Abstract
In a sheep model of intrauterine growth restriction (IUGR) produced from placental insufficiency, late gestation fetuses had smaller skeletal muscle mass, myofiber area, and slower muscle protein accretion rates compared with normally growing fetuses. We hypothesized that IUGR fetal muscle develops adaptations that divert amino acids (AAs) from protein accretion and activate pathways that conserve substrates for other organs. We placed hindlimb arterial and venous catheters into late gestation IUGR (n = 10) and control (CON, n = 8) fetal sheep and included an external iliac artery flow probe to measure hindlimb AA uptake rates. Arterial and venous plasma samples and biceps femoris muscle were analyzed by mass spectrometry-based metabolomics. IUGR fetuses had greater abundance of metabolites enriched within the alanine, aspartate, and glutamate metabolism pathway compared with CON. Net uptake rates of branched-chain AA (BCAA) were lower by 42%–73%, and muscle ammoniagenic AAs (alanine, glycine, and glutamine) were lower by 107%–158% in IUGR hindlimbs versus CON. AA uptake rates correlated with hindlimb weight; the smallest hindlimbs showed net release of ammoniagenic AAs. Gene expression levels indicated a decrease in BCAA catabolism in IUGR muscle. Plasma purines were lower and plasma uric acid was higher in IUGR versus CON, possibly a reflection of ATP conservation. We conclude that IUGR skeletal muscle has lower BCAA uptake and develops adaptations that divert AAs away from protein accretion into alternative pathways that sustain global energy production and nitrogen disposal in the form of ammoniagenic AAs for metabolism in other organs.
Keywords: fetal growth restriction, metabolomics, skeletal muscle
INTRODUCTION
Intrauterine growth restriction (IUGR) fetuses and small-for-gestational-age neonates have less lean muscle mass than their appropriately grown counterparts (41, 60). This smaller muscle mass persists throughout childhood and into adulthood (26, 33). Epidemiological studies link low birth weight and decreased muscle mass to insulin resistance (38, 59, 72), development of the metabolic syndrome and type 2 diabetes (1, 2, 78), and increased risk for adverse cardiovascular events (e.g., stroke and myocardial infarction) later in life (4). Thus, reduced skeletal muscle growth in utero may lead to lasting consequences that adversely affect lifelong metabolic health (3, 12, 17, 47). The mechanisms that lead to lower rates of skeletal muscle growth in IUGR fetuses and increase the risk of later-life metabolic disease remain incompletely understood.
Previously, we have shown in a sheep model of placental insufficiency-induced IUGR that reduced skeletal muscle protein accretion rates were associated with smaller muscle mass in IUGR fetuses when compared with normally growing controls (67). Net total amino acid (AA) uptake rates by the hindlimb were lower in IUGR compared with control fetuses, despite normal circulating plasma concentrations of nearly all AAs (67). Previous studies also have indicated that hindlimb metabolism largely consists of skeletal muscle-specific metabolism rather than bone or other tissues (6, 28, 30, 32). However, the mechanisms responsible for lower hindlimb net (total) AA uptake rates and lower skeletal muscle protein synthesis and accretion rates in the IUGR fetus are unknown.
The overall goal of this study was to identify metabolite differences using a metabolomics approach in fetal hindlimb skeletal muscle from IUGR and control late gestation fetal sheep. Using chronic surgical catheterization of the fetal abdominal aorta and femoral vein, AAs and metabolites were measured in the arterial influx and venous efflux from the hindlimb to determine differences in AA uptake and metabolite profiles that were associated with IUGR. Biceps femoris biopsies were concurrently obtained to measure relative concentrations of skeletal muscle-specific hydrophilic metabolites. We hypothesized that IUGR fetal muscle develops adaptations that divert AAs away from protein accretion into alternative metabolic pathways that conserve energy for the fetus and maintain cellular metabolism for other organs.
MATERIALS AND METHODS
Animal care and IUGR model.
Study protocols were approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee [no. 77617(10)E]. All experiments were performed in accordance with relevant guidelines and regulations from the Guide for the Care and Use of Laboratory Animals. Experimental details are reported according to the Animal in Research: Reporting In Vivo Experiments guidelines (40). The current study used animals from a cohort that was previously published in Rozance et al. (67).
Pregnant Columbia-Rambouillet mixed-breed sheep were randomized and housed in two different environmental chambers. Ewes were either housed in an environmental chamber with elevated ambient temperatures (40°C for 12 h; 35°C for 12 h) and 40% humidity from 38 days gestation (dGA; term = 147 dGA) to 116 dGA, which produces placental insufficiency and IUGR (IUGR group; n = 10) (5, 9), or an environmental chamber with normal ambient temperatures and humidity from 43 dGA to 120 dGA [control (CON) group; n = 8). After environmental treatment, all sheep were housed in normal ambient temperatures and humidity for the remainder of the studies. All sheep were given ad libitum access to water. Maternal feed intake was matched on an absolute basis between sheep in CON and IUGR groups. All fetuses in the study were with the product of singleton pregnancies, with the exception of one fetus in the IUGR group that was a product of a triplet pregnancy that was incidentally found at the time of necropsy. The catheterized triplet fetus (fetal weight: 1,466 g) was included in the analysis because it was not an outlier for any physiological parameters measured within the IUGR group as previously published (67).
Fetal surgical procedures.
Pregnant sheep underwent a surgical procedure at 127 ± 1 dGA for the placement of fetal and maternal catheters according to previously published methods (9, 67, 79). Sheep were fasted for 24 h and thirsted for 12 h before surgery. Anesthesia was induced with an intravenous dose of 0.2 mg/kg of diazepam and 20 mg/kg of ketamine and maintained with 2%–4% isoflurane for the duration of the surgical procedure. The fetal lamb was exposed by maternal laparotomy and hysterotomy. In the “study” hindlimb, a polyvinyl catheter was placed in the pudendoepigastric venous trunk with the tip advanced 1 cm into the common femoral vein (V). The deep circumflex iliac artery and vein and the pudendoepigastric arterial trunk were ligated to minimize collateral circulation to midline structures that might contaminate external iliac blood flow to the skin, bone, and muscle of the hindlimb (67). A transit time ultrasonic blood flow transducer (3 mm, Transonic Systems, Ithaca, NY) was placed around the external iliac artery of the study limb for continuous blood flow measurement. A distal arterial occluder was placed for zero-flow corrections. In the “nonstudy” hindlimb, a catheter was placed in the pedal artery with the tip positioned in the external iliac artery, just before the descending aorta (A). A 0.5-mL biopsy of the biceps femoris muscle was obtained at the time of surgery and frozen in liquid nitrogen for baseline isotope enrichment analysis used in a previous publication (67). Ampicillin (500 mg) was injected into the amniotic fluid before the uterus was sutured closed. Once the maternal linea alba and skin were closed, the maternal femoral artery and vein were catheterized. All the catheters and the flow probe were tunneled subcutaneously to the maternal flank and gathered into a pouch that was sutured to the ewe’s skin. After recovery from anesthesia, ewes received 2 days of postoperative treatment with analgesic (1.1 mg/kg of Banamine im, bid), probios (10 g of Probios po, bid) and a total of 5 days postoperative care before experimentation.
Fetal arterial and venous blood sample measurements.
Ewes were conscious and freestanding in their pens and were allowed free access to food and water. Food and water intakes were measured daily to ensure normal hydration and nutrition (67). Four paired, simultaneously drawn A and V blood samples were obtained over 1 h as the external iliac blood flow rate was recorded to establish steady-state conditions. An isovolemic transfusion of heparinized maternal blood (24 mL) was administered to the fetus during the sampling period to replace fetal blood sampled. The results of this metabolic study, including fetal hindlimb glucose, lactate, and total AA net uptake rates and protein metabolic rates, were previously published (67). Our goal was to evaluate the metabolic profile of the most severe IUGR phenotype. From the previously published data set of 13 IUGR fetuses (67), 3 IUGR fetuses fell within 2 standard deviations of the CON fetal weight mean; therefore, they were removed from this metabolomics study to produce a subset of 10 severely affected IUGR fetuses. For the AA net uptake rate measurements only, 2 of 10 IUGR fetuses were excluded because of flow probe malfunction. From this final subset of 10 IUGR and 8 CON fetuses, fetal arterial blood gas values (Radiometer ABL 800 Flex Blood Gas Analyzers, Copenhagen, Denmark), plasma glucose and lactate concentrations (YSI 2900 Biochemistry Analyzer, Yellow Springs, OH), and plasma insulin (7), IGF-1 (9), cortisol (46), and norepinephrine (48) concentrations are presented here to demonstrate the physiological characteristics of the fetuses included in the present study (Table 1).
Table 1.
Fetal blood and plasma parameters
| CON | IUGR | |
|---|---|---|
| Fetal weight, g | 3,324 ± 137 | 1,331 ± 132* |
| Fetal hindlimb weight, g | 344 ± 17 | 157 ± 14* |
| Biceps femoris weight, g | 18.1 ± 0.7 | 8.7 ± 0.9* |
| Total fetuses | 8 | 10 |
| Male fetus, % | 50 | 50 |
| Gestational age, days | 134 ± 0 | 134 ± 0 |
| pH | 7.36 ± 0.0 | 7.33 ± 0.1* |
| , mmHg | 50.4 ± 0.7 | 51.6 ± 0.8 |
| , mmHg | 20.6 ± 0.7 | 13.6 ± 0.9* |
| , % | 48.3 ± 1.6 | 22.0 ± 2.7* |
| O2 content, mmol/L | 3.3 ± 0.2 | 1.4 ± 0.2* |
| Hematocrit, % | 34.1 ± 1.0 | 32.3 ± 1.3 |
| Hemoglobin, mmol/L | 6.9 ± 0.2 | 6.5 ± 0.3 |
| Glucose, mg/dL | 17.9 ± 0.5 | 11.0 ± 1.0* |
| Lactate, mmol/L | 2.0 ± 0.1 | 2.5 ± 0.2 |
| Insulin, ng/mL | 0.4 ± 0.1 | 0.1 ± 0.0* |
| IGF-1, ng/mL | 108.2 ± 15.2 | 27.9 ± 6.0* |
| Cortisol, ng/mL | 20.2 ± 5.3 | 24.3 ± 6.1 |
| Norepinephrine, pg/mL# | 617.1 ± 252.5 | 5,670 ± 2,474* |
This cohort of animals is a subset of animals from a previously published study (67). CON (n = 8) and IUGR (n = 10). CON, control; IUGR, intrauterine growth restriction.
P ≤ 0.05, unpaired Student’s t tests. Data are represented as means ± SE;
Data were log transformed for analysis.
Fetal net hindlimb AA uptake rates.
Fetal plasma A and V AA concentrations were measured using HPLC as previously described (67). External iliac plasma flow was calculated by multiplying external iliac blood flow by (1-hct). Hindlimb (net) AA uptake rates were calculated by multiplying the mean A-V concentration difference from the four steady-state blood draws by the mean hindlimb plasma flow during the draw period. The essential AAs (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine) were summed to calculate the total essential AA uptake rate, and the remaining nonessential AAs (alanine, arginine, asparagine, aspartate, cysteine, glutamate, glutamine, glycine, ornithine, proline, serine, taurine, and tyrosine) were summed to calculate the total nonessential AA uptake rate. All hindlimb AA uptake rates were normalized to 100 g of fetal hindlimb weight and compared between CON and IUGR groups.
Fetal skeletal muscle collection.
After conclusion of the metabolic study, ewes received a dose of diazepam (0.2 mg/kg) and ketamine (20 mg/kg) intravenously, and fetuses were delivered via maternal laparotomy and hysterotomy. The biceps femoris muscle was exposed from the study hindlimb of the anesthetized fetus, and a biopsy was obtained and immediately frozen in liquid nitrogen. This sample was used for metabolomics analysis. A bolus dose of pentobarbital sodium (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI) was administered intravenously to both the mother and the fetus, after which the fetal weight, fetal hindlimb weight, and biceps femoris weight were obtained from the nonstudy hindlimb. No physical differences were observed between the study and the nonstudy hindlimb.
Hydrophilic metabolite extraction.
Frozen biceps femoris muscle samples were extracted in 15 mg/mL ice-cold lysis/extraction buffer (50% methanol, 30% acetonitrile, 20% water). Fetal plasma samples from the fourth paired A and V blood draw were extracted at 1:25 dilution with the same lysis/extraction buffer. Samples were agitated at 4°C for 30 min followed by centrifugation at 10,000 g for 15 min at 4°C. Protein and lipid pellets were discarded, whereas supernatants were injected into an ultra-HPLC system (Vanquish, Thermo, San Jose, CA) and run on a Kinetex C18 column (150 × 2.1 mm internal diameter, 1.7 μm particle size; Phenomenex, Torrance, CA) at 25°C using a 3-min isocratic method (5% Optima acetonitrile, 95% Optima H2O, 0.1% formic acid) flowing at 250 μL/min (56). The ultra-HPLC system was coupled online with a Q Exactive mass spectrometer (Thermo, Bremen, Germany), scanning in full mass spectrometer mode (2 μscans) at 70,000 resolution in the 60–900 mass-to-charge ratio range, 4 kV spray voltage, 15 sheath gas and 5 auxiliary gas, operated in negative and then positive ion mode (separate runs, 3 min each) (56). Mass spectrometer stability was assessed by determining peak area coefficient of variation for a technical mixture injected every 10 runs.
Metabolite and pathway analysis.
Metabolite assignments were determined using Maven (Princeton, NJ) (16) following conversion of .raw files into .mzXML format through MassMatrix (Cleveland, OH) and assignments confirmed against a subset of 650 light- and heavy-labeled standards (IROATech, Sigma-Aldrich, St. Louis, MO) as described previously (19).
Statistical and pathway analyses of metabolites were conducted with MetaboAnalyst 4.0 (15). For each metabolite, outliers were identified by Grubbs’ and Tukey’s test and replaced by the median of the individual group, and zero values were replaced by 50% of the lowest value from both groups. Data were then normalized by median, log transformed, and autoscaled. For the skeletal muscle, the groups analyzed were CON and IUGR. A Student's t test was used to determine significant differences in metabolites between groups (CON and IUGR). For the plasma samples, the groups analyzed were CON-A, CON-V, IUGR-A, and IUGR-V. A two-way ANOVA was conducted to analyze the effects of group (CON and IUGR), vessel (A and V), and their interaction. Tukey’s post hoc test was performed to determine differences among groups. The false discovery rate-adjusted P values [based on the Benjamini-Hochberg false discovery rate ≤10% (76)] were applied to adjust for multiple comparison testing (82). Significance was designated at an adjusted P ≤ 0.05, and marginal significance was designated at an adjusted P ≤ 0.06–0.15, mainly to maximize the number of potentially important metabolites involved in muscle metabolism for generation of pathway analysis.
For both the plasma and skeletal muscle, principal component analysis (PCA) was used as an unsupervised method to allow unbiased identification of patterns of metabolites between samples. In addition, partial least squares-discriminant analysis (PLS-DA), a supervised multivariate statistical method, was performed to identify metabolites that differ between groups to account for unwanted biological variation between animals in principal components when using PCA. Pathway analysis for both the plasma and skeletal muscle samples was performed with the top 30 metabolites with the highest variable importance in projection (VIP) scores regardless of adjusted P values. The homo sapiens Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway library was used. The pathway analysis algorithms used were the hypergeometric test for over representation analysis and relative-betweenness centrality for the pathway topology analysis.
Skeletal muscle gene expression analysis.
Total RNA was extracted and purified from snap-frozen fetal biceps femoris muscle (100 mg) using TRIzol LS (Invitrogen, Grand Island, NY) and RNeasy Mini Kit (Qiagen, Germantown, MD) with RNase-Free DNase Set (Qiagen) according to the manufacturer’s protocols. RNA (2 µg) was converted to complementary DNA (cDNA; 100 ng/μL) using SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s protocol. All cDNA samples (3–20 ng) were run in triplicate using FastStart Universal SYBR Green Master (Roche, Pleasanton, CA). The quantitative real-time PCR was performed with a relative standard curve of pooled skeletal muscle cDNA for relative quantification (Lightcycler 480 II; Roche Life Science, Indianapolis, IN) using the following conditions of amplification: 95°C for 5 min; 95°C for 15 s, 60°C for 30 s, 72°C for 30 s (40 cycles); and melting curve from 60°C to 95°C (10, 71). Primers (0.5 μM, final concentration) used were designed for SYBR green assays using either Ovis aries or Bos taurus genome sequence (Table 2) (8, 73, 74). The gene of interest was normalized to the average of three reference genes: β-actin (ACTB), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and ribosomal protein S15 (RPS15). Expression of reference genes was not different between treatment groups. For each gene, the expression data are presented as a fold change compared with the average of the CON group. The quantitative real-time PCR experiments and analysis were performed according to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines (11).
Table 2.
Primers used for real-time quantitative PCR assays
| Gene Symbol | Gene Name | Forward Primer | Reverse Primer | Accession Number | Species |
|---|---|---|---|---|---|
| ACTB | β-Actin | TGCAGAAAGAGATCACTGCC | GACAGCGAGGCAGGATGG | NM_001009784 | Ovis aries |
| ALT1 | Alanine aminotransaminase 1 | AGCCCTTCACCGAGGTCAT | CACGCCTGCAAGATGCGC | NM_001083740 | Bos taurus |
| AST | Aspartate transaminase | AAAGCTCCCGAGTTCTCCAT | CCCTGATAGGCCGAGTCAA | XM_004015042 | Ovis aries |
| BCAT1 | Branched-chain amino acid transaminase 1 | CATCCTGGACTTGGCACACA | CAGGCGGTACCTGAACCAAA | NM_001009444 | Ovis aries |
| BCAT2 | Branched-chain amino acid transaminase 2 | TGTCCTCCGTTTCCACAAGG | AGCTTTACACCGGGAGCATC | XM_027978581 | Ovis aries |
| BCKD | Branched-chain α-keto acid dehydrogenase | CGGCAGGGCCAGATCATC | GCCATAGTTGGTCATGTAGAA | XM_024984691 | Bos taurus |
| BCKDK | Branched-chain ketoacid dehydrogenase kinase | AAAGTGGGTGGACTTTGCCA | GCATCGGGATGAAGGGGAAA | XM_004020920 | Ovis aries |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | TGGAGGGACTTATGACCACTG | TAGAAGCAGGGATGATGTTCT | NM_001190390 | Ovis aries |
| GLS | Glutaminase | CCCAGAAGGCACAGACATGGTTGG | GGGCAGAAGCCACCATTAGCCA | XM_027962793 | Ovis aries |
| GS | Glutamine synthetase | GAAAGCCTGCAGAGACCAAT | GCCATTGGAAGGCCAACCA | NM_001040474 | Bos taurus |
| LDHA | Lactate dehydrogenase A | CATGGCCTGTGCCATCAGTA | GGAAAAGGCTGCCATGTTGG | XM_027959817 | Ovis aries |
| LDHB | Lactate dehydrogenase B | GAGGGAGCGATCCCAAACAA | CAGAATGCTGATGGCACACG | XM_027967824 | Ovis aries |
| PC | Pyruvate carboxylase | GCACAGCATGGGGCTTGGCT | AACTGGGCCAGGTCCCCCAC | XM_027959891 | Ovis aries |
| PDK4 | Pyruvate dehydrogenase kinase 4 | CCCAGAGGACCAAAAGGCAT | GGGTCAGCTGTACAGGCATC | XM_004007738 | Ovis aries |
| PDH | Pyruvate dehydrogenase | GTTAAGGGGGCTGCTAGGTG | AGCCACTGCGTACTGTGAAA | XM_004021953 | Ovis aries |
| PFK | Phosphofructokinase 1 | TGGTGGCTCCATGCTGGGGA | GCAGGGCGTGGATGCTGTGA | XM_004006406 | Ovis aries |
| PK | Pyruvate kinase | ACCACGCAGAGACCATCAAG | GGTCCTTTAGTGTCCAGGGC | XM_012180891 | Ovis aries |
| RPS15 | Ribosomal protein S15 | ATCATTCTGCCCGAGATGGTG | CGGGCCGGCCATGCTTTACG | XM_015096022 | Ovis aries |
All primer sequences are from 5′ to 3′. Accession numbers refer to the published gene sequences from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/gene) from which the primer sequences were designed.
Statistical analysis.
Analysis of metabolomics and pathway enrichment in CON versus IUGR are described above. For fetal physiological and blood/plasma parameters, individual AA uptake rates, and targeted gene expression analysis, the data are presented as mean values ± SE with consideration for statistical significance at P ≤ 0.05. Unpaired Student’s t tests were used for direct comparisons between CON and IUGR groups, and a Mann-Whitney test was used when variances were unequal (Graph Pad Prism 5). Relationships between various AA uptake rates [branched-chain amino acid (BCAA), alanine, glycine, glutamine] and fetal hindlimb weight and/or oxygen content were determined using simple linear regression analysis. Regression relationships were determined for all fetuses pooled, and P ≤ 0.05 was considered statistically significant. The total number of animals per group was not large enough to have sufficient power to fully evaluate for sex differences; thus, we did not include sex in our statistical model.
RESULTS
Fetal characteristics.
Table 1 shows the physiological measurements of the fetuses included in this study, which is a subset of animals from the previously published study (67). The fetal weights and hindlimb weights were 60% and 54% lighter, respectively, in the IUGR group compared with CON (P ≤ 0.05). Additionally, the fetal biceps femoris muscle weight was 52% less in IUGR compared with CON (P ≤ 0.05). IUGR fetal arterial pH, blood oxygen content, and plasma glucose, insulin, and IGF-1 concentrations were 40%–75% lower than CON (P ≤ 0.05). Fetal plasma norepinephrine concentrations were eightfold higher than CON (P ≤ 0.05) (67).
Metabolomics analysis of IUGR and CON fetal skeletal muscle.
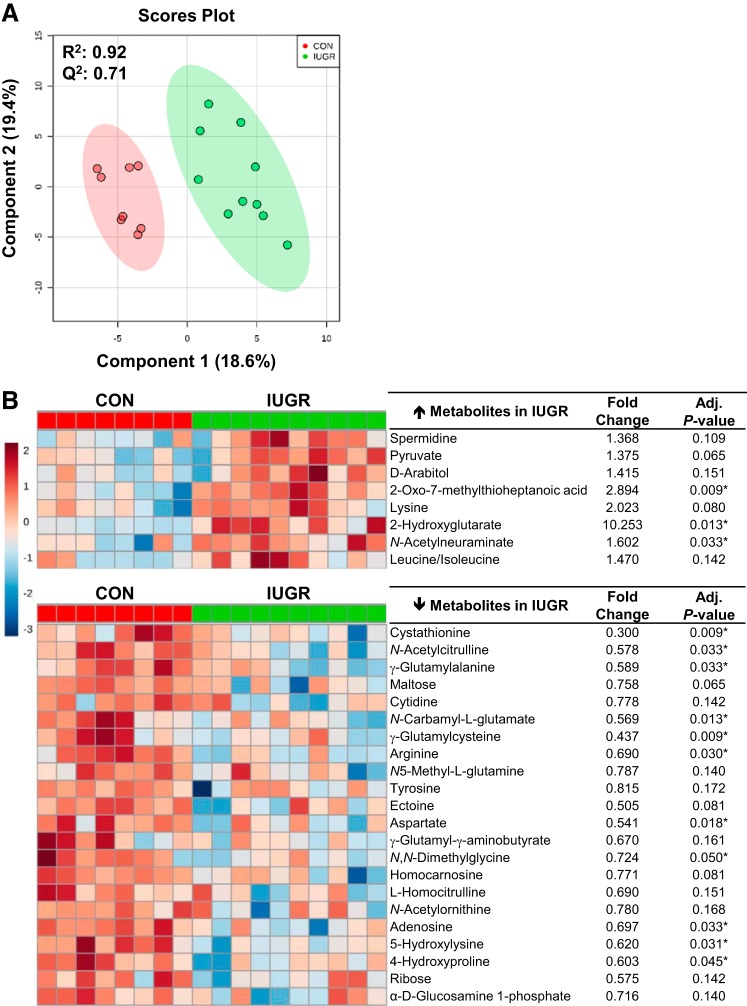
The PCA plots showed separation between the IUGR and CON groups (Supplemental Figure S1A; Supplemental Material is available online at https://doi.org/10.6084/m9.figshare.8009198). Subsequently, the top 30 metabolites were identified according to the VIP scores from the PLS-DA scores plot (R2 = 0.92, Q2 = 0.71) by comparing IUGR versus CON (Fig. 1 A). From the top 30 metabolites, 8 were higher and 22 were lower in IUGR muscle compared with CON muscle (Fig. 1B). In IUGR skeletal muscle, the relative enrichment of the metabolite 2-hydroxyglutarate was 10-fold higher when compared with the CON (adjusted P = 0.013). The metabolites that were lowest in the IUGR muscle compared with CON were 5-hydroxylysine (40%; adjusted P = 0.009) and arginine (30%; adjusted P = 0.009).
Fig. 1.
Metabolomic profiles of skeletal muscle of intrauterine growth restriction (IUGR) and control (CON) fetuses. A: partial least squares discriminant analysis was used to identify metabolites with changes in abundance that defined separation of samples between the IUGR (n = 10 fetuses) and CON (n = 8 fetuses) groups. B: heat map of the 30 metabolites with the highest variable importance in projection scores. Each square is representative of the mean levels of that metabolite for the individual animal. Row values are normalized for each metabolite, and quantitative changes are color coded from red (high) to blue (low). Student’s t test was conducted to detect differences in fold change. *False discovery rate-adjusted P ≤ 0.05.
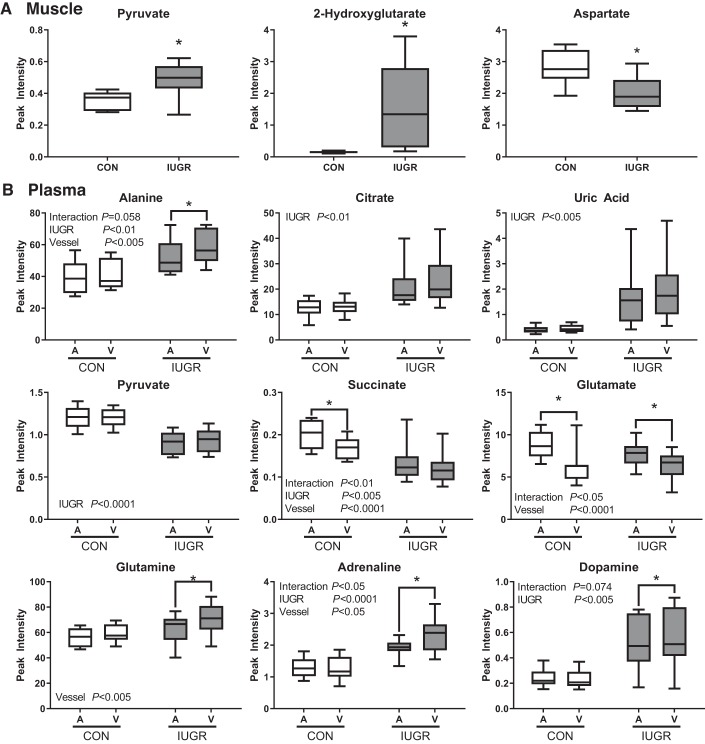
Skeletal muscle metabolic pathway analysis was performed using the top 30 VIP scores (Table 3). Pathways that were significantly enriched in IUGR skeletal muscle included arginine and proline metabolism; glycine, serine, and threonine metabolism; aminoacyl-RNA biosynthesis; and alanine, aspartate, and glutamate metabolic pathways (adjusted P ≤ 0.15). Selected significant metabolites (pyruvate, 2-hydroxyglutarate, and aspartate) highlighting the alanine, aspartate, and glutamate metabolism pathway in the IUGR and CON fetal skeletal muscle are shown in Fig. 2A.
Table 3.
Metabolic pathways identified in the arterial and venous plasma, and muscle of IUGR vs. control fetuses
| Total in Pathway | Hits | Adj. P Value |
Metabolites Up in IUGR vs. CON |
Metabolites Down in IUGR vs. CON |
|
|---|---|---|---|---|---|
| Skeletal muscle | |||||
| Arginine and proline metabolism | 77 | 8 | 0.0002 | Pyruvate, Spermidine | Arginine, Aspartate, N-Acetylornithine, Homocarnosine, 4-Hydroxyproline, g-Glutamyl-g-aminobutyrate |
| Glycine, serine, and threonine metabolism | 48 | 5 | 0.007 | Pyruvate | Aspartate, Cystathionine, Ectoine, Dimethylglycine, |
| Aminoacyl-tRNA biosynthesis | 75 | 6 | 0.007 | Lysine, Leucine, Isoleucine | Arginine, Aspartate, Tyrosine |
| Alanine, aspartate, and glutamate metabolism | 24 | 3 | 0.059 | Pyruvate, 2-Hydroxyglutarate | Aspartate |
| Valine, leucine, and isoleucine metabolism | 27 | 3 | 0.067 | Pyruvate, Leucine, Isoleucine | |
| Lysine metabolism | 32 | 3 | 0.091 | Lysine, 2-Hydroxyglutarate | Aspartate |
| Arterial and venous plasma | |||||
| Citrate cycle (TCA cycle) | 20 | 3 | 0.051 | Citrate | Pyruvate, Succinate |
| Taurine and hypotaurine metabolism | 20 | 3 | 0.051 | Taurine, Alanine | Pyruvate |
| Alanine, aspartate, and glutamate metabolism | 24 | 3 | 0.058 | Alanine | Pyruvate, Succinate |
| Pentose phosphate pathway | 32 | 3 | 0.101 | Glucose, Ribose, Pyruvate |
Targeted pathway analysis was performed using MetaboAnalyst. The hypergeometric test and the relative-betweeness centrality test were used for overrepresentation analysis and pathway topology analysis. P values were adjusted using false discovery rate. IUGR (n = 10), intrauterine growth restriction; CON (n = 8), control.
Fig. 2.
Selected features to highlight the tricarboxylic acid (TCA) cycle; alanine, aspartate, and glutamate metabolism; and other key metabolites. A: metabolites from intrauterine growth restriction (IUGR; n = 10 fetuses) and control (CON; n = 8 fetuses) fetal skeletal muscle. B: features from IUGR (shaded box) and CON (open box) fetal arterial (IUGR-A, CON-A) and venous (IUGR-V, CON-V) plasma samples. Data are from MetaboAnalyst as presented in Figs. 1 and 3 and analyzed using Student’s t test (A) or two-way ANOVA (B). *False discovery rate-adjusted P ≤ 0.1 by Tukey’s post hoc test. Box denotes 25th and 75th percentiles; bars denote minimum and maximum values. A, arterial; V, venous.
Metabolomics analysis of IUGR and CON fetal arterial and venous plasma.
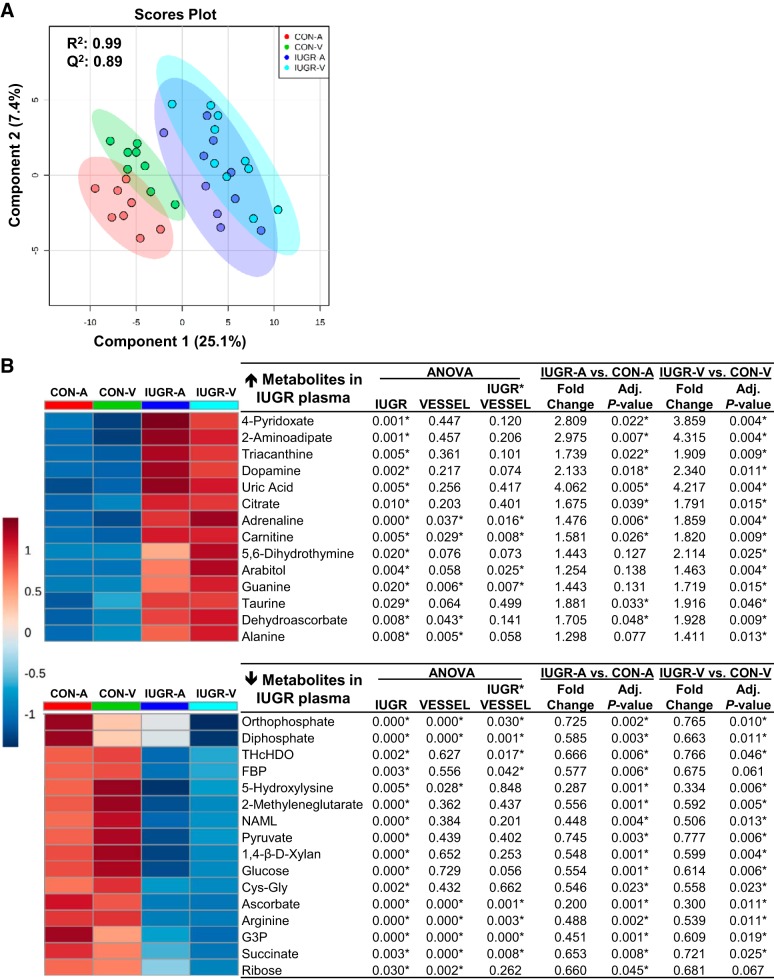
PCA plots showed separation between the IUGR and CON groups but not between vessel groups (Supplemental Figure S1B; https://doi.org/10.6084/m9.figshare.8009198). Using a PLS-DA scores plot (R2 = 0.99, Q2 = 0.89), four groups (CON-A, CON-V, IUGR-A, IUGR-V) were compared to generate VIP scores for each of the metabolites (Fig. 3A). The top 30 metabolites are shown in Fig. 3B, from which 14 metabolites were higher and 16 metabolites were lower in IUGR fetal plasma compared with CON (adj. P ≤ 0.05, group effect; Fig. 3B). Heat maps of the individual animal metabolite profile were generated for the skeletal muscle (Supplemental Figure S2A; https://doi.org/10.6084/m9.figshare.8009198) and the arterial and venous plasma samples (Supplemental Figure S2B; https://doi.org/10.6084/m9.figshare.8009198). The metabolites that were highest in IUGR fetal plasma compared with CON (all with adjusted P ≤ 0.05) included uric acid (4-fold), 2-aminoadipate (3-fold), 4-pyridoxate (3-fold), dopamine (2-fold), and 5,6-dihydrothymine (2-fold). The metabolites that were lowest in the IUGR fetal plasma compared with CON were ascorbate (80%) and 5-hydroxylysine (70%) (Fig. 3B).
Fig. 3.
Metabolomic profiles of arterial and venous plasma of intrauterine growth restriction (IUGR) and control (CON) fetuses. A: partial least squares-discriminant analysis was used to identify metabolites with changes in abundance that defined separation of samples between the IUGR (n = 10 fetuses) and CON (n = 8 fetuses) groups. B: heat map of the 30 metabolites with the highest variable importance in projection scores. For simplicity, each square is representative of the mean levels of that metabolite per group and vessel. Row values are normalized for each metabolite, and quantitative changes are color coded from red (high) to blue (low). A, arterial plasma; FBP, fructose 1,6-bisphosphate; G3P, sn-glycerol 3-phosphate; NAML, N-Acyl-d-mannosaminolactone; THcHDO, 3D-(3,5/4)-trihydroxycyclohexane-1,2-dione; V, venous plasma. ANOVA was performed on all 4 groups (CON-A, CON-V, IUGR-A, and IUGR-V). Student’s t test was performed separately on the arterial and venous plasma of IUGR versus CON. *False discovery rate-adjusted P ≤ 0.05.
A metabolic pathway analysis was performed on the top 30 VIP metabolites from the fetal plasma (Table 3). Significantly enriched pathways identified were the tricarboxylic acid (TCA) cycle; taurine and hypotaurine metabolism; and alanine, aspartate, and glutamate metabolism (adjusted P ≤ 0.15). Top features in the IUGR and CON fetal arterial and venous plasma highlighting the TCA cycle, alanine, aspartate, and glutamate metabolism and other key metabolites are shown in Fig. 2B.
Individual AA uptake rates across the fetal hindlimb.
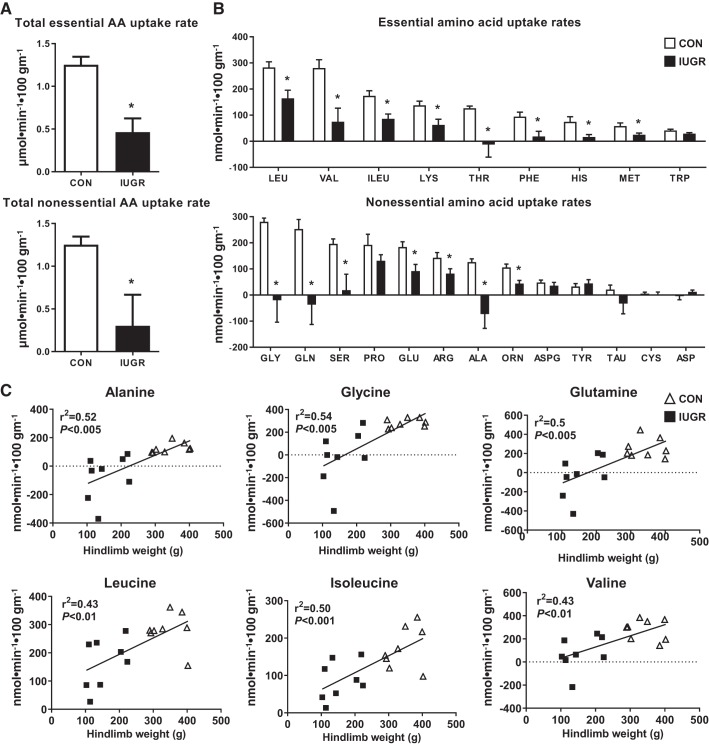
The total essential and nonessential net hindlimb AA uptake rates were lower by 63% and 81%, respectively, in IUGR compared with CON (Fig. 4A). Individual net hindlimb essential AA uptake rates for the BCAAs leucine, valine, and isoleucine were 42%, 73%, and 50% lower, respectively, in IUGR compared with CON (P ≤ 0.05). The remaining hindlimb essential AA uptake rates (lysine, threonine, phenylalanine, histidine, and tryptophan) were 54%–110% lower in IUGR compared with CON (P ≤ 0.05) (Fig. 4B). Nonessential net hindlimb AA uptake rates for alanine, glycine, and glutamine were 158%, 107%, and 115% lower, respectively, in IUGR compared with CON (P ≤ 0.05). The remaining nonessential AAs glutamate, serine, arginine, and ornithine were 42%–90% lower in IUGR compared with CON (P ≤ 0.05) (Fig. 4B).
Fig. 4.
Reduced hindlimb uptake of essential and nonessential amino acids in the intrauterine growth restriction (IUGR) fetus. A: total amino acid (AA) uptake rates. B: individual AA uptake rates. Data are represented as means ± SE; IUGR (n = 8) and control (CON; n = 8). Arg, arginine; Asp, aspartate; ASPG, asparagine; Cys, cysteine; Glu, glutamate; His; Lys, lysine; Met, methionine; Orn, ornithine; Phe, phenylalanine; Pro, proline; Ser, serine; Tau, taurine; Thr, threonine; Trp, tryptophan; Tyr, tyrosine. *Statistical significance at P ≤ 0.05 vs. CON, Student’s t test. C: linear relationship between hindlimb weight and alanine (Ala), glutamine (Gln), glycine (Gly), leucine (Leu), isoleucine (Ile), and valine (Val) uptake rates. IUGR fetuses and CON fetuses with R2 and P values are shown.
We further investigated the relationship between the BCAA, alanine, glycine, and glutamine uptake rates, hindlimb weight, and fetal arterial blood oxygen content. We found a positive association between BCAA, alanine, glycine, and glutamine net uptake rates with hindlimb weight, with the smallest hindlimbs demonstrating net release of alanine, glycine, and glutamine (Fig. 4C). Fetal arterial oxygen content correlated positively with the uptake rates of BCAAs (leucine, r2 = 0.43, P = 0.005; isoleucine, r2 = 0.40, P = 0.008; valine, r2 = 0.33, P = 0.02), alanine (r2 = 0.46, P = 0.004), glycine (r2 = 0.51, P = 0.002), and glutamine (r2 = 0.42, P = 0.007). Fetuses with the lowest oxygen content showed a release of alanine, glycine, and glutamine from the hindlimb (data not shown).
Quantitative real-time PCR analysis.
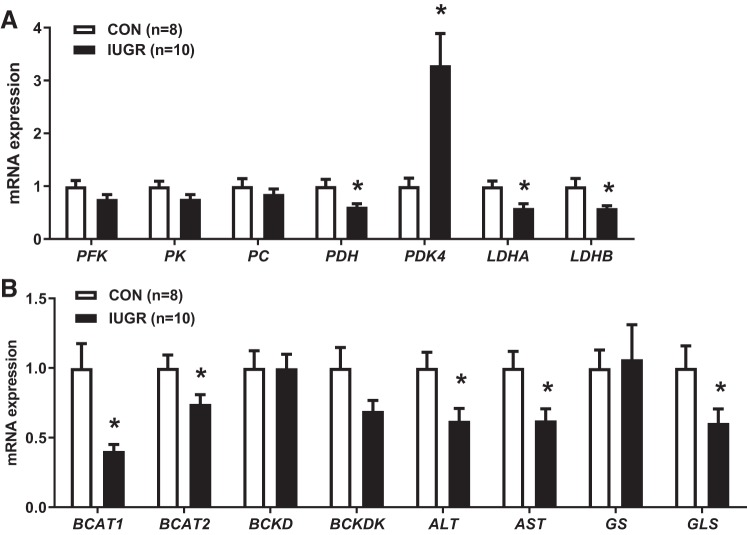
To understand the activation or repression of specific metabolic pathways in IUGR muscle as indicated by the pathway analysis, we measured expression of target genes involved in energy metabolism (rate-limiting steps of glycolysis and the TCA cycle) and AA catabolism (Fig. 5). Expressions of phosphofructokinase 1 (PFK), pyruvate kinase (PK), and pyruvate carboxylase (PC) were similar between groups. However, expression of pyruvate dehydrogenase (PDH), which decarboxylates pyruvate to acetyl-CoA, was 39% lower (P ≤ 0.05). Expression of pyruvate dehydrogenase kinase 4 (PDK4), an inhibitor of PDH activity, was 3.3-fold higher in IUGR compared with CON (P ≤ 0.05), as we have demonstrated previously (8). Gene expressions of lactate dehydrogenase A (LDHA) and lactate dehydrogenase B (LDHB) were both 42% lower in the IUGR compared with CON (P ≤ 0.05). Likewise, the expressions of branched-chain amino acid transaminase 1 (BCAT1) and branched-chain amino acid transaminase 2 (BCAT2), which transaminate BCAAs to their ketoacids, were 60% and 26% lower in IUGR compared with CON, respectively (P ≤ 0.05). We did not detect any significant difference in the expression of branched-chain α-keto acid dehydrogenase (BCKD), an enzyme that decarboxylates ketoacids. However, the gene expression of its inhibitor, branched-chain ketoacid dehydrogenase kinase (BCKDK), was lower by 31% (P = 0.07). Additionally, the expressions of both alanine aminotransaminase 1 (ALT1) and aspartate transaminase (AST) were lower by 38%, and glutaminase (GLS) expression was lower by 40% in IUGR compared with CON (P ≤ 0.05).
Fig. 5.
Gene expressions of enzymes in the fetal skeletal muscle. A: genes involving in the glycolysis and tricarboxylic acid (TCA) cycle pathways. B: branched-chain amino acid catabolism in the intrauterine growth restriction (IUGR; n = 10) versus control (CON; n = 8). *P ≤ 0.05, unpaired Student’s t tests. ALT, alanine aminotransaminase 1; AST, aspartate transaminase; BCAT1, branched-chain amino acid transaminase 1; BCAT2, branched-chain amino acid transaminase 2; BCKD, branched-chain α-keto acid dehydrogenase; BCKDK, branched-chain ketoacid dehydrogenase kinase; GS, glutamine synthetase; GLS, glutaminase; LDHA, lactate dehydrogenase A; LDHB, lactate dehydrogenase B; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; PDK4, pyruvate dehydrogenase kinase 4; PFK, phosphofructokinase; PK, pyruvate kinase.
DISCUSSION
Summary of key findings.
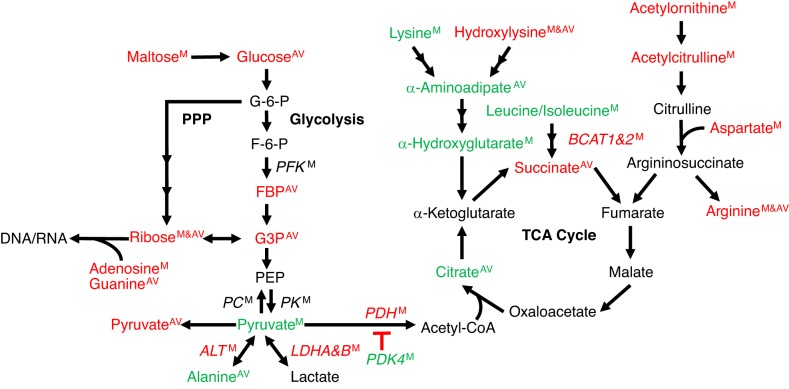
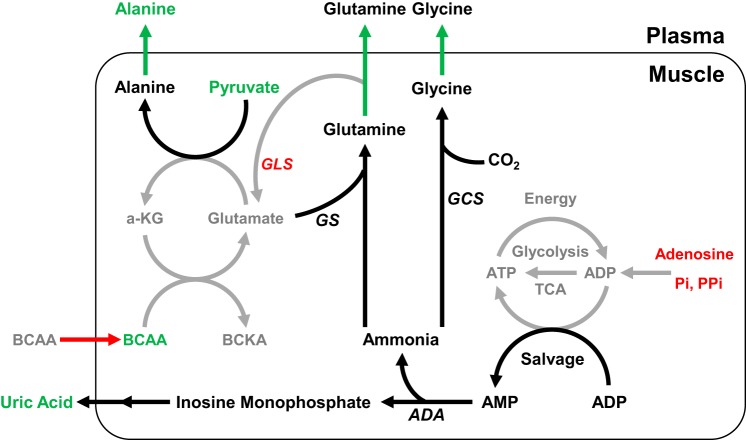
Our metabolomics approach demonstrated that the main metabolic (KEGG) pathways impacted in IUGR fetal skeletal muscle were the TCA cycle and AA metabolism. The shared metabolic pathway identified in both IUGR fetal plasma and in skeletal muscle was alanine, aspartate, and glutamate metabolism. The net uptake rates of the BCAAs alanine, glycine, and glutamine were lower in IUGR fetuses compared with CON and strongly correlated with fetal hindlimb weight. IUGR fetuses with the smallest hindlimbs demonstrated net release of alanine, glycine, and glutamine, potentially to shuttle gluconeogenic substrates or nitrogen out of the skeletal muscle and to the liver or other organs. In addition, both the metabolomics analysis and gene expression levels indicated that in the IUGR skeletal muscle, pyruvate may be used as a substrate for alanine production. Other metabolite differences indicated energy deficiency in the IUGR hindlimb, including lower phosphate concentrations in femoral arterial and venous plasma, lower intramuscular concentrations of adenosine, and higher femoral venous plasma concentrations of uric acid. The lower rate of BCAA uptake by the hindlimb is a likely contributor to the lower rate of skeletal muscle protein accretion and growth in the IUGR fetus compared with CON, shown previously (67). Limiting the energy cost of net protein accretion at the expense of skeletal muscle growth allows for energy conservation in the IUGR fetus. This is a positive fetal response to the lower supplies of nutrients and oxygen because of placental insufficiency and likely functions to promote fetal survival. These results support our hypothesis that IUGR fetal skeletal muscle develops adaptations that divert AAs away from those pathways that lead to protein accretion and into selective metabolic pathways that sustain global energy production and nitrogen disposal (Figs. 6 and 7).
Fig. 6.
Pathway diagram of glycolysis, pentose phosphate pathway (PPP), and tricarboxylic acid (TCA) cycle highlighting metabolites that differed between control (CON) and intrauterine growth restriction (IUGR) fetal skeletal muscle and plasma. Selected metabolites are shown from the top 30 variable importance in projection scores that were higher (green), lower (red), or unchanged (black) in IUGR (n = 10) fetal skeletal muscle (M) and/or femoral arterial and venous plasma (AV) when compared with CON (n = 8) fetuses. The gene expression of selected enzymes (italics) in the fetal skeletal muscle also are shown. PPP production of ribose was lower in IUGR muscle and AV, as was adenosine in IUGR muscle for DNA/RNA and ATP production. Despite lower glycolytic intermediates in IUGR AV, pyruvate concentrations were higher in IUGR muscle. LDHA, LDHB, and PDH expressions was lower in IUGR muscle, and alanine concentrations were higher in IUGR AV, supporting alanine release from muscle, as opposed to lactate production or conversion of pyruvate to acetyl-CoA for entry into the TCA cycle. Lysine and its catabolic product α-hydroxyglutarate were higher in IUGR muscle, yet α-ketoglutarate concentrations were unchanged; leucine/isoleucine concentrations also were higher in IUGR muscle, yet BCAT expression was lower, further supporting limited substrate entry into the TCA cycle in IUGR muscle. Finally, intermediates in the arginine synthesis pathway were lower in IUGR muscle. ALT, alanine aminotransaminase 1; BCAT1, branched-chain amino acid transaminase 1; BCAT2, branched-chain amino acid transaminase 2; FBP, fructose 1,6-bisphosphate; G3P, sn-glycerol 3-phosphate; LDHA, lactate dehydrogenase A; LDHB, lactate dehydrogenase B; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; PDK4, pyruvate dehydrogenase kinase 4; PFK, phosphofructokinase; PK, pyruvate kinase.
Fig. 7.
Summary of proposed metabolic adaptations in intrauterine growth restriction (IUGR) skeletal muscle that conserve energy utilization and result in the release of ammoniagenic amino acids (AAs; alanine, glutamine, glycine) and uric acid. Metabolites, AA uptake rates, and gene expressions of enzymes (italics) in the IUGR (n = 10) fetal skeletal muscle or plasma that were higher (green), lower (red), or unchanged (black) when compared with control (CON; n = 8) fetuses. Black and gray-shaded arrows and font show proposed increases and decreases, respectively, in metabolic pathway flux. Based on these analyses, we propose that reduced oxidative metabolism (through glycolysis and the TCA cycle) and reduced availability of phosphate and adenosine result in increased entry of ADP into a salvage pathway for ATP generation. AMP and ammonia are then overly produced in IUGR muscle. AMP is further processed to uric acid via AMP deaminase, and excess ammonia is converted and released into the plasma in the forms of glutamine and glycine. Alanine, which is a gluconeogenic substrate and released into the circulation, is produced as a result of a series of transamination reactions that alleviate the load of pyruvate and glutamate. α-KG, α-ketoglutarate; ADA, adenosine deaminase; BCAA, branched-chain amino acid; BCKA, branched-chain α-ketoacids; GCS, γ-glutamylcysteine synthetase; GLS, glutaminase; GS, glutamine synthetase; Pi, orthophosphate; PPi, diphosphate.
Lower AA uptake rates and the release of alanine, glycine, and glutamine from the IUGR hindlimb.
Glucose, oxygen, and AA availability promote protein synthesis and accretion rates in fetal skeletal muscle (6, 24). Previously, we demonstrated lower protein accretion rates in IUGR fetal skeletal muscle compared with normally growing CON fetal sheep (67). When normalized to the weight of the hindlimb, the hindlimb blood flow rates and net glucose and lactate uptake rates were similar between IUGR and CON fetal sheep; however, weight-normalized hindlimb oxygen delivery and consumption rates were 40% and 29% lower, respectively, in IUGR compared with CON (67). The net total hindlimb AA uptake rates were 55% lower in IUGR compared with CON, indicating that the uptake of AAs was reduced relative to glucose in muscle (67). Therefore, lower oxygen delivery to the hindlimb might limit the capacity of skeletal muscle to take up and metabolize AAs into anabolic pathways or the capacity for AA oxidation.
In the current study, we further evaluated the net hindlimb uptake rates of individual AAs to determine whether the lower total AA uptake rate included specific AAs or groups of AAs (essential vs. nonessential). The net uptake rates of nearly all of the AAs were lower in the IUGR than CON hindlimb. Specifically, the uptake rates of essential BCAAs (leucine, valine, and isoleucine) were lower in IUGR compared with CON. Human and rat studies have shown that BCAAs, especially leucine, independently stimulate muscle protein synthesis (28, 32). In addition, fetal sheep studies have shown that leucine uptake and oxidation rates are lower during hypoxic states, resulting in decreased protein accretion and use of leucine for energy production compared with normoxic controls (53). The decrease in BCAA uptake into IUGR fetal muscle also could be due to the limited capacity of the skeletal muscle for oxidative metabolism (8). In support of this concept, we found that the hindlimb uptake rates of BCAA were lower in the IUGR group and positively correlated with fetal arterial blood oxygen content. Furthermore, BCAA uptake by the skeletal muscle is directly related to circulating concentrations of insulin, a key stimulator of fetal growth (28, 29). Thus, the reduced protein synthesis rates, accretion rates, and overall slower growth of IUGR skeletal muscle, could be due to the lower skeletal muscle BCAA uptake rates.
Despite the lower essential BCAA uptake rates by the IUGR hindlimb, essential AA concentrations (lysine, leucine/isoleucine) were higher in the IUGR muscle compared with CON. This pattern of metabolites indicates a reduction in the intramuscular utilization of essential AAs for protein accretion and/or catabolic processes (84). We have previously shown that in the IUGR fetal hindlimb, there were lower protein synthesis and accretion rates compared with CON (67). In addition, lower gene expressions of BCAT1 and BCAT2 suggest a decrease in transamination of BCAAs to α-ketoacids, the first step in BCAA catabolism. Interestingly, lysine is one of the least oxidized AAs in the fetus (42, 44, 50), potentially resulting in accumulation within the muscle if it is not used for protein synthesis. However, catabolic intermediates in lysine metabolism were higher in both the IUGR muscle (2-hydroxyglutarate, 10-fold) and plasma (2-aminoadipic, 3-fold) compared with CON. Previous work in human primary cells (endothelial cells, smooth muscle cells, and lung fibroblasts) showed that hypoxic stress increases cellular 2-hydroxyglutarate concentrations (58). The significance and/or consequence of this striking increase in 2-hydroxyglutarate in the fetal IUGR muscle is yet to be determined.
Hindlimb uptake rates of alanine, glutamine, and glycine were lower in IUGR and strongly correlated with fetal hindlimb weight and arterial oxygen content. In fact, the IUGR fetuses with the lowest hindlimb weight had negative uptake rates of alanine, glutamine, and glycine, indicating net release of these AAs by the hindlimb. In human conditions of elevated ammonia, ammoniagenic AAs (alanine, glutamine, and glycine) are generated in the tissue to maintain nitrogen balance by carrying ammonia molecules to the liver where they can be converted to urea and excreted by the kidney (27, 68). In IUGR fetal skeletal muscle, several processes may be responsible for a net release of these three AAs. Ammonia is generated from transamination of BCAAs to their corresponding ketoacids. Consistent with this, one of the metabolic pathways that was enriched in both the skeletal muscle and plasma was the alanine, aspartate, and glutamate pathway. In skeletal muscle, alanine and glutamate are synthesized from BCAAs through the transfer of an amine group to pyruvate, oxaloacetate, or α-ketoglutarate, with release of their α-ketoacid products (Fig. 7). In adults, this process is enhanced especially during times of stress, including sepsis, starvation, burn injury, and trauma by increased activity of muscle BCAA aminotransferase (BCAT) (34, 35). Alternatively, in an environment of hypoxia and limited ATP availability as in the IUGR fetus, ammonia is generated from the recycling of ATP via ADP and adenylate kinase. This process generates not only ATP but also AMP, which is further processed to uric acid via AMP deaminase (75), thus resulting in higher levels of plasma uric acid and ammoniagenic AAs (excess tissue ammonia) in the IUGR fetus (Fig. 7).
Glutamine is further synthesized through the formation of an amide bond between ammonia and glutamate (31). Glutamine acts as a nitrogen shuttle among organs to deliver nitrogen to rapidly dividing cells or to eliminate ammonia from muscle (18). Consistent with this cycle, lower expression of GLS, an enzyme that converts glutamine to glutamate, and maintained expression of GS (glutamine synthase) indicates that the synthesis of glutamine was favored over glutamate in IUGR muscle. IUGR fetal skeletal muscle, therefore, preferentially forms alanine, glycine, and glutamine from the limited supply of BCAAs and releases them into the circulation, possibly to the liver for gluconeogenesis (39, 57) to sustain glucose availability to vital organs (77), or for disposal of nitrogen to the liver for urea production (31) (Fig. 7).
Our findings, however, differ from the classically described BCAA-alanine and glutamine cycle in adults, which is mediated by increased BCAT activity and decreased BCKD activity. We observed lower expression of BCAT1 and 2 with maintained expression of BCKD. There are several possible explanations for this difference. First, acute metabolic stressors described in the adult result in the stimulation of proteolysis to make BCAAs available to other organs (34, 35). Conversely, in placental insufficiency, the fetus adapts to a chronic and progressive decline in nutrient and oxygen availability by slowing protein synthesis as opposed to increasing protein breakdown (67). Second, lower BCAT1 and 2 expression in IUGR fetal muscle might be reflective of the overall reduction of BCAA uptake by the IUGR fetal hindlimb, another adaptation to long-standing reduction in AA supply from the placenta (13, 49, 64). Finally, lower BCAT1 and 2 expression might reflect developmentally regulated differences in AA metabolism between the fetus, when growth of the muscle is expendable, and the adult, when muscle is fully mature.
We also found higher concentrations of adrenaline and dopamine in IUGR fetal plasma, which is consistent with previous reports of increased catecholamines in IUGR and hypoxic fetuses (20, 37, 67, 70). Higher levels of catecholamines in the IUGR fetuses have been shown to suppress insulin secretion and stimulate hepatic gluconeogenesis (43, 85), which also supports interorgan communication between the IUGR fetal muscle and the liver.
Higher levels of pyruvate in the IUGR skeletal muscle.
Despite lower glycolytic intermediates, pyruvate concentration was higher in IUGR muscle compared with CON (Fig. 6). Pyruvate is the end-product of glycolysis and can enter multiple metabolic pathways, including conversion to lactate, alanine, or entry into the TCA cycle. IUGR fetuses have lower whole body fractional rates of glucose oxidation (8, 45). Increased gene expression of PDK4 and reduced gene expression of PDH suggest decreased PDH activation as a mechanism for limited glucose oxidation (8). However, despite these differences in gene expression, PDH activity is increased in IUGR skeletal muscle (61). Further studies are needed to measure pyruvate flux in the IUGR muscle and understand its role linking glycolysis and TCA cycle activity. Lower expression of LDHA and LDHB in IUGR muscle indicates limited interconversion between pyruvate and lactate, consistent with normal LDH activity in IUGR muscle (61). Gene expression of ALT, the transaminase that catalyzes the reversible reaction between pyruvate and alanine, also was lower in IUGR fetal skeletal muscle compared with CON. However, higher concentrations of circulating alanine in the venous pool, along with evidence of release of alanine from the smallest IUGR hindlimbs, supports increased glucose to alanine flux in the muscle via pyruvate and likely downregulation of ALT to prohibit conversion of alanine back to pyruvate (14, 57).
We speculate that hypoxia-inducible factor (HIF) signaling might play a role in how the IUGR fetal muscle responds to chronically lower substrate and oxygen delivery from the placenta. It has been proposed that HIF signaling is activated within mature muscle exposed to hypoxia (HIF-2α) and promotes the upregulation of glucose transporter 1 and 4, insulin-like growth factor binding protein-1, and the glycolytic enzymes (36, 63), all of which might explain the higher levels of pyruvate in IUGR fetal skeletal muscle. Likewise, it has been reported that immature skeletal muscle cells are more dependent on glucose and anaerobic metabolism versus mature cells (83). The potential activation of signaling pathways, such as HIF, which might link placental insufficiency and chronic fetal hypoxemia with muscle metabolic adaptations to maintain glycolysis and/or pyruvate metabolism in the context of lower plasma glucose concentrations requires further study.
Lower rates of arginine and proline metabolism.
The most significant metabolic pathway identified by KEGG analysis in the IUGR skeletal muscle was arginine and proline metabolism. In the IUGR muscle, both arginine and proline concentrations were lower compared with CON. In late gestation fetal sheep, the level of arginine concentration in the skeletal muscle is positively associated with muscle weight (69). In neonatal piglets, arginine supplementation enhances protein synthesis in the skeletal muscle (25) similar to the action of BCAAs. Therefore, lower concentrations of arginine observed in the IUGR fetal muscle might contribute to lower skeletal muscle growth rates compared with CON. As the precursor to nitric oxide, arginine serves as a nitrate reservoir in muscle (54, 62, 81). Additionally, metabolites involved in redox homeostasis were lower in the IUGR muscle, specifically dimethylglycine, cystathionine, and γ-glutamylcysteine (precursor to glutathione) (51, 52, 65). In the IUGR plasma, there were lower levels of ascorbate and Cys-Gly but higher levels of dehydroascorbate. Future studies are needed to investigate whether lower concentrations of arginine result in lower nitric oxide and impaired vasodilation of the microcirculation and whether an oxidative stress may contribute to reduced oxygen and nutrient delivery to skeletal muscle in the IUGR fetus.
Several metabolites identified within the arginine and proline metabolic pathway are also involved (directly or indirectly) in the urea cycle (e.g., spermidine, arginine, aspartate, acetylornithine, homocarnosine, and γ-glutamyl-GABA). In addition, metabolites acetylcitrulline, homocitrulline, and N-carbamyl-l-glutamate are related to urea cycle metabolism and were lower in IUGR muscle compared with CON (Fig. 6). The urea cycle primarily occurs in the liver and, to a lesser extent, the kidney and intestine. The skeletal muscle is not known to have complete urea cycle function, but enzymes in the cycle that are present in the muscle could contribute to metabolism of nitrogen-containing molecules as they relate to nitrogen balance, oxidative stress, and generation of intermediate AAs. These findings, and urea cycle enzymes as they relate to fetal skeletal muscle metabolism, warrant further investigation.
Evidence for lower energy status in IUGR muscle.
Several metabolites in the IUGR plasma and muscle indicate lower energy status, consistent with our previously observed reduction in hindlimb oxygen consumption rate (67). First, orthophosphate and diphosphate concentrations were lower in both the arterial and femoral venous plasma of IUGR fetuses, which could result in lower ATP production by the muscle. Second, the concentration of ribose was lower in both the IUGR plasma and skeletal muscle compared with CON (Fig. 6). Third, the purine adenosine was lower in the IUGR muscle, and purines are also used for the generation/synthesis of ATP (Fig. 7).
Low levels of ribose and purines (particularly adenosine) in the IUGR muscle could indicate an increased consumption of ribose sugars and purines as global energy currency, at the expense of local skeletal muscle energy metabolism. Consistent with this hypothesis, we observed a fourfold increase in uric acid in IUGR arterial and femoral venous plasma compared with CON. Uric acid is a catabolite of purines (e.g., adenosine and guanine) and is elevated, particularly in the fetus and neonate, in situations of extreme energy deficiency, including inborn errors of fat metabolism (e.g., medium-chain acyl-CoA dehydrogenase deficiency) and carbohydrate metabolism (e.g., glycogen storage diseases and hereditary fructose intolerance) (66). Glucose is metabolized through the pentose phosphate pathway to generate ribose 5-phosphate, NADPH, and erythrose 4-phosphate (80). Ribose phosphate is used in the synthesis of nucleotides and nucleic acids. It is possible that there is reduced pentose phosphate pathway flux in IUGR muscle in favor of glycolysis for pyruvate production, and consequently, decreased intramuscular synthesis of nucleic acids to support myoblast replication (71, 84). In addition, both purine and pyrimidine (e.g., adenosine and cytosine, respectively) levels were lower in the IUGR muscle compared with CON, suggesting that DNA and RNA contents might be lower as a result of slower rates of replication, transcription, and cellular growth. These results indicate that the IUGR muscle is deficient in both energy and growth and develops adaptations to provide substrates (such as alanine, glutamine, and uric acid) to be used as energy substrates globally, and to sustain cellular metabolism and support growth and essential metabolism of other organs, such as the brain or heart. Additionally, future work should focus on determining whether fetal sex plays a role in the adaptation of fetal skeletal muscle energy metabolism to an IUGR environment (22, 23).
Perspectives and Significance
Metabolomics and pathway analysis identified global metabolic changes unique to IUGR fetal hindlimb skeletal muscle. We conclude that IUGR skeletal muscle develops adaptations in an environment of low oxygen and nutrient availability to prioritize alanine and glutamine production to support the growth and metabolism of other organs in the fetus in lieu of protein accretion and growth. The consequent effects on skeletal muscle metabolism result in elevations in uric acid and other ammoniagenic AAs (e.g., glycine). Although this may help to sustain global energy needs, the consequences of reduced protein accretion in the IUGR fetal muscle can be seen in the phenotypes of reduced muscle mass and reduced myofiber size. The most concerning aspect of this phenotype in IUGR fetuses is that their small muscle mass and myofiber number will limit their postnatal hypertrophic muscle growth (26, 33, 41). In addition, the restricted growth of these fetuses not only affects the mass of the skeletal muscle, but also has a serious metabolic cost with more likelihood of developing adult metabolic diseases and cardiovascular events (e.g., diabetes, obesity, coronary disease, etc.) (21, 55). Determining changes in key metabolic pathways in the IUGR fetal skeletal muscle has potential to provide an experimental basis for potential targets for hormonal, nutrient, and oxygen supplements to increase IUGR muscle growth.
GRANTS
This research was supported by the National Institutes of Health Grants R01-HD-079404 (to L. D. Brown), R01-DK-088139 (to P. J. Rozance), and R01-DK-108910 (to S. R. Wesolowski) and by The Center for Women’s Health Research at the University of Colorado School of Medicine (to L. D. Brown).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R.W., W.W.H.J., P.J.R., and L.D.B. conceived and designed research; E.A.G., J.A.R., A.D., and L.D.B. performed experiments; E.I.C., S.R.W., E.A.G., J.A.R., and L.D.B. analyzed data; E.I.C., S.R.W., E.A.G., P.R.B., J.A.R., A.D., W.W.H.J., P.J.R., and L.D.B. interpreted results of experiments; E.I.C., P.R.B., J.A.R., and L.D.B. prepared figures; E.I.C. and L.D.B. drafted manuscript; E.I.C., S.R.W., E.A.G., P.R.B., J.A.R., A.D., W.W.H.J., P.J.R., and L.D.B. edited and revised manuscript; E.I.C., S.R.W., E.A.G., P.R.B., J.A.R., A.D., W.W.H.J., P.J.R., and L.D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank David Caprio, David Goldstrohm, Jenai Kailey, Dan LoTurco, Gates Roe, and Karen Trembler for expert technical assistance.
REFERENCES
- 1.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA; Members of the Florey Adelaide Male Ageing Study . Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism 58: 1013–1022, 2009. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36: 62–67, 1993. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 327: P1077–P1081, 1986. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 4.Basaria S, Bhasin S. Targeting the skeletal muscle-metabolism axis in prostate-cancer therapy. N Engl J Med 367: 965–967, 2012. doi: 10.1056/NEJMcibr1203160. [DOI] [PubMed] [Google Scholar]
- 5.Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol 9: 17–29, 1987. [PubMed] [Google Scholar]
- 6.Brown LD, Hay WW Jr. Impact of placental insufficiency on fetal skeletal muscle growth. Mol Cell Endocrinol 435: 69–77, 2016. doi: 10.1016/j.mce.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW Jr. Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab 296: E56–E63, 2009. doi: 10.1152/ajpendo.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown LD, Rozance PJ, Bruce JL, Friedman JE, Hay WW Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 309: R920–R928, 2015. doi: 10.1152/ajpregu.00197.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW Jr. Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab 303: E352–E364, 2012. doi: 10.1152/ajpendo.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown LD, Thorn SR, O’Meara MC, Lavezzi JR, Rozance PJ. A physiological increase in insulin suppresses muscle-specific ubiquitin ligase gene activation in fetal sheep with sustained hypoglycemia. Physiol Rep 2: e12045, 2014. doi: 10.14814/phy2.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622, 2009. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 12.Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care 41: 158–176, 2011. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carver TD, Quick AA, Teng CC, Pike AW, Fennessey PV, Hay WW Jr. Leucine metabolism in chronically hypoglycemic hypoinsulinemic growth-restricted fetal sheep. Am J Physiol Endocrinol Metab 272: E107–E117, 1997. doi: 10.1152/ajpendo.1997.272.1.E107. [DOI] [PubMed] [Google Scholar]
- 14.Chang TW, Goldberg AL. The origin of alanine produced in skeletal muscle. J Biol Chem 253: 3677–3684, 1978. [PubMed] [Google Scholar]
- 15.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46: W486–W494, 2018. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinformatics 14: 11, 2012. doi: 10.1002/0471250953.bi1411s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3: 19, 2009. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P. Molecular mechanisms of glutamine action. J Cell Physiol 204: 392–401, 2005. doi: 10.1002/jcp.20339. [DOI] [PubMed] [Google Scholar]
- 19.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 57: 325–336, 2017. doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 20.Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to alpha-adrenergic blockade in fetal sheep during late gestation. J Physiol 563: 611–620, 2005. doi: 10.1113/jphysiol.2004.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology 148: 1350–1358, 2007. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- 22.De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol 292: R875–R886, 2007. doi: 10.1152/ajpregu.00430.2006. [DOI] [PubMed] [Google Scholar]
- 23.Donovan EL, Buckels EJ, Hancock S, Smeitink D, Oliver MH, Bloomfield FH, Jaquiery AL. Twin conception in sheep leads to impaired insulin sensitivity and sexually dimorphic adipose tissue and skeletal muscle phenotypes in adulthood. Reprod Sci 24: 865–881, 2017. doi: 10.1177/1933719116670516. [DOI] [PubMed] [Google Scholar]
- 24.Fahey AJ, Brameld JM, Parr T, Buttery PJ. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J Anim Sci 83: 2564–2571, 2005. doi: 10.2527/2005.83112564x. [DOI] [PubMed] [Google Scholar]
- 25.Frank JW, Escobar J, Nguyen HV, Jobgen SC, Jobgen WS, Davis TA, Wu G. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J Nutr 137: 315–319, 2007. doi: 10.1093/jn/137.2.315. [DOI] [PubMed] [Google Scholar]
- 26.Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab 86: 267–272, 2001. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- 27.Garber AJ, Karl IE, Kipnis DM. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem 251: 836–843, 1976. [PubMed] [Google Scholar]
- 28.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr 135, Suppl: 1553S–1556S, 2005. doi: 10.1093/jn/135.6.1553S. [DOI] [PubMed] [Google Scholar]
- 29.Garlick PJ, Fern M, Preedy VR. The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochem J 210: 669–676, 1983. doi: 10.1042/bj2100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg AL, Chang TW. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc 37: 2301–2307, 1978. [PubMed] [Google Scholar]
- 31.Hakvoort TB, He Y, Kulik W, Vermeulen JL, Duijst S, Ruijter JM, Runge JH, Deutz NE, Koehler SE, Lamers WH. Pivotal role of glutamine synthetase in ammonia detoxification. Hepatology 65: 281–293, 2017. doi: 10.1002/hep.28852. [DOI] [PubMed] [Google Scholar]
- 32.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr 4: 409–454, 1984. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 33.Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics 102: E60, 1998. doi: 10.1542/peds.102.5.e60. [DOI] [PubMed] [Google Scholar]
- 34.Holeček M. The BCAA-BCKA cycle: its relation to alanine and glutamine synthesis and protein balance. Nutrition 17: 70, 2001. doi: 10.1016/S0899-9007(00)00483-4. [DOI] [PubMed] [Google Scholar]
- 35.Holeček M. Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition 18: 130–133, 2002. doi: 10.1016/S0899-9007(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 36.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol 26: 3514–3526, 2006. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jelinek J, Jensen A. Catecholamine concentrations in plasma and organs of the fetal guinea pig during normoxemia, hypoxemia, and asphyxia. J Dev Physiol 15: 145–152, 1991. [PubMed] [Google Scholar]
- 38.Jensen CB, Martin-Gronert MS, Storgaard H, Madsbad S, Vaag A, Ozanne SE. Altered PI3-kinase/Akt signalling in skeletal muscle of young men with low birth weight. PLoS One 3: e3738, 2008. doi: 10.1371/journal.pone.0003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones AK, Brown LD, Rozance PJ, Serkova NJ, Hay WW Jr, Friedman JE, Wesolowski SR. Differential effects of intrauterine growth restriction and a hypersinsulinemic-isoglycemic clamp on metabolic pathways and insulin action in the fetal liver. Am J Physiol Regul Integr Comp Physiol 316: R427–R440, 2019. doi: 10.1152/ajpregu.00359.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatr 86: 196–200, 1997. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- 42.Lemons JA, Adcock EW III, Jones MD Jr, Naughton MA, Meschia G, Battaglia FC. Umbilical uptake of amino acids in the unstressed fetal lamb. J Clin Invest 58: 1428–1434, 1976. doi: 10.1172/JCI108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 298: E770–E778, 2010. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limesand SW, Rozance PJ, Brown LD, Hay WW Jr. Effects of chronic hypoglycemia and euglycemic correction on lysine metabolism in fetal sheep. Am J Physiol Endocrinol Metab 296: E879–E887, 2009. doi: 10.1152/ajpendo.90832.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Limesand SW, Rozance PJ, Smith D, Hay WW Jr. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 46.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 47.Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum Dev 81: 745–751, 2005. doi: 10.1016/j.earlhumdev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Maliszewski AM, Gadhia MM, O’Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab 302: E1483–E1492, 2012. doi: 10.1152/ajpendo.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marconi AM, Paolini CL, Stramare L, Cetin I, Fennessey PV, Pardi G, Battaglia FC. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res 46: 114–119, 1999. doi: 10.1203/00006450-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Meier PR, Peterson RG, Bonds DR, Meschia G, Battaglia FC. Rates of protein synthesis and turnover in fetal life. Am J Physiol Endocrinol Metab 240: E320–E324, 1981. doi: 10.1152/ajpendo.1981.240.3.E320. [DOI] [PubMed] [Google Scholar]
- 51.Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci 15: 177–190, 1974. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- 52.Meister A. On the enzymology of amino acid transport. Science 180: 33–39, 1973. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- 53.Milley JR. Ovine fetal leucine kinetics and protein metabolism during decreased oxygen availability. Am J Physiol Endocrinol Metab 274: E618–E626, 1998. doi: 10.1152/ajpendo.1998.274.4.E618. [DOI] [PubMed] [Google Scholar]
- 54.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr 137, Suppl 2: 1602S–1609S, 2007. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 55.Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL, McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol 587: 4199–4211, 2009. doi: 10.1113/jphysiol.2009.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom 31: 663–673, 2017. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odessey R, Khairallah EA, Goldberg AL. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem 249: 7623–7629, 1974. [PubMed] [Google Scholar]
- 58.Oldham WM, Clish CB, Yang Y, Loscalzo J. Hypoxia-mediated increases in L-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab 22: 291–303, 2015. doi: 10.1016/j.cmet.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48: 547–552, 2005. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- 60.Padoan A, Rigano S, Ferrazzi E, Beaty BL, Battaglia FC, Galan HL. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am J Obstet Gynecol 191: 1459–1464, 2004. doi: 10.1016/j.ajog.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 61.Pendleton AL, Humphreys LR, Davis MA, Camacho LE, Anderson MJ, Limesand SW. Increased pyruvate dehydrogenase activity in skeletal muscle of growth-restricted ovine fetuses. Am J Physiol Regul Integr Comp Physiol 317: R513–R520, 2019. doi: 10.1152/ajpregu.00106.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piknova B, Park JW, Swanson KM, Dey S, Noguchi CT, Schechter AN. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 47: 10–16, 2015. doi: 10.1016/j.niox.2015.02.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol 295: R583–R595, 2008. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Umbilical uptakes and transplacental concentration ratios of amino acids in severe fetal growth restriction. Pediatr Res 73: 602–611, 2013. doi: 10.1038/pr.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richman PG, Orlowski M, Meister A. Inhibition of gamma-glutamylcysteine synthetase by L-methionine-S-sulfoximine. J Biol Chem 248: 6684–6690, 1973. [PubMed] [Google Scholar]
- 66.Roe TF, Kogut MD. The pathogenesis of hyperuricemia in glycogen storage disease, type I. Pediatr Res 11: 664–669, 1977. doi: 10.1203/00006450-197705000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Rozance PJ, Zastoupil L, Wesolowski SR, Goldstrohm DA, Strahan B, Cree-Green M, Sheffield-Moore M, Meschia G, Hay WW Jr, Wilkening RB, Brown LD. Skeletal muscle protein accretion rates and hindlimb growth are reduced in late gestation intrauterine growth-restricted fetal sheep. J Physiol 596: 67–82, 2018. doi: 10.1113/JP275230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudman D, Galambos JT, Smith RB III, Salam AA, Warren WD. Comparison of the effect of various amino acids upon the blood ammonia concentration of patients with liver disease. Am J Clin Nutr 26: 916–925, 1973. doi: 10.1093/ajcn/26.9.916. [DOI] [PubMed] [Google Scholar]
- 69.Sales F, Pacheco D, Blair H, Kenyon P, McCoard S. Muscle free amino acid profiles are related to differences in skeletal muscle growth between single and twin ovine fetuses near term. Springerplus 2: 483, 2013. doi: 10.1186/2193-1801-2-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simonetta G, Rourke AK, Owens JA, Robinson JS, McMillen IC. Impact of placental restriction on the development of the sympathoadrenal system. Pediatr Res 42: 805–811, 1997. doi: 10.1203/00006450-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 71.Soto SM, Blake AC, Wesolowski SR, Rozance PJ, Barthel KB, Gao B, Hetrick B, McCurdy CE, Garza NG, Hay WW Jr, Leinwand LA, Friedman JE, Brown LD. Myoblast replication is reduced in the IUGR fetus despite maintained proliferative capacity in vitro. J Endocrinol 232: 475–491, 2017. doi: 10.1530/JOE-16-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 96: 2898–2903, 2011. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 73.Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorn SR, Sekar SM, Lavezzi JR, O’Meara MC, Brown LD, Hay WW Jr, Rozance PJ. A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am J Physiol Regul Integr Comp Physiol 303: R861–R869, 2012. doi: 10.1152/ajpregu.00331.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van den Berghe G, Bontemps F, Vincent MF, Van den Bergh F. The purine nucleotide cycle and its molecular defects. Prog Neurobiol 39: 547–561, 1992. doi: 10.1016/0301-0082(92)90006-Z. [DOI] [PubMed] [Google Scholar]
- 76.van Iterson M, ’t Hoen PA, Pedotti P, Hooiveld GJ, den Dunnen JT, van Ommen GJ, Boer JM, Menezes RX. Relative power and sample size analysis on gene expression profiling data. BMC Genomics 10: 439, 2009. doi: 10.1186/1471-2164-10-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wesolowski SR, Hay WW Jr. Role of placental insufficiency and intrauterine growth restriction on the activation of fetal hepatic glucose production. Mol Cell Endocrinol 435: 61–68, 2016. doi: 10.1016/j.mce.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsén T, Grill V, Gudnason V, Hulman S, Hyppönen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 300: 2886–2897, 2008. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 79.Wilkening RB, Boyle DW, Teng C, Meschia G, Battaglia FC. Amino acid uptake by the fetal ovine hindlimb under normal and euglycemic hyperinsulinemic states. Am J Physiol Endocrinol Metab 266: E72–E78, 1994. doi: 10.1152/ajpendo.1994.266.1.E72. [DOI] [PubMed] [Google Scholar]
- 80.Wood T. Physiological functions of the pentose phosphate pathway. Cell Biochem Funct 4: 241–247, 1986. doi: 10.1002/cbf.290040403. [DOI] [PubMed] [Google Scholar]
- 81.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 37: 1–17, 2009. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 82.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics 55: 14.10.1–14.10.91, 2016. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 83.Xia Y, Warshaw JB, Haddad GG. Effect of chronic hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am J Physiol Regul Integr Comp Physiol 273: R1734–R1741, 1997. doi: 10.1152/ajpregu.1997.273.5.R1734. [DOI] [PubMed] [Google Scholar]
- 84.Yates DT, Cadaret CN, Beede KA, Riley HE, Macko AR, Anderson MJ, Camacho LE, Limesand SW. Intrauterine growth-restricted sheep fetuses exhibit smaller hindlimb muscle fibers and lower proportions of insulin-sensitive Type I fibers near term. Am J Physiol Regul Integr Comp Physiol 310: R1020–R1029, 2016. doi: 10.1152/ajpregu.00528.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yates DT, Green AS, Limesand SW. Catecholamines mediate multiple fetal adaptations during placental insufficiency that contribute to intrauterine growth restriction: lessons from hyperthermic sheep. J Pregnancy 2011: 1–9, 2011. doi: 10.1155/2011/740408. [DOI] [PMC free article] [PubMed] [Google Scholar]