Abstract
Activation of calpain 1 (CPN1) and calpain 2 (CPN2) contributes to cardiac injury during ischemia (ISC) and reperfusion (REP). Complex I activity is decreased in heart mitochondria following ISC-REP. CPN1 and CPN2 are ubiquitous calpains that exist in both cytosol (cs)-CPN1 and 2 and mitochondria (mit)-CPN1 and 2. Recent work shows that the complex I subunit (NDUFS7) is a potential substrate of the mit-CPN1. We asked whether ISC-REP led to decreased complex I activity via proteolysis of the NDUFS7 subunit via activation of mit-CPN1 and -2. Activation of cs-CPN1 and -2 decreases mitophagy in hepatocytes following ISC-REP. We asked whether activation of cs-CPN1 and -2 impaired mitophagy in the heart following ISC-REP. Buffer-perfused rat hearts underwent 25 min of global ISC and 30 min of REP. MDL-28170 (MDL; 10 µM) was used to inhibit CPN1 and -2. Cytosol, subsarcolemmal mitochondria (SSM), and interfibrillar mitochondria (IFM) were isolated at the end of heart perfusion. Cardiac ISC-REP led to decreased complex I activity with a decrease in the content of NDUFS7 in both SSM and IFM. ISC-REP also resulted in a decrease in cytosolic beclin-1 content, a key component of the autophagy pathway required to form autophagosomes. MDL treatment protected the contents of cytosolic beclin-1 and mitochondrial NDUFS7 in hearts following ISC-REP. These results support that activation of both cytosolic and mitochondrial calpains impairs mitochondria during cardiac ISC-REP. Mitochondria-localized calpains impair complex I via cleavage of a key subunit. Activation of cytosolic calpains contributes to mitochondrial dysfunction by impairing removal of the impaired mitochondria through depletion of a key component of the mitophagy process.
Keywords: calpain inhibitor, electron transport chain, mitochondria, NADH:ubiquinone oxidoreductase
INTRODUCTION
Mitochondrial dysfunction contributes to cell injury in multiple pathologic conditions, including ischemia (ISC) and reperfusion (REP). The mitochondrial electron transport chain (ETC) is impaired in hearts following ISC-REP (38). Complex I is the first respiratory complex of the ETC (35). Complex I is an L-shaped molecular complex, with the membrane arm embedded in the inner mitochondrial membrane and the other peripheral arm extending into the matrix compartment (47). The peripheral arm includes seven NADH:ubiquinone oxidoreductase core subunits (NDUFV1, NDUFV2, NDUFS1, NDUFS2, NDUFS3, NDUFS7, and NDUFS8) that are essential for NADH oxidation and subsequent electron transfer through the complex (47). The initial three subunits are required for NADH oxidation, and the last four subunits contribute key roles in transferring electrons to ubiquinone (47). NDUFS7 is a nuclear-encoded subunit that transfers electrons to the N2 subunit and subsequently to ubiquinone (62). Mutation of the NDUFS7 gene in humans is associated with Leigh syndrome with a severe complex I defect (36). A deficiency of the NDUFS7 also impairs the formation of the juncture between peripheral and membrane arms of complex I (59). Taken together, the NDUFS7 subunit is essential to maintain complex I activity.
Calpain 1 (CPN1) and calpain 2 (CPN2) are two ubiquitous Ca2+-dependent cysteine proteases (7, 52) existing in cytosol and mitochondria (7, 64). Activation of the mitochondria-localized CPN1 (mit-CPN1) and CPN2 (mit-CPN2) impairs mitochondria during ISC-REP (3, 11, 43, 49, 64, 70). In rat heart mitochondria, activation of mitochondrial CPN2 (mit-CPN2) sensitizes to mitochondrial permeability transition pore (MPTP) opening during REP (64). Activation of mit-CPN1 cleaves apoptotic inducing factor (AIF) in the mitochondrial intermembrane space to truncated AIF that is then released into the cytosol to activate caspase-independent cell death (11, 76). Pyruvate dehydrogenase (PDH) α1-subunit, located in the mitochondrial matrix, is also cleaved by mit-CPN1 during ISC-REP (70). The complex I subunit (NDUFS7) content is decreased in cardiac mitochondria incubated with exogenous calcium (25 μM) (12). Genetic elimination of CPN1 and CPN2 activities protects NDUFS7 in the calcium-treated mitochondria (12). These results support that activation of mitochondrial-localized calpains leads to decreased NDUFS7 subunit content. Complex I activity is decreased in rat heart mitochondria following ISC-REP (9). In the present study, we asked whether activated mitochondria-localized calpains led to decreased complex I activity during ISC-REP by cleaving the NDUFS7 subunit.
There are two populations of cardiac mitochondria: subsarcolemmal mitochondria (SSM) located underneath the plasma membrane and interfibrillar mitochondria (IFM) located among the myofibrils (40, 55). ISC-REP impairs the ETC in both SSM and IFM from rat hearts (9). The decreased complex I activity and increased MPTP susceptibility occur in both SSM and IFM following ISC-REP (10). Compared with SSM, IFM have a greater calcium tolerance (54) and are more resistant to ischemic damage (42). The contribution of mitochondrial-localized CPN1 and CPN2 on complex I impairment was studied. The role of calpains in the potential impairment of the mitophagy pathway was evaluated. Impaired mitophagy allows the complex I defect-laden mitochondria to persist during ISC-REP.
The impaired mitochondria are sources of cardiac injury during ISC-REP by increasing the generation of reactive oxygen species (ROS), opening of the MPTP, and the release of cytochrome c and AIF (apoptosis inducing factor) from mitochondria into cytosol to activate cell death programs (37, 38, 63). Thus, timely removal of the impaired mitochondria is critical to decrease cardiac injury (63). Mitophagy is a macroautophagic process that removes the dysfunctional mitochondria. Impaired mitophagy contributes to increased cell injury in liver during ISC-REP (24, 71) and the development of diabetic cardiomyopathy (77). Improvement of mitophagy with anesthetic postconditioning decreases cardiac injury during ISC-REP (75). Mitophagy is regulated by autophagy-related proteins, including beclin-1 and Atg7 (autophagy-related protein 7) (33). Both beclin1 and Atg7 are calpain substrates (33, 34). Activation of cytosolic calpains (78) impairs mitophagy in hepatocytes following ISC-REP by cleaving beclin-1 and Atg 7. Enhancement of mitophagy with overexpression of beclin-1 protects liver mitochondria during ISC-REP (33). These results indicate that calpain-mediated cleavage of beclin-1 contributes a key role in the impairment of mitophagy during ISC-REP. We asked whether inhibition of ubiquitous calpains using MDL-28170 (MDL) to inhibit CPN1 and -2 can decrease cardiac injury during ISC-REP by protection of components of the mitophagy pathway to hell.
METHODS
Preparation of rat hearts for perfusion.
The Institutional Animal Care and Use Committees of the McGuire VA Medical Center and Virginia Commonwealth University approved the study. Sprague-Dawley (SD) rats (3–4 mo of age) were anesthetized with pentobarbital sodium (100 mg/kg ip) and anticoagulated with heparin (1,000 IU/kg ip). Rat hearts were isolated and perfused in the Langendorff mode with modified Krebs-Henseleit (K-H) buffer (115 mM NaCl, 4.0 mM KCl, 2.5 mM CaCl2, 26 mM NaHCO3, 1.1 mM MgSO4, 0.9 mM KH2PO4, 5.5 mM glucose, and 5 IU insulin/L), oxygenated with 95% O2-5% CO2 (9). Hearts were paced at 300 beats/min during a 15-min equilibration period and after 15 min of REP. Pacing was discontinued during ISC and the initial 15 min of REP. Cardiac function was monitored during the entire experiment, with a balloon inserted into the left ventricle, and data were recorded digitally (AD Instruments, Colorado Springs, CO). In the untreated ISC-REP group, hearts were perfused for 15-min equilibration with K-H buffer, including 0.001% DMSO as vehicle, followed by 25 min of global ISC at 37°C and 30 min of REP. In the MDL-treated hearts, MDL (10 μM) was included in the perfusion buffer during the entire experimental period. The entire perfusion time in ISC-REP hearts with or without MDL treatment was 70 min. In the time control group, hearts were buffer perfused without ISC-REP. The entire perfusion time in time control group was 45 min (excluding 25 min ISC time). Coronary effluent was collected during the entire 30-min REP period in the untreated and MDL groups and during the last 30 min of the perfusion period and in the time control group for lactate dehydrogenase (LDH) measurement (9). At the end of the experiment, the heart was harvested for mitochondrial isolation.
Isolation of rat heart mitochondria.
The heart was removed from the perfusion cannula at the end of perfusion and placed into buffer A [100 mM KCl, 50 mM 3-(N-morpholino) propanesulfonic acid (MOPS), 1 mM EGTA, 5 mM MgSO4·7 H2O, and 1 mM ATP, pH 7.4] at 4°C. Mitochondria were isolated using the procedure of Palmer et al. (55), except that trypsin was used as the protease (6). Cardiac tissue was finely minced and placed in buffer A containing 0.2% bovine serum albumin and homogenized with a polytron tissue processor (Brinkman Instruments, Westbury, NY) for 2.5 s at the setting of 6.0. The polytron homogenate was centrifuged at 500 g, the supernatant was saved for isolation of SSM, and the pellet was washed. The combined supernatants were centrifuged at 3,000 g to sediment SSM. IFM were isolated by incubation of skinned myofibers, obtained after polytron treatment, with 5 mg/g (wet wt) trypsin for 10 min at 4°C. SSM and IFM were washed twice and then suspended in 100 mM KCl, 50 mM MOPS, and 0.5 mM EGTA. Mitochondrial protein content was measured by the Lowry method, using bovine serum album as a standard. To identify the localization of mit-CPN1 within mitochondria, mitochondrial outer membrane (OMM), intermembrane space (IMS), inner membrane (IMM), and matrix (MTR) were separated using our published methods (7).
Mitochondrial oxidative phosphorylation and enzyme activity.
Oxygen consumption in mitochondria was measured using a Clark-type oxygen electrode at 30°C, as previously described (42). Mitochondria were incubated in 80 mM KCl, 50 mM MOPS, 1 mM EGTA, 5 mM KH2PO4, and 1 mg/ml defatted, dialyzed bovine serum albumin at pH 7.4. Glutamate (20 mM, complex I substrate), succinate (20 mM) plus 7.5 μM rotenone (complex II substrate), and TMPD (N,N,N’,N’ tetramethyl p-phenylenediamine, 1 mM)-ascorbate (10 mM, complex IV substrate) plus rotenone were used. Enzyme activities of the ETC were determined using previously published methods (9).
Calcium retention capacity in isolated mitochondria.
Calcium retention capacity (CRC) was used to assess calcium-induced mitochondrial permeability transition pore opening in isolated mitochondria (53). Mitochondria (125 μg/ml) were incubated in medium containing 150 mM sucrose, 50 mM KCl, 2 mM KH2PO4, and 5 mM succinate in 20 mM Tris·HCl, pH 7.4, by sequential pulses of a known amount of calcium (5 nmol). The calcium retention capacity is greater in mitochondria oxidizing a complex II substrate compared with a complex I substrate (45, 46). Thus, succinate was used as substrate for CRC measurement. Extramitochondrial Ca2+ concentration was recorded with 0.5 µM Calcium Green-5N and fluorescence monitored with excitation and emission wavelengths set at 500 and 530 nm, respectively.
Western blotting.
Mitochondrial proteins were separated using 12 or 4–15% Tris-glycine gels (Bio-Rad, Hercules, CA) and transferred to PVDF membrane (Fisher Scientific, Hampton, NH) using semidry transfer (Bio-Rad). The molecular weight marker mix used was purchased form Bio-Rad Precision Plus Protein Dual Color Standards (10–250 kDa; catalog no. 1610374). The blots were incubated for 1 h at room temperature in 5% (wt/vol) nonfat dry milk (Bio-Rad) in TBST buffer (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween20), followed by an overnight incubation at 4°C with primary antibody. Primary antibody information is listed in Table 1. After 1-h incubation at room temperature with a 1:10,000 dilution of horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG F(ab)2 (GE Healthcare Life Sciences, Piscataway, NJ), blots were developed using ECL Plus Western Blotting Detection Reagents (GE Healthcare Life Sciences, Piscataway, NJ). Membranes were digitally analyzed (Bio-Rad, Hercules, CA) using Image Lab 6.0 software. Background intensity adjustment, if performed, was always adjusted for the entire membrane. For the preparation of figures, membranes were cut horizontally.
Table 1.
Antibodies used in the current manuscript
| Antibody Name | Company | Catalog No. | Concentration |
|---|---|---|---|
| AIF | Cell Signaling Technology | 4642 | 1:1,000 |
| Beclin 1 | Cell Signaling Technology | 3495 | 1:1,000 |
| Calpain 1 | ThermoFisher Scientific | MA3-940 | 1:1,000 |
| Complex IV (subunit 4) | Cell Signaling Technology | 4844 | 1:1,000 |
| Complex V | ThermoFisher Scientific | A-21351 | 1:2,500 |
| Cytochrome c | ThermoFisher Scientific | 710627 | 1:2,500 |
| GAPDH | Cell Signaling Technology | 5174 | 1:1,000 |
| LC3A/B | Cell Signaling Technology | 4108 | 1:1,000 |
| LC3B | Cell Signaling Technology | 2775 | 1:1,000 |
| NDUFS7 | ThermoFisher Scientific | PA5-19343 | 1:500 |
| PDHα1 | Cell Signaling Technology | C54G1 | 1:1,000 |
| Spectrin | Santa Cruz Biotechnology | csc-46696 | 1:100 |
| VDAC | Abcam | 14715 | 1:2,500 |
AIF, apoptotic inducing factor; LC3A/B, microtubule-associated protein 3A/B; NDUFS7, NADH:ubiquinone oxidoreductase core subunit S7; PDHα1, pyruvate dehydrogenase-α1; VDAC, voltage-dependent anion channel.
Statistical analysis.
Data are expressed as means ± SE (66). For all analyses, differences between groups (≥3 groups) were compared by one-way ANOVA. When a significant F value was obtained, means were compared using the Student-Newman-Keuls test of multiple comparisons. Differences between two groups were compared by unpaired Student’s t-test (SigmaStat 3.5; Systat, Richmond, CA). Statistical significance was defined as a value of P < 0.05.
RESULTS
Inhibition of calpain decreases cardiac injury in buffer-perfused hearts during ISC-REP.
There were no differences in left ventricular developed pressure (LVDP; mmHg) or left ventricular end-diastolic pressure (LVEDP; mmHg) before ISC between the time control (TC) and ISC-REP groups (Fig. 1, A and B). MDL treatment led to a slightly decreased LVDP and LVEDP compared with TC or ISC-REP before ISC (Fig. 1, A and B), suggesting that MDL (10 uM) had a slight inhibition of cardiac function before ISC. This mild inhibition of cardiac function by MDL before ISC might contribute to decreased cardiac injury during REP. ISC-REP impaired cardiac function, as shown by the decreased LVDP and the increased LVEDP during 30 min of REP. Compared with untreated ISC-REP hearts, MDL treatment improved cardiac function with better recovery of LVDP and LVEDP during REP (Fig. 1, A and B). Compared with time control, the cardiac injury in the untreated hearts was associated with increased LDH release into coronary effluent in untreated hearts following ISC-REP. The LDH content was significantly decreased in MDL-treated hearts (Fig. 1C). These results clearly support that inhibition of calpain decreases cardiac injury in rat hearts following ISC-REP.
Fig. 1.
MDL-28170 (MDL) treatment decreased cardiac injury during ischemia (ISC)-reperfusion (REP). Compared with time control (TC), MDL treatment slightly decreased left ventricular developed pressure (LVDP, mmHg) and left ventricular end-diastolic pressure (LVEDP, mmHg) before ischemia (A and B). ISC-REP markedly decreased LVDP (A) and increased LVEDP (B) compared with time control. MDL treatment improved LVDP and decreased LVEDP compared with vehicle-treated hearts following ISC-REP (A and B). ISC-REP markedly increased the release of LDH into the coronary effluent compared with time control. MDL treatment decreased the LDH release during REP (C). Data are expressed as means ± SE. *P < 0.05 vs. control; †P < 0.05 vs. untreated; n = 6 in each group. D shows the localization of the mitochondrial (mit)-calpain 1 (CPN1) in mitochondrial components isolated from rat heart mitochondria. The mit-CPN1 band (top band, over 75 kDa) was detected in the intermembrane space (IMS) and the matrix (MTR). Voltage-dependent anion channel (VDAC), cytochrome c, complex V, and pyruvate dehydrogenase (PDH) were used as markers of the outer membrane (OMM), the IMS, the inner membrane (IMM), and the MTR, respectively. EQ, equilibration; IR, ischemia-reperfusion; LDH, lactate dehydrogenase; MW, molecular weight.
Localization of calpain1 within mitochondria.
Calpain 1 includes an isoform-specific, 80 kDa large subunit and a common, 28 kDa small regulatory subunit (also known as calpain 4). The large subunit of CPN1 (mit-CPN1; arrow in Fig. 1D) was found in the mitochondrial intermembrane space (IMS) and the matrix (MTR). The mit-CPN1 was not found in the outer membrane (OMM) or inner membrane (IMM) (Fig. 1D). VDAC (voltage-dependent anion channel) was used as an OMM marker. Some VDAC was also found in the IMM. This may be due to the presence of contact sites where OMM and IMM merge (29). Subunit-α of complex V was used as the IMM maker that was detected only in the IMM. Cytochrome c and PDH were used as markers of the IMS and MTR, respectively (7).
MDL treatment protects mitochondrial function during ISC-REP.
ISC-REP markedly decreased the rate of oxidative phosphorylation in both SSM and IFM with glutamate as complex I substrate (Fig. 2, A and D), succinate as complex II substrate (Fig. 2, B and E), and TMPD-ascorbate as complex IV (Fig. 2, C and F) compared with time control. MDL treatment attenuated injury to mitochondria reflected in improved oxidative phosphorylation in both SSM and IFM with complexes I, II, and IV substrates (Fig. 2).
Fig. 2.
MDL-28170 (MDL) improved oxidative phosphorylation in both subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) following ischemia (ISC)-reperfusion (REP). Compared with time control, ISC-REP decreased the rate of oxidative phosphorylation in SSM using glutamate, succinate, and tetramethyl p-phenylenediamine (TMPD)-ascorbate as complex I (A), complex II (B), and complex IV (C) substrates, respectively. MDL treatment attenuated the ISC-REP-induced decreases in the rate of oxidative phosphorylation with each substrate (A–C). MDL treatment also protected oxidative phosphorylation in IFM following ISC-REP using complex I (D), II (E), and IV (F) substrates. Data are expressed as means ± SE. *P < 0.05 vs. time control; †P < 0.05 vs. vehicle-treated hearts; n = 6 in each group. IR, ischemia-reperfusion.
The activities of complex I and citrate synthase were measured in detergent-solubilized mitochondria. The complex I activity in both SSM and IFM was significantly decreased in mitochondria following ISC-REP compared with time control (Fig. 3, A and B). However, citrate synthase activity was not altered in SSM and IFM following ISC-REP (Fig. 3, C and D), indicating that the decreased complex I activity is not due to loss of mitochondrial content. MDL treatment dramatically protected complex I activity in both SSM and IFM compared with untreated hearts following ISC-REP (Fig. 3, A and B).
Fig. 3.
MDL-28170 (MDL) treatment protected complex I activity and decreased mitochondrial permeability transition pore (MPTP) opening in both subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) following following ischemia (ISC)-reperfusion (REP). Compared with time control (TC), ISC-REP led to decreased complex I activity in both SSM (A) and IFM (B). MDL treatment improved complex I activity in both SSM and IFM compared with vehicle-treated hearts (A and B). There were no differences in citrate synthase activity among groups (C and D). Compared with time control, ISC-REP increased MPTP opening in both SSM (E) and IFM (F). MDL decreased MPTP opening in both SSM and IFM when succinate was used as complex II substrate compared with vehicle-treated hearts (E and F). Data are expressed as means ± SE. *P < 0.05 vs. TC, †P < 0.05 vs. vehicle treatment; n = 6 in each group for mitochondria. CRC, calcium retention capacity; CS, citrate synthase.
Administration of MDL decreases the MPTP opening during ISC-REP.
Succinate was used to energize mitochondria in that complex II is relatively resistant to exogenous calcium-induced damage (45, 46). In the time control group, the CRC in IFM was greater than that in SSM, supporting that IFM can tolerate more calcium loading in the baseline state (56). Compared with time control, ISC-REP significantly decreased the CRC in both SSM and IFM (Fig. 3, E and F). MDL treatment significantly improved the CRC compared with untreated hearts following ISC-REP (Fig. 3, E and F). These results suggest that activation of calpains during ISC-REP sensitizes to MPTP opening in both SSM and IFM.
Administration of MDL decreases cytosolic and mitochondrial calpain activation during ISC-REP.
ISC-REP activates cytosolic calpains in a number of animal models (31). The cleaved product of spectrin is commonly used as a marker of cytosolic calpain activation. Although ISC-REP did not alter full-length spectrin content compared with time control, it increased the formation of the cleaved spectrin compared with time control (Fig. 4, A–C). MDL treatment decreased the formation of the cleaved spectrin in hearts following ISC-REP (Fig. 4, A and C).
Fig. 4.
MDL-28170 (MDL) prevented cytosolic and mitochondrial calpain activation. There were no differences in the content of full length of spectrin between groups (A and B). Ischemia (ISC)-reperfusion (REP) increased cytosolic calpain activity, as shown by the increased content of the cleaved spectrin in cytosol compared with time control (TC) (A and C). MDL prevented cytosolic calpain (CPN) activation, as shown by the decreased content of the cleaved spectrin (A and C). A decrease in apoptotic inducing factor (AIF) content is used as an indicator of mitochondrial (mit)-CPN1 activation. ISC-REP led to decreased AIF content in both subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) compared with TC (A, D, and E), indicating that ISC-REP activated the mit-CPN1. MDL treatment preserved the AIF content in both SSM and IFM, suggesting that MDL is sufficient to inhibit the mit-CPN1 (A, D, and E). Data are expressed as means ± SE. *P < 0.05 vs. time control, †P < 0.05 vs. vehicle treatment; n = 4 in each group. MW, molecular weight.
Activation of mitochondrial calpain 1 cleaves AIF to truncated AIF that is released into the cytosol. Thus, a decrease in mitochondrial AIF content is used as a marker of the mit-CPN1 activation. Compared with time control, ISC-REP led to decreased AIF content in both SSM and IFM (Fig. 4, A, D and E). MDL treatment tended to improve AIF content in SSM (P = 0.058). MDL treatment did improve the AIF content in IFM compared with untreated hearts (Fig. 4, A and E). These results indicate that MDL treatment decreases the activation of both cytosolic and mitochondrial calpain activation during ISC-REP. Protein contents of the cytosolic and mitochondrial CPN1 and CPN2 were not altered in hearts following ISC-REP (data not shown).
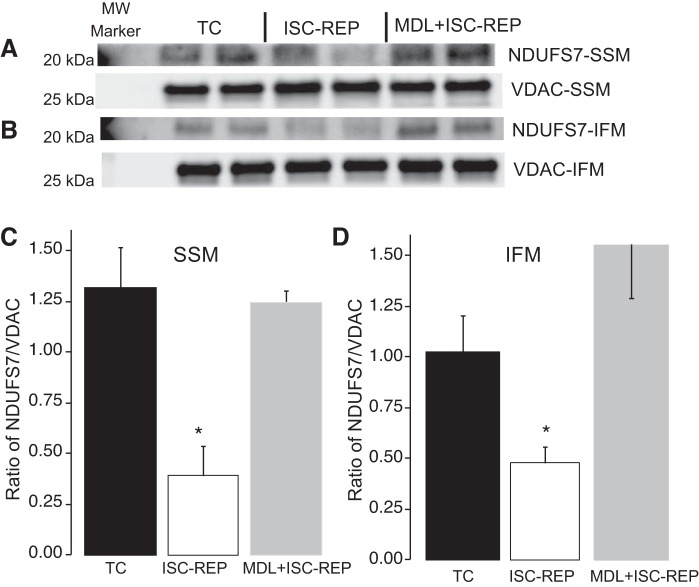
Administration of MDL prevents the cleavage of a complex I subunit during ISC-REP.
NDUFS7 is one of the iron/sulfur-containing protein subunits within complex I and a catalytic center that participates in electron flow through the complex (47). Compared with time control, ISC-REP decreased the content of the NDUFS7 subunit in both SSM (Fig. 5, A and C) and IFM (Fig. 5, B and D). MDL treatment protected the NDUFS7 subunit in both SSM and IFM following ISC-REP (Fig. 5). These results suggest that the calpain-mediated depletion of this complex I subunit contributes to the complex I impairment and decrease in activity during ISC-REP.
Fig. 5.
MDL-28170 (MDL) protected NADH:ubiquinone oxidoreductase core subunit S7 (NDUFS7) in both subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) following ischemia (ISC)-reperfusion (REP). ISC-REP decreased NDUFS7 content in both SSM (A and C) and IFM (B and D) compared with time control (TC). MDL protected the NDUFS7 content in SSM (A and C) and IFM (B and D) following ISC-REP. Because the intensity of the molecular weight (MW) marker used in the NDUFS7 blotting was too strong, only a partial marker was shown on the blotting image. Data are expressed as means ± SE. *P < 0.05 vs. TC; n = 4 in each group. VDAC, voltage-dependent anion channel.
Administration of MDL preserves the capacity for mitophagy during ISC-REP.
Activation of autophagy converts cytosolic microtubule-associated protein 1A/1B light chain 3 [LC3-A (I)] to a lipidated and membrane-bound form [LC3-B (II)]. Thus, an increased ratio of LC3-B/A is used as an indicatorof autophagy activation (23, 24). Beclin-1 is a key component required to form the autophagosome, and the decrease in beclin1 content is used as an indicator of impaired autophagy in liver (33, 34).
Compared with time control, ISC-REP markedly decreased the beclin-1 content in cytosol (Fig. 6, A and B). The ratio of LC3B/A was also decreased in cytosol following ISC-REP compared with time control (Fig. 6, A and C). These results indicated that the autophagy process was impaired in rat hearts following global ISC-REP. MDL treatment protected the beclin-1 content in hearts following ISC-REP (Fig. 6, A and B). MDL tended to increase the ratio of LC3B/A compared with untreated hearts following ISC-REP (Fig. 6, A and C). These results suggest that activation of CPN1 and -2 contributes to impairment of autophagy during ISC-REP via depletion of key proteins in the autophagy pathway.
Fig. 6.
MDL-28170 (MDL) preserved the capacity for mitophagy in subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) following ischemia (ISC)-reperfusion (REP). ISC-REP decreased beclin-1 content (A and B) and the ratio of microtubule-associated protein 3B (LC3B) to LC3A in cytosol (A and C) compared with time control (TC). MDL protected beclin-1 content and the ratio of LC3B/A in cytosol. ISC-REP decreased LC3B content in SSM (D and E) and IFM (D and F) compared with time control. MDL treatment increased LC3B content in SSM and IFM following ISC-REP. Data are expressed as means ± SE. *P < 0.05 vs. TC, †P < 0.05 vs. vehicle treatment; n = 4 in each group. IR, ischemia-reperfusion; MW, molecular weight.
LC3B is a lipidated protein that is conjugated with the membrane through interaction with phosphatidylethanolamine (23). LC3B is a key component of the phagophore that engulfs the dysfunctional mitochondria (23). Thus, LC3B plays a key role in the transport of the impaired mitochondria to the lysosome for degradation. LC3B content was determined in isolated SSM and IFM with or without ISC-REP. LC3B was found in both SSM and IFM from time control hearts (Fig. 6D). Compared with time control, LC3B content was markedly decreased in both SSM (Fig. 6, D and E) and IFM (Fig. 6, D and F) following ISC-REP. MDL treatment maintained the LC3B content in SSM and improved its content in IFM compared with untreated hearts. These results show that activation of the cytosolic CPN1/2 contributes to decreased LC3B content in mitochondria following ISC-REP.
DISCUSSION
Activation of cytosolic CPN1 and CPN2 increases cardiac injury during ISC-REP by cleaving cytosolic and membrane proteins (5, 11, 70). In the present study, we found that activation of the cytosolic CPN1 and CPN2 likely impaired the capacity for mitophagy in hearts following ISC-REP. Activation of mit-CPN1 and CPN2 contributes to mitochondrial impairment during ISC-REP (5, 11, 64, 70). In the current study, we found that MDL treatment improved the CRC in both SSM and IFM following ISC-REP, supporting that activation of mitochondrial calpains sensitizes to MPTP opening. Compared with untreated ISC-REP hearts, MDL treatment improves oxidative phosphorylation using substrates selective for complexes I, II, and IV, supporting that inhibition of mitochondrial calpains protects the ETC. In addition, we found that activation of the mit-CPN1/2 led to decreased complex I activity in both SSM and IFM accompanied by cleavage of the NDUFS7 subunit. Our study shows that activation of mit-CPN1/2 directly impairs the respiratory chain, whereas activation of the cytosolic CPN1/2 augments accumulation of damaged mitochondria by potentially impairing the removal of dysfunctional mitochondria. Pharmacologic inhibition of calpain can be an intriguing strategy to protect complex I during ISC-REP (Fig. 7).
Fig. 7.
Depiction of the role of activated cytosolic and mitochondrial calpain (CPN)1/2 in mitochondrial damage during ischemia (ISC)-reperfusion (REP). ISC-REP-mediated calcium overload activated cytosolic CPN1/2 (cCPN1/2) and mitochondrial CPN1/2 (mCPN1/2). Spectrin is a substrate of cCPN1/2, and an increased content of the cleaved spectrin is used as a marker of cCPN1/2 activation. The cCPN1/2 activation led to mitochondrial dysfunction by impairing mitophagy through degradation of cytosolic proteins, including beclin-1. The decreased content of beclin-1 caused less microtubule-associated protein 3B (LC3B) translocation to the mitochondria and impaired mitophagy during ISC-REP. Activation of mCPN1/2 in mitochondrial intermembrane space cleaved apoptotic inducing factor (AIF) to truncated AIF (t-AIF) that was released into cytosol and nucleus to induce caspase-independent apoptosis. The increased t-AIF content is used as a marker of the mCPN1 activation. Activation of mCPN1/2 in mitochondrial matrix impaired mitochondrial function by degrading metabolic enzymes and complex I subunits, including NADH:ubiquinone oxidoreductase core subunit S7 (NDUFS7). MDL-28170 treatment improved mitochondrial function by inhibiting cCPN1/2 and mCPN1/2 during ISC-REP.
ISC-REP leads to activation of cytosolic (31) and mitochondrial calpains (11). An increase in cytosolic cleaved spectrin content is used as an index of cytosolic CPN1 and CPN2 activation (31). In the present study, ISC-REP increased the formation of the cleaved spectrin, whereas MDL treatment decreased the cleaved spectrin content in isolated hearts. These results support that MDL is sufficient to prevent the activation of CPN1 and CPN2 during ISC-REP.
The mit-CPN1 is identified in the mitochondrial intermembrane space (52) and matrix (7). Our study confirms that mit-CPN1 is located in rat heart mitochondrial intermembrane space and matrix (Fig. 1D). Activation of mit-CPN1 within the mitochondrial intermembrane space augments cardiac injury during reperfusion by inducing a translocation of the truncated AIF from mitochondria to cytosol and nucleus (11). Therefore, a reduced AIF content or an increased truncated AIF content is an indicator of mit-CPN1 activation (11, 52), at least in the intermembrane space compartment. ISC-REP leads to a decreased AIF content in both SSM and IFM, whereas MDL treatment protects the AIF content in SSM and IFM following ISC-REP. These results show that ISC-REP activates the mit-CPN1 located in the intermembrane space compartment and that MDL treatment efficiently prevents the mit-CPN1 activation at this site.
Complex I activity is decreased in heart mitochondria following ex vivo and in vivo ISC-REP (18, 39, 61). In buffer-perfused rat hearts, ISC leads to a decrease in complex I activity (9). NADH dehydrogenase is the first component of complex I in the sequence of electron flow and is located in the matrix arm of the complex (Fig. 7) (50, 60). Ischemia does not decrease NADH dehydrogenase activity in either SSM or IFM in buffer-perfused rat hearts, indicating that the defect is located in sites distal to the NADH dehydrogenase portion of complex I (8, 9). The mechanisms of complex I impairment during ISC include subunit damage (60) and posttranslational modifications, including S-nitrosylation (2, 15, 48, 65), phosphorylation (13), intercellular acidification (74), and alteration of chaperone proteins (68). Complex I activity is also decreased by a conformational change leading to a transition from its “active” form to the “deactive” form (19). Tightly bound lipids, including cardiolipin, also play a role in stabilizing interactions between the subunits (16). Alteration of cardiolipin content during ISC-REP also decreases complex I activity in cardiac mitochondria (57). In isolated guinea pig hearts, ISC led to decreased complex I activity accompanied by decreased cardiolipin content (17). Interestingly, REP led to slightly recovered complex I activity compared with ISC alone. However, cardiolipin content was decreased further in mitochondria following REP (17). Thus, the decreased complex I activity is not solely dependent on cardiolipin content. The decreased complex I subunit (NDUFA9) may contribute to decreased complex I activity in this study (17). In the rabbit heart, ISC decreased cardiolipin content in mitochondria that was not associated with a decrease in complex I activity (41, 42). The complex I defect remains present in detergent-solubilized mitochondria in the presence of exogenous asolectin, a mimic of mitochondrial phospholipids. This result suggests that the decreased complex I activity is less likely due to the decreased cardiolipin content (42). Thus, although decreases in cardiolipin content clearly occur (17, 41, 57), other mechanisms likely account for the decrease in complex I activity. In the current study, we found that ISC-REP led to decreased complex I activity accompanied by decreased subunit NDUFS7 content in both SSM and IFM.
NDUFS7 is one of the iron/sulfur clusters within complex I oriented toward the mitochondrial matrix. The matrix-localized mit-CPN1 has the potential to access the NDUFS7 subunit located in the peripheral arm oriented into the matrix (Fig. 7). Inhibition of mit-CPN1 protected complex I activity and the NDUFS7 subunit content. These results provide evidence that activation of mit-CPN1 leads to complex I damage by cleaving NSDUFS7. In addition to SSM, these alterations were observed in IFM. IFM was isolated using protease treatment that removes potential cytosolic calpain contamination. Thus, the cleaved NDUFS7 is not due to the presence of cytosolic calpain contamination on the outer mitochondrial membrane. These results support that activation of the matrix-localized mit-CPN1 contributes to complex I damage in heart mitochondria. Although MDL-28170 inhibits mainly calpain 1, it also decreases calpain 2 activity (7). In addition, both calpain 1 and calpain 2 are found In heart mitochondria (64, 70). Thus, the potential role of the mit-CPN2 activation in complex I damage to NDUFS7 cannot be excluded. The use of a selective calpain 1 inhibitor or genetic deletion of calpain 1 will help to further clarify the role of the mit-CPN1 in complex I damage.
Complex I activity is also decreased by a conformational change (shifting from the normal “active” A-form to the “deactive” dormant D-form) during ISC (19, 20, 22). The A to D transition is potentially reversible, and its mechanism is a sulfhydryl oxidation of a key cysteine in complex I subunit 9 (19). Activation of the mit-CPN1 may alter complex I activity by facilitating A to D transition during ISC-REP. The effect of the posttranslational modification or A to D form transition on complex I activity is reversible. However, complex I activity is decreased during ISC and persists during REP. These results indicate that ISC-REP leads to persistent impairment to a proportion of the complex I that is beyond merely the A to D transition (9, 38). The current study shows that ISC-REP leads to decreased complex I activity through degradation of at least the NDUFS7 subunit. The proteolytic degradation of a complex I subunit may explain that ISC-REP leads to persistent impairment to complex I. The potential interrelationship between enhanced exposure of the NDUFS7 subunit to mit-CPN1 as a result of the conformational change in complex I to the deactive form will require further study.
MPTP opening is a key factor to trigger the cell death process during ISC-REP (25, 26, 73). Inhibition of MPTP opening using physiologic (1, 69) or pharmacologic approaches (27, 28, 69) decreased cardiac injury during ISC-REP. Genetic ablation of complex I subunits sensitizes to MPTP opening in cardiac mitochondria following myocardial pressure overload, suggesting that the damaged complex I favors MPTP opening (21, 32). Our study shows that protection of complex I activity using a calpain inhibitor leads to decreased MPTP opening. Inhibition of mit-CPN2 also decreases MPTP opening in cardiac mitochondria by protecting the complex I ND6 subunit (64). These results suggest that activation of mitochondrial calpains contributes to MPTP opening during ISC-REP by decreasing complex I activity. In addition to the complex I defect, impairment of mitophagy also sensitizes to MPTP opening in hepatocytes during ISC-REP (33, 34). MDL treatment preserves the capacity for improved mitophagy and inhibits the MPTP opening during ISC-REP.
Dysfunctional mitochondria are a key source of cardiac injury during ISC-REP. Timely removal of damaged mitochondria through mitophagy deceases cardiac injury during ISC-REP (58). ISC-REP leads to an increasingly depolarized inner mitochondrial membrane potential (10) that recruits PINK1, Parkin, and p62 to mitochondria (30). LC3B relocates to damaged mitochondria by interacting with p62 proteins (14, 23, 24). Relocation of LC3B to damaged mitochondria is an early step in the formation of the autophagosome that is transported to lysosome for degradation (23). An increase in LC3B association with mitochondria by expression of p66SHC stimulates mitophagy (51). Prevention of LC3B translocation to mitochondria impairs mitophagy during sepsis (Fig. 7) (67). These results indicate that translocation of LC3B to mitochondria is critical for mitophagy to occur. Beclin-1 contributes a key role in regulating mitophagy. ISC-REP leads to decreased mitophagy in hepatocytes by cleavage of beclin-1 through activation of the cytosolic CPN2. Stimulation of autophagy using carbamazepine decreases liver injury by increasing beclin-1 content via inhibition of calpains (34). Overexpression of beclin-1 increases mitophagy in cardiac myocytes (67, 72). In the current study, we find that ISC-REP leads to decreased cytosolic beclin-1 content, whereas inhibition of calpains using MDL restores beclin-1 content. A decreased LC3B content in dysfunctional mitochondria impairs mitophagy (67). LC3B content is decreased in both SSM and IFM following ISC-REP. The presence of LC3B in IFM indicates that LC3B is tightly bound with the mitochondrial membrane. A loosely associated LC3B will be removed during IFM isolation with trypsin treatment. Inhibition of calpain activation using MDL restores the LC3B content in cytosol and mitochondria, suggesting that inhibition of calpains promotes autophagy by increasing LC3B translocation to mitochondria.
Beclin-1 is important for LC3B translocation to mitochondria. Depletion of beclin-1 in cytosol prevents LC3B translocation to mitochondria, whereas overexpression of beclin-1 increases LC3B translocation to mitochondria (67). Our study shows that ISC-REP leads to decreased LC3B content by activating cytosolic calpains. Our results suggest that ISC-REP impairs mitophagy in part by decreasing LC3B translocation to mitochondria through depletion of cytosolic beclin-1 content. Inhibition of CPN1/2 may decrease cardiac injury by improving the mitophagy during ISC-REP.
There are limitations in the current study. Cytosolic LC3B content is decreased in rat hearts following ISC-REP (4). Our study also shows that ISC-REP decreases cytosolic LC3B content. However, we need to be very cautious regarding the explanation of these data. LC3B is degraded in the lysosome with acidification and the cathepsin B protease (58). Lysosome dysfunction can affect LC3B content. Since we did not use a lysosome inhibitor such as chloroquine to block autophagy flux (34), we cannot conclude that the ISC-REP decreases autophagy based on the alteration of cytosolic LC3B content. MDL is a classic inhibitor of calpains 1 and 2. However, MDL also has off-target effects, including inhibition of cathepsin B (7, 44). Thus, a genetic approach is needed to clarify the role of calpain activation in decreased mitophagy during ISC-REP in a future study.
Reversible inhibition of complex I decreases cardiac injury during ISC-REP (38), indicating that the manipulation of complex I activity is a valuable approach to decrease cardiac injury. However, persistent complex I damage increases cardiac injury during REP by impairing energy production, favoring ROS production, and increasing the probability of MPTP opening (38) that favors the activation of cell death programs. Inhibition of cytosolic and mitochondrial calpains decreases cardiac injury during ISC-REP by preserving mitochondrial function through protection of mitochondrial respiratory chain reinforced by preservation of the capability for mitophagy to remove those mitochondria that do sustain damage.
GRANTS
This work was supported by National Institute on Aging (NIA) Grant R21 AG054975-01 (Q. Chen), the Office of Research and Development, Medical Research Service Merit Review Award (2IO1BX001355-01A2; Q. Chen and E. J. Lesnefsky), Department of the Army, U.S. Department of Defense (PR151666, J. Dean and E. J. Lesnefsky), and the Pauley Heart Center, Virginia Commonwealth University (Q. Chen, J. Thompson, Y. Hu, and E. J. Lesnefsky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.C. and E.J.L. conceived and designed research; Q.C., J.T., Y.H., and J.D. performed experiments; Q.C., J.T., Y.H., and J.D. analyzed data; Q.C. and E.J.L. interpreted results of experiments; Q.C. prepared figures; Q.C. drafted manuscript; Q.C. and E.J.L. edited and revised manuscript; Q.C., J.T., Y.H., J.D., and E.J.L. approved final version of manuscript.
REFERENCES
- 1.Bradley JM, Li Z, Organ CL, Polhemus DJ, Otsuka H, Islam KN, Bhushan S, Gorodnya OM, Ruchko MV, Gillespie MN, Wilson GL, Lefer DJ. A novel mtDNA repair fusion protein attenuates maladaptive remodeling and preserves cardiac function in heart failure. Am J Physiol Heart Circ Physiol 314: H311–H321, 2018. doi: 10.1152/ajpheart.00515.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao T, Fan S, Zheng D, Wang G, Yu Y, Chen R, Song LS, Fan GC, Zhang Z, Peng T. Increased calpain-1 in mitochondria induces dilated heart failure in mice: role of mitochondrial superoxide anion. Basic Res Cardiol 114: 17, 2019. doi: 10.1007/s00395-019-0726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Hu LX, Dong T, Wang GQ, Wang LH, Zhou XP, Jiang Y, Murao K, Lu SQ, Chen JW, Zhang GX. Apoptosis and autophagy contribute to gender difference in cardiac ischemia-reperfusion induced injury in rats. Life Sci 93: 265–270, 2013. 10.1016/j.lfs.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277: 29181–29186, 2002. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther 316: 200–207, 2006. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Lesnefsky EJ. Heart mitochondria and calpain 1: Location, function, and targets. Biochim Biophys Acta 1852: 2372–2378, 2015. doi: 10.1016/j.bbadis.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294: C460–C466, 2008. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319: 1405–1412, 2006. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Paillard M, Gomez L, Li H, Hu Y, Lesnefsky EJ. Postconditioning modulates ischemia-damaged mitochondria during reperfusion. J Cardiovasc Pharmacol 59: 101–108, 2012. doi: 10.1097/FJC.0b013e31823827cc. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, Lesnefsky EJ. Activation of mitochondrial μ-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun 415: 533–538, 2011. doi: 10.1016/j.bbrc.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Thompson J, Hu Y, Hollander JM, Lesnefsky EJ. Activation of Mitochondrial Calpain 1 Leads to Degradation of PDH (Abstract) FASEB J 32: 543.547, 2018.29101220 [Google Scholar]
- 13.Chen R, Fearnley IM, Peak-Chew SY, Walker JE. The phosphorylation of subunits of complex I from bovine heart mitochondria. J Biol Chem 279: 26036–26045, 2004. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- 14.Colunga A, Bollino D, Schech A, Aurelian L. Calpain-dependent clearance of the autophagy protein p62/SQSTM1 is a contributor to ΔPK oncolytic activity in melanoma. Gene Ther 21: 371–378, 2014. doi: 10.1038/gt.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 117: 112–121, 2007. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, Sazanov LA. Atomic structure of the entire mammalian mitochondrial complex I. Nature 538: 406–410, 2016. doi: 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadicherla AK, Stowe DF, Antholine WE, Yang M, Camara AK. Damage to mitochondrial complex I during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine. Biochim Biophys Acta 1817: 419–429, 2012. doi: 10.1016/j.bbabio.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galan DT, Bito V, Claus P, Holemans P, Abi-Char J, Nagaraju CK, Dries E, Vermeulen K, Ventura-Clapier R, Sipido KR, Driesen RB. Reduced mitochondrial respiration in the ischemic as well as in the remote nonischemic region in postmyocardial infarction remodeling. Am J Physiol Heart Circ Physiol 311: H1075–H1090, 2016. doi: 10.1152/ajpheart.00945.2015. [DOI] [PubMed] [Google Scholar]
- 19.Galkin A, Abramov AY, Frakich N, Duchen MR, Moncada S. Lack of oxygen deactivates mitochondrial complex I: implications for ischemic injury? J Biol Chem 284: 36055–36061, 2009. doi: 10.1074/jbc.M109.054346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galkin A, Meyer B, Wittig I, Karas M, Schägger H, Vinogradov A, Brandt U. Identification of the mitochondrial ND3 subunit as a structural component involved in the active/deactive enzyme transition of respiratory complex I. J Biol Chem 283: 20907–20913, 2008. doi: 10.1074/jbc.M803190200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gharib A, De Paulis D, Li B, Augeul L, Couture-Lepetit E, Gomez L, Angoulvant D, Ovize M. Opposite and tissue-specific effects of coenzyme Q2 on mPTP opening and ROS production between heart and liver mitochondria: role of complex I. J Mol Cell Cardiol 52: 1091–1095, 2012. doi: 10.1016/j.yjmcc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Gorenkova N, Robinson E, Grieve DJ, Galkin A. Conformational change of mitochondrial complex I increases ROS sensitivity during ischemia. Antioxid Redox Signal 19: 1459–1468, 2013. doi: 10.1089/ars.2012.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol 299: C203–C210, 2010. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottlieb RA, Thomas A. Mitophagy and mitochondrial quality control mechanisms in the heart. Curr Pathobiol Rep 5: 161–169, 2017. doi: 10.1007/s40139-017-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res 61: 372–385, 2004. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 27.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta 1767: 1007–1031, 2007. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174: 167–172, 1997. doi: 10.1023/A:1006879618176. [DOI] [PubMed] [Google Scholar]
- 29.Hoppel CL, Kerner J, Turkaly P, Turkaly J, Tandler B. The malonyl-CoA-sensitive form of carnitine palmitoyltransferase is not localized exclusively in the outer membrane of rat liver mitochondria. J Biol Chem 273: 23495–23503, 1998. doi: 10.1074/jbc.273.36.23495. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One 6: e20975, 2011. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inserte J, Barba I, Hernando V, Abellán A, Ruiz-Meana M, Rodríguez-Sinovas A, Garcia-Dorado D. Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage. Cardiovasc Res 77: 782–790, 2008. doi: 10.1093/cvr/cvm082. [DOI] [PubMed] [Google Scholar]
- 32.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JS, Nitta T, Mohuczy D, O’Malley KA, Moldawer LL, Dunn WA Jr, Behrns KE. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology 47: 1725–1736, 2008. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JS, Wang JH, Biel TG, Kim DS, Flores-Toro JA, Vijayvargiya R, Zendejas I, Behrns KE. Carbamazepine suppresses calpain-mediated autophagy impairment after ischemia/reperfusion in mouse livers. Toxicol Appl Pharmacol 273: 600–610, 2013. doi: 10.1016/j.taap.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laughlin TG, Bayne AN, Trempe JF, Savage DF, Davies KM. Structure of the complex I-like molecule NDH of oxygenic photosynthesis. Nature 566: 411–414, 2019. doi: 10.1038/s41586-019-0921-0. [DOI] [PubMed] [Google Scholar]
- 36.Lebon S, Rodriguez D, Bridoux D, Zerrad A, Rötig A, Munnich A, Legrand A, Slama A. A novel mutation in the human complex I NDUFS7 subunit associated with Leigh syndrome. Mol Genet Metab 90: 379–382, 2007. doi: 10.1016/j.ymgme.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial metabolism in aging heart. Circ Res 118: 1593–1611, 2016. doi: 10.1161/CIRCRESAHA.116.307505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL. Mitochondrial Dysfunction and Myocardial Ischemia-Reperfusion: Implications for Novel Therapies. Annu Rev Pharmacol Toxicol 57: 535–565, 2017. doi: 10.1146/annurev-pharmtox-010715-103335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys 385: 117–128, 2001. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 40.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol 33: 1065–1089, 2001. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 41.Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 280: H2770–H2778, 2001. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- 42.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol 273: H1544–H1554, 1997. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Zhang L, Ni R, Cao T, Zheng D, Xiong S, Greer PA, Fan GC, Peng T. Disruption of calpain reduces lipotoxicity-induced cardiac injury by preventing endoplasmic reticulum stress. Biochim Biophys Acta 1862: 2023–2033, 2016. [Erratum in Biochim Biophys Acta 1863: 2409, 2017.] 10.1016/j.bbadis.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lubisch W, Hofmann HP, Treiber HJ, Möller A. Synthesis and biological evaluation of novel piperidine carboxamide derived calpain inhibitors. Bioorg Med Chem Lett 10: 2187–2191, 2000. doi: 10.1016/S0960-894X(00)00430-3. [DOI] [PubMed] [Google Scholar]
- 45.Madungwe NB, Zilberstein NF, Feng Y, Bopassa JC. Critical role of mitochondrial ROS is dependent on their site of production on the electron transport chain in ischemic heart. Am J Cardiovasc Dis 6: 93–108, 2016. [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuura TR, Bartos JA, Tsangaris A, Shekar KC, Olson MD, Riess ML, Bienengraeber M, Aufderheide TP, Neumar RW, Rees JN, McKnite SH, Dikalova AE, Dikalov SI, Douglas HF, Yannopoulos D. Early effects of prolonged cardiac arrest and ischemic postconditioning during cardiopulmonary resuscitation on cardiac and brain mitochondrial function in pigs. Resuscitation 116: 8–15, 2017. doi: 10.1016/j.resuscitation.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta 1817: 851–862, 2012. doi: 10.1016/j.bbabio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 42: 812–825, 2007. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB, Fan GC, Lu Y, Abel ED, Greer PA, Peng T. Mitochondrial calpain-1 disrupts ATP synthase and induces superoxide generation in type 1 diabetic hearts: a novel mechanism contributing to diabetic cardiomyopathy. Diabetes 65: 255–268, 2016. doi: 10.2337/db15-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J Bioenerg Biomembr 37: 1–15, 2005. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- 51.Onnis A, Cianfanelli V, Cassioli C, Samardzic D, Pelicci PG, Cecconi F, Baldari CT. The pro-oxidant adaptor p66SHC promotes B cell mitophagy by disrupting mitochondrial integrity and recruiting LC3-II. Autophagy 14: 2117–2138, 2018. doi: 10.1080/15548627.2018.1505153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozaki T, Tomita H, Tamai M, Ishiguro S. Characteristics of mitochondrial calpains. J Biochem 142: 365–376, 2007. doi: 10.1093/jb/mvm143. [DOI] [PubMed] [Google Scholar]
- 53.Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol 46: 902–909, 2009. doi: 10.1016/j.yjmcc.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys 236: 691–702, 1985. doi: 10.1016/0003-9861(85)90675-7. [DOI] [PubMed] [Google Scholar]
- 55.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731–8739, 1977. [PubMed] [Google Scholar]
- 56.Palmer JW, Tandler B, Hoppel CL. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am J Physiol 250: H741–H748, 1986. doi: 10.1152/ajpheart.1986.250.5.H741. [DOI] [PubMed] [Google Scholar]
- 57.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res 94: 53–59, 2004. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 58.Queliconi BB, Kowaltowski AJ, Gottlieb RA. Bicarbonate increases ischemia-reperfusion damage by inhibiting mitophagy. PLoS One 11: e0167678, 2016. doi: 10.1371/journal.pone.0167678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhein VF, Carroll J, Ding S, Fearnley IM, Walker JE. NDUFAF5 Hydroxylates NDUFS7 at an early stage in the assembly of human complex I. J Biol Chem 291: 14851–14860, 2016. doi: 10.1074/jbc.M116.734970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol 244: H743–H748, 1983. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- 61.Rouslin W, Ranganathan S. Impaired function of mitochondrial electron transfer complex I in canine myocardial ischemia: loss of flavin mononucleotide. J Mol Cell Cardiol 15: 537–542, 1983. doi: 10.1016/0022-2828(83)90329-2. [DOI] [PubMed] [Google Scholar]
- 62.Schuler F, Casida JE. Functional coupling of PSST and ND1 subunits in NADH:ubiquinone oxidoreductase established by photoaffinity labeling. Biochim Biophys Acta 1506: 79–87, 2001. doi: 10.1016/S0005-2728(01)00183-9. [DOI] [PubMed] [Google Scholar]
- 63.Sciarretta S, Maejima Y, Zablocki D, Sadoshima J. The role of autophagy in the heart. Annu Rev Physiol 80: 1–26, 2018. doi: 10.1146/annurev-physiol-021317-121427. [DOI] [PubMed] [Google Scholar]
- 64.Shintani-Ishida K, Yoshida K. Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia-reperfusion. Int J Cardiol 197: 26–32, 2015. doi: 10.1016/j.ijcard.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steel R, Torrie J. Principles and procedures of statistics. New York: Mc Graw-Hill, 1960. [Google Scholar]
- 67.Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, Levine B, Hill JA, Wolf SE, Minei JP, Zang QS. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation 138: 2247–2262, 2018. doi: 10.1161/CIRCULATIONAHA.117.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem 286: 29610–29620, 2011. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ. Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion 12: 180–189, 2012. doi: 10.1016/j.mito.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson J, Hu Y, Lesnefsky EJ, Chen Q. Activation of mitochondrial calpain and increased cardiac injury: beyond AIF release. Am J Physiol Heart Circ Physiol 310: H376–H384, 2016. doi: 10.1152/ajpheart.00748.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tong M, Sadoshima J. Mitochondrial autophagy in cardiomyopathy. Curr Opin Genet Dev 38: 8–15, 2016. doi: 10.1016/j.gde.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, Ikeda S, Shirakabe A, Sadoshima J. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res 124: 1360–1371, 2019. doi: 10.1161/CIRCRESAHA.118.314607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res 93: 292–301, 2003. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 74.Xu A, Szczepanek K, Maceyka MW, Ross T, Bowler E, Hu Y, Kenny B, Mehfoud C, Desai PN, Baumgarten CM, Chen Q, Lesnefsky EJ. Transient complex I inhibition at the onset of reperfusion by extracellular acidification decreases cardiac injury. Am J Physiol Cell Physiol 306: C1142–C1153, 2014. doi: 10.1152/ajpcell.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu P, Zhang J, Yu S, Luo Z, Hua F, Yuan L, Zhou Z, Liu Q, Du X, Chen S, Zhang L, Xu G. Protective effect of sevoflurane postconditioning against cardiac ischemia/reperfusion injury via ameliorating mitochondrial impairment, oxidative stress and rescuing autophagic clearance. PLoS One 10: e0134666, 2015. doi: 10.1371/journal.pone.0134666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297: 259–263, 2002. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 77.Yu W, Gao B, Li N, Wang J, Qiu C, Zhang G, Liu M, Zhang R, Li C, Ji G, Zhang Y. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim Biophys Acta Mol Basis Dis 1863: 1973–1983, 2017. doi: 10.1016/j.bbadis.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Q, Guo Z, Deng W, Fu S, Zhang C, Chen M, Ju W, Wang D, He X. Calpain 2-mediated autophagy defect increases susceptibility of fatty livers to ischemia-reperfusion injury. Cell Death Dis 7: e2186, 2016. doi: 10.1038/cddis.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]