Abstract
Multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multigene family with isoform-specific regulation of vascular smooth muscle (VSM) functions. In previous studies, we found that vascular injury resulted in VSM dedifferentiation and reduced expression of the CaMKIIγ isoform in medial wall VSM. Smooth muscle knockout of CaMKIIγ enhanced injury-induced VSM neointimal hyperplasia, whereas CaMKIIγ overexpression inhibited VSM proliferation and neointimal formation. In this study, we evaluated DNA cytosine methylation/demethylation as a mechanism for regulating CaMKII isoform expression in VSM. Inhibition of cytosine methylation with 5-Aza-2′-deoxycytidine significantly upregulated CaMKIIγ expression in cultured VSM cells and inhibited CaMKIIγ downregulation in organ-cultured aorta ex vivo. With the use of methylated cytosine immunoprecipitation, the rat Camk2g promoter was found hypomethylated in differentiated VSM, whereas injury- or cell culture-induced VSM dedifferentiation coincided with Camk2g promoter methylation and decreased expression. We report for the first time that VSM cell phenotype switching is accompanied by marked induction of thymine DNA glycosylase (TDG) protein and mRNA expression in injured arteries in vivo and in cultured VSM synthetic phenotype cells. Silencing Tdg in VSM promoted expression of CaMKIIγ and differentiation markers, including myocardin, and inhibited VSM cell proliferation and injury-induced neointima formation. This study indicates that CaMKIIγ expression in VSM is regulated by cytosine methylation/demethylation and that TDG is an important determinant of this process and, more broadly, VSM phenotype switching and function.
NEW & NOTEWORTHY Expression of the calcium calmodulin-dependent protein kinase II-γ isoform (CaMKIIγ) is associated with differentiated vascular smooth muscle (VSM) and negatively regulates proliferation in VSM synthetic phenotype (VSMSyn) cells. This study demonstrates that thymine DNA glycosylase (TDG) plays a key role in regulating CaMKIIγ expression in VSM through promoter cytosine methylation/demethylation. TDG expression is strongly induced in VSMSyn cells and plays key roles in negatively regulating CaMKIIγ expression and more broadly VSM phenotype switching.
Keywords: CaMKIIγ, CaM kinase II, cytosine methylation/demethylation, thymine DNA glycosylase, vascular injury, vascular smooth muscle
INTRODUCTION
Vascular smooth muscle (VSM) cells are a major component of the medial layer of blood vessels, and differentiated VSM (VSMDiff) provides mechanical support for conducting and regulating blood flow and pressure. VSM is not terminally differentiated (27, 28) and may revert to a “synthetic” phenotype during wound healing and some occlusive vascular diseases such as atherosclerosis (2, 26). Synthetic phenotype VSM (VSMSyn) properties include extracellular matrix remodeling, proinflammatory function, motility, and proliferation. Mechanisms that mediate VSMSyn function in vascular disease, as well as transcriptional, posttranscriptional and epigenetic mechanisms that underlie VSM phenotype plasticity are under active investigation.
Multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a mediator of Ca2+ and reactive oxygen species signaling in VSM and regulates VSMSyn functions such as proliferation and migration (14, 19, 32, 34). Four homologous and highly conserved CaMKII isoforms exist in mammals and functionally organize into large hetero-multimeric holoenzymes. CaMKIIα and -β isoforms are mainly expressed in neural tissues, whereas CaMKIIγ and -δ are more ubiquitous and are the predominant isoforms in VSM (36, 37). CaMKIIγ and -δ isoform expressions are reciprocally regulated during VSM phenotype modulation and function oppositely in regulating injury-induced vascular remodeling (13, 14, 32). CaMKIIδ promotes proliferation and motility of VSMSyn both in vitro and in vivo in response to vascular injury (14). Previous studies, including from this laboratory, reported that VSM CaMKIIδ expression is negatively regulated by miRNA-30 and miR-145 via binding to the CaMKIIδ mRNA 3′-untranslated region (6, 24). Conversely, CaMKIIγ is downregulated in VSMSyn and acts to suppress vascular remodeling and neointimal hyperplasia in response to vascular injury (32). Nothing has been reported about how CaMKIIγ expression is regulated in VSM.
Transcriptional control of the VSMDiff phenotype is driven by the “master regulator” myocardin (MYOCD), which, in concert with serum response factor (SRF), controls expression of a number of smooth muscle-specific contractile proteins, including smooth muscle myosin heavy chain (MYH11), which is considered a definitive smooth muscle marker gene. CaMKIIγ represents a class of genes positively associated with the VSMDiff phenotype but regulated independently of SRF/MYOCD (32).
Epigenetic regulation, including DNA cytosine methylation/demethylation, also plays important roles in vascular development and diseases (8, 38). Methylated DNA cytosines globally affect the chromatin landscape, and within CpG-rich gene promoter regions where cytosines are largely demethylated, specific cytosine methylations are typically repressive. Recently, one isoform of ten-eleven translocation (TET), which demethylates DNA cytosine by catalyzing formation of 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-fC), and 5-carboxylcytosine (5-caC) (17), has also been described as a “master regulator” of VSM phenotype. High levels of TET2 expression with 5-hydroxycytosine methylation at specific loci in MYOCD, SRF, and MYH11 promoters were associated with the VSMDiff phenotype and expression of these smooth muscle marker genes. Downregulation of TET2, decreased 5-hmC, and increased 5-mC was associated with VSMSyn, as well as increased human atherosclerotic lesion severity (21, 22).
Cycles of cytosine demethylation and methylation expected when VSM dynamically switches from a quiescent contractile phenotype to a proliferative synthetic phenotype requires removal of 5-hmC, 5-fC, and 5-caC by base excision and repair pathways, including by thymine DNA glycosylase (TDG), which has comparatively high affinity for 5-fC and 5-caC (11, 25). TDG is essential for development (7) and plays a key role in regulating intestinal tumorigenesis (41). There is very limited information on TDG expression and function in VSM. TDG was identified in a yeast two-hybrid screen to physically interact with MYOCD and through in vitro studies shown to negatively regulate MYOCD transactivating activity by inhibiting MYOCD/SRF interactions, independent of its enzymatic activity (44). MiR-29 has been shown to negatively regulate TET1–3 and TDG expression in VSM associated with global decreases of 5-hmC content (42). Beyond these preliminary reports that include no in vivo studies, the functional roles of TDG and TDG-mediated DNA cytosine demethylation in regulating VSMSyn phenotype gene expression and function are unknown.
In this study, we evaluated DNA cytosine methylation/demethylation as a mechanism regulating CaMKIIγ expression in VSM. Using methylated DNA immunoprecipitation (MeDIP), we determined that the Camk2g promoter is demethylated in VSMDiff correlating with CaMKIIγ expression, whereas injury- or enzymatic dispersion-induced VSM de-differentiation coincided with CaMKIIγ promoter methylation and decreased expression in VSMSyn cells. We report for the first time that VSM cell phenotype switching is accompanied by marked upregulation of TDG expression in injured arteries in vivo and in cultured VSMSyn cells. Silencing TDG expression in VSMSyn cells promotes expression of CaMKIIγ as well as differentiation markers, including Myocd and Myh11, and inhibits VSM cell proliferation both in vitro and in vivo. This study indicates that CaMKIIγ expression in VSM is regulated by promoter cytosine methylation/demethylation and identifies TDG as an important determinant of this process and, more broadly, VSM synthetic phenotype switching and function.
MATERIALS AND METHODS
Polyclonal antibodies against the COOH-terminus of CaMKIIγ (anti-LNVHYHCSGAPAAPL) and CaMKIIδ2 (anti-NFSGGTSLWQNI), which are conserved across species, were raised in rabbits as described previously and validated using recombinant proteins (10, 13, 14, 30). The antibodies for β-actin, GAPDH, and calponin were purchased from Sigma. The antibodies for TDG and MYH11 were purchased from Santa Cruz and Alpha Aesar, respectively. 5-Aza-2′-deoxycytidine (5-Aza) was purchased from Sigma. The 5-MeDIP kit was purchased from Active Motif.
Animal studies.
All animal use protocols were submitted to and approved by the Albany Medical Center Institutional Animal Care and Use Committee. All animal experiments were carried out in accordance with the National Institutes of Health and American Association for Accreditation of Laboratory Animal Care guidelines.
VSM cell dispersion and culture.
VSM cells were dispersed and cultured from rat descending thoracic aorta as described previously (23). Male Sprague-Dawley rats (150–200 g) were euthanized with CO2, and aorta medial layers were enzymatically digested with collagenase and elastase to free VSM cells. The dispersed VSM cells were cultured in DMEM-F12 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. All experiments were conducted on VSM cells from passages 3 to 6.
Rat carotid artery balloon injury.
The rat carotid artery balloon injury procedure has been described previously (43). Male Sprague-Dawley rats (400–430 g) were anesthetized with ketamine and xylazine by intraperitoneal injection. The left common carotid artery was dissected to the bifurcation and blood flow stopped by two vascular clips on the distal internal carotid artery and proximal common carotid artery. The external carotid artery was ligated and a small arteriotomy made followed by insertion of a 2-Fr Fogarty embolectomy catheter into the common carotid artery. The catheter was then inflated under the constant pressure of 1.5–1.8 atm, followed by pulling the inflated catheter through the common carotid three times. After injury, a complete ligation was performed between the bifurcation and the arteriotomy in the external branch, and carotid blood flow was restored by releasing the two vascular clips.
Lentivirus production and lentivirus infection.
Lentiviral constructs were produced using the pMD2.G envelop system (Addgene). In brief, human embryonic kidney 293 FT cells (Invitrogen) under passage 10 were grown in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin on 100-mm Petri dishes. When confluency reached 80–90%, cells were transfected with pRSV-Rev (1.5 μg), pMDLS-pRRE (1.5 μg), pMD2.G (1.5 μg), and lentiviral plasmid-encoding scramble RNA plasmid or all-in-one guide RNA (gRNA) plasmid (2 μg) using TransIT-2020 (36 μL) (Mirus Bio, WI). After a 16-h incubation, the culture media was changed to DMEM with 0.01% fetal bovine serum and 1% penicillin-streptomycin, followed by a 24-h incubation for virus production. The lentiviral particles were harvested from media and concentrated using Lenti-X concentrator (Clonetech, CA). Lentivirus titration was measured using the Lenti-X qRT-PCR Titration Kit following the manufacturer’s protocols (Clonetech, CA).
CRISPR-Cas9 targeting of TDG.
The single guide RNA CRISPR/Cas9 all-in-one plasmids targeting rat Tdg were purchased from ABM (cat. no. K6955005). The product provided three individual plasmids targeting Tdg exon 1, exon 2, and exon 3, respectively. We evaluated these and found the single guide RNA targeting the exon 3 sequence GATTCACCAACATGGTGGAA most effective (data not shown).
Western blot analysis and quantitative PCR.
VSM cells were washed with Dulbecco’s PBS twice and lysed in 1% Triton in Tris-buffered saline for Western blot analysis or TRIzol (1 mL) for RNA extraction. Ex vivo cultured aorta rings were homogenized in radioimmunoprecipitation assay buffer to extract protein for Western blot analysis. Cell lysates or tissue lysates were heated at 95°C for 5 min and resolved using 10% SDS-PAGE, followed by transfer onto nitrocellulose membranes (GE Healthcare). The membranes were blocked with 5% nonfat milk in Tris-buffered saline with 1% Tween 20 for 30 min and incubated with primary antibody overnight at 4°C. Signals were detected using the SuperSignal chemiluminescent substrate for 5 min and imaged on a Fuji LAS4000. Total RNA was extracted using TRIzol and chloroform by phase separation. After determining the concentration by Nanodrop (Thermo), the total RNA was reverse transcribed to cDNA using the Quantitech reverse transcription kit (Qiagen). Quantitative RT-PCR was carried out on an Mx3000P QPCR System using IQ Sybr green Supermix (Bio-Rad). Primers were as follows: Camk2g forward: 5′-GCCACCTGCACCCGCTTCAC-3′ and reverse: 5′-CTCCTGCTGCGACGTTTCTT-3′; Tagln forward: 5′-GAGCGGCTAGTGGAGTGGATTG-3′ and reverse: 5′-GTTCACCTTGCTCAGAATC-3′; Myh11 forward: 5′-GACACTATGTCAGGGAAAAC-3′ and reverse: 5′-CTTTGTGCAGGGCTGTGGTTGAC-3′; Gapdh forward: 5′-TCGTCTCATAGACAAGATGGT-3′ and reverse: 5′-GTAGTTGAGGTCAATGAAGGG-3′; and Tdg forward: 5′-ACTCTCCCTGACATTTTGACC and reverse: 5′-TCAACCCAGACATGAACAGAC.
Genomic DNA extraction.
Whole genomic DNA (gDNA) extraction was performed following the kit manufacturer's protocol (NucleoSpin Tissue kit, Machenrey-Nagel, Germany). Briefly, rat aorta, carotid artery, and cultured VSM cells were treated with proteinase K at 56°C for 1–3 h (cells) or overnight (tissues), and homogenized samples were lysed at 70°C for 10 min. Ethanol (33% of total volume) was used to precipitate DNA followed by DNA binding to a NucleoSpin column. After extensive washes, DNA was eluted. The concentration of gDNA was determined by Nanodrop (Invitrogen).
Methylated DNA immunoprecipitation.
Methylated DNA fragments were immunoprecipitated (MeDIP) followed by quantitative PCR (qPCR). The assay was performed using the manufacturer's protocol (Active Motif). Briefly, 20 μg genomic DNA isolated from VSM cells or arterial medial layers was fragmented to approximate 500 base pairs (bp) by sonication. The fragmented gDNA was denatured by incubation at 95°C for 10 min and then immediately iced to keep DNA as a single-stranded form with good accessibility to antibody. Anti-5-methylation cytosine antibody and magnetic protein G beads were mixed with the fragmented DNA (1 μg) and incubated overnight with end-to-end rotation at 4°C. After extensive washes, the DNA that bound to anti-5-mc antibody was eluted, and the enrichment of genes was examined by qPCR. For normalization, input DNA was 0.1 μg single-strand fragmented DNA and used as template for downstream qPCR. The MeDIP primers are listed below: Camk2g forward: 5′-GCTCTCCTGATAAAACCGCA-3′ and reverse: 5′-TGTACACTCGGGAGATGCCC-3′; Camk2d forward: 5′-TGGGAGCAGTAAACACGAAC and reverse: 5′-ACGGAAAGATGAGGGACAAC; and Myh11 forward: 5′-GGCTAAGGGACACAGAGATTG-3′ and reverse: 5′-CATCGTTGAAAAGCGGGTTG-3′.
Statistical analysis.
Quantitative data are presented as means ± SE. Student's unpaired t test was performed in the comparisons between two groups. Comparisons among multiple groups were carried out using ANOVA with Dunnet or Bonferroni post hoc tests. All statistical analysis was performed using the GraphPad Prism 5 program. Significance is presented as P < 0.05, P < 0.01, and P < 0.001, and n indicates the number of independent experiments.
RESULTS
CaMKIIγ is downregulated in synthetic phenotype VSM.
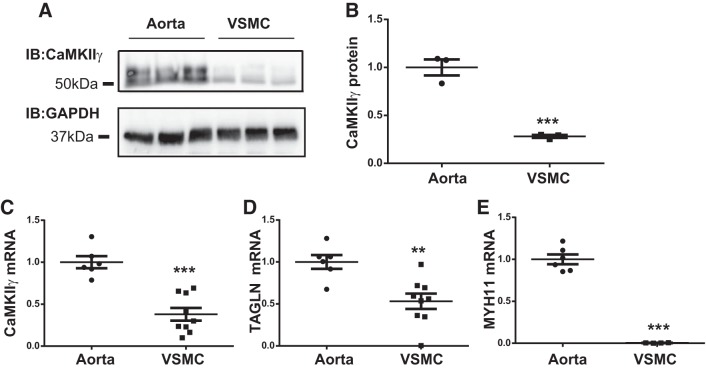
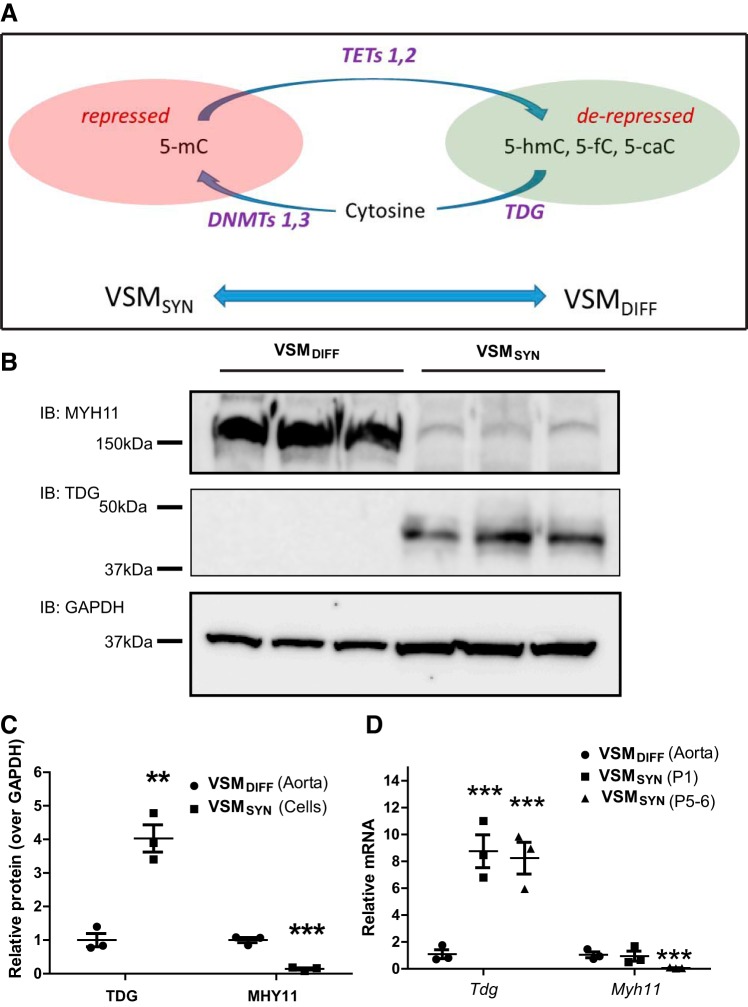
Previously, our laboratory documented CaMKIIγ expression dynamics in VSM following vascular injury in rat and mouse models (13, 14, 32) and used smooth muscle-specific Camk2g gene knockout to elucidate a protective role of CaMKIIγ against vascular injury-induced neointimal hyperplasia (32). In Fig. 1, we compare both CaMKIIγ protein and mRNA expression in aortic medial VSMDiff and in aortic VSMSyn in primary culture. CaMKIIγ signals appeared as a doublet, consistent with expression of γC (~52 kDa) and γB (~54 kDa) splice variants as previously demonstrated in VSM (13). Both CaMKIIγ protein (Fig. 1, A and B) and Camk2g mRNA (Fig. 1C) were reduced ~70% in VSMSyn cells compared with VSMDiff. As positive controls, the smooth muscle differentiation makers, smooth muscle Myh11 and transgelin or SM22Mα, were markedly decreased in cultured VSMSyn cells compared with VSMDiff (Fig. 1, D and E).
Fig. 1.
Differential Ca2+/calmodulin-dependent protein kinase IIγ (CaMKIIγ) expression in differentiated vascular smooth muscle (VSMDiff) and VSM synthetic phenotype (VSMSyn). Rat aorta medial layer VSM cells (VSMCs) were enzymatically dispersed and cultured for 3–6 passages. A and B: CaMKIIγ and GAPDH were analyzed by SDS-PAGE and immunoblotting (IB) comparing intact aorta medial layer VSM (VSMDiff) and primarily cultured aortic VSM cells (VSMSyn). C–E: quantitative PCR was used to measure Camk2g, Tagln, myosin heavy chain (Myh11), and Gapdh mRNA. All values were normalized over GAPDH expression. Values are shown as means ± SE, n = 3–7, and analyzed by unpaired t test. **P < 0.01 and ***P < 0.001.
CaMKIIγ is upregulated in VSMSyn after global cytosine demethylation.
To understand mechanisms underlying VSM phenotype-specific CaMKIIγ expression, we tested the hypothesis that DNA cytosine methylation/demethylation might regulate expression. Consistent with this possibility, inspection of the Camk2g gene revealed the presence of CpG islands, potential genomic DNA regulatory regions, including in the predicted promoter region of the human Camk2g gene and rat Camk2g genes (Supplemental Figs. S1 and S2, respectively; University of California, Santa Cruz genome browser; supplemental material may be found at https://doi.org/10.6084/m9.figshare.8193995).
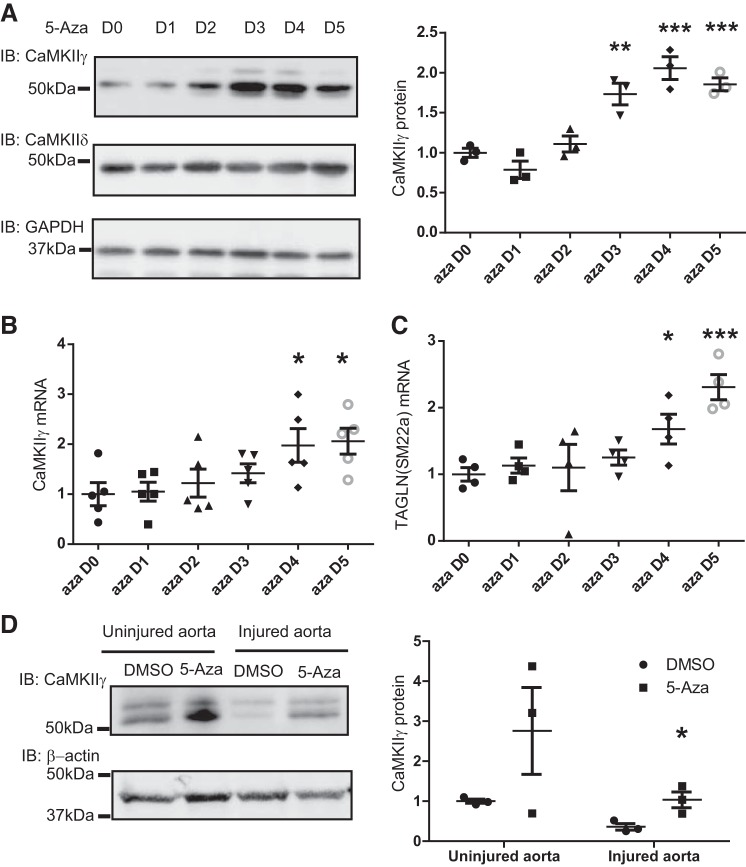
Cultured rat VSMSyn cells were treated with the DNA methyltransferase (DNMT) inhibitor 5-Aza for 1–5 days to globally inhibit DNA cytosine methylation (5). Both CaMKIIγ mRNA and protein levels were significantly increased up to twofold after 4 days of 5-Aza treatment (Fig. 2, A and B), suggesting that the Camk2g promoter is normally methylated in VSMSyn cells with a repressive effect on gene expression. CaMKIIδ expression was unaffected by 5-Aza treatment (Fig. 2A).
Fig. 2.
Global DNA demethylation with the DNA methyltransferase inhibitor 5-Aza-2'-deoxycytidine (5-Aza) increases Ca2+/calmodulin-dependent protein kinase IIγ (CaMKIIγ) expression in vascular smooth muscle synthetic phenotype (VSMSyn) cells. Cultured VSMSyn cells were exposed to 5-Aza (10 μM) for 0 to 5 days (D0–D5). A: CaMKIIγ and CaMKIIδ protein levels were examined by Western immunoblot (IB) using CaMKII isoform-specific antibodies and quantified by densitometric scanning (right). B and C: quantitative PCR was performed to determine Camk2g and Tagln mRNA levels. n = 3–5 analyzed by one-way ANOVA with Dunnett’s post hoc test. D: Rat thoracic aortas were freshly harvested and endothelial-injured or uninjured aortas cultured ex vivo with or without the presence of 10 μM 5-Aza for 5 days. CaMKIIγ protein content was tested by Western blot. n = 3 analyzed by 2-way ANOVA with Tukey post hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001.
To confirm the effect of 5-Aza on CaMKIIγ expression in medial wall VSM in situ, we used ex vivo organ culture of aortic tissue, in which we predicted a decrease in CaMKIIγ expression as the VSMDiff cells dedifferentiated in culture. One group of tissues was also deendothelialized to exacerbate the vascular “injury” simulated by organ culture (9). CaMKIIγ expression was markedly reduced in deendothelialized tissues compared with intact tissues after 5 days in organ culture (Fig. 2D, lane 1 vs. 3). Administration of 5-Aza significantly increased CaMKIIγ expression ~threefold in deendothelialized samples. A similar trend was observed in intact aortic segments treated with 5-Aza but did not reach statistical significance (Fig. 2D). Interestingly, the induced expression was most marked in the smaller CaMKIIγ variant. Although we do not understand the mechanism for this, cytosine DNA methylation has been shown to regulate some alternative splicing events (35).
Camk2g promoter is methylated in VSMSyn cells.
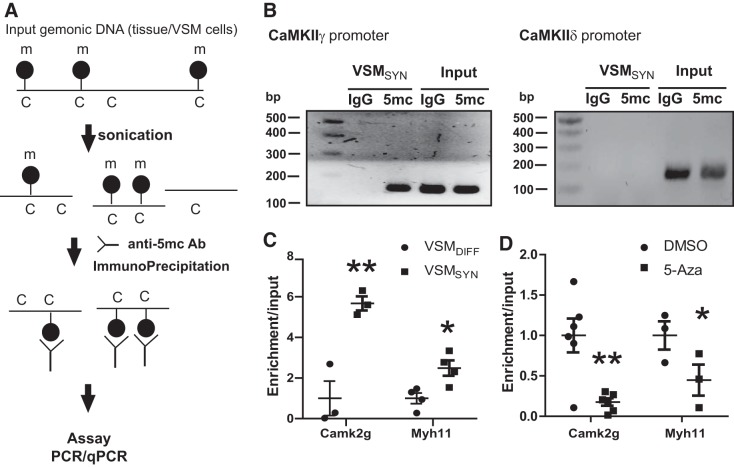
CaMKIIγ expression induced by 5-Aza could be a direct effect by decreasing Camk2g promoter methylation or an indirect effect of other 5-Aza targets regulating CaMKIIγ expression. Therefore, we directly tested promoter methylation in rat VSM using methylated gDNA immunoprecipitation (MeDIP) and specific primers to quantify the presence of immunoprecipitated Camk2g promoter sequence by qPCR (Fig. 3A). A Camk2g promoter region (−621−~472) containing predicted CpG islands was successfully amplified in cultured rat aortic VSMSyn cell input DNA (Fig. 3B, left), as well as in methylcytosine immunoprecipitations (Fig. 3B, left), indicating some degree of promoter methylation correlating with low levels of CaMKIIγ protein in these cells. The same strategy failed to amplify the Camk2d promoter region (Fig. 3B, right), suggesting that the Camk2d promoter is likely to be unmethylated. With the use of this assay, methylated Camk2g and Myh11 (positive control) promoters were significantly enriched sixfold and twofold, respectively, in the cultured VSMSyn cells when compared with VSMDiff from aortic tissue (Fig. 3C). Treatment of VSMSyn cells with the DNMT inhibitor 5-Aza significantly decreased Camk2g and Myh11 promoter methylation by 75 and 50%, respectively (Fig. 3D).
Fig. 3.
Camk2g promoter is methylated in vascular smooth muscle (VSM) synthetic phenotype (VSMSyn) cells. A: diagram of methylation DNA immunoprecipitation procedure. Genomic DNA (gDNA) is extracted from tissues or cultured cells and fragmented to approximate 500 bp by sonication. A specific methylated cytosine antibody is used to pull down methylated gDNA fragments and the presence and enrichment of specific genes are examined by quantitative PCR (qPCR). B: gDNA extracted from cultured VSMSyn cells was fragmented and immunoprecipitated. The presence of Camk2 isoform promoters in the methylated gDNA fraction was tested by PCR amplification of −621 to −472 in the Camk2g promoter and −577 to −442 in the Camk2d promoter. C: enrichment of Camk2g and myosin heavy chain (Myh11) in the methylated gDNA fractions was compared between aorta tissue with differentiated VSM (VSMDiff) and cultured VSM cells (VSMSyn). D: VSMSyn cells were treated with 5-Aza-2'-deoxycytidine (5-Aza) (10 μM) for 5 days and the enrichment of methylated Camk2g and Myh11 compared with untreated and treated cells. Values are shown as means ± SE, n = 3–4, and analyzed by 2-way ANOVA with Tukey post hoc test. *P < 0.05 and **P < 0.01. Ca2+/calmodulin-dependent protein kinase IIγ, CaMKIIγ.
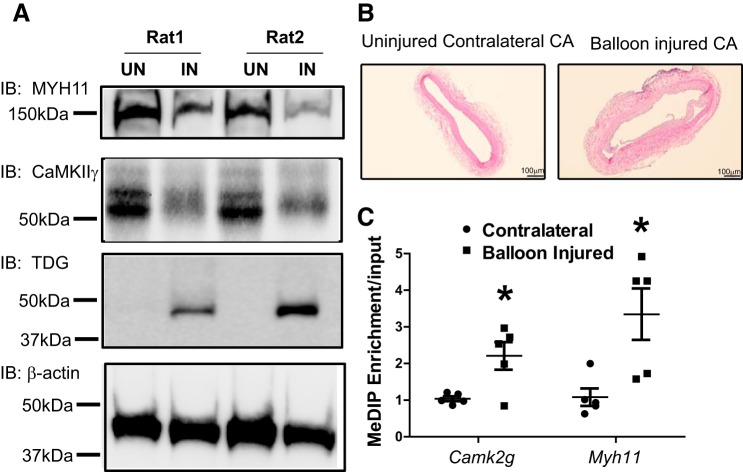
To study Camk2g promoter methylation during VSM phenotype switching in vivo, we employed the well-characterized rat carotid artery balloon injury model. As expected, based on previous studies, expression of CaMKIIγ and MYH11 were reduced in the injured left carotid artery compared with the uninjured contralateral control artery 14 days after injury (Fig. 4A), correlating with intimal hyperplasia (Fig. 4B). Camk2g and Myh11 promoter methylation were significantly enriched in the injured arteries (Fig. 4C), suggesting that cytosine methylation controls Camk2g and Myh11 expression during the VSM phenotype switch in vivo. Overall, these results indicate that Camk2g promoter cytosine methylation correlates inversely with expression, and increased promoter methylation contributes to decreased CaMKIIγ expression in VSMSyn cells.
Fig. 4.
Camk2g promoter is methylated in vascular smooth muscle synthetic phenotype (VSMSyn) cells in vivo. A: rat carotid arteries were intraluminally injured, and 14 days later, protein levels of smooth muscle myosin heavy chain (MYH11), Ca2+/calmodulin-dependent protein kinase IIγ (CaMKIIγ), thymine DNA glycosylase (TDG), and β-actin were measured by SDS-PAGE and Western immunoblotting (IB) using specific antibodies in uninjured contralateral (UN) and balloon-injured (IN) carotid arteries. B: representative sections were examined by hematoxylin-eosin staining. C: genomic DNA was extracted from uninjured and balloon-injured left CAs 14 days after injury. Enrichment of methylated Camk2g and Myh11 promoters was determined using methylated DNA immunoprecipitation (MeDIP) followed by quantitative PCR. Values are shown as means ± SE, n = 5, and analyzed by paired t test. *P < 0.05.
TDG is upregulated in VSMSyn suppressing CaMKIIγ expression.
DNA methylation dynamics are regulated by DNMTs, which methylate DNA cytosine, TETs that “demethylate” cytosine by catalyzing formation of 5-hmC, 5-fC, and 5-caC, and by base excision and repair mechanisms, including the enzyme TDG, that return modified cytosine to native cytosine (17, 39, 40). Global hypomethylation is associated with the contractile VSMDiff phenotype (22). TET2 is highly expressed in VSMDiff and significantly reduced in VSMSyn cells induced in a rodent vascular injury model and in human atherosclerotic coronary artery (21). As illustrated in the model depicted in Fig. 5A, switching from a hypomethylated state in a given gene in VSMDiff to a hypermethylated state in VSMSyn cells requires regeneration of unmodified cytosines to serve as substrates for DNMTs. TDG is one prominent DNA damage repair factor capable of excising 5-fmC and 5-caC from DNA (7) and can complex with DNMT3a/b to facilitate remethylation (20).
Fig. 5.
Thymine DNA glycosylase (TDG) is upregulated in vascular smooth muscle (VSM) synthetic phenotype (VSMSyn). A: diagram of a switch from a hypomethylated state in differential VSM (VSMDiff) to a hypermethylated state in VSMSyn cells following vascular injury or with vasculo-proliferative diseases. Oxidation of 5-methylcytosine (5-mC) by ten-eleven translocations (TETs) generates 5-hydroxymethylcytosine (5-hmc), 5-formylcytosine (5-fc), and 5-caC in VSMDiff. TDG is one prominent DNA damage repair factor capable of excising 5-fmC and 5-caC from DNA and can complex with DNA methyltransferase 3a/b to facilitate remethylation in VSMSyn. B: immunoblots (IB) of myosin heavy chain (MYH) 11, TDG, and GAPDH in rat aortic VSMDiff cells and cultured VSMSyn cells from 3 individual animals. C: quantitation of immunoblots in B. D: rat aorta medial layer VSM cells (VSMDiff) were enzymatically dispersed and cultured for 1–6 passages (VSMSyn). Quantitative PCR was employed to measure mRNA levels of Tdg, Myh11, and Gapdh in intact aorta and derived cells. Values were normalized over Gapdh and expressed as means ± SE. n = 3 different animals analyzed by unpaired t test for C or 2-way ANOVA for D. **P < 0.01 and ***P < 0.001.
We compared TDG expression (Fig. 5, B and C) and mRNA levels of Dnmt1, 3a, 3b, and Tet1, 2, 3 in VSMDiff from rat aorta and cultured VSMSyn cells from the same aortas (Supplemental Fig. S3). TDG was nearly undetectable in aorta containing VSMDiff by Western blot analysis but obviously detected in the cultured VSMSyn cells. Tdg mRNA levels were increased ~eightfold in VSMSyn cells compared with VSMDiff (Fig. 5D). Repeated passage had no additional effects on VSM TDG expression in culture. Compared with the levels in VSMDiff, DNMT1 mRNA expression was increased nearly twofold, but DNMT3a, Tet1, and Tet2 mRNA levels were all reduced, ranging from 50 to 80% in VSMSyn (Supplemental Fig. S3). Decreased TET2 expression in VSMSyn compared with VSMDiff is consistent with previously reported results (21). MYH11 expression levels are shown in the same samples to confirm the phenotype switch. Markedly induced arterial TDG expression was also observed in vivo in response to vascular injury (Fig. 4A), correlating with downregulation of CaMKIIγ, and the switch to the VSMSyn phenotype.
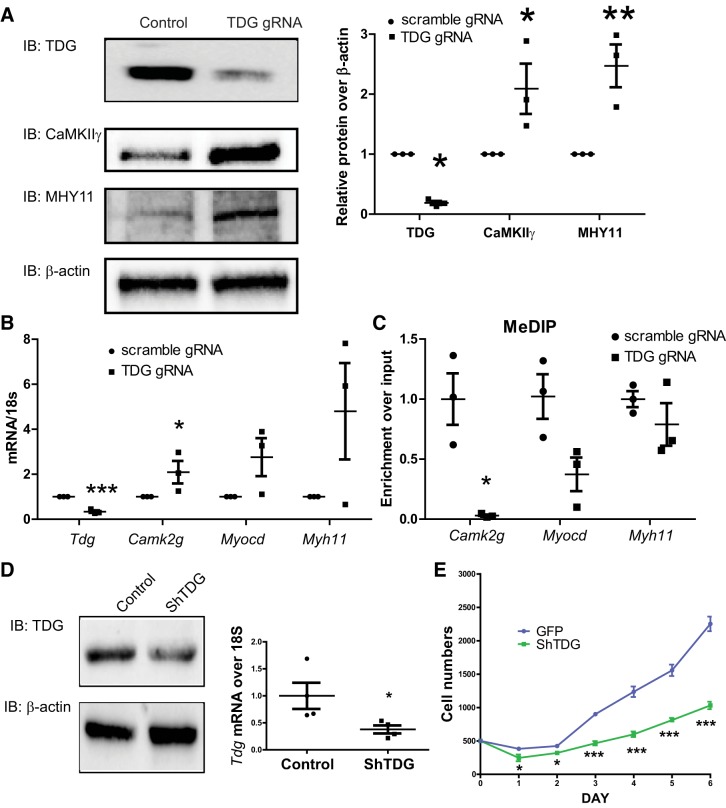
To further investigate TDG function in VSMSyn cells, we employed CRISPR/Cas9 technology with gRNAs targeting rat Tdg exon 3 to suppress TDG expression in the cultured VSM cells. TDG protein (Fig. 6A) and mRNA (Fig. 6B) were reduced 80% by the gRNA-Tdg/cas9 approach delivered by lentiviral vectors. Interestingly, CaMKIIγ, MYH11, and Myocd expression increased two- to sixfold in the TDG-silenced VSM cells (Fig. 6, A and B). Silencing Tdg dramatically reduced methylated Camk2g promoter enrichment. A similar trend of decreased methylated Myocd promoter enrichment was also observed but did not reach statistical significance. Enrichment of the Myh11 promoter was not altered by silencing TDG.
Fig. 6.
Thymine DNA glycosylase (TDG) suppression results in Ca2+/calmodulin-dependent protein kinase IIγ (CaMKIIγ) promoter hypomethylation and increased CaMKIIγ expression in vascular smooth muscle synthetic phenotype (VSMSyn). Crispr/Cas9 all-in-one constructs expressing Cas9, scrambled guide RNA (gRNA), or TDG gRNA targeting Tdg exon3 were introduced into VSMSyn cells using lentivirus (A–C). Three days after infection, cells were collected for analysis of protein, mRNA, and genomic DNA. A: protein levels of TDG, CaMKIIγ, myosin heavy chain (MYH) 11, and β-actin were analyzed using SDS-PAGE and Western blotting with specific antibodies. B: quantitative PCR (qPCR) was used to determine Tdg, Camk2g, Myocd, and Myh11 mRNA expression. C: total genomic DNA was extracted from cultured cells infected with lentivirus-expressing scrambled gRNA and TDG gRNA. Methylated genomic DNA was purified by methylated DNA immunoprecipitation (MeDIP), and the enrichment of Camk2g, Myocd, and Myh11 promoters was analyzed by qPCR and specific primers. D: control lentivirus-expressing green fluorescent protein and scrambled RNA (control or GFP) or shRNA targeting TDG shTDG was used to infect VSMSyn cells for 3 days. The knockdown efficiency was examined by immunoblotting and qPCR. E: infected cells were resuspended and replated with identical cell numbers 3 days after lentivirus infection. The starting cell number was 500 for each sample, and cell numbers were counted every 24 h for a total of 6 days. All quantification was normalized over β-actin. Values shown are means ± SE. n = 3–4 analyzed by unpaired t test and 2-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
Effects of TDG on VSMSyn cell proliferation and injury-induced neointima formation.
To assess TDG function in VSM proliferation and injury-induced neointimal formation, we used conventional shRNA targeting by lentivirus (85–91 MOI) to inhibit TDG expression in vitro (Fig. 6) and in vivo (Fig. 7) following vascular injury. Both TDG protein and mRNA were significantly reduced by lentiviral constructs expressing shRNA targeting Tdg in cultured VSMSyn (Fig. 6D). Interestingly, suppressing TDG expression by shRNA dramatically inhibited VSMSyn proliferation (Fig. 6E). Consistently, we also observed inhibition of proliferation of VSMSyn cells infected with lentivirus-expressing Cas9 and gRNA targeting Tdg exon3 (data not shown).
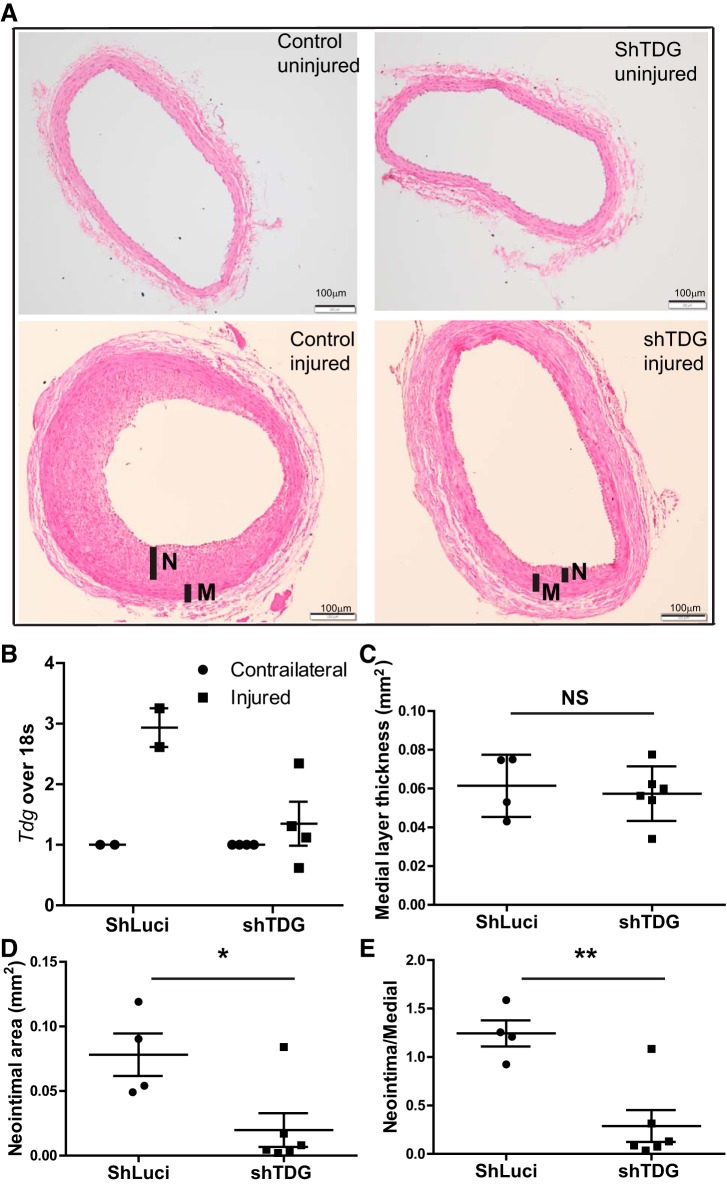
Fig. 7.
Thymine DNA glycosylase (TDG) suppression attenuates injury-induced neointima formation in vivo. Rat carotid artery was balloon injured and locally infected with lentivirus-encoding scrambled RNA or TDG shRNA. After 14 days, the carotid arteries were collected for analysis. A: representative 8-μm-fixed, hematoxylin and eosin-stained sections. B: analysis of Tdg mRNA expression by quantitative PCR normalized to 18S rRNA (n = 2–4). C–E: quantitative morphometric analysis (n = 4–6) using ImageJ software of medial layer area (C), neointimal area (D), and neointimal/medial area ratios. Individual values are shown with means ± SE. *P < 0.05 and **P < 0.01 by unpaired t test. M, medial layer area; N, neointimal area; NS, not significant.
Injury-induced neointima hyperplasia is mainly a consequence of VSMSyn cell proliferation (12). Since we demonstrated that silencing TDG significantly inhibited VSM proliferation in vitro, we tested effects of silencing TDG on vascular remodeling in vivo following rat carotid artery balloon injury (Fig. 7A). ShRNA targeting Tdg was introduced into the medial wall by lentiviral (1.5~1.65 × 108 infectious units in 30 μL) transduction immediately after carotid injury to prevent injury-induced TDG upregulation using previously published approaches (24). Consistent with our finding of TDG induction in cultured VSMSyn cells (Fig. 4), TDG expression was significantly elevated by balloon injury in the carotid arteries infected with control lentivirus-expressing Luciferase (Fig. 7B). On the other hand, shTdg transduction strongly inhibited injury-induced TDG upregulation. Functionally, shTdg had no effect on medial wall areas (Fig. 7C) but dramatically decreased neointimal areas and neointimal/medial wall area ratios greater than 90%.
DISCUSSION
Multifunctional CaMKII has been studied extensively in VSM (reviewed in Ref. 36). CaMKIIδ and γ isoforms are coexpressed in VSMDiff and are reciprocally regulated as a function of phenotype switching following vascular injury or cell culture (13, 32). Although CaMKIIδ has been reported to be regulated by miR-145 and miR-30 family members (6, 24), nothing is reported about CaMKIIγ regulation in VSM, and very little is known from other systems. Here we present evidence that CaMKIIγ expression is regulated by promoter cytosine methylation/demethylation in the context of VSM phenotype switching. Moreover, for the first time we show that TDG plays a key role in regulating cytosine methylation dynamics during VSM phenotype switching, regulating CaMKIIγ expression, and, independently, VSM marker gene expression, with potentially broad effects on VSM phenotype and function.
Our previous studies showed that CaMKIIγ expression was rapidly downregulated in response to VSM dedifferentiation induced by vascular injury or primary cell culture (13, 32). Although posttranscriptional regulation of CaMKIIγ expression by miR-219 with reduced protein expression has been reported in the spinal cord (2, 29), in preliminary studies we found no evidence for this mechanism regulating CaMKIIγ expression in VSMSyn cells (data not shown). Kim et al. (15) reported that the CAMK2B promoter is hypermethylated in breast cancer cells and that treatment with 5-Aza, an inhibitor of DNA cytosine methylation (5), significantly recovered CaMKIIβ expression. Inspection of the rat Camk2d and Camk2g gene promoters indicated CpG islands, potential sites for regulatory DNA cytosine methylations, suggesting that this mode of regulation could be common to multiple CaMKII isoforms. In fact, treatment of VSMSyn cells with 5-Aza strongly induced expression of both CaMKIIγ protein and mRNA levels in vitro and ex vivo, consistent with a role for cytosine methylation in regulating the Camk2g promoter in VSM (Fig. 2). The mechanism underlying 5-Aza inhibition of DNA methylation is by competition with cytosine during DNA synthesis (5). In our experiments, it took 3–4 days for VSM cells to upregulate CaMKIIγ expression after exposure to 5-Aza, which could be explained by gradual incorporation into newly synthesized DNA during VSM cell mitosis.
As noted in results, 5-Aza treatment appeared to change the relative expression levels of two CaMKIIγ variants (Fig. 2D), consistent with the possibility that cytosine methylation can regulate some alternative splicing events (35). We note that the human Camk2g gene contains a CpG island that covers an alternatively spliced exon, which is differentially methylated across cell types (Supplemental Fig. S1). Interestingly, 5-Aza treatment had no effect on CaMKIIδ expression in our experiments, suggesting that the previously demonstrated regulation by miRNAs might be a dominant mode of regulation for this isoform (24).
DNA cytosine methylation is pervasive in the human genome (3). However, CpG islands, defined by short stretches of genomic DNA with particularly enriched CG content, in promoter regions of active genes are generally hypomethylated, and selective methylation of relatively few key cytosine residues may repress gene transcription by modifying transcription factor accessibility (16, 31). To directly evaluate promoter methylation, we used a methylcytosine immunoprecipitation assay (MeDIP) with fragmented gDNA from VSM as input and specific primers targeting promoters of genes of interest, including Camk2g, to amplify the precipitated template. Importantly, this assay is reported to be specific for 5-mC (1, 33) compared with 5-hmC, 5-fC, and 5-caC, which are generally considered nonrepressive and/or potentially positive regulators of gene transcription. Using MeDIP, we found increased Camk2g promoter methylation, correlating with decreased CaMKIIγ expression, in dedifferentiated VSMSyn cells compared with VSMDiff where the promoter is demethylated and CaMKIIγ expression is high. Conversely, inhibiting methylation in VSMSyn cells with 5-Aza resulted in marked decreases in Camk2g promoter methylation and increased CaMKIIγ expression. Importantly, similar shifts in CaMKIIγ methylation and expression were observed in vivo, correlating with VSM phenotype switching following balloon catheter-induced carotid artery injury. As we demonstrated previously, CaMKIIγ expression decreases in this model following injury, and as shown here, this correlates with increased Camk2g promoter methylation. In parallel MeDIP analyses, the Camk2d promoter was not amplified from cultured VSM. This and the lack of effect of 5-Aza treatment on CaMKIIδ expression in cultured VSM suggest that the Camk2d promoter is demethylated in VSMSyn cells.
Although sequence conservation in specific CpG islands across species is low, CpG islands are identifiable in the Camk2g promoter region of multiple species. Interestingly, sequence identity analysis of Camk2g using Clustal 2.1 showed that human and mouse share 80% identity of the CpG islands located in the promoter region and the first exon and intron, and the sequence of rat CpG islands in these regions is 42% identical with human and mouse. Therefore, DNA methylation-dependent regulation of CaMKIIγ expression found here in rat VSM models might extend to humans, a hypothesis that requires further investigation.
MYOCD is the master transcriptional regulator of VSM differentiation (4). CaMKIIγ expression in cultured VSMSyn cells can be induced by MYOCD overexpression, albeit in an SRF-independent mechanism (32). The Myocd promoter itself can be regulated by methylation/demethylation (45), and 5-Aza treatment of cultured VSM cells has been reported to increase Myocd mRNA expression (45). Therefore, at this point we cannot conclude how much of the 5-Aza induced expression of CaMKIIγ in VSMSyn cells shown here can be attributed directly to Camk2g promoter demethylation compared with possible indirect effects of MYOCD through an SRF-independent mechanism.
A high degree of demethylated Camk2g promoter in intact VSMDiff is consistent with previous studies documenting high levels of TET2 expression regulating expression of key smooth muscle markers in VSMDiff compared with VSMSyn cells (21). We confirmed relative promoter demethylation of Myh11, considered a definitive smooth muscle marker, in VSMDiff compared with VSMSyn cells in culture or induced following vascular injury in vivo. Although induced expression of TET family proteins and promoter demethylation in VSMDiff can at least partly explain high levels of VSM marker gene expression, remethylation and suppressed expression of these genes in VSMSyn cells requires a process of active demethylation including enzymatic removal of 5-hmC, 5-fC, and 5-caC that result from actions of TETs, followed by cytosine methylation mediated by DMNTs (Fig. 5). TDG is a key enzyme capable of removing 5-fC- and 5-caC-modified cytosines (25). TDG has been shown to be essential for development (7) and plays a key role in regulating intestinal tumorigenesis (41). TDG was identified to physically interact with MYOCD and negatively regulate its transactivating activity in vitro (44). However, TDG expression dynamics and function are not known in VSM.
A major finding in this study is that TDG expression is regulated in VSM in association with phenotype with very low expression in VSMDiff compared with VSMSyn cells. Upon VSM cell dispersion, TDG mRNA increased rapidly within primary culture and first passage and was maintained at high expression levels with subsequent passages. Moreover, TDG protein expression in vivo was barely detected in VSMDiff isolated from the vascular wall but was strongly expressed in injured arteries containing significant neointimal hyperplasia and VSMSyn cells. This inverse correlation between TDG expression and VSM differentiation is consistent with previously reported results using serum deprivation as a protocol to induce partial differentiation of cultured VSM cells and in which TDG expression was found to inversely correlate with expression of VSM contractile protein marker genes (44). In our hands, silencing endogenous TDG expression in VSMSyn cells with shRNA or by CRISPR/Cas9 approaches resulted in increased CaMKIIγ and contractile protein marker expression, coincident with Camk2g and Myocd promoter methylation. In interpreting these results mechanistically, we cannot exclude the possibility that induced expression of contractile genes after TDG silencing might result, at least in part, from decreased physical interaction with MYOCD resulting in de-repression of its transactivating effects (44).
Based on the effect of 5-Aza to decrease Myh11 promoter methylation (Fig. 3D), we were surprised to find that TDG knockdown had no significant effect on Myh11 promoter methylation (Fig. 6C). One possible explanation for this is that 5-Aza may affect cytosine methylation globally compared with TDG, which likely acts locally and perhaps specifically within DNA binding complexes (5, 18). If so, this suggests that TDG may not be a primary regulator of MYH11 expression and that the observed increase in MYH11 expression following TDG knockdown is attributable to other mechanisms, for example increased MYOCD/SRF activity.
The functional consequences of TDG knockdown in vitro was a strong inhibition of VSMSyn cell proliferation. Administration of shTDG lentiviral constructs in vivo immediately following vascular injury inhibited TDG upregulation and strongly inhibited neointimal hyperplasia consistent with a requirement for TDG in VSM phenotype switching and/or subsequent proliferation of VSMSyn cells. Similarly, administration of 5-Aza to mice inhibited atherosclerotic plaque development and carotid ligation-induced neointimal hyperplasia, indicating a role for DNMT activity in VSM phenotype switching (45). Together, these results are consistent with a role for cytosine remethylation in promoting VSM phenotype switching from VSMDiff to VSMSyn. It is unlikely that upregulation of CaMKIIγ itself could affect contractile gene expression or phenotype switching, as our previous studies in smooth muscle CaMKIIγ knockouts indicated no effect on baseline contractile gene expression (32). Thus, although CaMKIIγ associates with the VSMDiff phenotype, we do not believe it is involved in regulating phenotype per se, but more likely VSMDiff functions such as contractility (36). Because CaMKIIγ overexpression is growth inhibitory in VSM in vitro and in vivo (32), it is also possible that growth inhibitory effects of TDG silencing are mediated by upregulation of CaMKIIγ. Because TDG knockdown would affect the methylation status of diverse genes, we expect that is mechanism is unlikely, but this requires further investigation.
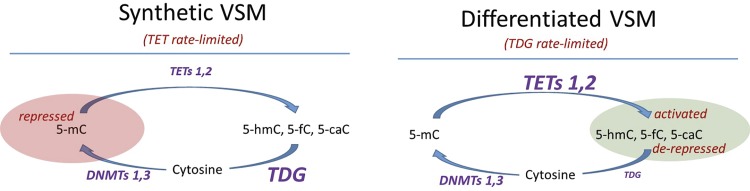
Based on previous studies of TET family function and dynamics in VSM (21) and our results, we have integrated TDG expression into a proposed model linking cytosine methylation/demethylation dynamics to VSM phenotype (Fig. 8). In proliferative synthetic phenotype VSM cells, relatively high expression of TDG and DNMTs compared with TETs results in a TET rate-limited state favoring accumulation of methylated cytosine in specific gene promoters, including Camk2g and contractile protein genes, suppressing their expression. Differentiation of VSM is associated with increased expression of TETs (21) and a strong reduction in TDG expression through currently unknown mechanisms. This results in a TDG rate-limited state that, coupled with cellular quiescence, leads to TET-mediated demethylation and accumulation of 5-hmC, 5-fC, and 5-caC modifications in these promoters, with the effect of enhanced expression of VSM contractile protein genes and other genes associated with the differentiated phenotype, exemplified here by CaMKIIγ. Dynamically switching back to a VSMSyn phenotype as a consequence of vascular disease or injury requires downregulation of TET expression, cell cycling to passively restore, and re-expression of TDG to actively restore, unmodified cytosine as substrates for DNMT with consequent remethylation and suppressed expression of specific genes. From this and previous studies and in the context of vascular injury and disease, mechanisms underlying DNMTs, TETs, and TDG expression and activity dynamics merit future investigation.
Fig. 8.
Model of DNA promoter cytosine methylation dynamics in vascular smooth muscle (VSM) phenotype switching. Left: in proliferative synthetic phenotype VSM cells, relatively high expression of thymine DNA glycosylase (TDG) compared with ten-eleven translocations (TETs) results in a TET rate-limited state favoring accumulation of methylated cytosine in specific genes, including Ca2+/calmodulin-dependent protein kinase IIγ and contractile protein genes, repressing their expression. Right: differentiation of VSM is associated with increased expression of TETs and a strong decrease in TDG expression, resulting in a TDG rate-limited state with TET-mediated demethylation and accumulation of 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-fC), and 5-carboxylcytosine (5-caC) modifications on the promoters of VSM-differentiated genes, leading to de-repression and/or activated expression. Dynamically switching back to a VSM synthetic phenotype as a consequence of vascular disease or injury requires downregulation of TET expression, cell cycling to passively restore and re-expression of TDG to actively restore, unmodified cytosine as substrates for DNA methyltransferase (DNMT) with consequent remethylation, and repressed expression of specific genes.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01-HL-049426 (to H. A. Singer), R56-HL-142807 (to D. Jourd'heuil), and R01-HL-139794 (to X. Long).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. and H.A.S. conceived and designed research; Y.L., D.V.S., W.Z., and F.L.J. performed experiments; Y.L. and H.A.S. analyzed data; Y.L., L.S., R.G., D.J., X.L., and H.A.S. interpreted results of experiments; Y.L. and H.A.S. prepared figures; Y.L. drafted manuscript; Y.L., R.G., and H.A.S. edited and revised manuscript; Y.L., L.S., D.V.S., R.G., W.Z., F.L.J., D.J., and H.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kathleen Martin, Yale School of Medicine, for generous gifts of plasmids for lentivirus production.
REFERENCES
- 1.Baker-Andresen D, Flavell CR, Li X, Bredy TW. Activation of BDNF signaling prevents the return of fear in female mice. Learn Mem 20: 237–240, 2013. doi: 10.1101/lm.029520.112. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res 118: 692–702, 2016. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev 16: 6–21, 2002. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345–1356, 2002. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 5.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21: 5483–5495, 2002. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 6.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460: 705–710, 2009. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, Abramowitz LK, Bartolomei MS, Rambow F, Bassi MR, Bruno T, Fanciulli M, Renner C, Klein-Szanto AJ, Matsumoto Y, Kobi D, Davidson I, Alberti C, Larue L, Bellacosa A. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 146: 67–79, 2011. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Findeisen HM, Kahles FK, Bruemmer D. Epigenetic regulation of vascular smooth muscle cell function in atherosclerosis. Curr Atheroscler Rep 15: 319, 2013. doi: 10.1007/s11883-013-0319-7. [DOI] [PubMed] [Google Scholar]
- 9.Finking G, Wolkenhauer M, Lenz C, Hanke H. Post-injury ex vivo model to investigate effects and toxicity of pharmacological treatment in rings of rabbit aortic vessels. ALTEX 17: 67–74, 2000. [PubMed] [Google Scholar]
- 10.Ginnan R, Pfleiderer PJ, Pumiglia K, Singer HA. PKC-delta and CaMKII-delta 2 mediate ATP-dependent activation of ERK1/2 in vascular smooth muscle. Am J Physiol Cell Physiol 286: C1281–C1289, 2004. doi: 10.1152/ajpcell.00202.2003. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto H, Hong S, Bhagwat AS, Zhang X, Cheng X. Excision of 5-hydroxymethyluracil and 5-carboxylcytosine by the thymine DNA glycosylase domain: its structural basis and implications for active DNA demethylation. Nucleic Acids Res 40: 10203–10214, 2012. doi: 10.1093/nar/gks845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herring BP, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell 6: 21, 2014. doi: 10.1186/2045-824X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.House SJ, Ginnan RG, Armstrong SE, Singer HA. Calcium/calmodulin-dependent protein kinase II-delta isoform regulation of vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol 292: C2276–C2287, 2007. doi: 10.1152/ajpcell.00606.2006. [DOI] [PubMed] [Google Scholar]
- 14.House SJ, Singer HA. CaMKII-delta isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol 28: 441–447, 2008. doi: 10.1161/ATVBAHA.107.156810. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Kim TW, Kim SJ. Downregulation of ARFGEF1 and CAMK2B by promoter hypermethylation in breast cancer cells. BMB Rep 44: 523–528, 2011. doi: 10.5483/BMBRep.2011.44.8.523. [DOI] [PubMed] [Google Scholar]
- 16.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 31: 89–97, 2006. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 502: 472–479, 2013. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavelle D, Saunthararajah Y, Desimone J. DNA methylation and mechanism of action of 5-azacytidine. Blood 111: 2485, 2008. doi: 10.1182/blood-2007-10-119867. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J Biol Chem 286: 7990–7999, 2011. doi: 10.1074/jbc.M110.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li YQ, Zhou PZ, Zheng XD, Walsh CP, Xu GL. Association of Dnmt3a and thymine DNA glycosylase links DNA methylation with base-excision repair. Nucleic Acids Res 35: 390–400, 2007. doi: 10.1093/nar/gkl1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128: 2047–2057, 2013. doi: 10.1161/CIRCULATIONAHA.113.002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu R, Leslie KL, Martin KA. Epigenetic regulation of smooth muscle cell plasticity. Biochim Biophys Acta 1849: 448–453, 2015. doi: 10.1016/j.bbagrm.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Sun LY, Singer DV, Ginnan R, Singer HA. CaMKIIδ-dependent inhibition of cAMP-response element-binding protein activity in vascular smooth muscle. J Biol Chem 288: 33519–33529, 2013. doi: 10.1074/jbc.M113.490870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YF, Spinelli A, Sun LY, Jiang M, Singer DV, Ginnan R, Saddouk FZ, Van Riper D, Singer HA. MicroRNA-30 inhibits neointimal hyperplasia by targeting Ca(2+)/calmodulin-dependent protein kinase IIδ (CaMKIIδ). Sci Rep 6: 26166, 2016. doi: 10.1038/srep26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiti A, Drohat AC. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. J Biol Chem 286: 35334–35338, 2011. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv 4: 104–111, 2011. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 28.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 29.Pan Z, Zhu LJ, Li YQ, Hao LY, Yin C, Yang JX, Guo Y, Zhang S, Hua L, Xue ZY, Zhang H, Cao JL. Epigenetic modification of spinal miR-219 expression regulates chronic inflammation pain by targeting CaMKIIγ. J Neurosci 34: 9476–9483, 2014. doi: 10.1523/JNEUROSCI.5346-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of vascular smooth muscle cell migration by calcium/ calmodulin-dependent protein kinase II-delta 2. Am J Physiol Cell Physiol 286: C1238–C1245, 2004. doi: 10.1152/ajpcell.00536.2003. [DOI] [PubMed] [Google Scholar]
- 31.Razin A, Cedar H. DNA methylation and gene expression. Microbiol Rev 55: 451–458, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saddouk FZ, Sun LY, Liu YF, Jiang M, Singer DV, Backs J, Van Riper D, Ginnan R, Schwarz JJ, Singer HA. Ca2+/calmodulin-dependent protein kinase II-γ (CaMKIIγ) negatively regulates vascular smooth muscle cell proliferation and vascular remodeling. FASEB J 30: 1051–1064, 2016. doi: 10.1096/fj.15-279158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Edelkraut U, Hoffmann A, Daniel G, Spengler D. Zac1 regulates astroglial differentiation of neural stem cells through Socs3. Stem Cells 31: 1621–1632, 2013. doi: 10.1002/stem.1405. [DOI] [PubMed] [Google Scholar]
- 34.Scott JA, Xie L, Li H, Li W, He JB, Sanders PN, Carter AB, Backs J, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. Am J Physiol Heart Circ Physiol 302: H1953–H1964, 2012. doi: 10.1152/ajpheart.00978.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shayevitch R, Askayo D, Keydar I, Ast G. The importance of DNA methylation of exons on alternative splicing. RNA 24: 1351–1362, 2018. doi: 10.1261/rna.064865.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer HA. Ca2+/calmodulin-dependent protein kinase II function in vascular remodelling. J Physiol 590: 1349–1356, 2012. doi: 10.1113/jphysiol.2011.222232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer HA, Benscoter HA, Schworer CM. Novel Ca2+/calmodulin-dependent protein kinase II gamma-subunit variants expressed in vascular smooth muscle, brain, and cardiomyocytes. J Biol Chem 272: 9393–9400, 1997. doi: 10.1074/jbc.272.14.9393. [DOI] [PubMed] [Google Scholar]
- 38.Wierda RJ, Geutskens SB, Jukema JW, Quax PH, van den Elsen PJ. Epigenetics in atherosclerosis and inflammation. J Cell Mol Med 14, 6A: 1225–1240, 2010. doi: 10.1111/j.1582-4934.2010.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev 25: 2436–2452, 2011. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet 18: 517–534, 2017. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Cortellino S, Tricarico R, Chang WC, Scher G, Devarajan K, Slifker M, Moore R, Bassi MR, Caretti E, Clapper M, Cooper H, Bellacosa A. Thymine DNA Glycosylase (TDG) is involved in the pathogenesis of intestinal tumors with reduced APC expression. Oncotarget 8: 89988–89997, 2017. doi: 10.18632/oncotarget.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang P, Huang B, Xu X, Sessa WC. Ten-eleven translocation (Tet) and thymine DNA glycosylase (TDG), components of the demethylation pathway, are direct targets of miRNA-29a. Biochem Biophys Res Commun 437: 368–373, 2013. doi: 10.1016/j.bbrc.2013.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Trebak M. Vascular balloon injury and intraluminal administration in rat carotid artery. J Vis Exp (94): 52045, 2014. doi: 10.3791/52045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou J, Blue EK, Hu G, Herring BP. Thymine DNA glycosylase represses myocardin-induced smooth muscle cell differentiation by competing with serum response factor for myocardin binding. J Biol Chem 283: 35383–35392, 2008. doi: 10.1074/jbc.M805489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang J, Luan P, Li H, Wang K, Zhang P, Xu Y, Peng W. The yin-yang dynamics of DNA methylation is the key regulator for smooth muscle cell phenotype switch and vascular remodeling. Arterioscler Thromb Vasc Biol 37: 84–97, 2017. doi: 10.1161/ATVBAHA.116.307923. [DOI] [PubMed] [Google Scholar]