Abstract
The HIV-1 capsid (CA) protein lattice encases viral genomic RNA and regulates steps essential to target cell invasion1. Cyclophilin A (CypA) has interacted with the CA of lentiviruses related to HIV-1 for millions of years2–7. Disruption of the CA-CypA interaction decreases HIV-1 infectivity in human cells8–12, but stimulates infectivity in non-human primate cells13–15. Genetic and biochemical data suggest that CypA protects HIV-1 from a CA-specific restriction factor in human cells16–20. Discovery of the CA-specific restriction factor tripartite-containing motif 5α (TRIM5α)21, and of multiple, independently-derived, TRIM5-CypA fusion genes4,5,15,22–26, pointed to human TRIM5α as the CypA-sensitive restriction factor. However, HIV-1 restriction by human TRIM5α in tumor cell lines is minimal21, and inhibition of such activity by CypA has not been detected27. Here, exploiting reverse genetic tools optimized for primary human blood cells, we demonstrate that disruption of the CA-CypA interaction renders HIV-1 susceptible to potent restriction by human TRIM5α, with the block occurring before reverse transcription. Endogenous TRIM5α associated with virion cores as they entered the cytoplasm, but only when the CA-CypA interaction was disrupted. These experiments resolve the long-standing mystery of the role of CypA in HIV-1 replication by demonstrating that this ubiquitous cellular protein shields HIV-1 from previously inapparent restriction by human TRIM5α.
To assess the role of TRIM5α and CypA in the primary human blood cell types that serve as targets for HIV-1 infection in vivo, lentiviral vectors were optimized for titer and knockdown efficiency in these cells27–31. Primary human macrophages, dendritic cells, and CD4+ T cells were transduced with lentivectors bearing a puromycin resistance cassette and shRNAs targeting either TRIM5 or luciferase (Luc) as a control. After three days of selection in puromycin, knockdown was confirmed by RT-qPCR for TRIM5 mRNA, and by rescue of N-MLV restriction (Extended Data Fig. 2a–c), as done previously27,28. TRIM5 and Luc control knockdown cells were then challenged with single-cycle, VSV G-pseudotyped, HIV-1-GFP reporter vectors. Three days later, the percentage of GFP+ cells was assessed by flow cytometry as a measure of infectivity (Extended Data Fig. 1 shows the gating strategy).
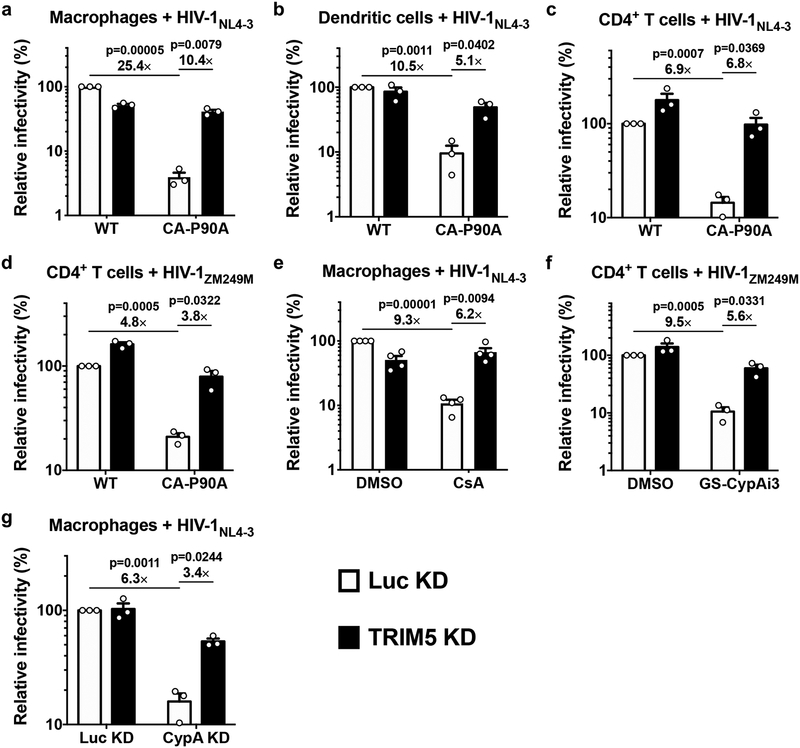
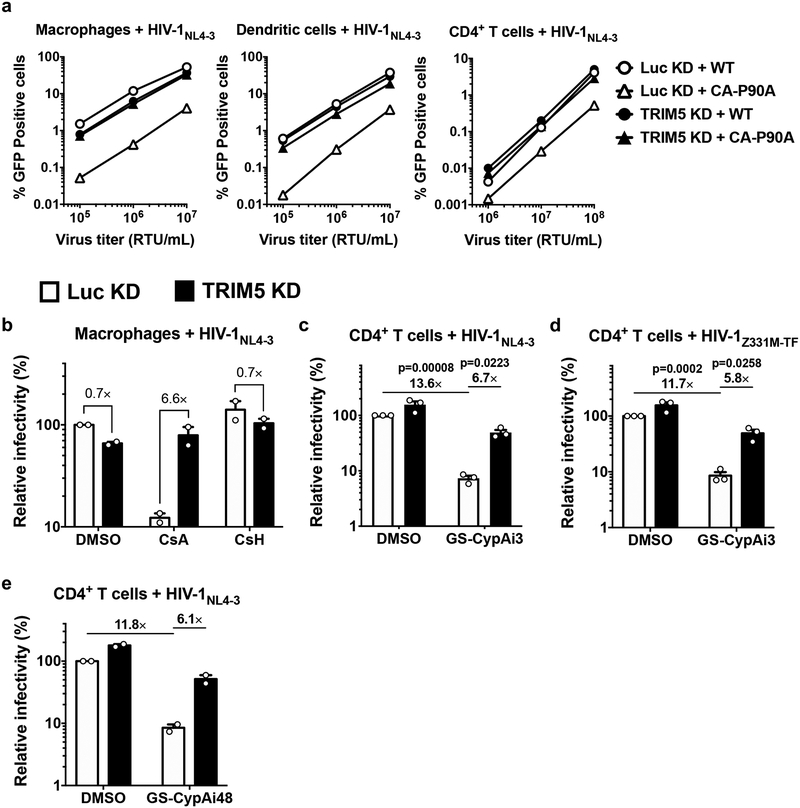
As compared with Luc control knockdown, TRIM5 knockdown had minimal effect on wild-type HIV-1 transduction efficiency in macrophages, dendritic cells, or CD4+ T cells (Fig. 1a–d; Extended Data Fig. 3a). Infectivity of HIV-1 CA-P90A, a mutant that disrupts CypA binding8,9, was significantly attenuated as compared to the wild type, in control knockdown cells generated with all three cell types (Fig. 1a–d). The effect was evident in cells from all blood donors tested (at least three blood donors per condition) and over a 100-fold range in challenge vector titer (Extended Data Fig. 3a). TRIM5 knockdown in macrophages, dendritic cells, or CD4+ T cells increased CA-P90A infectivity (Fig. 1a–d; Extended Data Fig 3a). Results were the same whether challenge was with a three-plasmid vector system based on the clade B HIV-1NL4–3 lab strain30,32 (Fig. 1a–c), or a two-plasmid vector system based on the clade C HIV-1ZM249M transmission-founder strain from Zambia30,33 (Fig. 1d).
Fig. 1. Disruption of the CA-CypA interaction in primary human blood cells renders HIV-1 susceptible to restriction by TRIM5.
a, Macrophages, b, dendritic cells, or c and d, CD4+ T cells, were selected after transduction with a lentivirus expressing shRNA targeting TRIM5 or Luc control, and challenged with single-cycle, VSV G-pseudotyped, HIV-1NL4–3GFP (a-c) or HIV-1ZM249MGFP (d), bearing WT CA or CA-P90A (mean ± SEM, n = 3 donors for each). e, TRIM5 knockdown or Luc knockdown macrophages were challenged with HIV-1NL4–3GFP in the presence of 8 μM CsA or DMSO solvent (mean ± SEM, n = 4 donors). f, TRIM5 knockdown or Luc knockdown CD4+ T cells were challenged with HIV-1ZM249MGFP in the presence of 2.5 μM GS-CypAi3 or DMSO solvent (mean ± SEM, n = 3 donors). g, Macrophages were transduced simultaneously with two vectors expressing shRNAs, as indicated, and selected with puromycin and blasticidin. Cells were then challenged with HIV-1NL4–3GFP (mean ± SEM, n = 3 donors). The percentage of GFP+ cells was assessed by flow cytometry and normalized to WT in Luc control knockdown cells in all cases. Significance was determined by two-tailed, paired t-test.
Given previous reports that endogenous human TRIM5α in immortalized cell lines has modest effect on HIV-1 infectivity, and that CypA and TRIM5α act independently to regulate HIV-1 transduction27, the relatively large magnitude rescue of CA-P90A infectivity by TRIM5 knockdown in primary human blood cells was surprising. Complementary pharmacologic and reverse genetic approaches were therefore taken to disrupt the CA-CypA interaction. For pharmacologic disruption, cells were incubated in media containing small molecules that compete with CA for binding to CypA2,8–10,34. As compared with DMSO solvent alone, cyclosporine A (CsA) reduced HIV-1 transduction efficiency in control Luc knockdown macrophages (Fig. 1e). In contrast, cyclosporine H, an analogue with 1,000-fold lower affinity for CypA35, caused only slight increase in HIV-1 infection (Extended Data Fig. 3b). Since CsA blocks T cell proliferation, two non-immunosuppressive CypA inhibitors derived from sanglifehrin A, GS-CypAi3 and GS-CypAi4834, were used instead on this cell type; these drugs decreased HIV-1 transduction efficiency in primary CD4+ T cells (Fig. 1f; Extended Data Fig. 3c–e). TRIM5 knockdown reversed the HIV-1 inhibition in macrophages caused by CsA (Fig. 1e), or in CD4+ T cells caused by the sanglifehrin A-derivatives (Fig. 1f; Extended Data Fig. 3c–e).
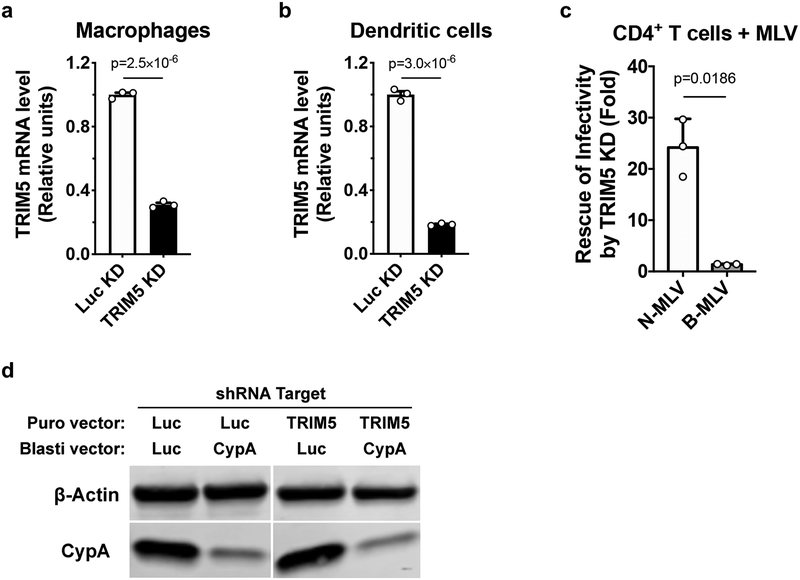
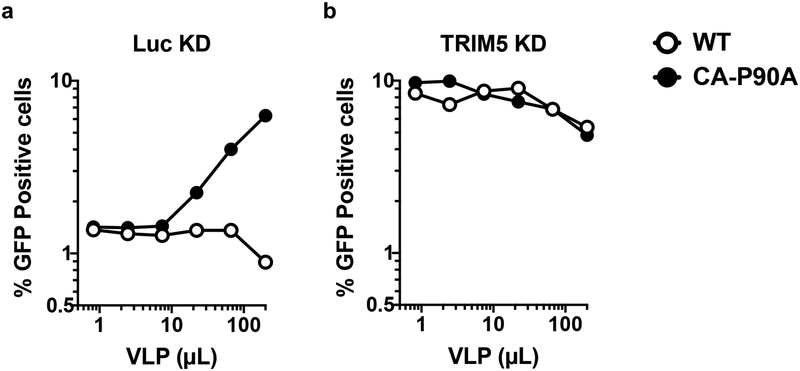
To disrupt the CA-CypA interaction using a genetic approach, macrophages were transduced with two vectors for knockdown of either TRIM5 or CypA, or both. The first vector conferred puromycin resistance and expressed shRNAs targeting either TRIM5 or Luc. The second vector conferred blasticidin resistance and expressed shRNAs targeting either CypA or Luc. After simultaneous transduction with pairs of these vectors, macrophages were selected for three days in both puromycin and blasticidin and then challenged with single-cycle, VSV G-pseudotyped, HIV-1-GFP reporter vector bearing wild-type CA. As compared with the control Luc knockdown, CypA knockdown reduced CypA protein levels ~70% (Extended Data Fig. 2d). Like CA-P90A and the small molecule inhibitors, CypA knockdown decreased transduction efficiency (Fig. 1g). This effect was rescued by simultaneous knockdown of TRIM5 (Fig. 1g) without restoring CypA protein levels (Extended Data Fig. 2d). Taken together, these data indicate that endogenous human TRIM5 is required for HIV-1 restriction in the absence of the CA-CypA interaction. Consistent with the previously reported CA-specific saturation of TRIM5 restriction activity13,27, the effective titer of HIV-1-GFP reporter vector bearing CA-P90A was increased in a dose-dependent manner by the addition of virus-like particles (VLPs) bearing CA-P90A, but not by VLPs bearing wild-type CA (Extended Data Fig. 4).
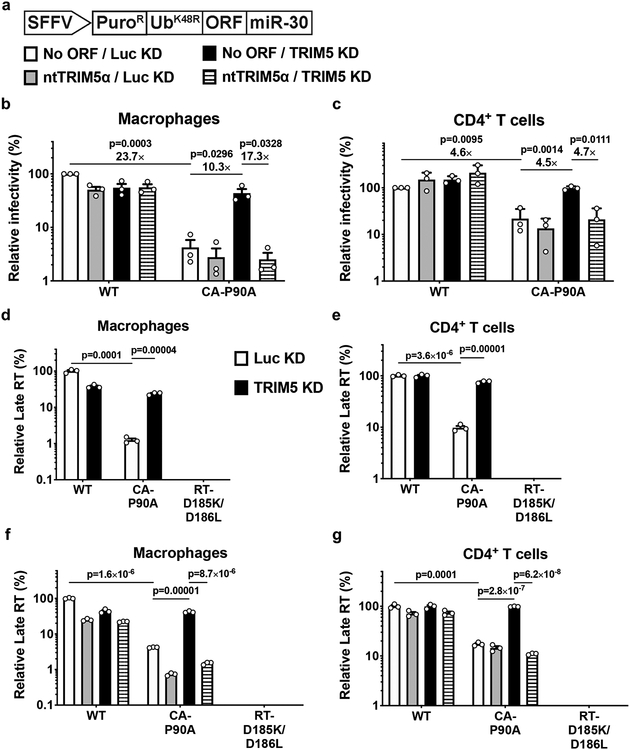
Though the shRNA that targets TRIM5 is distinguished from the next most similar sequence in the human genome (GRCh38) by multiple mismatches, off-target effects are theoretically possible. To test whether TRIM5 is sufficient to explain the HIV-1 restriction activity associated with CA-CypA disruption, a vector was designed based on the ubiquitin fusion technique36, that expresses a tripartite fusion of puromycin N-acetyl transferase (PuroR), ubiquitinK48R, and coding sequence for a protein of interest, in addition to an shRNA (Fig. 2a). Four variants of the plasmid were engineered in which the shRNA targeted either TRIM5 or Luc, with or without a TRIM5α coding sequence that bears mismatches in the shRNA target sequence (Fig. 2a).
Fig. 2. Human TRIM5α is sufficient to explain the inhibition of reverse transcription that results from disruption of CA-CypA interaction.
a, Schematic representation of all-in-one shRNA-rescue lentivector, in which the SFFV promoter expresses a tripartite fusion of puromycin N-acetyl transferase (PuroR), the K48R mutant of ubiquitin (UbK48R), and an open reading frame for a gene of interest (ORF), as well as a miR-30 based shRNA (miR-30). b and c, All-in-one lentivectors encoding empty control (No ORF), or non-targetable, shRNA-resistant TRIM5α coding sequence (ntTRIM5α), along with shRNA targeting Luc or TRIM5, as indicated in (a), were used to transduce macrophages (b) or CD4+ T cells (c). The percentage of GFP-expressing cells was measured by flow cytometry and normalized to the values for No cDNA/Luc KD cells challenged with WT CA; mean ± SEM, n = 3 donors for each. Significance was determined by two-tailed, paired t-test. d-g, TRIM5 knockdown or Luc knockdown macrophages (d) or CD4+ T cells (e), or macrophages (f), or CD4+ T cells (g) transduced with the all-in-one shRNA-rescue lentivectors described in a, were challenged with HIV-1NL4–3GFP containing WT CA or CA-P90A, as indicated. DNA was extracted 20 hrs post-challenge and late products of reverse transcription (RT) were assessed by qPCR (mean ± SEM, n = 3 biologically independent samples). RT-D185K/D186L mutant virus was used as a control for background. Significance was determined by two-tailed, unpaired t-test.
Macrophages (Fig. 2b) and CD4+ T cells (Fig. 2c) were transduced with each of the four variants of the shRNA tripartite fusion vector and selected for three days with puromycin. Cells were then challenged with the three-plasmid HIV-1-GFP reporter vector used in Fig. 1a, and assessed by flow cytometry for percent GFP positive cells three days later. As in Fig. 1, the infectivity of vector bearing wild-type CA was minimally affected by TRIM5 knockdown, or by TRIM5 overexpression (Fig. 2b, c). As compared to the wild-type, the infectivity of vector bearing CA-P90A was decreased (Fig. 2b, c), and the infectivity of this mutant was rescued by TRIM5 shRNA (Fig. 2b, c). In the presence of the shRNA targeting TRIM5, delivery of TRIM5α coding sequence bearing shRNA target-site mismatches restored restriction activity to the control level (Fig. 2b, c). These results demonstrate that, in primary human blood cells, human TRIM5α is sufficient to restrict HIV-1 transduction, but only when the CA-CypA interaction is disrupted.
To determine at which step in the virus life cycle human TRIM5α inhibits HIV-1 when the CA-CypA interaction is disrupted, HIV-1 cDNA resulting from reverse transcription was assessed by qPCR. Macrophages and CD4+ T cells were stably transduced and selected with vector expressing shRNAs targeting TRIM5 or Luc control (Fig 2d, e), or with each of the four variants of the shRNA tripartite fusion vector (Fig. 2f, g). Cells were then challenged with an HIV-1 reporter vector that has the 34 basepair loxP sequence in U3 to distinguish reporter vector transcripts from those of the shRNA lentivector37. DNA was collected 20 hrs post-challenge and qPCR was performed using primers specific for full-length linear HIV-1 cDNA (late RT). In all experiments, reporter vector bearing the RT-D185K/D186L loss-of-function mutation37 was included as a control for background signal not due to nascent reverse transcription (Fig. 2d–g).
In Luc control knockdown macrophages and CD4+ T cells, viral cDNA was reduced by CA-P90A, and this reduction was reversed by TRIM5 knockdown (Fig. 2d, e). Viral cDNA was also reduced by CA-P90A in either cell type transduced with the control shRNA tripartite fusion vector (Fig. 2f, g). TRIM5 shRNA rescued the cDNA (Fig. 2d–g), and rescue of TRIM5α with the non-targetable coding sequence again decreased the CA-P90A cDNA (Fig. 2f, g). These results demonstrate that, when the CA-CypA interaction is disrupted, human TRIM5α blocks HIV-1 at an early step of viral infection, prior to completion of reverse transcription.
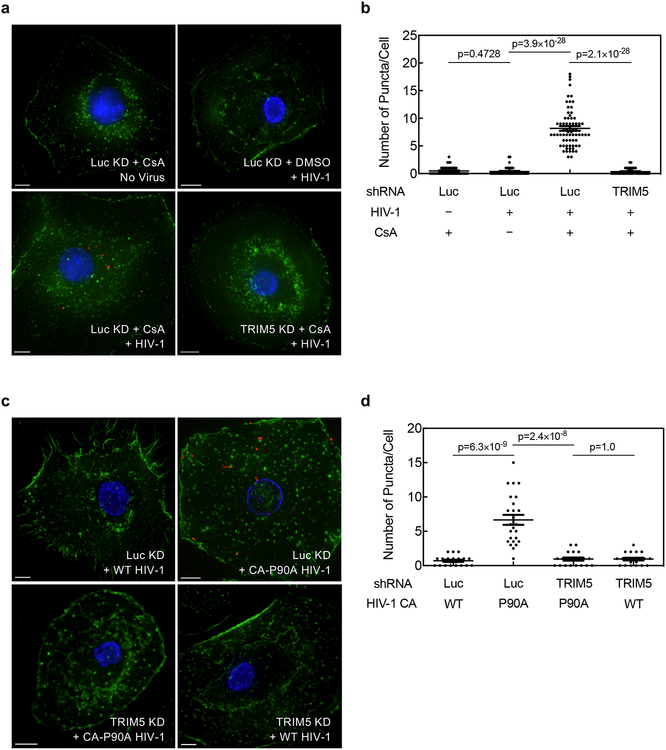
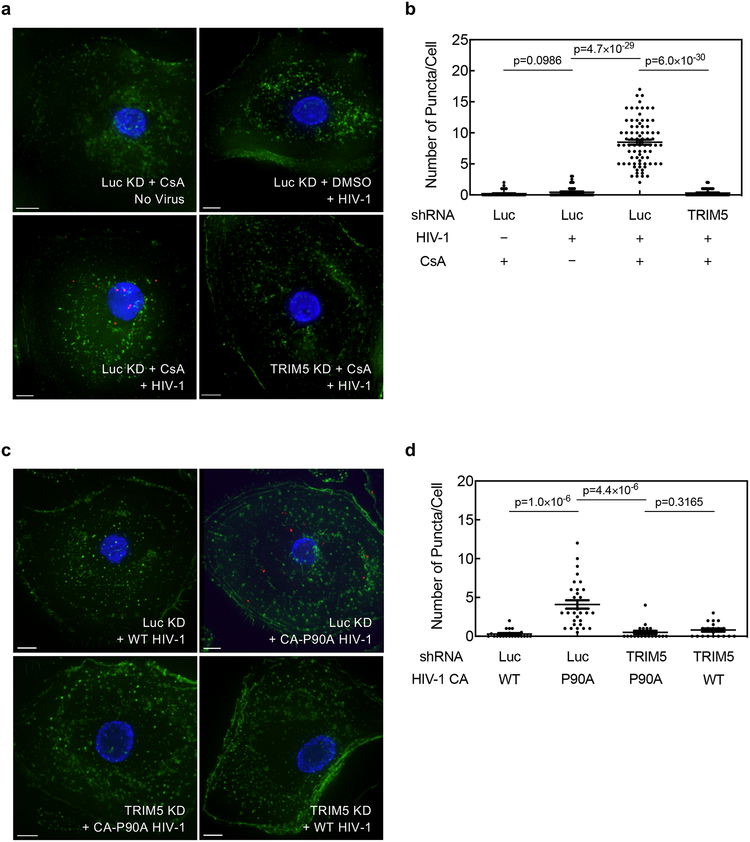
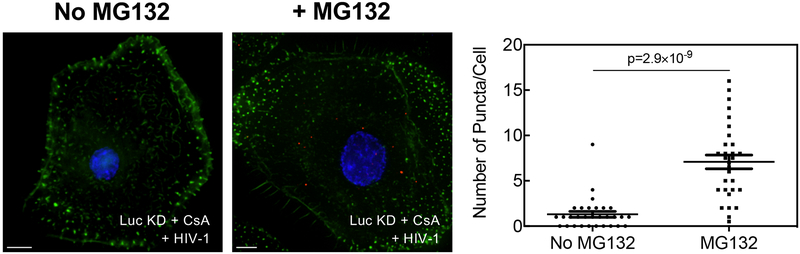
To determine if TRIM5α associates with HIV-1 CA in cells when the CA-CypA interaction is disrupted, primary human macrophages were stably transduced with TRIM5 shRNA or Luc shRNA and then challenged for 2 hrs with wild-type HIV-1 reporter vector in the presence or absence of CsA, or with HIV-1 vectors bearing wild-type CA or CA-P90A. Cells were fixed and subjected to the proximity ligation assay (PLA) with antibodies specific for HIV-1 CA and endogenous human TRIM5α. When cells were challenged with wild-type HIV-1 in the absence of CsA, very few puncta were detected (Fig. 3a–d; Extended Data Fig. 5a–d). Similarly, few puncta were detected when cells were treated with CsA in the absence of HIV-1 challenge (Fig. 3a, b; Extended Data Fig. 5a, b). In contrast, when cells were challenged with wild-type HIV-1 in the presence of CsA or with HIV-1 CA-P90A, multiple puncta were detected (Fig. 3a–d; Extended Data Fig. 5a–d), an increase of at least 10- and 20-fold in the average number of puncta per cell over the background, respectively (Fig. 3b, d; Extended Data Fig. 5b, d). TRIM5 knockdown eliminated the puncta (Fig. 3a–d; Extended Data Fig. 5a–d), indicating that the PLA signal was dependent upon TRIM5 expression. The PLA signal was also dependent on the proteasome inhibitor MG132 (Extended Data Fig. 6), a result consistent with the reported involvement of the proteasome in inhibition of reverse transcription by TRIM5α38. These results indicate that, in infection of primary human cells, endogenous TRIM5α associates with HIV-1 CA when the CA-CypA interaction is disrupted.
Fig. 3. Endogenous TRIM5α in primary human macrophages associates with HIV-1 CA after acute challenge but only when the CA-CypA interaction is disrupted.
a-d, Macrophages were transduced and selected with vector bearing shRNA targeting either TRIM5 or Luc. Cells were then challenged for 2 hrs with VSV G-pseudotyped, HIV-1NL4–3GFP, in the presence of 5 μM CsA or DMSO solvent (a and b), or challenged with HIV-1NL4–3GFP harboring WT CA or CA-P90A (c and d), as indicated. Cells were fixed and PLA was performed with antibodies against HIV-1 CA and TRIM5α. Representative images in a and c show PLA puncta (red), nuclei stained with Hoechst (blue), and actin filaments stained with phalloidin (green). The graphs in b and d show the number of PLA puncta per cell with mean ± SEM. b, Luc KD + CsA No Virus, n = 45 cells analyzed; Luc KD + DMSO + HIV-1, n = 45; Luc KD + CsA + HIV-1, n = 69; TRIM5 KD + CsA + HIV-1, n = 45. d, Luc KD + WT HIV-1, n = 20; Luc KD + CA-P90A HIV-1, n = 25; TRIM5 KD + CA-P90A HIV-1, n = 20; TRIM5 KD + WT HIV-1, n = 20. Significance was determined by two-tailed, unpaired t-test. Data shown are representative of four independent experiments using cells from four blood donors for each condition.
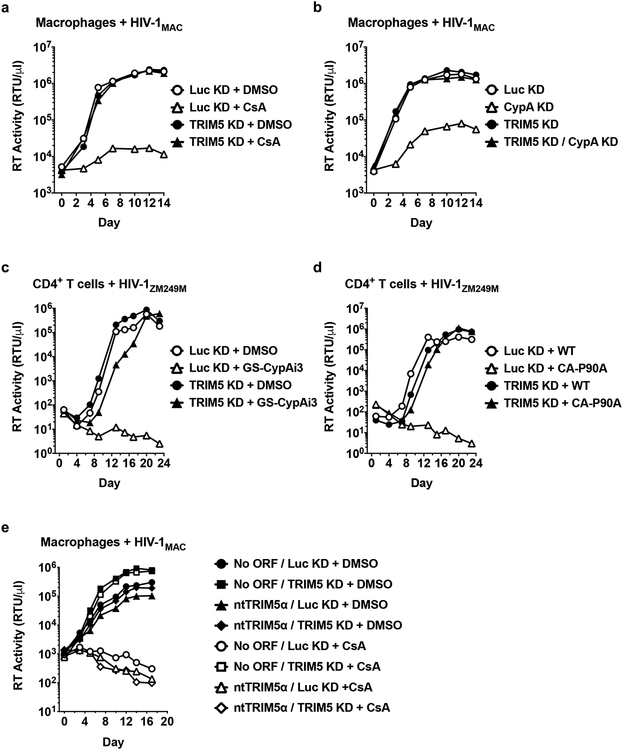
The above experiments used single-cycle HIV-1 vectors. The effect of CypA on HIV-1 restriction by human TRIM5α was therefore evaluated next using replication-competent HIV-1 in a context where the virus spreads cell-to-cell. Primary human macrophages were challenged with clade B HIV-1 bearing a macrophage-tropic env (HIV-1MAC) and replication was monitored for 14 days by measuring the accumulation of reverse transcriptase activity in the supernatant. As in the single cycle experiments (Fig. 1), TRIM5 knockdown itself had little effect on wild-type HIV-1 replication (Fig. 4a, b). Disruption of the CA-CypA interaction with CsA (Fig. 4a) or with shRNA targeting CypA (Fig. 4b) effectively suppressed viral spread in the culture, and, in both cases, replication kinetics was completely restored to the control level by shRNA targeting TRIM5 (Fig. 4a, b). Primary CD4+ T cells were then challenged with a clade C transmission/founder virus (HIV-1ZM249M). As observed in macrophages, TRIM5 knockdown alone had minimal effect on wild-type HIV-1 replication (Fig. 4c, d). No viral replication was detectable when the CA-CypA interaction was disrupted by the small molecule GS-CypAi3 (Fig. 4c) or by the presence of CA-P90A in HIV-1 (Fig. 4d); in both cases shRNA targeting TRIM5 rescued replication kinetics to the level of controls (Fig. 4c, d). Furthermore, the shRNA tripartite fusion vectors were exploited to rule out off-target effects of the shRNA, as well as to demonstrate that TRIM5α is sufficient to restrict HIV-1 replication under conditions in which the CA-CypA interaction is interrupted (Fig. 4e).
Fig. 4. Endogenous TRIM5α suppresses spreading infection of HIV-1 in primary human macrophages and CD4+ T cells when the CA-CypA interaction is disrupted.
a and b, Spreading infection of HIV-1MAC in TRIM5 or Luc knockdown macrophages with 5 μM CsA (a) or with vectors bearing shRNAs targeting CypA or Luc (b), as indicated. c and d, Spreading infection of HIV-1ZM249M in CD4+ T cells expressing shRNA targeting TRIM5 or Luc with 2.5 μM GS-CypAi3 (c) or when challenged with virus bearing CA-P90A (d), as indicated. e, Spreading infection of HIV-1MAC in macrophages transduced with all-in-one shRNA-rescue lentivectors described in Fig. 2, as indicated. HIV-1 replication was monitored by measuring reverse transcriptase (RT) activity in the culture supernatant over time. Data shown are representative of two independent experiments using cells from two blood donors for each condition.
The experiments presented here demonstrate that, in primary human blood cells, HIV-1 exploits CypA to evade CA recognition by, and the antiviral activity of, endogenous TRIM5α. The simplest model is that CypA sterically blocks TRIM5α from binding to CA. Alternatively, since CypA possesses peptidyl-prolyl isomerase activity39 and HIV-1 CA-P90 is a validated substrate40, perhaps CypA shifts CA conformation and thereby protects HIV-1 CA from recognition by TRIM5α. This answers the long-standing question of how CypA promotes HIV-1 infection and clearly establishes that, in the absence of CypA, human TRIM5α potently restricts HIV-1. Conservation of the lentiviral CA-CypA interaction across millions of years of evolution is likely a result of selective pressure applied by TRIM5α orthologues encoded by host species that are otherwise permissive for lentiviral replication. Contrasting with the results here, the observation that CypA promotes restriction in non-human primate cells13–15 likely reflects a different mode of CA-recognition by TRIM5α orthologues from these species. Finally, the results here, in which primary human blood cells were challenged by clones of HIV-1 that were isolated after human-to-human transmission, indicate that, by rendering HIV-1 susceptible to the potent antiviral activity of TRIM5α, non-immunosuppressive CypA inhibitors have potential to make an important contribution to anti-HIV-1 drug cocktails.
Methods
Plasmids
All plasmids used here are described in Supplementary Table 1, and are available, along with full sequences, at https://www.addgene.org/Jeremy_Luban/.
Human blood
Leukopaks were obtained from anonymous, healthy, blood donors (New York Biologics, Southhampton, NY). These experiments were reviewed by the University of Massachusetts Medical School Institutional Review Board, and declared non-human subjects research, according to NIH guidelines (http://grants.nih.gov/grants/policy/hs/faqs_aps_definitions.htm).
Cell culture
All cells were cultured in humidified, 5% CO2 incubators at 37°C. HEK293 cells (ATCC) were cultured in DMEM supplemented with 10% heat-inactivated FBS, 1 mM sodium pyruvate, 20 mM GlutaMAX™-I, 1× MEM non-essential amino acids, and 25 mM HEPES, pH 7.2 (DMEM-FBS complete). PBMCs were isolated from leukopaks by gradient centrifugation on Lymphoprep (Axis-Shield PoC As, Oslo, Norway, catalogue #AXS-1114546). To generate dendritic cells (DCs) or macrophages, CD14+ mononuclear cells were enriched by positive selection using anti-CD14 antibody microbeads (Miltenyi, San Diego, CA, catalogue #130-050-201). Enriched CD14+ cells were plated in RPMI-1640, supplemented with 5% heat-inactivated human AB+ serum (Omega Scientific, Tarzana, CA), 1 mM sodium pyruvate, 20 mM GlutaMAX™-I, 1× MEM non-essential amino acids, and 25 mM HEPES pH 7.2 (RPMI-HS complete), at a density of 106 cells/mL for macrophages or 2×106 cells/mL for DCs. To differentiate CD14+ cells into macrophages, 1:100 GM-CSF-conditioned media was added. To differentiate CD14+ cells into DCs, 1:100 cytokine-conditioned media containing human GM-CSF and human IL-4 was added. GM-CSF and IL-4 were produced from HEK293 cells transduced with pAIP-hGMCSF-co (Addgene #74168) or pAIP-hIL4-co (Addgene #74169), as previously described28,30. CD4+ T cells were isolated from CD14-depleted PBMCs using anti-CD4 antibody microbeads (Miltenyi, catalogue #130-045-101); enrichment was typically >90%, as assessed by measuring the percentage of CD3+/CD4+ cells via flow cytometry with FITC-anti-CD3 (Biolegend, San Diego, CA, catalogue #317306) and APC-anti-CD4 (Biolegend, catalogue #317416). The cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated FBS, 1 mM sodium pyruvate, 20 mM GlutaMAX™-I, 1× MEM non-essential amino acids, and 25 mM HEPES pH 7.2 (RPMI-FBS complete) with 50 U/mL hIL-2 (NIH AIDS Reagent Program, catalogue #136).
Virus production
24 hrs prior to transfection, 6 × 105 HEK293 cells were plated per well in 6-well plates. All transfections used 2.49 μg plasmid DNA with 6.25 μL TransIT LT1 transfection reagent (Mirus, Madison, WI), in 250 μL Opti-MEM (Gibco). 2.49 μg of replication-competent HIV-1 provirus DNA was transfected. For two-part, single-cycle vector, or for HIV-1 virus-like particles (VLPs) lacking a packageable genome, 2.18 μg of env-defective HIV-1 provirus or p8.9 NΔSB gag-pol plasmid was co-transfected with 0.31 μg pMD2.G VSV G plasmid, respectively. Three-part, single-cycle vectors were produced by co-transfecting 1.25 μg minimal lentivector genome plasmid (either pALPS-GFP, pWPTS-GFP, pLXIN-GFP, pAPM-CoE-D4-miR30, pABM-CoE-D4-miR30, or pPU-ORF-miR30), 0.93 μg gag-pol plasmid (either psPAX2, p8.9 NΔSB, pCIG3-N or pCIG3-B41), and 0.31 μg pMD2.G VSV G plasmid. Vpx-containing SIV-VLPs were produced by transfection of 2.18 μg pSIV3+ and 0.31 μg pMD2.G plasmid. 16 hrs post-transfection, culture media was changed to the media specific for the cells to be transduced. Viral supernatant was harvested at 72 hrs, passed through a 0.45 μm filter, and stored at −80°C.
Exogenous reverse transcriptase assay
5 μL transfection supernatant was mixed with 5 μL 0.25% Triton X-100, 50 mM KCl, 100 mM Tris-HCl pH 7.4, and 0.4 U/μL RiboLock RNase inhibitor, and then diluted 1:100 in 5 mM (NH4)2SO4, 20 mM KCl, and 20 mM Tris-HCl pH 8.3. 10 μL of this was then added to a single-step, RT-PCR assay with 35 nM MS2 RNA (IDT) as template, 500 nM of each primer (5’-TCCTGCTCAACTTCCTGTCGAG-3’ and 5’-CACAGGTCAAACCTCCTAGGAATG-3’), and 0.1 μL hot-start Taq DNA polymerase (Promega, Madison, WI) in 20 mM Tris-Cl pH 8.3, 5 mM (NH4)2SO4, 20 mM KCl, 5 mM MgCl2, 0.1 mg/ml BSA, 1/20,000 SYBR Green I (Invitrogen), and 200 μM dNTPs in total 20 μL reaction. The RT-PCR reaction was carried out in a Biorad CFX96 real-time PCR detection system with the following parameters: 42°C for 20 min, 95°C for 2 min, and 40 cycles [95°C for 5 sec, 60°C for 5 sec, 72°C for 15 sec, and acquisition at 80°C for 5 sec].
Transduction with lentiviral knockdown vectors
For DCs, 2 × 106 CD14+ monocytes/mL were transduced with 1:4 volume of SIV-VLPs and 1:4 volume of knockdown lentivector. For macrophages, 106 CD14+ monocytes/mL were transduced with 1:8 volume of SIV-VLPs and 1:8 volume of knockdown lentivector. The Vpx-containing SIV-VLPs were added to these cultures to overcome a SAMHD1 block to lentiviral transduction42,43. Transduced cells were selected with 3 μg/mL puromycin (InvivoGen, San Diego, CA, catalogue #ant-pr-1), 10 μg/mL blasticidin (InvivoGen, catalogue #ant-bl-1), or both, for 3 days, starting 3 days post-transduction.
Following isolation with magnetic beads, human CD4+ T cells were cultured at 2 to 3 × 106 cells/mL in RPMI-FBS complete, supplemented with 50 U/mL hIL-2, and stimulated with 5 μg/mL PHA-P (Sigma-Aldrich, catalogue #L-1668). Alternatively, CD4+ T cells at 106 cells/mL were stimulated with 25 μL/mL ImmunoCult™ Human CD3/CD28 T Cell Activator (STEMCELL Technologies, Vancouver, Canada, catalogue #10991). At day 3 post-stimulation, T cells were replated at 2 to 3 × 106 cells/mL in RPMI-FBS complete, with 50 U/mL hIL-2. Cells were transduced with 108 RT units of viral vector per 106 cells for 3 days, followed by selection with 2 μg/mL puromycin. After selection for 3 days, cells were re-stimulated with PHA-P, or with ImmunoCult™ Human CD3/CD28 T Cell Activator, for 3 days. The stimulated cells were then replated at 2 to 3 × 106 cells/mL (PHA) or at 106 cells/mL (CD3/CD28) in RPMI-FBS complete with 50 U/mL hIL-2, and challenged with lentiviral vectors for assessment of single-cycle infectivity or spreading infection. Fresh media containing hIL-2 was replenished every 2 to 3 days.
Infectivity assay using single-cycle viruses
For human DCs, 2.5 × 105 cells were seeded per well, in a 48-well plate, on the day of virus challenge. Media containing VSV G-pseudotyped lentiviral vector expressing GFP (HIV-1-GFP) was added to challenge cells in a total volume of 250 μL. For human macrophages, 2.5 × 105 cells were seeded per well in a 24-well plate, and challenged with HIV-1-GFP in a total volume of 500 μL. The cells were also simultaneously challenged with HIV-1 VLPs, as indicated. 1:50 volume of SIV VLPs was also added to the medium during virus challenge of DCs or macrophages. To challenge human CD4+ cells activated with PHA, 5 × 105 cells were plated per well in 96-well plate at 3 days after the second PHA stimulation. For CD4+ cells stimulated with CD3/CD28 activator, 2 × 105 cells were plated in each well of a 96-well plate, 3 days after secondary stimulation. Cells were then challenged with GFP reporter viruses in a total volume of 200 μL. For all three cell types, 4 dilutions of viral stocks, from 105 to 108 RT units/mL, were used to challenge cells. Where indicated, cells were pre-treated with 8 μM cyclosporine A (CsA), 8 μM cyclosporine H (CsH), or 2.5 μM of non-immunosuppressive CypA inhibitors from Gilead (GS-CypAi3 or GS-CypAi48)34, for 1 hr prior to virus challenge. In experiments using CsH treatment, the media was replaced after 16 hrs treatment in order to avoid CsH toxicity44. At 48 hrs post-challenge with 2-part HIV-1 vectors, or at 72 hrs post-challenge with 3-part lentiviral vectors, cells were harvested for flow cytometric analysis, by pipetting (CD4+ T cells) or scraping (DCs and macrophages). Cells were pelleted at 500 × g for 5 min, and fixed in a 1:4 dilution of BD Cytofix Fixation Buffer with phosphate-buffered saline (PBS) without Ca2+ and Mg2+, supplemented with 2% FBS and 0.1% NaN3.
Flow Cytometry
Data was collected on an Accuri C6 (BD Biosciences, San Jose, CA) and plotted with FlowJo software. Infectivity at each dilution, in each condition (CA mutant, CypA inhibitor, or CypA knockdown) was compared to the infectivity of WT CA in the control condition. Dilutions yielding infectivity greater than 30% GFP+ cells were excluded from analysis on the assumption that these were out of the linear range, according to the Poisson distribution.
Statistical analysis
Experimental n values and information regarding the statistical tests can be found in the figure legends. The data of infectivity assay using single-cycle viruses including at least three independent donors were statistically analyzed by using two-tailed paired t-test compared to the control condition or the indicated condition for each donor. The quantitative PCR data measuring viral cDNA level with three biologically independent samples for each condition were analyzed using two-tailed, unpaired t-test for comparison of two conditions as indicated in Fig. 2. The data of PLA quantification were assessed for statistical significance using two-tailed unpaired t-test to compare two conditions as indicated in Fig. 4. All statistical analyses were performed using PRISM 8.2 (GraphPad Software, La Jolla, CA).
Quantitative PCR for viral late reverse transcriptase product
Total DNA was extracted from cells using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s instruction. Late RT products were detected with TaqMan system using the primers pWPTS J1B fwd and pWPTS J2 rev with the late RT probe (LRT-P)45. Mitochondrial DNA was used for normalization with the following primer/probe set: MH533, MH534 and Mito probe46. The primer and probe sequences are specified in Supplementary Table 3. The quantitative PCR was performed in 20 μL reaction mix containing 1× TaqMan Gene Expression Master Mix (Applied Biosystems), 900 nM each primer, 250 nM TaqMan probe and 30 to 50 ng template DNA. After an initial incubation at 50 °C for 2 min and the second incubation at 95 °C for 10 min, 45 cycles of amplification were carried out at 95 °C for 15 sec followed by 1 min and 30 sec at 60 °C. Real-Time PCR reactions were run on a CFX96™ thermal cycler (Bio-Rad).
qRT-PCR
Total RNA was isolated in TRIzol reagent followed by RNA purification with RNeasy Plus Mini kit (Qiagen). First-strand cDNA was generated using SuperScript™ VILO™ Master Mix (Thermo Fisher) with random hexamers, in accordance with manufacturer’s instructions. Duplex qPCR was performed in 20 μL reaction mix containing 1× TaqMan Gene Expression Master Mix, 1× TaqMan Gene Expression Assay detecting TRIM5 (FAM dye-labeled, TaqMan probe ID #Hs01552559_m1), 1× TaqMan Gene Expression Assay targeting a housekeeping gene OAZ1 (VIC dye-labeled, primer-limited, TaqMan probe ID #Hs00427923_m1). Amplification was on a Biorad CFX96 real-time PCR detection system, using: 95 °C for 10 min, then 45 cycles of 95 °C for 15 sec and 60 °C for 60 sec.
Western blot
Cells were lysed in Hypotonic Lysis Buffer: 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM EDTA, 0.5% NP-40, 0.1% Triton X-100, and cOmplete mini protease inhibitor (Sigma-Aldrich) for 20 min on ice. The lysates were mixed 1:1 with 2 × Laemmli buffer containing 1:20-diluted 2-mercaptoethanol, boiled for 10 min, and centrifuged at 16,000 × g for 5 min at 4°C. Samples were run on 4–20% SDS-PAGE and transferred to nitrocellulose membranes. Membrane blocking, as well as antibody binding were in TBS Odyssey Blocking Buffer (Li-Cor, Lincoln, NE). Primary antibodies used were rabbit anti-CypA (1:10,000 dilution; Enzo Life Sciences, Farmingdale, NY, catalogue #BML-SA296) and mouse anti-β-actin (1:1,000 dilution; Abcam, Cambridge, UK, catalogue #ab3280). Goat anti-mouse-680 (Li-Cor, catalogue #925–68070) and goat anti-rabbit-800 (Li-Cor, catalogue #925–32211) as secondary antibodies were used at 1:10,000 dilutions. Blots were scanned on the Li-Cor Odyssey CLx.
Proximity ligation assay (PLA)
2.5 × 105 macrophages were plated on 12 mm coverslips (Warner Instrument, Hamden, CT, catalogue #CS-12R15) in 24-well plates. Cells were spinoculated at 1,200 × g using 6 × 108 RT unit/mL of 3-part lentiviral vector (pALPS-GFP, p8.9NΔSB, and pMD2.G, generated as above) at 13°C for 2 hrs. Media was replaced with RPMI-HS complete containing 2 μM MG132, and either DMSO or 5 μM CsA, and cells were incubated at 37° for 2 hrs. Coverslips were fixed with 3.7% formaldehyde (ThermoFisher) in 0.1 M PIPES, pH 6.8, for 5 min at room temperature, and then incubated at room temperature for 1 hr in PBS containing 0.1% saponin, 10% donkey serum, 0.01% sodium azide, mouse anti-TRIM5α antibody (NIH AIDS Reagent Program, catalogue #12271) at a 1:750 dilution and rabbit anti-HIV-1 CA (p24) antibody (Abcam, catalogue #ab32352) at a 1:400 dilution. The samples were processed further using a Duolink® In Situ Red kit (Sigma-Aldrich), following the instructions of the manufacturer. Then samples were incubated with 10 μM phalloidin (FITC) (Enzo Life Science) and 1 mg/mL Hoechst 33342 (Invitrogen), in PBS containing 10% donkey serum and 0.01% sodium azide for 30 min at room temperature. Coverslips were mounted on slides and stored at −20 °C. Interaction was detected as fluorescent spots (λexcitation/emission 598/634 nm). λexcitation/emission 475/523 nm and λexcitation/emission 390/435 nm were used to detect phalloidin and Hoechst, respectively. Z-stack images were collected with a DeltaVision wide-field fluorescent microscope (Applied Precision, GE) and deconvolved with SoftWoRx deconvolution software (Applied Precision, GE). All images were acquired under identical acquisition conditions and analyzed by Imaris 8.3.1 (Bitplane). Three dimensional representations were constructed by using the Easy 3D function (Imaris 8.3.1).
Challenge with replication-competent HIV-1
5 × 105 macrophages per well in 12-well plates were challenged with 108 RT units of HIV-1 for 2 hrs, in the presence of CsA or DMSO solvent, as indicated. Macrophage experiments used NL4–3MAC; pNL4–3, in which env was replaced from the end of the signal peptide to the env stop codon, with macrophage-tropic env from GenBank #U63632.1. 3 days after secondary stimulation with CD3/CD28, 106 CD4+ T cells per well in 48-well plates were challenged with 2 × 107 RT units of HIV-1 for 2 hrs, in the presence of GS-CypAi3 or DMSO solvent, as indicated. CD4+ T cell experiments used HIV-1ZM249M, a clade C transmission-founder strain. After HIV-1 challenge, cells were washed with fresh media and resuspended in 1 mL of RPMI-HS complete for macrophages or RPMI-FBS complete containing 50 U/mL hIL-2 for CD4+ T cells. Where indicated, culture media also contained CsA, GS-CypAi3, or DMSO solvent. Every 2–3 days, culture supernatant was harvested to measure RT activity.
Extended Data
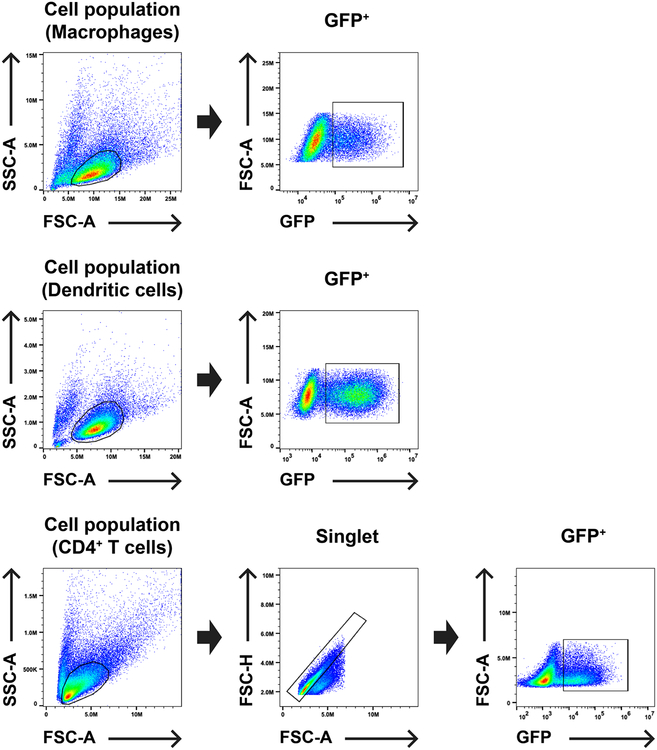
Extended Data Fig. 1. Gating strategy for flow cytometry experiments assessing single cycle infectivity.
Macrophage and dendritic cell populations, previously enriched as per the methods, were gated by SSC-A vs. FSC-A, as indicated, and then the GFP+ population was plotted vs. FSC-A. Enriched CD4+ T cells were gated by SSC-A vs FSC-A, as indicated, then a singlet population was gated from FSC-H vs. FSC-A, and finally GFP+ cells were plotted vs. FSC-A.
Extended Data Fig. 2. Assessment of shRNA-mediated knockdown in primary human blood cells.
a-c, Lentiviral vectors containing puromycin N- acetyltransferase (PuroR) and shRNA targeting TRIM5 or Luc were used to transduce macrophages (a), dendritic cells (b), or CD4+ T cells (c). At 3 days post-transduction, cells were selected with puromycin for 3 days. Total RNA was isolated from the macrophages and dendritic cells, followed by cDNA synthesis, and qPCR with TaqMan detection of TRIM5 and the housekeeping gene OAZ1, for normalization (mean ± SEM, n = 3 independent samples). Significance was determined by two-tailed, unpaired t-test (a and b). The selected CD4+ T cells were challenged with N- or B-MLV vector harboring GFP reporter for 3 days. Flow cytometry was used to assess the percentage of GFP+ cells. The infectivity of each vector in TRIM5 knockdown cells was normalized to the Luc control condition. Shown is mean ± SD (n = 3 donors for each). Significance was determined by two-tailed, paired t-test (c). d. Macrophages were simultaneously transduced with two lentiviral vectors, the first expressing shRNA targeting TRIM5 or Luc with PuroR, and the second expressing shRNA targeting CypA or Luc with blasticidin S-deaminase (bsd). After selection with both antibiotics, CypA and β-actin proteins were detected by western blot. Data shown is representative of three independent experiments using cells from three blood donors.
Extended Data Fig. 3. CA-CypA interaction promotes HIV-1 transduction by inhibiting TRIM5 activity in primary human blood cells.
a, Raw infectivity data for single cycle viruses, prior to normalization of infectivity to control condition. Shown are representative of three independent experiments using cells from three blood donors for each condition. b, Macrophages expressing shRNA targeting TRIM5 or Luc were challenged with single-cycle, VSV G-pseudotyped, HIV-1NL4–3GFP in the presence of 8 µM CsA, 8 µM CsH, or DMSO solvent (mean ± SEM, n = 2 donors). c and d, TRIM5 or Luc knockdown CD4+ T cells were challenged with single-cycle HIV-1NL4–3GFP (c) or HIV-1Z331M-TFGFP (d) in the presence of 2.5 µM GS-CypAi3 or DMSO solvent alone (mean ± SEM, n = 3 donors for each). e, HIV-1NL4–3GFP was used to challenge TRIM5 or Luc knockdown CD4+ T cells with 2.5 µM GS-CypAi48 or DMSO solvent alone (mean ± SEM, n = 2 donors). Flow cytometry was used to measure the percentage of GFP+ cells, followed by normalization to WT in Luc knockdown cells. Significance was determined by two-tailed, paired t- test for data generated with at least three donors (n = 3).
Extended Data Fig. 4. Saturation of TRIM5- mediated restriction in primary human macrophages.
Luc or TRIM5 knockdown macrophages were simultaneously challenged with a constant amount of single-cycle, VSV G-pseudotyped HIV-1NL4–3GFP containing CA-P90A and the indicated quantities of HIV- 1NL4–3 VLPs harboring either WT CA or CA-P90A. The percentage of GFP+ cells was assessed by flow cytometry at day 3 post-challenge. Data shown here are representative of four independent experiments performed on cells from four blood donors.
Extended Data Fig. 5. CA-CypA interaction prevents association of endogenous TRIM5α with HIV-1 CA in primary human macrophages.
a-d, TRIM5 or Luc knockdown macrophages from a different blood donor than that used in Fig. 4 were challenged with VSV G-pseudotyped, HIV-1NL4–3GFP in the presence of 5 µM CsA or DMSO solvent for 2 hrs (a and b), or challenged with HIV-1NL4–3GFP bearing WT CA or CA-P90A (c and d). PLA was then performed using anti-CA (p24) and anti-TRIM5α antibodies. Representative images (a and c) show PLA puncta (red), nuclei stained with Hoechst (blue), and actin filaments stained with phalloidin (green). The plots (b and d) are the number of PLA puncta per cell in the PLA with mean ± SEM. b, Luc KD + CsA No Virus, n = 45 cells analyzed; Luc KD + DMSO + HIV-1, n = 45; Luc KD + CsA + HIV-1, n = 80; TRIM5 KD + CsA + HIV-1, n = 45. d, Luc KD + WT HIV-1, n = 20; Luc KD + CA-P90A HIV-1, n = 30; TRIM5 KD + CA-P90A HIV-1, n = 20; TRIM5 KD + WT HIV-1, n = 20. Significance was determined by two-tailed, unpaired t-test.
Extended Data Fig. 6. The effect of proteasome inhibitor treatment on the proximity ligation assay for HIV-1 CA and endogenous TRIM5α.
Luc control knockdown macrophages treated with 5 µM CsA were challenged with VSV G-pseudotyped HIV-1NL4– 3GFP in the presence of 2 µM MG132 or DMSO solvent. Cells were fixed and proximity ligation assay (PLA) was performed with anti-CA (p24) and anti-TRIM5α antibodies. Representative images show PLA puncta (red), nuclei stained with Hoechst (blue), and actin filaments stained with phalloidin (green). The graph on the right shows the number of puncta per cell in the PLA, after analysis of 30 cells per condition (mean ± SEM). Significance was determined by two-tailed, unpaired t- test.
Supplementary Material
Acknowledgements
We thank Tomas Cihlar, Beatrice Hahn, Stephane Hausmann, Eric Hunter, Richard Mackman, Massimo Pizzato, and Stephen Yant for reagents. We are also grateful to anonymous blood donors who contributed leukocytes to this study. This work was supported by NIH Grants 5R01AI111809, 5DP1DA034990, 1R01AI117839, and 1R37AI147868 to J.L.
Footnotes
The authors declare no competing financial interests.
Supplementary information is available in the online version of the paper.
Data availability statement The plasmids described in Supplementary Table 1 are available at https://www.addgene.org/Jeremy_Luban/. All data generated or analyzed during this study are presented in the paper or in the Supplementary Information file, or are available from the corresponding author upon request.
References
- 1.Yamashita M & Engelman AN Capsid-Dependent Host Factors in HIV-1 Infection. Trends Microbiol. 25, 741–755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luban J, Bossolt KL, Franke EK, Kalpana GV & Goff SP Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73, 1067–1078 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Goldstone DC, Yap MW, Robertson LE, Haire LF, Taylor WR, Katzourakis A, Stoye JP & Taylor IA Structural and functional analysis of prehistoric lentiviruses uncovers an ancient molecular interface. Cell Host Microbe 8, 248–259 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Malfavon-Borja R, Wu LI, Emerman M & Malik HS Birth, decay, and reconstruction of an ancient TRIMCyp gene fusion in primate genomes. Proc. Natl. Acad. Sci. U. S. A 110, E583–92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mu D, Yang H, Zhu J-W, Liu F-L, Tian R-R, Zheng H-Y, Han J-B, Shi P & Zheng Y-T Independent birth of a novel TRIMCyp in Tupaia belangeri with a divergent function from its paralog TRIM5. Mol. Biol. Evol 31, 2985–2997 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Gilbert C, Maxfield DG, Goodman SM & Feschotte C Parallel germline infiltration of a lentivirus in two Malagasy lemurs. PLoS Genet. 5, e1000425 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzourakis A, Tristem M, Pybus OG & Gifford RJ Discovery and analysis of the first endogenous lentivirus. Proc. Natl. Acad. Sci. U. S. A 104, 6261–6265 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braaten D, Franke EK & Luban J Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol 70, 3551–3560 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke EK, Yuan HE & Luban J Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372, 359–362 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J & Göttlinger HG Functional association of cyclophilin A with HIV-1 virions. Nature 372, 363–365 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Braaten D & Luban J Cyclophilin A regulates HIV‐1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J (2001). <at http://emboj.embopress.org/content/20/6/1300.abstract> [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokolskaja E, Sayah DM & Luban J Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J. Virol 78, 12800–12808 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Towers GJ, Hatziioannou T, Cowan S, Goff SP, Luban J & Bieniasz PD Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med 9, 1138–1143 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Berthoux L, Sebastian S, Sokolskaja E & Luban J Cyclophilin A is required for TRIM5α-mediated resistance to HIV-1 in Old World monkey cells. Proc. Natl. Acad. Sci. U. S. A 102, 14849–14853 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayah DM, Sokolskaja E, Berthoux L & Luban J Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430, 569–573 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Luban J Cyclophilin A, TRIM5, and resistance to human immunodeficiency virus type 1 infection. J. Virol 81, 1054–1061 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayah DM & Luban J Selection for loss of Ref1 activity in human cells releases human immunodeficiency virus type 1 from cyclophilin A dependence during infection. J. Virol 78, 12066–12070 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebastian S & Luban J TRIM5α selectively binds a restriction-sensitive retroviral capsid. Retrovirology 2, 1 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebastian S, Sokolskaja E & Luban J Arsenic counteracts human immunodeficiency virus type 1 restriction by various TRIM5 orthologues in a cell type-dependent manner. J. Virol 80, 2051–2054 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI & Sodroski J Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proc. Natl. Acad. Sci. U. S. A 103, 5514–5519 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P & Sodroski J The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Wilson SJ, Webb BLJ, Ylinen LMJ, Verschoor E, Heeney JL & Towers GJ Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl. Acad. Sci. U. S. A 105, 3557–3562 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virgen CA, Kratovac Z, Bieniasz PD & Hatziioannou T Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc. Natl. Acad. Sci. U. S. A 105, 3563–3568 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman RM, Hall L, Kirmaier A, Pozzi L-A, Pery E, Farzan M, O’Neil SP & Johnson W Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog. 4, e1000003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan G, Kozyrev Y & Hu S-L TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl. Acad. Sci. U. S. A 105, 3569–3574 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boso G, Shaffer E, Liu Q, Cavanna K, Buckler-White A & Kozak CA Evolution of the rodent Trim5 cluster is marked by divergent paralogous expansions and independent acquisitions of TrimCyp fusions. Sci. Rep 9, 11263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokolskaja E, Berthoux L & Luban J Cyclophilin A and TRIM5alpha independently regulate human immunodeficiency virus type 1 infectivity in human cells. J. Virol 80, 2855–2862 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pertel T, Hausmann S, Morger D, Züger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grütter MG & Luban J TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472, 361–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellmann C, Hoffmann T, Sridhar V, Hopfgartner B, Muhar M, Roth M, Lai DY, Barbosa IAM, Kwon JS, Guan Y, Sinha N & Zuber J An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 5, 1704–1713 (2013). [DOI] [PubMed] [Google Scholar]
- 30.McCauley SM, Kim K, Nowosielska A, Dauphin A, Yurkovetskiy L, Diehl WE & Luban J Intron-containing RNA from the HIV-1 provirus activates type I interferon and inflammatory cytokines. Nat. Commun 9, 5305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yurkovetskiy L, Guney MH, Kim K, Goh SL, McCauley S, Dauphin A, Diehl WE & Luban J Primate immunodeficiency virus proteins Vpx and Vpr counteract transcriptional repression of proviruses by the HUSH complex. Nat Microbiol 3, 1354–1361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A & Martin MA Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol 59, 284–291 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH & Shaw GM Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med 206, 1273–1289 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackman RL, Steadman VA, Dean DK, Jansa P, Poullennec KG, Appleby T, Austin C, Blakemore CA, Cai R, Cannizzaro C, Chin G, Chiva J-YC, Dunbar NA, Fliri H, Highton AJ, Hui H, Ji M, Jin H, Karki K, Keats AJ, Lazarides L, Lee Y-J, Liclican A, Mish M, Murray B, Pettit SB, Pyun P, Sangi M, Santos R, Sanvoisin J, Schmitz U, Schrier A, Siegel D, Sperandio D, Stepan G, Tian Y, Watt GM, Yang H & Schultz BE Discovery of a Potent and Orally Bioavailable Cyclophilin Inhibitor Derived from the Sanglifehrin Macrocycle. J. Med. Chem 61, 9473–9499 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Husi H & Zurini MG Comparative binding studies of cyclophilins to cyclosporin A and derivatives by fluorescence measurements. Anal. Biochem 222, 251–255 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Varshavsky A in Methods in Enzymology 399, 777–799 (Academic Press, 2005). [DOI] [PubMed] [Google Scholar]
- 37.De Iaco A & Luban J Inhibition of HIV-1 infection by TNPO3 depletion is determined by capsid and detectable after viral cDNA enters the nucleus. Retrovirology 8, 98 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Anderson JL, Campbell EM, Joseph AM & Hope TJ Proteasome inhibitors uncouple rhesus TRIM5 restriction of HIV-1 reverse transcription and infection. Proceedings of the National Academy of Sciences 103, 7465–7470 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A & Luban J Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity 21, 189–201 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Bosco DA, Eisenmesser EZ, Pochapsky S, Sundquist WI & Kern D Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A. Proc. Natl. Acad. Sci. U. S. A 99, 5247–5252 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bock M, Bishop KN, Towers G & Stoye JP Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol 74, 7422–7430 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E, Yatim A, Emiliani S, Schwartz O & Benkirane M SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP & Skowronski J Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrillo C, Thorne LG, Unali G, Schiroli G, Giordano AMS, Piras F, Cuccovillo I, Petit SJ, Ahsan F, Noursadeghi M, Clare S, Genovese P, Gentner B, Naldini L, Towers GJ & Kajaste-Rudnitski A Cyclosporine H Overcomes Innate Immune Restrictions to Improve Lentiviral Transduction and Gene Editing In Human Hematopoietic Stem Cells. Cell Stem Cell 23, 820–832.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinhard C, Bottinelli D, Kim B & Luban J Vpx rescue of HIV-1 from the antiviral state in mature dendritic cells is independent of the intracellular deoxynucleotide concentration. Retrovirology 11, 12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butler SL, Hansen MS & Bushman FD A quantitative assay for HIV DNA integration in vivo. Nat. Med 7, 631–634 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.