Abstract
Whether the histone deacetylase (HDAC) and sirtuin families of protein deacetylases regulate insulin-stimulated glucose uptake, independent of their transcriptional effects, has not been studied. Our objective was to determine the nontranscriptional role of HDACs and sirtuins in regulation of skeletal muscle insulin action. Basal and insulin-stimulated glucose uptake and signaling and acetylation were assessed in L6 myotubes and skeletal muscle from C57BL/6J mice that were treated acutely (1 h) with HDAC (trichostatin A, panobinostat, TMP195) and sirtuin inhibitors (nicotinamide). Treatment of L6 myotubes with HDAC inhibitors or skeletal muscle with a combination of HDAC and sirtuin inhibitors increased tubulin and pan-protein acetylation, demonstrating effective impairment of HDAC and sirtuin deacetylase activities. Despite this, neither basal nor insulin-stimulated glucose uptake or insulin signaling was impacted. Acute reduction of the deacetylase activity of HDACs and/or sirtuins does not impact insulin action in skeletal muscle.
Keywords: glucose uptake, histone deacetylase, L6 myotube, sirtuin
INTRODUCTION
Insulin-mediated glucose uptake by skeletal muscle is posited to occur through phosphorylation-based signaling (9). Interestingly, various proteins within the insulin-signaling and GLUT4-trafficking pathways are reversibly acetylated on lysine residues (3, 11, 27), with direct changes in their acetylation altering their activity (3, 23, 27). Lysine acetylation is a balance between the activity of protein acetyltransferases and deacetylases, which add and remove acetyl groups, respectively (5). There are 18 known deacetylases that are divided into two families: the zinc-dependent histone deacetylases (HDACs) and NAD+-dependent sirtuins (5). Indeed, both the HDACs (17, 20, 22, 25, 26) and the sirtuins (1, 7, 13, 21, 24) have been implicated in the regulation of insulin-stimulated glucose uptake in skeletal muscle. Importantly, however, this regulation appears to occur primarily via acetylation of transcriptional regulators and/or histones and subsequent changes in the transcription of insulin-signaling proteins (17, 20, 21, 26). To our knowledge, the ability of these deacetylases to nontranscriptionally (i.e., “directly”) regulate insulin-stimulated glucose uptake, particularly in insulin-responsive tissues such as skeletal muscle, has not been thoroughly studied. Notably, while previous studies have demonstrated that pharmacological inhibition of deacetylases enhances insulin action in skeletal muscle (8, 17, 20, 22, 25), because incubation times were for multiple days, this enhancement could be via changes in transcription of insulin-signaling proteins, acetylation of cytosolic insulin-signaling proteins, or a combination of the two.
Thus the primary goal of this study was to investigate the nontranscriptional contribution of HDACs and sirtuins to insulin-mediated glucose uptake in skeletal muscle. For this, we assessed basal and insulin-stimulated glucose uptake in L6 myotubes and mouse skeletal muscle after brief (i.e., 1 h) pharmacological inhibition of HDACs and/or sirtuins. We hypothesized that, similar to chronic inhibition, acute inhibition of deacetylases would augment insulin-stimulated glucose uptake in skeletal muscle.
MATERIALS AND METHODS
Cell culture conditions.
L6 myoblasts were maintained in minimum essential medium-α (MEMα) with 10% fetal bovine serum (FBS) at 5% CO2 and 37°C. Myoblasts were differentiated into myotubes in MEMα with 2% fetal bovine serum for 4 days.
Inhibitors.
Trichostatin A (no. 9950S) was from Cell Signaling Technology, nicotinamide (no. 98092-0) was from Sigma-Aldrich, panobinostat (no. A8178) was from Apexbio Technology, and TMP195 (no. NC1452975) was from Selleck Chemical. The IC50 values for inhibitors used are shown in Table 1 (2, 4, 14).
Table 1.
IC50 values for inhibitors used
| Deacetylase Class |
||||
|---|---|---|---|---|
| I (HDACs) | II (HDACs) | III (Sirtuins) | Reference | |
| Trichostatin A | ~10 nM | ~1 µM | 2 | |
| Panobinostat | ~100 nM | ~100 nM | 2 | |
| TMP195 | >100 nM | ~100 nM | 14 | |
| Nicotinamide | ~100 µM | 4 | ||
HDAC, histone deacetylase.
Flow cytometry.
L6 myoblasts were serum-starved, in suspension, for 3 h in 0.5% fatty acid-free BSA in MEMα and then for 1.5 h in HEPES-buffered Krebs-Ringer bicarbonate buffer [10 mM HEPES, 4.8 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 0.5% fatty-acid free BSA in Dulbecco’s phosphate-buffered saline (DPBS)] with DMSO or inhibitors at 37°C. Myoblasts were fixed in 2% paraformaldehyde at room temperature (RT) for 30 min, washed 2× with DPBS, permeabilized with 0.5% saponin at RT for 30 min, and incubated with Alexa Fluor 647-labeled acetylated lysine (Ac-Lys, 1:20 dilution; no. 623405, Biolegend) at RT for 30 min. Cells were then washed 1× with DPBS and resuspended in 500 μL of DPBS. Flow cytometric acquisitions were performed using a ZE5 Cell Analyzer (Bio-Rad) and analyzed using FloJo (Treestar, Ashland, OR). In all cases, at least 5,000 cells were counted.
L6 myotube 2-deoxy-d-glucose uptake.
L6 myotubes were treated, as above, but adherent to 24-well plates. For the last 30 min in HEPES-buffered Krebs-Ringer bicarbonate buffer, 100 nM insulin was present; thereafter, the myotubes were incubated for 10 min with 1 mM [2-3H]-deoxy-d-glucose (2DOG, 6 mCi/mmol; PerkinElmer). Cells were washed twice with ice-cold DPBS, lysed with 1 M NaOH, and neutralized with 1 M HCl. Radioactivity of cell lysates was measured using a liquid scintillation counter. Radioactivity was normalized to the protein concentration of each sample using the Pierce BCA protein assay kit. Data were normalized to cells treated with DMSO and no insulin within each experiment.
Immunoprecipitation and immunoblotting.
Immunoprecipitation and immunoblotting were performed as previously described (16). For immunoblotting, all antibodies were used at a dilution of 1:1,000. Akt (no. CS 9272B), phosphorylated (T308) Akt (pAktT308; no. CS 9275), phosphorylated (S473) Akt (pAktS473; no. CS 4058), glycogen synthase kinase (GSK)-3α/β (GSK3α/β; no. CS 5676), phosphorylated (S21/9) GSK3α/β (pGSK3α/βS21/9; no. CS 9331), eukaryotic elongation factor 2 (eEF2; no. CS 2332), acetylated tubulin (no. CS 5335), and tubulin (no. CS 2148) were from Cell Signaling Technology. Densitometric analysis of immunoblots was performed using Image Lab (Bio-Rad, Hercules, CA). Phosphorylated and acetylated protein abundance was normalized to respective total protein abundance. Silver staining was performed and analyzed as previously described (16).
Mice.
Studies were conducted in female C57BL/6J mice housed in a conventional facility with a 12:12-h light-dark cycle. Procedures were carried out with the approval of, and in accordance with, the Animal Care Program and Institutional Animal Care and Use Committee at the University of California, San Diego.
Muscle 2DOG uptake.
Basal and insulin-stimulated 2DOG uptake was measured in soleus and extensor digitorum longus (EDL) muscles as previously described (16, 21) with some modifications. Paired soleus and EDL muscles were placed into a “preincubation” solution including DMSO or inhibitors for 1 h. Subsequently, muscles were transferred into an “incubation” solution that included 2DOG and [14C]mannitol for 30 min, with muscles from one side being exposed to insulin (0.36 nM) and the contralateral side exposed to no insulin. During this 30-min period, muscles continued to be incubated with DMSO or inhibitors.
Statistical analysis.
Statistical analyses were performed using Prism 8 (GraphPad Software, La Jolla, CA). L6 myotube 2DOG uptake, muscle 2DOG uptake, and phosphorylated proteins (insulin × inhibitor) were analyzed by two-way ANOVA. Acetylated tubulin in L6 myotubes and Ac-Lys flow cytometry were analyzed by one-way ANOVA with Tukey’s multiple-comparison test. Muscle insulin-stimulated 2DOG uptake and acetylated tubulin and Akt were analyzed by Student’s t test. All data are expressed as means ± SE.
RESULTS
Acute inhibition of HDACs does not affect insulin-stimulated glucose uptake in L6 myotubes.
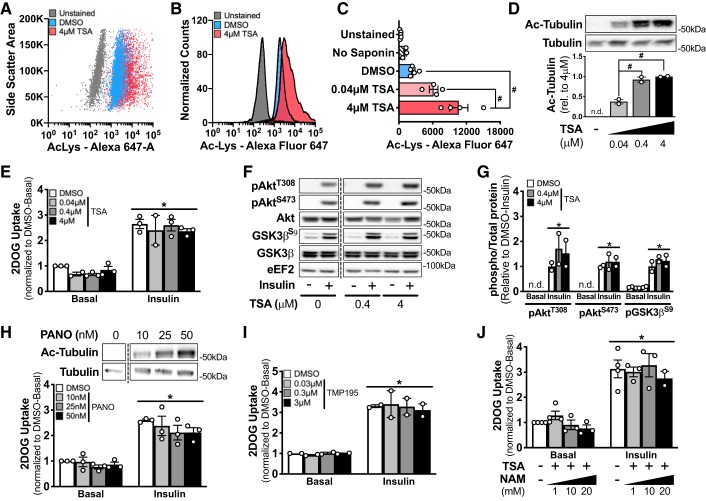
Treatment of L6 myoblasts for 1.5 h with the pan-HDAC inhibitor trichostatin A led to a dose-dependent increase in lysine-acetylated proteins, as assessed by flow cytometry (Fig. 1, A–C). In validation of this flow-based approach, the difference in signal between unstained L6 myoblasts and stained but unpermeabilized (i.e., no saponin) L6 myoblasts was not statistically significant (Fig. 1C). In addition to increasing pan-protein lysine acetylation, trichostatin A dose-dependently increased the acetylation of a cytosolic target of HDACs, tubulin, in L6 myotubes (Fig. 1D). Insulin increased glucose uptake ~2.5-fold in DMSO-treated L6 myotubes compared with the basal condition (Fig. 1E). One-hour pretreatment with increasing doses of trichostatin A did not impact basal or insulin-stimulated 2DOG uptake in L6 myotubes (Fig. 1E). Furthermore, basal and insulin-stimulated phosphorylation of AktT308, AktS473, and GSK3βS9 were comparable between DMSO and trichostatin A-treated L6 myotubes, regardless of the trichostatin A concentration (Fig. 1, F and G). Similarly, while pretreatment of L6 myotubes for 1 h with an alternate HDAC inhibitor, panobinostat, dose-dependently increased tubulin acetylation, basal and insulin-stimulated glucose uptake were comparable to that for DMSO-treated myotubes (Fig. 1H). Since we were interested in the nonnuclear effects of HDAC inhibition, we utilized TMP195, a potent inhibitor of the class IIa HDACs (14), which are, at least partly, found in the cytosol (10). Nevertheless, pretreatment for 1 h with TMP195 had no effect on basal or insulin-stimulated glucose uptake (Fig. 1I). Lastly, we utilized a combination of trichostatin A (4 μM) and nicotinamide (1 mM, 10 mM, 20 mM) to inhibit both the HDAC and sirtuin families in L6 myotubes. Preincubation of L6 myotubes with trichostatin A and nicotinamide for 1 h did not affect basal or insulin-stimulated 2DOG uptake compared with DMSO (Fig. 1J).
Fig. 1.
Acute inhibition of histone deacetylases (HDACs) increases acetylation but does not alter insulin-stimulated glucose uptake in L6 myotubes. A–C: representative experiment (A and B) and mean fluorescence intensity (C) for total acetylated proteins in unstained, stained without saponin, DMSO, and trichostatin A (TSA)-treated L6 myoblasts as analyzed by flow cytometry. Representative of data of 4 independent experiments. Ac-Lys, acetylated lysine. D: representative image and quantification for acetylated tubulin (Ac-tubulin) and total tubulin in L6 myotubes pretreated for 1 h with DMSO or TSA. E, H, I, and J: normalized basal and insulin (100 nM) 2-deoxy-d-glucose (2DOG) uptake in L6 myotubes pretreated for 1 h with DMSO or TSA (E), panobinostat (PANO; H), TMP195 (I), or TSA (4 μM; J) and nicotinamide (NAM). F and G: representative image (F) and quantification (G) of phosphorylated (p)AktT308 (pAktT308), pAktS473, total Akt, phosphorylated glycogen synthase kinase 3β (pGSK3βS9), and total GSK3β in basal and insulin-stimulated (− and +, respectively) L6 myotubes pretreated with or without TSA for 1 h. #P < 0.05, 1-way ANOVA with Tukey’s multiple comparison test. *P < 0.05, 2-way ANOVA, main effect of insulin. Trichostatin A treatment Western blots are representative of 2 independent experiments and 2DOG uptake assays are representative of 3 independent experiments. Data reported as means ± SE.
Concurrent inhibition of HDACs and sirtuins does not affect insulin-stimulated glucose uptake in skeletal muscle ex vivo.
To validate these in vitro findings, we studied mature mouse skeletal muscle. Pretreatment of mouse soleus muscle with trichostatin A (4 μM) and nicotinamide (20 mM) for 1 h resulted in a ~20% increase in total protein acetylation, as measured in immunoprecipitates using a pan acetyl-lysine antibody (Fig. 2A), and an ~8-fold increase in acetylated tubulin (Fig. 2B); however, there were no changes in acetylated Akt (Fig. 2B). Pretreatment of either the soleus or EDL with trichostatin A and nicotinamide did not affect basal, insulin, or insulin-stimulated (i.e., insulin 2DOG uptake minus basal 2DOG uptake) 2DOG uptake compared with DMSO treatment (Fig. 2, C and D). Likewise, basal and insulin-stimulated phosphorylation of AktT308, AktS473, and GSK3βS9 were comparable between DMSO and trichostatin A and nicotinamide-treated EDL muscles (Fig. 2E).
Fig. 2.
Concurrent inhibition of histone deacetylases (HDACs) and sirtuins does not affect insulin-stimulated glucose uptake in skeletal muscle ex vivo. A: silver stain of acetyl-lysine (Ac-Lys) immunoprecipitates from DMSO or trichostatin A and nicotinamide-treated soleus muscles (TSA+NAM, 4 µM TSA and 20 mM). B: quantitation and representative images of acetylated tubulin (Ac-tubulin), tubulin, acetylated Akt (Ac-Akt), and Akt from DMSO or TSA+NAM-treated soleus muscles. Ac-Akt was determined by immunoprecipitation (IP) with an acetyl-lysine antibody and subsequent immunoblotting (IB) with an Akt antibody. C and D: basal 2-deoxy-d-glucose uptake (2DOGU), insulin (0.36 nM) 2DOGU, and insulin-stimulated 2DOGU (I-Stim; calculated as insulin 2DOGU – basal 2DOGU) in isolated soleus (C) and extensor digitorum longus (EDL) muscles (D) from female C57BL/6J mice pretreated for 1 h with DMSO or TSA+NAM. E: representative image and quantification of phosphorylated (p)Akt (pAktT308), pAktS473, total Akt, phosphorylated glycogen synthase kinase 3β (pGSK3βS9), and total GSK3β in basal and insulin-stimulated (− and +, respectively) EDL muscles pretreated with DMSO or TSA+NAM for 1 h. DMSO (n = 5), TSA+NAM (n = 6). #P < 0.05, unpaired Student’s t test. *P < 0.05, 2-way ANOVA, main effect of insulin. Data reported as means ± SE.
DISCUSSION
Both the HDAC (17, 20, 22, 25, 26) and sirtuin (1, 7, 13, 21, 24) deacetylase families have been extensively studied for their role in the transcriptional modulation of skeletal muscle insulin action. However, as insulin-signaling and GLUT4-trafficking proteins can be reversibly acetylated (3, 11, 23), which alters their function (3, 23, 27), it is possible that HDACs and sirtuins could also impact skeletal muscle insulin action through nontranscriptional mechanisms. To address this, we acutely inhibited the HDAC and/or sirtuin deacetylase families for 1 h and assessed insulin-stimulated glucose uptake in L6 myotubes and mature mouse skeletal muscle. Our results demonstrate that brief inhibition of the HDAC and/or sirtuin families does not impact insulin-stimulated glucose uptake or signaling in skeletal muscle.
Using both knockdown and overexpression models, several of the 11 HDACs have been implicated in the transcriptional regulation of insulin-signaling proteins (17, 20, 22, 25, 26). For example, HDAC4 and HDAC9 work in conjunction with HDAC5 to transcriptionally repress the GLUT4 gene via deacetylation of GLUT4 enhancer factor and myocyte enhancer factor 2 (17, 20, 26). When considering inhibitor-based studies, 24 h of treatment with trichostatin A, a commonly used HDAC inhibitor, increases insulin-stimulated glucose uptake and phosphorylation of AktS473 and GSK3βS9 in C2C12 myotubes (22). This increase, however, is not manifested with 6 or 12 h of treatment (22). Likewise, at least 24 h of treatment with another HDAC inhibitor, scriptaid, increased GLUT4 mRNA expression in human primary muscle cells (17, 20), basal and insulin-stimulated glucose uptake in L6 myotubes (20, 25), and insulin-stimulated glucose uptake in isolated EDL muscles of obese mice (8). Importantly, however, treatment with scriptaid or trichostatin A for less than 24 h does not increase insulin-stimulated glucose uptake in L6 myotubes (25). In accordance with previous studies, we demonstrate that brief (1 h) pretreatment with HDAC inhibitors (trichostatin A, panobinostat, or TMP195) did not improve basal or insulin-stimulated glucose uptake in L6 myotubes. Furthermore, while acute treatment with trichostatin A was sufficient to increase both pan acetylation and acetylation of a cytosolic HDAC target (tubulin), it did not alter insulin-stimulated phosphorylation of AktT308, AktS473, or GSK3βS9 in L6 myotubes. Hence, our results, together with the previous literature, demonstrate that HDAC inhibitors likely improve insulin action through transcriptional mechanisms.
The contribution of the sirtuins to skeletal muscle insulin action has been extensively studied (1, 7, 13, 21, 24). In L6 myotubes, SIRT1 overexpression increases, while SIRT1 knockdown reduces, insulin-stimulated phosphorylation of Akt (7). Furthermore, prolonged incubation (6 h) of H4IIE rat hepatoma liver cells with nicotinamide reduces insulin-stimulated phosphorylation of Akt (27). Conversely, however, SIRT2 knockdown improves insulin-stimulated glucose uptake and phosphorylation of Akt and GSK3β in C2C12 myotubes grown in insulin-resistant conditions (1). In in vivo mouse models, knockout or overexpression of SIRT1 in skeletal muscle does not impact insulin sensitivity (21, 24), although SIRT1 does mediate calorie restriction-induced enhancement of skeletal muscle insulin sensitivity via a transcriptional mechanism (21). Furthermore, mice with whole body knockout of SIRT2 have impaired skeletal muscle insulin sensitivity, while mice with a knockout of SIRT2 (12) or SIRT3 (13) have exacerbated skeletal muscle insulin resistance while on a high-fat diet; however, due to the whole body nature of these models, it is not possible to determine the role of SIRT2 or SIRT3 in skeletal muscle, per se. Regardless, these studies do not discern whether sirtuins regulate insulin action via transcriptional or nontranscriptional mechanisms. Addressing this, we found that insulin-stimulated glucose uptake in L6 myotubes is not affected by acute incubation with trichostatin A and nicotinamide. Similarly, in mature mouse muscle, while acute treatment with trichostatin A and nicotinamide was sufficient to increase overall protein acetylation, including increased acetylated tubulin, there was no effect on insulin-stimulated glucose uptake, regardless of muscle fiber type. Furthermore, acute trichostatin A and nicotinamide treatment did not alter acetylated Akt levels or insulin-stimulated phosphorylation of AktT308, AktS473, or GSK3βS9. Hence, while there is evidence that both sirtuins and HDACs alter insulin action in skeletal muscle via transcriptional mechanisms, our data demonstrate that short-term, pan inhibition of deacetylases does not alter skeletal muscle insulin sensitivity.
While the purpose of the study was to determine the effects of acute deacetylase inhibition, a possible limitation was our choice of incubation time. Importantly, in both L6 myotubes and mature mouse muscle, pan-acetylated proteins, as well as acetylated tubulin, which is a cytosolic target of deacetylases (6), were significantly increased by only 1 h of treatment with deacetylase inhibitors. Furthermore, time course studies in C2C12 myotubes and SH-SY5Y neuroblastoma cells have established that 3–4 h of incubation with trichostatin A is the earliest time point at which there are significant changes in mRNA expression of HDAC target genes, with no significant changes at 1–2 h of treatment (15, 18, 19). Together with this, previous studies only demonstrate changes to insulin signaling and/or glucose uptake with at least 24 h of treatment with inhibitors of deacetylases (8, 22, 25). Therefore, we believe that the 1-h preincubation period used was sufficient to impair HDAC and/or sirtuin function and sufficiently short to investigate insulin action independent of effects on gene expression.
In summary, we studied the nontranscriptional role of HDACs and sirtuins in the regulation of insulin action in skeletal muscle. In contrast to our hypothesis, we found that acute, pan inhibition of HDACs and/or sirtuins in L6 myotubes or mature mouse muscle does not enhance insulin-stimulated signaling or glucose uptake. Taken together, our results suggest that deacetylases do not “directly” modulate skeletal muscle insulin action, but rather do so “indirectly” via transcriptional mechanisms.
GRANTS
This work was supported, in part, by National Institute on Aging Grant R01 AG043120 (to S. Schenk), National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant T32 AR060712 and National Institute of Diabetes and Digestive and Kidney Diseases Grant F30 DK115035 (to V. F. Martins), a University of California San Diego Frontiers of Innovation Scholars Program Grant (to S. Schenk), Graduate Student Research Support from the University of California San Diego Institute of Engineering in Medicine (to V. F. Martins), Swiss National Science Foundation Postdoctoral Fellowship P2BSP3-165311 and American Federation of Aging Research Postdoctoral Fellowship PD18120 (to K. Svensson), and a Medical Student Training in Aging Research Grant T35 AG26757 (to M. Begur).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.F.M., M.B., S.L., C.E.M., and S.S. conceived and designed research; V.F.M., M.B., S.L., K.S., J.P., and B.H. performed experiments; V.F.M., M.B., S.L., K.S., J.P., and B.H. analyzed data; V.F.M., M.B., S.L., K.S., and B.H. interpreted results of experiments; V.F.M., K.S., and J.P. prepared figures; V.F.M., M.B., and S.L. drafted manuscript; V.F.M., K.S., C.E.M., and S.S. edited and revised manuscript; V.F.M., M.B., S.L., K.S., J.P., B.H., C.E.M., and S.S. approved final version of manuscript.
REFERENCES
- 1.Arora A, Dey CS. SIRT2 negatively regulates insulin resistance in C2C12 skeletal muscle cells. Biochim Biophys Acta 1842: 1372–1378, 2014. doi: 10.1016/j.bbadis.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280: 233–241, 2009. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Belman JP, Bian RR, Habtemichael EN, Li DT, Jurczak MJ, Alcázar-Román A, McNally LJ, Shulman GI, Bogan JS. Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment. J Biol Chem 290: 4447–4463, 2015. doi: 10.1074/jbc.M114.603977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang YL, Lin H. An improved fluorogenic assay for SIRT1, SIRT2, and SIRT3. Org Biomol Chem 14: 2186–2190, 2016. doi: 10.1039/C5OB02609A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol 15: 536–550, 2014. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- 6.Denu JM. The Sir 2 family of protein deacetylases. Curr Opin Chem Biol 9: 431–440, 2005. doi: 10.1016/j.cbpa.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Fröjdö S, Durand C, Molin L, Carey AL, El-Osta A, Kingwell BA, Febbraio MA, Solari F, Vidal H, Pirola L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol 335: 166–176, 2011. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Gaur V, Connor T, Venardos K, Henstridge DC, Martin SD, Swinton C, Morrison S, Aston-Mourney K, Gehrig SM, van Ewijk R, Lynch GS, Febbraio MA, Steinberg GR, Hargreaves M, Walder KR, McGee SL. Scriptaid enhances skeletal muscle insulin action and cardiac function in obese mice. Diabetes Obes Metab 19: 936–943, 2017. doi: 10.1111/dom.12896. [DOI] [PubMed] [Google Scholar]
- 9.Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol 306: C879–C886, 2014. doi: 10.1152/ajpcell.00069.2014. [DOI] [PubMed] [Google Scholar]
- 10.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749, 2003. doi: 10.1042/bj20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaBarge S, Migdal C, Schenk S. Is acetylation a metabolic rheostat that regulates skeletal muscle insulin action? Mol Cells 38: 297–303, 2015. doi: 10.14348/molcells.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantier L, Williams AS, Hughey CC, Bracy DP, James FD, Ansari MA, Gius D, Wasserman DH. SIRT2 knockout exacerbates insulin resistance in high fat-fed mice. PLoS One 13: e0208634, 2018. doi: 10.1371/journal.pone.0208634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lantier L, Williams AS, Williams IM, Yang KK, Bracy DP, Goelzer M, James FD, Gius D, Wasserman DH. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes 64: 3081–3092, 2015. doi: 10.2337/db14-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, Baloglu E, Trump RP, Head MS, Hofmann GA, Murray-Thompson M, Schwartz B, Chakravorty S, Wu Z, Mander PK, Kruidenier L, Reid RA, Burkhart W, Turunen BJ, Rong JX, Wagner C, Moyer MB, Wells C, Hong X, Moore JT, Williams JD, Soler D, Ghosh S, Nolan MA. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol 9: 319–325, 2013. doi: 10.1038/nchembio.1223. [DOI] [PubMed] [Google Scholar]
- 15.Maehara K, Uekawa N, Isobe K. Effects of histone acetylation on transcriptional regulation of manganese superoxide dismutase gene. Biochem Biophys Res Commun 295: 187–192, 2002. doi: 10.1016/S0006-291X(02)00646-0. [DOI] [PubMed] [Google Scholar]
- 16.Martins VF, Dent JR, Svensson K, Tahvilian S, Begur M, Lakkaraju S, Buckner EH, LaBarge SA, Hetrick B, McCurdy CE, Schenk S. Germline or inducible knockout of p300 or CBP in skeletal muscle does not alter insulin sensitivity. Am J Physiol Endocrinol Metab 316: E1024–E1035, 2019. doi: 10.1152/ajpendo.00497.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGee SL, van Denderen BJW, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 57: 860–867, 2008. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 18.Nunes MJ, Milagre I, Schnekenburger M, Gama MJ, Diederich M, Rodrigues E. Sp proteins play a critical role in histone deacetylase inhibitor-mediated derepression of CYP46A1 gene transcription. J Neurochem 113: 418–431, 2010. doi: 10.1111/j.1471-4159.2010.06612.x. [DOI] [PubMed] [Google Scholar]
- 19.Nunes MJ, Moutinho M, Milagre I, Gama MJ, Rodrigues E. Okadaic acid inhibits the trichostatin A-mediated increase of human CYP46A1 neuronal expression in a ERK1/2-Sp3-dependent pathway. J Lipid Res 53: 1910–1919, 2012. doi: 10.1194/jlr.M027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raichur S, Teh SH, Ohwaki K, Gaur V, Long YC, Hargreaves M, McGee SL, Kusunoki J. Histone deacetylase 5 regulates glucose uptake and insulin action in muscle cells. J Mol Endocrinol 49: 203–211, 2012. doi: 10.1530/JME-12-0095. [DOI] [PubMed] [Google Scholar]
- 21.Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K, Olefsky JM. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121: 4281–4288, 2011. doi: 10.1172/JCI58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun C, Zhou J. Trichostatin A improves insulin stimulated glucose utilization and insulin signaling transduction through the repression of HDAC2. Biochem Pharmacol 76: 120–127, 2008. doi: 10.1016/j.bcp.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal 4: ra46, 2011. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 24.Svensson K, LaBarge SA, Martins VF, Schenk S. Temporal overexpression of SIRT1 in skeletal muscle of adult mice does not improve insulin sensitivity or markers of mitochondrial biogenesis. Acta Physiol (Oxf) 221: 193–203, 2017. doi: 10.1111/apha.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takigawa-Imamura H, Sekine T, Murata M, Takayama K, Nakazawa K, Nakagawa J. Stimulation of glucose uptake in muscle cells by prolonged treatment with scriptide, a histone deacetylase inhibitor. Biosci Biotechnol Biochem 67: 1499–1506, 2003. doi: 10.1271/bbb.67.1499. [DOI] [PubMed] [Google Scholar]
- 26.Weems J, Olson AL. Class II histone deacetylases limit GLUT4 gene expression during adipocyte differentiation. J Biol Chem 286: 460–468, 2011. doi: 10.1074/jbc.M110.157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem 282: 34356–34364, 2007. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]