Keywords: bile acid modulators, bile acid sequestrants, biomarkers, cannabinoid receptor agonists, individualized treatment, ion exchangers/transporters, linaclotide, opioid visceral analgesics
Abstract
The overall objectives of this review are to summarize actionable biomarkers for organic etiology of lower functional gastrointestinal disorders (FGIDs) that lead to individualized treatment for their FGIDs and to assess the pipeline for novel approaches to the management of constipation, diarrhea, and chronic abdominal pain in lower FGIDs. The new approaches to therapy include ion exchangers/transporters for functional constipation (sodium-glucose cotransporter 1, Na+/H+ exchanger 3, and solute carrier family 26 member 3 inhibitors), bile acid modulators for constipation such as ileal bile acid transporter inhibitors and fibroblast growth factor 19 analog for functional constipation, and bile acid sequestrants or farnesoid X receptor agonists for functional diarrhea. Treatment for chronic abdominal pain remains an unmet need in patients with lower FGIDs, and promising novel approaches include delayed-release linaclotide, nonclassical opioid visceral analgesics, and selective cannabinoid receptor agonists. The role of probiotics, fecal microbial transplantation, and possible future microbiome therapies is discussed.
INTRODUCTION
It is increasingly recognized that there are several specific disorders of function that result in clinical manifestations that overlap with lower functional gastrointestinal disorders (FGIDs). The symptom complexes that encompass FGIDs are classified using symptom-based criteria. These organic disorders of function presenting with symptoms of lower FGIDs such as diarrhea, constipation, bloating, and pain include pelvic floor dyssynergia, bile acid (BA) deficiency, colonic transit disorders, and disaccharidase deficiencies. Several actionable biomarkers have been identified to diagnose these organic disorders and provide individualized treatment.
At least one-third of patients presenting in secondary or tertiary care practice with functional constipation/constipation-predominant irritable bowel syndrome (IBS-C) have diverse rectal evacuation disorders, most frequently due to spastic pelvic floor dyssynergia, and, more rarely, descending perineum syndrome (49). Digital rectal examination with confirmation by abdominopelvic radiograph showing collection of gas and stool above the pelvic floor (53, 54), anorectal manometry (focused on resting anal sphincter pressure, anal relaxation, and rectoanal pressure gradient), and balloon expulsion test >30 s (13) provide the opportunity to guide the patient to biofeedback-assisted pelvic floor retraining at home or in the clinical setting (29, 57, 58).
Measurements of fasting serum 7-α-hydroxy-4-cholesten-3-one (7αC4) and 48-h fecal BA excretion (79) or, where available, 75Se-homocholic acid-taurine (75SeHCAT) retention can definitively identify BA diarrhea (BAD; excess synthesis or malabsorption), and specific therapeutic approaches are discussed below. Colonic transit measurements are useful to identify the predominant site of delayed transit in patients with constipation; in patients with predominant transit delay in the left colon at 48 h on scintigraphy or day 4 or 5 with the radiopaque marker transit test, the measurement of transit leads to other tests to exclude evacuation disorder (43, 61).
In addition to highly prevalent lactase deficiency (34), it is now recognized that although rare, congenital sucrose-isomaltase deficiency mutations with known defective disaccharidase properties are found more often in patients with IBS than controls, and a common sucrose-isomaltase variant (15Phe), which induces reduced enzymatic activity in vitro, is strongly associated with an increased risk of IBS (28).
The rest of this article addresses novel approaches for treatment of symptoms of lower FGIDs that are likely to be in the pipeline for approval and application in the foreseeable future. For each category, the discussion focuses on the biological rationale and proof of efficacy, with major focus on human studies where these are available.
MODULATING ION EXCHANGERS/TRANSPORTERS FOR FUNCTIONAL CONSTIPATION
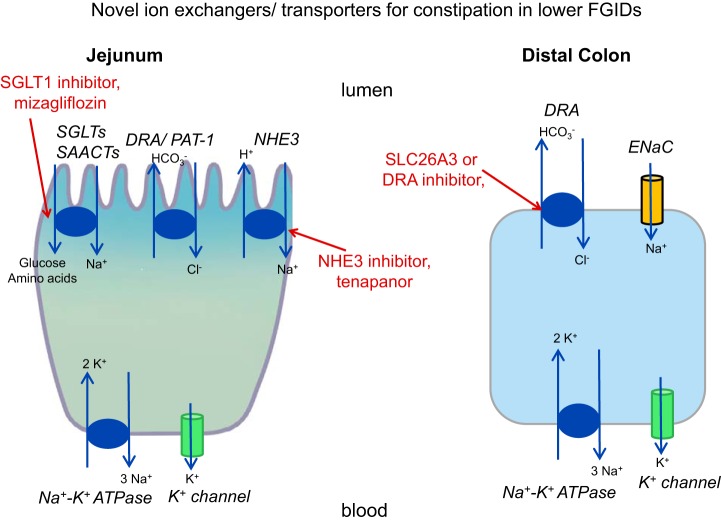
Three classes of drugs are being developed with the objective of reducing uptake of sodium ion from the lumen, resulting in obligatory retention of water in the lumen where these transporters are expressed in the small intestine and colon and ultimately resulting in looser stool consistency by delivering more water to the fecal stream (Fig. 1).
Fig. 1.
Novel ion exchangers/transporters for constipation in lower functional gastrointestinal disorders (FGIDs). DRA, downregulated in adenoma; ENaC, epithelial Na+ channel; NHE3, Na+/H+ exchanger 3; PAT-1, putative anion transporter-1; SAACTs, sodium-amino acid cotransporters; SGLTs, sodium-glucose cotransporters; SLC26A3, solute carrier family 26 member 3.
Sodium-Glucose Cotransporter 1 Inhibitor
Rationale.
The rationale for the sodium-glucose cotransporter 1 (SGLT1) inhibitor approach is that SGLT1 is found in both the small and large bowel. The SGLT1 inhibitor mizagliflozin is almost entirely degraded in the upper gut, with its site of action being in the small intestinal mucosa (52). Active cotransport of sodium with glucose or amino acids together with Na+/H+ exchanger 3 (NHE3) generates the osmotic gradients within the intestinal villi to drive rapid absorption of water. Even a small reduction in absorptive efficiency by blocking SGLT1 could lead to substantial increases in fluid entering the colon (25, 71).
Evidence from human studies.
In a randomized, placebo-controlled, double-blind, phase 2 trial in patients with constipation, 5- and 10-mg doses of mizagliflozin were both associated with benefits in stool response and number of spontaneous bowel movements (SBMs) per week in patients with either functional constipation or IBS-C. Treatment was efficacious compared with placebo from the first week and persisted throughout the 4-wk studies. In general, the effect of the 10-mg dose of mizagliflozin was more consistent than the effect of the 5-mg dose for the end points of complete SBMs (CSBMs) per week and stool consistency. The medication appears to be safe (25). The number needed to treat (NNT) for SBM responder at week 1 was 4 [95% confidence interval (95% CI), 3–11] for the 5-mg dose, and 3 (95% CI, 2–5) for the 10-mg dose of mizagliflozin.
NHE3 Inhibitor: Tenapanor
Rationale.
The human NHEs are encoded by the SLC9 gene family of the solute carrier (SLC) classification of transporters; NHE3 is encoded by SLC9A3, and a congenital mutation can result in congenital Na diarrhea or sudden infant death syndrome in humans (22). NHE3 is present in the sodium-absorptive cells of the mammalian small intestine, colon, gall bladder, renal proximal tubule, and thick and thin limbs of the loop of Henle (31). NHE3 is responsible for the majority of intestinal and renal Na absorption and participates in the proximal, low efficiency-high capacity systems called neutral NaCl absorption (92). In this process, NHE3 is linked to members of the SLC26A family of Cl−/ exchangers, particularly downregulated in adenoma (DRA, or SLC26A3, see below) and, to an unclear extent, putative anion transporter-1 (PAT-1, or SLC26A6) as well (35). These basic science studies provide the rationale for the application of a small-molecule inhibitor of the gastrointestinal NHE3, such as tenapanor, to reverse (sodium and water) absorption in the intestine and therefore relieve constipation in lower FGIDs. Tenapanor also reduced permeability (FITC-dextran flux) of primary human monolayer colonic cell cultures exposed to fecal supernatants (obtained from 9 patients with Rome III-positive IBS-C and 10 healthy controls and prepared by diluting 1 g stool in 0.3 mL Krebs–Ringer buffer, homogenizing, and filtering with 100-μm-pore size filters), suggesting additional mucosal effects that were speculated to reduce visceral pain by reducing access of potentially injurious molecules to the subepithelial layers (86).
Evidence from human studies.
Tenapanor is a first-in-class, small-molecule inhibitor of the gastrointestinal NHE3. Tenapanor dose-dependently increased stool sodium excretion and decreased urinary sodium excretion compared with placebo (59), and at doses of 15 mg twice a day, tenapanor did not have a clinically relevant impact on cytochrome P450 3A4 (CYP3A4) in humans (36). This is relevant given the large number of drugs metabolized by CYP3A4, and it suggests that it is unlikely that tenapanor will result in drug-drug interactions; that is, tenapanor can be coadministered with CYP3A4-metabolized drugs without affecting their exposure.
In a phase 2b, placebo-controlled, randomized trial of tenapanor, 356 subjects with IBS-C (Rome III criteria) had evidence of active disease as determined during a 2-wk screening period. They were enrolled in a comparative trial of 5, 20, or 50 mg of tenapanor or placebo twice a day for 12 consecutive weeks and were followed for an additional 4 wk (16).
The responder rates for CSBMs, composite responder rates, and abdominal symptoms (pain, discomfort, bloating, cramping, and fullness) were significantly higher in the group receiving 50 mg tenapanor twice a day than in the placebo group. As expected, diarrhea was the most frequent adverse event (tenapanor twice a day: 20 mg, 12.4%; 50 mg, 11.2%). In a network meta-analysis comparison of tenapanor with other secretory medications used in the treatment of IBS-C, tenapanor ranked first for decreasing bloating, but its efficacy was otherwise comparable to the other secretagogues (4). A preliminary report of a 26-wk, phase 3 trial confirmed efficacy of 50 mg tenapanor twice a day in IBS-C with estimated NNTs of 7–9 for different bowel function end points such as CSBM and combined CSBM and >30% reduction in pain; the NNT for abdominal pain reduction of >30% alone was 11 (17).
SLC26A3 Inhibitor
Rationale.
SLC26A3 (DRA) is a Cl−/ electroneutral exchanger expressed in the luminal membrane of intestinal epithelial cells, where it facilitates electroneutral NaCl absorption. A mutation in SLC26A3 is associated with congenital chloride diarrhea, and the exchanger is stimulated by cAMP (76). When administered intraluminally, an inhibitor of DRA (DRAinh-A250) blocked fluid absorption in mouse colonic loops and reversed loperamide-induced constipation (27).
Evidence from human studies.
No evidence from human studies is available.
BA MODULATORS FOR FUNCTIONAL CONSTIPATION OR FUNCTIONAL DIARRHEA
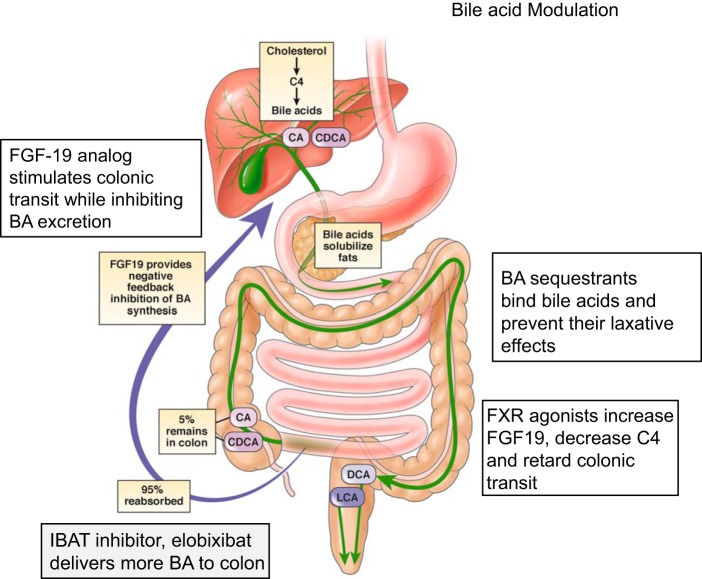
BAs facilitate uptake of fats within the small bowel; after the fat is absorbed, 95% of the free BAs are reabsorbed within the terminal ileum through the ileal bile acid transporter (IBAT). Having entered the ileal enterocytes, BAs activate the nuclear farnesoid X receptor (FXR), which increases the transcription of fibroblast growth factor 19 (FGF-19). FGF-19, acting as an endocrine hormone, reaches the liver through the portal venous system, binds to FGF receptor 4 and β-klotho (KLB) on the hepatocyte membrane, leading to inhibition of hepatic CYP7A1, the rate-limiting enzyme for synthesis of 7αC4 and the primary BAs, cholic and chenodeoxycholic acids (Fig. 2).
Fig. 2.
Bile acid modulation in lower functional gastrointestinal disorders with diarrhea or constipation. BA, bile acid; C4, 7-α-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxoycholic acid; FGF-19, fibroblast growth factor 19; FXR, farnesoid X receptor; IBAT, ileal bile acid transporter; LCA, lithocholic acid. [Reproduced in part from Vijayvargiya and Camilleri (79) with permission.]
BAD has been reported in 25–33% of patients who present with chronic diarrhea (77, 88). BAs are physiological laxatives. Conversely, a subgroup of patients with IBS-C have a deficiency of BAs in the colon (78).
IBAT Inhibition for Functional Constipation: Elobixibat
Rationale.
Elobixibat is an ileal BA transport inhibitor that accelerated colonic transit (90), decreased LDL cholesterol and LDL-to-HDL ratio, and increased circulating peak glucagon-like peptide-1 levels in humans (60). Elobixibat induced giant migrating contractions during defecation in dogs (73). These actions are considered to result from the effects of BAs to increase colonic secretion of water and electrolytes (41) and to stimulate high-amplitude propagated colonic contractions (3).
Evidence from human studies.
Multiple placebo-controlled, randomized trials of elobixibat, including mechanistic phase 2a trials (90), multicenter phase 2b trials in the United States (14) and Japan (45), and phase 3 trials including long-term safety and efficacy in Japan (46, 47), have been published. The drug is efficacious in the treatment of constipation, including severe constipation, and is safe and well tolerated (37). The estimated NNT of 10 mg elobixibat for CSBM at week 1 (difference from placebo, 35%) and week 2 of treatment (difference from placebo, 32%) is ~3 (46).
FGF-19 Analog for Functional Constipation: NGM282
Rationale.
NGM282 is an analog of FGF-19, a potent inhibitor of BA synthesis in animals and humans. In phase 2 trials in patients with type 2 diabetes and primary biliary cholangitis, NGM282 was associated with dose-related abdominal cramping and diarrhea. This induction of looser bowel movements was counterintuitive since an FGF-19 analog would be expected to reduce hepatocyte synthesis of primary BAs and would therefore be predicted to reduce colonic function. Human pharmacodynamic studies have proposed possible mechanisms that may underpin the effects in patients with functional constipation.
Evidence from human studies.
A phase 2, two-dose NGM282 (1 and 6 mg sc, daily), parallel-group, randomized, placebo-controlled, 14-day study was conducted in patients with functional constipation (Rome III criteria) and baseline colonic transit 24-h geometric center <3.0 (on a 1–5 scale, where 1 = ascending colon and 5 = stool). NGM282 altered bowel functions (increased number of bowel movements, looser stool form, and increased ease of passage), significantly accelerated gastric and colonic transit at 24 h, and reduced fecal total BA excretion, but no differences were observed in fecal fat or weight (51). Interestingly, there was treatment interaction with the KLB single-nucleotide polymorphism, with greater increase in colonic transit in participants with the minor A allele, which is associated with normal survival kinetics of the KLB protein (89).
The precise mechanism for the acceleration of colonic transit and increase in stool frequency and consistency is still unclear; potential mechanisms are as follows: repression of the apical sodium-dependent BA transporter (26), compensatory increase in the number or function of BA transporters or Takeda G protein-coupled receptor 5 as a result of reduced colonic BA concentrations, and stimulation of excitatory neural control, which is supported by the known effect of the FGF family of signaling molecules as regulatory factors in autocrine/endocrine signaling during development of enteric neurons (42).
BA Sequestrants for BAD Presenting as Functional Diarrhea: Cholestyramine and Colesevelam
Rationale.
Intraluminal sequestration of BAs has the potential to reduce the secretory, detergent, and motility-stimulating effects of BAs that result in diarrhea.
Evidence from human studies.
Although there are no large, randomized, controlled trials of BA sequestrants in patients with BAD, several lines of evidence based on comparisons of bowel functions between basal and treatment periods suggest that cholestyramine, colestipol, and colesevelam reduce diarrhea in patients with unexplained chronic diarrhea, postcholecystectomy diarrhea, and diarrhea-predominant IBS (IBS-D; 23, 30, 62). Further large, placebo-controlled, randomized studies are required. It has been demonstrated that formal diagnosis of BAD by 75SeHCAT testing or 48-h fecal BA excretion tests provides a means to identify potential responders to such BA sequestrants. Thus, among patients with functional diarrhea and increased fecal BA excretion, the response rate to BA sequestrants observed in clinical practice was ~70% (80, 88), in contrast to the 25% response rate in patients with functional diarrhea and normal fecal BA excretion who were empirically treated with such sequestrants (80). In relatively small pharmacodynamics trials, ascending colon transit retardation in response to colesevelam was correlated with the rank serum 7αC4 level, i.e., the hepatic BA synthesis rate (50), and higher sequestration of fecal BAs was positively correlated with improvement in stool frequency and consistency (81). Nevertheless, it is important to note that the optimal doses for these agents are still incompletely understood. Improvement in BA retention after cholestyramine occurred in 50% of patients with postcholecystectomy BAD, and normalization was achieved in only 20% of patients (62). Fifteen of 27 patients with BAD responded with colestipol treatment (2). Therefore, further dose-response studies with these agents are also required.
FXR Agonism for Functional Diarrhea: Obeticholic Acid and Tropifexor
Rationale.
FXR agonists stimulate the release of FGF-19 from ileal epithelial cells and activate the feedback inhibition of BA synthesis by hepatocytes. Therefore, the intraluminal concentration of BAs reaching the colon is reduced, thereby limiting the secretory and motor effects of BAs and relieving the diarrhea.
Evidence from human studies.
Two small placebo-controlled trials have demonstrated the pharmacodynamics efficacy of obeticholic acid (6-ethyl chenodeoxycholic acid) and tropifexor (a non-BA molecule), which improved clinical outcomes [stool index with obeticholic acid (85) or biochemical markers (increased serum FGF-19 levels and reduced 7αC4 levels) and ascending colon emptying with tropifexor] in patients with BAD (8).
NOVEL ANALGESIC APPROACHES
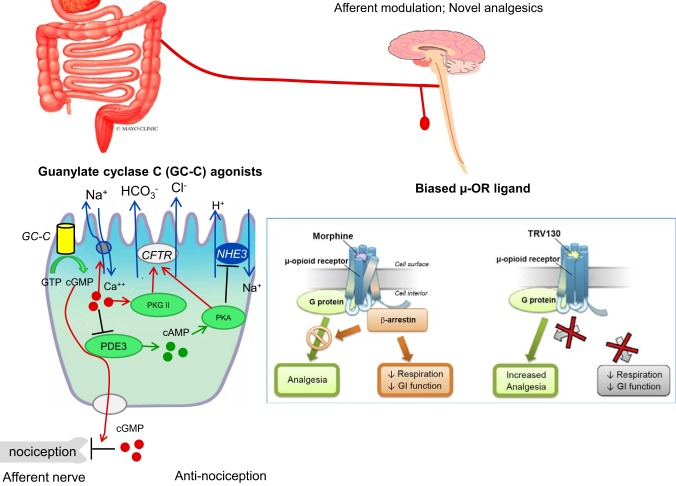
Novel analgesic approaches are shown in Fig. 3.
Fig. 3.
Afferent modulation and novel analgesics for pain in lower functional gastrointestinal disorders. CFTR, cystic fibrosis transmembrane regulator; GI, gastrointestinal; NHE3, Na+/H+ exchanger 3; PDE3, phosphodiesterase type 3; TRV130, oliceridine; µ-OR, µ-opioid receptor. [Reproduced with permission from Waldman and Camilleri (84) and Camilleri (7).]
Guanylate Cyclase C Agonists
Rationale.
Guanylate cyclase C (GC-C) agonists (84) mimic the activation by the endogenous paracrine hormones uroguanylin and guanylin and result in intracellular production of the downstream effector cGMP. In addition to stimulating chloride secretion through the cystic fibrosis transmembrane regulator (CFTR), the GC-C signaling axis modulates afferent pathways involved in gastrointestinal pain sensation. cGMP can be transported into the extracellular space through multidrug resistance-associated protein 4 cyclic nucleotide efflux pumps at the basolateral membrane of enterocytes or colonocytes (74), decreasing conduction of submucosal afferent nociceptive neurons and attenuating the sensation of visceral pain (11). Thus, uroguanylin and the GC-C agonist linaclotide inhibit colonic nociception in models of colonic visceral hypersensitivity (6), and cGMP increases pain thresholds in trinitrobenzene sulfonic acid-induced colitis (65). Chronic intracolonic administration of linaclotide reduces the increased activation of dorsal horn neurons in mice with colonic visceral hypersensitivity (10), and this effect is independent of any alteration in the function of the amygdala (38).
GC-C signaling may contribute to analgesia by indirectly reducing inflammation through maintenance of the intestinal barrier, restricting access of luminal factors to nociceptive or immune mechanisms in the lamina propria (64).
Evidence from human studies.
Linaclotide stimulates colonic transit and loosens stool consistency in patients with IBS-C in a dose-related manner (1). Both linaclotide and plecanatide relieve constipation and pain in patients with IBS-C with approximately equal efficacy, as shown in a network meta-analysis (4); the estimated NNTs for both medications were 11–12 (63).
Since all secretagogue laxatives had approximately equal efficacy in the network meta-analysis, it has previously been unclear whether the beneficial effects on pain were simply attributable to the relief of constipation. However, recent studies of targeted delivery of linaclotide to the intestine in patients with IBS-C demonstrated relief of pain, independent of the effect on constipation, since these colonic release formulations did not affect either stool frequency or stool consistency (15). Overall, these data suggest that there is a specific effect of GC-C agonists on visceral pain, and the observations from experimental animal studies suggest that the most likely mechanism responsible for the analgesic effect is the release of cGMP from the epithelial cells in the intestine and colon, reducing the function of the peripheral visceral afferents.
Nonclassical Opioid Analgesics
Novel peripheral visceral analgesics are reviewed extensively elsewhere (7). Two examples of ligands related to the µ-opioid receptors (µ-ORs) are briefly discussed here.
Biased µ-OR ligands.
rationale.
When conventional opioids bind to the µ-ORs and induce analgesia through activation of G protein-mediated pathways, they also recruit β-arrestin, which induces respiratory depression and inhibition of gut motility. A new class of µ-ORs called biased ligands activate the G protein pathway without activating β-arrestin. β-Arrestin-2 knockout mice have demonstrated enhanced analgesia with reduced gastrointestinal and respiratory dysfunction (5, 56). The analgesic efficacy without adverse effects has been demonstrated in vivo in male C57BL/6J mice or male Sprague-Dawley rats using hot-plate, tail-flick, and incisional pain studies (21). In the same studies, there was no significant effect on colon motility, based on glass bead expulsion time. Nevertheless, more recent studies suggest that the prototype medication in this class of biased ligands, TRV130 (oliceridine), continues to exhibit depression of respiratory function or abuse potential, possibly at higher doses than those achieving analgesia, as previously summarized elsewhere (48).
evidence from human studies with trv130 (oliceridine).
Several human clinical trials of oliceridine have been reported. The first two trials conducted in healthy volunteers showed that TRV130 produced greater analgesia than morphine with less reduction in respiratory drive and less severe nausea (68, 69). A separate phase 2 study in patients after bunionectomy showed that intravenous TRV130 rapidly produced profound analgesia in moderate-to-severe acute pain with analgesic effects that were superior to placebo and at least comparable to those of morphine (83); there were no serious adverse events, and tolerability was similar to morphine.
One phase 2b and two phase 3 trials have compared oliceridine and placebo as well as active treatment on severe acute pain in patients after undergoing abdominoplasty (66, 67, 82). All the trials confirmed that 0.35 and 0.5 mg iv oliceridine provided effective, rapid analgesia in patients with moderate-to-severe postoperative pain, with an acceptable safety and tolerability profile (e.g., lower rescue antiemetic use) and a potentially wider therapeutic window than for morphine. There are, as yet, no human studies in visceral pain.
Another medication in this class is PZM21 (40), which has not yet been tested in humans.
Targeting µ-OR under acidic conditions.
rationale.
Low pH is a hallmark of injured tissue, as occurs in inflammation. Spahn et al. (70) developed a pH-sensitive opioid that, because of its low pKa, selectively activates peripheral µ-ORs at the source of pain generation where there is lower pH. Acidosis augments the function of G protein-coupled receptors and affects the protonation of ligands, which is required for binding to the ORs. The prototype molecule, N-(3-fluoro-1-phenethylpiperidin-4-yl)-N-phenylpropionamide, is a fluorinated derivative of fentanyl; the fluorine serves to attract protons. This approach produced injury-restricted analgesia in rats with different types of inflammatory pain without respiratory depression, sedation, constipation, or addiction potential. The attraction of this approach is that the fentanyl is only active at sites of tissue acidosis such as inflammation, and therefore, it may target visceral pain associated with pathophysiological conditions, particularly inflammation, without activation of the fentanyl at other sites, where it might cause adverse effects such as respiratory depression.
evidence from human studies.
There are no human studies to date.
Cannabinoid Type 2 Receptor Agonist
Rationale.
Cannabinoid type 2 (CB2) receptors are G protein-coupled receptors that have amino acid sequence similar to that of CB1 and mediate effects on the immune system. Given CB2 receptors’ widespread tissue expression in the brain, peripheral nervous system, and gastrointestinal tract, they have potential as analgesics devoid of the centrally mediated effects of nonselective cannabinoids. Olorinab (formerly APD371), a highly selective full agonist of CB2 receptors, reduced pain in a model of pancreatitis (93) and also reduced visceral hypersensitivity (visceromotor response to colorectal distension) in an inflammatory bowel disease-like rat model of trinitrobenzene sulfonic acid-induced colitis and an IBS-like mouse model of chronic visceral hypersensitivity (12).
Evidence from human studies.
Olorinab was generally well tolerated with no central nervous system side effects reported in healthy volunteers. In a randomized, open-label, parallel-group, multicenter, phase 2a study of patients with quiescent Crohn’s disease experiencing average weekly abdominal pain score ≥4 on a scale of 0 (no pain) to 10 (worst ever), 14 participants were randomized to 25 or 100 mg oral olorinab 3 times a day for up to 8 wk. The average abdominal pain score improved from baseline at weeks 4 and 8 and was associated with a higher number of pain-free days per week and proportion of abdominal pain responders (11/13 at week 4 and 11/11 at week 8), as well as improvements in daily bowel movements, daily symptoms, daily coping, daily life, and emotional impact (91).
Other cannabinoid-related compounds have been tested in patients, but they appear to be less efficacious. In a pilot randomized controlled trial, Δ9-tetrahydrocannabinol was compared with placebo in three forms of chronic pain and was shown not to be superior to placebo (20).
Palmitoylethanolamide is an endogenous fatty acid amide, which binds to a nuclear receptor and has diverse biological functions related to chronic pain and inflammation. The effect of palmitoylethanolamide-polydatin, 20 or 200 mg twice a day, compared with placebo on abdominal pain/discomfort severity was tested in 54 patients with IBS and 12 healthy controls (19). Palmitoylethanolamide-polydatin treatment was effective in reducing the severity of abdominal pain/discomfort.
Micro-RNA-Based Treatments
Rationale.
Micro-RNAs (miRNAs) are short RNA molecules that do not code for protein production, but they are capable of posttranslational regulation of expression of several target mRNAs. They are released from cells in protected forms and therefore are stable for long periods in biological fluids. Elsewhere, we have summarized mechanisms of action and possible therapeutic roles of different miRNAs involved in IBS (9). For example, miRNA-24, which is upregulated in patients with IBS and in intestinal mucosa of a mouse model of IBS, targets the serotonin reuptake transporter and may therefore aggravate IBS by increasing local levels of serotonin with its effects on motility, secretion, and sensation. Other miRNAs are associated with increased mucosal permeability and inflammatory, pain, and motility pathways (9). In particular, decreased colonic miRNA-199 expression correlates with visceral pain through translational upregulation of transient receptor potential vanilloid type 1 in patients with IBS-D (94).
Experimental therapeutic studies have documented potential beneficial cytoprotective effects of galactooligosaccharides on rat colonic epithelial cells in a lipopolysaccharide-induced colitis model by upregulation of miR-19b associated with decrease in diarrhea (72). In other studies using an IBS-D rat model induced by chronic and acute stress, upregulation of miR-200a was accompanied by downregulation of cannabinoid receptor 1 and serotonin transporter in cultured colonic epithelial cells from the IBS-D rats. The authors suggested that miR-200a inhibition of the expression of cannabinoid receptor 1/serotonin transporter may result in hyperalgesia (32), although the studies to date are more suggestive of association than causation.
Evidence from human studies.
There are no therapeutic human studies targeting miRNAs in lower FGIDs to date.
GUT MICROBIOTA IN IBS: PRESENT AND FUTURE APPROACHES TARGETING THE MICROBIOME
Rationale
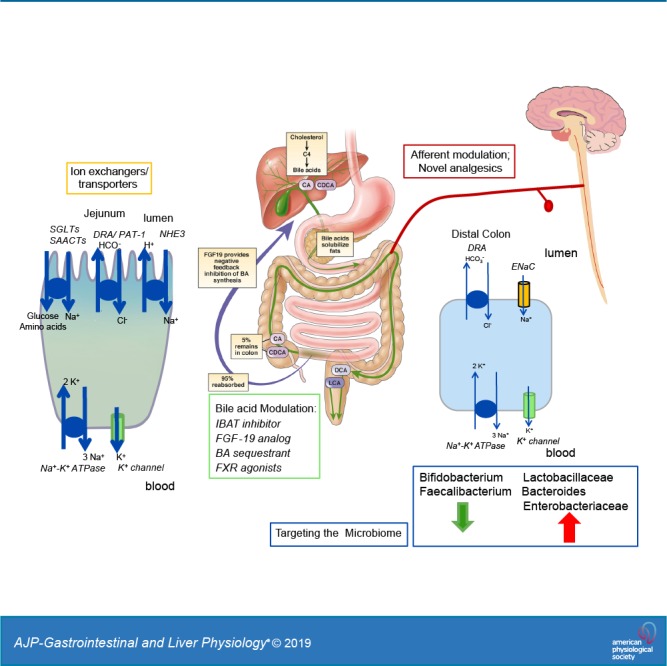
A thorough systematic review of the literature (55) comprehensively addressed microbiota at the level of phylum, order, family, genus, and species and appraised microbial diversity in IBS. The overall findings of this analysis are summarized in Table 1. The new finding from this analysis is that uncultured Clostridiales I, genus Faecalibacterium including Faecalibacterium prausnitzii, and genus Bifidobacterium were decreased in patients with IBS compared with controls. On the other hand, Lactobacillaceae, Bacteroides, and Enterobacteriaceae were increased in patients with IBS (55).
Table 1.
Summary of findings from systematic review of the literature addressing microbiota at the level of phylum, order, family, genus, and species and appraising microbial diversity in IBS
| Variable | Bacteria in Patients with IBS |
|---|---|
| 2 papers with increase in IBS | Aeromonas, Aneurinibacillus, Bacillus, Campylobacter, Clostridium difficile, Enterobacteriales, Helicobacter, Lactobacillus salivarius, Pediococcus, Pseudomonas aeruginosa, Ruminococcus gnavus |
| 3 papers with decrease in IBS | Uncultured Clostridiales I |
| 3 or more papers with no difference | Clostridium coccoides (4 papers) |
Summary of findings from systematic review of the literature (55). IBS, irritable bowel syndrome.
More in-depth analysis in the same comprehensive study showed variable results for genus Bacteroides in IBS-D: three of five articles demonstrated significant increase in IBS-D, whereas two of five showed insignificant results. Three of four studies evaluating genus Bifidobacterium and three of three papers evaluating genus Faecalibacterium showed significant decreases in patients with IBS-D. Only one small study of IBS-C alone (14 patients with IBS-C vs. 12 healthy controls) found differences. One study of 11 patients with postinfectious IBS, 11 healthy participants, and 12 patients with IBS-D documented that the differences in postinfectious IBS from healthy controls largely mirrored differences seen with IBS-D (55).
With regard to microbial diversity in IBS, only 9 of 24 papers provided α-diversity: 5 reported significant decrease and 4 reported no difference compared with healthy controls, with 1 study reporting no significant difference in nonspecific microbial diversity and 2 studies reporting insignificant differences in β-diversity (Jensen–Shannon distance and Bray–Curtis distance) and mean number of operational taxonomic units. Among 9 of the 10 studies that assessed total number of bacteria, there were no differences in patients with IBS and healthy controls, whereas 1 paper showed a significant increase in the number of bacteria from colonic tissue in Chinese patients with IBS (55).
The authors identified limitations in this comprehensive analysis of the microbiome in IBS, such as heterogeneity of microbiota assessment methods, unclear risk of bias due to selection of the control group, and lack of data in baseline characteristics to assess comparability of the cases and controls (55). In addition, this study conducted microbiome analyses according to IBS subtype and reported that any difference found between IBS and healthy controls was similar in both IBS-D (n = 117 patients) and IBS-C (n = 65 patients), with no inconsistency between the groups, whereas 5 other studies involving 117 patients with IBS-D and 77 patients with IBS-C did report discrepancies in microbiome profiles between the 2 IBS subtypes, but no mechanistic theme emerged regarding the differences in the microbiome. Six studies that evaluated the microbiome in 130 participants with IBS-mixed found no differences between IBS-mixed, IBS-C, and IBS-D (55).
Evidence from Human Studies
Present therapies based on microbiota modifications are shown below. These are summarized briefly to provide background on their potential as “pipeline” approaches in the foreseeable future.
Fecal Microbiota Transplantation for IBS
A systematic review and meta-analysis have comprehensively assessed efficacy of diverse forms of fecal microbiota transplantation for IBS based on administration by capsules, colonoscopy, or nasojejunal tube. The numbers of fecal microbiota transplantation trials and involved participants are small, but overall, two trials using colonoscopy showed an overall favorable risk ratio [0.63 (95% CI, 0.43–0.93)], whereas neither nasojejunal tube nor capsule delivery were significant (33) relative to placebo. Novel approaches that deliver more tailored microbial supplementation in a convenient and cost-effective manner are being explored.
Efficacy of Probiotics and Nonabsorbable Antibiotics in Treatment of IBS
Probiotics are living microorganisms, which commonly comprise gut-friendly bacteria and yeast, and they are ingested in the form of foodstuffs and supplements. A systematic review and meta-analysis assessed effects on global IBS symptoms or abdominal pain scores (24) and calculated the standard mean differences for combination probiotics versus placebo [−0.15 (95% CI, −0.31 to 0.01); P = 0.06], with nonsignificant effects on individual strains (Bifidobacterium, Saccharomyces, and Lactobacillus); similar effects were observed on flatulence. In addition, there were no significant effects on bloating with any of the combination or single-probiotic preparations (24).
The only nonabsorbable antibiotic tested in more than one trial was rifaximin, which was associated with a significant beneficial effect on persistence of IBS symptoms based on five randomized controlled trials [risk ratio = 0.84 (95% CI, 0.79–0.90)] in patients with nonconstipated IBS and a calculated NNT of 9. The analysis did not provide insights into efficacy for relief of pain (24).
Future Microbiota-Based Therapies: Prebiotics, Synbiotics, and Other Approaches
Given the failure of probiotics and the limited efficacy of antibiotic approaches for relief of IBS pain, other approaches targeting the microbiome are being developed and are summarized as follows (18): 1) Prebiotics are nonviable food components that improve host gut health by altering microbiota. 2) Customized probiotic cocktails consist of different cocktails of bacteria, fungi, and Archaebacteria intended to address individual deficiencies or dysbiosis. 3) Synbiotics are combinations of probiotics and prebiotics in food ingredients or supplements in a form of synergism. 4) Postbiotics are bioactive components that are nonviable soluble factors produced by beneficial bacteria via fermentation (such as bacterial cell wall components, enzymes, peptides such as glutathione, polysaccharides, organic acids, and short-chain fatty acids). 5) Parabiotics are inactivated probiotics that may stimulate immune responses. 6) Bacteriophages (viruses that infect bacteria) are naturally occurring entities that control microbial populations including the microbial communities in the gastrointestinal tract. On the basis of metagenomics analysis, ~1,200 viral genotypes, mostly siphophages and prophages, have been identified within bacterial genomes in fecal contents from healthy humans. Bacteriophage therapy is selective against specific bacteria. 7) Precision antimicrobials, such as specific targeted antimicrobial peptides, are biofilm-destroying compounds that facilitate antibiotics’ penetration into “resistant areas.” An alternative precision antimicrobial approach is the production of contractile nanotubes by certain bacteria, with binding of the nanotubes to receptors on bacterial cell surfaces and injection of a hollow tube into the cell wall, allowing ionic flux and killing of the bacterial cell or nanoparticles.
There is no evidence of efficacy of the novel approaches in patients with IBS to date.
CONCLUSION AND A LOOK TO THE FUTURE
The most significant advances in the management of lower FGIDs in the next 5 years are likely to result from more precise identification of the pathophysiology or phenotype of the lower FGIDs in order to select individualized treatment (87) and from novel pharmacological therapies targeting pain. Further interactions between basic and translational science and the pharmaceutical industry are essential to achieve these goals. Additional mechanisms that could be explored include diverse peripheral visceral afferent modulators, including agents acting on other ORs, and calcitonin gene-related peptide. These will require further exploration of the basic rationale and demonstration of efficacy in human clinical trials. There is experimental evidence [summarized by Ma et al. (39) and Thiagarajah et al. (75)] that antidiarrheal treatments (i.e., inhibitors of calcium-activated chloride channels and CFTR) have potential for acute secretory, infectious diarrheas typically associated with bacterial or viral enterotoxins. However, as yet, there are no published human studies or registered human studies at ClinicalTrials.gov (https://clinicaltrials.gov/, accessed August 9, 2019) or studies in experimental animal models of chronic diarrhea (which would be relevant for lower FGIDs) with these classes of medications. Therefore, this class of medication is not included among the present drugs in the pipeline for lower FGIDs. Moreover, other therapeutic approaches that may be introduced into clinical therapeutics beyond the coming 5 years are not reviewed in the present pipeline, though they are showing potential; an example is nanoparticle-based therapies, which provide effective drug delivery based on their physicochemical properties, allowing tissue penetration or cell-based uptake (44). To date, these have been studied in preclinical models of inflammatory bowel diseases, though it is conceivable that they could also be applied to lower FGIDs associated with altered barrier function or immune activation.
GRANTS
M. Camilleri’s research is supported by National Institutes of Health Grants RO1-DK-115950 and RO1-DK-67071.
DISCLOSURES
M. Camilleri has conducted research in studies cited in this article, including those pertaining to elobixibat, NGM282, and colesevelam, but received no personal remuneration for any study.
AUTHOR CONTRIBUTIONS
M.C. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Cindy Stanislav for excellent secretarial assistance.
REFERENCES
- 1.Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM, Currie MG, Zinsmeister AR. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 133: 761–768, 2007. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 2.Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 64: 84–92, 2015. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 3.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol 282: G443–G449, 2002. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 4.Black CJ, Burr NE, Quigley EM, Moayyedi P, Houghton LA, Ford AC. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology 155: 1753–1763, 2018. doi: 10.1053/j.gastro.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science 286: 2495–2498, 1999. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 6.Busby RW, Bryant AP, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Mahajan-Miklos S, Pierce CM, Solinga RM, Sun LJ, Tobin JV, Kurtz CB, Currie MG. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol 649: 328–335, 2010. doi: 10.1016/j.ejphar.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M. Toward an effective peripheral visceral analgesic: responding to the national opioid crisis. Am J Physiol Gastrointest Liver Physiol 314: G637–G646, 2018. doi: 10.1152/ajpgi.00013.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilleri M, Linker Nord S, Burton D, Oduyebo I, Zhang Y, Chen J, Bhad P, Badman MK, Sanders DS, Walters JR. A double-blind, randomized, placebo-controlled, crossover, multiple-dose study of tropifexor, a non bile acid FXR agonist, in patients with primary bile acid diarrhea. Gastroenterology 156, Suppl 1: S204–S205, 2019. doi: 10.1016/S0016-5085(19)37308-1. [DOI] [Google Scholar]
- 9.Camilleri M, Oduyebo I, Halawi H. Chemical and molecular factors in irritable bowel syndrome: current knowledge, challenges, and unanswered questions. Am J Physiol Gastrointest Liver Physiol 311: G777–G784, 2016. doi: 10.1152/ajpgi.00242.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro J, Harrington A, Maddern J, Schober G, Grundy LA, Ge P, Hannig G, Brierley S. Chronic intracolonic administration of linaclotide inhibits nociceptive signaling in a mouse model of chronic visceral hypersensitivity. Gastroenterology 156, Suppl 1: S570, 2019. doi: 10.1016/S0016-5085(19)38317-9. [DOI] [Google Scholar]
- 11.Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, Jin H, Jacobson S, Hannig G, Mann E, Cohen MB, MacDougall JE, Lavins BJ, Kurtz CB, Silos-Santiago I, Johnston JM, Currie MG, Blackshaw LA, Brierley SM. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 145: 1334–1346.e1, 2013. doi: 10.1053/j.gastro.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Castro J, Maddern J, Garcia-Caraballo S, Lumsden AL, Lindstrom B, Adams J, Brierley S. Olorinab (formerly APD371), a peripherally restricted, highly selective, full agonist of the cannabinoid receptor 2 (CB2), reduces visceral hypersensitivity in animal models. Gastroenterology 156, Suppl 1: S382, 2019. doi: 10.1016/S0016-5085(19)37805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chedid V, Vijayvargiya P, Halawi H, Park SY, Camilleri M. Audit of the diagnosis of rectal evacuation disorders in chronic constipation. Neurogastroenterol Motil 31: e13510, 2019. doi: 10.1111/nmo.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol 106: 1803–1812, 2011. [Erratum in Am J Gastroenterol 109: 782, 2014.] doi: 10.1038/ajg.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chey WD, Chamberlin P, Bochenek W, Tripp K, Higgins CS, Omniewski N, Fox SM, Hall M, Hashash A, Miller M, O’Dea CR, Currie M. Targeted delivery of linaclotide to specific areas of the intestine affects clinical efficacy in patients with irritable bowel syndrome with constipation (IBS-C). Gastroenterology 152, Suppl 1: S1314–S1315, 2017. doi: 10.1016/S0016-5085(17)34374-3. [DOI] [Google Scholar]
- 16.Chey WD, Lembo AJ, Rosenbaum DP. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol 112: 763–774, 2017. doi: 10.1038/ajg.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chey WD, Lembo AJ, Yan A, Rosenbaum DP. Efficacy and safety of tenapanor in patients with constipation predominant irritable bowel syndrome: a 6-month, double-blind, placebo-controlled phase 3 trial (T3MPO-2). Gastroenterology 154, Suppl 1: S1362, 2018. doi: 10.1016/S0016-5085(18)34452-4. [DOI] [Google Scholar]
- 18.Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The microbiome and irritable bowel syndrome: a review on the pathophysiology, current research and future therapy. Front Microbiol 10: 1136, 2019. [Erratum in Front Microbiol 10: 1870, 2019.] doi: 10.3389/fmicb.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremon C, Stanghellini V, Barbaro MR, Cogliandro RF, Bellacosa L, Santos J, Vicario M, Pigrau M, Alonso Cotoner C, Lobo B, Azpiroz F, Bruley des Varannes S, Neunlist M, DeFilippis D, Iuvone T, Petrosino S, Di Marzo V, Barbara G. Randomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment Pharmacol Ther 45: 909–922, 2017. doi: 10.1111/apt.13958. [DOI] [PubMed] [Google Scholar]
- 20.de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H; Pain and Nociception Neuroscience Research Group . Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol 15: 1079–1086.e4, 2017. doi: 10.1016/j.cgh.2016.09.147. [DOI] [PubMed] [Google Scholar]
- 21.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344: 708–717, 2013. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 22.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol Aspects Med 34: 236–251, 2013. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Bañares F, Esteve M, Salas A, Forné TM, Espinos JC, Martín-Comin J, Viver JM. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 46: 2231–2238, 2001. doi: 10.1023/A:1011927302076. [DOI] [PubMed] [Google Scholar]
- 24.Ford AC, Harris LA, Lacy BE, Quigley EM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther 48: 1044–1060, 2018. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 25.Fukudo S, Endo Y, Hongo M, Nakajima A, Abe T, Kobayashi H, Nakata T, Nakajima T, Sameshima K, Kaku K, Shoji E, Tarumi K, Nagaoka Y, Ooshima T, Ozawa K, Majima T, Kamata S, Tada T, Ishii H, Segawa Y, Miyazaki S, Yamamoto T, Yagi Y, Sawada H, Shirota S, Otsuka S, Yamada N, Suzuki R, Kurakata H, Nakai K, Syuji Y, Usui T, Yamamura M, Oishi T, Tanaka H; Mizagliflozin Study Group . Safety and efficacy of the sodium-glucose cotransporter 1 inhibitor mizagliflozin for functional constipation: a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Gastroenterol Hepatol 3: 603–613, 2018. doi: 10.1016/S2468-1253(18)30165-1. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh A, Chen F, Banerjee S, Xu M, Shneider BL. c-Fos mediates repression of the apical sodium-dependent bile acid transporter by fibroblast growth factor-19 in mice. Am J Physiol Gastrointest Liver Physiol 306: G163–G171, 2014. doi: 10.1152/ajpgi.00276.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haggie PM, Cil O, Lee S, Tan JA, Rivera AA, Phuan PW, Verkman AS. SLC26A3 inhibitor identified in small molecule screen blocks colonic fluid absorption and reduces constipation. JCI Insight 3: e121370, 2018. doi: 10.1172/jci.insight.121370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henström M, Diekmann L, Bonfiglio F, Hadizadeh F, Kuech EM, von Köckritz-Blickwede M, Thingholm LB, Zheng T, Assadi G, Dierks C, Heine M, Philipp U, Distl O, Money ME, Belheouane M, Heinsen FA, Rafter J, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Walter S, Simrén M, Karling P, Ohlsson B, Schmidt PT, Lindberg G, Dlugosz A, Agreus L, Andreasson A, Mayer E, Baines JF, Engstrand L, Portincasa P, Bellini M, Stanghellini V, Barbara G, Chang L, Camilleri M, Franke A, Naim HY, D’Amato M. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 67: 263–270, 2018. doi: 10.1136/gutjnl-2016-312456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum 50: 428–441, 2007. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med 281: 397–402, 1969. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 31.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996. doi: 10.1152/ajpgi.1996.270.1.G29. [DOI] [PubMed] [Google Scholar]
- 32.Hou Q, Huang Y, Zhang C, Zhu S, Li P, Chen X, Hou Z, Liu F. MicroRNA-200a targets cannabinoid receptor 1 and serotonin transporter to increase visceral hyperalgesia in diarrhea-predominant irritable bowel syndrome rats. J Neurogastroenterol Motil 24: 656–668, 2018. doi: 10.5056/jnm18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 50: 240–248, 2019. doi: 10.1111/apt.15330. [DOI] [PubMed] [Google Scholar]
- 34.Itan Y, Jones BL, Ingram CJ, Swallow DM, Thomas MG. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol Biol 10: 36, 2010. doi: 10.1186/1471-2148-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/ exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002. doi: 10.1053/gast.2002.31875. [DOI] [PubMed] [Google Scholar]
- 36.Johansson S, Rosenbaum DP, Ahlqvist M, Rollison H, Knutsson M, Stefansson B, Elebring M. Effects of tenapanor on cytochrome P450-mediated drug-drug interactions. Clin Pharmacol Drug Dev 6: 466–475, 2017. doi: 10.1002/cpdd.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumagai Y, Amano H, Sasaki Y, Nakagawa C, Maeda M, Oikawa I, Furuie H. Effect of single and multiple doses of elobixibat, an ileal bile acid transporter inhibitor, on chronic constipation: a randomized controlled trial. Br J Clin Pharmacol 84: 2393–2404, 2018. doi: 10.1111/bcp.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ligon CO, Hannig G, Ge P, Higgins CS, Greenwood-Van Meerveld B. GC-C agonism with linaclotide attenuates chronic stress induced colonic hypersensitivity independently of elevated Crf expression in the central nucleus of the amygdala. Gastroenterology 156, Suppl 1: S364, 2019. doi: 10.1016/S0016-5085(19)37748-0. [DOI] [Google Scholar]
- 39.Ma J, Ding X, Yin Y, Huang P. Peptide inhibitors of chloride channels for treating secretory diarrhea. Front Biosci 23: 1780–1788, 2018. doi: 10.2741/4672. [DOI] [PubMed] [Google Scholar]
- 40.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang XP, Sassano MF, Giguère PM, Löber S, Da Duan, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537: 185–190, 2016. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mekhjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest 50: 1569–1577, 1971. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memic F, Knoflach V, Morarach K, Sadler R, Laranjeira C, Hjerling-Leffler J, Sundström E, Pachnis V, Marklund U. Transcription and signaling regulators in developing neuronal subtypes of mouse and human enteric nervous system. Gastroenterology 154: 624–636, 2018. doi: 10.1053/j.gastro.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology 92: 40–47, 1987. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 44.Mohan LJ, Daly JS, Ryan BM, Ramtoola Z. The future of nanomedicine in optimising the treatment of inflammatory bowel disease. Scand J Gastroenterol 54: 18–26, 2019. doi: 10.1080/00365521.2018.1563805. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima A, Seki M, Taniguchi S. Determining an optimal clinical dose of elobixibat, a novel inhibitor of the ileal bile acid transporter, in Japanese patients with chronic constipation: a phase II, multicenter, double-blind, placebo-controlled randomized clinical trial. J Gastroenterol 53: 525–534, 2018. doi: 10.1007/s00535-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakajima A, Seki M, Taniguchi S, Ohta A, Gillberg PG, Mattsson JP, Camilleri M. Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol Hepatol 3: 537–547, 2018. doi: 10.1016/S2468-1253(18)30123-7. [DOI] [PubMed] [Google Scholar]
- 47.Nakajima A, Taniguchi S, Kurosu S, Gillberg PG, Mattsson JP, Camilleri M. Efficacy, long-term safety, and impact on quality of life of elobixibat in more severe constipation: post hoc analyses of two phase 3 trials in Japan. Neurogastroenterol Motil 31: e13571, 2019. doi: 10.1111/nmo.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Negus SS, Freeman KB. Abuse potential of biased mu opioid receptor agonists. Trends Pharmacol Sci 39: 916–919, 2018. doi: 10.1016/j.tips.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nullens S, Nelsen T, Camilleri M, Burton D, Eckert D, Iturrino J, Vazquez-Roque M, Zinsmeister AR. Regional colon transit in patients with dys-synergic defaecation or slow transit in patients with constipation. Gut 61: 1132–1139, 2012. doi: 10.1136/gutjnl-2011-301181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol 8: 159–165.e5, 2010. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oduyebo I, Camilleri M, Nelson AD, Khemani D, Nord SL, Busciglio I, Burton D, Rhoten D, Ryks M, Carlson P, Donato L, Lueke A, Kim K, Rossi SJ, Zinsmeister AR. Effects of NGM282, an FGF19 variant, on colonic transit and bowel function in functional constipation: a randomized phase 2 trial. Am J Gastroenterol 113: 725–734, 2018. doi: 10.1038/s41395-018-0042-7. [DOI] [PubMed] [Google Scholar]
- 52.Ohno H, Kojima Y, Harada H, Abe Y, Endo T, Kobayashi M. Absorption, disposition, metabolism and excretion of [14C]mizagliflozin, a novel selective SGLT1 inhibitor, in rats. Xenobiotica 49: 463–473, 2019. doi: 10.1080/00498254.2018.1449269. [DOI] [PubMed] [Google Scholar]
- 53.Park SY, Khemani D, Acosta A, Eckert D, Camilleri M. Rectal gas volume: defining cut-offs for screening for evacuation disorders in patients with constipation. Neurogastroenterol Motil 29: e13044, 2017. doi: 10.1111/nmo.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park SY, Khemani D, Nelson AD, Eckert D, Camilleri M. Rectal gas volume measured by computerized tomography identifies evacuation disorders in patients with constipation. Clin Gastroenterol Hepatol 15: 543–552.e4, 2017. doi: 10.1016/j.cgh.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, Moayyedi P. Gut microbiota in patients with irritable bowel syndrome: a systematic review. Gastroenterology 157: 97–108, 2019. doi: 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 56.Raehal KM, Walker JK, Bohn LM. Morphine side effects in β-arrestin 2 knockout mice. J Pharmacol Exp Ther 314: 1195–1201, 2005. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 57.Rao SS, Valestin J, Brown CK, Zimmerman B, Schulze K. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. Am J Gastroenterol 105: 890–896, 2010. doi: 10.1038/ajg.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao SS, Valestin JA, Xiang X, Hamdy S, Bradley CS, Zimmerman MB. Home-based versus office-based biofeedback therapy for constipation with dyssynergic defecation: a randomised controlled trial. Lancet Gastroenterol Hepatol 3: 768–777, 2018. doi: 10.1016/S2468-1253(18)30266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenbaum DP, Yan A, Jacobs JW. Pharmacodynamics, safety, and tolerability of the NHE3 inhibitor tenapanor: two trials in healthy volunteers. Clin Drug Investig 38: 341–351, 2018. doi: 10.1007/s40261-017-0614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudling M, Camilleri M, Graffner H, Holst JJ, Rikner L. Specific inhibition of bile acid transport alters plasma lipids and GLP-1. BMC Cardiovasc Disord 15: 75, 2015. doi: 10.1186/s12872-015-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadik R, Stotzer PO, Simrén M, Abrahamsson H. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil 20: 197–205, 2008. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 62.Sciarretta G, Furno A, Mazzoni M, Malaguti P. Post-cholecystectomy diarrhea: evidence of bile acid malabsorption assessed by SeHCAT test. Am J Gastroenterol 87: 1852–1854, 1992. [PubMed] [Google Scholar]
- 63.Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol 113: 329–338, 2018. doi: 10.1038/ajg.2017.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shailubhai K, Palejwala V, Arjunan KP, Saykhedkar S, Nefsky B, Foss JA, Comiskey S, Jacob GS, Plevy SE. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther 6: 213–222, 2015. doi: 10.4292/wjgpt.v6.i4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silos-Santiago I, Hannig G, Eutamene H, Ustinova EE, Bernier SG, Ge P, Graul C, Jacobson S, Jin H, Liong E, Kessler MM, Reza T, Rivers S, Shea C, Tchernychev B, Bryant AP, Kurtz CB, Bueno L, Pezzone MA, Currie MG. Gastrointestinal pain: unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain 154: 1820–1830, 2013. doi: 10.1016/j.pain.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 66.Singla N, Minkowitz HS, Soergel DG, Burt DA, Subach RA, Salamea MY, Fossler MJ, Skobieranda F. A randomized, Phase IIb study investigating oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res 10: 2413–2424, 2017. doi: 10.2147/JPR.S137952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singla NK, Skobieranda F, Soergel DG, Salamea M, Burt DA, Demitrack MA, Viscusi ER. APOLLO-2: a randomized, placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the μ-opioid receptor, for management of moderate to severe acute pain following abdominoplasty. Pain Pract 19: 715–731, 2019. doi: 10.1111/papr.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, Skobieranda F, Violin JD, Webster LR. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 155: 1829–1835, 2014. doi: 10.1016/j.pain.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, Violin JD, Lark MW. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol 54: 351–357, 2014. doi: 10.1002/jcph.207. [DOI] [PubMed] [Google Scholar]
- 70.Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein C. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 355: 966–969, 2017. doi: 10.1126/science.aai8636. [DOI] [PubMed] [Google Scholar]
- 71.Spiller R. Inhibiting glucose absorption to treat constipation. Lancet Gastroenterol Hepatol 3: 588–589, 2018. doi: 10.1016/S2468-1253(18)30214-0. [DOI] [PubMed] [Google Scholar]
- 72.Sun J, Liang W, Yang X, Li Q, Zhang G. Cytoprotective effects of galacto-oligosaccharides on colon epithelial cells via up-regulating miR-19b. Life Sci 231: 116589, 2019. doi: 10.1016/j.lfs.2019.116589. [DOI] [PubMed] [Google Scholar]
- 73.Taniguchi S, Yano T, Imaizumi M, Manabe N. Elobixibat, an ileal bile acid transporter inhibitor, induces giant migrating contractions during natural defecation in conscious dogs. Neurogastroenterol Motil 30: e13448, 2018. doi: 10.1111/nmo.13448. [DOI] [PubMed] [Google Scholar]
- 74.Tchernychev B, Ge P, Kessler MM, Solinga RM, Wachtel D, Tobin JV, Thomas SR, Lunte CE, Fretzen A, Hannig G, Bryant AP, Kurtz CB, Currie MG, Silos-Santiago I. MRP4 modulation of the guanylate cyclase-C/cGMP pathway: effects on linaclotide-induced electrolyte secretion and cGMP efflux. J Pharmacol Exp Ther 355: 48–56, 2015. doi: 10.1124/jpet.115.224329. [DOI] [PubMed] [Google Scholar]
- 75.Thiagarajah JR, Ko EA, Tradtrantip L, Donowitz M, Verkman AS. Discovery and development of antisecretory drugs for treating diarrheal diseases. Clin Gastroenterol Hepatol 12: 204–209, 2014. doi: 10.1016/j.cgh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tse CM, Yin J, Singh V, Sarker R, Lin R, Verkman AS, Turner JR, Donowitz M. cAMP stimulates SLC26A3 activity in human colon by a CFTR-dependent mechanism that does not require CFTR activity. Cell Mol Gastroenterol Hepatol 7: 641–653, 2019. doi: 10.1016/j.jcmgh.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut 65: 1951–1959, 2016. doi: 10.1136/gutjnl-2015-309889. [DOI] [PubMed] [Google Scholar]
- 78.Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol 16: 522–527, 2018. doi: 10.1016/j.cgh.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vijayvargiya P, Camilleri M. Current practice in the diagnosis of bile acid diarrhea. Gastroenterology 156: 1233–1238, 2019. doi: 10.1053/j.gastro.2018.11.069. [DOI] [PubMed] [Google Scholar]
- 80.Vijayvargiya P, Donato L, Calderon G, Lueke A, Camilleri M. Assessment of the impact of introducing fecal bile acid diagnostic test for bile acid diarrhea in 250 patients in clinical practice and on prediction of response to bile acid sequestrants. Gastroenterology 156, Suppl 1: S188–S189, 2019. doi: 10.1016/S0016-5085(19)37265-8. [DOI] [Google Scholar]
- 81.Vijayvargiya P, Donato LJ, Szarka LA, Acosta A, Lueke AJ, Harmsen WS, Camilleri M. Randomized, double-blind, placebo-controlled trial of colesevelam in patients with bile acid diarrhea: effects on fecal bile acids, colonic transit, and bowel functions. Gastroenterology 156, Suppl 1: S464–S465, 2019. doi: 10.1016/S0016-5085(19)38013-8. [DOI] [Google Scholar]
- 82.Viscusi ER, Skobieranda F, Soergel DG, Cook E, Burt DA, Singla N. APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the µ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J Pain Res 12: 927–943, 2019. doi: 10.2147/JPR.S171013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain 157: 264–272, 2016. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 84.Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut 67: 1543–1552, 2018. doi: 10.1136/gutjnl-2018-316029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther 41: 54–64, 2015. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Larauche MH, Siegel M, King AJ, Mayer EA, Tillisch K, Labus JS, Chang L, Caldwell JS. Tenapanor attenuates increased macromolecule permeability in human colon monolayer cultures induced by inflammatory cytokines and human fecal supernatants. Gastroenterology 154, Suppl 1: S326, 2018. doi: 10.1016/S0016-5085(18)31424-0. [DOI] [Google Scholar]
- 87.Wang XJ, Camilleri M. Personalized medicine in functional gastrointestinal disorders: understanding pathogenesis to increase diagnostic and treatment efficacy. World J Gastroenterol 25: 1185–1196, 2019. doi: 10.3748/wjg.v25.i10.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 30: 707–717, 2009. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 89.Wong BS, Camilleri M, Carlson PJ, Guicciardi ME, Burton D, McKinzie S, Rao AS, Zinsmeister AR, Gores GJ. A Klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology 140: 1934–1942, 2011. doi: 10.1053/j.gastro.2011.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol 106: 2154–2164, 2011. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]
- 91.Yacyshyn B, Ginsberg DC, Gilder K, Walsh B, English B, Turner SA, Klassen P, Hanauer SB, Barish CF, Higgins PD. Safety and efficacy of olorinab, a peripherally restricted, highly selective, cannabinoid receptor 2 agonist in a phase 2A study in chronic abdominal pain associated with Crohn’s disease. Gastroenterology 156, Suppl 1: S665, 2019. doi: 10.1016/S0016-5085(19)38567-1. [DOI] [Google Scholar]
- 92.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 93.Zhang L, Kline RH IV, McNearney TA, Johnson MP, Westlund KN. Cannabinoid receptor 2 agonist attenuates pain related behavior in rats with chronic alcohol/high fat diet induced pancreatitis. Mol Pain 10: 66, 2014. doi: 10.1186/1744-8069-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, Croce C, Verne GN. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 65: 797–805, 2016. doi: 10.1136/gutjnl-2013-306464. [DOI] [PMC free article] [PubMed] [Google Scholar]