Abstract
Doxorubicin (DOX) is a highly effective antitumor agent used for the treatment of a wide range of cancers. Unfortunately, DOX treatment results in cytotoxic side effects due to its accumulation within off-target tissues. DOX-induced cellular toxicity occurs as a result of increased oxidative damage, resulting in apoptosis and cell death. While there is no standard-of-care practice to prevent DOX-induced toxicity to healthy organs, exercise has been shown to prevent cellular dysfunction when combined with DOX chemotherapy. Endurance exercise stimulates numerous biochemical adaptations that promote a healthy phenotype in several vulnerable tissues without affecting the antineoplastic properties of DOX. Therefore, for the development of an effective strategy to combat the pathological effects of DOX, it is important to determine the appropriate exercise regimen to prescribe to cancer patients receiving DOX therapy and to understand the mechanisms responsible for exercise-induced protection against DOX toxicity to noncancer cells. This review summarizes the cytotoxic effects of DOX on the heart, skeletal muscle, liver, and kidneys and discusses the current understanding of the clinical benefits of regular physical activity and the potential mechanisms mediating the positive effects of exercise on each organ system.
Keywords: Adriamycin, anthracycline, antioxidants, chemotherapy, mitochondria
INTRODUCTION
Doxorubicin (DOX), an anthracycline antibiotic, is used in the treatment of a broad spectrum of human cancers, including acute leukemia, lymphomas, stomach, breast, and ovarian cancers, Kaposi’s sarcoma, and bone tumors (78). Unfortunately, the clinical use of this highly efficacious anticancer drug is limited because of its toxicity. DOX administration results in dose-dependent, irreversible side effects, several of which include the development of cardiomyopathy, dyspnea, exercise intolerance, hepatotoxicity, and nephropathy (7, 21, 26, 31, 67, 89). While work has been done to identify risk factors, develop less-toxic derivatives, and detect subclinical toxicity earlier, there is no consensus on the best approach to prevent anthracycline-induced cytotoxicity (118). Therefore, further advances in the molecular basis of DOX-induced pathology are needed to generate preventative strategies.
It is well established that exercise training provides robust protection during a variety of injurious conditions, and emerging evidence demonstrates that exercise is a protective therapy for patients undergoing DOX treatment (21, 69, 103, 105). This review presents the current understanding of the effects of exercise on the health of multiple tissues affected by DOX chemotherapy and existing information regarding the current understanding of the mechanisms responsible for exercise-induced protection against DOX-induced toxicity. This review begins with a brief summary of the cellular events leading to DOX-induced pathology followed by a broad description of the current understanding of the effects of exercise training on cardiac muscle, skeletal muscle, liver, and kidney function.
DOXORUBICIN: MODE OF ACTION
DOX (clinically known as Adriamycin) is a potent broad-spectrum chemotherapeutic agent, the antineoplastic effects of which are mediated primarily through direct interactions with DNA. Specifically, DOX has been demonstrated to inhibit tumor cell proliferation by 1) intercalation into DNA and inhibition of macromolecular biosynthesis, 2) interference with topoisomerase II and prevention of DNA replication, 3) inhibition of helicase and promotion of DNA unwinding, and 4) production of free radicals, resulting in damage to DNA and cellular membranes (114). Despite the efficacy of DOX as a chemotherapeutic agent, the clinical use of DOX in patients is generally discontinued once the total administered dose exceeds 550 mg/m2 body surface area, as a result of the drastic increase in the risk of irreversible cytotoxicity to healthy, noncancerous tissues (119).
DOX-INDUCED TOXICITY TO NONCANCER CELLS
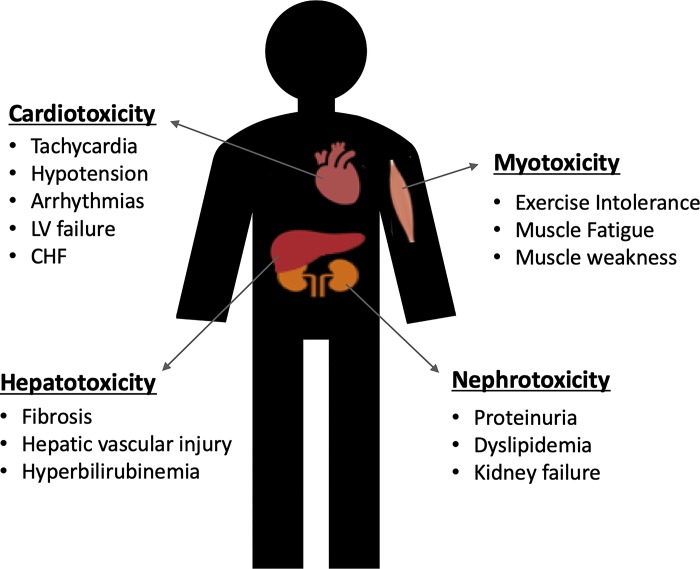
DOX chemotherapy is often administered to patients via three unique routes. Most commonly, DOX is given systemically by intravenous infusion (7). In addition, to treat ovarian cancer and peritoneal carcinomatosis, DOX is administered by intraperitoneal injection to target the abdominal cavity (27). Finally, in cases of unresectable limb tumors, soft tissue sarcoma, and melanoma, isolated limb perfusion may be used (45). Regardless of the route of administration, DOX has debilitating effects on healthy tissues as a result of nonspecific targeting of rapidly dividing cells. These cytotoxic effects are primarily responsible for the severe limitations in the use of this highly effective antineoplastic agent. Indeed, DOX has been demonstrated to have debilitating effects on a wide variety of tissues, including cardiac muscle, skeletal muscle, liver, and kidney (Fig. 1).
Fig. 1.
Potential side effects of doxorubicin chemotherapy on heart, skeletal muscle, liver, and kidneys. CHF, congestive heart failure; LV, left ventricle.
DOX-Induced Cardiotoxicity
The risk of cardiotoxicity is one of the greatest limiting factors to the clinical use of DOX. Indeed, repetitive administration of DOX is associated with the dose-dependent development of cardiomyopathy and heart failure. The effects of DOX on the heart vary between patients and can result in both acute and chronic cardiovascular events. Acute effects of DOX can develop within minutes to days after administration and normally manifest as chest pain as a result of hypotension, left ventricular failure, tachycardia, and various arrhythmias (12). The mechanisms for these immediate changes are not fully understood but are often transient and reversible in patients. Indeed, it is hypothesized that the acute effects of DOX on the heart occur as a result of reversible myocardial edema (112). It is estimated that ~11% of patients receiving DOX treatment will experience these acute symptoms of cardiac dysfunction (111, 112). The incidence of chronic cardiovascular effects after DOX administration is much lower (~1.7%) as a result of limiting the dose of DOX given to patients (119). Specifically, an analysis of the incidence of DOX cardiotoxicity estimated that the occurrence of cardiomyopathy increases from 4% to 36% when the administered dose of DOX increases from 500 to 600 mg/m2 (74). Patients presenting with chronic DOX cardiotoxicity normally demonstrate symptoms within 30 days after cessation of treatment; however, cardiomyopathy has been demonstrated 6–10 yr after the last dose of DOX is administered (12). The chronic effects of DOX include the insidious onset of cardiomyopathy, which often leads to congestive heart failure. Heart failure in DOX-treated patients results in systemic complications that can affect the health of many organs. Indeed, variation in adequate organ perfusion as a result of reduced cardiac output and hemodynamic disturbances is associated with skeletal muscle atrophy, liver failure, and chronic kidney disease (CKD) in heart failure patients (49, 80, 116). Furthermore, the development of DOX-induced congestive heart failure is extremely harmful, with ~50% mortality in patients (119).
DOX-Induced Skeletal Muscle Toxicity
Fatigue affects >75% of patients undergoing cancer treatment and is the most commonly reported symptom of patients undergoing chemotherapy (110). Fatigue is reported to cause extreme distress due to its negative impact on the activities of daily living, reduced quality of life, and increased morbidity and mortality (51, 86, 117). In cancer patients, fatigue can manifest as a perceived tiredness with a lack of energy and as physical weakness with a decrease in muscle strength (41, 86, 110). These disconcerting symptoms can result in discontinued or delayed treatment and often persist for years following chemotherapy treatment (31, 41, 115).
Preclinical models demonstrating the effects of DOX on skeletal muscle have established that severe myopathy occurs after treatment, with no preferential deficits demonstrated between fiber types (85). These models have described DOX-induced skeletal muscle weakness as defined by impaired muscle relaxation and contraction, reduction in skeletal muscle force production and maximal twitch force, and muscle fiber atrophy as early as 2 days following DOX exposure (38–40, 85). These findings are corroborated in the clinic, as reports have described lower-extremity weakness and loss of functional performance and muscle mass in patients after DOX chemotherapy (31, 103, 115). Locoregional DOX therapy in the form of hyperthermic isolated limb perfusion to treat limb sarcoma tumors has also been demonstrated to result in muscle dysfunction and atrophy, potentially due to neurotoxicity and the development of neuromuscular damage (8, 95). Remarkably, DOX can also be used clinically as a chemomyectomy agent to treat facial spasms localized to the eyelids. In these conditions, DOX is injected directly into the muscle to induce permanent skeletal muscle necrosis and decreased muscle mass to relieve involuntary muscle spasms (82, 83, 124).
DOX-Induced Hepatotoxicity
The liver plays a key role in the metabolism and elimination of DOX. Indeed, while DOX is taken up by many tissues, it appears to be rapidly taken up by the liver, where it accumulates before metabolism and elimination (71, 73). While ~50% of all DOX is unmodified before removal from the body (64), it is broken down in several ways. The majority of DOX is metabolized in the liver by a two-electron reduction to the metabolite doxorubicinol; this process is facilitated primarily by the hepatic reducing enzyme carbonyl reductase 1 (67, 114). In addition, DOX can undergo a one-electron reduction by NADPH cytochrome P-450 reductase to form a semiquinone free radical (6, 114). Finally, detoxification of reactive DOX metabolites can occur via hepatic microsomal reductases to form deoxyglycones (3, 71).
The metabolism of a high volume of DOX results in toxic effects to the liver: an estimated 40% of individuals undergoing treatment incur some form of liver injury (23, 35). In patients receiving DOX, liver injury has been classified by elevated levels of aminotransferases, hyperbilirubinemia, and hepatic vascular injury (4, 23, 63); in rodent models of DOX hepatotoxicity, liver damage presents as hepatocyte degeneration, parenchymal necrosis, proliferation of the biliary duct, thrombosis of the central vein, and liver fibrosis (84, 126).
In addition to direct DOX-induced hepatotoxicity, patients with preexisting liver disease are at a greater risk for DOX-induced toxicity as a result of elevated systemic concentrations of DOX. The rate of hepatic extraction from the circulation is determined by hepatic blood flow, hepatocyte mass, and hepatic function (71). In general, liver disease is associated with hepatocyte dysfunction and delayed excretion and elevated plasma concentrations of DOX, causing increased systemic toxicity and deterioration of liver function (35, 71). Importantly, it is recommended that patients identified to have abnormal liver function (as assessed by bilirubin levels) receive reduced dosages of DOX depending on the severity of the impairment (35, 71).
DOX-Induced Nephrotoxicity
While the majority (~40–50%) of DOX and its metabolites are excreted through bile, the kidneys also play a role in DOX excretion from the body (72). Specifically, metabolized DOX accumulates in the kidneys, and ~4–8% of administered DOX is slowly excreted through urine over several days (72, 113). Although the accretion of DOX within the kidneys is necessary for its elimination, renal failure has been demonstrated in patients undergoing Adriamycin chemotherapy (10, 44, 100). Indeed, the necessity to closely monitor kidney function in patients was first established in 1977 in a case study demonstrating that DOX therapy was associated with renal failure (10). DOX can cause nephropathy in cancer patients for a variety of reasons. For example, DOX-induced tumor lysis and collection within the kidneys can result in the increased deposition of potassium, phosphate, and uric acid into the renal tubules, as well as deposition of fibrin thrombi within the glomeruli and the need for maintenance dialysis (44, 100).
In addition to the findings within the patient population, it is well established that DOX causes renal failure in experimental animals, and DOX therapy is commonly used as a rodent model of CKD because of the rapid occurrence (within days) of renal injury following drug administration (13, 72, 96). Specifically, studies demonstrate that DOX induces direct toxicity to the kidneys, resulting in damage to the glomerulus, tubulointerstitial inflammation and fibrosis, podocyte effacement and elevated serum creatinine, reduced creatinine clearance, reduced serum albumin, dyslipidemia, and proteinuria (13, 42, 46, 60, 96, 121).
MECHANISMS OF DOX-INDUCED CYTOTOXICITY
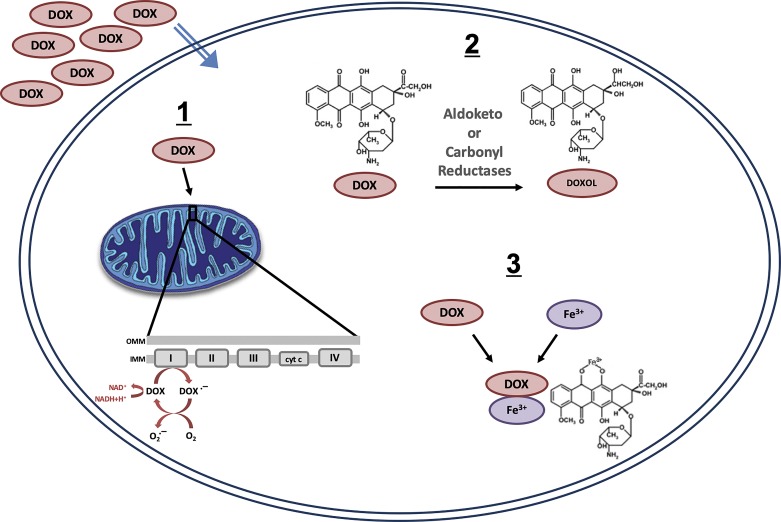
While the mechanisms responsible for DOX-induced toxicity to nontumor cells are not well understood, evidence suggests that the primary mediator of DOX toxicity is the induction of oxidative stress (30). There are several ways in which DOX can produce reactive oxygen species (ROS) (Fig. 2). First, a one-electron reduction in the DOX quinone structure leads to the formation of a semiquinone free radical intermediate. The unpaired electron of the semiquinone moiety can be donated to oxygen to form superoxide radicals (24). Second, metabolism of DOX via cleavage of the sugar residue and reduction in the carbonyl group at C-13 to produce doxorubicinol also results in ROS generation through redox cycling and increased levels of free iron (16). Third, DOX can generate ROS through a direct interaction with iron or other metal ions. Specifically, DOX can form a complex with iron that is capable of reacting with O2 or H2O2 to produce free radicals (48, 76). Superoxide and its decomposition products can initiate lipid peroxidation and cause injury to healthy tissue.
Fig. 2.
Primary mechanisms of doxorubicin (DOX)-induced reactive oxygen species production: 1-electron reduction in the DOX quinone structure (1), metabolic breakdown of DOX (2), and direct interaction of DOX with iron and/or other metal ions (3). IMM and OMM, inner and outer membranes of mitochondria, respectively.
While multiple potential sources of ROS exist, evidence indicates that the mitochondria are a significant source of oxidant production following DOX administration (120). Specifically, it is well established that DOX accumulates in the mitochondria because of its high affinity for cardiolipin, a phospholipid expressed on the inner mitochondrial membrane (65, 120). The complex that DOX forms with cardiolipin places it in close proximity to the electron transport chain, and redox cycling of DOX is mediated through its interaction with NADH dehydrogenase (24, 30). Elevated free radical production can lead to several damaging events in the mitochondria that, taken as a whole, can drastically diminish mitochondrial function and result in cell death (120).
In addition to the ability of DOX to damage mitochondria as a result of futile redox cycling, emerging evidence suggests that DOX-induced toxicity may be mediated by an interaction between DOX and topoisomerase IIβ (127). Specifically, it was demonstrated that genetic deletion prevented DOX cytotoxicity. Mechanistically, this protection occurred as a result of inhibition of DOX-induced mitochondrial dysfunction that occurs as a result of topoisomerase IIβ-induced activation of apoptosis. This was sufficient to elicit alterations in the transcriptome that increases mitochondrial ROS production and reduces the expression of genes necessary for mitochondrial biogenesis (127).
There are no clinically approved therapeutics to combat DOX-induced toxicity. However, as a nonpharmacological treatment, exercise is recommended at all points following cancer diagnosis (104). Indeed, exercise training has been demonstrated to have cytoprotective effects against DOX toxicity and may be a vital tool in the development of countermeasures to protect against the toxic effects.
EXERCISE PROTECTS AGAINST DOX TOXICITY
In 1979 Combs and colleagues provided the first evidence that exercise training elicits therapeutic effects that counteract the toxicity of DOX (20). Specifically, it was reported that an acute 30-min bout of swimming was sufficient to increase the survival rate of mice treated with Adriamycin (20). This finding was significant at the time, because it opposed the popular belief that exercise training would exacerbate the toxic effects of DOX as a result of increased ROS production and mitochondrial dysfunction. This initial finding has led to an abundance of work dedicated to determining the precise mechanisms by which exercise can reduce the off-target effects of DOX and to several clinical trials aimed at assessing the safety and efficacy of exercise as an adjuvant therapy for cancer patients receiving DOX chemotherapy. The following sections highlight some exciting findings within the patient population and detail what is currently known about the mechanisms for exercise protection.
CYTOPROTECTIVE EFFECTS OF EXERCISE
Heart
In 1983 reports detailing the first evidence that DOX enhances free radical damage to the heart in a dose-dependent manner led to subsequent investigation into the effects of exercise training on cardiac muscle morphology (24, 66). Kanter et al. exposed mice to a 21-wk training period and a 40 mg/kg cumulative dose of DOX (66). Assessment of cardiac muscle morphology following completion of the training period revealed significantly less cardiac damage in the exercise-trained than the drug-treated sedentary animals, leading to the conclusion that chronic aerobic exercise in combination with DOX chemotherapy elicits positive cardiac adaptations (66). Future studies support this work and have elucidated numerous preclinical endurance exercise paradigms that preserve cardiac function following DOX treatment. Specifically, both acute (75, 125) and chronic (17, 19, 55, 56, 75, 125) exercise preconditioning have been shown to ameliorate DOX-induced cardiac dysfunction, while further work has demonstrated that aerobic exercise initiated simultaneously with DOX treatment can also preserve cardiac function (18, 29, 54).
The majority of reports have focused on the beneficial effects of endurance exercise training to mitigate DOX cardiomyopathy. However, Pfannenstiel et al. (94) recently demonstrated that 12 wk of progressive resistance training before DOX treatment reduced oxidative stress in the heart and preserved cardiac function in a preclinical model. This finding is significant, because resistance exercise training is an important component of rehabilitation for patients with coronary artery disease to increase muscle strength and reduce perceived exertion (81), and recently it was demonstrated that resistance training may be equally beneficial in cancer rehabilitation (107, 123). Moreover, breast cancer patients who participated in supervised resistance training during chemotherapy reported improvements in quality of life compared with those who participated in endurance training and sedentary interventions (101).
Based on the numerous preclinical reports demonstrating exercise-induced cardioprotection against DOX toxicity, clinical trials investigating the effects of exercise on cardiac health in patients receiving DOX treatment are ongoing to determine safety, efficacy, and appropriate exercise prescriptions for cancer patients during treatment and rehabilitation following treatment. In 2003 Courneya et al. (21) completed a randomized controlled trial to determine the effects of a 15-wk exercise-training protocol following surgery, radiotherapy, and/or chemotherapy on postmenopausal breast cancer survivors, some of whom received DOX as part of their chemotherapy regimen. This study demonstrated improved cardiopulmonary function in the exercise-trained group compared with the nontrained group, which correlated to improvements in perceived quality of life (21). Subsequently, both a case study and a phase II randomized trial demonstrated that aerobic training in breast cancer patients is safe and well tolerated at appropriate levels (25, 52). Importantly, both studies also revealed a significant improvement in peak O2 uptake, which is significant because reduced fitness capacity is a predictor of anthracycline-induced left ventricular dysfunction and cardiovascular disease risk in breast cancer patients (25, 52). Furthermore, exercise-based rehabilitation in a group of 15 cancer survivors was shown to support the current physical activity guidelines for cancer survivors, as a 10-wk exercise intervention reduced fatigue and systemic oxidative stress (98). Finally, studies are ongoing and continue to evaluate the feasibility of exercise interventions in the prevention of DOX-induced cardiotoxicity (69).
Skeletal Muscle
Growing evidence supports the notion that participation in regular bouts of physical activity improves muscular strength, reduces fatigue, and improves quality of life in cancer survivors (104). Importantly, exercise programs initiated at any point following diagnosis can provide important benefits to cancer patients, and current exercise prescriptions exist to promote the safety and efficacy of exercise independent of the disease stage (104). Unfortunately, it has been reported that 80% of cancer care providers are unaware of the exercise guidelines for patients and, thus, do not implement them or provide necessary information to patients (88, 104). In fact, advice from oncology caregivers often suggests rest following treatment, which is now believed to promote muscle weakness and reduced functional capacity (102).
While past studies have assessed the efficacy of regular physical activity to reduce fatigue and improve quality of life following adjuvant therapy for breast cancer, the majority of which included DOX chemotherapy, Schwartz et al. (105) were the first to directly measure muscle strength. Patients from this randomized clinical trial were assigned to aerobic exercise, resistance exercise, or usual care, with exercise testing at the beginning of chemotherapy and again in 6 mo. Results from this trial demonstrated a significant increase in 12-min walk distance, greater muscle strength for single repetitions of the seated row and leg extension, and increased aerobic capacity in the aerobic exercise group compared with the other groups (105).
In addition to the work done in the patient population, preclinical animal studies focusing on DOX-induced skeletal muscle dysfunction have demonstrated that both short-term (2 wk) and long-term (10 wk) endurance exercise preconditioning programs and resistance exercise preconditioning are sufficient to protect against skeletal muscle atrophy, contractile dysfunction, and muscular fatigue in animals receiving a bolus dose of DOX (9, 87). However, while DOX-induced skeletal muscle dysfunction occurs independent of muscle fiber type composition, exercise appears to preferentially protect slow-twitch (soleus) and mixed (diaphragm) muscles compared with fast-twitch (extensor digitorum longus) muscles (9, 87). Finally, a recent study demonstrated that interval training throughout a chronic DOX dosing protocol was also sufficient to prevent DOX-induced atrophy of the soleus muscle (28). Therefore, exercise can protect against skeletal muscle atrophy and weakness when initiated before or at the start of DOX treatment.
Liver
Although evidence indicates that exercise has no detrimental effects on patients with liver disease, physical activity is often restricted because of the proposed risk of increased portal pressures, bleeding, or hepatic encephalopathy (5, 37, 77, 99). However, a recent systematic literature review elucidating the efficacy of exercise interventions on patients with cirrhosis concluded that exercise in liver failure patients should be considered safe and potentially beneficial (77). In addition, exercise training may also prevent liver disease in populations at risk for the development of fatty liver disease (108). Currently, the potential benefits of exercise on DOX-induced liver toxicity remain to be elucidated in the patient population.
Significantly, preclinical studies have demonstrated that aerobic exercise training can modify DOX-induced hepatotoxicity and prevent DOX-induced maladaptations to the liver. Specifically, preconditioning protocols utilizing treadmill exercise ranging from 6 wk to 5 days before DOX administration provided valuable modifications to hepatocyte signaling (1, 2, 50, 79, 128). These studies demonstrate improvements in oxidative stress signaling, mitochondrial function, iron dysregulation, and insulin-like growth factor signaling and support the inclusion of exercise training as an advantageous therapy to preclude DOX hepatotoxicity (1, 2, 50, 79, 128).
Kidneys
Pathological changes within the kidneys following DOX administration strongly resemble those observed in patients with chronic renal disease (89), and guidelines for management of CKD now recommend regular physical activity (59, 122). The physiological gains from exercise in patients with CKD extend beyond improvements in kidney function; they also include decreased systemic inflammation, increased muscular strength, and improved cardiovascular health (70, 122). In addition to improving the symptoms of kidney disease, participation in regular physical activity is associated with a reduced risk of developing CKD. Indeed, in a cohort of 17,979 patients, fitness level was inversely related to the risk of developing CKD (26). This finding is confirmed by several studies demonstrating that lack of regular exercise is a factor associated with the highest risk for proteinuria (36, 90). Therefore, exercise has the potential to reduce the incidence of CKD, reduce complications associated with chronic kidney dysfunction, and improve quality of life.
Although exercise is a protective strategy for the prevention and management of progressive renal dysfunction, few studies have investigated the effects of exercise on DOX-induced nephropathy. Only preclinical animal studies of DOX nephrotoxicity have reported on the effects of exercise on kidney health, with results from these studies predominantly demonstrating beneficial outcomes. Specifically, Robert Peng and colleagues demonstrated duration-dependent effects of swimming and treadmill exercise training on kidney function (14, 15, 92, 93). Indeed, DOX (7.5 mg/kg) administered before initiation of a 9-wk swim-training program (3 days/wk for 30 or 60 min/day) showed reductions in renal edema and inflammation, with the 60-min/day program providing improved restoration of kidney morphology compared with the 30-min/day program (92). In a follow-up to this study, 60 min/day of swimming exercise for 9 wk effectively improved glomerular filtration rate, reduced blood urea nitrogen, and increased urine creatinine clearance rate (14). Similar results were demonstrated between 30 and 60 min of treadmill exercise (3 days/wk for 11 wk) (14, 15, 93): the 60-min/day regimen elicited greater protective effects against DOX-induced CKD. Finally, reports have also demonstrated a protective effect against kidney dysfunction when exercise is performed before DOX administration (2.5 mg/kg) (32) and when it is initiated 2 wk following a 7-wk dosing protocol (2 mg·kg−1·wk−1) (11).
MECHANISM OF EXERCISE-INDUCED PROTECTION AGAINST DOX TOXICITY
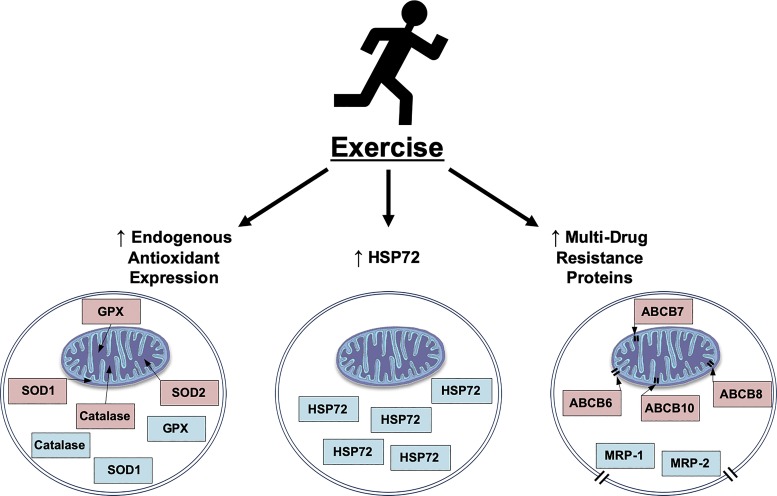
While it is well established that aerobic exercise training promotes cellular adaptations necessary to preclude DOX-induced toxicity, the mechanisms responsible for this cytoprotection remain unclear. Numerous signaling pathways have been hypothesized to promote the exercise-induced reduction in noncancerous tissue toxicity (Fig. 3).
Fig. 3.
Potential mechanisms responsible for exercise-induced cytoprotection against doxorubicin cellular toxicity include increased endogenous antioxidant enzyme expression, increased heat shock protein 72 (HSP72) expression, and/or increased multidrug resistance protein (MRP) expression. GPX, glutathione peroxidase; SOD, superoxide dismutase; ABCB, ATP-binding cassette transporter subfamily B.
Endogenous Antioxidants
The discovery that DOX can undergo redox cycling within the mitochondria to induce free radical production led to the first investigation into the effects of exercise training on endogenous antioxidant enzyme expression in DOX-treated animals (66). The study hypothesized that exercise could prevent DOX toxicity by inducing an increase in the activity of endogenous antioxidant enzymes, which would augment the oxidant-buffering capacity. This notion was supported by the finding that swim training in mice was sufficient to increase antioxidant enzyme activity in the heart and liver, which was maintained in swim-trained animals treated with DOX (66). Interestingly, further evaluation of endogenous antioxidant enzyme expression in the heart and liver following exercise and DOX administration has yielded mixed results. In the heart, several reports have revealed improved cardiac function following exercise independent of exercise-induced enhancement of antioxidant protein expression, leading to the possibility that increased antioxidant expression is not required for exercise-induced cardioprotection (17, 18, 62, 125). In contrast, exercise training induces an increase in antioxidant enzyme expression in the liver regardless of the exercise program (1, 128). Evidence for the requirement of exercise-induced antioxidant expression to prevent skeletal muscle dysfunction is mixed, as conflicting reports exist as to whether exercise induces antioxidant expression in skeletal muscle and, to date, reports that have evaluated skeletal muscle functional properties have not assessed antioxidant enzyme level (53, 109). Finally, increased antioxidant expression appears to facilitate the exercise-induced reduction in renal dysfunction, as long-term endurance exercise training was shown to protect against kidney dysfunction as a result of increased antioxidant expression (14).
Heat Shock Protein 72
Induction of heat shock protein (HSP) expression is a well-documented effect of endurance exercise training that can assist in the folding of damaged proteins, act as protein chaperones, and reduce cellular proteolysis (33, 106). HSP72, the highly conserved 72-kDa family member, has been shown to be upregulated following exercise in multiple organs, including the liver, kidneys, skeletal muscle, and heart (34, 47). The effects of combining DOX treatment with exercise training on HSP72 expression in the kidneys are unknown. However, significant increases in HSP72 expression have been reported in the liver, skeletal muscle, and cardiac muscle when exercise is combined with DOX treatment (1, 18, 68, 109).
While the necessity for exercise-induced HSP72 expression in the liver and skeletal muscle have not been elucidated, reports by Chicco et al. and Kavazis et al. revealed that HSP72 expression is not required for exercise-induced cardioprotection against DOX toxicity (17, 68). Indeed, using a training protocol designed to mimic walking exercise shown to benefit cancer patients during treatment, Chicco et al. (17) showed that low-intensity treadmill exercise during chronic DOX administration is sufficient to attenuate left ventricular dysfunction. However, this exercise-training protocol did not induce an increase in cardiac expression of HSP72. In addition, Kavazis et al. (68) discovered that inhibiting the exercise-induced expression of HSP72 in the heart by training animals in a cold environment abolished the protective effects of exercise against DOX-induced oxidative stress and mitochondrial dysfunction. Therefore, HSP72 expression does not appear to be required for exercise-induced cardioprotection against DOX toxicity. However, further work is needed to elucidate the role of HSP72 in the skeletal muscle, liver, and kidneys.
Multidrug Resistance Proteins
DOX administration results in nonspecific tissue accumulation, causing its buildup in noncancerous cells (65). After DOX enters the cell, its retention is mediated by the expression of drug efflux proteins on the cellular membrane (22). While the effects of exercise on liver and kidney accumulation of DOX are unknown, recent evidence revealed that exercise training is sufficient to reduce the cardiac and skeletal muscle accumulation of DOX (43, 61, 87, 91, 97). The ATP-binding cassette (ABC) proteins are a class of transporters that are capable of extruding xenobiotics from the cell, and previous reports have demonstrated that increased activity is sufficient to increase the expression of specific family members within the heart and skeletal muscle (87, 91, 97). Specifically, Parry and Hayward (91) demonstrated that 12 wk of voluntary wheel running resulted in a significant induction of the cardiac protein expression of the ABC transporters multidrug resistance proteins (MRP)-1 and MRP-2, which coincided with a significant reduction in left ventricle DOX accumulation. Similar to this finding, Quinn et al. (97) showed a significant reduction in DOX accumulation in the left ventricle following 10 wk of voluntary wheel running. However, in this study, protein expression of MRP-1, MRP-2, and MRP-7 was elevated in both the sedentary DOX-treated animals and the wheel-running animals, suggesting that exercise-induced increases in these proteins are not responsible for the exercise-induced reduction in DOX accumulation in the left ventricle (97). Additionally, this study also reported a significant reduction in DOX accumulation within the soleus muscle, and this decline was not associated with any change in ABC transporter protein expression (97).
A recent report by Morton et al. (87) showed that DOX accumulation is reduced not only within the cardiac and skeletal muscle but, specifically, from within the mitochondrial fraction of each of these tissues. In addition, the cardiac and diaphragm expression of the four mitochondria-localized ABC transporters was increased with exercise training (87). Therefore, the exercise-induced reduction in DOX accumulation in muscle may be due to increased expression of ABCB6, ABCB7, ABCB8, and/or ABCB10. Significantly, independent of exercise, in cardiac muscle, Ichikawa et al. demonstrated that overexpression of ABCB8 protein is sufficient to protect against cardiac DOX accumulation and cardiac dysfunction (57, 58). Therefore, these mitochondrial ABC transporters may play an important role in mediating exercise-induced cytoprotection against DOX toxicity. Further work is needed to elucidate the contribution of each mitochondrial ABC transporter to exercise-induced protection.
CONCLUSIONS
Given the effectiveness of DOX against a wide spectrum of solid tumors and hematological malignancies, it is important to find a countermeasure to protect against DOX-induced cytotoxicity. While it is established that DOX can induce cellular toxicity through the generation of mitochondrial ROS production, there are no suitable therapeutic approaches to combat DOX toxicity. Given the abundance of reports indicating that exercise can protect against DOX toxicity and that exercise training produces beneficial adaptations to multiple organ systems, it is necessary to elucidate a safe and effective exercise protocol for patients receiving DOX chemotherapy. In addition, continuing work is needed to elucidate the mechanisms responsible for exercise-induced cytoprotection to help develop pharmacological therapeutics to combat the toxic effects of DOX.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.S. drafted manuscript; A.J.S. edited and revised manuscript; A.J.S. approved final version of manuscript.
REFERENCES
- 1.Ahmadian M, Dabidi Roshan V, Leicht AS. Age-related effect of aerobic exercise training on antioxidant and oxidative markers in the liver challenged by doxorubicin in rats. Free Radic Res 52: 775–782, 2018. doi: 10.1080/10715762.2018.1470328. [DOI] [PubMed] [Google Scholar]
- 2.Alishahi A, Roshan VD, Hedayyati M. Pretreatment effects of regular aerobic training on the IGF system and hepatotoxicity induced by doxorubicin in rats. Asian Pac J Cancer Prev 14: 7427–7431, 2013. doi: 10.7314/APJCP.2013.14.12.7427. [DOI] [PubMed] [Google Scholar]
- 3.August DA, Verma N, Vaertan MA, Shah R, Brenner DE. An evaluation of hepatic extraction and clearance of doxorubicin. Br J Cancer 72: 65–71, 1995. doi: 10.1038/bjc.1995.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avilés A, Herrera J, Ramos E, Ambriz R, Aguirre J, Pizzuto J. Hepatic injury during doxorubicin therapy. Arch Pathol Lab Med 108: 912–913, 1984. [PubMed] [Google Scholar]
- 5.Bandi JC, García-Pagán JC, Escorsell A, François E, Moitinho E, Rodés J, Bosch J. Effects of propranolol on the hepatic hemodynamic response to physical exercise in patients with cirrhosis. Hepatology 28: 677–682, 1998. doi: 10.1002/hep.510280312. [DOI] [PubMed] [Google Scholar]
- 6.Berlin V, Haseltine WA. Reduction of Adriamycin to a semiquinone-free radical by NADPH cytochrome P-450 reductase produces DNA cleavage in a reaction mediated by molecular oxygen. J Biol Chem 256: 4747–4756, 1981. [PubMed] [Google Scholar]
- 7.Bielack SS, Erttmann R, Winkler K, Landbeck G. Doxorubicin: effect of different schedules on toxicity and anti-tumor efficacy. Eur J Cancer Clin Oncol 25: 873–882, 1989. doi: 10.1016/0277-5379(89)90135-1. [DOI] [PubMed] [Google Scholar]
- 8.Bonifati DM, Ori C, Rossi CR, Caira S, Fanin M, Angelini C. Neuromuscular damage after hyperthermic isolated limb perfusion in patients with melanoma or sarcoma treated with chemotherapeutic agents. Cancer Chemother Pharmacol 46: 517–522, 2000. doi: 10.1007/s002800000175. [DOI] [PubMed] [Google Scholar]
- 9.Bredahl EC, Pfannenstiel KB, Quinn CJ, Hayward R, Hydock DS. Effects of exercise on doxorubicin-induced skeletal muscle dysfunction. Med Sci Sports Exerc 48: 1468–1473, 2016. doi: 10.1249/MSS.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 10.Burke JF Jr, Laucius JF, Brodovsky HS, Soriano RZ. Doxorubicin hydrochloride-associated renal failure. Arch Intern Med 137: 385–388, 1977. doi: 10.1001/archinte.1977.03630150079022. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso DF, Coriolano HA, Duarte JA. Regular voluntary running has favorable histological effects on doxorubicin-induced kidney toxicity in Wistar rats. Cell Tissue Res 374: 177–187, 2018. doi: 10.1007/s00441-018-2840-z. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology 115: 155–162, 2010. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, Lee WH. Experimental focal segmental glomerulosclerosis in mice. Nephron 78: 440–452, 1998. doi: 10.1159/000044974. [DOI] [PubMed] [Google Scholar]
- 14.Chen KC, Hsieh CL, Peng CC, Peng RY. Exercise rescued chronic kidney disease by attenuating cardiac hypertrophy through the cardiotrophin-1 → LIFR/gp 130 → JAK/STAT3 pathway. Eur J Prev Cardiol 21: 507–520, 2014. doi: 10.1177/2047487312462827. [DOI] [PubMed] [Google Scholar]
- 15.Chen KC, Peng CC, Hsieh CL, Peng RY. Exercise ameliorates renal cell apoptosis in chronic kidney disease by intervening in the intrinsic and the extrinsic apoptotic pathways in a rat model. Evid Based Complement Alternat Med 2013: 1–13, 2013. doi: 10.1155/2013/368450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St. Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv 7: 147–156, 2007. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 17.Chicco AJ, Hydock DS, Schneider CM, Hayward R. Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol (1985) 100: 519–527, 2006. doi: 10.1152/japplphysiol.00148.2005. [DOI] [PubMed] [Google Scholar]
- 18.Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol 47: 182–189, 2006. doi: 10.1097/01.fjc.0000199682.43448.2d. [DOI] [PubMed] [Google Scholar]
- 19.Chicco AJ, Schneider CM, Hayward R. Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol 289: R424–R431, 2005. doi: 10.1152/ajpregu.00636.2004. [DOI] [PubMed] [Google Scholar]
- 20.Combs AB, Hudman SL, Bonner HW. Effect of exercise stress upon the acute toxicity of Adriamycin in mice. Res Commun Chem Pathol Pharmacol 23: 395–398, 1979. [PubMed] [Google Scholar]
- 21.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol 21: 1660–1668, 2003. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 22.Couture L, Nash JA, Turgeon J. The ATP-binding cassette transporters and their implication in drug disposition: a special look at the heart. Pharmacol Rev 58: 244–258, 2006. doi: 10.1124/pr.58.2.7. [DOI] [PubMed] [Google Scholar]
- 23.Damodar G, Smitha T, Gopinath S, Vijayakumar S, Rao Y. An evaluation of hepatotoxicity in breast cancer patients receiving injection doxorubicin. Ann Med Health Sci Res 4: 74–79, 2014. doi: 10.4103/2141-9248.126619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies KJ, Doroshow JH, Hochstein P. Mitochondrial NADH dehydrogenase-catalyzed oxygen radical production by Adriamycin, and the relative inactivity of 5-iminodaunorubicin. FEBS Lett 153: 227–230, 1983. doi: 10.1016/0014-5793(83)80153-7. [DOI] [PubMed] [Google Scholar]
- 25.de Paleville DT, Topp RV, Swank AM. Effects of aerobic training prior to and during chemotherapy in a breast cancer patient: a case study. J Strength Cond Res 21: 635–637, 2007. doi: 10.1519/R-19735.1. [DOI] [PubMed] [Google Scholar]
- 26.DeFina LF, Barlow CE, Radford NB, Leonard D, Willis BL. The association between midlife cardiorespiratory fitness and later life chronic kidney disease: the Cooper Center Longitudinal Study. Prev Med 89: 178–183, 2016. doi: 10.1016/j.ypmed.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Demicheli R, Bonciarelli G, Jirillo A, Foroni R, Petrosino L, Targa L, Garusi G. Pharmacologic data and technical feasibility of intraperitoneal doxorubicin administration. Tumori 71: 63–68, 1985. doi: 10.1177/030089168507100112. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson JM, D’Lugos AC, Mahmood TN, Ormsby JC, Salvo L, Dedmon WL, Patel SH, Katsma MS, Mookadam F, Gonzales RJ, Hale TM, Carroll CC, Angadi SS. Exercise protects skeletal muscle during chronic doxorubicin administration. Med Sci Sports Exerc 49: 2394–2403, 2017. doi: 10.1249/MSS.0000000000001395. [DOI] [PubMed] [Google Scholar]
- 29.Dolinsky VW, Rogan KJ, Sung MM, Zordoky BN, Haykowsky MJ, Young ME, Jones LW, Dyck JR. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol Metab 305: E243–E253, 2013. doi: 10.1152/ajpendo.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doroshow JH. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res 43: 460–472, 1983. [PubMed] [Google Scholar]
- 31.Elbl L, Vasova I, Tomaskova I, Jedlicka F, Kral Z, Navratil M, Smardova L, Wagnerova B, Vorlicek J. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma 47: 843–851, 2006. doi: 10.1080/10428190500402559. [DOI] [PubMed] [Google Scholar]
- 32.Faleiros CM, Francescato HD, Papoti M, Chaves L, Silva CG, Costa RS, Coimbra TM. Effects of previous physical training on Adriamycin nephropathy and its relationship with endothelial lesions and angiogenesis in the renal cortex. Life Sci 169: 43–51, 2017. doi: 10.1016/j.lfs.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Fehrenbach E, Niess AM. Role of heat shock proteins in the exercise response. Exerc Immunol Rev 5: 57–77, 1999. [PubMed] [Google Scholar]
- 34.Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol Regul Integr Comp Physiol 268: R28–R32, 1995. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- 35.Floyd J, Mirza I, Sachs B, Perry MC. Hepatotoxicity of chemotherapy. Semin Oncol 33: 50–67, 2006. doi: 10.1053/j.seminoncol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Fujibayashi K, Fukuda H, Yokokawa H, Haniu T, Oka F, Ooike M, Gunji T, Sasabe N, Okumura M, Iijima K, Hisaoka T, Isonuma H. Associations between healthy lifestyle behaviors and proteinuria and the estimated glomerular filtration rate (eGFR). J Atheroscler Thromb 19: 932–940, 2012. doi: 10.5551/jat.12781. [DOI] [PubMed] [Google Scholar]
- 37.García-Pagàn JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, Bosch J, Rodés J. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology 111: 1300–1306, 1996. doi: 10.1053/gast.1996.v111.pm8898644. [DOI] [PubMed] [Google Scholar]
- 38.Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St. Clair DK, Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol (1985) 107: 1935–1942, 2009. doi: 10.1152/japplphysiol.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilliam LA, Moylan JS, Callahan LA, Sumandea MP, Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve 43: 94–102, 2011. doi: 10.1002/mus.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilliam LA, Moylan JS, Ferreira LF, Reid MB. TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm weakness. Am J Physiol Lung Cell Mol Physiol 300: L225–L231, 2011. doi: 10.1152/ajplung.00264.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilliam LA, St. Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal 15: 2543–2563, 2011. doi: 10.1089/ars.2011.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo J, Ananthakrishnan R, Qu W, Lu Y, Reiniger N, Zeng S, Ma W, Rosario R, Yan SF, Ramasamy R, D’Agati V, Schmidt AM. RAGE mediates podocyte injury in Adriamycin-induced glomerulosclerosis. J Am Soc Nephrol 19: 961–972, 2008. doi: 10.1681/ASN.2007101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall SE, Smuder AJ, Hayward R. Effects of calorie restriction and voluntary exercise on doxorubicin-induced cardiotoxicity. Integr Cancer Ther 18: 1534735419843999, 2019. doi: 10.1177/1534735419843999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamner RW, Verani R, Weinman EJ. Mitomycin-associated renal failure. Case report and review. Arch Intern Med 143: 803–807, 1983. doi: 10.1001/archinte.1983.00350040193029. [DOI] [PubMed] [Google Scholar]
- 45.Hegazy MA, Kotb SZ, Sakr H, El Dosoky E, Amer T, Hegazi RA, Farouk O. Preoperative isolated limb infusion of doxorubicin and external irradiation for limb-threatening soft tissue sarcomas. Ann Surg Oncol 14: 568–576, 2007. doi: 10.1245/s10434-006-9138-1. [DOI] [PubMed] [Google Scholar]
- 46.Heikkilä E, Juhila J, Lassila M, Messing M, Perälä N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H. β-Catenin mediates Adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant 25: 2437–2446, 2010. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 47.Henstridge DC, Febbraio MA, Hargreaves M. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J Appl Physiol (1985) 120: 683–691, 2016. doi: 10.1152/japplphysiol.00811.2015. [DOI] [PubMed] [Google Scholar]
- 48.Hershko C, Pinson A, Link G. Prevention of anthracycline cardiotoxicity by iron chelation. Acta Haematol 95: 87–92, 1996. doi: 10.1159/000203954. [DOI] [PubMed] [Google Scholar]
- 49.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J; ADHERE Scientific Advisory Committee and Investigators . High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 13: 422–430, 2007. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Hinkley JM, Morton AB, Ichinoseki-Sekine N, Huertas AM, Smuder AJ. Exercise training prevents doxorubicin-induced mitochondrial dysfunction of the liver. Med Sci Sports Exerc 51: 1106–1115, 2019. doi: 10.1249/MSS.0000000000001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooke MC, McCarthy K, Taylor O, Hockenberry MJ. Fatigue and carnitine levels over multiple cycles of chemotherapy in children and adolescents. Eur J Oncol Nurs 19: 7–12, 2015. doi: 10.1016/j.ejon.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE II, Coan A, Gutierrez A, Hornsby KP, Hamilton E, Wilke LG, Kimmick GG, Peppercorn JM, Jones LW. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol 53: 65–74, 2014. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 53.Huang SC, Wu JF, Saovieng S, Chien WH, Hsu MF, Li XF, Lee SD, Huang CY, Huang CY, Kuo CH. Doxorubicin inhibits muscle inflammation after eccentric exercise. J Cachexia Sarcopenia Muscle 8: 277–284, 2017. doi: 10.1002/jcsm.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hydock DS, Lien CY, Jensen BT, Parry TL, Schneider CM, Hayward R. Rehabilitative exercise in a rat model of doxorubicin cardiotoxicity. Exp Biol Med (Maywood) 237: 1483–1492, 2012. doi: 10.1258/ebm.2012.012137. [DOI] [PubMed] [Google Scholar]
- 55.Hydock DS, Lien CY, Schneider CM, Hayward R. Effects of voluntary wheel running on cardiac function and myosin heavy chain in chemically gonadectomized rats. Am J Physiol Heart Circ Physiol 293: H3254–H3264, 2007. doi: 10.1152/ajpheart.00801.2007. [DOI] [PubMed] [Google Scholar]
- 56.Hydock DS, Lien CY, Schneider CM, Hayward R. Exercise preconditioning protects against doxorubicin-induced cardiac dysfunction. Med Sci Sports Exerc 40: 808–817, 2008. doi: 10.1249/MSS.0b013e318163744a. [DOI] [PubMed] [Google Scholar]
- 57.Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, Wu R, Khechaduri A, Jairaj Naik T, Ardehali H. Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci USA 109: 4152–4157, 2012. doi: 10.1073/pnas.1119338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124: 617–630, 2014. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 60.Jeansson M, Björck K, Tenstad O, Haraldsson B. Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol 20: 114–122, 2009. doi: 10.1681/ASN.2007111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jensen BT, Lien CY, Hydock DS, Schneider CM, Hayward R. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J Cardiovasc Pharmacol 62: 263–269, 2013. doi: 10.1097/FJC.0b013e3182982ce0. [DOI] [PubMed] [Google Scholar]
- 62.Ji LL, Mitchell EW. Effects of Adriamycin on heart mitochondrial function in rested and exercised rats. Biochem Pharmacol 47: 877–885, 1994. doi: 10.1016/0006-2952(94)90488-X. [DOI] [PubMed] [Google Scholar]
- 63.Joensuu H, Asola R, Minn H. Combination chemotherapy with dacarbazine and lomustine in disseminated malignant melanoma. Acta Radiol Oncol 25: 177–179, 1986. doi: 10.3109/02841868609136399. [DOI] [PubMed] [Google Scholar]
- 64.Joerger M, Huitema AD, Meenhorst PL, Schellens JH, Beijnen JH. Pharmacokinetics of low-dose doxorubicin and metabolites in patients with AIDS-related Kaposi sarcoma. Cancer Chemother Pharmacol 55: 488–496, 2005. doi: 10.1007/s00280-004-0900-4. [DOI] [PubMed] [Google Scholar]
- 65.Jung K, Reszka R. Mitochondria as subcellular targets for clinically useful anthracyclines. Adv Drug Deliv Rev 49: 87–105, 2001. doi: 10.1016/S0169-409X(01)00128-4. [DOI] [PubMed] [Google Scholar]
- 66.Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol (1985) 59: 1298–1303, 1985. doi: 10.1152/jappl.1985.59.4.1298. [DOI] [PubMed] [Google Scholar]
- 67.Kassner N, Huse K, Martin HJ, Gödtel-Armbrust U, Metzger A, Meineke I, Brockmöller J, Klein K, Zanger UM, Maser E, Wojnowski L. Carbonyl reductase 1 is a predominant doxorubicin reductase in the human liver. Drug Metab Dispos 36: 2113–2120, 2008. doi: 10.1124/dmd.108.022251. [DOI] [PubMed] [Google Scholar]
- 68.Kavazis AN, Smuder AJ, Min K, Tümer N, Powers SK. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am J Physiol Heart Circ Physiol 299: H1515–H1524, 2010. doi: 10.1152/ajpheart.00585.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keats MR, Grandy SA, Giacomantonio N, MacDonald D, Rajda M, Younis T. EXercise to prevent AnthrCycline-based Cardio-Toxicity (EXACT) in individuals with breast or hematological cancers: a feasibility study protocol. Pilot Feasibility Stud 2: 44, 2016. doi: 10.1186/s40814-016-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kiuchi MG, Chen S, Hoye NA. The effects of different physical activities on atrial fibrillation in patients with hypertension and chronic kidney disease. Kidney Res Clin Pract 36: 264–273, 2017. doi: 10.23876/j.krcp.2017.36.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koren G, Beatty K, Seto A, Einarson TR, Lishner M. The effects of impaired liver function on the elimination of antineoplastic agents. Ann Pharmacother 26: 363–371, 1992. doi: 10.1177/106002809202600311. [DOI] [PubMed] [Google Scholar]
- 72.Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011. doi: 10.1111/j.1440-1797.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- 73.Lee YT, Chan KK, Harris PA, Cohen JL. Distribution of Adriamycin in cancer patients: tissue uptakes, plasma concentration after IV and hepatic IA administration. Cancer 45: 2231–2239, 1980. doi:. [DOI] [PubMed] [Google Scholar]
- 74.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of Adriamycin cardiotoxicity. Cancer 32: 302–314, 1973. doi:. [DOI] [PubMed] [Google Scholar]
- 75.Lien CY, Jensen BT, Hydock DS, Hayward R. Short-term exercise training attenuates acute doxorubicin cardiotoxicity. J Physiol Biochem 71: 669–678, 2015. doi: 10.1007/s13105-015-0432-x. [DOI] [PubMed] [Google Scholar]
- 76.Link G, Tirosh R, Pinson A, Hershko C. Role of iron in the potentiation of anthracycline cardiotoxicity: identification of heart cell mitochondria as a major site of iron-anthracycline interaction. J Lab Clin Med 127: 272–278, 1996. doi: 10.1016/S0022-2143(96)90095-5. [DOI] [PubMed] [Google Scholar]
- 77.Locklear CT, Golabi P, Gerber L, Younossi ZM. Exercise as an intervention for patients with end-stage liver disease: systematic review. Medicine (Baltimore) 97: e12774, 2018. doi: 10.1097/MD.0000000000012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu P. Monitoring cardiac function in patients receiving doxorubicin. Semin Nucl Med 35: 197–201, 2005. doi: 10.1053/j.semnuclmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Mackay AD, Marchant ED, Munk DJ, Watt RK, Hansen JM, Thomson DM, Hancock CR. Multitissue analysis of exercise and metformin on doxorubicin-induced iron dysregulation. Am J Physiol Endocrinol Metab 316: E922–E930, 2019. doi: 10.1152/ajpendo.00140.2018. [DOI] [PubMed] [Google Scholar]
- 80.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 85: 1364–1373, 1992. doi: 10.1161/01.CIR.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 81.McCartney N. Role of resistance training in heart disease. Med Sci Sports Exerc 30, Suppl: S396–S402, 1998. doi: 10.1097/00005768-199810001-00008. [DOI] [PubMed] [Google Scholar]
- 82.McLoon LK, Bauer G, Wirtschafter J. Quantification of muscle loss in the doxorubicin-treated orbicularis oculi of the monkey. Effect of local injection of doxorubicin into the eyelid. Invest Ophthalmol Vis Sci 32: 1667–1673, 1991. [PubMed] [Google Scholar]
- 83.McLoon LK, Wirtschafter JD, Cameron JD. Muscle loss from doxorubicin injections into the eyelids of a patient with blepharospasm. Am J Ophthalmol 116: 646–648, 1993. doi: 10.1016/S0002-9394(14)73213-1. [DOI] [PubMed] [Google Scholar]
- 84.Mete R, Oran M, Topcu B, Oznur M, Seber ES, Gedikbasi A, Yetisyigit T. Protective effects of onion (Allium cepa) extract against doxorubicin-induced hepatotoxicity in rats. Toxicol Ind Health 32: 551–557, 2016. doi: 10.1177/0748233713504807. [DOI] [PubMed] [Google Scholar]
- 85.Min K, Kwon OS, Smuder AJ, Wiggs MP, Sollanek KJ, Christou DD, Yoo JK, Hwang MH, Szeto HH, Kavazis AN, Powers SK. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J Physiol 593: 2017–2036, 2015. doi: 10.1113/jphysiol.2014.286518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer 10: 389–398, 2002. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 87.Morton AB, Mor Huertas A, Hinkley JM, Ichinoseki-Sekine N, Christou DD, Smuder AJ. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion 45: 52–62, 2019. doi: 10.1016/j.mito.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nadler M, Bainbridge D, Tomasone J, Cheifetz O, Juergens RA, Sussman J. Oncology care provider perspectives on exercise promotion in people with cancer: an examination of knowledge, practices, barriers, and facilitators. Support Care Cancer 25: 2297–2304, 2017. doi: 10.1007/s00520-017-3640-9. [DOI] [PubMed] [Google Scholar]
- 89.Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M. Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int 29: 502–510, 1986. doi: 10.1038/ki.1986.28. [DOI] [PubMed] [Google Scholar]
- 90.Ong LM, Punithavathi N, Thurairatnam D, Zainal H, Beh ML, Morad Z, Lee SY, Bavanandan S, Kok LS. Prevalence and risk factors for proteinuria: the National Kidney Foundation of Malaysia Lifecheck Health Screening Programme. Nephrology (Carlton) 18: 569–575, 2013. doi: 10.1111/nep.12112. [DOI] [PubMed] [Google Scholar]
- 91.Parry TL, Hayward R. Exercise training does not affect anthracycline antitumor efficacy while attenuating cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol 309: R675–R683, 2015. doi: 10.1152/ajpregu.00185.2015. [DOI] [PubMed] [Google Scholar]
- 92.Peng CC, Chen KC, Hsieh CL, Peng RY. Swimming exercise prevents fibrogenesis in chronic kidney disease by inhibiting the myofibroblast transdifferentiation. PLoS One 7: e37388, 2012. doi: 10.1371/journal.pone.0037388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng CC, Chen KC, Lu HY, Peng RY. Treadmill exercise improved Adriamycin-induced nephropathy. J Biol Regul Homeost Agents 26: 15–28, 2012. [PubMed] [Google Scholar]
- 94.Pfannenstiel K, Hayward R. Effects of resistance exercise training on doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol 71: 332–339, 2018. doi: 10.1097/FJC.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 95.Pfeiffer T, Krause U, Thome U, Rajewski A, Skorzek M, Scheulen ME. Tissue toxicity of doxorubicin in first and second hyperthermic isolated limb perfusion—an experimental study in dogs. Eur J Surg Oncol 23: 439–444, 1997. doi: 10.1016/S0748-7983(97)93727-6. [DOI] [PubMed] [Google Scholar]
- 96.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009. doi: 10.1152/ajprenal.90421.2008. [DOI] [PubMed] [Google Scholar]
- 97.Quinn CJ, Gibson NM, Pfannenstiel KB, Bashore AC, Hayward R, Hydock DS. Effects of exercise on doxorubicin accumulation and multidrug resistance protein expression in striated muscle. Global J Med Res 16: 2016. [Google Scholar]
- 98.Repka CP, Hayward R. Effects of an exercise intervention on cancer-related fatigue and its relationship to markers of oxidative stress. Integr Cancer Ther 17: 503–510, 2018. doi: 10.1177/1534735418766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ritland S. Exercise and liver disease. Sports Med 6: 121–126, 1988. doi: 10.2165/00007256-198806020-00006. [DOI] [PubMed] [Google Scholar]
- 100.Sahni V, Choudhury D, Ahmed Z. Chemotherapy-associated renal dysfunction. Nat Rev Nephrol 5: 450–462, 2009. doi: 10.1038/nrneph.2009.97. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt T, Weisser B, Dürkop J, Jonat W, Van Mackelenbergh M, Röcken C, Mundhenke C. Comparing endurance and resistance training with standard care during chemotherapy for patients with primary breast cancer. Anticancer Res 35: 5623–5629, 2015. [PubMed] [Google Scholar]
- 102.Schwartz AL. Fatigue mediates the effects of exercise on quality of life. Qual Life Res 8: 529–538, 1999. doi: 10.1023/A:1008978611274. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz AL. Understanding and treating cancer-related fatigue. Oncology (Williston Park) 21: 30–34, 2007. [PubMed] [Google Scholar]
- 104.Schwartz AL, de Heer HD, Bea JW. Initiating exercise interventions to promote wellness in cancer patients and survivors. Oncology (Williston Park) 31: 711–717, 2017. [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum 34: 627–633, 2007. doi: 10.1188/07.ONF.627-633. [DOI] [PubMed] [Google Scholar]
- 106.Senf SM. Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Front Physiol 4: 330, 2013. doi: 10.3389/fphys.2013.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simonavice E, Liu PY, Ilich JZ, Kim JS, Arjmandi B, Panton LB. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl Physiol Nutr Metab 39: 730–739, 2014. doi: 10.1139/apnm-2013-0281. [DOI] [PubMed] [Google Scholar]
- 108.Smart NA, King N, McFarlane JR, Graham PL, Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis. Br J Sports Med 52: 834–843, 2018. doi: 10.1136/bjsports-2016-096197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced oxidative stress and proteolysis in skeletal muscle. J Appl Physiol (1985) 110: 935–942, 2011. doi: 10.1152/japplphysiol.00677.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer 79: 1479–1486, 1999. doi: 10.1038/sj.bjc.6690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97: 2869–2879, 2003. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 112.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49: 330–352, 2007. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Tavoloni N, Guarino AM. Disposition and metabolism of Adriamycin in the rat. Pharmacology 21: 244–255, 1980. doi: 10.1159/000137439. [DOI] [PubMed] [Google Scholar]
- 114.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 21: 440–446, 2011. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turner-Gomes SO, Lands LC, Halton J, Hanning RM, Heigenhauser GJ, Pai M, Barr R. Cardiorespiratory status after treatment for acute lymphoblastic leukemia. Med Pediatr Oncol 26: 160–165, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 116.van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail 16: 84–90, 2010. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 117.van Norren K, van Helvoort A, Argilés JM, van Tuijl S, Arts K, Gorselink M, Laviano A, Kegler D, Haagsman HP, van der Beek EM. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer 100: 311–314, 2009. [Erratum in Br J Cancer 100: 1014, 2009.] doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 64: 938–945, 2014. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 119.Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91: 710–717, 1979. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 120.Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol 93: 105–115, 2003. doi: 10.1034/j.1600-0773.2003.930301.x. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Wang YP, Tay YC, Harris DC. Progressive Adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 122.Watson EL, Gould DW, Wilkinson TJ, Xenophontos S, Clarke AL, Vogt BP, Viana JL, Smith AC. Twelve-week combined resistance and aerobic training confers greater benefits than aerobic training alone in nondialysis CKD. Am J Physiol Renal Physiol 314: F1188–F1196, 2018. doi: 10.1152/ajprenal.00012.2018. [DOI] [PubMed] [Google Scholar]
- 123.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv 6: 189–199, 2012. doi: 10.1007/s11764-011-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wirtschafter JD, McLoon LK. Long-term efficacy of local doxorubicin chemomyectomy in patients with blepharospasm and hemifacial spasm. Ophthalmology 105: 342–346, 1998. doi: 10.1016/S0161-6420(98)93484-4. [DOI] [PubMed] [Google Scholar]
- 125.Wonders KY, Hydock DS, Schneider CM, Hayward R. Acute exercise protects against doxorubicin cardiotoxicity. Integr Cancer Ther 7: 147–154, 2008. doi: 10.1177/1534735408322848. [DOI] [PubMed] [Google Scholar]
- 126.Yagmurca M, Bas O, Mollaoglu H, Sahin O, Nacar A, Karaman O, Songur A. Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch Med Res 38: 380–385, 2007. doi: 10.1016/j.arcmed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18: 1639–1642, 2012. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 128.Zolfagharzadeh F, Roshan VD. Pretreatment hepatoprotective effect of regular aerobic training against hepatic toxicity induced by doxorubicin in rats. Asian Pac J Cancer Prev 14: 2931–2936, 2013. doi: 10.7314/APJCP.2013.14.5.2931. [DOI] [PubMed] [Google Scholar]