Abstract
Carbonic anhydrase III (CAIII) is abundant in liver, adipocytes, and skeletal muscles, but not heart. A cytosolic enzyme that catalyzes conversions between CO2 and in the regulation of intracellular pH, its physiological role in myocytes is not fully understood. Mouse skeletal muscles lacking CAIII showed lower intracellular pH during fatigue, suggesting its function in stress tolerance. We created transgenic mice expressing CAIII in cardiomyocytes that lack endogenous CAIII. The transgenic mice showed normal cardiac development and life span under nonstress conditions. Studies of ex vivo working hearts under normal and acidotic conditions demonstrated that the transgenic and wild-type mouse hearts had similar pumping functions under normal pH. At acidotic pH, however, CAIII transgenic mouse hearts showed significantly less decrease in cardiac function than that of wild-type control as shown by higher ventricular pressure development, systolic and diastolic velocities, and stroke volume via elongating the time of diastolic ejection. In addition to the effect of introducing CAIII into cardiomyocytes on maintaining homeostasis to counter acidotic stress, the results demonstrate the role of carbonic anhydrases in maintaining intracellular pH in muscle cells as a potential mechanism to treat heart failure.
Keywords: acidosis, carbonic anhydrase, ex vivo working heart, heart, muscle
INTRODUCTION
Carbonic anhydrases (CAs) catalyze both forward and reverse reactions between CO2 and + H+. At least 16 CA isozymes have been identified in mammals with different tissue distribution and catalytic activity (25). The physiological functions of carbonic anhydrases include hydrase activity to maintain intracellular pH, antioxidative activity to protect muscle from oxidative stress, and oxidative phosphorylation, bone resorption, and taste preference (12, 36, 48, 57, 60).
CA isozyme 3 (CAIII) encoded by the Car3 gene is an ~30-kDa cytosolic protein (9) and is present at high levels in liver, adipocytes, and skeletal muscles (60). It is a low-activity enzyme among the CA isozymes (31, 32) and is resistant to sulfonamide inhibitors (54). The physiological roles of CAIII in various tissue and cell types are not fully understood. CAIII is expressed in preadipocytes, and its expression increases to high levels after differentiation into adipocytes (42), implicating a role in fatty acid metabolism (44). In rodent liver, CAIII appears to be nutritionally regulated, and its mRNA levels decrease during starvation (15, 72). CAIII may facilitate conversion of glycolytic intermediates to oxaloacetate and citrate in fatty acid synthesis. However, adipocyte CAIII expression in obese mice is lower than that in lean mice (43), and CAIII is not required for fatty acid synthesis and does not protect against high fat diet-induced obesity in mice (51).
CAIII is found to protect osteocytes from oxidative stress (58). It regulates peroxisome proliferation-activated receptor-γ2 in adipocytes, and its downregulation in preadipocyte enhances adipogenesis (46). Dietary stresses, such as protein depletion and alcohol consumption, decrease the mRNA and protein levels of CAIII in rodent liver (4, 7, 28, 52, 71). Reduction in its levels forms a biomarker for liver injury (10).
CAIII expresses at high levels in slow-twitch skeletal muscles (20, 59, 60, 65, 73). Car3 gene knockout in mice did not show apparent effects on normal development, growth, or life span despite reduced function of muscles, such as the soleus, that contain a high percentage of slow-twitch fibers (29). Further studies showed that CAIII in skeletal muscles plays a role in fatigue resistance through its function in regulating intracellular pH (16, 40).
The regulation of intracellular pH is crucial in cardiomyocytes for normal heart function and adaptation to physiological stress or pathological conditions. Uncompensated decrease of intracellular pH leads to reductions in cardiac muscle contractility, disruption of intracellular Ca2+ signaling, and arrhythmia (18, 19). Carbonic anhydrases in cardiomyocytes participate in the pH regulation (1, 38). In contrast to skeletal muscles, the heart does not express CAIII but has other carbonic anhydrases such as cytosolic CAII and extracellular anchored CAIV, CAIX, and CAXIV (21, 55, 68). As CAIII plays an antifatigue role by maintaining pH homeostasis in skeletal muscle, we tested in the present study a hypothesis that introduction of CAIII to cardiomyocytes may also increase the tolerance of cardiac muscle to the acidotic stress that occurs in heart failure. We created transgenic mice expressing CAIII in adult cardiomyocytes. Functional characterization showed that the acidotic pH-induced decrease in cardiac function of CAIII transgenic mouse hearts was significantly attenuated relative to that in wild-type control. The results demonstrate the role of carbonic anhydrases in maintaining intracellular pH as well as the effect of adding CAIII in cardiomyocytes on maintaining homeostasis under acidotic stress.
METHODS
Animals.
All animal procedures were approved by the Institutional Animal Care and Use Committee of Wayne State University and were conducted in accordance with Guiding Principles in the Care and Use of Animals, under the guidelines of the Council of The American Physiological Society.
A transgene DNA was constructed using the adult heart-specific promotor of the α-myosin heavy chain (α-MHC) gene to drive the expression of a cDNA encoding mouse CAIII cloned from liver RNA with reverse transcription-coupled PCR. After restriction enzyme mapping and DNA sequencing to verify the construct, the transgene DNA was used for microinjection of zygotes to produce transgenic mice on C57BL/6 background at the Northwestern University Gene Targeting and Transgenic Core Facility.
Transgenic founders and offspring were identified using PCR genotyping and back-bred with C57BL/6 mice purchased from the Jackson Laboratory for over seven generations before use in functional studies. Wild-type and transgenic male and female mice of 3–8 mo of age were used in the present study. The genotype of all individual mice used in functional studies was confirmed on postmortem tissue samples.
SDS-PAGE and Western blot analysis.
Fresh or frozen muscle samples were rapidly processed to homogenize at room temperature in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer containing 2% SDS and 3% 2-mercaptoethanol, pH 8.8, using a high-speed mechanical homogenizer (Pro 250; Pro Scientific) with three blends of 5 s each to extract total proteins. The homogenized SDS-gel samples were stored on ice for no more than 15 min before heating at 80°C for 5 min. The samples were then clarified by centrifugation at 14,000 g in a microcentrifuge at room temperature for 5 min.
The protein samples were resolved on 14% SDS-gel with an acrylamide-to-bisacrylamide ratio of 180:1 made in a modified Laemmli buffer system, in which both stacking and resolving gels were in pH 8.8 buffers. The use of pH 8.8 buffer in the stacking gel does not have notable impact on the stacking effect of discontinuous electrophoresis that is based on glycine’s slower mobility, yielding reproducible high-resolution results as shown in numerous publications of our laboratory including those cited in the present paper. On the other hand, the use of the same buffer in stacking and resolving gels simplifies the system and gives stability for bulk-casted SDS-gels to be stored at 4°C for several weeks by eliminating the diffusion between different buffers in the two gel layers as in the traditional Laemmli gel. Protein bands resolved in the SDS-gel were visualized by staining with Coomassie Blue R 250. Total protein input in each lane was quantified by ImageJ software to normalize the amount of sample loading.
The resolved protein bands in duplicate SDS-gels were electrophoretically transferred to nitrocellulose membranes using a Bio-Rad semidry electrical transfer device at 5 mA/cm2 for 15 min. The blotted membranes were blocked in 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS; 150 mM NaCl and 50 mM Tris, pH 7.5) with shaking at room temperature for 30 min. The blocked membrane was probed with anti-CAIII monoclonal antibody (mAb) (CP3; 26), diluted in TBS containing 0.1% BSA, with gentle rocking at 4°C overnight. The membrane was then washed three times with TBS containing 0.5% Triton X-100 and 0.05% SDS, incubated with alkaline phosphatase-labeled goat anti-mouse IgG secondary antibody (Santa Cruz Biotechnology), washed again as above, and developed in 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate solution to visualize the protein bands detected by the primary antibody.
Ex vivo mouse working-heart preparations.
Preparation of isolated mouse working hearts was done using a protocol described in our previous studies (17). Briefly, mouse was heparinized (100 U ip) 30 min before anesthesia with pentobarbital (100 mg/kg ip). After opening the chest, the heart was rapidly excised together with lung, trachea, and thoracic aorta. The heart was submerged in Krebs buffer at room temperature and carefully dissected with the large vessels remaining on for cannulations. Within 2 min after the opening of the chest, Langendorff retrograde perfusion of the heart using Krebs solution at 37°C was established from the aortic cannula connected to a 70–80-mmHg reservoir (Radnoti).
The left atrium was then cannulated via the pulmonary vein for switching the heart to working mode. The pulmonary artery was cannulated to collect and measure the volume of coronary outflow. A 1.2-F P-V catheter with 3.5-mm spacing between the volume electrodes (Transonic Scisense) was inserted into the left ventricular chamber through a track made at the apex using a sharp 30-g needle to record the intraventricular pressure and volume. A pair of laboratory-made pacing electrodes was placed on the surface of the right atrium to provide supraventricular electrical stimulation from an isolated stimulator (A365; World Precision Instruments). A pressure transducer was connected to the aortic cannula to record the development of aortic pressure. A pair of copper wires with one wire attached with an iron clip was placed under the outlet of aortic flow to record the aortic output in calibrated drops in real time. Another such drop detector was placed under the outlet of the pulmonary arterial cannula to monitor the coronary flow.
The left ventricular preload was set as the hydraulic height from the surface of the perfusant in the pulmonary vein-left atrium-perfusing reservoir to the left ventricle. The left ventricular afterload was set as the hydraulic height from the left ventricle to the outlet of the aortic outflow track. The signals of aortic pressure, left ventricular pressure and volume, aortic output, and coronary flow were digitized via an analog-to-digital interface (16-channel PowerLab; ADInstruments) and continuously collected using Chart 5 computer software (ADInstruments) for later analysis.
The perfusion solution was a modified Krebs-Henseleit bicarbonate buffer, containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 2.25 mM MgSO4, 2.25 mM CaCl2, 0.32 mM EGTA, 2 mM sodium pyruvate, and 15 mM d-glucose, equilibrated with 95% O2-5% CO2. NaHCO3 was added to adjust the pH to 7.36 at 37°C. The perfusion medium was filtered through a 0.45-μm membrane and was not circulated for reuse.
Functional studies of ex vivo mouse working hearts.
After all cannulation and electrodes were set up and the heart was switched to ejection mode by turning on the left ventricular preload flow, the ex vivo mouse working-heart preparation was stabilized at the baseline condition of 10-mmHg preload and 55-mmHg afterload at 37°C for 15–20 min. Baseline functions were recorded at a stably paced physiological heart rate of 480 beats/min. Afterload challenges were then applied at 60, 65, 70, 80, and 90 mmHg to assess the responses of cardiac function.
After examining the cardiac functions at normal pH, the perfusate was switched to a buffer of the same components but equilibrated with a higher CO2 level (90% O2–10% CO2) to lower the extracellular pH from 7.36 to 7.06 at 37°C as calculated using the Henderson–Hasselbalch equation. The changes in cardiac function during the first 20 min of acidosis treatment were recorded at 10-mmHg preload and 55-mmHg afterload. The tolerance of cardiac performance to acidosis was then further tested by repeating the afterload challenges.
Measurement of left ventricular ejection time of ex vivo working hearts.
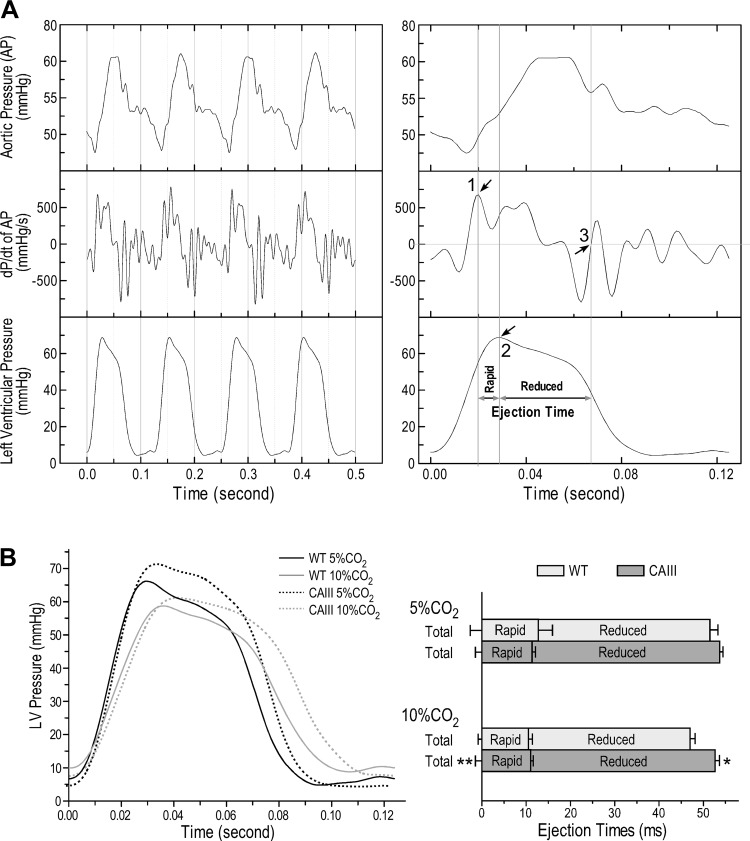
As previous described (17), cardiac ejection time was determined by aligning the traces of left ventricular pressure, aortic pressure, and rate of aortic pressure development (dP/dt of aortic pressure) collected from the ex vivo working-heart functional measurements. The start of left ventricular ejection is defined as the aortic valve opening at the peak rate of the increase of aortic pressure. The rapid ejection phase is the systolic ejection time from aortic valve opening to the peak of left ventricular pressure. The reduced ejection phase is the diastolic ejection time from the peak of left ventricular pressure to the closing of the aortic valve when the aortic dP/dt is zero.
Data analysis.
Densitometric quantification of CAIII Western blots was performed with images scanned at 600 dots/in. and normalized to the actin band in parallel SDS-gel. All quantitative data are presented as means ± SE. Student’s t test was performed for comparison between means, and ANOVA was used for comparison between curves. P < 0.05 was used to establish the level of significance.
RESULTS
Transgenic expression of CAIII in mouse hearts.
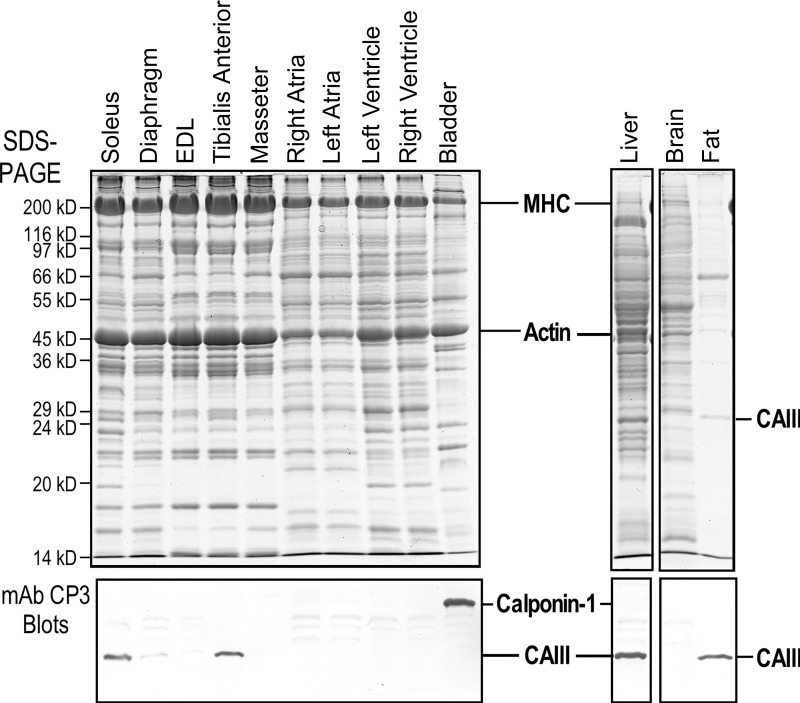
mAb CP3 was used in Western blot analysis to detect the expression of CAIII (16). The results in Fig. 1 show that CAIII is normally expressed at high levels in mouse liver and soleus muscle. In contrast, wild-type mouse extensor digitorum longus (EDL) muscle and heart showed no detectable endogenous CAIII.
Fig. 1.
The absence of carbonic anhydrase III (CAIII) in normal mouse heart. The SDS-gels and Western blots show that mAb CP3 recognizes CAIII in mouse soleus (slow twitch-type) muscle, liver, and fat. The fast twitch-type extensor digitorum longus (EDL) muscle and cardiac muscle show no detectable expression of CAIII. Since mAb CP3 was raised against chicken gizzard smooth muscle calponin 1 immunization, it also recognizes smooth muscle calponin 1 in mouse bladder. MHC, α-myosin heavy chain.
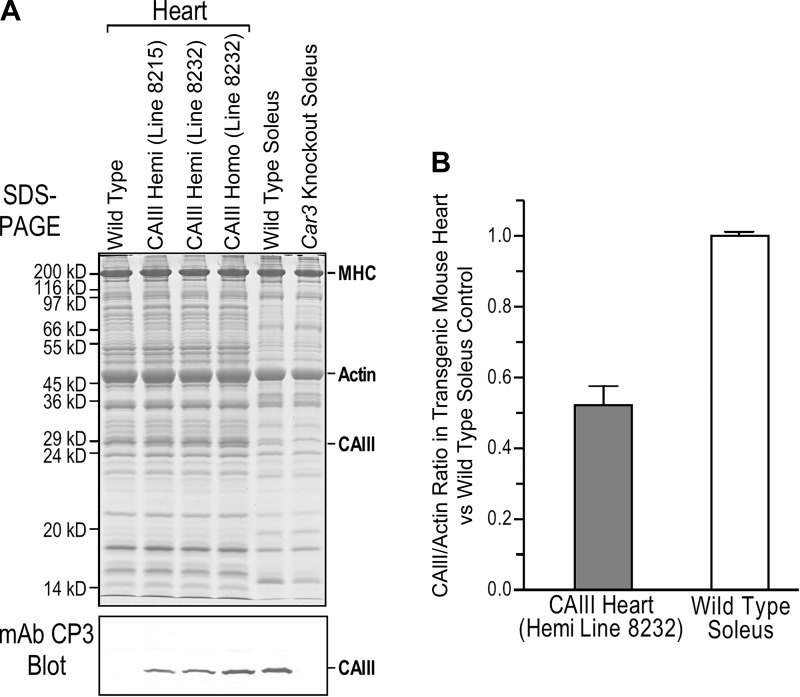
Two transgenic mouse lines were developed with α-MHC promotor-driven expression of mouse Car3 cDNA. Line 8215 and line 8232 exhibit low and high physiological levels of CAIII expressions in the heart, respectively, as shown in the mAb CP3 Western blot of total cardiac muscle protein extracts (Fig. 2A). Line 8232, with the higher level of expression in hemizygotes (~50% of the level in wild-type mouse soleus muscle, Fig. 2B), was used in functional studies. In the meantime, adult transgenic mouse hearts showed a profile of total proteins in SDS-PAGE similar to that of wild-type control (Fig. 2A). Line 8232 homozygotes expressing CAIII in the heart at a higher level closer to that in wild-type soleus muscle showed no negative impact on overall and cardiac phenotypes (data not shown), further justifying the use of this line as a physiological model for cardiac functional studies.
Fig. 2.
Transgenic mouse lines expressing carbonic anhydrase III (CAIII) in the heart. Adult heart-specific expression of CAIII was produced in mice engineered by introducing an α-myosin heavy chain (MHC) promotor-driven transgene. Two stable transgenic mouse lines were generated with low and high physiological levels of CAIII expression (lines 8215 and 8232, respectively) as confirmed with mAb CP3 Western blot of cardiac muscle protein extracts and densitometric analysis. The transgenic and wild-type control mouse hearts showed similar patterns of overall protein expression in the SDS-gel (A). Transgenic mouse line 8232, with a higher level of expression in hemizygotes (Hemi) at ~50% of the level in soleus muscle (B), was used for functional studies. Line 8232 homozygotes (Homo) expressed a higher level of CAIII in the heart reaching the level in soleus muscle (A) with no detectable negative impact (data not shown). Values are presented as means ± SE. Car3, carbonic anhydrase III gene.
Transgenic expression of CAIII in mouse hearts had no destructive impact.
On the basis of the fact that normal skeletal muscles could express CAIII at high or null levels, we did not anticipate any destructive impact from its physiological level transgenic expression in mouse cardiac muscle. This rationale was confirmed by the absence of any detectable difference in heart weight and body weight in young and older mice (Table 1) or in total protein profiles in SDS-PAGE of the heart (Fig. 2A) compared with that of wild-type controls. Separate analysis of CAIII transgenic mice for the older age group (8 mo) also did not detect cardiac hypertrophy or functional abnormality (data not shown) compared with age-matched wild-type controls.
Table 1.
Transgenic expression of CAIII in mouse heart does not alter cardiac development
| WT | CAIII | |
|---|---|---|
| n | 7 | 8 |
| Body weight, g | 26.2 ± 1.5 | 24.3 ± 1.7 |
| Heart weight, mg | 131.9 ± 8.4 | 127.9 ± 9.1 |
| HW/BW, mg/g | 5.0 ± 0.2 | 5.3 ± 0.1 |
Values are means ± SE; n = no. of mice. Transgenic mice (3–8 mo old) with α-myosin heavy chain promoter-driven expression of carbonic anhydrase III (CAIII) showed no difference in body weight, heart weight, or heart weight-to-body weight ratio (HW/BW) from those of wild-type (WT) control in the same age range, indicating no destructive effect of CAIII expression on the heart. Statistical analysis was performed using Student’s t test.
Transgenic expression of CAIII in mouse hearts did not alter baseline cardiac function.
Multiparameter ex vivo working-heart studies did not detect functional difference between CAIII transgenic and wild-type mice at baseline conditions of 10-mmHg preload, 55-mmHg afterload, and near-physiological heart rate of 480 beats/min (Table 2). The results further demonstrate that the physiological level of transgenic expression of CAIII in cardiac muscle did not have a negative impact on basic functions of the heart.
Table 2.
Transgenic expression of CAIII in mouse heart does not alter baseline functions
| WT | CAIII | |
|---|---|---|
| n | 4 | 3 |
| Heart rate, beats/min | 480 | 480 |
| LVPmax, mmHg | 69.7 ± 2.3 | 71.4 ± 1 |
| LVPmin, mmHg | 4.8 ± 0.8 | 4.6 ± 0.2 |
| APmax, mmHg | 60.4 ± 0.2 | 61.4 ± 0.4 |
| APmin, mmHg | 47.8 ± 0.3 | 46.6 ± 0.4 |
| SV, μL/mg | 0.164 ± 0.015 | 0.195 ± 0.010 |
| +dP/dt, mmHg/s | 3,749.8 ± 129.4 | 3,931.4 ± 93.3 |
| −dP/dt, mmHg/s | −2,697.0 ± 93.84 | −2,776.3 ± 115.6 |
Values are means ± SE; n = no. of mice. Ex vivo working-heart functions of 3-mo-old transgenic mice tested under baseline conditions at 10-mmHg preload, 55-mmHg afterload, and heart rate of 480 beats/min showed no difference from wild-type (WT) control. APmax and APmin, maximum and minimum aortic pressure, respectively; LVPmax and LVPmin, maximum and minimum left ventricular pressure, respectively; SV, left ventricular stroke volume. Statistical analysis was performed using Student’s t test.
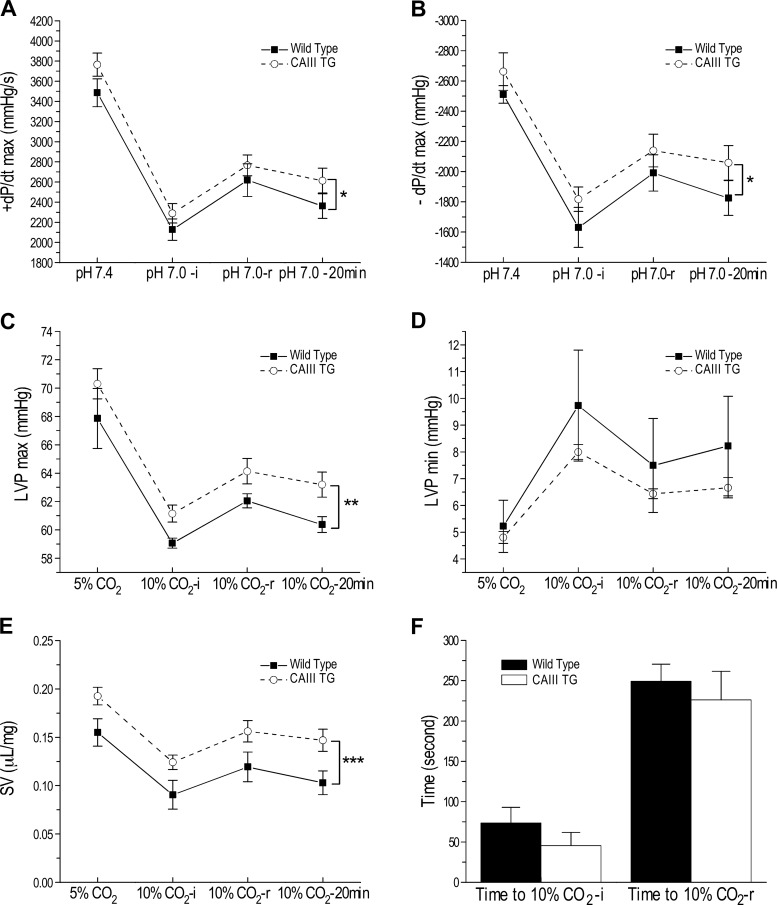
CAIII transgenic mouse hearts exhibit a higher resistance to acidosis than WT controls.
When the ex vivo working hearts were switched from perfusion with normal Krebs solution at pH 7.36 to the acidotic pH of 7.06, cardiac functions responded to the acute acidosis with rapid decreases. Cardiac functions then gradually and partially recovered to an intermediate level that plateaued through the end of the 20-min period of pH 7.06 perfusion (Fig. 3). When comparing cardiac functions during the acidotic treatment, the CAIII transgenic mouse hearts showed a trend of better performance at normal pH but sustained significantly higher functions under acidosis than did wild-type hearts (Fig. 3, A–E). Comparing the time courses of responding to acidosis, CAIII transgenic and wild-type hearts were not different in the initial response [time to pH 7.0-initial (i)] or the following adaptive partial recovery [time to pH 7.0-recovery (r); Fig. 3F].
Fig. 3.
Acidotic stress produced less decrease in baseline function in carbonic anhydrase III (CAIII) transgenic (TG) mouse hearts than that in wild-type control hearts. Baseline functions of ex vivo working hearts were studied at 10-mmHg preload, 55-mmHg afterload, and heart rate of 480 beats/min under normal perfusion with buffer equilibrated with 95% O2-5% CO2 (pH 7.36) and during 20-min acidotic perfusion with buffer equilibrated with 90% O2-10% CO2 (pH 7.06). The results of maximum systolic velocity (+dP/dt max, A), maximum diastolic velocity (−dP/dt max, B), left ventricular peak pressure (LVPmax, C), end-diastolic left ventricular pressure (LVPmin, D), and left ventricular stroke volume (SV, E) showed that after switching to acidotic perfusion, cardiac functions had a rapid initial decrease to reach a lowest point [10% CO2-initial (i)] and then recovered to an intermediate level [10% CO2-recovery (r)] that gradually stabilized at a slightly lower level at the end of the 20 min treatment (10% CO2-20 min). Although CAIII transgenic and wild-type groups had similar trends in the time course of response to acidotic stress (F), CAIII mouse hearts showed a higher tolerance to acidotic stress. Values are presented as means ± SE; n = 4 hearts in wild-type group, and n = 3 hearts in transgenic group. *P < 0.05, **P < 0.01, and ***P < 0.001 using two-way ANOVA with Tukey’s adjustment for mean comparisons.
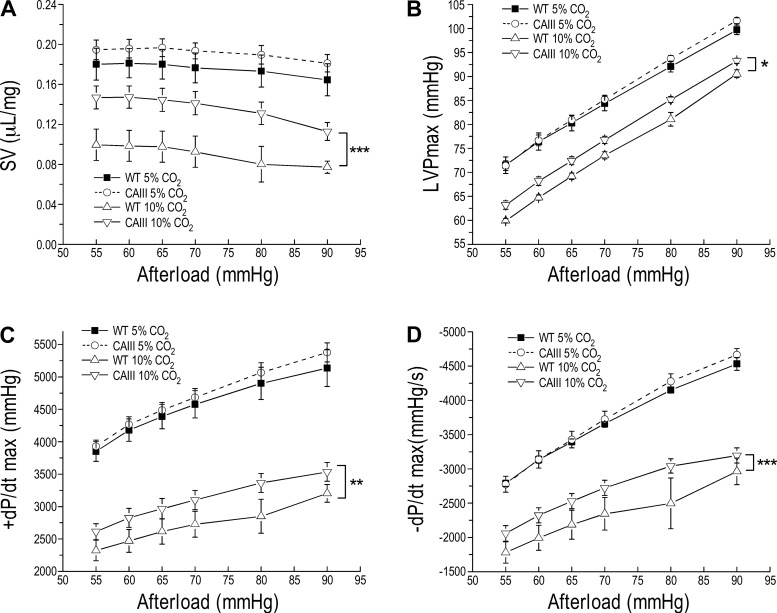
The better function and higher resistance to acidosis of CAIII transgenic mouse hearts are sustained at high afterload.
When challenged by increasing left ventricular afterload from 55 to 60–90 mmHg, the higher-than-wild type tolerance of CAIII transgenic mouse hearts to acidosis was sustained (Fig. 4). This feature of CAIII transgenic mouse hearts to maintain the homeostasis of intracellular pH in acidosis and at pressure overload is plausible since both conditions occur in heart failure.
Fig. 4.
The higher resistance of carbonic anhydrase III (CAIII) transgenic mouse hearts to acidosis is sustained at high afterload. Ex vivo working-heart studies at heart rate of 480 beats/min, 10-mmHg preload, and increased afterload from 55 to 90 mmHg perfused at 90% O2-10% CO2, pH 7.06, vs. 95% O2-5% CO2, pH 7.36, demonstrated that the stroke volume (SV, A), left ventricular peak pressure (LVPmax, B), and maximum contractile and relaxation velocities (+dP/dt max and −dP/dt max, C and D, respectively) of CAIII transgenic mouse hearts responded to high afterload similarly to those of wild-type (WT) hearts at physiological pH. However, CAIII hearts showed higher resistance to acidosis than that of WT hearts by sustaining better function at high afterloads. Values are presented as means ± SE; n = 4 hearts in WT group, and n = 3 hearts in transgenic group. *P < 0.05, **P < 0.01, and ***P < 0.001 using two-way ANOVA with Tukey’s adjustment for mean comparisons.
CAIII transgenic mouse hearts produced longer left ventricular ejection time to compensate for the reduction of stroke volume in acidosis.
The measurement of the time parameters of left ventricular ejection of ex vivo working hearts is shown in Fig. 5A. The representative left ventricular pressure development curves and ejection time analysis in Fig. 5B demonstrate that when perfused with Krebs buffer equilibrated with 10% CO2 (pH 7.06), the total ejection time of CAIII hearts was significantly elongated compared with that of wild-type control paced at the same heart rate of 480 beats/min. The rapid ejection time was not different between the two groups, but the CAIII hearts had significantly better sustained the reduced ejection time in acidosis (Fig. 5B). The effect of transgenic expression of CAIII in cardiomyocytes on myocardial contractile kinetics indicates a major contribution in compensating for cardiac stroke volume in acidosis.
Fig. 5.
Elongated reduced ejection time in carbonic anhydrase III (CAIII) transgenic mouse hearts contributed to the higher stroke volume at acidotic pH. Left ventricular ejection time of ex vivo mouse working heart was determined from the simultaneously recorded left ventricular (LV) pressure (LVP) curve, aortic pressure (AP) curve, and rate (dP/dt) of AP development as previous described (17). A: the rapid ejection phase is the systolic ejection time from aortic valve opening indicated by the peak rate of aortic pressure increase (arrow 1) to the peak of LVP (arrow 2). The reduced ejection phase is the diastolic ejection time from the peak of LVP to the closing of the aortic valve when dP/dt of AP was zero (arrow 3). B: the representative LVP traces demonstrate that at 10-mmHg preload, 55-mmHg afterload, and the same heart rate of 480 beats/min, CAIII mouse hearts showed a trend of longer duration of LVP development than that of wild-type (WT) control. Quantification of left ventricular ejection time showed that CAIII hearts had elongated total ejection time from longer reduced ejection phase than that of WT control in acidosis (90% O2-10% CO2, pH 7.06). Values are presented as mean ± SE; n = 4 hearts in WT group, and n = 3 hearts in transgenic group. *P < 0.05 and **P < 0.01 using Student’s t test vs. WT control.
DISCUSSION
Physiologic stresses or many pathologic conditions can alter intracellular pH in the heart, and compensatory mechanisms are required to return the cardiomyocytes back to physiological pH. CAIII is naturally expressed in various tissues including skeletal muscles, but not in the heart. On the basis of the known function of CAIII in regulating intracellular pH and improving the resistance of skeletal muscle to fatigue (16), we studied the effect of expressing CAIII in cardiomyocytes on adaptation of the heart to pH changes. By developing a novel transgenic mouse model postnatally expressing CAIII in the heart, the phenotypic studies showed that the expression of physiological levels of CAIII in mouse heart had no destructive effects and did not significantly alter the baseline cardiac function. When challenged with acidosis and high pressure load, CAIII transgenic mouse hearts exhibited less decrease in cardiac functions. The following findings and possible mechanisms may help to understand the role of CAIII in pH regulation in muscle cells and the potential implication for use in sustaining cardiac function in heart failure.
Transgenic expression of CAIII in mouse heart has no negative impact on cardiac development and baseline function.
Although CAIII is expressed at high levels in liver, adipocytes, and skeletal muscles (60), previous studies have shown that knockout of the Car3 gene in mice does not affect overall development, growth, fertility, and life span (29). Other studies found that CAIII is not required for de novo lipogenesis, and Car3 knockout mice do not differ from wild-type mice in responses to high-fat diet (51). The expression of CAIII in some but not all skeletal muscles does not show a clear correlation to specific fiber types or contractile features (16). Since other carbonic anhydrase isozymes coexist in liver, adipocytes, and skeletal muscle with higher specific activities than that of CAIII, its function is considered nonessential to lipid metabolism or muscle contraction.
Although the heart normally does not express or require CAIII for function, transgenic expression of CAIII in mouse heart did not have negative impacts on body weight, heart weight, or baseline cardiac function (Tables 1 and 2), demonstrating that the addition of CAIII in cardiomyocytes at an abundant level similar to that in normal soleus muscle has no deteriorating effect, providing a compensatory mechanism and safe approach to enhance the maintenance of intracellular pH homeostasis in physiologic stress and pathologic conditions.
Metabolic functions of carbonic anhydrases in the regulation of intracellular pH.
The regulation of intracellular pH of cardiomyocytes is mainly via sarcolemma H+-equivalent transporters that move H+, OH−, or ions in or out of the cell (67). At least five such transporters have been identified, including Na+/H+ exchanger (NHE), Na+- cotransporter (NBC), Cl−/ exchanger (CBE), Cl−/OH− exchanger (CHE), and lactate-H+ cotransporter [monocarboxylate transporter (MCT); 34, 35, 49, 62, 70]. By catalyzing reversible hydration of CO2, carbonic anhydrases contribute to the formation of and H+, of which is the substrate for NBC and CBE. Besides the expression of mitochondrial CAV (22) and cytosolic CAII (2), cardiomyocytes express membrane-anchored CAIV and transmembrane CAIX and CAXIV (55). Cytosolic CAII and transmembrane CAIX form complexes with NBCe1 in kidney (61) and heart (47), respectively. CAII binds to and enhances the activity of NHE1, which is critical to maintaining cardiac intracellular pH (13, 27, 33).
CA activity increases during ischemia, and inhibition of CA prevents and reverts cardiomyocyte hypertrophy (2). The catalytic activity of CA to generate and H+ substrates for NHE1, NBC, and CBE (14, 38, 61) supports the regulatory role of CA in the homeostasis of intracellular pH. CAII and CAIV are increased in hypertrophic and failing human hearts (3). CAII-deficient mice (37) showed physiological hypertrophy with an increase in heart weight-to-body weight ratio but no significant change in cardiac function (6). Overexpression of CAII in rat neonatal cardiomyocytes promotes hypertrophy, and cardiomyocytes from CAII-deficient mice do not respond to prohypertrophic stimulation (6). Skeletal muscle of mice deficient in CAII showed normal contractility at normal intracellular pH (5). Further testing CAII-deficient mouse hearts for adaption to acidosis may help to understand its role in regulating intracellular pH via its catalytic activity or through interactions with NBC3 (41) and NHE1 (66).
The biological role of CAIII is not fully understood. Although CAIII is not normally expressed in the heart, it can form a functional complex with NBCe1 when they are coexpressed in Xenopus oocytes (56), supporting its role in pH regulation shown in our experimental model of transgenic expression in cardiac muscle to protect heart function in acidosis and pressure overload. Possible mechanisms for its role in mouse hearts under acidosis and pressure overload may also involve its function as a scavenging protective protein against oxidative stress-induced apoptosis as shown in CAIII-transfected rat and mouse fibroblast cells (50, 53). Cys186 and Cys181, two cysteine residues in CAIII that are not present in CAI and CAII, undergo posttranslational S-glutathionylation (11, 39, 45) during early response to oxidative damage (63), underlying the antioxidative function of CAIII in muscle injury and recovery (69, 74). This unique feature of CAIII may contribute to its antiacidosis effect in transgenic mouse hearts in addition to the presence of CAII and other CA isoenzymes. CAIII has a low esterase activity compared with other CA isozymes (8, 30). Although the inhibition of esterase showed benefit in reducing cardiac and skeletal muscle ischemia-reperfusion injury (24, 64), no reports are found for the esterase function of CAIII in muscle contractile performance.
Expression of CAIII in cardiomyocytes increases the resistance to acidosis at baseline and at high pressure load.
Despite the controversy as to whether CAIII catalyzes the conversion of CO2 to in vivo based on its low enzyme activity (31, 32) and resistance to sulfonamide inhibitors (54), experimental evidence showed that CAIII plays a role in skeletal muscle’s resistance to fatigue where intracellular pH decreases due to ATP depletion and accumulation of metabolic products. CAIII-deficient gastrocnemius muscle exhibited decreases in ATP and pH with increases in Pi and ADP (40). An acidotic environment decreases the intracellular pH and increases Pi in muscle cells, resulting in slower relaxation of tetanic contraction (23). We previously showed in mice that CAIII-positive fast twitch fiber-type tibialis anterior (TA) muscle was more resistant to fatigue than was CAIII-negative fast twitch fiber-type EDL muscle and that this resistance to fatigue was diminished in Car3-knockout mouse TA muscle (16).
Because of the presence of multiple CA isoforms with various specific activities in cardiomyocytes, it is technically difficult to estimate the relative level of activity added by the transgenic expression of CAIII in a working heart. On the other hand, normal skeletal muscles can express high or null levels of CAIII (16), whereas the CAIII expression in our transgenic mouse hearts is close to the physiological level in normal soleus muscle (Fig. 2B). Therefore, although we did not quantitatively correlate the added CA activity to the effect on cardiac function, the results clearly show the benefit of adding CAIII to improve the resistance of cardiac muscle to acidosis.
Consistent with the role of CAIII in intracellular pH regulation in acidosis in skeletal muscle cells, the results of our present study demonstrated the function of CAIII in maintaining intracellular pH of cardiomyocytes in acidosis. CAIII is naturally expressed in abundance in many skeletal muscles, and its transgenic expression in heart has no destructive effect on physiological function. Therefore, our present study suggests that enhancing the antiacidosis function of CAs in cardiomyocytes by adding CAIII presents a potential approach to develop a new treatment for heart failure. The interesting finding that CAIII transgenic mouse hearts under acidosis treatment and fixed heart rate of 480 beats/min had better sustained left ventricular ejection time than wild-type control by significantly elongating the reduced ejection time in early diastole with no change in the rapid ejection time in systole (Fig. 5) suggests an underlying mechanism to sustain stroke volume when the systolic force and velocity are decreased in acidosis, which merits further investigation.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-127691 and HL-138007 (to J.-P. Jin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.-Z.F. and J.-P.J. conceived and designed research; H.-Z.F. performed experiments; H.-Z.F. and J.-P.J. analyzed data; H.-Z.F. and J.-P.J. interpreted results of experiments; H.-Z.F. and J.-P.J. prepared figures; H.-Z.F. and J.-P.J. drafted manuscript; H.-Z.F. and J.-P.J. edited and revised manuscript; H.-Z.F. and J.-P.J. approved final version of manuscript.
REFERENCES
- 1.Al-Samir S, Papadopoulos S, Scheibe RJ, Meißner JD, Cartron JP, Sly WS, Alper SL, Gros G, Endeward V. Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J Physiol 591: 4963–4982, 2013. doi: 10.1113/jphysiol.2013.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez BV, Johnson DE, Sowah D, Soliman D, Light PE, Xia Y, Karmazyn M, Casey JR. Carbonic anhydrase inhibition prevents and reverts cardiomyocyte hypertrophy. J Physiol 579: 127–145, 2007. doi: 10.1113/jphysiol.2006.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez BV, Quon AL, Mullen J, Casey JR. Quantification of carbonic anhydrase gene expression in ventricle of hypertrophic and failing human heart. BMC Cardiovasc Disord 13: 2, 2013. doi: 10.1186/1471-2261-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroor AR, Jackson DE, Shukla SD. Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats. Alcohol Clin Exp Res 35: 2128–2138, 2011. doi: 10.1111/j.1530-0277.2011.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekley MD, Wetzel P, Kubis HP, Gros G. Contractile properties of skeletal muscle fibre bundles from mice deficient in carbonic anhydrase II. Pflügers Arch 452: 453–463, 2006. doi: 10.1007/s00424-006-0048-7. [DOI] [PubMed] [Google Scholar]
- 6.Brown BF, Quon A, Dyck JR, Casey JR. Carbonic anhydrase II promotes cardiomyocyte hypertrophy. Can J Physiol Pharmacol 90: 1599–1610, 2012. doi: 10.1139/y2012-142. [DOI] [PubMed] [Google Scholar]
- 7.Caballero VJ, Mendieta JR, Giudici AM, Crupkin AC, Barbeito CG, Ronchi VP, Chisari AN, Conde RD. Alternation between dietary protein depletion and normal feeding cause liver damage in mouse. J Physiol Biochem 67: 43–52, 2011. doi: 10.1007/s13105-010-0047-1. [DOI] [PubMed] [Google Scholar]
- 8.Carter N, Jeffery S, Shiels A, Edwards Y, Tipler T, Hopkinson DA. Characterization of human carbonic anhydrase III from skeletal muscle. Biochem Genet 17: 837–854, 1979. doi: 10.1007/BF00504307. [DOI] [PubMed] [Google Scholar]
- 9.Carter N, Shiels A, Tashian R. Carbonic anhydrase III isoenzyme from human and bovine muscle Biochem Soc Trans 6: 552–553, 1978. doi: 10.1042/bst0060552. [DOI] [PubMed] [Google Scholar]
- 10.Carter WG, Vigneswara V, Newlaczyl A, Wayne D, Ahmed B, Saddington S, Brewer C, Raut N, Gerdes HK, Erdozain AM, Tooth D, Bolt EL, Osna NA, Tuma DJ, Kharbanda KK. Isoaspartate, carbamoyl phosphate synthase-1, and carbonic anhydrase-III as biomarkers of liver injury. Biochem Biophys Res Commun 458: 626–631, 2015. doi: 10.1016/j.bbrc.2015.01.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai YC, Jung CH, Lii CK, Ashraf SS, Hendrich S, Wolf B, Sies H, Thomas JA. Identification of an abundant S-thiolated rat liver protein as carbonic anhydrase III; characterization of S-thiolation and dethiolation reactions. Arch Biochem Biophys 284: 270–278, 1991. doi: 10.1016/0003-9861(91)90295-T. [DOI] [PubMed] [Google Scholar]
- 12.Chegwidden WR, Dodgson SJ, Spencer IM. The roles of carbonic anhydrase in metabolism, cell growth and cancer in animals. In: The Carbonic Anhydrases, edited by Chegwidden WR, Carter ND, Edwards YH. Basel, Switzerland: Birkhäuser, 2000, p. 343–363. doi: 10.1007/978-3-0348-8446-4_16. [DOI] [PubMed] [Google Scholar]
- 13.Cingolani HE, Ennis IL. Sodium-hydrogen exchanger, cardiac overload, and myocardial hypertrophy. Circulation 115: 1090–1100, 2007. doi: 10.1161/CIRCULATIONAHA.106.626929. [DOI] [PubMed] [Google Scholar]
- 14.Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J 417: 423–439, 2009. doi: 10.1042/BJ20081634. [DOI] [PubMed] [Google Scholar]
- 15.Dodgson SJ, Quistorff B, Ridderstråle Y. Carbonic anhydrases in cytosol, nucleus, and membranes of rat liver. J Appl Physiol (1985) 75: 1186–1193, 1993. doi: 10.1152/jappl.1993.75.3.1186. [DOI] [PubMed] [Google Scholar]
- 16.Feng HZ, Jin JP. Carbonic anhydrase III is expressed in mouse skeletal muscles independent of fiber type-specific myofilament protein isoforms and plays a role in fatigue resistance. Front Physiol 7: 597, 2016. doi: 10.3389/fphys.2016.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng HZ, Jin JP. Coexistence of cardiac troponin T variants reduces heart efficiency. Am J Physiol Heart Circ Physiol 299: H97–H105, 2010. doi: 10.1152/ajpheart.01105.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fliegel L. Molecular biology of the myocardial Na+/H+ exchanger. J Mol Cell Cardiol 44: 228–237, 2008. doi: 10.1016/j.yjmcc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Fliegel L. Regulation of the Na+/H+ exchanger in the healthy and diseased myocardium. Expert Opin Ther Targets 13: 55–68, 2009. doi: 10.1517/14728220802600707. [DOI] [PubMed] [Google Scholar]
- 20.Frémont P, Charest PM, Côté C, Rogers PA. Carbonic anhydrase III in skeletal muscle fibers: an immunocytochemical and biochemical study. J Histochem Cytochem 36: 775–782, 1988. doi: 10.1177/36.7.3133407. [DOI] [PubMed] [Google Scholar]
- 21.Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S. Human carbonic anhydrase XIV (CA14): cDNA cloning, mRNA expression, and mapping to chromosome 1. Genomics 61: 74–81, 1999. doi: 10.1006/geno.1999.5938. [DOI] [PubMed] [Google Scholar]
- 22.Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S. Human mitochondrial carbonic anhydrase VB. cDNA cloning, mRNA expression, subcellular localization, and mapping to chromosome x. J Biol Chem 274: 21228–21233, 1999. doi: 10.1074/jbc.274.30.21228. [DOI] [PubMed] [Google Scholar]
- 23.Geers C, Gros G. Effects of carbonic anhydrase inhibitors on contraction, intracellular pH and energy-rich phosphates of rat skeletal muscle. J Physiol 423: 279–297, 1990. doi: 10.1113/jphysiol.1990.sp018022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horstick G, Heimann A, Götze O, Hafner G, Berg O, Böhmer P, Becker P, Darius H, Rupprecht HJ, Loos M, Bhakdi S, Meyer J, Kempski O. Intracoronary application of C1 esterase inhibitor improves cardiac function and reduces myocardial necrosis in an experimental model of ischemia and reperfusion. Circulation 95: 701–708, 1997. doi: 10.1161/01.CIR.95.3.701. [DOI] [PubMed] [Google Scholar]
- 25.Imtaiyaz Hassan M, Shajee B, Waheed A, Ahmad F, Sly WS. Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem 21: 1570–1582, 2013. doi: 10.1016/j.bmc.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 26.Jin JP, Walsh MP, Resek ME, McMartin GA. Expression and epitopic conservation of calponin in different smooth muscles and during development. Biochem Cell Biol 74: 187–196, 1996. doi: 10.1139/o96-019. [DOI] [PubMed] [Google Scholar]
- 27.Karmazyn M, Sostaric JV, Gan XT. The myocardial Na+/H+ exchanger: a potential therapeutic target for the prevention of myocardial ischaemic and reperfusion injury and attenuation of postinfarction heart failure. Drugs 61: 375–389, 2001. doi: 10.2165/00003495-200161030-00006. [DOI] [PubMed] [Google Scholar]
- 28.Kharbanda KK, Vigneswara V, McVicker BL, Newlaczyl AU, Bailey K, Tuma D, Ray DE, Carter WG. Proteomics reveal a concerted upregulation of methionine metabolic pathway enzymes, and downregulation of carbonic anhydrase-III, in betaine supplemented ethanol-fed rats. Biochem Biophys Res Commun 381: 523–527, 2009. doi: 10.1016/j.bbrc.2009.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim G, Lee TH, Wetzel P, Geers C, Robinson MA, Myers TG, Owens JW, Wehr NB, Eckhaus MW, Gros G, Wynshaw-Boris A, Levine RL. Carbonic anhydrase III is not required in the mouse for normal growth, development, and life span. Mol Cell Biol 24: 9942–9947, 2004. doi: 10.1128/MCB.24.22.9942-9947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim G, Selengut J, Levine RL. Carbonic anhydrase III: the phosphatase activity is extrinsic. Arch Biochem Biophys 377: 334–340, 2000. doi: 10.1006/abbi.2000.1793. [DOI] [PubMed] [Google Scholar]
- 31.Koester MK, Pullan LM, Noltmann EA. The p-nitrophenyl phosphatase activity of muscle carbonic anhydrase. Arch Biochem Biophys 211: 632–642, 1981. doi: 10.1016/0003-9861(81)90499-9. [DOI] [PubMed] [Google Scholar]
- 32.Koester MK, Register AM, Noltmann EA. Basic muscle protein, a third genetic locus isoenzyme of carbonic anhydrase? Biochem Biophys Res Commun 76: 196–204, 1977. doi: 10.1016/0006-291X(77)91686-2. [DOI] [PubMed] [Google Scholar]
- 33.Kusumoto K, Haist JV, Karmazyn M. Na+/H+ exchange inhibition reduces hypertrophy and heart failure after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 280: H738–H745, 2001. doi: 10.1152/ajpheart.2001.280.2.H738. [DOI] [PubMed] [Google Scholar]
- 34.Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol 458: 361–384, 1992. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol 517: 159–180, 1999. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehenkari P, Hentunen TA, Laitala-Leinonen T, Tuukkanen J, Väänänen HK. Carbonic anhydrase II plays a major role in osteoclast differentiation and bone resorption by effecting the steady state intracellular pH and Ca2+. Exp Cell Res 242: 128–137, 1998. doi: 10.1006/excr.1998.4071. [DOI] [PubMed] [Google Scholar]
- 37.Lewis SE, Erickson RP, Barnett LB, Venta PJ, Tashian RE. N-ethyl-N-nitrosourea-induced null mutation at the mouse Car-2 locus: an animal model for human carbonic anhydrase II deficiency syndrome. Proc Natl Acad Sci USA 85: 1962–1966, 1988. doi: 10.1073/pnas.85.6.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Alvarez B, Casey JR, Reithmeier RA, Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J Biol Chem 277: 36085–36091, 2002. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- 39.Lii CK, Chai YC, Zhao W, Thomas JA, Hendrich S. S-thiolation and irreversible oxidation of sulfhydryls on carbonic anhydrase III during oxidative stress: a method for studying protein modification in intact cells and tissues. Arch Biochem Biophys 308: 231–239, 1994. doi: 10.1006/abbi.1994.1033. [DOI] [PubMed] [Google Scholar]
- 40.Liu M, Walter GA, Pathare NC, Forster RE, Vandenborne K. A quantitative study of bioenergetics in skeletal muscle lacking carbonic anhydrase III using 31P magnetic resonance spectroscopy. Proc Natl Acad Sci USA 104: 371–376, 2007. doi: 10.1073/pnas.0609870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loiselle FB, Morgan PE, Alvarez BV, Casey JR. Regulation of the human NBC3 Na+/ cotransporter by carbonic anhydrase II and PKA. Am J Physiol Cell Physiol 286: C1423–C1433, 2004. doi: 10.1152/ajpcell.00382.2003. [DOI] [PubMed] [Google Scholar]
- 42.Lynch CJ, Hazen SA, Horetsky RL, Carter ND, Dodgson SJ. Differentiation-dependent expression of carbonic anhydrase II and III in 3T3 adipocytes. Am J Physiol Cell Physiol 265: C234–C243, 1993. doi: 10.1152/ajpcell.1993.265.1.C234. [DOI] [PubMed] [Google Scholar]
- 43.Lynch CJ, McCall KM, Billingsley ML, Bohlen LM, Hreniuk SP, Martin LF, Witters LA, Vannucci SJ. Pyruvate carboxylase in genetic obesity. Am J Physiol Endocrinol Metab 262: E608–E618, 1992. doi: 10.1152/ajpendo.1992.262.5.E608. [DOI] [PubMed] [Google Scholar]
- 44.Lyons GE, Buckingham ME, Tweedie S, Edwards YH. Carbonic anhydrase III, an early mesodermal marker, is expressed in embryonic mouse skeletal muscle and notochord. Development 111: 233–244, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Mallis RJ, Poland BW, Chatterjee TK, Fisher RA, Darmawan S, Honzatko RB, Thomas JA. Crystal structure of S-glutathiolated carbonic anhydrase III. FEBS Lett 482: 237–241, 2000. doi: 10.1016/S0014-5793(00)02022-6. [DOI] [PubMed] [Google Scholar]
- 46.Mitterberger MC, Kim G, Rostek U, Levine RL, Zwerschke W. Carbonic anhydrase III regulates peroxisome proliferator-activated receptor-γ2. Exp Cell Res 318: 877–886, 2012. doi: 10.1016/j.yexcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orlowski A, De Giusti VC, Morgan PE, Aiello EA, Alvarez BV. Binding of carbonic anhydrase IX to extracellular loop 4 of the NBCe1 Na+/ cotransporter enhances NBCe1-mediated influx in the rat heart. Am J Physiol Cell Physiol 303: C69–C80, 2012. doi: 10.1152/ajpcell.00431.2011. [DOI] [PubMed] [Google Scholar]
- 48.Patrikainen M, Pan P, Kulesskaya N, Voikar V, Parkkila S. The role of carbonic anhydrase VI in bitter taste perception: evidence from the Car6−/− mouse model. J Biomed Sci 21: 82, 2014. doi: 10.1186/s12929-014-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole RC, Halestrap AP, Price SJ, Levi AJ. The kinetics of transport of lactate and pyruvate into isolated cardiac myocytes from guinea pig. Kinetic evidence for the presence of a carrier distinct from that in erythrocytes and hepatocytes. Biochem J 264: 409–418, 1989. doi: 10.1042/bj2640409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Räisänen SR, Lehenkari P, Tasanen M, Rahkila P, Härkönen PL, Väänänen HK. Carbonic anhydrase III protects cells from hydrogen peroxide-induced apoptosis. FASEB J 13: 513–522, 1999. doi: 10.1096/fasebj.13.3.513. [DOI] [PubMed] [Google Scholar]
- 51.Renner SW, Walker LM, Forsberg LJ, Sexton JZ, Brenman JE. Carbonic anhydrase III (Car3) is not required for fatty acid synthesis and does not protect against high-fat diet induced obesity in mice. PLoS One 12: e0176502, 2017. doi: 10.1371/journal.pone.0176502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ronchi VP, Conde RD, Guillemot JC, Sanllorenti PM. The mouse liver content of carbonic anhydrase III and glutathione S-tranferases A3 and P1 depend on dietary supply of methionine and cysteine. Int J Biochem Cell Biol 36: 1993–2004, 2004. doi: 10.1016/j.biocel.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Roy P, Reavey E, Rayne M, Roy S, Abed El Baky M, Ishii Y, Bartholomew C. Enhanced sensitivity to hydrogen peroxide-induced apoptosis in Evi1 transformed Rat1 fibroblasts due to repression of carbonic anhydrase III. FEBS J 277: 441–452, 2010. doi: 10.1111/j.1742-4658.2009.07496.x. [DOI] [PubMed] [Google Scholar]
- 54.Sanyal G, Swenson ER, Pessah NI, Maren TH. The carbon dioxide hydration activity of skeletal muscle carbonic anhydrase. Inhibition by sulfonamides and anions. Mol Pharmacol 22: 211–220, 1982. [PubMed] [Google Scholar]
- 55.Scheibe RJ, Gros G, Parkkila S, Waheed A, Grubb JH, Shah GN, Sly WS, Wetzel P. Expression of membrane-bound carbonic anhydrases IV, IX, and XIV in the mouse heart. J Histochem Cytochem 54: 1379–1391, 2006. doi: 10.1369/jhc.6A7003.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schueler C, Becker HM, McKenna R, Deitmer JW. Transport activity of the sodium bicarbonate cotransporter NBCe1 is enhanced by different isoforms of carbonic anhydrase. PLoS One 6: e27167, 2011. doi: 10.1371/journal.pone.0027167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah GN, Rubbelke TS, Hendin J, Nguyen H, Waheed A, Shoemaker JD, Sly WS. Targeted mutagenesis of mitochondrial carbonic anhydrases VA and VB implicates both enzymes in ammonia detoxification and glucose metabolism. Proc Natl Acad Sci USA 110: 7423–7428, 2013. doi: 10.1073/pnas.1305805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi C, Uda Y, Dedic C, Azab E, Sun N, Hussein AI, Petty CA, Fulzele K, Mitterberger-Vogt MC, Zwerschke W, Pereira R, Wang K, Pajevic PD. Carbonic anhydrase III protects osteocytes from oxidative stress. FASEB J 32: 440–452, 2018. doi: 10.1096/fj.201700485RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shima K. Human muscle carbonic anhydrase III (CA-III). Purification, immunohistochemical localization in the human skeletal muscle and its clinical application to the neuromuscular disease. Hokkaido Igaku Zasshi 59: 98–116, 1984. [PubMed] [Google Scholar]
- 60.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 64: 375–401, 1995. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 61.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 276: 47886–47894, 2001. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 62.Sun B, Leem CH, Vaughan-Jones RD. Novel chloride-dependent acid loader in the guinea-pig ventricular myocyte: part of a dual acid-loading mechanism. J Physiol 495: 65–82, 1996. doi: 10.1113/jphysiol.1996.sp021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas JA, Poland B, Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys 319: 1–9, 1995. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- 64.Toomayan GA, Chen LE, Jiang HX, Qi WN, Seaber AV, Frank MM, Urbaniak JR. C1-esterase inhibitor and a novel peptide inhibitor improve contractile function in reperfused skeletal muscle. Microsurgery 23: 561–567, 2003. doi: 10.1002/micr.10210. [DOI] [PubMed] [Google Scholar]
- 65.Väänänen HK, Paloniemi M, Vuori J. Purification and localization of human carbonic anhydrase. III. Typing of skeletal muscle fibers in paraffin embedded sections. Histochemistry 83: 231–235, 1985. doi: 10.1007/BF00953989. [DOI] [PubMed] [Google Scholar]
- 66.Vargas LA, Díaz RG, Swenson ER, Pérez NG, Álvarez BV. Inhibition of carbonic anhydrase prevents the Na+/H+ exchanger 1-dependent slow force response to rat myocardial stretch. Am J Physiol Heart Circ Physiol 305: H228–H237, 2013. doi: 10.1152/ajpheart.00055.2013. [DOI] [PubMed] [Google Scholar]
- 67.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol 46: 318–331, 2009. doi: 10.1016/j.yjmcc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 68.Waheed A, Pham T, Won M, Okuyama T, Sly WS. Human carbonic anhydrase IV: in vitro activation and purification of disulfide-bonded enzyme following expression in Escherichia coli. Protein Expr Purif 9: 279–287, 1997. doi: 10.1006/prep.1996.0691. [DOI] [PubMed] [Google Scholar]
- 69.Wroblewski K, Spalthoff S, Zimmerman UJ, Post RL, Sanger JW, Forster RE. The role of carbonic anhydrase in the recovery of skeletal muscle from anoxia. J Appl Physiol (1985) 99: 488–498, 2005. doi: 10.1152/japplphysiol.01409.2004. [DOI] [PubMed] [Google Scholar]
- 70.Xu P, Spitzer KW. Na-independent Cl−- exchange mediates recovery of pHi from alkalosis in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 267: H85–H91, 1994. doi: 10.1152/ajpheart.1994.267.1.H85. [DOI] [PubMed] [Google Scholar]
- 71.Yamada M, Shiroeda H, Hayashi R, Yano H, Sato K, Tsutsumi M, Arisawa T. Survival rates of early-stage HCV-related liver cirrhosis patients without hepatocellular carcinoma are decreased by alcohol. J Clin Biochem Nutr 48: 167–169, 2011. doi: 10.3164/jcbn.09-119GFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang F, Xu X, Zhou B, He Z, Zhai Q. Gene expression profile change and associated physiological and pathological effects in mouse liver induced by fasting and refeeding. PLoS One 6: e27553, 2011. doi: 10.1371/journal.pone.0027553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng A, Rahkila P, Vuori J, Rasi S, Takala T, Väänänen HK. Quantification of carbonic anhydrase III and myoglobin in different fiber types of human psoas muscle. Histochemistry 97: 77–81, 1992. doi: 10.1007/BF00271284. [DOI] [PubMed] [Google Scholar]
- 74.Zimmerman UJ, Wang P, Zhang X, Bogdanovich S, Forster R. Anti-oxidative response of carbonic anhydrase III in skeletal muscle. IUBMB Life 56: 343–347, 2004. doi: 10.1080/1521-6540400000850. [DOI] [PubMed] [Google Scholar]