Abstract
Exercise is a potent facilitator of long-term weight loss maintenance (WLM), whereby it decreases appetite and increases energy expenditure beyond the cost of the exercise bout. We have previously shown that exercise may amplify energy expenditure through energetically expensive nutrient deposition. Therefore, we investigated the effect of exercise on hepatic de novo lipogenesis (DNL) during WLM and relapse to obesity. Obese rats were calorically restricted with (EX) or without (SED) treadmill exercise (1 h/day, 6 days/wk, 15 m/min) to induce and maintain weight loss. After 6 wk of WLM, subsets of WLM-SED and WLM-EX rats were allowed ad libitum access to food for 1 day to promote relapse (REL). An energy gap-matched group of sedentary, relapsing rats (REL-GM) were provided a diet matched to the positive energy imbalance of the REL-EX rats. During relapse, exercise increased enrichment of hepatic DN-derived lipids and induced hepatic molecular adaptations favoring DNL compared with the gap-matched controls. In the liver, compared with both REL-SED and REL-GM rats, REL-EX rats had lower hepatic expression of genes required for cholesterol biosynthesis; greater hepatic expression of genes that mediate very low-density lipoprotein synthesis and secretion; and greater mRNA expression of Cyp27a1, which encodes an enzyme involved in the biosynthesis of bile acids. Altogether, these data provide compelling evidence that the liver has an active role in exercise-mediated potentiation of energy expenditure during early relapse.
Keywords: de novo lipogenesis, exercise, obesity/energy balance, nutrient regulation, weight gain
INTRODUCTION
America has an alarming prevalence of obesity, with more than one-third of adults and 17% of adolescents diagnosed as obese (29). At any given time, ~70% of obese women and 63% of obese men are trying to lose weight (6); unfortunately, less than 20% of these individuals maintain a 10% weight-reduction after 1 yr (20, 53). Several biological adaptations to weight loss have been implicated in this high incidence of weight regain (13, 51). Changes in neuroendocrine and metabolic systems act to defend a higher body weight during caloric restriction, resulting in a suppression of energy expenditure and an increase in appetite (24). This drive to regain weight and defend a higher body weight has been shown to be particularly robust and resolute in rodent models and may help explain the high rates of weight regain in obesity (21, 22). Addressing this powerful biological response and designing ways to ameliorate it is critical for successfully facilitating long-term weight loss maintenance (WLM) (27).
There is a growing body of evidence that supports exercise as an effective strategy to offset the biological drive to regain weight and promote WLM (7, 17, 36, 50). In preclinical models, we have demonstrated that exercise decreases the impetus to overeat and, when positive energy imbalance is controlled for, generates a beneficial thermogenic effect during relapse (43). Of note, on the first day of relapse, the energetic cost of the exercise bout was only 3.5 ± 0.3 kcals, yet exercisers expended ~10 kcals more than controls matched for positive energy imbalance. Moreover, resting energy expenditure and nonexercise activity were not different between relapsing exercisers and their gap-matched controls (43). These data indicated that resting and activity energy expenditure did not completely account for the difference in total energy expenditure (TEE) observed with exercise on the first day of relapse compared with controls in a similar positive energy imbalance. Rather, because TEE comprises resting energy expenditure, activity energy expenditure, and diet-induced thermogenesis (DIT), through process of elimination, these results suggested that exercise may increase TEE during relapse by potentiating DIT.
A component of DIT is the energetic cost of storing nutrients, and it has been previously shown that nutrient storage efficiency increases during weight regain (25). During a relapse from WLM, fat oxidation is suppressed and carbohydrate oxidation increases to meet energetic demands (19). This pattern of nutrient utilization facilitates the storage of dietary fat into triglyceride depots, a process that requires little energy (~98% efficiency) (19, 37). Alternatively, storing excess carbohydrates or proteins as fat through de novo lipogenesis (DNL) is an energetically expensive process (~75% efficiency) that may offset rapid weight regain by increasing energy expenditure (37, 40).
We previously observed greater dietary fat oxidation, greater retention of DN-derived lipids in liver and retroperitoneal (RP) adipose tissue, and a lower expression of genes that encode proteins in the DNL pathway in RP adipose tissue of relapsing exercising rats compared with sedentary controls in a similar positive energy imbalance. Because exercise during relapse lowered lipogenic gene expression in adipose tissue despite increasing retention of DN-derived lipids in adipose tissue and liver (18, 43), we hypothesized that regular exercise throughout WLM potentiates hepatic DNL during relapse. The current study utilizes tissues from our parent study (43) and uniquely examines the impact of regular exercise on hepatic molecular adaptations mediating DNL during WLM and early relapse. This study design was based on a rodent paradigm modeling weight regain after long-term WLM that included a ‘gap-match’ group, in which sedentary rats were given a food provision to match the positive energy imbalance that the relapsing exercise rats experienced. This approach allowed us to study the adaptive responses in the liver irrespective of exercise’s direct effects on appetite and expenditure.
RESEARCH DESIGN AND METHODS
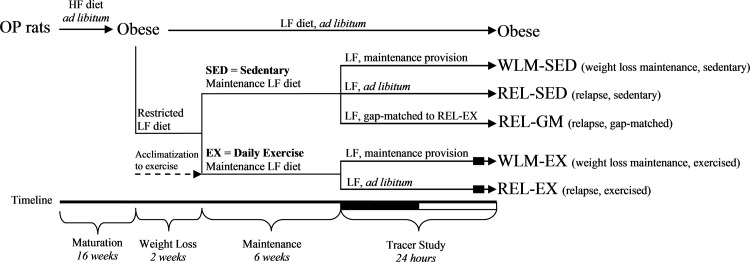
Experimental paradigm of weight loss maintenance and regain.
The current study utilizes tissues from animals in our previously published parent study, which has been described in detail (18, 43) and is depicted in Fig. 1. Briefly, male Wistar rats were individually housed (22–24°C; 12:12-h light-dark cycle) with free access to water. Obesity-prone rats were placed on an ad libitum high-fat diet (46% kcal fat, Research Diets; cat. no. D12344) for 12 wk to induce obesity. A subset of animals was switched to an ad libitum low-fat diet (LFD; 25% kcal fat, Research Diets, cat. no. D07091301) for the remainder of the study (obese group). The remaining animals were placed on the same LFD but were restricted to ~60% of the calories they had eaten ad libitum for 2 wk, which reduced body weight by ~17%, and were supplied enough diet to maintain this lower body weight for 6 wk. A subset of WLM rats was allowed ad libitum access to LFD for 24 h after the 6-wk maintenance period to promote relapse to obesity. Animals in the obese, relapsing sedentary, and relapsing exercise groups were not fasted before euthanization. However, only a single provision of food was dropped at the initiation of the dark cycle on the final day of study. WLM rats ate the entirety of their food provision in the first 3 h of the provision; thus, they were fasted for at least 20 h. Relapsing gap-matched rats ate the entirety of their food provision in the first 18 h of the provision; thus, they were without food for at least 6 h. The obese, relapsing sedentary, and relapsing exercise rats were provided food in excess. All procedures were approved by the Institutional Animal Care and Use Committee.
Fig. 1.
Experimental groups and study design. Study design to investigate the effect of exercise (EX) in weight loss maintenance (WLM) and relapsing (REL) conditions compared with sedentary (SED) rats. The REL-GM (relapsing, gap-matched) group was a sedentary group of weight-reduced rats that were given a specific amount of food on the tracer day so that their positive energy imbalance matched that of the REL-EX group. Rats were placed in the metabolic monitoring system for the final 4 days of the study. Exercise occurred within 3 h of the dark cycle, and estimated time of exercise is depicted as filled in boxes. HF diet, high-fat diet; LF diet, low-fat diet; OP, obesity prone. This figure is adapted from the initial study, originally published in Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011 (43).
Experimental groups.
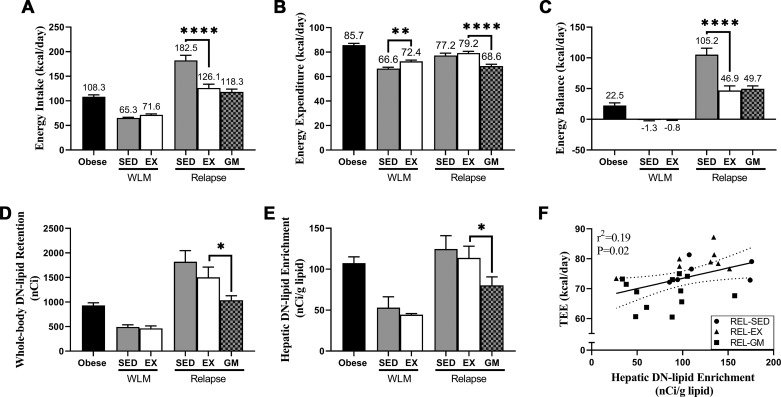
Study design is depicted in Fig. 1. At the initiation of weight loss, animals were assigned to either sedentary conditions or exercise interventions. After 6 wk of weight maintenance, subsets of exercise and sedentary rats were allowed to relapse to obesity (ad libitum access to LFD) during the final 24 h of study. A sedentary, relapsing group was gap-matched (REL-GM) to the same positive energy balance as relapsing exercise (REL-EX) rats. The amount of food provided to the REL-GM rats was determined by titrating down food availability in sedentary relapsing rats so that caloric excess (positive energy imbalance) matched relapsing exercising rats. Our planned comparisons included weight loss-maintained exercising (WLM-EX) versus weight loss-maintained sedentary (WLM-SED) rats, REL-EX versus relapsing sedentary (REL-SED) rats, and REL-EX versus REL-GM rats. Gap-matching is depicted in Fig. 2, A–C, whereby energy intake (Fig. 2A) in a subset of sedentary rats was limited so that when energy expenditure (Fig. 2B) was accounted for, REL-EX and REL-GM rats were in a similar positive energy imbalance (Fig. 2C).
Fig. 2.
Energetics and de novo (DN) lipid retention during weight regain after weight loss. Energy balance and the net retention of DN-derived lipid ([3H]triglyceride) was studied over 24 h in obese, weight loss maintained (WLM), and relapsing rats (REL), with WLM and REL rats remaining sedentary (SED) or performing a daily bout of treadmill exercise (EX). An additional group of relapsing sedentary animals was added to the study design, in which the amount of overfeeding was limited to match their positive energy imbalance with relapsing exercised animals [gap-matched (GM)]. A: energy intake was assessed over final 24 h. B: total energy expenditure (TEE) was calculated from indirect calorimetry and urinary nitrogen measurements. C: energy balance was assessed over the final 24 h of the experiment. D: total body DN-derived lipid was estimated by summing up the absolute amounts of 3H-lipid in the liver, skeletal muscle, and adipose tissue. E: hepatic 3H enrichment was assessed in liver lipid extracts at the end of the 24-h period. Data were analyzed by ANOVA, with planned comparisons (n = 7–12 per group; *P < 0.05; **P < 0.01; ****P < 0.0001). F: association between hepatic DN lipid enrichment and TEE in relapsing rats, as determined by Pearson’s product-moment correlation (n = 26). These data are adapted from the initial report of the dual-tracer study, originally published in Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011 (43).
Exercise regimen.
All exercise was performed as previously described (43). Briefly, rats were acclimatized over 2 wk to run on a treadmill at 15 m/min for 1 h/day, 6 days/wk. Acclimatization began at beginning of the weight loss phase, and exercise was performed throughout WLM and on the first day of relapse. All exercise was completed during the 3-h window before the start of the dark cycle (between 11:00 AM and 2:00 PM) or, for the final day of study, just before euthanization. Timing for exercise is depicted in Fig. 1.
Energy expenditure and balance.
Energy expenditure and balance was assessed during the final 4 days of the study for obese and WLM groups and the final day of study for the relapsing groups, as described in detail in the parent study (43). In short, rats were placed in a metabolic monitoring system modified for performing in vivo tracer studies in combination with indirect calorimetry (Oxymax CLAMS-8M; Columbus Instruments, Columbus, OH). Metabolic rate (MR) was calculated every 16 min using the formula: MR = [3.815 + 1.232 (carbon dioxide consumption/oxygen consumption)] × oxygen consumption. MR was extrapolated over 24 h to derive TEE. Data were not normalized to body mass; however, body weights and composition can be found in Supplemental Figure S1 (All Supplemental Material is available at https://doi.org/10.6084/m9.figshare.7824230.v4). Energy balance was calculated as energy intake minus TEE.
De novo lipogenesis.
In the current study, we outline previously unpublished findings on DN-derived lipids. The incorporation of tritium (3H2O) into lipid pools was used to estimate the net retention of carbons via lipogenesis, the majority of which are DN-derived over a 24-h period. Animals were administered a 250 μCi intraperitoneal injection of 3H2O 2 h before the start of the final dark period, which allows for equilibration with total body water (9). At the conclusion of the 24-h tracer study, tissues were harvested and lipid pools were extracted, as previously described, to determine 3H retention and enrichment in the lipid fractions of liver, skeletal muscle, and adipose tissue (19). Specifically, DN lipid enrichment is calculated as specific activity of 3H in extracted lipid, normalized to the weight of the extracted lipid. Net DN lipid retention is a calculation based off enrichment:
Western blot and analysis.
Frozen livers were pulverized (~80 mg) and homogenized in ice-cold lysis buffer (50 mM Tris, pH 7.4; 150 mM NaCl, 2.0 mM EDTA, 50 mM NaF, 5.0 mM sodium orthovanadate, 1% Triton X-100, 1% deoxycholate, 0.1% SDS) and supplemented with Halt Protease and Phosphatase Inhibitor cocktail (Thermo Scientific, Waltham, MA). Lysates were centrifuged for 20 min at 14,000 g at 4°C, and total protein concentration of the supernatant was determined by bicinchoninic acid assay (Pierce Thermo Fisher, Waltham, MA). Protein was identified and quantified by capillary electrophoresis size-based sorting via the WES, and data were analyzed with Compass software (ProteinSimple, San Jose, CA). Antibodies used included fatty acid synthase (FASN) (1:1,000, cat. no. 3180, Cell Signaling, Danvers, MA), ATP citrate lyase (ACLY) (1:200; cat. no. 4332, Cell Signaling), acetyl-CoA carboxylase 1 (ACC1) (1:200; cat. no. 4190, Cell Signaling), phospho-acetyl-CoA carboxylase [Serine (Ser)79] (1:200; cat. no. 3661, Cell Signaling), insulin receptor substrate 2 (IRS-2) (1:25; cat. no. 4502, Cell Signaling), Akt (1:50; cat. no. 9272, Cell Signaling), phospho-Akt (Ser473) (1:50; cat. no. 9271, Cell Signaling), forkhead box O1 (FoxO1) (1:25; cat. no. NBP2-31376, Novus Biologicals, Littleton, CO), phospho-FoxO1 (Ser256) (1:100; cat. no. NB-100-81927, Novus Biologicals), glycogen synthase kinase β (GSK3β) (1:50; cat. no. 12456, Cell Signaling), and phospho-GSK3β (1:50; cat. no. 5558, Cell Signaling). All proteins were normalized to either β-actin (1:50; cat. no. 4970, Cell Signaling) or Vinculin (1:15,000; cat. no. V9131, Sigma-Aldrich, St. Louis, MO). Phosphorylated proteins were further normalized to their respective protein content.
mRNA studies.
Frozen livers were pulverized (~40 mg) and homogenized in QIAzol, and RNA was subsequently isolated using the RNAeasy mini kit according to manufacturer’s instructions (Qiagen, Germantown, MD). Total RNA quality was determined using Agilent Tape Station 4200 (Agilent, Santa Clara, CA). Total RNA (100 ng) was used as input to construct mRNA libraries from the NuGEN Universal Plus mRNA-Seq protocol part no. 9133 (NuGEN, Redwood City, CA). Sequencing was done on an Illumina NovaSEQ6000 instrument using an S4 flow cell and 2×150 paired end sequencing (Illumina, San Diego, CA). Generated reads were mapped to the rat genome using gSNAP and Fragments Per Kilobase of transcript per Million mapped reads (FPKM) were derived using Cufflinks (54). Genes were removed if their FPKM expression was less than 1 in 13 of 15 samples or if their Ensembl identification did not have an associated Entrez identifier. mRNA sequencing data can be found at the GEO accession number GSE135700.
Histological analysis.
At euthanization, a portion of each liver was fixed in 10% formalin, embedded, and sectioned (4 µm). Sections were stained with hematoxylin and eosin. Periodic acid–Schiff +/− diastase stains were used to detect liver glycogen deposits. To measure lipid accumulation, livers were stained for adipophilin by immunofluorescence as follows: antigen retrieval was performed with Antigen Unmasking Solution no. H-3300 (Vector Laboratories, Burlingame, CA). Sections were treated with 0.2% glycine (30 min) and then blocked with 10% normal donkey serum and 0.1 mg/ml saponin in PBS (60 min). Sections were incubated with primary antibody adipophilin (1:100 in PBS; cat. no. 20R-AP002, Fitzgerald Industries Acton, MA) overnight at 4̊C. This was followed by incubation with Cy3-conjugated donkey anti-guinea pig (1:100; cat. no. 706-165-148, Jackson Immunoresearch, West Grove, PA) and then 0.5 g/ml DAPI for 45 min at room temperature.
Blood metabolite and endocrine analyses.
All blood metabolites and hormones were originally measured in our parent study (12, 43). Plasma insulin was determined using the Rat Endocrine Lincoplex Kit (RENDO-85K; Linco Research/Millipore, St. Charles, MO). Plasma cholesterol and glucose were determined via colorimetric assays (43).
Nuclear magnetic resonance on liver extracts.
Aqueous and lipid metabolites were extracted from liver tissues using 12% perchloric acid (Sigma-Aldrich) as previously described (33, 39). Two-dimensional (2D)-H, C-HSQC (heteronuclear single quantum correlation) spectra were obtained on a Bruker 500 MHz DRX spectrometer (Bruker Bisopin, Fremont, CA) using an inverse 5-mm TXI probe (38), metabolites were quantified as µmol/g of tissue according to Rudolph et al. (33), and tissue metabolite chemical shifts were verified relative to a chemical shift database of pure compounds (5).
Statistical analysis.
Data were analyzed by ANOVA using a Fisher’s least significant difference test on the planned comparisons in GraphPad Prism 8 (GraphPad Software, La Jolla, CA). Pearson product-moment correlations were used to measure associations. Differentially regulated pathways in the RNA sequencing data sets were assessed in the R programming environment (45) (version 3.6.0) using the Generally Applicable Gene-set Enrichment for Pathway Analysis (GAGE) package (23). Significance was set at P < 0.05. When applicable, data were expressed as means ± SE.
RESULTS
Whole body and hepatic de novo lipogenesis.
We estimated net retention of carbons from lipogenesis, the majority of which are DN-derived via tritium incorporation into lipid pools (43). In data adapted from our initial report, we found that total body DN-derived lipids (as estimated by summing net retention of DN lipids within skeletal muscle, adipose tissue, and liver tissue) were significantly elevated in the REL-EX rats compared with the REL-GM rats (Fig. 2D). DN lipid enrichment in liver was also significantly elevated in the REL-EX rats compared with that of the REL-GM rats (Fig. 2E). Interestingly, enrichment of DN-derived lipids in the liver positively correlated with TEE in relapsing animals (Fig. 2F), suggesting that hepatic DNL influences TEE when in a positive energy imbalance.
Hepatic lipogenic protein expression.
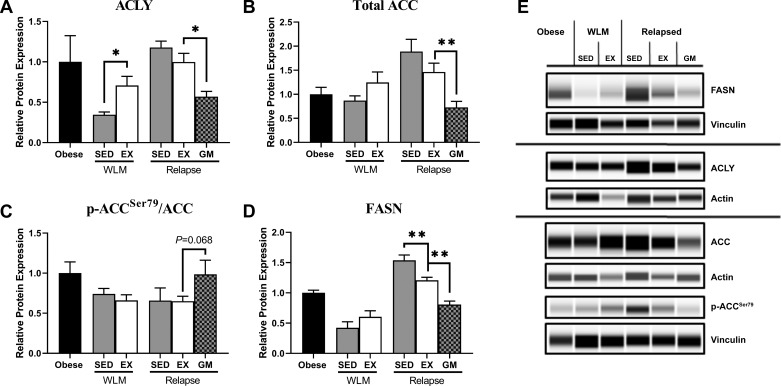
Increased enrichment of DN-derived lipid in hepatic tissue in the REL-EX animals prompted us to explore the DNL pathway. Protein expression of hepatic ACLY was elevated in the WLM-EX rats compared with the WLM-SED rats (Fig. 3, A and E). This exercise-induced expression was still apparent on the first day of relapse (REL-EX) when compared with REL-GM rats (Fig. 3, A and E). Protein expression of ACC and FASN was significantly higher in the REL-EX rats compared with the REL-GM rats, and the REL-SED rats had significantly higher expression of FASN than the REL-EX rats did (Fig. 3, B, D, and E). Phosphorylation of ACC at Ser79 trended toward being significantly lower in REL-EX rats compared with REL-GM rats (Fig. 3, C and E). Electropherograms are presented in Supplemental Figure S2. These data suggest that exercise can lead to molecular adaptations in the liver that favor activation of the energetically expensive DNL pathway.
Fig. 3.
Exercise potentiates hepatic de novo lipogenic signaling during relapse. Protein expression of ATP citrate lyase (ACLY; A), total acetyl-CoA carboxylase (ACC; B), phosphorylated ACC, serine79 (p-ACCSer79; C), and fatty acid synthase (FASN; D). Proteins were normalized to either β-actin or vinculin and total protein if applicable and expressed as a proportion of the obese values. Representative images of FASN, ACLY, p-ACCSer79, and ACC from whole cell liver lysates are shown (E). Data were analyzed by ANOVA, with planned comparisons (n = 4 per group; *P < 0.05; **P < 0.01). EX, exercise; GM, gap-matched; SED, sedentary; WLM, weight loss maintenance.
Effect of exercise on hepatic insulin signaling.
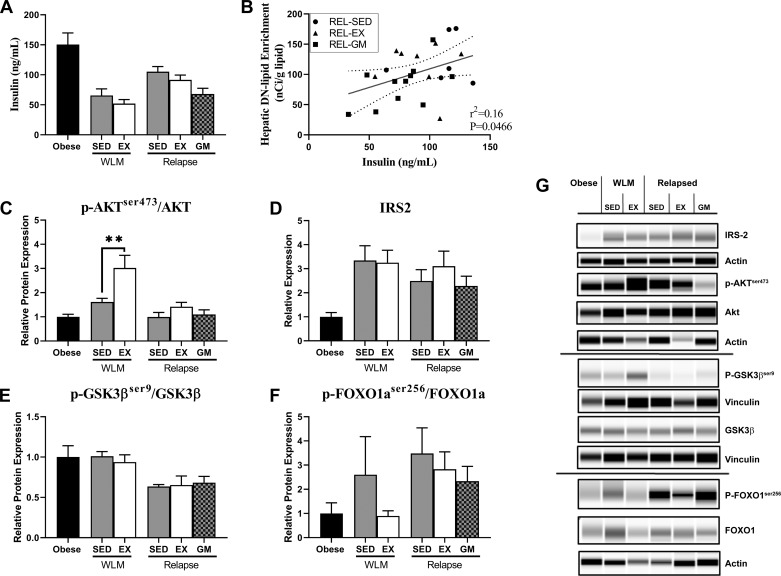
Insulin is capable of activating the DNL pathway (49). Although there were no differences in serum insulin observed between the planned comparisons (Fig. 4A), serum insulin positively correlated with hepatic DN lipid enrichment in relapsing rats (Fig. 4B). This relationship was supported by positive correlations between circulating insulin levels and hepatic FASN protein expression as well as net retention of DN-derived lipids in the liver of WLM and REL rats (FASN, P = 0.005; Retention, P = 0.0002; Supplemental Table S1).
Fig. 4.
Effect of exercise on hepatic insulin signaling. A: plasma insulin adapted from the initial report of the dual-tracer study, originally published in Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011 (43). B: association between circulating insulin and hepatic de novo (DN) lipid enrichment in relapsing rats (n = 26), as determined by Pearson’s product-moment correlation. C: protein expression of phosphorylated protein kinase B, serine 473 (p-AKTSer473). D: protein expression of insulin receptor substrate (IRS) 2. E: protein expression of phosphorylated glycogen synthase kinase β, serine 9 (p-GSK3βSer9). F: protein expression of phosphorylated forkhead transcription factor 1, serine 256 (p-FoxO1Ser256). Proteins were normalized to either β-actin or vinculin and total protein if applicable and expressed as a proportion of the obese values. G: representative images of IRS, AKT, p-AKTSer473, GSK3β, p-GSK3βSer9, FOXO1, and p-FOXO1Ser256. Data were analyzed by ANOVA, with planned comparisons (n = 4 per group; **P < 0.01). EX, exercise; GM, gap-matched; SED, sedentary; WLM, weight loss maintenance.
These data led us to further investigate mechanisms of insulin regulation of lipogenesis in this model. During WLM, exercise significantly increased Akt phosphorylation at serine 473 (Ser473) (WLM-EX vs. WLM-SED; Fig. 4, C and G). However, in the relapsing rats, Akt phosphorylation did not change with exercise (Fig. 4, C and G). Furthermore, in our planned comparisons, we did not find any effect of exercise on IRS-2 expression, phosphorylation of GSK3β at the inhibitory Ser9 site, or phosphorylation of FOXO1 at the Akt-targeted inhibitory site, Ser256 (Fig. 4, D–G). Electropherograms and quantitation of total protein levels are available in Supplemental Figure S2.
RNA sequencing in relapsing rats.
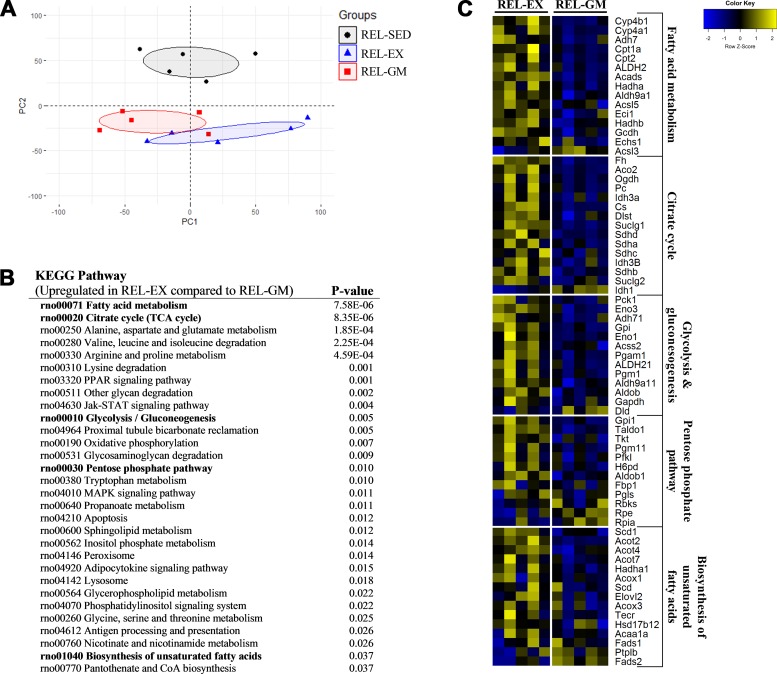
We then examined the transcriptome of relapsing rats using next generation RNA sequencing (RNA-seq). Principle component analysis revealed a distinct transcriptional profile of the REL-SED rats (Fig. 5A). In a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis between REL-EX and REL-GM rats, 56 pathways were differentially regulated (Supplemental Table S2). Of note, some of the most upregulated pathways were involved in nutrient catabolism and anabolism (Fig. 5B). Extracting the most differentially regulated genes from these pathways confirms their upregulation in REL-EX compared with REL-GM rats (Fig. 5C). KEGG pathway analysis between REL-SED and REL-EX rats can be found in Supplemental Table S3.
Fig. 5.
Liver transcriptome during relapse. A: principle component analysis of transcriptome from relapsing rats. Ellipses represent 95% confidence interval. B: 30 most upregulated Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways by exercise during relapse (REL-EX) compared with relapsing energy gap-matched sedentary (REL-GM) rats). Pathways are ranked in ascending order based on P value. C: top 15 most differentially regulated genes from select KEGG pathways from Fig. 5B (in bold). Not all genes are significantly different. If KEGG pathways comprised ≤15 genes, all genes in the pathway were included. Genes are sorted in descending order based on fold change between REL-EX and REL-GM (n = 5 per group). PC, principle component; SED, sedentary.
Transcription factors regulating de novo lipogenesis.
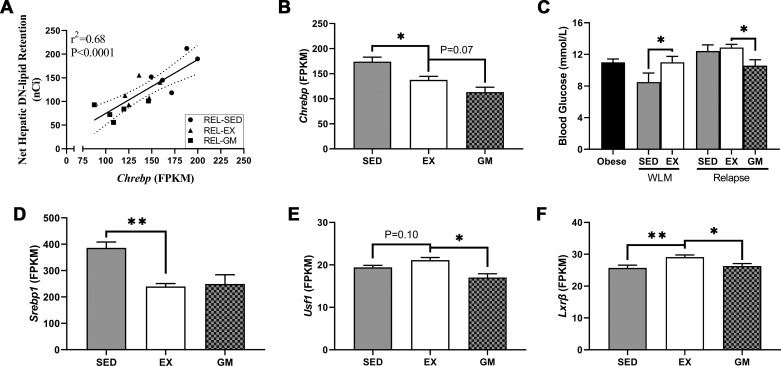
Next, we mined the RNA-seq data set for transcription factors that are known to mediate DNL (49). There was a strong positive correlation between carbohydrate-response element-binding protein mRNA (Chrebp) and hepatic DN lipid retention in all relapsing rats (Fig. 6A). Chrebp was upregulated in REL-SED compared with REL-EX, and there was a trend for greater Chrebp expression in REL-EX compared with REL-GM rats (Fig. 6B). Additionally, plasma glucose was found to be elevated with exercise during WLM and relapse when compared with REL-GM (Fig. 6C). Sterol regulatory element-binding protein 1 mRNA (Srebp1) was upregulated in REL-SED rats but not differentially expressed between REL-EX and REL-GM (Fig. 6D). Upstream stimulatory factor 1 mRNA (Usf1) was upregulated in REL-EX compared with REL-GM rats (Fig. 6E). Liver X receptor β mRNA (Lxrβ) was upregulated in REL-EX compared with both sedentary groups (Fig. 6F).
Fig. 6.
Transcriptional regulation of de novo lipogenesis (DNL) during relapse. RNA-sequencing data from relapsing rats were mined to determine differential expression of genes that encode transcription factors that mediate DNL. A: association between hepatic carbohydrate-response element-binding protein (Chrebp) expression and net hepatic DN lipid retention (n = 15), as determined by Pearson’s product-moment correlation. B: mRNA expression of Chrebp. C: plasma glucose adapted from the initial report of the dual-tracer study, originally published in Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011 (43). D: mRNA expression of sterol regulatory element-binding protein 1 (Srebp1). E: mRNA expression of upstream stimulatory factor 1 (Usf1). F: mRNA expression of liver X receptor β (Lxrβ). Data were analyzed by ANOVA, with planned comparisons (n = 5 per group for gene expression data, n = 7–12 for plasma glucose data; *P < 0.05; **P < 0.01). EX, exercise; FPKM, Fragments Per Kilobase of transcript per Million mapped reads; GM, gap-matched; REL, relapse; SED, sedentary; WLM, weight loss maintenance.
Exercise-induced regulation of genes required for cholesterol and bile acid biosynthesis during relapse.
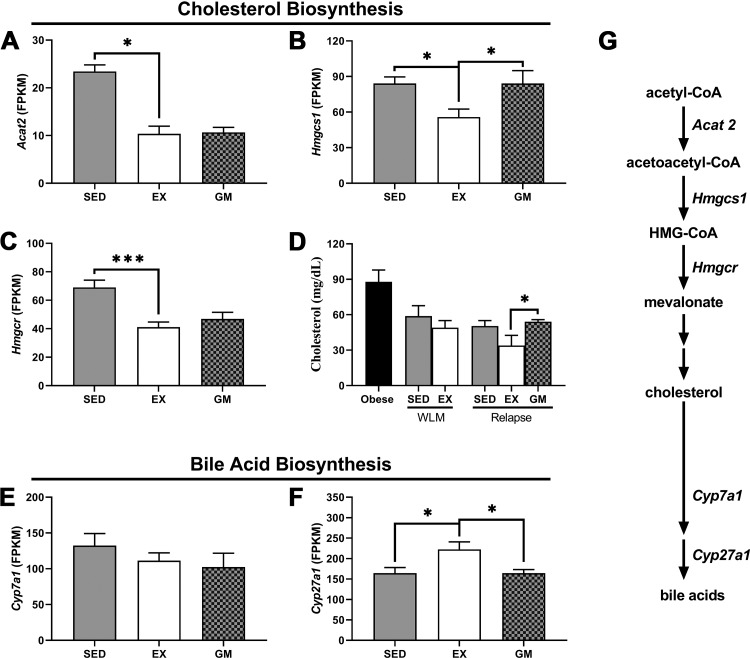
In the process of DNL, cytoplasmic acetyl-CoA can be shuttled toward fatty acid biosynthesis or the mevalonate pathway, which generates cholesterol. The mevalonate pathway appeared to be downregulated with exercise during relapse, as acetyl-Coenzyme A acetyltransferase 2 mRNA (Acat2) was lower in REL-EX compared with REL-SED (Fig. 7A); hydroxymethylglutaryl-CoA synthase 1 mRNA (Hmgcs1) was lower with exercise compared with both sedentary relapsing controls (Fig. 7B); and hydroxymethylglutaryl-CoA reductase mRNA (Hmgcr) was lower in REL-EX compared with REL-SED (Fig. 7C). Moreover, serum cholesterol was lower in REL-EX compared with REL-SED (Fig. 7D). Despite an apparent decrease in the pathway that produces cholesterol, which is a precursor to bile acid synthesis, sterol 27-hydroxylase mRNA (Cyp27a1) was upregulated in REL-EX compared with both sedentary groups (Fig. 7F). Cholesterol 7 α-hydroxylase (Cyp7a1) was not differentially regulated (Fig. 7E). A simplified version of the mevalonate pathway and downstream bile acid synthesis is depicted in Fig. 7G.
Fig. 7.
Cholesterol and bile acid biosynthesis during relapse. RNA-sequencing data from relapsing rats were mined to determine differential expression of genes that encode proteins in the mevalonate or bile acid biosynthesis pathway. Upper mevalonate pathway regulation was assessed by investigating acetyl-Coenzyme A acetyltransferase 2 (Acat2; A), hydroxymethylglutaryl-CoA synthase 1 (Hmgcs1; B) and hydroxymethylglutaryl-CoA reductase (Hmgcr; C) mRNA expression. Plasma cholesterol levels adapted from the initial report of the dual-tracer study, originally published in Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011 (43), are shown (D). Bile acid biosynthesis was assessed by investigating cholesterol 7 α-hydroxylase (Cyp7a1; E) and sterol 27-hydroxylase (Cyp27a1; F) mRNA expression. Data were analyzed by ANOVA, with planned comparisons (n = 5 per group for gene expression data, n = 7–12 for plasma cholesterol data; *P < 0.05;***P < 0.001). Simplified diagram of the mevalonate pathway and downstream cholesterol and bile acid biosynthesis is shown (G). EX, exercise; GM, gap-matched; SED, sedentary; WLM, weight loss maintenance.
Central role for hepatic de novo lipogenesis in whole body de novo-derived lipid content.
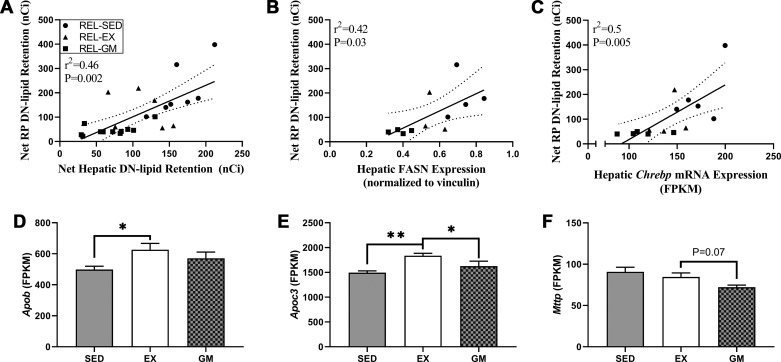
In data adapted from our parent study, we found significantly greater net retention of DN-derived lipids in RP adipose tissue of REL-EX rats compared with REL-GM rats (18). However, in this same cohort of rats, we found significantly lower mRNA expression of Chrebp and Fasn in RP adipose tissue of REL-EX compared with REL-GM rats (18). These data suggested that the greater DN lipid retention in RP adipose tissue of REL-EX compared with REL-GM rats was likely derived from another lipogenic tissue. Therefore, we investigated the relationship between hepatic DNL and retention of DN-derived lipids in RP adipose tissue. In relapsing rats, net retention of DN-derived lipids in the liver positively correlated with net retention of DN-derived lipids in retroperitoneal adipose tissue (Fig. 8A). Net retention of DN-derived lipids in retroperitoneal adipose tissue also positively correlated with FASN protein abundance and Chrebp mRNA expression in the liver of relapsing rats (Fig. 8, B and C). We also mined the RNA-seq data set for genes involved in very low-density lipoprotein (VLDL) synthesis and secretion and found that apolipoprotein B mRNA (Apob) was greater in REL-EX compared with REL-GM rats (Fig. 8D); apolipoprotein C3 mRNA (Apoc3) was greater in REL-EX compared with both relapsing controls (Fig. 8E); and microsomal triglyceride transfer protein mRNA (Mttp) was trending toward being upregulated in REL-EX compared with REL-GM rats (Fig. 8F). In all, these data suggest that hepatic DN-derived lipids are secreted into circulation and taken up by peripheral tissues.
Fig. 8.
Central role for hepatic de novo lipogenesis (DNL) in peripheral DN lipid retention during relapse (REL). Association between net retention of DN-derived lipids in the liver and net retention of DN-derived lipids in retroperitoneal (RP) adipose tissue (n = 25) is shown (A). Association between fatty acid synthase (FASN) protein abundance in the liver and net retention of DN-derived lipids in RP adipose tissue (n = 11) is shown (B). Relationship between carbohydrate-response element-binding protein (Chrebp) mRNA expression in the liver and net retention of DN-derived lipids in RP adipose tissue (n = 11) is shown (C). All associations determined by Pearson’s product-moment correlation (A–C). RNA-sequencing data were mined to assess expression of genes mediating very low-density lipoprotein synthesis and secretion, including apolipoprotein B (Apob; D) apolipoprotein C3 (Apoc3; E) and microsomal triglyceride transfer protein (Mttp; F) mRNA expression. Data were analyzed by ANOVA, with planned comparisons (n = 5 per group; *P < 0.05; **P < 0.01). EX, exercise; GM, gap-matched; SED, sedentary; WLM, weight loss maintenance.
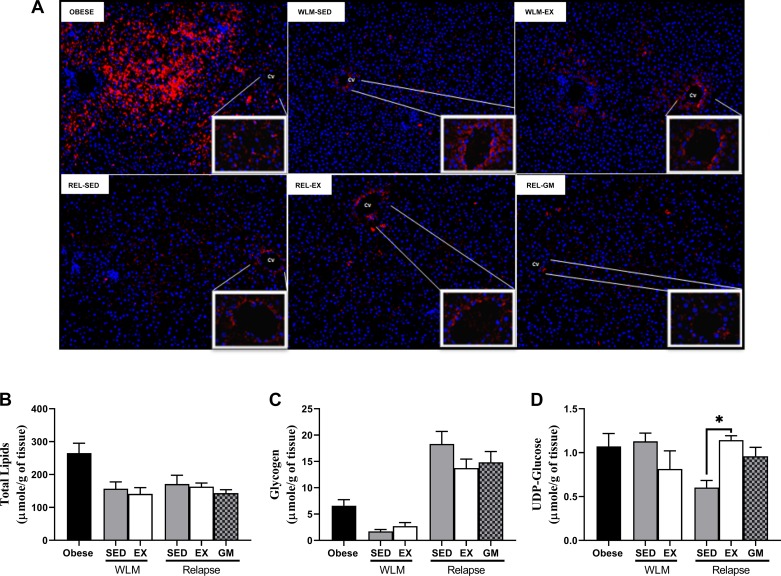
Hepatic lipid accumulation, as visualized by adipophilin immunofluorescence, was qualitatively similar among the weight-reduced rats and relapsing rats (Fig. 9A). Consistent with the immunofluorescence images (Fig. 9A), total liver lipids [as determined by nuclear magnetic resonance (NMR)] were similar across all WLM and relapsing groups (Fig. 9B). We observed no significant change in glycogen levels (as measured via NMR) (Fig. 9C) in any of our planned comparisons, which was consistent with the similar levels of phosphorylated GSK3β at ser9 (Fig. 4C), a negative regulator of glycogen synthase. This data is qualitatively reflected through Periodic acid–Schiff staining +/− diastase of the livers (Supplemental Fig. S3). However, we also measured UDP-glucose, an active glucose donor for the synthesis of glycogen, and found significantly higher levels of UDP-glucose in the REL-EX rats compared with both the REL-SED (P < 0.001) and REL-GM rats (P < 0.05, Fig. 9D).
Fig. 9.
Hepatic lipid and glycogen during weight loss maintenance (WLM) and weight regain. A: hepatic lipid was visualized by immunofluorescence employing an adipophilin/perilipin 2 (plin2) primary antibody, specific for lipid droplets, and a DAPI stain for nuclei. B: liver lipid. C: glycogen. D: UDP-glucose. n = 4–8 for all panels; *P < 0.05. EX, exercise; GM, gap-matched; REL, relapse; SED, sedentary.
DISCUSSION
The novel observation of this study is that exercise potentiates hepatic lipogenic capacity during the relapse to obesity in a preclinical paradigm of weight regain, as demonstrated by increased hepatic protein expression and activation of critical enzymes involved in the DNL pathway. This suggests that the liver is not simply a passive organ in exercise-induced shifts in nutrient trafficking during weight regain. The liver, like skeletal muscle and adipose tissue, displays distinct changes in metabolic pathways at the molecular level that suggest its direct involvement in mediating the effects of exercise. This unique metabolic context juxtaposes enhanced hepatic lipogenic capacity with suppressed lipogenic capacity in adipose tissue (18), while muscle exhibits an enhanced capacity to utilize fat for energy production (43). Taken together, these data suggest that regular exercise alters metabolic pathways in liver, muscle, and adipose tissue during weight regain in a manner that directs the deposition of excess energy through the energetically expensive pathway of hepatic DNL. This favorable shift in fuel utilization may explain, in part, why exercise counters the biological drive to regain weight and facilitates long-term WLM (26).
In our parent study, we found a significant increase in the oxidation of dietary fat and reduced trafficking of dietary fat toward adipose tissue storage in exercising compared with the sedentary rats with the same energy imbalance (43). These findings were associated with an increase in the expression of genes that favor the uptake, mobilization, and oxidation of fat in skeletal muscle, which suggest a shift in fuel utilization that favors fat for energy production in response to excess nutrients. The energetic expense of depositing glucose and protein that must be converted to fat through DNL is substantially higher than that of storing dietary fat and, as such, relapsing exercised animals may have expended more energy over the day to store the same nutrient excess. Supporting this notion, we also found differential expression of lipogenic pathway genes in both liver and adipose tissue. Adipose tissue exhibited a decrease in expression of genes that stimulate lipid uptake and triglyceride synthesis, but at the end of the 24-h relapse period, adipose tissue was enriched with DN-derived lipids (18). Here, we found that regular bouts of exercise increased gene and protein expression of enzymes required for DNL in the liver, indicating that exercise potentiated hepatic lipogenic capacity during a relapse. Consistent with the signaling changes observed in hepatic tissue, we found that the liver lipids were enriched with tritium, a surrogate marker for DN-derived lipids.
Although our observations show an increase in the lipogenic capacity of the liver, it is less clear how exercise redirects this liver-driven nutrient trafficking. Regulation of hepatic DNL may occur through transcriptional regulation of enzymes involved in fatty acid synthesis via activation of insulin signaling or increasing concentrations of glucose in response to overfeeding (34, 49). Some of our data implicate the involvement of both glucose and insulin signaling in mediating an increase in hepatic DNL with exercise. Hepatic ChREBP, a transcription factor that transcribes genes in the DNL pathway, is activated in response to a postprandial rise in hepatocyte glucose levels and metabolites from the pentose phosphate pathway (1, 34). We found that hepatic Chrebp mRNA trended toward being elevated with REL-EX compared with REL-GM and, in all relapsing rats, positively correlated with DN lipid retention in the liver. Moreover, during relapse, exercise increased plasma glucose and expression of genes in the pentose phosphate pathway compared with gap-matched controls. As for insulin signaling, phosphorylation of AKT at Ser473 was significantly higher in response to exercise during WLM, and plasma insulin positively correlated with hepatic DN lipid enrichment during relapse. However, we did not observe changes in phosphorylation of proteins known to be downstream of AKT. Additionally, Srebp1 mRNA, which increases in response to insulin (16), was upregulated in REL-SED versus REL-EX rats but not differentially regulated in REL-EX compared with REL-GM rats, suggesting that its transcription was mediated by energy balance rather than exercise. Further research is required to determine a role for insulin and glucose signaling, and their downstream targets ChREBP and SREBP1, in exercise-induced DNL.
We also investigated transcription factors that coordinate with SREBP1 and ChREBP to promote lipogenic programming. We found that Lxrβ and Usf1 mRNA are upregulated with exercise during relapse irrespective of energy balance. LXRs are known to regulate lipogenesis via direct activation of lipogenic genes (49) and upregulation of SREBP1 and ChREBP (8, 31). USF1 recruits SREBP1 to lipogenic promoter regions and is required for insulin-mediated transcription of FASN (47, 48). Although our data do not explain why Lxrβ and Usf1 are upregulated with exercise, their upregulation may explain the potentiation of DNL with exercise during relapse when energy balance is controlled.
Our pathway analysis between REL-EX and REL-GM groups indicates an increased flux of nutrients available to produce substrates and cofactors for DNL (increased fatty acid metabolism, citric acid cycle, glycolysis/gluconeogenesis, and pentose phosphate pathways). Of note, these data, along with the elevated ACLY, suggest an increase in cytosolic acetyl-CoA, a substrate that is a precursor required for fatty acid, cholesterol, or bile acid biosynthesis. Prior research has demonstrated that exercise can decrease cholesterol biosynthesis and increase bile acid biosynthesis (14, 28). Our data support these previous findings, as we found that genes involved in cholesterol biosynthesis were downregulated and genes involved in bile acid biosynthesis were upregulated with exercise during relapse. Meanwhile, genes and proteins specific to fatty acid biosynthesis were upregulated in REL-EX compared with REL-GM, suggesting that, with exercise, acetyl-CoA may be selectively shuttled toward fatty acid biosynthesis.
DNL is a source of intrahepatocellular lipids in the pathogenesis of nonalcoholic fatty liver disease under specific conditions (40); thus, it is conceivable that exercise might increase susceptibility to nonalcoholic fatty liver disease during relapse to obesity. However, our study examines the independent effects of regular exercise before a brief period of overfeeding, designed to mimic the process of weight regain or relapse to obesity, and is not a model in which the liver is primed for pathological lipid accumulation. In accordance, despite finding an increase in DNL in REL-EX compared with REL-GM, we measured total lipid levels via NMR at the conclusion of the study and found a lack of hepatic lipid accumulation. Moreover, we found a greater expression of genes required for VLDL synthesis and secretion, suggesting that lipids are being secreted into circulation and made available to metabolically active tissues. Our previous reports are consistent with these findings, where we observed enrichment of DN-derived lipid in adipose and skeletal muscle lipid pools of relapsing exercising rats, despite lower expression of genes that encode proteins in the DNL pathway (skeletal muscle unpublished observations) (18, 43). Even so, we do not have any direct measures of hepatic VLDL secretion to confirm this hypothesis in the present studies. Our studies simply measure the retention of DN-derived lipid in the liver and peripheral tissues at a given time point. Further studies are needed to more clearly elucidate the flux of nutrients in and out of the liver throughout the day in both the weight-reduced and weight-regain metabolic conditions.
Other tissues and mechanisms may also be responsible for the exercise-induced increase in energy expenditure. Brown/beige fat and skeletal muscle are thought to have a role in resting metabolic rate and dietary-induced thermogenesis (11, 15, 32), and both tissues are favorably impacted by exercise. Exercise triggers an upregulation of uncoupling proteins in adipose tissue (41, 42) and may increase skeletal muscle mitochondrial uncoupling capacity (3, 4). Additionally, exercise training has been shown to potentiate sympathetic signaling after weight loss (10, 44), which may impact total energy expenditure with a bout of overfeeding. However, increasing adipose uncoupling protein 1 expression or activating the sympathetic nervous system does not appear to increase energy expenditure during weight regain (12, 30). Regardless, further analyses of these tissues and mechanisms are required to dissect their importance during weight regain.
This study is not without limitations, one of which is the timing of the exercise intervention. Circadian rhythm is known to have a powerful influence over metabolism, and recent studies have demonstrated that time of exercise impacts systemic metabolism differently (35, 46). We exercised rats during the light cycle just before the dark cycle, which would be equivalent to early morning exercise. Interestingly, it has been shown that exercise in the morning leads to greater weight loss (52). Moreover, morning exercise might decrease energy intake more than exercise later in the day (2); therefore, the timing of our exercise intervention may, in part, explain the lesser energy intake we observed between REL-EX and REL-SED rats. An additional concern is that the time of exercise chosen may interfere with the sleep cycle; however, in our studies, we consistently find that sedentary animals are awake during the time period we exercise rats (unpublished observations). Nevertheless, future studies are warranted to determine the effect of exercise timing in a weight loss and weight regain paradigm.
Overall, we have provided preclinical evidence that regular exercise defends a lower body weight during weight regain by not only reducing food intake, but increasing energy expenditure beyond the cost of the exercise as well. We uniquely demonstrated that chronic exercise increases the lipogenic capacity of the liver during relapse, which directs nutrients to energetically inefficient pathways of deposition. Altogether, these data provide evidence that the liver has an active role in exercise-mediated potentiation of energy expenditure during early relapse and improves our understanding of exercise’s impact on nutrient trafficking when in a positive energy imbalance.
GRANTS
This work was supported by grants from the National Institutes of Health (NIH; Grant Nos. DK-38088 and DK-67403 to P. S. MacLean). We also utilized the Energy Balance and Metabolic core laboratories within the Colorado Clinical Nutrition Research Unit, which receives funding from the NIH (Grant No. DK-48520).
DISCLAIMERS
P. S. MacLean is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M.P., L.A.C., M.R.J., A.J.S., M.C.R., and P.S.M. conceived and designed research; D.M.P., L.A.C., E.D.G., J.A. Houck, P.G.W., A.J.S., G.C.J., and P.S.M. performed experiments; D.M.P., L.A.C., K.L.J., M.C.R., and P.S.M. analyzed data; D.M.P., L.A.C., M.R.J., J.A. Higgins, K.L.J., E.D.G., M.C.R., and P.S.M. interpreted results of experiments; D.M.P. and L.A.C. prepared figures; D.M.P., L.A.C., and K.L.J. drafted manuscript; D.M.P., L.A.C., M.R.J., J.A. Higgins, K.L.J., E.D.G., J.A. Houck, M.C.R., and P.S.M. edited and revised manuscript; D.M.P., L.A.C., M.R.J., J.A. Higgins, E.D.G., J.A. Houck, P.G.W., G.C.J., M.C.R., and P.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Katrina Diener of the Genomics Core at the University of Colorado Anschutz Medical Campus for performing RNA sequencing.
REFERENCES
- 1.Abdul-Wahed A, Guilmeau S, Postic C. Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab 26: 324–341, 2017. doi: 10.1016/j.cmet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh Z, Younespour S, Rajabian Tabesh M, Haghravan S. Comparison between the effect of 6 weeks of morning or evening aerobic exercise on appetite and anthropometric indices: a randomized controlled trial. Clin Obes 7: 157–165, 2017. doi: 10.1111/cob.12187. [DOI] [PubMed] [Google Scholar]
- 3.Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257–31266, 2007. doi: 10.1074/jbc.M706129200. [DOI] [PubMed] [Google Scholar]
- 4.Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA 105: 16701–16706, 2008. doi: 10.1073/pnas.0808889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrends M, Hirose R, Serkova NJ, Coatney JL, Bedolli M, Yardi J, Park YH, Niemann CU. Mild hypothermia reduces the inflammatory response and hepatic ischemia/reperfusion injury in rats. Liver Int 26: 734–741, 2006. doi: 10.1111/j.1478-3231.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 6.Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW III, Khan LK. Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes Res 13: 596–607, 2005. doi: 10.1038/oby.2005.64. [DOI] [PubMed] [Google Scholar]
- 7.Catenacci VA, Grunwald GK, Ingebrigtsen JP, Jakicic JM, McDermott MD, Phelan S, Wing RR, Hill JO, Wyatt HR. Physical activity patterns using accelerometry in the National Weight Control Registry. Obesity (Silver Spring) 19: 1163–1170, 2011. doi: 10.1038/oby.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha J-Y, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem 282: 743–751, 2007. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 9.Commerford SR, Pagliassotti MJ, Melby CL, Wei Y, Hill JO. Inherent capacity for lipogenesis or dietary fat retention is not increased in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 280: R1680–R1687, 2001. doi: 10.1152/ajpregu.2001.280.6.R1680. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge L, Moreira EA, Martin CK, Ravussin E; Pennington CALERIE Team . Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity (Silver Spring) 18: 414–416, 2010. doi: 10.1038/oby.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucet E, Tremblay A, Simoneau JA, Joanisse DR. Skeletal muscle enzymes as predictors of 24-h energy metabolism in reduced-obese persons. Am J Clin Nutr 78: 430–435, 2003. doi: 10.1093/ajcn/78.3.430. [DOI] [PubMed] [Google Scholar]
- 12.Dulloo AG, Seydoux J, Girardier L. Dissociation of enhanced efficiency of fat deposition during weight recovery from sympathetic control of thermogenesis. Am J Physiol 269: R365–R369, 1995. doi: 10.1152/ajpregu.1995.269.2.R365. [DOI] [PubMed] [Google Scholar]
- 13.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 6: 67–85, 2005. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 14.Farahnak Z, Magri Tomaz L, Bergeron R, Chapados N, Lavoie JM. The effect of exercise training on upregulation of molecular markers of bile acid metabolism in the liver of ovariectomized rats fed a cholesterol-rich diet. ARYA Atheroscler 13: 184–192, 2017. [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, Le Lièpvre X, Berthelier-Lubrano C, Spiegelman B, Kim JB, Ferré P, Foufelle F. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol 19: 3760–3768, 1999. doi: 10.1128/MCB.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foright RM, Presby DM, Sherk VD, Kahn D, Checkley LA, Giles ED, Bergouignan A, Higgins JA, Jackman MR, Hill JO, MacLean PS. Is regular exercise an effective strategy for weight loss maintenance? Physiol Behav 188: 86–93, 2018. doi: 10.1016/j.physbeh.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giles ED, Steig AJ, Jackman MR, Higgins JA, Johnson GC, Lindstrom RC, MacLean PS. Exercise decreases lipogenic gene expression in adipose tissue and alters adipocyte cellularity during weight regain after weight loss. Front Physiol 7: 32, 2016. doi: 10.3389/fphys.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, MacLean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol 294: R1117–R1129, 2008. doi: 10.1152/ajpregu.00808.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, Sciamanna CN. Long-term weight loss maintenance in the United States. Int J Obes 34: 1644–1654, 2010. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 278: R231–R237, 2000. doi: 10.1152/ajpregu.2000.278.1.R231. [DOI] [PubMed] [Google Scholar]
- 22.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 10: 161, 2009. doi: 10.1186/1471-2105-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol 301: R581–R600, 2011. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R1306–R1315, 2004. doi: 10.1152/ajpregu.00463.2004. [DOI] [PubMed] [Google Scholar]
- 26.MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, Giles ED, Brown IE, Hill JO. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 297: R793–R802, 2009. doi: 10.1152/ajpregu.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, Levin BE, Perri MG, Rolls BJ, Rosenbaum M, Rothman AJ, Ryan D. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 23: 7–15, 2015. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 218: 323–329, 2011. doi: 10.1016/j.atherosclerosis.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 29.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311: 806–814, 2014. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Presby DM, Jackman MR, Rudolph MC, Sherk VD, Foright RM, Houck JA, Johnson GC, Orlicky DJ, Melanson EL, Higgins JA, MacLean PS. Compensation for cold-induced thermogenesis during weight loss maintenance and regain. Am J Physiol Endocrinol Metab 316: E977–E986, 2019. doi: 10.1152/ajpendo.00543.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev 14: 2819–2830, 2000. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowland LA, Maurya SK, Bal NC, Kozak L, Periasamy M. Sarcolipin and uncoupling protein 1 play distinct roles in diet-induced thermogenesis and do not compensate for one another. Obesity (Silver Spring) 24: 1430–1433, 2016. doi: 10.1002/oby.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph MC, McManaman JL, Phang T, Russell T, Kominsky DJ, Serkova NJ, Stein T, Anderson SM, Neville MC. Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics 28: 323–336, 2007. doi: 10.1152/physiolgenomics.00020.2006. [DOI] [PubMed] [Google Scholar]
- 34.Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc 91: 452–468, 2016. doi: 10.1111/brv.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato S, Basse AL, Schönke M, Chen S, Samad M, Altıntaş A, Laker RC, Dalbram E, Barrès R, Baldi P, Treebak JT, Zierath JR, Sassone-Corsi P. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab 30: 92–110.e4, 2019. doi: 10.1016/j.cmet.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr 66: 551–556, 1997. doi: 10.1093/ajcn/66.3.551. [DOI] [PubMed] [Google Scholar]
- 37.Schutz Y. Concept of fat balance in human obesity revisited with particular reference to de novo lipogenesis. Int J Obes Relat Metab Disord 28, Suppl 4: S3–S11, 2004. doi: 10.1038/sj.ijo.0802852. [DOI] [PubMed] [Google Scholar]
- 38.Serkova NJ, Jackman M, Brown JL, Liu T, Hirose R, Roberts JP, Maher JJ, Niemann CU. Metabolic profiling of livers and blood from obese Zucker rats. J Hepatol 44: 956–962, 2006. doi: 10.1016/j.jhep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31: 15–24, 2007. doi: 10.1152/physiolgenomics.00028.2007. [DOI] [PubMed] [Google Scholar]
- 40.Solinas G, Borén J, Dulloo AG. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol Metab 4: 367–377, 2015. doi: 10.1016/j.molmet.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stallknecht B, Andersen PH, Vinten J, Bendtsen LL, Sibbersen J, Pedersen O, Galbo H. Effect of physical training on glucose transporter protein and mRNA levels in rat adipocytes. Am J Physiol Endocrinol Metab 265: E128–E134, 1993. doi: 10.1152/ajpendo.1993.265.1.E128. [DOI] [PubMed] [Google Scholar]
- 42.Stallknecht B, Vinten J, Ploug T, Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am J Physiol Endocrinol Metab 261: E410–E414, 1991. doi: 10.1152/ajpendo.1991.261.3.E410. [DOI] [PubMed] [Google Scholar]
- 43.Steig AJ, Jackman MR, Giles ED, Higgins JA, Johnson GC, Mahan C, Melanson EL, Wyatt HR, Eckel RH, Hill JO, MacLean PS. Exercise reduces appetite and traffics excess nutrients away from energetically efficient pathways of lipid deposition during the early stages of weight regain. Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011. doi: 10.1152/ajpregu.00212.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev 36: 391–397, 1996. doi: 10.1051/rnd:19960405. [DOI] [PubMed] [Google Scholar]
- 45.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.r-project.org. [Google Scholar]
- 46.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Sul HS. Upstream stimulatory factor binding to the E-box at -65 is required for insulin regulation of the fatty acid synthase promoter. J Biol Chem 272: 26367–26374, 1997. doi: 10.1074/jbc.272.42.26367. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Sul HS. Upstream stimulatory factors bind to insulin response sequence of the fatty acid synthase promoter. USF1 is regulated. J Biol Chem 270: 28716–28722, 1995. doi: 10.1074/jbc.270.48.28716. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Viscarra J, Kim S-J, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol 16: 678–689, 2015. [Erratum in Nat Rev Mol Cell Biol 17: 64, 2016.] doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE. Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr 75: 499–504, 2002. doi: 10.1093/ajcn/75.3.499. [DOI] [PubMed] [Google Scholar]
- 51.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999-2002. Am J Prev Med 33: 34–40, 2007. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 52.Willis EA, Creasy SA, Honas JJ, Melanson EL, Donnelly JE. The effects of exercise session timing on weight loss and components of energy balance: midwest exercise trial 2. Int J Obes. In press. doi: 10.1038/s41366-019-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 82, Suppl: 222S–225S, 2005. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 54.Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26: 873–881, 2010. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]