Abstract
Chronic heart failure (CHF) is associated with global oxidative stress, which contributes to sympathoexcitation. Increased reactive oxygen species in the brain accumulate within neurons and lead to enhanced neuronal excitability. Exercise training (ExT) is associated with a reduction of oxidative stress by upregulation of antioxidant enzymes. The link between ExT and antioxidant enzyme expression in the brain of animals with CHF is not clear. We hypothesized that ExT enhances transcription and translation of the nuclear factor erythroid 2-related factor 2 (Nrf2) gene, a master transcription factor that modulates antioxidant enzyme gene expression, in the rostral ventrolateral medulla (RVLM) of mice with CHF. Mice were divided into the following groups: Sham sedentary (Sham-Sed), Sham-ExT, CHF-Sed, and CHF-ExT. After 8 wk of ExT, we measured Nrf2 and NAD(P)H dehydrogenase [quinone] 1 (NQO-1) message and protein expression along with maximal exercise tolerance and urinary norepinephrine (NE) excretion. We found that Nrf2 and NQO-1 mRNA and protein expression in the RVLM were lower in CHF-Sed mice compared with Sham-Sed. ExT attenuated the CHF-induced reduction of Nrf2 and NQO-1 mRNA and protein expression in the RVLM. NE excretion was higher in CHF-Sed mice compared with Sham-Sed (666.8 ± 79.3 ng/24 h, n = 6 vs. 397.8 ± 43.7 ng/24 h, P = 0.04). CHF-ExT mice exhibited reduced urinary NE excretion compared with CHF-Sed (360.7 ± 41.7 ng, n = 4 vs. 666.8 ± 79.3 ng, n = 6; P = 0.03). We conclude that ExT-induced upregulation of Nrf2 in the RVLM contributes to the beneficial effects of ExT on sympathetic function in the heart failure state.
NEW & NOTEWORTHY This study provide evidence for an important role for exercise training in the modulation of antioxidant enzyme production in the rostral ventrolateral medulla (RVLM) in the heart failure state. We show here a correlation between exercise training and the expression of the antioxidant transcription factor Nrf2 in the RVLM. Exercise training reduced sympathetic function (norepinephrine excretion) and upregulated both Nrf2 and the antioxidant enzyme NQO-1. We conclude that Nrf2 in the RVLM may be an important target for controlling sympathetic outflow in heart failure.
Keywords: antioxidant mechanism, exercise training, rostral ventrolateral medulla, sympathetic regulation
INTRODUCTION
Chronic heart failure (CHF) is a major cause of death, affecting more than 26 million people worldwide and nearly 6 million in the United States. Increased sympathetic tone is a hallmark of the CHF state in humans and animals with experimental CHF (9, 14, 18). Sympathoexcitation plays an essential role in the progression, morbidity, and mortality of CHF. Targeting sympathoexcitation is an important therapeutic strategy in this syndrome (29). The underlying mechanisms of sympathoexcitation in CHF are not completely understood. It is well accepted that central mechanisms such as an increased discharge of presympathetic neurons and alterations in excitatory membrane receptors are responsible for elevated neurohumoral stimulation in CHF (22, 39, 56). Importantly, a growing body of literature implicates increased oxidative stress as a major mediator of central sympathoexcitation (8, 20).
An imbalance between the production of reactive oxygen species (ROS) and antioxidant protective mechanisms results in accumulation of ROS and thereby oxidative stress. Increased oxidative stress in presympathetic neurons is likely to change the activity of multiple ion channels, contributing to enhanced neuronal excitability and thus sympathetic outflow (6, 55). In CHF, the central nervous system is subjected to increased oxidative stress, which evokes major alterations in neuronal structure and function leading to enhanced neural excitability (20). Previous data from our laboratory (20, 21) demonstrated that elevated ROS in the rostral ventrolateral medulla (RVLM), a major region of presympathetic neurons that project to the spinal cord, plays an important role in sympathoexcitation in rabbits with CHF. We found that an increase in central superoxide anion () due to administration of diethyldithiocarbamic acid, a superoxide dismutase (SOD) inhibitor, significantly increased sympathetic outflow in chronically instrumented, conscious sham and CHF rabbits. In contrast, a decrease of central by tempol, a SOD mimetic, reduced sympathetic outflow in rabbits with CHF (20).
Phase II antioxidant enzymes, whose genes contain antioxidant response elements (AREs) on the promotor region, play an essential role in maintaining redox homeostasis. The balance between oxygen radical production and antioxidant scavenging enzymes is one of the primary endogenous mechanisms that protect cells and organs from oxidative damage. Multiple studies have shown that impaired antioxidant defense mechanisms play an essential role in oxidative stress and sympathetic hyperactivity in CHF and hypertension (7, 21). The underlying mechanisms behind reduction in the expression and activity of antioxidant enzymes in CHF (especially in the brain) are not clear. However, an intriguing mechanism that may be important in the setting of CHF is the modulation of the redox sensitive transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) (23, 44). Nrf2 plays an important role in the transcriptional regulation of phase II antioxidant enzymes through its binding to their AREs (44), thus indirectly reducing oxidative stress.
Exercise training (ExT) is an effective therapeutic regimen in the CHF state (33, 34). A previous analysis from the HF-ACTION database (15, 37) showed that ExT is associated with a significant reduction in cardiovascular mortality and hospitalization, as well as an enhancement in quality of life. Additionally, studies carried out on CHF patients have shown a reduction in sympathetic tone, measured by either direct recording of muscle sympathetic nerve activity or urinary norepinephrine (NE) excretion following an ExT regimen (17, 52). The central mechanisms behind these beneficial effects are not completely understood; however, the data suggest that they are mediated, in part, by changes in the expression of antioxidant enzymes. For instance, previous data from this laboratory in rabbits with pacing-induced CHF demonstrated that ExT reduced sympathoexcitation in part due to suppression of oxidative stress and enhancement of antioxidant enzymes in the RVLM (21). Although the mechanisms behind central reduction of antioxidant enzymes in the CHF state are not well characterized, this may be mediated by a decrease in Nrf2. We have previously shown that selective deletion of the Nrf2 gene in the RVLM of otherwise normal mice resulted in a remarkable decrease of heme oxygenase 1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO-1), SOD2, and catalase protein expression (23). As such, changes in the expression of central antioxidant enzymes after ExT in the CHF state may be driven by a similar alteration in central Nrf2.
A role for Nrf2 in the modulation of central antioxidant enzyme expression after ExT in the setting of CHF remains to be elucidated. Therefore, in the present study we used the mouse myocardial infarction (MI) model to determine the influence of ExT on central Nrf2, antioxidant enzyme expression, and sympathetic outflow in CHF. We hypothesized that mice with CHF express lower Nrf2 message and protein in the RVLM, resulting in sympathoexcitation, and that ExT attenuates the CHF-induced reduction of Nrf2 in the RVLM.
MATERIALS AND METHODS
Animals
All animal experiments in this study were approved by the Institutional Animal Care and Use committee at the University of Nebraska Medical Center. These experiments were carried out under the guidelines of the National institute of Health Guide for the Care and Use of Laboratory Animals.
C57BL/6 mice were purchased from Jackson laboratory (Bay Harbor, ME) at 8 wk of age and allowed to acclimate at the University of Nebraska Medical Center animal facility for 1 wk. Food and water were provided ad libitum and the mice were maintained on a 12:12-h light-dark cycle. For induction and maintenance of anesthesia, isoflurane (2%) was administered via inhalation, and Buprenorphine (0.05 mg/kg) was provided on the day of surgery and for 3 days postsurgery to reduce the pain stress. Because almost all published studies regarding the effect of ExT on cardiovascular disease have utilized male mice, we chose this gender, which allows for comparison with the current literature. At the conclusion of the study and within 24 h after the last exercise session, the mice were fully anesthetized with urethane (800 mg/kg) and α- chloralose (40 mg/kg), followed by euthanasia by heart excision which allowed us to harvest tissues for biochemical analysis.
Because of the tiny amount of RVLM, multiple cohorts were used in this study, which can be summarized as follows: morphological and Nrf2/NQO-1 immunoblotting data (Sham-Sed, n = 6; Sham-ExT, n = 5; CHF-Sed, n = 6; Sham-Sed, n = 4); Nrf2 and NQO-1 mRNA data (Sham Sed, n = 6; Sham-ExT, n = 6; CHF-Sed, n = 5; CHF-ExT, n = 6); echocardiography data (Sham-Sed, n = 6; Sham-ExT, n = 5; CHF-Sed, n = 5; CHF-ExT, n = 6); hemodyamics data (Sham-Sed, n = 5; Sham-ExT, n = 6; CHF-Sed, n = 6; CHF-ExT, n = 7).
Induction of Heart Failure
CHF was induced by permanent left anterior descending coronary artery ligation, as previously described (51). Briefly, under 2% isoflurane anesthesia, mice were intubated and ventilated using a mechanical respirator (Mouse Ventilator miniVent model 845, Harvard Apparatus; tidal volume: 150 uL; frequency: 200 breaths/min) throughout the surgery. A thoracotomy was performed between the fourth and fifth intercostal space to expose the heart. In the CHF group, the left anterior descending branch of the coronary artery was permanently ligated with 7-0 suture. After these procedures, the lungs were reinflated, the chest was closed with 6-0 suture, and the skin was closed with 4-0 suture. Sham mice underwent the same procedures, but the coronary artery was not ligated. Once the animals began to recover from anesthesia, the trachea was extubated. Mice were then placed on a 30–32°C heating pad and room air was supplemented with 100% oxygen (1 L/min) for 3 days after the surgery to improve survival rate. Mice were assigned randomly into four groups: sham sedentary (Sham-Sed), sham exercise-trained (Sham-ExT), CHF sedentary (CHF-Sed), and CHF exercise-trained (CHF-ExT).
Echocardiography
Four weeks after MI or sham surgery, echocardiography was carried out on all mice. Using a 40-MHz probe, hearts were imaged on a Visual Sonics Vevo 3100 ultrasound. B-mode images were acquired in the long and short axis under isoflurane anesthesia (1–2%). All animals were echoed when heart rates were above 400 beats/min. Left ventricular volumes [left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV)] and diameters [left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD)] were measured. Ejection fraction (EF) was calculated by the following standard formula: [(LVEDV − LVESV)/LVEDV] × 100. Fractional shortening was calculated as follows: [(LVEDD − LVESD)/LVEDD] × 100.
In Vivo Experiments
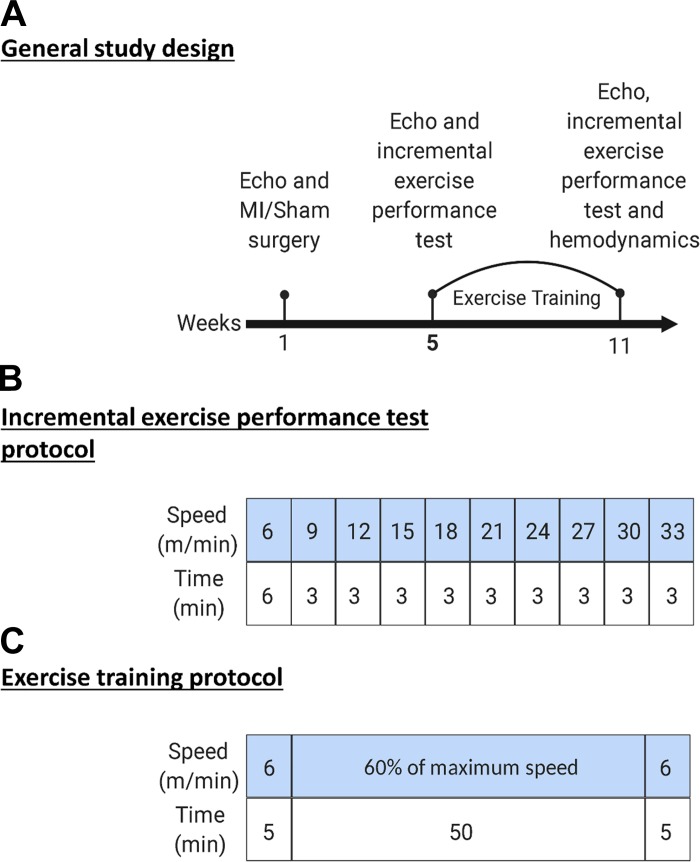
Figure 1A shows the general study design. After a 1-wk acclimatization period, the mice were echoed and underwent either sham or MI surgery. Four weeks after surgery, the mice were echoed again and subjected to an exercise capacity test followed by aerobic training on a treadmill. After 8 wk of training, the mice were echoed and subjected again to exercise capacity test to evaluate the change in exercise capacity.
Fig. 1.
Study design, exercise testing, and training protocols. A: schematic of general study design. B: incremental exercise capacity testing protocol. C: exercise training regimen including warm-up, exercise intensity, duration, and cool down. MI, myocardial infarction.
Maximal Exercise Capacity Testing
Figure 1B shows the exercise capacity testing protocol. We performed exercise familiarization and capacity testing in a manner similar to that described previously (50), with minor modifications. Mice were familiarized to a motorized treadmill (Columbus Instruments, Columbus, OH). Familiarization consisted of a 10-min period where the treadmill and speed were set to 0 with the shock grid setting at 1.2 mA and 2 Hz. The treadmill speed was then increased to 6 m/min on the next day for an additional 10 min. On the following day, mice were subjected to an exercise capacity test, which consists of a warm-up period at speed of 6 m/min for 6 min. The speed was increased by 3 m/min every 3 min at 5% inclination until exhaustion. At the termination of the test, the total distance and the peak workload were recorded. Based on the peak workload, individual workloads corresponding to 60% the maximum speed were determined.
We developed strict criteria for exhaustion. These criteria include the following: 1) 5 consecutive seconds on the electric grid without reengaging the treadmill and 2) inability to keep up with the treadmill speed for 3 min. Every mouse was removed from his lane once one or both criteria were reached. After 8 wk of training, we repeated this test to evaluate changes in exercise capacity. Distance run (meters run before exhaustion) and work performed (calculated as the product of body weight and vertical distance) were recorded. The vertical distance was calculated as the product of the distance run and the angle of inclination of the treadmill, as outlined previously (32).
Exercise Training
Mice assigned to exercise training groups were subjected to 8 wk of treadmill running at 60% of the maximal workload achieved during the initial exercise capacity test. As shown in Fig. 1C, the training protocol consisted of a warm-up period at 6 m/min for 5 min. The training intensity was set at 14 m/min for the Sham-ExT group and 11 m/min for the CHF-ExT. These intensities corresponded to 60% of the maximal speed calculated for each group, respectively during the initial exercise capacity test. Training sessions occurred between 1600 and 1800, 5 days/wk. Sedentary mice were habituated to the experimental environment on the treadmill at 0 speed 2–3 days/wk.
Hemodynamics
Under 2% isoflurane anesthesia, mice were placed on a metal heating pad in the supine position. The appropriate level of anesthesia was determined by absence of reflex withdrawal to a painful stimulus such as foot pinch. The right common carotid artery was dissected, and a pressure transducer (SPR-1000; Millar Instruments, Houston, TX) was advanced into the ascending aorta and left ventricle for the measurement of blood pressure, left ventricular pressure, the first derivative of left ventricular pressure (dP/dt), and heart rate. All signals were processed by a PowerLab data acquisition system (Model 8S) and analyzed using LabChart 7 software (ADInstruments, Colorado Springs, CO).
Ex Vivo Experiments
mRNA expression.
Total RNA was extracted using TRIzol reagent (Invitrogen) from RVLM punches (coordinates: 1.25–1.75 mm lateral to the midline, 1.25–2.00 mm ventral to the dorsal surface of the brain stem, 1.16–1.52 mm cranial from the obex) (19). RNA was reverse transcribed into double-stranded cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories). cDNA templates (50 ng) were then subjected to real-time polymerase chain reaction in triplicate using a thermocycler (PTC-200 Peltier thermal cycler with CHROMO4 continuous fluorescence detector; Bio-Rad Laboratories) and Brilliant III ultra-FastQPCRmastermix (Agilent Technologies). Mouse Nrf2 primers (Mm.PT.58.29108649) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Mm.PT.39a.1) from the Integrated DNA Technologies (Coralville, IA) were used for semiquantification of Nrf2 mRNA expression. Nrf2 mRNA was normalized to GAPDH mRNA as Δ cycle, and then the relative expression was compared with the control group using ΔΔ cycle threshold method for quantification with Opticon Monitor software (Bio-Rad laboratories).
Western blot analysis.
The target proteins and their antibodies were Nrf2 (ab62352 R-IgG) and NQO-1 (ab80588 R-IgG). GAPDH (cat. no. 15738 M-IgG, Thermo Fisher Scientific, Rockford, IL) served as an internal loading control. Total protein was extracted using radioimmunoprecipitation assay buffer. Protein concentration was measured using Pierce bicinchoninic acid (BCA) protein assay kit (cat. no. 23225, Thermo Fisher Scientific). The protein concentration was then adjusted by adding 4% sodium dodecyl sulfate buffer to obtain equal concentration among the samples. Protein (30 μg) from each sample was loaded on a 10% SDS-PAGE gel before being subjected to electrophoresis. The gel was then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was probed with the primary antibodies of the target proteins and then with GAPDH primary antibody. After primary antibody incubation, the membrane was probed with the secondary antibodies and followed by treatment with chemiluminescence substrate (Pierce). A UVP BioImaging System (Epi Chemi II Darkroom, Analytik Jena US, Upland, CA) was used to visualize and analyze the proteins bands. The data are presented as the target proteins band densities divided by the GAPDH density.
Nrf2 antibody validation by blocking peptide.
Because Nrf2 is highly translationally modified, we felt it necessary to validate the Nrf2 anibody used in our laboratory. The Nrf2 antibody was diluted in the blocking buffer (1:1,000; 10 mL), which was equally divided into two tubes (5 mL each). In the first tube, 2 times excess blocking peptide (ab167152) was added (10 uL); in the second tube, equivalent amount of buffer only was added. RVLM punch samples (n = 4) from normal C57BL/6 mice were loaded on the first 4 lanes on a 10% SDS-PAG gel, and the same samples were loaded again on the next 4 lanes before being subjected to electrophoresis. After the gel was transferred into PVDF membrane, the membrane was cut into two halves. The first half (containing the first 4 lanes) was incubated with the control unblocked antibody, and the second half of the membrane (containing identical samples) was incubated with the blocked antibody. The two membranes were probed with the secondary antibodies followed by treatment with chemiluminescence substrate (Thermo Fisher Scientific). A UVP BioImaging System (Epi Chemi II Darkroom, Analytik Jena US) was used to visualize and analyze the proteins bands. The data are presented as the target proteins band densities divided by the GAPDH density.
Urinary norepinephrine.
Mice were placed in metabolic cages (Harvard Apparatus, Holliston, MA) and acclimated to the environment for 2–3 days. Daily water intake and urine excretion were measured. Urinary NE concentration was measured using a NE Enzyme Immunoassay kit (Labor Diagnostika Nord KG, Nordhorn, Germany). The samples were diluted 20-fold, from which 10 uL was used for NE measurement according to the kit instructions. Each sample was measured in duplicate. The excretion rate was calculated by multiplying NE concentration by 24-h urine volume.
Citrate synthase.
Citrate synthase (CS) activity was carried out as previously described (53). Briefly, left soleus muscle samples were placed on ice-cold lysis buffer (MSM-EDTA), homogenized, and centrifuged at 14,000 g for 10 min. The supernatants were collected, and protein concentration was measured using the Pierce BCA method (Thermo Fisher Scientific, Rockford, IL). The assays were carried out in duplicate using a spectrophotometer (DU Series 640; Beckman Instruments, Fullerton, CA). The CS activity was determined by measuring the appearance of yellow product [5,5′-dithiobis (2-nitrobenzoic); DTNB], which was detected spectrophotometrically by measuring absorbance at 412 nm. The absorbance changes were measured every 15 s over 1 min at 412 nm for determination of the CS activity. The CS activity of each specimen was expressed as specific activity normalized to total protein (μg).
Statistical Analysis
All data are expressed as means ± SE. Ordinary two-way ANOVA analysis was used to perform between-group comparisons. To perform within group comparisons, repeated two-way ANOVA analysis was used. Tukey’s post hoc test was used for analyzing the difference among the 4 groups using GraphPad Prism 7.03 software. A P value of <0.05 was considered statistically significant.
RESULTS
Body Weight, Organ Weight, and Echocardiographic and Hemodynamics Data
Because of the small amount of RVLM tissue, we were unable to do all experiments in a single cohort of animals; therefore, multiple animal cohorts were used.
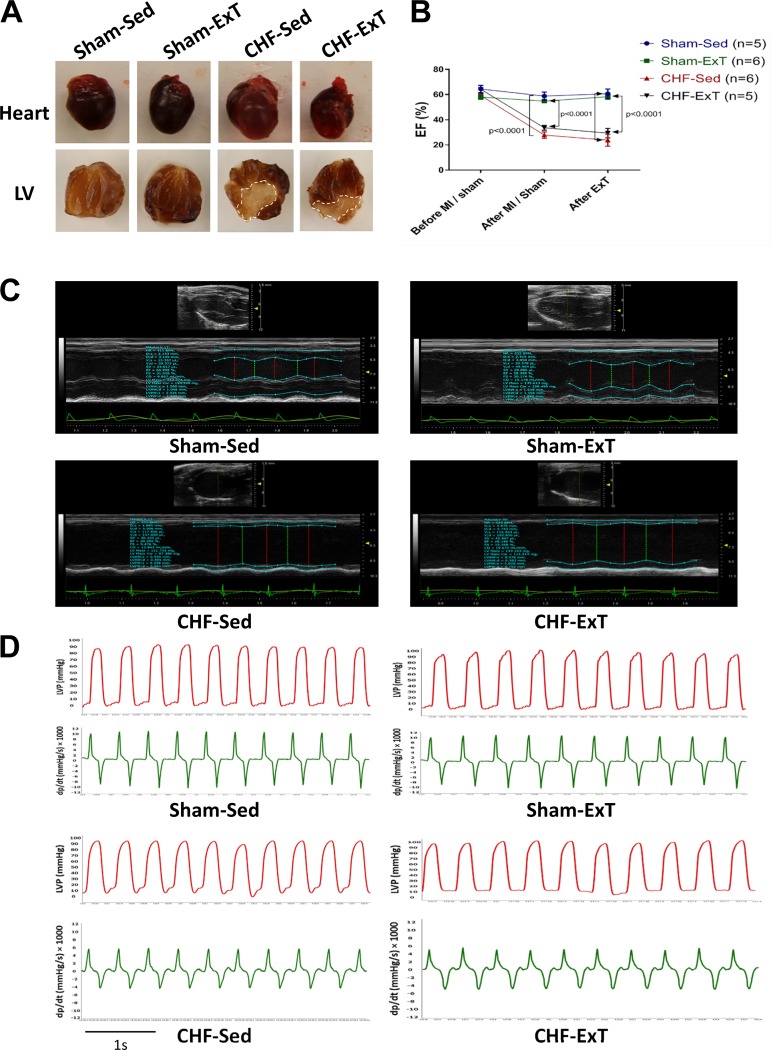
The morphological data shown in Table 1 are from the cohort used to measure Nrf2 and NQO-1 proteins in RVLM punches (Sham-Sed, n = 6; Sham-ExT, n = 5; CHF-Sed, n = 6; CHF-ExT, n = 4). Although the body weight was not different among the four groups, the heart weight was significantly higher in CHF-Sed and CHF-ExT groups compared with the Sham-Sed group, suggesting cardiac hypertrophy in the CHF state. There was no detectable damage to the hearts of Sham mice, whereas CHF mice showed marked myocardial infarcts (infarct size: 33 ± 5.4% and 30 ± 5.5% in the Sed and ExT groups, respectively) (Fig. 2A).
Table 1.
Morphological data of exercise-trained and sedentary mice
| Sham-Sed | Sham-ExT | CHF-Sed | CHF-ExT | |
|---|---|---|---|---|
| n | 6 | 5 | 6 | 4 |
| Body weight, g | 28.7 ± 0.4 | 29.4 ± 0.5 | 28.9 ± 0.8 | 29.5 ± 0.7 |
| Heart weight, mg | 140.3 ± 8.8 | 149.3 ± 6.6 | 239.9 ± 33.8* | 248.1 ± 23.5# |
| (P = 0.0167) | (P = 0.0454) | |||
| Heart weight/tibial length, mg/mm | 8.3 ± 0.5 | 9.0 ± 0.4 | 14.3 ± 2.1* | 14.8 ± 1.3 |
| (P = 0.0175) | ||||
| Wet lung weight, mg | 204.8 ± 4.1 | 189.6 ± 9.9 | 219.3 ± 8.1 | 202.5 ± 5.2 |
| Infarct size, % of LV | 0 | 0 | 33.0 ± 5.4**** | 30.0 ± 5.5### |
| (P < 0.0001) | (P = 0.0004) |
Data are expressed as means ± SE; n = no. of mice. CHF-ExT, chronic heart failure exercise-trained; CHF-Sed, chronic heart failure sedentary; LV, left ventricle; Sham-ExT, sham exercise-trained; Sham-Sed, sham sedentary.
and
CHF-Sed vs. Sham-Sed;
and
CHF-ExT vs. Sham-ExT.
Fig. 2.
Infarct size, echocardiography, and hemodynamic evaluation. A: example of a left ventricular (LV) infarct (dotted line) compared with a sham heart at 12 wk after myocardial infarction (MI). Sham sedentary (Sham-Sed), n = 6; sham exercise-trained (Sham-ExT), n = 5; chronic heart failure sedentary (CHF-Sed), n = 6; and CHF-ExT, n = 4. B: ejection fraction (EF) at 3 time points: before MI or sham surgery, 4 wk after surgery, and after exercise training (ExT). Sham-Sed, n = 5; Sham-ExT, n = 6; CHF-Sed, n = 6; CHF-ExT, n = 5. C: representative M-mode, long-axis, and two-dimensional echocardiography images of the LV from each group. D: representative traces (1 s) of left ventricular pressure (LVP) and left ventricular dP/dt. Sham-Sed, n = 5; Sham-ExT, n = 6; CHF-Sed, n = 6; CHF-ExT, n = 7. n = no. of mice.
The echocardiographic data are summarized in Table 2 and derived from a cohort used to measure Nrf2 in the whole brain stem (data not shown; Sham-Sed, n = 5; Sham-ExT, n = 6; CHF-Sed, n = 6; CHF-ExT n = 5). In this cohort, echocardiography was done at 3 time points: before MI/Sham surgery, 4 wk after surgery, and 8 wk after ExT (Fig. 2B). Both CHF-Sed and CHF-ExT exhibited signs of impaired cardiac function. EF and fractional shortening were significantly lower in CHF animals 12 wk after MI compared with sham animals. Furthermore, left ventricular volume and chamber diameter were greater in CHF animals compared with their respective Sham groups. The left ventricular anterior wall thickness was significantly lower in CHF animals; however, left ventricular posterior wall thickness was not altered among the four groups. Each of the four groups showed nearly similar EF after ExT compared with measurements before ExT, suggesting that ExT did not influence cardiac function in this model of CHF (Fig. 2C).
Table 2.
Echocardiographic data of exercise-trained and sedentary mice
| Sham-Sed | Sham-ExT | CHF-Sed | CHF-ExT | |
|---|---|---|---|---|
| n | 5 | 6 | 6 | 5 |
| EF, % | 60.9 ± 2.3 | 58.3 ± 2.1 | 23.8 ± 4.8**** | 29.4 ± 3.8#### |
| (P < 0.0001) | (P < 0.0001) | |||
| FS, % | 31.2 ± 3.3 | 29.2 ± 1.9 | 11.2 ± 2.7*** | 14.6 ± 2.4## |
| (P = 0.0002) | (P = 0.0044) | |||
| EDV, μL | 46.8 ± 11.9 | 63 ± 4.5 | 192.5 ± 34.2** | 161 ± 23.6# |
| (P = 0.0012) | (P = 0.0298) | |||
| ESV, μL | 26 ± 5.9 | 25.2 ± 2.6 | 153.5 ± 30.3** | 111.8 ± 24.3# |
| (P = 0.0016) | (P = 0.0336) | |||
| LVAWd, mm | 1.1 ± 0.1 | 1 ± 0.1 | 0.5 ± 0.1** | 0.7 ± 0.1 |
| (P = 0.0027) | ||||
| LVAWs, mm | 1.4 ± 0.1 | 1.5 ± 0.1 | 0.6 ± 0.1** | 0.8 ± 0.1## |
| (P = 0.0017) | (P = 0.0055) | |||
| LVPWd, mm | 0.9 ± 0.1 | 1 ± 0.1 | 0.9 ± 0.1 | 1 ± 0.1 |
| LVPWs, mm | 1.2 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| LVIDd, mm | 3.5 ± 0.2 | 3.4 ± 0.4 | 6.1 ± 0.5** | 5.6 ± 0.4## |
| (P = 0.0014) | (0.0063) | |||
| LVIDs, mm | 2.5 ± 0.2 | 2.6 ± 0.1 | 5.5 ± 0.6*** | 5.1 ± 0.5## |
| (P = 0.0004) | (P = 0.0024) |
Data are expressed as means ± SE; n = no. of mice. CHF-ExT, chronic heart failure exercise-trained; CHF-Sed, chronic heart failure sedentary; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; FS, fractional shortening; LVAW, left ventricular anterior wall, LVPW, left ventricular posterior wall, LVID, left ventricular interior diameter; Sham-ExT, sham exercise-trained; Sham-Sed, sham sedentary; d, diastolic; s, systolic.
,
, and
CHF-Sed vs. Sham-Sed;
,
, and
CHF-ExT vs. Sham-ExT.
Hemodynamic data are shown in Table 3 and are from two cohorts; the first cohort was used to measure Nrf2 in the whole brain stem (data not shown), the other cohort was used to measure Nrf2 and NQO-1 mRNA from the RVLM punches (Sham-Sed, n = 6; Sham-ExT, n = 6; CHF-Sed, n = 5; CHF-ExT, n = 6). Left ventricular end-diastolic pressure was elevated in CHF mice compared with Sham. Maximal and minimal dP/dt were decreased in CHF mice (Fig. 2D).
Table 3.
Hemodynamic data of exercise-trained and sedentary mice
| Sham-Sed | Sham-ExT | CHF-Sed | CHF-ExT | |
|---|---|---|---|---|
| n | 5 | 6 | 6 | 7 |
| LVEDP, mmHg | 3.4 ± 0.8 | 2.8 ± 0.4 | 12.5 ± 1.4* | 11.6 ± 2.9# |
| (P = 0.0181) | (P = 0.0122) | |||
| dP/dt max, mmHg/s | 9,067 ± 479.1 | 9,366 ± 519.2 | 4,648 ± 679.2*** | 6,381 ± 487.7## |
| (P = 0.0001) | (P = 0.0039) | |||
| dP/dt min, mmHg/s | −9,553 ± 695.5 | −9,983 ± 662.2 | −5,080 ± 866.6** | −5,475 ± 579.4### |
| (P = 0.0019) | (P = 0.0007) | |||
| Heart rate, beats/min | 472 ± 35.6 | 466 ± 29.7 | 487 ± 28.5 | 504 ± 23.9 |
Data are expressed as means ± SE; n = no. of mice. CHF-ExT, chronic heart failure exercise-trained; CHF-Sed, chronic heart failure sedentary; LVEDP, left ventricular end diastolic pressure; Sham-ExT, sham exercise-trained; Sham-Sed, sham sedentary.
,
, and
comparisons between CHF-Sed and Sham-Sed;
,
, and
comparisons between CHF-ExT and Sham-ExT.
Exercise Capacity and Citrate Synthase Activity
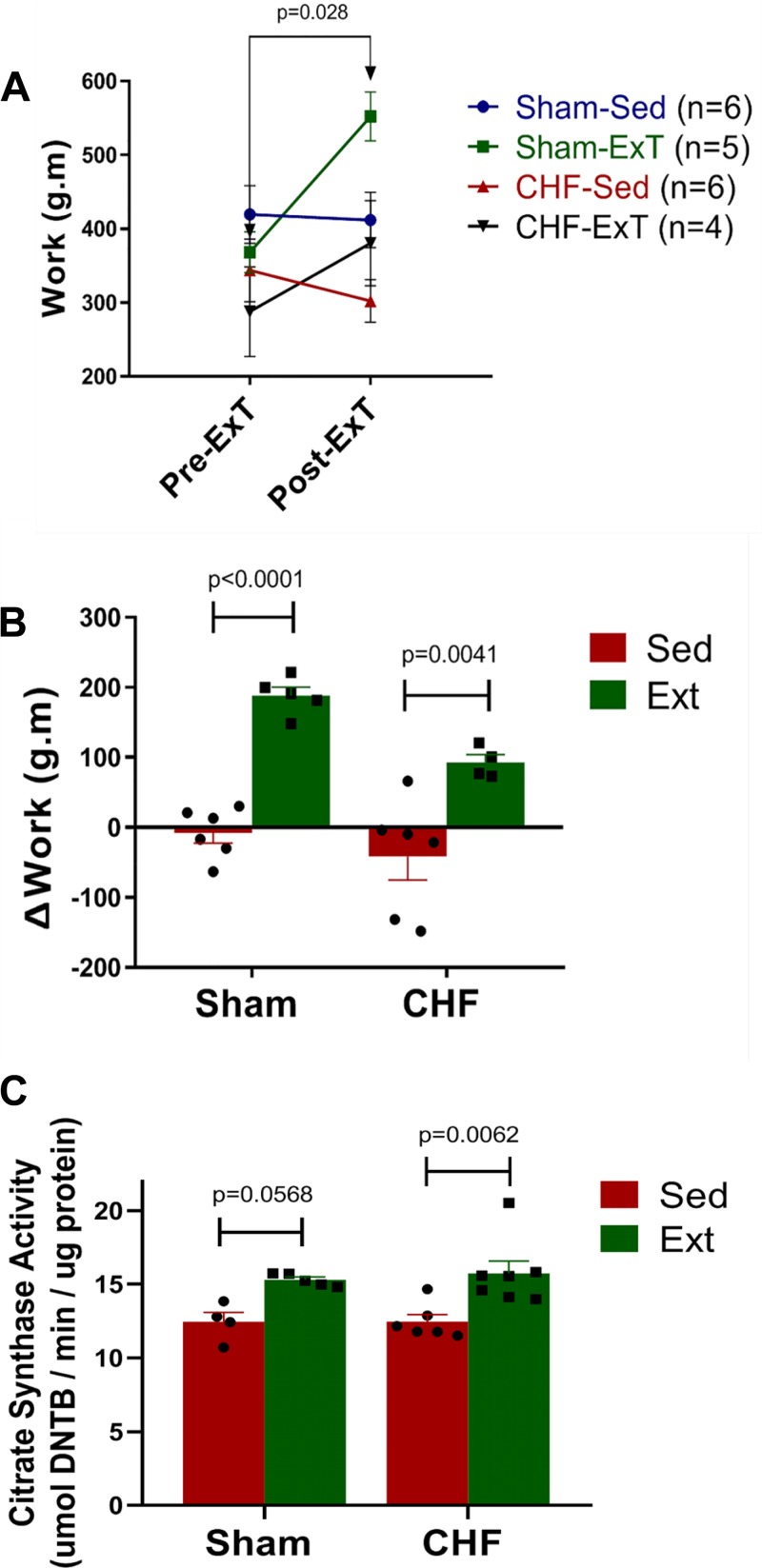
To document a training effect, mice were subjected to exercise capacity testing. Exercise capacity was evaluated at 2 time points, 0, and 8 wk post-ExT. These time points corresponded to 4- and 12-wk post MI/Sham surgery. As shown in Fig. 3A, Sham mice exhibited a significantly higher level of work after 8 wk of ExT. Although there was a trend for a higher work level in the CHF-ExT group, it did not reach significance. The sedentary groups of Sham and CHF mice showed either no improvement or a lower capacity over time. Although the absolute work level after ExT was not different among the groups, the delta change in the work level was significantly higher in the exercise groups of both sham and CHF compared with their respective sedentary groups (Fig. 3B) To further confirm the training adaptation at the molecular level, we measured CS activity in the soleus muscle (Fig. 3C). After 8 wk of training, the CHF-ExT group showed a significantly higher CS activity compared with the CHF-Sed group; however, there was no difference between the CHF-Sed group compared with the Sham-Sed groups. CS activity was also increased in the Sham-ExT group.
Fig. 3.
Exercise training increases work performance and citrate synthase activity in skeletal muscle. A: absolute work performed before and after exercise training (ExT). B: delta (Δ) change in the work performed before and after ExT. Sham sedentary (Sham-Sed), n = 6; Sham-ExT, n = 5; chronic heart failure sedentary (CHF-Sed), n = 6; CHF-ExT n = 4. C: citrate synthase activity in soleus muscle. Sham-Sed, n = 4; Sham-ExT, n = 5; CHF-Sed, n = 6; CHF-ExT, n = 7. n = no. of mice.
Exercise Training Upregulates Nrf2 and NQO-1 mRNA and Protein Expression in the RVLM
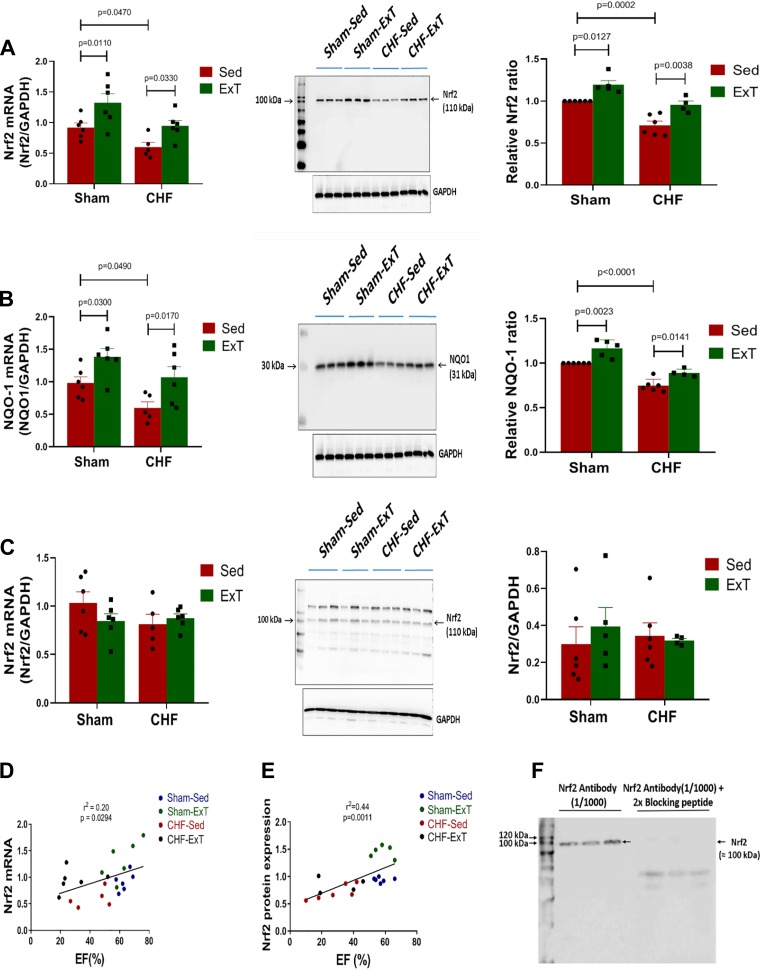
Figure 4, A and B, shows expression of Nrf2 and one of its target (NQO-1) mRNA and protein from tissue punches of the RVLM. Compared with Sham-Sed, the CHF-Sed group expressed lower mRNA and protein for both Nrf2 and NQO-1. ExT increased expression of both mRNA and protein, suggesting that antioxidant defenses are impaired in CHF and that ExT had a beneficial effect on Nrf2 activation. Interestingly, ExT also upregulated Nrf2 and NQO-1 mRNA and protein in Sham mice, suggesting that ExT enhances antioxidant mechanisms even in the healthy condition. We also evaluated the effect of ExT on Nrf2 expression in the visual cerebral cortex, an area that does not include, to the best of our knowledge, autonomic neurons. As shown in Fig. 4C, ExT did not alter Nrf2 mRNA and protein expression in Sham or CHF mice. Nrf2 mRNA and protein expression in the RVLM of mice with varying degrees of cardiac function exhibited a positive correlation with EF (Fig. 4, D and E).
Fig. 4.
Exercise training attenuates heart failure-induced downregulation of nuclear factor erythroid 2-related factor 2 (Nrf2) and NAD(P)H quinone dehydrogenase 1 (NQO-1) in the rostral ventrolateral medulla (RVLM). A: Nrf2 mRNA [Sham sedentary (Sham-Sed), n = 6; sham exercise-trained (Sham-ExT), n = 6; chronic heart failure sedentary (CHF-Sed), n = 5; CHF-ExT n = 6)] and Nrf2 protein (Sham-Sed, n = 6; Sham-ExT, n = 5; CHF-Sed, n = 6; CHF-ExT, n = 4) in the RVLM. B: NQO-1 mRNA and protein expression in the RVLM. C: Nrf2 mRNA and protein expression in the visual cerebral cortex. D: correlation between Nrf2 mRNA in the RVLM with ejection fraction (EF). E: correlation between Nrf2 protein expression in the RVLM with EF. F: Nrf2 antibody validation by immunoblot after preabsorption with a blocking peptide. n = no. of mice.
Figure 4F shows validation of the Nrf2 antibody (ab62352) using immunoblotting. The bands observed using Nrf2 antibody were not present when the same blot was probed after preabsorption with the blocking peptide.
Exercise Training Decreased Norepinephrine Excretion in CHF
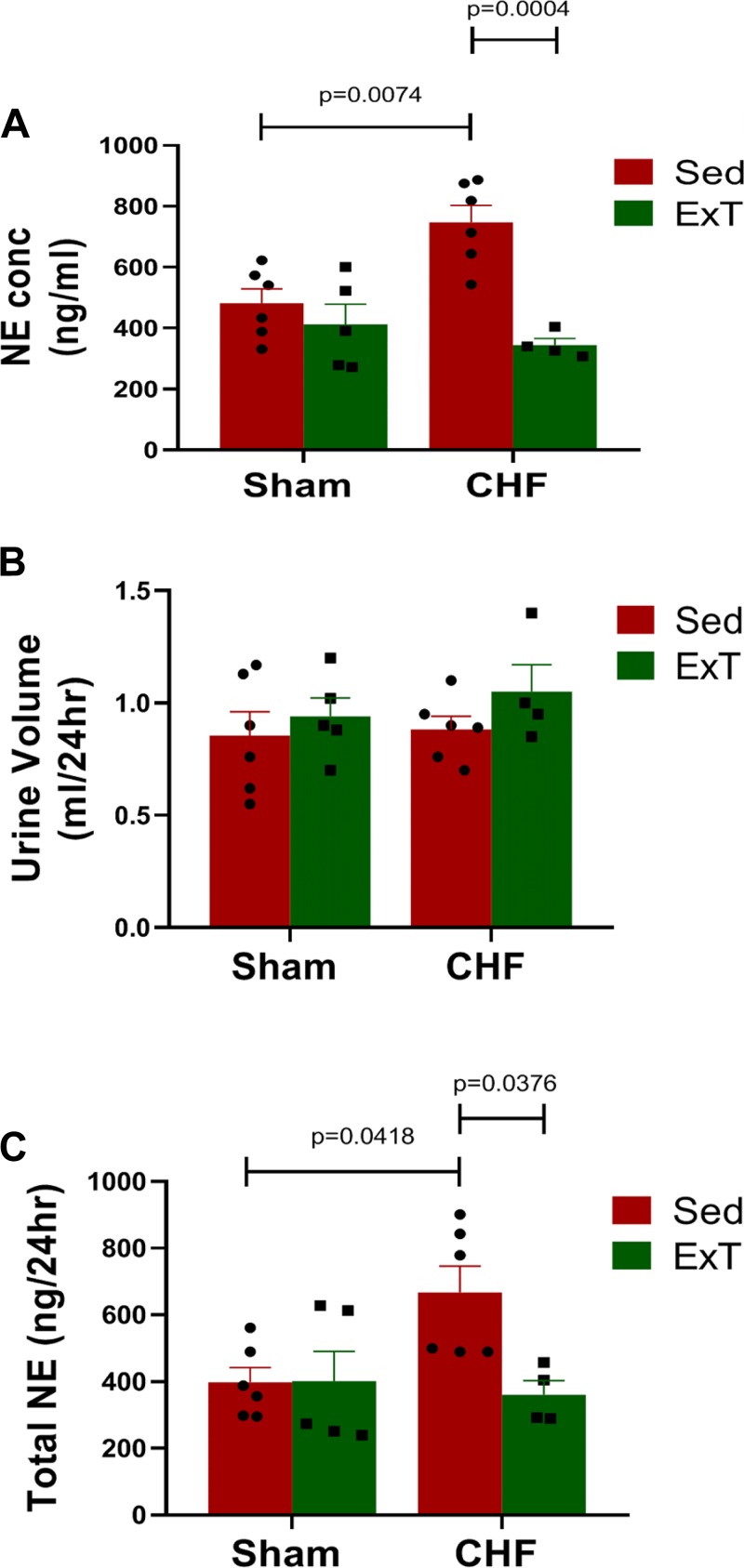
As an indirect index of sympathetic nerve activity in conscious mice, urinary NE excretion was measured 12 wk following MI and after 3 days of acclimatization to a metabolic cage. As shown in Fig. 5A, urinary NE concentration was significantly higher in the CHF-Sed group compared with Sham-Sed. ExT prevented the increase in NE concentration; however, the urinary NE concentration does not reflect global sympathetic nerve activity and therefore we measured urinary NE excretion. The amount of urinary volume in 24 h was not different among groups (Fig. 5B), and the increase in NE excretion rate in CHF was prevented by ExT, which is similar to the previous findings (21, 26).
Fig. 5.
Urinary norepinephrine (NE) levels. Urinary NE concentration (A), 24 h urine volume (B), and urinary NE excretion rate (C). Sham sedentary (Sham-Sed), n = 6; sham exercise-trained (Sham-ExT), n = 5; chronic heart failure sedentary (CHF-Sed), n = 6; CHF-ExT, n = 4. n = no. of mice. n = no. of mice.
DISCUSSION
In the present study, we tested the hypothesis that ExT increases Nrf2 and NQO-1 expression in the RVLM of mice with CHF subjected to a moderate intensity ExT regimen. This study demonstrates that Nrf2 and NQO-1 are downregulated in the RVLM of mice with CHF, which was accompanied by elevated urinary NE excretion. ExT normalized the expression of both Nrf2 and NQO-1 as well as NE excretion. These findings suggest that ExT in CHF activates a central Nrf2 antioxidant signaling pathway and upregulates antioxidant enzymes to regulate redox balance and sympathetic outflow.
Although ExT only slightly improved the increased left ventricular end-diastolic pressure and the decreased pressure change (dP/dt), it did not improve the decreased EF associated with CHF. The fact that ExT did not improve the cardiac function in mice with CHF should not be unexpected. The beneficial effects of ExT in patients with CHF may be primarily due to improvement in neurohumoral drive and therefore the cardiovascular regulation and fluid balance (40). One of ExT’s adaptations is an increase in end-diastolic volume, which results in increased cardiac contractility via Frank–Starling mechanism (3). However, ExT failed to improve cardiac function of sham mice in this study. We believe that the exercise intensity was not high enough to stress these young and healthy mice. Alternatively, it is possible that anesthesia masks the improvement in the cardiac functions in this group of mice.
Citrate synthase (CS) is a pace-making enzyme in the Krebs cycle; as such, it is considered a reliable indicator of cellular aerobic metabolism (35). After ExT, CHF-ExT mice exhibited significantly higher activity of CS compared with CHF-Sed mice; however, CS activity was not different at baseline in CHF-Sed compared with Sham-Sed. Despite the fact that CHF has been characterized by skeletal myopathy (42) (usually an advanced symptom), we did not observe a reduction in body weight or soleus weight (data not shown) in CHF mice. Reduction of CS activity in skeletal muscle may suggest that de novo ATP synthase during exercise is impaired; therefore, increased CS activity in Sham-ExT (P = 0.05) and CHF-ExT (P = 0.006) suggests that aerobic metabolism is improved in both trained groups. Overall, these data show a significant training effect in both Sham and CHF mice.
The RVLM is a major brainstem nucleus and considered to be a common projection pathway of presympathetic neurons descending to the spinal cord to regulate sympathetic outflow and cardiovascular function (10, 25). In addition, the RVLM receives inhibitory projections from the nucleus tractus solitarius and the caudal ventrolateral medulla, which modulates arterial baroreflex regulation of HR, peripheral resistance, and sympathetic nerve activity (43). We have previously shown that selective deletion of the Nrf2 gene in the RVLM of normal Nrf2 floxed mice leads to an increase in RVLM reactive oxygen species (ROS) concomitant with a downregulation of antioxidant enzymes (23). Importantly, these mice exhibited significant elevation of blood pressure, urinary NE, and renal sympathetic nerve activity. Conversely, in a recent study we showed that overexpression of Nrf2 in the RVLM of mice with CHF reduced sympathetic outflow along with a reduction in ROS and an increase in several Nrf2-dependent antioxidant enzymes (31). These data strongly suggest that in the RVLM Nrf2 gene manipulation contributes to alterations in ROS, blood pressure, and sympathoexcitation. The present data showing downregulation of Nrf2 in the RVLM of CHF mice suggest that decreased antioxidant enzymes and sympathoexcitation in CHF may be mediated, in part, by impaired Nrf2 signaling in the RVLM. This is consistent with previous data in the heart, where Nrf2 appears to also be downregulated in CHF (47) and may contribute to the cardiac remodeling process (41). However, no studies have examined the effects of ExT on Nrf2 expression in the RVLM in the CHF state.
The results of this study further support increasing evidence that antioxidant enzymes such as NQO-1 are decreased in sympathoregulatory areas of the brain in CHF (20, 21). In addition, these data clearly show that ExT upregulates Nrf2 and NQO-1 mRNA and protein expression in the RVLM of mice with CHF. Exercise may provide a stimulus for upregulation of antioxidant enzymes in regions remote from the exercising muscle. In addition, these data are consistent with previous reports that demonstrate upregulation of antioxidant enzymes in the brain and other regions in response to ExT (21, 28, 45). However, the precise mechanism that converts ExT into changes in antioxidant enzymes is not known. One possibility, for which there is a growing body of literature, is the idea that extracellular vesicles shed from exercising skeletal muscle can influence remote tissues, including the brain, by transferring their cargo and regulating oxidative stress (12). Indeed, in a recent study from our laboratory (47), we clearly showed that exosomes released from cardiac myocytes contain microRNAs that are specific to the translation of Nrf2.
In a review, Finkel and Holbrook (13) propose that the best strategy to increase antioxidant defense mechanisms may be to periodically increase sublethal oxidative stress. Therefore, it is possible that some of the beneficial effects of ExT are derived from such a stress-tolerance mechanism. One of the major benefits associated with nonexhaustive ExT is induction of mild oxidative stress that stimulates expression of antioxidant enzymes integral to several redox signaling pathways (27). During ExT, skeletal muscle generates a large amount of ROS (5, 11) that, in part through lipid peroxidation, may provide a stimulus for other tissues, including the RVLM within the brain stem. Such a mechanism may condition presympathetic neurons to cope with oxidative stress. Presumably, this adaptation occurs because of cumulative effects of repeated bouts of exercise on gene expression of antioxidant enzymes. The intracellular mechanisms triggered by ExT to upregulate antioxidant enzymes are still unclear. One possible mechanism is that an exercise-associated increase in metabolic activity of presympathetic neurons (36) triggers ROS production, which in turn activates Nrf2.
There are several redox sensitive transcription factors, such as NF-kB and Nrf2 (30, 48, 49), that may be involved in the transduction mechanism by which ExT alters protein transcription in the brain. ExT has been shown to modulate both of these proteins (1, 44, 46). Previous data from the literature show that the prooxidant NF-kB and antioxidant Nrf2 transcription factors compete for binding to the nuclear creb binding protein (30); as such, both transcription factors are critical for regulating intracellular oxidative stress. We have previously shown that ExT decreased NF-kB signaling in the brain of rabbits with CHF (21). Although there are no studies showing this competition in the RVLM, we speculate that in CHF, NF-kB reduces the ability of Nrf2 to bind to the CBP, which further reduces its ability to stimulate downstream antioxidant response elements and thus antioxidant enzymes. Data in the present study showing upregulation of Nrf2 in the RVLM may indicate that the ExT-induced upregulation of antioxidant enzymes is mediated, in part, by Nrf2 through restoration of the balance between Nrf2 and NF-kB.
Although the findings in this study showed an association between Nrf2 and NQO-1 in response to ExT in the CHF state, it is not completely clear if the change in the expression of NQO-1 is mediated by Nrf2. Furthermore, we cannot determine whether ExT-induced reduction of urinary NE excretion in CHF mice is Nrf2 dependent. To determine whether ExT decreases sympathetic nerve activity and upregulates antioxidant enzymes in the RVLM by an Nrf2-dependent mechanism in CHF, future experiments on Nrf2-floxed mice treated with RVLM Cre virus will have to be carried out. However, as indicated above (31), when Nrf2 was overexpressed in the RVLM following the delivery of Cre virus to Keap1 floxed mice with CHF, marked sympathoinhibition was observed.
One potential limitation in this study is the lack of evaluation of oxidative stress in the RVLM. Our laboratory has published several papers showing that up- and downregulation of Nrf2 in the RVLM of both normal and CHF mice results in an inverse relationship between oxidative stress and Nrf2 expression (31, 38, 50). The fact that ExT reduces oxidative stress is not new; a more important question is to determine whether the reduction of oxidative stress in the RVLM after an ExT regimen in CHF state is Nrf2 dependent.
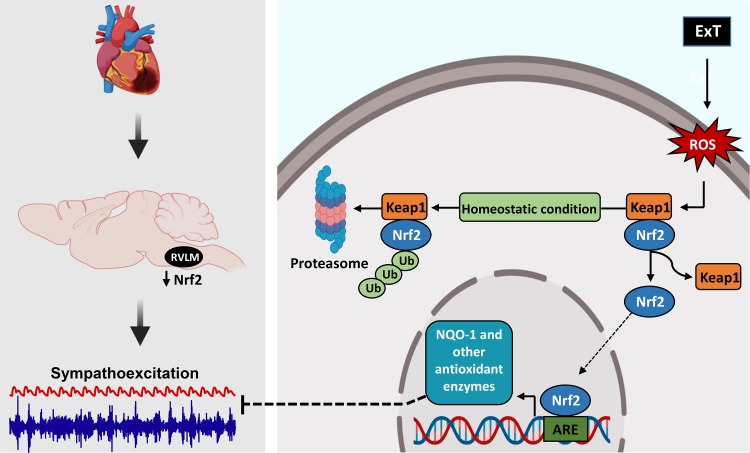
Some of the mechanisms by which ExT upregulates antioxidant enzymes and reduces sympathoexcitation in CHF state are summarized in Fig. 6. In CHF, antioxidant enzyme reduction within the RVLM contributes to oxidative stress and sympathoexcitation. A reduction of Nrf2 protein in CHF reduces the influence of this transcription factor on AREs of a series of genes. We have shown in this study that Nrf2 reduction in the RVLM is partially mediated by reduced transcription; however, it is also possible that the decreased Nrf2 in the CHF state is mediated by enhanced ubiquitination and/or reduced nuclear translocation.
Fig. 6.
A schematic of the nuclear factor erythroid 2-related factor 2 (Nrf2)/Keap1 signaling pathway and some of the potential mechanisms by which exercise training (ExT) impacts Nrf2, antioxidant enzymes, oxidative stress, and sympathetic nerve activity. ARE, antioxidant response element; NQO-1, NAD(P)H quinone dehydrogenase 1; ROS, reactive oxygen species; RVLM, rostral ventrolateral medulla.
Phase II antioxidant enzymes are induced by AREs and activated by many oxidative stressors (4). Although multiple transcription factors act on AREs, Nrf2 is probably the most important regulator of AREs and Phase II antioxidant enzyme production (54). ROS, which are produced during exercise, have been shown to oxidize sulfhydryl groups on cysteine residues of Keap1 (16), thus dissociating from Nrf2 and allowing Nrf2 to move to the nucleus and initiate transcription of antioxidant enzymes by binding to AREs.
ExT in the CHF state is currently an area of intense investigation. Although many studies (2, 24) have shown that ExT is beneficial in CHF, the mechanism by which this occurs is largely unknown. This study revealed a new potential mechanism through which ExT upregulates antioxidant enzyme and decreases sympathetic nerve activity in CHF. The data provided here suggest that ExT in CHF provides a stimulus for a central mechanism through which antioxidant enzymes are normalized, most likely due to a change in Nrf2 expression. These studies further suggest that reduction in sympathetic nerve activity in the CHF state can be mediated through ExT or activation of the Nrf2 pathway with precisely targeted small molecule activators of Nrf2.
GRANTS
This project was supported by a grant from the National Heart, Lung, and Blood Institute P01 HL-62222. A. Wafi was supported by the Ministry of Education Scholarship, Jazan University (Jazan, Saudi Arabia).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.H.Z. conceived and designed research; A.M.W., L.Y., and L.G. performed experiments; A.M.W. and L.Y. analyzed data; A.M.W., L.G., and I.H.Z. interpreted results of experiments; A.M.W. prepared figures; A.M.W. drafted manuscript; A.M.W., L.G., and I.H.Z. edited and revised manuscript; A.M.W., L.Y., L.G., and I.H.Z. approved final version of manuscript.
REFERENCES
- 1.Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol 293: F914–F919, 2007. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- 2.Belardinelli R, Georgiou D, Scocco V, Barstow TJ, Purcaro A. Low intensity exercise training in patients with chronic heart failure. J Am Coll Cardiol 26: 975–982, 1995. doi: 10.1016/0735-1097(95)00267-1. [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol 45: 169–189, 1983. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- 4.Boddupalli S, Mein JR, Lakkanna S, James DR. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: perspectives in maintaining the antioxidant activity of vitamins a, C, and e. Front Genet 3: 7, 2012. doi: 10.3389/fgene.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady PS, Brady LJ, Ullrey DE. Selenium, vitamin E and the response to swimming stress in the rat. J Nutr 109: 1103–1109, 1979. doi: 10.1093/jn/109.6.1103. [DOI] [PubMed] [Google Scholar]
- 6.Chan SH, Chan JY. Brain stem oxidative stress and its associated signaling in the regulation of sympathetic vasomotor tone. J Appl Physiol (1985) 113: 1921–1928, 2012. doi: 10.1152/japplphysiol.00610.2012. [DOI] [PubMed] [Google Scholar]
- 7.Chan SH, Tai MH, Li CY, Chan JY. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic Biol Med 40: 2028–2039, 2006. doi: 10.1016/j.freeradbiomed.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Chao YM, Lai MD, Chan JY. Redox-sensitive endoplasmic reticulum stress and autophagy at rostral ventrolateral medulla contribute to hypertension in spontaneously hypertensive rats. Hypertension 61: 1270–1280, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00469. [DOI] [PubMed] [Google Scholar]
- 9.Cohn JN. Abnormalities of peripheral sympathetic nervous system control in congestive heart failure. Circulation 82, Suppl 2: I59–I67, 1990. [PubMed] [Google Scholar]
- 10.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 11.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982. doi: 10.1016/S0006-291X(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 12.Delezie J, Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front Neurol 9: 698, 2018. doi: 10.3389/fneur.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 14.Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am Coll Cardiol 22, Suppl A: 72A–84A, 1993. doi: 10.1016/0735-1097(93)90466-E. [DOI] [PubMed] [Google Scholar]
- 15.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DWJJ, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP; HF-ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1451–1459, 2009. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem 285: 8463–8471, 2010. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrão CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail 9: 630–636, 2007. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Francis GS. Neurohumoral mechanisms involved in congestive heart failure. Am J Cardiol 55: 15A–21A, 1985. doi: 10.1016/0002-9149(85)90791-X. [DOI] [PubMed] [Google Scholar]
- 19.Franklin KBJ, Paxinos G. Paxinos and Franklin’s the Mouse Brain In Stereotaxic Coordinates. Cambridge, MA: Academic Press, 2013. [Google Scholar]
- 20.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 21.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension 52: 708–714, 2008. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Zimmerman MC, Biswal S, Zucker IH. Selective Nrf2 gene deletion in the rostral ventrolateral medulla evokes hypertension and sympathoexcitation in mice. Hypertension 69: 1198–1206, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geyssant A, Geelen G, Denis C, Allevard AM, Vincent M, Jarsaillon E, Bizollon CA, Lacour JR, Gharib C. Plasma vasopressin, renin activity, and aldosterone: effect of exercise and training. Eur J Appl Physiol Occup Physiol 46: 21–30, 1981. doi: 10.1007/BF00422171. [DOI] [PubMed] [Google Scholar]
- 25.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 26.Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106: 1763–1774, 2010. doi: 10.1161/CIRCRESAHA.109.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci 959: 82–92, 2002. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawler JM, Kwak HB, Kim JH, Suk MH. Exercise training inducibility of MnSOD protein expression and activity is retained while reducing prooxidant signaling in the heart of senescent rats. Am J Physiol Regul Integr Comp Physiol 296: R1496–R1502, 2009. doi: 10.1152/ajpregu.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leimbach WN Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986. doi: 10.1161/01.CIR.73.5.913. [DOI] [PubMed] [Google Scholar]
- 30.Liu GH, Qu J, Shen X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta 1783: 713–727, 2008. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Ma A, Hong J, Shanks J, Rudebush T, Yu L, Hackfort BT, Wang H, Zucker IH, Gao L. Upregulating Nrf2 in the RVLM ameliorates sympatho-excitation in mice with chronic heart failure. Free Radic Biol Med 141: 84–92, 2019. doi: 10.1016/j.freeradbiomed.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol 288: R1006–R1013, 2005. doi: 10.1152/ajpregu.00476.2004. [DOI] [PubMed] [Google Scholar]
- 33.McConnell TR. A review to develop an effective exercise training for heart failure patients. Eura Medicophys 41: 49–56, 2005. [PubMed] [Google Scholar]
- 34.Mears S. The importance of exercise training in patients with chronic heart failure. Nurs Stand 20: 41–47, 2006. doi: 10.7748/ns.20.31.41.s54. [DOI] [PubMed] [Google Scholar]
- 35.Moreira JBN, Bechara LRG, Bozi LHM, Jannig PR, Monteiro AWA, Dourado PM, Wisløff U, Brum PC. High- versus moderate-intensity aerobic exercise training effects on skeletal muscle of infarcted rats. J Appl Physiol (1985) 114: 1029–1041, 2013. doi: 10.1152/japplphysiol.00760.2012. [DOI] [PubMed] [Google Scholar]
- 36.Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol 34: 377–384, 2007. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JAJJ, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL, HF-ACTION Investigators; HF-ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1439–1450, 2009. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan YX, Gao L, Wang WZ, Zheng H, Liu D, Patel KP, Zucker IH, Wang W. Exercise training prevents arterial baroreflex dysfunction in rats treated with central angiotensin II. Hypertension 49: 519–527, 2007. doi: 10.1161/01.HYP.0000256955.74461.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel KP. Neural regulation in experimental heart failure. Baillieres Clin Neurol 6: 283–296, 1997. [PubMed] [Google Scholar]
- 40.Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol 302: H527–H537, 2012. doi: 10.1152/ajpheart.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piao CS, Gao S, Lee GH, Kim DS, Park BH, Chae SW, Chae HJ, Kim SH. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial KATP channels. Pharmacol Res 61: 342–348, 2010. doi: 10.1016/j.phrs.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Piepoli MF, Crisafulli A. Pathophysiology of human heart failure: importance of skeletal muscle myopathy and reflexes. Exp Physiol 99: 609–615, 2014. doi: 10.1113/expphysiol.2013.074310. [DOI] [PubMed] [Google Scholar]
- 43.Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20: 1675–1688, 2002. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Reuland DJ, McCord JM, Hamilton KL. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc Sport Sci Rev 41: 162–168, 2013. doi: 10.1097/JES.0b013e3182948a1e. [DOI] [PubMed] [Google Scholar]
- 45.Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000. doi: 10.1152/ajpheart.2000.279.5.H2068. [DOI] [PubMed] [Google Scholar]
- 46.Sousa e Silva T, Longui CA, Rocha MN, Faria CD, Melo MR, Faria TG, de Souza JA, Rizzo LV. Prolonged physical training decreases mRNA levels of glucocorticoid receptor and inflammatory genes. Horm Res Paediatr 74: 6–14, 2010. doi: 10.1159/000313586. [DOI] [PubMed] [Google Scholar]
- 47.Tian C, Gao L, Zimmerman MC, Zucker IH. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am J Physiol Heart Circ Physiol 314: H928–H939, 2018. doi: 10.1152/ajpheart.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turpaev KT. Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc) 78: 111–126, 2013. doi: 10.1134/S0006297913020016. [DOI] [PubMed] [Google Scholar]
- 49.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wafi AM, Hong J, Rudebush TL, Yu L, Hackfort B, Wang H, Schultz HD, Zucker IH, Gao L. Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle. J Appl Physiol (1985) 126: 477–486, 2019. doi: 10.1152/japplphysiol.00654.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension 58: 1057–1065, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yousufuddin M, Shamim W, Chambers JS, Henein M, Amin FR, Anker SD, Kemp M, Hooper J, Coats AJ. Superiority of endothelin-1 over norepinephrine in exercise-induced alterations of the conduit artery tone of the non-exercised arm in patients with chronic heart failure. Int J Cardiol 73: 15–25, 2000. doi: 10.1016/S0167-5273(99)00200-4. [DOI] [PubMed] [Google Scholar]
- 53.Yuan H, Niu Y, Liu X, Yang F, Niu W, Fu L. Proteomic analysis of skeletal muscle in insulin-resistant mice: response to 6-week aerobic exercise. PLoS One 8: e53887, 2013. doi: 10.1371/journal.pone.0053887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 100: 30–47, 2013. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45: 717–723, 2005. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]
- 56.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension 48: 1005–1011, 2006. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]