Abstract
Nur77 is an orphan nuclear receptor implicated in the regulation of a wide range of biological processes, including the maintenance of systemic blood vessel homeostasis. Although Nur77 is known to be expressed in the lung, its role in regulating pulmonary vascular functions remains entirely unknown. In this study, we found that Nur77 is expressed at high levels in the lung, and its expression is markedly upregulated in response to LPS administration. While the pulmonary vasculature of mice that lacked Nur77 appeared to function normally under homeostatic conditions, we observed a dramatic decrease in its barrier functions after exposure to LPS, as demonstrated by an increase in serum proteins in the bronchoalveolar lavage fluid and a reduction in the expression of endothelial junctional proteins, such as vascular endothelial cadherin (VE-cadherin) and β-catenin. Similarly, we found that siRNA knockdown of Nur77 in lung microvascular endothelial cells also reduced VE-cadherin and β-catenin expression and increased the quantity of fluorescein isothiocyanate-labeled dextran transporting across LPS-injured endothelial monolayers. Consistent with Nur77 playing a vascular protective role, we found that adenoviral-mediated overexpression of Nur77 both enhanced expression of VE-cadherin and β-catenin and augmented endothelial barrier protection to LPS in cultured cells. Mechanistically, Nur77 appeared to mediate its protective effects, at least in part, by binding to β-catenin and preventing its degradation. Our findings demonstrate a key role for Nur77 in the maintenance of lung endothelial barrier protection to LPS and suggest that therapeutic strategies aimed at augmenting Nur77 levels might be effective in treating a wide variety of inflammatory vascular diseases of the lung.
Keywords: acute lung injury, endothelial cells, lung, Nur77, orphan nuclear receptor, permeability
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a severe inflammatory lung disorder that causes respiratory failure and leads to substantial morbidity and mortality in the majority of patients (14). One mechanism by which respiratory failure develops in ARDS is through immune-mediated disruption of the endothelial barrier and the subsequent influx of protein-rich fluid in the gas-exchange units of the lung (6, 13). Currently, therapeutic strategies to reduce endothelial barrier disruption are believed to be important for decreasing the onset and/or severity of ARDS and other inflammatory vascular conditions.
The lung endothelium serves as a formidable barrier against the influx of cells and the leakage of proteins and other circulating materials in the lung. The maintenance of endothelial barrier function is dependent on the expression of numerous proteins, which serve as the “glue” that binds endothelial cells together. One important component of this glue is the cell-cell junctional protein called vascular endothelial cadherin (VE-cadherin). VE-cadherin is one of the most abundant proteins in endothelial junctions and is known to play a pivotal role in maintaining endothelial barrier integrity in response to different types of pulmonary insults (23). This function is achieved, in large part, by its binding to the cytoplasmic domain of other intracellular molecules, such as the catenin family of proteins, which together serve to stabilize and prevent the degradation of these binding partners (4, 18). Indeed, genetic manipulations that enhance VE-cadherin-catenin protein interactions have been shown to dramatically increase endothelial barrier protection, whereas downregulation in VE-cadherin weakens endothelial cell-cell interactions in the mouse lung (18).
Nur77, together with Nurr1 and NOR1, comprise the nuclear receptor 4A (NR4A) subfamily of proteins (22). These proteins are often referred to as orphan nuclear receptors because their activating ligands have not yet been identified. Like other types of nuclear receptors, members of the NR4A subfamily have an NH2-terminal transactivation domain (TAD), a central 2-zinc-finger DNA-binding domain (DBD), and a COOH-terminal ligand-binding domain (LBD; see Ref. 33). In systemic endothelial cells, the expression of NR4A proteins increases in response to a wide range of stimuli, including hypoxia, tumor necrosis factor-α (TNF-α), and the growth factor vascular endothelial growth factor (VEGF; see Refs. 3, 27, 28, and 30). Moreover, this upregulation appears to be critical for regulating a range of homeostatic functions, such as the activation of progrowth and prosurvival pathways (2, 30). In addition, we recently demonstrated that Nur77 suppresses the expression of leukocyte adhesion molecules in systemic endothelium by blocking nuclear factor-κB (NF-κB) activation (10, 28), and others have also shown that Nur77 can act to enhance expression of junctional proteins in systemic endothelial cells (34).
Although Nur77 has recently been shown to be expressed in the mouse lung, its role in mediating pulmonary vascular functions has yet to be explored. In this study, we hypothesized that Nur77 might regulate critical pulmonary vascular functions, namely protect the lung from plasma leakage in response to pulmonary insult. To test this hypothesis, we employed a variety of tools to systematically dissect the role of Nur77 in lung endothelial biology. In brief, our findings indicate that Nur77 regulates endothelial barrier protection in the lung under inflammatory conditions, specifically in response to the bacterial toxin LPS.
MATERIALS AND METHODS
Mice.
Nur77−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Knockout and control mice used in our studies were generated using a two-step breeding strategy in which knockout mice were first bred with wild-type mice to generate Nur77+/− mice, followed by the breeding of Nur77+/− to generate knockout and age-matched controls. Before the initiation of any studies, animal protocols were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. Lipopolysaccharide from Escherichia coli 0111:B4 was purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and transfection.
Mouse lung microvessel endothelial cells (MLMECs) were purchased from Cell Biologics (Chicago, IL). Cells were cultured in EBM-2 medium (Lonza) supplemented with the EGM-2 BulletKit (Lonza) at 37°C in a humidified 5% CO2 incubator, except 24 h before LPS exposure when cells were cultured in 1% FBS EBM-2 in the absence of any growth factors. Nur77 knockdown was performed in MLMECs using an siRNA approach per published protocols (12).
Murine ALI model.
ALI was induced as previously described (19). In brief, anesthetized mice (8–12 wk, 20–25 g) were suspended from a sloped board, and a one-time dose of LPS (100 μg) was instilled in posterior oropharyngeal space of anesthetized mice. After LPS administration (24 h), lung tissues were harvested for further analysis.
Pulmonary microvascular permeability.
Lung permeability was assessed by several measures, including by quantifying the extravasation of Evans blue dye and by measuring lung wet-to-dry weight ratios. Evans blue (20 mg/kg; Sigma-Aldrich) was injected intravenously 2 h before euthanasia. Before lungs were extracted from the thorax, the pulmonary vasculature was gently perfused by slowly injecting saline in the spontaneously beating right ventricle. Extraction of Evans blue dye was performed by incubating tissues at 65°C with formamide (2 ml/g tissue) overnight. Lung tissues were then centrifuged (12,000 g for 30 min), and Evans blue dye concentration in supernatant was determined spectrophotometrically by measuring absorption at 620 nm and correcting for the presence of heme pigments: A620 (corrected) = A620 − (1.426 × A740 − 0.030) where A620 is absorbance at 620 nm and A740 is absorbance at 740 nm (19). The lung wet-to-dry (W/D) weight ratio was calculated as previously described (19).
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) was performed by cannulating the trachea with a blunt 22-gauge needle and instilling the same 1 ml of sterile PBS back and forth in the airways three times (19). Total cell counts were performed using a TC20 automated cell counter (Bio-Rad Laboratories, Hercules, CA), and differential cell counts were performed after cytocentrifuging cells on glass slides and staining with HEMA 3 (Fisher Scientific, Tustin, CA). Total protein and albumin concentrations were determined as previously described (19).
Enzyme-linked immunosorbent assay.
KC, TNF-α, and IgM levels were quantified in BALF using commercially available DuoSet ELISA kits (R&D Systems) according to the manufacturer's instructions and published protocols (19). Briefly, Nalgene Nunc Maxisorp plates were coated overnight with antibodies to TNF-α (4 μg/ml), KC/CXCL1 (2 μg/ml), or IgM (1 μg/ml), and the next morning plates were washed and blocked for 2 h. Samples were added to the wells at various dilutions, followed by incubation with detection antibody for 2 h. Plates were subsequently washed, and streptavidin-horseradish peroxidase conjugate antibody was added to each well for 20 min. This was followed by an additional wash step, before plates were incubated with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (R&D Systems). Enzymatic reactions were quantified by measuring absorbance at 450 nm using a standard plate reader (Biotek Instrument).
Immunoprecipitation assays.
Cell lysates were incubated with antibodies against β-catenin, Nur77, or control IgG at 4°C overnight, and then immune complexes were resolved by SDS-PAGE. Protein complexes were next transferred to nitrocellulose membranes (Bio-Rad) for detection by Western blot as previously described (17).
Real-time quantitative PCR.
Real-time quantitative PCR (qRT-PCR) analysis was performed as described previously (29). RNA was extracted from cells and treated with DNase I using the RNeasy Micro kit (Qiagen). mRNA levels of β-catenin were determined by qRT-PCR using cDNA obtained from the reverse transcription reactions as template, with the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad) and HotStart-IT SYBR Green One-Step qRT-PCR Master Mix Kit (Affymetrix). The primer sequences are described as follows: human β-catenin: forward, 5′-AGCTTCCAGACACGCTATCAT-3′ and reverse, 5′-CGGTACAACGAGCTGTTTCTAC-3′; human GAPDH: forward, 5′-AGGGCTTTTAACTCTGGT-3′ and reverse, 5′-CCCCACTTGATTTTGGAGGGA-3′. Human GAPDH served as a control for the amount of cDNA present in each sample. Data were analyzed using the comparative difference in cycle number (ΔCT) method according to the manufacturer's instructions.
Western blotting.
Cell/tissues were homogenized in ice-cold Nonidet P-40 (NP-40) extraction buffer (50 mmol/L Tris·HCl, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% deoxycholate, 1% NP-40, and 1% Triton X-100 in PBS with pH adjusted to 7.4) containing protease inhibitor cocktail (Sigma) and P2/P3 phosphatase inhibitor cocktail (Sigma). Cell/tissue lysates were incubated at 4°C for 10 min to solubilize proteins and then centrifuged for 15 min at 12,000 rpm to pellet cell debris. Protein concentration was determined using the bicinchoninic acid assay method (Pierce). Samples were subjected to SDS gel electrophoresis and transferred to nitrocellulose membrane for immunoblotting. Membranes were blocked for 1 h at room temperature in a solution containing 5% BSA in Tris-buffered saline, and before overnight incubation at 4°C with primary antibodies against rabbit anti-Nur77 (catalog no. 12335–1-AP; 1:1,000 dilution; Proteintech), rabbit anti-NOR1 (catalog no. sc-393902; 1:500 dilution; Santa Cruz), rabbit anti-Nurr1 (catalog no. sc-990, 1:500 dilution; Santa Cruz), mouse anti-VE-cadherin (catalog no. sc-9989, 1:1,000 dilution; Santa Cruz), rabbit anti-β-catenin (catalog no. 9562; 1:1,000 dilution; Cell Signaling Technology), mouse anti-lamin A/C (catalog no. ab8984; 1:1,000 dilution; Abcam), or mouse anti-GAPDH (catalog no. sc-32233; 1:1,000 dilution; Santa Cruz). This step was followed by incubation with goat anti-rabbit IgG [heavy chain (H) + light chain (L), DyLight 680 conjugated; Thermo Scientific] or goat anti-mouse IgG (H + L, DyLight 800 conjugated; Thermo Scientific) for 1 h, and immunoblots were then visualized using the Odyssey Imaging System (LI-COR) and quantified using Image J software (17).
Adenovirus construction.
Wild-type flag-tagged Nur77 (Ad-Nur77) was made as previously described (28). Briefly, Ad293 cells were cotransfected with pBHGlox△E1,3Cre and the pDC shuttle vector, which contained the gene of interest. Viral purification was performed using CsCl banding. Briefly, 5 ml virus supernatant obtained from HEK 293 cells was loaded on the top of CsCl step gradients (bottom layer: 0.5 ml 1.5 g/ml CsCl, middle layer: 3.0 ml 1.35 g/ml CsCl, and top layer: 3.0 ml 1.25 g/ml CsCl). After centrifugation at 35,000 rpm for 1 h at 10°C, the virus band was collected and transferred to an ultracentrifugation tube, and 1.35 g/mL CsCl was added to top up the tube. After centrifugation at 35,000 rpm for 20 h at 4°C, the virus band was collected by puncturing the tube under the band using a needle and syringe. The collected virus was dialyzed against 20 mmol/L Tris-buffered saline with 10% glycerol. Viral titers were determined using the Adeno-X Rapid Titer kit (Clontech) according to the manufacturer’s instructions.
Immunofluorescence staining.
For the purposes of our studies, MLMECs were cultured on laminin-coated glass cover slips. At specified time points, cells were fixed and permeabilized using 4% paraformaldehyde and 0.25% Triton X-100, respectively. Immunostaining for VE-cadherin was performed using a primary polyclonal rabbit antibody (1:200; Santa Cruz) incubated overnight at 4°C followed by a 1-h exposure at room temperature to a TRITC-conjugated anti-rabbit IgG secondary antibody (1:250; Invitrogen). Cell nuclei were visualized using DAPI stain. To examine the colocalization of Nur77 with β-catenin, human lung endothelial cells were plated on an eight-well Laboratory-Tek II Chamber Slide (Thermo Fisher Scientific, Waltham, MA). After infection with Ad-Nur77 (20 multiplicity of infection) for 48 h, cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Next, cells were permeabilized with 0.1% Triton X-100 for 10 min, blocked with 1.5% BSA for 1 h, and incubated with anti-β-catenin antibody (catalog no. 610154, 1:500 dilution; BD Biosciences) and anti-Nur77 antibody (catalog no. NB10Q-56745, 1:100 dilution; NOVUSBIO) at 4°C overnight. Cells were washed with PBS three times and incubated with Alexa 488 Donkey anti-mouse (catalog no. A21202, 1:1,000 dilution; Thermo Fisher Scientific) or Alexa 555 goat anti-rabbit (catalog no. A21428, 1:1,000 dilution; Thermo Fisher Scientific) secondary antibody at room temperature for 1 h. To visualize the nuclei, cells were stained with 1 µg/mL of DAPI (Thermo Fisher Scientific) for 15 min and mounted with ProLong Gold Antifade reagent (Thermo Fisher Scientific). Images were captured with a Nikon A1R laser scanning confocal microscope (Nikon, Tokyo, Japan) and analyzed by NIS-Elements Cofocal software (Nikon).
Subcellular fractionation.
Subcellular fractions were prepared by differential centrifugation of cell homogenates as described previously (26). Briefly, cells were homogenized manually in hypotonic buffer [10 mmol/L Tris·HCl (pH 7.5), 1 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotinin]. The cytoplasmic fraction was obtained from low-speed centrifugation (500 g), and the pellets were then washed with phosphate-buffered saline (PBS) three times and dissolved in hypertonic buffer [20 mmol/L HEPES (pH 7.9), 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 25% glycerol, and the abovementioned protease inhibitors]. After high-speed centrifugation at 12,800 g for 10 min, the supernatants (nuclear extracts) were stored at −80°C until use.
Lung histology.
Lung histology was performed on paraformaldehyde-fixed tissues embedded in paraffin wax. Sections (5 μm) were placed on positively charged glass slides, and tissues were deparaffinized before undergoing hematoxylin and eosin (H&E) staining. Tissues were visualized using standard light microscopy.
Endothelial monolayer permeability assay.
Endothelial permeability assays were performed as previously described (11). Briefly, MLMECs (1 × 106/plate) were cultured on 0.4-μm pore size polycarbonate membranes in six-well Transwell plates (Corning). After transfection (48 h) with an adenoviral vector or a siRNA, cells were cultured in low-serum medium for 12 h, followed by exposure to LPS for varying durations. Endothelial leak was quantified by assessing the amount of fluorescein isothiocyanate (FITC)-dextran (molecular weight 70,000; Sigma Aldrich) migrating across endothelial monolayers. The concentration of FITC-dextran in the bottom chamber was determined by using a BioTek plate reader (480/520 nm).
Statistical analysis.
Statistics were performed using GraphPad Prism 5.0 software. Two-group comparisons were analyzed by unpaired Student's t test, and multiple-group comparisons were performed using one-way analysis of variance followed by the Tukey post hoc analysis. Statistical significance was achieved when P < 0.05, representing the 95% confidence interval.
RESULTS
Nur77 expression is upregulated in the mouse lung after LPS administration.
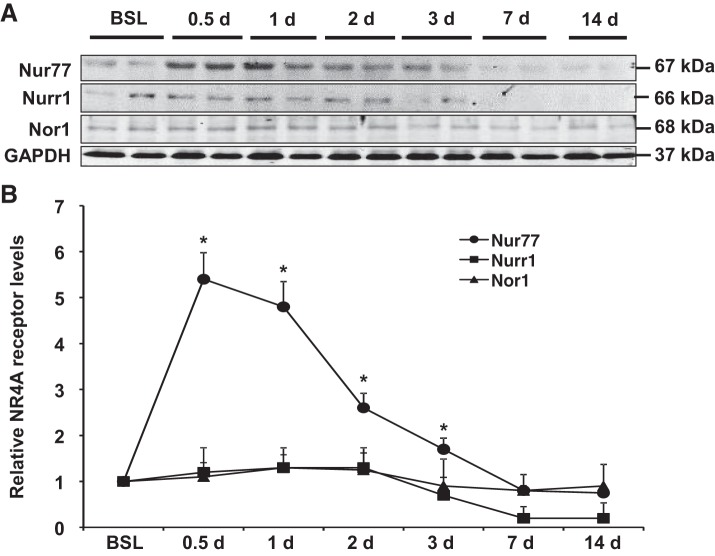
Administration of LPS in the mouse lung leads to a highly reproducible response characterized by an early and sustained influx of immune cells, an accumulation of protein-rich material from the circulation, and the expression of high levels of proinflammatory cytokines. Typically, these features develop within hours after injury and persist for days, before ultimately subsiding by 7 days after LPS exposure (19). With this time course in mind, we sought to determine whether expression of individual NR4A receptors changes in the lung after intrapulmonary LPS administration. As shown in Fig. 1, we found that protein levels of Nur77 were dramatically increased in whole lung homogenates relatively soon after injury, as demonstrated by a nearly 5.4-fold increase in protein levels at 12 h. Moreover, we found that this increase persisted for ~3 days (Fig. 1) before ultimately returning to baseline levels at around day 5 after injury. Interestingly, we did not detect any significant changes in levels of Nor1 or Nurr1 at any time point after LPS, perhaps suggesting a specific role for Nur77 in the regulation of lung injury responses to LPS.
Fig. 1.
Nur77 expression is increased in the lung after LPS-induced injury. A: Western blot analysis showing expression of Nur77, Nor1, and Nurr1 in whole lung tissues at various time points after LPS (100 µg). B: densitometry analysis of Nur77, Nor1, and Nurr1 expression. BSL, baseline. *P < 0.05 vs. baseline group, n = 5.
Nur77 deficiency exacerbates LPS-induced barrier disruption in the lung.
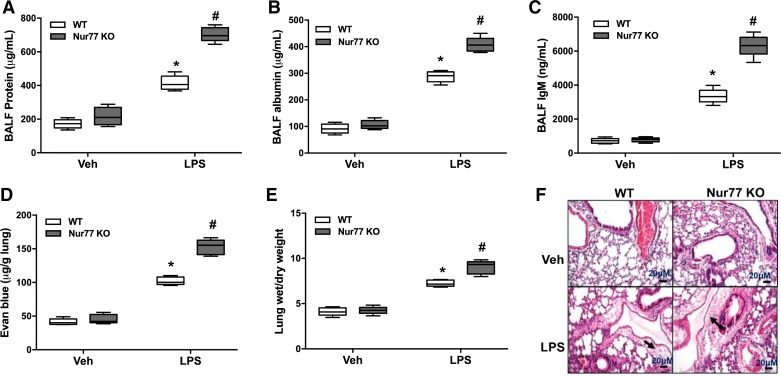
To test the role of Nur77 in acute lung injury responses, we instilled LPS in the airways of wild-type mice and mice that globally lack Nur77 expression. To quantify lung injury responses in these mice, we first measured total protein levels in the BALF since this is often used as a surrogate of pulmonary vascular leakage in mice. Consistent with the published literature, we found that instillation of LPS resulted in a dramatic increase in BALF protein levels in all mice. However, we also found that protein levels were nearly 40% higher in the BALF of Nur77−/− mice relative to age-matched controls. Moreover, these findings were associated with a marked increase in the concentration of albumin and IgM in the BALF, indicating greater leakage of both small- and large-molecular-weight proteins in the lung (Fig. 2, A–C). Consistent with an increase in pulmonary vascular leakage, we also detected a greater amount of Evans blue dye extravasation in the lung after LPS exposure (Fig. 2D), and we found that lung wet-to-dry weight ratios and the amount of fluid surrounding pulmonary blood vessels were increased in LPS-injured Nur77−/− mice (Fig. 2, E and F). Notably, BALF protein levels were not increased in Nur77−/− mice in the absence of any insult, indicating that deficiency in Nur77 does not appear to alter lung endothelial barrier functions under homeostatic conditions.
Fig. 2.
Nur77 deficiency augments vascular leak after LPS administration. Total protein (A), albumin (B), and IgM (C) concentrations in bronchoalveolar lavage fluid (BALF) at baseline and after LPS stimulation in wild-type (WT) and Nur77−/− mice. P < 0.05 vs. WT vehicle (Veh) group (*) and WT group challenged with LPS (#), n = 7. D: Evans blue dye extravasation in lung of control and LPS-injured WT and Nur77−/− mice. E: lung wet-to-dry weight ratio in control and LPS-injured WT and Nur77−/− mice. P < 0.05 vs. WT Veh group (*) and WT group challenged with LPS (#), n = 7. F: representative images of hematoxylin and eosin (H&E)-stained lung sections from WT and Nur77 −/− mice after Veh or LPS exposure. Black arrowheads indicate location of perivascular fluid accumulation. KO, knockout. Bars = 20 μm.
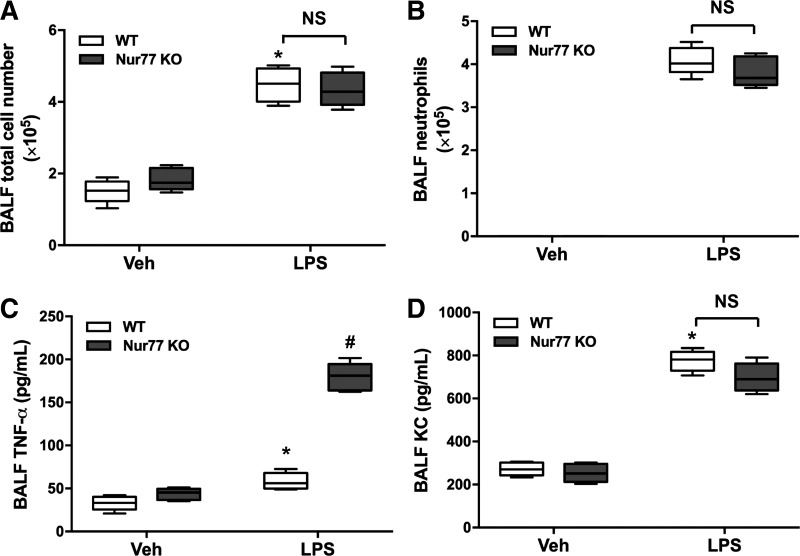
Nur77 deficiency does not significantly alter leukocyte entry in the lung in response to LPS.
Because loss of barrier protection is often accompanied by an influx of immune cells in the lung, we next hypothesized that leukocyte entry in the lung would also be increased in Nur77−/− mice after exposure to LPS. Surprisingly, we found that total leukocyte counts and neutrophil numbers in the BALF were largely the same in wild-type and Nur77−/− mice at 24 h after LPS (Fig. 3, A and B). Moreover, levels of the neutrophil chemotactic factor KC also did not significantly differ between wild-type and Nur77−/− mice (Fig. 3C). However, we did detect a significant increase in TNF-α levels in the BALF of Nur77−/− (Fig. 3D), indicating that inflammatory responses to LPS were not entirely normal in these mice.
Fig. 3.
Nur77 deficiency does not alter leukocyte entry in the LPS-injured lung. Total leukocyte (A) and neutrophil (B) counts in bronchoalveolar lavage fluid (BALF) of wild-type (WT) and Nur77−/− mice after vehicle (Veh) or LPS (n = 7). ELISA for TNF-α (C) and KC (D) and in BALF at 24 h after Veh or LPS administration (n = 7). KO, knockout. P < 0.05 vs. WT baseline group (*) and LPS-challenged WT group (#).
Nur77 regulates expression of junctional proteins and enhances endothelial barrier function to LPS.
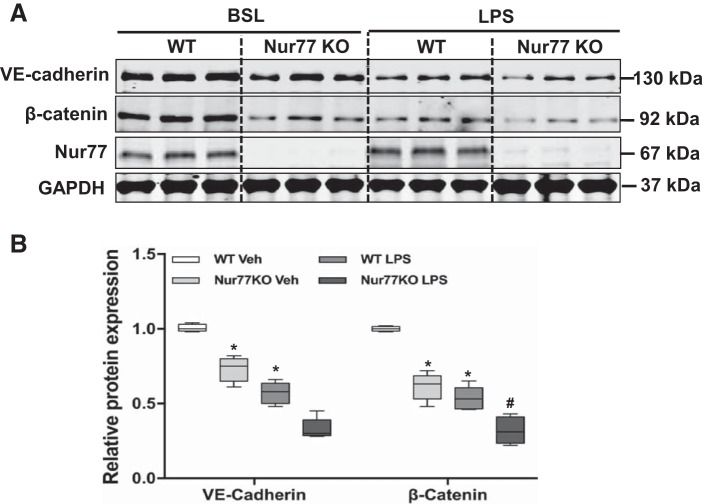
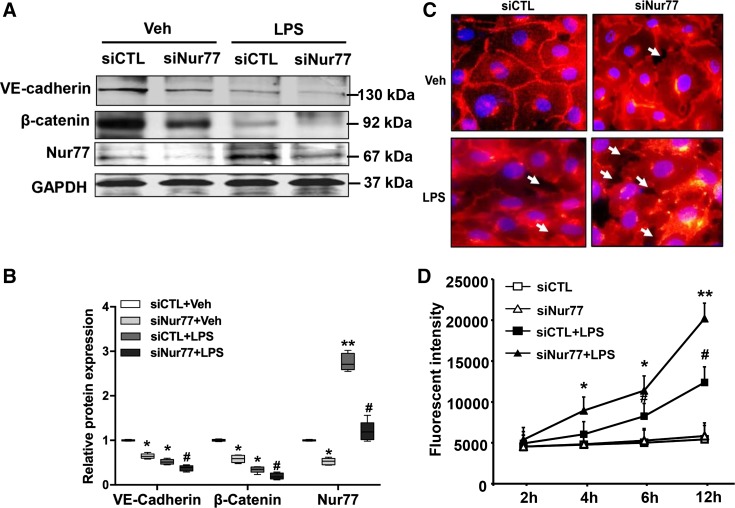
To elucidate the mechanism by which Nur77 regulates barrier protection in the lung, we first examined levels of VE-cadherin and β-catenin in whole lung homogenates from wild-type and Nur77−/− mice. As shown in Fig. 4, we detected a dramatic reduction in levels of VE-cadherin and β-catenin in the lungs of Nur77−/− mice even in the absence of any LPS administration (Fig. 4). Moreover, we found that expression of these proteins was further reduced in the lungs of Nur77−/− mice at 24 h after LPS administration. To explore whether Nur77 regulates the expression of these proteins, we performed loss-of-function studies in MLMECs using the siRNA knockdown approach to examine the effects on expression of junctional proteins. Importantly, siRNA knockdown was highly effective in reducing Nur77 expression, as demonstrated by the nearly 80% reduction in Nur77 protein levels in MLMECs (Fig. 5A). Moreover, we found that siRNA knockdown resulted in a dramatic reduction in levels of VE-cadherin and β-catenin in MLMECs, and this was further exacerbated in cells that were also exposed to LPS (Fig. 5, A and B). Furthermore, we found that these biochemical changes resulted in a marked reduction in endothelial barrier functions to LPS, as judged by a dramatic decrease in histologically visible endothelial cell-cell contacts (Fig. 5C) and by a dramatic increase in the quantity of FITC-labeled dextran transporting across endothelial cell monolayers (Fig. 5D).
Fig. 4.
Expression of endothelial junction proteins in the lungs of wild-type (WT) and Nur77 −/− mice. A: Western blot for vascular endothelial cadherin (VE-cadherin), β-catenin, and Nur77 in WT and Nur77 −/− mice 24 h after LPS (100 µg). B: densitometry analysis of VE-cadherin and β-catenin (n = 5). BSL, baseline; KO, knockout. P < 0.05 vs. baseline group (*) and WT group challenged with LPS (#).
Fig. 5.
Nur77 knockdown promotes LPS-induced barrier dysfunction in lung microvascular endothelial cells. A: expression of vascular endothelial cadherin (VE-cadherin), β-catenin, and Nur77 in LPS-injured mouse lung microvessel endothelial cells (MLMECs) transfected with siCTL or siNur77. B: densitometry analysis of VE-cadherin, β-catenin, and Nur77 expression (n = 4). Veh, vehicle. *P < 0.05 or **P < 0.01 vs. siCTL without LPS. #P < 0.05 vs. siCTL with LPS treatment. C: representative images of VE-cadherin in control and LPS-injured MLMECs after transfection with either siCTL or siNur77. Red color represents VE-cadherin; blue color corresponds to DAPI nuclear stain. D: levels of FITC-labeled dextran in the lower well of control and LPS-injured endothelial cell monolayers that have been transfected with either siCTL or siNur77 (100 ng/mL). FITC-dextran was measured using a fluorescence plate reader (BioTek) at 480/520 nm at the indicated time points. P < 0.05 vs. siCTL without LPS treatment (*) and siCTL with LPS treatment (#). **P < 0.01 vs. siCTL without LPS treatment.
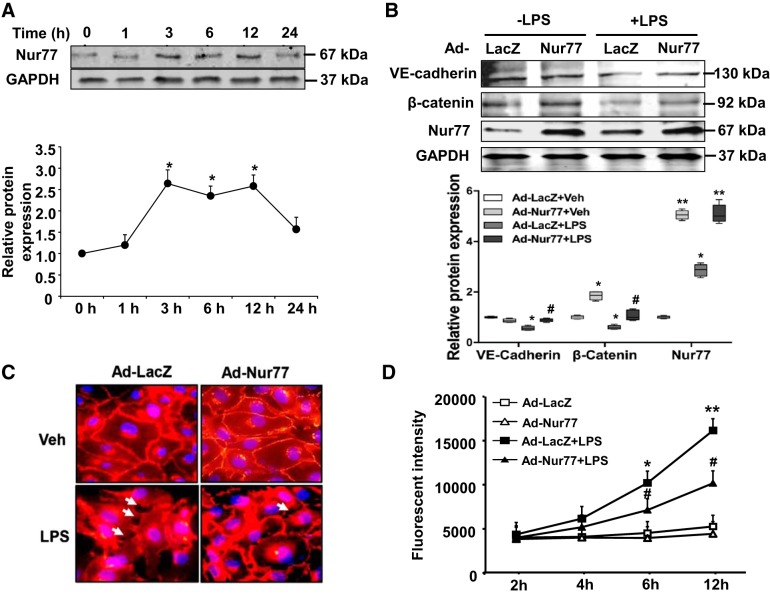
Because downregulation of Nur77 was found to exacerbate endothelial barrier dysfunction to LPS, we next sought to determine whether augmenting its expression would accomplish the opposite. Indeed, LPS stimulation significantly increased Nur77 expression in MLMECs (Fig. 6A), but not in lung epithelial cells and macrophages as determined by qPCR (data not shown). As shown in Fig. 6B, adenovirus-mediated overexpression of Nur77 had little to no effect on baseline levels of VE-cadherin but significantly increased β-catenin levels in our culture cells. Moreover, we found that both VE-cadherin and β-catenin levels were significantly increased after overexpression of Nur77 in LPS-exposed cells. Furthermore, we found that enhanced expression of these proteins associated with an increase in the number of visible endothelial cell-cell contacts (Fig. 6C) and an increase in endothelial barrier function under LPS stimulation conditions, as judged by a decrease in the amount of FITC-labeled dextran transporting across LPS-exposed cell monolayers (Fig. 6D).
Fig. 6.
Overexpression of Nur77 prevents LPS-induced endothelial permeability in mouse lung microvessel endothelial cells (MLMECs). A: time-dependent effect of LPS (100 ng/mL) on Nur77 expression in MLMECs as determined by Western blot. Densitometry analysis for Nur77 (n = 3). *P < 0.05 vs. 0 h of the treatment. B: Western blot analysis for vascular endothelial cadherin (VE-cadherin), β-catenin, and Nur77 in MLMECs transduced with either Ad-LacZ or Ad-Nur77 for 48 h before LPS (100 ng/ml) exposure for 12 h. Densitometry analysis of VE-cadherin, β-catenin, and Nur77 expression (n = 4). *P < 0.05 or **P < 0.01 vs. Ad-LacZ without LPS treatment group. #P < 0.05 vs. Ad-LacZ with LPS treatment. C: representative images of MLMECs stained for VE-cadherin. Red color represents VE-cadherin; blue corresponds to DAPI nuclear stain; white arrowheads indicate areas were there appears to be a disruption of endothelial cell-cell contacts. D: levels of fluorescein isothiocyanate (FITC)-labeled dextran in the lower well of control and LPS-injured endothelial cell monolayers that have been transfected with either Ad-LacZ or Ad-Nur77. FITC-dextran was measured using a fluorescence plate reader (BioTek) at 480/520 nm at the indicated time points. Veh, vehicle. P < 0.05 vs. Ad-LacZ without (*) and with (#) LPS treatment. **P < 0.01 vs Ad-LacZ without LPS treatment.
Nur77 regulates β-catenin protein stability in MLMECs.
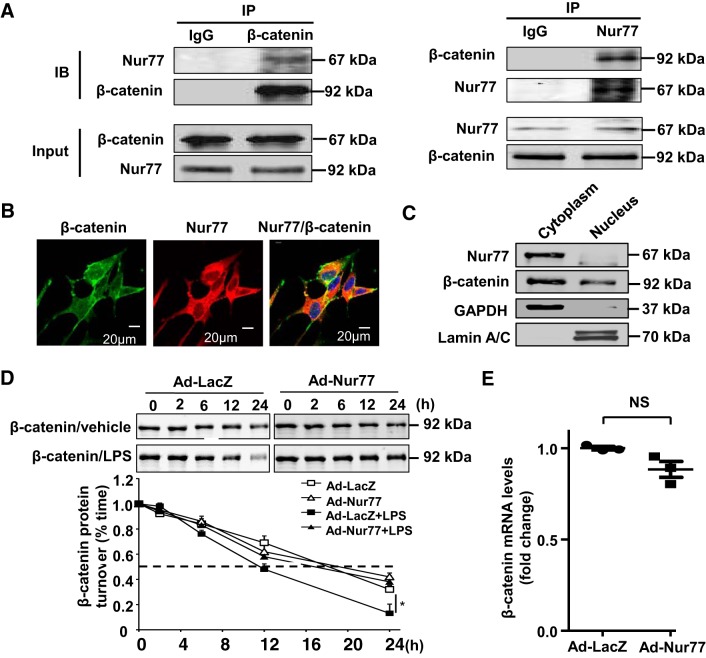
Next, we sought to delineate the mechanisms by which Nur77 enhances endothelial barrier protection to LPS in cultured MLMECs. Because Nur77 deficiency was associated with a downregulation of endothelial junctional proteins, we speculated that Nur77 may be important for preventing the degradation of junctional proteins after LPS. To test this, we performed coimmunoprecipitation assays to evaluate whether Nur77 associates with β-catenin. As shown in Fig. 7A, we found that immunoprecipitation of Nur77 also led to detectable quantities of β-catenin in immunoblots. Likewise, immunoprecipitation of β-catenin led to detectable quantities of Nur77 in immunoblots. Furthermore, we find that Nur77 was mainly localized in the cytoplasm and colocalized with β-catenin in human lung endothelial cells (Fig. 7, B and C). Because these findings support the notion of a physical association between Nur77 and β-catenin, we next sought to determine whether expression of Nur77 impacts the stability of β-catenin in MLMECs. To test this, we overexpressed Nur77 in MLMECs and examined the half-life of β-catenin protein under control and LPS injury conditions. As shown in Fig. 7D, we found that overexpression of Nur77 had little to no effect on the half-life of β-catenin in cycloheximide-treated cells in the absence of LPS stimulation. However, a marked increase in the biological half-life of β-catenin (11.5 ± 1.4 to 16.4 ± 1.3 h, P < 0.05, n = 4) was observed in cycloheximide-treated cells after LPS stimulation (Fig. 7D). Furthermore, we found that overexpression of Nur77 had no effect on the mRNA levels of β-catenin in human lung endothelial cells (Fig. 7E). Together, these results indicate that Nur77 augments lung endothelial barrier protection to LPS, at least in part, by augmenting β-catenin protein stability and enhancing cell-cell junctional integrity.
Fig. 7.
Nur77 associates with β-catenin in mouse lung microvessel endothelial cells (MLMECs). A: total cell lysates from MLMECs were immunoprecipitated (IP) with anti-β-catenin, anti-Nur77 antibodies, or control IgG, and then immunocomplexes were separated by 10% SDS-PAGE. The transferred membrane was immunoblotted (IB) with either anti-Nur77 or anti-β-catenin antibodies. B: colocalization of Nur77 (red color) and β-catenin (green color) in human lung endothelial cells. C: subcellular fractions isolated from human lung endothelial cells were assessed by Western blotting to examine the localization in Nur77 and β-catenin. Cytosolic marker, GAPDH; nuclear marker, lamin A/C. E: MLMECs were infected with Ad-LacZ or Ad-Nur77 for 48 h and then stimulated with LPS as described above. Expression of β-catenin was determined by Western blotting at indicted time points after addition of cycloheximide (5 μg/mL). Densitometry analysis of β-catenin expression is shown in graph on the bottom (n = 4). *P < 0.05 vs. Ad-LacZ without LPS treatment or Ad-Nur77 with LPS treatment. E: human lung endothelial cells were transduced with Ad-LacZ and Ad-Nur77 (multiplicity of infection = 10) for 48 h. Expression of β-catenin was determined by real-time PCR.
DISCUSSION
Recent studies indicate that the NR4A subfamily of proteins plays an important role in regulating endothelial homeostasis in the systemic circulation (2, 15, 17, 28, 30, 32), but the importance of these proteins in controlling pulmonary vascular homeostasis has not been investigated. In the present study, we show that, among the three NR4A family members, only the expression of Nur77 is dynamically regulated in the lung in response to LPS. Moreover, we show that deficiency in Nur77 expression renders mice more susceptible to LPS-induced pulmonary vascular leakage and that Nur77 mediates its endothelial barrier protection, in part, by reducing the degradation of the endothelial junction protein β-catenin.
The major finding of this study is that Nur77 enhances endothelial barrier function in the lung by preventing degradation of endothelial junctional proteins. We show that Nur77 deficiency results in a significant reduction in the expression of VE-cadherin and β-catenin and that overexpression of Nur77 attenuates degradation of these proteins in the LPS-exposed lung. Mechanistically, we found that Nur77 associates with β-catenin in lung endothelial cells, suggesting a role in limiting its degradation. Importantly, these findings are consistent with recently published reports showing that β-catenin plays an important role in maintaining endothelial barrier integrity in various extrapulmonary vascular beds (4, 8, 24). Although we believe that interactions between Nur77/β-catenin are important for enhancing β-catenin expression, we also recognize that other mechanisms might be contributing, such as changes on transcript stability. Moreover, we also recognize that other reports have shown that β-catenin levels are reduced in response to Nur77 in cell types outside the lung (5, 20, 21, 25). Perhaps this might be explained by cell-specific differences in the association of Nur77 with intracellular proteins or by differences in the nature of the injury stimulus. Consistent with findings in our study, Zhao et al. recently showed that Nur77 exacerbates VEGF-induced vascular permeability in systemic endothelial cells. However, in this study, increased vascular permeability was attributed to elevated endothelial nitric oxide synthase activity rather than to associations between Nur77 and β-catenin (32). That said, our results are consistent with work in human colon cancer cells showing that Nur77/β-catenin associations are important for initiating a positive feedback loop to hypoxia (21).
Although our findings suggest that Nur77 enhances endothelial barrier function by augmenting the expression of junctional adhesion proteins, we recognize that other mechanisms may also be contributing to an increase in pulmonary vascular leakage to LPS. For example, we recently reported that Nur77 suppresses the expression of leukocyte adhesion proteins (E-selectin and VCAM) outside the lung. Moreover, we found that these adhesion molecules are also increased in the lungs of Nur77−/− mice after LPS (data not shown). Together, these findings suggest that increased leukocyte binding and rolling along the endothelium might also contribute to enhanced plasma leakage in the lung after LPS exposure (1). Alternatively, we cannot exclude the possibility that Nur77 deficiency affects the functioning of other vascular cell types in the lung, such as smooth muscle cells, or somehow reduces the clearance of fluid from the lung after entry. In Nur77 knockout mice, the level of β-catenin is significantly reduced under basal conditions compared with that in wild-type mice, and this relative reduction persists after exposure to LPS. However, decreases in basal levels did not appear to affect lung function, as assessed by histology or levels of plasma proteins in the lung, indicating that levels of β-catenin in Nur77 knockout mice are sufficient to possibly meet the minimum requirement (β-catenin-VE-cadherin interaction) to maintain barrier function under basal conditions. In response to LPS, although Nur77 is increased in wild-type mice, we suspect this increase is not sufficient to prevent the degradation of β-catenin by other mechanisms. Our results indicated that Nur77 functions as a negative feedback regulator of vascular permeability, and its deficiency will further lead to β-catenin degradation and exacerbate barrier disruption in the lung in response to LPS stimulation. Future studies exploring these alternative hypotheses will be important for understanding the full impact of Nur77 deficiency on injury responses in the lung.
Despite an increase in pulmonary vascular leakage in the lungs of Nur77−/− mice after LPS administration, we did not detect an increase in leukocyte entry in the lungs. We speculate that this might be explained by the fact that levels of leukocyte chemoattractant signals (e.g., KC) were not increased in response to Nur77 deficiency. Alternatively, it could be that Nur77 deficiency impairs the ability of leukocytes to migrate in the alveolar space. Although immune cell infiltration was not increased, we did detect a marked increase in TNF-α levels in the lungs of Nur77−/− mice after LPS administration. Perhaps this might be explained by an increase in inflammatory responses to higher levels of plasma-rich materials in the lung or by the fact that Nur77 deficiency might alter the inflammatory response to LPS in the lung. Consistent with this latter mechanism, it has recently been shown that Nur77 deficiency polarizes macrophages to an M1 proinflammatory phenotype (7), suggesting the possibility that increased numbers of M1 macrophages might have contributed to elevated TNF-α concentrations in our study.
In summary, this study is the first to suggest that Nur77 is important for protecting the lung from injury and for maintaining lung endothelial barrier protection. Collectively, our findings suggest that Nur77 activators, such as cytosporone B, hyperoside (9, 16, 31), and others, might have therapeutic potential in ameliorating the onset and/or progression of inflammatory vascular diseases of the lung.
GRANTS
This work was supported by the Natural Science Foundation of Shanghai (18ZR1438800) and Shanghai Municipal Health Commission (201540217) to N. Zhu, and National Institutes of Health (National Heart, Lung, and Blood Institute Grant R01 HL103869 and National Institute of General Medical Sciences Grant R01 GM123047) and American Heart Association Established Investigator Award 16EIA27710023 to J. Sun.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.Z., G.-X.Z., B.Y., Z.-F.G., R.S., and J.S. conceived and designed research; N.Z., G.-X.Z., B.Y., Z.-F.G., S.J., Y.Y., F.Y., and J.S. performed experiments; N.Z., G.-X.Z., B.Y., S.J., Y.Y., F.Y., R.S., and J.S. analyzed data; N.Z., G.-X.Z., R.S., and J.S. interpreted results of experiments; N.Z., G.-X.Z., Z.-F.G., and J.S. prepared figures; N.Z., R.S., and J.S. drafted manuscript; S.J., R.S., and J.S. edited and revised manuscript; N.Z., G.-X.Z., B.Y., Z.-F.G., Y.Y., F.Y., R.S., and J.S. approved final version of manuscript.
REFERENCES
- 1.Alevriadou BR. CAMs and Rho small GTPases: gatekeepers for leukocyte transendothelial migration. Focus on “VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration”. Am J Physiol Cell Physiol 285: C250–C252, 2003. doi: 10.1152/ajpcell.00189.2003. [DOI] [PubMed] [Google Scholar]
- 2.Arkenbout EK, de Waard V, van Bragt M, van Achterberg TA, Grimbergen JM, Pichon B, Pannekoek H, de Vries CJ. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation 106: 1530–1535, 2002. doi: 10.1161/01.CIR.0000028811.03056.BF. [DOI] [PubMed] [Google Scholar]
- 3.Bonta PI, Pols TW, de Vries CJ. NR4A nuclear receptors in atherosclerosis and vein-graft disease. Trends Cardiovasc Med 17: 105–111, 2007. doi: 10.1016/j.tcm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol 162: 1111–1122, 2003. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HZ, Liu QF, Li L, Wang WJ, Yao LM, Yang M, Liu B, Chen W, Zhan YY, Zhang MQ, Cai JC, Zheng ZH, Lin SC, Li BA, Wu Q. The orphan receptor TR3 suppresses intestinal tumorigenesis in mice by downregulating Wnt signalling. Gut 61: 714–724, 2012. doi: 10.1136/gutjnl-2011-300783. [DOI] [PubMed] [Google Scholar]
- 6.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med 17: 293–307, 2011. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res 110: 416–427, 2012. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem 276: 12301–12309, 2001. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- 9.Huo Y, Yi B, Chen M, Wang N, Chen P, Guo C, Sun J. Induction of Nur77 by hyperoside inhibits vascular smooth muscle cell proliferation and neointimal formation. Biochem Pharmacol 92: 590–598, 2014. doi: 10.1016/j.bcp.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail H, Mofarrahi M, Echavarria R, Harel S, Verdin E, Lim HW, Jin ZG, Sun J, Zeng H, Hussain SN. Angiopoietin-1 and vascular endothelial growth factor regulation of leukocyte adhesion to endothelial cells: role of nuclear receptor-77. Arterioscler Thromb Vasc Biol 32: 1707–1716, 2012. doi: 10.1161/ATVBAHA.112.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederer PA, Zhou T, Chen W, Epshtein Y, Wang H, Mathew B, Jacobson JR. Attenuation of murine acute lung injury by PF-573,228, an inhibitor of focal adhesion kinase. Vascul Pharmacol 110: 16–23, 2018. doi: 10.1016/j.vph.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, Zhu N, Yi B, Wang N, Chen M, You X, Zhao X, Solomides CC, Qin Y, Sun J. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res 113: 1117–1127, 2013. doi: 10.1161/CIRCRESAHA.113.301306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomiyama T, Zhao Y, Gizard F, Findeisen HM, Heywood EB, Jones KL, Conneely OM, Bruemmer D. Deficiency of the NR4A neuron-derived orphan receptor-1 attenuates neointima formation after vascular injury. Circulation 119: 577–586, 2009. doi: 10.1161/CIRCULATIONAHA.108.822056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, Mielenz D, Tomcik M, Fürnrohr BG, Scholtysek C, Dees C, Beyer C, Krönke G, Metzger D, Distler O, Schett G, Distler JH. Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis. Nat Med 21: 150–158, 2015. doi: 10.1038/nm.3777. [DOI] [PubMed] [Google Scholar]
- 17.Qin Q, Chen M, Yi B, You X, Yang P, Sun J. Orphan nuclear receptor Nur77 is a novel negative regulator of endothelin-1 expression in vascular endothelial cells. J Mol Cell Cardiol 77: 20–28, 2014. doi: 10.1016/j.yjmcc.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte D, Küppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J 30: 4157–4170, 2011. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah D, Romero F, Stafstrom W, Duong M, Summer R. Extracellular ATP mediates the late phase of neutrophil recruitment to the lung in murine models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 306: L152–L161, 2014. doi: 10.1152/ajplung.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Z, Cao X, Jiang MM, Qiu Y, Zhou H, Chen L, Qin B, Wu H, Jiang F, Chen J, Liu J, Dai Y, Chen HF, Hu QY, Wu Z, Zeng JZ, Yao XS, Zhang XK. Inhibition of β-catenin signaling by nongenomic action of orphan nuclear receptor Nur77. Oncogene 31: 2653–2667, 2012. doi: 10.1038/onc.2011.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.To SK, Zeng WJ, Zeng JZ, Wong AS. Hypoxia triggers a Nur77-β-catenin feed-forward loop to promote the invasive growth of colon cancer cells. Br J Cancer 110: 935–945, 2014. doi: 10.1038/bjc.2013.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Tiel CM, de Vries CJ. NR4All in the vessel wall. J Steroid Biochem Mol Biol 130: 186–193, 2012. doi: 10.1016/j.jsbmb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol 19: 8–15, 2009. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 1778: 794–809, 2008. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Lin Y, Li W, Sun Z, Gao W, Zhang H, Xie L, Jiang F, Qin B, Yan T, Chen L, Zhao Y, Cao X, Wu Y, Lin B, Zhou H, Wong AS, Zhang XK, Zeng JZ. Regulation of Nur77 expression by β-catenin and its mitogenic effect in colon cancer cells. FASEB J 25: 192–205, 2011. doi: 10.1096/fj.10-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan G, Zhu N, Huang S, Yi B, Shang X, Chen M, Wang N, Zhang GX, Talarico JA, Tilley DG, Gao E, Sun J. Orphan nuclear receptor Nur77 inhibits cardiac hypertrophic response to beta-adrenergic stimulation. Mol Cell Biol 35: 3312–3323, 2015. doi: 10.1128/MCB.00229-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo YG, Na TY, Yang WK, Kim HJ, Lee IK, Kong G, Chung JH, Lee MO. 6-Mercaptopurine, an activator of Nur77, enhances transcriptional activity of HIF-1alpha resulting in new vessel formation. Oncogene 26: 3823–3834, 2007. doi: 10.1038/sj.onc.1210149. [DOI] [PubMed] [Google Scholar]
- 28.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res 104: 742–749, 2009. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 29.You X, Guo ZF, Cheng F, Yi B, Yang F, Liu X, Zhu N, Zhao X, Yan G, Ma XL, Sun J. Transcriptional up-regulation of relaxin-3 by Nur77 attenuates β-adrenergic agonist-induced apoptosis in cardiomyocytes. J Biol Chem 293: 14001–14011, 2018. doi: 10.1074/jbc.RA118.003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med 203: 719–729, 2006. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, Li G, Xu Q, Zhang M, Weimer BC, Chen D, Cheng Z, Zhang L, Li Q, Li S, Zheng Z, Song S, Huang Y, Ye Z, Su W, Lin SC, Shen Y, Wu Q. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol 4: 548–556, 2008. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Qin L, Bourbon PM, James L, Dvorak HF, Zeng H. Orphan nuclear transcription factor TR3/Nur77 regulates microvessel permeability by targeting endothelial nitric oxide synthase and destabilizing endothelial junctions. Proc Natl Acad Sci USA 108: 12066–12071, 2011. doi: 10.1073/pnas.1018438108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol 30: 1535–1541, 2010. doi: 10.1161/ATVBAHA.109.191163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Howatt DA, Gizard F, Nomiyama T, Findeisen HM, Heywood EB, Jones KL, Conneely OM, Daugherty A, Bruemmer D. Deficiency of the NR4A orphan nuclear receptor NOR1 decreases monocyte adhesion and atherosclerosis. Circ Res 107: 501–511, 2010. doi: 10.1161/CIRCRESAHA.110.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]