2: Sonic Concepts H-209) and a custom-designed transducer (transducer 3). H-149 and H-209 were commercially available models that we identified as potentially appropriate for BBB opening applications in humans. These transducers were chosen as examples of small and large f-number, respectively (0.64 vs. 1.27). The custom-designed transducer (outer diameter: 110 mm, radius of curvature: 110 mm, f-number: 1) was derived after multiple iterations of different designs, with emphasis on the outer diameter (search space: 60–140 mm) and radius of curvature (search space: 70–120 mm). To allow for insertion of a PCD transducer or a receiving ultrasound array, an inner gap of 44 mm in diameter was applied in all transducer designs.
Table 1:
Transducer characteristics used in numerical simulations.
| Transducer | Center frequency (MHz) | Outer diameter (mm) | Inner diameter (mm) | Radius of curvature (mm) |
|---|---|---|---|---|
| 1 | 0.2 | 110 | 44 | 70 |
| 2 | 0.35 | 60 | 44 | 76 |
| 3 | 0.25 | 110 | 44 | 110 |
A human CT skull DICOM file from the Cancer Imaging archive (Cancer Imaging Archive 2017) (Head-Neck Cetuximab demo) was used as input in our simulations. Hounsfield CT units were converted to sound speed and medium density, as described previously (Aubry et al. 2003; Wu et al. 2018). Sound speed, medium density, and attenuation coefficient within the brain were set to be equal to water at 37°C (i.e. 1,524 m/s, 1,000 kg/m3, and 3.5×10−4 dB/MHz cm respectively). The transducers were positioned close to the skull in an effort to place the focal volume as close to the brain median plane as possible, while maintaining a reasonable radius of curvature and realistic housing dimensions (Table 1). A number of axial offsets were tested (range: −30 mm to +30 mm, step 10 mm), in order to determine the evolution of focal shifts across different depths. In the case of axial offset of 0 mm, the transducer’s nominal focus was positioned at the human brain midline. The primary aim of these simulations was to evaluate the effect of different focusing depths on the focal volume distortion, therefore we only tested axial offsets and not lateral. Introducing lateral offsets would produce a large variation in the incidence angle, deviating significantly from the desirable 90° incidence. Therefore, the lateral position of the FUS transducer center was fixed at y = 0 mm for all simulations. We have also tested pulses of different lengths (i.e., 1, 5, 25, and 2,500 cycles) to investigate the effects of interference and standing waves within the human skull. To calculate the theoretical ultrasound transmission coefficient through the human skull, we repeated the simulations with different pulse lengths in free field, by replacing the human skull with water. The simulation grid was equal to 300 mm × 300 mm, at 1 mm spatial resolution, while the temporal resolution was 143 ns with a total of 7,000 time steps or exposure time of 1 ms. For the pulse length of 2,500 cycles, the simulation consisted of 70,000 time steps or 10 ms, to enable comparison with the treatment scheme typically used for in vivo BBB opening. Shear waves were not taken into account in these simulations. Axial (i.e., x) and lateral (i.e., y) axes were defined with respect to the FUS transducer, and had left to right and anterior to posterior directions, respectively.
Clinical system description
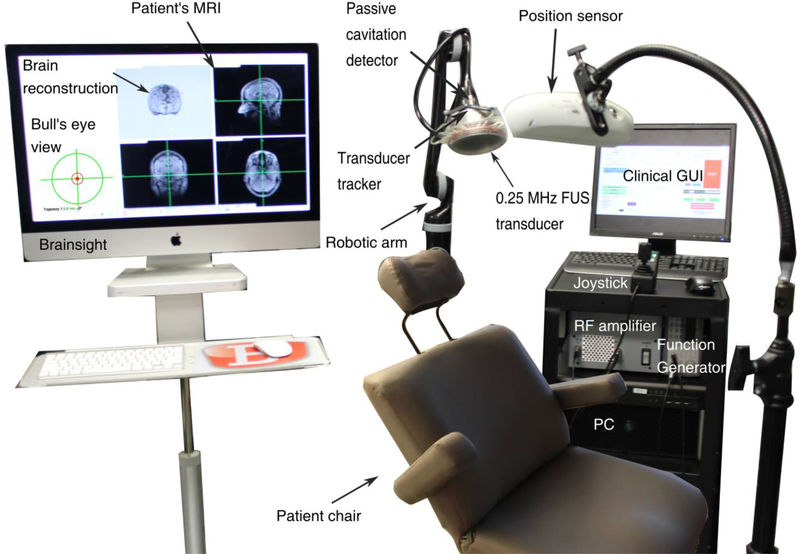
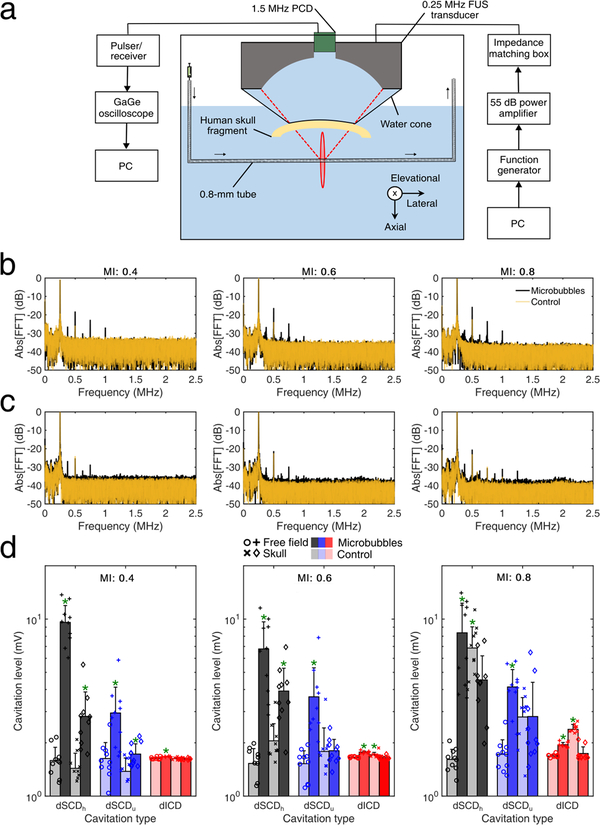
In the proposed clinical system (Fig. 1), we chose a low center frequency (i.e., 0.25 MHz) to reduce the attenuation caused by the human skull and decrease the pressure threshold for cavitation induction (Apfel and Holland 1991). The first step was to refine the dimensions and characteristics of the single-element spherical-segment transducer based on numerical simulations. We then constructed the chosen single-element FUS transducer (part number: H-231, center frequency: 0.25 MHz; Sonic Concepts, Bothell, WA, USA) and attached it onto a robotic arm (Kinova Jaco2; Kinova, Boisbriand, QC, Canada). The robotic arm had 4 degrees of freedom, maximum mid-range loading capacity of 4.4 kg, and was controlled via a joystick. The whole construct was fixed onto a wheeled cart, making the system portable to any location.
Fig. 1 :
Clinical setup with a single-element transducer and neuronavigation guidance.
The clinical FUS transducer was driven by a function generator (33500B Series; Agilent technologies, Santa Clara, CA, USA) through a 55 dB RF power amplifier (A150; E&I, Rochester, NY, USA) using clinically-relevant parameters (table 2). A water degassing system (WDS105+; Sonic Concepts, Bothell, WA, USA) was used to fill the transducer cone with degassed water and inflate or deflate the cone according to the sonicated location. Reflective beads were attached to the transducer to enable real-time tracking of its location through an infrared camera acting as a position sensor and neuronavigation guidance (BrainSight; Rogue Research, Montreal, QC, Canada). Using the bull’s eye view function, we achieved high targeting accuracy with spatial error lower than 2 mm (Wu et al. 2018).
Table 2:
Clinically-relevant ultrasound parameters for blood-brain barrier opening in vivo using the neuronavigation-guided focused ultrasound system.
| Parameter | Value |
|---|---|
| Centre frequency | 0.25 MHz |
| Derated peak-negative pressure | 0.2 MPapk-neg |
| Mechanical Index | 0.4 |
| Definity microbubble dose | 10 μl/kg (1x clinical dose) |
| Pulse length | 10 ms or 2,500 cycles |
| Pulse repetition frequency | 2 Hz |
| Sonication duration | 2 min |
Microbubble acoustic emissions were recorded (sampling frequency: 50 MHz, capture length: 10 ms) with a 1.5 MHz passive cavitation detector (diameter: 32 mm, focal depth: 114 mm; ndtXducer, Northborough, MA, USA). PCD provides information about the cavitation magnitude, duration, and mode within the focal volume, using either separate transducers (Tung et al. 2010) or a therapeutic transducer alone (Heymans et al. 2017). Cavitation signals also provide indirect information about the microbubble velocity through the Doppler effect, that can be captured either with a single-element PCD (Pouliopoulos and Choi 2016) or using an array of receivers (Pouliopoulos et al. 2017). Here, we used PCD to define the cavitation mode in vitro and in vivo, by calculating the stable cavitation dose (SCD) and inertial cavitation dose (ICD), as described before (Tung et al. 2010). Briefly, the recorded time-domain signals were transformed into the frequency domain through a Fast Fourier Transform (segment size: 524,288 data points), performed in Matlab (The Mathworks, Natick, MA, USA). Three spectral areas were filtered to derive the relevant cavitation levels or cavitation dose per pulse:
harmonic peaks, fh.n = nfc;
ultraharmonic peaks fu,n = (n –1 / 2)fc;
broadband emissions fb with fh,n + 10kHz < f < fu,n −10kHz and fu,n + 10kHz < fb < fh,n+1−10kHz,
where fc was the center frequency (i.e., 0.25 MHz) and n the harmonic number (n = 3,4,5,…,10). Fundamental and second harmonics were excluded from the calculations, due to strong skull reflections at these frequencies in control experiments.
Stable harmonic (dSCDh), stable ultraharmonic (dSDCu), and inertial cavitation (dICD) levels were then calculated as the mean root-mean-square (RMS) of the maximum absolute FFT amplitude in each frequency region and for each acoustic pulse: , and . The total cavitation dose in vivo was calculated as the sum of all the cavitation levels throughout the FUS treatment: , and . The total sonication duration was T = 2 min.
In vitro characterization
Skull-induced aberrations were characterized in a water tank. A capsule hydrophone (HGL-0200, ± 3 dB frequency range: 0.25 to 40 MHz, electrode aperture: 200 μm; Onda Corporation, Sunnyvale, CA, USA) was used to measure the emitted pressure profiles in free field and with a human skull fragment in the beam path. The skull fragment was submerged in water and degassed before the experiment using a vacuum pump, to reduce the gas content within the bone. Raster scans around the focal point were performed at a spatial resolution of 0.1 mm laterally and 1 mm axially. The scans had lateral/elevational and axial ranges of 10 mm and 60 mm, respectively, and were centered at the geometric focus of the FUS transducer (110 mm from transducer surface). Shifts along the lateral and elevational dimensions were averaged, assuming an axisymmetric distortion of the beam. Ultrasound pressure transmission coefficient through the human skull was calculated (in %) by dividing the maximum pressure of the focal volume after the skull placement by the maximum pressure of the focal volume in free field, for both simulations and experiments. Transcranial pressure loss was calculated as 100% - transmission coefficient. To determine the ultrasound attenuation through a NHP skull, we used the same setup replacing the human skull fragment with a monkey skull fragment. The human and primate skull fragments were positioned right on top of the water cone and at a perpendicular incidence angle, to imitate the clinical scenario. Pressure profiles and transcranial loss were expected to be extremely sensitive to the incidence angle and distance from the transducer surface. Here, we only tested one incidence angle (i.e., ~90°) and transducer surface-skull distance (i.e., 62 mm), which are clinically-relevant for treatment of dorsolateral prefrontal cortex. Pressure profiles and losses were estimated at skull locations of variable thickness (n = 10, thickness range: 3 – 7.5 mm, measured with a caliper), since attenuation depends on the skull thickness (Gerstenmayer et al. 2018). All reported pressure values refer to the derated peak-negative pressure.
Cavitation detection through the human skull was also conducted within a water tank. A 0.8-mm silicon elastomer tube was submerged and fixed at a horizontal position within the focal volume of the clinical transducer (120 mm from transducer surface). The tube was filled with either water, which served as a control, or Definity microbubbles (0.2 ml microbubbles/L of solution) flowing at a rate of 1.8 ml/min. Measurements were conducted both in free field and with the human skull fragment in the beam path, positioned 62 mm away from the transducer surface. We tested three derated acoustic pressures, 200 kPa, 300kPa and 400 kPa, corresponding to MI of 0.4, 0.6 and 0.8, respectively. Cavitation levels were calculated across the experimental conditions (n=10 consecutive pulses per condition) to establish the ability of the PCD transducer to detect cavitation signals through the human skull at each acoustic pressure.
In separate experiments, a tissue-implantable type-T thermocouple (Physitemp instruments, Clifton, NJ, USA) was attached to the skull surface to measure the heating profile during clinically-relevant FUS exposure (MI: 0.4–0.8, duty cycle: 2%, Table 2). A positive control sonication at a higher duty cycle (20% at MI of 0.8) was conducted to compare with the low duty cycle BBB opening scheme. Temperature data was recorded at a sampling rate of 100 samples/second. Temperature increase on the skull surface was calculated by subtracting the temperature before FUS exposure from the measured value during FUS exposure (n=3).
In vivo feasibility
All animal experiments were reviewed and approved by the local Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health (NIH) guidelines for animal welfare. Two male adult Rhesus macaques (weight: 8–11 kg, age: 12–20 y.o.) were treated with the clinical FUS transducer, targeting the thalamus (NHP 1) and the dorsolateral prefrontal cortex (NHP 2), in order to examine the performance of the system at both cortical and subcortical regions. To accommodate the NHP experiment, the patient chair (Fig. 1) was replaced with a surgical table equipped with a stereotactic apparatus for head fixation. NHPs were initially sedated with a mixture of ketamine (10 mg/kg) and atropine (0.02 mg/kg) through intramuscular injection. Once sedated, the animals were intubated and catheterized via the saphenous vein. Anesthesia was induced and maintained throughout the experiment using inhalable isoflurane mixed with oxygen (1–2%).
These ultrasound parameters used here (table 2) were identical to those approved by the FDA for use in Alzheimer’s patients using our system (derated peak-negative pressure: 0.2 MPa, pulse length: 10 ms, pulse repetition frequency: 2 Hz, total sonication duration: 2 min). We maintained the MI below the FDA-approved limit for ultrasound imaging applications with Definity microbubbles to avoid compromising safety. BBB opening in the NHP model was attempted at a peak-negative pressure of 0.2 MPa or at MI of 0.4. This MI is approximately 5 times lower that the maximum MI approved by the FDA for imaging applications (i.e., MI of 1.9), twice lower than the BBB opening threshold found by Carpentier et al. in humans (Carpentier et al. 2016), and similar to the threshold found in our previous NHP studies (Wu et al. 2018). In contrast to our previous studies that used 4–5μm size-isolated microbubbles at a dose of 2.5×108 microbubbles/kg (Karakatsani et al. 2017; Wu et al. 2018), here we used commercially available Definity microbubbles at the FDA-approved clinical dose for ultrasound imaging applications (i.e., 10 μl/kg). Definity microbubbles were infused as a bolus via a single injection, upon the treatment initiation.
BBB opening was assessed approximately 60 min post-sonication with T1-weighted MRI (3-D Spoiled Gradient-Echo, TR/TE = 20/1.4 ms; flip angle: 30°; NEX = 2; spatial resolution: 500 × 500 μm2; slice thickness: 1 mm with no inter-slice gap). T1-weighted scans were acquired before and after IV administration of 0.2 ml/kg gadodiamide MRI contrast agent (Omniscan; GE Healthcare, Bronx, NY, USA), which is normally impermeable to the BBB (molecular weight: 591.7 Da). BBB opening was quantified by comparing the pre- and post-contrast administration T1 scans. Safety outcomes were assessed with axial T2-weighted MRI (TR/TE = 3000/80 ms; flip angle: 90°; NEX = 3; spatial resolution: 400 × 400 μm2; slice thickness: 2 mm with no inter-slice gap) and susceptibility-weighted imaging (SWI; TR/TE = 19/27 ms; flip angle: 15°; NEX = 1; spatial resolution: 400 × 400 μm2; slice thickness: 1 mm with no inter-slice gap). All scans were performed in a 3T clinical MRI scanner.
BBB opening quantification
We developed a graphics user interface in Matlab for BBB opening quantification and analysis. To calculate the BBB opening volume, the pre-contrast T1 scan was subtracted from the post-contrast T1 scan. An intensity threshold was set to isolate the BBB opening area in the difference image, and a contour plot was applied to the pixels above the threshold within the selected region of interest. The area of the BBB opening contour was calculated for each coronal MRI slice, and the total BBB opening volume in mm3 was found by summing the BBB opening areas in all slices.
Statistical analysis
All measurements presented here are shown as mean ± standard deviation. Simulations were performed for n = 4 pulse lengths and n = 6 transducer axial positions. Cavitation detection was established by comparing control and microbubble-seeded cavitation levels in free field and through the human skull, using a two-sample t-test in Matlab (n =10 pulses). Statistically significant differences were assumed at p < 0.05.
RESULTS
Numerical simulations
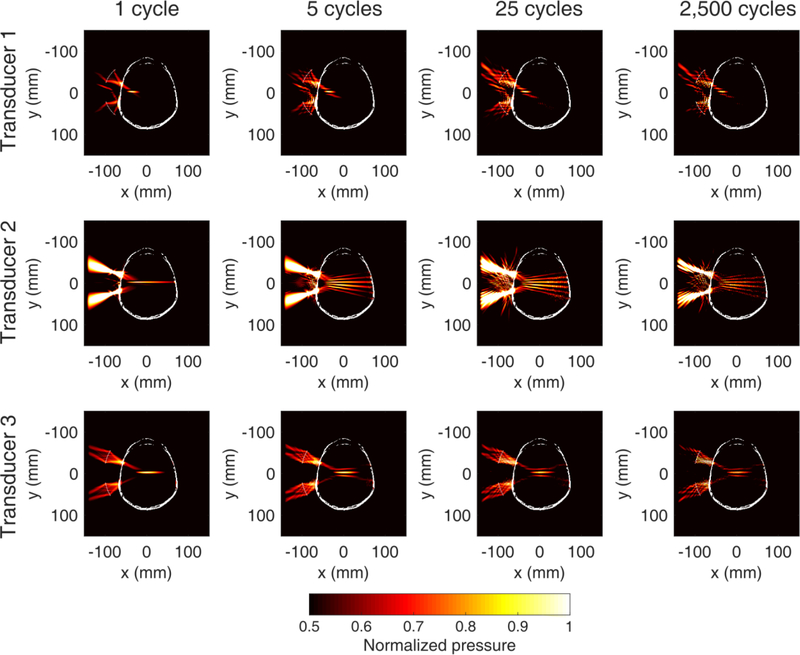
Numerical simulations showed that transducer 3 was able to target the brain median plane while maintaining a tightly focused beam, without multiple sidelobes (Fig. 2). Transducer 1 did not have sufficient radius of curvature to produce a long enough focal depth for the human skull, due to its low f-number. Transducer 2 produced multiple sidelobes of similar amplitude to the main lobe due to the large f-number and the low outer-to-inner diameter ratio. Furthermore, the focal volume was subject to greater distortion due to the higher center frequency compared to transducers 1 and 3 (0.35 MHz vs. 0.2 MHz and 0.25 MHz). We found that in the case of a single-element transducer, an f-number of 1 (transducer 3) was more suitable for applications in the human brain, compared to lower or larger f-numbers within the subset we have tested.
Fig. 2 :
Numerical simulations of ultrasound propagation with different single-element transducers (top to bottom: 1, 2, 3) emitting pulses of variable length (left to right: 1, 5, 25, 2,500 cycles). Transducer 3 was the only configuration that was able to treat deep structures without presenting multiple sidelobes within the human skull. Color bar: normalized focal pressure. Each pressure profile was self-normalized to the maximum acoustic pressure within the skull to illustrate the −3dB focal volume. Pressure values refer to the maximum instantaneous pressure at each location.
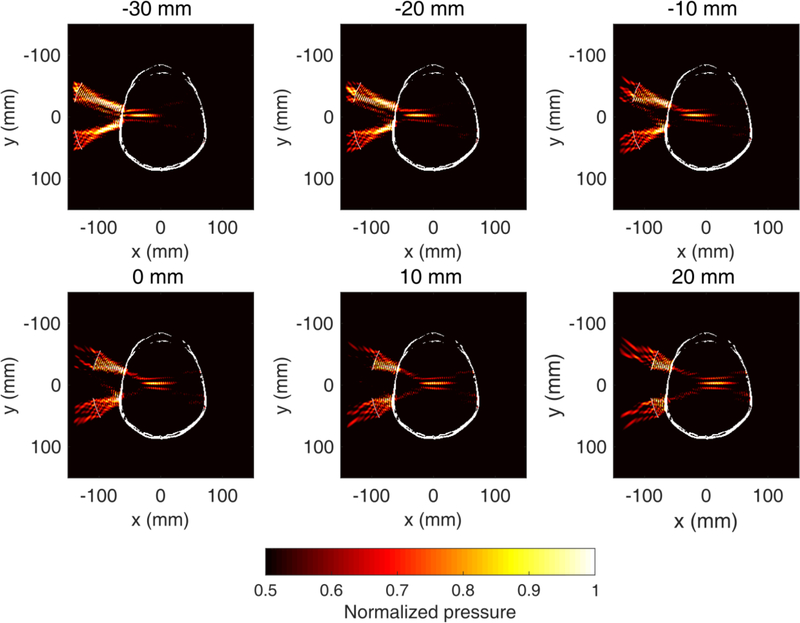
Such a transducer design allows targeting of both superficial cortical areas and deeper subcortical areas (Fig. 3). By physically moving the FUS transducer towards/away the skull surface, one can achieve a treatment envelope of up to 80 mm in depth. Simulations showed that the focal dimensions, pressure profile, and skull-induced focal shift depend on the transducer axial offset and the pulse length (Fig. 4). The transducer axial offset was defined as the distance of the free-field focus from the simulation center (x = 0 mm). Intracranial acoustic pressures only moderately changed throughout the axial offsets. Highest pressures were observed near the skull center, while there was a decrease of up to 7% towards the proximal and distal skull. The amplitude of lateral sidelobes increased with pulse length, from 49% of the main lobe at 1 cycle to 76% of the main lobe at 2,500 cycles. All pressure profiles shown in figures 2 and 3 were normalized to the maximum pressure within the skull and plotted in the range [0.5, 1], in order to visualize the −3dB focal volume following transcranial ultrasound propagation.
Fig. 3 :
Numerical simulations of ultrasound propagation with the clinical focused ultrasound (FUS) transducer targeting structures of variable depth within a human skull. Examples are shown for transducer axial offset of −30 mm to 20 mm (offset = 0mm when the focus in free field coincides with the midline). Center frequency: 0.25 MHz, pulse length: 2,500 cycles. Color bar: normalized focal pressure. Each pressure profile was self-normalized to the maximum acoustic pressure within the skull to illustrate the −3dB focal volume. Pressure values refer to the maximum instantaneous pressure at each location.
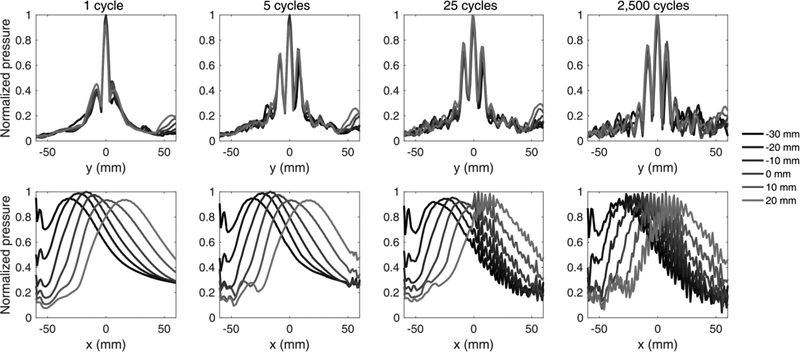
Fig. 4:
Lateral (top) and axial (bottom) profiles of the simulated pressure field within a human skull. Lateral sidelobes and interference patterns emerge for pulse lengths larger than 1 cycle. The spatial length of interference away from the distal skull bone increases linearly with the pulse length.
Pulse lengths longer than 1 cycle produced constructive and destructive interference at the distal part of skull, with nodes and antinodes appearing at a spacing of half wavelength (i.e., 3 mm). The interference spatial extent was equal to half the spatial length of the acoustic pulse (e.g., 2.5 cycles or 15 mm for a pulse length of 5 cycles or 30 mm). For the clinically-relevant pulse length of 2,500 cycles, the interference profile reached equilibrium and extended throughout the interior of the human skull. The theoretical limit for standing wave generation at 0.25 MHz and a skull size of 130 mm is 43 cycles.
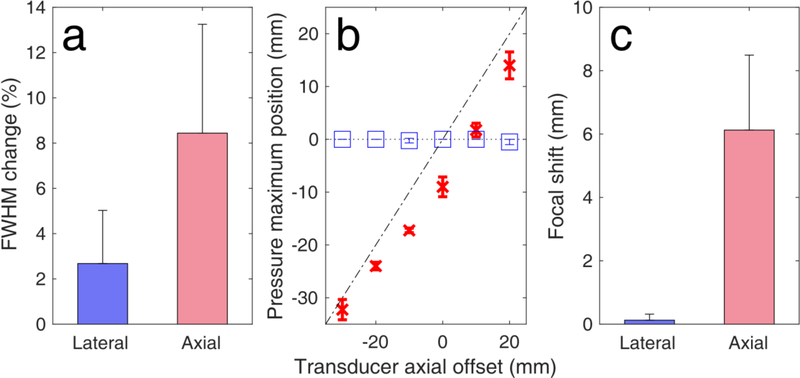
The presence of the human skull distorted and shifted the simulated focal volume (Fig. 5). In water medium without the human skull, the axial and lateral full widths at half maximum (FWHM) were simulated to be 65.5 mm × 5.6 mm. The focal width and length were reduced by 2.7 ± 2.4 % and by 8.4 ± 4.8 % along the lateral and axial dimensions, respectively, due to skull-induced aberrations (n = 4 pulse lengths and n = 6 transducer positions). The focus was also negatively shifted towards the transducer [Fig. 5(b)]. Axial shifts depended on the transducer position. Interestingly, shifts were smaller for larger offsets. In other words, the farther the focus from the brain midline, the smaller the axial shift. On average, the axial and lateral focal shifts were 6.1 ± 2.4 mm and 0.1 ± 0.2 mm, respectively [Fig. 5(c)]. Pressure attenuation due to the human skull was simulated to be 36.1 ± 3.4 % (n = 10 different CT slices).
Fig. 5 :
Simulated human skull-induced focal distortion. (a) Full width at half maximum (FHWM) change due to the presence of the human skull. FWHM changes were first averaged across the pulse lengths for each axial offset (n = 4 pulse lengths), and then averaged across all depths (n=6 axial offsets). (b) Simulated focal shifts along the axial (red crosses) and lateral (blue boxes) dimensions. Diagonal dotted-dashed line and parallel dotted line denote axial and lateral shifts equal to zero, respectively (n=4 pulse lengths). (c) Average focal shifts across the lateral and axial dimensions (n=6 axial offsets). Data presented as mean ± standard deviation.
In vitro characterization
To confirm the simulation findings, we performed a detailed estimation of the 2D beam profiles along the lateral/elevational and lateral/axial dimensions, with and without the presence of a human skull fragment (Fig. 6). Using the capsule hydrophone and the 3D positioning system [Fig. 6(a)], we measured the pressure profiles along the axial, lateral and elevational dimensions. The free-field focal length and width were 47.6 mm × 5.6 mm (Figs. 6b-left and 6c-left). These values were close to the nominal focal dimensions of 49 mm × 6 mm, provided by the manufacturer. Ultrasound propagation through the human skull was expected to attenuate and shift the acoustic focus. Inserting the skull fragment within the beam path attenuated the pressure amplitude by 44.4 ± 1.3 % and distorted the focal region [Fig. 6(b)-right and 6(c)-right]. The lateral and axial FWHM decreased by 3.3 ± 1.5 % and 3.9 ± 1.8 %, respectively [Fig. 6(d)]. Experimental focal shifts along the lateral and axial dimensions were 0.5 ± 0.4 mm and 2.1 ± 1.1 mm, respectively [Fig. 6(e)].
Fig. 6:
Experimental human skull-induced focal distortion. (a) Experimental setup for measuring focal distortion using a hydrophone. A raster scan was performed to measure the focal volume in (b, c-left) free field and (b, c-right) with a human skull fragment. Pressure maximum was 10 mm closer to the transducer compared to the geometric focus. White crosses denote the position of the free-field focus. Green crosses denote the position of the focus following transcranial propagation. (d) full width at half maximum change and (e) focal shifts along the lateral and axial dimensions. Data presented as mean ± standard deviation (n=10 scans with ultrasound propagating through skull segments of different thickness).
Passive cavitation detection measurements confirmed that the 1.5MHz PCD transducer can detect cavitation signals through the human skull (Fig. 7). Using the in vitro setup described earlier [Fig. 7(a)], we detected stationary reflections at the fundamental and the second harmonic for the control experiment, both from the tube and the human skull [Fig. 7(b)]. When Definity microbubbles were flowing through the vessel phantom, we observed a rise in the higher harmonics (up to the 5th harmonic or 1.25 MHz) and ultraharmonics (up to the 3rd ultraharmonic or 0.825 MHz).
Fig. 7:
Passive cavitation detection through the human skull. (a) In vitro setup for passive cavitation detection. A 0.8-mm tube filled with Definity microbubbles was used as a vessel-mimicking phantom. (b) Spectra of control (transparent orange line) and microbubble (black line) acoustic emissions for mechanical index (MI) of 0.4 (left), 0.6 (middle), and 0.8 (right) in free field. (c) Spectra of control and microbubble acoustic emissions through the human skull. (d) Cavitation levels in free-field (circles, plus signs) and through the human skull (crosses, diamonds), for control (light bars) and microbubbles (dark bars), at MI of 0.4 (left), 0.6 (middle), and 0.8 (right). Data presented as mean ± standard deviation (n=10 pulses). *: p < 0.05.
Higher acoustic pressures led in general to higher harmonic and ultraharmonic peaks. In figure 7(d) light-color bars represent control sonications, while dark-color bars represent sonications with Definity microbubbles. The two lefts bars in each cavitation dose represent free-field sonications, while the right two bars represent sonications through the human skull fragment. Ten distinct therapeutic pulses were emitted for each condition. Harmonic stable cavitation levels were significantly higher for microbubbles than the control, for MI of 0.4 and 0.6 in both free-field and through the human skull [Fig. 7(d)]. Ultraharmonic stable cavitation levels with microbubbles were significantly higher than the control at MI of 0.4 and 0.6 only in free-field. There was a significant difference through the human skull at MI of 0.4, but a non-significant increase at MI of 0.6. At the highest acoustic pressure, stable harmonic and inertial cavitation levels were significantly higher for the control than for microbubbles. This was likely due to inadequate degassing of the human skull fragment, which resulted in intracranial cavitation nuclei in the control experiment. Inertial cavitation levels rose considerably above the noise level at all MIs in free-field, and also during the control experiments at the presence of skull for MI of 0.6 and 0.8.
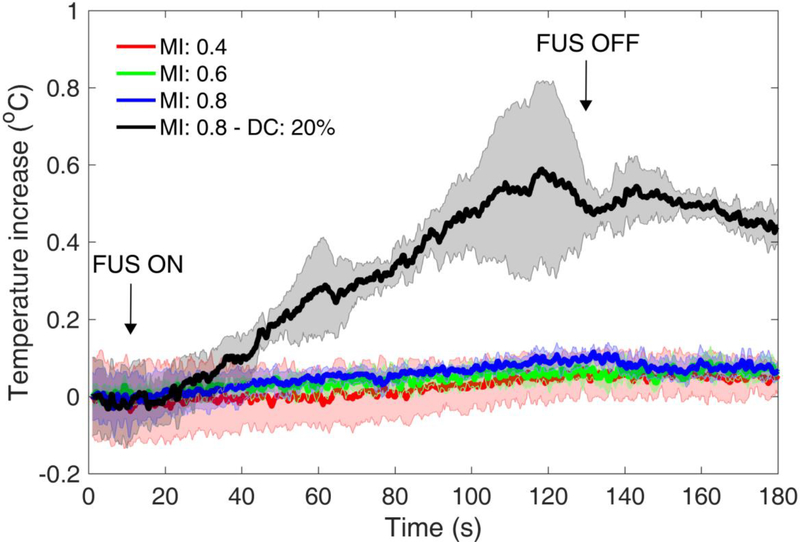
Next, we measured the ultrasound-induced heating during clinically-relevant ultrasound exposure. A wire thermocouple was attached below the human skull fragment and within the ultrasound beam path. To simulate the clinical scenario, 2-min sonications were performed using the parameters intended for the clinic (Table 2). Maximum temperature increase was between 0.11 ± 0.05 and 0.16 ± 0.03 °C (n = 3) during sonication at MI of 0.4–0.8 (Fig. 8). This negligible heating was expected, given the low duty cycle of ultrasonic pulse sequences used in BBB opening (i.e., 2 %). A control sonication at 10x higher duty cycle (i.e., 20%) and MI of 0.8 did increase the temperature by 0.59 ± 0.23 °C.
Fig. 8:
Skull heating using the clinical focused ultrasound transducer at mechanical index (MI) of 0.4 (red line), 0.6 (green line), and 0.8 (blue line) and clinically-relevant ultrasound parameters (center frequency: 0.25 MHz, pulse length: 2,500 cycles or 10 ms, pulse repetition frequency: 2 Hz, duty cycle: 2%, total duration: 2 minutes). A higher duty cycle (i.e., 20%) was used as a positive control for heating (black line). Data presented as mean ± standard deviation (n=3).
In vivo feasibility
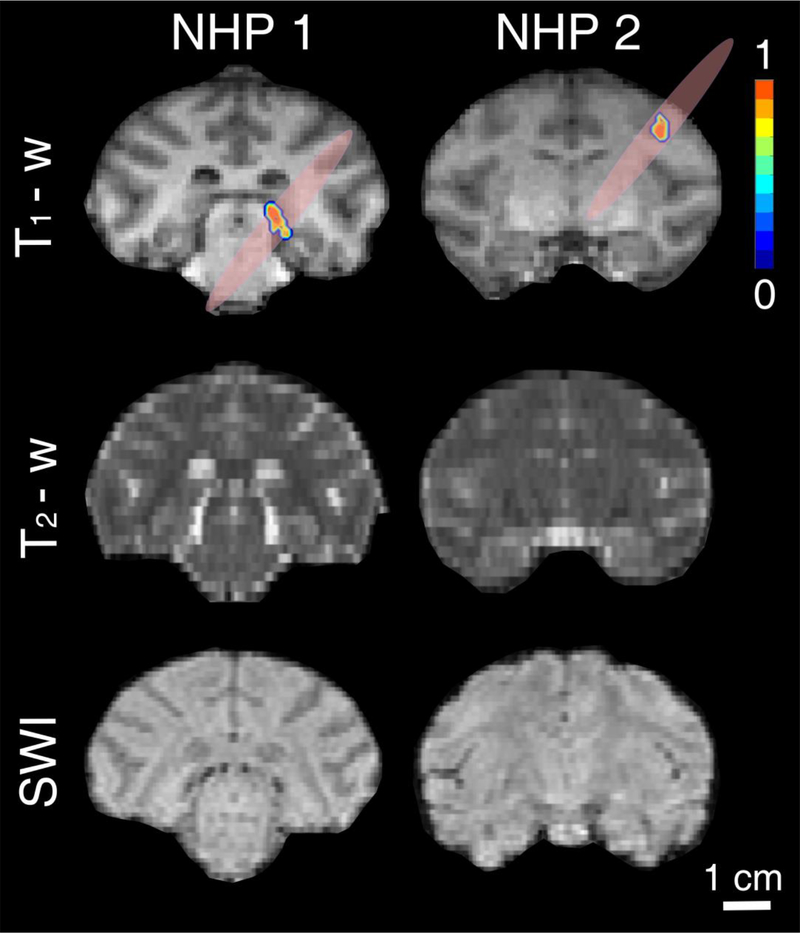
Finally, we tested the proposed clinical system in a NHP model to perform non-invasive and targeted BBB opening at peak-negative pressure of 200 kPa or at MI of 0.4, using the clinically-recommended Definity dose (10 μl/kg). Two NHPs were treated targeting the thalamus (NHP 1) and the dorsolateral prefrontal cortex (NHP 2). The two targets were selected as examples of deep and superficial structures, respectively. Despite the low pressure and microbubble dose, we achieved BBB opening in both targeted structures (Fig. 9). BBB opening was more pronounced in the gray matter rather than in the white matter tracts, as shown before (Karakatsani et al. 2017). The total BBB opening volume was 153 mm3 for NHP 1 and 164 mm3 for NHP 2. Safety was evaluated with T2-weighted MRI and SWI (Fig. 9). There was neither a hyper-intense region in T2 scans nor a hypo-intense region in SWI an hour post-sonication, suggesting lack of hemorrhage or edema in the sonicated region. Although this was an acute safety evaluation, we did not expect any long-term effects at this low-pressure regime, based on our previous studies (Downs et al. 2015).
Fig. 9:
In vivo feasibility in a non-human primate (NHP) model. Coronal T1-weighted, T2-weighted and susceptibility-weighted imaging (SWI) for NHPs 1 (left) and 2 (right). T1-weighted MRI confirmed blood-brain barrier opening in the thalamus (NHP 1) and dorsolateral prefrontal cortex (NHP 2), using the clinical focused ultrasound (FUS) transducer with clinically relevant parameters (MI: 0.4) and FDA-approved Definity microbubble dose (10 μl/kg). T2-weighted and SWI showed that there is no acute hemorrhage or edema after the FUS treatment. Color bar: normalized contrast enhancement. Scale bar: 1 cm.
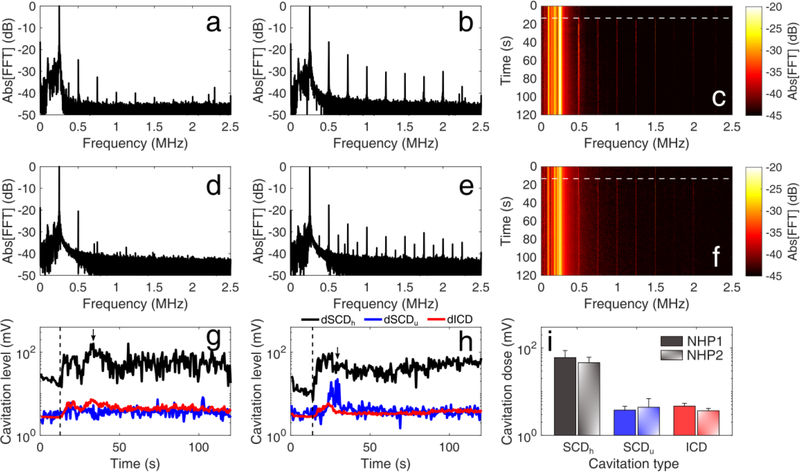
Safety outcomes were corroborated by the captured PCD data which confirmed in realtime the absence of violent cavitation events within the focal volume (Fig. 10). Before microbubble administration, the spectral content of the received signals included the fundamental frequency (i.e., 0.25 MHz) and the first 2–3 harmonics [Fig. 10(a) and 10(d)]. Following microbubble bolus injection, there was an increase of higher harmonics and - for NHP 2 - ultraharmonics [Fig. 10(b) and 10(e)]. However, there was no considerable increase of the broadband signal floor following microbubble administration (white dashed line), as shown in the spectrograms of both FUS treatments [Fig. 10(c) and 10(f)]. These qualitative traits were quantified with SCD and ICD [Fig. 10(g)–(i)]. SCDh increased by 5.44 ± 1.16 fold upon microbubble infusion (t > 15s), while SCDu and ICD increased by 1.46 ± 0.01 and 1.48 ± 0.21 fold, respectively. We can thus infer that microbubbles underwent stable and recurrent oscillations, with stable cavitation dominating over transient and inertial cavitation throughout treatment. On average, the total cavitation dose was 1.37 ± 0.17 × 104 mV.
Fig. 10:
In vivo passive cavitation detection measurements confirmed that stable cavitation dominated throughout ultrasound treatment at clinically-relevant conditions. Spectral amplitude (a, d) before and (b, e) after microbubble injection, for non-human primate (NHP) 1 (a, b) and NHP 2 (d, e). Spectrogram of the entire treatment session for NHP 1 (c) and NHP 2 (f). Higher harmonic emissions were detected, with no substantial increase in the broadband floor after microbubble entrance into the focal volume (white dashed lines). (g)-(h) Stable harmonic cavitation levels (black line) rose right after microbubble administration (dashed line) and remained relatively constant throughout the sonication, for both NHP 1 (g) and 2 (h). Stable ultraharmonic (blue line) and inertial cavitation levels (red line) had a moderate increase, indicating absence of violent cavitation events at MI of 0.4. Arrows indicate the time points shown in (b) and (e). (i) Average stable harmonic (black), stable ultraharmonic (blue) and inertial (red) cavitation dose during focused ultrasound treatment for NHP 1 (filled bars) and NHP 2 (patterned bars), following microbubble administration (t > 15s). Data presented as mean ± standard deviation (n = 210 pulses).
DISCUSSION
A clinical system using a single-element transducer and neuronavigation guidance for BBB opening offers distinct advantages compared to alternative approaches. First, BBB opening can be achieved in a non-invasive manner which is preferable especially for long-term repeated treatments required in AD or PD. Second, such system can provide access to both shallow (i.e., cortical) and deep (i.e., subcortical) brain regions (Figs. 3, 4, 5), although at the expense of a large axial to lateral focal size ratio and variable focal distortion in different depths (Fig. 5). Also, there is no need for an MRI scanner during treatment which may be a costly and formidable hurdle for widespread use of FUS-mediated treatments. Neuronavigation systems are typically available for neurosurgical procedures (Grunert et al. 2003), so the only additional cost for hospitals is the single-element transducer, the driving electronics and the robotic arm. The targeting and sonication procedure is efficient and simple (< 30 min) as opposed to MR-guided FUS treatment (3–4 hours). Moreover, the NgFUS is portable so treatment can take place at any location without the need of an MRI unit. Finally, low-frequency and low-duty-cycle treatment leads to limited skull-induced aberrations (Figs. 5 and 6) and FUS-induced skull heating (Fig. 8), respectively.
Lower frequencies favor cavitation-mediated bioeffects at low acoustic pressures (Apfel and Holland 1991; Ilovitsh et al. 2018). We have shown here that the BBB can be opened in a NHP model at MI of 0.4 (Fig. 9), which is twice lower than the minimum MI required using the unfocused implanted 1.05-MHz transducer in humans (Carpentier et al. 2016) and similar to other NHP studies (Downs et al. 2015; Karakatsani et al. 2017; McDannold et al. 2012; Wu et al. 2018). Low-pressure treatments not only ensure safety (Fig. 9), but also facilitate regulatory approval because they are compatible with routinely used ultrasound imaging protocols. Such acoustic pressure instigates cavitation activity that is detectable in real-time with the co-aligned PCD transducer (Fig. 7), with stable cavitation emissions dominating the spectra during FUS treatment in a NHP model (Fig. 10). Clinically-relevant parameters (table 2) are thus not expected to lead to violent inertial cavitation, which was detected in higher MI sonication (Fig. 7).
Here, we demonstrated successful BBB opening using 10-ms-long pulses. However, such pulse lengths produce interference and standing waves within the human skull (O’Reilly et al. 2010), as shown here (Figs. 2, 3, 4), and promote primary (Dayton et al. 2002; Koruk et al. 2015) and secondary (Dayton et al. 1997; Lazarus et al. 2017) acoustic radiation forces, which may indirectly compromise safety. In our future work, we aim to use either coded-excitation (Kamimura et al. 2015) or shorter pulses on the order of ps (< 50 cycles) to avoid standing wave formation (O’Reilly et al. 2011). Such pulses have been shown to produce more uniform cavitation activity within the focal area, by extending the microbubble lifetime (Pouliopoulos et al. 2014), avoiding cluster formation and spreading the microbubble activity in space and time (Pouliopoulos et al. 2016; Pouliopoulos 2017). In vivo, rapid short-pulse sequences produce uniform BBB openings that last for less than 10 minutes and do not allow extravasation of inflammation-inducing proteins, such as albumin, into the brain parenchyma (Morse et al. 2019). On the other hand, it is more difficult to deliver large therapeutic molecules using short pulses (Choi et al. 2011).
Short pulses also allow for improved passive mapping of cavitation signals, through the synchronization of the therapeutic and imaging processes (i.e., using absolute time-of-flight information) (Burgess et al. 2018; Gateau et al. 2011). PAM in either time (Coviello et al. 2015; Gyöngy and Coussios 2010) or frequency (Arvanitis et al. 2015a; Arvanitis et al. 2017; Haworth et al. 2012; Haworth et al. 2017; Salgaonkar et al. 2009) domain can be achieved by replacing the single-element PCD transducer with a multi-element linear array operating in receive mode. Using a PAM array, one can account for skull-induced aberrations in receive and localize acoustic cavitation activity in a more precise manner (Arvanitis et al. 2015b; Jones et al. 2013; Jones et al. 2015; O’Reilly et al. 2014). Furthermore, we plan to use pulse inversion in the short therapeutic pulses, to be able to detect weak cavitation emissions through the thick human skull (Pouliopoulos et al. 2018). Future efforts will finally focus on using short pulses and PAM in closed-loop control (Jones et al. 2018; Kamimura et al. 2018; Patel et al. 2018; Sun et al. 2017) to improve the spatiotemporal control of acoustic cavitation activity within the brain.
The proposed system is limited by a number of factors. First, the axial focal length is 8 times larger than the lateral focal width. The elongated focus was necessary to increase the targetable brain coverage or treatment envelope, however comes at the expense of potentially asymmetric BBB opening. Hemispherical arrays provide lower axial-to-lateral focal beam width even at large steering angles, in both emission and reception modes. Second, real-time single-element PCD monitoring does not allow for either sub-focal volume localization of cavitation activity or detection of cavitation signals over a large bandwidth. The clinical transducer was designed with an inner diameter of 44 mm to allow for insertion of not only single-element transducers but also multi-element arrays for passive mapping of cavitation activity. Third, the proposed system is not able to either compensate for skull-induced aberrations in a way similar to multi-element hemispherical arrays or target complex 3D brain structures such as the hippocampus in a single treatment. One possible solution is to use 3D printed holographic phase plates tailored to each skull contour and targeted structure (Ferri et al. 2018; Maimbourg et al. 2018; Melde et al. 2016). Accurate knowledge of the intracranial pressure is impossible, therefore one needs to simulate the pressure field within the targeted location on a patient-by-patient basis using head CT scans. However, this remains an approximation and emitted pressures should remain below the safety limits assuming the lowest attenuation coefficient possible. Finally, the robotic system used to hold and position the transducer in place for treatment has only 4 degrees of freedom, which limit the achievable range of incidence angles. Subsequent improvements will include a robotic arm with 6 degrees of freedom.
In this study, numerical simulations were performed in 2D space, assuming axisymmetric beam profiles along the axial dimension. However, the human skull is asymmetric and highly inhomogeneous in 3D space, therefore the simulated profiles are a first-order approximation. The single-element transducer was simulated in k-Wave as a collection of 1-mm point sources firing simultaneously. This may be the cause of the over-estimation of the axial beam width in the simulations compared to the experiment (65.53 mm vs. 47.57 mm in free field). Also, we used a human skull fragment for our experiments as opposed to a complete human skull. In previous reports from our group, we have investigated the effect of varying incidence angles at the BBB opening volume (Karakatsani et al. 2017). Here, our primary interest was to study the effects of focusing the therapeutic beam at different depths (Figs. 3, 4, 5), thus only one incidence angle (approximately 90°) was set for both simulations and experiments. For this reason, the lateral position of the FUS transducer remained constant in the numerical simulations. Yet, there was a discrepancy between the simulated and experimental pressure loss following transcranial propagation (36 % vs. 44.4 %), which can be reduced by using 3D simulations, finer grids and time steps, and identical skull shapes/dimensions. In upcoming clinical trials, 3D simulations will be performed for each patient, using a grid with isotropic resolution of 0.5 mm, a specific beam trajectory, and a well-defined target within the prefrontal cortex.
On average, axial shifts were higher in the simulations than in the experiments (Figs. 5 and 6). Averaging in the simulations was conducted over different pulse lengths and focusing depths (Fig. 5), whereas experimental measurements (Fig. 6) were conducted with a single pulse length (i.e., 25 cycles) and fixed transducer-skull distance (i.e., 62 mm). The axial shift in the simulation which resembled the experimental skull-transducer distance (i.e., axial offset of −30 mm) was 2.25 ± 1.92 mm (Fig. 5), similar to the experimentally-derived shift of 2.1 ± 1.1 mm (Fig. 6). The in vitro cavitation detection experiment was conducted using a single 0.8 mm vessel-mimicking tube, which does not capture the complexity and variability of the in vivo vasculature. Finally, whilst all simulations and bench top experiments focused on the human skull, the initial in vivo feasibility testing of the NgFUS system was conducted using two NHPs. Although this model is the closest resemblance to humans, the safety and performance of this approach remains to be tested in the clinic.
CONCLUSIONS
In conclusion, we developed a clinical setup for BBB opening based on a single-element transducer with neuronavigation guidance and real-time cavitation monitoring. Using this system, one can achieve non-invasive and targeted BBB opening with limited focal distortion and induced skull heating. Lateral and axial shifts were experimentally measured to be 0.5 ± 0.4 mm and 2.1 ± 1.1 mm, and were simulated as 0.1 ± 0.2 mm and 6.1 ± 2.4 mm. We found that the focal volume decreased by 3.3 ± 1.4 % and 3.9 ± 1.8 % along the lateral and axial dimensions, respectively, following transmission through a human skull fragment. Maximum temperature increase on the skull surface was 0.16 ± 0.03 °C. Using this clinical system, we produced a 153 ± 5.5 mm3 BBB opening in a NHP model with clinically relevant parameters and without any detectable damage. Ongoing work is focused on the short- and long-term safety profile of the current clinical system, including histopathology and behavioral studies in multiple NHPs, before progressing into human applications. In our future work, we plan to use the NgFUS system, which was recently granted an investigational device exemption (IDE G180140) by the FDA, to achieve non-invasive and targeted BBB opening in AD patients.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grants 5R01EB009041 and 5R01AG038961. The authors acknowledge the members of the Ultrasound Elasticity and Imaging laboratory (UEIL) at Columbia University for their contribution in the development this system. A.N.P. would also like to thank Javier Cudeiro for the advice on the numerical simulations, Cindy Han for the guidance on regulatory issues and Rogue Research Inc. for assistance with the BrainSight platform.
REFERENCES
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol 1991;17:179–85. [DOI] [PubMed] [Google Scholar]
- Arvanitis C, McDannold N, Clement G. Fast passive cavitation mapping with angular spectrum approach. J Acoust Soc Am Acoustical Society of America, 2015a;138:1845–1845. [Google Scholar]
- Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, Bernabeu MO, Fukumura D, McDannold N, Jain RK. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc Natl Acad Sci National Academy of Sciences, 2018;115:201807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis CD, Clement G, McDannold N. Transcranial assessment and visualization of acoustic cavitation: modeling and experimental validation. IEEE Trans Med Imaging 2015b;34:1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis CD, Crake C, McDannold N, Clement GT. Passive Acoustic Mapping with the Angular Spectrum Method. IEEE Trans Med Imaging 2017;36:983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled Ultrasound-Induced Blood-Brain Barrier Disruption Using Passive Acoustic Emissions Monitoring Muñoz-Barrutia A, ed. PLoS One 2012;7:e45783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquier N, Bouchoux G, Canney M, Martin C, Law-Ye B, Leclercq D, Chapelon J-Y, Lafon C, Idbaih A, Carpentier A. Blood-brain barrier disruption in humans using an implantable ultrasound device: quantification with MR images and correlation with local acoustic pressure. J Neurosurg JNS 2019;1–9. [DOI] [PubMed] [Google Scholar]
- Aubry J-F, Tanter M, Pernot M, Thomas J-L, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am Acoustical Society of America, 2003;113:84–93. [DOI] [PubMed] [Google Scholar]
- Burgess MT, Apostolakis I, Konofagou EE. Power cavitation-guided blood-brain barrier opening with focused ultrasound. Phys Med Biol 2018;63:065009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Imaging Archive. https://wiki.cancerimagingarchive.net/display/Public/Head-Neck+Cetuximab. 2017.
- Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon J-Y, Capelle L, Cornu P, Sanson M, Hoang-Xuan K, Delattre J-Y, Idbaih A. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 2016;8:343re2 LP-343re2. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med Biol 2007;33:95–104. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci USA 2011;108:16539–16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol 2002;47:1219. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Harvey C. Clinical uses of microbubbles in diagnosis and treatment. Med Biol Eng Comput 2009;47:813–26. [DOI] [PubMed] [Google Scholar]
- Coussios CC, Roy RA. Applications of Acoustics and Cavitation to Noninvasive Therapy and Drug Delivery. Annu Rev Fluid Mech Annual Reviews, 2008;40:395–420. [Google Scholar]
- Coviello C, Kozick R, Choi J, Gyöngy M, Jensen C, Smith PP, Coussios C-C. Passive acoustic mapping utilizing optimal beamforming in ultrasound therapy monitoring. J Acoust Soc Am Acoustical Society of America, 2015;137:2573. [DOI] [PubMed] [Google Scholar]
- Dayton P, Morgan K, Klibanov A, Brandenburger G, Nightingale K, Ferrara K. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control 1997;44:1264–1277. [Google Scholar]
- Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am 2002;112:2183–2192. [DOI] [PubMed] [Google Scholar]
- Downs ME, Buch A, Sierra C, Karakatsani ME, Chen S, Konofagou EE, Ferrera VP. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One 2015;10:e0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, Schwartz M, Hynynen K, Lozano AM, Shah BB, Huss D, Dallapiazza RF, Gwinn R, Witt J, Ro S, Eisenberg HM, Fishman PS, Gandhi D, Halpern CH, Chuang R, Butts Pauly K, Tierney TS, Hayes MT, Cosgrove GR, Yamaguchi T, Abe K, Taira T, Chang JW. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med Massachusetts Medical Society, 2016;375:730–739. [DOI] [PubMed] [Google Scholar]
- Fan Z, Liu H, Mayer M, Deng CCX. Spatiotemporally controlled single cell sonoporation. Proc Natl Acad Sci 2012;109:16486–16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri M, Bravo JM, Redondo J, Sánchez-Pérez JV. Enhanced Numerical Method for the Design of 3-D-Printed Holographic Acoustic Lenses for Aberration Correction of Single-Element Transcranial Focused Ultrasound. Ultrasound Med Biol 2018; [DOI] [PubMed] [Google Scholar]
- Gateau J, Aubry J-F, Pernot M, Fink M, Tanter M. Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011. pp. 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenmayer M, Fellah B, Magnin R, Selingue E, Larrat B. Acoustic Transmission Factor through the Rat Skull as a Function of Body Mass, Frequency and Position. Ultrasound Med Biol Elsevier, 2018;44:2336–2344. [DOI] [PubMed] [Google Scholar]
- Goldwirt L, Canney M, Horodyckid C, Poupon J, Mourah S, Vignot A, Chapelon JY, Carpentier A. Enhanced brain distribution of carboplatin in a primate model after blood-brain barrier disruption using an implantable ultrasound device. Cancer Chemother Pharmacol Springer Berlin Heidelberg, 2016;77:211–216. [DOI] [PubMed] [Google Scholar]
- Graham SM, Carlisle R, Choi JJ, Stevenson M, Shah AR, Myers RS, Fisher K, Peregrino M, Seymour L, Coussios CC. Inertial cavitation to non-invasively trigger and monitor intratumoral release of drug from intravenously delivered liposomes. J Control release The Authors, 2014;178:101–7. [DOI] [PubMed] [Google Scholar]
- Grunert P, Darabi K, Espinosa J, Filippi R. Computer-aided navigation in neurosurgery. Neurosurg Rev 2003;26:73–99; discussion 100–1. [DOI] [PubMed] [Google Scholar]
- Gyöngy M, Coussios C-C. Passive cavitation mapping for localization and tracking of bubble dynamics. J Acoust Soc Am 2010;128:EL175–80. [DOI] [PubMed] [Google Scholar]
- Haworth KJ, Bader KB, Rich KT, Holland CK, Mast TD. Quantitative Frequency-Domain Passive Cavitation Imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64:177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth KJ, Mast TD, Radhakrishnan K, Burgess MT, Kopechek J a., Huang S-L, McPherson DD, Holland CK. Passive imaging with pulsed ultrasound insonations. J Acoust Soc Am 2012;132:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans SV., Martindale CF, Suler A, Pouliopoulos AN, Dickinson RJ, Choi JJ. Simultaneous Ultrasound Therapy and Monitoring of Microbubble-Seeded Acoustic Cavitation Using a Single-Element Transducer. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64:1234–1244. [DOI] [PubMed] [Google Scholar]
- Horodyckid C, Canney M, Vignot A, Boisgard R, Drier A, Huberfeld G, François C, Prigent A, Santin MD, Adam C, Willer J-C, Lafon C, Chapelon J-Y, Carpentier A. Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J Neurosurg 2017;126:1351–1361. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001;220:640–646. [DOI] [PubMed] [Google Scholar]
- Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, Asquier N, Law-Ye B, Leclercq D, Bissery A, Rycke Y De, Trosch C, Capelle L, Sanson M, Hoang-Xuan K, Dehais C, Houillier C, Laigle-Donadey F, Mathon B, André A, Lafon C, Chapelon J-Y, Delattre J-Y, Carpentier A. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin Cancer Res American Association for Cancer Research, 2019;clincanres.3643.2018. [DOI] [PubMed] [Google Scholar]
- Ilovitsh T, Ilovitsh A, Foiret J, Caskey CF, Kusunose J, Fite BZ, Zhang H, Mahakian LM, Tam S, Butts-Pauly K, Qin S, Ferrara KW. Enhanced microbubble contrast agent oscillation following 250 kHz insonation. Sci Rep Nature Publishing Group, 2018;8:16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Deng L, Leung K, McMahon D, O’Reilly MA, Hynynen K. Three-Dimensional Transcranial Microbubble Imaging for Guiding Volumetric Ultrasound-Mediated Blood-Brain Barrier Opening. Theranostics 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, O’Reilly M a, Hynynen K. Transcranial passive acoustic mapping with hemispherical sparse arrays using CT-based skull-specific aberration corrections: a simulation study. Phys Med Biol 2013;58:4981–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, O’Reilly MA, Hynynen K. Experimental demonstration of passive acoustic imaging in the human skull cavity using CT-based aberration corrections. Med Phys American Association of Physicists in Medicine, 2015;42:4385–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura HA, Flament J, Valette J, Cafarelli A, Aron Badin R, Hantraye P, Larrat B. Feedback control of microbubble cavitation for ultrasound-mediated blood-brain barrier disruption in non-human primates under magnetic resonance guidance J Cereb Blood Flow Metab SAGE PublicationsSage UK: London, England, 2018;0271678X1775351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura HAS, Wang S, Chen H, Wang Q, Aurup C, Acosta C, Antonio AO, Konofagou EE. Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1 . 9 MHz. Med Phys 2016;43:5730–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura HAS, Wang S, Wu S-Y, Karakatsani ME, Acosta C, Carneiro AAO, Konofagou EE. Chirp- and random-based coded ultrasonic excitation for localized blood-brain barrier opening. Phys Med Biol 2015;60:7695–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakatsani MEM, Samiotaki GM, Downs ME, Ferrera VP, Konofagou EE. Targeting Effects on the Volume of the Focused Ultrasound-Induced Blood-Brain Barrier Opening in Nonhuman Primates in Vivo. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64:798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konofagou EE. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics 2012;2:1223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koruk H, El Ghamrawy A, Pouliopoulos AN, Choi JJ. Acoustic Particle Palpation for Measuring Tissue Elasticity. Appl Phys Lett 2015;107:223701–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotopoulis S, Dimcevski G, Gilja OH, Hoem D, Postema M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Med Phys 2013;40:072902. [DOI] [PubMed] [Google Scholar]
- Lazarus C, Pouliopoulos AN, Tinguely M, Garbin V, Choi JJ. Clustering dynamics of microbubbles exposed to low-pressure 1-MHz ultrasound. J Acoust Soc Am Acoustical Society of America, 2017;142:3135–3146. [DOI] [PubMed] [Google Scholar]
- Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun Nature Publishing Group, 2018;9:2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, Hynynen K, Lozano AM. MR-guided focused ultrasound thalamotomy for essential tremor: A proof-of-concept study. Lancet Neurol Elsevier, 2013;12:462–468. [DOI] [PubMed] [Google Scholar]
- Maimbourg G, Houdouin A, Deffieux T, Tanter M, Aubry JF. 3D-printed adaptive acoustic lens as a disruptive technology for transcranial ultrasound therapy using single-element transducers. Phys Med Biol IOP Publishing, 2018;63. [DOI] [PubMed] [Google Scholar]
- Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, Perry J, Hynynen K. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci Rep Nature Publishing Group, 2019;9:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet F, Tung YS, Teichert T, Ferrera VP, Konofagou EE. Noninvasive, transient and selective Blood-Brain barrier opening in Non-Human primates in vivo. PLoS One 2011;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res American Association for Cancer Research, 2012;72:3652–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melde K, Mark AG, Qiu T, Fischer P. Holograms for acoustics. Nature Nature Publishing Group, 2016;537:518–522. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. Contrast-agent gas bodies enhance hemolysis induced by lithotripter shock waves and high-intensity focused ultrasound in whole blood. Ultrasound Med Biol 1996;22:1089–95. [DOI] [PubMed] [Google Scholar]
- Morse SV, Pouliopoulos AN, Chan TG, Copping MJ, Lin J, Long NJ, Choi JJ. Rapid Short-pulse Ultrasound Delivers Drugs Uniformly across the Murine Blood-Brain Barrier with Negligible Disruption. Radiology Radiological Society of North America, 2019;291:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M a., Hynynen K. Blood-Brain Barrier: Real-time Feedback-controlled Focused Ultrasound Disruption by Using an Acoustic Emissions-based Controller. Radiology 2012;263:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M a., Waspe AC, Ganguly M, Hynynen K. Focused-Ultrasound Disruption of the Blood-Brain Barrier Using Closely-Timed Short Pulses: Influence of Sonication Parameters and Injection Rate. Ultrasound Med Biol 2011;37:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MA, Huang Y, Hynynen K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model. Phys Med Biol 2010;55:5251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MA, Jones R, Hynynen K. Three-Dimensional Transcranial Ultrasound Imaging of Microbubble Clouds Using a Sparse Hemispherical Array. IEEE Trans Biomed Eng 2014;61:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Schoen SJ Jr, Arvanitis CD. Closed Loop Spatial and Temporal Control of Cavitation Activity with Passive Acoustic Mapping. IEEE Trans Biomed Eng 2018;016971:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliopoulos A, Smith C, El Ghamrawy A, Tang M, Choi J. Doppler passive acoustic mapping for monitoring microbubble velocities in ultrasound therapy. J Acoust Soc Am Acoustical Society of America, 2017;141:3491–3491. [Google Scholar]
- Pouliopoulos AN. Controlling microbubble dynamics in ultrasound therapy. 2017.
- Pouliopoulos AN, Bonaccorsi S, Choi JJ. Exploiting flow to control the in vitro spatiotemporal distribution of microbubble-seeded acoustic cavitation activity in ultrasound therapy. Phys Med Biol 2014;59:6941–6957. [DOI] [PubMed] [Google Scholar]
- Pouliopoulos AN, Burgess MT, Konofagou EE. Pulse inversion enhances the passive mapping of microbubble-based ultrasound therapy. Appl Phys Lett 2018;113:044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliopoulos AN, Choi JJ. Superharmonic microbubble Doppler effect in ultrasound therapy. Phys Med Biol 2016;61:6154–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliopoulos AN, Li C, Tinguely M, Garbin V, Tang M-X, Choi JJ. Rapid short-pulse sequences enhance the spatiotemporal uniformity of acoustically driven microbubble activity during flow conditions. J Acoust Soc Am Acoustical Society of America, 2016;140:2469–2480. [DOI] [PubMed] [Google Scholar]
- Salgaonkar VA, Datta S, Holland CK, Mast TD. Passive cavitation imaging with ultrasound arrays. J Acoust Soc Am 2009;126:3071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamout FE, Pouliopoulos AN, Lee P, Bonaccorsi S, Towhidi L, Krams R, Choi JJ. Enhancement of Non-invasive Trans-membrane Drug Delivery Using Ultrasound and Microbubbles during Physiologically Relevant Flow. Ultrasound Med Biol 2015;41:2435–2448. [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med Biol 2008;34:1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Zhang Y, Power C, Alexander PM, Sutton JT, Aryal M, Vykhodtseva N, Miller EL, McDannold NJ. Closed-loop control of targeted ultrasound drug delivery across the blood-brain/tumor barriers in a rat glioma model. PNAS National Academy of Sciences, 2017;114:E10281–E10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeby BE, Cox BT. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J Biomed Opt 2010;15:021314. [DOI] [PubMed] [Google Scholar]
- Treeby BE, Jaros J, Rendell AP, Cox BT. Modeling nonlinear ultrasound propagation in heterogeneous media with power law absorption using a k -space pseudospectral method. J Acoust Soc Am Acoustical Society of America, 2012;131:4324–4336. [DOI] [PubMed] [Google Scholar]
- Tung Y-S, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys Med Biol 2010;55:6141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Lani SW, Hwang GM. Ultrasonic modulation of neural circuit activity. Curr Opin Neurobiol 2018;50:222–231. [DOI] [PubMed] [Google Scholar]
- Wei K-C, Tsai H-C, Lu Y-J, Yang H-W, Hua M-Y, Wu M-F, Chen P-Y, Huang C-Y, Yen T-C, Liu H-L. Neuronavigation-guided focused ultrasound-induced blood-brain barrier opening: a preliminary study in swine. AJNR Am J Neuroradiol American Journal of Neuroradiology, 2013;34:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-Y, Aurup C, Sanchez CS, Grondin J, Zheng W, Kamimura H, Ferrera VP, Konofagou EE. Efficient Blood-Brain Barrier Opening in Primates with Neuronavigation-Guided Ultrasound and Real-Time Acoustic Mapping. Sci Rep Nature Publishing Group, 2018;8:7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JZ, Xie FL, Ran LF, Xie XP, Fan YM, Wu F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol 2012;38. [DOI] [PubMed] [Google Scholar]