Abstract
Chemerin is a contractile adipokine, produced in liver and fat, and removal of the protein by antisense oligonucleotides (ASO) lowers blood pressure in the normal Sprague Dawley rat. In humans, chemerin is positively associated with blood pressure and obesity so we hypothesized that in a model of hypertension derived from high-fat (HF) feeding, the chemerin ASO would reduce blood pressure more than a high-salt (HS) model. Male Dahl S rats were given a HF (60% kcal fat; age 3–24 wk) or HS diet (4% salt; age 20–24 wk to match age and blood pressure of HF animals). Scrambled control, whole body, or liver-specific ASOs that knock down chemerin were delivered subcutaneously once per week for 4 wk with tissue and blood collected 2 days after the last injection. Conscious blood pressure was measured 24 h/day by radiotelemetry. By the end of whole body ASO administration, blood pressure of HF animals had fallen 29 ± 2 mmHg below baseline, while blood pressure of HS-diet animals fell by only 12 ± 4 mmHg below baseline. Administration of a liver-specific ASO to HF Dahl S resulted in a 6 ± 2 mmHg fall in blood pressure below baseline. Successful knockdown of chemerin in both the whole body and liver-specific administration was confirmed by Western and PCR. These results suggest that chemerin, not derived from liver but potentially from adipose tissue, is an important driver of hypertension associated with high fat. This knowledge could lead to the development of antihypertensive treatments specifically targeted to obesity-associated hypertension.
Keywords: blood pressure, chemerin, high-fat, pharmacology
INTRODUCTION
While the diagnosis of hypertension is important because it identifies an individual as having a higher risk of a potentially fatal secondary event like a stroke or heart attack, different circumstances can lead to the same elevation in blood pressure. One subpopulation with an important connection to hypertension are people with obesity (22). Up to 75% of primary hypertension can be attributed to visceral adiposity (17). Hypertension and obesity are essential elements to the diagnosis of metabolic syndrome, which includes alterations in waist circumference, fasting glucose, blood pressure, plasma HDL, and triglycerides (1).
Chemerin is a protein that has recently emerged in the epidemiological literature because of positive associations of circulating chemerin with blood pressure (9, 11, 19, 23, 29, 30, 38), glucose levels (33), and obesity (5, 6, 9, 11, 29). These associations led to chemerin being proposed as a marker for metabolic syndrome (4, 16). Studies are beginning to provide mechanistic support for these epidemiological associations. First, chemerin is an adipokine, with primary production in the liver and adipose tissue (4), that enhances the development of adipocytes as well as maintenance of mature adipocytes (15). Second, chemerin is contractile in vasculature of both humans and rats with the literature attributing this mechanism to its actions on the endothelium (24, 27), smooth muscle cells (12, 21), and local nerves (8). Despite the in vitro vasoactivity of chemerin, few studies have evaluated the role of chemerin on blood pressure regulation. Recently, we demonstrated that antisense oligonucleotide (ASO)-mediated reductions of whole body chemerin resulted in small, yet statistically significant blood pressure reductions in normal chow-fed Sprague Dawley (SD) male rats (13).
Through Watson and Crick pairing, ASOs bind to target pre-mRNA in the nucleus of cells, facilitating its destruction, thereby preventing its protein translation (20). ASOs can be delivered subcutaneously and have a half-life on the order of weeks to months. Following systemic administration, ASOs distribute to various tissues except for the central nervous system (2); the primary sites of systemic tissue uptake and ASO potency include liver and kidney, followed by adipose tissue. Ligands can be covalently attached to the ASO to enhance delivery into specific cell types. Notable to this project is the use of N-acetylgalactosamine (GalNAc) ligand that enhances uptake into hepatocytes via the asialoglycoprotein receptor (ASGPR), thereby markedly reducing uptake into non-ASGPR-expressing cells such as adipose tissue (26, 28). In our previous study, administration of the liver-specific chemerin ASO abolished circulating plasma levels of chemerin but did not decrease chemerin production from fat or blood pressure as did the whole body chemerin ASO in lean SD male rats (13). This makes it doubtful that the liver or plasma contribute chemerin that regulates blood pressure. Chemerin’s classification as an adipokine suggested that the fat may be an important contributor to chemerin’s blood pressure effects. Fat-specific ASOs are not available, so to make inferences about the relation of fat-derived chemerin and blood pressure, we can target the other major source of chemerin (liver) and compare different etiologies of hypertension [high-fat (HF) versus high-salt (HS) diet].
For the current study, we hypothesized that rats made hypertensive by HF diet would demonstrate a reduction of blood pressure with the whole body ASO against chemerin but not with the liver-specific ASO against chemerin. To determine whether this effect might be specific for fat-associated hypertension rather than a general response of hypertensive animals, we also tested the effect of the same ASO treatment on blood pressure in lean animals made hypertensive from ingestion of a HS diet (another recognized risk factor for hypertension).
The Dahl salt-sensitive (Dahl S) rat was chosen as a model of both normal-weight hypertension and fat-associated hypertension. The Dahl S HF-diet rat is unique as one of the few diet-induced models of obesity-associated hypertension among both mice and rats. Dahl S animals fed a HS (31) or HF (3, 14) diet will develop high blood pressure on their respective diet versus an animal fed a control diet. Dahl S HF animals also have increased visceral adiposity compared with control diet controls (14). Even though the mechanism of elevated blood pressure in a Dahl S animal fed HF diet is still under investigation, this model is advantageous because animals of the same genetic background and age develop similar levels of hypertension with different stimuli. If chemerin secreted from fat is unique to the etiology of hypertension in the HF Dahl S rat, the chemerin ASO will reduce blood pressure to a greater degree in the HF animal than the HS animal. These data suggest a major role of chemerin in HF, but not HS-induced hypertension.
METHODS
Animal handling.
All procedures that involved animals were performed in accordance with the institutional guidelines and animal use committee of Michigan State University. Animals were maintained on a 12:12 h light-dark cycle at a temperature of 22–25°C.
Rat models of disease.
For the HF model, Dahl S rats were purchased from Charles River Laboratories (Portage, MI) and placed on a HF diet (60% kcal from fat, 0.3% NaCl; D12492, Research Diets, New Brunswick, NJ), ad libitum, from weaning at 3 wk of age for 21 wk before ASOs were administered. Rats were single-housed and remained on their respective diets for the duration of the experiment.
For the HS model, Dahl S rats were purchased from Charles River Laboratories and placed on a HS diet (12% kcal from fat, 4% NaCl; D17013, Research Diets), ad libitum, starting at 20 wk of age for 4 wk until ASO treatment began. HS diet was continued through the duration of the experiment.
Radiotelemetry and ASO administration.
Catheters for radiotelemetry were placed in the femoral artery and the radiotelemeter body was placed subcutaneously (HD-S10, DSI). Catheters for both diet groups were implanted between 16 and 18 wk of age. Rats were allowed to recover for at least 1 wk, and baseline measurements were collected over 4 days before ASO administration. Physiologic measures were sampled for 10 s every 10 min for the duration of the experiment. Data are represented as 24 h averages of the aforementioned sampling.
ASO synthesis and sequences are exactly as previously described (13). The following are lists of the scrambled control, liver-specific and whole body ASOs used in this study: Scrambled control (5′-3′), GGCCAATACGCCGTCA; liver-specific (5′-3′), GalNAc-ACAGTTTTATTAGCCTGGAG; whole body (5′-3′), GTTTTATTAGCCTGGA.
ASOs were designed to avoid any perfect match rat mRNA and where chosen from >300 ASOs as being well tolerated and potent. In silico off-target analysis of the liver-specific 20 mer ASO shows no off-targets with zero- or one-base mismatches, and only 1 off-target (Zfp804b) with 2 base mismatches. The 16 mer whole body chemerin ASO does have 22 one-base mismatch targets, this is common given its shorter size. None of these targets are regulators of blood pressure or adipocyte development.
Experiments were carried out in three cohorts, separated in time, and pooled for a final result: 1) HF Dahl S with control and whole body ASO, 2) HS Dahl S with control and whole body ASO, and 3) HF Dahl S with whole body and liver-specific ASO. To administer ASOs, animals were anesthetized with 1–2% isoflurane, weighed, and subcutaneously injected with 25 mg/kg control ASO, 25 mg/kg whole body ASO, or 10 mg/kg liver-specific ASO on days 0, 7, 14, and 19. Samples were collected on day 21, and euthanasia was performed under 1–2% isoflurane with pneumothorax. Kidney, liver, epididymal fat, retroperitoneal fat (dissected from the posterior abdominal wall), and heart were dissected and weighed. Samples of blood (collected in EDTA), liver, epididymal fat, retroperitoneal fat, mesenteric fat (taken from the distal superior mesenteric artery avoiding mesenteric resistance vessels), and aortic fat were collected for protein and mRNA analysis. Blood was stored at 4°C and tissue was placed in liquid nitrogen.
Western blots.
Protein analysis for plasma and tissue was performed as described (13). Blood was centrifuged for 20 min at 4°C and 500 g. Plasma was collected and diluted 1:25 before we performed a bicinchoninic acid assay for total protein (#BCA1, Sigma Chemical Co., St. Louis, MO; RRID:SCR_008988). Fat pads were homogenized in an Omni Bead Ruptor 24 (5.65 m/s, 2 cycles, 30 s cycles, 30 s between cycles, 6°C; Omni International, Kennesaw, GA). We added 100 μg of protein to a 15% polyacrylamide gel and ran it at 120 V. Protein was transferred to a PVDF-FL membrane (#IPFL00010; EMD Millipore, Billerica, MA) for 1 h at 100 V. Blots were dried, Total Protein Stain (#926-11011, Li-Cor) was added and reverted and then blocked with chick egg ovalbumin for 3 h. Chemerin antibody (1:1,000; Abcam Cat. #ab112520, RRID:AB_10864055, Cambridge, MA) was incubated with blots for 48 h at 4°C, and the secondary antibody (1:1,000; IRDye 800 anti-Mouse, LI-COR Biosciences Cat. #926-32210, RRID:AB_621842, Lincoln, NE) was incubated with blots for 1 h at 4°C. Total Protein Stain was the loading control for tissues. Total protein quantification is becoming a preferred method of performing a loading control because it does not rely on the expression of one protein and can more ubiquitously control for leading over a large range of tissue types (10). Blots were visualized with the Odyssey CLx Infrared Imaging (Odyssey CLx, RRID:SCR_014579) and quantified with Image Studio (5.2.5, LI-COR Image Studio Software, RRID:SCR_015795). When quantifying signal within Image Studio, we find that adjustment of LUTs does not affect the signal result.
PCR.
Analysis for mRNA was performed as described in our previous study (13). Quantitative qRT-PCR (qPCR) mRNA analysis was performed with TaqMan primer probes (Thermo Fisher-Applied Biosystems, Foster City, CA). Total RNA was extracted from whole tissue with the RNeasy RNA isolation kit (Qiagen, Valencia, CA; RRID:SCR_008539). Samples (50 ng total RNA) were subjected to qPCR analysis with commercial reagents (Thermo Fisher-Invitrogen, Carlsbad, CA) and analyzed with the ABI StepOne Plus Sequence Detector (Thermo Fisher-Applied Biosystems). TaqMan primers and probe for chemerin are as follows: Forward Sequence, CAGGAGATCGGTGTGGACAGT; Reverse Sequence, GAGCTTAAATTCC-AGCCTCACAA; Probe Sequence, TGATGACCTGTTCTTCTCAGCTGGCACCX. The PCR probes were labeled with 5′-FAM (a 6- carboxyfluorescein reporter) and 3′-TAMRA [a 5 (6)-carboxytetramethyl rhodamine quencher]. After 40 amplification cycles, absolute values were obtained with SDS analysis software (Thermo Fisher-Applied Biosystems). Values were normalized to total RNA via Ribogreen measurement (Thermo Fisher-Invitrogen). The use of total RNA as a calibration control is validated (18).
Analyses and statistics.
After sample collection, researchers performing the Western and PCR analysis were blinded as to the treatment of the animal. In PCR, averages of normalized (total RNA) control ASO were used to establish the percentage of mRNA expression with whole body and liver-specific treatments. Westerns were normalized to total protein. Contrast and brightness have been adjusted on Western images (whole, not in part), but these adjustments do not affect the measurements in the Image Studio software. Statistical analysis was performed on GraphPad Prism (7.0c, Graph Pad, La Jolla, CA; RRID:SCR_002798). Data are reported as means ± SE. Comparisons of one variable were made by one-way ANOVA and Tukey’s correction for multiple comparisons (if more than two groups were being compared) or Student’s t test (if only two groups were being compared). Comparisons involving two variables (treatment and time) were performed by a two-way ANOVA with a Tukey correction for multiple comparisons. Statistics used for each analysis are shown in each figure legend.
RESULTS
Chemerin ASOs knockdown mRNA expression and translated protein.
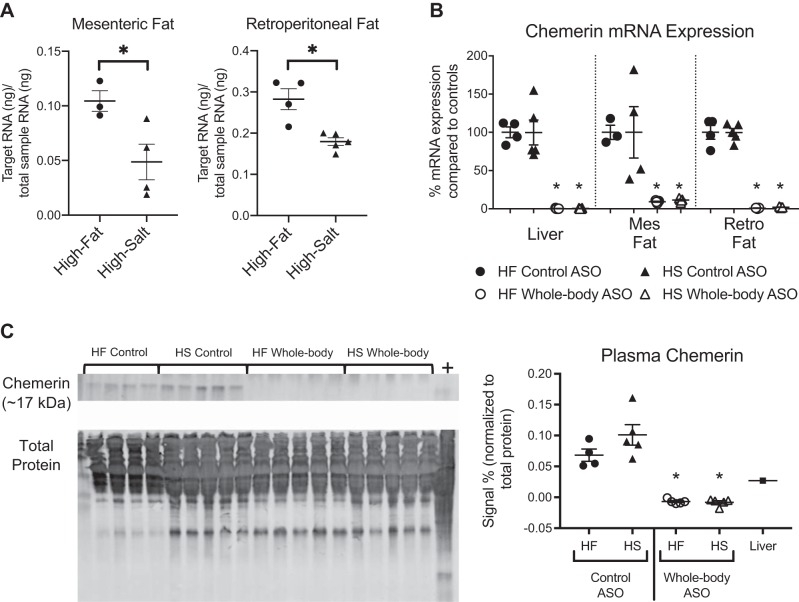
Absolute mRNA levels of chemerin from animals given control ASO (Fig. 1A; measurements made against a standard curve relative to total RNA in each sample) indicate that chemerin expression was higher in the mesenteric and retroperitoneal fat of the HF-fed animals versus the HS-fed animals. In both models of hypertension (HF and HS), chemerin mRNA (Fig. 1B) was present and detectable in rats that received scrambled control ASOs. We have previously validated that chemerin mRNA expression in tissue is representative of protein expression in that tissue (13). We have also validated that while mesenteric chemerin mRNA expression is more variable than the others, chemerin protein is highly expressed with administration of control ASOs (13). In both HF and HS animals, whole body chemerin ASOs completely abolished chemerin mRNA expression in the liver, mesenteric fat, and retroperitoneal fat relative to levels in diet-matched control ASO animals. Circulating chemerin levels as measured by Western blot (Fig. 1C) were undetectable in animals treated with the whole body chemerin ASO in both diets and maintained in rats that received control ASO.
Fig. 1.
Antisense oligonucleotides (ASOs) knocked down chemerin expression in high-fat (HF) and high-salt (HS) diet-fed animals. Absolute quantifications of animals receiving control ASO measure chemerin mRNA expression using standard curves normalized to total RNA in the sample. In both mesenteric and retroperitoneal fat, chemerin expression is higher in the HF fed animals versus the HS fed animals (A). When HF animals, along with HS animals, are given scrambled control or whole body ASOs against chemerin, mRNA expression (as measured by PCR) was abolished in liver, mesenteric (mes) fat, and retroperitoneal (retro) fat (B). Plasma chemerin protein levels (measured by Western blot) are also completely knocked down with whole body ASO (C). In all Western blots, the chemerin band (~17 kDa) is shown on top and total protein stain (used as loading control) on bottom. Positive control (+) for Westerns was liver homogenate isolated from normal SD animals. Negative numbers in Westerns represent instances where the band at the specified molecular weight was lighter than the average background signal for that lane. For HF Control ASO, n = 4; HF Whole body ASO n = 5; HS Control ASO, n = 5; HS Whole body ASO, n = 5 where n represents biological replicates. Scatter plots display data distribution as well as means ± SE *P < 0.05 statistically significant change in the indicated group compared with its control as measured by a one-way ANOVA and Tukey multiple-comparisons test (if more than two groups are compared) or by Student’s t test (if only two groups are being compared).
Organ analysis of HF versus HS Dahl S animals.
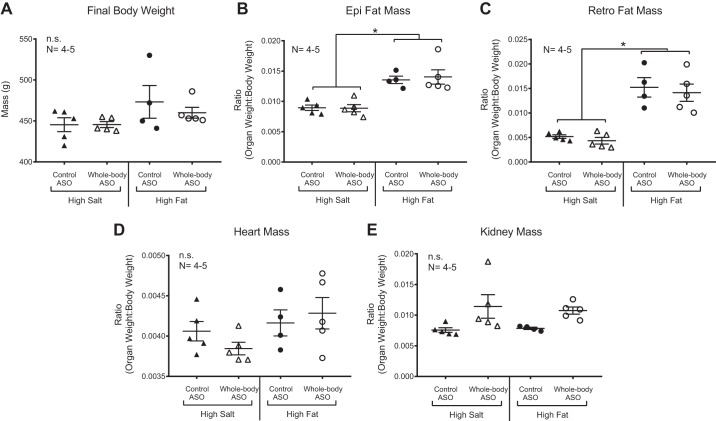
Age-matched Dahl S animals fed a HF diet were the same final weight as HS animals at the end of ASO administration (Fig. 2A). Total body weight as a difference from baseline did not change over the course of the drug treatment (data not shown). When controlled for overall body weight, HF animals had more epididymal and retroperitoneal fat mass than their HS counterparts (Fig. 2, B and C). The epididymal and retroperitoneal fat masses are prime examples of white fat depots in the rat and are relatively easy to dissect upon euthanasia. While they are not as easily measured, we expect that other white adipose depots, like the mesenteric adipose tissue, are also increased relative to body weight with HF feeding. We have previously validated, in our hands, that HF Dahl S rats have an expanded visceral fat depot compared with control diet Dahl S rats (14). Neither heart nor kidney weight (indexed to total body weight) changed with administration of chemerin ASO (Fig. 2, D and E).
Fig. 2.
High-fat animals have increased proportional fat mass. On day 21, rats were euthanized and body weight was recorded (A). Epididymal (epi; B) and retroperitoneal (retro; C) fat pads were increased in mass when controlled for total body weight. Heart (D) and kidney (E) mass were not increased. Scatter plots are representative of the biological replicate (n) listed in the figure and show means ± SE. *P < 0.05; n.s., no significance by one-way ANOVA with Tukey’s multiple-comparison test.
Chemerin ASOs in HF Dahl S animals cause a fall in blood pressure.
A one-way ANOVA of the average blood pressure at baseline in the four treatment groups (HS Control, HS Whole body, HF Control, HF Whole body; Supplemental Table S1; https://figshare.com/s/ccd53be88eaefd81f466) revealed no significant differences with a 0.83 power. After the first administration of the whole body ASO, mean arterial pressure (MAP) reached its lowest point in 48 h (Fig. 3A). Previous studies with ASOs of the same backbone confirm that knockdown of the RNA persists through acute changes of blood pressure (26). Additionally, the homeostatic response we observed is typical of virtually all antihypertensive drugs. In the HF animal, this lowest point after first injection was 21 ± 2 mmHg below baseline, while in the HS animal, it was only 6 ± 2 mmHg below baseline. Over the course of the entire experiment, the nadir of MAP captured in the HF animals was 29 ± 2 mmHg below baseline and was 12 ± 4 mmHg below baseline in the HS animals. MAP in the HS control ASO animals continued to rise throughout the experiment given the continuing HS diet. The largest difference in MAP between HS rats receiving control ASO and HS rats receiving chemerin ASO was 19 ± 4 mmHg, statistically lower than the difference seen in the HF animals, 30 ± 3 mmHg (by P < 0.05; Fig. 3A).
Fig. 3.
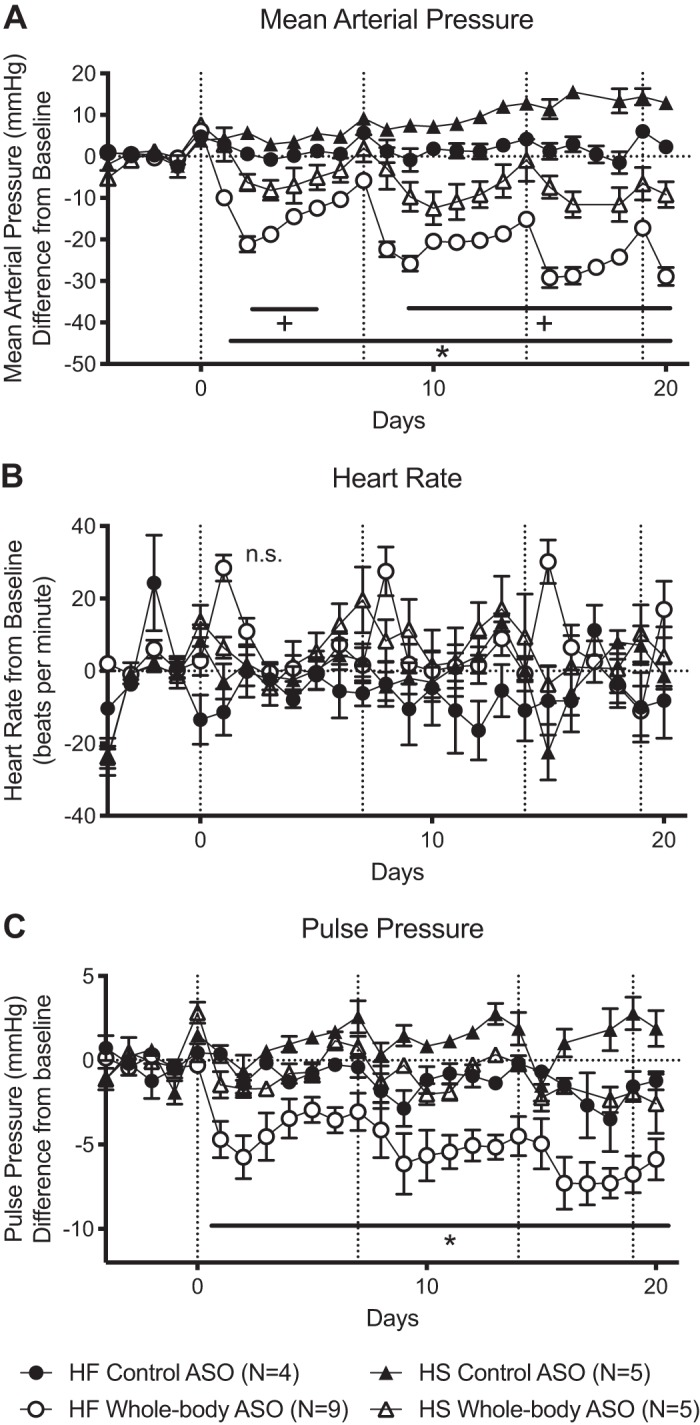
High-fat diet-fed (HF) animals experience a profound drop in blood pressure with whole body chemerin antisense oligonucleotide (ASO). Dahl S animals receiving HF diet experienced a larger fall in mean arterial pressure (MAP) with whole body ASO compared with Dahl S high-salt diet-fed (HS) animals, even when the difference is calculated from the control ASO of the same diet (A). Heart rate (HR) did not change with ASO or diet (B). Pulse pressure (PP) was decreased in HF animals receiving whole body ASO compared with all other groups (C). Dotted vertical lines represent dates of ASO injection and dotted horizontal lines represent baseline. Baseline values are listed in Supplemental Table S1 (https://figshare.com/s/ccd53be88eaefd81f466). *P < 0.05 of the HF whole body group compared with all other groups; +P < 0.05 of the HS whole body ASO group compared with all other groups. Points and error bars represent means ± SE. Statistics were measured by 2-way ANOVA and Tukey’s multiple-comparison test. n values represent biological replicates and are listed in the figures. Cardiovascular measures of HF animals administered whole body ASO in the cohort also receiving the liver-specific ASO were pooled and included in this figure. Because analysis of Westerns and PCR should not be pooled outside of their respective blots or plates, they are not included in the Fig. 2 but were still analyzed and are shown in Fig. 4.
HF whole body ASO rats experienced a temporary spike in heart rate (HR) the day after ASO administration. Overall, none of the treatment groups experienced a significant change in heart rate over the course of the experiment (Fig. 3B). Pulse pressure (PP; difference between systolic and diastolic blood pressure) was significantly reduced in the HF animals treated with chemerin ASO versus all other treatment groups (Fig. 3C).
Liver-specific ASOs have a minor effect on fat-associated hypertension.
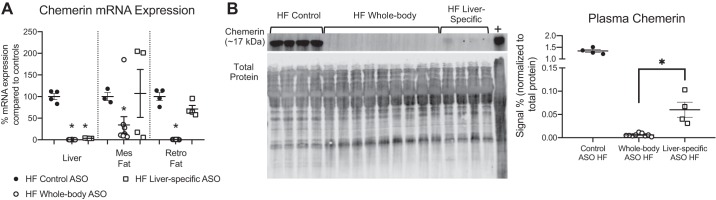
HF animals were given the liver-specific ASO (GalNAc), paired with rats given whole body ASO. These data were combined with other individual data points from previous cohorts given the same treatment scheme to make comparisons between whole body ASO and liver-specific ASO. However, Western blots were rerun with samples from multiple cohorts to make comparisons of plasma chemerin levels. With the liver-specific ASO, chemerin mRNA expression in the mesenteric fat and retroperitoneal fat remained at control levels but was abolished in the liver (Fig. 4A). Plasma chemerin protein levels of the Dahl S HF animals given liver-specific ASO were decreased by 96% ± 1% compared with those in control ASO-treated rats but remained at levels above those observed in whole body ASO-treated rats (Fig. 4B).
Fig. 4.
Liver-specific antisense oligonucleotides (ASOs) knock down chemerin in liver but not completely in plasma. Liver-specific ASOs given to HF Dahl S animals reduced chemerin expression (measured by PCR) in liver but not mesenteric (mes) fat or retroperitoneal (retro) fat (A). Plasma chemerin protein (measured by Western blot) was eliminated in animals given whole body ASO (some samples used in Fig. 2D were run again in this blot), levels of circulating chemerin were markedly reduced after liver-specific ASO treatment; however, residual levels were still present (B). The chemerin band (~17 kDa) is shown on top and total protein stain (used as loading control) on bottom. Positive control (+) for Westerns was liver homogenate isolated from normal SD animals. For Control ASO HF, n = 4; Whole body ASO HF, n = 9; Liver-specific ASO HF, n = 4 where n represents biological replicates. Matching HF whole body ASO plasma samples from Fig. 2 were pooled and run again for this figure. Scatter plots display data distribution as well as means ± SE *P < 0.05 statistically significant change in the indicated groups as calculated by an unpaired Student’s t test.
After the first injection, the lowest point of blood pressure occurred in 48 h. Rats given liver-specific ASO exhibited a MAP only 3 ± 1 mmHg below baseline (Fig. 5A). The MAP nadir was 6 ± 2 mmHg below baseline for the liver-specific ASO versus 29 ± 2 mmHg in the HF whole body ASO. Neither HR nor PP was significantly affected in rats given the liver-specific ASO compared with the HF whole body ASO or HF control ASO (Fig. 5, B and C).
Fig. 5.
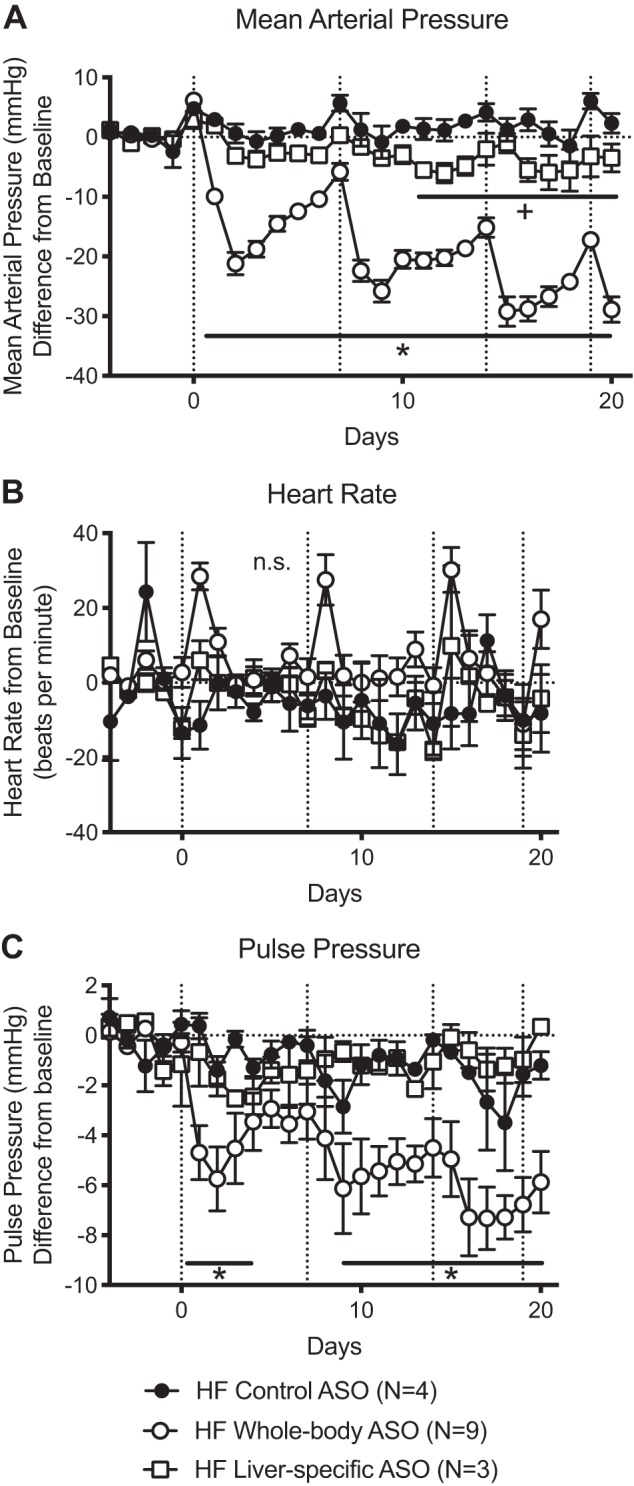
Liver-specific antisense oligonucleotides (ASOs) in HF animals result in a modest fall in blood pressure. Dahl S animals receiving high-fat (HF) diet and the liver-specific ASO displayed a modest fall in mean arterial pressure (MAP) that did not approach the large fall in blood pressure observed with the whole body ASO (A). Heart rate (HR) did not change with any of the treatments (B). Pulse pressure (PP) was significantly decreased with the whole body ASO but not the liver-specific ASO (C). Dotted vertical lines represent dates of ASO injection and dotted horizontal lines represent baseline. Baseline values are listed in Supplemental Table S1 (https://figshare.com/s/ccd53be88eaefd81f466). Points and error bars represent means ± SE. *P < 0.05 of the HF whole body group compared with all other groups; +P < 0.05 of the HF liver-specific ASO group compared with all other groups. HF whole body ASO and control ASO data were pooled with matching HF data from Fig. 3. Statistics were measured by 2-way ANOVA and Tukey’s multiple-comparison test. n values represent biological replicates and are listed in the figures.
DISCUSSION
Chemerin’s influence on HF diet hypertension.
The magnitude of blood pressure fall in the HF Dahl S rat treated with a whole body ASO supports a significant role for chemerin in the regulation of blood pressure. Comparing responses in the HS Dahl S rat and the HF Dahl S controls for a number of important variables that could influence the magnitude of blood pressure response to chemerin ASOs. The similarity obtained here between groups in genetic background, age, species, strain, supplier, and pretreatment blood pressure is not usually available when comparing blood pressure responses to treatment in models with different pathophysiology. Our results with two different Dahl S models allow us to conclude that 1) fat-associated hypertension is caused, at least in part, by a mechanism distinct from mechanisms responsible for salt-associated hypertension; and 2) chemerin plays a significant role in the pathology of HF but not HS hypertension.
Despite substantial changes in blood pressure, the gross appearance and weight of the heart and kidney did not change with any ASO treatment (in rats on either diet). Our failure to observe a lower heart or kidney weight in animals with markedly lower blood pressures probably reflects an inadequate time for structural cardiac adaptations in response to a lower blood pressure to occur, but we cannot rule out a direct effect of chemerin on cardiac or renal structure.
Whole body ASO did reduce blood pressure in the HS Dahl S rat when compared with the control ASO. The magnitude from baseline (6 ± 2 mmHg) was similar to what we previously observed in normotensive SD rats (13). However, there are two ways to interpret blood pressure data: 1) a difference from the baseline of that treatment group and 2) a difference from a control treatment group (scrambled control ASO). Even with the increasing baseline of the HS control ASO group, the control ASO versus whole body ASO in Dahl S HS animals (19 ± 4 mmHg) and the whole body versus control ASO in the Dahl S HF (30 ± 3 mmHg) were significantly different. The fall in blood pressure in the HF animal versus control ASO (a difference of 30 ± 3 mmHg) is likely due to a mechanism specific to the HF diet and not the hypertension alone.
Source of the chemerin matters.
In the experiments described here, we used the same liver-specific ASO as in our previous study (13), but now in the Dahl S HF model of fat-associated hypertension. While giving the HS Dahl S rat a liver-specific ASO would satisfy all the permutations of the variables we are manipulating (diet and ASO type), it would not answer the goals of this study, which were to assess whether blood pressure changes due to chemerin in a HF model are due to general blood pressure status (comparison to HS model) or contributions of the other major producer of chemerin (liver in the HF rat). In the Dahl S HF rat, treatment with a liver-specific ASO reduced MAP by 6 ± 2 mmHg versus control ASO. This was a fraction of the 30 ± 3 mmHg fall in blood pressure observed with the whole body ASO versus control ASO. This relatively small decrease in blood pressure with the liver-specific ASO emphasizes that circulating chemerin from the liver (96% of total circulating chemerin) has only modest effects on blood pressure, since plasma chemerin was nearly eliminated by the liver-specific ASO. This observation is in agreement with our previous study using the liver-specific ASOs in male SD rats (13) where we showed that the liver is the main source of chemerin in the circulation but does not contribute to the chemerin responsible for maintaining blood pressure.
Both the endothelium and smooth muscle cells contain the chemerin receptor (34), and primary smooth muscle cells are able to respond with a calcium flux directly in response to both the chemerin analog (chemerin-9) and recombinant chemerin (12). Given that chemerin is produced in such high amounts by the fat (4, 15), it is probable that the chemerin secreted from perivascular adipose tissue can act on smooth muscle cells without entering the circulation. Especially if the chemerin in the circulation is quickly degraded (36) or of a different isoform (37) than that produced by the fat, this is a mechanism by which blood pressure can be regulated by chemerin independently of the circulation.
A recent study testing male Wistar rats fed HF diet found that circulating chemerin protein levels did not increase compared with normal-diet rats. However, in these same animals, expression of chemerin protein and the chemerin receptor in the vasculature and perivascular adipose tissue was increased (35). Our study supports the idea that chemerin mRNA expression is increased in fat with HF feeding and that plasma chemerin protein does not influence blood pressure. This study provides the first mechanistic proof that while chemerin does directly influence blood pressure, blood pressure is not directly associated with plasma levels of the chemerin protein, at least in the rat. Together, our study and the previous study with Wistar rats suggest that the actions of chemerin may be local.
Dahl S rats fed HF diet do exhibit increased renal injury compared with control-diet rats in the form of hyaline casts, interstitial fibrosis, glomerular sclerosis, and tubular atrophy (14). However, a specific local role of chemerin in the kidney is unlikely given that the Chemerin1 receptor is not expressed in the kidney (4, 15). Another possibility is that chemerin from fat is influencing the mesenteric resistance vessels that influence blood pressure (7, 32). The perivascular adipose tissue (PVAT) around these resistance vessels is one of the components of visceral adipose tissue implicated in the positive correlation between high blood pressure and fat (17, 22). We know that chemerin is produced in this mesenteric PVAT and that these mesenteric resistance vessels have the ability to contract to chemerin (34). The present study is one approach to determine if adipose tissue is important to chemerin-dependent changes in blood pressure. Here, we increased fat burden to increase (local) chemerin burden. Another tactic would be to target fat directly. Future in vivo studies should focus on targeting the ASO to the fat. Because of limitations in drug delivery, in vitro studies may be needed to differentiate chemerin derived from different locations of white adipose tissue.
Future studies also need to assess the target of the chemerin derived from the fat. In an isolated tissue bath, chemerin is contractile to the vasculature (12, 24, 34), but there are other influences chemerin could have in the whole body to support blood pressure. We have previously shown that the Chemerin1 receptor antagonist alone (CCX832) reduces electrical field-stimulated contracted blood vessels in a PVAT-dependent manner (8). This points toward a possible role of the sympathetic nervous system in the actions of chemerin as well as affirming the importance of local chemerin production. In support of that idea, chemerin and the Chemerin1 receptor are also present in the adrenal gland (25). Sympathetic mechanisms of blood pressure modification by chemerin need to be considered as a possible means through which chemerin could affect blood pressure.
Limitations
HF Dahl S treatment was replicated in different sets to confirm the large magnitude of effect produced by the whole body ASO. The HF Dahl S animals used here were first employed in a 1 mo oral estradiol treatment protocol. The current study was, in part, an experiment of opportunity where instrumented Dahl S rats fed HF diet for 6 mo, seemingly unaffected by previous intervention, were available for study with the chemerin ASO. Estradiol treatment of the HF animals did not affect their blood pressure, and the animals were allowed to recover for 3 wk before the initiation of the present study. Orally administered estradiol has a half-life of 13–20 h so we believe a 3 wk washout period was sufficient to clear any remaining drug. Studies with the Dahl S fed HS were designed to complement the data from the HF rats. Dahl S animals on normal diet were not used for this study but have been used previously to confirm the model of Dahl HF hypertension (14).
Additionally, because the animals receiving liver-specific ASO were in a separate cohort from most other animals (but still containing some animals receiving control and whole body ASO), the mean of the liver-specific treatment group baseline blood pressures was slightly lower than the other treatment groups. When combined with all other treatment groups, baseline blood pressures of this group were not statistically different than the others, but the reduced biological replicates in this group may have skewed the power analysis. The purpose of this smaller liver-specific group was to follow up on a similar experiment done on normal Sprague Dawley rats (13) where we saw similar trends in both groups.
Conclusions
Adiposity is an established risk factor for hypertension. This study suggests that biological actions of the adipokine chemerin could be an explanation for this association, although it is likely that chemerin is only one part of a network of vasoactive factors found in enlarged and dysfunctional adipocytes, specifically, fat that is adjacent to blood vessels. In the large number of human subjects with obesity-associated hypertension, manipulation of chemerin production may be uniquely effective in the treatment of hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-117847 and HL-143937.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.F., H.G., A.E.M., G.D.F., and S.W.W. conceived and designed research; D.J.F., E.D.F., H.G., S.T.Y., S.R., and S.W.W. performed experiments; D.J.F., E.D.F., S.T.Y., and S.R. analyzed data; D.J.F., A.E.M., G.D.F., and S.W.W. interpreted results of experiments; D.J.F. prepared figures; D.J.F. drafted manuscript; D.J.F., E.D.F., H.G., S.T.Y., S.R., A.E.M., G.D.F., and S.W.W. edited and revised manuscript; D.J.F., E.D.F., H.G., S.T.Y., S.R., A.E.M., G.D.F., and S.W.W. approved final version of manuscript.
REFERENCES
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-CC, James WPT, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; national Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645, 2009. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Bennett CF, Baker BF, Pham N, Swayze E, Geary RS. Pharmacology of Antisense Drugs. Annu Rev Pharmacol Toxicol 57: 81–105, 2017. doi: 10.1146/annurev-pharmtox-010716-104846. [DOI] [PubMed] [Google Scholar]
- 3.Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension 60: 404–410, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148: 4687–4694, 2007. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 5.Chakaroun R, Raschpichler M, Klöting N, Oberbach A, Flehmig G, Kern M, Schön MR, Shang E, Lohmann T, Dreßler M, Fasshauer M, Stumvoll M, Blüher M. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 61: 706–714, 2012. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Cheon DY, Kang JG, Lee SJ, Ihm SH, Lee EJ, Choi MG, Yoo HJ, Kim CS. Serum chemerin levels are associated with visceral adiposity, independent of waist circumference, in newly diagnosed type 2 diabetic subjects. Yonsei Med J 58: 319–325, 2017. doi: 10.3349/ymj.2017.58.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res 38: 1–12, 2001. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- 8.Darios ES, Winner BM, Charvat T, Krasinksi A, Punna S, Watts SW. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am J Physiol Heart Circ Physiol 311: H498–H507, 2016. doi: 10.1152/ajpheart.00998.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern Med 50: 1093–1097, 2011. doi: 10.2169/internalmedicine.50.5025. [DOI] [PubMed] [Google Scholar]
- 10.Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One 8: e72457, 2013. doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X, Li P, Zhou C, Jia X, Kang J. Elevated levels of serum chemerin in patients with obstructive sleep apnea syndrome. Biomarkers 17: 248–253, 2012. doi: 10.3109/1354750X.2012.658864. [DOI] [PubMed] [Google Scholar]
- 12.Ferland DJ, Darios ES, Neubig RR, Sjögren B, Truong N, Torres R, Dexheimer TS, Thompson JM, Watts SW. Chemerin-induced arterial contraction is Gi- and calcium-dependent. Vascul Pharmacol 88: 30–41, 2017. doi: 10.1016/j.vph.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferland DJ, Seitz B, Darios ES, Thompson JM, Yeh ST, Mullick AE, Watts SW. Whole-Body but Not Hepatic Knockdown of Chemerin by Antisense Oligonucleotide Decreases Blood Pressure in Rats. J Pharmacol Exp Ther 365: 212–218, 2018. doi: 10.1124/jpet.117.245456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes R, Garver H, Harkema JR, Galligan JJ, Fink GD, Xu H. Sex Differences in Renal Inflammation and Injury in High-Fat Diet-Fed Dahl Salt-Sensitive Rats. Hypertension 72: e43–e52, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282: 28175–28188, 2007. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 16.Gu P, Jiang W, Lu B, Shi Z. Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin Exp Hypertens 36: 326–332, 2014. doi: 10.3109/10641963.2013.827697. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 116: 991–1006, 2015. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto JG, Beadles-Bohling AS, Wiren KM. Comparison of RiboGreen and 18S rRNA quantitation for normalizing real-time RT-PCR expression analysis. Biotechniques 36: 54–56, 2004. doi: 10.2144/04361BM06. [DOI] [PubMed] [Google Scholar]
- 19.Hu W, Feng P. Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res Clin Pract 91: 159–163, 2011. doi: 10.1016/j.diabres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Hung G, Xiao X, Peralta R, Bhattacharjee G, Murray S, Norris D, Guo S, Monia BP. Characterization of target mRNA reduction through in situ RNA hybridization in multiple organ systems following systemic antisense treatment in animals. Nucleic Acid Ther 23: 369–378, 2013. doi: 10.1089/nat.2013.0443. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AJ, Yang P, Read C, Kuc RE, Yang L, Taylor EJA, Taylor CW, Maguire JJ, Davenport AP. Chemerin Elicits Potent Constrictor Actions via Chemokine-Like Receptor 1 (CMKLR1), not G-Protein-Coupled Receptor 1 (GPR1), in Human and Rat Vasculature. J Am Heart Assoc 5: e004421, 2016. doi: 10.1161/JAHA.116.004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens 23: 1170–1178, 2010. doi: 10.1038/ajh.2010.172. [DOI] [PubMed] [Google Scholar]
- 23.Kukla M, Zwirska-Korczala K, Hartleb M, Waluga M, Chwist A, Kajor M, Ciupinska-Kajor M, Berdowska A, Wozniak-Grygiel E, Buldak R. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand J Gastroenterol 45: 235–242, 2010. doi: 10.3109/00365520903443852. [DOI] [PubMed] [Google Scholar]
- 24.Lobato NS, Neves KB, Filgueira FP, Fortes ZB, Carvalho MHC, Webb RC, Oliveira AM, Tostes RC. The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway. Life Sci 91: 600–606, 2012. doi: 10.1016/j.lfs.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Moore A, Zabel BA, Burnett R, Watts SW. Chemerin Peptide Releases Catecholamines from Rat Adrenal Medulla. Pharmacologica 7: 290–295, 2016. doi: 10.5567/pharmacologia.2016.290.295. [DOI] [Google Scholar]
- 26.Mullick AE, Yeh ST, Graham MJ, Engelhardt JA, Prakash TP, Crooke RM. Blood Pressure Lowering and Safety Improvements With Liver Angiotensinogen Inhibition in Models of Hypertension and Kidney Injury. Hypertension 70: 566–576, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09755. [DOI] [PubMed] [Google Scholar]
- 27.Neves KB, Lobato NS, Lopes RA, Filgueira FP, Zanotto CZ, Oliveira AM, Tostes RC. Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: a link to vascular dysfunction in obesity? Clin Sci (Lond) 127: 111–122, 2014. doi: 10.1042/CS20130286. [DOI] [PubMed] [Google Scholar]
- 28.Prakash TP, Yu J, Migawa MT, Kinberger GA, Wan WB, Østergaard ME, Carty RL, Vasquez G, Low A, Chappell A, Schmidt K, Aghajan M, Crosby J, Murray HM, Booten SL, Hsiao J, Soriano A, Machemer T, Cauntay P, Burel SA, Murray SF, Gaus H, Graham MJ, Swayze EE, Seth PP. Comprehensive Structure-Activity Relationship of Triantennary N-Acetylgalactosamine Conjugated Antisense Oligonucleotides for Targeted Delivery to Hepatocytes. J Med Chem 59: 2718–2733, 2016. doi: 10.1021/acs.jmedchem.5b01948. [DOI] [PubMed] [Google Scholar]
- 29.Shin HY, Lee DC, Chu SH, Jeon JY, Lee MK, Im JA, Lee JW. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin Endocrinol (Oxf) 77: 47–50, 2012. doi: 10.1111/j.1365-2265.2011.04217.x. [DOI] [PubMed] [Google Scholar]
- 30.Stepan H, Philipp A, Roth I, Kralisch S, Jank A, Schaarschmidt W, Lössner U, Kratzsch J, Blüher M, Stumvoll M, Fasshauer M. Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul Pept 168: 69–72, 2011. doi: 10.1016/j.regpep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension 17, Suppl: I61–I68, 1991. doi: 10.1161/01.HYP.17.1_Suppl.I61. [DOI] [PubMed] [Google Scholar]
- 32.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 122: 1–12, 2012. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett 582: 573–578, 2008. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol 33: 1320–1328, 2013. doi: 10.1161/ATVBAHA.113.301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng C, Shen Z, Li X, Jiang W, Peng L, Yuan H, Yang K, Wang J. Effects of chemerin/CMKLR1 in obesity-induced hypertension and potential mechanism. Am J Transl Res 9: 3096–3104, 2017. [PMC free article] [PubMed] [Google Scholar]
- 36.Wittamer V, Grégoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem 279: 9956–9962, 2004. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- 37.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem 280: 34661–34666, 2005. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 38.Zylla S, Pietzner M, Kühn JP, Völzke H, Dörr M, Nauck M, Friedrich N. Serum chemerin is associated with inflammatory and metabolic parameters-results of a population-based study. Obesity (Silver Spring) 25: 468–475, 2017. doi: 10.1002/oby.21735. [DOI] [PubMed] [Google Scholar]