Abstract
Mediation analysis was used to investigate the role of white matter integrity in the relationship between injury severity and verbal memory performance in participants with chronic pediatric traumatic brain injury (TBI). DTI tractography was used to measure fractional anisotropy (FA) within the corpus callosum, fornix, cingulum bundles, perforant pathways, and uncinate fasciculi. Injury severity was indexed using Glasgow Coma Scale (GCS) scores obtained at the time of the injury. Verbal memory was measured by performance on the long-delay free recall (LDFR) trial of the California Verbal Learning Test–Children’s version. Participants were between the ages of 10–18 and included 21 children with TBI (injured before age 9) and 19 typically-developing children (TDC). Children with TBI showed lower FA across all pathways and poorer LDFR performance relative to TDC. Within the TBI group, mediation analysis revealed neither a significant total effect of GCS on LDFR nor significant direct effects of GCS on LDFR across pathways; however, the indirect effects of GCS on LDFR through FA of the corpus callosum, left perforant pathway, and left uncinate fasciculus were significant and opposite in sign to their respective direct effects. These results suggests that the predictive validity of GCS for LDFR is initially suppressed by the substantial variance accounted for by FA, which is uncorrelated with GCS, and the predictive validity of GCS increases only when FA is considered, and the opposing path is controlled. These findings illustrate the complex associations between acute injury severity, white matter pathways, and verbal memory several years following pediatric TBI.
Keywords: pediatric traumatic brain injury, diffusion tensor imaging tractography, fractional anisotropy, injury severity, verbal memory
Introduction
Traumatic brain injury (TBI) is the most common cause of long-term disability in children and adolescents, both in the United States and in other countries (Cuff et al. 2007; Dewan et al. 2016). Several studies have investigated the efficacy of pediatric TBI severity indices as predictors of functional outcome and structural imaging, yet the literature examining the long-term consequences of pediatric TBI in relation to changes in white matter integrity and cognitive function is scarce. Furthermore, an investigation into the long-term effects that various levels of injury severity have on verbal memory in relation to the microstructural integrity of associated white matter pathways following pediatric TBI has yet to be published in the literature.
The Glasgow Coma Scale (GCS; Teasdale & Jennett, 1974) score is the most widely used clinical measure of acute injury severity, where a lower GCS indicates greater severity. In the months following a pediatric TBI, GCS is a good predictor of cerebral atrophy, which is a sensitive measure of white matter volume loss (Ghosh et al. 2009). An inverse relationship between injury severity and cognitive outcome is consistently documented in pediatric TBI, where verbal memory is shown to be a key predictor of long-term academic success (Lajiness-O’Neill et al. 2011). In terms of more specific cognitive outcome, Anderson and Catroppa (2007) demonstrated a negative relationship between pediatric TBI severity and performance on measures of verbal learning and memory.
Diffusion tensor imaging (DTI) is useful in detecting subtle changes in white matter integrity in adults and children with TBI (Niogi and Mukherjee 2010; Voelbel et al. 2012; Wilde et al. 2012) by measuring the direction of water molecule movement along tracts organized within the brain. White matter integrity can be quantified using DTI-derived metrics, such as fractional anisotropy (FA), a scalar measure that ranges from 0 (isotropy) to 1 (anisotropy) (Alexander et al. 2007; Hunter et al. 2012).
In adults with TBI, Palacios et al. (2011) found evidence for unique FA reduction patterns in both long-and short-associative and commissural fibers to be related to different types of memory impairments. Specifically, a positive relationship was found between working memory performance and FA of the superior longitudinal fasciculus, the arcuate fasciculus, the fornix, and the corpus callosum, while declarative memory performance was restricted to a positive relationship with FA of the fornix and the corpus callosum. Further analysis of long-term memory deficits revealed decreased cortical thickness and compromised white matter integrity of the left parietal lobe to be the main contributor of these deficits in adults with chronic TBI (Palacios et al. 2013).
An investigation of the integrity of the dorsal cingulum bundle in adults with chronic TBI, using DTI tractography, revealed a positive correlation with cingulum integrity and memory performance (Baek et al. 2013). Wilde et al. (2010) further demonstrated reduced integrity of the cingulum to be related to injury severity and cognitive control impairment in pediatric TBI compared to typically developing children. The association fibers that run through the ventral arm of C-shaped cingulum make up the perforant pathway, which plays a critical role for memory consolidation in the healthy brain, as the fibers extend from the major sensory cortices to various intra-hippocampal regions (Augustinack et al. 2010). Studies utilizing DTI have shown relationships between perforant pathway degradation and delayed verbal memory recall in healthy brains (Yassa et al. 2010), mild cognitive impairment and dementia (Kalus et al. 2006; Rogalski et al. 2009), and TBI (Christidi et al. 2011). In the latter study, Christidi and colleagues (2011) demonstrated significantly decreased FA in the perforant pathway in both hemispheres and a significant relationship between perforant pathway integrity and performance on tasks of long-term retrieval and delayed recall.
Mabbott and colleagues (2009) found broad white matter compartments to be influential in declarative memory for healthy adolescents. Specifically, the authors implicated the proficiency of auditory-verbal declarative memory with the integrity of the left uncinate fasciculus and broader parietal regions. Sepulcre et al. (2008) used a non-aprioristic approach to investigate which disrupted white matter pathways contributed to declarative verbal memory storage and retrieval in adults with multiple sclerosis. Per their results, white matter regions within the left temporal lobe, the left thalamic region, the anterior limb of the left internal capsule, and the right temporal stem were associated with both storage and retrieval of declarative verbal memory. Retrieval was further associated with temporo-parieto-frontal paramedian bundles, particularly the dorsal cingulum bundle, the corpus callosum, and temporal portions of the fronto-occipital fasciculi.
The literature discussed above demonstrates increasing support for the direct, bivariate relationships between injury severity and verbal memory dysfunction, injury severity and the integrity of various white matter pathways, and the influence of white matter integrity on declarative memory following brain injury. However, there are no published studies to date that have investigated the role that white matter integrity plays as a mediator of the relationship between injury severity and verbal memory during the chronic phase of recovery following pediatric TBI. The present study aims to address this gap in the literature through the initial detection of any differences in verbal memory performance and white matter integrity between typically-developing children (TDC) and those who have suffered from pediatric TBI, and finally through an investigation of the mediating role of white matter integrity in the relationship between injury severity and verbal memory performance following pediatric TBI. It is hypothesized that poorer memory outcomes in the TBI group will be related to reduced microstructural integrity of their white matter. Further, in those with pediatric TBI, it is hypothesized that greater injury severity will predict poorer verbal memory performance, and this relationship will be mediated by decreased microstructural integrity of relevant white matter pathways.
Methods
Participants
Inclusion criteria for the TBI group consisted of a complicated mild, moderate, or severe closed head injury sustained during young childhood (between ages 1–10) and a minimum of a 5-year post-injury interval (i.e., time between injury and current age), such that current age at scanning was 6–18 years. Injury severity was defined by the lowest post-resuscitation GCS score obtained at the time of the injury (Teasdale and Jennett 1974; Williams et al. 1990), where those with scores between 13–15, 9–12, and 3–8 are considered to have suffered from mild, moderate, and severe injuries, respectively. Due to the difficulty in using the GCS to assess injury severity in preverbal children, pediatric variants of the GCS (Kirkham et al. 2008), which assess age-appropriate verbal abilities (e.g., babbling, cooing, crying) were used in those injured at ages 1–3 years. All children with TBI enrolled in the study had one or more positive intracranial findings on initial clinical imaging (see Table 1). Exclusion criteria for the TDC group included any history of head trauma, and exclusion criteria for both groups included any history of childhood abuse, previous diagnosis of psychotic disorder, contraindications to MRI, and ages older than 18 years at the time of the study. Children in the TDC group were selected to match the participants with TBI on age (within one year), sex, and maternal education. See Table 1 for details pertaining to participant characteristics. Written informed consent was obtained from participants who were 18 years old and from a parent or legally authorized representative of participants under 18 years of age.
Table 1.
Abnormal day-of-injury CT findings
| ID | Intracranial Lesion | Skull Fracture | |||
|---|---|---|---|---|---|
| Type | Side | Location | Side | Location | |
| 1 | EDH | L | Frontal, Parietal | L | Temporal |
| 2 | SAH | Mid | Lateral Ventricle, Fourth Ventricle | ||
| SDH | R | Frontal, Temporal | |||
| 3 | SAH | L | Temporal | ||
| 4 | EDH | R | Frontal, Parietal | R | Frontal, Parietal |
| 5 | SDH | R | Parietal | L | Parietal, Temporal, Basilar |
| 6 | SDH | L | Temporal | R | Frontal |
| Contusion | R | ||||
| 7 | SDH | L | Cerebellar | ||
| 8 | SAH | L | |||
| SDH | L | ||||
| IVH | L | ||||
| Edema | L | ||||
| 9 | SDH | R | Temporal | Bi | Occipital, Basilar |
| Contusion | R | Temporal | |||
| 10 | IPH | L | |||
| SDH | R | Parietal | |||
| Contusion | Bi | Frontal, Temporal, Subcortical | |||
| Shearing injury | Bi | Frontal, Temporal, Subcortical | |||
| 11 | Contusion | Bi | Occipital | ||
| 12 | SDH | L | Parietal, Occipital | Bi | Parietal, Temporal, Basliar |
| SAH | L | Frontal, Parietal, Temporal, Occipital | |||
| 13 | Contusion | L | Parietal | L | Parietal, Occipital |
| 14 | IVH | R | Lateral Ventricle | ||
| 15 | HC | R | Frontal | L | Basilar |
| 16 | SDH | L | Frontal, Cerebellar | Mid | Occipital |
| Encephalomalacia | L | Frontal, Occipital | |||
| 17 | IVH | Bi | Lateral Ventricle (Occipital Horn) | ||
| 18 | HC | R | Temporal | ||
| 19 | CH | R | Cerebellar | Bi | Temporal, Basilar |
| IVH | R | Lateral Ventricle (Occipital Horn) | |||
| 20 | HC | L | Frontal | R | Temporal |
| SAH | Basal Cistern | ||||
| SDH | Cerebellar | ||||
| 21 | IPH | L | Parietal | L | Parietal |
EDH = epidural hematoma. SAH = subarachnoid hematoma. SDH = subdural hematoma. IPH = intraparenchymal hemorrhage. IVH = intraventricular hemorrhage. HC = hemorrhagic contusion. CH = cerebellar hematoma. L = left. Mid = midline. R = right. Bi = bilateral.
Diffusion Tensor Imaging
Image acquisition and preprocessing
A Philips 3.0 Tesla Intera scanner (Philips, Cleveland, OH) was used to acquire MRI data for all participants at the Texas Children’s Hospital in Houston and at the University of Arkansas for Medical Sciences in Little Rock. Comparable platforms and imaging software were used at the two scanner locations. American College of Radiology (ACR) phantom testing and quality assurance testing was regularly performed on both scanners, and both scanners were consistently noted to be within range throughout the duration of the study. The stability of the scanners over time were confirmed with similar value ranges for Weisskoff’s stability measurements (i.e., minimum 1/SNR index, peak-to-peak and RMS stability) (Weisskoff 1996) obtained on the day of each participant’s scan.
A spin echo technique was used for the acquisition of diffusion-weighted images, along with multiple slice, single-short, and EPI sequences. An 8-channel head coil was used to acquire 55 slices in the transverse plane, and diffusion was measured along 15 directions (number of b-factors = 2, bmax = 860). The following parameters were additionally used: TR = 10150.5 ms, TE = 90 ms, slice thickness/gap = 2.7/0 mm, FOV = 256, RFOV = 100%, measured voxel size (M × P × S) = 2.67 × 2.69 × 2.70 mm, reconstructed voxel size (M × P × S) = 2.0 × 2.0 × 2.7 mm, total scan duration = 345 seconds.
All data were carefully inspected for any artifacts or irregularities that may have compromised its accuracy or reliability. The Philips PRIDE-registration tool (Netsch 2001) was used to remove shear and eddy current distortion as well as head motion artifact prior to calculating FA maps with Philips fiber tracking 4.1v3 Beta 2 software (Cleveland, OH). To improve the signal-to-noise ratio via removal of eddy current distortion and motion artifact, two acquisitions were obtained and averaged using Philips diffusion affine registration tool. Each acquisition took approximately 290 seconds, and 70 slices were acquired.
Tractography
Based on a review of the previous literature, the total corpus callosum, the total fornix, the right and left cingulum bundles, the right and left perforant pathways, and the right and left uncinate fasciculi were deemed relevant white matter pathways and included in the present analysis. Although measurements were taken of the genu, splenium, and total corpus callosum, only the total corpus callosum is included here, owing to the limited statistical power resulting from the small sample size. The total fornix was measured using two seed points placed along the pathway in two coronal slices where the fornix body is visible as one bundle. After all ROIs were created, fiber tracts passing through the cingulum bundles, perforant pathways, and uncinate fasciculi were determined bilaterally, consistent with previously published protocols (Christidi et al. 2011; Levin et al. 2008; Wilde et al. 2006; Wilde et al. 2010).
Mean FA of all pathways was measured using Philips PRIDE software v4.0. Tractography was implemented using the Philips PRIDE v4.0 Fiber tracking 4.1 and the automated Philips 3D fiber tracking tool. The algorithm for fiber tracking is based upon the fiber assignment by continuous tracking (FACT) method (Mori et al. 1999). Tract termination occurred when the FA of a voxel fell below 0.2 or if the angle between adjacent voxels along the track was greater than 7 degrees; mean FA of each pathways was used as the quantitative measure for all DTI variables. Intra-and inter-rater reliabilities were calculated using Shrout-Fleiss reliability statistics (Shrout and Fleiss 1979). To examine intra-rater agreement, FA of each pathway was measured twice; intra-class correlation coefficients exceeded 0.98 in all cases. Inter-rater agreement was also assessed by the measurement of each structure by two different raters in three separate cases for both groups; intra-class correlation coefficients again exceeded 0.98 in all cases.
Neuropsychological Assessment
The Children’s version of the California Verbal Learning Test (CVLT-C; Delis et al., 1994) is a valid and reliable measure of episodic verbal learning and memory in children with considerable criterion validity in TBI (Levin et al. 2000; Roman et al. 1998; Yeates et al. 1995), as it is sensitive to mild to severe disabilities in learning, attention, and declarative memory. Mottram and Donders (2005) analyzed the latent structure of the CVLT-C when used with pediatric TBI populations and found that the highest factor loading on a construct of delayed recall was performance on the long-delay free recall (LDFR) trial (r = .98), providing support for its construct validity as the primary outcome measure in the present study. Furthermore, performance on the LDFR trial of the CVLT-C has been shown to be sensitive to parameters of TBI severity, including length of coma and neuroimaging findings (Mottram and Donders 2006).
Statistical Analyses
All statistical analyses were completed using Stata 15.1 (2017; College Station, TX: StataCorp LLC). All variables were initially screened for missing data, and those with greater than 5% of data missing were assessed for systematic patterns of missing data relating to LDFR performance; such a relationship was not found for any variable. Any variables with values exceeding +/− two interquartile ranges from the median value were considered univariate outliers and fenced accordingly. Homogeneity of variance in participant characteristics between TBI and TDC groups was be confirmed via Levene’s test prior to between-group comparisons using an independent sample t-test for age and Fisher’s exact test for sex, race/ethnicity, and handedness.
Levene’s test was also used to assess homogeneity of variance prior to between-group comparisons across outcome measures (i.e., FA of each pathway and LDFR score) using independent sample t-tests; Welch’s two sample t-test (Welch 1947) was used as a more reliable comparison method for variables with unequal variances (Ruxton 2006). Hedges’s g was utilized as an unbiased estimate of effect size for differences between group means (Keselman et al. 2008; Peng and Chen 2014), where g ≥ .20, .50, and .80 are interpreted as small, medium, and large, respectively (Hedges 1981). In an effort to reduce the type-I error rate after testing multiple comparisons, the threshold for significance was set to a two-tailed α = .01, and the results of all between-group analyses are reported along with 95% confidence intervals (CIs) in Table 3.
Table 3.
Results of between-group comparisons for outcome measures
| Outcome Measure | TDC | TBI | MD | t | df | 95% CI | Hedges’s g | |||
|---|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | n | M (SD) | LL | UL | |||||
| FA | ||||||||||
| CCa | 19 | .482 (.019) | 21 | .455 (.039) | .027** | 2.821 | 30.9 | .001 | .047 | .848 |
| FX | 17 | .351 (.018) | 20 | .325 (.024) | .026** | 3.747 | 35.0 | .012 | .040 | 1.210 |
| Right CBa | 19 | .396 (.026) | 21 | .378 (.045) | .018 | 1.563 | 33.8 | −.005 | .041 | .473 |
| Left CBa | 19 | .425 (.030) | 21 | .407 (.048) | .018 | 1.427 | 35.7 | −.008 | .043 | .433 |
| Right PPa | 19 | .342 (.016) | 21 | .326 (.039) | .016 | 1.737 | 27.7 | −.003 | .035 | .520 |
| Left PP | 19 | .329 (.024) | 21 | .319 (.036) | .010 | 1.024 | 38.0 | −.010 | .030 | .318 |
| Right UF | 18 | .379 (.020) | 20 | .368 (.033) | .010 | 1.153 | 36.0 | −.008 | .029 | .367 |
| LeftUF | 18 | .394 (.028) | 16 | .381 (.037) | .013 | 1.129 | 32.0 | −.010 | .036 | .378 |
| LDFR rawa | 19 | 12.00 (2.29) | 21 | 10.19 (3.75) | 1.809 | 1.862 | 34.9 | −.164 | 3.783 | .564 |
| LDFR z-score | 19 | 0.42 (0.99) | 21 | −0.25 (1.41) | .671 | 1.729 | 38.0 | −.115 | 1.457 | .536 |
TDC = typically developing children. TBI = traumatic brain injury. FA = fractional anisotropy. CC = corpus callosum. FX = fornix. CB = cingulum bundle. PP = perforant pathways. UF = uncinate fasciculus. LDFR = total score on the long-delay free recall trial of the California Verbal Learning Test – Children’s version (Delis et al. 1994). Hedges’s g ≥ .20, .50, and .80 are considered small, medium, and large effect sizes, respectively (Hedges 1981)
p < .01
Welch’s two-sample t-test (Welch 1947) was used to compare means with unequal variances
Mediation analysis was utilized to assess the role of FA in the relationship between GCS and LDFR. In the present model, GCS is the initial predictor, LDFR is the outcome variable, and FA of the white matter pathway of interest is included as a mediator (Figure 1). Only the TBI group was included in the mediation analyses, for those in the TDC group lack the examined data used for prediction in the model (i.e., GCS score). Our mediation analysis procedure followed the modified causal approach outlined by Shrout and Bolger (2002), which allows for further investigation following a nonsignificant total effect when two conditions are met: (1) it is likely that the effect is distal in nature and (2) the inclusion of the mediator allows for a more powerful test of the association than a test of the association between the predictor and outcome would be otherwise.
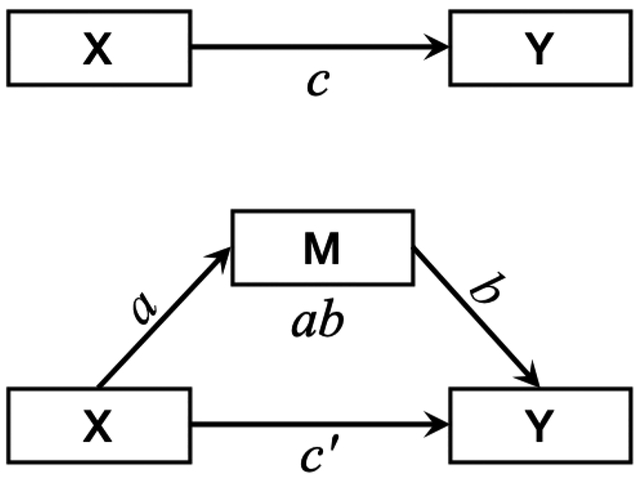
Fig. 1.
Mediation models demonstrating the total effect (c) of a predictor variable (X) on an outcome variable (Y; top) and the indirect effect (ab) of X on Y through a mediator variable (M; bottom). Path a represents the bivariate relationship between and X and M, where a one-unit change in X is associated with a change of a units in M. Path b represents the bivariate relationship between M and Y, where a one-unit change in M is associated with a change of b units in Y when X is held constant. Path c’ represents the direct effect of X on Y when M is included in the model but held constant. When mediation occurs, the direct effect is smaller than the total effect (c’ < c). When suppression occurs, the direct effect is smaller than the total effect and opposite in sign to the indirect effect
The total effect of GCS on LDFR (path c) and the three regression paths of the mediation model (paths a, b, c’) were initially estimated in Stata, and the indirect effect of GCS on LDFR through FA (path ab) in each pathway was estimated in R (v. 3.4.2) using the RMediation statistical package (Tofighi and MacKinnon 2011). Effect size was estimated using R2, which was calculated according to the modified squared semi-partial correlation approach suggested by de Haus (2012; see also Fairchild et al., 2009). The threshold for significance was set to an α = .05, and the results of all mediation analyses are reported along with 95% CIs in Table 4, and the full mediation models for all eight white matter pathways are presented in Figure 2.
Table 4.
Results of mediation analyses across all pathways
| Path | β | (SE) | 95% CI | R2 | ||
|---|---|---|---|---|---|---|
| LL | UL | |||||
| c | GCS → LDFR | .21 | (.21) | −.19 | .62 | .05 |
| Corpus Callosum | ||||||
| a | GCS → FA | .45 | (.16)* | .13 | .77 | .20 |
| b | FA → LDFR | .71 | (.14)*** | .43 | .99 | .40 |
| c’ | GCS → LDFR | −.11 | (.18) | −.46 | .25 | .01 |
| ab | GCS → FA → LDFR | .32 | (.13)** | .12 | .56 | .01 |
| Fornix | ||||||
| a | GCS → FA | .54 | (.15)*** | .25 | .83 | .29 |
| b | FA → LDFR | .37 | (.23) | −.09 | .82 | .10 |
| c’ | GCS → LDFR | .01 | (.25) | −.47 | .50 | .00 |
| ab | GCS → FA → LDFR | .20 | (.14) | −.01 | .45 | .03 |
| Right Cingulum Bundle | ||||||
| a | GCS → FA | .35 | (.19) | −.02 | .71 | .12 |
| b | FA → LDFR | .26 | (.21) | −.16 | .68 | .06 |
| c’ | GCS → LDFR | .12 | (.22) | −.31 | .55 | .01 |
| ab | GCS → FA → LDFR | .09 | (.10) | −.03 | .27 | .01 |
| Left Cingulum Bundle | ||||||
| a | GCS → FA | .40 | (.17)* | .06 | .75 | .16 |
| b | FA → LDFR | .37 | (.21) | −.03 | .78 | .12 |
| c’ | GCS → LDFR | .06 | (.22) | −.36 | .49 | .00 |
| ab | GCS → FA → LDFR | .15 | (.11) | .00 | .36 | .02 |
| Right Perforant Pathway | ||||||
| a | GCS → FA | .47 | (.16)** | .15 | .78 | .22 |
| b | FA → LDFR | .26 | (.23) | −.19 | .71 | .05 |
| c’ | GCS → LDFR | .09 | (.23) | −.37 | .55 | .01 |
| ab | GCS → FA → LDFR | .12 | (.12) | −.05 | .34 | .01 |
| Left Perforant Pathway | ||||||
| a | GCS → FA | .48 | (.16)** | .17 | .79 | .23 |
| b | FA → LDFR | .71 | (.15)*** | .42 | 1.00 | .39 |
| c’ | GCS → LDFR | −.13 | (.19) | −.49 | .24 | .01 |
| ab | GCS → FA → LDFR | .34 | (.13)** | .14 | .58 | .09 |
| Right Uncinate Fasciculus | ||||||
| a | GCS → FA | .51 | (.16)*** | .20 | .81 | .26 |
| b | FA → LDFR | .09 | (.26) | −.41 | .59 | .01 |
| c’ | GCS → LDFR | .07 | (.26) | −.44 | .57 | .00 |
| ab | GCS → FA → LDFR | .05 | (.14) | −.17 | .28 | .00 |
| Left Uncinate Fasciculus | ||||||
| a | GCS → FA | .62 | (.14)*** | .34 | .89 | .38 |
| b | FA → LDFR | .77 | (.20)*** | .38 | 1.15 | .37 |
| c’ | GCS → LDFR | −.31 | (.24) | −.78 | .16 | .06 |
| ab | GCS → FA → LDFR | .47 | (.16)*** | .23 | .76 | .14 |
Note. c = total effect. c’ = direct effect. ab = indirect effect. GCS = Glasgow Coma Scale score. FA = fractional anisotropy. LDFR = total z-score on the long-delay free recall trial of the California Verbal Learning Test – Children’s version (Delis et al. 1994) Bold text indicates indirect effects where GCS predicts LDFR through suppression by FA
p < .05
p < .01
p < .05
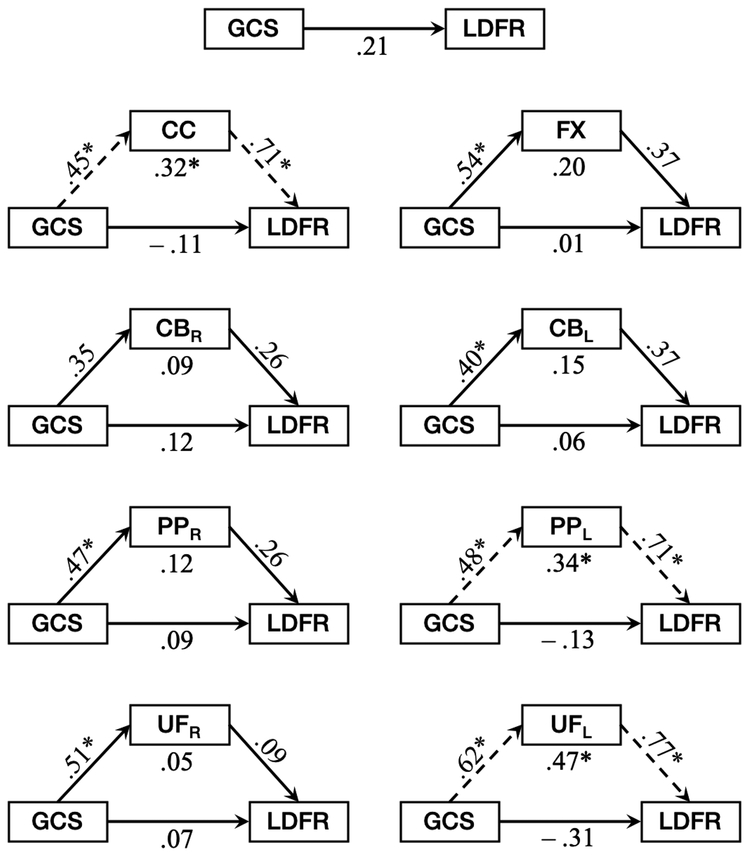
Fig. 2.
Mediation models demonstrating the total effect of injury severity (GCS) on verbal memory (LDFR; top) and the indirect effect of GCS on LDFR through fractional anisotropy (FA; lower eight) of the corpus callosum (CC), fornix (FX), right and left cingulum bundle (CBR, CBL), right and left perforant pathway (PPR, PPL), and right and left uncinate fasciculus (UFR, UFL). For each white matter pathway, bivariate and linear regression models were used to assess the bivariate relationship between GCS and FA, the bivariate relationship between FA and LDFR, holding GCS constant, and the direct effect of GCS on LDFR, holding FA constant. Each estimate along the path represents the standardized β coefficient from the regression model. Asterisks reflect statistically significant relationships (p < .05), and dashed arrows are indicative of indirect effects where GCS predicts LDFR through suppression by FA
Results
Participant Characteristics
Twenty-one participants were included in the TBI group (29% female) with ages ranging from 10 to 18 years (M = 13.57, SD = 2.32) at the time of participation. Participants in the TBI group sustained head injuries between 1 to 8 years of age (M = 4.10, SD = 2.02) as the result of a motor vehicle-accident, fall, or blunt force trauma, and the post-injury interval ranged from 5 to 15 years (M = 9.48, SD = 2.86). The TDC comparison group consisted of 19 healthy participants (32% female), between the ages of 9 and 17 years (M = 13.19, SD = 2.32). No significant differences in participant characteristics existed between TDC and TBI groups. Demographic and clinical characteristics are reported in Table 2.
Table 2.
Demographic and clinical characteristics between groups
| TDC | TBI | ||||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | All TBI | ||
| n | 19 | 3 | 4 | 14 | 21 |
| Sex | |||||
| Female | 6 | 0 | 2 | 4 | 6 |
| Male | 13 | 3 | 2 | 10 | 15 |
| Dominant Hand | |||||
| Right | 16 | – | 2 | 13 | 15 |
| Left | 3 | – | 2 | 1 | 3 |
| Mechanism | |||||
| MVA | – | 0 | 2 | 6 | 8 |
| Fall | – | 3 | 0 | 4 | 7 |
| BFT | – | 0 | 2 | 4 | 6 |
| Mean (SD) | |||||
| Age at testing | 13.19 (2.3) | 11.33 (2.5) | 13.75 (2.2) | 13.14 (2.4) | 13.57 (2.3) |
| Age at injury | – | 3.33 (1.5) | 4.75 (2.2) | 4.07 (2.1) | 4.10 (2.0) |
| Years post-injury | – | 11.33 (2.5) | 9.00 (3.7) | 9.47 (2.8) | 9.48 (2.9) |
| GCS | – | 14.33 (1.2) | 10.50 (1.3) | 5.71 (2.3) | 7.62 (3.8) |
| LDFR raw | 12.00 (2.3) | 10.67 (4.2) | 12.00 (4.8) | 9.57 (3.5) | 10.19 (3.8) |
| LDFR z-score | 0.42 (1.0) | −0.17 (1.6) | 0.25 (1.9) | −0.46 (1.4) | −0.25 (1.4) |
TDC = typically developing children. TBI = traumatic brain injury. MVA = motor vehicle accident. BFT = blunt force trauma. GCS = Glasgow Coma Scale score. LDFR = total score on the long-delay free recall trial of the California Verbal Learning Test – Children’s version (Delis et al. 1994)
Between-group Comparisons
In the assessment of differences in FA and LDFR between TBI and TDC groups, the results of independent t-tests (Table 3) revealed significant differences in FA of the corpus callosum and fornix, where FA was significantly lower in the TBI group for both pathways. There were no other significant differences in FA between groups; however, Hedges’s g estimates revealed small to medium effect sizes across all pathways, where FA was consistently lower in the TBI group relative to the TDC group. A medium effect size was also demonstrated for poorer verbal memory performance in the TBI group relative to the TDC group through lower LDFR scores in the former, although this difference was not statistically significant.
Mediation Analyses
The results of the mediation analysis (Table 3; Fig. 2) revealed a nonsignificant total effect (path c) of GCS as a predictor of LDFR in the TBI cohort. An estimate of the bivariate relationship between GCS and FA (path a) for each pathway demonstrated that GCS was a significant predictor of FA for the corpus callosum, the fornix, the left cingulum bundle, the right and left perforant pathways, and the right and left uncinate fasciculi. Significant effects of FA on LDFR, while holding the direct effect of GCS on LDFR constant (path b), were seen in the corpus callosum, the left perforant pathway, and the left uncinate fasciculus. The direct effect of GCS on LDFR, holding the relationship between FA and LDFR constant (path c’), was not significant for any pathway. Using RMediation software, our results demonstrated significant indirect effects of GCS on LDFR through FA (path ab) of the corpus callosum, the left perforant pathway, and the left uncinate fasciculus.
Discussion
The complex interplay between injury-and recovery-related factors, such as injury severity and white matter integrity, and their influence on cognitive functioning following pediatric TBI is poorly understood in the current literature. The present study sought to detect differences in FA of relevant white matter pathways and verbal memory performance between children who have suffered an early brain insult relative to those in the TDC group. In particular, the impact of injury severity and reduced FA on verbal memory performance was examined. Given that a greater extent of traumatic axonal injury is commonly seen in more severe injuries, it was hypothesized that FA mediates the association between injury severity and verbal memory in the chronic phase of recovery following pediatric TBI.
Relevant white matter pathways were chosen based on their involvement in verbal memory performance, as evidenced by previous literature implicating decreased microstructural integrity with increased injury severity and memory impairment in both pediatric (Mabbott et al. 2009; Wilde et al. 2010) and adult TBI (Christidi et al. 2011; Palacios et al. 2011; Palacios et al. 2013; Tomaiuolo et al. 2005). Pathways of interest included the corpus callosum, fornix, and the right and left cingulum bundles, perforant pathways, and uncinate fasciculi. In healthy populations, the cingulum bundles and perforant pathways are known to be important for memory consolidation, long-term retrieval, and delayed verbal memory recall (Augustinack et al. 2010; Yassa et al. 2010), while the corpus callosum, fornix, and uncinate fasciculi play a critical role in auditory-verbal declarative memory (Mabbott et al. 2009).
Between-group comparisons revealed significantly decreased FA in the corpus callosum and fornix of those with pediatric TBI in comparison to TDC. Although no other significant differences were found for FA or LDFR score, small to large estimates of effect size were demonstrated across all measures, such that the TBI group consistently demonstrated poorer outcomes than the TDC group (refer to results in Table 2). The results of the mediation analyses (Table 3; Fig. 2) demonstrated no significant total effect of GCS as a predictor of LDFR. This is likely due to the distal nature of such an effect, as the deficits in verbal memory develop over time following injury, and chronic outcome was of interest in the present study.
In support of the hypotheses that injury severity would be negatively associated with FA, and that FA would mediate the relationship between GCS and LDFR in chronic pediatric TBI, the present results revealed that GCS was a significant, positive predictor of FA of the corpus callosum, fornix, left cingulum bundle, and the right and left perforant pathways and uncinate fasciculi. Within these pathways, FA in the corpus callosum, left perforant pathway, and left uncinate fasciculus significantly predicted LDFR. No significant direct effects of GCS on LDFR were seen, however the direct effects were negative for the corpus callosum, left perforant pathway, and left uncinate fasciculus. In each of these pathways, significant indirect effects of GCS on LDFR were present through FA of the respective pathway, and all were positive, thus opposite in sign to their respective direct effects.
Within a mediation model, a suppression effect is considered to be present when the statistical control of variance in the mediator results in an increase in the magnitude of the effect that a predictor has on the outcome variable; suppression effects are represented by direct and indirect effects with opposing signs (MacKinnon et al. 2000; Paulhus et al. 2004). Anthony Conger (1974) defined a suppressor variable as “a variable which increases the predictive validity of another variable by its inclusion in the regression equation” (p. 36). This may occur in one of two ways: (1) classic suppression, where the only function of the suppressor variable is to remove criterion-irrelevant variance from the outcome variable, or (2) as a predictor that is uncorrelated with the other predictor, and whose only function is to account for a significant amount of variance in the outcome variable (Tzelgov and Henik 1991). In accordance with the latter case, our results suggest that FA of the corpus callosum, the left perforant pathway, and the left uncinate fasciculus acts as a suppressor of the effect that GCS has on LDFR; suppression occurred significantly in the corpus callosum, left perforant pathway, and left uncinate fasciculus. The opposing signs of the direct and indirect effects in the present results demonstrate that the predictive validity of GCS for LDFR is undermined by the substantial criterion-related variance arising from the relationship between FA and LDFR in these pathways, and the extent to which this occurs is evident by the effect size, represented by the value of R2. When FA is considered in this relationship and the opposing path is controlled, the contribution of FA to the variance in LDFR is revealed, and the direct effect of GCS on LDFR is estimated more accurately (Davis 1985; Paulhus et al. 2004).
An important limitation to the present study is the heterogeneity in demographic and injury-related characteristics between those with mild, moderate, and severe pediatric TBI. Although characteristics of the TBI group did not differ from those of the TDC group, the variability across levels of injury severity in the TBI group may have contributed to the lack of statistically significant differences in verbal memory performance and in FA of several white matter pathways. However, greater traumatic axonal injury is consistently associated with greater injury severity throughout the literature (Adnan et al. 2013; Ewing-Cobbs et al. 2008; Ghosh et al. 2009; Schonberger et al. 2009; Wilde et al. 2012); therefore, we are hesitant to rely too heavily on the present results, as they were obtained from a small sample size with insufficient power. Heterogeneity in the injury mechanism, pathophysiology, and the interval of time post-injury are also limitations in the present study. Finally, the present sample size is too small to consider the impact that age at injury, time post-injury, and primary injury pathology had on the development of neuronal networks. It is very possible that a relationship exists between such key developmental or injury-related factors and the recovery of damaged white matter in the developing brain, and this relationship should be addressed in future studies.
Regardless of these limitations, the results of the mediation analyses within the TBI group support our primary hypothesis, further suggesting that the predictive validity of acute injury severity for verbal memory outcome several years post-injury is magnified when FA of the corpus callosum, left perforant pathway, and left uncinate fasciculus is considered following pediatric TBI. Future studies with larger samples and a more homogenous distribution of patients across severity levels are necessary to provide more conclusive results. Additionally, future studies may consider using diffusion metrics, such as FA and radial diffusivity as proxies of injury severity, especially in studies where an extended period of time post-injury has elapsed. Finally, to reduce the likelihood of type-I error, we decided a priori to only use LDFR as the memory outcome measure. It is quite possible that there are additional important associations between specific white matter pathways and other aspects of memory and learning that should be investigated, even when limited to the CVLT-C.
Conclusions
This is the first investigation into the influence of microstructural damage to white matter on the relationship between injury severity and long-term verbal memory outcome following pediatric TBI. It is well known that extensive structural damage and persistent cognitive dysfunction are more likely to result from more severe injuries (Anderson et al. 2012; Faber et al. 2016; Ghosh et al. 2009; Lajiness-O’Neill et al. 2011; Wilde et al. 2012; Wilde et al. 2015; Yeates et al. 2017), but the knowledge of the specific contributions of these factors toward cognitive outcome is sparse. This is particularly true concerning damage to the developing brain, even though it is the most common cause of childhood disability (Cuff et al. 2007). Structural and functional outcomes following childhood injury differ greatly from those seen in adult TBI. Childhood injuries are more likely to result in extensive, long-lasting deficits and poorer prognosis relative to TBIs sustained in adulthood. Thus, a greater understanding of the primary mechanisms underlying from development following injury to the developing brain is essential.
The present study demonstrates that the efficacy of acute injury severity for predicting verbal memory after pediatric TBI is greatly enhanced when the integrity of relevant white matter pathways is considered at the time of follow-up. Support for this relationship is further provided by our findings that, amongst the pathways exhibiting the greatest influence on this relationship are those known to be the most vulnerable to focal impact and traumatic axonal injury following brain injury, including the corpus callosum and uncinate fasciculi (Bigler 2007; Levin 1993; Wilde et al. 2005), and those known to be largely involved in memory, including the cingulum bundles and perforant pathways (Baek et al. 2013; Christidi et al. 2011; Wilde et al. 2010).
Additionally, a pattern is seen in the results of our mediation analyses, where the direct effect of FA on LDFR is specific to pathways of the left hemisphere. As verbal abilities are predominately organized within the left hemisphere in most individuals, this pattern may represent further support for the relationship between white matter integrity and verbal memory after pediatric TBI. Leftward asymmetry across these pathways has been noted in healthy children (Dennis et al. 2015; Hasan et al. 2009; Wilde et al. 2009; Wilde et al. 2010; Yu et al. 2014) and is interpreted as an indication of the effects of maturation on the organization and myelination of fibers in the developing brain. Although no statistical analyses were conducted to control for hemispheric differences in FA, a follow-up analysis of the abnormal day-of-injury CT findings demonstrated no relationship between injury severity and the frequency of injuries to the right versus left hemispheres in our sample. It is possible, however, that the more rapidly developing pathways within the left hemispheric are simply more vulnerable to the effects of pediatric TBI than those of the right hemisphere (Ewing-Cobbs et al. 2016).
Although the present findings are by no means definitive when considered alone, they represent an important first step toward expanding the knowledge within the field and have important implications for understanding the course of recovery following insult to the developing brain.
Acknowledgments
We wish to express our gratitude to our participants and their family members. The study was funded by the National Institute of Health grant R21 NS065937 (‘Trauma to Developing Brain: Model Refinement and Therapeutic Intervention’; Levin, PI and Noble, PI). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest
Hannah M. Lindsey, Sanam Jivani Lalani, Jonathan Mietchen, Shawn D. Gale, Elisabeth A. Wilde, Jessica Faber, Marianne MacLeod, Jill V. Hunter, Zili D. Chu, Mary E. Aitken, Linda Ewing-Cobbs, and Harvey S. Levin declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
References
- Adnan A, Crawley A, Mikulis D, Moscovitch M, Colella B, & Green R (2013). Moderate-severe traumatic brain injury causes delayed loss of white matter integrity: Evidence of fornix deterioration in the chronic stage of injury. Brain Inj, 27(12), 1415–1422, doi: 10.3109/02699052.2013.823659. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, & Field AS (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316–329, doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V, & Catroppa C (2007). Memory outcome at 5 years post-childhood traumatic brain injury. Brain Inj, 21(13–14), 1399–1409, doi: 10.1080/02699050701785070. [DOI] [PubMed] [Google Scholar]
- Anderson V, Godfrey C, Rosenfeld JV, & Catroppa C (2012). Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics, 129(2), e254–261, doi: 10.1542/peds.2011-0311. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, Helmer K, Huber KE, Kakunoori S, Zollei L, & Fischl B (2010). Direct visualization of the perforant pathway in the human brain with ex vivo diffusion tensor imaging. Frontiers in Human Neuroscience, 4, 42, doi: 10.3389/fnhum.2010.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SO, Kim OL, Kim SH, Kim MS, Son SM, Cho YW, et al. (2013). Relation between cingulum injury and cognition in chronic patients with traumatic brain injury: Diffusion tensor tractography study. NeuroRehabilitation, 33(3), 465–471, doi: 10.3233/NRE-130979. [DOI] [PubMed] [Google Scholar]
- Bigler ED (2007). Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology, 21(5), 515–531, doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Christidi F, Bigler ED, McCauley SR, Schnelle KP, Merkley TL, Mors MB, et al. (2011). Diffusion tensor imaging of the perforant pathway zone and its relation to memory function in patients with severe traumatic brain injury. Journal of Neurotrauma, 28(5), 711–725, doi: 10.1089/neu.2010.1644. [DOI] [PubMed] [Google Scholar]
- Conger AJ (1974). A revised definition for suppressor variables: A guide to their identification and interpretation. Educational and Psychological Measurement, 34(1), 35–46. [Google Scholar]
- Cuff S, DiRusso S, Sullivan T, Risucci D, Nealon P, Haider A, et al. (2007). Validation of a relative head injury severity scale for pediatric trauma. Journal of Trauma, 63(1), 172–177; discussion 177–178, doi: 10.1097/TA.0b013e31805c14b1. [DOI] [PubMed] [Google Scholar]
- Davis JA (1985). The logic of causal order (Vol. 55). Thousand Oaks, CA: SAGE Publishing. [Google Scholar]
- de Heus P (2012). R squared effect-size measures and overlap between direct and indirect effect in mediation analysis. Behavioral Research Methods, 44(213–221). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, & Ober B (1994). Manual for the California Verbal Learning Test-Children’s Version. San Antonio, Texas: The Psychological Corporation. [Google Scholar]
- Dennis EL, Jin Y, Villalon-Reina JE, Zhan L, Kernan CL, Babikian T, et al. (2015). White matter disruption in moderate/severe pediatric traumatic brain injury: advanced tract-based analyses. Neuroimage Clin, 7, 493–505, doi: 10.1016/j.nicl.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan MC, Mummareddy N, Wellons JC 3rd, & Bonfield CM (2016). Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurg, 91, 497–509 e491, doi: 10.1016/j.wneu.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Johnson CP, Juranek J, DeMaster D, Prasad M, Duque G, et al. (2016). Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: Impact of age at injury and time since injury on pathway integrity. Hum Brain Mapp, 37(11), 3929–3945, doi: 10.1002/hbm.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS Jr., Fletcher JM, et al. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. NeuroImage, 42(4), 1305–1315, doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber J, Wilde EA, Hanten G, Ewing-Cobbs L, Aitken ME, Yallampalli R, et al. (2016). Ten-year outcome of early childhood traumatic brain injury: Diffusion tensor imaging of the ventral striatum in relation to executive functioning. Brain Inj, 30(13–14), 1635–1641, doi: 10.1080/02699052.2016.1199910. [DOI] [PubMed] [Google Scholar]
- Fairchild AJ, Mackinnon DP, Taborga MP, & Taylor AB (2009). R2 effect-size measures for mediation analysis. Behavioral Research Methods, 41(2), 486–498, doi: 10.3758/BRM.41.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Wilde EA, Hunter JV, Bigler ED, Chu Z, Li X, et al. (2009). The relation between Glasgow Coma Scale score and later cerebral atrophy in paediatric traumatic brain injury. Brain Inj, 23(3), 228–233, doi: 10.1080/02699050802672789. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, et al. (2009). Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res, 1276, 67–76, doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV (1981). Distribution theory for glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128. [Google Scholar]
- Hunter JV, Wilde EA, Tong KA, & Holshouser BA (2012). Emerging imaging tools for use with traumatic brain injury research. Journal of Neurotrauma, 29(4), 654–671, doi: 10.1089/neu.2011.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, et al. (2006). Examining the gateway to the limbic system with diffusion tensor imaging: The perforant pathway in dementia. NeuroImage, 30(3), 713–720, doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Algina J, Lix LM, Wilcox RR, & Deering KN (2008). A generally robust approach for testing hypotheses and setting confidence intervals for effect sizes. Psychological Methods, 13(2), 110–129, doi: 10.1037/1082-989X.13.2.110. [DOI] [PubMed] [Google Scholar]
- Kirkham FJ, Newton CR, & Whitehouse W (2008). Paediatric coma scales. Developmental Medicine & Child Neurology, 50(4), 267–274. [DOI] [PubMed] [Google Scholar]
- Lajiness-O’Neill R, Erdodi L, & Bigler ED (2011). Demographic and injury-related moderators of memory and achievement outcome in pediatric TBI. Applied Neuropsychology, 18(4), 298–308, doi: 10.1080/09084282.2011.595457. [DOI] [PubMed] [Google Scholar]
- Levin HS (1993). Head trauma. Curr Opin Neurol, 6(6), 841–846. [DOI] [PubMed] [Google Scholar]
- Levin HS, Song J, Scheibel RS, Fletcher JM, Harward HN, & Chapman SB (2000). Dissociation of frequency and recency processing from list recall after severe closed head injury in children and adolescents. Journal of Clinical and Experimental Neuropsychology, 22(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, et al. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil, 23(4), 197–208, doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott DJ, Rovet J, Noseworthy MD, Smith ML, & Rockel C (2009). The relations between white matter and declarative memory in older children and adolescents. Brain Res, 1294, 80–90, doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, & Lockwood CM (2000). Equivalence of the mediation, confounding and suppression effect. Prevention Science, 1(4), 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, & van Zijl PC (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45(2), 265–269. [DOI] [PubMed] [Google Scholar]
- Mottram L, & Donders J (2005). Construct validity of the California Verbal Learning Test–Children’s Version (CVLT-C) after pediatric traumatic brain injury. Psychological Assessment, 17(2), 212–217, doi: 10.1037/1040-3590.17.2.212. [DOI] [PubMed] [Google Scholar]
- Mottram L, & Donders J (2006). Cluster subtypes on the California Verbal Learning Test–Children’s Version after pediatric traumatic brain injury. Developmental Neuropsychology, 30(3), 865–883, doi: 10.1207/s15326942dn3003_6. [DOI] [PubMed] [Google Scholar]
- Netsch T (2001). Towards real-time multi-modality 3-D medical image registration Paper presented at the International Conference on Computer Vision, Vancouver, Canada. [Google Scholar]
- Niogi SN, & Mukherjee P (2010). Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil, 25(4), 241–255, doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- Palacios EM, Fernandez-Espejo D, Junque C, Sanchez-Carrion R, Roig T, Tormos JM, et al. (2011). Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol, 11, 24, doi: 10.1186/1471-2377-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios EM, Sala-Llonch R, Junque C, Fernandez-Espejo D, Roig T, Tormos JM, et al. (2013). Long-term declarative memory deficits in diffuse TBI: Correlations with cortical thickness, white matter integrity, and hippocampal volume. Cortex, 49(3), 646–657, doi: 10.1016/j.cortex.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Paulhus DL, Robins RW, Trzesniewski KH, & Tracy JL (2004). Two replicable suppressor situations in personality research. Multivariate Behavioral Research, 39(2), 303–328, doi: 10.1207/s15327906mbr3902_7. [DOI] [PubMed] [Google Scholar]
- Peng C-YJ, & Chen L-T (2014). Beyond Cohen’s d: Alternative effect size measures for between-subject designs. The Journal of Experimental Education, 82(1), 22–50, doi: 10.1080/00220973.2012.745471. [DOI] [Google Scholar]
- Rogalski EJ, Murphy CM, deToledo-Morrell L, Shah RC, Moseley ME, Bammer R, et al. (2009). Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment: A diffusion tensor imaging study. Behavioral Neurology, 21(1), 51–61, doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman MJ, Delis DC, Willerman L, Magulac M, Demadura TL, de la Peña JL, et al. (1998). Impact of pediatric traumatic brain injury on components of verbal memory. Journal of Clinical and Experimental Neuropsychology, 20(2), 245–258. [DOI] [PubMed] [Google Scholar]
- Ruxton GD (2006). The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behavioral Ecology, 17(4), 688–690. [Google Scholar]
- Schonberger M, Ponsford J, Reutens D, Beare R, & O’Sullivan R (2009). The relationship between age, injury severity, and MRI findings after traumatic brain injury. Journal of Neurotrauma, 26, 2157–2167, doi:10.1089=neu.2009.0939 [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Masdeu JC, Sastre-Garriga J, Goni J, Velez-de-Mendizabal N, Duque B, et al. (2008). Mapping the brain pathways of declarative verbal memory: Evidence from white matter lesions in the living human brain. NeuroImage, 42(3), 1237–1243, doi: 10.1016/j.neuroimage.2008.05.038. [DOI] [PubMed] [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods, 7(4), 422–445. [PubMed] [Google Scholar]
- Shrout PE, & Fleiss JL (1979). Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin, 86(2), 420–428. [DOI] [PubMed] [Google Scholar]
- Teasdale G, & Jennett B (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet, 2(7872), 81–84. [DOI] [PubMed] [Google Scholar]
- Tofighi D, & MacKinnon DP (2011). RMediation: An R package for mediation analysis confidence intervals. Behavioral Research Methods, 43(3), 692–700, doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F, Worsley KJ, Lerch J, Di Paola M, Carlesimo GA, Bonanni R, et al. (2005). Changes in white matter in long-term survivors of severe non-missile traumatic brain injury: A computational analysis of magnetic resonance images. Journal of Neurotrauma, 22(1), 76–82. [DOI] [PubMed] [Google Scholar]
- Tzelgov J, & Henik A (1991). Suppression situations in psychological research: Definitions, implications, and applications. Psychological Bulletin, 109(3), 524–536. [Google Scholar]
- Voelbel GT, Genova HM, Chiaravalotti ND, & Hoptman MJ (2012). Diffusion tensor imaging of traumatic brain injury review: Implications for neurorehabilitation. NeuroRehabilitation, 31(3), 281–293, doi: 10.3233/NRE-2012-0796. [DOI] [PubMed] [Google Scholar]
- Weisskoff RM (1996). Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magnetic Resonance Medicine, 36(4), 643–645. [DOI] [PubMed] [Google Scholar]
- Welch BL (1947). The generalisation of student’s problems when several different population variances are involved. Biometrika, 34(1–2), 28–35. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Ayoub KW, Bigler ED, Chu ZD, Hunter JV, Wu TC, et al. (2012). Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: Changes within an 18 month post-injury interval. Brain Imaging Behav, 6(3), 404–416, doi: 10.1007/s11682-012-9150-y. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma, 23(10), 1412–1426, doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, et al. (2005). Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma, 22(3), 333–344, doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Chu Z, Hunter JV, Bigler ED, Yallampalli R, et al. (2009). Diffusion tensor imaging of hemispheric asymmetries in the developing brain. J Clin Exp Neuropsychol, 31(2), 205–218, doi: 10.1080/13803390802098118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Jivani S, Hanten G, Faber J, & Gale SD (2015). Neuropsychological consequences of paediatric brain injury: Executive Function In Reed J, Byard K, & Fine H (Eds.), Neuropsychological Rehabilitation of Childhood Brain Injury: A Practical Guide. New York, NY: MacMillan Publishers. [Google Scholar]
- Wilde EA, Ramos MA, Yallampalli R, Bigler ED, McCauley SR, Chu Z, et al. (2010). Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Developmental Neuropsychology, 35(3), 333–351, doi: 10.1080/87565641003696940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DH, Levin HS, & Eisenberg HM (1990). Mild head injury classification. Neurosurgery, 27(3), 422–428. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, & Stark CE (2010). Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences USA, 107(28), 12687–12691, doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Blumenstein E, Patterson CM, & Delis DC (1995). Verbal learning and memory following pediatric closed-head injury. Journal of the International Neuropsychological Society, 1(1), 78–87. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Levin HS, & Ponsford J (2017). The Neuropsychology of traumatic brain injury: Looking back, peering ahead. Journal of the International Neuropsychological Society, 23(9–10), 806–817, doi: 10.1017/S1355617717000686. [DOI] [PubMed] [Google Scholar]
- Yu Q, Peng Y, Mishra V, Ouyang A, Li H, Zhang H, et al. (2014). Microstructure, length, and connection of limbic tracts in normal human brain development. Front Aging Neurosci, 6, 228, doi: 10.3389/fnagi.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]