Abstract
The neonatal Fc receptor (FcRn) has been shown to be required for antigen presentation in dendritic cells, and global knockout of FcRn attenuates immune-mediated kidney disease. Podocytes express interleukin-6 (IL-6) receptor and produce IL-6 under proinflammatory conditions. Here we examined the role of FcRn in the IL-6-mediated inflammatory response in podocytes. We examined IL-6 production by ELISA and expression by qPCR in wild type (WT) and FcRn knockout (KO) podocytes after treatment with proinflammatory stimuli as well as IL-6-mediated signaling via the JAK/STAT pathway. We also examined podocyte motility in cultured WT and KO podocytes after a proinflammatory challenge. We found that FcRn KO podocytes produced minimal amount of IL-6 after treatment with albumin, IgG, or immune complexes whereas WT podocytes had a robust response. FcRn KO podocytes also had minimal expression of IL-6 compared with WT. By Western blotting, there was significantly less phosphorylated STAT3 in KO podocytes after treatment with IFNγ or immune complexes. In a scratch assay, FcRn KO podocytes showed increased motility comparted KO, suggesting a defect in actin dynamics. Cultured FcRn KO podocytes also demonstrated abnormal stress fibers compared with WT and the defect could be rescued by IL-6 treatment. This study shows that in podocytes, FcRn modulates the IL-6 mediated response to proinflammatory stimuli and regulates podocytes actin structure, motility and synaptopodin expression.
Keywords: cytoskeleton, immune mediated kidney disease, interleukin-6, neonatal Fc receptor, podocyte
INTRODUCTION
The podocyte, a highly specialized and differentiated cell, is an essential part of the glomerular filtration barrier (GFB) that has a crucial role in preventing protein loss into the urine. The GFB comprises three components: the fenestrated endothelial cell, the glomerular basement membrane, and the podocyte slit diaphragm (23, 35). Injury to the podocyte results in actin cytoskeletal derangements, podocyte foot process effacement and detachment from the basement membrane and is a key factor contributing to loss of glomerular function and progressive kidney damage (24).
The neonatal Fc receptor (FcRn), a major histocompatibility-like class I protein, is expressed on glomerular endothelial cells, podocytes, and proximal tubular cells (17, 30). FcRn has been shown to be required in proximal tubular cells for albumin recycling from the ultrafiltrate (40). In podocytes, FcRn is required for the correct trafficking of IgG but not albumin (1, 9, 32). FcRn has also been shown to be required for antigen presentation in dendritic cells (27, 28) and lack of FcRn ameliorates systemic as well as renal immune-mediated diseases (2, 26, 33), linking FcRn to immunomodulatory pathways (30).
In a previous study we found that podocytes release cytokines including interleukin-6 (IL-6) when exposed to albumin (25). IL-6 is a potent inflammatory cytokine with pleiotropic effects involved in inflammation and the immune response (20, 34). IL-6 signals via two different mechanisms: In the classic pathway, IL-6 binds to a heterotetramer of the membrane bound IL-6 receptor and glycoprotein 130 (gp130) (34). While gp130 is ubiquitously expressed, the IL-6 receptor is expressed on only a few cell types including podocytes, macrophages, neutrophils, CD4+-T cells and hepatocytes (34). IL-6 is able to signal to multiple different cell types via the trans-signaling pathway in which IL-6 forms a complex with the soluble IL-6 receptor and binds membrane bound gp130 (13). Activation of either the classical or trans pathway leads to signaling mediated by the JAK/STAT3 or Src homology region 2 (SH2)-containing protein tyrosine phosphatase 2 (SHP2)/Grb2-associated binders (Gab)/MAPK cascades (31).
Previous studies have shown both a protective and a harmful effect of IL-6 signaling in immune-mediated glomerulonephritis, depending on the model used. Karkar et al. (21) demonstrated that in the nephrotoxic nephritis (NTN) model in rats, infusion of IL-6 was protective while Luig et al. (22) showed that inhibition of IL-6 signaling worsened NTN in mice. Similarly, Hagenstein et al. (16) have found a complex role for IL-6-mediated signaling in CD4+ T cells in NTN disease initiation and progression in mice. In some lupus models in mice, IL-6 infusion worsened disease (12), whereas a different study showed that lupus-prone IL-6-deficient mice had a significant reduction in proteinuria and hematuria and a concomitant reduction in inflammatory infiltrates within the kidney (18).
Since podocytes can both secrete and respond directly to IL-6 and IL-6 modulates immune-mediated glomerular disease, we sought to examine IL-6 production and signaling in wild-type podocytes and podocytes lacking FcRn. Here we show that cultured podocytes lacking FcRn do not produce IL-6 in response to inflammatory or immune stimuli, that STAT3 signaling in response to immune stimuli is impaired in FcRn knockout (KO) podocytes, and that dysregulated IL-6 signaling leads to alterations in the actin cytoskeleton and podocyte motility in vitro.
MATERIALS AND METHODS
Cell culture.
Podocytes were isolated from WT or global FcRn KO mice as previously described (8) and then conditionally immortalized using a thermosensitive SV40 antigen (9). Briefly, primary podocytes were isolated from WT and FcRn KO mice and were then infected with a thermosensitive SV40. Podocytes were cultured on type I collagen-coated flasks or dishes. After transformation, replication occurred at 33°C. To induce differentiation, the cells were cultured at 37°C for 8–10 days. All experiments were performed using differentiated and confluent podocytes. To examine IL-6 production after treatment with proinflammatory or immune complexes, WT and FcRn KO cells were treated with RPMI containing either 5 mg/mL human albumin (Sigma, St. Louis, MO), 1.5 mg/mL human immunoglobulin G (Sigma), 100 U/mL recombinant mouse IFNɣ (cat. no. MI-485, R&D systems, MN), or 4-hydroxy-3-nitrophenylacetyl hapten (NP)-ovalbumin (cat. no. N-5051-100, Bioresearch, CA; final concentration 40 µg/mL) plus IFNγ. To examine the effects of IL-6 on the podocyte actin cytoskeleton, cells were treated with 100 ng/mL recombinant mouse IL-6 (cat. no. 575702, Biolegend, CA) for the indicated times.
Western blot analysis.
WT or FcRn KO podocytes were harvested by scraping into RIPA buffer. Lysate protein concentration was measured by BCA protein assay (Thermo Fisher Scientific). Samples were run on 10% polyacrylamide gel (Bio-Rad) and transferred to nitrocellulose membrane (GE Healthcare Life Sciences, Germany). Membranes were blocked (5% of nonfat milk, 60 min) and were then incubated overnight with a 1:1,000 dilution of primary antibodies including: rabbit anti-mouse STAT3 antibody (cat. no. 12640s, Cell Signaling Technology), rabbit anti-mouse phospho-STAT3 (Y705) (cat. no. 9145s, Cell Signaling Technology), rabbit anti-mouse STAT1 antibody (cat. no. 14994S, Cell Signaling Technology), rabbit anti-mouse phospho-STAT 1 antibody (cat. no. 9167S, Cell Signaling Technology), rabbit anti-mouse synaptopodin antibody (cat. no. 21064-1-AP, Proteintech), rabbit anti-mouse Ras homolog gene family, member A (RhoA) antibody (cat. no. 2117s, Cell signaling Technology) rabbit anti-mouse kidney ankyrin repeat-containing protein 2 (KANK2) antibody (cat. no. 21733-1-AP, Proteintech), and rabbit anti-mouse inverted formin 2 (INF2) antibody (cat. no. 20466-1-AP, Proteintech), at 4°C. The appropriate anti-IgG with horseradish peroxidase-labeled secondary antibodies (cat. no. 31431 and 65-6120, Thermo Fisher Scientific) were used at a concentration of 1:7,000. After incubation with Supersignal West Dura (Thermo Fisher Scientific) the intensity signal was detected by chemiluminescence (Bio-Rad). β-Actin (cat. no. A1978, Sigma-Aldrich) was used a loading control. Quantitative analysis of band density was performed by Image J software (NIH Image, Bethesda, MD) and protein expression was normalized to β-actin. All antibodies used in this study were obtained from commercial sources with validation performed by these sources.
Enzyme-linked immunosorbent assay for IL-6.
Confluent, differentiated WT or FcRn KO podocytes were treated with albumin, IgG, or inflammatory stimuli for the indicated times and then stored at −80°C until processing. The IL-6 concentration was measured by using a commercially available ELISA kit (cat. no. 431304, Biolegend, CA) according to the manufacturer’s instructions.
Real-time polymerase chain reaction.
Total ribonucleic acid (RNA) was extracted from cultured WT and FcRn KO podocytes after treatment with inflammatory stimuli using RNeasy Mini Kit (cat. no. 74136, Qiagen, Valencia, CA). The complementary deoxyribonucleic acid (cDNA) was synthesized from 1.0 μg of total RNA with SuperMix (cat. no. 10142-786, Quantabio). PCR was performed using Power SYBR Green PCR Master Mix (cat. no. 4368702, Thermo Fisher Scientific) and the Applied Biosystems Real-time PCR system. Real-time PCR was measured with three reactions in each gene target (triple replication technique). The PCR primer used for IL-6 was as follows: 5′-GAA ATG AGA AAA GAG TTG TGC AAT G-3′ and reverse 5′-GGT ACT CCA GAA GAC CAG AGG-3′. The gene expression was calculated using comparative cycle threshold method and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The relative fold change of IL-6 expression compared with WT treated with regular media (as the reference group).
Cell migration assay.
Differentiated, confluent WT and FcRn KO podocytes were treated with different inflammatory stimuli. The cell monolayer was then scored with a 200 µl pipette tip. To evaluate cell motility, images were taken at 0 and 8 h by phase-contrast microscope. Images were analyzed using by ImageJ software (NIH Image, Bethesda, MD).
Actin cytoskeleton structure analysis.
WT and FcRn KO podocytes were seeded onto 35 mm2 collagen-coated glass bottom dishes (MatTek, MA) and differentiated at 37°C. Differentiated podocytes were treated with or without IL-6 (100 ng/mL) containing media for 48 h. After treatment, podocytes were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) with 0.5% Triton X-100 (20 min; room temperature). Actin filaments were stained with Alexa Flour 488 phalloidin (1:1,000, cat. no. A-11006, Invitrogen, Thermo Fisher Scientific), and nuclei were stained with Hoechst 33342 (1:1,000, cat. no. H3570, Thermo Fisher Scientific). Confocal microscopy images were performed using a Zeiss 780 laser-scanning confocal microscope (Zeiss, Thornwood, NY). Stress fiber length was quantitated in three independent experiments using ImageJ software (NIH Image, Bethesda, MD).
RhoA activity assay.
RhoA activity was measured in untreated WT or FcRn KO podocytes and WT or FcRn podocytes treated with IFNɣ, NP-ovalbumin or IFNɣ + NP-ovalbumin for 30 min. Cell lysate was harvested and RhoA activity was measured using the RhoA G-LISA Activation Assay Kit (Colorimetric format) from Cytoskeleton Inc. (CO).
Statistical analysis.
Data were analyzed using Prism software (GraphPad, San Diego, CA) and are shown as means ± SE. Statistical analysis was performed using t tests for two groups and one-way analysis of variance for three or more groups. Tukey’s post hoc t test was applied to the ANOVA data. A P value of <0.05 was considered statistically significant.
RESULTS
FcRn KO podocytes produced and expressed a minimal amount of IL-6 after an immune challenge whereas WT podocytes had a robust response.
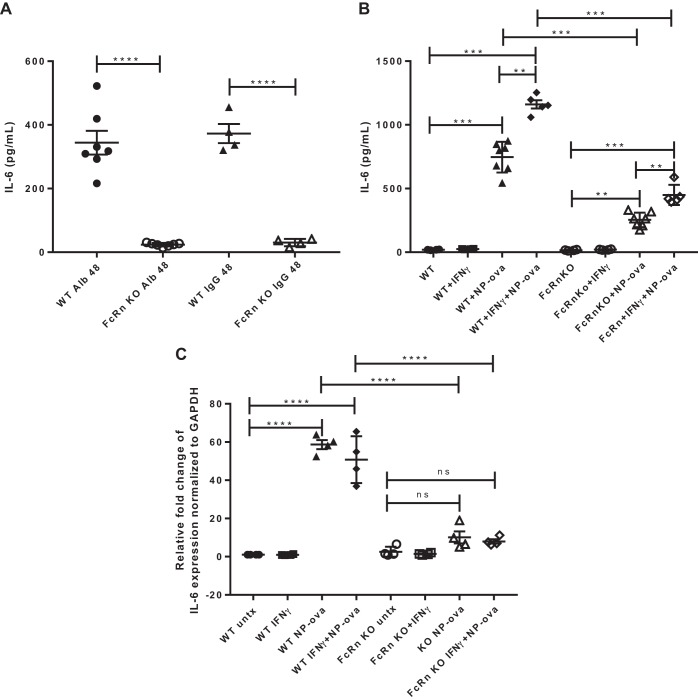
In a previous study, we have shown that our conditionally immortalized WT and FcRn KO podocytes express the podocyte markers podocin and WT-1 and that FcRn is expressed in WT podocytes and is absent in the KO (9). To evaluate whether WT or FcRn KO podocytes respond differently to proinflammatory stimuli, podocytes were treated with albumin, IgG, IFNɣ, and immune complexes (NP-ovalbumin). The amount of IL-6 released into the supernatant was assayed by ELISA, and gene expression was measured by qPCR. After treatment with albumin and IgG for 48 h, FcRn KO podocytes produced a minimal amount of IL-6 whereas WT podocytes had a robust response. The amount of IL-6 produced by WT podocytes was ~15-fold that produced by KO cells (Fig. 1A). WT podocytes also produced and expressed significantly more IL-6 than KO in response to NP-ovalbumin alone or NP-ovalbumin + IFNɣ (Fig. 1B, indicating that the KO have impaired IL-6 production in response to proinflammatory stimuli. The significant decrease in IL-6 was seen also at the mRNA level as FcRn KO podocytes had significantly lower levels of IL-6 mRNA after treatment with NP-ova or IFNɣ + NP-ova compared with WT (Fig. 1C).
Fig. 1.
Neonatal Fc receptor (FcRn) knockout (KO) podocytes produce significantly less IL-6 than wild type (WT) in response to albumin, IgG, or proinflammatory stimuli. A: IL-6 concentration in the supernatant harvested from WT or FcRn KO podocytes treated with albumin (Alb) or IgG for 48 h (n ≥ 4 experiments). B: IL-6 concentration in supernatant of WT and FcRn KO podocytes untreated (WT or KO) or treated with IFNɣ, 4-hydroxy-3-nitrophenylacetyl hapten-ovalbumin (NP-ova), or NP-ova plus IFNɣ for 48 h (n = ≥ 4 experiments). C: the relative fold change of IL-6 mRNA expression normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level for untreated WT or KO podocytes (untx) or treated with IFNɣ or IFNɣ + NP-ova for 48 h (n = 4 experiments). Data are presented as means ± SE; ns, not statistically significant. In A–C, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Phosphorylated STAT3 is expressed in WT but not KO podocytes after an immune challenge.
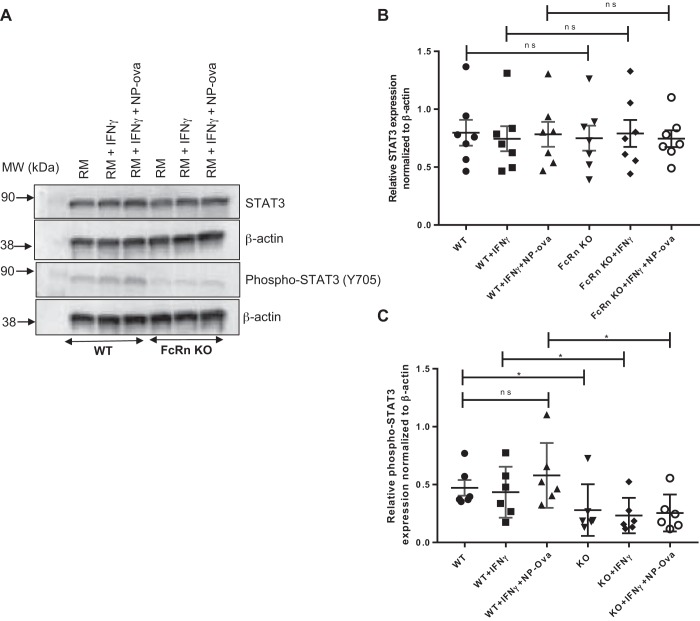
Activation of either the classic or trans IL-6 pathway leads to activation of the JAK/STAT3 cascades and phosphorylation of STAT3 which mediates downstream effects of this pathway. We examined STAT3 and phospho-STAT3 levels in WT and FcRn KO podocytes in response to IFNγ, NP-ovalbumin alone, or NP-ovalbumin + IFNɣ. At baseline and after treatment with proinflammatory stimuli, both WT and FcRn KO podocytes expressed the same level of STAT3 (Fig. 2, A and B). FcRn KO podocytes expressed significantly less both phospho-STAT3 at baseline and after treatment with proinflammatory stimuli compared with WT (P < 0.01, Fig. 2, A and C), consistent with the significant decrease in IL-6 production in the KO.
Fig. 2.
Neonatal Fc receptor (FcRn) knockout (KO) podocytes have significantly less phospho-STAT3 compared with wild type (WT) in response to proinflammatory stimuli. A: representative Western blot of STAT3, phospho-STAT3, and β-actin expression in WT and FcRn KO podocytes after treatment with regular media (RM), IFNɣ, or 4-hydroxy-3-nitrophenylacetyl hapten-ovalbumin (NP-ova), plus IFNɣ for 6 h (n = 6 experiments). B and C: densitometric analysis of STAT3 and phospho-STAT3 normalized to β-actin as quantified by ImageJ (n = 6 experiments). Data are presented as means ± SE. In B and C, ns, not statistically significant, and *P < 0.05.
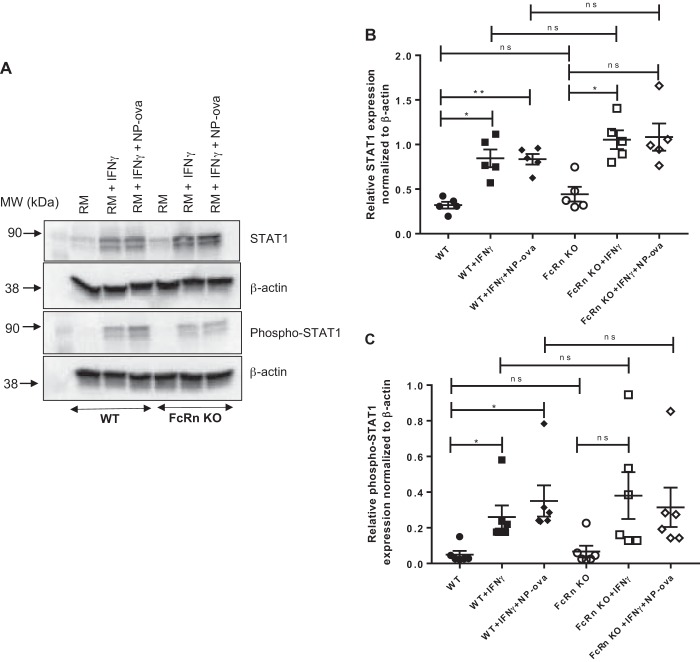
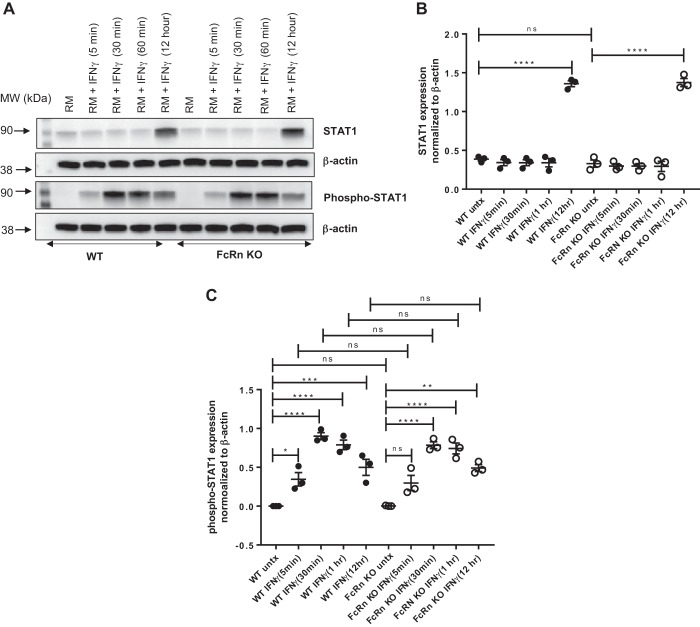
We noted that FcRn KO podocytes expressed significantly less phospho-STAT3 in response to IFNɣ treatment for 48 h. IFNɣ is known to signal via the JAK/STAT1 pathway (19). We examined STAT1 and phospho-STAT1 expression in WT and FcRn KO podocytes after treatment with proinflammatory stimuli. We found that there was no significant difference in STAT1 or phospho-STAT1 expression between WT and KO podocytes after treatment with IFNɣ or IFNɣ + NP-ova for 48 h (Fig. 3). We examined the IFNɣ response at shorter time periods and found that there was no significant difference between WT and KO podocytes in response to IFNɣ treatment for treatment periods ranging from 5 min to 12 h (Fig. 4). Thus the differences in IL-6-mediated signaling between WT and KO are primarily mediated via the JAK/STAT3 pathway.
Fig. 3.
There is no difference in phospho-STAT1 expression in wild type (WT) versus neonatal Fc receptor (FcRn) knockout (KO) podocytes in response to proinflammatory stimuli. A: Western blot of STAT1 and phospho-STAT1 in WT and FcRn KO podocytes treated with regular media (RM), IFNɣ, or IFNɣ plus 4-hydroxy-3-nitrophenylacetyl hapten-ovalbumin (NP-ova), for 6 h (n = 5 experiments). B and C: quantitative analysis of STAT1 and phospho-STAT1 expression normalized to β-actin as quantified by ImageJ (n = 5 experiments). Data are presented as means ± SE. In B and C, ns, not statistically significant, *P < 0.05, and **P < 0.01.
Fig. 4.
There is no significant difference in phospho-STAT1 in wild type (WT) or knockout (KO) podocytes treated with IFNɣ for different amounts of time. A: Western blot of STAT1 and phospho-STAT1 in WT and neonatal Fc receptor (FcRn) KO podocytes after treatment with regular media (RM) or IFNɣ for indicated times: 5 min, 30 min, 1 h, and 12 h. B and C: quantitative analysis of STAT1 and phospho-STAT1 expression normalized to β-actin as quantified by ImageJ (n = 3 experiments). Data are presented as means ± SE. In B and C, ns, not statistically significant, *P < 0.05, **P < 0.01,***P < 0.001, and ****P < 0.0001.
FcRn KO podocyte can phosphorylate STAT3 in response to IL-6.
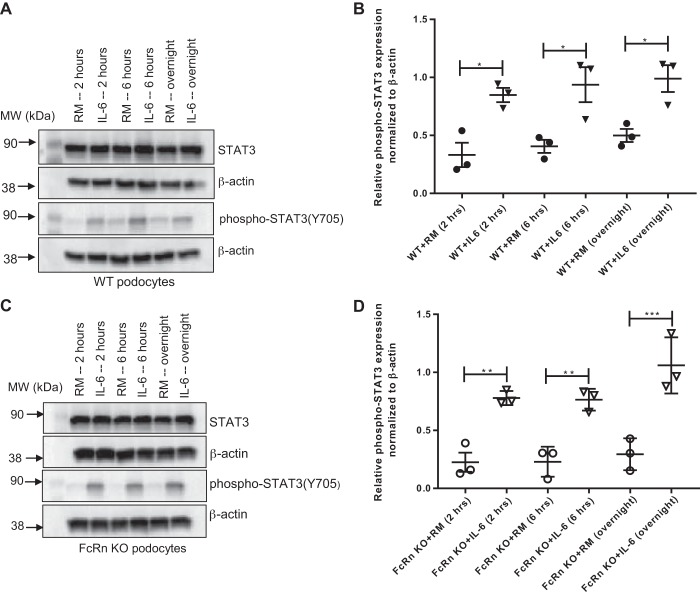
To examine whether FcRn KO podocytes can activate the JAK/STAT3 signaling cascade in response to IL-6, podocytes were treated with IL-6 for varying amounts of time and phospho-STAT3 levels were measured. Both WT and KO podocytes demonstrated a similar increase in phospho-STAT3 levels in response to IL-6 (Fig. 5), indicating that the lack of phospho-STAT3 in response to proinflammatory stimuli was due to a lack of IL-6 and not due to an intrinsic defect in the IL-6 signaling pathway in KO.
Fig. 5.
Neonatal Fc receptor (FcRn) knockout (KO) podocytes can phosphorylate STAT3 in response to IL-6. A and C: Western blot of STAT3 and phospho-STAT3 in wild type (WT) and FcRn KO podocytes after treatment with regular media (RM) with or without IL-6 for indicated times: 2 h, 6 h, and overnight. B and D: quantitative analysis of phospho-STAT3 expression of WT and FcRn KO podocytes, respectively. The graph represents the relative expression of phospho-STAT3 normalized to β-actin (n = 3 experiments for all conditions). Data are presented as mean ± SE. In B and D, *P < 0.05, **P < 0.01, and ***P < 0.001.
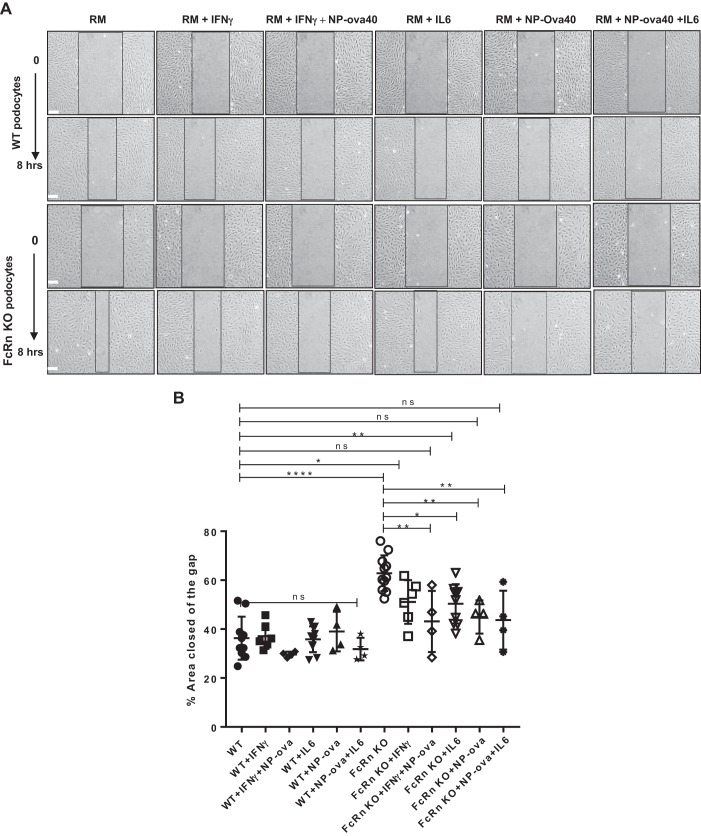
FcRn KO podocytes are hypermotile compared with WT podocytes.
IL-6-mediated signaling via the JAK/STAT3 pathway has been shown to be involved in cell motility and migration (38, 39, 42). To examine motility in WT and FcRn KO podocytes, we scratched differentiated, confluent WT or KO podocytes treated with proinflammatory stimuli and determined the percentage of the scored area closed after 8 h. We found that at baseline, KO podocytes had a significant increase in the percentage of the scored area closed compared with WT (P < 0.0001, Fig. 6, A and B), indicating that at baseline, KO podocytes are hypermotile compared with WT. Treatment of KO podocytes with IFNγ, NP-ovalbumin, IFNɣ + NP-ovalbumin, or IL-6 decreased the percent area closed at 8 h (Fig. 6, A and B), returning the motility of the FcRn KO podocytes to a phenotype more closely resembling that of WT.
Fig. 6.
Neonatal Fc receptor (FcRn) knockout (KO) podocytes are hypermotile compared with wild type (WT) and motility can be restored towards normal after treatment with proinflammatory stimuli. A: images of WT and FcRn KO podocytes immediately (time (t) = 0) and 8 h after being scratched, treated with regular media (RM), IFNɣ, IFNɣ plus 4-hydroxy-3-nitrophenylacetyl hapten-ovalbumin (NP-ova), IL-6, NP-ova, or IL-6 plus NP-ova at 0 and 8 h (n ≥ 4 experiments). Scale bar: 300 µm. B: quantitative analysis of the percentage area of the gap closed 8 h after scratching. Data were analyzed by using ImageJ. Data are presented as mean ± SE. In B, ns, not statistically significant, *P < 0.05, **P < 0.01, and ****P < 0.0001.
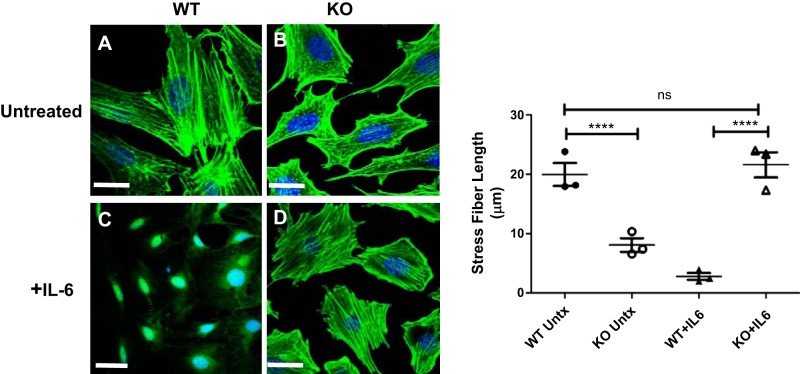
The effect of IL-6 on actin cytoskeleton structure in WT and FcRn KO podocytes.
Since STAT3 signaling has been shown to modulate actin structure and cell migration (38, 39, 42), we examined the actin cytoskeleton in WT and FcRn KO podocytes. At baseline, we found that FcRn KO podocytes had short and bunched F-actin fibers and were lacking elongated stress fibers whereas the WT podocytes had long F-actin fibers (Fig. 7, A and B). Since FcRn KO podocytes can phosphorylate and activate STAT3 in response to IL-6 (Fig. 5), we examined the effect of IL-6 treatment on FcRn KO podocyte actin structure. Treatment with IL-6 for 48 h restored stress fibers in FcRn KO podocytes (Fig. 7D), whereas WT podocytes treated with IL-6 lost stress fibers and demonstrated complete loss of actin cytoskeletal structure (Fig. 7C). Taken together, these results suggest that IL-6 tunes the actin cytoskeleton in podocytes via STAT3-mediated signaling.
Fig. 7.
Neonatal Fc receptor (FcRn) knockout (KO) podocytes have decreased stress fibers at baseline compared with wild type (WT) and treatment with interleukin-6 (IL-6) restores stress fiber formation. A and B: actin in untreated WT and FcRn KO podocytes was stained with 635 phalloidin (green). Nuclei were stained with Hoechst (blue). C and D: images were obtained after treatment with IL-6 for 48 h (n = 3 experiments for all conditions). Scale bar: 20 µm. Quantitation of stress fiber length shows that at baseline, FcRn KO podocytes have stress fibers that are significantly shorter than WT. After treatment with IL-6, there are almost no stress fibers in the WT whereas KO podocyte stress fiber length is restored to that of WT. Data are presented as means ± SE from 3 independent experiments. In B, ns, not statistically significant, and ****P < 0.0001.
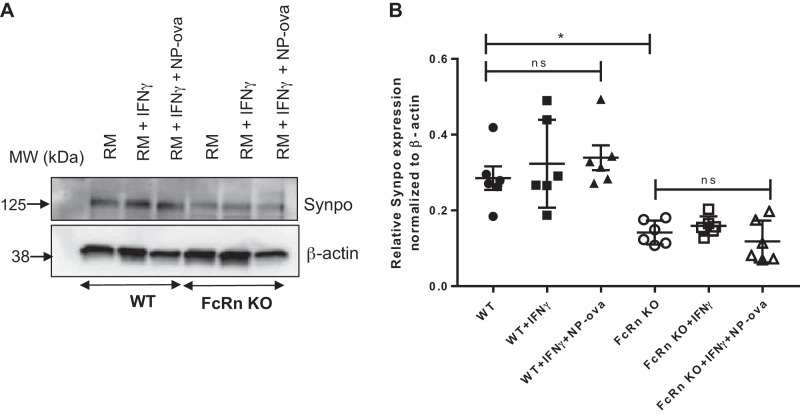
FcRn KO podocytes have reduced synaptopodin expression compared with WT.
Synaptopodin is an actin-associated protein which plays a role in the actin bundling in podocytes (3, 4). Since FcRn KO podocytes demonstrate abnormalities in the actin cytoskeleton, we examined synaptopodin expression in WT and KO podocytes at baseline and after treatment with proinflammatory stimuli. We found that FcRn KO podocytes had significantly decreased expression of synaptopodin compared with WT cells at baseline and after treatment with proinflammatory stimuli for 48 h (Fig. 8, A and B). Treatment with immune stimuli did not alter synaptopodin expression in the KO compared with baseline alone, suggesting that either the amount of IL-6 produced by the KO during the treatment period was not enough to stimulate synaptopodin expression.
Fig. 8.
Neonatal Fc receptor (FcRn) knockout (KO) podocytes have decreased synaptopodin expression at baseline and after treatment with proinflammatory stimuli. A: Western blot synaptopodin (Synpo) expression in wild type (WT) and FcRn KO podocytes treated with regular media (RM), IFNɣ, or IFNɣ plus 4-hydroxy-3-nitrophenylacetyl hapten-ovalbumin (NP-ova). B: quantitative analysis of synaptopodin expression normalized to β-actin as quantified by ImageJ (n = 6 experiments). Data are presented as means ± SE. In B, ns, not statistically significant, and *P < 0.05.
RhoA expression and activity is similar in WT and FcRn KO podocytes.
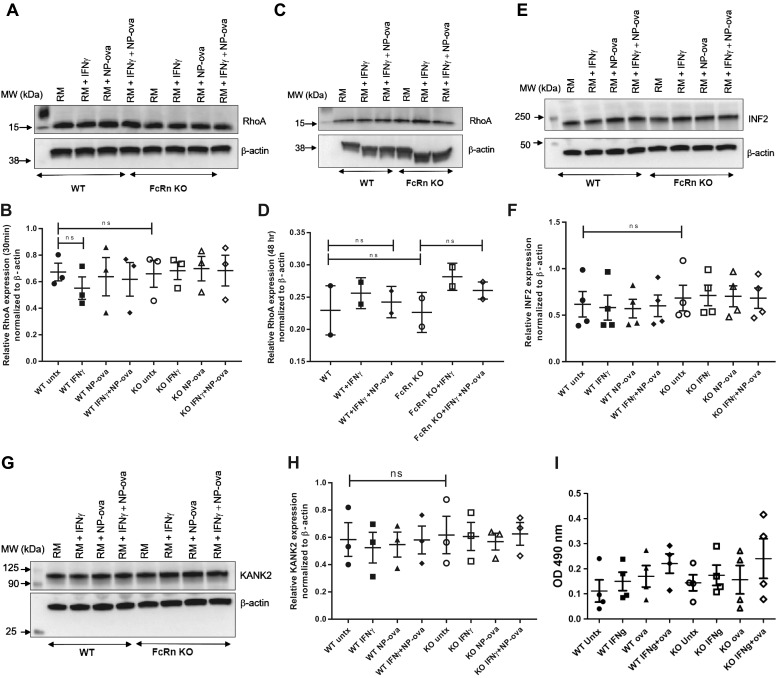
Rho A is an actin associated-actin G protein that modulates the actin cytoskeleton and podocyte morphology and motility (11) and is regulated by synaptopodin (4). Alterations in RhoA activity cause reorganization of the podocyte actin cytoskeleton (10, 44). Based on our results, we further investigated whether KO of FcRn alters RhoA expression in podocytes at baseline and after treatment with proinflammatory stimuli. We found no significant difference in RhoA expression at baseline or after treatment with proinflammatory stimuli for 30 min and 48 h (Fig. 9, A–D). There was also no significant difference in RhoA activity in WT versus KO podocytes after treatment with proinflammatory stimuli (Fig. 9I).
Fig. 9.
There is no difference in RhoA, inverted formin 2 (INF2), and kidney ankyrin repeat-containing protein 2 (KANK2) expression in wild type (WT) or knockout (KO) podocytes at baseline or after treatment with proinflammatory stimuli. A–D: Western blot analysis of RhoA in WT and neonatal Fc receptor (FcRn) KO podocytes after treatment with regular media (RM), IFNɣ, 4-hydroxy-3-nitrophenylacetyl hapten-ovalbumin (NP-ova), or IFNɣ plus NP-ova for 30 min (A and B, n = 3 experiments) and 48 h (C and D, n = 2 experiments), respectively. Data are presented as means ± SE. E and F: Western blotting of INF2 expression after treatment with regular media (RM), IFNɣ, NP-ova, or IFNɣ plus NP-ova for 30 min (n = 4 experiments). Data are presented as means ± SE. G and H: Western blotting of KANK2 expression after treatment with regular media (RM), IFNɣ, NP-ova or IFNɣ plus NP-ova for 30 min (n = 3 experiments). Data are presented as means ± SE. In B, D, F, and H, ns, not statistically significant. I: RhoA activity in WT and FcRn KO podocytes after treatment with regular media, IFNɣ, NP-ova, or NP-ova + IFNɣ for 30 min.
INF2 expression in WT and FcRn KO podocytes.
Inverted formin 2 (INF2) helps to maintain actin dynamics in podocytes and is the antagonist of RhoA signaling. Knockout of INF2 leads to altered actin dynamics and protein trafficking within the slit diaphragm (36). To investigate whether FcRn KO alters INF2 expression, we performed Western blotting of WT and FcRn KO podocytes after challenge with inflammatory stimuli. We found that INF2 expression was not significantly different between WT and FcRn KO podocytes at baseline and after challenge with inflammatory stimuli (Fig. 9, E and F).
KANK2 expression in WT and FcRn KO podocytes.
Previous studies have shown that KANK2 (KN motif and ankyrin repeat domains) localizes to podocytes and regulates Rho GTPase activity. Knockout of KANK2 in podocyte resulted in increases of RhoA activity and reduced cell migration (14). To determine whether FcRn KO effect KANK2 in cultured podocytes, we examined KANK2 expression in WT and FcRn KO cells after inflammatory challenge. Our results demonstrated that there were not significant differences of KANK2 expression in WT and FcRn KO podocytes (Fig. 9, G and H).
DISCUSSION
Podocytes are a target of injury in many immune-mediated kidney diseases, but podocyte-specific therapies are limited as there is little understanding of how podocytes respond to immune challenges. Podocytes have been proposed to be nontraditional antigen-presenting cells (15) and have been shown to produce cytokines including IL-6 after challenge with proinflammatory stimuli (25). Podocytes also express the neonatal Fc receptor, FcRn, which plays a crucial role in trafficking of IgG (1, 9, 32), antigen presentation (15), and regulating the cellular and humoral immune response (7, 41). Little is known, however, about how antigen trafficking in podocytes affects cytokine production or response. Here we demonstrate the knockout of FcRn in podocytes results in a significant reduction in IL-6 production which in turn leads to altered signaling via STAT3 as well as alterations in podocyte motility and the actin cytoskeleton. To the best of our knowledge, this is the first study linking trafficking of immune complexes, cytokine production, and actin modulation in podocytes.
Abnormal signaling via the JAK/STAT pathway has been implicated in glomerular diseases such as diabetic nephropathy and focal segmental glomerulosclerosis (FSGS). Zhang et al. (43) have found that overexpression of JAK2 in podocytes leads to increased albuminuria and worsened diabetic histology in mice. In individuals with FSGS, JAK/STAT signaling is upregulated in both peripheral blood mononuclear cells as the glomerular and tubular compartments in the kidney (37). The molecular events initiating abnormal signaling through the JAK/STAT pathway in glomerular diseases need to be elucidated, but our study would suggest that aberrant cellular trafficking may play a significant role.
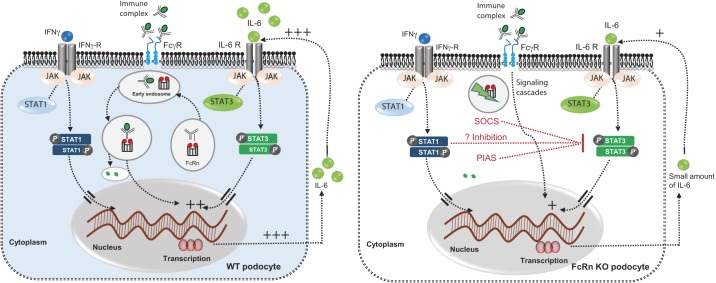
While IL-6-mediated signaling via STAT3 was impaired in FcRn KO podocytes, IFNɣ signaling via STAT1 was not significantly altered in the KO, suggesting that alterations in FcRn-mediated trafficking cause defects specifically in IL-6-mediated pathways. Previous work has also shown that STAT1 and STAT3 can compete for the same phosphotyrosine motif. Thus, in the FcRn KO podocyte, unopposed signaling via IFNɣ may lead to additional inhibition of the signaling through the STAT3 pathway (Fig. 10).
Fig. 10.
Model of differential STAT1 and STAT3 signaling pathways in wild type (WT) and neonatal Fc receptor (FcRn) knockout (KO) podocytes. Left: in WT podocytes, antigen/antibody (immune complexes) bind FcRn in the late endosome. Immune complexes (ICs) bound to FcRn are trafficked to the lysosome for proteolytic degradation. This trafficking pathway leads to IL-6 production via an unknown mechanism. IL-6 in turns binds to the IL-6 receptor (IL-6 R) on podocytes and activates the JAK/STAT3 pathway. IFNɣ signals via the IFNɣ receptor and the JAK/STAT1 pathway. Right: in FcRn KO podocytes, lack of FcRn leads to significantly less IL-6 production and thus little activation of the JAK/STAT3 pathway. Signaling through the JAK/STAT1 pathway via IFNɣ is unaffected in the KO. Unopposed signaling via phospho-STAT1 may lead to further inhibition of signaling via phospho-STAT3. SOCS, suppressor of cytokine signaling; PIAS, protein inhibitor of activated STAT.
A limitation of the present study is that the work was performed in cultured podocytes. Certain features of cultured podocytes such as stress fibers may not be found in podocytes in vivo (29). While cultured podocytes do not fully replicate the phenotype in vivo, they do allow for more detailed mechanistic studies which are often difficult to perform in vivo given podocytes’ unique location within the filtration barrier.
We have found that knockout of FcRn suppresses both IL-6 transcription and translation. The molecular basis underlying IL-6 transcription is not well understood and thus it is not clear how knockout of FcRn, which is a protein that sorts and traffics immune complexes, could lead to IL-6 suppression. Our work indicates that FcRn KO in podocytes does not lead to impaired signaling through the IL-6 receptor when sufficient IL-6 is present, as treating FcRn KO podocytes with IL-6 resulted in comparable levels of phosphorylated STAT3 as wild type.
Previous work has shown that the IL-6-mediated JAK/STAT3 pathway plays a role in cell motility (38, 39, 42). In accordance with these findings, we have found that knockout of FcRn in podocytes leads to podocyte hypermotility and that this increased motility in the KO can be corrected by treatment with exogenous IL-6. Since actin-associated proteins and cytoskeletal structure influence podocyte motility and migration (5), we examined the role of FcRn in podocyte structure and dynamics. Our results demonstrated that knockout of FcRn results in a reduction of synaptopodin expression in vitro. Interestingly, FcRn KO podocytes had abnormal F-actin-bundles and stress fibers and these abnormalities in F-actin could be rescued by IL-6 treatment. These findings provide additional evidence that FcRn plays a key role in actin cytoskeletal organization and podocyte motility and provides a link between the sorting of IgG and immune complexes in podocytes and podocyte structure.
RhoA, a protein in the Rho family of GTPases, regulates cytoskeleton cell branching morphology, which modulates cell dynamics (11). Previous work has shown that synaptopodin regulates actin stress fiber and migration of podocytes via RhoA signaling (4) by blocking of proteasomal degradation (6). We have found that although knockout of FcRn in podocytes leads to a reduction in synaptopodin expression, there was no change in the expression of RhoA, INF2, or KANK2, which have been shown to regulate RhoA activity. Similarly, we did not find a significant difference in RhoA activity in KO podocytes compared with WT after treatment with proinflammatory stimuli. Thus knockout of FcRn may alter the cytoskeletal structure in podocytes via non-RhoA-mediated mechanisms. Further work will be required to elucidate precisely how knockout of FcRn alters the podocyte actin cytoskeleton.
In conclusion, this study demonstrates that knockout of FcRn abrogates IL-6 production and signaling in response to immune complexes and proinflammatory stimuli which also alters podocyte motility and cytoskeletal structure. Our work suggests that FcRn links the trafficking of immune complexes in podocytes to podocyte structure and function and may be a key player in the podocyte response to immune challenges.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK104264 to J. Blaine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.B. conceived and designed research; P.T., J.D., L.L., and J.B. performed experiments; P.T., J.D., L.L., and J.B. analyzed data; P.T., J.D., and J.B. interpreted results of experiments; P.T. and J.B. prepared figures; P.T., J.D., L.L., and J.B. drafted manuscript; P.T., J.D., L.L., and J.B. edited and revised manuscript; P.T., J.D., L.L., and J.B. approved final version of manuscript.
REFERENCES
- 1.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA 105: 967–972, 2008. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian D. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest 113: 1328–1333, 2004. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198, 2005. doi: 10.1172/JCI200523371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 5.Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 94: 235–263, 2014. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- 6.Buvall L, Rashmi P, Lopez-Rivera E, Andreeva S, Weins A, Wallentin H, Greka A, Mundel P. Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics. Nat Commun 4: 2863, 2013. doi: 10.1038/ncomms3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervenak J, Bender B, Schneider Z, Magna M, Carstea BV, Liliom K, Erdei A, Bosze Z, Kacskovics I. Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J Immunol 186: 959–968, 2011. doi: 10.4049/jimmunol.1000353. [DOI] [PubMed] [Google Scholar]
- 8.Dobrinskikh E, Lewis L, Doctor RB, Okamura K, Lee MG, Altmann C, Faubel S, Kopp JB, Blaine J. Shank2 regulates renal albumin endocytosis. Physiol Rep 3: e12510, 2015. doi: 10.14814/phy2.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dylewski J, Dobrinskikh E, Lewis L, Tonsawan P, Miyazaki M, Jat PS, Blaine J. Differential trafficking of albumin and IgG facilitated by the neonatal Fc receptor in podocytes in vitro and in vivo. PLoS One 14: e0209732, 2019. doi: 10.1371/journal.pone.0209732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 420: 629–635, 2002. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 12.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest 94: 585–591, 1994. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, Scheller J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev 23: 85–97, 2012. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, Zhou W, Lovric S, Fang H, Nettleton M, Zhu JY, Hoefele J, Weber LT, Podracka L, Boor A, Fehrenbach H, Innis JW, Washburn J, Levy S, Lifton RP, Otto EA, Han Z, Hildebrandt F. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125: 2375–2384, 2015. doi: 10.1172/JCI79504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldwich A, Burkard M, Ölke M, Daniel C, Amann K, Hugo C, Kurts C, Steinkasserer A, Gessner A. Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol 24: 906–916, 2013. doi: 10.1681/ASN.2012020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagenstein J, Melderis S, Nosko A, Warkotsch MT, Richter JV, Ramcke T, Herrnstadt GR, Scheller J, Yan I, Mittrücker HW, Kluger MA, Steinmetz OM. A novel role for IL-6 receptor classic signaling: induction of RORγt+Foxp3+ Tregs with enhanced suppressive capacity. J Am Soc Nephrol 30: 1439–1453, 2019. doi: 10.1681/ASN.2019020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haymann JP, Levraud JP, Bouet S, Kappes V, Hagège J, Nguyen G, Xu Y, Rondeau E, Sraer JD. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 11: 632–639, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, Avalos-Díaz E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus 7: 154–158, 1998. doi: 10.1191/096120398678919949. [DOI] [PubMed] [Google Scholar]
- 19.Horvath CM. The Jak-STAT pathway stimulated by interferon gamma. Sci STKE 2004: tr8, 2004. doi: 10.1126/stke.2602004tr8. [DOI] [PubMed] [Google Scholar]
- 20.Jones SA, Fraser DJ, Fielding CA, Jones GW. Interleukin-6 in renal disease and therapy. Nephrol Dial Transplant 30: 564–574, 2015. doi: 10.1093/ndt/gfu233. [DOI] [PubMed] [Google Scholar]
- 21.Karkar AM, Smith J, Tam FW, Pusey CD, Rees AJ. Abrogation of glomerular injury in nephrotoxic nephritis by continuous infusion of interleukin-6. Kidney Int 52: 1313–1320, 1997. doi: 10.1038/ki.1997.456. [DOI] [PubMed] [Google Scholar]
- 22.Luig M, Kluger MA, Goerke B, Meyer M, Nosko A, Yan I, Scheller J, Mittrücker HW, Rose-John S, Stahl RA, Panzer U, Steinmetz OM. Inflammation-induced IL-6 functions as a natural brake on macrophages and limits GN. J Am Soc Nephrol 26: 1597–1607, 2015. doi: 10.1681/ASN.2014060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miner JH. The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagata M. Podocyte injury and its consequences. Kidney Int 89: 1221–1230, 2016. doi: 10.1016/j.kint.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Okamura K, Dummer P, Kopp J, Qiu L, Levi M, Faubel S, Blaine J. Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS One 8: e54817, 2013. doi: 10.1371/journal.pone.0054817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olaru F, Luo W, Suleiman H, St. John PL, Ge L, Mezo AR, Shaw AS, Abrahamson DR, Miner JH, Borza DB. Neonatal Fc receptor promotes immune complex-mediated glomerular disease. J Am Soc Nephrol 25: 918–925, 2014. doi: 10.1681/ASN.2013050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J Immunol 194: 4595–4603, 2015. doi: 10.4049/jimmunol.1403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA 105: 9337–9342, 2008. doi: 10.1073/pnas.0801717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinschen MM, Gödel M, Grahammer F, Zschiedrich S, Helmstädter M, Kretz O, Zarei M, Braun DA, Dittrich S, Pahmeyer C, Schroder P, Teetzen C, Gee H, Daouk G, Pohl M, Kuhn E, Schermer B, Küttner V, Boerries M, Busch H, Schiffer M, Bergmann C, Krüger M, Hildebrandt F, Dengjel J, Benzing T, Huber TB. A multi-layered quantitative in vivo expression atlas of the podocyte unravels kidney disease candidate genes. Cell Rep 23: 2495–2508, 2018. doi: 10.1016/j.celrep.2018.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7: 715–725, 2007. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 31.Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb Perspect Biol 10: a028415, 2018. doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, Quigg RJ. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol 20: 1941–1952, 2009. doi: 10.1681/ASN.2008090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sesarman A, Sitaru AG, Olaru F, Zillikens D, Sitaru C. Neonatal Fc receptor deficiency protects from tissue injury in experimental epidermolysis bullosa acquisita. J Mol Med (Berl) 86: 951–959, 2008. doi: 10.1007/s00109-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 34.Su H, Lei CT, Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol 8: 405, 2017. doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol 9: 470–477, 2013. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun H, Schlondorff J, Higgs HN, Pollak MR. Inverted formin 2 regulates actin dynamics by antagonizing Rho/diaphanous-related formin signaling. J Am Soc Nephrol 24: 917–929, 2013. doi: 10.1681/ASN.2012080834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, Hartman J, Wang W, Kretzler M, Lafayette RA. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int 94: 795–808, 2018. doi: 10.1016/j.kint.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng TS, Lin B, Manser E, Ng DC, Cao X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J Cell Sci 122: 4150–4159, 2009. doi: 10.1242/jcs.057109. [DOI] [PubMed] [Google Scholar]
- 39.Teng Y, Ross JL, Cowell JK. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAK-STAT 3: e28086, 2014. doi: 10.4161/jkst.28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, Kaldenbach M, Boor P, Fuss A, Uhlig S, Lanzmich R, Willemsen B, Dijkman H, Grepl M, Wild K, Kriz W, Smeets B, Floege J, Moeller MJ. Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol 24: 1966–1980, 2013. doi: 10.1681/ASN.2013010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Végh A, Farkas A, Kövesdi D, Papp K, Cervenak J, Schneider Z, Bender B, Hiripi L, László G, Prechl J, Matkó J, Kacskovics I. FcRn overexpression in transgenic mice results in augmented APC activity and robust immune response with increased diversity of induced antibodies. PLoS One 7: e36286, 2012. doi: 10.1371/journal.pone.0036286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Z, Jiang W, Wang H, Li H, Tang B, Liu B, Jiang H, Sun X. The IL-6/STAT3 pathway regulates adhesion molecules and cytoskeleton of endothelial cells in thromboangiitis obliterans. Cell Signal 44: 118–126, 2018. doi: 10.1016/j.cellsig.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Nair V, Saha J, Atkins KB, Hodgin JB, Saunders TL, Myers MG Jr, Werner T, Kretzler M, Brosius FC. Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int 92: 909–921, 2017. doi: 10.1016/j.kint.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]