Abstract
A comprehensive understanding of the dynamic regulatory networks that govern postnatal alveolar lung development is still lacking. To construct such a model, we profiled mRNA, microRNA, DNA methylation, and proteomics of developing murine alveoli isolated by laser capture microdissection at 14 predetermined time points. We developed a detailed comprehensive and interactive model that provides information about the major expression trajectories, the regulators of specific key events, and the impact of epigenetic changes. Intersecting the model with single-cell RNA-Seq data led to the identification of active pathways in multiple or individual cell types. We then constructed a similar model for human lung development by profiling time-series human omics data sets. Several key pathways and regulators are shared between the reconstructed models. We experimentally validated the activity of a number of predicted regulators, leading to new insights about the regulation of innate immunity during lung development.
Keywords: alveolar development, laser capture microdissection, time-series omics data
INTRODUCTION
Lung development starts from a lung bud arising from the foregut in the human embryo at ~5 wk postconception, resulting eventually in a highly complex organ optimized to support gas exchange, as well as important roles in immune regulation and metabolism. Initially consisting of two cell layers (epithelium and mesenchyme), the lung eventually includes multiple specialized cell types. Lung development is relatively conserved among mammalian species, and much of what we know about human lung development is extrapolated from observations of mouse lung development, although there are structural and functional differences in the development of mouse and human lungs.
The development of the lung occurs in overlapping histological stages, consisting of a pseudoglandular stage [human: 5–17 wk gestation, mouse: embryonic (E) day 9.5–16.6], canalicular stage (human: 16–25 wk, mouse: E16.6–17.4), saccular stage [human: 24–32 or 36 wk; mouse: E17.4–postnatal (P) day 5], and the alveolar stage (human: late fetal period to adolescence; mouse: P5–P36). The transition from saccular to alveolar stages occurs prenatally in humans and postnatally in mice and has important functional consequences; decreased numbers and abnormal structure of alveoli cause significant morbidity in premature infants who develop bronchopulmonary dysplasia (BPD). Impaired pulmonary function (associated with too few alveoli) in young adulthood greatly increases the risk of chronic obstructive pulmonary disease (COPD) (22); activation of developmental pathways is also observed in chronic parenchymal lung disease (28). Normal pulmonary alveolar development is a dynamic coordinated process that requires accurate spatial and temporal integration of signals. Despite significant progress in our understanding of alveolar development, we still lack a comprehensive understanding of the dynamic regulatory networks that govern this process. For instance, although we are aware that the regulation of alveolar development depends on integration of numerous signals from multiple pathways (e.g., Wnt, TGF-β, hedgehog, retinoid) (7), we have little understanding of how these pathways interact in the complex and rapidly varying microenvironment of the developing lung. Similarly, we do not have a detailed understanding of the hierarchy of transcriptional regulation during lung development, including temporal changes in epigenetic regulation, expression of regulatory RNAs such as microRNAs, and changes in transcription factors (TFs). These different types of regulatory information need to be jointly collected at the right time points and then integrated to reconstruct an accurate and detailed model of lung development.
To provide such integrated model of alveolar development, as part of the LungMAP consortium (www.lungmap.net), we generated multiomic profiles of microdissected murine and human alveoli and the tools to analyze and visualize them. We first identified the most informative time points in murine postnatal alveolar tissue by applying time point selection (TPS; see Ref. 18). We then isolated alveolar parenchyma by laser capture microdissection (LCM) and performed RNA-Seq, miRNA profiling, genomewide CpG methylation, and global protein analysis at the selected time points. Next, we extended methods for the integration of time-series and static interaction data so that additional types of data (including proteomics and DNA methylation) could be used to improve the reconstruction of a regulatory model. The extended method, interactive Dynamic Regulatory Events Miner (iDREM) reconstructs a dynamic model that identifies the grouping of genes during the development process; the transcription factors (TF), miRNAs, and methylation events that regulate them; and the timing of these regulatory events. We then developed an interactive tool that allows users to query and use the model to focus on specific genes, regulators, miRNAs, methylation values, and more and also enables the integration of other types of data, including single-cell and -sorted cell RNA-Seq data with the model. Applying iDREM to mouse and human omics data led to a comprehensive interactive model revealing both the major genes and functions involved in alveolar development and identifying new roles for several TFs and miRNAs in regulating various stages of this process.
METHODS
Mouse Lung Samples
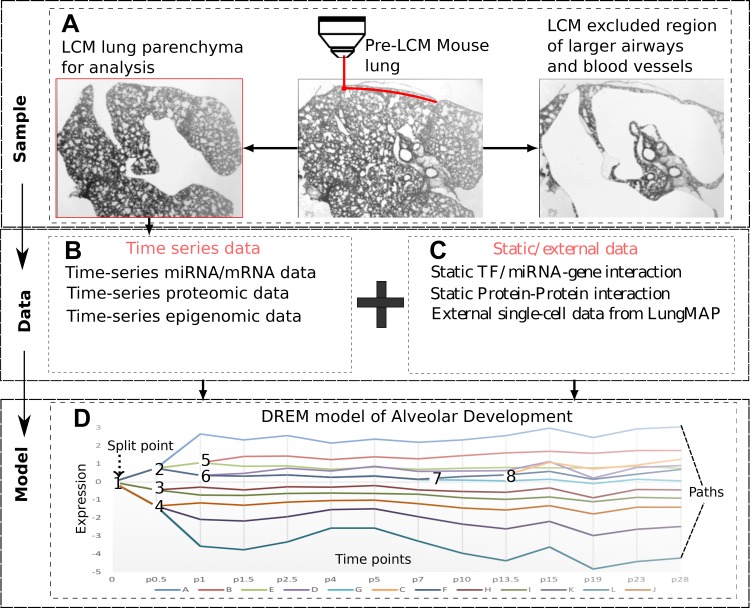
Standard operating procedures and all data from this and other LungMAP projects are publicly available at www.lungmap.net (https://lungmap.net/resources/sop-search-page/). Animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Lung tissue used in this study was obtained from mouse pups born to C57BL/6 timed-pregnant females purchased from Jackson Laboratories (Bar Harbor, ME). Tissue specimens were immediately embedded in optimal cutting temperature compound (no. 4583; Tissue-Tek) after dissection, snap-frozen in liquid N2, and transferred at −80°C for storage. See Fig. 1 for the study design and Data Supplement Supporting Methods (all supplemental materials for this article are available at https://doi.org/10.5281/zenodo.3332781) for details.
Fig. 1.
Study design. A: samples were obtained from laser capture microdissected (LCM) lungs. B: mRNA, miRNA, and protein levels were profiled at 14 time points selected by the time point selection (TPS) method and methylation at a subset of 6 time points. C: in addition to the condition-specific temporal data the modeling method integrates general static information about protein-DNA and protein-protein interactions and can also use single-cell RNA-Seq (scRNA-Seq) data. D: time-series and static data are integrated using interactive Dynamic Regulatory Events Miner (iDREM). The resulting dynamic model assigns genes to paths and regulators [miRNAs, transcription factors (TFs)] to the paths they regulate. See Fig. 2 for a more detailed version of our model.
Human Lung Samples
Postmortem human lung samples were supplied by the LungMAP Human Tissue Core (University of Rochester Medical Center). Sample collection, processing, and handling of the human lung samples are described on LungMAP.net (https://lungmap.net/resources/sop-search-page/). The Human Tissue Core procured, processed, deposited, and distributed normal neonatal and early childhood human lung tissue for LungMAP working with the United Network for Organ Sharing. Transplantation quality tissues were collected through the International Institute for the Advancement of Medicine and the National Disease Research Interchange. See Data Supplement Supporting Methods for details (https://doi.org/10.5281/zenodo.3332781).
mRNA Analysis
Mice.
Total RNA was isolated from LCM samples at 14 selected time points: (E16.5, P0.5, P1, P1.5, P2.5, P4, P5, P7, P10, P13.5, P15, P19, P23, and P28) using the miRneasy Micro Kit (217084; Qiagen). RNA concentration and integrity were measured using NanoDrop ND-2000 and a 2200 Tape Station. Total RNA (100 ng) from each time point were used for RNA Sequencing.
Human.
Total RNA was isolated from LCM of 19 human samples from ages day 1 to 9 yr using the miRneasy Micro Kit (217084; Qiagen). Total RNA (20 ng) from each sample were used for RNA Sequencing. See Data Supplement Supporting Methods (https://doi.org/10.5281/zenodo.3332781) for complete details.
miRNA Analysis
RNA extraction was performed by the miRNeasy MicroKit (Qiagen) following the manufacturer’s protocol. RNA concentration and integrity were measured by using NanoDrop ND-2000 and a 2200 Tape Station. Comprehensive NanoString prebuilt miRNA panels for mouse (mouse v1.5 miRNA assay) and human (human v3 miRNA expression panel) were used. The nCounter Gene Expression Assay was performed using 50 ng total RNA. Data normalization was performed using nSolver from Nanostring.
DNA Methylation Analysis
Mouse genomic DNA from lung tissues was isolated from LCM samples at six different time points (P0.5, P2.5, P7, P10, P19, and P28). Three biological samples per time point were analyzed by MeDIP-seq. DNA methylation from human samples was profiled using the Illumina Infinium Methylation EPIC Kit, which profiles 850,000 methylation sites at single-nucleotide resolution. See also Data Supplement Supporting Methods (https://doi.org/10.5281/zenodo.3332781).
Proteomic Analysis
Proteins were extracted using tissue protein extraction reagent (T-PER; Thermo). Protein concentrations were determined with the EZQ protein assay (Life Sciences). Analysis of liquid chromatography mass spectrometry (LCMS)-generated data was carried out using Expressionist (Genedata), which assigns high-confidence protein identifications to each ion and aligns mass and time tags from ion plots generated from the post-LCMS run. Following the modeling of the entire data set, the peptide and protein probabilities were set at >80% and >99%, respectively, with false discovery rates based on reverse concatenated searches set at <1%.
Validation Experiments
Real-time RT-PCR was used to measure gene expression of Irf1, Nfatc4, and Meg3 in wild-type non-germ-free mouse and germ-free lungs at P0, P3, P7, P14, and P28. Germ-free mouse pups were obtained from the University of Alabama at Birmingham’s gnotobiotic mouse core and euthanized immediately on receipt.
For the IRF-1 in situ hybridization experiment, lung tissue specimens were isolated from C57BL/6 wild-type mice at selected time points, fixed 12–16 h in normal buffered formalin (10% NBF), embedded in paraffin, freshly cut on Superfrost Plus slides, and kept at 4°C. In situ hybridization was performed using RNAscope Multiplex Fluorescent V2 assay (Advanced Cell Diagnostics, Newark, CA). See Data Supplement Supporting Methods (https://doi.org/10.5281/zenodo.3332781) for complete details.
Specific mirVana microRNA inhibitors (Ambion, Carlsbad, CA) were used for in vivo miR-590–3p or miR-539–5p knockdown, along with scramble control. The combination of miR-539–5p and miR-590–3p was also evaluated. Mice were treated with 5 µg/g of inhibitors on P1, P4, P8, and P12 by intranasal administration as described earlier (25). The 5 µg/g dose was found to reduce the miRNA concentration in mouse lung homogenates at P12 by 50%. Increasing the miRNA inhibitor dose to 10 µg/g or administration intraperitoneally did not increase inhibition. At P14, anesthetized mice were evaluated for lung mechanics on a flexiVent and then euthanized, and lungs were fixed in inflation-fixation for morphometry (25). Our standard procedure for lung development measurements are for at least six mice per group and an evaluation of six random lung fields from each mouse for mean linear intercepts and radical alveolar counts (24, 25). The statistics were calculated by two-way ANOVA.
Data Integration and Modeling
Selection of time points to profile.
Past work, including our own prior work (28), has relied on ad hoc methods to determine which time points to profile. We have recently developed the TPS method, which optimally selects time points to profile (18). Here we used TPS to select the set of points for the RNA-Seq, miRNA, and proteomics experiments and to select a subset of these points for DNA methylation analysis.
The Dynamic Regulatory Events Miner.
The Dynamic Regulatory Events Miner (DREM; see Refs. 15, 27, and 28) integrates static, general, TF-DNA binding interactions data, and condition-specific (in our case lung development) time-series gene and miRNA expression data to determine the set of TFs and miRNAs that control gene expression over time. The basic idea behind DREM is to use the static interaction data to explain why a set of coexpressed genes starts to diverge at a specific point in time. This allows the method to identify both the TFs and miRNAs controlling these divergence events and to associate a specific time with these (initially static) interactions (Fig. 2, A and B). To identify these bifurcation points and the set of TFs and miRNAs that control them, DREM combines a machine-learning method termed Input Output Hidden Markov Model with a logistic regression classifier. To address the issue that TFs and their partners may be posttranscriptionally regulated and so would not show increase in abundance, iDREM relies not just on the expression of the regulators or their partners but also on the activity of the targets in the next time point. Specifically, the method can identify TFs as active even if their own expression (and the expression of their partners) does not change at all if the expression of their targets changes. We have shown previously that such an approach indeed allows us to identify active TFs even if they are not transcriptionally regulated (5, 11).
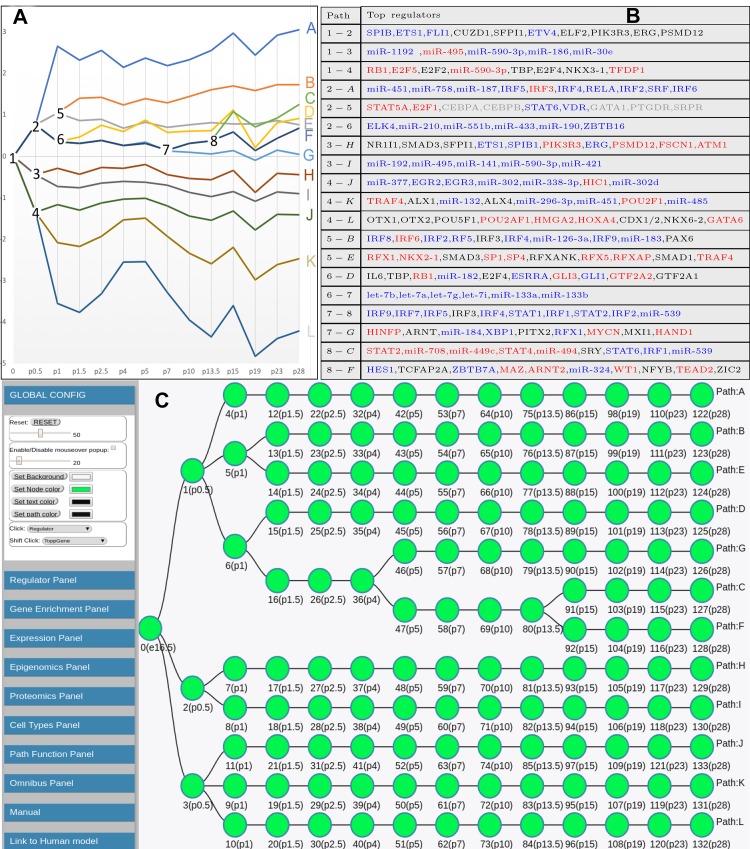
Fig. 2.
Learned interactive Dynamic Regulatory Events Miner (iDREM) model for mouse lung development. A and B: detailed model displaying expression trajectories (A) and the regulators controlling specific temporal events (B). The transcription factors (TFs) with increased expression are marked in blue and TFs with decreased expression are marked in red in B. C: schematic view of the iDREM lung model used in the interactive visualization tool. See text for detailed discussion of paths, branching, TFs, and miRNA predicted to regulate various stages in the model.
Integrating proteomics and protein-protein interactions.
Most work on the reconstruction of dynamic regulatory networks has focused on the use of RNA-Seq and to a lesser extent miRNA data. In this study, we have also profiled complementary time-series proteomics and methylation data sets that were integrated to refine the model. We used the proteomics data to improve our ability to detect the time of TF activation. Specifically, we look for two lines of evidence to determine such activity. The first is the level of the protein itself, and the second is the likelihood of a posttranslational interaction or modification that leads to its activation. For the former, we use the proteomics data directly. For the latter, we combine protein-interaction data with the proteomics data; we determine the protein levels of its interacting partners and use this to increase or decrease our belief in the activity of the TF. Specifically, we set the activity level of a TF at each time point to:
where ATF(x,t) is the inferred activity of TF x at time t, Y denotes the set of proteins that interact with x, and PPI(x,y) is the static protein-protein interaction strength between TF x and y from STRING (34). P(x,t), P(y,t) represent the protein level for TF x and partner y, respectively, at time t, and wATF is the logistic function steepness parameter. This activity is then used as a dynamic prior by our model.
Integrating Epigenetics Data
While it is challenging to obtain time-series TF binding data, since these require several additional experiments, we can often obtain global indirect information about such events. Methylation data were shown to correlate with other epigenetic data sets and with binding for several TFs (46). We thus used DNA methylation data to obtain a dynamic prior for TFs. Our use of the DNA methylation data is based on several published studies, by us and others, that have demonstrated a strong link between methylation and TF binding (11, 41, 47). One function of DNA methylation is restricting TF access to promoter regions by changing chromatin structure (16). We use time-series methylation data to identify “silenced” TFs. These are TFs that, while active, may seem to be inactive for some targets because their binding sites for these targets are methylated. In the original DREM method, such TFs would be assigned a low score (since several of their targets are inactive) and may be wrongly removed from the model. To overcome this, we revised the static prior interaction map used by DREM and reduced the interaction likelihood for genes with methylated promoters. This reduction places more weight on nonmethylated targets when compared with methylated ones and so may allow a better identification of active TFs. Specifically, we use the following as the methylation score:
where Mr(y,t) represents the regulation “score” for gene y at time t based on the given methylation data. methyl(y,t) is the average methylation of the promoter of gene y at time point t. We next discuss how we combine this score with the other interaction and activity values to infer a comprehensive regulatory model.
Inferring a Combined Dynamic Prior
We next combined the different static and dynamic data sources to derive a dynamic prior for the regulation of a gene by a TF. This dynamic prior was then combined with the dynamic gene expression information of the target gene to group genes in paths and infer TF activity. For the dynamic prior, we combined the TF activity value [ATF(x,t)] derived from the time-series proteomics data, the methylation score Mr(y,t), and a static TF-DNA prior Rstatic(x,y) (Fig. 1B). For this, we first combined the two terms that are based on DNA accessibility (the static protein-DNA interaction score and the methylation score) and then combined the resulting score with the activity value derived from the proteomics data. See Data Supplement Supporting Methods (https://doi.org/10.5281/zenodo.3332781) for details.
Interactive visualization of the reconstructed model.
iDREM provides an interactive display for the reconstructed developmental network (Fig. 2C). The new visualization framework provides additional capabilities for intersecting other data sets with the current model, including data from time-series single-cell RNA-Seq studies and data from sorted cells at various time points. See Data Supplement Supporting Methods (https://doi.org/10.5281/zenodo.3332781) for details.
Comparison with Human Models
Grouping of human samples.
We combined the following four sets of human samples: birth (days 1 and 5), early infancy (24 days and 2 and 3 mo), toddler (7, 19, 20, and 21 mo and 3 and 4 yr), and school age (8 and 9 yr). Subsequent analysis used the median of each gene for each set.
Aligning mouse and human data.
We extracted human genes with mouse orthologs based on Ensembl (2). We used splines to represent continuous gene expression and tested a number of continuous alignment methods for mapping human to mouse time points. The best function we found was an exponential alignment y = 2.9log(x) + 5.1 y = 2.9log(x) + 5.1, where y is the mouse day and x is the human day. We calculated the P value for this function using permutation testing. Note that, because the number of time points is limited, some of the permutations are actually very close to the correct ordering itself, which is why the P value cannot be much lower. See Data Supplement Supporting Methods (https://doi.org/10.5281/zenodo.3332781) for details.
Reconstructing human developmental model.
We used iDREM to integrate the different human data sets. We combined the human data (time-series mRNA, miRNA, proteomics and DNA methylation data, and static TF-gene and PPI human interaction data) in the same way as we did for mice.
Data and Software Availability
The data that support the findings of this study have been deposited in the LungMAP consortium (www.lungmap.net). The accession numbers are included in Table 1.
Table 1.
Multiomics data availability
| URL | |
|---|---|
| RNA-seq mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001630 |
| RNA-seq (AmpliSeq) human LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001631 |
| Nanostring microRNA mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000000227 |
| Nanostring microRNA human LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000003682 |
| MeDIP-Seq mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001224 |
| Methylation array human LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001628 |
| Proteomics mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000000180 |
| Proteomics human LCM no. 1 | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000000863 |
| Proteomics human LCM no. 2 | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001605 |
LCM, laser capture microdissection.
Standard operating procedures and all data and associated metadata (e.g., mouse: sex, weight; human: postnatal age, gestational age, sex, health status, etc.) from this and other LungMAP projects are publicly available at www.lungmap.net. The interactive model is available at our supporting website (https://www.cs.cmu.edu/~jund/idrem_lung/), and the software is freely available at GitHub (https://github.com/phoenixding/idrem).
RESULTS
General Description of OMICS Results
For mouse, we profiled mRNA, miRNA, and proteomics data from LCM alveolar parenchyma obtained at 14 selected time points (E16.5, P0.5, P1, P1.5, P2.5, P4, P5, P7, P10, P13.5, P15, P19, P23, and P28, with 3 biological replicates per time point) and DNA methylation in a subset of six of these time points (P0.5, P2.5, P7, P10, P19, and P28; 3 biological replicates each). These 14 time points were selected using the TPS approach (18), which guaranteed that the reconstructed profiles capture as much of the underlying regulatory activity as possible while minimizing the number of samples (methods). We also profiled LCM of alveolar parenchyma of human lungs at 13 time points between 1 day and 9 yr (methods). For human samples, we profiled mRNA, proteomics, and methylation. Supplemental Fig. S1 (https://doi.org/10.5281/zenodo.3332781) provides clustering heatmaps that summarize all expression data collected for mouse and human. From the mouse data, we identified 18,567 genes in at least one of the time points. Of these, 12,209 (65.8%) displayed a fold change of at least 2 between their highest and lowest expression values, and these were used for the model. We identified 599 expressed miRNAs and 1,004 proteins with varying expression levels across the time points. Methylation data were genomewide, although the model focused on the upstream regions of the genes (methods). For the human data, we used the top 5,000 genes with mouse orthologs for the modeling (methods). Human proteomics data included 2,254 proteins. We observed similar average methylation levels (genomewide) for the mouse and human data.
Constructing a Dynamic Mouse Developmental Model
We used iDREM to reconstruct a mouse lung developmental regulatory network. iDREM integrates time-series and static “omics” data to infer a dynamic network model in which genes are grouped into paths (Fig. 1). Splits (nodes where two sets of previously coexpressed genes start to diverge) are annotated with the regulators that are predicted to control them. The resulting model (Fig. 2A) has 12 paths (labeled A–L) with 132 nodes. Each path represents a set of coexpressed genes (ranging from 164 genes assigned to path L to >2,000 genes in path H). Divergence events (splits into two or more paths) occur mostly at early time points, with only one after P4, and were identified for only 5 of the 14 time points profiled.
The top regulators predicted for the splits in the reconstructed networks (Figs. 2B and 3A) included many TFs and miRNAs that are known mediators of normal lung development (e.g., NKX2-1, CEBPA, and GLI1) and many that have not so far been considered as involved in lung development (e.g., TMEM37, ARHGEF7, and POU2F1). Other predicted regulators highlighted potential novel mechanisms for controlling lung development. For example, miR-127 and miR-433 are predicted to regulate path D, which contains the Meg3 long non-coding RNA (lncRNA). These two miRNAs are actually part of the Meg3 chr12qF1 locus and were previously associated with regulating immune response functions (21) and glucocorticoid signaling (31), consistent with the function of genes on path D. Global evaluation of the predicted regulators suggests that some pathways (primarily the ones with increased expression over time, e.g., A, B, E, and D) are regulated primarily by TFs, other paths (primarily the ones that show mild decrease in expression, e.g., I, J, and K) are regulated mostly by microRNAs, while some pathways (e.g., G, C, F, and H) are regulated by both TFs and microRNAs. The activity of regulators is complex, with many regulators modulating multiple pathways, and either “initiating” or “maintaining” regulation along a pathway. We also analyzed the predicted regulators using gene set enrichment analysis; we found significant enriched terms for all paths except path I, which is only regulated by miRNAs (Supplemental Table S1; https://doi.org/10.5281/zenodo.3332781). We found that the top paths (A, B, C, D, and F) are enriched for regulators of immune- and defense-related functions, while the bottom paths (J and K) are more enriched for cell cycle regulators.
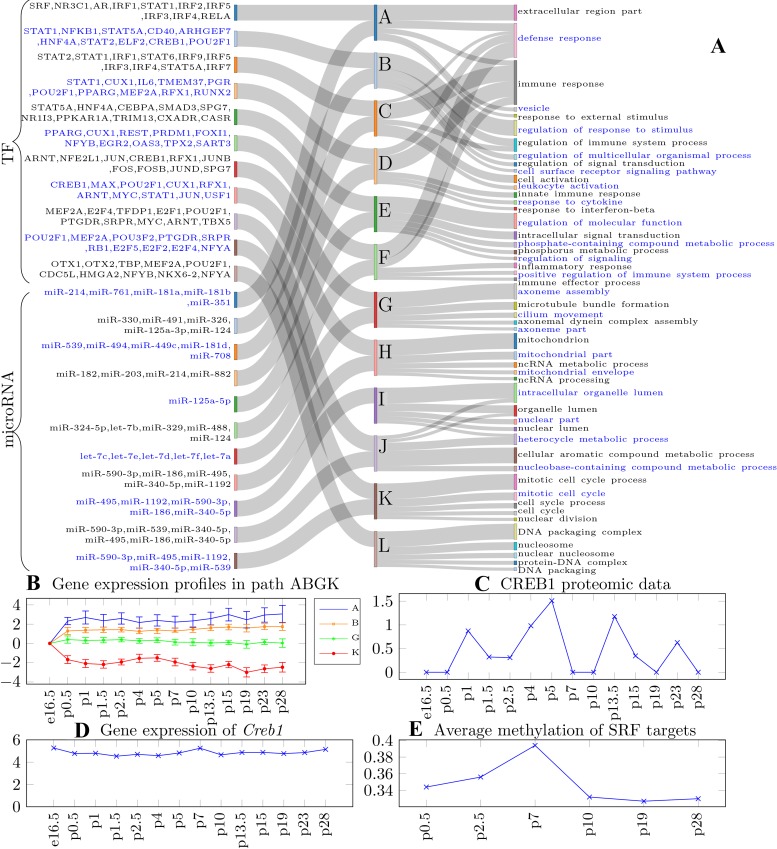
Fig. 3.
A: path Sankey diagram. The middle column represents the paths; the left column shows the regulators for each path; the right column shows the enriched Gene Ontology (GO) terms associated with each path; blue text and gray text are used to separate the regulators of different paths. TF, transcription factors. B: expression profiles of genes assigned to the top 2 (A and B), middle (G), and bottom (K) paths. Lines represent mean gene expression in the path while the error bar denotes standard deviation. While differences between the overall expression of these genes are clear, without the use of the regulatory information it would have been hard to correctly assign them to the different paths due to overlap in their expression profiles. C and D: gene expression and protein level for the known regulator CREB1. E: methylation data used to infer the activity of serum response factor (SRF) in regulating cell proliferation.
Several of the paths contain genes that are significantly associated with key functions related to lung development (Fig. 3A and Supplemental Table S2; https://doi.org/10.5281/zenodo.3332781). The top paths (A, B, C, D, and F) (increasing expression) are significantly enriched for immune and defense response genes (P value = 1.72 × 10−32), which are known to increase with advancing postnatal age. Middle paths are primarily enriched for cell-type-specific functions. For example, path E is enriched for cell migration (1.33 × 10−11), which is an essential process during organ development, growth, and repair (17). Path G is enriched for axoneme-related functions such as axoneme assembly (2.4 × 10−8) and cilium movement (6.5 × 10−5). The bottom paths (decreasing expression) seem to represent processes related to the reduction in cell proliferation with advancing lung maturation and differentiation. Path K is enriched for repressed cell cycle genes (8.43 × 10−46), and path L is enriched for repressed nucleosome assembly genes (4.24 × 10−42). Gene expression profiles (relative expression to the first time point) for several paths are shown in Fig. 3B.
Proteomics, Epigenetics, and Single-cell Data Improve the Ability to Model Developmental Regulation
While prior methods relied primarily on mRNA and miRNA data for reconstructing lung developmental networks (28), here we also used time-series epigenetics and proteomics to reconstruct the models. This enabled iDREM to identify key regulators that would have been missed without the use of such data. For example, as can be seen in Supplemental Fig. S2 (https://doi.org/10.5281/zenodo.3332781), RUNX2 (29), CASR (8), TRIM13 (23), CREB1 (3), and TBX5 (4), all reported to regulate lung development, are missing from a model that is only based on the transcriptomics data. More generally, several other TFs identified only when using the proteomics data are known regulators of development. GO analysis performed on the set of TFs that are not found when only using transcriptomics data identifies “developmental process” as the most significant GO term (6.27 × 10−8). See Data Supplement Supporting Results (https://doi.org/10.5281/zenodo.3332781) for details. Similarly, methylation data has allowed iDREM to identify serum response factor (SRF) as the regulator for path K. Compared with other paths, path K is enriched with low methylated SRF target genes at the early developmental stage (time point p0.5 to time point p3), allowing the method to infer the activity of SRF on this path (Fig. 3C).
One important limitation of our method using LCM data is that the alveolar parenchyma is a mixture of cells in close proximity, and the mRNA, miRNA, and proteomic profile is generated from multiple adjacent cells. We therefore used available time-series single-cell RNA-Seq data (13) to determine if different paths in the model are associated with specific cell types. We found that epithelial cells at P1 localize to node 4 (path A) and node 5 (paths B and E), while they localize to node 53 (path A) and node 55 (path E) at P7. Endothelial cells also localize to these paths (A, B, and E). On the other hand, matrix fibroblasts localized to paths A, H, and I while myofibroblasts/smooth muscle cells localize to path I. T lymphocytes localized to paths K and L. The sharing of certain paths among different cell types (e.g., between epithelial and endothelial cells) suggests that, although cell lineage may be specified much earlier, regulatory mechanisms may have much in common across cell types.
Comparing Mouse and Human Developmental Programs
To determine which of the pathways and regulators are shared between human and mouse, and to identify correlations between human and mouse lung development, we also profiled human tissues at distinct stages of alveolar development. Because the human data were obtained from different individuals and were likely noisier than the mouse data, we grouped the 13 time points into 4 time segments representing birth (1–5 days), early infancy (24 days–3 mo), toddlers (7 mo–4 yr), and school-age children (8–9 yr). Each of these groups contained between two and six measurements that were averaged (methods).
To compare human and mouse development, we first needed to align the human data to the mouse data. Alignment of time-series omics data within and between species is often based on methods that select transformations that minimize the difference in gene expression of orthologs between the two species (5). Because the sampling times are not expected to directly match, such methods require that the data be first represented by a continuous function (usually splines; see Ref. 1) and then that a transformation function be applied to convert one set of time points (in humans) to another (mouse). For this, we tested several different types of functions (methods). We used an iterative algorithm that, in addition to determining the temporal alignment parameters, can also select the set of genes that is the most similar following the alignment such that differences related to specific background expression or noise can be minimized (methods).
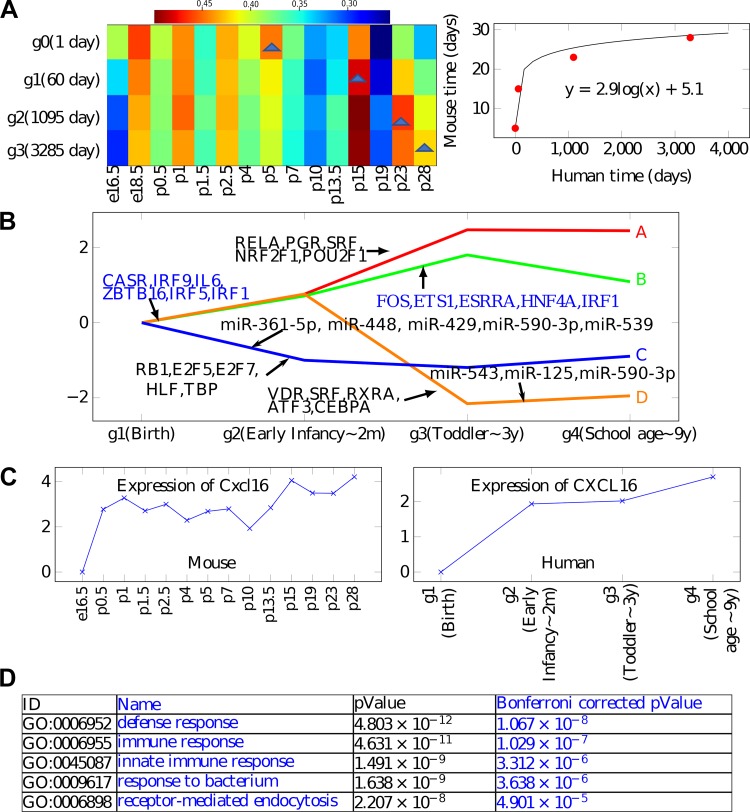
We found that an exponential alignment leads to the best fit for our data (Fig. 4A). The alignment assigned human day 1 (birth) to mouse day 5 (P5), human 2 mo (early infancy) to mouse P15, 3 yr (toddlers) to mouse P23, and 9 yr (school-age) to mouse P28 (the last time point for mice). Please refer to Supplemental Table S3 (https://doi.org/10.5281/zenodo.3332781) for the detailed correlation table shown in Fig. 4A. The resulting agreement between genes following the alignment was significant (P value = 0.029 based on randomization tests). Importantly, genes that best agreed with the alignment were highly related to biological functions that are expected to be active during development, including circulatory system development (corrected P value 1 × 10−10) and regulation of cell proliferation (corrected P value = 1 × 10−11). See also Supplemental Table S4 (https://doi.org/10.5281/zenodo.3332781).
Fig. 4.
Analysis of human data. A: optimal alignment obtained for mouse and human expression data. The color represents the correlation. B: reconstructed human model showing both paths (expression trajectories) and regulators. Note agreement between several of the transcription factors (TFs) and miRNAs in the human and mouse models. C: CXCL16 shows similar expression pattern between the human and mouse data sets and is assigned to a similar path (topmost path) in both models. See Supplemental Fig. S3 (https://doi.org/10.5281/zenodo.3332781) for additional examples of similarly expressed genes. D: Gene Ontology (GO) enrichment for the top path (A) in the human model.
We next combined the human RNA-Seq, microRNA, proteomics, and methylation data to reconstruct a human developmental model with four time points (Fig. 4B). Although it is less detailed than the mouse model (because of the smaller number of time points, and from later in lung development, since human samples were collected only from term infants in the alveolar stage of development while mouse lungs were collected from saccular-stage lungs), several of the paths and TFs are in agreement between the models. Specifically, the top path in the human model is also enriched for “defense response” and “immune response” (corrected P value = 5 × 10−10), while the lower (repressed) path is enriched for genes associated with proliferation and extracellular matrix. As for TFs, we again see members of the IRF TF family regulating the top paths, indicating that innate immunity is part of the human developmental program as well. We also see other immune factors, including IL-6 and RELA, as shared regulators of top paths in both human and mouse. As for the lower paths, in both human and mouse, we identify RB1, a repressor TF, as regulating the lower (repressed expression paths). RB1 is linked to cell cycle repression, which agrees well with the reduced proliferation observed at later stages of lung development. Several genes and TFs appear in similar paths/split nodes between the two models. For example, CXCL16 (Fig. 4C), CD74, PRELP, CYP4B1, SFTPD, and many others appear in the topmost path in both human and mouse models (see Supplemental Fig. S3). Similar to the mouse model, we also used profiled microRNAs to identify potential miRNA regulators of paths in the human model. Several miRNAs are predicted by iDREM to regulate human lung development (Fig. 4B). Two of these, miR-590–3p and miR-539 (5p), match top predictions for the mouse model. In both models, they are predicted to regulate paths in the bottom half (decreasing expression).
Experimental Validation of Predicted Regulators
Supplemental Table S5 (https://doi.org/10.5281/zenodo.3332781) lists literature support for at least one of the predicted regulators for each path in our model. Several of the predicted regulatory relationships (miRNA-mRNA, DNA methylation-mRNA, and TF-known targets) have also been reported or verified in the literature (Supplemental Table S6; https://doi.org/10.5281/zenodo.3332781). In addition to the literature support for the predicted regulators, we also experimentally validated selected novel model predictions.
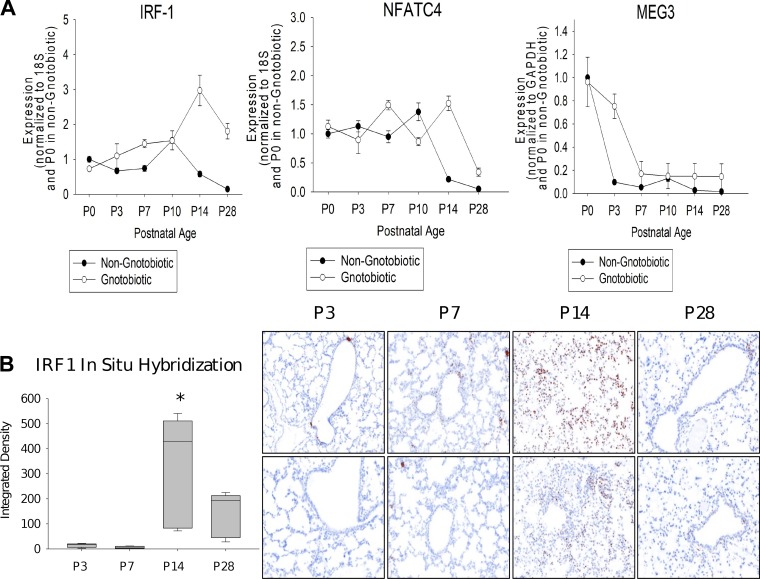
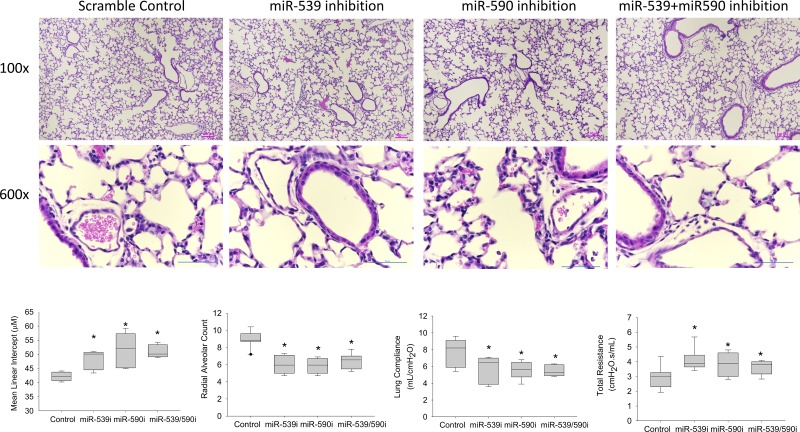
Several of the regulators of the top paths in both models, as well as genes assigned to these paths, were significantly associated with immune response. Specifically, the IRF (Fig. 3A) and STAT families of TFs were associated with these paths, and paths were significantly enriched (P = 8.29 × 10−25) for immune response genes. The prediction of the model regarding the activation of an innate immunity program at the early stages can be interpreted in two different ways. It may be a response program (not directly related to development), which is activated following exposure of the developing lung to the postnatal environmental microbiome. An alternative option is that the activation of innate immunity pathways, regardless of the pathogens the lung is exposed to, is an inherent part of the developmental program (42). To test this, we evaluated the expression of key immune TFs predicted by the model in lungs of gnotobiotic (germ-free) mice. We focused regulators of the top paths (IRF1 and NFATC4) and a regulator of node 80 (lncRNA Meg3, which contains miR-9, a regulator of node 80), a key split node at P13.5 (the only split after P4). We observed (Fig. 5A) similar expression profiles for both TFs and the lncRNA in the gnotobiotic mice even though these were never exposed to pathogens (in some cases expression was even higher than for the exposed mice), indicating that postnatal exposure to microbes did not drive expression of IRF1 and NFATC4. We also observed by in situ hybridization that, while IRF1 was expressed in only a few bronchiolar epithelial cells at earlier time points (P3, P7), it was expressed diffusely at a much higher magnitude in the alveolar parenchyma at P14, and subsequently had reduced expression at P28, similar to that at early time points (Fig. 5B). These results support model assignments for these regulators and indicate that the activation of these immune response programs is an inherent part of the developmental program. We performed another experiment to validate novel predictions regarding the role of miR-539 (mmu-miR-539–5p) and miR-590 (mmu-miR-590–3p) as regulators of lung development (predicted to regulate paths C, J, and K; Fig. 3A). As shown in Fig. 6, inhibition of miR-539 and miR-590 significantly reduces alveolar development, with a reduction in radial alveolar count and lung compliance by ~20% (P value < 0.05) and an increase in mean linear intercept and total lung resistance by ~10% (P value < 0.05) compared with scramble control, supporting model predictions for their roles.
Fig. 5.
Experimental validations of predicted regulators. A: expression of two transcription factors (TFs) and a long non-coding RNA (lncRNA) predicted to regulate key split nodes in the model. As can be seen, even though the model was learned using data from wild-type (WT) mice, we see a comparable increase (for IRF1 and NFATC4) or decrease (Meg3) in the gnotobiotic (germ-free) mice indicating that these regulators are activated or repressed as part of the general developmental program rather than in response to specific pathogens (n = 6 per time point; mean + SE). B: IRF1 localization by in situ hybridization (ISH) in mouse lungs at postnatal day 3 (P3), P7, P14, and P28. Left: image analysis quantitation of IRF1 ISH (n = 3 per time point; box-and-whiskers plot with box indicating 25th–75th percentiles, line across box indicating median, and whiskers indicating 5th–95th percentiles. *P < 0.05 vs. control by 2-way ANOVA followed by Tukey’s test). Right: photomicrographs of ISH demonstrating low concentration of IRF1 at P3 and P7 in a few bronchiolar epithelial and alveolar cells. In contrast, we observe high concentrations at P14 (seen diffusely in many alveolar cells), followed by a decline at P28 with staining only in a few bronchiolar epithelial cells.
Fig. 6.
Effects of scramble control, miR-539 inhibition, miR-590 inhibition, and combined miR-539 and miR-590 inhibition on alveolar development. Top: lung histology (×100; calibration bar 100 µm). Middle: lung histology at higher magnification (×600; calibration bar 50 µm). Bottom: graphs indicate magnitude of lung development as estimated by mean linear intercept (MLI; lower MLI indicates better alveolar growth) and radial alveolar count (RAC; higher RAC indicates more alveolar septation) and lung compliance and resistance as measured on a flexiVent. It can be observed that both miR-539 and miR-590 inhibition, as well as combined miR-539 and miR-590 inhibition, increased MLI and lung resistance by ~10% and reduced RAC and lung compliance by ~20% (*P < 0.05 vs. scramble control). n = 6 per time point; box-and-whisker plot with box indicating 25th–75th percentiles, line across box indicating median, and whiskers indicating 5th–95th percentiles.
DISCUSSION
We reconstructed a detailed molecular description of the regulatory processes involved in normal postnatal lung alveolar development. Our study has several important novel attributes and strengths. First, we did not analyze bulk lung tissue, nor did we focus on predefined isolated cells; instead, we used LCM to select the alveolar regions, avoiding larger bronchi, blood vessels, or immune cell aggregates. While this did not provide a cell-specific profile, it allowed a holistic profile of the alveolar unit and permitted identification of both cell-specific and cross-cell-type transcriptional programs operational at different time points during lung development. The use of single-cell transcriptomic data from lungs at the same time points (available at lungmap.net) enables the identification of cell types associated with these regulatory events. Second, while a number of previous studies have profiled time-series high-throughput data sets during lung development [including mRNA (9, 37), miRNA (28), proteomics (9), epigenetics (10), and most recently single-cell RNA-seq (35)], these studies did not focus on the alveolus as a unit, were limited to one molecular technology or species, and did not provide a comprehensive systems biology model of the tightly regulated molecular and cellular events during alveolar development. In contrast, we profiled several “omics” data types simultaneously and used novel computational methods to provide a comprehensive dynamic regulatory network. Third, instead of arbitrarily selecting time points, we used a computational approach developed by us to identify time points that would be most informative to the determination of regulatory events. Fourth, we performed specific experiments that validated some of the model regulatory predictions and revealed that the upregulation of immune networks in the alveolar septa was not in response to microbial exposure but in fact a predetermined innate developmental response. Fifth, we profiled both human and mouse data and aligned the developmental regulatory stages to discover common regulatory hubs. This also enabled the first objective estimation of which time points in mouse lung development correspond to human lung development (P5 = human term; P15 = 2 mo; P23 = 3 yr; P28 = 9 yr), which is very valuable information for investigators who use mouse experimental models for disorders of lung development, such as BPD. Last, we developed an interactive model interface (http://www.cs.cmu.edu/~jund/idrem_lung/) that allows the immediate sharing of our results and insights. The ease of exploration and wealth of information enables investigators to interrogate the role of multiple molecules in lung development and to generate and test novel hypotheses regarding putative regulators.
A limitation of this study is that, even when using LCM, the alveolar regions consist of multiple cell types (epithelial, endothelial, fibroblast, immune cells, etc.). The use of single-cell analyses may help determine cell-specific signatures of some of the molecular signals we profiled. However, high-throughput techniques for several data types (for example, proteomics) have not yet been optimized for single-cell analyses. Even for data that can be profiled in single cells (mRNA or epigenetic data), to date these were not concurrently obtained, requiring downstream integration of multiple cells (14). In addition, for lung data, the focus on single cells excludes the critical role of the extracellular matrix in lung development (45). Although LCM could potentially cause RNA damage, the quality control (QC) of all samples used for analysis indicated excellent RNA quality. We thus view the LCM-derived model as an important complement to single-cell data, enabling us to focus on alveolar parenchyma, including the extracellular matrix and soluble factors associated with the matrix. As we show, by combining the global model with single-cell RNA-Seq and -sorted cell mRNA data, we are able to identify pathways that change in specific cell types or across cell types. Another issue is that, while our analyses include mouse lung samples from the saccular and alveolar stage, all human lungs were in the alveolar stage, since fetal lung samples could not be used in the NIH LungMAP project. We are therefore unable to comment on the correspondence of saccular lung development in human lungs versus the mouse.
The number of replicates was small for mice (n = 3) and particularly so for humans (n = 2–6). We note that the mouse time points and replicates were selected based on extensive analysis and experiments. Unlike prior work in the literature, we did not empirically select time points and number of replicates. Instead, we used the TPS method (18), which to the best of our knowledge is the only analytical method that has been proven to correctly determine such experimental design requirements. Based on TPS, we concluded that, for the time points selected for the mouse data, the expected reconstruction error is less than the expected repeat error. Thus, we believe that our results are as robust as any other previously published time series for mouse. As for human data, normal human lung samples are a much more limited resource, and the human model was used primary to validate the mouse model and evaluate concordance with the mouse model, rather than as a stand-alone model with a large number of replicates at each time point. It is likely that the human data can only capture the strongest signals and may miss minor variations associated with sex and race. Also, analysis of sex-specific differences in lung development is a separate ongoing project, which is difficult to integrate with this study.
The existing literature on lung development, which has been reviewed in detail by Warburton et al. (36) and more recently by Whitsett et al. (38), has primarily focused on descriptive and mechanistic studies of known cells and pathways involved in alveolar development (7, 33) and recently on mapping single-cell expression in the developing lung (13, 14). There have been relatively few attempts at generating a systems biology-based integrated understanding of late lung development. Beauchemin et al. (6) generated genomewide transcriptomic data for 26 pre- and postnatal time points in three mouse strains and identified both strain-independent and -dependent patterns of gene expression. The regression modeling of the principal components in this study was consistent with the stages of lung development and major biological processes (6). Cox et al. (9) performed mathematical modeling of protemic profiles with corresponding transcriptomic data in mouse lung organogenesis, and Dong et al. (12) performed comprehensive miRNA and mRNA profiling and integrated these data with an existing protein database. These analyses demonstrated dynamic regulation of multiple molecules during lung development. More recently, we integrated miRNA and mRNA expression at five different time points during mouse development using the miRNA Dynamic Regulatory Events Miner (mirDREM) (28). Our current work on iDREM extends mirDREM by inclusion of information from proteomic data and DNA methylation data to further inform the model and, importantly, also develops a first ever systems biology model of human lung development and identifies correspondence between the mouse and human models.
Similar to branching morphogenesis, an earlier step in lung development, alveolar development is modular, being driven by directed network processes that are hierarchical, deterministic, and stepwise. One of the major findings from our iDREM model is how this hierarchy works during alveolar septation. Key divergence events (“splits”) occurred at only 5 of the 14 time points profiled. This finding is even more impressive considering that we selected these 14 time points through a systemic evaluation of 42 time points. The divergence nodes occurred at E16.5, P0.5, P1, P7, and P13.5. Considering that morphologic alveolar development begins at P5 in the mouse, our results suggest that the “master routine,” subroutines, and local parameters (to borrow computer engineering terms) essential for alveolar development and septation are in place and initiated soon after birth and before morphological changes are evident. Intriguingly, we did not find signals that would indicate cessation of alveolar development. Alveolar development is mostly complete by P28 in the mouse, but there were no nodes to indicate a regulatory “brake” on continued alveolar growth. It is unclear whether this happens because the brakes are activated at P13.5 or because other unmeasured phenomena such as mechanical forces (e.g., limited thoracic volume) may serve to inhibit continued lung growth. Additional investigation is required to determine which of the regulators predicted by our model at the early nodes is necessary and sufficient for the initiation and termination of alveolarization.
The main putative regulators predicted by iDREM for the critical nodes include many that are well known to be involved in lung development [e.g., NKX2-1 (44), CEBPA (40), GLI1 (43), SMAD3 (39), and HMGA2 (30)] but also many that are not usually considered as relevant to lung development (e.g., NKX3-1, ETS1). However, a careful review of the existing literature indicates potential roles for these molecules in later lung development that needs additional investigation for confirmation. For example, NKX3-1, which regulates proliferation of glandular epithelium and ductal formation in the minor salivary glands and prostate, has also been found to be expressed in the lung and augmented in the absence of sonic hedgehog (26), and ETS1 has been shown to be important for lung branching morphogenesis in the late fetal period (19), and its expression in the developing lung regulates hypoxia-induced mitogenic factor (20).
Evaluation of the terminal nodes of paths C and F resulting from the final split at P13.5 (Fig. 3A) suggests that these paths are primarily immune related. Therefore, many of the later changes in lung transcriptional or regulatory activity may deal with maturation of the immune system. Our experiments indicating comparable activation of IRF1, NFATC4, and MEG3 in both gnotobiotic (germ-free) and mouse lungs are consistent with a relatively late autonomous subroutine in the activation of immune response and potentially other effects in alveolar septa that are independent of microbiome exposure.
Another important observation is that multiple pathways are regulated by microRNAs. For example, miR-1192, miR-495, miR-141, miR-590–3p, and miR-421 are major initiators and regulators of path I. Of note, myofibroblasts/smooth muscle cells localize to path I when single-cell RNA signatures are considered, which suggests that these miRNAs regulate myofibroblasts and smooth muscle cells in the developing lung. Another observation that requires further investigation is the changing expression profile of lncRNAs (e.g., Meg3, Malat1) during alveolar development. Marked reductions of Meg3 and increases in Malat1 at the later time points may potentially contribute to pulmonary arterial smooth muscle cell proliferation and vascular development (32).
Some of the paths are shared among different cell types (e.g., paths A, B, and E by epithelial and endothelial cells), which suggests that regulatory mechanisms may overlap significantly. It is known that alveolar development depends on both epithelial cells and endothelial cells (in addition to myofibroblasts), and it is possible that their maturation is coregulated to a certain extent, perhaps by growth factors in the local environment.
To conclude, in this study, we provide the most detailed molecular description to date of the regulatory processes underlying the development of a complex organ. The interactive model reconstructed by iDREM enables rapid and facile identification and visualization of the pattern of gene expression of specific genes, regulators, and their methylation status at multiple time points during normal lung development. We believe that this model will also enable a better understanding of deviations from normal during development (e.g., BPD, congenital diaphragmatic hernia, alveolar capillary dysplasia) or aberrant reinduction of developmental pathways such as idiopathic pulmonary fibrosis, COPD or even lung cancer.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants U01 HL122626 and R01 HL127349.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S.H., N.K., Z.B.-J., and N.A. conceived and designed research; F.A., C.R.E., D.C., T.N., X.Y., C.V.L., J.S.H., N.K., and N.A. performed experiments; J.D., C.R.E., Z.B.-J., and N.A. analyzed data; F.A. and N.A. interpreted results of experiments; F.A. and N.A. prepared figures; J.D., F.A., J.S.H., N.K., Z.B.-J., and N.A. drafted manuscript; J.D., F.A., C.R.E., J.S.H., N.K., Z.B.-J., and N.A. edited and revised manuscript; J.D., F.A., C.R.E., D.C., T.N., X.Y., C.V.L., J.S.H., N.K., Z.B.-J., and N.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the LungMAP consortium (www.lungmap.net) and Dr. Charles Dela-Cruz for helpful comments and Dr. Nelida Olave for assistance with the evaluation of miR-539 and -590 in mouse lung development.
REFERENCES
- 1.Ahlberg JH, Nilson EN, Walsh JL. The Theory of Splines and Their Applications: Mathematics in Science and Engineering: A Series of Monographs and Textbooks. Amsterdam: Elsevier, 2016. [Google Scholar]
- 2.Aken BL, Achuthan P, Akanni W, Amode MR, Bernsdorff F, Bhai J, Billis K, Carvalho-Silva D, Cummins C, Clapham P, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Juettemann T, Keenan S, Laird MR, Lavidas I, Maurel T, McLaren W, Moore B, Murphy DN, Nag R, Newman V, Nuhn M, Ong CK, Parker A, Patricio M, Riat HS, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Wilder SP, Zadissa A, Kostadima M, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Cunningham F, Yates A, Zerbino DR, Flicek P. Ensembl 2017. Nucleic Acids Res 45, D1: D635–D642, 2017. doi: 10.1093/nar/gkw1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antony N, McDougall AR, Mantamadiotis T, Cole TJ, Bird AD. Creb1 regulates late stage mammalian lung development via respiratory epithelial and mesenchymal-independent mechanisms. Sci Rep 6: 25569, 2016. doi: 10.1038/srep25569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet 8: e1002866, 2012. doi: 10.1371/journal.pgen.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Joseph Z, Gitter A, Simon I. Studying and modelling dynamic biological processes using time-series gene expression data. Nat Rev Genet 13: 552–564, 2012. doi: 10.1038/nrg3244. [DOI] [PubMed] [Google Scholar]
- 6.Beauchemin KJ, Wells JM, Kho AT, Philip VM, Kamir D, Kohane IS, Graber JH, Bult CJ. Temporal dynamics of the developing lung transcriptome in three common inbred strains of laboratory mice reveals multiple stages of postnatal alveolar development. PeerJ 4: e2318, 2016. doi: 10.7717/peerj.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res 57: 38R–46R, 2005. doi: 10.1203/01.PDR.0000159630.35883.BE. [DOI] [PubMed] [Google Scholar]
- 8.Brennan SC, Wilkinson WJ, Tseng HE, Finney B, Monk B, Dibble H, Quilliam S, Warburton D, Galietta LJ, Kemp PJ, Riccardi D. The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep 6: 21975, 2016. doi: 10.1038/srep21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox B, Kislinger T, Wigle DA, Kannan A, Brown K, Okubo T, Hogan B, Jurisica I, Frey B, Rossant J, Emili A. Integrated proteomic and transcriptomic profiling of mouse lung development and Nmyc target genes. Mol Syst Biol 3: 109, 2007. doi: 10.1038/msb4100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuna A, Halloran B, Faye-Petersen O, Kelly D, Crossman DK, Cui X, Pandit K, Kaminski N, Bhattacharya S, Ahmad A, Mariani TJ, Ambalavanan N. Alterations in gene expression and DNA methylation during murine and human lung alveolar septation. Am J Respir Cell Mol Biol 53: 60–73, 2015. doi: 10.1165/rcmb.2014-0160OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Hagood JS, Ambalavanan N, Kaminski N, Bar-Joseph Z. iDREM: Interactive visualization of dynamic regulatory networks. PLOS Comput Biol 14: e1006019, 2018. doi: 10.1371/journal.pcbi.1006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J, Jiang G, Asmann YW, Tomaszek S, Jen J, Kislinger T, Wigle DA. MicroRNA networks in mouse lung organogenesis. PLoS One 5: e10854, 2010. doi: 10.1371/journal.pone.0010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Guo M, Whitsett JA, Xu Y. ‘LungGENS’: a web-based tool for mapping single-cell gene expression in the developing lung. Thorax 70: 1092–1094, 2015. doi: 10.1136/thoraxjnl-2015-207035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, Pryhuber GS, Mariani TJ, Bhattacharya S, Guo M, Potter SS, Dexheimer P, Aronow B, Jobe AH, Whitsett JA, Xu Y. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax 72: 481–484, 2017. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst J, Vainas O, Harbison CT, Simon I, Bar-Joseph Z. Reconstructing dynamic regulatory maps. Mol Syst Biol 3: 74, 2007. doi: 10.1038/msb4100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmston N, Lenhard B. Chromatin and epigenetic features of long-range gene regulation. Nucleic Acids Res 41: 7185–7199, 2013. doi: 10.1093/nar/gkt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter MV, Fernandez-Gonzalez R. Coordinating cell movements in vivo: junctional and cytoskeletal dynamics lead the way. Curr Opin Cell Biol 48: 54–62, 2017. doi: 10.1016/j.ceb.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Kleyman M, Sefer E, Nicola T, Espinoza C, Chhabra D, Hagood JS, Kaminski N, Ambalavanan N, Bar-Joseph Z. Selecting the most appropriate time points to profile in high-throughput studies. eLife 6: 6, 2017. doi: 10.7554/eLife.18541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kola I, Brookes S, Green AR, Garber R, Tymms M, Papas TS, Seth A. The Ets1 transcription factor is widely expressed during murine embryo development and is associated with mesodermal cells involved in morphogenetic processes such as organ formation. Proc Natl Acad Sci USA 90: 7588–7592, 1993. doi: 10.1073/pnas.90.16.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Lager J, Wang D, Li D. Ets-1 participates in and facilitates developmental expression of hypoxia-induced mitogenic factor in mouse lung. Front Biosci 12: 2269–2278, 2007. doi: 10.2741/2229. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Ge YL, Li M, Fang XZ, Yuan YP, Liang L, Huang SQ. miR-127 contributes to ventilator-induced lung injury. Mol Med Rep 16: 4119–4126, 2017. doi: 10.3892/mmr.2017.7109. [DOI] [PubMed] [Google Scholar]
- 22.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med 375: 871–878, 2016. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 23.Narayan K, Waggoner L, Pham ST, Hendricks GL, Waggoner SN, Conlon J, Wang JP, Fitzgerald KA, Kang J. TRIM13 is a negative regulator of MDA5-mediated type I IFN production. J Virol 88: 10748–10757, 2014. doi: 10.1128/JVI.02593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B, Olave N, Bulger A, Oparil S, Chen YF. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol 301: L125–L134, 2011. doi: 10.1152/ajplung.00074.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos PV, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. doi: 10.1152/ajplung.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider A, Brand T, Zweigerdt R, Arnold H. Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech Dev 95: 163–174, 2000. doi: 10.1016/S0925-4773(00)00355-5. [DOI] [PubMed] [Google Scholar]
- 27.Schulz MH, Devanny WE, Gitter A, Zhong S, Ernst J, Bar-Joseph Z. DREM 2.0: Improved reconstruction of dynamic regulatory networks from time-series expression data. BMC Syst Biol 6: 104, 2012. doi: 10.1186/1752-0509-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz MH, Pandit KV, Lino Cardenas CL, Ambalavanan N, Kaminski N, Bar-Joseph Z. Reconstructing dynamic microRNA-regulated interaction networks. Proc Natl Acad Sci USA 110: 15686–15691, 2013. doi: 10.1073/pnas.1303236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi N, Zhang J, Chen SY. Runx2, a novel regulator for goblet cell differentiation and asthma development. FASEB J 31: 412–420, 2017. doi: 10.1096/fj.201600954R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh I, Mehta A, Contreras A, Boettger T, Carraro G, Wheeler M, Cabrera-Fuentes HA, Bellusci S, Seeger W, Braun T, Barreto G. Hmga2 is required for canonical WNT signaling during lung development. BMC Biol 12: 21, 2014. doi: 10.1186/1741-7007-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SS, Dole NS, Franceschetti T, Hrdlicka HC, Delany AM. MicroRNA-433 dampens glucocorticoid receptor signaling, impacting circadian rhythm and osteoblastic gene expression. J Biol Chem 291: 21717–21728, 2016. doi: 10.1074/jbc.M116.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Z, Nie X, Sun S, Dong S, Yuan C, Li Y, Xiao B, Jie D, Liu Y. Long non-coding RNA MEG3 downregulation triggers human pulmonary artery smooth muscle cell proliferation and migration via the p53 signaling pathway. Cell Physiol Biochem 42: 2569–2581, 2017. doi: 10.1159/000480218. [DOI] [PubMed] [Google Scholar]
- 33.Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 313: L1101–L1153, 2017. doi: 10.1152/ajplung.00343.2017. [DOI] [PubMed] [Google Scholar]
- 34.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43, Suppl D1: D447–D452, 2015. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509: 371–375, 2014. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J. Lung organogenesis. : Current Topics in Developmental Biology. Amsterdam: Elsevier, 2010, p. 73–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng T, Chen Z, Jin N, Gao L, Liu L. Gene expression profiling identifies regulatory pathways involved in the late stage of rat fetal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1027–L1037, 2006. doi: 10.1152/ajplung.00435.2005. [DOI] [PubMed] [Google Scholar]
- 38.Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol Rev 99: 513–554, 2019. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu B, Chen H, Xu W, Zhang W, Buckley S, Zheng SG, Warburton D, Kolb M, Gauldie J, Shi W. Molecular mechanisms of MMP9 overexpression and its role in emphysema pathogenesis of Smad3-deficient mice. Am J Physiol Lung Cell Mol Physiol 303: L89–L96, 2012. doi: 10.1152/ajplung.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Wang Y, Besnard V, Ikegami M, Wert SE, Heffner C, Murray SA, Donahue LR, Whitsett JA. Transcriptional programs controlling perinatal lung maturation. PLoS One 7: e37046, 2012. doi: 10.1371/journal.pone.0037046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin Y, Morgunova E, Jolma A, Kaasinen E, Sahu B, Khund-Sayeed S, Das PK, Kivioja T, Dave K, Zhong F. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 356: eaaj2239, 2017. doi: 10.1126/science.aaj2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yun Y, Srinivas G, Kuenzel S, Linnenbrink M, Alnahas S, Bruce KD, Steinhoff U, Baines JF, Schaible UE. Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS One 9: e113466, 2014. doi: 10.1371/journal.pone.0113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Wang H, Teng H, Shi J, Zhang Y. Expression of SHH signaling pathway components in the developing human lung. Histochem Cell Biol 134: 327–335, 2010. doi: 10.1007/s00418-010-0738-2. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Rath N, Hannenhalli S, Wang Z, Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF, Morrisey EE. GATA and Nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development 134: 189–198, 2007. doi: 10.1242/dev.02720. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, Bitterman PB, Corley RA, Ding BS, Engler AJ, Hansen KC, Hagood JS, Kheradmand F, Lin QS, Neptune E, Niklason L, Ortiz LA, Parks WC, Tschumperlin DJ, White ES, Chapman HA, Thannickal VJ. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol 73: 77–104, 2018. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet 39: 61–69, 2007. doi: 10.1038/ng1929. [DOI] [PubMed] [Google Scholar]
- 47.Zuo Z, Roy B, Chang YK, Granas D, Stormo GD. Measuring quantitative effects of methylation on transcription factor–DNA binding affinity. Sci Adv 3: eaao1799, 2017. doi: 10.1126/sciadv.aao1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study have been deposited in the LungMAP consortium (www.lungmap.net). The accession numbers are included in Table 1.
Table 1.
Multiomics data availability
| URL | |
|---|---|
| RNA-seq mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001630 |
| RNA-seq (AmpliSeq) human LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001631 |
| Nanostring microRNA mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000000227 |
| Nanostring microRNA human LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000003682 |
| MeDIP-Seq mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001224 |
| Methylation array human LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001628 |
| Proteomics mouse LCM | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000000180 |
| Proteomics human LCM no. 1 | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000000863 |
| Proteomics human LCM no. 2 | https://lungmap.net/breath-omics-experiment-page/?experimentId=LMEX0000001605 |
LCM, laser capture microdissection.
Standard operating procedures and all data and associated metadata (e.g., mouse: sex, weight; human: postnatal age, gestational age, sex, health status, etc.) from this and other LungMAP projects are publicly available at www.lungmap.net. The interactive model is available at our supporting website (https://www.cs.cmu.edu/~jund/idrem_lung/), and the software is freely available at GitHub (https://github.com/phoenixding/idrem).