Abstract
The activity of cardiac and skeletal muscles depends upon the ATP-coupled actin-myosin interactions to execute the power stroke and muscle contraction. The goal of this review article is to provide insight into the function of myosin II, the molecular motor of the heart and skeletal muscles, with a special focus on the role of myosin II light chain (MLC) components. Specifically, we focus on the involvement of myosin regulatory (RLC) and essential (ELC) light chains in striated muscle development, isoform appearance and their function in normal and diseased muscle. We review the consequences of isoform switching and knockout of specific MLC isoforms on cardiac and skeletal muscle function in various animal models. Finally, we discuss how dysregulation of specific RLC/ELC isoforms can lead to cardiac and skeletal muscle diseases and summarize the effects of most studied mutations leading to cardiac or skeletal myopathies.
Introduction
The striated muscle sarcomere contains a system of interdigitating myosin II-containing thick filaments and actin/tropomyosin (Tm)/troponin(Tn)-containing thin filaments (Schiaffino et al. 1996). Myofibrillar contraction involves the sliding of the thick filaments past the thin filaments (Huxley 1957, Huxley 1969), and this process is tightly controlled by changes in the cytosolic concentrations of Ca2+ that bind to Tm-Tn regulatory complex (Ebashi 1974). Specifically, binding of Ca2+ to TnC triggers a series of conformational changes in actin-Tm-Tn thin filaments, allowing for ATP-dependent cyclic interactions of myosin cross-bridges with actin and muscle contraction (Huxley 1985, Geeves et al. 2005). With every beat of the heart or flexing of the biceps, it is myosin II that is responsible for producing the contractile force. Chemical energy from ATP hydrolysis is converted into the mechanical force driving muscle contraction.
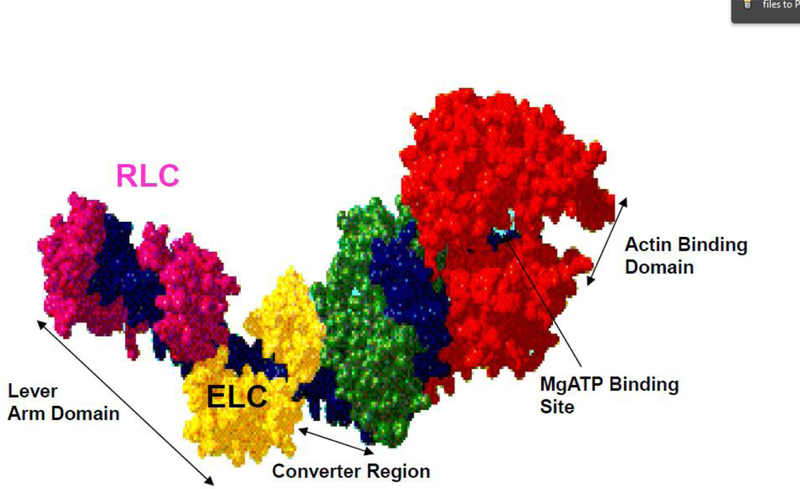
Myosin II is a hexameric molecule ~150 nm in length and is composed of two heavy chains (MHCs), two regulatory (RLC) and two essential (ELC) light chains (Rayment et al. 1993, Trybus 1994, Szczesna-Cordary 2003). The sequence of the myosin rod, which directs thick filament assembly, shows the classic seven-residue heptad repeat that is considered the hallmark of coiled-coil structures (Taylor et al. 2015). Important domains of the myosin head (cross-bridge) include the motor domain containing the ATP and actin binding sites, and the lever arm, which undergoes large rotational motions that drive the power stroke (Rayment et al. 1993, Geeves 2002). Both myosin light chains, RLC and ELC, are localized at their respective IQ (IQxxxRGxxxR) motifs of the myosin lever arm region (Figure 1).
Figure 1.
The myosin head (S1) derived from the atomic structure by Rayment et al., 1993. The heavy chain is shown in green, red, and blue to highlight functional domains. The essential light chain and regulatory light chain function to support the myosin neck region and are colored in yellow (ELC) and magenta (RLC) (pdb: 2MYS).
To fully understand the function of myosin and how it powers cardiac or skeletal muscle, it is essential to have insight into the role of myosin light chain (MLC) components. This review focuses on the involvement of myosin RLC and ELC in striated muscle development and their function in cardiac and skeletal muscle in health and disease.
I. Diverse appearance and functions of MLCs
Our bodies contain three types of muscles: smooth, skeletal and cardiac muscles expressed in their specific locations, with each type having explicit structural and functional characteristics. Cardiac and skeletal muscles are striated muscles due to their myofibrillar appearance of repeating Z-disk-originating sarcomeres. They share many contractile and regulatory similarities, but they also differ with respect to cell shapes, rhythmic patterns, mitochondria number, and tissue-specific protein expression. Cardiac myocytes (or cardiomyocytes) make up the majority of cardiac cell types, and they are responsible for involuntary contractions of ventricles necessary for pumping of blood to the organs (Rehman et al. 2018). Skeletal muscles are under the voluntary control of the nervous system, but they are similarly organized and regulated as cardiac muscles.
The myosin regulatory and essential light chains structurally support the neck region of myosin motor (cross-bridge) that powers cardiac and skeletal muscle contraction via chemomechanical transduction of ATP free energy used to produce the power stroke and drive striated muscle contraction (Geeves et al. 2005). The mechanical coupler is the lever arm that rotates cyclically to impel bound actin (Wang et al. 2013, Wang et al. 2014, Wang et al. 2016). RLC and ELC are inherent components of myosin lever arm (Eldin et al. 1994, Alamo et al. 2017) (Figure 1), but they are also capable of modulating myosin motor activity, and thus muscle contraction in a light chain-dependent manner (reviewed in (Szczesna-Cordary 2003, Hernandez et al. 2007)). Structurally, both RLC and ELC belong to the EF-hand calcium-binding protein family, whose members include calmodulin and TnC, each containing a homologous helix-loop-helix region composed of a 12-amino acid Ca2+-binding loop flanked by two perpendicular α-helices (EF-hand motifs) (Figure 2). The EF-hand homolog superfamily of proteins contains a multitude of different Ca2+-modulated proteins, which have a broad range of functions (Villalobo et al. 2019).
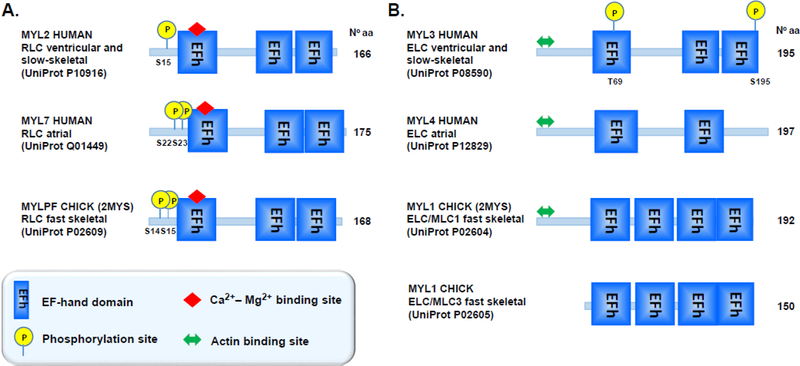
Figure 2.
Domain organization of human isoforms of RLC (A) and ELC (B) proteins. Both light chains contain the EF-hand Ca2+ binding motifs (depicted with blue boxes) computed by UniProt (accession numbers listed in parentheses). Out of all EF-hand Ca2+ binding sites on RLC and ELC, only first EF-hand site on the RLC can bind Ca2+ and/or Mg2+. The N-terminal region of RLC contains phosphorylatable Serine(s) (Ser14/Ser15), targets of myosin light chain kinase (MLCK). The ELC contains two potential phosphorylation sites (T69 and S195), identified by proteomics. In addition to myosin heavy chain binding site, the N-terminus ELC contains additional actin binding site, allowing ELC to make molecular contacts with actin during contraction.
I.1. Myosin RLC (MLC-2)
Myosin RLC (~19 kDa) is a primary regulatory subunit of smooth muscle myosin and a modulator of the Ca2+-Tm-Tn – regulated striated muscle contraction (Sweeney et al. 1993, Szczesna-Cordary 2003). RLC belongs to the EF-hand calcium binding protein superfamily, and its N-terminal EF-hand domain can bind calcium or magnesium at the Ca2+-Mg2+ binding site (Figure 2A). RLC also contains a highly conserved N-terminal phosphorylatable serine constituting a myosin light chain kinase (MLCK)-dependent phosphorylation site (Sweeney et al. 1993, Muthu et al. 2012). The Ca2+-calmodulin-activated MLCK phosphorylation of this site in smooth muscle activates contraction (Sobieszek 1977, Hartshorne et al. 1982), while in skeletal and cardiac muscle, phosphorylation of RLC modulates contraction by increasing the Ca2+ sensitivity and the level of contractile force and also by modulating the kinetics of force-generating myosin cross-bridges (reviewed in (Sweeney et al. 1993)).
Cardiac RLC in mouse myocardium can be either singly or doubly phosphorylated at Ser14 or Ser15, while human cardiac RLC has only one functional phosphorylatable site at Ser15 (Scruggs et al. 2010, Gregorich et al. 2017) (Figure 2A). At the level of protein, phosphorylation of RLC at Ser15 was shown to alter the secondary structure (α-helical content) and Ca2+ binding affinity of the human ventricular RLC protein (Szczesna et al. 2001). Adjacent to Ser15, the human cardiac RLC contains Asn14, which can be deamidated to aspartic acid, thereby creating a charge similar to that of RLC phosphorylation (Scruggs et al. 2010). Phosphorylation of Ser15 on myosin RLC has been widely recognized to play an important role in cardiac muscle contraction under both normal and disease conditions, and this modification receives a lot of recognition as a potential novel therapeutic target to battle heart disease (Muthu et al. 2012, Muthu et al. 2014, Yuan et al. 2015, Yadav et al. 2019).
Striated muscle RLC or MLC-2 appears in three different isoforms depending on where they are expressed in tissues. The MYL2 gene encodes the RLC protein in heart ventricles and in slow-twitch skeletal muscle, while the MYL7 gene encodes the cardiac atrial isoform of RLC. The MYLPF gene encodes for the fast-twitch skeletal muscle isoform, designated as MLC-2f, and its expression is restricted to fast skeletal muscles (Figure 2A).
I.2. Myosin ELC (MLC-1)
Myosin ELC (~22 kDa) also belongs to the EF-hand Ca2+ binding protein family but unlike RLC, it has lost the ability to bind Ca2+(only scallop myosin ELC binds Ca2+) (Kwon et al. 1990, Grabarek 2006) (Figure 2B). The N-terminus of ELC (N-ELC) draws a lot of attention in myofilament research because of its importance in actin-myosin interaction and ability to modulate force. Numerous studies indicate that during striated muscle contraction, there is a direct interaction between the N-ELC and actin (Winstanley et al. 1977, Henry et al. 1985, Milligan et al. 1990, Morano et al. 1995). It is speculated that the positively charged N-ELC, that exclusively occurs in the heart, can make contacts with the negative C-terminus of actin (Sutoh 1982, Timson et al. 1999) and lead to potential alterations in force development during muscle contraction (Morano et al. 1995, Sweeney 1995, Rarick et al. 1996, Miller et al. 2005, Haase et al. 2006). Three-dimensional maps of vertebrate muscle thin filaments obtained by cryo-electron microscopy revealed that the N-terminal portion of the myosin ELC is in a position to make molecular contacts with the C-terminus of the actin monomer (Milligan et al. 1990). Structural modeling study from Morano’s group (Aydt et al. 2007) depicts the N-ELC as a rod-like 91 Å-long extension that can function as a bridge between the ELC core of the myosin head and the binding site of the ELC on the actin filament. Using electron cryomicroscopy in conjunction with light-scattering and fluorescence analyses, Lowey et al. demonstrated the binding of the N-terminal extension of the ELC to the SH3 domain of MHC and subsequently to actin (Lowey et al. 2007). Utilizing 1H NMR techniques, Timson et al. revealed that the major actin binding region occurred in the very N-terminal four residues (APKK) of ELC (Timson et al. 1999). It was postulated that the N-ELC-actin interaction can alter the function of myosin as the molecular motor, change binding of the myosin head (S1) to actin and influence the cross-bridge cycling kinetics. This ultimately can lead to changes in force production and muscle contraction (for review see (Timson 2003) and (Hernandez et al. 2007)). N-ELC was proposed to function as a tether between the myosin head and actin capable of regulating the thick and thin filament interactions and force production in the heart (Miller et al. 2005, Kazmierczak et al. 2009). Studies from the Burghardt laboratory proposed a novel mechanism of N-ELC mediated step frequency modulation to control myosin power output (Burghardt et al. 2015, Wang et al. 2016, Wang et al. 2018). Despite these multilevel investigations, the questions as to whether these protein-protein contacts between the N-ELC and actin promote or inhibit the affinity of myosin for actin, force production and muscle contraction are still to be determined. Studies from the Szczesna-Cordary group revealed that the lack of the N-terminal ELC extension in Tg-Δ43 mice, expressing a 43 aa truncated ELC, led to changes in myosin head orientation, positioning it closer to the actin filaments (Muthu et al. 2011). The important function of the N-terminal extension of the ELC protein was also demonstrated in another study using Tg-Δ43 animals that showed that the length-dependent activation in Tg-Δ43 muscle strips was blunted (Michael et al. 2013). This could eventually lead to hypertrophy in Tg-Δ43 mice, but of non-pathological nature (Kazmierczak et al. 2014). The C-terminal region of myosin ELC was shown to interact with MHC and possibly with RLC (Houdusse et al. 1996). Using pharmacologically preconditioned cardiomyocytes, the Van Eyk’s group identified Ser195 and Thr69 on myosin ELC as phosphorylatable residues (Arrell et al. 2001) (Figure 2). The physiological significance of Ser195-ELC phosphorylation was demonstrated in zebrafish system using C-terminally truncated mutant (lazm647) of ELC, where severe contractile insufficiency and early embryonic death were observed (Meder et al. 2009, Scheid et al. 2016). Phosphorylation of ELC was also thought to be an important mechanism controlling the intracellular action of matrix metalloproteinase 2 (MMP-2) during myocardial ischemia/reperfusion injury and promoting degradation of ELC (Cadete et al. 2012). In contrast to these reports, no phosphorylation of ventricular or atrial ELC isoforms was found in either human or swine myocardium by top-down mass spectrometry by the Ge group (Gregorich et al. 2017).
ELC is known to exist in several isoforms that are expressed in specific locations and differ in their amino acid sequences (Figure 2B). In the heart, the atria and ventricles express two different ELC isoforms encoded by the MYL4 (atrial ELC) and MYL3 (ventricular ELC) genes. In fast-twitch skeletal muscles, two ELC variants are expressed by the MYL1 gene (Buckingham et al. 1998), but due to a differential splicing event, they differ by ~43 amino acids at the N-terminus (long MLC1 and short MLC3). In addition to ventricular ELC, the MYL3 gene also encodes the slow-skeletal ELC isoform (Stuart et al. 2016). All ELC proteins (except MLC3) express the long form of ELC that contain the extended N-terminus (Fodor et al. 1989), known to bind to the C-terminus of actin (Trayer et al. 1987, Aydt et al. 2007).
In summary, both MLCs are key components of myosin, critically important to myosin motor function evidenced by many to play a role in the regulation of striated muscle contraction. As such, it is necessary to examine how each MLC isoform is expressed throughout development in cardiac and skeletal muscle tissues (Figure 3).
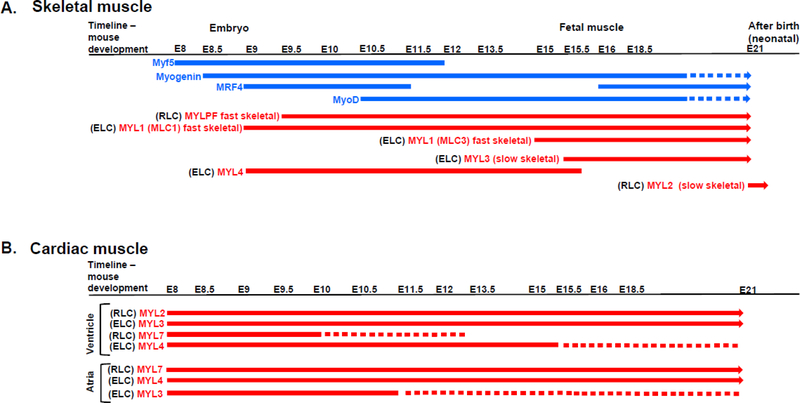
Figure 3.
Developmental expression of myogenic factors and different isoforms of myosin light chains in skeletal (A) and cardiac (B) muscles of mice. E denotes embryonic day. Abbreviations of MLCs isoforms as in Fig. 2. Dashed lines indicate decreased transcript expression. For references, see paragraph II. Myogenesis of striated muscle components.
II. Myogenesis of striated muscle components
During striated muscle development, a complex array of gene expression generates the diversity of muscle types found in the adult vertebrate (Kelly et al. 1995). Cardiac and skeletal muscles originate from the mesodermal cell layer, more precisely from the lateral plate mesoderm and paraxial mesoderm of somites, respectively (Tirosh-Finkel et al. 2006).
II.1. Myogenesis in skeletal muscles
In skeletal muscles, myogenesis starts with the determination of multipotential mesodermal cells to give rise to myoblasts to eventually form skeletal muscle fibers (or myocytes) (Furst et al. 1989). Regulation of the majority of sarcomeric genes is under transcriptional control mediated by several families of regulatory proteins, including those of the MyoD and MEF2 transcription factors (Weintraub, 1993; Yu et al., 1992). The key transcription factors regulating mouse skeletal myogenesis during embryonic development and the time-line expression of MLCs in skeletal muscles are summarized in Figure 3A.
Regulation of many sarcomeric genes is mediated by several families of regulatory proteins belonging to the basic helix-loop-helix (bHLH) category of myogenetic regulatory factors (MRF) and comprise 4 members: Myf5, Myogenin, MRF4 and MyoD (Murre et al. 1989). Myf-5, pax3 and pax7 are the first transcription factors to be detected during embryonic development inducing the formation of primary myotomes which express specific cytoskeletal proteins such as the slow skeletal MHC (Myh7), embryonic MHC (Myh3) and myosin light chain 1 (Myl1) (Babai et al. 1990, Kelly et al. 1997, Biressi et al. 2007). Following this, expression of an array of transcription factors (myf-5. MyoD, myogenin and MRF4) are observed, leading to fetal fiber formation expressing markers for fast MHC isoforms (Van Horn et al. 1989) and myosin light chain 3 (myl3) (Messina et al. 2010) which will give rise to myocytes via myogenin regulation, ultimately forming myofibers (modulation) (Hasty et al. 1993).
More specifically, skeletal myogenesis begins with the expression of Myf-5 at embryonic day 8 (E8) (high levels at E9.25) in the dermomyotome of somites before formation of myotome. Myf-5 gradually decreases from E11.5 (Ott et al. 1991). Myogenin starts to accumulate in the myotomes of somites from E8.5 until E17.5 and occurs at the same time as cardiac actin expression (Sassoon et al. 1989). MRF4 is an interesting transcription factor as it shows a biphasic pattern of expression during mouse development. Arnold et al. showed that MRF4 is first detected in the somites of embryo from E9 to E11, and its expression declines from E11.5 to E15 and increases again from E16 to birth, where it becomes highly expressed (Bober et al. 1991). MRF4 is the most predominant form of MRF in adult muscle (Hinterberger et al. 1991). MyoD is the last transcription factor regulating myogenesis in skeletal muscle and is not detectable in the myotome until E10.5 where its expression rapidly increases until E17.5 and continues to be expressed at high levels in embryonic and fetal development (Sassoon et al. 1989, Ott et al. 1991). A summary of transcription factors involved in RLC and ELC expression as well as the timeline of expression during skeletal muscle development is provided in Figure 3A.
II.1.1. Expression of RLC in skeletal muscles development
RLC exists in three major isoforms, each with distinct temporal and spatial expression pattern during myogenesis. MLC-2f (MYLPF gene) is restricted to fast-twitch skeletal muscle of neonatal and adult vertebrates. The expression of MLC-2f is initiated in the myotomal regions of roastral somites at E9.5, the time by which myogenin is already expressed (Faerman et al. 1993). Surprisingly, at this stage, MLC-2f is also expressed in the cardiomyocytes, but this fades away at later stages. MYL2, encoding the slow-twitch skeletal RLC isoform was shown to be only detectable after birth in hind limb muscle, and its expression peaked at 10 days after birth (Wang et al. 2007) (Figure 3A).
II.1.2. Expression of ELC in skeletal muscles development
The expression of ELC transcripts has been extensively studied by Lyons et al. (Lyons et al. 1990). The authors demonstrated that each transcript has a specific pattern of expression in mouse embryonic skeletal muscles. For instance, MLC1f begins to be expressed at E9 while MLC3f only starts to be expressed in skeletal muscles at E15.5. Study by Ontell et al. however, reports on MLC3f expression at E13.5 (Ontell et al. 1993). Interestingly, cardiac-specific ELC isoforms have also been detected in skeletal muscles and demonstrate distinct pattern of expression (Figure 3A). MYL4 (atrial ELC) is the most predominant gene expressed at E9.5, but its expression in skeletal muscle decreases at E15.5. MYL3 (ventricular ELC) is not detected in the skeletal muscle until E15.5 and continues to be expressed until after birth (Figure 3A).
II.2. Myogenesis in cardiac muscles
The heart is the first organ to develop in the body, and its normal function is necessary for maintaining life. Mouse heart development resembles human development and is divided into 5 major stages, which are finely tuned and are tightly regulated by a variety of transcription factors that are essential for mouse cardiac myogenesis (Table 1). These developmental stages will eventually give rise to chambers and associated heart structures necessary for appropriate pumping of blood to the organs. The five stages of mouse heart development are differentiated by embryonic days and start with the formation of the cardiac crescent at E7.5. At this stage, Mesp½ is considered as one of the earliest markers of cardiac myogenesis, and it allows migration of cardiac precursor cells to their appropriate location (Saga et al. 1999, Bruneau 2013). These cells include two major cell populations referred to as heart fields, in which the first heart field (FHF) will give rise to the left ventricle and parts of the atria while the second heart field (SHF) will primarily induce formation of the arterial pole of the heart (Buckingham et al. 2005). Another factor contributing to the cardiac crescent formation is Nkx2–5 (part of the NK-class homeodomain-containing transcription factor), considered as a master gene playing important roles in the differentiation and morphogenesis in early heart development (Lints et al. 1993, Lyons et al. 1995). The second stage occurs around E8 and results from migration of cardiac precursor cells to form a linear heart tube governed by an array of transcription factors including the GATA family of zinc finger-containing transcription factors (GATA4 and GATA6) (Charron et al. 1999), Mesp½ (Kitajima et al. 2000), Hand1 and Hand2 (bHLH family of transcription factors) (Srivastava et al. 1995) and MEF2C (Edmondson et al. 1994). Following this, the heart begins to loop around E8.5–9.5, and the beginning of chamber formation is noticeable with the single ventricle that bulges out of the looping heart, following right ventricle and atria (Bruneau 2013). At this stage, in addition to the previously mentioned transcription factors, Tbx5, a member of the T-transcription factor family is expressed and plays key roles in cardiomyocytes maturation (Steimle et al. 2017). Next, around E10-E12, the chambers form with the appearance of outflow tract and both atria in a process regulated by Nkx2–5, Tbx5, GATA4, Hand1–2 (Bruneau 2002). Finally, from E12 to birth, ventricles and atria mature and septate as well as the valves and interventricular septum (IVS) begin to form under the control of Nkx2–5, Tbx5 and GATA4, which are the most prominent factors regulating this final process (Schott et al. 1998, Koshiba-Takeuchi et al. 2009, Bruneau 2013). The timeline of expression of the RLC and ELC isoforms in the ventricles and atria are summarized in Figure 3B.
Table 1.
Major stages of mouse heart development and key transcription factors implicated in cardiac development in mice.
| Stage in the heart development | Embryonic day | Transcription factors |
|---|---|---|
| Cardiac crescent | E7.5 | Nkx2–5, Mesp1, Mesp2 |
| Linear heart tube | E8.0 | GATA4, MEF2C, Mesp1, Mesp2, Hand1, Hand2 |
| Cardiac looping | E8.5-E9.5 | Nkx2–5, Tbx5, GATA4, MEF2C, Hand1, Hand2 |
| Chamber formation | E10-E12 | Nkx2–5, Tbx5, Hand1, Hand2, GATA4 |
| Maturation/septation | E12-birth | Nkx2–5, Tbx5, GATA4 |
Abbreviations: Nkx2–5 - NK2 homeobox 5 transcription factor, Mesp1/Mesp2 - mesoderm posterior bHLH (basic helix-loop-helix) transcription factor 1/2, GATA4 - zinc finger protein 4 that bind the consensus DNA sequence (T/A)GATA(A/G), MEF2C - myocyte enhancer binding factor 2C, Hand1/Hand2 - heart- and neural crest derivatives-expressed protein 1/2, Tbx5- T-box transcription factor 5. E denotes embryonic day.
II.2.1. Expression of cardiac RLC isoforms
During mammalian cardiac-specific myogenesis in murine embryogenesis, expression of ventricular RLC is restricted to the ventricular segment of the linear heart tube as early as day 8.0 postcoitum (p.c.) and shows regionalization to the ventricular compartment by day 11 (O’Brien et al. 1993) (Figure 3B). It remains restricted to the ventricular chamber throughout embryonic development into adulthood, and this restriction occurs prior to septation, suggesting that the molecular cues dictating MYL2 gene expression are independent of physiological cues governing distinct cardiac chamber formation (O’Brien et al. 1993). Ablation of MYL2 in mice resulted in embryonic lethality at E12.5, and was associated with ultrastructural defects in ventricular sarcomere assembly, despite an increase in the atrial RLC isoform expression and its thick filament incorporation (Chen et al. 1998). During embryogenesis, the atrial RLC isoform (MYL7 gene) has been shown to be expressed earlier (E7.5) than the ventricular RLC (MYL2), and uniformly expressed throughout the heart tube formation. Its expression is selectively downregulated in the ventricular chamber beginning at E10 and is undetectable in ventricular chambers by E12 (Kubalak et al. 1994) (Figure 3B).
II.2.2. Expression of cardiac ELC isoforms
According to Lyons et al., the expression of MYL3 (ventricular ELC) in the slow-twitch skeletal muscle starts at E15 and continues throughout adulthood (Lyons et al. 1990). The ventricular ELC isoform (MYL3 gene) is expressed in all myocardial cells including atrial cells, but its expression in the atria decreases from E11.5 to 15.5 d p.c., when its expression becomes restricted to the ventricular cells (Lyons et al. 1990, Lyons et al. 1990). Just like ventricular ELC, the atrial ELC (MYL4 gene) is expressed in all myocardial cells up until day 15.5 p.c., when it is mainly expressed in the atria (Lyons et al. 1990) (Figure 3B). The mRNA levels of atrial ELC gradually decrease, but its expression is still detectable in ventricular cells until a few days after birth (Lyons et al. 1990). Although both ELC isoforms (ventricular and atrial) are encoded by different genes, the DNA and amino acid sequence homology is high (Kurabayashi et al. 1988). Petzhold et al. reported ~80% identity between the sequences of ventricular and atrial ELC; however, their affinities to actin were not the same, despite having similar amino acid residues in the critical actin binding region on the N-terminus ELC (Petzhold et al. 2014).
III. Effects of genetic MLC ablation/isoform switch in RLC and ELC animal models
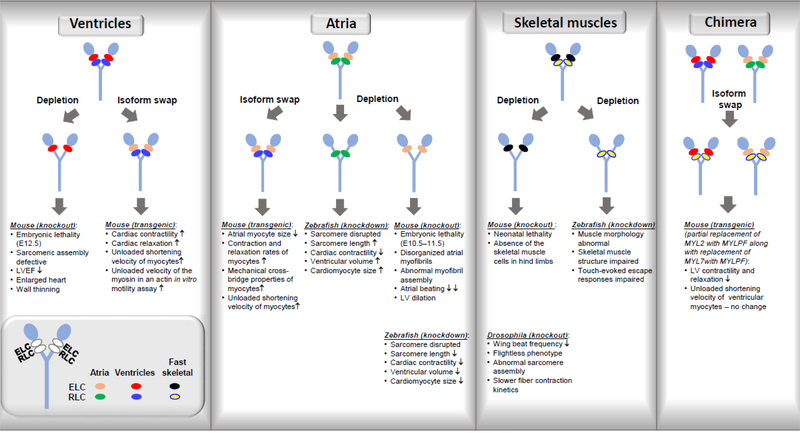
The highly conserved amino acid sequences of individual MLC isoforms reflect a unique functional requirement for the specific RLC and ELC isoforms to be expressed in distinct muscle cell types. The consequences of MLC isoform switching/depletion in striated muscle myosin on the ability of myosin to generate force and motion and on function of cardiac and skeletal muscle are summarized in Figure 4.
Figure 4.
Effect of genetic manipulations in myosin light chains of cardiac and skeletal muscle observed in various animal models.
III.1. Effects of genetic manipulations in ventricles
The effects of genetic depletion of myosin RLC in the ventricles of mice have been examined by Chen et al. over two decades ago (Chen et al. 1998). Lack of the ventricular RLC isoform in knock-out mice resulted in embryonic lethality at E12.5 even though the level of atrial RLC was increased reaching levels comparable to the ventricular RLC. Ultrastructural analysis revealed defects in sarcomeric assembly and an embryonic form of DCM characterized by reduced left ventricular ejection fraction in mutant embryos compared with wild-type littermates (Chen et al. 1998). This seminal study clearly demonstrated a unique function of ventricular myosin regulatory light chain in ventricular muscle cells and its indispensable function in sarcomeric assembly during mammalian cardiogenesis and the maintenance of cardiac contractility (Chen et al. 1998) (Figure 4).
The specific functions of the ventricular versus atrial isoforms of the myosin ELC were tested in transgenic mice by the Robbins laboratory (Fewell et al. 1998). Specifically, the authors examined the effects of replacement of ventricular ELC isoform by atrial ELC in the ventricles of mice (Figure 4). This is because pathological cardiac phenotypes are often associated with increased atrial ELC expression in the ventricles of patients with ischemic, dilated and hypertrophic cardiomyopathies (Schaub et al. 1984). The data showed that unloaded velocities and the ability of myosin to translocate actin filaments in the in vitro motility assay were significantly increased as a result of the ELC isoform substitution. In addition, this isoform switch led to increased cardiac contractility/relaxation at the whole organ level, without any hypertrophic response (Fewell et al. 1998). The authors concluded that the increased ELC atrial expression in the ventricles of mice was advantageous to the heart. These data are in accord with the human study by Abdelaziz et al. (Abdelaziz et al. 2004) who showed that the incorporation of the atrial ELC isoform into the failing myocardium acted as a compensatory response by enhancing shortening velocity and recovering mechanical power output (Palmer 2005).
Addressing the functional significance of actin-ELC interaction and the role of the N-terminal region of ELC in this interaction, Robbins and colleagues generated transgenic mice in which the mouse atrial ELC or ventricular ELC contained a 10-amino acid deletion at positions 5–14 of the N-terminus of ELC (Sanbe et al. 2001). Surprisingly, when the kinetics of skinned fibers isolated from ELCvΔ5–14 or ELCaΔ5–14 mice were examined, no alterations in either the unloaded shortening velocity or maximum shortening velocity were observed. Myofibrillar ATPase activity was also unchanged in these preparations (Sanbe et al. 2001). However, in the following work of Miller et al. (Miller et al. 2005) who employed osmotically compressed muscle strips from these ELCvΔ5–14 mice, a decreased maximum isometric tension without a change in calcium sensitivity was observed, suggesting that the interaction between residues 5–14 of the N-ELC and actin enhances cardiac performance (Miller et al. 2005).
III.2. Effects of genetic manipulations in atria
To understand the role of the atrial isoform of RLC (MYL7 gene), Huang et al. generated MYL7 deficient mice, lacking one of the major structural components of the atrial myofibrillar apparatus (Huang et al. 2003) (Figure 4). Homozygous null mice demonstrated embryonic lethality at E10.5–11.5 due to cardiovascular insufficiency (defects in formation of intraembryonic vasculature) and the lack of atrial myofibrillar organization followed by diminished atrial contraction, suggesting that atrial function is essential for embryogenesis. In contrast, left ventricles of null mice contracted normally, and displayed normal myofilament assembly (Huang et al. 2003). These results were consistent with previous studies from this group, which demonstrated that although atrial RLC is initially expressed throughout the linear heart tube before becoming restricted to the atria at E12.5 (Kubalak et al. 1994), RLC atrial protein is not incorporated into left ventricle structures (Chen et al. 1998).
The effects of atrial RLC replacement by ventricular RLC isoform have been studied in atrial myocytes of transgenic mice (Figure 4). Exchanging atrial RLC with ventricular isoform led to enhancement of mechanical properties of transgenic atrial myocytes to the level seen in ventricular myocytes of control animals. In addition, ventricular myocytes isolated from transgenic mice were morphologically indistinct from those of controls, while atrial myocytes were significantly shorter than atrial myocytes similarly isolated from non-transgenic littermates (Pawloski Dahm et al. 1998). The authors also showed that the amplitude of Ca2+ signals were lower in atrial transgenic myocytes compared to non-transgenic atrial myocytes. Using the same animal model, Buck et al. showed an increase in shortening velocity in transgenic atrial myocytes expressing ventricular RLC compared to wild-type mice. These data suggested that the presence of ventricular RLC isoform in atria of mice is responsible for stronger contractile force. The greater shortening velocity of atrial myocytes is consistent with greater power-generating requirements of ventricular compartment compared with atrial tissue (Buck et al. 1999).
Using zebrafish model, Chen et al. investigated the specific functions of the two main ELC and RLC orthologues in zebrafish heart development (Chen et al. 2008) with cmlc1 most closely related to MYL4 (atrial ELC) and cmlc2 to MYL7 (atrial RLC) (Rottbauer et al. 2006). Removal of cmlc1 or cmlc2 led to disruption of sarcomeres and decreased cardiac contractility. Interestingly, cmlc1 morphants exhibited an increase in cardiomyocyte size and number, sarcomere length, and ventricular chamber volume, while removal of cmlc2 led to decreased sarcomere length, ventricular volume and cardiomyocyte size, suggesting that ELC and RLC have distinct roles in cardiac function and development (Chen et al. 2008) (Figure 4).
III.3. Effects of genetic manipulations in skeletal muscles
Total absence of fast-twitch skeletal RLC isoform in mouse skeletal muscles led to neonatal lethality of mice presumably due to the respiratory failure as their diaphragms did not develop (Wang et al. 2007). Furthermore, this resulted in total absence of the skeletal muscle cells in hind limb, and the lack of skeletal muscle formation in the null mice did not affect the development of other organs including the heart (Figure 4). In addition, the authors found that wild-type mice did not express the ventricular/slow skeletal RLC isoform until after birth, while it was expressed normally in the embryonic heart. These results suggested the total dependence of skeletal muscle development on the presence of fast-twitch RLC isoform during embryogenesis (Wang et al. 2007).
The absence of RLC in skeletal muscles was also studied using a null mutation of the myosin RLC locus of Drosophila melanogaster by Warmke et al. (Warmke et al. 1992). Mlc2E38 null conferred dominant flightless behavior that was associated with reduced wing beat frequency. Heterozygotes flies exhibited a 50% reduction of RLC mRNA concentration in adult thoracic musculature, which resulted in a proportionate reduction of RLC protein in the indirect flight muscles that also showed aberrant myofilament structures at the periphery. Ca2+-activated fibers exhibited slower contraction kinetics compared with wild-type. These results indicated that a proper RLC stoichiometry is required for normal indirect flight muscle assembly and function (Warmke et al. 1992). Interestingly, study by Gulick showed that partial replacement of MYL2 by MYLPF along with replacement of MYL7 with MYLPF led to decreased left ventricular contractility and relaxation while unloaded shortening velocity of the ventricular myocytes was not affected (Gulick et al. 1997).
The importance of skeletal ELC isoforms for the interaction of myosin with actin was demonstrated in the study by VanBuren et al., where selective removal of ELC from chicken skeletal myosin reduced isometric force by 50% (VanBuren et al. 1994). More recently, removal of skeletal muscle ELC in zebrafish (myl1) via generation of morpholino knockdown resulted in abnormal muscle morphology, disrupted muscle structure and impaired touch-evoked escape responses, indicating that skeletal muscle fast-twitch specific myosin ELC is critical for appropriate muscle development and function (Ravenscroft et al. 2018).
IV. Involvement of striated MLC isoforms in disease
The process of myogenesis in cardiac and skeletal muscles has gained much attention in the field of myopathies where understanding of detailed developmental expression of sarcomeric genes, including myosin light chains, is critical to elucidating the effects of hereditary sarcomeric mutations on the structure and function of skeletal and cardiac muscle and in development of various forms of myopathies (Wang et al. 2018). The pathogenesis of these mutations (mostly point mutations) that can lead to cardiac and skeletal myopathies has been extensively studied in the last 50 years, and the most-studied mutations in MLCs are summarized below.
IV.1. Skeletal and cardiac myopathies associated with MYL2
Albeit minimal, skeletal muscle involvement has been described in idiopathic cardiomyopathy in studies dating back as early as 1949 (Evans 1949, Gaunt et al. 1956). In 1971, Shafiq et al. conducted the first detailed electromyographic and histological study to describe several unusual patterns of skeletal muscle involvement in patients with idiopathic cardiomyopathy such as selective atrophy of type II fibers, pronounced non-selective myopathy and hypotrophy of type I fibers (Shafig et al. 1972). A plethora of studies have since shown the existence of skeletal similar abnormalities in cardiomyopathies patients (Hootsmans et al. 1971, Isaacs et al. 1975, Smith et al. 1976, Lochner et al. 1981, Przybojewski et al. 1981, Dunnigan et al. 1984, Dunnigan et al. 1987). Skeletal muscle abnormalities such as myogenic myopathy, with an absence of neurogenic abnormalities, have been described in patients with dilated and hypertrophic cardiomyopathy (Caforio et al. 1989).
Infantile fiber-type disproportion with type I fiber hypotrophy, cardiomyopathy and myofibrillar lysis in type I muscle fibers was previously reported in three Dutch families and described as a progressive myopathy with onset shortly after birth and a cardiomyopathy leading to early death in infants (Barth et al. 1998). Only recently, Wetermen et al. identified the genetic cause of this disease and reported three different recessive mutations in MYL2 for this ‘light chain myopathy’ (Weterman et al. 2013). A homozygous splice site mutation (c.403–1G>C) was detected in the last acceptor splice site of the MYL2 gene and mRNA analysis showed a cryptic splice site 23 nucleotides upstream of the original splice site, thereby resulting in a frameshift mutation and replacement of the last 32 codons by 20 different codons and thus altering the C-terminal part of the protein. In addition, whole exome sequencing of the Italian patient revealed compound heterozygosity for two other mutations, c.431delC; p.Pro144LeufsX2 and c.432delT; p.Asp145ThrfsX2, that affected two adjacent nucleotides in the last exon of the MYL2 gene. Both mutations had resulted in a frame shift and premature termination in the third codon of the shifted open reading frame (original codon 146), leading to mutant truncated proteins that are 20 amino acids shorter than the normal protein (Weterman et al. 2013). Using recombinant human cardiac IVS6–1 RLC protein in reconstituted porcine cardiac system, Zhou et al. showed reduced ATPase activity as well as stopped-flow dissociation kinetics, and decreased binding to MHC as well as and reduced binding of IVS6–1-reconstituted myosin to actin in rigor (Zhou et al. 2016). In skinned porcine cardiac muscles, RLC-depleted and IVS6–1 reconstituted muscle strips displayed a significant decrease in maximal contractile force and a significantly increased Ca2+ sensitivity, both hallmarks of hypertrophic cardiomyopathy (HCM)-associated mutations in MYL2. More recently, R58Q mutation, shown by population studies to cause HCM and sudden cardiac death in families of various ethnic origins (Flavigny et al. 1998, Kabaeva et al. 2002, Yadav et al. 2019), have been reported to manifest functional, structural, and energetic changes in slow twitch soleus skeletal muscles, presumably via protein–protein and cellular interactions (Kazmierczak et al. 2019).
Ever since the very first discovery of A13T, E22K and P95A during RLC screening in HCM patients with pronounced mid cavity obstruction, a plethora of mutations in ventricular MYL2 have since been associated with one and more types of familial cardiomyopathies. F18L, M20L, I44M, N47K, R58Q, K104E, E134A, I158L, G162R, D166A, D166V and IVS5–2 have been associated with HCM, while G57E and D94A have been linked to RCM and DCM, respectively (detailed review in (Yadav et al. 2019)). These mutations, depending upon the location and nature, often lead to biophysical and biochemical alterations, thereby altering the enzymatic, binding, and force production capability of myosin. Because of genetic heterogeneity and phenotypic variability in HCM, there is no clear correlation between the location of the mutation on the RLC gene and the consequent phenotypes. With the exception of R58Q-RLC mutation (Yadav et al. 2019), HCM mutations in myosin RLC induce a hypercontractility phenotype (Spudich 2014) and are associated with an increase in Ca2+ sensitivity of force (Poggesi et al. 2014) and occurrences of fibrosis and myofilament disarray (Teekakirikul et al. 2012). The only studied DCM-associated D94A-RLC mutation was shown to induce the hypocontractile activity of myosin motors and resulted in the rightward shift of the force-pCa dependence curve (Yuan et al. 2018).
IV.2. Skeletal and cardiac myopathies linked to MYL1/MYL3
Congenital myopathies following mutations in MYL1 have recently been identified in two probands (Ravenscroft et al. 2018). The group found a homozygous essential splice acceptor variant (c.479–2A>G), predicted to induce exon 5 skipping and a homozygous missense substitution (c.488T>G, p.M163R) in two Turkish patients. Analysis of muscle pathology revealed increased myopathic features with infiltration of fatty acids, small type II myofibers in proband 1 (age 5 months) while proband 2 (age 8 months) showed hypotonic muscles, marked axial weakness and almost absence of type II myofibers. This study also revealed an absence of MYL1 in skeletal muscle biopsies of both patients via Western blotting. The authors concluded that remodeling of MYL1 in the proband with the M163R mutation may affect the binding of ELC to MHC, ultimately impacting myosin motor function in skeletal muscles. In addition, the group also showed that knockdown of myl1 in zebrafish led to abnormal morphology and disrupted muscle structure, further strengthening the idea that myl1 is essential for skeletal muscle function (Ravenscroft et al. 2018).
Analyses of skeletal tissues with individuals with mutations in MYL3 have been performed, and a study by Poetter et al. (Poetter et al. 1996) found that biopsy obtained from soleus and deltoid muscles of patients with the M149V-ELC mutation showed myopathic changes associated with increased accumulation of mitochondria and ragged red fiber (RRF) pattern with no abnormality of cytochrome oxidase. Pathological phenotypes in MYL3 may also occur in response to change in its local expression. Shi et al. indicated an increase of ventricular ELC expression in the atria of children with different forms of congenital heart diseases (Chaudhuri et al.), i.e. tetralogy of Fallot (TOF) and perimembranous ventricular septal defects (Shi et al. 1991).
Mutations in the ventricular ELC isoform (MYL3 gene) have been identified by population studies to cause the HCM phenotypes. Among them, 5 missense mutations are located in exon 3 (E56G, A57G, R63C, V79I, R81H), 6 in exon 4 (G128C, E143K-HCM with restrictive physiology, M149V, E152K, R154H, H155D) and 2 in exon 5 (M173V, E177G). The phenotypic characterization of these HCM-ELC mutations has been reviewed in detail in (Yadav et al. 2019). As it is discussed there, for many of the mutations, there is a profound lack of mechanistic in vitro studies using recombinant ELC proteins and very few studies show the data from animal models (Muthu et al. 2011, Kazmierczak et al. 2013, Kazmierczak et al. 2014, Yuan et al. 2017). Further studies on transgenic ELC mutant animals would help in understanding the unknown mechanisms behind some of these poorly studied mutations.
Changes in expression of atrial ELC (MYL4 gene) in other regions apart from the atrium postnatally have been associated with congenital heart defects (CHDs) and cardiomyopathies and linked with pressure overload (England et al. 2013). Children with tetralogy of Fallot were shown to express large amounts of atrial ELC in their ventricles, replacing the endogenous ventricular ELC (MYL3) of this region (Auckland et al. 1986). Pathological phenotypes associated with atrial ELC were also found to occur in response to a change in its expression in ventricles of patients with ischemic, dilated and hypertrophic cardiomyopathies (Schaub et al. 1984, Sutsch et al. 1992). A study by Morano et al. showed that increased expression of atrial ELC in patients with different CHDs resulted in higher shortening velocity of isometric tension production in skinned fibers obtained from the right ventricular infundibulum (Morano et al. 1996). In a recent study by Orr et al., the authors identified a novel E11K mutation in atrial ELC in a family with a previously unreported syndrome characterized by early-onset atrial fibrillation AF (age <35 years), conduction disease and signs of a primary atrial myopathy (Orr et al. 2016). To test pathogenicity of this MYL4 mutation, the authors generated a transgenic zebrafish line expressing the corresponding mutation in the zebrafish ortholog cmlc1 under control of the atrial-specific myh6 promoter. Mutant zebrafish showed disruption of sarcomeric structure, atrial enlargement and electrical abnormalities, the phenotype closely mirroring human AF (Orr et al. 2016). The whole-genome sequencing of the Icelandic population found a recessive frameshift deletion mutation in MYL4 (c.234delC, p.Cys78Trpfs*29) that causes early-onset AF (Gudbjartsson et al. 2015). In the following study by Gudbjartsson et al., the authors reported on eight homozygous carriers of this mutation, all of whom had early-onset AF. Six of the homozygotes were diagnosed by the age of 30 and the remaining two in their 50s. Three of the homozygotes had received pacemaker implantations due to sick sinus syndrome, while three had suffered an ischemic stroke, and one suffered sudden cardiac death (Gudbjartsson et al. 2017).
Summary, conclusions and future directions
The goal of this review was to summarize the role of myosin regulatory and essential light chains in cardiac and skeletal muscle development and function in normal and disease states. RLC and ELC are essential constituents of myosin, which stabilize the lever arm and modulate myosin motor activity and contribute to force production. MLCs are expressed in several isoforms depending on whether they are expressed in atria, ventricles or skeletal muscles where they contribute to tissue-specific function. Cardiac and skeletal myogenesis refer to the process of converting progenitor cells to myocytes via expression of specific transcription factors necessary for determination of unique RLC and ELC isoforms. Understanding this process is crucial in dissecting specific functions of MLCs in regulating cardiac or skeletal muscle contraction. We reviewed the consequences of isoform switching and knockout of specific MLC isoforms in the heart and skeletal muscles on cardiac and skeletal muscle function in various animal models.
We also focused on the effects of MLC mutations that lead to cardiac or skeletal myopathies with an emphasis on atrial and skeletal MLCs isoforms as the ventricular isoforms’-linked myopathies have been recently addressed in our 2019 review (Yadav et al. 2019). Research on mouse models of cardiac disease greatly furthers the understanding of the role of myosin RLC and ELC in cardiac disease, allowing for physiological and biochemical studies in controlled genetic environment. However, mouse hearts have very different physiology than human hearts (heart rate, different ion channels, different Ca2+ handling machinery, and different MHC isoforms), and thus, transgenic mouse models do not always recapitulate the human disease phenotype and pharmacological response. Future approaches may utilize cardiac tissues generated from human induced pluripotent stem cells (iPSCs) that can serve as platforms for patient/mutation-specific studies of physiology and disease to give unique insights into the disease pathogenesis that cannot be recapitulated in other systems.
Acknowledgments
Funding: This work was supported by the National Institutes of Health R01-HL123255 (DSC), and the American Heart Association 17PRE33650085 (SY).
References
- Abdelaziz A, Segaric J, Bartsch H, Petzhold D, Schlegel W-P, Kott M, Seefeldt I, Klose J, Bader M, Haase H and Morano I (2004). “Functional characterization of the human atrial essential myosin light chain (hALC-1) in a transgenic rat model.” Journal of Molecular Medicine 82(4): 265–274. [DOI] [PubMed] [Google Scholar]
- Alamo L, Ware JS, Pinto A, Gillilan RE, Seidman JG, Seidman CE and Padron R (2017). “Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes.” Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrell DK, Neverova I, Fraser H, Marban E and Van Eyk JE (2001). “Proteomic analysis of pharmacologically preconditioned cardiomyocytes reveals novel phosphorylation of myosin light chain 1.” Circ Res 89(6): 480–487. [DOI] [PubMed] [Google Scholar]
- Auckland LM, Lambert SJ and Cummins P (1986). “Cardiac myosin light and heavy chain isotypes in tetralogy of Fallot.” Cardiovasc Res 20(11): 828–836. [DOI] [PubMed] [Google Scholar]
- Aydt EM, Wolff G and Morano I (2007). “Molecular modeling of the myosin-S1(A1) isoform.” Journal of Structural Biology 159(1): 158–163. [DOI] [PubMed] [Google Scholar]
- Babai F, Musevi-Aghdam J, Schurch W, Royal A and Gabbiani G (1990). “Coexpression of alphasarcomeric actin, alpha-smooth muscle actin and desmin during myogenesis in rat and mouse embryos I. Skeletal muscle.” Differentiation 44(2): 132–142. [DOI] [PubMed] [Google Scholar]
- Barth PG, Wanders RJ, Ruitenbeek W, Roe C, Scholte HR, van der Harten H, van Moorsel J, Duran M and Dingemans KP (1998). “Infantile fibre type disproportion, myofibrillar lysis and cardiomyopathy: a disorder in three unrelated Dutch families.” Neuromuscul Disord 8(5): 296–304. [DOI] [PubMed] [Google Scholar]
- Biressi S, Tagliafico E, Lamorte G, Monteverde S, Tenedini E, Roncaglia E, Ferrari S, Ferrari S, Cusella-De Angelis MG, Tajbakhsh S and Cossu G (2007). “Intrinsic phenotypic diversity of embryonic and fetal myoblasts is revealed by genome-wide gene expression analysis on purified cells.” Dev Biol 304(2): 633–651. [DOI] [PubMed] [Google Scholar]
- Bober E, Lyons GE, Braun T, Cossu G, Buckingham M and Arnold HH (1991). “The muscle regulatory gene, Myf-6, has a biphasic pattern of expression during early mouse development.” J Cell Biol 113(6): 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau BG (2002). “Transcriptional regulation of vertebrate cardiac morphogenesis.” Circ Res 90(5): 509–519. [DOI] [PubMed] [Google Scholar]
- Bruneau BG (2013). “Signaling and transcriptional networks in heart development and regeneration.” Cold Spring Harb Perspect Biol 5(3): a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck SH, Konyn PJ, Palermo J, Robbins J and Moss RL (1999). “Altered kinetics of contraction of mouse atrial myocytes expressing ventricular myosin regulatory light chain.” Am J Physiol 276(4 Pt 2): H1167–1171. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Kelly R, Tajbakhsh S and Zammit P (1998). “The formation and maturation of skeletal muscle in the mouse: the myosin MLC1F/3F gene as a molecular model.” Acta Physiol Scand 163(3): S3–5. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S and Zaffran S (2005). “Building the mammalian heart from two sources of myocardial cells.” Nat Rev Genet 6(11): 826–835. [DOI] [PubMed] [Google Scholar]
- Burghardt TP, Sun X, Wang Y and Ajtai K (2015). “In vitro and in vivo single myosin step-sizes in striated muscle.” J Muscle Res Cell Motil 36(6): 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadete VJ, Sawicka J, Jaswal JS, Lopaschuk GD, Schulz R, Szczesna-Cordary D and Sawicki G (2012). “Ischemia/reperfusion-induced myosin light chain 1 phosphorylation increases its degradation by matrix metalloproteinase 2.” FEBS J 279(13): 2444–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caforio AL, Rossi B, Risaliti R, Siciliano G, Marchetti A, Angelini C, Crea F, Mariani M and Muratorio A (1989). “Type 1 fiber abnormalities in skeletal muscle of patients with hypertrophic and dilated cardiomyopathy: evidence of subclinical myogenic myopathy.” J Am Coll Cardiol 14(6): 1464–1473. [DOI] [PubMed] [Google Scholar]
- Charron F and Nemer M (1999). “GATA transcription factors and cardiac development.” Semin Cell Dev Biol 10(1): 85–91. [DOI] [PubMed] [Google Scholar]
- Chaudhuri T, Mukherjea M, Sachdev S, Randall JD and Sarkar S (2005). “Role of the fetal and alpha/beta exons in the function of fast skeletal troponin T isoforms: correlation with altered Ca2+ regulation associated with development.” J Mol Biol 352(1): 58–71. [DOI] [PubMed] [Google Scholar]
- Chen J, Kubalak SW, Minamisawa S, Price RL, Becker KD, Hickey R, Ross J Jr. and Chien KR (1998). “Selective Requirement of Myosin Light Chain 2v in Embryonic Heart Function.” J. Biol. Chem 273(2): 1252–1256. [DOI] [PubMed] [Google Scholar]
- Chen Z, Huang W, Dahme T, Rottbauer W, Ackerman MJ and Xu X (2008). “Depletion of zebrafish essential and regulatory myosin light chains reduces cardiac function through distinct mechanisms.” Cardiovasc Res 79(1): 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnigan A, Pierpont ME, Smith SA, Breningstall G, Benditt DG and Benson DW Jr. (1984). “Cardiac and skeletal myopathy associated with cardiac dysrhythmias.” Am J Cardiol 53(6): 731–737. [DOI] [PubMed] [Google Scholar]
- Dunnigan A, Staley NA, Smith SA, Pierpont ME, Judd D, Benditt DG and Benson DW Jr. (1987). “Cardiac and skeletal muscle abnormalities in cardiomyopathy: comparison of patients with ventricular tachycardia or congestive heart failure.” J Am Coll Cardiol 10(3): 608–618. [DOI] [PubMed] [Google Scholar]
- Ebashi S (1974). “Regulatory mechanism of muscle contraction with special reference to the Ca-troponin-tropomyosin system.” Essays Biochem 10: 1–36. [PubMed] [Google Scholar]
- Edmondson DG, Lyons GE, Martin JF and Olson EN (1994). “Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis.” Development 120(5): 1251–1263. [DOI] [PubMed] [Google Scholar]
- Eldin P, Le Cunff M, Vosberg HP, Mornet D and Leger JJ (1994). “Mapping of the actomyosin interfaces.” Proc Natl Acad Sci U S A 91(7): 2772–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England J and Loughna S (2013). “Heavy and light roles: myosin in the morphogenesis of the heart.” Cell Mol Life Sci 70(7): 1221–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W (1949). “Familial cardiomegaly.” Br Heart J 11(1): 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faerman A and Shani M (1993). “The expression of the regulatory myosin light chain 2 gene during mouse embryogenesis.” Development 118(3): 919–929. [DOI] [PubMed] [Google Scholar]
- Fewell JG, Hewett TE, Sanbe A, Klevitsky R, Hayes E, Warshaw D, Maughan D and Robbins J (1998). “Functional significance of cardiac myosin essential light chain isoform switching in transgenic mice.” J Clin Invest 101(12): 2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavigny J, Richard P, Isnard R, Carrier L, Charron P, Bonne G, Forissier JF, Desnos M, Dubourg O, Komajda M, Schwartz K and Hainque B (1998). “Identification of two novel mutations in the ventricular regulatory myosin light chain gene (MYL2) associated with familial and classical forms of hypertrophic cardiomyopathy.” J Mol Med (Berl) 76(3–4): 208–214. [DOI] [PubMed] [Google Scholar]
- Fodor WL, Darras B, Seharaseyon J, Falkenthal S, Francke U and Vanin EF (1989). “Human ventricular/slow twitch myosin alkali light chain gene characterization, sequence, and chromosomal location.” J Biol Chem 264(4): 2143–2149. [PubMed] [Google Scholar]
- Furst DO, Osborn M and Weber K (1989). “Myogenesis in the mouse embryo: differential onset of expression of myogenic proteins and the involvement of titin in myofibril assembly.” J Cell Biol 109(2): 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt RT and Lecutier MA (1956). “Familial cardiomegaly.” Br Heart J 18(2): 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves MA (2002). “Molecular motors: Stretching the lever-arm theory.” Nature 415(6868): 129–131. [DOI] [PubMed] [Google Scholar]
- Geeves MA and Holmes KC (2005). “The molecular mechanism of muscle contraction.” Adv Protein Chem 71: 161–193. [DOI] [PubMed] [Google Scholar]
- Grabarek Z (2006). “Structural Basis for Diversity of the EF-hand Calcium-binding Proteins.” Journal of Molecular Biology 359(3): 509–525. [DOI] [PubMed] [Google Scholar]
- Gregorich ZR, Cai W, Lin Z, Chen AJ, Peng Y, Kohmoto T and Ge Y (2017). “Distinct sequences and post-translational modifications in cardiac atrial and ventricular myosin light chains revealed by topdown mass spectrometry.” J Mol Cell Cardiol 107: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson DF, Helgason H, Gudjonsson SA, Zink F, Oddson A, Gylfason A, Besenbacher S, Magnusson G, Halldorsson BV, Hjartarson E, Sigurdsson GT, Stacey SN, Frigge ML, Holm H, Saemundsdottir J, Helgadottir HT, Johannsdottir H, Sigfusson G, Thorgeirsson G, Sverrisson JT, Gretarsdottir S, Walters GB, Rafnar T, Thjodleifsson B, Bjornsson ES, Olafsson S, Thorarinsdottir H, Steingrimsdottir T, Gudmundsdottir TS, Theodors A, Jonasson JG, Sigurdsson A, Bjornsdottir G, Jonsson JJ, Thorarensen O, Ludvigsson P, Gudbjartsson H, Eyjolfsson GI, Sigurdardottir O, Olafsson I, Arnar DO, Magnusson OT, Kong A, Masson G, Thorsteinsdottir U, Helgason A, Sulem P and Stefansson K (2015). “Large-scale whole-genome sequencing of the Icelandic population.” Nat Genet 47(5): 435–444. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Holm H, Sulem P, Masson G, Oddsson A, Magnusson OT, Saemundsdottir J, Helgadottir HT, Helgason H, Johannsdottir H, Gretarsdottir S, Gudjonsson SA, Njolstad I, Lochen ML, Baum L, Ma RC, Sigfusson G, Kong A, Thorgeirsson G, Sverrisson JT, Thorsteinsdottir U, Stefansson K and Arnar DO (2017). “A frameshift deletion in the sarcomere gene MYL4 causes early-onset familial atrial fibrillation.” Eur Heart J 38(1): 27–34. [DOI] [PubMed] [Google Scholar]
- Gulick J, Hewett TE, Klevitsky R, Buck SH, Moss RL and Robbins J (1997). “Transgenic remodeling of the regulatory myosin light chains in the mammalian heart.” Circ Res 80(5): 655–664. [DOI] [PubMed] [Google Scholar]
- Haase H, Dobbernack G, Tunnemann G, Karczewski P, Cardoso C, Petzhold D, Schlegel W-P, Lutter S, Pierschalek P, Behlke J and Morano I (2006). “Minigenes encoding N-terminal domains of human cardiac myosin light chain-1 improve heart function of transgenic rats.” FASEB J. 20(7): 865–873. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ and Mrwa U (1982). “Regulation of smooth muscle actomyosin.” Blood Vessels 19(1): 1–18. [DOI] [PubMed] [Google Scholar]
- Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN and Klein WH (1993). “Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene.” Nature 364(6437): 501–506. [DOI] [PubMed] [Google Scholar]
- Henry GD, Winstanley MA, Dalgarno DC, Scott GM, Levine BA and Trayer IP (1985). “Characterization of the actin-binding site on the alkali light chain of myosin.” Biochim Biophys Acta 830(3): 233–243. [DOI] [PubMed] [Google Scholar]
- Hernandez OM, Jones M, Guzman G and Szczesna-Cordary D (2007). “Myosin essential light chain in health and disease.” Am J Physiol Heart Circ Physiol 292(4): H1643–1654. [DOI] [PubMed] [Google Scholar]
- Hinterberger TJ, Sassoon DA, Rhodes SJ and Konieczny SF (1991). “Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development.” Dev Biol 147(1): 144–156. [DOI] [PubMed] [Google Scholar]
- Hootsmans WJ and Meerschwam IS (1971). “Electromyography in patients with hypertrophic obstructive cardiomyopathy.” Neurology 21(8): 810–816. [DOI] [PubMed] [Google Scholar]
- Houdusse A and Cohen C (1996). “Structure of the regulatory domain of scallop myosin at 2 A resolution: implications for regulation.” Structure 4(1): 21–32. [DOI] [PubMed] [Google Scholar]
- Huang C, Sheikh F, Hollander M, Cai C, Becker D, Chu PH, Evans S and Chen J (2003). “Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis.” Development 130(24): 6111–6119. [DOI] [PubMed] [Google Scholar]
- Huxley AF (1957). “A hypothesis for the mechanism of contraction of muscle.” Prog. Biophys. Biophys. Chem. 7: 255–318. [PubMed] [Google Scholar]
- Huxley HE (1969). “The Mechanism of Muscular Contraction.” Science 164(3886): 1356–1366.4181952 [Google Scholar]
- Huxley HE (1985). “The Crossbridge Mechanism of Muscular Contraction and its Implications.” J Exp Biol 115(1): 17–30. [DOI] [PubMed] [Google Scholar]
- Isaacs H and Muncke G (1975). “Idiopathic cardiomyopathy and skeletal muscle abnormality.” Am Heart J 90(6): 767–773. [DOI] [PubMed] [Google Scholar]
- Kabaeva ZT, Perrot A, Wolter B, Dietz R, Cardim N, Correia JM, Schulte HD, Aldashev AA, Mirrakhimov MM and Osterziel KJ (2002). “Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy.” Eur J Hum Genet 10(11): 741–748. [DOI] [PubMed] [Google Scholar]
- Kazmierczak K, Liang J, Yuan CC, Yadav S, Sitbon YH, Walz K, Ma W, Irving TC, Cheah JX, Gomes AV and Szczesna-Cordary D (2019). “Slow-twitch skeletal muscle defects accompany cardiac dysfunction in transgenic mice with a mutation in the myosin regulatory light chain.” FASEB J 33(3): 3152–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, Paulino EC, Huang W, Muthu P, Liang J, Yuan CC, Rojas AI, Hare JM and Szczesna-Cordary D (2013). “Discrete effects of A57G-myosin essential light chain mutation associated with familial hypertrophic cardiomyopathy.” Am J Physiol Heart Circ Physiol 305(4): H575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, Xu Y, Jones M, Guzman G, Hernandez OM, Kerrick WGL and Szczesna-Cordary D (2009). “The Role of the N-Terminus of the Myosin Essential Light Chain in Cardiac Muscle Contraction.” J Mol Biol 387(3): 706–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, Yuan C-C, Liang J, Huang W, Rojas AI and Szczesna-Cordary D (2014). “Remodeling of the heart in hypertrophy in animal models with myosin essential light chain mutations.” Frontiers in Physiology 5:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Alonso S, Tajbakhsh S, Cossu G and Buckingham M (1995). “Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice.” J Cell Biol 129(2): 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RG, Zammit PS, Schneider A, Alonso S, Biben C and Buckingham ME (1997). “Embryonic and fetal myogenic programs act through separate enhancers at the MLC1F/3F locus.” Dev Biol 187(2): 183–199. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Takagi A, Inoue T and Saga Y (2000). “MesP1 and MesP2 are essential for the development of cardiac mesoderm.” Development 127(15): 3215–3226. [DOI] [PubMed] [Google Scholar]
- Koshiba-Takeuchi K, Mori AD, Kaynak BL, Cebra-Thomas J, Sukonnik T, Georges RO, Latham S, Beck L, Henkelman RM, Black BL, Olson EN, Wade J, Takeuchi JK, Nemer M, Gilbert SF and Bruneau BG (2009). “Reptilian heart development and the molecular basis of cardiac chamber evolution.” Nature 461(7260): 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalak SW, Miller-Hance WC, O’Brien TX, Dyson E and Chien KR (1994). “Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis.” J Biol Chem 269(24): 16961–16970. [PubMed] [Google Scholar]
- Kurabayashi M, Komuro I, Tsuchimochi H, Takaku F and Yazaki Y (1988). “Molecular cloning and characterization of human atrial and ventricular myosin alkali light chain cDNA clones.” J Biol Chem 263(27): 13930–13936. [PubMed] [Google Scholar]
- Kwon H, Goodwin EB, Nyitray L, Berliner E, O’Neall-Hennessey E, Melandri FD and SzentGyorgyi AG (1990). “Isolation of the Regulatory Domain of Scallop Myosin: Role of the Essential Light Chain in Calcium Binding.” PNAS 87(12): 4771–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints TJ, Parsons LM, Hartley L, Lyons I and Harvey RP (1993). “Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants.” Development 119(3): 969. [DOI] [PubMed] [Google Scholar]
- Lochner A, Hewlett RH, O’Kennedy A, van der Walt JJ, Tiedt FA, Hoffman H, de Graaf AS, Przybojewski JZ and Torrington M (1981). “A study of a family with inherited disease of cardiac and skeletal muscle. Part II. Skeletal muscle morphology and mitochondrial oxidative phosphorylation.” S Afr Med J 59(13): 453–461. [PubMed] [Google Scholar]
- Lowey S, Saraswat LD, Liu H, Volkmann N and Hanein D (2007). “Evidence for an Interaction between the SH3 Domain and the N-terminal Extension of the Essential Light Chain in Class II Myosins.” Journal of Molecular Biology 371(4): 902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons GE, Ontell M, Cox R, Sassoon D and Buckingham M (1990). “The expression of myosin genes in developing skeletal muscle in the mouse embryo.” J Cell Biol 111(4): 1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons GE, Schiaffino S, Sassoon D, Barton P and Buckingham M (1990). “Developmental regulation of myosin gene expression in mouse cardiac muscle.” J Cell Biol 111(6 Pt 1): 2427–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L and Harvey RP (1995). “Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5.” Genes Dev 9(13): 1654–1666. [DOI] [PubMed] [Google Scholar]
- Meder B, Laufer C, Hassel D, Just S, Marquart S, Vogel B, Hess A, Fishman MC, Katus HA and Rottbauer W (2009). “A Single Serine in the Carboxyl Terminus of Cardiac Essential Myosin Light Chain1 Controls Cardiomyocyte Contractility In Vivo.” Circ Res 104(5): 650–659. [DOI] [PubMed] [Google Scholar]
- Messina G, Biressi S, Monteverde S, Magli A, Cassano M, Perani L, Roncaglia E, Tagliafico E, Starnes L, Campbell CE, Grossi M, Goldhamer DJ, Gronostajski RM and Cossu G (2010). “Nfix regulates fetal-specific transcription in developing skeletal muscle.” Cell 140(4): 554–566. [DOI] [PubMed] [Google Scholar]
- Michael JJ, Gollapudi SK, Ford SJ, Kazmierczak K, Szczesna-Cordary D and Chandra M (2013). “Deletion of 1–43 amino acids in cardiac myosin essential light chain blunts length dependency of Ca(2+) sensitivity and cross-bridge detachment kinetics.” Am J Physiol Heart Circ Physiol 304(2): H253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Palmer BM, Ruch S, Martin LA, Farman GP, Wang Y, Robbins J, Irving TC and Maughan DW (2005). “The Essential Light Chain N-terminal Extension Alters Force and Fiber Kinetics in Mouse Cardiac Muscle.” J. Biol. Chem 280(41): 34427–34434. [DOI] [PubMed] [Google Scholar]
- Milligan RA, Whittaker M and Safer D (1990). “Molecular structure of F-actin and location of surface binding sites.” Nature 348(6298): 217–221. [DOI] [PubMed] [Google Scholar]
- Morano I, Ritter O, Bonz A, Timek T, Vahl CF and Michel G (1995). “Myosin light chain-actin interaction regulates cardiac contractility.” Circ Res 76(5): 720–725. [DOI] [PubMed] [Google Scholar]
- Morano M, Zacharzowski U, Maier M, Lange PE, Alexi-Meskishvili V, Haase H and Morano I (1996). “Regulation of Human Heart Contractility by Essential Myosin Light Chain Isoforms.” J. Clin. Invest. 98(2): 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C, McCaw PS and Baltimore D (1989). “A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins.” Cell 56(5): 777–783. [DOI] [PubMed] [Google Scholar]
- Muthu P, Huang W, Kazmierczak K and Szczesna-Cordary D (2012). “Functional Consequences of Mutations in the Myosin Regulatory Light Chain Associated with Hypertrophic Cardiomyopathy” In: Veselka J (Ed.) Cardiomyopathies – From Basic Research to Clinical Management. Ch. 17. InTech, Croatia: pp 383–408. [Google Scholar]
- Muthu P, Kazmierczak K, Jones M and Szczesna-Cordary D (2012). “The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts.” J Cell Mol Med 16(4): 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu P, Liang J, Schmidt W, Moore JR and Szczesna-Cordary D (2014). “In Vitro Rescue Study of a Malignant Familial Hypertrophic Cardiomyopathy Phenotype by Pseudo-Phosphorylation of Myosin Regulatory Light Chain.” Arch Biochem Biophys 552–553(15 June–1 July 2014): 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu P, Wang L, Yuan CC, Kazmierczak K, Huang W, Hernandez OM, Kawai M, Irving TC and Szczesna-Cordary D (2011). “Structural and functional aspects of the myosin essential light chain in cardiac muscle contraction.” FASEB J 25(12): 4394–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TX, Lee KJ and Chien KR (1993). “Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube.” Proc Natl Acad Sci U S A 90(11): 5157–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontell MP, Sopper MM, Lyons G, Buckingham M and Ontell M (1993). “Modulation of contractile protein gene expression in fetal murine crural muscles: emergence of muscle diversity.” Dev Dyn 198(3): 203–213. [DOI] [PubMed] [Google Scholar]
- Orr N, Arnaout R, Gula LJ, Spears DA, Leong-Sit P, Li Q, Tarhuni W, Reischauer S, Chauhan VS, Borkovich M, Uppal S, Adler A, Coughlin SR, Stainier DY and Gollob MH (2016). “A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation.” Nat Commun 7: 11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott MO, Bober E, Lyons G, Arnold H and Buckingham M (1991). “Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo.” Development 111(4): 1097–1107. [DOI] [PubMed] [Google Scholar]
- Palmer B (2005). “Thick Filament Proteins and Performance in Human Heart Failure.” Heart Failure Reviews 10(3): 187–197. [DOI] [PubMed] [Google Scholar]
- Pawloski Dahm CM, Song G, Kirkpatrick DL, Palermo J, Gulick J, Dorn GW 2nd, Robbins J and Walsh RA (1998). “Effects of total replacement of atrial myosin light chain-2 with the ventricular isoform in atrial myocytes of transgenic mice.” Circulation 97(15): 1508–1513. [DOI] [PubMed] [Google Scholar]
- Petzhold D, Simsek B, Meißner R, Mahmoodzadeh S and Morano I (2014). “Distinct interactions between actin and essential myosin light chain isoforms.” Biochemical and Biophysical Research Communications 449(3): 284–288. [DOI] [PubMed] [Google Scholar]
- Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, Rayment I, Sellers JR, Fananapazir L and Epstein ND (1996). “Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle.” Nat Genet 13(1): 63–69. [DOI] [PubMed] [Google Scholar]
- Poggesi C and Ho CY (2014). “Muscle dysfunction in hypertrophic cardiomyopathy: what is needed to move to translation?” J Muscle Res Cell Motil 35(1): 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybojewski JZ, Hoffman H, de Graaf AS, van der Walt JJ, Tiedt FA, O’Kennedy A, Torrington M, Lochner A and Hewlett R (1981). “A study of a family with inherited disease of cardiac and skeletal muscle. Part I. Clinical, electrocardiographic, echocardiographic, haemodynamic, electrophysiological and electron microscopic studies.” S Afr Med J 59(11): 363–373. [PubMed] [Google Scholar]
- Rarick HM, Opgenorth TJ, von Geldern TW, Wu-Wong JR and Solaro RJ (1996). “An essential myosin light chain peptide induces supramaximal stimulation of cardiac myofibrillar ATPase activity.” J Biol Chem 271(43): 27039–27043. [DOI] [PubMed] [Google Scholar]
- Ravenscroft G, Zaharieva IT, Bortolotti CA, Lambrughi M, Pignataro M, Borsari M, Sewry CA, Phadke R, Haliloglu G, Ong R, Goullee H, Whyte T, Consortium UK, Manzur A, Talim B, Kaya U, Osborn DPS, Forrest ARR, Laing NG and Muntoni F (2018). “Bi-allelic mutations in MYL1 cause a severe congenital myopathy.” Hum Mol Genet 27(24): 4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G and Holden HM (1993). “Three-dimensional structure of myosin subfragment-1: a molecular motor.” Science 261(5117): 50–58. [DOI] [PubMed] [Google Scholar]
- Rehman I and Rehman A (2018). Anatomy, Thorax, Heart. StatPearls; Treasure Island (FL). [PubMed] [Google Scholar]
- Rottbauer W, Wessels G, Dahme T, Just S, Trano N, Hassel D, Burns CG, Katus HA and Fishman MC (2006). “Cardiac Myosin Light Chain-2. A Novel Essential Component of Thick-Myofilament Assembly and Contractility of the Heart.” Circ Res: 01.RES.0000234807.0000216034.fe. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J and Inoue T (1999). “MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube.” Development 126(15): 3437–3447. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Gulick J, Fewell J and Robbins J (2001). “Examining the in Vivo Role of the Amino Terminus of the Essential Myosin Light Chain.” J. Biol. Chem 276(35): 32682–32686. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H and Buckingham M (1989). “Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis.” Nature 341(6240): 303–307. [DOI] [PubMed] [Google Scholar]
- Schaub MC, Tuchschmid CR, Srihari T and Hirzel HO (1984). “Myosin isoenzymes in human hypertrophic hearts. Shift in atrial myosin heavy chains and in ventricular myosin light chains.” Eur Heart J 5 Suppl F: 85–93. [DOI] [PubMed] [Google Scholar]
- Scheid LM, Mosqueira M, Hein S, Kossack M, Juergensen L, Mueller M, Meder B, Fink RH, Katus HA and Hassel D (2016). “Essential light chain S195 phosphorylation is required for cardiac adaptation under physical stress.” Cardiovasc Res 111(1): 44–55. [DOI] [PubMed] [Google Scholar]
- Schiaffino S and Reggiani C (1996). “Molecular diversity of myofibrillar proteins: gene regulation and functional significance.” Physiological Reviews 76(2): 371–423. [DOI] [PubMed] [Google Scholar]
- Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE and Seidman JG (1998). “Congenital heart disease caused by mutations in the transcription factor NKX2–5.” Science 281(5373): 108–111. [DOI] [PubMed] [Google Scholar]
- Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ and Buttrick PM (2010). “A novel, in-solution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain.” Mol Cell Proteomics 9(9): 1804–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafig SA, Sande MA, Carruthers RR, Killip T and Milhorat AT (1972). “Skeletal muscle in idiopathic cardiomyopathy.” J Neurol Sci 15(3): 303–320. [DOI] [PubMed] [Google Scholar]
- Shi QW, Danilczyk U, Wang JX, See YP, Williams WG, Trusler GA, Beaulieu R, Rose V and Jackowski G (1991). “Expression of ventricular myosin subunits in the atria of children with congenital heart malformations.” Circ Res 69(6): 1601–1607. [DOI] [PubMed] [Google Scholar]
- Smith ER, Heffernan LP, Sangalang VE, Vaughan LM and Flemington CS (1976). “Voluntary muscle involvement in hypertrophic cardiomyopathy. A study of eleven patients.” Ann Intern Med 85(5): 566–572. [DOI] [PubMed] [Google Scholar]
- Sobieszek A (1977). “Ca-linked phosphorylation of a light chain of vertebrate smooth-muscle myosin.” Eur J Biochem 73(2): 477–483. [DOI] [PubMed] [Google Scholar]
- Spudich James A. (2014). “Hypertrophic and Dilated Cardiomyopathy: Four Decades of Basic Research on Muscle Lead to Potential Therapeutic Approaches to These Devastating Genetic Diseases.” Biophysical Journal 106(6): 1236–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, Cserjesi P and Olson EN (1995). “A subclass of bHLH proteins required for cardiac morphogenesis.” Science 270(5244): 1995–1999. [DOI] [PubMed] [Google Scholar]
- Steimle JD and Moskowitz IP (2017). “TBX5: A Key Regulator of Heart Development.” Curr Top Dev Biol 122: 195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart CA, Stone WL, Howell ME, Brannon MF, Hall HK, Gibson AL and Stone MH (2016). “Myosin content of individual human muscle fibers isolated by laser capture microdissection.” Am J Physiol Cell Physiol 310(5): C381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K (1982). “Identification of myosin-binding sites on the actin sequence.” Biochemistry 21(15): 3654–3661. [DOI] [PubMed] [Google Scholar]
- Sutsch G, Brunner UT, von Schulthess C, Hirzel HO, Hess OM, Turina M, Krayenbuehl HP and Schaub MC (1992). “Hemodynamic performance and myosin light chain-1 expression of the hypertrophied left ventricle in aortic valve disease before and after valve replacement.” Circ Res 70(5): 1035–1043. [DOI] [PubMed] [Google Scholar]
- Sweeney HL (1995). “Function of the N terminus of the myosin essential light chain of vertebrate striated muscle.” Biophys J 68(4 Suppl): 112S–118S; discussion 118S-119S. [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF and Stull JT (1993). “Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function.” Am J Physiol 264(5 Pt 1): C1085–1095. [DOI] [PubMed] [Google Scholar]
- Szczesna-Cordary D (2003). “Regulatory light chains of striated muscle myosin. Structure, function and malfunction.” Curr Drug Targets Cardiovasc Haematol Disord 3(2): 187–197. [DOI] [PubMed] [Google Scholar]
- Szczesna D, Ghosh D, Li Q, Gomes AV, Guzman G, Arana C, Zhi G, Stull JT and Potter JD (2001). “Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2+ binding, and phosphorylation.” J Biol Chem 276(10): 7086–7092. [DOI] [PubMed] [Google Scholar]
- Taylor KC, Buvoli M, Korkmaz EN, Buvoli A, Zheng Y, Heinze NT, Cui Q, Leinwand LA and Rayment I (2015). “Skip residues modulate the structural properties of the myosin rod and guide thick filament assembly.” Proc Natl Acad Sci U S A 112(29): E3806–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teekakirikul P, Padera RF, Seidman JG and Seidman CE (2012). “Hypertrophic cardiomyopathy: translating cellular cross talk into therapeutics.” J Cell Biol 199(3): 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timson DJ (2003). “Fine tuning the myosin motor: the role of the essential light chain in striated muscle myosin.” Biochimie 85(7): 639–645. [DOI] [PubMed] [Google Scholar]
- Timson DJ, Trayer HR, Smith KJ and Trayer IP (1999). “Size and charge requirements for kinetic modulation and actin binding by alkali 1-type myosin essential light chains.” J Biol Chem 274(26): 18271–18277. [DOI] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Elhanany H, Rinon A and Tzahor E (2006). “Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract.” Development 133(10): 1943–1953. [DOI] [PubMed] [Google Scholar]
- Trayer IP, Trayer HR and Levine BA (1987). “Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study.” Eur J Biochem 164(1): 259–266. [DOI] [PubMed] [Google Scholar]
- Trybus KM (1994). “Role of myosin light chains.” J Muscle Res Cell Motil 15(6): 587–594. [DOI] [PubMed] [Google Scholar]
- Van Horn R and Crow MT (1989). “Fast myosin heavy chain expression during the early and late embryonic stages of chicken skeletal muscle development.” Dev Biol 134(2): 279–288. [DOI] [PubMed] [Google Scholar]
- VanBuren P, Waller GS, Harris DE, Trybus KM, Warshaw DM and Lowey S (1994). “The essential light chain is required for full force production by skeletal muscle myosin.” Proc Natl Acad Sci U S A 91(26): 12403–12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobo A, Gonzalez-Munoz M and Berchtold MW (2019). “Proteins with calmodulin-like domains: structures and functional roles.” Cell Mol Life Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Geist J, Grogan A, Hu LR and Kontrogianni-Konstantopoulos A (2018). “Thick Filament Protein Network, Functions, and Disease Association.” Compr Physiol 8(2): 631–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ajtai K and Burghardt TP (2013). “The Qdot-labeled actin super-resolution motility assay measures low-duty cycle muscle myosin step size.” Biochemistry 52(9): 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ajtai K and Burghardt TP (2014). “Ventricular myosin modifies in vitro step-size when phosphorylated.” J Mol Cell Cardiol 72: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ajtai K, Kazmierczak K, Szczesna-Cordary D and Burghardt TP (2016). “N-Terminus of Cardiac Myosin Essential Light Chain Modulates Myosin Step-Size.” Biochemistry 55(1): 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szczesna-Cordary D, Craig R, Diaz-Perez Z, Guzman G, Miller T and Potter JD (2007). “Fast skeletal muscle regulatory light chain is required for fast and slow skeletal muscle development.” FASEB J 21(9): 2205–2214. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yuan CC, Kazmierczak K, Szczesna-Cordary D and Burghardt TP (2018). “Single cardiac ventricular myosins are autonomous motors.” Open Biol 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke J, Yamakawa M, Molloy J, Falkenthal S and Maughan D (1992). “Myosin light chain-2 mutation affects flight, wing beat frequency, and indirect flight muscle contraction kinetics in Drosophila.” J Cell Biol 119(6): 1523–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weterman MA, Barth PG, van Spaendonck-Zwarts KY, Aronica E, Poll-The BT, Brouwer OF, van Tintelen JP, Qahar Z, Bradley EJ, de Wissel M, Salviati L, Angelini C, van den Heuvel L, Thomasse YE, Backx AP, Nurnberg G, Nurnberg P and Baas F (2013). “Recessive MYL2 mutations cause infantile type I muscle fibre disease and cardiomyopathy.” Brain 136(Pt 1): 282–293. [DOI] [PubMed] [Google Scholar]
- Winstanley MA, Trayer HR and Trayer IP (1977). “Role of the myosin light chains in binding to actin.” FEBS Lett 77(2): 239–242. [DOI] [PubMed] [Google Scholar]
- Yadav S, Kazmierczak K, Liang J, Sitbon YH and Szczesna-Cordary D (2019). “Phosphomimeticmediated in vitro rescue of hypertrophic cardiomyopathy linked to R58Q mutation in myosin regulatory light chain.” FEBS J 286(1): 151–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Sitbon YH, Kazmierczak K and Szczesna-Cordary D (2019). “Hereditary heart disease: pathophysiology, clinical presentation, and animal models of HCM, RCM, and DCM associated with mutations in cardiac myosin light chains.” Pflugers Arch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CC, Kazmierczak K, Liang J, Kanashiro-Takeuchi R, Irving TC, Gomes AV, Wang Y, Burghardt TP and Szczesna-Cordary D (2017). “Hypercontractile mutant of ventricular myosin essential light chain leads to disruption of sarcomeric structure and function and results in restrictive cardiomyopathy in mice.” Cardiovasc Res 113(10): 1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CC, Kazmierczak K, Liang J, Zhou Z, Yadav S, Gomes AV, Irving TC and SzczesnaCordary D (2018). “Sarcomeric perturbations of myosin motors lead to dilated cardiomyopathy in genetically modified MYL2 mice.” Proc Natl Acad Sci U S A 115(10): E2338–E2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CC, Muthu P, Kazmierczak K, Liang J, Huang W, Irving TC, Kanashiro-Takeuchi RM, Hare JM and Szczesna-Cordary D (2015). “Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice.” Proc Natl Acad Sci U S A 112(30): E4138–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Huang W, Liang J and Szczesna-Cordary D (2016). “Molecular and Functional Effects of a Splice Site Mutation in the MYL2 Gene Associated with Cardioskeletal Myopathy and Early Cardiac Death in Infants.” Front Physiol 7: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]