Keywords: dysbiosis, homeostasis, microbiome, microbiota
Abstract
Advances in data collection technologies reveal that an imbalance (dysbiosis) in the composition of host-associated microbial communities (microbiota) is linked to many human illnesses. This association makes dysbiosis a central concept for understanding how the human microbiota contributes to health and disease. However, it remains problematic to define the term dysbiosis by cataloguing microbial species names. Here, we discuss how incorporating the germ-organ concept, ecological assumptions, and immunological principles into a theoretical framework for microbiota research provides a functional definition for dysbiosis. The generation of such a framework suggests that the next logical step in microbiota research will be to illuminate the mechanistic underpinnings of dysbiosis, which often involves a weakening of immune mechanisms that balance our microbial communities.
INTRODUCTION
In the wake of advances in data collection and analysis technologies, the 21st century has seen the birth of a new discipline devoted to studying microbial communities inhabiting our body (the human microbiota). Pioneering work cataloguing microbial species reveals that an imbalance in the human microbiota is linked to various noncommunicable diseases (11), thereby raising the hope that investment into microbiota research might yield new therapeutic approaches for many human illnesses. However, there is a growing consensus that information gained from cataloguing microbial species names is reaching an asymptote, where additional work will increase the size of available databases without bringing about revolutionary advances in understanding what constitutes a healthy microbiome (54). Considering this problem in microbiome research, we should reflect on how we arrived at this juncture and assess where the field may be headed.
CATALOGUING THE MICROBIOME TO DEFINE HOMEOSTASIS AND DYSBIOSIS
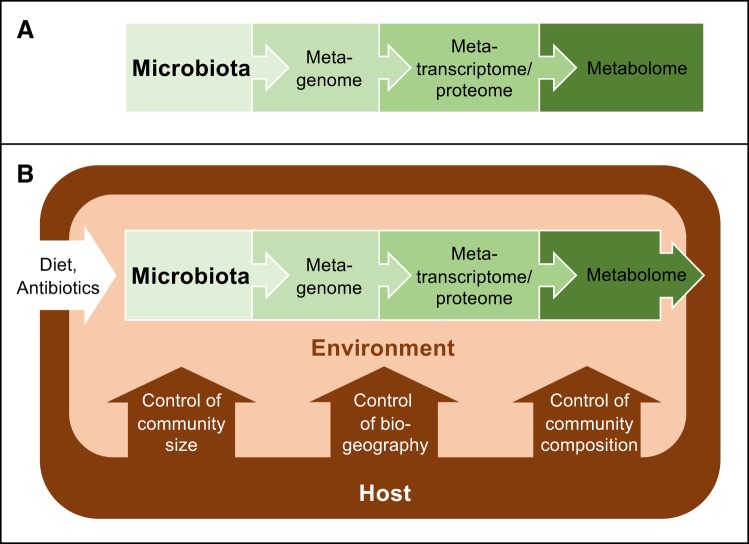
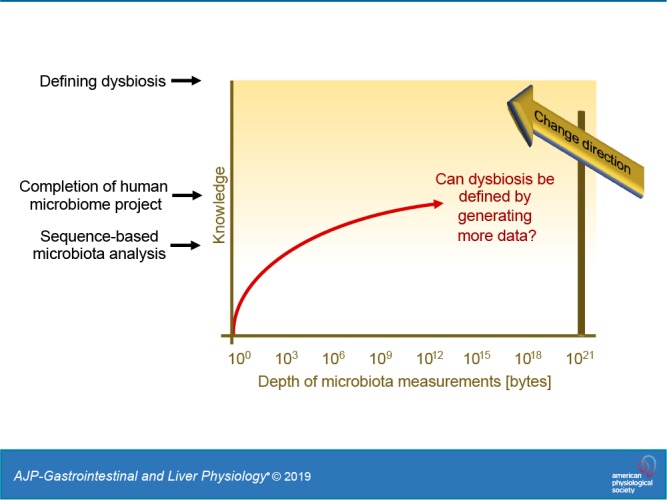
One reason for the strong emphasis that microbiota research has placed on cataloguing microbial genes and species names is the concept that host-associated microbial communities form an entity that is separate from the rest of the body (54). This viewpoint is reflected in an early definition of the term microbiome credited to Joshua Lederberg, who suggested that the expression should be used to describe the collective genome of our resident microbes (28). Building on this concept, the microbiome is commonly defined as the microbiota, its genes, and gene products, which puts the focus entirely on the microbes (Fig. 1A). This inward-looking view of host-associated microbial communities nurtures an expectation that cataloguing microorganisms, microbial genes, and microbiota-derived metabolites in sufficient depth will provide a complete understanding of the microbiome eventually. To accomplish this goal, the Human Microbiome Project, launched in 2007 by the National Institutes of Health, set out to identify elements that are common to a healthy microbiome (30, 31). Initial efforts to catalog genes present in the large intestine failed to define a core microbiome (74), which prompted calls for additional studies with increased sample sizes (44). This call was heeded through the generation of 42 terabytes of multiomic data by the Human Microbiome Project, which yielded a wealth of community resources (31). However, the remarkable depth of microbiota measurements achieved by the Human Microbiome Project in 2019 did not reveal what constitutes a healthy microbiome (54), which illustrates that this approach has limitations. As cataloguing microbial species and their genes does not reveal what represents a balanced microbial community, some go as far as to suggest that the balance concept is a holdover from prescientific thought, as it cannot be measured and is, therefore, not useful for microbiome research (52).
Fig. 1.
Graphical representation of different microbiome concepts. A: The organ metaphor suggests that the microbiota contains our second genome, which spawned the concept that the microbiome is composed of the microbiota, its genes, and gene products. B: ecological frameworks define the microbiome as the microbiota and its environment. The host shapes the microbial environment using habitat filters that control the size, biogeography, and species composition of the microbiota. Thus, ecologically, the microbiome can be viewed as a host-microbe chimera.
Our inability to define balance, in turn, makes it problematic to specify what constitutes an imbalance in the microbiota, commonly referred to as dysbiosis, a major organizing concept in microbiome research (52). Dysbiosis can be described as a compositional and functional alteration in the microbiota in individuals with disease compared with healthy subjects (35). Dysbiosis can feature a loss of beneficial microorganisms, an expansion of potentially harmful microbes, and/or a loss of overall microbial diversity (53). But since researchers still do not agree on what constitutes a healthy microbiome, it is not clear how to define an impaired one (54). Despite its elusiveness, the term dysbiosis is gaining popularity among scientists, as indicated by a rapidly growing number of articles using this expression (Fig. 2), including some published by the authors of this review. Recently, after publishing an article discussing the dysbiosis concept (38), one of us received an email from an eminent microbiome researcher urging us not to use this “unscientific” expression because “the gut microbiota is dynamic and changing and the term dysbiosis cannot be applied to it, it has no units and cannot even be defined.” We believe this criticism points to the larger underlying problem that data-driven research has been ineffective in translating terabytes of information into a theoretical framework. As a result, the terminology and organizing concepts generated through technology-based empiricism remain elusive (7, 52). Increasing the amount of available multiomic microbiome profiling data from terabytes to petabytes holds little promise for clarifying how to define dysbiosis, which raises the question of where microbiome science should go from here.
Fig. 2.
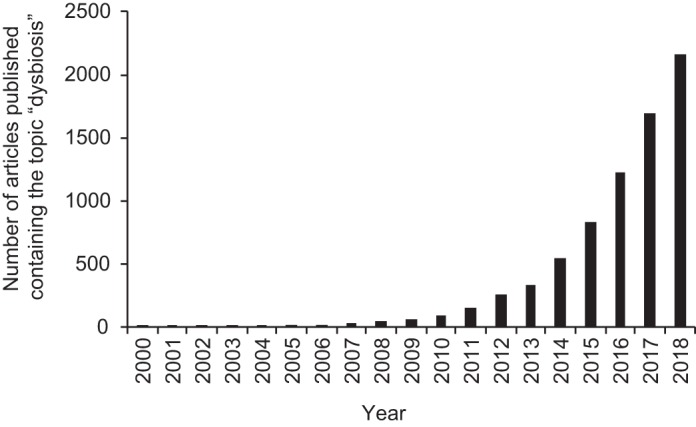
Increasing popularity of the term dysbiosis in the scientific literature. The graph shows the annual number of articles deposited in the Web of Science database that contain the search topic “dysbiosis”.
A CASE FOR CHANGING THE COURSE OF MICROBIOME SCIENCE BY WIDENING ITS SCOPE
As cataloguing the microbiome in greater depth is not likely to provide decisive breakthroughs, the elephant in the room becomes that microbiome research has to move in a new direction to meet expectations of generating novel interventions for treating diseases that are linked to dysbiosis. At the conclusion of the Human Microbiome Project, Gilbert and Lynch wrote a perspective on the future direction of microbiota research, which highlights the necessity to incorporate community ecology as a framework for microbiome studies (24). There is a growing appreciation for the need to understand the ecological and evolutionary relationships microbes have with each other and with their hosts (54). Notably, studies investigating both the human host and the microbiota are rare, but neglecting the host omits how we interact with our resident microbial communities (41). Additionally, the host should be considered a foundation species because of its substantial role in structuring the ecosystem through nutrient flow, the immune system, and by comprising many of its habitats. Thus, omitting the host from microbiome studies is inadmissible within this framework. Incorporating ecological principles into microbiota research provides an important impetus for expanding research beyond the microbes to include an influence from the host, because in ecological terms, the microbiome is defined as the microbiota and its environment (69) (Fig. 1B). This is a much broader definition than the one suggested by Joshua Lederberg (28), as it includes the environment inhabited by the microbiota, which is shaped by the human host (10, 17). In other words, incorporating ecology as a theoretical framework suggests that the microbiome is a host-microbe chimera composed of the microbiota and host factors that influence the microbial environment (8). Thus, whereas cataloguing microbial species and their genes can shine a light on an important piece of the microbiome puzzle (Fig. 1A), this approach alone cannot provide a complete picture of the microbiome (Fig. 1B), which explains why further increasing the depth of microbiota measurements is not likely to create future breakthroughs. To obtain a complete picture of the microbiome, it will be necessary to change our approach by incorporating how the host shapes the environment inhabited by the microbiota into a conceptual framework.
The behavior of the host, including choice of habitat and diet, influences microbiota composition by altering host habitat patches in the intestine (47). In addition, host cells and host tissues employ numerous control mechanisms that serve as habitat filters for sculpting the microbiota (Fig. 1B). Some host control mechanisms limit the size of microbial communities, which includes physical defenses, such as the mucus escalator in the lung (55), or nonspecific chemical defenses, such as gastric acid (61). Paneth cells of the small bowel can influence the biogeography of microbial communities by secreting antimicrobial peptides, such as human defensin 5, which limits growth of segmented filamentous bacteria (phylum Firmicutes) that occupy a spatial niche in direct contact with the epithelial surface (60). Similarly, MUC2 mucin secretion by goblet cells shapes the biogeography in the large intestine by forming a densely packed inner mucus layer that is largely devoid of bacteria (32). Finally, some host control mechanisms function in shaping the species composition of the microbiota, which is exemplified by the breakdown of host-derived glycogen by human α-amylase in vaginal mucosal fluid to nourish members of the genus Lactobacillus (phylum Firmicutes) (66), a taxon that dominates the microbiota in the female reproductive tract (13, 56). Whereas this list of examples is by no means complete, it will suffice to illustrate that the host has control over the environment inhabited by the microbiota (10). The question arising from this concept is for what purpose does the host control the size, biogeography, and species composition of the microbiota.
ACKNOWLEDGING HOST CONTROL OVER THE ECOSYSTEM DEFINES DYSBIOSIS FUNCTIONALLY
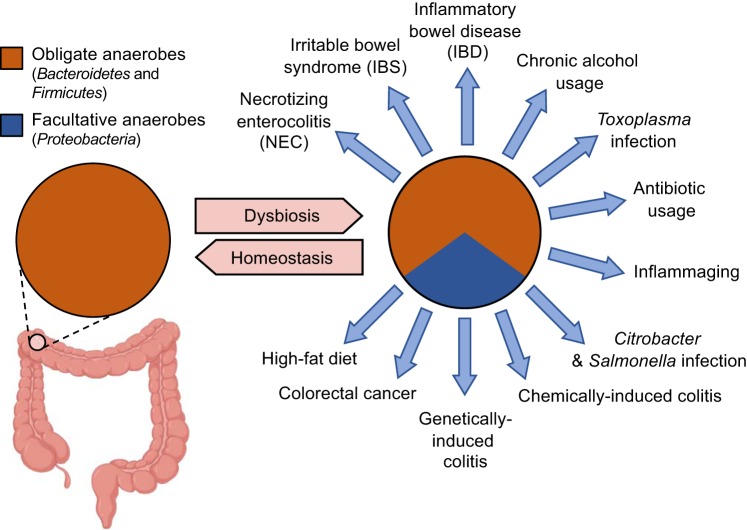
Ecological theory suggests that host control over the microbial environment shapes the microbiota to be beneficial (17). In some cases, host control mechanisms that limit the size of microbial communities or keep microorganisms at a distance from the epithelium might serve simply to prevent harm to mucosal surfaces. However, microbial communities also provide direct benefit to the host, and these functions need to be maintained to preserve homeostasis. For instance, obligate anaerobic bacteria belonging to the classes Clostridia (phylum Firmicutes) and Bacteroidia (phylum Bacteroidetes), which dominate the microbiota of the large intestine (30), perform an important digestive function for the host. Complex carbohydrates, which escape digestion by host enzymes in the upper gastrointestinal tract, are broken down in the colon by Clostridia and Bacteroidia into fermentation products, such as short-chain fatty acids, which are absorbed by the host for nutrition (14, 16, 72). Furthermore, microbial metabolites, such as short-chain fatty acids, also provide benefit by contributing to immune education (2, 4, 21, 64). Because of the benefit it provides to the host, the colonic microbiota could be viewed as an organ-like collection of microbes (11, 45, 51). To ensure functionality of its microbial organ, the host limits the amount of oxygen diffusing into the intestinal lumen by maintaining the colonic epithelium in a state of physiological hypoxia (<1% O2) (75). Hypoxia is actively maintained through epithelial peroxisome proliferator-activated receptor-γ signaling, which increases cellular oxygen consumption by activating mitochondrial oxidative phosphorylation (9). Since oxygen can diffuse across biological membranes, epithelial hypoxia limits the amount of oxygen entering the intestinal lumen, which helps to maintain anaerobiosis, thereby driving a dominance of obligate anaerobic Clostridia and Bacteroidia (9, 58) (Fig. 3). This series of events enables the host to maintain functionality of its microbial organ by shaping the composition of the gut microbiota toward a dominance of species capable of performing digestive functions that confer benefit to the host, a concept termed the germ-organ theory (7). Notably, the germ-organ theory provides a functional definition for what constitutes a healthy microbiome in the colon by suggesting that the host maintains homeostasis by limiting the availability of a critical resource, oxygen, to preserve the digestive function of its microbial organ (7).
Fig. 3.
An expansion of Proteobacteria is a microbial signature of dysbiosis in the fecal microbiota. The fecal microbiota of healthy individuals is dominated by obligate anaerobic bacteria belonging to the phyla Firmicutes and Bacteroidetes (30). A dysbiotic expansion in the fecal microbiota of facultative anaerobic bacteria of the phylum Proteobacteria is observed in patients with necrotizing enterocolitis (50), irritable bowel syndrome (12, 33), inflammatory bowel disease (18), colorectal cancer (3), or in individuals consuming a high-fat diet (46), with chronic alcohol usage (15), or undergoing inflammaging (48). A dysbiotic expansion of Proteobacteria in the large intestine is also observed in mouse models of chemically induced colitis (43), genetically induced colitis (23), antibiotic treatment (6, 59), and infection with Salmonella enterica (68), Citrobacter rodentium (43), or Toxoplasma gondii (27).
Changes in energy metabolism that reduce oxygen consumption in the colonic epithelium are associated with a loss of epithelial hypoxia, which, in turn, disrupts anaerobiosis in the colon (38). A disruption of anaerobiosis is associated with an expansion of facultative anaerobic bacteria belonging to the phylum Proteobacteria (57), which can use oxygen to outgrow obligate anaerobic Clostridia and Bacteroidia in the colon (9, 25, 29, 42, 58). An expansion of facultative anaerobic Proteobacteria is a microbial signature of dysbiosis in the colon (62) (Fig. 3). As Proteobacteria lack the capability of breaking down complex carbohydrates (16), their expansion provides no digestive benefit to the host. The emerging picture suggests that epithelial hypoxia is a host component of the microbiome that maintains homeostasis in the colon, whereas changes in epithelial energy metabolism that impair this host control mechanism trigger dysbiosis (39). Thus, the colonic epithelium functions as a control switch that governs a shift between homeostatic and dysbiotic microbial communities (38). These considerations identify dysbiosis in the colon as a microbial signature of a weakened epithelial control over the microbial ecosystem (39), a functional definition that becomes measurable, for example, by quantifying epithelial oxygen consumption (9). In conclusion, by expanding the definition of the microbiome to include host control mechanisms that shape the microbial ecosystem (Fig. 1B), it becomes possible to define homeostasis and dysbiosis functionally. However, epithelial hypoxia is a host control mechanism that is specific to the colon, and digestive functions of the microbiota are limited to the gastrointestinal tract, which raises the question whether functional definitions for homeostasis and dysbiosis can be adapted to other body surfaces.
A QUEST FOR CAUSATION AS THE NEXT PHASE IN MICROBIOME RESEARCH
A benefit conferred by the microbiota that is relevant to all body surfaces is its ability to repel pathogens, a property known as colonization resistance (36, 37). Colonization resistance is part of the larger phenomenon of microbiota resistance, an ability to remain unchanged in the face of a disturbance, which is mediated through mechanisms that are entrenched in community ecology (65). To coexist, each member of the microbiota must occupy a different nutrient-niche defined by critical resources that the occupant can utilize better than any other microorganism present in the microbial community (19). Each occupant of a nutrient-niche gains an advantage over new arrivals that compete for the same resources through niche preemption or niche modification, a principle known as historical contingency (20, 67). Colonization resistance against pathogens is mediated through priority effects, resulting from niche preemption or niche modification (40, 70, 71). Whereas niche preemption signifies a competition for resources, niche modification encompasses the production of microbiota-derived metabolites that antagonize pathogens (1, 22). Notably, our microbial organ and host control mechanisms that maintain its functionality could be viewed as being part of our immune system, because colonization resistance against pathogens represents a nonspecific immune function (8). This part of the immune system has been dubbed microbiota-nourishing immunity, because some host control mechanisms literally nourish the microbiota, a feature that distinguishes this part of the immune system from sterilizing immunity, which functions in detection and removal of microorganisms from host tissue, but never provides nourishment to microbes (8).
Microbiota-nourishing immunity encompasses the microbiota and host control mechanisms that shape the microbial ecosystem, thereby covering all relevant aspects of the microbiome (8) (Fig. 1B). This theoretical framework, therefore, enables microbiome science to adopt well-established immunological concepts, such as immunocompetence and immunodeficiency. For example, using this theoretical framework, we can define a healthy microbiome simply as a state of immunocompetence in microbiota-nourishing immunity. Conversely, a weakening of microbiota-nourishing immunity could be viewed as an immunodeficiency associated with dysbiosis (8). Consistent with the immunodeficiency concept, a disruption of the microbial component of microbiota-nourishing immunity through antibiotic therapy is associated with opportunistic infections caused by Clostridioides difficile (phylum Firmicutes) (34). Similarly, a weakening of a host component of microbiota-nourishing immunity in cystic fibrosis, which is caused by mutations in the CFTR (cystic fibrosis transmembrane-conductance regulator) gene that impair the mucus escalator, is linked to opportunistic infections with Pseudomonas aeruginosa (phylum Proteobacteria) (26). Notably, accepting the immunodeficiency concept suggests that dysbiosis is a symptom of a weakening in microbiota-nourishing immunity (8). This concept steers microbiome research in a new direction, because it suggests that a description of dysbiosis should always be followed by studies aimed at identifying the nature of the underlying immunodeficiency. Thus, after the initial cataloguing of microbial genes and species names is complete, the vision of microbiota-nourishing immunity provides microbiome science the next goal to seize as it enters its second phase of identifying potential treatment targets for diseases associated with dysbiosis.
CONCLUDING REMARKS
Increasing the depth of microbiome measurements has failed to provide genuinely needed definitions for homeostasis and dysbiosis (52), which reflects the lack of an overarching conceptual framework in microbiome science (7). Here, we discuss the idea that incorporating concepts from community ecology, immunology, and the germ-organ theory into microbiome research provides a functional definition for dysbiosis and helps to guide microbiome science toward investigating an underlying weakening in microbiota-nourishing immunity. When cataloguing microbial species names, one should keep in mind that not every microbial change is necessarily functionally consequential for the host and that the presence and expression of functionally relevant metabolic pathways might be conserved across species barriers. Our theoretical framework suggests that the resident microbiota is dysbiotic if it exhibits impaired functionality, as indicated by a weakening in colonization resistance or a decline in its ability to perform digestive tasks, immune education, or other beneficial duties.
A remaining ambiguity of the terminological framework is that it does not distinguish between dysbiosis being the cause or the result of disease (52). Dysbiosis can be the cause of disease if microbiota-nourishing immunity becomes weakened by a direct disruption of its microbial component, which is a common side effect of antibiotic treatment. For instance, fecal microbiota transplants are effective in treating opportunistic C. difficile infections (5), because this therapy targets the underlying cause, which is an antibiotic-mediated disruption of the microbiota. However, in case of illnesses in which a host component of microbiota-nourishing immunity is weakened, dysbiosis is secondary to an immunodeficiency of the host (8), which supports the idea that dysbiosis should refer to the result of disease, rather than its cause (52). Between these two extremes lie conditions in which dysbiosis is secondary to an underlying host immunodeficiency, but the resulting change in the microbial community structure exacerbates disease. Thus, it will remain important to determine experimentally in each case whether dysbiosis associated with a human illness makes a causal contribution to the disease process, for example by adopting Koch’s postulates for microbiome research (49, 63, 73). Determining the nature of the immunodeficiency that underlies dysbiosis and resolving whether dysbiosis makes a causal contribution to disease will be relevant for identifying new therapeutic targets to treat illnesses associated with changes in the microbiota composition.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.T. and A.J.B. edited and revised manuscript; C.R.T. and A.J.B. approved final version of manuscript; A.J.B. drafted manuscript.
ACKNOWLEDGMENTS
Work in A. J. Bäumler’s laboratory is supported by the U.S. Department of Agriculture/National Institute of Food and Agriculture award 2015-67015-22930 and by Public Health Service Grants AI044170, AI096528, AI112445, and AI112949.
REFERENCES
- 1.Antunes LC, McDonald JA, Schroeter K, Carlucci C, Ferreira RB, Wang M, Yurist-Doutsch S, Hira G, Jacobson K, Davies J, Allen-Vercoe E, Finlay BB. Antivirulence activity of the human gut metabolome. MBio 5: e01183-–e14., 2014. doi: 10.1128/mBio.01183-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120–123, 2012. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341, 2011. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, Moore TA, Russell G, Surawicz C; Fecal Microbiota Transplantation Workgroup . Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 9: 1044–1049, 2011. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohnhoff M, Drake BL, Miller CP. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med 86: 132–137, 1954. doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- 7.Byndloss MX, Bäumler AJ. The germ-organ theory of non-communicable diseases. Nat Rev Microbiol 16: 103–110, 2018. doi: 10.1038/nrmicro.2017.158. [DOI] [PubMed] [Google Scholar]
- 8.Byndloss MX, Litvak Y, Bäumler AJ. Microbiota-nourishing immunity and its relevance for ulcerative colitis. Inflamm Bowel Dis 25: 811–815, 2019. doi: 10.1093/ibd/izz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Bäumler AJ. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357: 570–575, 2017. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byndloss MX, Pernitzsch SR, Bäumler AJ. Healthy hosts rule within: ecological forces shaping the gut microbiota. Mucosal Immunol 11: 1299–1305, 2018. doi: 10.1038/s41385-018-0010-y. [DOI] [PubMed] [Google Scholar]
- 11.Cani PD. Gut microbiota—at the intersection of everything? Nat Rev Gastroenterol Hepatol 14: 321–322, 2017. doi: 10.1038/nrgastro.2017.54. [DOI] [PubMed] [Google Scholar]
- 12.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 24: 521–530, 2012. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, Xie H, Chen X, Zeng C, Wen B, Zeng L, Du H, Tang H, Xu C, Xia Y, Xia H, Yang H, Wang J, Wang J, Madsen L, Brix S, Kristiansen K, Xu X, Li J, Wu R, Jia H. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 8: 875, 2017. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, Govorun VM. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 5: 141, 2017. doi: 10.1186/s40168-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11: 497–504, 2013. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 17.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature 548: 43–51, 2017. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13,780–13,785, 2007. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freter R, Brickner H, Fekete J, Vickerman MM, Carey KE. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun 39: 686–703, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst 46: 1–23, 2015. doi: 10.1146/annurev-ecolsys-110411-160340. [DOI] [Google Scholar]
- 21.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450, 2013. [Erratum in Nature 506: 254, 2014.] doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 22.García C, Tebbji F, Daigneault M, Liu NN, Köhler JR, Allen-Vercoe E, Sellam A. The human gut microbial metabolome modulates fungal growth via the TOR signaling pathway. MSphere 2: e00555-17, 2017. doi: 10.1128/mSphere.00555-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 8: 292–300, 2010. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert JA, Lynch SV. Community ecology as a framework for human microbiome research. Nat Med 25: 884–889, 2019. doi: 10.1038/s41591-019-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillis CC, Hughes ER, Spiga L, Winter MG, Zhu W, Furtado de Carvalho T, Chanin RB, Behrendt CL, Hooper LV, Santos RL, Winter SE. Dysbiosis-associated change in host metabolism generates lactate to support Salmonella growth. Cell Host Microbe 23: 54–64.e6, 2018. [Erratum in Cell Host Microbe 23: 570, 2018.] doi: 10.1016/j.chom.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60: 539–574, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One 7: e35988, 2012. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science 292: 1115–1118, 2001. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 29.Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Büttner L, Smoot MP, Behrendt CL, Cherry S, Santos RL, Hooper LV, Winter SE. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 21: 208–219, 2017. doi: 10.1016/j.chom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214, 2012. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Integrative HMP (iHMP) Research Network Consortium The integrative human microbiome project. Nature 569: 641–648, 2019. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol 9: 95, 2009. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuijper EJ, Coignard B, Tüll P; ESCMID Study Group for Clostridium difficile; EU Member States; European Centre for Disease Prevention and Control . Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect 12, Suppl 6: 2–18, 2006. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 35.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol 17: 219–232, 2017. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 36.Libertucci J, Young VB. The role of the microbiota in infectious diseases. Nat Microbiol 4: 35–45, 2019. doi: 10.1038/s41564-018-0278-4. [DOI] [PubMed] [Google Scholar]
- 37.Litvak Y, Bäumler AJ. The founder hypothesis: A basis for microbiota resistance, diversity in taxa carriage, and colonization resistance against pathogens. PLoS Pathog 15: e1007563, 2019. doi: 10.1371/journal.ppat.1007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litvak Y, Byndloss MX, Bäumler AJ. Colonocyte metabolism shapes the gut microbiota. Science 362: eaat9076, 2018. doi: 10.1126/science.aat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litvak Y, Byndloss MX, Tsolis RM, Bäumler AJ. Dysbiotic proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr Opin Microbiol 39: 1–6, 2017. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Litvak Y, Mon KKZ, Nguyen H, Chanthavixay G, Liou M, Velazquez EM, Kutter L, Alcantara MA, Byndloss MX, Tiffany CR, Walker GT, Faber F, Zhu Y, Bronner DN, Byndloss AJ, Tsolis RM, Zhou H, Bäumler AJ. Commensal Enterobacteriaceae protect against Salmonella colonization through oxygen competition. Cell Host Microbe 25: 128–139.e5, 2019. doi: 10.1016/j.chom.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloréns-Rico V, Raes J. Tracking humans and microbes. Nature 569: 632–633, 2019. doi: 10.1038/d41586-019-01591-y. [DOI] [PubMed] [Google Scholar]
- 42.Lopez CA, Miller BM, Rivera-Chávez F, Velazquez EM, Byndloss MX, Chávez-Arroyo A, Lokken KL, Tsolis RM, Winter SE, Bäumler AJ. Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science 353: 1249–1253, 2016. doi: 10.1126/science.aag3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2: 204, 2007. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Marchesi JR. Human distal gut microbiome. Environ Microbiol 13: 3088–3102, 2011. doi: 10.1111/j.1462-2920.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 45.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut 65: 330–339, 2016. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, Darfeuille-Michaud A, Barnich N. Western diet induces dysbiosis with increased E. coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 63: 116–124, 2014. doi: 10.1136/gutjnl-2012-304119. [DOI] [PubMed] [Google Scholar]
- 47.Miller ET, Svanbäck R, Bohannan BJM. Microbiomes as metacommunities: understanding host-associated microbes through metacommunity ecology. Trends Ecol Evol 33: 926–935, 2018. doi: 10.1016/j.tree.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Doré J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 72: 1027–1033, 2006. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neville BA, Forster SC, Lawley TD. Commensal Koch’s postulates: establishing causation in human microbiota research. Curr Opin Microbiol 42: 47–52, 2018. doi: 10.1016/j.mib.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Normann E, Fahlén A, Engstrand L, Lilja HE. Intestinal microbial profiles in extremely preterm infants with and without necrotizing enterocolitis. Acta Paediatr 102: 129–136, 2013. doi: 10.1111/apa.12059. [DOI] [PubMed] [Google Scholar]
- 51.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 7: 688–693, 2006. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olesen SW, Alm EJ. Dysbiosis is not an answer. Nat Microbiol 1: 16228, 2016. doi: 10.1038/nmicrobiol.2016.228. [DOI] [PubMed] [Google Scholar]
- 53.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 16: 1024–1033, 2014. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proctor L. Priorities for the next 10 years of human microbiome research. Nature 569: 623–625, 2019. doi: 10.1038/d41586-019-01654-0. [DOI] [PubMed] [Google Scholar]
- 55.Quie PG. Lung defense against infection. J Pediatr 108: 813–816, 1986. doi: 10.1016/S0022-3476(86)80750-8. [DOI] [PubMed] [Google Scholar]
- 56.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108, Suppl 1: 4680–4687, 2011. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J 7: 1256–1261, 2013. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera-Chávez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, Xu G, Velazquez EM, Lebrilla CB, Winter SE, Bäumler AJ. Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19: 443–454, 2016. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saito K. [Studies on the habitation of pathogenic Escherichia coli in the intestinal tract of mice. I. Comparative experiments on the habitation of each type of resistant pathogenic Escherichia coli under an administration of streptomycin]. Paediatr Jpn 65: 385–393, 1961. [PubMed] [Google Scholar]
- 60.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11: 76–82, 2010. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarker SA, Ahmed T, Brüssow H. Hunger and microbiology: is a low gastric acid-induced bacterial overgrowth in the small intestine a contributor to malnutrition in developing countries? Microb Biotechnol 10: 1025–1030, 2017. doi: 10.1111/1751-7915.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33: 496–503, 2015. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Singh VP, Proctor SD, Willing BP. Koch’s postulates, microbial dysbiosis and inflammatory bowel disease. Clin Microbiol Infect 22: 594–599, 2016. doi: 10.1016/j.cmi.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 64.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 15: 630–638, 2017. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 66.Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, Spear WW, Landay A, Micci S, Lee BH, Hamaker BR. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 210: 1019–1028, 2014. doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sprockett D, Fukami T, Relman DA. Role of priority effects in the early-life assembly of the gut microbiota. Nat Rev Gastroenterol Hepatol 15: 197–205, 2018. doi: 10.1038/nrgastro.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5: 2177–2189, 2007. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tipton L, Darcy JL, Hynson NA. A developing symbiosis: enabling cross-talk between ecologists and microbiome scientists. Front Microbiol 10: 292, 2019. doi: 10.3389/fmicb.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vannette RL, Fukami T. Historical contingency in species interactions: towards niche-based predictions. Ecol Lett 17: 115–124, 2014. doi: 10.1111/ele.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velazquez EM, Nguyen H, Heasley KT, Saechao CH, Gil LM, Rogers AWL, Miller BM, Rolston MR, Lopez CA, Litvak Y, Liou MJ, Faber F, Bronner DN, Tiffany CR, Byndloss MX, Byndloss AJ, Bäumler AJ. Endogenous Enterobacteriaceae underlie variation in susceptibility to Salmonella infection. Nat Microbiol 4: 1057–1064, 2019. doi: 10.1038/s41564-019-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Velázquez OC, Lederer HM, Rombeau JL. Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol 427: 123–134, 1997. [PubMed] [Google Scholar]
- 73.Vonaesch P, Anderson M, Sansonetti PJ. Pathogens, microbiome and the host: emergence of the ecological Koch’s postulates. FEMS Microbiol Rev 42: 273–292, 2018. doi: 10.1093/femsre/fuy003. [DOI] [PubMed] [Google Scholar]
- 74.Yang X, Xie L, Li Y, Wei C. More than 9,000,000 unique genes in human gut bacterial community: estimating gene numbers inside a human body. PLoS One 4: e6074, 2009. doi: 10.1371/journal.pone.0006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol 309: C350–C360, 2015. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]