Abstract
Insulin modulates vasomotor tone through vasodilator and vasoconstrictor signaling pathways. The purpose of the present work was to determine whether insulin-stimulated vasoconstriction is a pathophysiological phenomenon that can result from a combination of persistent insulin signaling, suppressed phosphatidylinositol-3 kinase (PI3K) activation, and an ensuing relative increase in MAPK/endothelin-1 (ET-1) activity. First, we examined previously published work from our group where we assessed changes in lower-limb blood flow in response to an oral glucose tolerance test (endogenous insulin stimulation) in lean and obese subjects. The new analyses showed that the peak rise in vascular resistance during the postprandial state was greater in obese compared with lean subjects. We next extended on these findings by demonstrating that insulin-induced vasoconstriction in isolated resistance arteries from obese subjects was attenuated with ET-1 receptor antagonism, thus implicating ET-1 signaling in this constriction response. Last, we examined in isolated resistance arteries from pigs the dual roles of persistent insulin signaling and blunted PI3K activation in modulating vasomotor responses to insulin. We found that prolonged insulin stimulation did not alter vasomotor responses to insulin when insulin-signaling pathways remained unrestricted. However, prolonged insulinization along with pharmacological suppression of PI3K activity resulted in insulin-induced vasoconstriction, rather than vasodilation. Notably, such aberrant vascular response was rescued with either MAPK inhibition or ET-1 receptor antagonism. In summary, we demonstrate that insulin-induced vasoconstriction is a pathophysiological phenomenon that can be recapitulated when sustained insulin signaling is coupled with depressed PI3K activation and the concomitant relative increase in MAPK/ET-1 activity.
NEW & NOTEWORTHY This study reveals that insulin-induced vasoconstriction is a pathophysiological phenomenon. We also provide evidence that in the setting of persistent insulin signaling, impaired phosphatidylinositol-3 kinase activation appears to be a requisite feature precipitating MAPK/endothelin 1-dependent insulin-induced vasoconstriction.
Keywords: diabetes, endothelin-1, MAPK, obesity, selective insulin resistance
INTRODUCTION
Insulin is a vasoactive hormone that stimulates vasodilation across mammalian species (1, 3, 5, 12, 19, 26, 33, 45, 48). The vasodilator actions of insulin contribute to insulin delivery, glucose disposal and glycemic control (4, 5, 18). At the endothelial cell level, insulin activates two primary signaling cascades, the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt) pathway and the Ras/mitogen-activated protein kinase (MAPK) pathway. Activation of the PI3K/Akt pathway stimulates production of the vasodilator nitric oxide (NO), whereas activation of the MAPK pathway stimulates production of the vasoconstrictor endothelin-1 (ET-1). The net effect of insulin on vascular tone is influenced by the activation of these two signaling cascades (22, 31).
Obesity and insulin resistance are associated with impaired insulin-stimulated vasodilation (10, 23, 24, 33, 34, 36, 38, 39, 41, 42) and limited delivery of insulin and glucose to the target organs (4–6, 18). Although it is less frequently observed, insulin-stimulated vasoconstriction has also been reported in obese rats and humans (13, 16). A primary mechanism implicated in this pathological adaptation is the discriminatory imbalance in endothelial insulin signaling, portrayed by a decrease in PI3K/Akt with either no change or heightened MAPK/ET-1 signaling (6, 12, 13, 21, 22, 29, 31, 36, 39, 41). This imbalance has been termed “selective insulin resistance” (22, 52). Hyperinsulinemia develops as a compensatory mechanism to offset insulin resistance, but because activation of MAPK remains intact, chronic insulin signaling can have deleterious vascular consequences. It is possible that the functional interplay between basal hyperinsulinemia and selective insulin resistance underlies insulin-induced vasoconstriction in obesity. Thus, the purpose of the present work was to determine whether insulin-induced vasoconstriction is a pathophysiological phenomenon that can result from a combination of persistent insulin signaling, suppressed PI3K activation, and an ensuing relative increase in MAPK/ET-1 activity. First, we tested the hypothesis that peripheral vasoconstriction during endogenous insulin stimulation occurs in the setting of obesity. To extend on these findings, we next determined if ET-1 signaling is implicated in insulin-induced vasoconstriction in isolated arteries from obese subjects undergoing abdominal surgery. Lastly, to understand the dual roles of persistent insulin signaling and blunted PI3K activation in modulating vasomotor responses to insulin, we performed a series of functional experiments in isolated arteries from pigs. Specifically, we tested the hypothesis that prior insulinization coupled with diminished PI3K activity evokes insulin-induced vasoconstriction through a relative enhancement of the countercurrent MAPK/ET-1 pathway.
METHODS
Human Studies
All human study procedures conformed to the Declaration of Helsinki and were approved by the Institutional Review Board at The University of Western Ontario or University of Missouri. All subjects provided written, informed consent before participation in the study.
In vivo experiment.
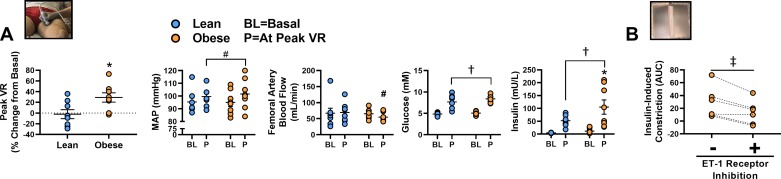
Hemodynamic data from a previously published study from our group were analyzed retrospectively for determination of peak increases in lower-limb vascular resistance during an oral glucose tolerance test (OGTT, endogenous insulin stimulation). Briefly, as previously described here (34), beat-by-beat noninvasive blood pressure was monitored from the left middle finger by photoplethysmographic methods, and brachial blood pressure was estimated using waveform reconstruction and regression equations (Finometer Model 1, Finapress Medical Systems). Additionally, superficial femoral artery blood flow was measured at the midpoint between the patella and iliac crest using Doppler ultrasound (4.7–10 MHz linear array probe; System 5; GE/Vingmed). Hemodynamics were studied at baseline and every 30 min for 2 h during the OGTT in lean (n = 8 women; age = 24 ± 1 yr, BMI = 21 ± 0 kg/m2, fasting blood glucose = 4.9 ± 0.1 mmol/L) and obese (n = 8 women; age = 24 ± 1 yr, BMI = 30 ± 2 kg/m2, fasting blood glucose = 5.1 ± 0.2 mmol/L) subjects. The Matsuda index of insulin sensitivity was calculated (28). In the original study, lower-limb blood flow and vascular conductance responses were reported at each time point, and in the present article hemodynamic variables are displayed at baseline and at peak vascular resistance. Vascular resistance was calculated as the quotient of mean arterial pressure and arterial blood flow (40). This analysis provided evidence that endogenous insulin stimulation can induce vasoconstriction in the setting of obesity.
Ex vivo experiments.
To expand on the above observations, we examined whether the vasoconstrictor properties of insulin in obesity relate to ET-1 signaling. This was accomplished by assessing the effect of ET-1 receptor blockade in isolated omental adipose tissue resistance arteries from obese subjects displaying insulin-induced vasoconstriction. Arteries from six obese subjects that underwent abdominal surgery at the University of Missouri Hospital were included in this analysis. Determination of type 2 diabetes and hypertension was made by a physician, and this information was obtained from the medical history. Briefly, isolated resistance arteries were mounted on 40-µm stainless steel wires in oxygenated physiological saline solution (PSS, 95% O2-5% CO2) in a small vessel wire myograph for isometric tension recording (Danish Myo Technology, Aarhus, Denmark), as previously described (7, 30). After vessels were warmed to 37°C and equilibration, normalization was performed (43), and vessels were stretched to achieve an internal circumference corresponding to a transmural pressure of 70 mmHg. The viability of each artery was assessed by exposure to 60 mM KCl for 5 min. Thereafter, arteries remained untreated or were incubated with an ET-1 receptor antagonist (bosentan,10 µM; Product HY-A0013; MedChemExpress, Monmouth Junction, NJ) for 30 min, before being preconstricted with 60 mM KCl and undergoing a dose-response curve to insulin (whole log doses; 1e−9–1e−6 M). Vasomotor responses were expressed as percent vasoconstriction relative to preconstriction. The area under the curve (net incremental; AUC) was calculated to approximate the net contraction response for each experimental condition.
Studies in Pig-Isolated Arteries and Western Blot Analysis Experiments
The animal protocol conformed to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (2011) and was approved by the University of Missouri Animal Care and Use Committee. Female farm pigs (n = 14, age = 3 ± 0 mo, mass = 29 ± 1 kg) were housed under temperature-controlled conditions, with a 12-h:12-h light-dark cycle and consumed a standard commercially available chow diet (5L80, Laboratory Diet; 2.98 kcal/g; 70% carbohydrate, 21% protein, and 9% fat). Pigs were anesthetized with telozol (5 mg/kg im)-xylazine (2.25 mg/kg im), followed by 5% inhaled isoflurane for 20 min, and then euthanized by removal of the heart or exsanguination. The brachial arteries and lateral head of the triceps were then harvested for ex vivo experiments. Experiments were designed to study the dual roles of persistent insulin stimulation and blunted PI3K activation in modulating vasomotor responses to insulin. We used wortmannin, a PI3K inhibitor, to recapitulate the phenotype of selective insulin resistance (i.e., to produce an imbalance between Akt and MAPK signaling). This imbalance in insulin signaling was verified by measuring activation of Akt and MAPK via Western blot analysis in whole artery segments (from the brachial artery), as well as in cultured endothelial cells, i.e., human umbilical vein endothelial cells (HUVECs; experimental details in figure legend). Resistance arteries from the triceps were used for vasomotor function experiments. Briefly, arteries were dissected from the muscle, transferred to a plexiglass chamber filled with PSS and cannulated with two glass micropipettes (60–75 µm) filled with PSS (with 10 g/L albumin added). The chambers were transferred to the stage of an inverted microscope (Nikon Diaphot 200) attached to a video camera (Javelin Electronics, Los Angeles, CA), video micrometer (Microcirculation Research Inst., Texas A&M University) and a Powerlab data acquisition system (ADInstruments, Colorado Springs, CO), as previously described (32, 33, 35, 39, 51). Fluid-filled reservoirs were used to set intraluminal pressure at 60 mmHg, and luminal diameter was monitored throughout the experiment. Arteries were allotted 30 min to stabilize, at which point maximal arterial vasoconstriction in response to 80 mM KCl was determined. Thirty minutes later, arteries were incubated for 3 h with versus without insulin (10 nM) in the presence or absence of the PI3K inhibitor wortmannin (100 nM, Cat. No.: 19545-26-7, Sigma-Aldrich; 2% DMSO used as vehicle control) (25). Thereafter, arteries were washed and wortmannin or vehicle control, but not insulin, were readded to the bath. Arteries were then preconstricted with a thromboxane A2 analog (U-46619, 1e−7–1e−4 M to achieve 20–40% tone) and underwent a dose-response curve for insulin (whole log doses; 1e−9–1e−6 M). While plasma insulin during the OGTT was elevated by ~10-fold and almost reached the nanomolar range in obese subjects, it should be acknowledged that the insulin concentration used for these ex vivo vasoreactivity studies ranged from high physiological (i.e., 1 nM) to supraphysiological levels (i.e., 1 µM). After the experiment, all vessels were washed twice with Ca2+ free PSS to determine maximal passive diameter. Vasomotor responses were expressed as percent vasodilation (i.e., the quotient of ∆ diameter - baseline diameter and ∆ maximal Ca2+ free diameter - baseline diameter, multiplied by 100) or vasoconstriction (quotient of ∆diameter - baseline diameter and baseline diameter). The area under (or over) the curve (net incremental; AUC) was calculated to approximate the net dilatory or constrictor response for each experimental condition (17, 38).
A subsequent experimental series was conducted to examine the role of MAPK and ET-1 in mediating insulin-induced constriction. To accomplish this, additional arteries were harvested from the pig triceps and underwent the same preparatory steps as described above. Briefly, arteries incubated with insulin and the PI3K inhibitor wortmannin for 3 h were cotreated with versus without a MAPK inhibitor (PD98059, 50 µM; Cat No.: 167869-21-8, Cell Signal) (14) or an ET-1 receptor inhibitor (tezosentan, 3 µM) (10). Thereafter, arteries were washed and all drugs except insulin were readded to the bath. Arteries were then preconstricted and underwent a dose-response curve for insulin, as described above. The sample size for each experiment is indicated in the figure legend. Vessel characteristics for all isolated pig forelimb arteries are as follows: average KCl-induced vasoconstriction = 72 ± 3%, average passive lumen diameter = 124 ± 4 µm, and average wall thickness = 23 ± 1 µm.
Western blot analysis.
Triton X-100 cell and tissue lysates were prepared in Laemmli buffer. Protein samples (6 µg/lane) were separated via Criterion Tris-Glycine eXtended-PAGE precast gels (Bio-Rad). Proteins were next transferred onto polyvinylidene difluoride membranes and blocked with 5% nonfat dry milk or BSA. Membranes were probed for total p44/42 MAPK (1:500; Cat. No. 4695, Cell Signaling) and phosphorylated p44/42 MAPK(Thr202/Tyr204) (1:250, Cat. No. 4370, Cell Signaling) as well as total Akt (1:500; Cat. No.4691, Cell Signaling) and phosphorylated Akt(Ser473) (1:250; No. 4060, Cell Signaling). Molecular weights of protein bands were confirmed by comparison to a visual ladder. Intensity of individual protein bands was quantified via densitometry using the Bio-Rad ChemiDoc XRS+ System (Bio-Rad, Hercules, CA) and Image Laboratory Software (version 6.0.1) and expressed as the ratio of phosphorylated to total Akt and MAPK.
Statistical Analyses
For human in vivo data, hemodynamics and blood parameters were analyzed using a mixed model repeated measures ANOVA with a prior comparisons for baseline and peak vascular resistance. Peak rise in vascular resistance (relative to baseline) between groups was analyzed by unpaired two-tailed t-test. Experiments in isolated arteries from humans with obesity were analyzed using a paired t-test (untreated vs. ET-1 receptor antagonism conditions). For Western blot analysis data and functional experiments in pig isolated arteries, protein expression and AUC were analyzed using ANOVA. Pairwise comparisons were performed using a post hoc Student-Newman-Keul test. The significance level was set at P < 0.05. Data are presented as means ± SE.
RESULTS
Human Studies
For the original description of these data, see Olver and colleagues (34). New data from the current analyses show that during the OGTT, the peak increase in lower-limb vascular resistance was greater in obese versus lean subjects (P < 0.05; Fig. 1A). There were no differences in basal MAP or lower-limb blood flow (P ≥ 0.88). Relative to baseline, at peak vascular resistance during the OGTT, increases in MAP approached significance (main effect of time point, P = 0.09) and reductions in lower-limb blood flow approached significance in the obese group only (P = 0.09). As also displayed in Fig. 1A, the OGTT elicited an increase in blood glucose and insulin concentrations in both groups (P < 0.01). Fasting and postprandial blood glucose at peak vascular resistance were similar between groups (P ≥ 0.15). Fasting plasma insulin was similar between groups (P = 0.76), but postprandial plasma insulin at peak vascular resistance was greater in the obese group (P = 0.02). The Matsuda index of insulin sensitivity was not different between groups (lean = 8.6 ± 0.9 vs. obese = 6.2 ± 1.5; P = 0.18). In isolated omental arteries from obese subjects undergoing abdominal surgery, insulin-induced vasoconstriction was attenuated with ET-1 receptor antagonism (P < 0.05; Fig. 1B). Participant characteristics for the obese subjects undergoing abdominal surgery are presented in Table 1.
Fig. 1.
Insulin-induced vasoconstriction in obesity and the role of endothelin-1 (ET-1) signaling. A: peak lower-limb vascular resistance (VR) during a 2-h oral glucose tolerance test (OGTT; 75 g glucose load) in lean (n = 8) and obese (n = 8) subjects. Plasma glucose and insulin, mean arterial pressure (MAP), and femoral artery blood flow at baseline and at peak VR are also presented; individual responses are displayed. B: ET-1 receptor inhibitor (bosentan, 10 µM) attenuates insulin-induced vasoconstriction in isolated omental adipose tissue resistance arteries from obese subjects (n = 6; individual responses presented). AUR, area under curve. *P < 0.05, difference between groups; #P = 0.09, trend for effect of time point; †P < 0.05, effect of time point; ‡P < 0.05, effect of ET-1 receptor inhibition. Data are expressed as means ± SE.
Table 1.
Patient characteristics
| Sex (women/men) | 5/1 |
| Age, yr | 56 ± 8 |
| Diabetes (yes/no) | 4/2 |
| Hypertension (yes/no) | 5/1 |
| Body mass index, kg/m2 | 49 ± 3 |
Values of age and body mass index are means + SE.
Studies in Pig-Isolated Arteries and Western Blot Analysis Experiments
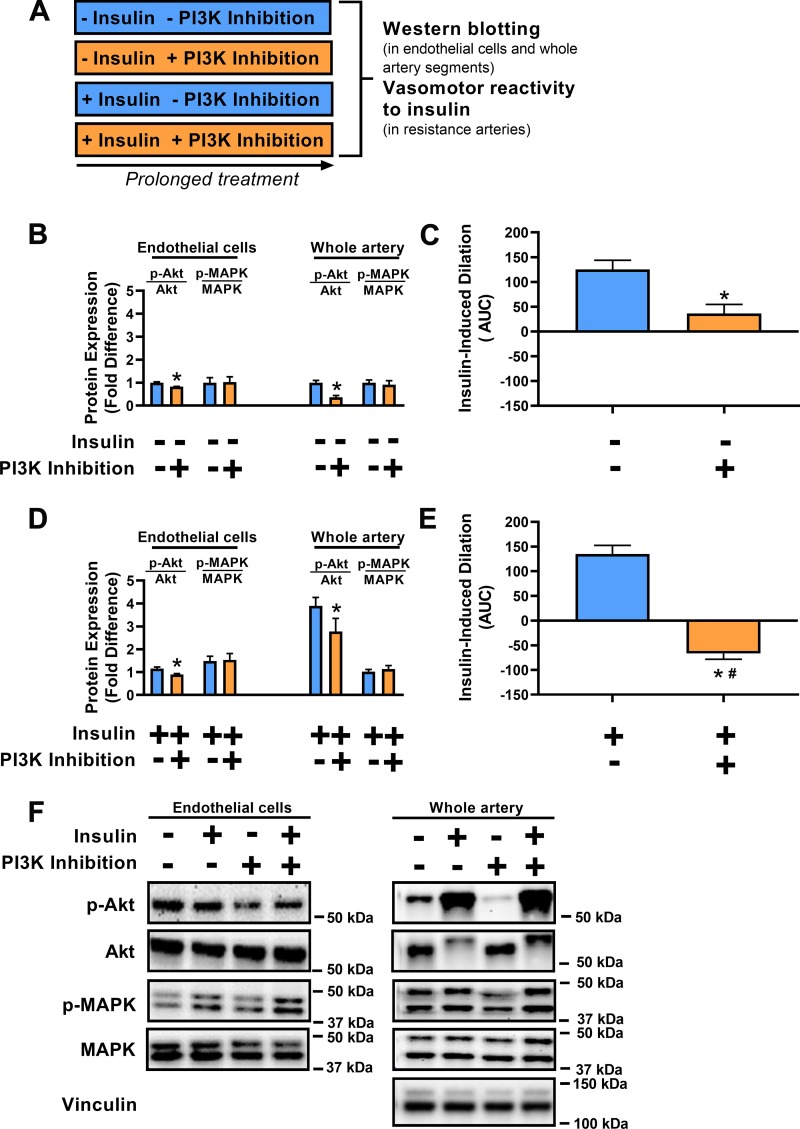
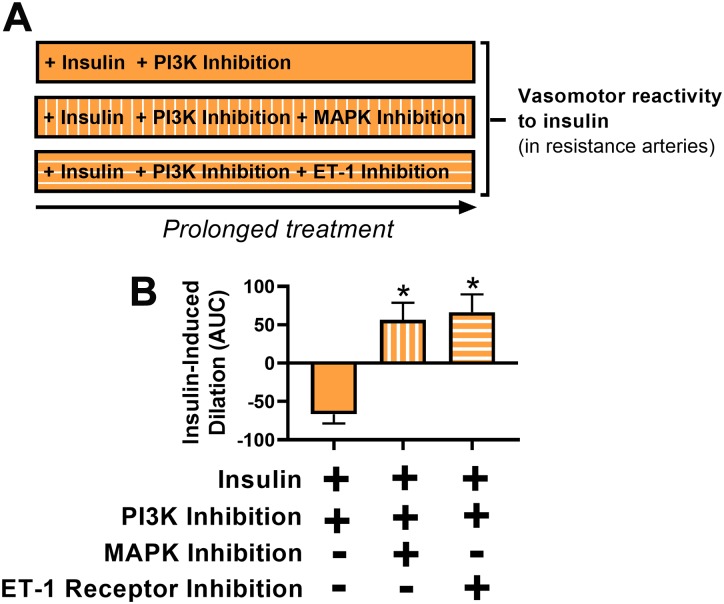
As intended, in HUVECs and isolated pig arterial segments, PI3K inhibition using wortmannin attenuated Akt activation (P < 0.05) without altering MAPK activation (P ≥ 0.68; Fig. 2, B and F). Accordingly, exposure of pig resistance arteries to PI3K inhibition for 3 h blunted insulin-induced vasodilation (P < 0.05; Fig. 2C). Similarly, in HUVECs as well as in isolated pig arterial segments that were insulin-treated, PI3K inhibition blunted Akt activation (P < 0.05) without disrupting MAPK activation (P ≥ 0.52; Fig. 2, D and F). While insulin stimulation increased phosphorylation of Akt, it also attenuated total Akt in pig arterial segments (P < 0.05). Expression of the housekeeping protein vinculin was similar across conditions (P = 0.89). Notably, while 3 h of insulin stimulation alone did not alter vasomotor function in response to insulin (P = 0.20), the combined exposure of insulin and PI3K inhibition promoted insulin-stimulated vasoconstriction (P < 0.05; Fig. 2E). The vasoconstrictor effect of insulin stimulation combined with PI3K inhibition was abolished by MAPK inhibition as well as by ET-1 receptor antagonism (P < 0.05; Fig. 3). Collectively, these data indicate that when PI3K signaling is interrupted, prolonged insulin stimulation results in MAPK/ET-1-dependent insulin-induced vasoconstriction.
Fig. 2.
Persistent insulin signaling coupled with restricted phosphatidylinositol-3 kinase (PI3K) activation causes insulin-induced vasoconstriction. A: illustration of 4 experimental conditions. B: Akt and MAPK 44/42 activation in human umbilical vein endothelial cells (HUVECs; n = 6/condition) and porcine brachial artery homogenates (whole artery; n = 5/condition). HUVECs (CC-2519, Lonza, passages 3–7 in VascuLife EnGS medium, serum starved for 24 h with 0.5% FBS) and whole artery (~5-mm segments placed in DMEM + 0.1% FBS and allowed to acclimate for 1 h at 37°C on a rocking platform) were treated with vs. without the PI3K inhibitor wortmannin (100 nM) for 48 and 3 h, respectively. Data expressed as fold difference from the PI3K inhibitor-untreated condition. C: insulin-induced dilation in isolated porcine triceps resistance arteries following treatment with vs. without the PI3K inhibitor wortmannin (100 nM) for 3 h (n = 8/condition). AUC, area under curve. D: Akt and MAPK 44/42 activation in HUVECs (n = 6/condition) and porcine brachial artery homogenates (whole artery; n = 5/condition). HUVECs and whole artery segments were treated with vs. without the PI3K inhibitor wortmannin (100 nM) for 48 and 3 h, respectively, in the presence of insulin (100 nM for HUVECs and 10 nM for whole artery). Data expressed as fold difference from the insulin-untreated and PI3K inhibitor-untreated condition in A. E: insulin-induced dilation in isolated porcine triceps resistance arteries following treatment with vs. without the PI3K inhibitor wortmannin (100 nM) for 3 h in the presence of insulin (10 nM) (n = 14/condition). F: representative Western blot images of HUVECs and whole artery segments. *P < 0.05, statistical significance from the PI3K inhibitor-untreated condition. #P < 0.05, statistical significance from the insulin-untreated condition in B. Data are expressed as means ± SE.
Fig. 3.
MAPK and endothelin-1 (ET-1) receptor blockade rescue insulin-induced vasoconstriction caused by persistent insulin signaling in the setting of restricted phosphatidylinositol-3 kinase (PI3K) activation. A: illustration of the 3 experimental conditions. B: insulin-induced vasomotor responses of isolated porcine triceps resistance arteries following insulin stimulation (10 nM) coupled with PI3K inhibition (wortmannin, 100 nM) for 3 h with vs. without cotreatment of MAPK inhibitor (PD98059, 50 µM) or ET-1 receptor inhibitor (tezosentan, 3 µM); n = 6–14/condition. AUC, area under curve. *P < 0.05, statistical significance from the first bar. Data are expressed as means ± SE.
DISCUSSION
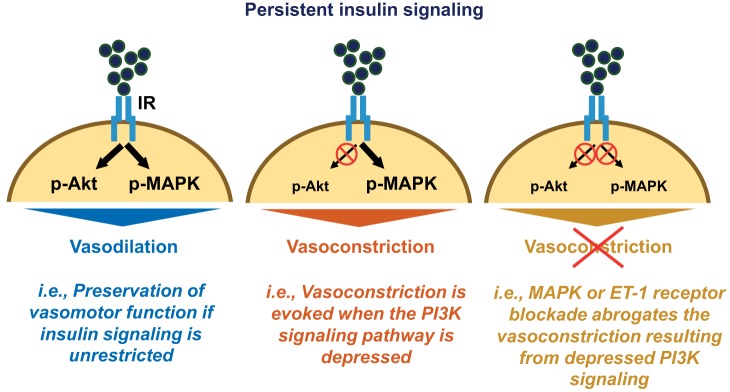
Findings from the present investigation support the hypothesis that insulin-induced vasoconstriction is a pathophysiological phenomenon that may result from simultaneous insulin stimulation and suppression of PI3K signaling (i.e., selective vascular insulin resistance), leading to an imbalance between Akt and MAPK/ET-1 activation in endothelial cells (Fig. 4; summary illustration). Indeed, examination of our existing human data revealed that vasoconstriction in the lower limb during an OGTT and attendant insulin stimulation is greater in the setting of obesity. In addition, the ex vivo arterial function experiments from obese humans indicated that this may be related, in part, to ET-1 signaling. Notably, findings in isolated arteries from pigs demonstrated that persistent insulin stimulation did not alter vasomotor responses to insulin when insulin signaling pathways remained preserved. However, prolonged insulinization coupled with restricted PI3K activity resulted in insulin-stimulated vasoconstriction, instead of vasodilation. This aberrant vascular response was rescued by either MAPK inhibition or ET-1 receptor antagonism, suggesting that MAPK/ET-1 signaling mediates insulin-induced vasoconstriction when PI3K/Akt signaling is dampened.
Fig. 4.
Schematic summarizing the main findings of the study. Persistent (and unrestricted) insulin signaling does not alter vasomotor function in response to insulin (left); however, persistent insulin signaling in the setting where phosphatidylinositol-3 kinase (PI3K)/Akt signaling is depressed causes insulin-induced vasoconstriction (middle), thus recapitulating the pathophysiological phenomenon. This insulin-induced vasoconstriction is abolished with concurrent MAPK inhibition or endothelin-1 (ET-1) receptor antagonism (right). IR, insulin receptor.
The retrospective analyses of human data provide evidence of the vasoconstrictor effects of endogenous insulin. Of note, typically both endogenous (4, 28, 34, 37) and exogenous (9, 26, 44, 48) insulin mediate a NO-dependent vasodilation. The dilatory response to insulin is blunted in insulin-resistant conditions, such as in obesity and T2D (10, 23, 24, 33, 34, 36, 38, 39, 41, 42). However, relative to the dilatory effects of insulin, the constrictor effects have not been well characterized. Previously, Gudbjörnsdottir and colleagues (16) reported that insulin stimulation increases forearm vascular resistance in obese, hypertensive subjects. The current analyses extend these initial observations and indicate that endogenous insulin-induced vasoconstriction can occur in the lower limb during an OGTT. Furthermore, given this response was observed in young obese subjects with no underlying cardiovascular disease, it may reflect an early pathophysiological alteration in the course of obesity-related vascular dysfunction. The mechanisms responsible for insulin-stimulated vasoconstriction in vivo may involve both neurohumoral inputs (i.e., increased sympathetic-mediated vasoconstriction) (2, 11, 26, 46, 47, 49, 50) and endothelial-derived signals (i.e., reduced NO bioavailability tied with increased MAPK/ET-1 signaling) (6, 8, 21, 22, 31, 33, 36, 41). Our finding that insulin-induced vasoconstriction was attenuated by ET-1 receptor antagonism in isolated resistance arteries from obese humans provides support for endothelial cell involvement in this vascular response.
Because hyperinsulinemia and impaired PI3K activation are shared features of insulin resistance (15), here we interrogated the interplay between these two variables as a potential driver of insulin-induced vasoconstriction. As expected on the basis of previous work by others (12), we found that pharmacological inhibition of PI3K signaling alone impaired insulin-induced dilation. Notably, persistent insulin stimulation under control conditions (i.e., unrestricted insulin signaling) did not alter vasomotor responses to insulin; however, persistent insulin stimulation accompanied by inhibition of PI3K produced a vasoconstriction response. Collectively, these data indicate that restriction of the PI3K pathway may be a requisite to unmask the deleterious effects of sustained insulin signaling. Thus, diminished PI3K activation may be a critical step underpinning the increased insulin-induced vasoconstriction in the setting of obesity and compensatory hyperinsulinemia.
Recently, it was demonstrated that acute hyperinsulinemia increases endothelial NO synthase phosphorylation and ET-1 protein in human skeletal muscle homogenates (27). Perhaps the maintenance of both PI3K/Akt and MAPK/ET-1 signaling during prolonged insulin incubation may help explain why prior insulin stimulation alone did not impair subsequent insulin-induced vasodilation in isolated arteries. Importantly, as intended by design, PI3K inhibition reduced Akt phosphorylation without altering MAPK activity, thereby promoting an imbalance in favor of increased MAPK relative to Akt signaling. Notably, we found that insulin-induced vasoconstriction in our experimental ex vivo model of selective insulin resistance was rescued with MAPK inhibition (which targets one side of the imbalance directly) and with ET-1 receptor antagonism (which targets the net vasoconstrictor effect owing to the imbalance). These findings extend on previous work from our group (39, 51) as well as others (12, 13) and suggest that restoring the normal equilibrium between insulin-stimulated PI3K and MAPK activation may be a viable target for the treatment of vascular insulin resistance. In this regard, recent work demonstrates that selective activation of the insulin receptor PI3K branch, leaving the MAPK branch largely inactive, results in protection from atherosclerosis in a mouse model of metabolic syndrome (20).
In aggregate, this work provides evidence that insulin-induced vasoconstriction is a pathophysiological phenomenon. Furthermore, we report that, in the setting of persistent insulin signaling, impaired PI3K activation appears to be a requisite feature precipitating MAPK/ET-1-dependent insulin-induced vasoconstriction.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants K01-HL-125503 and R01-HL-137769 (to J. Padilla), R01-HL-088105 (to L. A. Martinez-Lemus), K08-HL-129074 (to C. Manrique-Acevedo), and R01-HL-112998 (to C. A. Emter); a Faculty Research grant from College of Veterinary Medicine-Committee on Research (to C. A. Emter); a Discovery grant from Natural Sciences and Engineering Research Council of Canada (to J. K. Shoemaker); FAPESP Grant 2017/25613-6) (to A. R. K. Sales); and Saskatchewan Health Research Foundation Establishment Grant 4522 (to T. D. Olver). Bosentan was purchased with funds provided by the graduate research grant from the Sports, Cardiovascular, and Wellness Nutrition dietetic practice group of the Academy of Nutrition and Dietetics (to Z. I. Grunewald).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.D.O., Z.I.G., P.W.R.L., J.K.S., and J.P. conceived and designed research; T.D.O., Z.I.G., T.G., R.M.R., L.K.P., and P.K.T. performed experiments; T.D.O., Z.I.G., T.G., and A.R.K.S. analyzed data; T.D.O., Z.I.G., T.G., R.M.R., A.R.K.S., L.K.P., P.K.T., R.R.G., C.A.E., P.W.R.L., J.K.S., C.M.-A., L.A.M.-L., and J.P. interpreted results of experiments; T.D.O., Z.I.G., and J.P. prepared figures; T.D.O. and J.P. drafted manuscript; T.D.O., Z.I.G., T.G., R.M.R., A.R.K.S., L.K.P., P.K.T., R.R.G., C.A.E., P.W.R.L., J.K.S., C.M.-A., L.A.M.-L., and J.P. edited and revised manuscript; T.D.O., Z.I.G., T.G., R.M.R., A.R.K.S., L.K.P., P.K.T., R.R.G., C.A.E., P.W.R.L., J.K.S., C.M.-A., L.A.M.-L., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jenna Edwards for technical assistance.
REFERENCES
- 1.Abramson DI, Schkloven N, Margolis MN, Mirsky IA. Influence of massive doses of insulin on peripheral blood flow in man. Am J Physiol 128: 124–132, 1939. doi: 10.1152/ajplegacy.1939.128.1.124. [DOI] [Google Scholar]
- 2.Allwood MJ, Ginsburg J, Paton A. The effect of insulin hypoglycaemia on blood flow in intact and sympathectomized extremities in man. J Physiol 139: 97–107, 1957. doi: 10.1113/jphysiol.1957.sp005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allwood MJ, Hensel H, Papenberg J. Muscle and skin blood flow in the human forearm during insulin hypoglycaemia. J Physiol 147: 269–273, 1959. doi: 10.1113/jphysiol.1959.sp006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab 70: 1525–1533, 1990. doi: 10.1210/jcem-70-6-1525. [DOI] [PubMed] [Google Scholar]
- 5.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96: 786–792, 1995. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301: E252–E263, 2011. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Heart Circ Physiol 309: H574–H582, 2015. doi: 10.1152/ajpheart.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 100: 820–825, 1999. doi: 10.1161/01.CIR.100.8.820. [DOI] [PubMed] [Google Scholar]
- 9.Creager MA, Liang CS, Coffman JD. Beta adrenergic-mediated vasodilator response to insulin in the human forearm. J Pharmacol Exp Ther 235: 709–714, 1985. [PubMed] [Google Scholar]
- 10.Crissey JM, Padilla J, Jenkins NT, Martin JS, Rector RS, Thyfault JP, Harold Laughlin M. Metformin does not enhance insulin-stimulated vasodilation in skeletal muscle resistance arteries of the OLETF rat. Microcirculation 20: 764–775, 2013. doi: 10.1111/micc.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dela F, Stallknecht B, Biering-Sørensen F. An intact central nervous system is not necessary for insulin-mediated increases in leg blood flow in humans. Pflugers Arch 441: 241–250, 2000. doi: 10.1007/s004240000444. [DOI] [PubMed] [Google Scholar]
- 12.Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56: 464–471, 2002. doi: 10.1016/S0008-6363(02)00593-X. [DOI] [PubMed] [Google Scholar]
- 13.Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 293: E1134–E1139, 2007. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 14.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes 58: 2238–2245, 2009. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95: 2195–2204, 1995. doi: 10.1172/JCI117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudbjörnsdottir S, Elam M, Sellgren J, Anderson EA. Insulin increases forearm vascular resistance in obese, insulin-resistant hypertensives. J Hypertens 14: 91–97, 1996. [PubMed] [Google Scholar]
- 17.Inocencio IM, Polglase GR, Miller SL, Sehgal A, Sutherland A, Mihelakis J, Li A, Allison BJ. Effects of maternal sildenafil treatment on vascular function in growth-restricted fetal sheep. Arterioscler Thromb Vasc Biol 39: 731–740, 2019. doi: 10.1161/ATVBAHA.119.312366. [DOI] [PubMed] [Google Scholar]
- 18.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins NT, Padilla J, Martin JS, Crissey JM, Thyfault JP, Rector RS, Laughlin MH. Differential vasomotor effects of insulin on gastrocnemius and soleus feed arteries in the OLETF rat model: role of endothelin-1. Exp Physiol 99: 262–271, 2014. doi: 10.1113/expphysiol.2013.074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanter JE, Kramer F, Barnhart S, Duggan JM, Shimizu-Albergine M, Kothari V, Chait A, Bouman SD, Hamerman JA, Hansen BF, Olsen GS, Bornfeldt KE. A novel strategy to prevent advanced atherosclerosis and lower blood glucose in a mouse model of metabolic syndrome. Diabetes 67: 946–959, 2018. doi: 10.2337/db17-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award lecture. Diabetes 65: 1462–1471, 2016. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 85: 1844–1852, 1990. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Xing W, Wang Y, Mi C, Zhang Z, Ma H, Zhang H, Gao F. Upregulation of caveolin-1 contributes to aggravated high-salt diet-induced endothelial dysfunction and hypertension in type 1 diabetic rats. Life Sci 113: 31–39, 2014. doi: 10.1016/j.lfs.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Doherty JU, Faillace R, Maekawa K, Arnold S, Gavras H, Hood WB Jr. Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest 69: 1321–1336, 1982. doi: 10.1172/JCI110572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoud AM, Szczurek MR, Blackburn BK, Mey JT, Chen Z, Robinson AT, Bian JT, Unterman TG, Minshall RD, Brown MD, Kirwan JP, Phillips SA, Haus JM. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol Rep 4: 1–15, 2016. doi: 10.14814/phy2.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, Uptergrove GM, Deo SH, Kim A, Kanaley JA, Fadel PJ, Thyfault JP. Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. Journal of Applied Physiology 111: 657–664, 2011. doi: 10.1152/japplphysiol.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 277: 1794–1799, 2002. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 30.Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension 66: 988–997, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 32.Olver TD, Edwards JC, Ferguson BS, Jessica A, Thorne PK, Hill MA, Laughlin MH, Emter CA. Chronic interval exercise training prevents BK Ca -channel mediated coronary vascular dysfunction in aortic-banded mini-swine. J Appl Physiol. 125: 86–96, 2018. doi: 10.1152/japplphysiol.01138.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olver TD, Grunewald ZI, Jurrissen TJ, MacPherson RE, LeBlanc PJ, Schnurbusch TR, Czajkowski AM, Laughlin MH, Rector RS, Bender SB, Walters EM, Emter CA, Padilla J. Microvascular insulin resistance in skeletal muscle and brain occurs early in the development of juvenile obesity in pigs. Am J Physiol Regul Integr Comp Physiol 314: R252–R264, 2018. doi: 10.1152/ajpregu.00213.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olver TD, Hazell TJ, Hamilton CD, Shoemaker JK, Lemon PW. Impaired superficial femoral artery vasodilation and leg blood flow in young obese women following an oral glucose tolerance test. Appl Physiol Nutr Metab 37: 176–183, 2012. doi: 10.1139/h11-148. [DOI] [PubMed] [Google Scholar]
- 35.Olver TD, Hiemstra JA, Edwards JC, Schachtman TR, Heesch CM, Fadel PJ, Laughlin MH, Emter CA. The loss of female sex hormones exacerbates cerebrovascular and cognitive dysfunction in aortic banded mini-swine through a NPY-BKCa-NO mediated mechanism. JAHA 6: e007409, 2017. doi: 10.1161/JAHA.117.007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olver TD, Laughlin MH. Endurance, interval sprint, and resistance exercise training: impact on microvascular dysfunction in type 2 diabetes. Am J Physiol Heart Circ Physiol 310: H337–H350, 2016. doi: 10.1152/ajpheart.00440.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olver TD, Mattar L, Grisé KN, Twynstra J, Noble EG, Lacefield JC, Shoemaker JK. Glucose-stimulated insulin secretion causes an insulin-dependent nitric oxide-mediated vasodilation in the blood supply of the rat sciatic nerve. Am J Physiol Regul Integr Comp Physiol 305: R157–R163, 2013. doi: 10.1152/ajpregu.00095.2013. [DOI] [PubMed] [Google Scholar]
- 38.Olver TD, McDonald MW, Grisé KN, Dey A, Allen MD, Medeiros PJ, Lacefield JC, Jackson DN, Rice CL, Melling CW, Noble EG, Shoemaker JK. Exercise training enhances insulin-stimulated nerve arterial vasodilation in rats with insulin-treated experimental diabetes. Am J Physiol Regul Integr Comp Physiol 306: R941–R950, 2014. doi: 10.1152/ajpregu.00508.2013. [DOI] [PubMed] [Google Scholar]
- 39.Olver TD, McDonald MW, Klakotskaia D, Richardson RA, Jasperse JL, Melling CW, Schachtman TR, Yang HT, Emter CA, Laughlin MH. A chronic physical activity treatment in obese rats normalizes the contributions of ET-1 and NO to insulin-mediated posterior cerebral artery vasodilation. Journal of Applied Physiology 122: 1040–1050, 2017. doi: 10.1152/japplphysiol.00811.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olver TD, Reid SM, Smith AR, Zamir M, Lemon PWR, Laughlin MH, Shoemaker JK. Effects of acute and chronic interval sprint exercise performed on a manually propelled treadmill on upper limb vascular mechanics in healthy young men. Physiol Rep 4: e12861, 2016. doi: 10.14814/phy2.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padilla J, Olver TD, Thyfault JP, Fadel PJ. Role of habitual physical activity in modulating vascular actions of insulin. Exp Physiol 100: 759–771, 2015. doi: 10.1113/EP085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. Journal of Applied Physiology 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richard V, Kaeffer N, Tron C, Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation 89: 1254–1261, 1994. doi: 10.1161/01.CIR.89.3.1254. [DOI] [PubMed] [Google Scholar]
- 44.Richter EA, Kiens B, Mizuno M, Strange S. Insulin action in human thighs after one-legged immobilization. Journal of Applied Physiology 67: 19–23, 1989. doi: 10.1152/jappl.1989.67.1.19. [DOI] [PubMed] [Google Scholar]
- 45.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. Journal of Applied Physiology 66: 876–885, 1989. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- 46.Sartori C, Trueb L, Nicod P, Scherrer U. Effects of sympathectomy and nitric oxide synthase inhibition on vascular actions of insulin in humans. Hypertension 34: 586–589, 1999. doi: 10.1161/01.HYP.34.4.586. [DOI] [PubMed] [Google Scholar]
- 47.Scherrer U, Vollenweider P, Randin D, Jéquier E, Nicod P, Tappy L. Suppression of insulin-induced sympathetic activation and vasodilation by dexamethasone in humans. Circulation 88: 388–394, 1993. doi: 10.1161/01.CIR.88.2.388. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinberg HO. Effects of Insulin on the Vascular System. In: Diabetes and Cardiovascular Disease. Contemporary Cardiology, ed. By Johnstone MT, Veves A. Totowa, NJ: Humana, 2005, p. 265–284. [Google Scholar]
- 50.Vollenweider P, Randin D, Tappy L, Jéquier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest 93: 2365–2371, 1994. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh LK, Ghiarone T, Olver TD, Medina-Hernandez A, Edwards JC, Thorne PK, Emter CA, Lindner JR, Manrique-Acevedo C, Martinez-Lemus LA, Padilla J. Increased endothelial shear stress improves insulin-stimulated vasodilatation in skeletal muscle. J Physiol 597: 57–69, 2019. doi: 10.1113/JP277050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104: 447–457, 1999. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]