Abstract
Although it is now well established that heart failure with preserved ejection fraction (HFpEF) is associated with marked inflammation and a prooxidant state that is accompanied by vascular dysfunction, whether acute antioxidant (AO) administration can effectively target these disease-related decrements has not been evaluated. Thus, the present study sought to evaluate the efficacy of an acute over-the-counter AO cocktail (600 mg α-lipoic acid, 1,000 mg vitamin C, and 600 IU vitamin E) to mitigate inflammation and oxidative stress, and subsequently improve nitric oxide (NO) bioavailability and vascular function, in patients with HFpEF. Flow-mediated dilation (FMD) and reactive hyperemia (RH) were evaluated to assess conduit vessel and microvascular function, respectively, 90 min after administration of either placebo (PL) or AO in 16 patients with HFpEF (73 ± 10 yr, EF 54–70%) using a double-blind, crossover design. Circulating biomarkers of inflammation (C-reactive protein, CRP), oxidative stress (malondialdehyde and protein carbonyl), free radical concentration (EPR spectroscopy), antioxidant capacity, ascorbate and NO bioavailability (plasma nitrate, , and nitrite, ) were also assessed. FMD improved following AO administration (PL: 3.49 ± 0.7%, AO: 5.83 ± 1.0%), whereas RH responses were similar between conditions (PL: 428 ± 51 mL, AO: 425 ± 51 mL). AO administration decreased CRP (PL: 4,429 ± 705 ng/mL, AO: 3,664 ± 520 ng/mL) and increased ascorbate (PL: 30.0 ± 2.9 µg/mL, AO: 45.1 ± 3.7 µg/mL) and (PL: 182 ± 21 nM, AO: 213 ± 24 nM) but did not affect other biomarkers. Together, these data suggest that acute AO administration can exert anti-inflammatory effects and improve conduit artery vasodilation, but not microvascular function, in patients with HFpEF.
Keywords: flow-mediated dilation, HFpEF, hemodynamics, inflammation, vascular function
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) accounts for greater than 50% of all heart failure cases nationwide, and the prevalence relative to heart failure with reduced ejection fraction (HFrEF) continues to rise 1% per year (37). Currently, the clinical presentation of HFpEF is defined by dyspnea upon exertion and severe exercise intolerance (23), symptoms that are unlikely due to a simple deficit in cardiac mechanics. Indeed, the contribution of vascular dysfunction to exercise intolerance in HFpEF has recently been identified (3), highlighting the importance of disease-related changes in the peripheral circulation to the HFpEF pathophysiology. Whereas vascular dysfunction has been well documented in HFrEF (12, 19, 25, 42), considerable uncertainty exists regarding disease-related changes in vascular health in HFpEF. Indeed, recent studies have provided evidence for (11, 21, 29) and against (14, 16) an impairment in vascular function in patients with HFpEF. Our group recently identified an attenuation in vascular function in HFpEF at the microcirculatory level, as determined by reactive hyperemia (RH), with an apparent preservation of conduit artery function, as assessed by flow-mediated vasodilation (FMD) testing (27). Despite a lack of consensus, it is clear that some manner of peripheral vascular dysfunction is likely present in HFpEF that may represent a therapeutic target in this patient group, who have proven challenging to treat.
One of the many unique features of HFpEF is the presence of multiple comorbidities, many of which are characterized by a proinflammatory and prooxidant state. Indeed, given the widely recognized chronic inflammation associated with noncardiac comorbidities in HFpEF such as obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), and chronic kidney disease, it is not surprising that the emerging paradigm in HFpEF is focused on the role of these multiple comorbidities in producing microvascular endothelial inflammation (32). The axis of inflammation, oxidative stress, and vascular function has been well described, whereby, as inflammation can both produce free radicals and diminish cellular antioxidant capacity, this increase in reactive oxygen species leads to endothelial nitric oxide synthase (eNOS) uncoupling, the formation of superoxide anions, reduced NO bioavailability, and ultimately impaired vascular function (7). However, many uncertainties remain regarding the nature of redox imbalance and whether pro- and antioxidant balance can be restored in HFpEF.
Although there are many potential strategies for combating the damaging effects of inflammation and oxidative stress on peripheral vascular function, antioxidant (AO) administration has emerged as a simple but effective approach. Indeed, our group has demonstrated the efficacy of acute AO administration (vitamins C, E, and α-lipoic acid) to attenuate oxidative stress and improve vascular function, as assessed by FMD testing, in the elderly (43) as well as in COPD (35) and HFrEF (42) patient groups. Given the widely recognized presence of inflammation and oxidative stress in HFpEF, it is likely that AO administration could also prove beneficial in targeting these aspects of HFpEF pathophysiology, yet this remains a largely unexplored area of research. In fact, to our knowledge, not a single study to date has examined the potential benefit of AO administration on vascular function in HFpEF.
Therefore, this study sought to evaluate the impact of acute AO administration on both conduit vessel and microvascular function in HFpEF and also to determine whether inflammation and redox balance would be improved by such an intervention. We hypothesized that AO administration in HFpEF would improve both FMD and RH and would decrease biomarkers of inflammation, oxidative stress, and free radicals while increasing biomarkers of NO and antioxidant capacity.
METHODS
Patients.
All patients (3 M/13 F, 73 ± 10 yr, EF: 54–70%) were recruited from the heart failure (HF) clinic at the University of Utah, the Salt Lake City Veterans Affairs Medical Center (VAMC), and the greater Salt Lake City community. HFpEF patient inclusion criteria were consistent with the TOPCAT trial (9), which uses the following: 1) HF, defined by the presence of ≥1 symptom at the time of screening (paroxysmal nocturnal dyspnea, orthopnea, dyspnea on exertion) and one sign (edema, elevation in jugular venous distention) in the previous 12 mo; 2) left ventricular ejection fraction (LVEF) ≥45%; 3) controlled systolic blood pressure; and 4) either ≥1 hospitalization in the previous 12 mo for which HF was a major component of hospitalization or B-type natriuretic peptide (BNP) ≥100 pg/mL, in the previous 60 days. Exclusion criteria included significant valvular heart disease, acute atrial fibrillation, or a body mass index (BMI) >45. All participants were nonsmokers. All females studied were postmenopausal and not currently on hormone replacement therapy. All procedures were approved by the University of Utah and Salt Lake City Veterans Affairs (VA) Medical Center Institutional Review Boards, and studies were performed at the VA Salt Lake City Geriatric Research, Education, and Clinical Center in the Utah Vascular Research Laboratory. The patients were informed and gave written consent to the study.
AO supplementation.
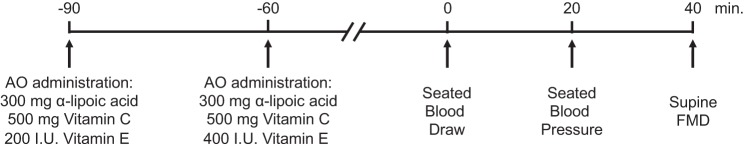
All patients reported to the laboratory twice within 1 wk (range: 2–7 days, mean time between study visits: 5 ± 2 days) with at least 48 h between visits to ensure a proper washout period and received an AO cocktail or placebo (PL) in a balanced, double-blind, crossover design. These visits were similar in duration and time of day, with only administration of either AO or PL varying. Supplements were taken in two doses, separated by 30 min to improve absorption, consumed 90 and 60 min before the blood draw and FMD protocol (Fig. 1). The first dose consisted of 300 mg of α-lipoic acid, 500 mg of vitamin C, and 200 IU of vitamin E. The second dose consisted of 300 mg of α-lipoic acid, 500 mg of vitamin C, and 400 IU of vitamin E. Placebo microcrystalline cellulose capsules of similar taste, color, and appearance were likewise consumed in two doses within the same time frame. Patients consumed a standardized breakfast (255 g of cornflakes and 200 mL of skim milk) 5 h before reporting to the laboratory. A single investigator performed all analyses, blinded to the randomization of the study visits.
Fig. 1.
Schematic diagram of study timeline. AO, antioxidant; FMD, flow-mediated dilation.
Cardiovascular measurements and analyses.
Patients provided a venous blood sample 90 min after the first AO or PL dose, and seated arterial blood pressure was determined after 20 min of seated rest (Fig. 1). Patients were then moved to a supine position and rested for an additional 20 min. FMD testing was then performed in accordance with current guidelines (13). Briefly, baseline measurements of right brachial artery diameter and blood velocity were taken for 1 min. Immediately after baseline measurements, a blood pressure cuff, placed on the right arm, proximal to the elbow and distal to the Doppler probe measurement site, was inflated to a suprasystolic pressure (>250 mmHg) for 5 min. The cuff was then rapidly deflated, and brachial artery diameter and blood velocity measurements were obtained continuously for 2 min. Blood velocity and vessel diameter recording as well as brachial artery blood flow, shear rate, and RH were assessed as previously reported (27).
Blood biomarker analyses.
Blood was sampled from the antecubital vein 90 min. after the first AO or PL administration (Fig. 1), and plasma and serum samples were stored at −80°C for subsequent analyses. Biomarkers associated with HF were assessed, including BNP, NH2-terminal (NT)-proBNP, ST2, galectin-3, and cystatin C, according the manufacturer’s instructions (Invitrogen, Waltham, MA). NO metabolite concentrations ( and ) were measured by chemiluminescence using a Sievers NO analyzer (NOA 280i; Zysense, Weddington, NC). Plasma free radical concentration was directly assessed by spin trapping (α-phenyl-tert-butylnitrone, PBN) and electron paramagnetic resonance (EPR) spectroscopy (Ruker, Billerica, MA) (6). The efficacy of the AO cocktail to acutely raise plasma ascorbate levels in the blood was determined (CosmoBio, Carlsbad, CA). Endogenous antioxidant capacity, assessed by catalase and superoxide dismutase activity, was also assayed in the plasma (Cayman Chemical, Ann Arbor, MI). Protein carbonyl, a marker of oxidant damage, was measured with a protein carbonyl ELISA (kit NWK-PCK01; Northwest Life Science Specialties, Vancouver, WA). Lipid peroxidation, a marker of oxidant damage, was assessed by plasma malondialdehyde (MDA) levels (LPO-586; Bioxytech, Foster City, CA). Serum CRP was measured using a liquid-phase, double-antibody radioimmunoassay (R&D Systems: Minneapolis, MN). High sensitivity IL-6 and TNF-α concentrations were measured using solid phase sandwich ELISA kits (kits HS600B and DTA00C, respectively, R&D Systems). Total antioxidant capacity was evaluated by determining the ferric-reducing ability of plasma (FRAP).
Statistical analyses.
Statistics were performed using commercially available software (SigmaStat 3.13; Systat Software, Point Richmond, CA). Paired Student’s t tests and two-way repeated-measures analyses were performed, Shapiro-Wilk normality test confirmed normal distribution, and the Bonferroni test was used for post hoc analysis when a significant main effect was found. Given the variation in chronic pharmaceutical drug use among patients, exploratory analyses were performed to evaluate the impact on pharmacotherapy on the change in FMD and RH. Patients were grouped according to β-blocker, angiotensin-converting-enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), statin, loop diuretic, aldosterone antagonists, or nitrate usage, and two-sample equal variance t tests were performed for each drug grouping. Patient characteristics are expressed as means ± SD, and all other data are expressed as means ± SE. Significance was established at P < 0.05 for all tests.
RESULTS
Patient characteristics.
Anthropometric data and clinical biomarkers are presented in Table 1, and disease characteristics and medications are documented in Table 2. Echocardiographic characteristics are recorded in Table 3, and circulating plasma biomarkers are reported in Table 4. Patients presented with the typical HFpEF phenotype of older individuals (73 ± 10 yr) who were overweight or obese (BMI 35 ± 6 kg/m2) and primarily female (3 M/13 F) (26). Additionally, a H2FpEF score of 6.7 ± 0.6 provides high confidence (>90%) for an accurate diagnosis of HFpEF (33).
Table 1.
Patient characteristics and clinical biomarkers
| Anthropometric Data | |
| Sex, M/F | 3 M/13 F |
| Age, yr | 73 ± 10 |
| Height, cm | 165 ± 12 |
| Weight, kg | 97 ± 23 |
| BMI, kg/m2 | 35 ± 6 |
| BSA, m2 | 2.1 ± 0.3 |
| Heart rate, beats/min | 66 ± 10 |
| Mean arterial pressure, mmHg | 89 ± 11 |
| Clinical biomarkers | |
| BNP, pg/mL | 70 ± 20 |
| NT-proBNP, pg/mL | 914 ± 249 |
| ST2, ng/mL | 50 ± 7 |
| Galectin-3, ng/mL | 15 ± 1 |
| Cystatin C, ng/mL | 19 ± 1 |
Values are means ± SD. BMI, body mass index; BNP, B-type natriuretic peptide; BSA, body surface area; NT-proBNP, NH2-terminal pro-B-type natriuretic peptide; ST2, suppression of tumorigenicity 2.
Table 2.
Patient disease characteristics and medications
| n, % | |
|---|---|
| Disease Characteristics | |
| NYHA class II | 12, 75 |
| NYHA class III | 4, 25 |
| NYHA class IV | 0, 0 |
| Diabetes | 3, 19 |
| COPD | 2, 13 |
| CAD | 4, 25 |
| Hypertension | 9, 56 |
| Hyperlipidemia | 7, 44 |
| Medications | |
| β-Blockers | 7, 44 |
| ACEi | 3, 19 |
| ARB | 5, 31 |
| Loop diuretics | 12, 75 |
| Aldosterone antagonists | 12, 75 |
| Statin | 9, 56 |
| Nitrates | 2, 13 |
| Ca2+ channel blocker | 6, 38 |
| Mean Rx types taken, n | 3.5 ± 1.4 |
Patients taking prescription (Rx), % 100 ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NYHA class, New York Heart Association functional classification.
Table 3.
Patient echocardiogram characteristics
| HFpEF | Reference Range | |
|---|---|---|
| Ejection fraction, % | 63 ± 7 | ≥55 |
| LV IVSD, cm | 1.2 ± 0.2 | 0.6–1.1 |
| LV PWD, cm | 1.0 ± 0.2 | 0.6–0.9 |
| LV ID diastole, cm | 4.6 ± 0.4 | 3.9–5.3 |
| LV ID systole, cm | 3.1 ± 0.4 | 2.0–4.0 |
| Peak E wave, cm/s | 11 ± 3 | ≤50 |
| Peak A wave, cm/s | 8 ± 4 | 12–36 |
| E/A ratio | 1.6 ± 1.1 | 0.6–1.32 |
| E′ lateral wall, cm/s | 9 ± 5 | 13–28 |
| E/E′ lateral ratio | 17.0 ± 11.5 | ≤8 |
| Mitral E wave deceleration time, ms | 221 ± 76 | 142–258 |
| RVSP, mmHg | 37 ± 3 | 15–45 |
| LA area (4C), cm2 | 26 ± 2 | <20 |
Values are means ± SD. HFpEF, heart failure with preserved ejection fraction; LV, left ventricle; IVSD, interventricular septum thickness at end-diastole; PWD, posterior wall thickness; ID, internal dimension; E wave, peak velocity of early diastolic transmitral flow; A wave, peak velocity of late transmitral flow; E′, peak velocity of early diastolic mitral annular motion; RVSP, right ventricular systolic pressure; LA area 4C, left atrial area from 4-chamber view.
Table 4.
Patient circulating plasma biomarkers
| PL | AO | CV, % | |
|---|---|---|---|
| Nitric oxide bioavailability | |||
| Nitrate, μM | 65.3 ± 15.0 | 57.9 ± 8.5 | 11 |
| Nitrite, nM | 182 ± 21 | 213 ± 24* | 26 |
| Free radicals | |||
| EPR, AU | 4.9 ± 1.2 | 4.9 ± 1.3 | 9 |
| Antioxidants | |||
| Ascorbate, µg/mL | 30.0 ± 2.9 | 45.1 ± 3.7* | 11 |
| Catalase, nmol·min−1·min−1 | 45.8 ± 6.4 | 46.2 ± 8.5 | 10 |
| SOD, U/mL | 1.56 ± 0.26 | 1.56 ± 0.23 | 9 |
| Oxidative stress | |||
| MDA, μM | 5.86 ± 0.76 | 5.64 ± 0.79 | 7 |
| Protein carbonyl, nmol/mg | 0.18 ± 0.02 | 0.20 ± 0.03 | 6 |
| Antioxidant capacity | |||
| FRAP, mM | 3.75 ± 0.41 | 3.97 ± 0.41 | 5 |
| Inflammation | |||
| CRP, ng/mL | 4,429 ± 705 | 3,664 ± 520* | 6 |
| IL-6, pg/mL | 5.38 ± 1.06 | 5.24 ± 0.72 | 5 |
| TNFα, pg/mL | 2.57 ± 0.41 | 2.65 ± 0.41 | 7 |
Values are means ± SE. EPR, electron paramagnetic resonance; MDA, malondialdehyde; CRP, C-reactive protein; IL-6, interleukin-6; TNFα, tumor necrosis factor-α; FRAP, ferric-reducing ability of plasma; superoxide dismutase, SOD; PL, placebo; AO, antioxidant; CV, mean intra-assay coefficient of variation.
P < 0.05 vs. PL.
Flow-mediated dilation.
FMD responses are illustrated in Fig. 2. Baseline brachial artery diameter (PL: 4.23 ± 0.15 mm, AO: 4.19 ± 0.16 mm) was not significantly different between PL and AO study visits. The change in brachial artery diameter was greater following AO, whether expressed as percent change (Fig. 2A) or absolute change (Fig. 2B). Likewise, when FMD was normalized for shear rate, AO administration increased both %FMD/Shear (Fig. 3A) and absolute change in FMD/Shear (Fig. 3B). The sum of shear at peak diameter was not different between PL and AO conditions (PL: 4.6 ± 2.5 AU, AO: 4.5 ± 2.5 AU). Exploratory analyses did not reveal any effect of standard HFpEF pharmacotherapy (β-blocker, ACE inhibitor, ARB, statin, loop diuretic, aldosterone antagonists, or nitrate) on the change in FMD from PL to AO (Table 5).
Fig. 2.
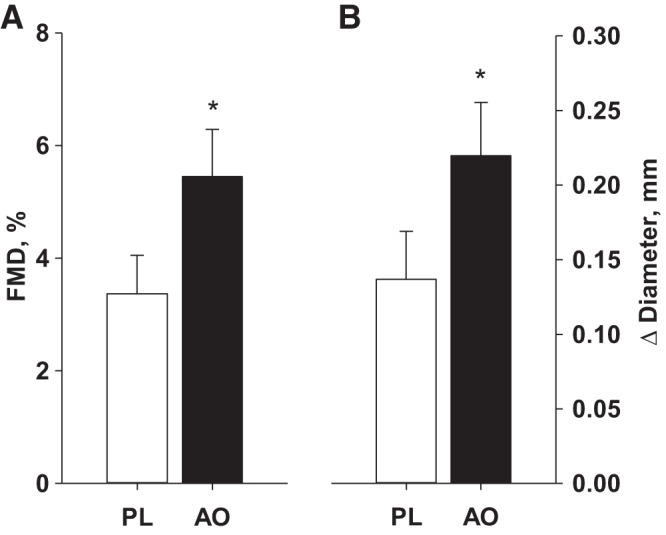
Brachial artery flow-mediated dilation (FMD), expressed as percent change (A) and absolute change (B) from baseline following placebo (PL) and antioxidant (AO) administration. *Significant difference between PL and AO, P < 0.05.
Fig. 3.
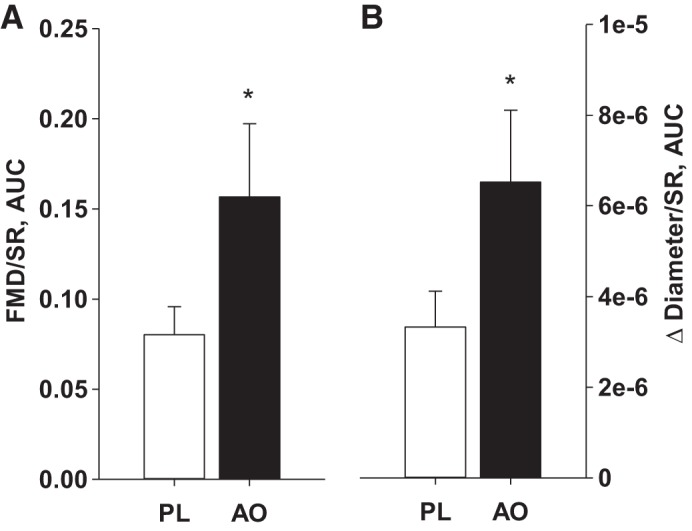
Brachial artery flow-mediated dilation (FMD) normalized for shear rate area under the curve (SR AUC), expressed as percent change (A) and absolute change (B) from baseline following placebo (PL) and antioxidant (AO) administration. *Significant difference between PL and AO, P < 0.05.
Table 5.
Vascular function changes from placebo to antioxidant visits in patients with HFpEF grouped by medication usage
| FMD, ∆% |
RH, ∆mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| Medication | Nonusers, n | Users, n | Nonuser | User | P Value | Nonuser | User | P Value |
| β-Blockers | 9 | 7 | 2.3 ± 0.6 | 2.8 ± 1.2 | 0.29 | 6 ± 43 | −32 ± 57 | 0.60 |
| ACEi | 13 | 3 | 1.7 ± 0.3 | 2.6 ± 0.7 | 0.61 | 9 ± 35 | −81 ± 99 | 0.30 |
| ARB | 11 | 5 | 2.6 ± 0.7 | 2.1 ± 1.0 | 0.64 | −25 ± 45 | 22 ± 49 | 0.53 |
| Loop diuretics | 4 | 12 | 2.0 ± 1.1 | 2.6 ± 0.7 | 0.73 | −62 ± 15 | 5 ± 41 | 0.44 |
| Aldosterone antagonists | 4 | 12 | 2.6 ± 1.1 | 2.4 ± 0.7 | 0.87 | −29 ± 22 | −2 ± 46 | 0.73 |
| Statins | 7 | 9 | 1.4 ± 0.7 | 3.2 ± 0.8 | 0.13 | −8 ± 43 | −9 ± 50 | 0.99 |
| Nitrates | 14 | 2 | 2.5 ± 0.6 | 1.9 ± 2.3 | 0.73 | −1 ± 38 | 63 ± 32 | 0.54 |
Values are means ± SE. ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; FMD, flow-mediated dilation change from placebo to antioxidant visits; RH, reactive hyperemia area under the curve change from placebo to antioxidant visits.
Reactive hyperemia.
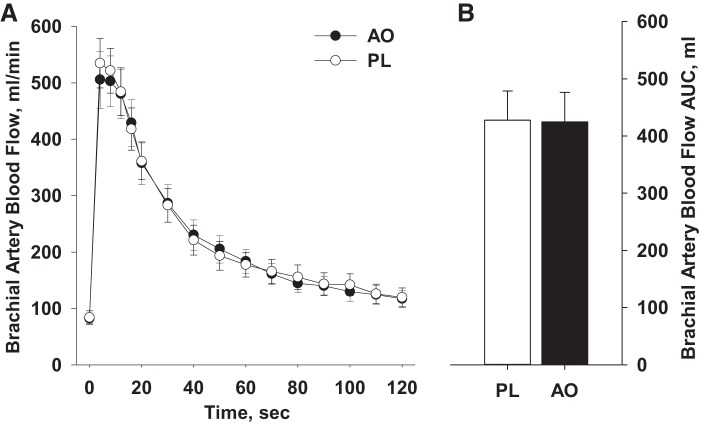
Blood flow at baseline was similar between conditions (PL: 84 ± 12 mL/min, AO: 82 ± 8 mL/min) and did not differ at any time point following cuff occlusion (Fig. 4A). Likewise, RH, assessed as AUC, was unchanged by AO (PL: 427 ± 51 mL, AO: 423 ± 51 mL; Fig. 4B). Exploratory analyses did not reveal any effect of standard HFpEF pharmacotherapy (β-blocker, ACE inhibitor, ARB, statin, loop diuretic, aldosterone antagonists, or nitrate) on the change in RH from PL to AO (Table 5).
Fig. 4.
Post-occlusion reactive hyperemia, expressed as both absolute blood flow (A) and blood flow area under the curve (AUC; B), following placebo (PL) and antioxidant (AO) administration.
Circulating biomarkers.
Circulating biomarkers of NO bioavailability, free radical concentration, antioxidant capacity, oxidative stress, and inflammation following PL and AO administration are documented in Table 4. Plasma ascorbate concentration was significantly elevated (55%) following AO administration compared with PL. Plasma was unaltered by the AO administration, but increased significantly (≈20%) following AO administration compared with PL. CRP decreased significantly (≈17%) following AO administration compared with PL. All other markers of oxidative stress, free radicals, and antioxidant capacity were not different in the PL and AO conditions. Intra-assay assessment of coefficient of variation (CV) identified good reproducibility across duplicate/triplicate samples (Table 4).
DISCUSSION
This study sought to evaluate the efficacy of acute, enteral AO administration to improve vascular function and RH and to decrease inflammation and oxidative stress in HFpEF. In support of our hypothesis, conduit vessel vasodilation, as assessed by brachial artery FMD, improved significantly following AO administration, a response that was evident even when dilation was normalized to the shear stimulus. However, RH, a measure of microvascular function, was unchanged following AO administration. Improvements in FMD were accompanied by a marked increase in plasma nitrite () and reduction in CRP, but additional biomarkers of oxidative stress, plasma free radical concentration, and antioxidant capacity were unaltered by AO administration. Taken together, these findings support the efficacy of an over-the-counter AO cocktail to exert systemic anti-inflammatory effects and improve peripheral vascular function independently of alterations in global markers of oxidative stress in HFpEF, providing new insight into the therapeutic potential of AO administration to improve vascular health in this population.
FMD and AO in HFpEF.
Vasodilation of the brachial artery following a period of blood flow occlusion, a noninvasive index of endothelium-dependent vasodilation, has been utilized in both healthy and diseased populations (4, 13) and is predictive of future cardiovascular events (45). Unlike the well-documented vascular dysfunction in HFrEF (12, 19, 25, 42), FMD studies to assess vascular function in HFpEF have been rather equivocal. Indeed, whereas earlier work failed to identify an impairment in arm (14) and leg (16) FMD in HFpEF compared with old healthy individuals, more recent studies have reported lower FMD values in HFpEF compared with healthy age-matched (21) or hypertensive (11, 29) control subjects. Our group has also recently provided evidence of decreased FMD in HFpEF compared with healthy age-matched controls, but when FMD was normalized for conduit artery shear rate, the difference between groups was abolished (27). Therefore, although not without contradictions, the majority of the literature appears to support some manner of decrement in vascular function in HFpEF, with discrepancies among studies attributable to variations in control group, degree of adherence to standardized guidelines for FMD testing (13), and the approach taken in interpretation of results. In the current investigation, we observed an improvement in brachial artery FMD after AO administration (Fig. 2, A and B) as well as FMD normalized for shear (Fig. 3, A and B), supporting the efficacy of this intervention to acutely improve conduit vessel dilation in HFpEF. Given the established link among inflammation, oxidative stress, and vascular dysfunction, it is somewhat surprising that the potential therapeutic benefit of AO administration has, until now, remained unexplored in this patient group. In fact, only one clinical trial is currently underway focused on this aspect of HFpEF pathophysiology. The “CoQ10 and d-Ribose in Patients With Diastolic Heart Failure” study (NCT03133793) will examine the efficacy of ubiquinol, the reduced form of coenzyme Q10, which acts as an AO, to improve symptom severity and cardiac function in HFpEF. However, this trial is still in the recruitment phase, with no interim results. Thus, to our knowledge, this is the first study to evaluate the impact of acute AO administration in HFpEF and to report a favorable effect on vascular function in this patient group.
The observed improvement in FMD extends previous findings from our group in a variety of clinical populations characterized by overt vascular dysfunction. Indeed, using the same AO formulation as in the present study, we have previously demonstrated the efficacy of this intervention to acutely disrupt oxidative stress and improve FMD in the elderly (43), as well as patients with COPD (18), and HFrEF (41). Interestingly, the magnitude of increase in FMD following AO administration in these previous studies (~50–60%) (18, 41, 43) is strikingly similar to the ~60% improvement in FMD in the present study (Fig. 2, A and B). Taken together, these previous and current data provide clear evidence for the efficacy of a simple, over-the-counter AO formulation to improve vascular function across the cardiovascular disease spectrum. The significance of such a marked increase in endothelial function should not be underestimated. Indeed, in a recent meta-analysis, Inaba et al. (17) reported that a 1% decrease in brachial artery FMD, adjusted for confounding risk factors, was associated with an 8% increase in risk for future cardiovascular events. In this context, the ~2% increase in FMD following AO consumption observed in the present study appears to represent a clinically meaningful improvement in vascular health with the potential to mitigate cardiovascular risk in patients with HFpEF, although additional studies utilizing a longitudinal design are needed to explore this interesting possibility. Furthermore, whereas this assessment was performed on the brachial artery, our group has previously identified a similar or greater magnitude of vascular reactivity in the lower compared with upper limbs (31), which gives credence to the observed changes in the brachial artery possibly being generalized to other arterial beds.
RH and AO in HFpEF.
Whereas the FMD test provides an assessment of vascular function in the conduit artery, it fails, per se, to provide information about vascular function at the level of the microcirculation. RH, however, provides an index of microvascular function, quantifying the overall increase in blood flow following arterial occlusion, and can serve to complement results from FMD testing by providing an evaluation of microvascular function. Like FMD, RH is inversely related to cardiovascular disease risk and is predictive of future cardiovascular events, such as myocardial infarction, unstable angina, and all-cause mortality (30), in healthy and diseased populations (15), and therefore is viewed as a clinically relevant vascular assessment.
Unlike results from studies focused on FMD, there is a general consensus regarding the presence of microvascular dysfunction in HFpEF. Marechaux et al. (29) reported a lower peak hyperemic response to FMD as well as attenuated microvascular function in HFpEF. Furthermore, Akiyama et al. (1) utilized peripheral arterial tonometry to identify a decrement in RH in HFpEF, which was attributed to microvascular dysfunction, despite a preserved FMD response. That investigation identified an association between microvascular function in HFpEF and adverse cardiovascular outcomes, further highlighting the importance of microvascular health in HFpEF (1). More recently, our group confirmed these previous findings, identifying a marked (≈30%) reduction in RH in HFpEF compared with healthy, age-matched controls (27). Together, these studies provide clear evidence of microvascular dysfunction in HFpEF and raises the question whether AO administration might favorably influence this decline in vascular health in this patient population.
Contrary to our hypothesis, in the present investigation, post-occlusion RH was unchanged following AO administration (Fig. 4, A and B), suggesting this intervention is insufficient to favorably influence microvascular function in HFpEF. Interestingly, this lack of effect agrees with previous studies from our group that also failed to document improvements in RH following AO administration in the elderly, patients with COPD, and heart transplant patients (18, 41, 43). Although the discrepancy between the impact of AO administration on conduit vessels (Figs. 2 and 3) and the microvasculature (Fig. 4) are not immediately obvious, it is likely related to the unique mechanisms governing these distinct regions of the arterial tree. In contrast to FMD, the assessment of RH quantifies the overall increase in blood flow following a period of vascular occlusion, and recent work suggests that the factors mediating this response are quite complex and likely involve primarily non-NO pathways (27). Therefore, when we viewed it in conjunction with the increase in plasma concentration, we observed that, following AO administration (Table 4), it is perhaps not surprising that the AO intervention utilized in the present study selectively improved conduit artery vascular function without a change in RH, findings that are aligned with the purported pathways underlying the two tests.
Inflammation, oxidative stress, and NO bioavailability.
In this study, we evaluated a host of plasma biomarkers to comprehensively evaluate the potential of AO administration to favorably influence inflammation and oxidative stress in HFpEF. While plasma ascorbate levels were higher than previously reported in HFrEF or age-matched controls in the PL condition, the magnitudes of increase in plasma ascorbate following AO administration were similar to previously reported increases (50%), showing the validity of the AO cocktail administration to increase plasma ascorbate concentrations (42). Interestingly, following AO administration we observed a marked reduction in CRP (a marker of inflammation) and an increase in (a NO bioavailability marker), whereas additional biomarkers of oxidative stress, free radical concentration, and antioxidant status were unchanged (Table 4). The anti-inflammatory effect may be particularly meaningful in this patient group, as there is increasing evidence that chronic inflammation, driven by comorbidities such as obesity, hypertension, diabetes mellitus, COPD, and chronic kidney disease, may represent an important aspect of HFpEF etiology. Furthermore, a decrease in CRP was recently found to be associated with a decrease in 3-year death risk only in decompensated acute heart failure but not HFrEF, suggesting a reduction in CRP and inflammation is clinically meaningful and should not be taken lightly, especially for patients with HFpEF (28). While the conventional target for the AOs utilized in the present study was O2-centered free radicals (34), it is noteworthy that α-lipoic acid (20), vitamin C (10), and vitamin E (36) have each been shown to be anti-inflammatory, as assessed by plasma CRP in various cardiovascular diseases. Thus, while the observed AO-induced reduction in inflammation was not hypothesized, there is some precedent for this noncanonical, anti-inflammatory effect of AO administration. Also noteworthy is that following AO administration there was a significant increase in plasma , a biomarker of NO, reflecting the degree of vascular dysfunction and correlating with cardiovascular risk factors (24). Recently, NO metabolites have been shown to be reduced in patients with HFpEF but not HFrEF (5), further suggesting endothelial dysfunction and NO deficiencies in patients with HFpEF, which may be improved with AO administration. Although it was somewhat surprising to observe this improvement in NO2 in the absence of changes in plasma free radical concentration or biomarkers of oxidative stress, it nonetheless supports the efficacy of AO administration to acutely improve NO signaling in a cohort with recognized impairments in this important regulatory pathway.
Experimental considerations.
In this study, we enrolled patients with HFpEF on optimized pharmacotherapy, and no medications were withheld on experimental days. Importantly, exploratory analyses that stratified responses according to drug class failed to identify any effect of pharmacotherapy on the observed changes in FMD or RH. We also enrolled patients regardless of comorbidities such as hypertension, diabetes, and coronary artery disease. Although this approach may have introduced some heterogeneity for our baseline parameters, it provided an opportunity to study the typical HFpEF pathophysiology, which was representative of the diverse nature of this patient population. We also acknowledge that circulating biomarkers assessed from venous blood samples are unable to detect potential regional differences in plasma concentration at the local level. It is noteworthy that some discrepancy between plasma concentrations of NT-pro-BNP and BNP was observed, which may be attributed to the significantly longer plasma half-life of NT-pro-BNP compared with BNP (8). The duration between study visits was between 2 and 7 days. While we recognize the possibility that this nonstandardized time interval could have contributed to the variability of FMD results (39), the use of a randomized study design minimized the possibility that the observed effect of AO administration on vascular assessments could be explained by intersubject variation in time between study visits.
Conclusions.
The current investigation has identified, for the first time, the efficacy of an acute, over-the-counter dose of AOs to mitigate inflammation and improve conduit artery vasodilation in HFpEF, providing new insight into the mechanisms that govern peripheral vascular dysfunction in this patient group.
Perspectives and Significance
Exercise intolerance is the hallmark symptom of HFpEF, and exercise training is widely believed to improve vascular health across the disease spectrum. However, it is interesting to note that several long-duration training interventions have been ineffective at improving FMD in HFpEF (2, 22, 38). Acute AO consumption has previously been shown to improve skeletal muscle perfusion during exercise, albeit in healthy older individuals, giving further credibility to the use of AOs to improve exercise capacity among those with chronic inflammation (44). Additionally, reducing systemic inflammation with the chronic use of the immunosuppressant anakinra improved aerobic capacity in patients with HFpEF (40), further suggesting that chronic inflammation is a critical component of exercise intolerance in HFpEF. Attenuating inflammation in patients with HFpEF may therefore be necessary to improve endothelial function and subsequent exercise tolerance.
GRANTS
This work was funded in part by the National Heart, Lung, and Blood Institute (HL-118313) the U.S. Department of Veterans Affairs (RX001311, RX001697, RX009275, CX001183), and the American Heart Association (18POST33960192).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.M.R., H.L.C., and D.W.W. conceived and designed research; S.M.R., H.L.C., J.R.G., D.T.L.S., T.S.T., K.B., J.K.A., and D.W.W. performed experiments; S.M.R., J.R.G., D.T.L.S., T.S.T., and K.B. analyzed data; S.M.R. interpreted results of experiments; D.W.W. prepared figures; S.M.R., J.K.A., R.S.R., J.B.W., J.J.R., and D.W.W. drafted manuscript; S.M.R., H.L.C., J.R.G., D.T.L.S., T.S.T., K.B., J.K.A., R.S.R., J.B.W., J.J.R., and D.W.W. edited and revised manuscript; S.M.R., H.L.C., J.R.G., D.T.L.S., T.S.T., K.B., J.K.A., R.S.R., J.B.W., J.J.R., and D.W.W. approved final version of manuscript.
REFERENCES
- 1.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 60: 1778–1786, 2012. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 2.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985) 119: 753–758, 2015. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56: 845–854, 2010. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 5.Chirinos JA, Akers SR, Trieu L, Ischiropoulos H, Doulias PT, Tariq A, Vasim I, Koppula MR, Syed AA, Soto-Calderon H, Townsend RR, Cappola TP, Margulies KB, Zamani P. Heart failure, left ventricular remodeling, and circulating nitric oxide metabolites. J Am Heart Assoc 5: 004133, 2016. [Erratum in J Am Heart Assoc 6: e002139, 2017.] doi: 10.1161/JAHA.116.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifton HL, Machin DR, Groot HJ, Frech TM, Donato AJ, Richardson RS, Wray DW. Attenuated nitric oxide bioavailability in systemic sclerosis: evidence from the novel assessment of passive leg movement. Exp Physiol 103: 1412–1424, 2018. doi: 10.1113/EP086991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosentino F, Lüscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res 43: 274–278, 1999. doi: 10.1016/S0008-6363(99)00134-0. [DOI] [PubMed] [Google Scholar]
- 8.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet 362: 316–322, 2003. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 9.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 162: 966–972.e10, 2011. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Ellulu MS, Rahmat A, Patimah I, Khaza’ai H, Abed Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther 9: 3405–3412, 2015. doi: 10.2147/DDDT.S83144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrero M, Blanco I, Batlle M, Santiago E, Cardona M, Vidal B, Castel MA, Sitges M, Barbera JA, Perez-Villa F. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 7: 791–798, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000942. [DOI] [PubMed] [Google Scholar]
- 12.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 26: 65–69, 2005. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 13.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci 68: 161–167, 2013. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, Stewart KP, Link KM, Herrington DM, Kitzman DW. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol 292: H1427–H1434, 2007. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 17.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 18.Ives SJ, Harris RA, Witman MA, Fjeldstad AS, Garten RS, McDaniel J, Wray DW, Richardson RS. Vascular dysfunction and chronic obstructive pulmonary disease: the role of redox balance. Hypertension 63: 459–467, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol 19: 918–925, 1992. doi: 10.1016/0735-1097(92)90271-N. [DOI] [PubMed] [Google Scholar]
- 20.Khabbazi T, Mahdavi R, Safa J, Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J Ren Nutr 22: 244–250, 2012. doi: 10.1053/j.jrn.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto S, Kajikawa M, Maruhashi T, Iwamoto Y, Matsumoto T, Iwamoto A, Oda N, Matsui S, Hidaka T, Kihara Y, Chayama K, Goto C, Aibara Y, Nakashima A, Noma K, Higashi Y. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int J Cardiol 231: 181–187, 2017. doi: 10.1016/j.ijcard.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol 62: 584–592, 2013. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288: 2144–2150, 2002. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 40: 295–302, 2006. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 84: 1589–1596, 1991. doi: 10.1161/01.CIR.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 26.Lam CSP, Chandramouli C. Fat, female, fatigued: features of the obese HFpEF Phenotype. JACC Heart Fail 6: 710–713, 2018. doi: 10.1016/j.jchf.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Lee JF, Barrett-O’Keefe Z, Garten RS, Nelson AD, Ryan JJ, Nativi JN, Richardson RS, Wray DW. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart 102: 278–284, 2016. doi: 10.1136/heartjnl-2015-308403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lourenço P, Pereira J, Ribeiro A, Ferreira-Coimbra J, Barroso I, Guimarães JT, Leite-Moreira A, Bettencourt P. C-reactive protein decrease associates with mortality reduction only in heart failure with preserved ejection fraction. J Cardiovasc Med (Hagerstown) 20: 23–29, 2019. doi: 10.2459/JCM.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 29.Maréchaux S, Samson R, van Belle E, Breyne J, de Monte J, Dédrie C, Chebai N, Menet A, Banfi C, Bouabdallaoui N, Le Jemtel TH, Ennezat PV. Vascular and microvascular endothelial function in heart failure with preserved ejection fraction. J Card Fail 22: 3–11, 2016. doi: 10.1016/j.cardfail.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Matsuzawa Y, Li J, Aoki T, Guddeti RR, Kwon TG, Cilluffo R, Widmer RJ, Gulati R, Lennon RJ, Lerman LO, Lerman A. Predictive value of endothelial function by noninvasive peripheral arterial tonometry for coronary artery disease. Coron Artery Dis 26: 231–238, 2015. doi: 10.1097/MCA.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiyama SK, Wray DW, Richardson RS. Sex and limb-specific ischemic reperfusion and vascular reactivity. Am J Physiol Heart Circ Physiol 295: H1100–H1108, 2008. doi: 10.1152/ajpheart.00318.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 33.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138: 861–870, 2018. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292: H1516–H1522, 2007. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 35.Rossman MJ, Trinity JD, Garten RS, Ives SJ, Conklin JD, Barrett-O’Keefe Z, Witman MA, Bledsoe AD, Morgan DE, Runnels S, Reese VR, Zhao J, Amann M, Wray DW, Richardson RS. Oral antioxidants improve leg blood flow during exercise in patients with chronic obstructive pulmonary disease. Am J Physiol Heart Circ Physiol 309: H977–H985, 2015. doi: 10.1152/ajpheart.00184.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr 25: 151–174, 2005. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC; Get With the Guidelines Scientific Advisory Committee and Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 126: 65–75, 2012. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka S, Sanuki Y, Ozumi K, Harada T, Tasaki H. Heart failure with preserved vs reduced ejection fraction following cardiac rehabilitation: impact of endothelial function. Heart Vessels 33: 886–892, 2018. doi: 10.1007/s00380-018-1128-2. [DOI] [PubMed] [Google Scholar]
- 39.van Mil AC, Greyling A, Zock PL, Geleijnse JM, Hopman MT, Mensink RP, Reesink KD, Green DJ, Ghiadoni L, Thijssen DH. Impact of volunteer-related and methodology-related factors on the reproducibility of brachial artery flow-mediated vasodilation: analysis of 672 individual repeated measurements. J Hypertens 34: 1738–1745, 2016. doi: 10.1097/HJH.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 40.Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol 113: 321–327, 2014. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witman MA, Fjeldstad AS, McDaniel J, Ives SJ, Zhao J, Barrett-O’Keefe Z, Nativi JN, Stehlik J, Wray DW, Richardson RS. Vascular function and the role of oxidative stress in heart failure, heart transplant, and beyond. Hypertension 60: 659–668, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witman MA, McDaniel J, Fjeldstad AS, Ives SJ, Zhao J, Nativi JN, Stehlik J, Wray DW, Richardson RS. A differing role of oxidative stress in the regulation of central and peripheral hemodynamics during exercise in heart failure. Am J Physiol Heart Circ Physiol 303: H1237–H1244, 2012. doi: 10.1152/ajpheart.00568.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O’Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol 297: H1870–H1875, 2009. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]