Abstract
Mitogen-activated protein kinase (MAPK) phosphatase 5 (MKP-5) is a member of the dual-specificity family of protein tyrosine phosphatases that negatively regulates p38 MAPK and the JNK. MKP-5-deficient mice exhibit improved muscle repair and reduced fibrosis in an animal model of muscular dystrophy. Here, we asked whether the effects of MKP-5 on muscle fibrosis extend to other tissues. Using a bleomycin-induced model of pulmonary fibrosis, we found that MKP-5-deficient mice were protected from the development of lung fibrosis, expressed reduced levels of hydroxyproline and fibrogenic genes, and displayed marked polarization towards an M1-macrophage phenotype. We showed that the profibrogenic effects of the transforming growth factor-β1 (TGF-β1) were inhibited in MKP-5-deficient lung fibroblasts. MKP-5-deficient fibroblasts exhibited enhanced p38 MAPK activity, impaired Smad3 phosphorylation, increased Smad7 levels, and decreased expression of fibrogenic genes. Myofibroblast differentiation was attenuated in MKP-5-deficient fibroblasts. Finally, we found that MKP-5 expression was increased in idiopathic pulmonary fibrosis (IPF)-derived lung fibroblasts but not in whole IPF lungs. These data suggest that MKP-5 plays an essential role in promoting lung fibrosis. Our results couple MKP-5 with the TGF-β1 signaling machinery and imply that MKP-5 inhibition may serve as a therapeutic target for human lung fibrosis.
Keywords: fibroblast, homeostasis, kinase, MKP-5, phosphatase, pulmonary fibrosis
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a devastating chronic lung disease that culminates into a fatal outcome within 3–3.5 yr after initial diagnosis (25). Despite extensive research efforts, its pathogenesis still remains poorly understood. Current pathogenetic hypotheses support the notion that aberrant wound healing of the alveolar epithelium in response to repetitive injury occurs in genetically predisposed individuals and accelerates later in aged individuals (21, 33, 45). After years without a treatment, the Food and Drug Administration approved pirfenidone and nintedanib because of evidence that these drugs slowed disease progression (15, 26, 37). The evidence that nintedanib slows down disease progression in IPF strongly supports the rationale for investigating the role of signaling pathways regulated by tyrosyl phosphorylation in lung fibrosis (10). Our group has demonstrated that the nonreceptor tyrosine phosphatase, Src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP-2), was downregulated in IPF lungs and specifically, lung fibroblasts, and its inhibition promoted fibroblast-to-myofibroblast differentiation (38). SHP-2 reduced fibroblast responsiveness to profibrotic stimuli through negative regulation of major signal transduction kinase-controlled pathways. Lentiviral overexpression of SHP-2 exerted antifibrotic properties in the bleomycin model of lung fibrosis, supporting a role for mimicking SHP-2 activity as a therapeutic option in pulmonary fibrosis (38). It has also been reported that the mice deficient for the expression of the receptor protein tyrosine phosphatase-α are protected from bleomycin-induced pulmonary fibrosis (3). These observations support the idea that protein tyrosine phosphatases are involved in the pathogenesis of lung fibrosis.
The mitogen-activated protein kinase (MAPK) phosphatases (MKPs) are members of the family of dual-specificity protein tyrosine phosphatases (DUSPs) (5, 7). The MKPs directly dephosphorylate and thereby inactivate the MAPKs (34). MKP-5, encoded by the DUSP10 gene, serves to specifically dephosphorylate the stress-responsive MAPKs, p38 MAPK, and the c-Jun NH2-terminal kinase (5, 7). Recent data in an animal model of muscular dystrophy demonstrated that MKP-5-deficient (MKP-5−/−) mice exhibited resistance from the development of dystrophic muscle disease that was, in part, a result of enhanced muscle stem cell activation (12). Additionally, Duchenne muscular dystrophic mice lacking MKP-5 also exhibited markedly reduced levels of skeletal muscle fibrosis (32). These observations raised the possibility that MKP-5 could be involved in the development of fibrosis. We hypothesized that MKP-5 functions in the regulation of skeletal muscle fibrosis might be extended to other tissues.
In this study, we asked whether MKP-5-deficient mice were also protected from the development of fibrosis using the experimental mouse model of bleomycin-induced lung fibrosis. We found that MKP-5 deficient mice (MKP-5−/−) were resistant to bleomycin-induced lung fibrosis with reduced collagen deposition and displayed an M1-macrophage phenotype, traditionally considered to suppress fibroproliferation (42). As expected, MKP-5−/− lung fibroblasts displayed enhanced MAPK activity. Mechanistically, MKP-5-deficient lung fibroblasts exhibited reduced responsiveness to the profibrotic actions of the transforming growth factor-β1 (TGF-β1) signal transduction pathway with impaired Smad3 phosphorylation and Smad7 upregulation. Finally, fibroblasts derived from the lungs of patients with IPF exhibited increased mRNA and protein expression levels of MKP-5 compared with normal human lung fibroblasts (NHLFs). Taken together, these results identify MKP-5 as having profibrotic properties in the lung through a pathway involving regulation of TGF-β1 signaling.
MATERIALS AND METHODS
Cell cultures, transfection, and knockdown experiments.
Cell culture experiments in normal human lung fibroblasts NHLF (Lonza CC2512) were performed as follows: NHLFs were first starved overnight with Opti-MEM reduced serum culture medium and then transfected at 70% confluency with a specific siRNA (DUSP10 Smart Pool 11221; ON-TARGETplus; Ref.: 2578138G; Dharmacon) at a final concentration of 10 nM using the Lipofectamine 2000 reagent (10 μL per reaction; Life Technologies) for 24 h. As a nonspecific control, a nontargeting siRNA from Dharmacon was used (D-001810-01-05) at a final concentration of 10 nM. Primary mouse lung fibroblasts were isolated from C57BL/6, 9- to 12-week-old female MKP-5 knockout mice (MKP-5−/−) or wild-type littermates (MKP-5+/+; described below) and in vitro cultured, according to standard protocols (Cell Biologics). Cells were then stimulated with 10 ng/mL TGF-β1 (R&D Systems) for 30 min or 6 h, as indicated, and then harvested for RNA and protein extraction.
Proliferation and migration (scratch wound) assays.
The Quick Cell Proliferation Assay kit (ab65473; Abcam) was used to measure mouse lung fibroblast proliferation, according to the manufacturer’s protocol. Briefly, mouse lung fibroblasts (1 × 104), isolated from MKP-5 knockout and wild-type mice, were plated into 96-well plates in 100 μL medium and incubated for 18 h at 37°C in a humidified atmosphere of 5% CO2. Eighteen hours after incubation, cells were treated with PDGF-BB (25 ng/mL) for 6 h. After a total of 24 h of incubation, the reaction was terminated in one-half of the wells using 10 μL/well 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate [a water-soluble tetrazolium (WST-1)], and absorbance was measured at 450/690 nm (time point 0). Twenty-four hours later, the reaction was terminated in the remaining wells using 10 μL/well WST-1, and absorbance was measured at 450/690 nm (time point 24 h). The ratio of median absorbance between the two time points (24:0 h) was used to compare proliferation between treatment groups. For in vitro cell culture wound-closure assays, cells were plated into six-well plates at a confluency of 70%, and a wound was created by using a 200-μL pipette tip that was pressed firmly against the surface of the tissue culture dish. The distance of one side of the wound to the other at time point 0 and 24 h after was measured and analyzed by using ImageJ software, as previously described (14).
Protein extraction and immunoblot analysis.
Proteins from tissues or cultured cells were extracted, transferred onto PVDF membranes, incubated overnight with appropriate primary antibodies, and visualized using the Gel Doc XR+ system (Bio-Rad Laboratories), according to the manufacturer’s protocol. Proteins from tissues or cultured cells were extracted in M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific, Rockford, IL), containing protease inhibitors, according to the manufacturer’s protocol. Protein concentration was measured with the bicinchoninic acid protein assay kit (Thermo Fisher Scientific) for immunoblotting. Protein cell lysates were first diluted 3:1 with 4× Laemmli sample buffer (Bio-Rad Laboratories) containing β-mercaptoethanol, heated for 10 min at 70°C, and then transferred onto PVDF membranes using the Trans-Blot Turbo Transfer System (Bio-Rad Laboratories) and hybridized overnight with appropriate primary antibodies as follows: 1) DUSP10/MKP-5 rabbit polyclonal antibody (1/1,000; 87842; Abcam); 2) α-smooth muscle actin (α-SMA) rabbit polyclonal antibody (1/1,000; 5694; Abcam); 3) phospho (p)-Smad3 (S423/S425) rabbit monoclonal antibody (1/2,000; 52903; Abcam); 4) Smad3 rabbit monoclonal antibody (1/2,000; 40854; Abcam); 5) p-stress-activated protein kinase/JNK (Thr183/Tyr185) rabbit polyclonal antibody (1:1,000; #9251); 6) stress-activated protein kinase/JNK rabbit polyclonal antibody (1:1,000; #9252); 7) p-Erk1/2 (Thr202/Tyr204; 20G11) rabbit monoclonal antibody (1:1,000; #4376); 8) Erk1/2 rabbit polyclonal antibody (1:1,000; #9102); 9) p-p38 MAPK (Thr180/Tyr182) rabbit polyclonal antibody (1:1,000; #9211); 10) p38 MAPK rabbit polyclonal antibody (#9212; Cell Signaling Technology); 11) MADH7/Smad7 rabbit polyclonal antibody (1/2,000; ab226872; Abcam); and 12) β-actin (1/2,000; sc-47778; Santa Cruz Biotechnology) was used as internal control. Membranes were then incubated with appropriate secondary antibodies at specific dilutions (1:1,000–1:2,000) and visualized using an enhanced chemiluminescent detection kit (Perkin Elmer, Waltham, MA). Visualization of immunoblot data and analysis was performed using the Gel Doc XR+ system (Bio-Rad Laboratories).
Immunofluorescence staining.
NHLFs were plated at a density of 0.5 × 106 cells/well on coverslips on eight-well culture dishes. After TGF-β1 (10 ng/mL) stimulation for 6 h, slides were washed three times with Ca2+/Mg2+-free PBS, fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X (Sigma-Aldrich) for 20 min, blocked with 5% normal goat serum for 1 h, and then incubated overnight with specific fluorochrome primary antibodies to α-SMA (5694; Abcam) at a 1:200 dilution. Cells were treated with an Alexa Fluor-488-conjugated secondary antibody (1:300; Life Technologies) and Phalloidin Green primary antibody at 12 μL/500 μL PBS for 1 h, according to the manufacturer’s instructions. The nuclei were stained with DAPI at a dilution of 1:5,000. NHLFs were imaged on an inverted microscope (Nikon USA).
Experimental model of pulmonary fibrosis.
All animal studies were conducted in accordance with the NIH guidelines for humane treatment of animals and were approved by the Institutional Animal Care and Use Committee, Yale University. All animal models were performed twice, and data were pooled and analyzed collectively. All animals treated were included in the analysis. Bleomycin challenge was not blinded, but analysis of animal samples was. C57BL/6, 9- to 12-week-old female mice were purchased (Taconic Biosciences, Hudson, NY). MKP-5−/− mice were previously described (19, 32). Genetic deletion of MKP-5 does not exhibit any apparent abnormal lung phenotypes. Mice were anesthetized by placing them in a chamber and having paper towels soaked with 40% isoflurane solution diluted with 1,2-propanediol. Mice were randomly assigned (day 0) to receive intratracheally either 1.5 U/kg bleomycin (Hospira, Lake Forest, IL) or an equivalent volume (50 μL) of 0.9% normal saline, as previously described (20). Mice were euthanized at day 14. For the bleomycin-induced IPF analyses, we used eight (n = 8) mice per group, which was the minimum number of animals that were used to achieve a statistical power of 0.8 with a 5% error (P < 0.05), meaning an 80% chance to accurately determine a 20% statistical difference between two groups of animals. This was based on the following: 1) an expected mortality rate of 10% or less, derived generally from complications of anesthesia, and 2) the minimum statistical difference we aimed to detect between two groups was 20%. A conservative estimate of standard deviation with intratracheal delivery of bleomycin was 10–15%. Independent samples Student’s t-test was used for two-groups comparison and one-way ANOVA for comparisons between three groups or more.
Hydroxyproline assay.
Lung hydroxyproline was analyzed with a hydroxyproline colorimetric assay kit from BioVision (Milpitas, CA), following the manufacturer’s instruction. Briefly, the lungs from control and MKP-5−/− mice were dried until constant weight and hydrolyzed in 12 N HCl for 3 h at 120°C. The digestions were reacted with Chloramine-T reagent and visualized by p-dimethylaminobenzaldehyde reagent. The absorbance was measured at 560 nm in a microplate reader. Data are expressed as μγ of hydroxyproline/right lung.
Quantitative real-time RT-PCR.
Gene expression was determined by employing TaqMan (Life Technologies, Thermo Fisher Scientific), according to the manufacturer’s instruction. In brief, total RNA was isolated with an miRNeasy kit (Qiagen, Valencia, CA) from cell cultures, mouse and human lung tissue. The real-time PCR reaction mix used TaqMan RNA-to-Ct 1-Step Kit (Life Technologies). All reactions were done on the ViiA 7 Real-Time PCR System (Life Technologies, Thermo Fisher Scientific). Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) and β-glucuronidase (GUSB) were used as an internal standard control, and the specific primers and probes (Mkp5, acta2, col1a1, col3a1, Fn1, Nos2, Fizz1, Arg1, Mrc1, Chil1) were all obtained from Life Technologies, Thermo Fisher Scientific. Each reaction was performed in triplicate. Relative gene expression was then normalized to a value of 1.0 for the unstimulated control group. Control reactions without cDNA were done as a negative control. Fold change was calculated by taking the mean of the controls as the baseline.
Human samples and lung fibroblasts.
Human lung samples were obtained through the University of Pittsburgh Health Sciences Tissue Bank and Yale University Pathology Tissue service, which were surgical remnants of biopsies or lungs explanted from patients with IPF who underwent pulmonary transplantation. IPF (n = 4) and normal human lung fibroblasts (n = 4) were obtained from Carol Feghali Bostwick, Division of Rheumatology and Immunology, Medical University of South Carolina.
Statistical analyses.
Statistical analysis was performed using GraphPad Prism 7 software. The results were analyzed by unpaired two-tailed t-test for comparisons between two groups or by ANOVA between three or more groups with Sidak multiple comparison tests, unless otherwise specified. Data are presented as means ± SD and were considered statistically significant at P < 0.05.
RESULTS
MKP-5 knockout mice are resistant to bleomycin-induced pulmonary fibrosis.
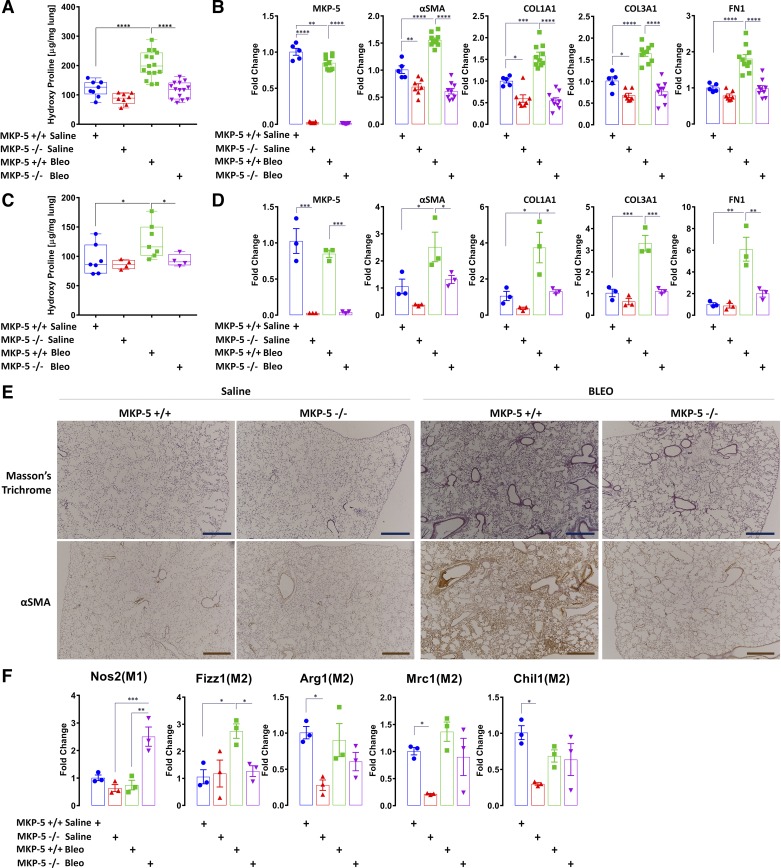
Nine- to twelve-week-old C57BL/6 mice with genetic deletion of MKP-5 (MKP-5−/−) and wild-type littermates (MKP-5+/+) were challenged with bleomycin (1.5 U/kg) or saline. The levels of hydroxyproline were comparable between saline-treated wild-type and MKP-5−/− mice (Fig. 1A). When wild-type mice were treated with bleomycin, we observed, as expected, a significant increase in the lung hydroxyproline content (Fig. 1A). In contrast, bleomycin-treated MKP-5−/− mice were resistant to the accumulation of hydroxyproline content in the lung, exhibiting a significant reduction of nearly twofold compared with wild-type mice at both time points of established fibrosis—days 14 and 21—following bleomycin challenge (Fig. 1, A and C). Next, we assessed the expression levels of profibrotic components, such as α-smooth muscle actin (α-SMA), collagen 1a1 (COL1A1), collagen 3a1 (COL3A1), and fibronectin (FN1), before and following bleomycin treatment of wild-type and MKP-5−/− mice. The levels of mRNA expression of α-SMA, COL1A1, and COL3A1 were all significantly reduced, on average, by at least 1.5-fold in saline-treated MKP-5−/− mice compared with wild-type littermate controls. Furthermore, after bleomycin treatment, MKP-5−/− mice showed, on average, a 2.5-, 2.9-, 2.2-, and 1.8-fold reduction of α-SMA, COL1A1, COL3A1, and FN1, respectively, compared with wild-type littermate controls at day 14 post-treatment. Similarly, at day 21 post-bleomycin treatment, MKP-5−/− mice showed, on average, a 1.9-, 2.9-, 3.0-, and 3.1-fold decrease of α-SMA, COL1A1, COL3A1, and FN1, respectively (Fig. 1, B and D). These results were supported by immunohistochemical analyses that revealed reduced collagen deposition in the lungs of MKP-5−/− mice compared with wild-type littermates, as assessed by Masson’s trichrome and α-SMA staining (Fig. 1E). These results demonstrate that MKP-5−/− mice are resistant to bleomycin-induced lung fibrosis.
Fig. 1.
MAPK phosphatase 5 (MKP-5) knockout (MKP-5−/−) mice display attenuated fibrotic responses to bleomycin (Bleo) challenge. A: decreased collagen deposition, assessed by hydroxyproline content, following bleomycin challenge at day 14 in MKP-5−/− mice compared with wild-type littermates (MKP-5+/+; 116.14 ± 27.99 vs. 202.25 ± 45.98). Data (μg/mg right lung) are presented as box and whisker plots, with horizontal bars representing means ± SD, n = 8–15 mice from each group-treated animals. One-way ANOVA with Sidak correction for stepwise comparison, ****P < 0.0001. B: relative changes following bleomycin challenge at day 14 in dual-specificity protein tyrosine phosphatase 10 (DUSP10)/MKP-5, α-smooth muscle actin (α-SMA), collagen 1A1 (COL1A1), collagen 3A1 (COL3A1), and fibronectin (FN1) mRNA expression levels. Each bar represents mean ± SD expression of n = 5/10/7/9 mice from each group (biological replicates). Bars are shown for the relative changes (fold) by setting the indicated control level to 1.0. One-way ANOVA with Sidak correction for stepwise comparison, *P < 0.05, **P < 0.005, ***P < 0.0002, ****P < 0.0001. C: decreased collagen deposition, assessed by hydroxyproline content, following bleomycin challenge at day 21 in MKP-5−/− mice compared with MKP-5+/+ (93.23 ± 10.38 vs. 126.67 ± 29.73). Data (micrograms/milligrams right lung) are presented as box and whisker plots, with bars representing means ± SD, n = 7 for MKP-5+/+ and n = 4 for MKP-5−/− mice group-treated animals. One-way ANOVA with Sidak correction for stepwise comparison, *P < 0.03. D: relative changes following bleomycin challenge at day 21 in DUSP10/MKP-5, α-SMA, COL1A1, COL3A1, and FN1 mRNA expression levels. Each bar represents mean ± SD expression of n = 3 from each group sample (biological replicates). Bars are shown for the relative changes (fold) by setting the indicated control level to 1.0. One-way ANOVA with Sidak correction for stepwise comparison, *P < 0.05, **P < 0.005, ***P < 0.0008. E: Masson’s trichrome (top) and α-SMA (bottom) staining of representative lung sections (n = 4) from each group of treated mice. Decreased collagen deposition and α-SMA staining are observed in the interstitium, peribronchiolar or perivascular space, in MKP-5−/− mice compared with MKP-5+/+ mice. Scale bars, 100 μΜ. F: relative changes following bleomycin challenge at day 14 of M1/M2 macrophage markers. mRNA expression of nitric oxidase synthase-2 (Nos2) and resistin-like molecule-α (Fizz1), arginase 1 (Arg1), c-type mannose receptor 1 (Mrc1), and chitinase-like 3 protein 1 (Chil1), following challenge with bleomycin. Each bar represents mean ± SD expression of n = 3 from each group samples (biological replicates). Bars are shown for the relative changes (fold) by setting the indicated control level to 1.0. One-way ANOVA with Sidak correction for stepwise comparison, *P < 0.05, **P < 0.002, ***P < 0.001.
With the consideration that the bleomycin model is also a model of inflammation, and inflammatory cells play pivotal roles during lung fibrosis, we focused our next series of experiments to determine the effects of MKP-5 deficiency on macrophages in response to bleomycin treatment. Despite the fact that MKP-5−/− mice were protected from the development of lung fibrosis, we observed a marked polarization of macrophages toward an M1 proinflammatory phenotype compared with an M2-profibrotic phenotype of wild-type littermates. This was manifested by increased mRNA expression levels of nitric oxidase synthase 2 (Nos2), resistin-like molecule-α (Fizz1), arginase 1 (Arg1), c-type mannose receptor 1 (Mrc1), and chitinase-like 3 protein 1 (Chil1), following challenge with bleomycin (Fig. 1F). These results suggest that MKP-5 deficiency skews the M1–M2 population toward an M1 phenotype, traditionally considered to suppress fibroproliferation (42).
MKP-5 inhibition attenuates fibroblast responsiveness to profibrotic stimuli in vitro.
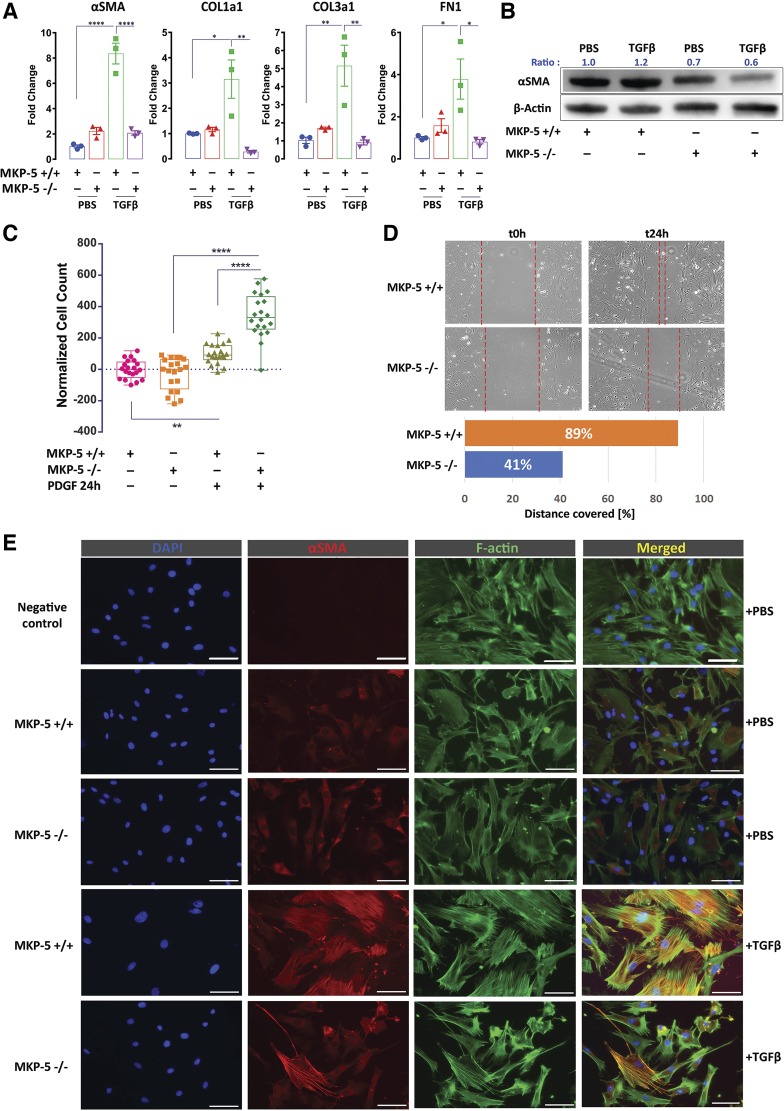
To define the mechanism through which MKP-5 mediates fibrosis in the lung, we isolated primary mouse lung fibroblasts from MKP-5−/− and wild-type (MKP-5+/+) mice and assessed their responsiveness to TGF-β1, a potent profibrotic stimulus (29, 30). When MKP-5+/+ mouse lung fibroblasts were isolated and stimulated with TGF-β1 (10 ng/mL) for 6 h, the mRNA expression levels of α-SMA, FN1, and COL1A1 and COL1A3 were significantly increased (Fig. 2A). However, MKP-5−/− mouse lung fibroblasts failed to induce the expression of either α-SMA, FN1, COL1A1, and COL1A3 in response to TGF-β1 stimulation at the RNA level (Fig. 2A). Additionally, α-SMA was suppressed at the protein level in extracts from MKP-5−/− fibroblasts stimulated with TGF-β1 (Fig. 2B). Next, we assessed the proliferative and migratory capacities of MKP-5−/− mouse lung fibroblasts. In response to PDGF-BB (25 ng/mL) for 24 h, MKP-5−/− mouse lung fibroblasts displayed significant increases in proliferation rates (2.2-fold) compared with wild-type littermates (Fig. 2C). Despite the observation that the proliferative rates of MKP-5−/− lung-derived fibroblasts were increased, their migratory capacity was significantly impaired (2.1-fold reduction) compared with MKP-5+/+ lung-derived fibroblasts (Fig. 2D). Finally, consistent with the observed defective TGF-β1 activation of fibrogenic genes, MKP-5 −/− lung-derived fibroblasts formed reduced stress fibers and expressed lower levels of α-SMA compared with MKP-5+/+ lung-derived fibroblast (Fig. 2E). These results suggest that MKP-5 is required for TGF-β1-mediated fibrogenic gene expression and the progression of fibroblast-to-myofibroblast differentiation.
Fig. 2.
MAPK phosphatase 5 (MKP-5) inhibition attenuates fibroblast-to-myofibroblast differentiation and migration and increases proliferation of mouse lung fibroblasts. Primary mouse lung fibroblasts were isolated from C57BL/6, 9- to 12-week-old mice. MKP-5 knockout mice (MKP-5−/−) or wild-type mice (MKP-5+/+) were stimulated with transforming growth factor β1 (TGF-β1; 10 ng/mL) and then harvested for RNA and protein extraction. Normal human lung fibroblasts were transfected at 70% confluency with a specific dual-specificity protein tyrosine phosphatase 10/MKP-5 siRNA (10 nM) and a nontargeting siRNA (10 nM) as a control for 24 h. A: relative change in α-smooth muscle actin (α-SMA), collagen 1A1 (COL1A1), collagen 3A1 (COL3A1), and fibronectin (FN1) mRNA levels following stimulation with TGF-β1 or PBS. Each bar represents mean expression of 3 samples (in duplicates). Bars (means ± SD) are shown for the relative changes (fold) by setting the indicated control level to 1.0. One-way ANOVA with Sidak correction for stepwise comparison, *P < 0.02, **P < 0.004, ****P < 0.0001. B: immunoblot analyses of α-SMA and β-actin. Each lane represents an individual mouse lung fibroblast preparation (biological replicates). Blots were stripped and reblotted using an anti-β-actin antibody as loading control. Ratio indicates protein densitometry ratio of the target/control protein. C: MKP-5−/− mouse lung fibroblasts exhibited increased proliferation rates following stimulation with PDGF-BB (25 ng/mL) for 6 h compared with wild type (MKP-5+/+). Data analyzed at 24 h post-treatment (ratio of 24 h/0 hr absorbance) are presented as box–whisker plots with bars representing means ± SD, n = 20. One-way ANOVA with Sidak correction for stepwise comparison, **P < 0.085, ****P < 0.0001. D: scratch wound assay was used to quantify the migration capacity of MKP-5+/+ and MKP-5−/− fibroblasts at different time points: baseline (t0h) and at 24 h (t24h). The areas between the dotted lines define the migratory boundary. Bottom: bar plot depicts quantitation of percentage of actual distance covered. E: double immunofluorescence analysis showing colocalization (merged/yellow) of α-SMA (red) with F-actin (green) in MKP-5+/+ and MKP-5−/− mouse lung fibroblasts following stimulation with TGF-β1 or PBS. MKP-5−/− mouse lung fibroblasts exhibited reduced TGF-β1-induced differentiation to myofibroblasts, as indicated by reduced formation of stress fibers and α-SMA expression compared with MKP-5+/+. Scale bars, 50 μΜ.
MKP-5 is required for TGF-β1-mediated Smad3 activation.
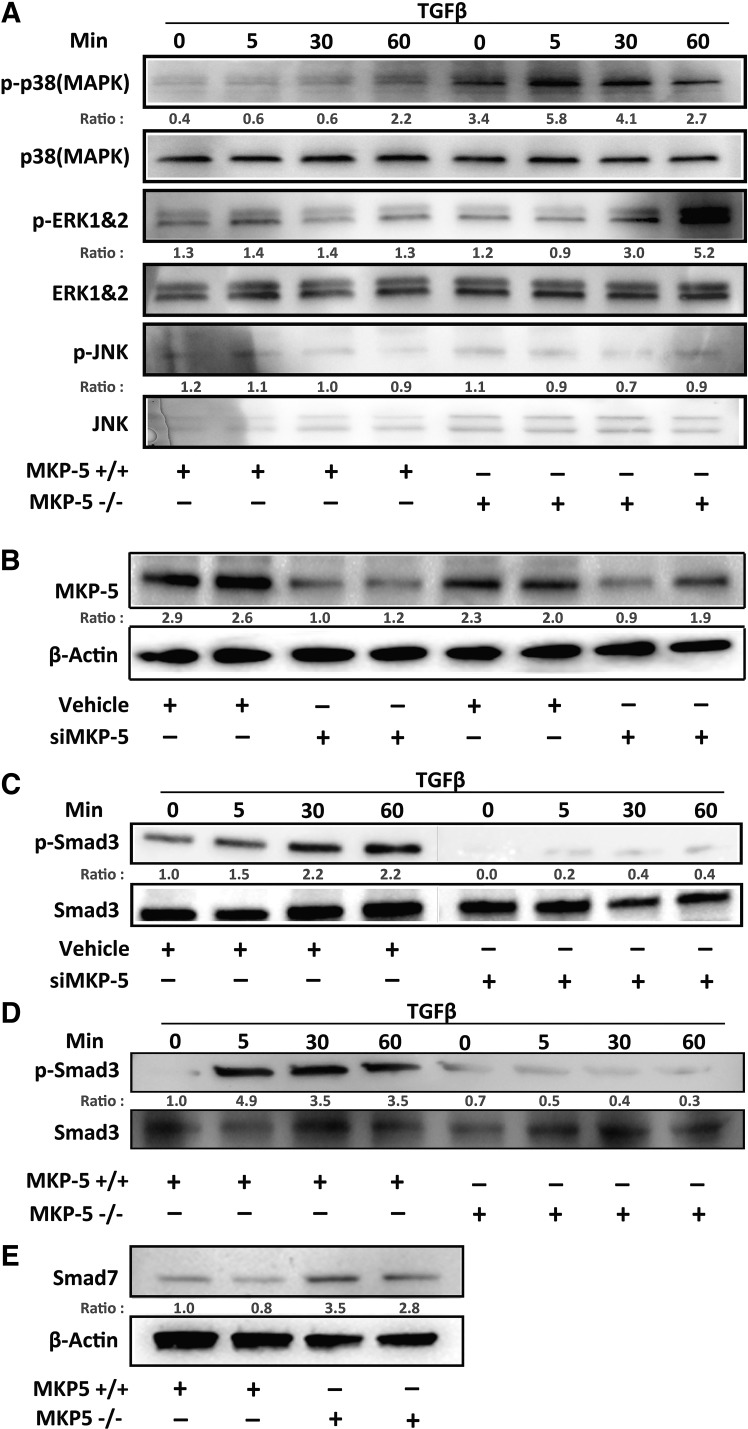
To define the mechanism through which MKP-5 regulates TGF-β1 signaling and thus provides insight into its actions in lung fibrosis, we assessed the phosphorylation levels of p38 MAPK, ERK1/2, and JNK following TGF-β1 stimulation in isolated lung-derived fibroblasts from MKP-5+/+ and MKP-5−/− mice. When lung-derived fibroblasts from MKP-5−/− mice were stimulated with TGF-β1, we observed that basal as well as stimulated p38 MAPK phosphorylation was enhanced compared with MKP-5+/+ lung-derived fibroblasts (Fig. 3A). Phosphorylation of ERK1/2 was increased in TGF-β1-stimulated MKP-5−/− lung-derived fibroblasts at the latest time point (60 min); however, JNK1/2 phosphorylation levels remain unaltered (Fig. 3A). Next, we investigated the effects of MKP-5 genetic ablation or knockdown on the phosphorylation states of Smad3, which functions as a critical, proximal signaling component of TGF-β1 signaling. As expected, transfection of normal human lung fibroblasts (NHLFs) with a specific DUSP10/MKP-5 siRNA (10 nM) or a nontargeting siRNA for 24 h (10 nM) resulted in an almost twofold decrease in MKP-5 protein expression (Fig. 3B). MKP-5 knockdown, in NHLF, resulted in an attenuated induction of TGF-β1-induced Smad3 phosphorylation (Fig. 3C). Similarly, Smad3 phosphorylation was robustly induced upon treating MKP-5+/+ lung-derived fibroblasts with TGF-β1 (Fig. 3D). However, MKP-5−/− lung-derived fibroblasts were markedly inhibited in their ability to phosphorylate Smad3 in response to TGF-β1 stimulation (Fig. 3D). Interestingly, the feedback inhibitor of TGF-β1 signaling, Smad7, which directly prevents Smad3 from becoming phosphorylated and associating with Smad4 (23, 40), was found to be upregulated in MKP-5−/− lung-derived fibroblasts compared with wild-type controls (Fig. 3E). Taken together, these results demonstrate that MKP-5 is required for TGF-β1-dependent activation of Smad3 in lung fibroblasts, and its absence was associated with upregulation of the antifibrotic Smad7 that attenuates the fibrotic effect of TGF-β (23, 40).
Fig. 3.
MAPK phosphatase 5 (MKP-5) inhibition negatively regulates transforming growth factor β1 (TGF-β1)-induced Smad3 fibrotic pathway. Primary mouse lung fibroblasts were isolated from C57BL/6, 9- to 12-week-old female mice in MKP-5 knockout (MKP-5−/−) or wild-type mice (MKP-5+/+), which were then stimulated with TGF-β1 (10 ng/mL) for 5, 30, and 60 min and then harvested for protein extraction and immunoblot analysis. Normal human lung fibroblasts (NHLFs) were transfected at 70% confluency with dual-specificity protein tyrosine phosphatase 10 (DUSP10)/MKP-5 siRNA (10 nM) for 24 h or a control nontargeting siRNA (10 nM). Cells were stimulated with TGF-β1 (10 ng/mL) for 5, 30, and 60 min. A: immunoblot analyses for phosphorylated (p-)MAPKs (p-JNK, p-ERK1/2, and p-p38 MAPK), along with their respective MAPK totals at the indicated time points of primary mouse lung fibroblast preparation from MKP-5+/+ and MKP-5−/− mice. B: immunoblot analyses for MKP-5 expression following transfection with DUSP10/MKP-5 siRNA (siMKP-5; 10 nM) or a nontargeting siRNA for 24 h (10 nM). Each lane represents an individual NHLF preparation. C: the same cell lysates were immunoblotted and analyzed for p-Smad3 and total Smad3 following TGF-β1 stimulation. D: immunoblot analysis for p-Smad3 and total Smad3 following TGF-β1 stimulation in primary lung fibroblasts from MKP-5+/+ and MKP-5−/− mice. E: immunoblot analysis for Smad7 in primary lung fibroblasts from MKP-5+/+ and MKP-5−/− fibroblasts. Ratio indicates protein densitometry ratio of the target/control protein.
MKP-5 is upregulated in IPF lung fibroblasts but not in whole IPF lungs.
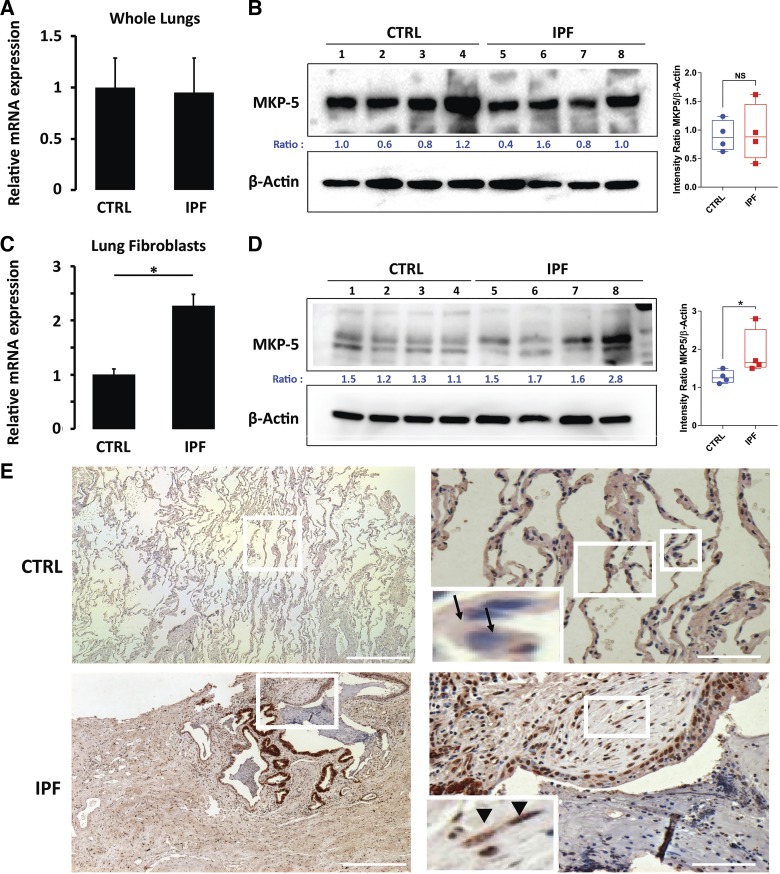
To demonstrate human relevance, we measured MKP-5 mRNA and protein levels in whole lung extracts as well as in lung fibroblasts derived from patients with IPF and controls. MKP-5 levels, both mRNA (Fig. 4A) and protein (Fig. 4B), showed no differences in whole lungs derived from patients with IPF compared with control lung. In contrast, IPF lung fibroblasts demonstrated significantly increased expression of MKP-5 mRNA (Fig. 4C) and protein (Fig. 4D) levels compared with NHLF. Immunohistochemical studies revealed increased MKP-5 expression in areas of active fibroblastic foci and the surrounding alveolar epithelium within the fibrotic lung, whereas minimal expression was observed in control lungs (Fig. 4E).
Fig. 4.
MAPK phosphatase 5 (MKP-5) expression is increased in idiopathic pulmonary fibrosis (IPF) lung fibroblasts but not in whole IPF lungs. A: relative change in MKP-5 mRNA levels in whole lung IPF. Each bar represents the mean expression of 3 samples (in triplicates). Bars (means ± SD) are shown for the relative fold changes by setting the indicated control (CTRL) level to 1.0. Mann-Whitney U-test for independent samples (left). B: immunoblot analysis showing MKP-5 protein expression in IPF lungs compared with controls vs. β-actin protein levels and corresponding relative densitometry. Ratio indicates ratio values of the target/control protein (left); box plots (right) summarize all 4 experimental samples of each group. NS, not significant. C: relative change in MKP-5 mRNA levels in IPF lung fibroblasts compared with normal human lung fibroblasts (NHLFs). Each bar represents mean expression of 3 samples (in triplicates). Bars (means ± SD) are shown for the relative changes (fold) by setting the indicated control level to 1.0. Mann-Whitney U-test for independent samples, *P < 0.05 (left). D: immunoblot analysis showing protein levels of MKP-5 in IPF lung fibroblasts compared with NHLF and corresponding relative densitometry. β-Actin protein levels serve as loading control. Ratio indicates ratio values of the target/control protein (left); box plots (right) summarize all 4 experimental samples of each group. *P < 0.05. E: immunohistochemistry analysis in representative lung samples (n = 5) showing increased MKP-5 expression within the fibrotic interstitium, as well as the surrounding alveolar epithelium, in IPF lungs compared with normal alveolar epithelium in control lung samples. Boxed regions are enlarged at right and as insets on the left. A nonimmune immunoglobulin of the same isotype and concentration as the primary antibody was used as negative control. Arrows indicate alveolar epithelial cells with minimal expression of MKP-5 (blue color). Arrowheads indicate spindle-shaped, fibroblast-like cells within fibroblastic foci in the fibrotic lung stained positive for MKP-5 (brown color).
DISCUSSION
Our study demonstrates a novel role of MKP-5 inhibition in experimental lung fibrosis, through, at least in part, downregulation of the TGF-β1 signal transduction pathway. Based on the previous observations that genetic deletion of MKP-5 resulted in improved regenerative myogenesis and reduced muscle fibrosis in a mouse model of dystrophic muscle disease (32), we aimed to determine whether this phenomenon could be extended to also curtail lung fibrosis. We discovered that MKP-5−/− mice displayed an attenuated fibrotic response to bleomycin challenge compared with wild-type littermates at two different time points of established fibrosis. Interestingly, MKP-5−/− lungs exhibited a marked polarization toward a proinflammatory antifibrotic M1 and suppression of the profibrotic M2 macrophage phenotype. Our in vivo findings were complemented with in vitro evidence demonstrating that either genetic deletion or MKP-5 knockdown in lung fibroblasts produced a highly proliferative fibroblast phenotype with impaired migratory and fibroblast-to-myofibroblast differentiative capacity. Interestingly, either genetic deletion or knockdown of MKP-5 reduced the responsiveness of lung fibroblasts to the profibrotic stimuli of TGF-β1. IPF lung fibroblasts displayed increased expression levels of MKP-5 compared with NHLF. Collectively, these results further support a role for MKP-5 in the development of fibrosis and extend this observation to lung disease.
MKP-5 is a dual-specificity phosphatase that specifically dephosphorylates the MAPKs and lies at the core of several immune-mediated diseases (17). Studies have shown that MKP-5 plays a protective role in regulating macrophage and T cell functions, including proliferation, differentiation, and production of cytokines (12). Deficiency in MKP-5 has been associated with aggravated lung injury due to uncontrolled T cell or macrophage-mediated inflammation (24, 46). Despite extensive data implicating MKP-5 in immune-related diseases (17), specific physiological properties of MKP-5 have not been defined for the maintenance and repair of lung tissue. Recent studies have shown an important role for MKP-5 in muscle regeneration through a mechanism that involved negative regulation of muscle stem cell homeostasis (32). MKP-5-deficient mice exhibited increased muscle stem cell proliferation and differentiation and consequently improved myogenesis. When a mouse model of Duchenne muscular dystrophy was generated to lack the expression of MKP-5, it was found that dystrophic muscle disease, including the development of fibrosis, was attenuated (19, 31, 32). These observations suggested the involvement of MKP-5 in skeletal muscle fibrosis.
We have identified that genetic deletion of MKP-5 rendered mice resistant to bleomycin-induced lung fibrosis. This could be explained not only by reduced fibroblast-dependent collagen deposition but also by a marked polarization of an antifibrotic, proinflammatory M1 macrophage phenotype and impaired profibrotic M2 phenotype. This is in line with the central concept that M1 macrophages suppress, and M2 macrophages promote fibroproliferation and uncontrolled repair through multiple cellular mechanisms, including matrix metalloproteinases and profibrotic mediators, respectively (2, 42). A recent study has also shown that patients with IPF, undergoing acute exacerbation, display elevated local levels of M2 macrophages (28), and nintedanib skews polarization of macrophages toward an M1 phenotype by major suppression of M2 markers in a concentration-dependent manner (4).
These data are in agreement with our previous observation that MKP-5 deficiency protects from the development of muscle fibrosis in a model of dystrophic muscle disease and add to the knowledge that MKP-5 may exert its profibrotic properties through multiple pathways in a cell type-dependent manner (32). Perhaps MKP-5 inhibition may lead to repolarization of alveolar and/or interstitial macrophages with major therapeutic impacts on experimental and human lung fibrosis, as it has been shown by inactivation of a ubiquitously expressed tyrosine phosphatase, SHP-2, which skewed M2 polarization and augmented bleomycin-induced fibrosis (36). Further studies are needed to support this hypothesis (9).
To provide mechanistic insights and with the consideration that fibroblasts represent one of the key cells of lung fibrosis, we focused our next series of experiments on fibroblast phenotypes and fibrosis-relevant signal transduction pathways. We performed loss-of-function experiments and discovered that MKP-5−/− mouse lung fibroblasts acquired an enhanced proliferative phenotype and responded weakly to profibrotic stimuli in vitro, as indicated by attenuated, TGF-β1-induced myofibroblast differentiation, migration, and extracellular matrix production. These findings are in line with current literature demonstrating that IPF lung fibroblasts are characterized by a hypersecretory phenotype with decreased proliferation and resistance to apoptosis compared with age-matched controls (1, 44).
As MKP-5 has been shown to dephosphorylate directly and thereby inactivate p38 MAPK, JNK, and to a much lesser extent, ERK1/2 activity (35), we examined whether MKP-5 is required to inactivate these MAPKs in primary mouse lung fibroblasts in the context of the profibrotic stimulus, TGF-β1. MKP-5−/− lung fibroblasts exhibited enhanced basal and sustained activation of p38 MAPK in response to TGF-β1 stimulation. Although ERK1/2 activity was seen to be modestly enhanced at later time points following TGF-β1 stimulation, JNK activation was completely unaffected. The effects of either MKP-5 genetic deletion or knockdown on TGF-β1-induced Smad3 phosphorylation were evaluated. These experiments revealed that TGF-β1 requires MKP-5 to effectively phosphorylate and thus activate Smad3. In line with this, we provide evidence that genetic ablation of MKP-5 was associated with upregulation of endogenous Smad7 levels, the feedback inhibitor of the TGF-β1 signaling machinery (11, 23). Smad7 exerts its inhibitory effects by blocking receptor activity, inducing receptor degradation, interfering with Smad–DNA binding, or competing with Smad4 to associate with Smad2/3 (43). Although the exact mechanism through which loss of MKP-5 upregulates negative regulators of the TGF-β1 signaling pathway is currently unknown, these are novel and important findings, implicating for the first time a dual-specificity phosphatase in the regulation of TGF-β1 signaling in the context of lung fibrosis. Importantly, our findings were complemented by human data showing increased MKP-5 expression levels, particularly in IPF lung fibroblasts, compared with normal human lung fibroblasts, suggesting that MKP-5 is required for fibroblast differentiation to a more fibrotic phenotype.
Our results implicate a novel role for MKP-5 in TGF-β1 signaling. TGF-β1 responses are not only the result of Smad activation but are also dependent on the cross-signaling interactions between Smad and other noncanonical intracellular signal-transduction pathways that may either promote or antagonize the TGF-β1/Smad canonical pathway in a cell type-specific manner (8). The antifibrotic actions of MKP-5 can be explained, at least in part, through its apparent requirement for TGF-β1-mediated fibrogenic signaling. The precise mechanism through which MKP-5 is required for TGF-β1-mediated signaling remains to be precisely defined. However, the mechanism likely involves the ability of MKP-5 to promote TGF-β1-mediated Smad phosphorylation by dephosphorylating a MAPK family member, presumably p38 MAPK. The presumptive dephosphorylation of p38 MAPK by MKP-5 serves either directly or indirectly to facilitate Smad phosphorylation in response to TGF-β1. It is noteworthy that MKP-5 is expressed in both the cytosol and the nucleus (35). Therefore, the site of MKP-5/Smad interference could occur at both the level of phosphorylation at the TGF-β1 receptor in addition to nuclear-related mechanisms. Interestingly, it has been shown that the protein tyrosine phosphatases, PTP4A1 and PTP4A2, promote dermal fibrosis through TGF-β1 signaling (27). Similarly, the receptor phosphatase, RPTPα, has been reported to promote profibrotic signaling and fibrosis in the lung through a TGF-β1-mediated Smad pathway (3). Although those studies implicate a role for protein tyrosine phosphatases in TGF-β1-mediated fibrogenesis, our work provides insight at the level of Smad7 activation and Smad3 phosphorylation to reveal an unappreciated connection between an MKP and TGF-β1-mediated fibrogenesis.
At this juncture, it is unknown whether MKP-5 deletion or inhibition moves toward compensatory activation or inactivation of other MKPs leading to deactivation of TGF-β1-mediated, fibrogenesis-induced Smad3 signaling. It is also conceivable that MKP-5 may regulate noncanonical TGF-β1-mediated fibrogenesis pathways in a cell type-specific manner (13). In particular, it has been shown that the Ras-MAPK pathway mediates phosphorylation of the linker region of the Smad2/3 auxiliary to the activation regions, leading to sequestration to the cytoplasm and attenuation of TGF-β1 signaling (16). This could explain why some cells with hyperactive Ras signaling do not respond to TGF-β1 stimulation (6). As such, it is conceivable that hyperactivation of p38 MAPK in the absence of MKP-5 following TGF-β1 activation may also result in a similar sequestration of Smad2. Additional potential mechanisms also include studies in which it has been demonstrated that both JNK and p38 MAPK positively regulate Smad7 expression, leading to deactivation of TGF-β1–Smad signaling (39). Our study raises interesting questions regarding the effects of MKP-5 on fibrogenesis, such as whether or not the antifibrotic effects of MKP-5 inhibition are mediated through a direct interaction of MKP-5 with the TGF-β1/Smad canonical pathway. Enhanced MAPK activity (p38 MAPK) in the absence of MKP-5 was associated with deactivation of key fibrotic signal transduction pathways and attenuated fibrotic responses both in vivo and in vitro. This is contrary to other reports demonstrating a profibrotic role of all three MAPKs (p38 MAPKs, JNK, ERK1/2) in both preclinical models of lung fibrosis and patients with IPF (18, 22, 41). These differences highlight the complexity of the MKP-5/MAPK signaling node and the necessity to investigate further the cross-signaling interactions between canonical and noncanonical TGF-β1 pathways in lung fibrosis.
In summary, our data identifies MKP-5 as a novel, positive regulator of fibroblast homeostasis by mediating TGF-β1-induced fibrotic responses and that its loss confers resistance to profibrotic stimuli. Our results couple MKP-5 function to the fibrogenic signaling machinery and identify MKP-5 inhibition as a promising therapeutic strategy for experimental and human lung fibrosis.
GRANTS
Support for the work was, in part, provided by National Institutes of Health (National Heart, Lung, and Blood Institute Grants R01HL095397 and R01HL127349; N. Kaminski), American Lung Association Award RT 350419, Marie Sklodowska/Curie, ERS/EU-RESPIRE 2-8860-2015 grant (A. Tzouvelekis), and Hellenic Thoracic Society Unrestricted Grant (T. Karampitsakos).
DISCLOSURES
A. Tzouvelekis and N. Kaminski are inventors on a pending patent on use of the thyroid hormone as an antifibrotic agent entitled “Novel Methods of Treating or Preventing Fibrotic Lung Diseases” [OCR 6368-047162-7029P1 (00219)]. A. Tzouvelekis consulted for Boehringer Ingelheim and Hoffmann LaRoche. N. Kaminski consulted for Biogen Idec, Boehringer Ingelheim, Numedii, MMI, and Pliant and has an ongoing collaboration with MiRagen, all outside the submitted work. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
N.X., K.M., F.A., J.D.H.-M., T.K., V.A., L.B., A.M.B., N.K., and A.T. conceived and designed research; N.X., K.M., F.A., G.Y., J.D.H.-M., T.K., V.A., L.B., and A.T. performed experiments; N.X., K.M., F.A., J.D.H.-M., T.K., V.A., L.B., A.M.B., N.K., and A.T. analyzed data; N.X., K.M., F.A., J.D.H.-M., T.K., V.A., L.B., A.M.B., N.K., and A.T. interpreted results of experiments; N.X., K.M., and A.T. prepared figures; N.X., K.M., D.B., A.M.B., N.K., and A.T. drafted manuscript; N.X., K.M., D.B., A.M.B., N.K., and A.T. edited and revised manuscript; N.X., K.M., F.A., G.Y., J.D.H.-M., T.K., V.A., L.B., D.B., A.M.B., N.K., and A.T. approved final version of manuscript.
REFERENCES
- 1.Álvarez D, Cárdenes N, Sellarés J, Bueno M, Corey C, Hanumanthu VS, Peng Y, D’Cunha H, Sembrat J, Nouraie M, Shanker S, Caufield C, Shiva S, Armanios M, Mora AL, Rojas M. IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 313: L1164–L1173, 2017. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arras M, Huaux F, Vink A, Delos M, Coutelier JP, Many MC, Barbarin V, Renauld JC, Lison D. Interleukin-9 reduces lung fibrosis and type 2 immune polarization induced by silica particles in a murine model. Am J Respir Cell Mol Biol 24: 368–375, 2001. doi: 10.1165/ajrcmb.24.4.4249. [DOI] [PubMed] [Google Scholar]
- 3.Aschner Y, Khalifah AP, Briones N, Yamashita C, Dolgonos L, Young SK, Campbell MN, Riches DW, Redente EF, Janssen WJ, Henson PM, Sap J, Vacaresse N, Kapus A, McCulloch CA, Zemans RL, Downey GP. Protein tyrosine phosphatase α mediates profibrotic signaling in lung fibroblasts through TGF-β responsiveness. Am J Pathol 184: 1489–1502, 2014. doi: 10.1016/j.ajpath.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamri N, Morzadec C, Joannes A, Lecureur V, Wollin L, Jouneau S, Vernhet L. Alteration of human macrophage phenotypes by the anti-fibrotic drug nintedanib. Int Immunopharmacol 72: 112–123, 2019. doi: 10.1016/j.intimp.2019.03.061. [DOI] [PubMed] [Google Scholar]
- 5.Boutros M, Kiger AA, Armknecht S, Kerr K, Hild M, Koch B, Haas SA, Paro R, Perrimon N; Heidelberg Fly Array Consortium . Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science 303: 832–835, 2004. doi: 10.1126/science.1091266. [DOI] [PubMed] [Google Scholar]
- 6.Calonge MJ, Massagué J. Smad4/DPC4 silencing and hyperactive Ras jointly disrupt transforming growth factor-beta antiproliferative responses in colon cancer cells. J Biol Chem 274: 33637–33643, 1999. doi: 10.1074/jbc.274.47.33637. [DOI] [PubMed] [Google Scholar]
- 7.Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J 280: 489–504, 2013. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapnick DA, Warner L, Bernet J, Rao T, Liu X. Partners in crime: the TGFβ and MAPK pathways in cancer progression. Cell Biosci 1: 42, 2011. doi: 10.1186/2045-3701-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai O, Winkler J, Minasyan M, Herzog EL. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med (Lausanne) 5: 43, 2018. doi: 10.3389/fmed.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimminger F, Günther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J 45: 1426–1433, 2015. doi: 10.1183/09031936.00149614. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 89: 1165–1173, 1997. doi: 10.1016/S0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 12.Huang CY, Tan TH. DUSPs, to MAP kinases and beyond. Cell Biosci 2: 24, 2012. doi: 10.1186/2045-3701-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene 24: 5742–5750, 2005. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 14.Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp (88): 2014. doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 16.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev 13: 804–816, 1999. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases–regulating the immune response. Nat Rev Immunol 7: 202–212, 2007. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka H, Arai T, Mori M, Goya S, Kida H, Morishita H, Fujiwara H, Tachibana I, Osaki T, Hayashi S. A p38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 283: L103–L112, 2002. doi: 10.1152/ajplung.00187.2001. [DOI] [PubMed] [Google Scholar]
- 19.Min K, Lawan A, Bennett AM. Loss of MKP-5 promotes myofiber survival by activating STAT3/Bcl-2 signaling during regenerative myogenesis. Skelet Muscle 7: 21, 2017. doi: 10.1186/s13395-017-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery RL, Yu G, Latimer PA, Stack C, Robinson K, Dalby CM, Kaminski N, van Rooij E. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol Med 6: 1347–1356, 2014. doi: 10.15252/emmm.201303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora AL, Rojas M, Pardo A, Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov 16: 755–772, 2017. [Erratum in Nat Rev Drug Discov 16: 810, 2017.] doi: 10.1038/nrd.2017.170. [DOI] [PubMed] [Google Scholar]
- 22.Moran N. p38 Kinase inhibitor approved for idiopathic pulmonary fibrosis. Nat Biotechnol 29: 301, 2011. doi: 10.1038/nbt0411-301. [DOI] [PubMed] [Google Scholar]
- 23.Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 389: 631–635, 1997. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 24.Qian F, Deng J, Gantner BN, Flavell RA, Dong C, Christman JW, Ye RD. Map kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 302: L866–L874, 2012. doi: 10.1152/ajplung.00277.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 27.Sacchetti C, Bai Y, Stanford SM, Di Benedetto P, Cipriani P, Santelli E, Piera-Velazquez S, Chernitskiy V, Kiosses WB, Ceponis A, Kaestner KH, Boin F, Jimenez SA, Giacomelli R, Zhang ZY, Bottini N. PTP4A1 promotes TGFβ signaling and fibrosis in systemic sclerosis. Nat Commun 8: 1060, 2017. doi: 10.1038/s41467-017-01168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schupp JC, Binder H, Jäger B, Cillis G, Zissel G, Müller-Quernheim J, Prasse A. Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS One 10: e0116775, 2015. doi: 10.1371/journal.pone.0116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheppard D. Pulmonary fibrosis: a cellular overreaction or a failure of communication? J Clin Invest 107: 1501–1502, 2001. doi: 10.1172/JCI13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 3: 413–417, 2006. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Gatzke F, Molle JM, Lee HB, Helm ET, Oldham JJ, Zhang L, Gerrard DE, Bennett AM. Mice lacking MKP-1 and MKP-5 reveal hierarchical regulation of regenerative myogenesis. J Stem Cell Regen Biol 1: 1–7, 2015. doi: 10.15436/2741-0598.15.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi H, Verma M, Zhang L, Dong C, Flavell RA, Bennett AM. Improved regenerative myogenesis and muscular dystrophy in mice lacking Mkp5. J Clin Invest 123: 2064–2077, 2013. doi: 10.1172/JCI64375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spagnolo P, Sverzellati N, Rossi G, Cavazza A, Tzouvelekis A, Crestani B, Vancheri C. Idiopathic pulmonary fibrosis: an update. Ann Med 47: 15–27, 2015. doi: 10.3109/07853890.2014.982165. [DOI] [PubMed] [Google Scholar]
- 34.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75: 487–493, 1993. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 35.Tanoue T, Moriguchi T, Nishida E. Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J Biol Chem 274: 19949–19956, 1999. doi: 10.1074/jbc.274.28.19949. [DOI] [PubMed] [Google Scholar]
- 36.Tao B, Jin W, Xu J, Liang Z, Yao J, Zhang Y, Wang K, Cheng H, Zhang X, Ke Y. Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J Immunol 193: 2801–2811, 2014. doi: 10.4049/jimmunol.1303463. [DOI] [PubMed] [Google Scholar]
- 37.Tzouvelekis A, Bouros E, Tzilas V, Bouros D. Pirfenidone in idiopathic pulmonary fibrosis “RECAP-itulating safety into the real world”. Respiration 94: 405–407, 2017. doi: 10.1159/000480299. [DOI] [PubMed] [Google Scholar]
- 38.Tzouvelekis A, Yu G, Lino Cardenas CL, Herazo-Maya JD, Wang R, Woolard T, Zhang Y, Sakamoto K, Lee H, Yi JS, DeIuliis G, Xylourgidis N, Ahangari F, Lee PJ, Aidinis V, Herzog EL, Homer R, Bennett AM, Kaminski N. SH2 domain-containing phosphatase-2 is a novel antifibrotic regulator in pulmonary fibrosis. Am J Respir Crit Care Med 195: 500–514, 2017. doi: 10.1164/rccm.201602-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida K, Suzuki H, Ohashi T, Nitta K, Yumura W, Nihei H. Involvement of MAP kinase cascades in Smad7 transcriptional regulation. Biochem Biophys Res Commun 289: 376–381, 2001. doi: 10.1006/bbrc.2001.5984. [DOI] [PubMed] [Google Scholar]
- 40.Ulloa L, Doody J, Massagué J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature 397: 710–713, 1999. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 41.van der Velden JL, Ye Y, Nolin JD, Hoffman SM, Chapman DG, Lahue KG, Abdalla S, Chen P, Liu Y, Bennett B, Khalil N, Sutherland D, Smith W, Horan G, Assaf M, Horowitz Z, Chopra R, Stevens RM, Palmisano M, Janssen-Heininger YM, Schafer PH. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin Transl Med 5: 36, 2016. doi: 10.1186/s40169-016-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, Chen YG. Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-β (TGF-β)/Smad signaling. J Biol Chem 291: 382–392, 2016. doi: 10.1074/jbc.M115.694281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Zeische R, Fraifeld VE. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY) 7: 664–672, 2015. [Erratum in Aging (Albany NY) 7: 1022, 2015.] doi: 10.18632/aging.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, de Castro JPW, DeIuliis G, Ahangari F, Woolard T, Aurelien N, Arrojo EDR, Gan Y, Graham M, Liu X, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 24: 39–49, 2018. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD, Flavell RA, Dong C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 430: 793–797, 2004. doi: 10.1038/nature02764. [DOI] [PubMed] [Google Scholar]