Abstract
The risk of cerebrovascular disease in stroke-prone spontaneously hypertensive rats (SHR-A3/SHRSP) arises from naturally occurring genetic variation. In the present study we show the involvement of SHR genetic variation that affects antibody formation and function in the pathogenesis of stroke. We have tested the involvement in susceptibility to stroke of genetic variation in IgH, the gene encoding the immunoglobulin heavy chain by congenic substitution. This gene contains functional natural variation in SHR-A3 that diverges from stroke-resistant SHR-B2. We created a SHR-A3 congenic line in which the IgH gene was substituted with the corresponding haplotype from SHR-B2. Compared with SHR-A3 rats, congenic substitution of the IgH locus [SHR-A3(IgH-B2)] markedly reduced cerebrovascular disease. Given the role in antibody formation of the IgH gene, we investigated the presence of IgG and IgM autoantibodies and their targets using a high-density protein array containing ~20,000 recombinant proteins. High titers of autoantibodies to key cerebrovascular stress proteins were detected, including FABP4, HSP70, and Wnt signaling proteins. Serum levels of these autoantibodies were reduced in the SHR-A3(IgH-B2) congenic line.
Keywords: HSP70, hypertension, immunoglobulin, SHRSP, stroke
INTRODUCTION
Susceptibility to end organ injury varies among the hypertensive population, and heritable factors appear to play an important role in end organ disease risk. The challenge of identifying the genetic variation that drives end organ disease susceptibility in the human population is impeded by a number of factors (4). Susceptibility is polygenic, reducing the statistical power of genetic association studies; it also demonstrates locus heterogeneity: the underlying genetic variation creating disease risk in some individuals, pedigrees, and populations is different from that in others. Inbred animal models of genetic susceptibility offer insight into genetic causes and pathogenetic mechanisms in which these confounding factors are reduced. The spontaneously hypertensive rat (SHR) was produced by selective breeding from outbred Wistar stock to aggregate pre-existing natural genetic variation that resulted in animals with high blood pressure (30). The subsequent generation of genetically isolated lineages of SHR (originally designated by the SHR-A, -B, and -C suffixes) gave rise to fully inbred SHR lines. Limited segregation of the blood pressure (BP) trait is observed when distinct inbred SHR lines are crossed with other SHR lines (3, 28), confirming the expectation from genealogy that underlying genetic variation producing hypertension in SHR lines is shared across SHR lines.
The unique emergence and subsequent genetic fixation of stroke susceptibility in the SHR-A lineage are documented (31). Selective breeding of SHR-A animals whose progenitors manifested stroke was performed to aggregate stroke susceptibility alleles. In males of the SHR-A3 line, often referred to as the stroke-prone SHR (SHRSP), stroke is fully penetrant and occurs rapidly when salt intake is increased (28, 31). Distinct lineages of SHR that share genetic susceptibility to hypertension via an overlapping set of genetic variants yet diverge in susceptibility to stroke provide an opportunity for genetic discovery of stroke susceptibility alleles that are largely free of the difficulties encountered in addressing these questions in outbred human populations. Technical approaches to isolate and confirm stroke susceptibility genetic variation are facilitated by the fact that the stroke-susceptible SHR-A3 and stroke-resistant SHR-B2 genomes are closely related because they descend from recent shared ancestors. Our whole genome sequencing and variant analysis of the genomes of SHR-A3 and SHR-B2 (5) indicate that the lines share DNA that has been inherited from a single common ancestor and is therefore identical by descent (39). The extent of the two genomes that is identical by descent is 87% (3). The remaining 13% of the genome of SHR-A3 comprises haplotype blocks containing genetic variation that differs from the corresponding parts of the SHR-B2 genome.
The genome sequences encoding the immunoglobulin heavy chain (IgH) in SHR-A3 and SHR-B2 are highly divergent from each other (16). This variation in IgH affects the concentration of immunoglobulin in the serum and alters the germ-line immunoglobulin repertoire, affecting the capacity to make immunoglobulins containing specific rat immunoglobulin V gene segments (9, 16). The identification of important genetic variation affecting antibody function in SHR-A3 suggested the involvement of antibodies in stroke occurrence. Here we examine the role of germ-line immunoglobulin heavy chain variation in stroke susceptibility by congenic substitution of IgH from SHR-B2 into the SHR-A3 genetic background. Given the function of IgH in antibody formation, we also performed a discovery study to compare serum IgG and IgM autoreactive antibodies in SHR-A3 versus SHR-B2 and to identify the protein targets of antibodies. Finally, we determined whether congenic substitution of IgH affects the abundance of these antibodies.
METHODS
Animals and treatments.
The Institutional Animal Welfare Committee prospectively reviewed and approved all animal experiments and protocols. Studies were performed on male animals from the injury-susceptible spontaneously hypertensive-A3 (SHR-A3, SHRSP/Bbb) and the injury-resistant SHR-B2 rat lines, maintained in our Association for Assessment and Accreditation of Laboratory Animal Care International-approved specific pathogen-free facility. Female SHR-A3 rats have a low rate of cardiovascular end organ disease and were not included in this study. These lines and their origins before transfer to our laboratory have been recorded at the Rat Genome Database (RGD, https://rgd.mcw.edu/), which has applied the following identifiers: SHR-A3 – RGD ID # = 8142383, symbol = SHRSP/BbbUtx; SHR-B2 – RGD ID # = 8142385, symbol = SHR/Utx. Animals were provided a standard rodent chow diet and drinking water ad libitum.
IgH congenic line creation.
We have previously described the generation of the SHR-A3(IgH-B2) congenic line (9). Targeted genotyping of the proximal and distal ends of IgH locus was performed to confirm transfer of the entire SHR-B2 chromosomal segment containing IgH (see supplemental data). The IgH locus determines serum immunoglobulin levels that are highly divergent between SHR-A3 and SHR-B2 lines. Serum immunoglobulin subclass levels in the SHR-A3(IgH-B2) congenic line were similar to SHR-B2, which provided further phenotypic confirmation of congenic substitution of the SHR-B2 IgH locus (9). Marker-assisted genome-wide SNP genotyping in the congenic line verified that, outside the IgH locus, all markers in the congenic contained the SHR-A3 genotype (Supplemental data published at https://zenodo.org/record/3405240).
BP measurement.
At 16–18 wk of age, male SHR-A3 and SHR-A3(IgH-B2) rats were implanted with radiotelemetry devices (Data Sciences, St. Paul, MN) to record BP as described previously (3). Animals were allowed to recover for 2 wk before initiating BP recordings at age 20 wk. Baseline BP was recorded 24 h before salt loading. Subsequent BP measurements were taken 1 day per week throughout the study. BP was measured by continuous sampling for 30 s every 30 min for 24 h.
Stroke induction and assessment.
Salt loading (1% NaCl in drinking water with standard 0.4% Na Purina rodent chow) was used to accelerate the development of cerebrovascular lesions and was initiated at age 20 wk, 2 wk after telemetry probe implantation surgery. Rats were weighed twice per week to monitor stroke onset. Animals exhibiting rapid weight loss, loss of coordination, reduced motor activity, paralytic gait, and sudden death were considered stroke-sign positive. The Yamori classification scheme was used to score neurological symptoms and stroke severity at euthanasia (1, 10). Score 1: normal; 2: hyperirritability, aggressiveness, piloerection, hyperkinesia, jumping, 3: lethargy with hypersensitivity to painful stimuli; and 4: lethargy with hyposensitivity to painful stimuli, akinesia, paralytic gait, emaciation. Rats losing over 15% of their highest body weight were euthanized. At the end of the treatment, rats were perfused transcardially (under 2.5% isoflurane) with saline followed by 4% buffered formalin. Perfusion pressure was maintained constant at ~80 mmHg. Brains were fixed in 4% buffered formalin for 24 h. Brain weight and maximal transverse and coronal diameters were measured following fixation. The brains were sectioned into 2 mm slices using a rat brain matrix (Kent Scientific), and gross morphological assessments were performed to detect microbleeds and hemorrhages, aided as needed by a dissecting stereomicroscope.
HuProt antigen microarray studies.
These studies were performed in the Genomics and Microarray Core Facility, University of Texas Southwestern Medical School. HuProt v3.1 arrays (CDI Laboratories, Mayaguez, PR) spotted in duplicate with ~20,000 full-length, well-folded, recombinant human proteins representing >80% of the human proteome were used according to the manufacturer’s protocol. Each individual array was HuProt array was blocked 5% BSA in 1× Tris-buffered saline + Tween 20 (TBST) followed by incubation with pooled serum collected from six individual animals SHR-A3 animals aged 16 wk and consuming normal 0.4% Na content chow and drinking water. Pools were also created from six animals that had drinking water replaced with 1% NaCl at 20 wk of age using serum collected after 8 wk of salt loading or serum obtained before euthanasia for animals exhibiting significant neurological injury. Similar serum pools were also analyzed from 28 wk old SHR-A3(IgH-B2) congenic animals at the conclusion of 8 wk of salt loading. After incubation with serum and rinsing, arrays were developed with detection antibodies targeting rat IgM and rat IgG (Goat polyclonal anti-Rat IgM-Heavy chain conjugated with Alexa Fluor 647, and Goat polyclonal anti-Rat IgG (H+L) conjugated with Cyanine3, both from Invitrogen). Arrays were further rinsed and fluorescence signals were obtained using a fluorescent slide reader (GenePix 4400A Microarray Scanner). Appropriate positive and negative controls for serum immunoglobulin profiling were provided on the array by the manufacturer. Z score normalization was used to minimize interarray variability and permit comparison of autoantibody signals across arrays. Intra-array reproducibility was assessed by analyzing correlation of signals between duplicate spots printed for each protein.
Dot blot for serum HSP70 immunoreactivity.
Rat sera were tested for autoantibodies against Hsp70 by the dot blot technique. Briefly, 1 μL of rat recombinant HSP70 protein (Enzo Biochem, New York, NY) was spotted on nitrocellulose membranes at a concentration of 0.68 μg/μL followed by serial dilutions (1:2, 1:4, 1:8, 1:16). All incubations were carried out at room temperature with shaking. Spots were air-dried for 5 min followed by blocking with protein-free blocking buffer (Pierce Biotech, Rockford, IL) for 30 min. Membranes were washed twice (15 min each wash) with 1× TBST. A second blocking step was performed with 1× PBS-5% BSA buffer for 30 min. Protein-free Block buffer was used for dilutions of sera and secondary antibodies in further steps. The membrane was washed (1× TBST, 15 min each) followed by incubation with diluted (1:300) sera from SHR-A3 and SHR-(IgH-B2) (pooled samples for each line, n = 6) for 30 min. Membranes were washed three times (1× TBST, 15 min each) followed by incubation with detection antibody (Goat-anti rat IgM/ Donkey anti-rat IgG-horseradish peroxidase conjugated, diluted 1:20,000) for 30 min. Membranes were washed four times, and antibody signal was detected using an ECL detection system.
Statistical analysis.
Comparison of data from SHR-A3 and congenic rats was analyzed by the Student’s t test using the Prism 7 software (GraphPad Software, La Jolla, CA). ANOVA with Tukey’s post hoc test was used to compare data from multiple groups. A value of P < 0.05 was considered statistically significant, with n = 7–10 independent animals per group and 5–7 replicates for in vitro studies per group.
RESULTS
Effect of IgH gene variation on stroke.
The IgH locus comprises a haplotype block in which SHR-A3 and SHR-B2 have fixed in homozygosity extensive divergent sequence variation arising from two distinct ancestors. This haplotype block is genetically isolated at the distal end of chromosome 6 with the remainder of chromosome 6 being largely identical by descent between SHR-A3 and SHR-B2 (3). The IgH gene comprises a complex set of immunoglobulin gene segments encoding the numerous variables, diversity, and joining segments that recombine and undergo mutational maturation to form the heavy chain antigen recognition site. It also contains exons encoding the constant regions that define the immunoglobulin classes (IgM, IgD, IgG1, IgG2a, IgG2b, IgG2c, IgE, and IgA). We have previously detailed the extensive genetic variation in IgH among SHR lines (16). To test the role of IgH in stroke occurrence in SHR-A3, we compared SHR-A3 with a congenic line in which the SHR-B2 IgH locus was backcrossed into the SHR-A3 genetic background (9). The congenic substitution of IgH between SHR-B2 (donor) and SHR-A3 (recipient) transfers inherited aspects of IgH function determined by this locus (for example, differences in serum immunoglobulin levels (9)) to recipient animals whose genomes retain >99% of the SHR-A3 background.
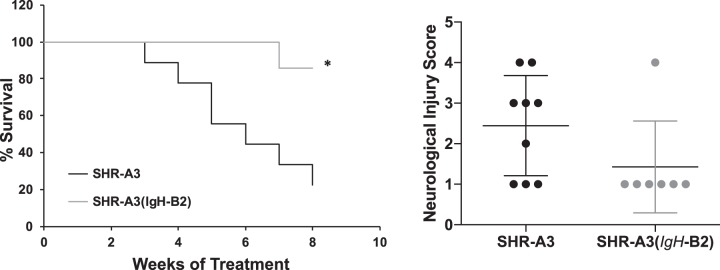
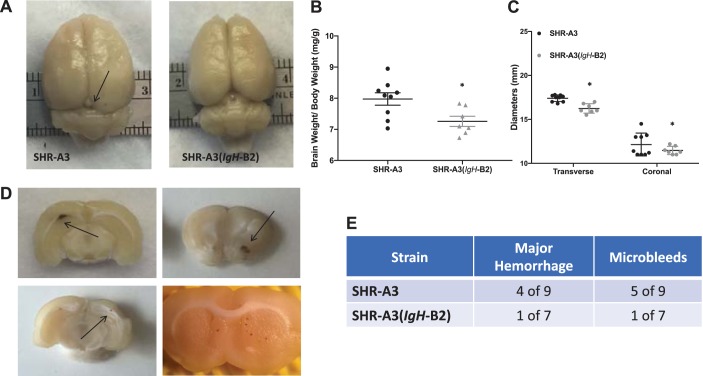
We measured BP by implanted telemetry in SHR-A3(IgH-B2) before salt loading (18–20 wk of age) and during 8 subsequent weeks when drinking water was replaced with 1% NaCl (Fig. 1). No significant BP difference between this congenic line and SHR-A3 was present before or during the salt loading period. However, this result is affected by the high rate of loss of SHR-A3 animals during the course of salt loading. Only 1 SHR-A3(IgH-B2) was euthanized during sodium loading and six of seven animals survived this treatment without experiencing stroke or reaching animal welfare criteria requiring euthanasia (Fig. 2). At the conclusion of the study, the surviving SHR-A3(IgH-B2) congenic animals all had Yamori scores of 1, indicating no neurological deficits (Fig. 2). Cerebral edema was significantly greater in SHR-A3 compared with SHR-A3(IgH-B2) (Fig. 3). Cerebral hemorrhagic lesions were absent in the brains from SHR-A3(IgH-B2), except for the single animal that was euthanized early in which both microbleeds and major hemorrhage were observed (Fig. 3).
Fig. 1.
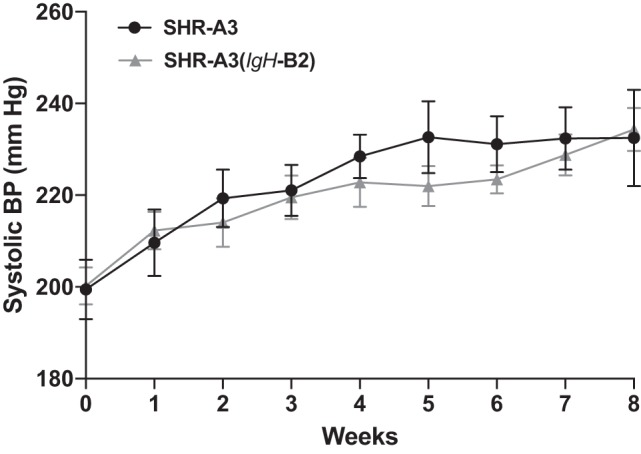
Blood pressure. SHR-A3 and SHR-A3(IgH-B2) rats were maintained for 8 wk on a stroke-inducing diet (1% NaCl in drinking water) starting at 20 wk of age. Systolic blood pressure (BP) was measured by telemetry immediately before and weekly during 8 wk of salt loading in SHR-A3 and SHR-A3(IgH-B2) congenic rats. BP was elevated during salt loading in both lines, and no significant difference was observed either before or during salt loading. Note, however, that progressive loss of SHR-A3 occurred during salt loading as a result of stroke or clinical phenotypes that reached our euthanasia threshold. In SHR-A3 animals, systolic BPs approaching 230 mmHg were predictive of imminent stroke or clinical pathology related to cerebrovascular disease. SHR-A3 n = 9, SHR-A3(IgH-B2), n = 7.
Fig. 2.
Left: Kaplan Meier curves for survival during 8 wk of salt loading. Right: the severity of neurological symptoms scored with the Yamori classification scheme at euthanasia in stroke sign-positive rats or at the end of the treatment protocol in surviving rats. *P < 0.05.
Fig. 3.
Brain morphometrics. A: gross morphology of the brains from SHR-A3 rats revealed global cerebral edema (top), evidenced by the filling in of major brain sulci (indicated by arrows) in SHR-A3 rats. Cerebral edema was quantified by measuring fixed brain weights normalized to initial body weights (before salt loading) (B), and transverse and coronal diameters (C). D: representative image of a brain slices showing a hemorrhagic lesions (top, arrows) in SHR-A3 rats and single and multiple microbleeds (bottom, arrows). *P < 0.05 vs SHR-A3 with n = 9 (SHR-A3), n = 7 (SHR-A3(IgH-B2). E: occurrence of microbleeds and major hemorrhage in salt-loaded rats.
Emergence of autoreactive antibodies in salt-loaded SHR-A3 and SHR-A3(IgH-B2).
To identify antigens that are potential targets for pathogenic antibodies in SHR-A3 serum we examined fluorescence signals attributable to IgM and IgG binding to the HuProt v3.1 protein array, a printed array containing nearly 20,000 unique full-length, recombinant human proteins expressed in a eukaryotic host (41). Serum was pooled from six animals, with pools representing the following groups: 16 wk old SHR-A3 without salt loading, 28 wk old salt-loaded SHR-A3(IgH-B2), and salt-loaded SHR-A3 animals at collected at 28 wk of age (n = 2) or at euthanasia due to salt loading-induced cerebrovascular disease (n = 4).
We first sorted the array signals from salt-loaded SHR-A3 (normalized as Z scores) to eliminate proteins that generated Z scores of <3 (per array manufacturer’s recommendation, ~99% of all antigens) and by selecting the 35 proteins generating the highest antibody binding signals. This was done for both IgM and IgG signals independently. We then compared the Z scores obtained for these antigens using pooled serum from 16 wk old SHR-A3 consuming normal salt intake. We eliminated antigens that had higher Z scores in 16 wk old animals than in the salt-loaded animals. We then examined similar serum pools from SHR-A3(IgH-B2) that were obtained after salt loading, identifying those antigens that had reduced Z scores in the salt-loaded congenic line compared with the levels observed in salt-loaded SHR-A3. We further determined which antigens replicated this pattern of elevation in SHR-A3 salt loading with lower levels in SHR-A3(IgH-B2) congenics for both IgG and IgM antibodies.
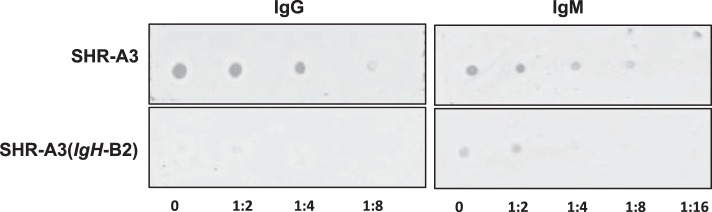
There was functional overlap among the antigens producing both IgG and IgM signals lower in congenic animals than in SHR-A3 (Table 1). Among the seven antigens identified with reactive IgM and IgG, two antigens (Hspa1L and Hspa6) were members of the HSP70 family of stress-responsive heat shock proteins. Human Hspa1L is closely homologous to rat Hspa1L (Blast score 1254). Hspa6 is a human protein not present in the rat or mouse genome, with high homology to rat Hspa1a (HSP70, Blast homology score = 1090). Pooled serum from SHR-A3 and SHR-A3(IgH-B2) was tested for antibodies to HSP70 by dot blot using rat recombinant HSP70. Serum from SHR-A3 rats showed strong immunoreactivity for both IgG and IgM, while only weak signals were observed in serum from SHR-A3(IgH-B2) rats (Fig. 4).
Table 1.
Antigens with reactivity to both IgM and IgG
| IgM Z Score |
IgG Z Score |
||||||
|---|---|---|---|---|---|---|---|
| Name | Array ID | SHR-A3 16 wk (no salt load) | SHR-A3 28 wk | SHR-A3(IgH SHR-B2) 28 wk | SHR-A3 16 wk (no salt load) | SHR-A3 28 wk | SHR-A3(IgH SHR-B2) 28 wk |
| HSPA1L | JHU04079.P043D09 | 22.31 | 50.18 | 11.75 | 8.55 | 23.82 | 4.69 |
| LETM1 | JHU01877.P176F10 | 8.01 | 29.57 | 0.09 | 2.30 | 10.45 | 0.07 |
| EAF1 | JHU05677.P060G08 | 10.82 | 19.47 | 13.55 | 6.28 | 10.32 | 5.69 |
| GARS | JHU11709.P122C09 | 3.53 | 19.46 | 0.54 | 7.44 | 8.42 | 0.29 |
| OLA1 | JHU01004.P011C03 | 5.27 | 16.57 | 2.98 | 2.57 | 8.57 | 1.53 |
| FAM131C | JHU05680.P060H09 | 10.78 | 13.60 | 12.94 | 4.80 | 8.09 | 6.57 |
| IST1 | JHU15907.P167B08 | 7.50 | 12.59 | 8.78 | 3.00 | 9.33 | 2.91 |
| LRRFIP1 | JHU25415.P241B12 | −0.27 | 11.96 | −0.22 | −0.14 | 12.36 | −0.16 |
| HSPA6 | JHU18279.P210A07 | 1.42 | 10.86 | 0.28 | 0.74 | 7.43 | 0.13 |
Fig. 4.
Serum HSP70 immunoreactivity. Pooled sera (diluted 1:300) from SHR-A3 and SHR-A3(IgH-B2) rats were tested for immunoreactivity to HSP70 by dot blot. Serial dilutions of rat recombinant HSP70 (starting concentration 0.68 μg/ml) were spotted on to nitrocellulose membranes, and serum immunoreactivity was detected with secondary antibodies to IgG (left) and IgM (right).
Other antigens with both IgM and IgG reactivity that are elevated in salt-loaded SHR-A3, but reduced in the congenic line include: Letm1, Lrrfip1, and Eaf1. These are a group of proteins involved in Wnt/beta-catenin signaling and consequently may be related to brain vascularization and blood-brain barrier function (19, 22, 25, 42). The largest signal observed in 28 wk old SHR-A3 was IgG binding to fatty acid binding protein 4 (Fabp4). This binding was negligible in the congenic line (Supplemental Data).
DISCUSSION
Identification of genetic variation contributing to stroke heritability promises mechanistic insight into pathogenesis. Large-scale human population genetics studies intended to uncover the genetic variants that create cerebrovascular injury have revealed numerous marker associations, some of which can be resolved to specific genes but are of limited effect size and reveal little mechanistic cohesion (11, 27). Despite their extensive genetic identity (87% of the genome), SHR-A3 and SHR-B2 are highly divergent in the immunoglobulin heavy chain gene (16). The rat IgH gene extends over 5 Mb and contains large numbers of variable (V), diversity (D), and joining (J) gene segments. Expression of random combinations of these gene segments results from genomic recombination events, rather than alternative splicing, providing a collection of B cells with a broad initial range of antigen reactivity, including self-reactivity (26). These initial antibodies are expressed as IgM and are inserted into the B cell membrane where they interact with antigens and, with help from T cells, control the development of B cell lineages and drive antibody affinity maturation by further germ line IgH mutation.
The broad range of antigen recognition capabilities initially presented by B cells is shaped by differences in the presence or absence of specific VDJ sequences in the germ line, by VDJ sequence polymorphism within a gene segment present in both lines, and by differences in copy number variation of IgH gene segments, all of which we have shown to occur between SHR-A3 and SHR-B2 (16). Our observation here that genetic variation in IgH influences the emergence of cerebrovascular disease in SHR-A3 may reflect effects of IgH genetic variation on the likelihood that particular antigens will be recognized by germline IgH gene products. While some of the extensive genetic divergence in the IgH gene between SHR-A3 and SHR-B2 has been cataloged (16), the divergence is likely to include extensive structural variation including numerous segmental duplication events. At present a more complete knowledge of IgH genetic variation in the rat will be required to allow a precise genetic mechanism influencing stroke to be defined.
The presence of antibody-mediated immune disease in and the beneficial effects of IgH transfer from SHR-B2 into SHR-A3 indicate a potential pathogenic role of antibodies in the occurrence of stroke. Pathogenic antibodies can create disease in a number of ways. For example, antibodies to the angiotensin receptor can activate it and raise BP (8, 29, 37). Autoantibodies can also create functional deficits by sequestering proteins (6, 33). Identification of targets of autoantibodies has recently improved through the use of recombinant protein arrays that allow the screening of antibody binding against very large numbers of specific targets (12). The results we obtained with the HuProt array are potentially interesting because they are enriched with antibody targets with functions linked to cerebrovascular disease. For example, cerebral ischemia induces expression of HSP70 family stress proteins, and this has protective effects against ischemic injury (23, 36, 47). This stress adaptation may arise in SHR-A3 as BP begins to exceed the range of autoregulatory control of blood flow in the brain (14). The formation of neutralizing autoantibodies to HSP70 in SHR-A3 may limit adaptation to ischemic stress. Similarly, three antigens for which IgG and IgM autoantibodies were recognized participate in Wnt/β-catenin signaling. Lrrfip1 is upregulated in cerebral ischemia and activates β-catenin to regulate prosurvival pathways (17, 22). Eaf1 binds to β-catenin and regulates Wnt signaling (25). Letm1 downregulation leads to downregulation of β-catenin (19, 46). All three genes are expressed in brain. A very large IgG signal was detected for Fabp4 in SHR-A3 but was not present in the IgM screen (Supplementary Data, https://zenodo.org/record/3248943). The Fabp4 IgG signal was much reduced in the congenic line. Fabp4 is a marker of stroke risk (7), a prognostic indicator of stroke outcome (24, 40), and a proangiogenic factor expressed in vascular endothelial cells (13). The HuProt array presents limitations that should be recognized: first, the arrayed proteins are human, not rat proteins. This may enrich the discovered autoantigens for proteins most highly conserved between rats and humans. Second, the arrays are estimated to contain ~80% of the human proteome, so antigens not represented will not be detected by SHR autoantibodies. Finally, pathogenic antibodies whose targets are not proteins cannot be uncovered. Nevertheless, the array is a useful discovery tool to identify and screen for a large number of preliminary autoantibody targets that will require further additional targeted study for full validation and confirmation.
Efforts to map stroke susceptibility loci have been reported using F2 progeny of intercrosses between SHRSP and SHR lines (15, 34, 35). Linked loci have been identified on chromosomes 1, 4, 5, and 18, and some further efforts have been reported to refine and substantiate these mapped loci. Despite the relatively large phenotypic effect on stroke risk, the IgH locus did not emerge as a stroke-linked locus by mapping in these previously reported crosses. There are a number of possible reasons for this. First, there is no knowledge regarding whether the IGH locus shares the same underlying genetic variation in the stroke-resistant SHR lines used in these crosses. In contrast, we have shown major functional immunoglobulin differences attributable to underlying genetic variation in IgH across our lines (16, 18). Some of these crosses have used both male and female F2 animals. SHR-A3 animals are resistant to stroke, reducing the power of mapping in crosses in which females are phenotyped (31). Furthermore, there are strong interactions between estrogen and expression of IgH genes, producing potentially contradictory effects in males and females and reducing the power to identify the locus (20, 21). In addition, the phenotypic assessments used in mapping were not fully overlapping with those used in the present study (e.g., cerebral edema, microbleeds). Finally, the density, distribution, and uniqueness of mapping markers used has not been ideal; for example, only two markers on chromosome 6 were used in one mapping study (35) and five in another (15), one of which reports variation at more than one genomic location.
Our model organism studies that can be considered in light of heritability of risk of stroke in humans. The use of genome-wide association study (GWAS) and whole exome sequencing as means of detecting association between genetic variation and disease has certain intrinsic limitations. These are particularly relevant to IgH. Some population studies “immortalize” the source of subject DNA by constructing lymphoblastoid cell lines. These cell lines are created by infecting B cells with Epstein-Barr virus. B cells recombine genomic IgH DNA to create diverse IgH messages. Consequently, it is unlikely that any lymphoblastoid cell line can provide genomic DNA that reflects the full, unrecombined, germ-line IgH sequence (44). GWASs are also limited in their ability to uncover disease associations attributable to structural variation in the genome (2). Structural variation is a major characteristic of IgH in both humans and rats (16, 43, 45). In our SHR studies, IgH structural variation comprises two highly divergent haplotypes. IgH haplotypes can be expected from existing knowledge to be highly varied in human populations (43–45). However, IgH has only been associated with disease in a single GWAS (32). This may reflect unsurmounted technical impediments to discover association in the IgH locus rather than actual lack of association (44).
The relationship between stroke and BP in humans is well recognized (38, 48). Diversity in stroke phenotype and pathogenesis in human stroke is far greater than in SHR. SHR has ischemic stroke with hemorrhagic features. It does not have atherosclerotic disease. There is no evidence to suggest that atrial fibrillation contributes to stroke in rats, and there is no known propensity for thrombotic disease in SHR. The broad phenotypic scope of stroke in humans is an impediment to discovery of underlying causative genetic variation. Additionally, genetic variants that influence hypertensive end organ disease in tissues other than the brain, such as the kidneys, can indirectly affect stroke susceptibility. The acute emergence of stroke also limits the ability to inform genetic studies about the hypertension status of study subjects, which must often rely on correlated phenotypes (such as history of antihypertensive medication).
In conclusion, we have shown that genetic variation in SHR-A3 affecting IgH contributes to risk of hypertensive cerebrovascular disease. The role of IgH as a major component of antibodies indicate that the pathogenesis of stroke in SHR-A3 involves pathogenic antibodies. Congenic substitution of IgH is associated with reduction in the level of serum IgM and IgG reactive to autoantigen targets that may be involved in adaptation to cerebral ischemia occurring as BP exceeds the cerebral autoregulatory range.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK-069632 and R01DK-081866 to P. A. Doris and American Heart Association postdoctoral fellowship award (AHA 17POST33660779) to I. S. Dhande.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.W., M.C.B., and P.A.D. conceived and designed research; I.S.D., S.C.K., A.S.J., Y.Z., and P.A.D. performed experiments; I.S.D., S.C.K., Y.Z., M.J.H., S.E.W., M.C.B., and P.A.D. analyzed data; I.S.D., Y.Z., M.J.H., S.E.W., M.C.B., and P.A.D. interpreted results of experiments; I.S.D. prepared figures; I.S.D., M.C.B., and P.A.D. drafted manuscript; M.J.H., M.C.B., and P.A.D. edited and revised manuscript; M.C.B. and P.A.D. approved final version of manuscript.
REFERENCES
- 1.Akiguchi I, Horie R, Ohtaka M, Yamori Y, Kawai C. Symptomatologic analysis of stroke in stroke-prone SHR [proceedings] [proceedings]. Jpn Heart J 18: 547–548, 1977. doi: 10.1536/ihj.18.547. [DOI] [PubMed] [Google Scholar]
- 2.Alkan C, Coe BP, Eichler EE. Genome structural variation discovery and genotyping. Nat Rev Genet 12: 363–376, 2011. doi: 10.1038/nrg2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell R, Herring SM, Gokul N, Monita M, Grove ML, Boerwinkle E, Doris PA. High-resolution identity by descent mapping uncovers the genetic basis for blood pressure differences between spontaneously hypertensive rat lines. Circ Cardiovasc Genet 4: 223–231, 2011. doi: 10.1161/CIRCGENETICS.110.958934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehme AK, Esenwa C, Elkind MSV. Stroke Risk Factors, Genetics, and Prevention. Circ Res 120: 472–495, 2017. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun MC, Herring SM, Gokul N, Monita M, Bell R, Zhu Y, Gonzalez-Garay ML, Wenderfer SE, Doris PA. Hypertensive renal injury is associated with gene variation affecting immune signaling. Circ Cardiovasc Genet 7: 903–910, 2014. doi: 10.1161/CIRCGENETICS.114.000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne SK, Holland SM. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol 10: 534–541, 2010. doi: 10.1097/ACI.0b013e3283402b41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang L, Zhang J, Liu L, Huang Z, Han Y, Zhu Y. Fatty acid binding protein 4 is associated with stroke risk and severity in patients with acute ischemic stroke. J Neuroimmunol 311: 29–34, 2017. doi: 10.1016/j.jneuroim.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Dechend R, Viedt C, Müller DN, Ugele B, Brandes RP, Wallukat G, Park J-K, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 107: 1632–1639, 2003. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 9.Dhande IS, Cranford SM, Zhu Y, Kneedler SC, Hicks MJ, Wenderfer SE, Braun MC, Doris PA. Susceptibility to hypertensive renal disease in the spontaneously hypertensive rat is influenced by 2 loci affecting blood pressure and immunoglobulin repertoire. Hypertension 71: 700–708, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhande IS, Zhu Y, Braun MC, Hicks MJ, Wenderfer SE, Doris PA. Mycophenolate mofetil prevents cerebrovascular injury in stroke-prone spontaneously hypertensive rats. Physiol Genomics 49: 132–140, 2017. doi: 10.1152/physiolgenomics.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dichgans M, Malik R, König IR, Rosand J, Clarke R, Gretarsdottir S, Thorleifsson G, Mitchell BD, Assimes TL, Levi C, O’Donnell CJ, Fornage M, Thorsteinsdottir U, Psaty BM, Hengstenberg C, Seshadri S, Erdmann J, Bis JC, Peters A, Boncoraglio GB, März W, Meschia JF, Kathiresan S, Ikram MA, McPherson R, Stefansson K, Sudlow C, Reilly MP, Thompson JR, Sharma P, Hopewell JC, Chambers JC, Watkins H, Rothwell PM, Roberts R, Markus HS, Samani NJ, Farrall M, Schunkert H, Gschwendtner A, Bevan S, Chen Y-C, DeStefano AL, Parati EA, Quertermous T, Ziegler A, Boerwinkle E, Holm H, Fischer M, Kessler T, Willenborg C, Laaksonen R, Voight BF, Stewart AFR, Rader DJ, Hall AS, Kooner JS; METASTROKE Consortium; CARDIoGRAM Consortium; C4D Consortium; International Stroke Genetics Consortium . Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 45: 24–36, 2014. doi: 10.1161/STROKEAHA.113.002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte JG, Blackburn JM. Advances in the development of human protein microarrays. Expert Rev Proteomics 14: 627–641, 2017. doi: 10.1080/14789450.2017.1347042. [DOI] [PubMed] [Google Scholar]
- 13.Elmasri H, Ghelfi E, Yu CW, Traphagen S, Cernadas M, Cao H, Shi G-P, Plutzky J, Sahin M, Hotamisligil G, Cataltepe S. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis 15: 457–468, 2012. doi: 10.1007/s10456-012-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fantini S, Sassaroli A, Tgavalekos KT, Kornbluth J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 3: 031411, 2016. doi: 10.1117/1.NPh.3.3.031411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandolgor T-A, Ohara H, Cui Z-H, Hirashima T, Ogawa T, Saar K, Hübner N, Watanabe T, Isomura M, Nabika T. Two genomic regions of chromosomes 1 and 18 explain most of the stroke susceptibility under salt loading in stroke-prone spontaneously hypertensive rat/Izm. Hypertension 62: 55–61, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00488. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Garay ML, Cranford SM, Braun MC, Doris PA. Diversity in the preimmune immunoglobulin repertoire of SHR lines susceptible and resistant to end-organ injury. Genes Immun 15: 528–533, 2014. doi: 10.1038/gene.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubern C, Camós S, Hurtado O, Rodríguez R, Romera VG, Sobrado M, Cañadas R, Moro MA, Lizasoain I, Serena J, Mallolas J, Castellanos M. Characterization of Gcf2/Lrrfip1 in experimental cerebral ischemia and its role as a modulator of Akt, mTOR and β-catenin signaling pathways. Neuroscience 268: 48–65, 2014. doi: 10.1016/j.neuroscience.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 18.Herring SM, Gokul N, Monita M, Bell R, Boerwinkle E, Wenderfer SE, Braun MC, Doris PA. Immunoglobulin locus associates with serum IgG levels and albuminuria. J Am Soc Nephrol 22: 881–889, 2011. doi: 10.1681/ASN.2010111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang B, Zhang J, Zhang X, Huang C, Hu G, Li S, Xie T, Liu M, Xu Y. Suppression of LETM1 by siRNA inhibits cell proliferation and invasion of bladder cancer cells. Oncol Rep 38: 2935–2940, 2017. doi: 10.3892/or.2017.5959. [DOI] [PubMed] [Google Scholar]
- 20.Jones BG, Penkert RR, Xu B, Fan Y, Neale G, Gearhart PJ, Hurwitz JL. Binding of estrogen receptors to switch sites and regulatory elements in the immunoglobulin heavy chain locus of activated B cells suggests a direct influence of estrogen on antibody expression. Mol Immunol 77: 97–102, 2016. doi: 10.1016/j.molimm.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BG, Sealy RE, Penkert RR, Surman SL, Maul RW, Neale G, Xu B, Gearhart PJ, Hurwitz JL. Complex sex-biased antibody responses: estrogen receptors bind estrogen response elements centered within immunoglobulin heavy chain gene enhancers. Int Immunol 31: 141–156, 2019. doi: 10.1093/intimm/dxy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labbé P, Faure E, Lecointe S, Le Scouarnec S, Kyndt F, Marrec M, Le Tourneau T, Offmann B, Duplaà C, Zaffran S, Schott JJ, Merot J. The alternatively spliced LRRFIP1 Isoform-1 is a key regulator of the Wnt/β-catenin transcription pathway. Biochim Biophys Acta Mol Cell Res 1864: 1142–1152, 2017. doi: 10.1016/j.bbamcr.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke 32: 2905–2912, 2001. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Bi P, Zhao W, Lian Y, Zhu H, Xu D, Ding J, Wang Q, Yin C. Prognostic Utility of Fatty Acid-Binding Protein 4 in Patients with Type 2 Diabetes and Acute Ischemic Stroke. Neurotox Res 33: 309–315, 2018. doi: 10.1007/s12640-017-9792-z. [DOI] [PubMed] [Google Scholar]
- 25.Liu J-X, Zhang D, Xie X, Ouyang G, Liu X, Sun Y, Xiao W. Eaf1 and Eaf2 negatively regulate canonical Wnt/β-catenin signaling. Development 140: 1067–1078, 2013. doi: 10.1242/dev.086157. [DOI] [PubMed] [Google Scholar]
- 26.Lucas JS, Murre C, Feeney AJ, Riblet R. The Structure and Regulation of the Immunoglobulin Loci, in Molecular Biology of B Cells (Alt FW, Honjo T, Radbruch A, Reth M, editors). (2nd ed.). London: Academic Press, 2015, p. 1–11. [Google Scholar]
- 27.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese A-K, van der Laan SW, Gretarsdottir S, Anderson CD, Chong M, Adams HHH, Ago T, Almgren P, Amouyel P, Ay H, Bartz TM, Benavente OR, Bevan S, Boncoraglio GB, Brown RD Jr, Butterworth AS, Carrera C, Carty CL, Chasman DI, Chen W-M, Cole JW, Correa A, Cotlarciuc I, Cruchaga C, Danesh J, de Bakker PIW, DeStefano AL, den Hoed M, Duan Q, Engelter ST, Falcone GJ, Gottesman RF, Grewal RP, Gudnason V, Gustafsson S, Haessler J, Harris TB, Hassan A, Havulinna AS, Heckbert SR, Holliday EG, Howard G, Hsu F-C, Hyacinth HI, Ikram MA, Ingelsson E, Irvin MR, Jian X, Jiménez-Conde J, Johnson JA, Jukema JW, Kanai M, Keene KL, Kissela BM, Kleindorfer DO, Kooperberg C, Kubo M, Lange LA, Langefeld CD, Langenberg C, Launer LJ, Lee JM, Lemmens R, Leys D, Lewis CM, Lin WY, Lindgren AG, Lorentzen E, Magnusson PK, Maguire J, Manichaikul A, McArdle PF, Meschia JF, Mitchell BD, Mosley TH, Nalls MA, Ninomiya T, O’Donnell MJ, Psaty BM, Pulit SL, Rannikmäe K, Reiner AP, Rexrode KM, Rice K, Rich SS, Ridker PM, Rost NS, Rothwell PM, Rotter JI, Rundek T, Sacco RL, Sakaue S, Sale MM, Salomaa V, Sapkota BR, Schmidt R, Schmidt CO, Schminke U, Sharma P, Slowik A, Sudlow CLM, Tanislav C, Tatlisumak T, Taylor KD, Thijs VNS, Thorleifsson G, Thorsteinsdottir U, Tiedt S, Trompet S, Tzourio C, van Duijn CM, Walters M, Wareham NJ, Wassertheil-Smoller S, Wilson JG, Wiggins KL, Yang Q, Yusuf S, Bis JC, Pastinen T, Ruusalepp A, Schadt EE, Koplev S, Björkegren JLM, Codoni V, Civelek M, Smith NL, Trégouët DA, Christophersen IE, Roselli C, Lubitz SA, Ellinor PT, Tai ES, Kooner JS, Kato N, He J, van der Harst P, Elliott P, Chambers JC, Takeuchi F, Johnson AD, Sanghera DK, Melander O, Jern C, Strbian D, Fernandez-Cadenas I, Longstreth WT Jr, Rolfs A, Hata J, Woo D, Rosand J, Pare G, Hopewell JC, Saleheen D, Stefansson K, Worrall BB, Kittner SJ, Seshadri S, Fornage M, Markus HS, Howson JMM, Kamatani Y, Debette S, Dichgans M; AFGen Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium; International Genomics of Blood Pressure (iGEN-BP) Consortium; INVENT Consortium; STARNET; BioBank Japan Cooperative Hospital Group; COMPASS Consortium; EPIC-CVD Consortium; EPIC-InterAct Consortium; International Stroke Genetics Consortium (ISGC); METASTROKE Consortium; Neurology Working Group of the CHARGE Consortium; NINDS Stroke Genetics Network (SiGN); UK Young Lacunar DNA Study; MEGASTROKE Consortium . Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 50: 524–537, 2018. doi: 10.1038/s41588-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaoka A, Iwatsuka H, Suzuoki Z, Okamoto K. Genetic predisposition to stroke in spontaneously hypertensive rats. Am J Physiol 230: 1354–1359, 1976. doi: 10.1152/ajplegacy.1976.230.5.1354. [DOI] [PubMed] [Google Scholar]
- 29.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol 302: R1197–R1201, 2012. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27: 282–293, 1963. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto K, Yamori Y, Nagaoka A. Establishment of the Stroke-prone Spontaneously Hypertensive Rat (SHR). Circ Res 14: I143–I153, 1974. [Google Scholar]
- 32.Parks T, Mirabel MM, Kado J, Auckland K, Nowak J, Rautanen A, Mentzer AJ, Marijon E, Jouven X, Perman ML, Cua T, Kauwe JK, Allen JB, Taylor H, Robson KJ, Deane CM, Steer AC, Hill AVS; Pacific Islands Rheumatic Heart Disease Genetics Network . Association between a common immunoglobulin heavy chain allele and rheumatic heart disease risk in Oceania. Nat Commun 8: 14946, 2017. doi: 10.1038/ncomms14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg JM, Maccari ME, Barzaghi F, Allenspach EJ, Pignata C, Weber G, Torgerson TR, Utz PJ, Bacchetta R. Neutralizing Anti-Cytokine Autoantibodies Against Interferon-α in Immunodysregulation Polyendocrinopathy Enteropathy X-Linked. Front Immunol 9: 544, 2018. doi: 10.3389/fimmu.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubattu S, Hubner N, Ganten U, Evangelista A, Stanzione R, Di Angelantonio E, Plehm R, Langanki R, Gianazza E, Sironi L, D’Amati G, Volpe M. Reciprocal congenic lines for a major stroke QTL on rat chromosome 1. Physiol Genomics 27: 108–113, 2006. doi: 10.1152/physiolgenomics.00086.2006. [DOI] [PubMed] [Google Scholar]
- 35.Rubattu S, Volpe M, Kreutz R, Ganten U, Ganten D, Lindpaintner K. Chromosomal mapping of quantitative trait loci contributing to stroke in a rat model of complex human disease. Nat Genet 13: 429–434, 1996. doi: 10.1038/ng0896-429. [DOI] [PubMed] [Google Scholar]
- 36.Sharp FR, Kinouchi H, Koistinaho J, Chan PH, Sagar SM. HSP70 heat shock gene regulation during ischemia. Stroke 24, Suppl: I72–I75, 1993. [PubMed] [Google Scholar]
- 37.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 55: 386–393, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sörös P, Whitehead S, Spence JD, Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol 9: 174–178, 2013. doi: 10.1038/nrneurol.2012.255. [DOI] [PubMed] [Google Scholar]
- 39.Thompson EA. Identity by descent: variation in meiosis, across genomes, and in populations. Genetics 194: 301–326, 2013. doi: 10.1534/genetics.112.148825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tu W-J, Zeng X-W, Deng A, Zhao S-J, Luo D-Z, Ma G-Z, Wang H, Liu Q. Circulating FABP4 (Fatty Acid-Binding Protein 4) Is a Novel Prognostic Biomarker in Patients With Acute Ischemic Stroke. Stroke 48: 1531–1538, 2017. doi: 10.1161/STROKEAHA.117.017128. [DOI] [PubMed] [Google Scholar]
- 41.Venkataraman A, Yang K, Irizarry J, Mackiewicz M, Mita P, Kuang Z, Xue L, Ghosh D, Liu S, Ramos P, Hu S, Bayron Kain D, Keegan S, Saul R, Colantonio S, Zhang H, Behn FP, Song G, Albino E, Asencio L, Ramos L, Lugo L, Morell G, Rivera J, Ruiz K, Almodovar R, Nazario L, Murphy K, Vargas I, Rivera-Pacheco ZA, Rosa C, Vargas M, McDade J, Clark BS, Yoo S, Khambadkone SG, de Melo J, Stevanovic M, Jiang L, Li Y, Yap WY, Jones B, Tandon A, Campbell E, Montelione GT, Anderson S, Myers RM, Boeke JD, Fenyö D, Whiteley G, Bader JS, Pino I, Eichinger DJ, Zhu H, Blackshaw S. A toolbox of immunoprecipitation-grade monoclonal antibodies to human transcription factors. Nat Methods 15: 330–338, 2018. doi: 10.1038/nmeth.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Cho C, Williams J, Smallwood PM, Zhang C, Junge HJ, Nathans J. Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance. Proc Natl Acad Sci USA 115: E11827–E11836, 2018. [Erratum in Proc Natl Acad Sci USA 116: 3934, 2019] doi: 10.1073/pnas.1813217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson CT, Breden F. The immunoglobulin heavy chain locus: genetic variation, missing data, and implications for human disease. Genes Immun 13: 363–373, 2012. doi: 10.1038/gene.2012.12. [DOI] [PubMed] [Google Scholar]
- 44.Watson CT, Matsen FA IV, Jackson KJL, Bashir A, Smith ML, Glanville J, Breden F, Kleinstein SH, Collins AM, Busse CE. Comment on “A Database of Human Immune Receptor Alleles Recovered from Population Sequencing Data”. J Immunol 198: 3371–3373, 2017. doi: 10.4049/jimmunol.1700306. [DOI] [PubMed] [Google Scholar]
- 45.Watson CT, Steinberg KM, Huddleston J, Warren RL, Malig M, Schein J, Willsey AJ, Joy JB, Scott JK, Graves TA, Wilson RK, Holt RA, Eichler EE, Breden F. Complete haplotype sequence of the human immunoglobulin heavy-chain variable, diversity, and joining genes and characterization of allelic and copy-number variation. Am J Hum Genet 92: 530–546, 2013. doi: 10.1016/j.ajhg.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Huang B, Li S, Zhang X, Xie T, Xu Y. Knockdown of LETM1 inhibits proliferation and metastasis of human renal cell carcinoma cells. Oncol Lett 16: 6377–6382, 2018. doi: 10.3892/ol.2018.9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsp70 protein. Brain Res 1234: 183–197, 2008. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Thijs L, Staessen JA. Blood pressure lowering for primary and secondary prevention of stroke. Hypertension 48: 187–195, 2006. doi: 10.1161/01.HYP.0000231939.40959.60. [DOI] [PubMed] [Google Scholar]