Abstract
We sought to investigate the role of group III/IV muscle afferents in limiting endurance exercise performance, independently of their role in optimizing locomotor muscle O2 delivery. While breathing 100% O2 to ensure a similar arterial O2 content () in both trials, eight male cyclists performed 5-km time trials under control conditions (HCTRL) and with lumbar intrathecal fentanyl (HFENT) impairing neural feedback from the lower limbs. After each time trial, common femoral artery blood flow (FBF) was quantified (Doppler ultrasound) during constant-load cycling performed at the average power of the preceding time trial. The assessment of end-tidal gases, hemoglobin content and saturation, and FBF facilitated the calculation of leg O2 delivery. Locomotor muscle activation during cycling was estimated from vastus lateralis EMG. With electrical femoral nerve stimulation, peripheral and central fatigue were quantified by pre- to postexercise decreases in quadriceps twitch torque (ΔQtw) and voluntary activation (ΔVA), respectively. FBF (~16 mL·min−1·W−1; P = 0.6), (~24 mL O2/dL; P = 0.9), and leg O2 delivery (~0.38 mL O2·min−1·W−1; P = 0.9) were not different during HCTRL and HFENT. Mean power output and time to completion were significantly improved by 9% (~310 W vs. ~288 W) and 3% (~479 s vs. ~463 s), respectively, during HFENT compared with HCTRL. Quadriceps muscle activation was 9 ± 7% higher during HFENT compared with HCTRL (P < 0.05). ΔQtw was significantly greater in HFENT compared with HCTRL (54 ± 8% vs. 39 ± 9%), whereas ΔVA was not different (~5%; P = 0.3) in both trials. These findings reveal that group III/IV muscle afferent feedback limits whole body endurance exercise performance and peripheral fatigue by restricting neural activation of locomotor muscle.
NEW & NOTEWORTHY Group III/IV muscle afferent feedback facilitates endurance performance by optimizing locomotor muscle O2 delivery but also limits performance by restricting neural drive to locomotor muscle. To isolate the performance-limiting effect of these sensory neurons, we pharmacologically attenuated their central projection during a cycling time trial while controlling for locomotor muscle O2 delivery. With no difference in leg O2 delivery, afferent blockade attenuated the centrally mediated restriction in motoneuronal output and improved cycling performance.
Keywords: blood flow, central fatigue, exercise limitation, metaboreflex, neural feedback
INTRODUCTION
Within skeletal muscle, contraction-induced mechanical and chemical stimuli activate thinly myelinated (group III) and unmyelinated (group IV) nerve fibers (1, 31, 32) that project, via the dorsal horn of the spinal cord (18), to various sites within the central nervous system. These include areas that regulate the cardiovascular and ventilatory response to exercise (e.g., ventral lateral medulla, nucleus tractus solitarii) but also neural circuits that determine central fatigue (e.g., motor cortex, insular or cingulate cortex) (17, 35, 36). As such, group III/IV afferent feedback from locomotor muscle plays an important role in the exercising human as it mediates critical determinants of whole body endurance performance.
Indeed, when group III/IV muscle afferent feedback from the locomotor muscles to the central nervous system is pharmacologically attenuated during exercise, the ventilatory (2, 3, 22) and cardiovascular (7, 8, 29) responses are substantially attenuated, and muscle efficiency is decreased (12) compared with exercise performed with intact neural feedback. This results in lower arterial oxygenation and compromised O2 delivery to the locomotor muscles that together exacerbate the development of peripheral fatigue (44) and limit performance (3, 47). Conversely, temporary blockade of group III/IV muscle afferents during cycling exercise concomitantly attenuates central fatigue (a centrally mediated restraint of motoneuronal output), resulting in an increase in voluntary locomotor muscle activation (10), which would be expected to facilitate an improvement in exercise performance. However, this expected improvement in performance is not actually evident because of the opposing, negative consequences of hindering oxygen delivery and muscle efficiency.
Previous experiments clearly highlight two opposing consequences of group III/IV muscle afferent feedback, with one improving endurance exercise performance by minimizing the development of peripheral fatigue and the other limiting performance by promoting central fatigue. The net effect of temporarily blocking these sensory neurons on whole body exercise performance, therefore, depends on the balance between these two opposing mechanisms. Overall, recent work has documented a compromised (3) or, at best, no change in performance with the blockade of group III/IV muscle afferent feedback (10, 47). For example, during a 5-km cycling time trial performed with attenuated group III/IV muscle afferent feedback, power output was, compared with the control trial performed with intact afferent feedback, ~10% greater during the first half and ~10% lower during the second half (10). Interestingly, although the pacing strategy adopted by the participants was drastically different and end-exercise peripheral fatigue substantially greater during the time trial with attenuated feedback, overall exercise performance was nearly identical compared with the control trial. It was therefore concluded that neither of the opposing consequences of the afferent blockade prevailed, with the net effect being no difference in performance time in both conditions.
Because of the “double-edged sword” nature of group III/IV muscle afferent feedback in terms of exercise performance, the actual relevance and magnitude of effect of these sensory neurons remain unclear. To isolate the central fatigue-mediated impact of muscle afferents on endurance performance, the consequences of manipulating afferent feedback on arterial oxygenation and limb O2 delivery need to be controlled. To address this issue, we asked healthy participants to perform 5-km cycling time trials with intact and blocked group III/IV muscle afferent feedback while ensuring similar locomotor muscle O2 delivery during both trials by providing a hyperoxic inspirate. This approach eliminated the impact of afferent blockade on exaggerating the development of peripheral fatigue and ensured a suitable scenario to study the central fatigue-mediated impact of muscle afferents on endurance performance. We hypothesized that, compared with the control time trial performed with an intact neural feedback system, the time trial performed with blocked group III/IV muscle afferents would yield improved exercise performance and that this would result in greater end-exercise peripheral fatigue.
METHODS
Subjects
Eight healthy, recreationally trained or competitive (19) male cyclists participated in this study (age: 29 ± 6 yr, height: 177 ± 2 cm, body mass: 71 ± 7 kg, maximal O2 consumption: 54 ± 5 mL·min−1·kg−1, peak power output: 317 ± 36 W). The subjects were nonsmokers, were not medicated, and were asymptomatic for cardiovascular or respiratory disease, and each refrained from vigorous exercise, caffeine, and alcohol for 24 h before the studies. The subjects did not take dietary supplements or performance-enhancing drugs before and during the course of the study. Written informed consent was obtained from each participant before the beginning of the study. All experimental procedures were reviewed and approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Department of Veterans Affairs Medical Center and conducted according to the Declaration of Helsinki for human experimentation.
Experimental Protocol
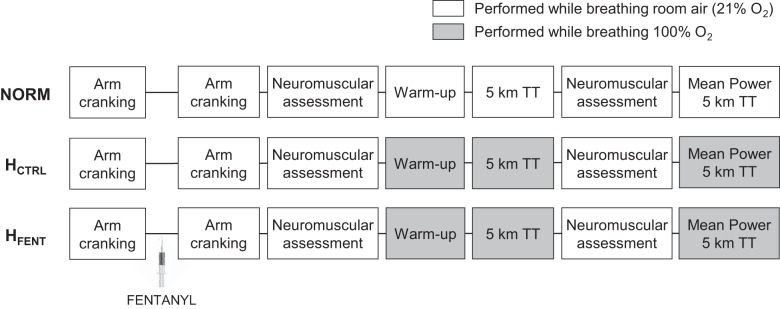
During preliminary visits, anthropometric measurements were collected and subjects were thoroughly familiarized with the neuromuscular testing procedures. During the first preliminary session, subjects performed a maximal incremental exercise test (20 W + 25 W/min) on a cycle ergometer (Velotron, Elite Model; RacerMate, Seattle, WA) to determine peak power output and maximal O2 consumption. In two or three familiarization sessions, the subjects practiced 5-km cycling time trials during which they were able to alter power output by changing the gear ratio and/or pedaling frequency ad libitum. To avoid an initial peak in power output, the time trial commenced once the subjects reached and held 80–90% of peak power output for 2–3 s. During the time trial, subjects were blinded to exercise time, power output, pedaling frequency, and gearing ratio but received visual feedback regarding distance completed. Participants were given strong verbal encouragement throughout exercise but were instructed to remain seated during the time trial. Familiarization with the time trial exercise modality was considered acceptable when the difference in time to completion between two successive practice trials was <2% (50). Although seven of the eight participants archived this goal with two sessions, one participant needed three practice sessions. Then, on separate days and in random order, subjects performed three 5-km time trials (Fig. 1). One of the time trials was performed while the subjects, blinded to the respective fraction of inspired oxygen (), breathed a normoxic gas mixture (NORM, = 0.21), and two time trials were performed with a 100% O2 inspirate. The hyperoxic trials were completed with intact (HCTRL) and with blocked (lumbar intrathecal fentanyl; HFENT) afferent feedback from the locomotor muscle. To test for the effects of intrathecal fentanyl on resting quadriceps function and to evaluate whether fentanyl migrated beyond the cervical level, resting neuromuscular quadriceps function and ventilatory responses to arm cranking (15 and 30 W for 3 min each), respectively, were assessed before and after drug administration (2). Subjects started the time trial ~30 min after fentanyl administration. Every time trial was preceded by a standardized warm-up (50, 75, and 100 W for 3 min each; referred to as “workload” below) performed under the same (0.21 or 1.0) as the subsequent time trial. To quantify exercise-induced locomotor muscle fatigue, neuromuscular quadriceps function was assessed before and again 30 s after the end of exercise. The during the assessment of neuromuscular function was always 0.21. After the postexercise neuromuscular assessment, all participants performed a 3-min constant-load cycling bout with the same as the preceding 5-km time trial and at the power output corresponding to the average power output achieved during that time trial. Femoral arterial blood flow (FBF) was continuously assessed during the warm-up and the 3-min constant-load cycling bout. The experimental sessions were separated by 3–7 days and performed at the same time on each day.
Fig. 1.
Schematic illustration of the experimental design. NORM, normoxia; HCTRL, control hyperoxia; HFENT, fentanyl hyperoxia; TT, time trial.
Neuromuscular Function
Contractile properties and voluntary activation of quadriceps.
For the assessment of neuromuscular function, subjects were seated on a custom-made bench, arms folded across the chest, with a trunk/thigh angle of 120° and a right knee joint angle of 90°. A noncompliant cuff attached to a calibrated linear strain gauge (MLP 300; Transducer Techniques, Temecula, CA) was connected to the subject’s right ankle, just superior to the malleoli. A self-adhesive electrode (3 × 3 cm, Ag-AgCl; Nikomed, Huntingdon Valley, PA) was placed at the stimulation site that resulted in the maximal torque response. The cathode was placed on the femoral triangle, and the anode, a 3 × 3-cm self-adhesive stimulation electrode, was placed on the greater trochanter. A constant-current stimulator (model DS7AH; Digitimer, Welwyn Garden City, UK) delivered a square-wave stimulus (200 μs). To ensure supramaximality during these tests, the stimulation intensity (164 ± 24 mA) was set to 120% of the intensity eliciting the maximal spatial recruitment of the quadriceps, as confirmed by both maximal torque response and maximal vastus lateralis muscle compound action potential (Mmax). For the evaluation of quadriceps function, potentiated quadriceps twitch torques were measured after each 3-s maximal voluntary contraction (MVC). Three MVCs, separated by 30 s, were performed before and again 30 s after the completion of each trial. Quadriceps twitch torque (Qtw) was evoked by single electrical stimulation of the femoral nerve 2 s after each MVC. The peak torques for all Qtw and MVC were averaged over the three measurements. Superimposed twitches were delivered during the peak torque of each MVC to calculate voluntary activation (VA) of the quadriceps (39). Fatigue indexes were expressed as a percent reduction from pre- to postexercise values. To evaluate the effect of intrathecal fentanyl on resting quadriceps function, the assessment procedure was performed before and ~15 min after the drug administration.
Surface electromyography.
Electrical activity of the vastus lateralis of the right quadriceps muscle was recorded from two pairs of Ag-AgCl surface electrodes (diameter = 10 mm; interelectrode distance = 20 mm) placed on the muscle belly connected to an electromyography (EMG) system (16-bit Micro 1401 mk-II; Cambridge Electronic Design, Cambridge, UK). EMG electrodes were placed according to SENIAM recommendations (25). The skin was shaved, abraded with emery paper, and cleaned with alcohol. The position of the electrodes was marked with indelible ink to ensure identical placement at subsequent visits. EMG signals were amplified (500×), filtered (bandwidth frequency 20–2,000 Hz), and recorded (sampling frequency 2 kHz) with commercially available software (Spike 2; Cambridge Electronic Design, Cambridge, UK). With a custom-made MATLAB (MATLAB 7.12; MathWorks, Natick, MA) algorithm, each burst onset and offset of the rectified EMG signal, recorded during the time trials, was determined. The criteria for the onset and offset values were based on a minimum threshold of two standard deviations from the resting baseline and a minimum burst duration of 100 ms. Careful visual inspection confirmed accurate identification of the timing of each burst. The root mean square (RMS) of the EMG signal recorded during each time trial was calculated and normalized to the RMS recorded during the preexercise MVCs. RMS during each MVC was calculated as the average value over a 0.5-s interval during the plateau phase of the MVC. To minimize movement artifacts during exercise, all cables were secured to the leg with medical tape and the subjects wore a fishnet tight to prevent cable movement throughout the trial.
Femoral Blood Flow and Leg O2 Delivery
With the participants exercising in upright position (i.e., with the handlebars raised) to facilitate access to the femoral artery, FBF was measured with ultrasound Doppler and averaged over the last minute of each workload. Specifically, measurements of common femoral artery blood velocity and artery diameter, 2–3 cm proximal to the superficial/deep bifurcation, were performed with an ultrasound Doppler system in duplex mode (Logiq 7; General Electric Medical Systems, Milwaukee, WI) equipped with a linear array transducer functioning at an imaging frequency of 10 MHz. Artery diameter, measured as internal diameter, was assessed during the peak of the electrocardiogram R wave (average of 4 or 5 measurements). Femoral artery blood velocity was assessed with a Doppler frequency of 5 MHz operated in the high-pulsed repetition frequency mode (2–25 mHz), as described previously (9). Blood velocity was assessed with an insonation angle of ≤60° (42), with the sample size maximized and centered according to vessel size and position in real time. All ultrasound data were calculated with Logiq 7 software. Ultimately, FBF (mL/min) was calculated with the equation FBF = [(mean blood velocity) × π (vessel radius)2 × 60], where mean blood velocity is expressed in centimeters per second and radius is expressed in centimeters.
Arterial O2 saturation () was measured with a pulse oximeter (Nellcor OxiMax, Pleasanton, CA) with optodes placed on the forehead, and hemoglobin concentration ([Hb]) was obtained from a venous blood draw (15.9 ± 0.8 g/dL). Arterial () partial pressure of O2 (Po2) was estimated by subtracting an assumed alveolar-arterial Po2 difference of 20 mmHg from end-tidal Po2 (5). Arterial O2 content () was calculated as follows: (mL/dL) = ([Hb] × 1.39 × /100) + ( × 0.003). Leg O2 delivery was calculated as the product of FBF and . Even if there was a 15-mmHg difference in alveolar-arterial Po2 difference between the hyperoxic trials, the discrepancy in would have been negligible (5).
Exercise Responses
Pulmonary ventilation (V̇e) was measured during the time trials and during the arm cranking test with a calibrated open-circuit calorimetry system (Innocor; Innovision, Glamsbjerg, Denmark) with custom configuration to allow data acquisition in hyperoxia. Heart rate (HR) was measured from the R-R interval recorded at 1 kHz with a 12-lead electrocardiogram (CardioCard; Nasiff Associates, Central Square, NY). was measured during warm-up and all time trials.
Intrathecal Fentanyl
Subjects were seated in a flexed position, and 0.025 mg of fentanyl was delivered intrathecally at the vertebral interspace L3–L4 as previously described (6). Intrathecal fentanyl eliminates approximately half of the central projection mediated by group III/IV muscle afferents (29).
Arm Cranking Test
The migration of fentanyl within the cerebrospinal fluid beyond the cervical level and the resulting effect of the drug on µ-opioid receptors located within brain structures mediating breathing and central fatigue would complicate the interpretation of the findings (33). Therefore, to evaluate whether a cephalad drug migration to the brain occurred, the ventilatory response to arm cranking (15 W and 30 W for 3 min each; Monark-Crescent, Varberg, Sweden) was assessed before and 10 min after fentanyl injection (2). To ensure a similar protocol across all testing days, the arm cranking assessment was also performed on the HCTRL and NORM days.
Statistical Analysis
Normality of every dependent variable and sphericity of the variance of the distributions (equal variance) were confirmed with the Kolmogorov–Smirnov test and the Mauchly test, respectively. A Greenhouse–Geisser correction was used when sphericity was violated. Student’s paired t tests with Bonferroni correction for multiple comparisons were used to determine the effect of afferent blockade on MVC, Qtw, VA, HR, and V̇e. Two-way ANOVA with repeated measures (condition × distance) was used to test differences during exercise in power output, surface EMG, V̇e, and HR. Two-way ANOVA with repeated measures (condition × time) was used to test differences before and after exercise on fatigue parameters (i.e., MVCs, peripheral and central fatigue). Two-way ANOVA with repeated measures (condition × workload) was used to test differences during warm-up and trials in FBF and O2 transport. When a significant difference was found, multiple-comparison analysis was performed with Tukey’s honestly significant difference test. Statistical analyses were conducted with Statistica 8.0 (StatSoft, Tulsa, OK). Coefficients of variation (CVs) and intraclass coefficient correlations (ICCs) were calculated to assess reproducibility of the 5-km time trial between familiarization sessions. Data presented in results are expressed as means ± SD except in the figures, where SE is used for clarity. Statistical significance was set at P < 0.05.
RESULTS
Resting Neuromuscular Quadriceps Function and Cardiorespiratory Response to Arm Exercise
In the resting state, intrathecal fentanyl had no effect on quadriceps MVC (221 ± 53 vs. 215 ± 51 Nm; P = 0.2), VA (91 ± 7% vs. 91 ± 7%; P = 0.8), or Qtw (61 ± 22 vs. 59 ± 22 Nm; P = 0.2). Additionally, both V̇e and HR during arm cranking at 15 W (V̇e: 26 ± 3 vs. 27 ± 5 L/min, HR: 91 ± 11 vs. 88 ± 12 beats/min; P > 0.2) and 30 W (V̇e: 33 ± 5 vs. 34 ± 5 L/min, HR: 102 ± 10 vs. 99 ± 11 beats/min; P > 0.2) were not different before and after the fentanyl injection.
Femoral Blood Flow and Leg O2 Delivery
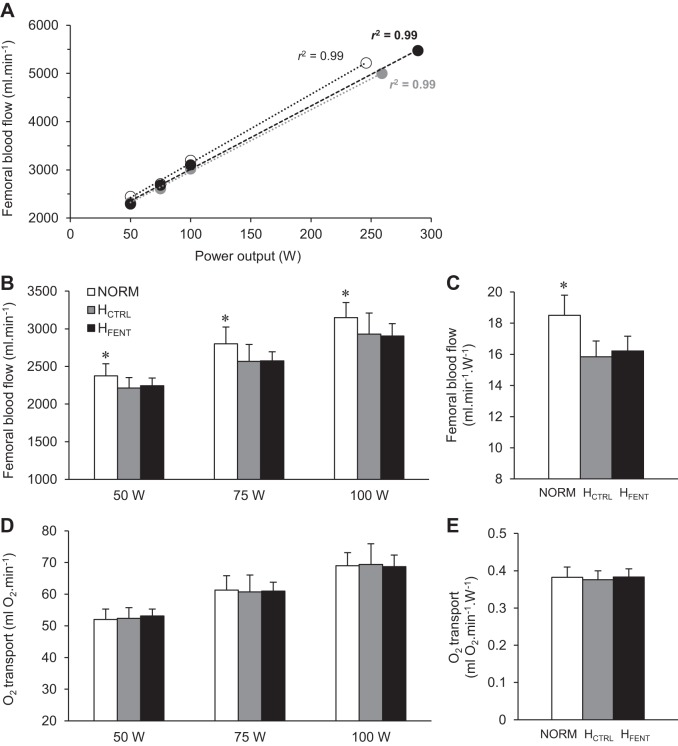
Within each condition, FBF increased in a linear fashion with exercise intensity (P < 0.001), as supported by high linear regression coefficients (r2: 0.95–0.99), calculated on an individual basis (Fig. 2A). As illustrated in Fig. 2B, FBF at the three submaximal workloads was not different between HCTRL and HFENT (P = 0.89), but these data were all ~8% lower than NORM (P < 0.001). At the differing mean power outputs achieved during the time trials, FBF was 4,942 ± 1,421 mL/min in NORM (261 ± 38 W), 4,677 ± 1,291 mL/min in HCTRL (288 ± 57 W), and 5,130 ± 1,175 mL/min in HFENT (310 ± 49 W). However, when normalized for the mean power achieved during the time trials (wattage), FBF was not different between HCTRL and HFENT (P = 0.9), but these FBFs were significantly lower than NORM (Fig. 2C). was not different in HCTRL and HFENT (24 ± 1 mL O2/dL vs. 24 ± 1 mL O2/dL; P = 0.9), but these values were higher than NORM (22 ± 1 mL O2/dL; P < 0.001). Leg O2 delivery was not different in all three conditions (P = 0.9; Fig. 2, D and E).
Fig. 2.
Femoral artery blood flow (FBF) and leg O2 delivery during cycling exercise. A: the linear relationship between FBF and exercise intensity of a representative subject with the associated linear regression coefficients calculated for each condition and on an individual basis (r2 range across all subjects: 0.95–0.99). B and D: blood flow response and O2 delivery during 3 different workloads. NORM, normoxia; HCTRL, control hyperoxia; HFENT, fentanyl hyperoxia. C and E: as the average wattage during the 3 time trials was different between conditions, FBF and O2 delivery were normalized for the mean power output achieved in each trial (NORM 261 W, HCTRL 288 W, HFENT 310 W). *P < 0.05 vs. HCTRL and HFENT.
Time Trial Performance: Time to Completion, Quadriceps EMG, and Power Output
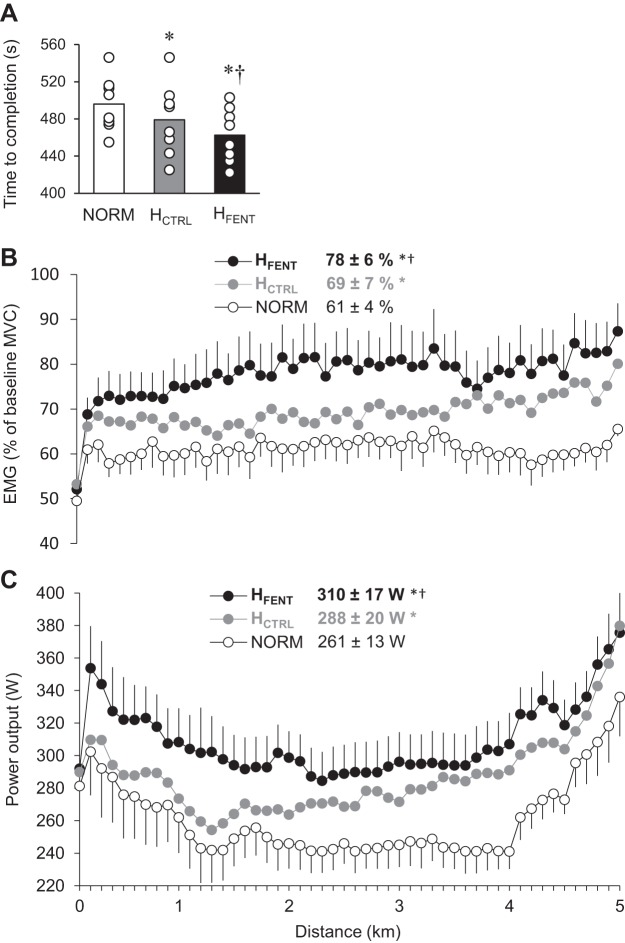
Based upon the time trial performances achieved during the familiarization trials (in normoxia), reliability for both time to completion (P = 0.9, CV = 0.6 ± 0.5%, ICC = 0.99) and power output (P = 0.5, CV = 1.4 ± 1.3%, ICC = 0.99) were “excellent” (according to the criteria set by Cicchetti et al., i.e., ICC > 0.90) (15).
Time to completion of each time trial is illustrated in Fig. 3A. HCTRL was, on average, 17 ± 12 s (3.5 ± 2.4%) faster than NORM (P < 0.01), and HFENT was, on average, 16 ± 13 s (3.3 ± 2.5%) faster than HCTRL (P < 0.01). Importantly, all eight participants improved their time to completion from HCTRL to HFENT (range: 0.2–7.9%; Fig. 3). Vastus lateralis EMG was augmented by 8 ± 5% from NORM to HCTRL and again by 9 ± 7% from HCTRL to HFENT (Fig. 3B). Maximal vastus lateralis muscle compound action potential remained unchanged in the three conditions (P = 0.8), reflecting invariant membrane excitability across experimental conditions. As illustrated in Fig. 3C, power output in all three trials followed the classic “U” shape, with significant variations in wattage throughout each trial (P < 0.001), a typical characteristic of the pacing strategy adopted in this exercise modality. No interaction effect (distance × condition) was evident (P = 0.6), indicating that there was no difference in pacing strategy between conditions. However, mean power output was augmented by 10 ± 8% from NORM to HCTRL (P < 0.01) and again by 9 ± 7% from HCTRL to HFENT (P < 0.05).
Fig. 3.
Time to completion, muscle activation, and power output during the 5-km cycling time trials. A: time to complete the 5-km time trials. NORM, normoxia; HCTRL, control hyperoxia; HFENT, fentanyl hyperoxia. B: vastus lateralis EMG normalized to the EMG response recorded during preexercise maximal voluntary contraction (MVC). C: power output during the time trials. *P < 0.05 vs. NORM, †P < 0.05 vs. HCTRL.
Exercise-Induced Locomotor Muscle Fatigue
Baseline neuromuscular quadriceps function was not different between conditions (MVC ~218 Nm, Qtw ~6 Nm, VA ~91%, RMS MVC ~1.1 mV; P ≥ 0.2). Postexercise quadriceps torque-generating capacity was significantly reduced after all time trials (Fig. 4). The decrease in MVC was not different in NORM and HCTRL (P = 0.9) but was significantly greater in HFENT (Fig. 4A). Similarly, the decrease in Qtw was not different in NORM and HCTRL (P = 0.6) but was greater in HFENT (P < 0.01). The documented exercise-induced decrease in VA was not different in all three conditions (P = 0.3).
Fig. 4.
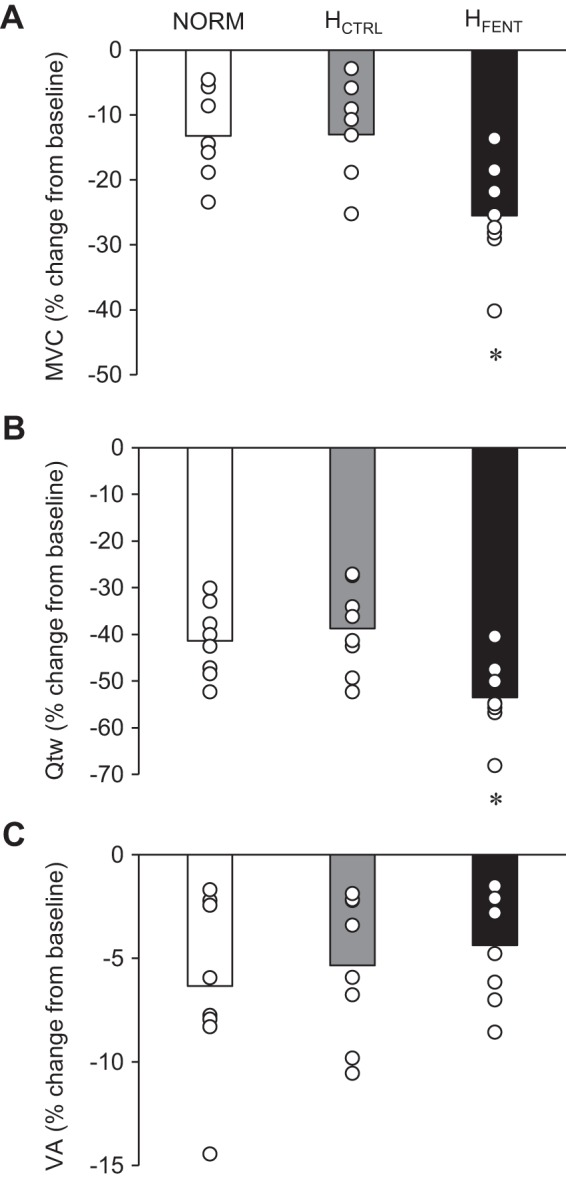
Neuromuscular fatigue following the three 5-km cycling time trials. A: maximal voluntary contraction (MVC). NORM, normoxia; HCTRL, control hyperoxia; HFENT, fentanyl hyperoxia. B: quadriceps twitch torque (Qtw). C: voluntary quadriceps activation (VA). *P < 0.05 vs. NORM and HCTRL.
Cardioventilatory Responses to Exercise
HR and V̇e during the 5-km time trials are presented in Table 1. HR increased linearly throughout the 5-km time trials (P < 0.001), without any differences between conditions (P = 0.48). V̇e also increased throughout all trials (P < 0.001). Compared with NORM, V̇e in HCTRL and HFENT was 13 ± 10% and 12 ± 10% lower throughout the exercise (P < 0.05). However, despite the ~10% greater power output in HFENT, V̇e was not different from HCTRL (P = 0.99). Because of the hyperoxic inspirate, measurements of O2 consumption and CO2 production were not feasible.
Table 1.
Cardioventilatory responses during 5-km cycling time trials
| Start | 1st km | 2nd km | 3rd km | 4th km | 5th km | |
|---|---|---|---|---|---|---|
| V̇e, L/min | ||||||
| NORM | 70 ± 33 | 112 ± 23 | 142 ± 31 | 147 ± 37 | 150 ± 33 | 165 ± 26 |
| HCTRL | 57 ± 23 | 99 ± 26 | 121 ± 30* | 126 ± 29* | 136 ± 31 | 154 ± 39 |
| HFENT | 54 ± 16 | 92 ± 27 | 118 ± 37* | 128 ± 34* | 139 ± 38 | 152 ± 28 |
| HR, beats/min | ||||||
| NORM | 115 ± 17 | 145 ± 11 | 158 ± 14 | 165 ± 13 | 170 ± 12 | 176 ± 12 |
| HCTRL | 122 ± 22 | 146 ± 14 | 156 ± 15 | 163 ± 12 | 173 ± 17 | 178 ± 14 |
| HFENT | 117 ± 16 | 147 ± 13 | 161 ± 10 | 167 ± 11 | 173 ± 11 | 179 ± 11 |
Data are presented as group means ± SD (n = 8 subjects). HCTRL, 5-km cycling time trial, intact afferent feedback, 100% O2; HFENT, 5-km cycling time trial, blocked afferent feedback, 100% O2; HR, heart rate; NORM, 5-km cycling time trial, intact afferent feedback, room air; V̇e, minute ventilation.
P < 0.05 vs. NORM.
DISCUSSION
Because of the double-edged sword nature of group III/IV muscle afferent feedback with regard to exercise performance, the actual relevance and magnitude of effect of these sensory neurons remain unclear. To address this issue, we asked healthy male cyclists to perform 5-km cycling time trials with intact and with blocked group III/IV muscle afferent feedback while minimizing potential differences in locomotor muscle O2 delivery during HCTRL and HFENT by providing a hyperoxic inspirate. The temporary blockade of group III/IV muscle afferents attenuated the centrally mediated restriction in motoneuronal output (i.e., less central fatigue), which resulted in significantly greater muscle activation and a substantial improvement in exercise performance. The faster time to completion in HFENT was associated with greater end-exercise peripheral fatigue compared with the (slower) time trial performed with intact neural feedback (i.e., HCTRL). These findings indicate that group III/IV muscle afferents limit cycling performance and peripheral fatigue by restricting the neural activation of locomotor muscle. It is important, however, to reemphasize that the experimental exposure of the performance-limiting aspect of these sensory neurons requires careful control of locomotor muscle O2 delivery.
Role of Group III/IV Muscle Afferents in Regulating Endurance Performance and Neuromuscular Fatigue
Blockade of group III/IV muscle afferent increased muscle activation (i.e., reduced central fatigue) during cycling exercise in earlier studies but also resulted in hypoventilation (6, 10) and attenuated limb blood flow (7), which, in combination, compromise locomotor muscle O2 delivery and exaggerate the development of peripheral fatigue (3). The net effect of the blockade-induced attenuation in central fatigue combined with the simultaneous exaggeration of peripheral fatigue was an unchanged exercise performance (6, 10). In light of these previous findings, the present study used hyperoxia to facilitate arterial oxygenation and verified comparable locomotor muscle O2 delivery, both with and without fentanyl, by quantifying and FBF during HCTRL and HFENT. As a consequence of this approach, the development of peripheral fatigue was not affected by a difference in O2 delivery between the two conditions, and the blockade-induced decrease in central fatigue resulted in higher power output and a faster time to completion. The exact mechanisms accounting for this centrally mediated improvement in locomotor muscle activation and endurance performance were not elucidated here. However, potential explanations include an attenuation of the group III/IV-mediated inhibition of voluntary descending drive “upstream” of the motor cortex (51) and/or a reduction of the afferent-mediated depression of corticospinal pathway excitability (37, 48).
Although substantially augmented during HFENT compared with HCTRL, and with no significant interaction effect for wattage, power output followed, in both conditions, the classic U-shaped pattern characteristic for time trials (Fig. 3C). The fact that power output during HFENT was, compared with HCTRL, significantly greater within 10 s of the start of the time trial emphasizes the significance of group III/IV muscle afferents in limiting exercise almost immediately and, interestingly, at a point at which intramuscular metabolic perturbations are likely minimal. Given the presumably low metabolic stimuli at this early stage of the time trial, this observation might be indicative of a significant role, in terms of limiting performance, for the mechanosensitive group III/IV muscle afferents, which are stimulated by muscle length and tension as well as being sensitive to fentanyl blockade (16, 24). Regardless, the early and considerable effect of afferent blockade is consistent with the sharp increase in both group III and IV afferent firing immediately at the onset of even light-intensity exercise (1) and the significance of these neurons in determining the spontaneous cardiovascular and ventilatory response at the onset of whole body exercise at any given intensity (2).
Similar to earlier studies (6, 10), end-exercise peripheral fatigue was greater after HFENT compared with HCTRL but was not different between the two trials with intact afferent feedback (i.e., NORM and HCTRL) (Fig. 4). This finding reemphasizes the centrally mediated role of group III/IV muscle afferents in limiting locomotor muscle activation, likely to restrict the development of peripheral fatigue to a “critical threshold,” a level of muscle fatigue that is not exceeded during exercise with an intact neural feedback system. Briefly, the concept of the critical threshold of peripheral fatigue is based on the idea that a negative feedback loop operates to protect the muscles of the exercising limb from severe threats to muscle homeostasis and fatigue (4, 14, 27). Afferent blockade temporarily suspends this regulatory mechanism, and the brain allows a larger degree of intramuscular metabolic perturbation and a resultant increase in the level of locomotor muscle fatigue. Of note, the explanation for the greater end-exercise peripheral fatigue following the time trial with fentanyl blockade in earlier studies (i.e., similar performance compared with the control time trial) (6, 10) was the blockade-induced decrease in leg O2 delivery and the resulting exaggerated rate of development of peripheral fatigue (3, 44). This difference in locomotor muscle O2 delivery was eliminated in the present study (Fig. 2, D and E) and therefore did not contribute to the difference in fatigue between HFENT and HCTRL. In fact, in the present study the most likely explanation for the augmented end-exercise peripheral fatigue following HFENT was the greater power output characterizing the time trial performed with blocked muscle afferents (Fig. 3C).
The exercise-induced decrease in VA was comparable after HFENT and HCTRL. Although this similarity (i.e., no difference in the degree of central fatigue after exercise) does not directly support the increased motoneuronal output and muscle activation during HFENT compared with HCTRL (Fig. 3B; i.e., attenuated central fatigue during HFENT), the finding is not surprising, as both central motor drive and afferent feedback contribute to exercise-induced decreases in VA (28). Specifically, afferent blockade increased central motor drive while lowering afferent feedback during HFENT and therefore altered two determinants of central fatigue at the same time. Although somewhat speculative, the facilitative effect of increased motor drive on central fatigue might have counterbalanced the attenuating influence related to the reduced afferent feedback, with the net effect of no difference between ΔVA in HFENT and HCTRL.
The relationship between the submaximal workload (20–80% peak workload) and FBF was linear in all trials (Fig. 2A) and comparable to some (40, 41), but not all (52), previously documented FBF values quantified by dye dilution or the constant-infusion thermodilution technique. As expected (23, 43, 52), FBF was higher at any given workload in NORM compared with both hyperoxic conditions (Fig. 2, B and C). However, the remarkably similar, and experimentally fortuitous, FBF in HCTRL and HFENT was rather unexpected, as it differs from the previously documented decrease in FBF during single-leg knee-extension exercise performed under normoxic conditions with blocked group III/IV lower limb muscle afferents (7). As a consequence, leg O2 delivery was not different between trials (Fig. 2, D and E). Although this serendipitously similar FBF response in the hyperoxic conditions certainly simplified our efforts to match leg O2 delivery in HCTRL and HFENT, the explanation for the apparently different FBF responses to afferent blockade during single-leg knee extension compared with cycling exercise remains unclear. While we recognize that hyperoxia may have played a role, further studies are needed to address the influence of group III/IV muscle afferents on regulating FBF during both small muscle mass and whole body exercise.
Finally, in addition to augmenting and convective O2 transport during exercise, hyperoxia can also alter peripheral and cerebral blood flow through its vasoactive properties (13), impair skeletal muscle mitochondrial efficiency (34), lower muscle sympathetic nervous activity (49), and suppress neural activity in the brain (46). Although these systemic effects might have contributed to the overall consequence of hyperoxia for the time to complete the time trial (i.e., NORM vs. HCTRL), the gain in performance resulting from afferent blockade was likely independent of the hyperoxia-related systemic repercussions, as arterial oxygenation was similar in HFENT and HCTRL.
Significance of Group III/IV-Mediated Limitation to Endurance Exercise
To highlight the substantial magnitude of the improvement in exercise performance following afferent blockade, it should be noted that the enhancement in power output (+9%) and time to completion (−4%) from HCTRL to HFENT was six times greater than the day-to-day variability observed during preliminary practice sessions. Indeed, the improvement was comparable to the considerable performance-enhancing effect of the ~10% increase in arterial oxygenation from NORM to HCTRL ( 22 vs. 24 mL O2/dL, respectively) (Fig. 3) or, from the literature, the result of blood doping (i.e., a 400-mL red blood cell infusion) before a 10-km running race (11). Furthermore, the magnitude of the improvement in performance resulting from the afferent blockade is twice that reported for caffeine [5 mg/kg; 4-km cycling time trial (21)] and acetaminophen [1.5 g; 16-km cycling time trial (38)] ingestion. Finally, it should be emphasized that the improvement in endurance exercise performance as a result of blocking the group III/IV muscle afferents documented here is likely an underestimation of the true potential impact, as intrathecal fentanyl only eliminates approximately half of the central projection mediated by these muscle afferents (29). Collectively, these observations highlight the substantial effect of group III/IV muscle afferent feedback in limiting whole body endurance performance.
Finally, although the influence of sex on the development of neuromuscular fatigue during exercise remains unclear (26, 45, 53), exercise pressor reflex function has been documented to differ between men and women (20, 30). Therefore, as afferent blockade might have different cardiovascular consequences in men versus women, we chose to limit this investigation to male participants. The generalizability of the present findings might therefore be limited.
Conclusions
Group III/IV muscle afferents limit whole body endurance performance and peripheral fatigue by imposing a centrally mediated restriction on the neural activation of the locomotor musculature during exercise. Given the multifaceted role of group III/IV muscle afferent feedback, the experimental exposure of the performance-limiting aspect of these neurons requires careful control of locomotor muscle O2 transport and the resulting consequences for peripheral fatigue.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute (HL-116579) and the Department of Veterans Affairs (E1697-R, E6910-R, and E9275-L).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.J.H. and M.A. conceived and designed research; T.J.H., J.C.W., T.S.T., H.-Y.W., J.R.G., J.E.J., and M.J.B. performed experiments; T.J.H. analyzed data; T.J.H., J.C.W., T.S.T., H.-Y.W., J.R.G., J.E.J., M.J.B., R.S.R., and M.A. interpreted results of experiments; T.J.H. prepared figures; T.J.H. and M.A. drafted manuscript; T.J.H., J.C.W., T.S.T., H.-Y.W., J.R.G., J.E.J., M.J.B., R.S.R., and M.A. edited and revised manuscript; T.J.H., J.C.W., T.S.T., H.-Y.W., J.R.G., J.E.J., M.J.B., R.S.R., and M.A. approved final version of manuscript.
REFERENCES
- 1.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol (1985) 82: 1811–1817, 1997. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589: 5299–5309, 2011. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586: 161–173, 2008. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575: 937–952, 2006. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa TC, Vianna LC, Fernandes IA, Prodel E, Rocha HN, Garcia VP, Rocha NG, Secher NH, Nobrega AC. Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. J Physiol 594: 715–725, 2016. doi: 10.1113/JP271335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304: H162–H169, 2013. doi: 10.1152/ajpheart.00603.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315, 2016. doi: 10.1113/JP272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brien AJ, Simon TL. The effects of red blood cell infusion on 10-km race time. JAMA 257: 2761–2765, 1987. doi: 10.1001/jama.1987.03390200101022. [DOI] [PubMed] [Google Scholar]
- 12.Broxterman RM, Hureau TJ, Layec G, Morgan DE, Bledsoe AD, Jessop JE, Amann M, Richardson RS. Influence of group III/IV muscle afferents on small muscle mass exercise performance: a bioenergetics perspective. J Physiol 596: 2301–2314, 2018. doi: 10.1113/JP275817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brugniaux JV, Coombs GB, Barak OF, Dujic Z, Sekhon MS, Ainslie PN. Highs and lows of hyperoxia: physiological, performance, and clinical aspects. Am J Physiol Regul Integr Comp Physiol 315: R1–R27, 2018. doi: 10.1152/ajpregu.00165.2017. [DOI] [PubMed] [Google Scholar]
- 14.Burnley M, Vanhatalo A, Jones AM. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol (1985) 113: 215–223, 2012. doi: 10.1152/japplphysiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- 15.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6: 284–290, 1994. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- 16.Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 594: 641–655, 2016. doi: 10.1113/JP271714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773, 1994. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- 18.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190, 2000. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 19.De Pauw K, Roelands B, Cheung SS, de Geus B, Rietjens G, Meeusen R. Guidelines to classify subject groups in sport-science research. Int J Sports Physiol Perform 8: 111–122, 2013. doi: 10.1123/ijspp.8.2.111. [DOI] [PubMed] [Google Scholar]
- 20.Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol (1985) 80: 245–251, 1996. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- 21.Felippe LC, Ferreira GA, Learsi SK, Boari D, Bertuzzi R, Lima-Silva AE. Caffeine increases both total work performed above critical power and peripheral fatigue during a 4-km cycling time trial. J Appl Physiol (1985) 124: 1491–1501, 2018. doi: 10.1152/japplphysiol.00930.2017. [DOI] [PubMed] [Google Scholar]
- 22.Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 606–615, 2012. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 23.González-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes SG, McCord JL, Koba S, Kaufman MP. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J Physiol 587: 873–882, 2009. doi: 10.1113/jphysiol.2008.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G. SENIAM 8: European Recommendations for Surface ElectroMyoGraphy. Enschede, The Netherlands: SENIAM, 1999. [Google Scholar]
- 26.Hunter SK. Sex differences in fatigability of dynamic contractions. Exp Physiol 101: 250–255, 2016. doi: 10.1113/EP085370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hureau TJ, Olivier N, Millet GY, Meste O, Blain GM. Exercise performance is regulated during repeated sprints to limit the development of peripheral fatigue beyond a critical threshold. Exp Physiol 99: 951–963, 2014. doi: 10.1113/expphysiol.2014.077974. [DOI] [PubMed] [Google Scholar]
- 28.Hureau TJ, Romer LM, Amann M. The ‘sensory tolerance limit’: A hypothetical construct determining exercise performance? Eur J Sport Sci 18: 13–24, 2018. doi: 10.1080/17461391.2016.1252428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Wray DW, Amann M. Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. J Physiol 596: 1373–1384, 2018. doi: 10.1113/JP275465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011. doi: 10.1152/ajpregu.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman MP, Hayes SG, Adreani CM, Pickar JG. Discharge properties of group III and IV muscle afferents. Adv Exp Med Biol 508: 25–32, 2002. doi: 10.1007/978-1-4615-0713-0_4. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: I60–I65, 1987. [PubMed] [Google Scholar]
- 33.Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164: 160–167, 2008. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Layec G, Bringard A, Le Fur Y, Micallef JP, Vilmen C, Perrey S, Cozzone PJ, Bendahan D. Opposite effects of hyperoxia on mitochondrial and contractile efficiency in human quadriceps muscles. Am J Physiol Regul Integr Comp Physiol 308: R724–R733, 2015. doi: 10.1152/ajpregu.00461.2014. [DOI] [PubMed] [Google Scholar]
- 35.Liu JZ, Dai TH, Sahgal V, Brown RW, Yue GH. Nonlinear cortical modulation of muscle fatigue: a functional MRI study. Brain Res 957: 320–329, 2002. doi: 10.1016/S0006-8993(02)03665-X. [DOI] [PubMed] [Google Scholar]
- 36.Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an FMRI study. J Neurophysiol 90: 300–312, 2003. doi: 10.1152/jn.00821.2002. [DOI] [PubMed] [Google Scholar]
- 37.Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586: 1277–1289, 2008. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauger AR, Jones AM, Williams CA. Influence of acetaminophen on performance during time trial cycling. J Appl Physiol (1985) 108: 98–104, 2010. doi: 10.1152/japplphysiol.00761.2009. [DOI] [PubMed] [Google Scholar]
- 39.Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proctor DN, Miller JD, Dietz NM, Minson CT, Joyner MJ. Reduced submaximal leg blood flow after high-intensity aerobic training. J Appl Physiol (1985) 91: 2619–2627, 2001. doi: 10.1152/jappl.2001.91.6.2619. [DOI] [PubMed] [Google Scholar]
- 41.Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol (1985) 94: 1859–1869, 2003. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo RJ, Sandager G, Astleford P, Payne K, Peterson-Kennedy L, Flinn WR, Yao JS. Mesenteric flow velocity variations as a function of angle of insonation. J Vasc Surg 11: 688–694, 1990. doi: 10.1016/0741-5214(90)90215-V. [DOI] [PubMed] [Google Scholar]
- 43.Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- 44.Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol 290: R365–R375, 2006. doi: 10.1152/ajpregu.00332.2005. [DOI] [PubMed] [Google Scholar]
- 45.Senefeld J, Yoon T, Bement MH, Hunter SK. Fatigue and recovery from dynamic contractions in men and women differ for arm and leg muscles. Muscle Nerve 48: 436–439, 2013. doi: 10.1002/mus.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheng M, Liu P, Mao D, Ge Y, Lu H. The impact of hyperoxia on brain activity: A resting-state and task-evoked electroencephalography (EEG) study. PLoS One 12: e0176610, 2017. doi: 10.1371/journal.pone.0176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE, Amann M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128: 44–55, 2017. doi: 10.1016/j.clinph.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidhu SK, Weavil JC, Thurston TS, Rosenberger D, Jessop JE, Wang E, Richardson RS, McNeil CJ, Amann M. Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J Physiol 596: 4789–4801, 2018. doi: 10.1113/JP276460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol 586: 1743–1754, 2008. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone MR, Thomas K, Wilkinson M, St Clair Gibson A, Thompson KG. Consistency of perceptual and metabolic responses to a laboratory-based simulated 4,000-m cycling time trial. Eur J Appl Physiol 111: 1807–1813, 2011. doi: 10.1007/s00421-010-1818-7. [DOI] [PubMed] [Google Scholar]
- 51.Taylor JL, Petersen N, Butler JE, Gandevia SC. Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol 525: 793–801, 2000. doi: 10.1111/j.1469-7793.2000.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977. doi: 10.1152/jappl.1977.42.3.385. [DOI] [PubMed] [Google Scholar]
- 53.Yacyshyn AF, Nettleton J, McNeil CJ. The effects of sex and motoneuron pool on central fatigue. Med Sci Sports Exerc 50: 1061–1069, 2018. doi: 10.1249/MSS.0000000000001536. [DOI] [PubMed] [Google Scholar]