Abstract
Adiponectin, a highly abundant polypeptide hormone in plasma, plays an important role in the regulation of energy metabolism in a wide variety of tissues, as well as providing important beneficial effects in diabetes, inflammation, and cardiovascular disease. To act on target tissues, adiponectin must move from the circulation to the interstitial space, suggesting that vascular permeability plays an important role in regulating adiponectin action. To test this hypothesis, fluorescently labeled adiponectin was used to monitor its biodistribution in mice with streptozotocin-induced diabetes (STZD). Adiponectin was, indeed, found to have increased sequestration in the highly fenestrated liver and other tissues within 90 min in STZD mice. In addition, increased myocardial adiponectin was detected and confirmed using computed tomography (CT) coregistration. This provided support of adiponectin delivery to affected cardiac tissue as a cardioprotective mechanism. Higher adiponectin content in the STZD heart tissues was further examined by ex vivo fluorescence molecular tomography (FMT) imaging, immunohistochemistry, and Western blot analysis. In vitro mechanistic studies using an endothelial monolayer on inserts and three-dimensional microvascular networks on microfluidic chips further confirmed that adiponectin flux was increased by high glucose. However, in the in vitro model and mouse heart tissue, high glucose levels did not change adiponectin receptor levels. An examination of the tight junction (TJ) complex revealed a decrease in the TJ protein claudin (CLDN)-7 in high glucose-treated endothelial cells, and the functional significance of this change was underscored by increased endothelium permeability upon siRNA-mediated knockdown of CLDN-7. Our data support the idea that glucose-induced effects on permeability of the vascular endothelium contribute to the actions of adiponectin by regulating its transendothelial movement from blood to the interstitial space. These observations are physiologically significant and critical when considering ways to harness the therapeutic potential of adiponectin for diabetes.
Keywords: adiponectin, diabetes, endothelial, fluorescence molecular tomography, heart, vascular permeability
INTRODUCTION
Extensive research on adiponectin has validated this hormone as a biomarker for cardiometabolic diseases and as a therapeutic target with enormous potential (43). To harness the numerous beneficial effects of adiponectin, it is essential to fully understand the mechanisms governing adiponectin action (29, 31, 39). To date, the vast majority of studies have focused on correlating changes in circulating adiponectin levels and disease markers (1). As a result, reduced adiponectin levels are well established to inversely correlate with diabetes and cardiovascular diseases (43). Adiponectin acts via binding to membrane receptors AdipoR1, AdipoR2, and T-cadherin (19, 45), and reductions in their expression or posttranslational modification in disease states has been proposed to lead to adiponectin resistance (11, 36, 42). However, another critical factor that likely determines adiponectin action is vascular permeability (25, 26, 46). More specifically, to directly elicit a response in a target tissue, adiponectin must transit from the circulation to interstitial space across the endothelial barrier (46).
The monolayer of endothelial cells lining the circulatory system acts as a barrier that regulates the movement of blood-borne factors to the interstitial space. In turn, the barrier properties of the endothelium are regulated by the transcellular pathway (i.e., solute transport across endothelial cells), as well as the paracellular pathway (i.e., solute movement between adjacent endothelial cells) (46). In general, larger macromolecules move via facilitated transcellular trafficking, while the paracellular route typically restricts solutes in the range of 3 nm radius (16). Tight junctions (TJ) provide a selective barrier to solute movement between cells, and altered expression of components, including zona occludens-1 (ZO-1), occludin, tricellulin, and claudins alter TJ structure to make a leakier or tighter barrier for paracellular movement of solutes (15). Numerous previous studies, particularly focusing on insulin, have shown that vascular permeability can play an important role in contributing to the development of diabetes and heart failure (25, 46). It is important to note that endothelial permeability varies widely throughout the body in a tissue (and intra-tissue)-specific manner (46). It has also been shown that endothelial barriers control adiponectin transport in a cell- and tissue-specific manner (35), and we have reported that transendothelial movement of adiponectin was reduced by glucocorticoids (9).
We believe that the role of endothelial permeability as an important determinant of adiponectin action has been somewhat underappreciated. In this study, we further considered a link between vascular permeability, adiponectin flux, and hyperglycemia/diabetes by producing recombinant adiponectin, which we then conjugated with a near-infrared probe to track its biodistribution in live wild-type control and diabetic mice using fluorescence molecular tomography (FMT). We also examined the effects of high glucose levels on adiponectin flux across isolated arteries and cultured monolayers of endothelial cells. Finally, because the cardioprotective actions of adiponectin are currently the focus in our laboratory (6–8, 30, 32), we considered the role of the vascular barrier and diabetes on adiponectin access and action in the heart. These studies provide a novel and integrated view on the movement of adiponectin across the endothelial barrier under conditions that relate to diabetes and offers new insight on the impact that vascular permeability has on the actions of adiponectin on key organs.
MATERIALS AND METHODS
Experimental animal model.
Male nu/nu homozygous mice and C57BL6 aged 8–13 wk were used for assessing adiponectin biodistribution. All mice were maintained with access to water and low-fluorescent chow [Teklad Global 18% protein rodent diet (Irradiated), Harlan Laboratories, Indianapolis, IN] throughout each experimental period. Age- and weight-matched pairs were grouped for each injection (averaging 23–25 g). After 12 h of starvation, diabetes was induced by single intraperitoneal injection of 150 mg/kg (body weight) streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO). Experiments were performed 4 days after STZ injection, and diabetes was diagnosed when mice exhibited a blood glucose level >14 mmol/l at this time, while all control mice received only a saline injection. Circulating adiponectin was detected in serum before and after infusion of VivoTag-750-conjugated adiponectin using a mouse adiponectin ELISA kit with a sensitivity of 3.12–200 ng/ml (Immunodiagnostics, Hong Kong, China), which detects all adiponectin oligomeric forms.
Fluorescence molecular tomography to detect VivoTag-750-adiponectin.
Recombinant adiponectin prepared in our laboratory was labeled with VivoTag-S 750 (NEV10123, Perkin Elmer, Boston, MA). Importantly, this recombinant protein formed oligomeric forms in a ratio, which closely mirrors those found in circulation (see Supplemental Figs. S1 and S2; all Supplemental material is available at https://doi.org/10.5281/zenodo.3332774). Labeling efficiency was assessed by running labeled full-length adiponectin on a SDS-PAGE gel and then scanned on a LI-COR Odyssey infrared imaging system to visualize the three adiponectin isoforms. Labeled proteins were transferred from the gels onto nitrocellulose membranes before probing with adiponectin-specific antibodies to assess fluorescence-to-total protein ratio. Labeled adiponectin was infused into lightly anesthetized mice (1–2% isoflurane) via a surgically placed permanent jugular vein cannula. For cannulation insertion, the mice were first anesthetized with 5% isoflurane then maintained at 2% isoflurane. The surgical site was cleaned with iodine solution and alcohol before a longitudinal incision of ~15 mm was made in the skin at the neck of the animal, just above the right front leg. Connective tissue surrounding the jugular vein was carefully separated before two sutures of 6-0 Ethilon surgeon’s silk (Johnson & Johnson, Brussels, Belgium) were placed ~5–10 mm apart on the vein. A fine lateral incision was made between the sutures using microscissors, which allowed insertion of a saline-filled polyethylene (PE-10) tube into the vein between the sutures. When placement was confirmed, the caudal suture was released, and the cannula was slowly fed caudally ~5 mm into the vein toward (but not entering) the heart. To confirm correct placement, 50 μL saline was delivered through the cannula using a 31-gauge insulin needle. The cannula was then securely tied to the jugular vein with silk suture and 85 µg of adiponectin conjugated with VivoTag-S was administered followed by a 50-µL saline flush. The cannula was sealed using a heated hemostat, the skin incision was closed using silk suture, and a serum sample was collected from the tail vein within 2 min of adiponectin infusion. Mice were maintained at 2% isoflurane, while being positioned in a glass mouse imaging cassette, then scanned 10, 30, 60, and 90 min postadiponectin infusion with a fluorescence molecular tomography (FMT) 2500 LX quantitative tomography system (VisEn Medical, Perkin Elmer, Downers Grove, IL) using the 750-nm near-infrared channel (750/800 nm excitation/emission). Once FMT scanning was completed, mice in the imaging cassette were immediately taken for a computed tomography (CT) scan (Locus eXplore MicroCT, GE Healthcare, London, ON, Canada and XRAD 225Cx, Precision X-Ray, North Banford, CT) under constant anesthesia to ensure identical mouse positioning and accurate alignment between CT and FMT. FMT software (TrueQuant, Perkin Elmer) reconstructs a quantitative three-dimensional (3D) data set, in which fluorescence/voxel is expressed in nanomoles per liter. FMT and CT data sets (dicom format) were imported into Inveon Research Workplace (Siemens Healthcare, Erlangen, Germany) or AMIDE (http://amide.sourceforge.net) for FMT-CT coregistration to permit accurate localization of the fluorescent signal.
FMT analysis of tissues ex vivo.
To avoid potential scatter from adjacent tissue, heart, liver, kidney, pancreas, and skeletal muscle, those tissues were excised from mice after cervical dislocation immediately following FMT and CT scanning (~100–120 min postadiponectin infusion). Two-dimensional (2D) epifluorescence FMT images were taken of these tissues on an opaque resin imaging block in the FMT imaging cassette. Quantitative analysis of the fluorescent signal was performed using the FMT software (TrueQuant, Perkin Elmer).
Ex vivo vascular permeability of adiponectin assay.
Five-week-old Wistar rats were used. After 2 wk of standard diet in the animal facility, rats were fasted 5 h before induction of diabetes by a single intraperitoneal injection of streptozotocin (STZ; Sigma-Aldrich) at a dose of 100 mg/kg body wt (∼200 µL/rat). Diabetes was diagnosed when hyperglycemia was higher than 10 mmol/L (180 mg/dL). Rats developed diabetes within 7 days of STZ injection. A control group of rats was injected with an equivalent volume of the vehicle solution (citrate buffer 0.5 mM, pH 4.5). Rats that failed to respond to STZ injection were not used for the study. Control or diabetic rats were anesthetized with isoflurane, and a midline laparotomy was performed to expose and remove the mesenteric bed. Isolated mesenteric arteries were mounted on a glass cannula immersed in a 2-mL organ bath filled with a physiological salt solution (PSS; in mmol/L: 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, 14.9 NaHCO3, 1.6 CaCl2, 0.023 EDTA, 10 glucose; pH 7.4) aerated with 12% O2; 5% CO2; 83% N2 at 37°C following a method previously described (33). Arterial segments were pressurized at 80 mmHg in no-flow conditions and equilibrated for 30 min before starting experiments. Ten micrograms per milliliter adiponectin (200 µL) was added to the arterial perfusate, which circulated at a flow rate of 2 µL/min for 90 min at a constant pressure of 80 mmHg. At 0, 30, 60, and 90 min, 100-µL samples of the extra-vascular bath containing transported adiponectin were collected, and an equivalent volume of physiological solution was added to the bath. Adiponectin levels were quantitatively determined by ELISA kit (Immunodiagnostics).
Cell culture.
Human dermal microvascular endothelial cells (HDMEC; American Type Culture Collection, Manassas, VA) were grown in vascular cell basal medium (American Type Culture Collection) supplemented with 10% FBS at 37°C, 5% CO2. All cells were used at passages 3 and 4 from the supplier. 500K HDMECs were seeded onto Transwell inserts (Corning, Tewksbury, MA) sized for 12-well plates having 3.0-μm pore sizes. Hyperglycemia was induced with 25 mM glucose in vascular cell basal medium supplemented with 2% FBS for 6 days. To adjust for osmotic pressure differences, control cells were treated with 5.5 mM glucose and 19.5 mM mannitol.
Measurement of transendothelial electric resistance.
Measurements of transendothelial electric resistance (TEER) were conducted using STX-2 chopstick electrodes attached to an epithelial voltohmmeter (EVOM; World Precision Instruments, Sarasota, FL). All TEER measurements were corrected for background by subtracting TEER recorded across a blank membrane primed with appropriate cell culture medium. Resistance measurements were taken at day 0 and day 6 of treatment with 25 mM glucose and control (5.5 mM glucose + 19.5 mM mannitol).
Permeability assay using endothelial monolayers.
HDMECs were seeded onto Transwell inserts and treated with 25 mM glucose and 5.5 mM glucose with 19.5 mM mannitol for 5 days before the start of experiments. At the start of the experiment, 10 μg/mL of adiponectin with the 25 mM glucose or 5.5 mM glucose with 19.5 mM mannitol, including 2% FBS vascular cell basal medium (American Type Culture Collection PCS-100-030; Cedarlane, Burlington, ON, Canada), was applied to the apical chamber only. After 24 h, apical and basolateral media were assessed for adiponectin concentration by gel electrophoresis after concentrating with 10,000 Da MWCO (molecular weight cutoff) filter (EMD Millipore, Billerica, MA) or by fluorescence intensity reader, respectively, and concentrations were calculated by comparison to standard curves prepared in culture medium.
Quantitative RT-PCR.
Using aliquots of total extracted RNA, we synthesized cDNA, and we performed quantitative RT-PCR (qRT-PCR) reactions using the SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA). Gene-specific primer sets were designed: human CLDN7, forward: 5′-GGAGACGACAAAGTGAAGAAGG-3′ and reverse: 5′-GGACAGGAACAGGAGAGCAG-3′; human GAPDH, forward: 5′-AACATCATCCCTGCCTCTACTG-3′ and reverse: 5′-CCTGCTTCACCACCTTCTTG-3′; murine AdipoR1, forward: 5′-ACGTTGGAGAGTCATCCCGTAT-3′and reverse: 5′-CTCTGTGTGGATGCGGAAGAT-3′; murine AdipoR2, forward: 5′-TCCCAGGAAGATGAAGGGTTTAT-3′ and reverse: 5′-TTCCATTCGTTCCATAGCATGA-3′; murine adiponectin, forward: 5′-TGTTCCTCTTAATCCTGCCCA-3′ and reverse: 5′-CCAACCTGCACAAGTTCCCTT-3′; murine CLDN7, forward: 5′-GGACCTGCCATCTTTATCGGC-3′ and reverse: 5′-AGCTTTGCTTTCACTGCCTGG-3′; murine ZO-1, forward: 5′-GTCCCTGTGAGTCCTTCAGCTG-3′ and reverse: 5′-ACTCAACACACCACCATTGCTG-3′; murine 18s RNA, forward: 5′-AGTGAAACTGCGAATGGCTCA-3′ and reverse: 5′-CGAGCGACCAAAGGAACCA-3′.
Claudin-7 knockdown by shRNA in HDMEC.
Claudin-7 gene expression was knocked-down in HDMECs using a pGPU6/Neo-claudin-7 plasmid, as shown previously (9) using the following shRNA target sequence: 5′-GGCCATCAG ATTGTCACAGAC-3′ (GenePharma, Shanghai, China). Lipofectamine 3000 reagent (Life Technologies, Carlsbad, CA) was used for all transfections following the manufacturer’s instructions. A nontargeted control (NTC) shRNA plasmid was designed with a nonspecific scrambled sequence. The transfected cells were stabilized for 24 h with 10% FBS medium and subjected to antibiotic selection (500 μg/mL G418; Sigma-Aldrich) for 2 days. During antibiotic selection, transfected cells were seeded on Transwell inserts or six-well plates for further experiments.
Western blot analysis.
Proteins from mouse tissues were homogenized by TissueLyser II (QIAGEN, Hilden, Germany) in RIPA buffer with 80 mM Tris·HCl (pH 6.8), 2% (wt/vol) SDS, 20% glycerol, 3.3% (vol/vol) β-mercaptoethanol, 0.01% (wt/vol) bromophenol blue, 30 mM HEPES (pH 7.4), 2.5 mM EGTA, 3 mM EDTA, 70 mM KCl, 20 mM β-glycerolphosphate, 20 mM NaF, 1 mM Na3VO4, 200 μM PMSF, 10 μM E64, 1 μM leupeptin, 1 μM pepstatin A, 0.1% NP40, and 0.1 μM okadaic acid. To detect the three forms of adiponectin, samples were prepared without β-mercaptoethanol or heating. For detection of other protein targets, samples were lysed in complete RIPA buffer and denatured at 95°C for 10 min. Transported adiponectin was collected from basolateral HDMEC medium and concentrated using Amicon Ultra-4 centrifugal filter units with Ultracel-10K (EMD Millipore, Billerica, MA). To detect the three adiponectin oligomers, concentrated adiponectin samples were prepared without denaturation or reduction. Prepared samples were run on SDS-PAGE gels and then transferred onto PVDF membranes (Bio-Rad Laboratories, Hercules CA), before incubation with primary antibody and detection by chemiluminescence. Polyclonal primary antibody of rabbit anti-adiponectin (dilution 1:1,000) is produced in-house (28). Rabbit anti-AdipoR1/2 antibodies were from Phoenix Biotech (Toronto, ON, Canada) (32). Mouse anti-FLAG M2 (dilution 1:1,,000, F1804; Sigma, Oakville, ON, Canada), rabbit anti-claudin-7 (dilution 1:500, cat. no. 34-9100; Thermo Fisher Scientific, Waltham, MA), rabbit anti-α/β-tubulin (dilution 1:1,000, cat. no. 2148; Cell Signaling, Danvers, MA), and rabbit anti-GAPDH(14C10) (dilution 1:1,000, cat. no. 2118; Cell Signaling) were purchased. The appropriate HRP-conjugated secondary antibody [anti-rabbit IgG-HRP (dilution 1:5,000, cat. no. 7074), anti-mouse IgG-HRP (dilution 1:5,000; cat. no. 7076)] was used from Cell Signaling. Bands were quantified by densitometry using Fiji software and normalized to relevant loading controls as indicated.
Immunofluorescence.
Mouse hearts were isolated following isoflurane anesthesia, and the ventricles were excised and then cross-sectioned at the midline with a surgical blade before being embedded into a mold with optimal cutting temperature compound (OCT) (Sakura Finetek, Torrance, CA) and frozen on dry ice. Five-μm-thick cryosections were made using a cryostat and mounted onto positively charged glass slides (Superfrost; Thermo Fisher Scientific). Mounted slides were fixed in 4% PFA solution for 10 min to stain adiponectin or fixed in 100% ice-cold acetone, air-dried at room temperature, and rehydrated in distilled water followed by washing with PBS buffer. Permeabilization was followed by 0.5% Triton X-100. Before permeabilization, cell border staining was performed by incubating with 5 µg/mL Alexa Fluor 488-conjugated wheat germ agglutinin (WGA; Thermo Fisher Scientific) for 10 min in adiponectin staining. Sections were then incubated with adiponectin primary antibody (1:100), Texas-red secondary antibody (1:250), and DAPI for nuclear visualization. For vasculature staining, primary antibodies directed against claudin-7 (dilution 1:50, cat. no. 34-9100; Thermo Fisher Scientific), VE-cadherin (dilution 1:100; cat. no. PA5-17401; Thermo Fisher Scientific), PECAM-1 (dilution 1:50; cat. no. SC-376764; Santa Cruz Biotech, Mississauga, CA), ZO-1 (dilution 1:100, cat. no. 61-7300; Thermo Fisher Scientific), α-SMA (1:100; α-SM1, a kind gift from Dr. Giulio Gabbiani, University of Geneva, Switzerland), and desmin (1:30, cat. no. M076029; Dako, Burlington, ON, Canada) were used. Isotype-specific secondary antibodies anti-rabbit IgG-TRITC (1:100; Sigma), Alexa Fluor 568-conjugated IgG (1:100; Abcam, Cambridge, MA), Alexa Fluor 647-conjugated IgG2a (1:100; Molecular Probes, LifeTechnologies), and anti-mouse IgG1-FITC (1:200; SouthernBiotech, Birmingham, AL), and DAPI (Vectashield mounting medium with DAPI, cat. no. H-1200; Vector Laboratories, Burlingame, CA) were used. Images were acquired using a LSM700 (Zeiss, Jena, Germany) or LSM780 (Zeiss) confocal microscope and an upright Zeiss Axio Observer M35 epifluorescence microscope equipped with structured illumination (Apotome) and Axiocam HR camera (Carl Zeiss). All images were processed using Fiji or Adobe Photoshop CS5 (Adobe System, San Jose, CA). Contrast and brightness were enhanced consistently for all representative images used in this article.
Fabrication of microfluidic devices and cell culture on a chip.
To recapitulate 3D microvascular networks in vitro, we used a microfluidic chip that included cells embedded in hydrogel. Previous studies have described the fabrication process of the chips in detail (37). Briefly, silicon-based organic PDMS microfluidic device fabrication includes steps of soft lithography with SU-8 silicon wafer mold (A-Med, Conroe, TX) and PDMS (1:10; Dow Corning, Midland, MI), generating reservoirs with 1–4-mm diameter biopsy punches (KAI Medical, Rockville, MD), and device assembly by plasma bonding (Femto Science, Somerset, NJ) to a coverslip (glass; Duran). The devices were sterilized by 15 min of sonication (Uil Ultrasonic, Wa-dong, South Korea) in 70% EtOH before the bonding process. An 80°C drying oven was used for curing PDMS mixture, dehydration of devices before plasma treatment, and recovering hydrophobicity after bonding (24 h in advanced cell seeding). In this study, a 120-μm depth of microchannel devices was used.
Human umbilical vein endothelial cells (HUVECs; Lonza) were grown in endothelial cell growth medium (EGM-2, Lonza) supplemented with 5% FBS at 37°C, 5% CO2. All cells were used at passages 6–8 from the supplier. After harvesting of trypsinized cells by centrifugation (125 g, 7 min), cells were resuspended in a concentration of 1.4 × 107 cells/mL within a 4 U/mL thrombin solution. We injected a 1:1 mixture of cells (1.4 × 107 cells/mL) and fibrinogen solution (5 mg/mL) into a gel inlet to fill it up. After incubating the chips at 37°C in a humid chamber for 15 min to produce gelation, we injected cell culture medium into the media channels. The cells in the microfluidic chips were incubated for 4 days with daily replacement of medium. Self-assembly of HUVECs into a perfusable vascular network (vasculogenesis) was induced by adding 50 ng/mL VEGF to EGM from day 0 (D0) to day 4 (D4).
Vascular permeability assay in microfluidic chips.
After a 4-day culture, the medium was washed out with a quick PBS rinse before a fluorescent molecule solution was infused. Because of the hydrostatic pressure drop between the two media channels, the solution in the media channel flows along with the perfusable microvascular networks to the opposite media channel. We monitored the diffusion of fluorescent molecules into the fibrin gel (extracellular matrix, ECM) through the vessel wall, and the images were captured at 6–10-s intervals for 3 min via a fluorescence microscope (Axio Observer Z1, Zeiss). The images were quantified using ImageJ.
Perfusibility test in three-dimensional microvascular network.
At day 4, endothelial growth medium was quickly washed with PBS by filling up two reservoirs in only one side channel to rinse out any particles from the 3D vessel lumen efficiently using hydrostatic pressure differences. When a desired field of view was found, bright-field images were captured. Then, 5.0–5.9 μm of FITC surface-labeled beads (0.1% wt/vol, F1CP-50-2; Spherotech) was infused into one media channel and time-lapse images were taken at 1.4-s intervals for 2 min using the fluorescence microscope (Axio, Observer Z1; Zeiss). The fluorescent images were overlaid with bright-field orientation image, and processed to video with ImageJ.
Statistical analysis.
Data are expressed as means ± SE, and the significant differences were determined where P < 0.05 resulted from performing statistical analysis with Student’s t-test or multiple t-test and one-way ANOVA.
RESULTS
Diabetes increases biodistribution of NIR-labeled full-length adiponectin into target tissues.
To accurately investigate adiponectin biodistribution, we developed a model using FMT in which three-dimensional localization of fluorescently tagged adiponectin could be tracked in short time intervals in vivo. Full-length adiponectin comprising high-, medium-, and low-molecular weight oligomers was tagged with a near-infrared (NIR) peptide (VivoTag-S 750) detectable at 750 nm [full-length adiponectin (fAd)-VT750]. Using this wavelength and feeding mice with AIN-76A low-chlorophyll chow diet (Harlan, Indianapolis IN) allowed us to avoid confounding effects of autofluorescence (2). Western blot and fluorescent imaging confirmed that all three oligomers of adiponectin were fluorophore conjugated (Supplemental Fig. S1A, https://doi.org/10.5281/zenodo.3332774), and we demonstrated a linear relationship between adiponectin concentration and fluorescence measurement (Supplemental S1, A–C).
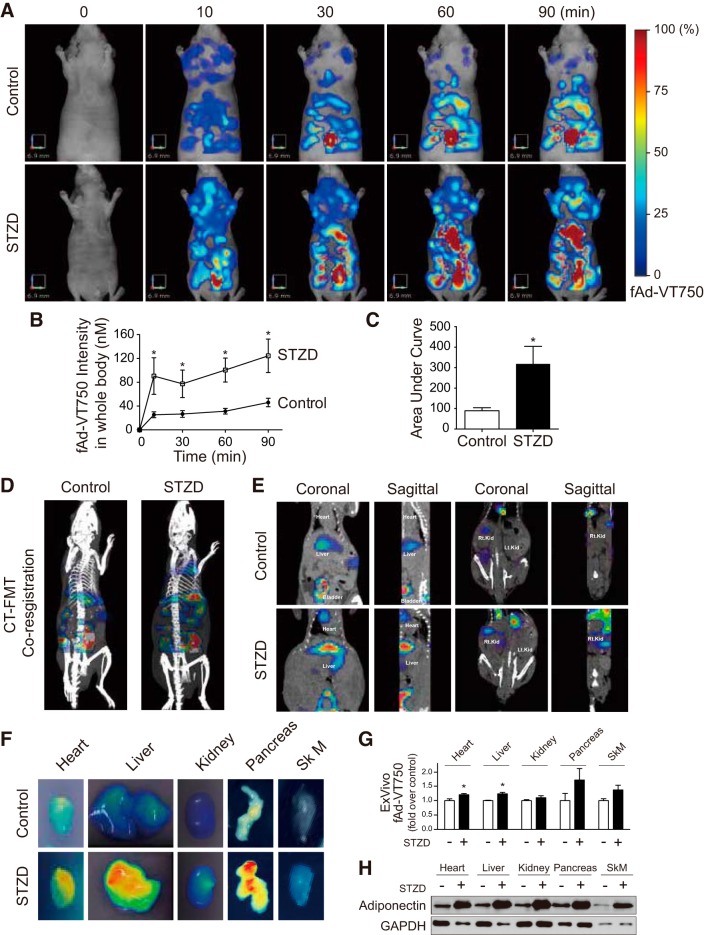
To test the hypothesis that hyperglycemia could alter adiponectin biodistribution, we infused fAd-VT750 (85 µg, via jugular vein) into STZ-injected (STZD) or control nu/nu mice, and then we monitored whole body fluorescence in mice by FMT imaging. We found no difference in circulating adiponectin levels between control and STZD mice and also observed the same level of fAd-VT750 in serum of either STZD or wt mice 2 min after injection via jugular vein (Supplemental Fig. S2A). Consecutive FMT images at 0, 10, 30, 60, and 90 min after fAd-VT750 injection showed increasing tissue accumulation of fluorescent adiponectin throughout the body, which appeared to be faster or higher in STZD mice (Fig. 1, A–C), particularly showing enhanced liver and/or heart accumulation relative to controls. FMT imaging confers the added advantage of providing quantitative analysis of whole body fluorescence and clearly measured an increased overall fluorescence intensity in STZD mice. As a control, infused unconjugated VT750 displayed a completely different biodistribution pattern, clearing quickly through the kidneys and localized primarily to the bladder within 60 min, with very little signal coming from other peripheral tissues (Supplemental Fig S3).
Fig. 1.
Fluorescence molecular tomography shows in vivo biodistribution of full-length adiponectin is increased in diabetic conditions. A: 85 µg of near-infrared labeled full-length adiponectin (fAd-VT750) was injected via cannulated jugular vein into control or diabetic (streptozotocin-induced diabetes, STZD) nu/nu mice. Representative images from a control and STZD mouse before injection and at 10, 30, 60, and 90 min postinjection are shown. B and C: total signal intensity from whole body three-dimensional (3D) field of view was calculated as nM (n ≥ 8; *P < 0.05 from multiple t-test). Time course data were converted to area under curve (AUC) (*P < 0.05 from Student’s t-test). D: coregistration of fluorescence molecular tomography (FMT) (color) and computed tomography (CT) data (white/gray). E: coronal and sagittal view of FMT-CT coregistration with organs of interest identified. F: ex vivo two-dimensional (2D) FMT analysis of fAd-VT750 from isolated tissues [heart, liver, kidney, pancreas, and skeletal muscle (tibialis anterior)]. G: quantification of these 2D images using TrueQuant software. *P < 0.05, compared with its control (n ≥ 4). Statistical analysis was performed using Student’s t-test. H: cardiac homogenates from control and STZD mice were analyzed by Western blot analysis under nondenaturing conditions to compare total amount of reduced (i.e., monomeric, 30 kDa) adiponectin.
To conduct further analysis of the tissue localization of fluorescent signal, mice also underwent X-ray computed tomography (CT) imaging for fluorescence coregistration (Fig. 1D). Coronal and sagittal sections of FMT/CT coregistration data showed accumulation of fAd-VT750 in the heart, liver, kidney, and bladder (Fig. 1E). To eliminate potential tissue-scattering effects, major target tissues of adiponectin action were removed 90 min following fAd-VT750 infusion and imaged ex vivo by FMT. Heart, liver, kidney, pancreas, and skeletal muscle (Sk M) of STZD mice exhibited a higher fluorescent signal when compared with control (Fig. 1F), although only heart and liver reached significance upon quantitation (Fig. 1G). In agreement with this, Western blot analysis of reduced tissue homogenates, to allow detection of all adiponectin as monomers, clearly showed increased levels in all of these target tissues from STZD versus wild-type control mice at 90 min post fAd-VT750 infusion (Fig. 1H).
Adiponectin accumulation in the heart is upregulated by STZD.
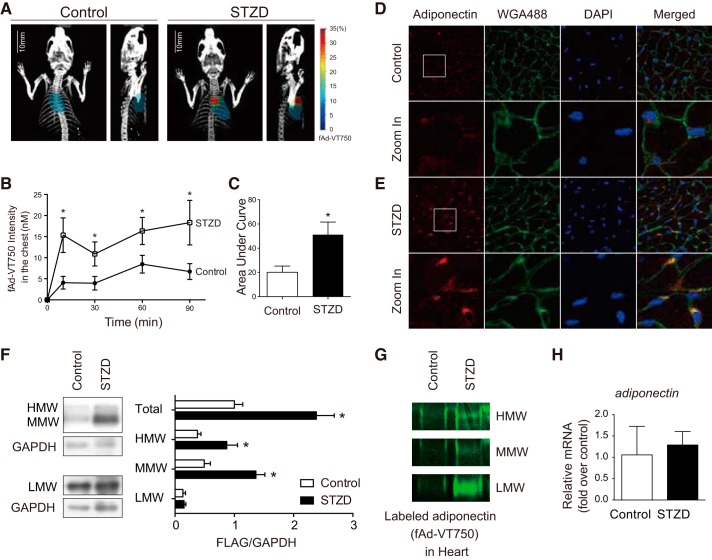
Because the main research focus of our laboratory is the cardioprotective effect of adiponectin, we then used FMT-CT coregistered images to quantify the fluorescence contained within a three-dimensional region of interest mapped to the heart (Fig. 2A). FMT quantitation shows that within 10 min, fAd-VT750 level in STZD hearts was significantly greater than that in wild-type control mice (Fig. 2B). Accordingly, area under the curve analysis showed greater accumulation of fAd-VT750 in the hearts of STZD mice (Fig. 2C). Immunofluorescent analysis of cross sections from myocardium also indicated increased fAd-VT750 in STZD mice 90 min postinfusion compared with wild-type control mice (Fig. 2, D and E). Interestingly, higher magnification revealed an apparent colocalization of fAd-VT750 with WGA, suggesting a near-membrane location.
Fig. 2.
Adiponectin uptake into the heart is increased by diabetes. A: representative heart regions of interest (ROIs) generated by alignment of computed tomography (CT) and fluorescence molecular tomography (FMT) three-dimensional (3D) images using Inveon Research Workplace software. B: quantitation of fAd-VT750 within mapped ROIs. Fluorescence intensity of localized fAd-VT750 (nM) was calculated over time by FMT-CT coregistration analysis (n ≥ 8). *P < 0.05 with respect to control at each time point, and the P values were obtained from the multiple t-test. C: area under the curve analysis of fAd-VT750 within the heart ROI (*P < 0.05). D and E: immunofluorescence analysis of transverse heart cryosections stained with adiponectin (red), wheat germ agglutinin (WGA; green), and DAPI (blue). Colocalization of adiponectin and WGA appears orange. F: cardiac homogenates from C57BL/6 mice 90 min following Flag-tagged adiponectin infusion, run under nondenaturing conditions by SDS-PAGE to detect different adiponectin oligomers: high-molecular weight (HMW; >250 kDa), middle-molecular weight (MMW; ~180 kDa), and low-molecular weight (LMW; ~90 kDa). Total adiponectin combines densitometry from all three oligomers, adjusted to GAPDH as a loading control (n = 5; *P < 0.05). The quantification analyzed by multiple t-test. G: detection of fAd-VT750 by SDS-PAGE in heart homogenates from nu/nu mice 90 min post fAd-VT750 infusion. H: real-time quantitative PCR analysis of heart homogenates 90 min postinjection adjusted for GAPDH (n = 5).
Because molecular weight could be an important determinant in the movement of the adiponectin isoforms from circulation into the heart, we assessed the amount of fAd-VT750 in perfused heart homogenates from control and STZD mice under nondenaturing conditions. There was an increased presence of all three oligomeric adiponectin isoforms in STZD heart homogenates when compared with those of wild-type control mice (Fig. 2G). To verify that increased fAd-VT750 localization was not influenced by the presence of the VivoTag-750 fluorophore, Flag-tagged and nonfluorescent recombinant adiponectin was injected into wild-type C57BL/6 mice treated with or without STZ. Similar to fAd-VT750, there was increased total heart localization of Flag-adiponectin in STZ-treated mice compared with controls (Fig. 2F). Quantitative PCR analysis showed no difference in adiponectin mRNA levels between control and STZD cardiac homogenates (Fig. 2H).
High glucose levels regulate claudin-7 expression to increase paracellular movement of adiponectin.
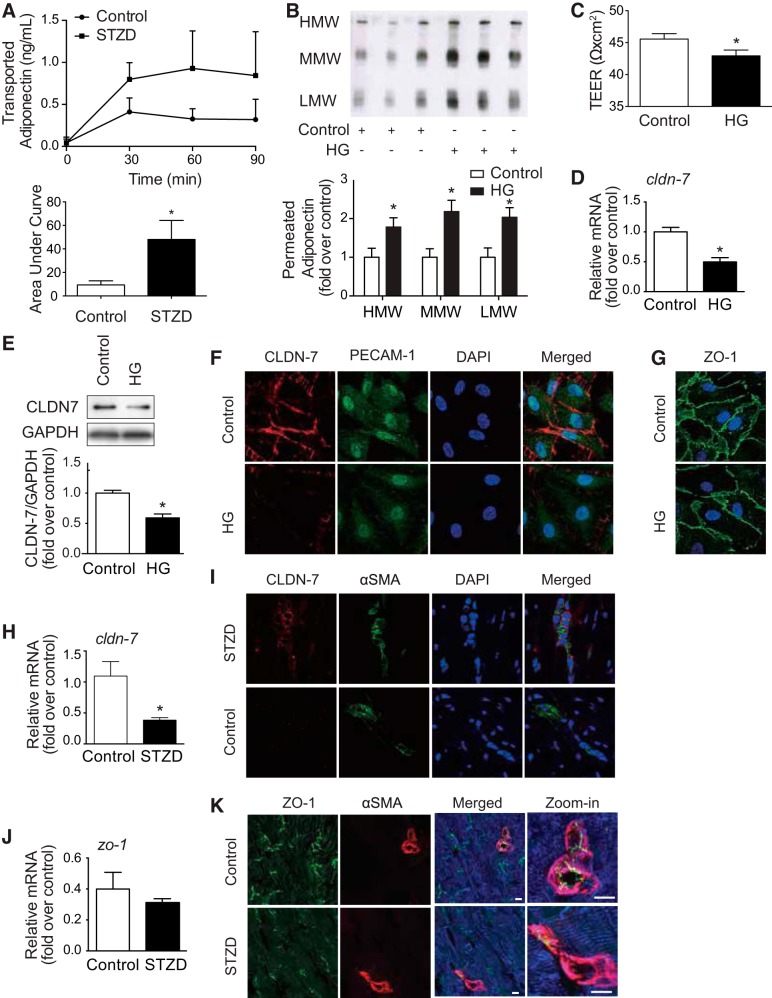
We next tested vascular permeability of adiponectin using mesenteric arteries isolated from STZD or control mice. Similar to our in vivo findings, adiponectin flux across the arterial wall of STZD mesenteric arteries was greater compared with those of wild-type control mice (Fig. 3A). We then exposed a monolayer of human dermal endothelial cells (HDMECs) cultured on Transwell inserts to high glucose and found that the flux of adiponectin, added to the apical chamber, was higher than in normal glucose conditions. Figure 3B shows a Western blot, with quantitation below, indicating a significant increase in flux of high-molecular weight (HMW)-, middle-molecular weight (MMW)-, and low-molecular weight (LMW)-adiponectin from apical to basolateral side in cells grown under hyperglycemic conditions (Fig. 3B). Immunoblotting of the adiponectin receptors AdipoR1 and AdipoR2 showed that their expression in HDMEC monolayers was not affected by high glucose (Supplemental Fig. S4C). Accordingly, we found that endothelial barrier tightness measured by transendothelial electrical resistance (TEER) was decreased in high glucose-treated cells (Fig. 3C). This corresponded to a decrease in claudin-7 (cldn-7) mRNA and protein expression following high glucose treatment (Fig. 3, D and E). Immunofluorescence imaging indicated a decrease in cell membrane expression of cldn-7 in high glucose-treated cells compared with control (Fig. 3F), whereas there was no difference in expression of another tight junction-related protein, ZO-1, between groups (Fig. 3G). This observation was mirrored when heart homogenates were analyzed. Protein and mRNA levels of adiponectin receptors were unchanged by STZD (Supplemental Fig. S4, A and B). In cardiac tissue sections analyzed by qPCR for mRNA and immunohistochemistry, ZO-1 level was not significantly lower (Fig. 3, J and K), while cldn-7 expression was significantly decreased (Fig. 3, H and I) in STZD hearts compared with that in wild-type control mice.
Fig. 3.
Hyperglycemia reduces claudin-7 expression and permits greater flux of adiponectin movement across the endothelium. A: mesenteric arteries extracted from control or streptozotocin-induced diabetes (STZD) rats were perfused with flag-tagged fAd, with samples of extravascular fluid taken at 30, 60, and 90 min postadiponectin and analyzed by ELISA. Area under the curve (AUC) analysis shows greater adiponectin flux from STZD arteries vs. control (n ≥ 5; *P < 0.05). B: human dermal microvascular endothelial cells (HDMEC) were cultured in a Transwell insert before treatment with high glucose (HG) or control. Adiponectin was added to the apical surface, and then basolateral media were collected 24 h following adiponectin administration. Samples were run under nondenaturing conditions; n = 3; *P < 0.05, with respect to each adiponectin isomer’s control. C: TEER (transendothelial electrical resistance) was lower in hyperglycemia significantly (*P < 0.05; n ≥ 11). Claudin-7 in hyperglycemia-treated endothelial cells also decreased at cldn-7 mRNA expression (*P < 0.05; n = 4) (D), as well as claudin-7 (CLDN-7) protein expression (*P < 0.05; n = 3) (E). F: lower CLDN-7 was localized on the cell membrane in hyperglycemia. CLDN-7 (red) and PECAM-1 (green; endothelial marker) costain in HDMEC. G: however, zona occludens (ZO-1; green), aligning under the cell membrane, was not changed by high glucose (HG) treatment. H: cldn-7 mRNA level decreased in the hearts of mice with STZ-induced diabetes (n = 5; *P < 0.05). J: no significant difference was shown in zo-1 mRNA level (n = 4). Statistical analysis was performed using the Student’s t-test. I: lower CLDN-7(red) surrounded by α-SMA (green) was observed in heart tissue sections from STZD (K), although there was no difference of ZO-1. Immunostaining for ZO-1 (green) to identify endothelial cell-specific tight junction structure, α-SMA (red) to identify vascular smooth muscle cells, and desmin (blue) to identify cardiomyocytes. Shown are representative images of n = 3 mice per experimental group. High-magnification images show junction formation between endothelial cells in small vessels. Scale bars: 50 μm and 150 μm, respectively.
Reduced claudin-7 expression decreased TEER and enhanced adiponectin movement across the endothelial monolayer.
To test the functional importance of cldn-7 in adiponectin flux across an HDMEC monolayer, knockdown of cldn-7 was induced by transfection of shRNA (shCLDN7), which resulted in ~50% decrease in cldn-7 protein compared with cells that received a scrambled shRNA sequence not targeting cldn-7 (shNTC) (Fig. 4A). TEER was significantly decreased in monolayers comprising shCLDN7 cells compared with shNTC (Fig. 4B). Furthermore, there was increased flux of LMW-adiponectin from apical to basolateral side of monolayers comprising shCLDN7 cells compared with shNTC controls (Fig. 4C).
Fig. 4.
Reduced claudin-7 (CLDN-7) expression decreased transendothelial electric resistance (TEER) and increased low-molecular-weight (LMW) adiponectin movement across the monolayer of human dermal microvascular endothelial cells (HDMECs). A: one of the major components of tight junction proteins, CLDN-7, was targeted to knock down by shRNA transfection after 96 h. Transfected HDMECs were transferred into the transmembrane culturing system (0.9 cm2) and formed a monolayer 72 h after transfection. B: the cells with lower CLDN-7 expression (**P < 0.01; n = 3) showed significantly lower TEER (*P < 0.05; n ≥ 7). TEER was measured at 96 h after transfection had started. C: monolayer of CLDN-7 knockdown HDMECs was used for adiponectin permeability assay for 24 h. The decreased CLDN-7 led the adiponectin movement increase, especially in LMW form with statistical significance (*P < 0.05; n ≥ 5). The values were expressed as means ± SE, and statistical analysis was performed using Student’s t-test.
Functional 3D perfusable microvascular networks allowed to confirm hyperglycemia effect on permeability of dextran and adiponectin.
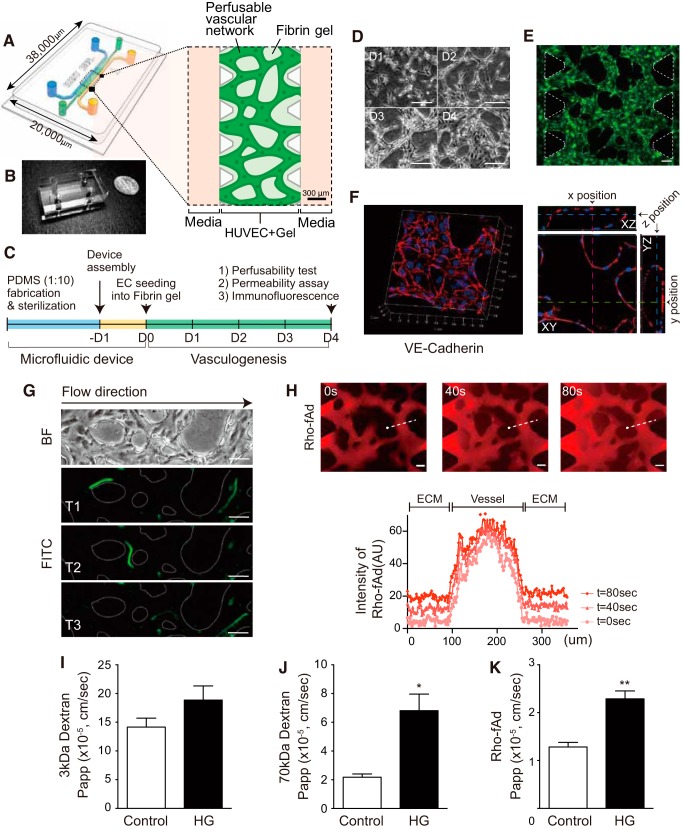
Finally, we tested the hyperglycemia effect in microfluidic microvascular network of HUVECs. This in vitro platform of 3D vasculature-on-a-chip was generated in the ECM mimetic environment with fibrin hydrogel. PDMS (polydimethylsiloxane) microfluidic device is useful to induce perfusable microvasculature, as well as to monitor the event inside of the highly branched vascular structure. The PDMS mold has a central gel channel containing HUVECs and fibrin hydrogel bordered by triangular posts and two media fluid channels (Fig. 5, A and B). 1.4 × 107 cells/mL of HUVECs were seeded with the fibrin hydrogel at D0 and incubated for 4 days to form endothelial monolayers of in vivo-like vasculature naturally (Fig. 5C). Additional VEGF was used to increase the dynamic alignment of HUVECs from D0 to D4 (Fig. 5D). At day 4, the formation of open-ended EC monolayer 3D vessels between two PDMS posts were completed. GFP transfected HUVECs showed highly branched features of microvascular networks in a device (Fig. 5E) and vascular endothelial cadherin (VE-cadherin)-positive-stained structures indicated that vessel-like EC monolayer barrier allows the perfusion of fluids (Fig. 5F; Supplemental Video S3). To confirm the functionality of this vasculature, we performed perfusibility test with 5-μm beads conjugated with FITC, and time-lapse images were taken under fluorescence exposure at every 1.4 s (Fig. 5G, Supplemental Video S4). Next, we wanted to test the effect of hyperglycemia on permeability of 3D microvascular networks using fAd and changes of paracellular movement across this barrier with 70 kDa dextran at several time intervals. Immunofluorescent detection of VE-C showed that the integrity of interconnected vascular structures appeared not to loosen in hyperglycemia compared with control (Supplemental Fig. S5). To assess the permeability, 10 μg/mL fAd conjugated with rhodamine was injected into one reservoir of a device, and fluorescent images were captured over time. The apparent permeability (Papp, cm/s) was calculated by the fluorescent intensity measurement from a linear region of interest (ROI) spanning the microvessel-ECM interface. Because the perfused fluorescent molecules were transferred to the ECM from the vessels via the concentration gradient, the intensity increase was observed in ECM area over time (Fig. 5H). Permeability of rhodamine-labeled fAd (Rho-fAd) increased in hyperglycemia (1.285 × 10−5 ± 0.09363 × 10−5 cm/s) compared with 19.5 mM mannitol with 5.5 mM glucose-treated control (2.290 × 10−5 ± 0.1651 × 10−5 cm/s) (Fig. 5K). 70 kDa dextran, which is smaller than LMW adiponectin (around 90 kDa), also showed significantly higher permeability in high glucose-treated condition (Papp of HG was 6.812 × 10−5 ± 1.152 × 10−5 cm/s and control Papp was 2.180 × 10−5 ± 0.2277 × 10−5 cm/s) (Fig. 5J). However, for the small size of dextran (3 kDa-FITC), hyperglycemia did not significantly change the permeability (Fig. 5I). These suggested that paracellular movement regulates transendothelial accessibility depending on the size of molecules. It also confirmed that hyperglycemia in vitro with 25 mM glucose altered the movement of full-length adiponectin in microfluidic vessels-on-a-chip.
Fig. 5.
Functional three-dimensional (3D) microvascular networks demonstrated size-dependent permeability and the effect of hyperglycemia on adiponectin transport. A: schematic representation of 3D perfusable microvascular network model with human umbilical vein endothelial cells (HUVECs) in a polydimethylsiloxane (PDMS) microfluidic device. The PDMS mold has a central gel channel containing HUVEC cells and fibrin hydrogel bordered by triangle posts. The HUVEC/fibrin gel channel is flanked on both sides by fluid channels, leading to four reservoirs filled with culture medium. B: a device photo is shown with an area dimension of 20,000 μm × 38,000 μm; a dime is shown alongside of it as a size reference. C: experimental timeline is shown. D: daily images (D1–D4) of HUVECs in the 3D fibrin gel shows cellular alignment dynamics during vasculogenesis (×10 magnification was used). E: continuously green fluorescent protein (GFP)-expressed HUVECs showed the perfusable vasculature on day 4. F: confocal reconstructed 3D image of VE-cadherin (red) stained with nucleus (DAPI, blue). A single plane from the stacked images showed three ortho-positions (x position/pink at 570 μm, y position/green at 700 μm, and z position/blue at 18 μm) of continuous endothelial hollow. G: to confirm the generated 3D vessels were perfusable, 5-μm beads conjugated with FITC were loaded into one reservoir, and time-lapse images were taken under fluorescence using Axio Observer Z1 microscope. Time interval average was 1.4 s. H: rhodamine-labeled, full-length adiponectin (Rho-fAd, red) was injected with a concentration of 10 μg/ml. Fluorescent images were captured every 6–10 s for 3 min from two or three different fields of view. Three representative images are shown at 0, 40, and 80 s postinfusion. To assess permeability, fluorescent signal intensity (arbitrary units, AU) was quantified along a linear region of interest (ROI) spanning the microvessel-ECM interface as shown and depicted as a distance from the ROI origin (●). Permeabilized fluorescent signals were observed in the extracellular matrix (ECM) (out of vessel) area over time. Apparent permeability (Papp) was quantified using ImageJ with measurement from randomly selected ROIs. Hyperglycemia with 25 mM glucose (J) did not change the permeability of 3 kDa-FITC dextran. However, 70-kDa Texas red dextran (I) and Rho-fAd (K) increased Papp significantly in high glucose (HG)-treated condition. D, E, G, H: scale bar indicates 100 μm. I–K: data presented are means ± SE. Statistical evaluation was done by Student’s t-test with the values from 15 to 25 ROIs (four to six different fields of view from two or three devices) for each condition. *P = 0.0005. **P < 0.0001.
DISCUSSION
Adiponectin has been shown to mediate widespread physiological effects with resulting antidiabetic, anti-inflammatory, and cardioprotective effects (12). Hence, there has been great interest within academic institutes and pharmaceutical companies to develop therapeutics targeting adiponectin action. To accomplish this, it is critical to fully understand the mechanisms regulating adiponectin’s cellular effects in various tissues. Although we have learned much about adiponectin receptor-mediated signaling mechanisms, few studies have focused on examining mechanisms regulating the transit of adiponectin from the circulation to the interstitial space and, thus, target cells, such as cardiomyocytes, hepatocytes, β-cells, and other cells. We previously proposed that vascular permeability was likely to be an important, and underappreciated, determinant of adiponectin’s physiological actions (46). This is particularly relevant since adiponectin is a complex molecule comprising multiple oligomeric forms, each with a different molecular weight and radial size estimated to be 3.96 to 10.1 nm (35). This is exactly the range within which tight junctions of around 4 nm in dimension can be expected to strongly contribute to dictating adiponectin flux. Indeed, we recently demonstrated that glucocorticoid-mediated decreases in transcellular endothelial permeability-restricted adiponectin transit across endothelial monolayers (9). The observation that adiponectin in cerebrospinal fluid is almost completely in the small trimeric form, and the total amount is only 0.1% of that in peripheral circulation (27), suggests that the tight blood-brain barrier restricts movement of adiponectin oligomers. Conversely, many studies focusing on metabolic effects of adiponectin have focused on liver, which has a highly fenestrated leaky vasculature, and found that the HMW adiponectin is highly bioactive and correlates well with clinical readouts of liver function (5, 38). Hence, this study was designed to use FMT to investigate distribution of fluorescently labeled adiponectin after systemic introduction in diabetic versus wild-type control mice. Given the critical need for adiponectin to access target tissues and the well-established regulatory role of vascular permeability in both physiology and therapeutics, we believe this work has widespread relevance.
We used a real-time noninvasive approach to monitor and quantify the biodistribution of exogenously administered fluorescent adiponectin. The use of FMT as a noninvasive approach to examine biodistribution of a circulating hormone, such as adiponectin, has strong advantages (40). The ability to monitor individual mice noninvasively for time-course studies vastly reduces the number of animals required and avoids issues, such as interanimal metabolic variation and variability in fluorophore injection. In addition, time and expense of isolating numerous tissues followed by analysis by Western blot can be a significant limiting factor to the identification of novel target tissues. Having a clear, temporal, whole body indicator of kinetics can provide a wealth of mechanistic insight, potentially leading to new therapeutics. In this study, we complemented FMT imaging with CT, which offered us high-resolution anatomical detail to improve the spatial and temporal localization of adiponectin. Analysis of coregistered FMT-CT data improves upon methodologies, which offer only 2D fluorescence acquisition. Through 3D analysis, we were able to precisely quantify fAd-VT750 in the heart through creation of CT-guided 3D ROIs. This is vital for accuracy, as many factors can impair rough localization of a fluorophore, including mouse orientation, variability in organ size, potential “bleed over” from highly targeted tissues (e.g., liver), and spatial overlap of several tissues, such as the lungs, heart, and thymus, from certain mouse orientations. Analysis of our in vivo findings were substantially corroborated by ex vivo scanning of target tissues and classic molecular biology techniques, including Western blot analysis and immunofluorescence imaging. For fluorescence in vivo imaging of whole body biodistribution, it is important to avoid autofluorescence, which requires near-infrared (NIR) labeling of a target (2). Probes and fluorophores are available in this range, from 680 to 750 nm and 800 nm, offering the potential for some creative multiplexing studies such as cotracking of insulin and adiponectin in circulation under various disease models. Imaging at 750–800 nm wavelengths completely avoids autofluorescence, and imaging at 680 nm only requires the use of low-chlorophyll chow to minimize confounding gastrointestinal background fluorescence.
Using this approach, we studied mice with or without streptozotocin-induced diabetes. Interestingly, an accumulation of adiponectin occurred in the peripheral tissues, particularly the liver, and to a greater extent in STZD mice. This was confirmed by quantitative analysis of in vivo FMT images, ex vivo imaging, and by analyzing tissue homogenates by Western blot. It was also interesting to note that we found no difference in endogenous adiponectin between control and STZD animals. As we are interested in the cardioprotective effects of adiponectin (11, 14), when we focused on myocardial adiponectin, we again found greater levels in STZD mice. The increased total amount of fAd-VT750 appeared to be accounted for by MMW, plus HMW and to a lesser extent LMW oligomeric forms. It is worth noting that the relative tightness of an endothelial barrier, whether in vivo, ex vivo, or in vitro, and the amount of change induced by a given treatment (e.g., high glucose), can lead to seemingly contradictory results, such as the data presented here (Fig. 2E vs. Fig. 4C). This may be explained through an understanding of protein flux dynamics at the cell junction level: slight opening of a tight endothelial barrier may lead to increased flux of only low-molecular weight proteins, while with a leakier barrier, the flux of low-molecular weight proteins may already be maximum. In this case, further opening of a leaky barrier would manifest as increased flux of only higher-molecular weight proteins. STZD is a common diabetic animal model, yet data must be interpreted with caution, since numerous endogenous changes beyond hyperglycemia may impact vascular permeability (3, 4, 34, 42). It would be of great interest to examine the same phenomenon studied here in models such as a high-fat diet feeding or in genetically obese models such as ob/ob mice (22). Nevertheless, to extend our observations further, we examined flux of adiponectin upon its addition to isolated mesenteric vessels from wild-type control or STZD animals. We observed again that vascular permeability of adiponectin was higher in STZD vessels. Furthermore, using an endothelial cell monolayer system in vitro, we found that flux of adiponectin was significantly greater in monolayers cultured in high glucose media versus normal glucose. Collectively, the data suggest that in the STZD model, hyperglycemia is likely to be one of the principal determinants of altered vascular permeability of adiponectin.
In this study, we also focused on alterations in tight junction proteins as a potential mechanism underlying the effects observed here. We focused on claudin-7 based on the rationale from previous studies, which showed that shRNA-mediated reduction in endothelial cell claudin-7 levels increased flux of adiponectin across endothelial monolayers (9). Indeed, our data indicated that a decrease in claudin-7 may be one important alteration leading to increased vascular permeability in STZD mice. Furthermore, we found ZO-1 was not significantly changed in STZD hearts, although previously, hyperglycemia was shown to increase permeability of the blood-brain barrier (17), and retinal pigment epithelial cells (17, 41) through downregulation of occludin and ZO-1. Because AdipoR-mediated endocytosis has been shown to have important cellular signaling consequences, we also believed that this may contribute to a transcellular route of transport (10, 46). However, the data in Supplemental Fig. S4, showing no significant change in the level of cardiac AdipoR1 or AdipoR2 after hyperglycemia in mice or high glucose level in HDMEC, suggest that this is likely not a major player.
We used advanced in vitro platform of 3D microvascular networks on a chip to confirm the observation from 2D Transwell endothelial monolayer responses in a high-glucose environment. Generating endothelial monolayer of 3D vessels with multiple branches using programmed cellular dynamics in hydrogel allows one to better mimic in vivo vascular morphology (23). Another strength of the showing 3D microvasculature in microfluidic devices is perfusibility, which allows one to test the functionality of the vessels on a chip, as well as to better mimic the physiological environment with application of fluidic shear stress (21, 24, 44). Using paracellular tracers, many researchers have shown that the basal permeability of comparably large molecules (40–70 kDa dextran) was lower than the permeability of smaller molecules (4–10 kDa dextran) (13, 18, 20). In this study, using the platform, we confirmed size-selective transport behavior and showed for the first time that movement of rhodamine-labeled full-length adiponectin was significantly impacted by hyperglycemia-mediated alteration of endothelial permeability.
In summary, we have shown that biodistribution of adiponectin can be altered in a diabetic environment. Data from a combination of temporal noninvasive imaging in mice, isolated vessels ex vivo and in vitro endothelial cell monolayers suggest that hyperglycemia increased vascular flux of adiponectin. We believe this may be an important determinant of adiponectin’s physiological actions and could provide rationale for further investigation and be exploited from a therapeutic perspective.
GRANTS
This work was supported by a grant to G. Sweeney from Natural Sciences and Engineering Research Council (NSERC) and Canadian Institutes of Health Research (CIHR). G. Sweeney also acknowledges support from the Heart & Stroke Foundation via a Career Investigator Award. The research of B. Hinz was supported by the Canadian Institutes of Health Research (Grant 375597), the Canadian Foundation for Innovation, and the Ontario Research Foundation (Grant 36050). J. S. Jeon acknowledges support from Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2017R1D1A1B03030428).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.D., E.T., J.S.J., J.J., T.D.M., J.G., J.D.P., and G.S. conceived and designed research; N.Y., K.D., T.D., T.C., N.N., A.R., and S.K. performed experiments; N.Y., K.D., N.N., B.H., and S.K. analyzed data; N.Y., K.D., E.T., J.S.J., S.P.K., and G.S. interpreted results of experiments; N.Y., K.D., N.N., and S.K. prepared figures; N.Y. drafted manuscript; N.Y., K.D., T.D., T.C., N.N., A.R., E.T., S.K., J.S.J., J.J., T.D.M., J.G., J.D.P., S.P.K., and G.S. approved final version of manuscript; K.D., B.H., E.T., J.S.J., J.J., T.M., J.G., J.D.P., S.P.K., and G.S. edited and revised manuscript.
REFERENCES
- 1.Alipoor E, Mohammad Hosseinzadeh F, Hosseinzadeh-Attar MJ. Adipokines in critical illness: A review of the evidence and knowledge gaps. Biomed Pharmacother 108: 1739–1750, 2018. doi: 10.1016/j.biopha.2018.09.165. [DOI] [PubMed] [Google Scholar]
- 2.Bhaumik S, DePuy J, Klimash J. Strategies to minimize background autofluorescence in live mice during noninvasive fluorescence optical imaging. Lab Anim (NY) 36: 40–43, 2007. doi: 10.1038/laban0907-40. [DOI] [PubMed] [Google Scholar]
- 3.Capucci MS, Hoffmann ME, De Groot A, Natarajan AT. Streptozotocin-induced toxicity in CHO-9 and V79 cells. Environ Mol Mutagen 26: 72–78, 1995. doi: 10.1002/em.2850260111. [DOI] [PubMed] [Google Scholar]
- 4.Chang KS, Stevens WC. Endothelium-dependent increase in vascular sensitivity to phenylephrine in long-term streptozotocin diabetic rat aorta. Br J Pharmacol 107: 983–990, 1992. doi: 10.1111/j.1476-5381.1992.tb13395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combs TP, Marliss EB. Adiponectin signaling in the liver. Rev Endocr Metab Disord 15: 137–147, 2014. doi: 10.1007/s11154-013-9280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol (Lausanne) 2: 62, 2011. doi: 10.3389/fendo.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadson K, Turdi S, Boo S, Hinz B, Sweeney G. Temporal and molecular analyses of cardiac extracellular matrix remodeling following pressure overload in adiponectin-deficient mice. PLoS One 10: e0121049, 2015. doi: 10.1371/journal.pone.0121049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadson K, Turdi S, Hashemi S, Zhao J, Polidovitch N, Beca S, Backx PH, McDermott JC, Sweeney G. Adiponectin is required for cardiac MEF2 activation during pressure overload induced hypertrophy. J Mol Cell Cardiol 86: 102–109, 2015. doi: 10.1016/j.yjmcc.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Dang TQ, Yoon N, Chasiotis H, Dunford EC, Feng Q, He P, Riddell MC, Kelly SP, Sweeney G. Transendothelial movement of adiponectin is restricted by glucocorticoids. J Endocrinol 234: 101–114, 2017. doi: 10.1530/JOE-16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Q, Wang Z, Chen Y. Endocytosis of adiponectin receptor 1 through a clathrin- and Rab5-dependent pathway. Cell Res 19: 317–327, 2009. doi: 10.1038/cr.2008.299. [DOI] [PubMed] [Google Scholar]
- 11.Engin A. Adiponectin-resistance in obesity. Adv Exp Med Biol 960: 415–441, 2017. doi: 10.1007/978-3-319-48382-5_18. [DOI] [PubMed] [Google Scholar]
- 12.Esmaili S, Hemmati M, Karamian M. Physiological role of adiponectin in different tissues: a review. Arch Physiol Biochem Nov. 18: 1–7, 2018. doi: 10.1080/13813455.2018.1493606. [DOI] [PubMed] [Google Scholar]
- 13.Funamoto K, Yoshino D, Matsubara K, Zervantonakis IK, Funamoto K, Nakayama M, Masamune J, Kimura Y, Kamm RD. Endothelial monolayer permeability under controlled oxygen tension. Integr Biol 9: 529–538, 2017. doi: 10.1039/C7IB00068E. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med 6: 27–35, 2009. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Günzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol 2: 1819–1852, 2012. doi: 10.1002/cphy.c110045. [DOI] [PubMed] [Google Scholar]
- 16.Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 50: 202–211, 2007. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 18.Ho YT, Adriani G, Beyer S, Nhan PT, Kamm RD, Kah JCY. A facile method to probe the vascular permeability of nanoparticles in nanomedicine applications. Sci Rep 7: 707, 2017. doi: 10.1038/s41598-017-00750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwabu M, Okada-Iwabu M, Yamauchi T, Kadowaki T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech Dis 1: 15013, 2015. doi: 10.1038/npjamd.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci USA 112: 214–219, 2015. [Erratum in Proc Natl Acad Sci USA. 112: E818, 2015.] doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M, Kamm RD. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol 6: 555–563, 2014. doi: 10.1039/C3IB40267C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AM, Costanzo A, Gareau MG, Armando AM, Quehenberger O, Jameson JM, Olefsky JM. High-fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLoS One 10: e0122195, 2015. doi: 10.1371/journal.pone.0122195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Kim W, Lim S, Jeon JS. Vasculature-on-a-chip for in vitro disease models. Bioengineering (Basel) 4: E8, 2017. doi: 10.3390/bioengineering4010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13: 1489–1500, 2013. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 25.Kolka CM, Bergman RN. The barrier within: endothelial transport of hormones. Physiology (Bethesda) 27: 237–247, 2012. doi: 10.1152/physiol.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolka CM, Bergman RN. The endothelium in diabetes: its role in insulin access and diabetic complications. Rev Endocr Metab Disord 14: 13–19, 2013. doi: 10.1007/s11154-012-9233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 50: 634–642, 2007. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu A, Sweeney G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes 64: 36–48, 2015. doi: 10.2337/db14-0267. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Sweeney G. Adiponectin action in skeletal muscle. Best Pract Res Clin Endocrinol Metab 28: 33–41, 2014. doi: 10.1016/j.beem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Palanivel R, Ganguly R, Turdi S, Xu A, Sweeney G. Adiponectin stimulates Rho-mediated actin cytoskeleton remodeling and glucose uptake via APPL1 in primary cardiomyocytes. Metabolism 63: 1363–1373, 2014. doi: 10.1016/j.metabol.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev 18: 631–644, 2013. doi: 10.1007/s10741-012-9337-8. [DOI] [PubMed] [Google Scholar]
- 32.Park M, Youn B, Zheng XL, Wu D, Xu A, Sweeney G. Globular adiponectin, acting via AdipoR1/APPL1, protects H9c2 cells from hypoxia/reoxygenation-induced apoptosis. PLoS One 6: e19143, 2011. doi: 10.1371/journal.pone.0019143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raignault A, Bolduc V, Lesage F, Thorin E. Pulse pressure-dependent cerebrovascular eNOS regulation in mice. J Cereb Blood Flow Metab 37: 413–424, 2017. doi: 10.1177/0271678X16629155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raza H, John A. Streptozotocin-induced cytotoxicity, oxidative stress and mitochondrial dysfunction in human hepatoma HepG2 cells. Int J Mol Sci 13: 5751–5767, 2012. doi: 10.3390/ijms13055751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutkowski JM, Halberg N, Wang QA, Holland WL, Xia JY, Scherer PE. Differential transendothelial transport of adiponectin complexes. Cardiovasc Diabetol 13: 47, 2014. doi: 10.1186/1475-2840-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sente T, Van Berendoncks AM, Hoymans VY, Vrints CJ. Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J Cachexia Sarcopenia Muscle 7: 261–274, 2016. doi: 10.1002/jcsm.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD, Chung S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat Protoc 7: 1247–1259, 2012. doi: 10.1038/nprot.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao C, Sifuentes A, Holland WL. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic β cells and adipocytes. Best Pract Res Clin Endocrinol Metab 28: 43–58, 2014. doi: 10.1016/j.beem.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 55: 2319–2326, 2012. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 40.Vasquez KO, Casavant C, Peterson JD. Quantitative whole body biodistribution of fluorescent-labeled agents by non-invasive tomographic imaging. PLoS One 6: e20594, 2011. doi: 10.1371/journal.pone.0020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villarroel M, García-Ramírez M, Corraliza L, Hernández C, Simó R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp Eye Res 89: 913–920, 2009. doi: 10.1016/j.exer.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Ma XL, Lau WB. Cardiovascular adiponectin resistance: the critical role of adiponectin receptor modification. Trends Endocrinol Metab 28: 519–530, 2017. doi: 10.1016/j.tem.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol 8: 93–100, 2016. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whisler JA, Chen MB, Kamm RD. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng Part C Methods 20: 543–552, 2014. doi: 10.1089/ten.tec.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab 28: 15–23, 2014. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Yoon N, Dang TQ, Chasiotis H, Kelly SP, Sweeney G. Altered transendothelial transport of hormones as a contributor to diabetes. Diabetes Metab J 38: 92–99, 2014. doi: 10.4093/dmj.2014.38.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]