Abstract
Postnatal growth of lean mass is commonly blunted in preterm infants and may contribute to short- and long-term morbidities. To determine whether preterm birth alters the protein anabolic response to feeding, piglets were delivered at term or preterm, and fractional protein synthesis rates (Ks) were measured at 3 days of age while fasted or after an enteral meal. Activation of signaling pathways that regulate protein synthesis and degradation were determined. Relative body weight gain was lower in preterm than in term. Gestational age at birth (GAB) did not alter fasting plasma glucose or insulin, but when fed, plasma insulin and glucose rose more slowly, and reached peak value later, in preterm than in term. Feeding increased Ks in longissimus dorsi (LD) and gastrocnemius muscles, heart, pancreas, and kidney in both GAB groups, but the response was blunted in preterm. In diaphragm, lung, jejunum, and brain, feeding increased Ks regardless of GAB. Liver Ks was greater in preterm than term and increased with feeding regardless of GAB. In all tissues, changes in 4EBP1, S6K1, and PKB phosphorylation paralleled changes in Ks. In LD, eIF4E·eIF4G complex formation, phosphorylation of TSC2, mTOR, and rpS6, and association of mammalian target of rapamycin (mTOR1) complex with RagA, RagC, and Rheb were increased by feeding and blunted by prematurity. There were no differences among groups in LD protein degradation markers. Our results demonstrate that preterm birth reduces weight gain and the protein synthetic response to feeding in muscle, pancreas, and kidney, and this is associated with blunted insulin- and/or amino acid-induced translation initiation signaling.
Keywords: amino acids, insulin, mammalian target of rapamycin, nutrition, preterm
INTRODUCTION
Worldwide each year, ∼15 million infants are born preterm, including nearly 10% of babies in the US (4). The neonatal period is a critical time for growth, and growth restriction during this period can lead to both short- and long-term adverse consequences, such as an increased risk for motor and cognitive impairment as well as metabolic syndrome, including obesity and insulin resistance (31, 32). However, despite improvements in their care and nutritional management, many premature infants experience extrauterine growth faltering and are discharged from the hospital weighing less than the 10th percentile for age (12, 24). At term-equivalent age, infants born prematurely frequently demonstrate lower body weight and lean mass than infants born at term (35). Thus, to develop strategies to improve postnatal outcomes for premature infants, it is essential to understand the mechanisms that regulate growth in the perinatal period and how they are influenced by stage of maturity.
In the human infant, ethical concerns preclude the use of the invasive techniques necessary to investigate the mechanisms by which feeding regulates tissue protein synthesis and growth. The neonatal piglet is an appropriate model of prematurity because its metabolism, physiology, and development are similar to that of the human infant (51, 57). In both newborn humans and piglets, growth is driven largely by the stimulation of protein synthesis in the whole body following a feed, and in the neonatal piglet, this response is greatest for skeletal muscle (14, 21). As a result, relative growth velocity during the neonatal period is higher than at any other stage of postnatal life (14). Importantly, viable pigs can be delivered by cesarean section before term gestation, and recent studies show that end points of growth, organ function, physical activity, and arousal are diminished or delayed in preterm versus term pigs similarly to preterm human babies (1, 28).
In neonatal pigs born at term gestation, the postprandial increases in both insulin and amino acids independently stimulate protein synthesis in skeletal muscle and are critical for supporting the rapid growth rate observed in early postnatal life (14, 69). The feeding-induced rise in insulin and amino acids stimulates independent signaling pathways that activate the mammalian target of rapamycin complex 1 (mTORC1), a key nutrient sensor that regulates translation initiation (69). Insulin-induced activation of the insulin receptor (IR) leads to tyrosine phosphorylation of the insulin receptor substrate-1 (IRS-1) (59). The resulting activation of phosphoinositide 3-kinase (PI 3-kinase) and protein kinase B (PKB) inhibits tuberous sclerosis complex (TSC) 1/2, enabling the activation of Ras homolog enriched in brain (Rheb) and mTORC1. mTORC1 then phosphorylates the 70-kDa ribosomal protein S6 kinase 1 (S6K1), as well as eukaryotic translation initiation factor (eIF)4E-binding protein 1 (4EBP1). Phosphorylation of 4EBP1 releases eIF4E to form the active complex with eIF4G, which binds to mRNA and initiates translation (39, 58). Translation initiation is also controlled by phosphorylation of ribosomal protein S6 (rpS6) and eukaryotic initiation factor 2α (eIF2α), whereas eukaryotic elongation factor 2 (eEF2) activity regulates peptide chain elongation (5, 23). The mechanisms by which amino acids stimulate mTORC1 and translation initiation are less well understood. A proposed model suggests that amino acids signal independently of PKB to induce recruitment of mTORC1 to the lysosomal surface by Ras-related GTP-binding protein A/B (RagA/B) and RagC/D heterodimeric complexes. Here, mTORC1 can be activated through the action of the lysosomal Ragulator complex consisting of five late endosomal/lysosomal adaptor, MAPK, and mTOR activator/regulator (LAMTOR) subunits and mammalian target of rapamycin complex (mTOR) interaction with the GTP-loaded Rheb (39). Downstream of mTORC1, the signaling pathways activated by amino acids are similar to those of insulin.
Protein degradation is regulated by multiple proteolytic systems, including the ubiquitin-proteosome and autophagy-lysosome systems. Both insulin and amino acids can inhibit muscle protein degradation (64). Insulin inhibits protein degradation through PKB activation, resulting in phosphorylation of forkhead box O (FoxO) transcription factors and downregulation of components of ubiquitin-proteosome activity in muscle, including atrogin-1 and muscle-specific RING finger (MuRF-1) (77, 78). Insulin, via mTORC1, also downregulates microtubule-associated protein light chain 3 (LC3), a key component of the autophagy-lysosome system (78). Amino acids appear to inhibit protein degradation by suppressing expression of atrogin-1 and MuRF-1 and by blocking autophagy though activation of mTORC1 (30, 77).
We have demonstrated that in skeletal muscle these pathways are developmentally regulated as a result of changes in the abundance and activity of key signaling molecules. These changes result in a decrease in the responsiveness of protein synthesis to the anabolic effects of feeding with maturation. It is unknown whether the activation of these signaling pathways by feeding is altered by preterm birth. The objective of this study was to determine whether prematurity alters the protein synthetic response of skeletal muscle and other tissues to feeding in a piglet model and to assess whether alterations in the protein synthetic response are due to blunted insulin- and/or amino acid-induced translation initiation signaling. Because prematurity is often associated with reduced lean mass at term-equivalent age (35), we hypothesized that prematurity decreases muscle protein synthesis by blunting the protein anabolic response to feeding.
MATERIALS AND METHODS
Animals and surgeries.
The experimental protocol was approved by the Institutional Animal Care and Use Committee, Baylor College of Medicine, and conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. All surgeries were performed under general anesthesia using sterile technique. Pregnant sows (Yorkshire × Landrace × Duroc × Hampshire) were obtained from a commercial farm (Burton, TX) and housed at the USDA/ARS Children’s Nutrition Research Center (Houston, TX) with ad libitum access to food and water. Piglets were delivered by cesarean section (27, 57) on gestation day 103 (preterm) or 112 (term). Day 103 was selected as the delivery day for premature piglets because at this age piglets clearly demonstrate features of prematurity, but lungs are adequately developed to allow for spontaneous respiration. Term piglets were delivered on day 112 to prevent natural delivery, which occurs at or around 114 days of gestation. Briefly, sows were injected with atropine (0.04 mg/kg; Henry Schein Animal Health, Dublin, OH), followed by a mixture of ketamine (20 mg/kg; Henry Schein Animal Health) and xylazine (2 mg/kg im; Akorn Animal Health, Lake Forest, IL). Paravertebral blockage was induced by injection of 40–50 mL of 2% lidocaine (Henry Schein Animal Health) along the lumbar vertebrae. Sows were intubated and maintained on anesthesia with isoflurane inhalation (2–5% in oxygen; Henry Schein Animal Health). The uterus was exposed via midline laparotomy, and a hysterotomy incision was made on the anti-mesenteric uterine surface. Piglets were delivered sequentially after the umbilical cord was ligated and transected and immediately resuscitated by suctioning the nasopharynx free of fluid and utilizing intermittent bag-mask ventilation to aid respiration. Once fully resuscitated, piglets were placed in individual incubators maintained at 30–35°C. Within 6 h of delivery (day of life 0), all viable piglets were implanted with jugular venous catheters, as previously described (13). Piglets were monitored every hour, with full clinical assessment every 12 h. They were administered injections of iron dextran (30–100 mg; Henry Schein Animal Health) and Meloxicam SR (0.6 mg/kg; Zoo Pharm, Laramie, WY) before surgery and received infusions of 4, 5, and 7 mL/kg sterile sow plasma at 6, 12, and 24 h after birth, respectively, for passive immunological protection.
Nutritional support and study design.
Total parenteral nutrition (TPN) was formulated to meet or exceed the nutritional requirements of neonatal pigs (49, 63) and consisted of a complete mixture of amino acids, dextrose, electrolytes, vitamins, and trace minerals. Postsurgery, TPN was administered via the jugular catheter at 6 mL·kg−1·h−1 and gradually increased over 48 h to 10 mL·kg−1·h−1. Piglets were weighed on day 0 (at birth), day 2, and day 3, with infusion rates adjusted accordingly. Infusion rates on day 1 were calculated according to birth weight (day 0). On day 2, all piglets received full amounts of nutrition (Table 1) (62, 63). On day 3, all surviving piglets were fasted for 4 h, and stratified randomization was performed to assign piglets by birth weight to fast for 1 additional hour or be fed a complete elemental meal by oral gavage [4 groups: PF (preterm fasted; 13 males, 10 females); PP (preterm postprandial; 14 males, 11 females); TF (term fasted; 10 males, 11 females); TP (term postprandial; 16 males, 5 females)]. Macronutrient composition of the enteral meal mimicked sow’s milk on day 3 of lactation (Table 1) (34) and provided one-sixth of the daily nutrient requirement, including electrolytes, vitamins, and trace minerals. Blood samples were collected into EDTA tubes from the jugular catheter immediately before feeding (0 min) and after 15, 30, 45, and 60 min. Plasma was collected after centrifugation and frozen at −20°C until analysis. Following euthanasia at 60 min, samples of the longissimus dorsi (LD), gastrocnemius, diaphragm, and heart muscles were collected as well as the pancreas, kidney, lung, jejunum, liver, and brain. The LD, gastrocnemius, kidney, liver, and heart were weighed, and all tissues were immediately snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Table 1.
Nutrient intake and macronutrient composition of total parenteral nutrition and the enteral meal
| TPNa,c,d,e,f,g | Enteral Mealb,c,d,e,f,g | |
|---|---|---|
| Fluid intake, mL | 240 | 40 |
| Energy, kcal | 189 | 31.5 |
| Carbohydrate, g | 22 | 1.3 |
| Amino acids, g | 16 | 2.7 |
| Lipid, g | 5 | 1.6 |
TPN, total parenteral nutrition
U·kg body wt−1·day−1 when provided to meet 100% of nutrient requirements;
U·kg body wt−1·meal−1;
carbohydrate supplied as dextrose, and amino acids as crystalline l-amino acids (63);
amino acids supplied as crystalline l-amino acids (g/L): alanine, 3.34; arginine, 2.89; aspartic acid, 5.14; cysteine, 1.02; glutamic acid, 6.42; glutamine, 5.14; glycine, 2.51; histidine, 1.67; isoleucine, 3.73; leucine, 6.62; lysine, 5.01; methionine, 1.67; phenylalanine, 3.41; proline, 4.82; serine, 3.61; threonine, 4.05; tryptophan, 0.78; tyrosine, 0.78; valine, 4.05 (63);
lipid provided as 20% Intralipid and contains 20% soybean oil, 1.2% egg yolk phospholipids, 2.25% glycerin, and water for injection;
electrolyte composition (mmol/L): sodium chloride, 17.2; potassium acetate, 13.8; potassium phosphate, 19.7; magnesium sulfate, 2.9; calcium gluconate, 4.5; sodium hydroxide, 13.0;
vitamins and trace minerals (U·kg body wt−1·day−1 when provided to meet 100% of nutrient requirements): vitamin A, 234 µg; vitamin D, 1.7 µg; vitamin E, 1.36 mg; vitamin B6, 0.396 mg; thiamin B1, 3.96 mg; riboflavin B2, 0.198 mg; folic acid, 0.016 mg, vitamin B12, 0.158 ppm, niacin B3, 3.96 mg; pantothenol, 0.396 mg; sulfur, 145 mg; zinc, 1,056 µg; copper, 422 µg; manganese, 106 µg; selenium, 21.1 µg; chromium, 4.22 µg.
Plasma insulin, glucose, and amino acids.
Insulin and glucose concentrations were determined in plasma collected at 0, 15, 30, 45, and 60 min and amino acids at 0, 30, and 60 min. Insulin concentrations were determined using a porcine insulin radioimmunoassay kit (MilliporeSigma, Burlington, MA). Glucose concentrations were measured using the glucose oxidase method (model 2300; Yellow Springs Instruments, Yellow Springs, OH). High-performance liquid chromatography (HPLC; PICO-TAG reverse-phase column; Waters, Milford, MA) was used to determine free amino acid concentrations following deproteinization and derivatization with phenylisothiocyanate (7).
Plasma GLP1, IGF-I, and urea.
Commercial kits were used to measure plasma GLP1 [GLP-1 (active) ELISA; MilliporeSigma], IGF-I (human IGF-I Quantikine ELISA Kit; R & D Systems, Inc., Minneapolis, MN), and urea (Urea Assay Kit; Sigma-Aldrich, St. Louis, MO) at 60 min.
Tissue protein synthesis.
Fractional rates of protein synthesis in muscle, visceral tissues, and the brain were measured with a flooding dose of l-[4-3H]phenylalanine (0.15 mmol Phe/kg, 0.5 mCi of l-[4-3H] Phe/kg−1; American Radiolabeled Chemicals, St. Louis, MO) injected 30 min before euthanasia (26). Blood samples were collected 5, 15, and 30 min after the l-[4-3H] Phe injection and stored at −20°C for measurement of the radioactivity of the Phe blood pool. Free and protein-bound tissue phenylalanine was measured by HPLC using an anion exchange column (PA1 column; Dionex, Sunnyvale, CA) (18). Radioactivity associated with the phenylalanine peak from each sample was measured in a liquid scintillation counter (Tri-Carb 2500TR; Packard Instrument Company, Meriden, CT). Fractional rates of protein synthesis (Ks; %protein mass synthesized/day) for each tissue were calculated as Ks = [(SAbound Phe/SAfree Phe)·1,440/t]·100, where SAbound phe is the specific radioactivity of the protein-bound phenylalanine, SAfree phe is the specific radioactivity of the average tissue free phenylalanine calculated by correcting the value at time of collection by the linear regression of the blood-specific radioactivity of the animal against time, 1,440 is the minutes-to-days conversion, and t is the time (min) of labeling of the specific tissue (13, 16). Protein synthetic capacity (Cs; mg RNA/g protein) and efficiency (KRNA; g protein synthesized/g RNA) were determined after protein content by the Pierce bicinchoninic acid assay (43) and quantification of total tissue RNA concentration by the method of Munro and Fleck (48) were measured.
Protein immunoblot analysis.
Proteins from tissue homogenates were subjected to Western blot analysis, as previously described (25, 65, 70). Values for total protein abundance were corrected and normalized for loading with GAPDH. Values for protein phosphorylation were normalized by their corresponding total protein abundance after stripping the blots in stripping buffer (Pierce Biotechnology, Rockford, IL) and re-probing with corresponding nonphosphospecific antibodies, as previously described (65). Primary antibodies were purchased from Bethyl Laboratories, Inc. (Montgomery, TX; 4EBP1 total), bioWORLD (Dublin, OH; eIF2α total), Biorbyt LCC (San Francisco, CA; IR phosphorylated Tyr1185), Cell Signaling Technology [Danvers, MA; 4EBP1 phosphorylated Thr70, AMP-activated kinase (AMPK) total and phosphorylated Thr172, DEP domain-containing mTOR-interacting protein (DEPTOR), eEF2 total and phosphorylated Thr56, FoxO3 total and phosphorylated Ser253, LAMTOR1, LAMTOR2, LC3 A/B, mTOR total and phosphorylated Ser2448, PKB total and phosphorylated Ser473 and Thr308, RagA, RagC, regulatory-associated protein of mTOR (Raptor), Rheb, rpS6 total and phosphorylated Ser235/236, Ser240/244, and TSC2 total and phosphorylated Thr1462], EMC Biosciences LLC (Versailles, KY; atrogin-1), Millipore-Sigma (eIF2α-phosphorylated Ser51), Proteintech Group, Inc. (Rosemont, IL; GAPDH, S6K1 total), R & D Systems, Inc. (MuRF-1- and S6K1-phosphorylated Thr389), and Santa Cruz Biotechnology (Dallas, TX; IR total).
Immunoprecipitation of IRS-1 and PI 3-kinase proteins.
To determine the tyrosine phosphorylation of IRS-1 and the association of PI 3-kinase (p85) with IRS-1, protein samples from tissue extracts were immunoprecipitated with anti-IRS-1 (Cell Signaling Technology), followed by immunoblotting with phospho-tyrosine antibody (Cell Signaling Technology), anti-IRS-1 (Cell Signaling Technology), and anti-PI 3-kinase (p85; Cell Signaling Technology), as previously described (68).
Quantification of eIF4E·eIF4G, mTOR·RagA, mTOR·RagC, mTOR·Rheb, and Sestrin2·Gator2 complex abundance.
The eIF4E·eIF4G complex was immunoprecipitated, as previously described (25). Amounts of eIF4G (Millipore-Sigma, Burlington, MA) were corrected for the amount of eIF4E (Cell Signaling Technology) recovered in the immunoprecipitate. mTOR·RagA, mTOR·RagC, and mTOR·Rheb complex abundance was determined by immunoprecipitation of homogenates with mTOR (Cell Signaling Technology) (22). The immunoprecipitated samples were subjected to immunoblot analysis using RagA, RagC, or Rheb primary antibody (Cell Signaling Technology), and results were normalized for mTOR abundance in the precipitate. To determine Sestrin2·Gator2 complex abundance, homogenates were incubated with Sestrin2 antibody (Cell Signaling Technology), followed by immunoblotting with anti-Mios (Gator2 subunit) antibody (Cell Signaling Technology) (66). Protein complexes were normalized by total precipitate Sestrin2 abundance.
Statistical analysis.
Statistical analysis was performed using SAS software (version 9.4; SAS Institute, Cary, NC). Comparisons between preterm and term piglets for body weight gain, nutrient delivery, and tissue weight were made using an independent t-test. Comparisons between groups (preterm versus term and fasted versus fed) were made using two-way ANOVA. Only when a significant interaction was found were means compared using Tukey’s post hoc test. ANOVA for repeated measures was used to assess plasma concentrations of glucose, insulin, and amino acids. Data are presented as least-square means ± SE. Differences were considered significant at P < 0.05.
RESULTS
Birth weight, body weight gain, and tissue weights.
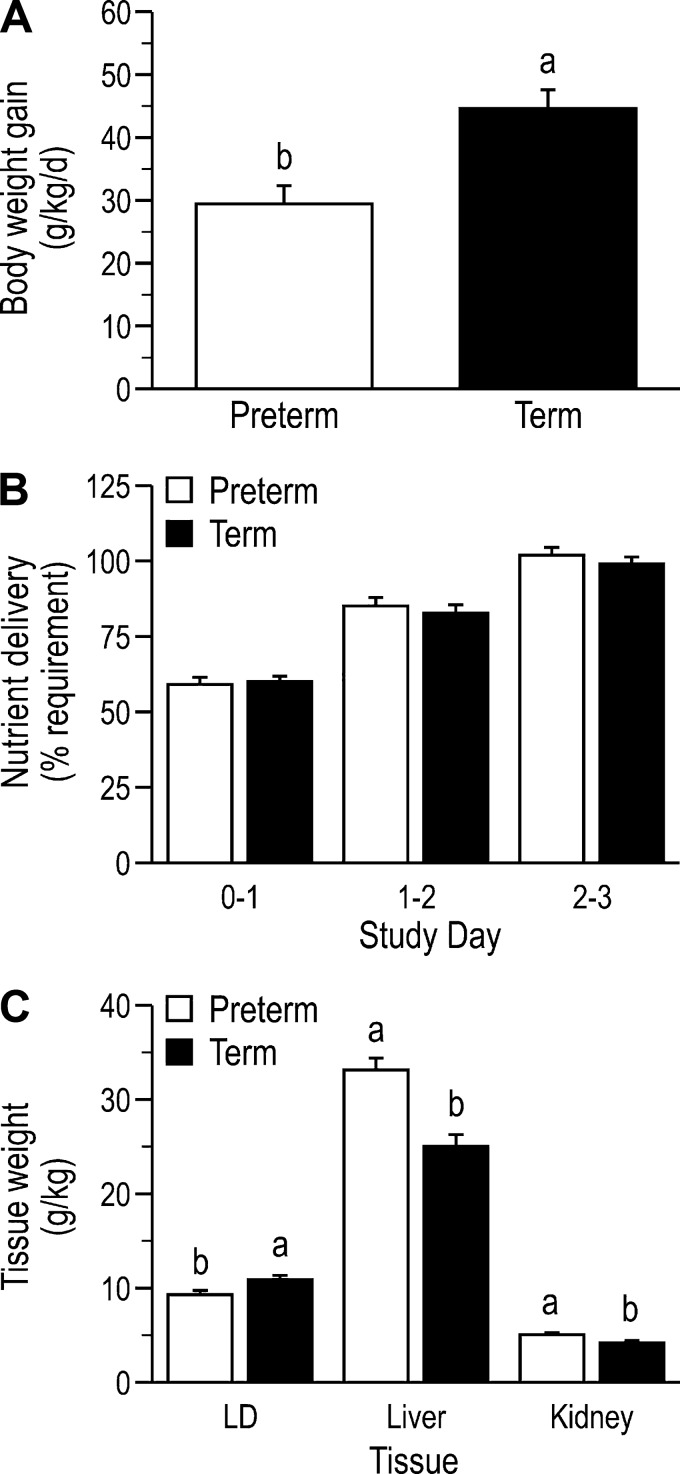
Birth weight of preterm pigs (n = 48; 4 litters) was lower (881.5 ± 33.6 vs. 1,208.0 ± 40.5 g, P < 0.01) than that of term (n = 42; 4 litters) pigs. Body weight gain velocity was lower (P = 0.01; Fig. 1A) in preterm than term pigs despite equivalent daily nutrient delivery (P = 0.91; Fig. 1B). On study day 3, preterm pigs had greater kidney (P = 0.03) and liver (P < 0.01) masses (relative to body weight) but lower (P = 0.04) LD mass than term pigs (Fig. 1C). The relative masses of the gastrocnemius and heart did not differ between preterm and term pigs (data not shown).
Fig. 1.
Body weight gain (A), nutrient delivery expressed relative to requirement (B), and tissue weights (C) of piglets born at preterm or term gestation. Values are presented as means ± SE; n = 42–48/group. Means without a common letter differ for body weight gain (P < 0.001), longissimus dorsi (LD) weight (P = 0.01), liver weight (P < 0.01), and kidney weight (P = 0.02).
Plasma glucose, insulin, and amino acid concentrations.
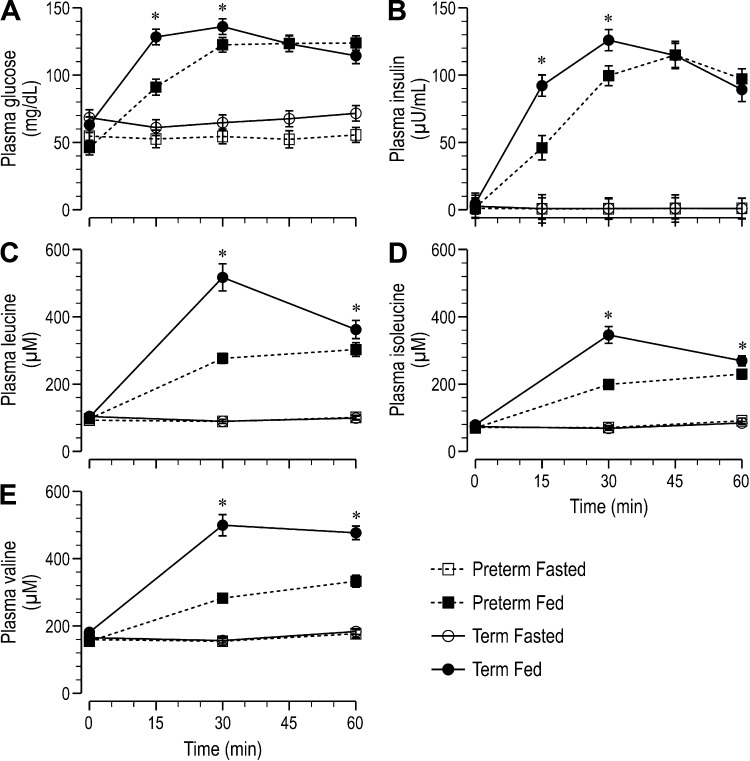
Circulating concentrations were determined before and over the 60-min period immediately after feeding. There were no differences among groups in plasma insulin or glucose at 0 min. Gestational age at birth (GAB) did not affect plasma glucose or insulin of fasted pigs, but among fed pigs, both plasma glucose (Fig. 2A) and insulin (Fig. 2B) rose more slowly and reached peak value later in preterm than in term pigs. Both plasma glucose and insulin were lower in fed preterm than fed term pigs at 15 and 30 min (P < 0.05), but these differences were no longer significant by 45 min after feeding (Fig. 2, A and B). Net incremental area under the curve (AUC) for both glucose (6,329.5 ± 280.1 vs. 7,143.3 ± 269.6 mg·h−1·dL−1, P = 0.04) and insulin (4,647.7 ± 356.9 vs. 5,693.2 ± 347.1 µU·h−1·mL−1, P = 0.04) was lower among fed preterm than fed term pigs. Similarly, circulating levels of the branched-chain amino acids did not differ between preterm and term fasted pigs (Fig. 2, C–E). Moreover, the feeding-induced rise in the plasma branched-chain amino acids, leucine (Fig. 2C), isoleucine (Fig. 2D), and valine (Fig. 2E) was blunted by prematurity, with their levels being significantly lower in fed preterm than fed term pigs at both 30 and 60 min (P < 0.05) despite being equivalent at 0 min. There were no differences in plasma concentrations of other indispensable or dispensable amino acids among fasted pigs (data not shown). Among fed pigs, plasma levels of the other amino acids mirrored those of the branched-chain amino acids, with levels significantly less in preterm than term pigs at both 30 and 60 min (data not shown).
Fig. 2.
Plasma glucose (A), insulin (B), leucine (C), isoleucine (D), and valine (E) concentrations of fasted or fed piglets born at preterm or term gestation. Values are least-square means ± SE; n = 21–25/group. Repeated-measures ANOVA for glucose: gestational age at birth (GAB) P = 0.04, feeding status (feed) P < 0.001, and GAB × feed P = 0.03; insulin: GAB P < 0.01, feed P < 0.001, and GAB × feed P = 0.02; leucine: GAB P < 0.001, feed P < 0.001, and GAB × feed P < 0.001; isoleucine: GAB P < 0.001, feed P < 0.001, and GAB × feed P < 0.001; valine: GAB P < 0.001, feed P < 0.001, and GAB × feed P < 0.001. *P < 0.05, term fed vs. preterm fed.
Plasma GLP1, IGF-I, and urea concentrations.
Circulating concentrations of plasma GLP1, IGF-I, and urea were assessed 60 min after the feed. Plasma GLP1 was not affected by GAB but was lower in fasted than in fed pigs (11.0 ± 2.4 vs. 33.3 ± 2.3 pM, respectively, P < 0.01). Plasma IGF-I was lower in preterm than term pigs (25.0 ± 0.6 vs. 29.9 ± 0.9 ng/mL, respectively, P = 0.045), with no effect of feeding. There was no effect of GAB or feeding on plasma urea (29.1 ± 2.2 mg/dL, P = 0.92).
Fractional protein synthesis rates in tissues.
There was no effect of GAB on fasting values of Ks for any tissues, except the liver. Feeding increased Ks in all of the tissues analyzed from both GAB groups, but the response in the LD, gastrocnemius, heart, and pancreas was lower, and the kidney was marginally lower in preterm than term pigs (Table 2). Ks increased to a similar extent with feeding regardless of GAB in the diaphragm, lung, jejunum, and brain (Table 2). Liver Ks was greater in preterm than in term pigs both in fasted and fed states, but the extent of the increase was similar for both groups (Table 2).
Table 2.
Tissue fractional protein synthesis rates (Ks; %/day) of fasted or fed piglets born at preterm or term gestation
| Preterm |
Term |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Tissue | Fasted | Fed | Fasted | Fed | GAB | Feed | GAB × feed |
| LD | 14.48 ± 1.28c | 33.98 ± 1.23b | 16.78 ± 1.34c | 42.86 ± 1.38a | <0.001 | <0.001 | 0.01 |
| Gastrocnemius | 15.86 ± 1.03c | 31.87 ± 0.99b | 16.66 ± 1.07c | 41.01 ± 1.07a | <0.001 | <0.001 | <0.001 |
| Diaphragm | 17.52 ± 1.14 | 36.11 ± 1.07 | 16.78 ± 1.16 | 37.97 ± 1.16 | NS | <0.001 | NS |
| Heart | 15.85 ± 0.86c | 26.57 ± 0.82b | 15.08 ± 0.90c | 34.12 ± 0.90a | <0.001 | <0.001 | <0.001 |
| Lung | 23.6 ± 1.67 | 30.5 ± 1.66 | 20.3 ± 1.70 | 30.1 ± 1.70 | NS | <0.001 | 0.08 |
| Jejunum | 33.5 ± 2.83 | 46.2 ± 2.84 | 35.2 ± 2.84 | 51.7 ± 2.90 | NS | <0.001 | NS |
| Liver | 63.9 ± 1.80 | 74.4 ± 1.77 | 53.0 ± 1.75 | 63.7 ± 1.77 | 0.02 | <0.001 | NS |
| Pancreas | 49.5 ± 3.34c | 75.4 ± 3.32b | 53.5 ± 3.41c | 95.9 ± 3.42a | 0.03 | <0.001 | <0.001 |
| Kidney | 29.5 ± 1.77 | 39.5 ± 1.77 | 34.1 ± 1.79 | 46.1 ± 1.80 | 0.06 | <0.001 | NS |
| Brain | 18.4 ± 1.20 | 25.9 ± 1.21 | 19.3 ± 1.23 | 26.8 ± 1.24 | NS | <0.001 | NS |
Values are least square means ± SE; n = 21–25/group. Feed, feeding status; GAB, gestational age at birth; LD, longissimus dorsi; NS, not statistically significant, P > 0.05. Two-way ANOVA followed by Tukey’s post hoc test.
a,
b,
Means without a common letter differ, P < 0.05.
Protein synthetic capacity (Cs), as indicated by total RNA concentration, was greater in the heart, lung, jejunum, liver, and kidney of preterm than in term pigs regardless of feeding status (Table 3). In the pancreas, Cs was similar for the term and preterm and increased with feeding (Table 3). There were no differences in Cs among groups in the LD, gastrocnemius, diaphragm, or brain (Table 3). Protein synthetic efficiency, i.e., the efficiency with which the ribosomes translated mRNA into protein (KRNA), was increased by feeding in all tissues of both preterm and term pigs (Table 3). The feeding-induced increase was blunted by prematurity in the LD, gastrocnemius, and heart but not in the diaphragm, lung, liver, or brain (Table 3). There was a tendency for a blunted response in the jejunum and pancreas. In the kidney, feeding increased KRNA of both preterm and term pigs, but regardless of feeding status, preterm pigs had lower KRNA than term pigs (Table 3).
Table 3.
Cs and KRNA of muscle, visceral organs, and brain from fasted or fed piglets born at preterm or term gestation
| Preterm |
Term |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Tissue | Fasted | Fed | Fasted | Fed | GAB | Feed | GAB × feed |
| Cs, mg RNA/g protein | |||||||
| LD | 27.6 ± 1.26 | 28.0 ± 1.25 | 28.9 ± 1.27 | 27.5 ± 1.28 | NS | NS | NS |
| Gastrocnemius | 29.7 ± 1.19 | 29.3 ± 1.15 | 28.5 ± 1.24 | 31.0 ± 1.25 | NS | NS | NS |
| Diaphragm | 22.5 ± 1.00 | 21.2 ± 1.02 | 20.4 ± 1.07 | 20.0 ± 1.06 | NS | NS | NS |
| Heart | 30.3 ± 1.01 | 31.8 ± 1.01 | 26.8 ± 1.05 | 27.4 ± 1.04 | 0.03 | 0.07 | NS |
| Lung | 57.3 ± 1.21 | 58.0 ± 1.20 | 48.8 ± 1.31 | 49.5 ± 1.26 | 0.002 | NS | NS |
| Jejunum | 65.8 ± 1.46 | 63.7 ± 1.45 | 59.7 ± 1.52 | 58.6 ± 1.53 | 0.02 | NS | NS |
| Liver | 80.8 ± 1.82 | 81.2 ± 1.82 | 71.0 ± 1.88 | 71.0 ± 1.86 | 0.01 | NS | NS |
| Pancreas | 79.5 ± 3.71 | 88.5 ± 3.68 | 84.3 ± 3.83 | 92.9 ± 3.75 | NS | 0.001 | NS |
| Kidney | 50.6 ± 0.92 | 50.9 ± 0.90 | 46.4 ± 0.96 | 48.2 ± 0.96 | <0.001 | NS | NS |
| Brain | 29.5 ± 1.63 | 29.3 ± 1.61 | 27.5 ± 1.64 | 27.4 ± 1.65 | NS | NS | NS |
| KRNA, g protein synthesized/g RNA | |||||||
| LD | 5.37 ± 0.59c | 12.45 ± 0.54b | 5.86 ± 0.62c | 16.82 ± 0.64a | <0.001 | <0.001 | 0.002 |
| Gastrocnemius | 5.31 ± 0.53c | 11.23 ± 0.53b | 5.88 ± 0.54c | 13.86 ± 0.57a | 0.004 | <0.001 | 0.05 |
| Diaphragm | 8.18 ± 0.75 | 17.44 ± 0.71 | 8.66 ± 0.77 | 19.50 ± 0.77 | 0.09 | <0.001 | NS |
| Heart | 5.23 ± 0.37c | 8.47 ± 0.35b | 5.59 ± 0.40c | 12.55 ± 0.39a | 0.009 | <0.001 | <0.001 |
| Lung | 4.11 ± 0.24b | 5.26 ± 0.24a | 4.04 ± 0.25b | 6.13 ± 0.25a | NS | <0.001 | 0.009 |
| Jejunum | 5.22 ± 0.26 | 7.28 ± 0.26 | 6.03 ± 0.27 | 8.71 ± 0.28 | 0.08 | <0.001 | 0.05 |
| Liver | 8.03 ± 0.20 | 9.23 ± 0.19 | 7.42 ± 0.20 | 9.01 ± 0.19 | 0.04 | <0.001 | NS |
| Pancreas | 6.34 ± 0.25c | 8.48 ± 0.24b | 6.39 ± 0.27c | 10.3 ± 0.27a | 0.10 | <0.001 | <0.001 |
| Kidney | 5.83 ± 0.23 | 7.74 ± 0.22 | 7.17 ± 0.24 | 9.57 ± 0.24 | 0.008 | <0.001 | NS |
| Brain | 6.33 ± 0.42 | 9.00 ± 0.42 | 6.98 ± 0.44 | 9.95 ± 0.44 | NS | <0.001 | NS |
Values are least-square means ± SE; n = 21–25/group. Cs, protein synthetic capacity; feed, feeding status; GAB, gestational age at birth; KRNA, protein synthetic efficiency; LD, longissimus dorsi; NS, not statistically significant, P > 0.05. Two-way ANOVA followed by Tukey’s post hoc test.
a,
b,
Means without a common letter differ, P < 0.05.
Activation of insulin- and amino acid-signaling pathways.
To identify the mechanism responsible for the blunted protein anabolic response of muscle to feeding in the premature piglets, the abundance and activation of signaling components within the insulin- and amino acid-induced protein synthetic pathways were analyzed in the LD at 60 min. Subsets of these indices were assessed at 60 min in other muscles as well as in visceral organs and brain.
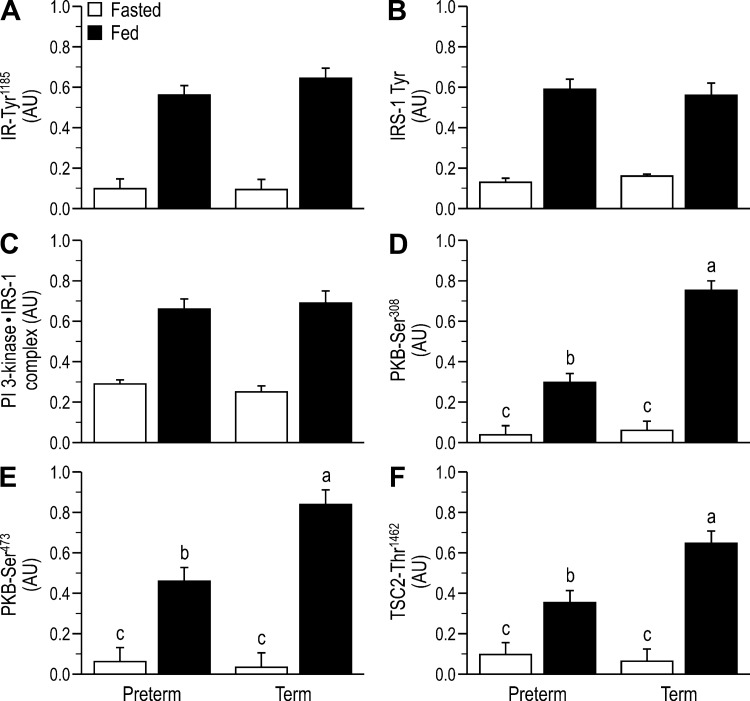
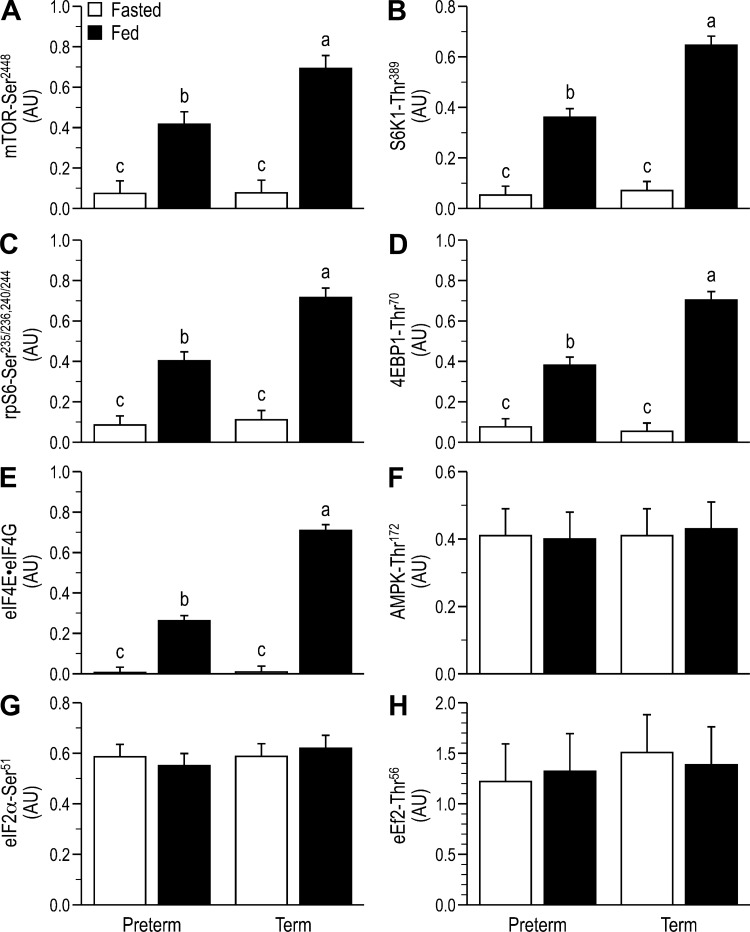
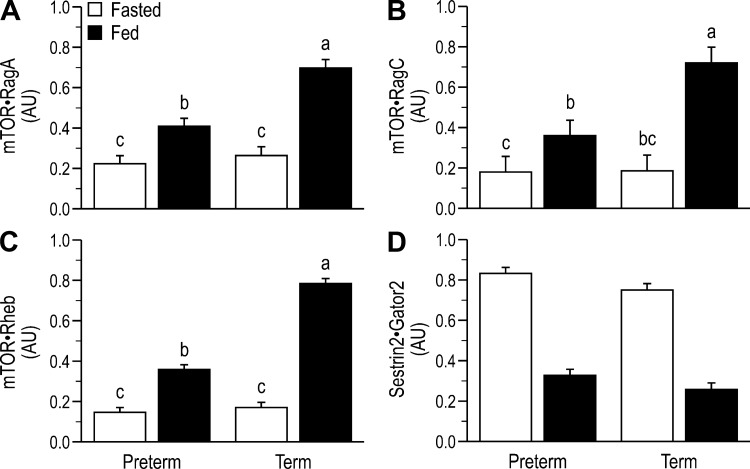
For key components of the insulin-signaling pathway that are upstream of PKB, there was no effect of GAB on activation in the fasted state. Feeding increased IR phosphorylation (Tyr1185; Fig. 3A), Tyr phosphorylation of IRS-1 (Fig. 3B), and PI 3-kinase·IRS-1 complex abundance (Fig. 3C) to the same level in both preterm and term piglets. Although feeding also increased the phosphorylation of PKB Thr308 (Fig. 3D), PKB Ser473 (Fig. 3E), and TSC2 Thr1462 (Fig. 3F), the response was blunted by prematurity. Feeding also increased the phosphorylation of mTORC1-dependent translation initiation factors, including mTOR Ser2448 (Fig. 4A), S6K1 Thr389 (Fig. 4B), rpS6 Ser235/236,240/244 (Fig. 4C), and 4EBP1 Thr70 (Fig. 4D) and the abundance of the eIF4E·eIF4G complex (Fig. 4E), but these responses were blunted by prematurity. Neither feeding nor GAB affected phosphorylation of AMPK (Thr172; Fig. 4F), eIF2α Ser51 (Fig. 4G), or eEF2 Thr56 (Fig. 4H). Within the amino acid-signaling pathway, the associations of mTOR with RagA (Fig. 5A), RagC (Fig. 5B), and Rheb (Fig. 5C) were enhanced with feeding, and these effects were blunted by prematurity. Formation of the Sestrin2·Gator2 complex decreased with feeding and tended to be higher in preterm than term (Fig. 5D).
Fig. 3.
Relative abundance of the phosphorylated insulin receptor (IR; A), insulin receptor substrate-1 (IRS-I; B), association of phosphatidylinositol 3-kinase (PI 3-kinase p85) with IRS-1 (C), phosphorylation of protein kinase B (PKB; D and E), and tuberous sclerosis 2 (TSC2; F) in the longissimus dorsi (LD) muscle of fasted or fed piglets born at preterm or term gestation. Values are least-square means ± SE; n = 21–25/group. Two-way ANOVA followed by Tukey’s post hoc test for IR Tyr1185: feeding status (feed) P < 0.01; IRS-I Tyr: feed P < 0.01; PI 3-kinase with IRS-1: feed P < 0.01; PKB Thr308: gestational age at birth (GAB) P < 0.01, feed P < 0.01, and GAB × feed P < 0.01; PKB SER473: GAB P < 0.01, feed P < 0.01, and GAB × feed P < 0.01; TSC2 Thr1462: GAB P < 0.01, feed P < 0.01, and GAB × feed P < 0.01. Means without a common letter differ, P < 0.05. AU, arbitrary units.
Fig. 4.
Relative abundance of phosphorylated mammalian target of rapamycin (mTOR; A), S6 kinase 1 (S6K1; B), ribosomal protein S6 (rpS6; C), eukaryotic initiation factor 4E-binding protein 1 (4EBP1; D), abundance of the active eukaryotic initiation factor 4E·eukaryotic initiation factor 4G complex (eIF4E·eIF4G; E), relative abundance of phosphorylated AMP-activated kinase (AMPK; F), eukaryotic initiation factor-2α (eIF2α; G), and eukaryotic elongation factor 2 (eEF2; H) in the longissimus dorsi (LD) muscle of fasted or fed piglets born at preterm or term gestation. Values are least-square means ± SE; n = 21–25/group. Two-way ANOVA followed by Tukey’s post hoc test for mTOR: gestational age at birth (GAB) P < 0.01, feeding status (feed) P < 0.01, and GAB × feed P < 0.01; S6K1: GAB P < 0.01, feed P < 0.01, and GAB × feed P < 0.01; rpS6: GAB P < 0.01, feed P < 0.01, and GAB × feed P < 0.01; 4EBP1: GAB P < 0.01, feed P < 0.01, and GAB × feed P < 0.01; eIF4E·eIF4G: GAB P < 0.01, feed P < 0.01, and GAB × feed P < 0.01. Means without a common letter differ, P < 0.05. AU, arbitrary units.
Fig. 5.
Relative abundance of mammalian target of rapamycin complex 1 (mTORC1) with Ras-related GTP-binding protein (Rag)A (A), RagC (B), Ras homolog enriched in brain (Rheb; C), and abundance of the Sestrin2·Gator2 complex (D) in the longissimus dorsi (LD) muscle of fasted or fed piglets born at preterm or term gestation. Values are least-square means ± SE; n = 21–25/group. Two-way ANOVA followed by Tukey’s post hoc test for mTOR·RagA: gestational age at birth (GAB) P < 0.001, feeding status (feed) P < 0.001, and GAB × feed P < 0.001; mTOR·RagC: GAB P < 0.01, feed P < 0.001, and GAB × Feed P < 0.01; mTOR·Rheb: GAB P < 0.001, feed P < 0.001, and GAB × feed P < 0.001; Sestrin2·Gator2: GAB P = 0.08 and feed P < 0.001. Means without a common letter differ, P < 0.05. AU, arbitrary units.
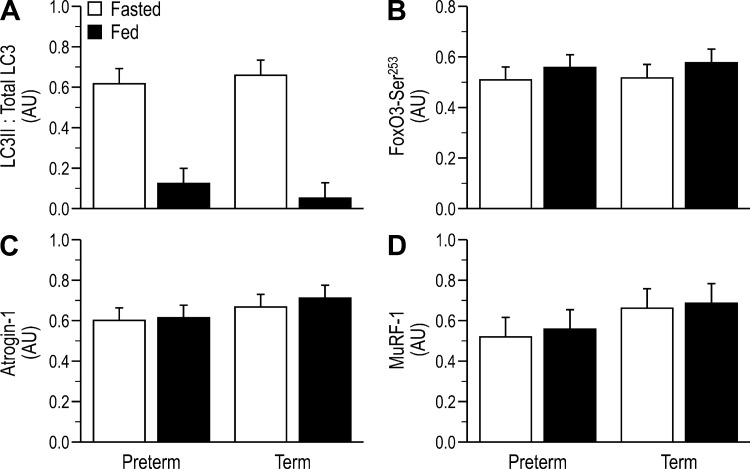
In addition to activation of the protein synthetic machinery, markers of protein degradation were also assessed for the LD. There were no differences among groups in total abundance of LC3 (data not shown), but LC3-II-to-total LC3 ratio decreased to a similar extent with feeding, regardless of GAB (Fig. 6A). There were no significant differences among groups in phosphorylation of FOXO3 Ser253 (Fig. 6B) or in abundance of atrogin-1 (Fig. 6C) and MuRF-1 (Fig. 6D). There also were no differences among groups in total abundances of GAPDH (loading control), insulin receptor, IRS-1, PI 3-kinase, PKB, TSC2, mTOR, S6K1, 4EBP1, rpS6, eIF4E, eIF2α, eEF2, Sestrin2, Gator2, LAMTOR1, LAMTOR2, Raptor, Rheb, RagA, RagC, Deptor, or FOXO3 (data not shown).
Fig. 6.
Indices of protein degradation, including the ratio of light chain 3 (LC3) II to total LC3 (A), the relative abundance of phosphorylated forkhead box O3 (FoxO3; B), and total abundances of atrogin-1 (C) and muscle RING-finger protein-1 (MuRF-1; D) in the longissimus dorsi (LD) muscle of fasted or fed piglets born at preterm or term gestation. Values are least-square means ± SE; n = 21–25/group. Two-way ANOVA, followed by Tukey’s post hoc test for LC3II: Total LC3: feeding status (feed), P < 0.001. Means without a common letter differ, P < 0.05. AU, arbitrary units.
For the gastrocnemius, phosphorylation of 4EBP1 Thr70, S6K1 Thr389, and PKB Ser473, as well as the abundance of the active eIF4E·eIF4G complex, was increased by feeding in both preterm and term pigs, but this activation was blunted by prematurity (Table 4). Similarly, phosphorylation of 4EBP1 Thr70, S6K1 Thr389, and PKB Ser473 increased with feeding but was blunted by prematurity in the heart (Table 4). For the diaphragm, phosphorylation of 4EBP1 Thr70, S6K1 Thr389, and PKB Ser473 and abundance of the eIF4E·eIF4G complex was increased by feeding and was similar for both GAB groups (Table 4). For the lung, jejunum, and brain, phosphorylation of 4EBP1 Thr70, S6K1 Thr389, and PKB Ser473 was increased with feeding and not influenced by GAB (Table 5). In the pancreas and kidney, phosphorylation of these proteins increased with feeding in both preterm and term pigs, but this response mostly was blunted by prematurity (Table 5). By contrast, feeding increased phosphorylation of 4EBP1 Thr70, S6K1 Thr389, and PKB Ser473 to a greater extent in the liver of preterm than term pigs (Table 5).
Table 4.
Relative abundances of phosphorylated signaling proteins within the gastrocnemius, diaphragm, and heart and abundance eIF4E·eIF4G in the gastrocnemius and diaphragm of fasted or fed piglets born at preterm or term gestation
| Preterm |
Term |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Tissue Protein |
Fasted | Fed | Fasted | Fed | GAB | Feed | GAB × feed |
| Gastrocnemius | |||||||
| 4EBP1 Thr70 | 0.06 ± 0.03c | 0.41 ± 0.03b | 0.07 ± 0.03c | 0.71 ± 0.03a | <0.001 | <0.001 | <0.001 |
| S6K1 Thr389 | 0.02 ± 0.04c | 0.29 ± 0.04b | 0.03 ± 0.04c | 0.66 ± 0.04a | <0.001 | <0.001 | <0.001 |
| PKB Ser473 | 0.06 ± 0.03c | 0.41 ± 0.03b | 0.09 ± 0.03c | 0.81 ± 0.03a | <0.001 | <0.001 | <0.001 |
| eIF4E·eIF4G | 0.35 ± 0.43c | 3.53 ± 0.42b | 0.23 ± 0.44c | 5.30 ± 0.47a | <0.001 | <0.001 | <0.001 |
| Diaphragm | |||||||
| 4EBP1 Thr70 | 0.06 ± 0.03 | 0.65 ± 0.03 | 0.05 ± 0.04 | 0.70 ± 0.04 | NS | <0.001 | NS |
| S6K1 Thr389 | 0.05 ± 0.06 | 0.64 ± 0.06 | 0.04 ± 0.06 | 0.68 ± 0.06 | NS | <0.001 | NS |
| PKB Ser473 | 0.09 ± 0.03 | 0.66 ± 0.03 | 0.10 ± 0.04 | 0.69 ± 0.04 | NS | <0.001 | NS |
| eIF4E·eIF4G | 0.14 ± 0.18 | 1.70 ± 0.18 | 0.15 ± 0.19 | 1.86 ± 0.20 | NS | <0.001 | NS |
| Heart | |||||||
| 4EBP1 Thr70 | 0.16 ± 0.02c | 0.48 ± 0.02b | 0.12 ± 0.02c | 0.86 ± 0.02a | <0.001 | <0.001 | <0.001 |
| S6K1 Thr389 | 0.08 ± 0.02c | 0.39 ± 0.02b | 0.09 ± 0.02c | 0.78 ± 0.02a | <0.001 | <0.001 | <0.001 |
| PKB Ser473 | 0.09 ± 0.02c | 0.35 ± 0.02b | 0.09 ± 0.02c | 0.83 ± 0.02a | <0.001 | <0.001 | <0.001 |
Data presented as least-square means ± SE; n = 21–25/group; values are expressed as arbitrary units. 4EBP1, eukaryotic initiation factor 4E binding protein 1; eIF4E, eukaryotic initiation factor 4E; eIF4G, eukaryotic initiation factor 4G; feed, feeding status; GAB, gestational age at birth; NS, not statically significant, P > 0.05; PKB, protein kinase B; S6K1, S6 kinase 1. Two-way ANOVA followed by Tukey’s post hoc test.
a,
b,
Means without a common superscripted letter differ.
Table 5.
Relative abundances of phosphorylated signaling proteins in visceral organs and brain of fasted or fed piglets born at preterm or term gestation
| Preterm |
Term |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Tissue (Protein) | Fasted | Fed | Fasted | Fed | GAB | Feed | GAB × Feed |
| Lung | |||||||
| 4EBP1 Thr70 | 0.08 ± 0.01 | 0.56 ± 0.04 | 0.07 ± 0.01 | 0.60 ± 0.04 | NS | <0.001 | NS |
| S6K1 Thr389 | 0.08 ± 0.01 | 0.69 ± 0.05 | 0.08 ± 0.01 | 0.72 ± 0.04 | NS | <0.001 | NS |
| PKB Ser473 | 0.14 ± 0.02 | 0.63 ± 0.04 | 0.13 ± 0.01 | 0.62 ± 0.04 | NS | <0.001 | NS |
| Jejunum | |||||||
| 4EBP1 Thr70 | 0.14 ± 0.03 | 0.72 ± 0.03 | 0.13 ± 0.04 | 0.77 ± 0.04 | NS | <0.001 | NS |
| S6K1 Thr389 | 0.13 ± 0.04 | 0.59 ± 0.04 | 0.11 ± 0.04 | 0.55 ± 0.04 | NS | <0.001 | NS |
| PKB Ser473 | 0.14 ± 0.04 | 0.59 ± 0.04 | 0.13 ± 0.04 | 0.62 ± 0.04 | NS | <0.001 | NS |
| Liver | |||||||
| 4EBP1 Thr70 | 0.10 ± 0.01c | 0.67 ± 0.04a | 0.09 ± 0.01c | 0.48 ± 0.03b | 0.03 | <0.001 | <0.001 |
| S6K1 Thr389 | 0.10 ± 0.01c | 0.79 ± 0.03a | 0.09 ± 0.01c | 0.57 ± 0.03b | 0.03 | <0.001 | <0.001 |
| PKB Ser473 | 0.13 ± 0.01c | 0.77 ± 0.04a | 0.10 ± 0.01d | 0.50 ± 0.03b | 0.01 | <0.001 | <0.001 |
| Pancreas | |||||||
| 4EBP1 Thr70 | 0.14 ± 0.02c | 0.49 ± 0.02b | 0.15 ± 0.02c | 0.81 ± 0.02a | <0.001 | <0.001 | <0.001 |
| S6K1 Thr389 | 0.08 ± 0.03c | 0.34 ± 0.03b | 0.09 ± 0.03c | 0.65 ± 0.03a | 0.005 | <0.001 | <0.001 |
| PKB Ser473 | 0.13 ± 0.04c | 0.44 ± 0.04b | 0.12 ± 0.04c | 0.69 ± 0.04a | 0.05 | <0.001 | <0.001 |
| Kidney | |||||||
| 4EBP1 Thr70 | 0.12 ± 0.02c | 0.45 ± 0.02b | 0.13 ± 0.03c | 0.72 ± 0.03a | 0.003 | <0.001 | <0.001 |
| S6K1 Thr389 | 0.09 ± 0.03c | 0.49 ± 0.03b | 0.12 ± 0.03c | 0.77 ± 0.03a | 0.005 | <0.001 | <0.001 |
| PKB Ser473 | 0.09 ± 0.05c | 0.35 ± 0.05b | 0.08 ± 0.06c | 0.59 ± 0.06a | NS | <0.001 | <0.001 |
| Brain | |||||||
| 4EBP1 Thr70 | 0.11 ± 0.03 | 0.48 ± 0.03 | 0.10 ± 0.03 | 0.51 ± 0.03 | NS | <0.001 | NS |
| S6K1 Thr389 | 0.13 ± 0.03 | 0.58 ± 0.03 | 0.15 ± 0.04 | 0.63 ± 0.04 | NS | <0.001 | NS |
| PKB Ser473 | 0.23 ± 0.05 | 0.75 ± 0.05 | 0.18 ± 0.05 | 0.70 ± 0.05 | NS | <0.001 | NS |
Values are least square means ± SE; n = 21–25/group. Values are expressed as arbitrary units. 4EBP1, eukaryotic initiation factor 4E binding protein 1; feed, feeding status; GAB, gestational age at birth; NS, not statistically significant, P > 0.05; PKB, protein kinase B; S6K1, S6 kinase 1. Two-way ANOVA followed by Tukey’s post hoc test.
a,
b,
c,
Means without a common superscripted letter differ, P < 0.05.
DISCUSSION
Many of the ∼15 million infants born prematurely worldwide each year experience postnatal growth faltering (4, 12, 24). A significant component of the growth faltering in preterm infants is due to diminished growth of lean tissue, which is comprised mainly of skeletal muscle (14). In contrast, adipose tissue deposition is maintained (73) and may contribute to an increase in morbidities over the long term. To investigate the mechanistic basis for the suboptimal growth of preterm infants, we used neonatal piglets to examine whether prematurity modifies the protein synthetic response of skeletal muscle and visceral tissues to feeding. We also assessed whether changes in this feeding response are mediated by alterations in insulin- and/or amino acid-regulated translation initiation and protein degradation signaling pathways. Our results provide novel evidence that prematurity blunts the protein anabolic response to feeding as measured by protein synthesis and translation initiation signaling pathways in several key organs and tissues that have been associated with adult metabolic diseases, namely skeletal muscle, pancreas, heart, and kidney.
Despite equivalent nutrient delivery, the relative body weight gain of preterm pigs was lower than that of the term pigs. The higher kidney mass of preterm versus term pigs on day 3 of life is consistent with human infants, in which total kidney volume (33, 36) and weight (71) are greater in preterm than term infants when corrected for body weight. For both the kidney and liver, the large organ mass relative to body weight in preterm pigs may be appropriate for gestational age or indicate asymmetrical growth or a protein-sparing effect. That is, in these preterm animals, energy and nutrient resources may be directed preferentially to vital organs at the expense of tissue such as skeletal muscle (61). Indeed, in the present study, prematurity did not affect relative mass of all tissues equally in that the LD mass (per kg body wt) of preterm pigs was significantly less than that of term pigs, again suggesting that vital organs such as the liver and kidney may have been spared at the expense of skeletal muscle. Given that muscle mass represents 12, 19, and 24% of birth weight in human infants born at 25, 34, and 40 wk gestation, respectively (47), the differences observed in the present study appear to be appropriate for gestational age.
In the present study, prematurity blunted the feeding-induced rise in plasma glucose, amino acids, and insulin, with these values rising more slowly and having a lower AUC in preterm than in term pigs. The enteral diet was formulated to mimic the composition of sow’s milk on day 3 of lactation (34) and was provided as a gavage with volume delivered based on piglet body weight. Because the diet was elemental, it is unlikely that differences in digestive capacity would affect plasma levels of these nutrients. Thus, the differences observed are likely due to differences in gastric emptying times, given that gastrointestinal motor activity development is not mature until after full-term birth (10), or to differences in absorption efficiency. Relative to absorptive capacities, intestinal glucose uptake of preterm pigs is reduced compared with that of term pigs (54), which is likely due to the combined effects of reduced intestinal mass and glucose transport capacity. Indeed, sodium glucose-linked transporter 1 (SGLT1) gene expression is greater in term than in either preterm feeding-naive or fed pigs (54). In human infants, glucose absorption capacity correlates positively with GAB; infants born prematurely demonstrate lower Vmax of d-glucose absorption compared with those born at term (45, 60), and absorption of leucine is reduced by 47% in preterm versus term pigs (54). In addition to the effects of gastric emptying and nutrient absorptive capacities, the effect of first-pass metabolism on plasma nutrient levels must also be considered. High epithelial cell turnover and metabolism in these young animals will incur a greater requirement for nutrients to maintain gut structural and functional integrity. Thus, because gastrointestinal nutrient utilization significantly impacts plasma nutrient concentration, the reduced/delayed appearance of both glucose and amino acids in the plasma of preterm versus term pigs may be partially explained by their greater epithelial metabolism compared with term pigs (9).
GLP1 is produced by enteroendocrine cells of the small intestine in response to the presence of nutrients and stimulates a reduction in blood glucose via augmented insulin secretion (56). However, despite the lower concentrations of plasma glucose and amino acids in preterm versus term pigs, plasma GLP1 of fed preterm and term pigs was similar in the present study. This similarity may reflect the time point sampled, as plasma glucose was similar between preterm and term pigs, and plasma amino acids were approaching similar levels, at the 60-min sampling. Consistent with these results, neither fed nor fasted plasma GLP1 level is affected by GAB in human infants (55), and GLP1 rises with the first oral feed regardless of GAB (56). Thus, GLP1 secretion may develop early in gestation as a means to promote the postprandial insulin response. In contrast to GLP1, plasma IGF-I was lower in preterm than in term pigs regardless of feeding status. This is consistent with data in human preterm infants (44) as well as in neonatal piglets, in which plasma IGF-1 increases with development (1, 13), and may be simply indicative of the younger developmental age of the preterm piglets (40–42, 52).
In the present study, feeding stimulated Ks in all tissues of both preterm and term pigs, but the response was blunted by prematurity in the skeletal muscle, heart, pancreas, and kidney. There are a number of possible explanations for the blunted response of these tissues. 1) Their capacity for protein synthesis may develop late in gestation; 2) they may be resistant to insulin- and/or amino acid-induced stimulation of translation initiation signaling; and 3) nutrients may be directed preferentially to other tissues such as the liver, diaphragm, lung, jejunum, or brain, in which GAB did not impact Ks. In animals born at term gestation, protein synthesis of various tissues is high in the first week of postnatal life and declines with age, an effect that is most pronounced in skeletal muscle (8, 9, 13, 15, 17, 18). This is consistent with the inverse relationship between conceptional age and whole body protein synthesis in human infants (50) but contrasts with the lower protein synthesis values observed in prematurely born piglets in the present study. The detrimental effect of prematurity on skeletal muscle Ks suggests that this tissue may have a lower priority as compared with vital organs such as the diaphragm, lung, jejunum, and brain in terms of nutrient partitioning during early development (2, 8) and leaves skeletal muscle most vulnerable to nutrient deficiency.
The discrepancies in protein synthesis observed between preterm and term animals could be ascribed to differences in both the capacity and the efficiency of translation (38). Tissue protein synthetic capacity (Cs) is determined by ribosomal abundance, which in the preterm pigs met or exceeded that of term pigs in all tissues measured, indicating that the blunted fractional rate of protein synthesis observed in preterm pigs was not due to a deficit in ribosomal number. This is consistent with the enhanced ribosomal abundance (RNA:protein ratio) observed in young versus older animals (13). Conversely, translational efficiency was increased with feeding in all tissues measured, but this increase was blunted by prematurity in the LD, gastrocnemius, heart, jejunum, and pancreas, which thereby explains the blunted rates of protein synthesis in these tissues of preterm pigs. This blunted translational efficiency in skeletal muscle may be indicative of insulin and/or amino acid resistance in the preterm animals because both insulin and amino acid infusion increase translational efficiency in term-born neonatal pigs (15, 29, 74, 75).
Like the developmental decline in skeletal muscle protein synthesis in term-born pigs that we documented previously, the feeding-induced activation of translation initiation signaling proteins in muscle also declines with development after term birth. This suggests that the decline in the acute stimulation of protein synthesis by feeding as muscles mature may be driven in part by the reduced activation or abundance of signaling proteins necessary to stimulate translation initiation (19, 20, 37, 53, 67, 68). In the present study, the feeding-induced increase in activation of many components of the insulin- and amino acid-signaling pathways leading to mTORC1 activation in LD muscle was blunted by prematurity. Upstream of mTOR, we could not detect differences due to GAB in phosphorylation of the IR (Tyr1185), IRS-1 (Tyr), or AMPK (Thr172) or in formation of the PI 3-kinase·IRS-1 complex. However, phosphorylation of PKB (Thr308 and Ser473) and TSC2 (Thr1462) in response to feeding was blunted by prematurity in LD muscle. Reduced insulin-stimulated phosphorylation of PKB (Ser473), without affecting the phosphorylation of IR or IRS-1, a hallmark of insulin resistance in skeletal muscle, has also been observed in skeletal muscle of premature as compared with term-born baboons (3). The reduced phosphorylation of TSC2 may be a direct consequence of the reduced phosphorylation of PKB in the preterm animals, in turn precipitating the reduced phosphorylation and activation of S6K1 and 4EBP1.
The abundance of mTOR and the amino acid-signaling proteins Sestrin2, Gator2, LAMTOR1, LAMTOR2, Raptor, Rheb, RagA, RagC, and Deptor were not affected by GAB. The binding of the leucine sensor, Sestrin2 with Gator2, an association that inhibits amino acid signaling (58), tended to be higher in preterm than term and decreased with feeding. The association of mTOR with RagA, RagC, and Rheb was lower postfeeding in LD muscle of premature compared with term-born pigs and is indicative of reduced amino acid signaling. For the LD, prematurity also blunted the activation of the translation initiation signals downstream of mTOR. Specifically, phosphorylation of mTOR (Ser2445), S6K1 (Thr389), rpS6 (Ser235/236,240/244), and 4EBP1 (Thr70) and formation of the active eIF4E·eIF4G complex were reduced. However, there was no effect of GAB on phosphorylation of eIF2α (Ser51) or eEF2 (Thr56), indicating that differences observed in protein synthesis are independent of the eIF2B-regulated arm of translation initiation (38) or elongation signaling (5). In contrast to the blunted insulin and amino acid signaling observed both upstream and downstream of mTOR, no effect of GAB was observed on indices of the ubiquitin-proteosome or autophagy-lysosome protein degradation systems, including the LC3-II-to-total LC3 ratio, phosphorylation of FOXO3 (Ser253), or abundance of Atrogin1 or MuRF-1 within the LD.
Similarly to the LD, phosphorylation of 4EBP1 (Thr70), S6K1 (Thr389), and PKB (Ser473) was increased by feeding in the gastrocnemius, heart, kidney, and pancreas of both preterm and term pigs, but this effect was blunted by prematurity. This pattern of signaling protein activation directly paralleled rates of protein synthesis observed in these individual tissues. Furthermore, activation of the signaling proteins directly reflected protein synthetic rates of all analyzed tissues; that is, in the diaphragm, lung, brain, and jejunum, phosphorylation of 4EBP1 (Thr70), S6K1 (Thr389), and PKB (Ser473) was increased by feeding regardless of GAB, possibly reflective of the critical role mTOR pathway activation plays in development (11, 46, 72, 76). Within the liver, activation of these signaling proteins was greater in preterm than in term pigs and was increased by feeding regardless of GAB. The liver was the only tissue in which feeding increased activation of signaling components more in preterm than in term pigs and is reflected in the larger relative mass of the liver of preterm versus term pigs. One possible explanation for this, as well as the Ks of the preterm liver being higher than term liver, is potentially related to the well-developed liver synthetic capacity observed in premature infants (6) in combination with the first-pass extraction of nutrients from the portal drainage.
Taken together, data from the present study indicate that multiple tissues of preterm pigs may be insulin and/or amino acid resistant, which results in a blunting of the protein synthesis response to feeding and subsequent reduced growth. To our knowledge, this study is the first to directly compare growth, fractional protein synthesis, and translation initiation signaling in the muscle, visceral organs, and brain of preterm and term animals. These results are of direct relevance for the bedside care of preterm infants, as current nutritional support is unable to match the growth and body composition attained in utero. Future studies should focus on elucidating the factors that are responsible for blunting the stimulatory effects of insulin and amino acids on translation initiation signaling in skeletal muscle and vital organs of preterm and term piglets and in applying this information to the development of targeted nutritional therapies to enhance lean growth in premature infants while avoiding the potential complications associated with the provision of excessive nutrient loads.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants HD-085573 and HD-072891, and Department of Agriculture Grants CRIS 6250-51000-055 and NIFA 2013-67015-20438.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.G.B., M.L.F., and T.A.D. conceived and designed research; J.K.N., A.S., H.V.N., A.H.-G., S.M.C., P.E.L., O.O.O., B.S., and T.A.D. performed experiments; J.K.N., A.S., H.V.N., and T.A.D. analyzed data; J.K.N., A.S., H.V.N., M.L.F., and T.A.D. interpreted results of experiments; J.K.N. prepared figures; J.K.N. and T.A.D. drafted manuscript; A.S., H.V.N., D.G.B., M.L.F., and T.A.D. edited and revised manuscript; J.K.N., A.S., H.V.N., A.H.-G., S.M.C., P.E.L., O.O.O., B.S., D.G.B., M.L.F., and T.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank E. O. Smith for statistical assistance, A. C. Gillum for support with graphics, and R. D. Parada for expert technical assistance.
REFERENCES
- 1.Andersen AD, Sangild PT, Munch SL, van der Beek EM, Renes IB, Ginneken C, Greisen GO, Thymann T. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am J Physiol Regul Integr Comp Physiol 310: R481–R492, 2016. doi: 10.1152/ajpregu.00349.2015. [DOI] [PubMed] [Google Scholar]
- 2.Bauman DE, Eisemann JH, Currie WB. Hormonal effects on partitioning of nutrients for tissue growth: role of growth hormone and prolactin. Fed Proc 41: 2538–2544, 1982. [PubMed] [Google Scholar]
- 3.Blanco CL, McGill-Vargas LL, Gastaldelli A, Seidner SR, McCurnin DC, Leland MM, Anzueto DG, Johnson MC, Liang H, DeFronzo RA, Musi N. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology 156: 813–823, 2015. doi: 10.1210/en.2014-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379: 2162–2172, 2012. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 5.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem 269: 5360–5368, 2002. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 6.Bunt JE, Rietveld T, Schierbeek H, Wattimena JL, Zimmermann LJ, van Goudoever JB. Albumin synthesis in preterm infants on the first day of life studied with [1-13C]leucine. Am J Physiol Gastrointest Liver Physiol 292: G1157–G1161, 2007. doi: 10.1152/ajpgi.00300.2006. [DOI] [PubMed] [Google Scholar]
- 7.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37: 593–599, 1995. doi: 10.1203/00006450-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Burrin DG, Davis TA, Fiorotto ML, Reeds PJ. Hepatic protein synthesis in suckling rats: effects of stage of development and fasting. Pediatr Res 31: 247–252, 1992. doi: 10.1203/00006450-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Burrin DG, Davis TA, Fiorotto ML, Reeds PJ. Stage of development and fasting affect protein synthetic activity in the gastrointestinal tissues of suckling rats. J Nutr 121: 1099–1108, 1991. doi: 10.1093/jn/121.7.1099. [DOI] [PubMed] [Google Scholar]
- 10.Cavell B. Gastric emptying in preterm infants. Acta Paediatr Scand 68: 725–730, 1979. doi: 10.1111/j.1651-2227.1979.tb18446.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Long F. mTOR signaling in skeletal development and disease. Bone Res 6: 1, 2018. doi: 10.1038/s41413-017-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 111: 986–990, 2003. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 13.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am J Physiol 270: E802–E809, 1996. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- 14.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12: 78–85, 2009. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis TA, Fiorotto ML, Beckett PR, Burrin DG, Reeds PJ, Wray-Cahen D, Nguyen HV. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 280: E770–E779, 2001. doi: 10.1152/ajpendo.2001.280.5.E770. [DOI] [PubMed] [Google Scholar]
- 16.Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol 277: E103–E109, 1999. doi: 10.1152/ajpendo.1999.277.1.E103. [DOI] [PubMed] [Google Scholar]
- 17.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol 265: R334–R340, 1993. doi: 10.1152/ajpregu.1993.265.2.R334. [DOI] [PubMed] [Google Scholar]
- 18.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol 257: R1141–R1146, 1989. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- 19.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279: E1226–E1234, 2000. doi: 10.1152/ajpendo.2000.279.6.E1226. [DOI] [PubMed] [Google Scholar]
- 20.Davis TA, Suryawan A, Orellana RA, Nguyen HV, Fiorotto ML. Postnatal ontogeny of skeletal muscle protein synthesis in pigs. J Anim Sci 86, Suppl: E13–E18, 2008. doi: 10.2527/jas.2007-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatr Res 30: 23–27, 1991. doi: 10.1203/00006450-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 286: 8287–8296, 2011. doi: 10.1074/jbc.M110.209171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2α kinases: their structures and functions. Cell Mol Life Sci 70: 3493–3511, 2013. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol 27: 302–310, 2003. doi: 10.1016/S0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 25.El-Kadi SW, Suryawan A, Gazzaneo MC, Srivastava N, Orellana RA, Nguyen HV, Lobley GE, Davis TA. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab 302: E674–E686, 2012. doi: 10.1152/ajpendo.00516.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192: 719–723, 1980. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoneim N, Bauchart-Thevret C, Oosterloo B, Stoll B, Kulkarni M, de Pipaon MS, Zamora IJ, Olutoye OO, Berg B, Wittke A, Burrin DG. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS One 9: e106888, 2014. doi: 10.1371/journal.pone.0106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen CF, Thymann T, Andersen AD, Holst JJ, Hartmann B, Hilsted L, Langhorn L, Jelsing J, Sangild PT. Rapid gut growth but persistent delay in digestive function in the postnatal period of preterm pigs. Am J Physiol Gastrointest Liver Physiol 310: G550–G560, 2016. doi: 10.1152/ajpgi.00221.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-García AD, Columbus DA, Manjarín R, Nguyen HV, Suryawan A, Orellana RA, Davis TA. Leucine supplementation stimulates protein synthesis and reduces degradation signal activation in muscle of newborn pigs during acute endotoxemia. Am J Physiol Endocrinol Metab 311: E791–E801, 2016. doi: 10.1152/ajpendo.00217.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herningtyas EH, Okimura Y, Handayaningsih AE, Yamamoto D, Maki T, Iida K, Takahashi Y, Kaji H, Chihara K. Branched-chain amino acids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell line. Biochim Biophys Acta 1780: 1115–1120, 2008. doi: 10.1016/j.bbagen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, Cutfield WS. Premature birth and later insulin resistance. N Engl J Med 351: 2179–2186, 2004. doi: 10.1056/NEJMoa042275. [DOI] [PubMed] [Google Scholar]
- 32.Hovi P, Andersson S, Eriksson JG, Järvenpää AL, Strang-Karlsson S, Mäkitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med 356: 2053–2063, 2007. doi: 10.1056/NEJMoa067187. [DOI] [PubMed] [Google Scholar]
- 33.Huang HP, Tsai IJ, Lai YC, Cheng CH, Tsau YK. Early postnatal renal growth in premature infants. Nephrology (Carlton) 12: 572–575, 2007. doi: 10.1111/j.1440-1797.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 34.Hurley WL. Composition of sow colostrum and milk. In: The Gestating and Lactating Sow, edited by Farmer C. Wageningen, The Netherlands: Wageningen Academic Publishers, 2015. [Google Scholar]
- 35.Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 130: e640–e649, 2012. doi: 10.1542/peds.2011-3379. [DOI] [PubMed] [Google Scholar]
- 36.Kandasamy Y, Smith R, Wright IM, Lumbers ER. Extra-uterine renal growth in preterm infants: oligonephropathy and prematurity. Pediatr Nephrol 28: 1791–1796, 2013. doi: 10.1007/s00467-013-2462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimball SR, Farrell PA, Nguyen HV, Jefferson LS, Davis TA. Developmental decline in components of signal transduction pathways regulating protein synthesis in pig muscle. Am J Physiol Endocrinol Metab 282: E585–E592, 2002. doi: 10.1152/ajpendo.00269.2001. [DOI] [PubMed] [Google Scholar]
- 38.Kimball SR, Jefferson LS. Cellular mechanisms involved in the action of insulin on protein synthesis. Diabetes Metab Rev 4: 773–787, 1988. doi: 10.1002/dmr.5610040806. [DOI] [PubMed] [Google Scholar]
- 39.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126: 1713–1719, 2013. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CY, Bazer FW, Etherton TD, Simmen FA. Ontogeny of insulin-like growth factors (IGF-I and IGF-II) and IGF-binding proteins in porcine serum during fetal and postnatal development. Endocrinology 128: 2336–2344, 1991. doi: 10.1210/endo-128-5-2336. [DOI] [PubMed] [Google Scholar]
- 41.Lee CY, Chung CS, Simmen FA. Ontogeny of the porcine insulin-like growth factor system. Mol Cell Endocrinol 93: 71–80, 1993. doi: 10.1016/0303-7207(93)90141-6. [DOI] [PubMed] [Google Scholar]
- 42.Louveau I, Combes S, Cochard A, Bonneau M. Developmental changes in insulin-like growth factor-I (IGF-I) receptor levels and plasma IGF-I concentrations in large white and Meishan pigs. Gen Comp Endocrinol 104: 29–36, 1996. doi: 10.1006/gcen.1996.0137. [DOI] [PubMed] [Google Scholar]
- 43.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 44.Martos-Moreno GA, Barrios V, Sáenz de Pipaón M, Pozo J, Dorronsoro I, Martínez-Biarge M, Quero J, Argente J. Influence of prematurity and growth restriction on the adipokine profile, IGF1, and ghrelin levels in cord blood: relationship with glucose metabolism. Eur J Endocrinol 161: 381–389, 2009. doi: 10.1530/EJE-09-0193. [DOI] [PubMed] [Google Scholar]
- 45.McNeish AS, Ducker DA, Warren IF, Davies DP, Harran MJ, Hughes CA. The influence of gestational age and size on the absorption of D-xylose and D-glucose from the small intestine of the human neonate. Ciba Found Symp 70: 267–280, 1979. doi: 10.1002/9780470720530.ch15. [DOI] [PubMed] [Google Scholar]
- 46.Meng D, Frank AR, Jewell JL. mTOR signaling in stem and progenitor cells. Development 145: dev152595, 2018. doi: 10.1242/dev.152595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modi N, Hutton JL. Urinary creatinine excretion and estimation of muscle mass in infants of 25-34 weeks gestation. Acta Paediatr Scand 79: 1156–1162, 1990. doi: 10.1111/j.1651-2227.1990.tb11404.x. [DOI] [PubMed] [Google Scholar]
- 48.Munro HN, Fleck A. Analysis of tissues and body fluids for nitrogenous constituents. In: Mammalian Protein Metabolism, edited by Munro HN. New York: Academic Press, 1969, vol III, p. 465–483. [Google Scholar]
- 49.National Research Council Nutrient Requirements of Swine (11th Revised Edition). Washington, DC: National Academies, 2012. [Google Scholar]
- 50.Nissim I, Yudkoff M, Pereira G, Segal S. Effects of conceptual age and dietary intake on protein metabolism in premature infants. J Pediatr Gastroenterol Nutr 2: 507–516, 1983. doi: 10.1097/00005176-198302030-00019. [DOI] [PubMed] [Google Scholar]
- 51.Odle J, Lin X, Jacobi SK, Kim SW, Stahl CH. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu Rev Anim Biosci 2: 419–444, 2014. doi: 10.1146/annurev-animal-022513-114158. [DOI] [PubMed] [Google Scholar]
- 52.Ohkawa N, Shoji H, Ikeda N, Suganuma H, Shimizu T. Relationship between insulin-like growth factor 1, leptin and ghrelin levels and catch-up growth in small for gestational age infants of 27-31 weeks during neonatal intensive care unit admission. J Paediatr Child Health 53: 62–67, 2017. doi: 10.1111/jpc.13307. [DOI] [PubMed] [Google Scholar]
- 53.Orellana RA, Wilson FA, Gazzaneo MC, Suryawan A, Davis TA, Nguyen HV. Sepsis and development impede muscle protein synthesis in neonatal pigs by different ribosomal mechanisms. Pediatr Res 69: 473–478, 2011. doi: 10.1203/PDR.0b013e3182176da1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Østergaard MV, Cilieborg MS, Skovgaard K, Schmidt M, Sangild PT, Bering SB. Preterm birth reduces nutrient absorption with limited effect on immune gene expression and gut colonization in pigs. J Pediatr Gastroenterol Nutr 61: 481–490, 2015. doi: 10.1097/MPG.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 55.Padidela R, Patterson M, Sharief N, Ghatei M, Hussain K. Elevated basal and post-feed glucagon-like peptide 1 (GLP-1) concentrations in the neonatal period. Eur J Endocrinol 160: 53–58, 2009. doi: 10.1530/EJE-08-0807. [DOI] [PubMed] [Google Scholar]
- 56.Salis ER, Reith DM, Wheeler BJ, Broadbent RS, Medlicott NJ. Insulin resistance, glucagon-like peptide-1 and factors influencing glucose homeostasis in neonates. Arch Dis Child Fetal Neonatal Ed 102: F162–F166, 2017. doi: 10.1136/archdischild-2015-309174. [DOI] [PubMed] [Google Scholar]
- 57.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci 91: 4713–4729, 2013. doi: 10.2527/jas.2013-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saxton RA, Sabatini DM. mTOR Signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. [Erratum in: Cell 169: 361–371, 2017.] doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmelzle K, Kane S, Gridley S, Lienhard GE, White FM. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes 55: 2171–2179, 2006. doi: 10.2337/db06-0148. [DOI] [PubMed] [Google Scholar]
- 60.Shulman RJ. In vivo measurements of glucose absorption in preterm infants. Biol Neonate 76: 10–18, 1999. doi: 10.1159/000014126. [DOI] [PubMed] [Google Scholar]
- 61.Stephens AS, Bentley JP, Taylor LK, Arbuckle SM. Diagnosis of fetal growth restriction in perinatal deaths using brain to liver weight ratios. Pathology 47: 51–57, 2015. doi: 10.1097/PAT.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, Burrin DG. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr 140: 2193–2200, 2010. doi: 10.3945/jn.110.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoll B, Puiman PJ, Cui L, Chang X, Benight NM, Bauchart-Thevret C, Hartmann B, Holst JJ, Burrin DG. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. JPEN J Parenter Enteral Nutr 36: 538–550, 2012. doi: 10.1177/0148607112444756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suryawan A, Davis TA. Regulation of protein degradation pathways by amino acids and insulin in skeletal muscle of neonatal pigs. J Anim Sci Biotechnol 5: 8, 2014. doi: 10.1186/2049-1891-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol (1985) 109: 1448–1454, 2010. doi: 10.1152/japplphysiol.00428.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suryawan A, Davis TA. Amino acid- and insulin-induced activation of mTORC1 in neonatal piglet skeletal muscle involves sestrin2-GATOR2, rag A/C-mTOR, and RHEB-mTOR complex formation. J Nutr 148: 825–833, 2018. doi: 10.1093/jn/nxy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 291: E849–E859, 2006. doi: 10.1152/ajpendo.00069.2006. [DOI] [PubMed] [Google Scholar]
- 68.Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 281: E908–E915, 2001. doi: 10.1152/ajpendo.2001.281.5.E908. [DOI] [PubMed] [Google Scholar]
- 69.Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab 293: E1597–E1605, 2007. doi: 10.1152/ajpendo.00307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71: 324–331, 2012. doi: 10.1038/pr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, Hoy WE, Bertram JF, Black MJ. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 22: 1365–1374, 2011. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takei N, Nawa H. mTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci 7: 28, 2014. doi: 10.3389/fnmol.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uthaya S, Thomas EL, Hamilton G, Doré CJ, Bell J, Modi N. Altered adiposity after extremely preterm birth. Pediatr Res 57: 211–215, 2005. doi: 10.1203/01.PDR.0000148284.58934.1C. [DOI] [PubMed] [Google Scholar]
- 74.Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140: 264–270, 2010. doi: 10.3945/jn.109.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson FA, Suryawan A, Orellana RA, Gazzaneo MC, Nguyen HV, Davis TA. Differential effects of long-term leucine infusion on tissue protein synthesis in neonatal pigs. Amino Acids 40: 157–165, 2011. doi: 10.1007/s00726-010-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Yu B, He J, Chen D. From nutrient to microRNA: a novel insight into cell signaling involved in skeletal muscle development and disease. Int J Biol Sci 12: 1247–1261, 2016. doi: 10.7150/ijbs.16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao J, Brault JJ, Schild A, Goldberg AL. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy 4: 378–380, 2008. doi: 10.4161/auto.5633. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Y, Wang Y, Zhu WG. Applications of post-translational modifications of FoxO family proteins in biological functions. J Mol Cell Biol 3: 276–282, 2011. doi: 10.1093/jmcb/mjr013. [DOI] [PubMed] [Google Scholar]