Abstract
Objective:
To compare a sample of adolescents with sport-related concussion (SRC) who were prescribed rest with 2 arms of a randomized controlled trial comparing aerobic exercise with placebo-like stretching. We also compared sex differences across the 3 approaches to treatment.
Design:
Quasi-experimental trial.
Setting:
University concussion management clinics.
Participants:
Adolescent athletes (aged 13-18 years) presenting within 10 days of SRC (mean, 5 days after injury) received a recommendation for rest (rest group [RG], n=48, 15.4±1y, 25% female). Their outcomes were compared with matched samples of adolescents assigned to aerobic exercise (exercise group [EG], n=52, 15.3±2y, 46% female) or placebo-like stretching (placebo group [PG], n=51, 15.4±2y, 47% female) (N=151).
Main Outcome Measures:
The primary outcome was median days from injury to recovery. The secondary outcome was proportion classified as normal recovery (<30d) or delayed recovery (≥30d).
Results:
The RG recovered in 16 days (interquartile range, 9.25-23.25d), which was significantly delayed (P=.020) compared with EG (13d; interquartile range, 10-18.5d). The PG recovered in 17 days (interquartile range, 13-23d). Four percent of the EG, 14% of the PG, and 13% of the RG had delayed recovery (P=.190). There was no difference in recovery time or delayed recovery between male participants and female participants across groups. Female participants prescribed rest experienced an increase in symptoms vs the other groups (P=.013).
Conclusion:
Relative rest and a placebo-like stretching program were very similar in days to recovery and symptom improvement pattern after SRC. Both conditions were less effective than subsymptom threshold aerobic exercise. Female adolescents appear to be susceptible to symptom increase when prescribed rest.
Keywords: Adolescent, Brain concussion, Exercise, Exercise test, Postconcussion syndrome, Rehabilitation
Concussion, a subtype of mild traumatic brain injury, is the result of sudden deceleration and rotational forces applied to the brain that trigger an acute and subacute pathophysiological metabolic response in the absence of gross structural changes to the brain.1,2 The incidence of sport-related concussion (SRC) is high in contact sports. In the United States alone, it is estimated that there are 1.6 million to 3.8 million sport-related traumatic brain injuries a year, the majority of them being concussion.3 The management of concussion has changed significantly over the years. The first summary and agreement statement of the 2001 consensus conference on concussion in sport held in Vienna recommended rest followed by a graduated return to play.4 This statement, combined with animal research that showed exercise interfered with upregulation of brain-derived neurotrophic factor (BDNF) and impeded brain recovery,5 provided practitioners with a cornerstone for concussion management: rest until asymptomatic.
Despite the fact that prescribed rest has been the treatment of choice for almost 20 years,6 there has been surprisingly little research to support it.7 Three systematic reviews on treatment for SRC concluded that there is little evidence to support the value of rest.7-9 Two uncontrolled investigations from the same concussion clinic reported benefit of prescribed rest to the cognitive performance with patients delayed recovery.6,10,11 In a randomized controlled trial (RCT) on concussed adolescents from the emergency department, however, Thomas et al12 found that participants prescribed 5 days of strict rest reported more symptoms and had slower symptom resolution than those prescribed usual care (1 or 2 days of rest followed by stepwise return to activity).
In a recent RCT of subsymptom threshold aerobic exercise vs a placebo-like progressive stretching program prescribed within 1 week of SRC, Leddy et al13 demonstrated that aerobic exercise safely reduced recovery time for adolescent athletes. While this study represents the strongest evidence to date for an active early concussion treatment, it did not evaluate recovery for the most commonly prescribed treatment: rest. The Leddy study also did not investigate the influence of sex. Research on sex differences in concussion recovery has produced conflicting results.13,14 Some studies indicate that girls and women recover more slowly, whereas other studies show no differences between the sexes. It should be noted that none of these studies of sex differences compare outcomes from specific treatments.
The primary purpose of the current study was to add a third comparison group to the 2 treatment arms of the Leddy13 RCT to compare recovery times across the 3 treatment interventions. The third group, from an earlier investigation,14 had identical inclusion criteria and included participants treated with prescribed relative rest, which was consistent with the standard of care at the time. Relative rest is defined as recommending against vigorous exercise and avoiding any activities that exacerbate symptoms. Relative rest is distinguished from strict rest, cognitive rest, or cocooning, all of which discourage all cognitive and athletic activities, including going to school and use of devices.6 The secondary purpose of this study was to compare the recovery trajectory of female patients with male patients. We hypothesized that subthreshold aerobic exercise would speed recovery relative to the other interventions and that female patients in each group would take longer to recover than male patients.
Methods
Study design
This study was approved by the University at Buffalo Institutional Review Board. This was not a randomized trial, but rather a comparison of cohorts who were recruited at different time points. The prescribed rest group (rest group [RG]) was recruited between March 2013 and February 2015 (clinicaltrials.gov: ),15 whereas the placebo stretching group (placebo group [PG]) and the aerobic exercise group (exercise group [EG]) were recruited between September 2015 and June 2018 (clinicaltrials.gov: ). Each cohort comprised adolescents presenting to a university concussion management clinic within 10 days of SRC. Sports medicine physicians diagnosed concussion based on international guidelines,16 which included a concussion history, a symptom questionnaire,17 a cognitive evaluation,18 and a concussion-specific physical examination.19,20 The physical examination used in this study was developed to standardize the examination across physicians and clinics. A patient receives 1 point for each sign (abnormal oculomotor response, vestibular response or signs of neck injury) or indication that performance of test item caused symptoms. If the participant was eligible, a research assistant explained the study and obtained written consent the same day. All groups were prescribed treatment at the initial clinic visit and followed up with the physician weekly for the first 4 weeks or until recovered, whichever came first.
Participants
Participants included male and female adolescents (aged 13-18 years) who sustained SRC within 10 days of initial visit. Exclusion criteria were the following: (1) evidence of focal neurologic deficit; (2) history of moderate or severe traumatic brain injury; (3) current diagnosis of attention deficit hyperactivity disorder, learning disorder, depression, anxiety, or history of >3 prior concussions because these factors are associated with delayed recovery21; (4) inability to understand English; and (5) having a symptom severity score of <5 on initial clinic visit symptom questionnaire. Once the participant had joined the study, we excluded those who did not complete at least 75% of daily symptom reports, and those who missed 3 or more days in a row were considered to have withdrawn from the study (because this interfered with determining the exact date of recovery).
Daily symptom reporting
Participants reported symptoms on a password-protected online data form each day between 7 and 10 PM. To ensure compliance, participants received daily e-mail or text message reminders. The online symptom questionnaire was the Sport Concussion Assessment Tool 3 Post Concussion Symptom Scale (PCSS),22 a validated instrument with normative data for male patients and female patients.23 The PCSS is a 22-item questionnaire, and each item is scored on a 7-point Likert scale (0-6) for a maximum possible symptom severity score of 132.
Treatment interventions
Exercise group
Participants were provided a subthreshold aerobic exercise prescription that was calculated as 80% of the heart rate achieved at symptom exacerbation or voluntary exhaustion on the Buffalo Concussion Treadmill Test (BCTT).24 Previous studies have shown that the BCTT is a reliable method to determine the symptom exacerbation exercise threshold in concussed patients and that performing it within 1 week of injury does not affect recovery.15 Participants were instructed to perform aerobic exercise (eg, walking, jogging, or biking) at home or in a gym under supervision each day for 20 minutes on a treadmill or stationary bike at the prescribed heart rate, with a 5-minute warm-up and a 5-minute cooldown. They were instructed to stop exercising if their symptoms got worse or at 20 minutes, whichever came first. Sports and exercise that rapidly increased heart rate were prohibited. Participants were instructed to perform daily activities (eg, watching television, using phones) as tolerated. Participants were given a Polar heart rate monitora to measure their heart rate at home during exercise. A new target heart rate was determined by weekly clinic BCTT performance for as long as the participant remained symptomatic. Further details about the subthreshold exercise prescription have been reported previously.13
Placebo group
Participants were prescribed a progressive stretching program that included breathing exercises and gentle whole body stretches that would not significantly elevate heart rate. They were provided a booklet (with pictures and instructions) and instructed to perform the exercises for 20 minutes per day. Each week the stretching exercises were slightly more difficult to perform. As with the aerobic exercise group, they were provided with Polar heart rate monitors and were instructed to stop exercising if their symptoms got worse or if their heart rate showed a notable increase from their resting heart rate. They were given the same instructions regarding sports and cognitive pacing as the aerobic exercise group. They performed the BCTT weekly at each clinic visit. Additional details about the placebo stretching program have been reported previously.13
Rest group
Participants were prescribed relative rest according to the previous standard of care.25 They were told that rest was recommended to give their concussed brain a chance to heal. Rest was described as not participating in any sports or other forms of exercise, including gym class. They were also told to limit activities that could exacerbate symptoms such as watching television or using their phones. They were not instructed to remain in a dark room or avoid social interaction. The participants in the rest group had weekly visits with the clinic physician and were also assessed on the BCTT. Half of the participants in the rest group were assessed on the BCTT on their first clinic visit as part of a study of the safety of the BCTT.14
Outcomes
The primary outcome measure was days to recovery from date of injury. Recovery was defined as symptom resolution to baseline, confirmed by a normal physical examination (normal neurologic examination including normal vestibular and oculomotor systems), and further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms on the BCTT.26 Symptom resolution was defined as having no symptoms or return to baseline symptoms, which is defined as a symptom severity score≤7 on the PCSS for 3 consecutive days without return.27 The first day of symptom resolution was considered to be the date of recovery (if confirmed by the independent assessments of the treating physician and the BCTT results). For participants not recovering within 30 days, date of recovery was determined through follow-up and medical record review. Secondary outcome measures were incidence of delayed recovery, which is defined as recovery requiring>30 days in adolescents,16,28 and daily symptom scores.
Sample size
We used data from a pilot study of exercise vs stretching in concussed patients29 and estimated 4.2 and 7.8 as the standard deviation of the days to recovery for the aerobic exercise and stretching groups, respectively. We used an underlying normal distribution to simulate time to recovery data with the above standard deviations. Using a 2-sample 2-sided t test, we calculated an 80% chance to detect a clinically significant mean difference of 3.7 days in recovery time between groups with 50 participants in each group.
Statistical analysis
Analyses were based on per protocol analysis. Baseline characteristics were analyzed to assess cluster differences between groups (exercise vs placebo vs rest). We assessed groupwise differences in normally distributed variables (age, total symptom severity scores on initial visit, and days to initial visit) using analysis of variance (ANOVA). Chi-square test was used to assess groupwise differences in sex, prior concussions, number of physical examination signs, and incidence of delayed recovery. Because the main outcome measure (days to recovery) was not normally distributed, a test of medians was used to evaluate group differences in days to recovery. Independent samples t test was used to compare male participants and female participants in each group in age, days to initial visit, and symptom severity scores. Equality of variance was assessed. Chi-square tests were used to compare male participants and female participants in each group for concussion history, physical examination findings, and incidence of delayed recovery. Test of medians was used to compare male participants and female participants in each group for days to recovery. Days to recovery from start of treatment was demonstrated by Kaplan-Meier curves. The equality of the survivor function across treatment groups was evaluated by log-rank test.
For secondary outcomes, we used a test of proportions to evaluate the proportion of subjects with delayed recovery in each group. The effect of treatment was further evaluated by means of a mixed effects linear regression model to account for repeated measures of symptom scores. The outcome was daily symptom score and the model included sex to account for differences in sex across treatment groups. Missing values for symptoms were calculated as the mean of day-before and day-after scores. A P value<.05 determined statistical significance and all tests were 2-sided. Statistical analyses were performed using SPSS 24b and Stata 14.c
Results
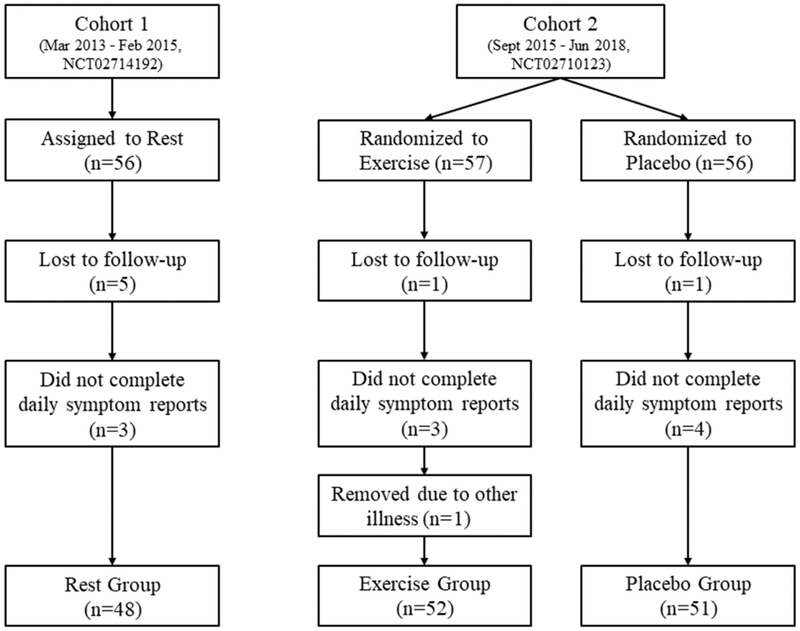
A total of 169 participants consented for the study, as shown in figure 1. One participant from EG, 1 from PG, and 5 from RG did not return to the clinic. Three participants from EG, 4 from PG, and 3 from RG were removed because they did not report their daily symptoms, and date of recovery could not be accurately determined. One participant from EG was removed because of influenza infection during recovery, and the participant stopped the exercise intervention. Hence, 52 participants made up EG, 51 participants PG, and 48 participants RG. Participant demographics are presented in table 1. Except for sex, there were no statistically significant differences in initial presentation between the treatment groups.
Fig 1.
Participant inclusion.
Table 1.
Participant demographics
| Characteristic | Exercise Group n = 52 | Placebo Group n = 51 | Rest Group n = 48 | P Values |
|---|---|---|---|---|
| Age (y), mean ± SD | 15.3±1.6 | 15.4±1.7 | 15.4±1.4 | .939 |
| Female sex, n (%) | 24 (46) | 24 (47) | 12 (25) | .041* |
| Previous concussion | .301 | |||
| 0 | 26 | 29 | 34 | |
| 1 | 16 | 12 | 11 | |
| 2 | 9 | 8 | 3 | |
| 3 | 1 | 2 | 0 | |
| Days to initial visit, mean ± SD | 4.9±2.2 | 4.8±2.4 | 4.3±2.0 | .400 |
| Initial visit symptom severity (PCSS), mean ± SD | 30.8±16.5 | 33.3±19.7 | 31.8±18.9 | .780 |
| Initial visit physical examination findings (BCPE), mean ± SD | 2.0±1.6 | 2.9±1.7 | 2.4±1.8 | .233 |
Abbreviation: BCPE, Buffalo concussion physical examination.
P<.05.
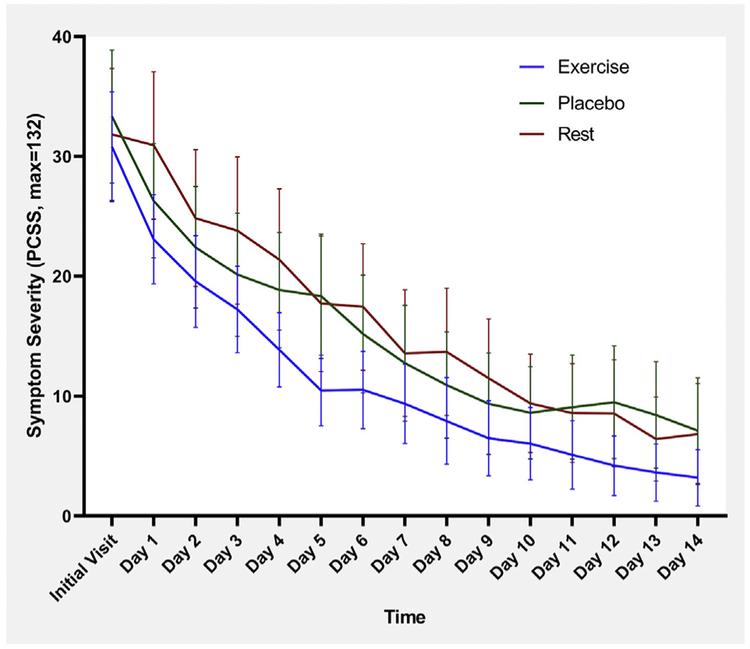
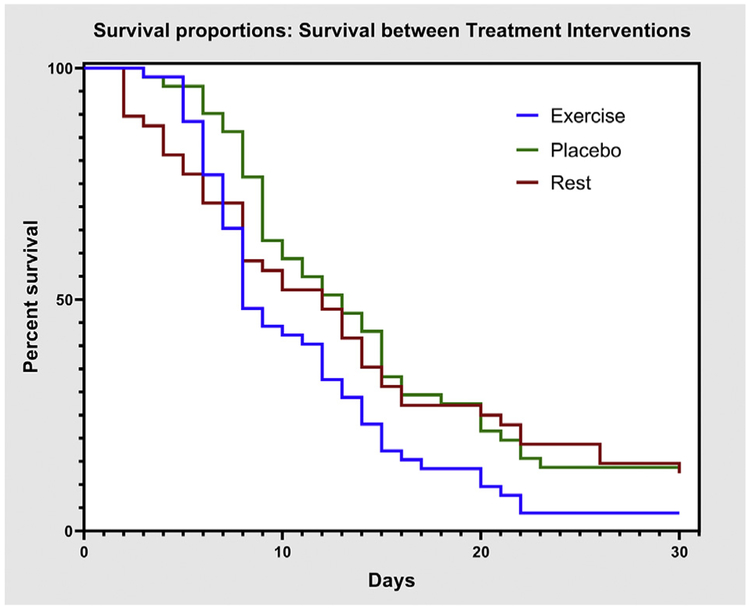
Median duration of clinical recovery and incidence of delayed recovery are presented in table 2. Median recovery time was significantly lower in EG (P=.020). Mean daily symptom score with 95% CI for the initial visit and the first 14 days are presented in figure 2. There was no significant difference between groups on repeated measures ANOVA (P=.343). Kaplan-Meier estimates of time to recovery are presented in figure 3. There was a significant difference (P=.039) on log-rank test between treatment groups. Proportional hazards assumption was met for Cox regressions, which showed a significant difference with treatment (P=.015) but not sex (P=.367).
Table 2.
Recovery time
| Recovery | Exercise Group n = 52 |
Placebo Group n = 51 |
Rest Group n = 48 |
P Value |
|---|---|---|---|---|
| Recovery time (d), median (IQR) | 13 (10-18.75) | 17 (13-23) | 16 (9.25-23.25) | .020* |
| Delayed recovery, n (%) | 2 (3.8) | 7 (13.7) | 6 (12.5) | .190 |
Abbreviation: IQR, interquartile range.
P<.05.
Fig 2.
Mean daily symptom scores on Post-Concussion Symptom Scale with 95% CI for exercise, placebo, and rest groups. NOTE. Repeated measures ANOVA does not show a significant difference between groups (Wilk’s lambda=0.806, P=.343).
Fig 3.
Kaplan-Meier estimates of time to recovery for exercise, placebo, and rest groups.
Participant demographics and recovery times, separated by sex, for each intervention arm are presented in table 3. Although female participants reported higher symptoms at the initial visit than male participants in every group, these differences were not statistically significant. Male and female participants did not significantly differ in recovery times or incidence of delayed recovery.
Table 3.
Participant demographics and recovery times by sex
| Group | Male | Femalec | P Value |
|---|---|---|---|
| Exercise group | n = 28 | n = 24 | |
| Age (y), mean ± SD | 15.3±1.5 | 15.3±1.9 | .949 |
| Previous concussion, mean ± SD | 0.86±0.9 | 0.54±0.7 | .291 |
| Days to initial visit ± SD | 4.9±2.5 | 4.8±1.8 | .876* |
| Initial visit symptom severity (PCSS), mean ± SD | 26.6±12.4 | 35.7±19.3 | .056* |
| Initial visit physical examination findings (BCPE), mean ± SD | 2.0±1.4 | 2.0±1.7 | .409 |
| Recovery time (d), median (IQR) | 12.5 (9.25-16.75) | 13 (11-19) | .948 |
| Delayed recovery, n (%) | 0 (0) | 2 (8.3) | .119 |
| Placebo group | n = 27 | n = 24 | |
| Age (y), mean ± SD | 15.3±1.6 | 15.6±1.8 | .505 |
| Previous concussion, mean ± SD | 0.93±1.0 | 0.38±0.7 | .071 |
| Days to initial visit, mean ± SD | 4.8±2.8 | 4.8±1.8 | .978 |
| Initial visit symptom severity (PCSS), mean ± SD | 31.2±23.1 | 35.8±15.1 | .407 |
| Initial visit physical examination findings (BCPE), mean ± SD | 3.1±1.6 | 2.6±1.9 | .530 |
| Recovery time (d), median (IQR) | 18 (12-29) | 16.5 (14-21.5) | .313 |
| Delayed recovery, n (%) | 5 (18.5) | 2 (8.3) | .291 |
| Rest group | n = 36 | n = 12 | |
| Age (y), mean ± SD | 15.4±1.4 | 15.5±1.7 | .774 |
| Previous concussion, mean ± SD | 0.33±0.6 | 0.42±0.5 | .149 |
| Days to initial visit, mean ± SD | 4.4±2.0 | 4.1±1.8 | .619 |
| Initial visit symptom severity (PCSS), mean ± SD | 30.2±20.6 | 36.8±11.8 | .178* |
| Initial visit physical examination findings (BCPE), mean ± SD | 2.4±1.9 | 2.3±1.7 | .481 |
| Recovery time (d), median (IQR) | 16 (8.25-20.75) | 15 (11.25-28.25) | .868 |
| Delayed recovery, n (%) | 4 (11.1) | 2 (16.7) | .614 |
Abbreviations: BCPE, Buffalo concussion physical examination;IQR, interquartile range.
Levene test for equality of variance <0.05, hence variance not assumed equal.
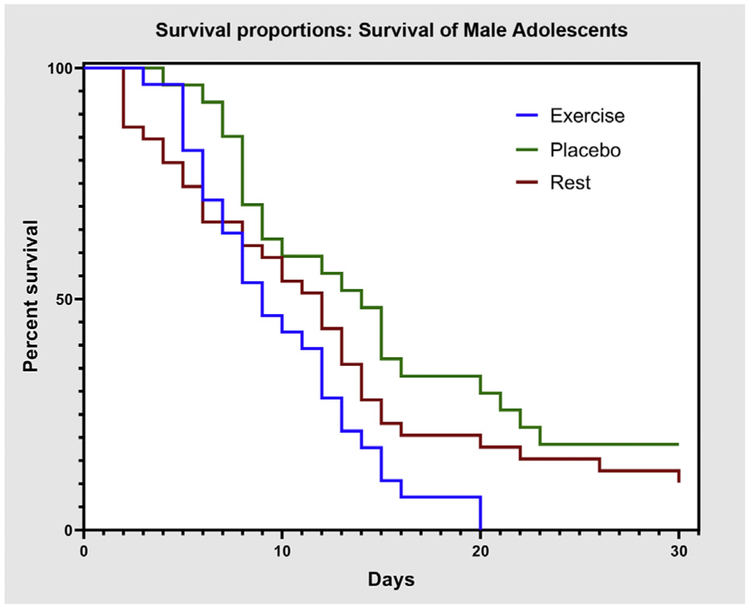
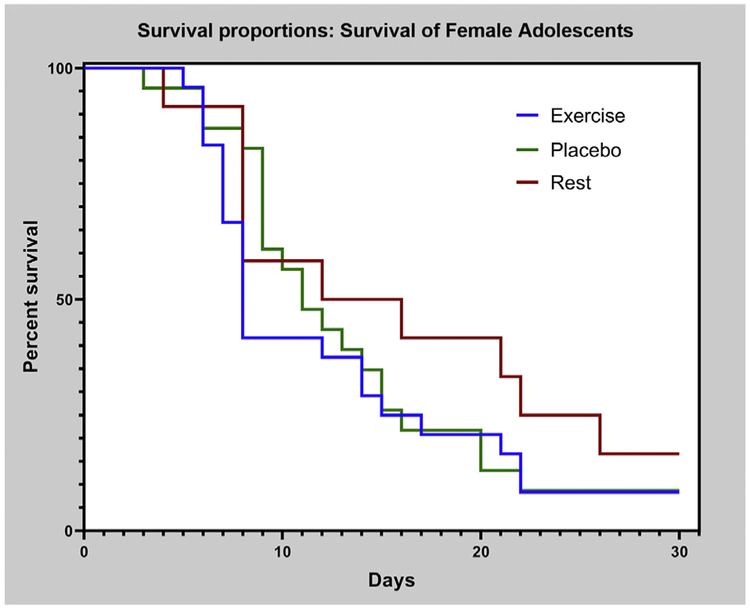
Kaplan-Meier estimates of time to recovery for male and female participants are presented in figures 4 and 5, respectively. Log-rank test on Kaplan-Meier curves showed a significant difference between treatment and recovery time for male (P=.019) but not female participants (P=.412).
Fig 4.
Kaplan-Meier estimates of time to recovery for male participants in exercise, placebo, and rest groups.
Fig 5.
Kaplan-Meier estimates of time to recovery for female participants in exercise, placebo, and rest groups.
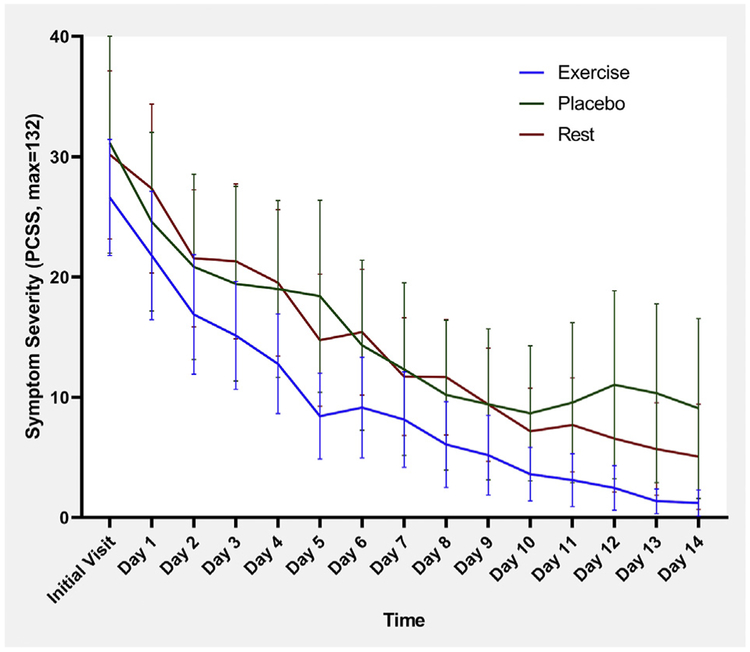
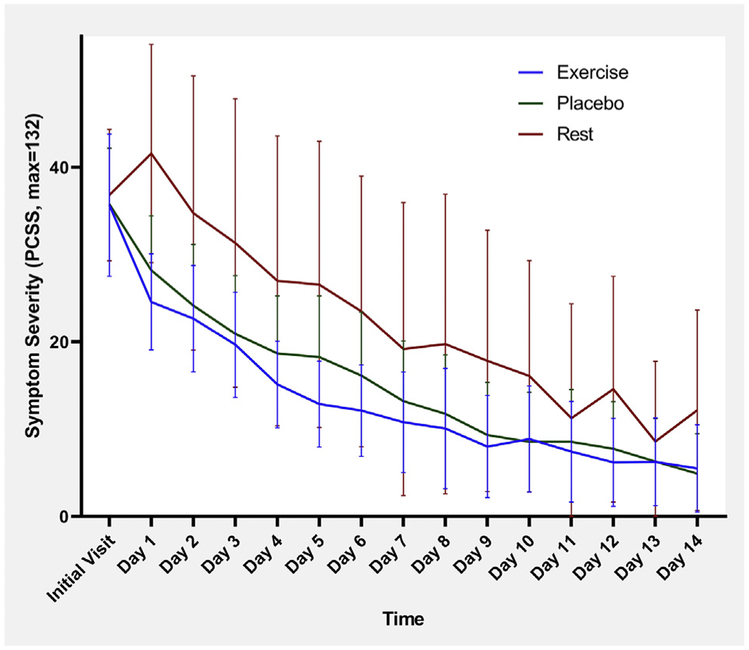
Mean daily symptom severity scores, with 95% CI, for male and female participants are presented in figures 6 and 7, respectively. Regarding symptom change over time, repeated measures ANOVA showed that there was a significant difference for female participants (P=.013) but not for male participants (P=.712).
Fig 6.
Mean daily symptom severity score with 95% CI for male participants. NOTE. Repeated measures ANOVA does not show a significant difference between groups (Wilk’s lambda=0.750, P=.712).
Fig 7.
Mean daily symptom severity score with 95% CI for female participants.NOTE. Repeated measures ANOVA shows a significant difference between groups (Wilk’s lambda = 0.389, P=.013).
Discussion
For almost 2 decades, the treatment of choice for concussion has been strict rest. Moser et al6,11 provides the most detailed description of cognitive rest, which includes not attending school, no tests, no note-taking, no homework, no household chores, no vacation travel, no driving, no social visits, no television, no video games, no computer use, no reading, and no aerobic exercise. The study by Arborgast et al30 suggests that most primary care providers recommend cognitive rest to their patients, which appears be similar to the Moser et al6 description. In the current study, we prescribed relative rest, which meant that the adolescent could participate in many of the activities listed above but do less of them, especially if the activity exacerbated symptoms. Even when we examine the effects of relative rest, participants took longer to recover than those prescribed subsymptom threshold aerobic exercise. Participants prescribed relative rest had outcomes largely indistinguishable from the PG. We conclude that relative rest may be less effective than aerobic exercise but may not be especially harmful, as was suggested in the review by Silverberg and Iverson.7 We did not study complete cognitive rest and isolation as described by Moser et al6, which apparently has been the treatment of choice for many primary care providers.
Consistent with previous research,13 the current study showed that prescribing an active treatment (ie, subsymptom threshold aerobic exercise) in the week following injury helped adolescents recover from SRC faster than relative rest. Furthermore, the incidence of delayed recovery was lower for the exercise group (4%) yet almost identical for the rest and placebo groups (13% and 14%, respectively). This result did not reach statistical significance. In an epidemiologic study with a large sample size, Grool et al31 found that of 2413 children with concussion, 30.4% had delayed recovery. Our study was underpowered to detect rare events such as delayed recovery, but the clinical significance of 4% vs 14% vs 30% cannot be overlooked.32 Further research in larger samples should evaluate the effect of prescribed subthreshold aerobic exercise on delayed recovery in adolescents after SRC.
There are several possible explanations for the benefits of aerobic exercise over rest. On the cellular level, animal studies have shown that voluntary aerobic exercise increases neural plasticity33 and proliferation of neuronal stem cells34 while reducing apoptotic cell behavior and subsequent neural degeneration.35 Aerobic exercise training enhances neuroplasticity by increasing serum BDNF levels,36 which may explain why it is effective for the treatment of mild cognitive impairment.37 On the macrophysiological level, autonomic nervous system dysfunction38 after concussion appears to limit or blunt the appropriate response to physiological stressors, as revealed by measures of cerebrovascular vasoreactivity,39 blood pressure regulation,39 heart rate variability,40 and CO2 sensitivity.41,42 Exercise is one type of stressor that elicits symptoms when the stress level exceeds a tolerable level. Thus, subsymptom threshold aerobic exercise training may stress physiological systems within the body’s autoregulatory capabilities after concussion to incrementally restore autonomic nervous system control to normal.43,44
Another aim of this study was to assess the effect of sex on recovery after SRC. Previous research has been mixed, with some studies finding female patients take longer to recover45 while others do not.46 While male patients prescribed rest or placebo took slightly longer (~ 1 day) to recover than female patients, we found no statistically significant difference in recovery time between the sexes in any intervention group. Female patients had higher symptom scores at the initial visit vs male patients but the small female sample size in the rest group limited the statistical significance of this finding. Greater female symptom reporting is consistent with previously published research.47 We observed a sharp rise in symptom scores the day after the initial visit in female patients who had been prescribed relative rest. Conversely, there was a sharp decline of symptoms in female patients who were prescribed aerobic exercise or placebo, which was not observed in the male patients. The mechanism for this observation is not clear, but it is possible that female patients advised to rest after SRC may ruminate, which increased symptoms. Studies48,49 have shown that female patients tend to ruminate about medical conditions more than male patients, with one report50 linking increased rumination and depressive symptoms after mild traumatic brain injury in female patients to BDNF gene polymorphisms. It may be particularly important for medical providers to recommend active treatment for female patients early after SRC to avoid early exacerbation of symptoms. Further research into sex differences in concussion recovery is needed, and it is recommended that such differences be examined within the context of treatment.
Study limitations
The historical sample prescribed relative rest was recruited several years prior to the other 2 groups. The RG had a smaller proportion of female participants. The relatively small sample size limited our power to detect rare events and thus draw any conclusions about the effect of the different treatments on delayed recovery. The disproportionately small sample of female participants in the RG also limits the generalizability of findings of the female symptom analysis. Further, we did not obtain any information about the menstrual cycle or hormonal levels of female patients, something we believe essential to a study that examines clinical outcomes of female patients following SRC. Finally, we had no objective measure as to whether the rest group participants were compliant with their treatment recommendation. Daily symptom reporting and self-reported compliance with treatment intervention, however, in the exercise and placebo groups were 83% and 86%, respectively,13 so it is reasonable to assume that patients prescribed relative rest also followed their physician’s recommendations.
Conclusions
This study showed that a recommendation for relative rest was not as effective as prescribing subsymptom threshold aerobic exercise to adolescents after acute SRC. The RG participants’ recovery was almost identical to the PG. We did not find significant differences in recovery from SRC between male and female patients, regardless of intervention, which suggests that future research on sex differences should account for the advice given or treatment recommendations made to concussed adolescents. Female patients prescribed rest had a rather dramatic increase in symptoms the day after being seen in the clinic, suggesting that a prescription for rest after SRC may be particularly problematic for female adolescents.
Acknowledgments
Supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (award no. 1R01NS094444) and the National Center for Advancing Translational Sciences of the National Institutes of Health (award no. UL1TR001412) to the State University of New York at Buffalo. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of abbreviations:
- ANOVA
analysis of variance
- BCTT
Buffalo Concussion Treadmill Test
- BDNF
brain-derived neurotrophic factor
- EG
exercise group (adolescents prescribed subthreshold aerobic exercise)
- PCSS
Post Concussion Symptom Scale
- PG
placebo group (adolescents prescribed stretching exercises)
- RCT
randomized controlled trial
- RG
rest group (adolescents recommended to rest after diagnosis of concussion)
- SRC
sport-related concussion
Footnotes
Polar heart rate monitor; H7 Polar USA.
SPSS 24; IBM, Armonk, NY, United States.
Stata 14; Stata Corp, College Station, Texas, United States.
Presented to the Interagency Conference on TBI, June 12, 2018, Washington, DC.
Clinical Trial Registrations Nos.: and .
Disclosures: none.
References
- 1.Barth JT, Freeman JR, Broshek DK, Varney RN. Acceleration-deceleration sport-related concussion: the gravity of it all. J Athl Train 2001;36:253–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R 2011;3(10 Suppl 2):359–68. [DOI] [PubMed] [Google Scholar]
- 3.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21:375–8. [DOI] [PubMed] [Google Scholar]
- 4.Aubry M, Cantu R, Dvorek J, et al. Concussion in sport group: recommendations for the improvement of safety and helth of athletes who may suffer concussive injuries. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001. Br J Sports Med 2002;36:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griesbach GS, Hovda D, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 2004;125:129–39. [DOI] [PubMed] [Google Scholar]
- 6.Moser RS, Schatz P, Glenn M, Kollias KE, Iverson GL. Examining prescribed rest as treatment for adolescents who are slow to recover from concussion. Brain Inj 2015;29:58–63. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg ND, Iverson GL. Is rest after concussion “the best medicine?”: recommendations for activity resumption following concussion in athletes, civilians, and military service members. J Head Trauma Rehabil 2013;28:250–9. [DOI] [PubMed] [Google Scholar]
- 8.Schneider KJ, Iverson GL, Emery CA, McCrory P, Herring SA, Meeuwisse WH. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med 2013;47:304–7. [DOI] [PubMed] [Google Scholar]
- 9.Schneider KJ, Leddy JJ, Guskiewicz KM, et al. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med 2017;51:930–4. [DOI] [PubMed] [Google Scholar]
- 10.Lempke L, Jaffri A, Erdman N. The effects of early physical activity compared to early physical rest on concussion symptoms. J Sport Rehabil 2019;28:99–105. [DOI] [PubMed] [Google Scholar]
- 11.Moser RS, Glatts C, Schatz P. Efficacy of immediate and delayed cognitive and physical rest for treatment of sports-related concussion. J Pediatr 2012;161:922–6. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015;135:213–23. [DOI] [PubMed] [Google Scholar]
- 13.Leddy JJ, Haider MN, Ellis MJ, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr 2019;173:319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med 2017;51:941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leddy JJ, Hinds AL, Miecznikowski J, et al. Safety and prognostic utility of provocative exercise testing in acutely concussed adolescents: a randomized trial. Clin J Sport Med 2018;28:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 2017;51:838–47. [DOI] [PubMed] [Google Scholar]
- 17.McCrory P, Meeuwisse W, Johnston K, et al. SCAT2. Br J Sports Med 2009;43(Suppl 1):i85–8. [DOI] [PubMed] [Google Scholar]
- 18.McCrea M, Randolph C, Kelly J. Standardized Assessment of Concussion (SAC): manual for administration, scoring and interpretation. Waukesha, WI: CNS Inc; 2000. p 2. [Google Scholar]
- 19.Haider MN, Leddy JJ, Du W, Viera K, Willer B. Practical management: brief physical examination for sport-related concussion in the outpatient setting. Clin J Sport Med 2018. November 7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leddy J, Lesh K, Haider MN, et al. Derivation of a focused, brief concussion physical examination for adolescents with sport-related concussion. Clin J Sport Med 2018. October 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med 2013;47:289–93. [DOI] [PubMed] [Google Scholar]
- 23.Chin EY, Nelson LD, Barr WB, McCrory P, McCrea MA. Reliability and validity of the Sport Concussion Assessment Tool—3 (SCAT3) in high school and collegiate athletes. Am J Sports Med 2016;44:2276–85. [DOI] [PubMed] [Google Scholar]
- 24.Haider MN, Leddy JJ, Wilber CG, et al. The predictive capacity of the Buffalo Concussion Treadmill Test after sport-related concussion in adolescents. Front Neurol 2019;10:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the Second International Conference on Concussion in Sport, Prague 2004. Phys Sportsmed 2005;33:29–44. [DOI] [PubMed] [Google Scholar]
- 26.Haider MN, Leddy JJ, Pavlesen S, et al. A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med 2018;52:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol 2006;13:166–74. [DOI] [PubMed] [Google Scholar]
- 28.Davis GA, Anderson V, Babl FE, et al. What is the difference in concussion management in children as compared with adults? A systematic review. Br J Sports Med 2017;51:949–57. [DOI] [PubMed] [Google Scholar]
- 29.Leddy JJ, Cox JL, Baker JG, et al. Exercise treatment for postconcussion syndrome: a pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. J Head Trauma Rehabil 2013;28:241–9. [DOI] [PubMed] [Google Scholar]
- 30.Arbogast KB, McGinley AD, Master CL, Grady MF, Robinson RL, Zonfrillo MR. Cognitive rest and school-based recommendations following pediatric concussion: the need for primary care support tools. Clin Pediatr 2013;52:397–402. [DOI] [PubMed] [Google Scholar]
- 31.Grool AM, Aglipay M, Momoli F, et al. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA 2016;316:2504–14. [DOI] [PubMed] [Google Scholar]
- 32.Overall JE. Sample size required to observe at least k rare events. Psychol Rep 1967;21:70–2. [DOI] [PubMed] [Google Scholar]
- 33.Jacotte-Simancas A, Costa-Miserachs D, Coll-Andreu M, Torras-Garcia M, Borlongan CV, Portell-Cortés I. Effects of voluntary physical exercise, citicoline, and combined treatment on object recognition memory, neurogenesis, and neuroprotection after traumatic brain injury in rats. J Neurotrauma 2015;32:739–51. [DOI] [PubMed] [Google Scholar]
- 34.Itoh T, Imano M, Nishida S, et al. Exercise increases neural stem cell proliferation surrounding the area of damage following rat traumatic brain injury. J Neural Transm (Vienna) 2011;118:193–202. [DOI] [PubMed] [Google Scholar]
- 35.Itoh T, Imano M, Nishida S, et al. Exercise inhibits neuronal apoptosis and improves cerebral function following rat traumatic brain injury. J Neural Transm (Vienna) 2011;118:1263–72. [DOI] [PubMed] [Google Scholar]
- 36.Leddy JJ, Haider MN, Ellis M, Willer BS. Exercise is medicine for concussion. Curr Sports Med Rep 2018;17:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki T, Shimada H, Makizako H, et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PloS One 2013;8:e61483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esterov D, Greenwald BD. Autonomic dysfunction after mild traumatic brain injury. Brain Sci 2017;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrador J, Tosto J, Reyes L, Blatt M, Ghobreal B, Falvo M. Cerebrovascular regulation is impaired immediately post concussion and associated with increased estimated ICP. FASEB J 2015;29(1 Suppl). 800.11. [Google Scholar]
- 40.Bishop S, Dech R, Aravinthan K, et al. Acute stages of concussion: suppression of blood pressure during postural hemodynamic drives. J Cereb Blood Flow Metab 2016;36:292.26661174 [Google Scholar]
- 41.Blake TA, McKay CD, Meeuwisse WH, Emery CA. The impact of concussion on cardiac autonomic function: a systematic review. Brain Inj 2016;30:132–45. [DOI] [PubMed] [Google Scholar]
- 42.Clausen M, Pendergast DR, Wilier B, Leddy J. Cerebral blood flow during treadmill exercise is a marker of physiological postconcussion syndrome in female athletes. J Head Trauma Rehabil 2016;31:215–24. [DOI] [PubMed] [Google Scholar]
- 43.Leddy J, Baker JG, Haider MN, Hinds A, Willer B. A physiological approach to prolonged recovery from sport-related concussion. J Athl Train 2017;52:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leddy JJ, Kozlowski K, Fung M, Pendergast DR, Willer B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: implications for treatment. NeuroRehabilitation 2007;22:199–205. [PubMed] [Google Scholar]
- 45.Henry LC, Elbin RJ, Collins MW, Marchetti G, Kontos AP. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery 2016;78:232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frommer LJ, Gurka KK, Cross KM, Ingersoll CD, Comstock RD, Saliba SA. Sex differences in concussion symptoms of high school athletes. J Athl Train 2011;46:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker JG, Leddy JJ, Darling SR, Shucard J, Makdissi M, Willer BS. Gender differences in recovery from sports-related concussion in adolescents. Clin Pediatr (Phila) 2016;55:771–5. [DOI] [PubMed] [Google Scholar]
- 48.Johnson DP, Whisman MA. Gender differences in rumination: a meta-analysis. Pers Individ Diff 2013;55:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohaus A, Vierhaus M, Frevert A, et al. Rumination and symptom reports in children and adolescents: results of a cross-sectional and experimental study. Psychol Health 2013;28:1032–45. [DOI] [PubMed] [Google Scholar]
- 50.Gabrys RL, Dixon K, Holahan MR, Anisman H. Self-reported mild traumatic brain injuries in relation to rumination and depressive symptoms: moderating role of sex differences and a brain-derived neurotrophic factor gene polymorphism. Clin J Sport Med 2016. November 21 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]