Abstract
Pulmonary arterial hypertension (PAH) is a fatal disease with a median survival of only 5–7 yr. PAH is characterized by remodeling of the pulmonary vasculature causing reduced pulmonary arterial compliance (PAC) and increased pulmonary vascular resistance (PVR), ultimately resulting in right ventricular failure and death. Better therapies for PAH will require a paradigm shift in our understanding of the early pathophysiology. PAC decreases before there is an increase in the PVR. Unfortunately, present treatment has little effect on PAC. The loss of compliance correlates with extracellular matrix remodeling and fibrosis in the pulmonary vessels, which have been linked to chronic perivascular inflammation and immune dysregulation. However, what initiates the perivascular inflammation and immune dysregulation in PAH is unclear. Alteration of the gut microbiota composition and function underlies the level of immunopathogenic involvement in several diseases, including atherosclerosis, obesity, diabetes mellitus, and depression, among others. In this review, we discuss evidence that raises the possibility of an etiologic role for changes in the gut and circulating microbiome in the initiation of perivascular inflammation in the early pathogenesis of PAH.
Keywords: dysbiosis, endotoxin, heart failure, inflammation, short-chain fatty acids
INTRODUCTION
Pulmonary arterial hypertension (PAH) continues to be a fatal and costly disease despite progress in understanding the pathogenesis and treatment over the last two decades. Median survival is only 5–7 yr (6, 36). In-hospital mortality continues to be high at 6% (3). Moreover, the economic burden for PAH-related health care costs in the United States is estimated to be approximately $29 billion per year (3). PAH is characterized by remodeling of the pulmonary vasculature causing reduced pulmonary arterial compliance (PAC) and increased pulmonary vascular resistance (PVR), ultimately resulting in right ventricular (RV) failure and death (34, 94). The Food and Drug Administration has approved 11 PAH-specific drug therapies for the treatment of PAH. These target three different pathways: the endothelin pathway, the nitric oxide pathway, and the prostacyclin pathway (28). These PAH-specific therapies are predominantly pulmonary vasodilators, which reduce PVR but have little effect on PAC (95). These drug therapies increase exercise capacity modestly and reduce hospital admission but are expensive and not curative (3, 28). Since 2005, no new therapeutic pathways have been identified for the treatment of PAH (34). Therefore, it is imperative that we reconsider the paradigms in our understanding of PAH and explore additional mechanisms that may be involved in the pathophysiology of this disease.
PAC is a measure of the stiffness of the pulmonary arteries. It represents the pulsatile afterload of the right ventricle, which accounts for approximately one-fourth of the RV afterload. In contrast, PVR is a measure of the steady afterload of the RV, which accounts for the remaining three-fourths of the RV afterload. In PAH, PAC decreases and PVR increases, leading to increased RV afterload. Whereas the decrease in PAC is attributed to the extracellular matrix remodeling of the pulmonary vasculature, the increase in PVR results from the intimal endothelial cell proliferation and medial smooth muscle hypertrophy/proliferation causing narrowing of the luminal cross-sectional area of the vasculature.
There is clear evidence in both preclinical and clinical studies that the decrease in PAC due to extracellular matrix remodeling precedes the increase in PVR due to intimal endothelial cell proliferation and medial smooth muscle hypertrophy. In fact, there is growing evidence that the decrease in PAC due to extracellular matrix remodeling causes intimal endothelial cell proliferation and medial smooth muscle hypertrophy. The extracellular matrix remodeling and fibrosis in the pulmonary vessels, which correlate with loss of compliance, have been linked to chronic perivascular inflammation and immune dysregulation (92). In fact, perivascular inflammation has been described in PAH from the earliest pathological studies (32). Many papers provide evidence of systemic inflammation in patients with PAH (35, 83). However, events and factors that initiate and/or exacerbate the immune dysregulation and perivascular inflammation in PAH remain unknown. Identifying the important factors that initiate and/or exacerbate maladaptive inflammatory responses in PAH will help us to understand the early pathophysiology, to diagnose patients earlier, and to develop novel therapies for PAH. In this review, we discuss evidence stimulating the hypothesis that there is an etiologic role for changes in gut and circulating microbiota in the initiation of perivascular inflammation in the early pathogenesis of PAH.
INFLAMMATION IN PAH
There is ample clinical and experimental evidence indicating a role for inflammation in PAH (63). Abnormalities in both adaptive and innate immunity have been described in PAH. Clinically, PAH is associated with autoimmune diseases, including scleroderma and systemic lupus erythematosus, and also with infectious diseases, such as human immunodeficiency virus and schistosomiasis (79). There is perivascular accumulation of inflammatory cells including macrophages, T cells, and B cells in the pulmonary arteries in patients with PAH (85). PAH mortality is associated with increased levels of circulating cytokines (35, 83). Interestingly, patients with idiopathic PAH have increased serum autoantibody levels such as antinuclear antibodies (66). Lower circulating natural killer cells are associated with poor survival in patients with PAH, suggesting that they have a protective role (23, 55).
In addition to these observations in patients with PAH, which demonstrate an association between inflammation and PAH, several animal experimental studies prove that a “cause-and-effect” relationship between inflammation and PAH is possible. First, PAH can be induced experimentally in animals by exposure to various immune stimuli, including human immunodeficiency virus, schistosomiasis, and IL-6 overexpression (2, 30, 86). Second, depletion of inflammatory macrophages in chronic hypoxic calves and rats prevents remodeling of the pulmonary vascular extracellular matrix and pulmonary hypertension (PH; 26). Third, an imbalance of cluster of differentiation 4 (CD4) helper T (Th) cell subsets occurs in PAH lungs. While there is an increase in Th1, Th2, and Th17 CD4 cells that induces inflammatory responses, the number of regulatory T cells (Tregs) with anti-inflammatory effects is decreased. Athymic rats deficient in T cells and B cells develop more severe pulmonary vascular disease in response to Sugen and hypoxia. Immune reconstitution of athymic rats with Tregs reduces the severity of PH in response to Sugen and hypoxia, suggesting a role for Treg deficiency in the pathogenesis of PAH (89). Moreover, induction of endogenous Tregs attenuates development of pulmonary vascular inflammation and PH in mice following transverse aortic constriction (98). Finally, autoantibodies from monocrotaline-treated rats cause PAH in naïve rats, supporting the importance of abnormal B cells in the pathogenesis of PAH (20). Taken together, these data strongly suggest a major role for inflammation in the pathogenesis of PAH. However, an important unanswered question remains: What initiates and/or exacerbates the immune dysregulation and perivascular inflammation in PAH?
GUT MICROBIOTA AND IMMUNE DYSREGULATION
The gut contains trillions of microorganisms including various bacteria, fungi, viruses, and parasites, collectively called the “gut microbiota.” This microbiota is integral to the host physiology and contributes to many physiological functions, including maintenance of the mucosal barrier in the intestine, systemic immune regulation, energy homeostasis and metabolism, vitamin synthesis and degradation, and neurological development, among others (21). Drugs, diet, exercise, and genetic factors determine the individual gut microbiota composition and function (10, 21, 31, 47, 67, 80).
Alteration in the gut microbiota composition and function associated with a disease process is called “gut dysbiosis.” Changes in the gut microbiome (i.e., genomic composition of the gut microbiota) have been linked to the immunopathogenesis of numerous diseases, such as atherosclerotic vascular disease, obesity, diabetes mellitus (both type 1 and type 2), depression, alcoholic and nonalcoholic steatohepatitis, multiple sclerosis, and chronic lung allograft rejection, among others (9, 17, 21, 40, 41, 50, 51, 74, 102). The gut microbiota influences the host immune system through several different mechanisms. Gut dysbiosis can increase gut permeability (51), which may allow increased bacterial translocation and release of endotoxin into the splanchnic circulation. This in turn triggers activation of innate and adaptive immune elements, including macrophages and T cells, through engagement of microbe-associated molecular pattern receptors (16, 33, 59).
The gut microbiota can impact the host immune system through release of immunomodulatory bacterial metabolites. Some of the most studied such products are short-chain fatty acids (SCFAs), such as butyrate, propionate, and acetate, which result from microbial fermentation of fiber in the diet. These SCFAs have anti-inflammatory activity. They activate or induce Tregs, either through activation of G protein-coupled receptors (GPCRs: GPCR43, GPCR41, and GPR109A) or through epigenetic modifications by inhibiting histone deacetylases (59, 90, 103). Microbial production of SCFAs promotes generation of Tregs in the colon (81). In addition, reduction of SCFA-producing bacteria levels in the gut decreases the circulating levels of SCFAs (71). Notably, butyrate also stimulates peroxisome proliferator-activated receptor-γ (PPARγ), which drives the colonic epithelium toward β-oxidation and maintenance of an anaerobic environment, which favors the obligate anaerobes in the colon (12). In contrast, the absence of butyrate allows increased expression of the inducible nitric oxide synthase and expansion of Proteobacteria, which are facultative anaerobes capable of nitrate respiration and are characteristically more abundant in dysbiotic states (12, 77).
Microbial products of tryptophan catabolism (indole, tryptamine, indolepropionic acid, indolelactic acid, indolealdehyde, indoleacrylic acid, etc.) constitute another group of metabolites that modulate various aspects of gastrointestinal physiology, including the gut barrier function and immunity, which ultimately may affect the pulmonary vasculature. Several tryptophan catabolites strengthen the mucosal barrier by enhancing expression of epithelial junctional complex molecules, increasing mucus production, and altering the balance of cytokines toward a less inflammatory state (4, 76). Some of these effects are mediated through the pregnane X receptor (PXR). PXR-deficient mice have dysregulated Toll-like receptor 4 (TLR4) expression, increased intestinal permeability, and heightened sensitivity toward different intestinal injury challenges, including nonsteroidal anti-inflammatory drugs, inflammation-induced edema, ischemia-reperfusion, and endotoxic shock (97). Catabolites of tryptophan also interact with the aryl hydrocarbon receptor (AhR), which is widely expressed at all barrier sites, including the gut, where it plays important roles in intestinal homeostasis (88). Specifically, AhR signaling is essential in maintenance of intraepithelial and innate lymphoid cell populations, promoting differentiation of Th17 cells and Tregs, and production of IL-22, a cytokine that participates in multiple barrier functions, including epithelial cell proliferation, mucus production, and production of antimicrobial peptides.
In addition, a causal link exists between increased risk of atherosclerotic vascular disease and circulating levels of trimethylamine N-oxide (TMAO; 91, 105), which has a vascular proinflammatory effect through oxidative stress (45). Plasma TMAO is produced in the liver by the oxidation of trimethylamine, which is generated by the gut bacteria from dietary components such as choline and carnitine. Thus, the potential for TMAO production is dependent on the individual composition of the intestinal microbiota and diet.
MICROBIAL TRANSLOCATION IN THE GUT AND THE CIRCULATING MICROBIOME
The pulmonary vasculature is vulnerable not only to endotoxin and bacterial metabolites but also to microbes that may translocate from the gut into the splanchnic venous circulation. Multiple indirect assays have been used historically to assess the gut barrier function, including measurements of oral probes of different sizes in serum or urine, as well as assessment of macromolecular flux across isolated segments of gastrointestinal tissue and morphometric investigations of the epithelial junction components (14). Altered intestinal epithelial integrity also correlates with plasma measurements of intestinal epithelial junctional components, such as zonulin and claudin-3, as well as markers of enterocyte damage, such as intestinal fatty acid-binding protein (58, 78, 87). These biomarkers are also typically correlated with circulating levels of endotoxin and various cytokines.
Recently, the next-generation sequencing technologies, which have enabled culture-independent studies of complex microbial communities, have also been increasingly applied to studies of the microbial DNA in blood, leading to the conceptual development of both healthy and dysbiotic human blood microbiota (15). The study of any low-biomass microbiota is technically challenging given the potential for contamination from the environment, reagents, and even venipuncture. However, this problem can be addressed by using appropriate controls, and there is reasonable agreement among different laboratories with regard to the composition of the circulating microbiome in healthy individuals (22, 29, 56, 100). Interestingly, the major phyla in the circulating microbiome are Proteobacteria and Actinobacteria, followed by Firmicutes and Bacteroidetes, whereas Firmicutes and Bacteroidetes are by far the dominant bacterial phyla in stool. However, Proteobacteria and Actinobacteria are more abundant in the small intestine and potentially more capable of translocation (25, 106). In addition, it is possible that other parts of the body including the mouth, skin, or lungs may contribute to the circulating microbiome (57). Patients with liver disease have decreased capacity for hepatic filtering of portal blood; therefore, as could be expected, the peripheral blood carries a higher burden of microbial DNA (19, 42, 60, 96, 104). At this time, the studies remain too preliminary to define any specific dysbiotic signature based on a bacterial community structure of the circulating microbiome. However, differences have been found in liver disease and other diseases, including cardiovascular disease, sepsis, chronic kidney disease, acute pancreatitis, and type 2 diabetes (22, 29, 44, 61, 73). The majority of bacteria found in blood are in the white blood cell compartment (56), which may be explained by their internalization at the barrier sites before entry into the bloodstream. It is not known what fraction of these bacteria is viable; however, even nonviable microbial products can stimulate the immune system (38).
EVIDENCE LINKING GUT DYSBIOSIS AND PAH
The etiologic role of the gut microbiota and altered circulating microbiome in the pathogenesis of PAH is unknown. However, there are several compelling observations, in preclinical models of PAH and in patients with PAH, that suggest a possible mechanistic role for gut and circulating microbiome dysbiosis.
First, gut dysbiosis occurs in experimental PAH. Wistar rats treated with Sugen and chronic hypoxia to cause PH demonstrate gut dysbiosis (13). On taxonomy-based analysis, compared with control rats, Sugen-hypoxia PAH rats have a threefold increase in the Firmicutes-to-Bacteroidetes ratio, which is also commonly associated with obesity (13, 43). This altered Firmicutes-to-Bacteroidetes ratio is primarily driven by less abundant Bacteroidetes families in PAH rats, with no significant changes in the relative presence of Firmicutes families (13). In addition, Sugen-hypoxia PAH rats have decreased acetate-producing bacterial genera in the gut and corresponding decreased serum acetate levels, but not butyrate or lactate levels, compared with controls (13). Although these data strongly associate PAH with gut dysbiosis, it is uncertain whether the gut microbiome changes in PAH rats are secondary to right heart failure resulting from PAH or whether they play a causative role in the early pathogenesis of immune dysregulation and pulmonary vascular remodeling. In Sugen-hypoxia PAH rats, gut dysbiosis and serum metabolite changes are observed within 2 wk of exposure to Sugen and hypoxia (13). This indicates that the gut microbiota changes, in a temporal sense, might have a causative role in the early pathogenesis of PAH.
Second, gut dysbiosis leads to increased gut permeability allowing translocation of gut bacteria and/or bacterial products, such as endotoxin. This innate immune phenomenon has been implicated in the pathogenesis of PAH. Serum endotoxin levels are elevated in some experimental models of pulmonary vascular disease (65, 93). Common bile duct ligation (CBDL) in a rat recapitulates human pulmonary vascular disease related to cirrhosis (93). CBDL rats develop the classic hepatopulmonary syndrome with biliary cirrhosis, hypoxemia with an increased alveolar-arterial gradient, alveolar capillary dilatation, and increased lung microvascular density. In addition, our prior work described an increased medial thickness and loss of lumen of the resistance pulmonary arterioles in CBDL rats compared with sham animals (93). In this experimental model, circulating endotoxin levels are elevated, which in turn recruits and activates pulmonary intravascular macrophages. In the presence of cirrhosis, portosystemic shunting may occur, in which case the endotoxin released from the gut into the splanchnic venous circulation enters directly into the pulmonary circulation, where it can activate intravascular macrophages (27). Endotoxin interacts with TLR4 in the macrophages and activates the nuclear factor-κB (NF-κB) signaling pathway leading to increased secretion of cytokines and growth factors (93). The muscular and nonmuscular pulmonary arteries in CBDL rat lungs show accumulation of CD68+ macrophages with near occlusion of some of the resistance arterioles. These macrophages are in an activated inflammatory stage as demonstrated by positive NF-κB staining in the nucleus (93). NF-κB is a transcription factor that translocates into the nucleus during inflammatory activation. In contrast, sham animals have no pulmonary intravascular macrophage accumulation and have low levels of tissue macrophages, which are also not activated. Treatment of CBDL rats with the antibiotic norfloxacin decreases pulmonary intravascular macrophage accumulation and reduces pulmonary vascular remodeling (62). Furthermore, depletion of the pulmonary intravascular macrophages in the CBDL rats prevents, as well as reverses, the pulmonary vascular changes (93). These data together suggest that increased circulating microbial products originating in the gut, such as endotoxin, play a central role in the pathogenesis of pulmonary vascular changes due to cirrhosis through activation of macrophages. Monocrotaline-induced PAH rats also have increased gut permeability, increased circulating levels of endotoxin in the portal vein, and increased circulating levels of soluble CD14, a marker of macrophage activation, in the systemic venous blood (65).
Mutations in the Bmpr2 gene encoding bone morphogenic protein receptor type 2 underlie 80% of heritable PAH, but disease penetrance is only 10–20%, indicating a requirement for an additional trigger or triggers (49, 53). Similar to humans carrying a BMPR2 mutation, Bmpr2+/− mice also have only mild or no elevation of pulmonary artery pressures at baseline (7). However, chronic administration of lipopolysaccharide (LPS; endotoxin is the lipid A portion of the LPS) causes PH in Bmpr2+/− mice compared with littermate controls, suggesting that endotoxin-induced inflammation can be an additional trigger for disease penetrance (82). Compared with littermate controls, LPS-administered Bmpr2+/− mice have increased lung IL-6 cytokine expression, coupled with an increase in the phosphorylation of signal transducer and activator of transcription 3 (STAT3), which is the major downstream signaling pathway of IL-6 (82). Likewise, in in vitro studies, acute administration of LPS increases IL-6 production and phospho-STAT3 activation in both mouse Bmpr2+/− and human BMPR2 mutant pulmonary artery smooth muscle cells that are haplo-insufficient for BMPR2 (82). This is associated with increased expression of TLR4 and increased superoxide levels (82). These data suggest that Bmpr2 deficiency predisposes to inflammation. Thus, gut dysbiosis leading to increased gut permeability and raised circulating endotoxin levels may be a potential additional trigger for phenotypic manifestation of PAH in BMPR2 mutation carriers.
Translocation of gut bacteria, serum endotoxemia, and activation of macrophages occur in human PAH as well. Patients with idiopathic PAH and heritable PAH have increased serum levels of endotoxin and soluble CD14 compared with healthy controls (65). In patients with PAH, the increase in serum CD14 levels parallels the increase in serum endotoxin levels (65). Furthermore, patients with PAH have increased blood TLR4 expression compared with healthy controls (18). The Abernethy malformation is a congenital anomaly of the splanchnic vasculature in which portal venous blood is diverted into the inferior vena cava creating a congenital portosystemic shunt. Patients with the Abernethy malformation often develop pulmonary vascular disease, including portopulmonary hypertension and hepatopulmonary syndrome (46, 75, 84). In the Abernethy malformation there is no hemodynamic left-to-right shunt, such as an atrial septal defect or ventricular septal defect, to explain the PH. Commensal gut bacteria and endotoxin released from the gut bypass the liver through the portosystemic shunt, avoiding hepatic uptake and inactivation, pass through the right heart, and enter the pulmonary vasculature. In the lungs they activate macrophages, leading to pulmonary arteriovenous malformations, capillary dilatation, and proliferative arteriopathy of the distal pulmonary arteries (27, 93). Liver transplantation with the correction of the portosystemic shunt reverses the pulmonary vascular changes in patients with the Abernethy malformation (37). These observations strongly suggest an etiologic role even for the normal gut microbiota in the pathogenesis of PAH.
Exercise training, by itself, improves exercise tolerance and functional capacity in patients with PAH (11). In fact, the improvement in 6-min walk distance seen with exercise training is significantly higher compared with the improvement in 6-min walk distance observed with PAH-specific vasodilator therapies (72 vs. ~40 m, respectively; 11, 48). Interestingly, there is emerging evidence that exercise-induced gut microbiota changes enhance exercise capacity (72). Exercise increases the gut Veillonella genus, which in turn improves exercise performance through its metabolic conversion of exercise-induced lactate into propionate (72). Thus, it is possible that the improvement in exercise capacity with exercise training in PAH might be mediated at least in part by gut microbiota changes.
Finally, gut dysbiosis can cause several immune dysregulations that have been reported in the pathogenesis of PAH. Increased TLR4 activation has been documented to occur in PAH (5). Gut dysbiosis has been associated with activation of Th1 and Th17 CD4 cells, increased secretion of IL-17, and downregulation of Tregs (8, 17, 50). Interestingly, Th1 and Th17 CD4 cell activation as well as Treg dysfunction have been implicated in the pathogenesis of PAH (63, 99). On the basis of these collective observations, it is certainly possible that gut dysbiosis plays a role in initiating immune dysregulation and early perivascular inflammation in PAH.
MECHANISMS BY WHICH GUT AND CIRCULATING MICROBIOME DYSBIOSIS MAY CAUSE PAH
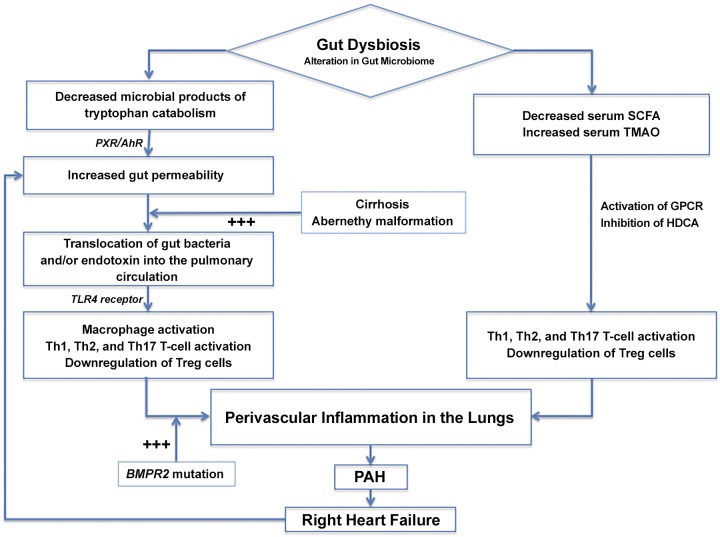
There are two possible, non-mutually exclusive, mechanistic ways in which altered microbial composition in the gut and circulating microbiome might initiate perivascular inflammation in the pulmonary vasculature (Fig. 1): 1) an increase in gut permeability allowing microbial translocation in the gut and increased levels of circulating microbes or microbial products and 2) an altered microbial community structure that generates a proinflammatory metabolome (either an increase in proinflammatory metabolites or a decrease in anti-inflammatory metabolites). The loss of hepatic filtration and the detoxification of circulating microbial products, such as endotoxin, secondary to either cirrhosis or portosystemic shunts, may also favor the development of PAH. Likewise, increased genetic susceptibility, e.g., BMPR2 mutation, may synergize with the above mechanisms, through increased sensitivity to proinflammatory signals, to trigger PAH pathogenesis.
Fig. 1.
Proposed mechanisms through which gut and circulating microbiome dysbiosis causes lung perivascular inflammation in pulmonary arterial hypertension. AhR, aryl hydrocarbon receptor; BMPR2, bone morphogenic protein receptor type 2 gene; GPCR, G protein-coupled receptor; HDCA, histone deacetylase; PAH, pulmonary arterial hypertension; PXR, pregnane X receptor; SCFA, short-chain fatty acid; Th, helper T; TLR, Toll-like receptor; TMAO, trimethylamine N-oxide; Treg, regulatory T cell.
CHRONIC RIGHT HEART FAILURE AND SERUM ENDOTOXIN LEVELS
Chronic right heart failure, by itself, can also lead to increased circulating endotoxin levels in PAH. Chronic right heart failure causes intestinal congestion and reduced bowel perfusion leading to intestinal ischemia. This increases intestinal permeability, allowing translocation of gut bacteria and/or endotoxin (52, 69). In support of this, patients with untreated PAH with right heart failure have higher levels of circulating endotoxin and soluble CD14 compared with patients with treated PAH who are not in chronic right heart failure (65). Similarly, diuretic treatment has been shown to normalize serum endotoxin levels in patients with congestive heart failure. Hence, it is possible that the translocation of gut bacteria and/or endotoxin and activation of macrophages in patients with PAH may occur because of primary gut dysbiosis, right heart failure, or a combination of both (69, 70).
GUT MICROBIOTA AS A POTENTIAL THERAPEUTIC TARGET TO TREAT PAH
On the basis of the collective evidence suggesting a possible link between gut dysbiosis and the pathogenesis of PAH, one can conclude that modulation of the gut microbiota may be a potential therapeutic option to treat PAH. The composition of the gut microbiota can be modulated by diet, antibiotics, prebiotics, probiotics, and/or intestinal microbiota transplantation (IMT), also known as fecal microbiota transplantation (39). IMT is arguably the most potent way to alter a patient’s intestinal microbiota, and it is currently used clinically to treat antibiotic-refractory Clostridiodes difficile infection. However, multiple unknowns need to be considered in designing microbiota-targeting interventions to treat PAH. For example, in the case of an IMT-based strategy it may be important to optimize patient and donor selection, as well as explore the effects of such a treatment on the small bowel, where microbial translocation is more likely to occur. It is also necessary to consider more conventional pharmacologic treatments that recapitulate the beneficial effects of microbial metabolites. Some of these may already be available and are widely used for other conditions. For example, 5-aminosalicylic acid, which is commonly used to treat ulcerative colitis, activates PPARγ, mimicking similar actions induced by butyrate (68). Also, some of the beneficial effects of metformin, currently one of the first-line drugs to treat type 2 diabetes, are likely mediated via the intestinal microbiota through expansion of butyrate-producing bacteria (24, 101). Intriguingly, metformin has been shown to attenuate experimental PH (1, 54).
FUTURE DIRECTIONS
Although there are persuasive findings linking gut dysbiosis to the early pathogenesis of PAH, there are several pivotal unanswered questions. These need to be addressed before modulation of the gut microbiota is introduced as a therapeutic option to treat PAH. First, although gut dysbiosis has been described in experimental PAH, it has not been studied in human patients with PAH. Thus, the gut and circulating microbiota should be characterized in patients with PAH compared with healthy controls. Second, rigorous experimental studies should be conducted to ascertain the causative role of gut dysbiosis and altered circulating microbiome in the pathogenesis of PAH. Antibiotic therapy to modulate gut microbiota composition, alteration of diet to increase production of SCFAs such as acetate or butyrate, and transplantation of germ-free mice with the gut microbiota from experimental models of PAH or human patients with PAH are various approaches that can be undertaken to prove a cause-and-effect relationship between gut dysbiosis and PAH. Third, future studies should determine whether the increase in the circulating microbiome and/or endotoxin in PAH is secondary to gut dysbiosis, to right heart failure, or to a combination of both. Addressing these questions can aid in developing potential interventional strategies to target the microbiota therapeutically in patients with PAH.
CONCLUSION
Ample evidence confirms that inflammation plays an important role in the early pathogenesis of PAH. However, what initiates the early perivascular inflammation in PAH is unclear. In a recent in-depth review of the early pathogenesis of PAH, the authors state that “although the complexity of inflammatory response in the pathogenesis of PAH is recognized, the initial trigger of inflammation that is involved in PAH development is not identified” (64). We propose that the gut dysbiosis and circulating microbiome changes could be an initiating event causing perivascular inflammation in PAH. Changes in the gut and circulating microbiome have been implicated in the immunopathogenesis of many chronic disorders. There is strong circumstantial evidence to suggest an etiologic link between changes in the gut and circulating microbiota and the pathogenesis of PAH. Changes in the gut microbiota may also be important in exacerbating other forms of PH (World Health Organization groups 2–5).
Well-designed studies are needed in the future to prove a causative role for gut dysbiosis in the pathobiology of PAH. Further exploration of the gut and circulating microbiome changes in PAH has a huge potential to yield significant breakthroughs in the development of novel therapeutic tools for the treatment of PAH.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 1-R01-HL-139797 (to Y. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.T., A.K., and E.K.W. prepared figures; T.T., A.K., and E.K.W. drafted manuscript; T.T., A.K., Y.C., and E.K.W. edited and revised manuscript; T.T., A.K., Y.C., and E.K.W. approved final version of manuscript.
REFERENCES
- 1.Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, Loirand G, Pacaud P. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol 158: 1285–1294, 2009. doi: 10.1111/j.1476-5381.2009.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC; Lung HIV Study . Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc 8: 308–312, 2011. doi: 10.1513/pats.201006-046WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand V, Roy SS, Archer SL, Weir EK, Garg SK, Duval S, Thenappan T. Trends and outcomes of pulmonary arterial hypertension-related hospitalizations in the United States: analysis of the Nationwide Inpatient Sample Database from 2001 through 2012. JAMA Cardiol 1: 1021–1029, 2016. doi: 10.1001/jamacardio.2016.3591. [DOI] [PubMed] [Google Scholar]
- 4.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA 107: 228–233, 2010. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med 18: 1509–1518, 2012. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 142: 448–456, 2012. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 7.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L1241–L1247, 2004. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskaran N, Quigley C, Paw C, Butala S, Schneider E, Pandiyan P. Role of short chain fatty acids in controlling Tregs and immunopathology during mucosal infection. Front Microbiol 9: 1995, 2018. doi: 10.3389/fmicb.2018.01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology 152: 1671–1678, 2017. doi: 10.1053/j.gastro.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 10.Bretler T, Weisberg H, Koren O, Neuman H. The effects of antipsychotic medications on microbiome and weight gain in children and adolescents. BMC Med 17: 112, 2019. doi: 10.1186/s12916-019-1346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buys R, Avila A, Cornelissen VA. Exercise training improves physical fitness in patients with pulmonary arterial hypertension: a systematic review and meta-analysis of controlled trials. BMC Pulm Med 15: 40, 2015. doi: 10.1186/s12890-015-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Bäumler AJ. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357: 570–575, 2017. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callejo M, Mondejar-Parreño G, Barreira B, Izquierdo-Garcia JL, Morales-Cano D, Esquivel-Ruiz S, Moreno L, Cogolludo Á, Duarte J, Perez-Vizcaino F. Pulmonary arterial hypertension affects the rat gut microbiome. Sci Rep 8: 9681, 2018. doi: 10.1038/s41598-018-27682-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24: 503–512, 2012. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo DJ, Rifkin RF, Cowan DA, Potgieter M. The healthy human blood microbiome: fact or fiction? Front Cell Infect Microbiol 9: 148, 2019. doi: 10.3389/fcimb.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro A, Bemer V, Nóbrega A, Coutinho A, Truffa-Bachi P. Administration to mouse of endotoxin from gram-negative bacteria leads to activation and apoptosis of T lymphocytes. Eur J Immunol 28: 488–495, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 17.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, Crabtree-Hartman E, Sand IK, Gacias M, Zhu Y, Casaccia P, Cree BA, Knight R, Mazmanian SK, Baranzini SE. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA 114: 10713–10718, 2017. [Erratum in Proc Natl Acad Sci USA 114: E8943, 2017.] doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesné J, Danger R, Botturi K, Reynaud-Gaubert M, Mussot S, Stern M, Danner-Boucher I, Mornex JF, Pison C, Dromer C, Kessler R, Dahan M, Brugière O, Le Pavec J, Perros F, Humbert M, Gomez C, Brouard S, Magnan A; COLT Consortium . Systematic analysis of blood cell transcriptome in end-stage chronic respiratory diseases. PLoS One 9: e109291, 2014. doi: 10.1371/journal.pone.0109291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho EJ, Leem S, Kim SA, Yang J, Lee YB, Kim SS, Cheong JY, Cho SW, Kim JW, Kim SM, Yoon JH, Park T. Circulating microbiota-based metagenomic signature for detection of hepatocellular carcinoma. Sci Rep 9: 7536, 2019. doi: 10.1038/s41598-019-44012-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, Yeager ME. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med 188: 1126–1136, 2013. doi: 10.1164/rccm.201302-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cresci GA, Bawden E. Gut microbiome: what we do and don’t know. Nutr Clin Pract 30: 734–746, 2015. doi: 10.1177/0884533615609899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinakaran V, Rathinavel A, Pushpanathan M, Sivakumar R, Gunasekaran P, Rajendhran J. Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One 9: e105221, 2014. doi: 10.1371/journal.pone.0105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards AL, Gunningham SP, Clare GC, Hayman MW, Smith M, Frampton CM, Robinson BA, Troughton RW, Beckert LE. Professional killer cell deficiencies and decreased survival in pulmonary arterial hypertension. Respirology 18: 1271–1277, 2013. doi: 10.1111/resp.12152. [DOI] [PubMed] [Google Scholar]
- 24.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O; MetaHIT consortium . Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528: 262–266, 2015. [Erratum in Nature 545: 116, 2017.] doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785, 2007. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol 7: 425–442, 2015. doi: 10.4254/wjh.v7.i3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37: 67–119, 2016. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 29.Gosiewski T, Ludwig-Galezowska AH, Huminska K, Sroka-Oleksiak A, Radkowski P, Salamon D, Wojciechowicz J, Kus-Slowinska M, Bulanda M, Wolkow PP. Comprehensive detection and identification of bacterial DNA in the blood of patients with sepsis and healthy volunteers using next-generation sequencing method: the observation of DNAemia. Eur J Clin Microbiol Infect Dis 36: 329–336, 2017. doi: 10.1007/s10096-016-2805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham BB, Chabon J, Kumar R, Kolosionek E, Gebreab L, Debella E, Edwards M, Diener K, Shade T, Bifeng G, Bandeira A, Butrous G, Jones K, Geraci M, Tuder RM. Protective role of IL-6 in vascular remodeling in Schistosoma pulmonary hypertension. Am J Respir Cell Mol Biol 49: 951–959, 2013. doi: 10.1165/rcmb.2012-0532OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet 18: 690–699, 2017. doi: 10.1038/nrg.2017.63. [DOI] [PubMed] [Google Scholar]
- 32.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 18: 533–547, 1958. doi: 10.1161/01.CIR.18.4.533. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162: 3749–3752, 1999. [PubMed] [Google Scholar]
- 34.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 53: 1801887, 2019. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 151: 1628–1631, 1995. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 36.Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jaïs X, Parent F, Savale L, Natali D, Günther S, Chaouat A, Chabot F, Cordier JF, Habib G, Gressin V, Jing ZC, Souza R, Simonneau G; French Pulmonary Arterial Hypertension Network . Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 36: 549–555, 2010. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 37.Iida T, Ogura Y, Doi H, Yagi S, Kanazawa H, Imai H, Sakamoto S, Okamoto S, Uemoto S. Successful treatment of pulmonary hypertension secondary to congenital extrahepatic portocaval shunts (Abernethy type 2) by living donor liver transplantation after surgical shunt ligation. Transpl Int 23: 105–109, 2010. doi: 10.1111/j.1432-2277.2009.00964.x. [DOI] [PubMed] [Google Scholar]
- 38.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 20: 197–216, 2002. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 39.Khoruts A, Brandt LJ. Fecal microbiota transplant: a rose by any other name. Am J Gastroenterol 114: 1176, 2019. doi: 10.14309/ajg.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 40.Kitai T, Tang WH. Gut microbiota in cardiovascular disease and heart failure. Clin Sci (Lond) 132: 85–91, 2018. doi: 10.1042/CS20171090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15: 36–59, 2018. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lelouvier B, Servant F, Païssé S, Brunet AC, Benyahya S, Serino M, Valle C, Ortiz MR, Puig J, Courtney M, Federici M, Fernández-Real JM, Burcelin R, Amar J. Changes in blood microbiota profiles associated with liver fibrosis in obese patients: a pilot analysis. Hepatology 64: 2015–2027, 2016. doi: 10.1002/hep.28829. [DOI] [PubMed] [Google Scholar]
- 43.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023, 2006. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Wang C, Tang C, Zhao X, He Q, Li J. Identification and characterization of blood and neutrophil-associated microbiomes in patients with severe acute pancreatitis using next-generation sequencing. Front Cell Infect Microbiol 8: 5, 2018. doi: 10.3389/fcimb.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, Chen Y, Gua C, Li X. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol 8: 350, 2017. doi: 10.3389/fphys.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin KY, Chen H, Yu L. Pulmonary arterial hypertension caused by congenital extrahepatic portocaval shunt: a case report. BMC Cardiovasc Disord 19: 141, 2019. doi: 10.1186/s12872-019-1124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Liu HY, Zhou H, Zhan Q, Lai W, Zeng Q, Ren H, Xu D. Moderate-intensity exercise affects gut microbiome composition and influences cardiac function in myocardial infarction mice. Front Microbiol 8: 1687, 2017. doi: 10.3389/fmicb.2017.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macchia A, Marchioli R, Tognoni G, Scarano M, Marfisi R, Tavazzi L, Rich S. Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J 159: 245–257, 2010. doi: 10.1016/j.ahj.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 49.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA III, Newman J, Williams D, Galiè N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L, Loyd JE, Trembath RC, Nichols WC. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 68: 92–102, 2001. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mariño E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, Krishnamurthy B, Nefzger CM, Macia L, Thorburn A, Baxter AG, Morahan G, Wong LH, Polo JM, Moore RJ, Lockett TJ, Clarke JM, Topping DL, Harrison LC, Mackay CR. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 18: 552–562, 2017. [Erratum in Nat Immunol 18: 951, 2017.] doi: 10.1038/ni.3713. [DOI] [PubMed] [Google Scholar]
- 51.Mokkala K, Röytiö H, Munukka E, Pietilä S, Ekblad U, Rönnemaa T, Eerola E, Laiho A, Laitinen K. Gut microbiota richness and composition and dietary intake of overweight pregnant women are related to serum zonulin concentration, a marker for intestinal permeability. J Nutr 146: 1694–1700, 2016. doi: 10.3945/jn.116.235358. [DOI] [PubMed] [Google Scholar]
- 52.Nagatomo Y, Tang WH. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail 21: 973–980, 2015. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA III, Loyd JE. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med 345: 319–324, 2001. doi: 10.1056/NEJM200108023450502. [DOI] [PubMed] [Google Scholar]
- 54.Omura J, Satoh K, Kikuchi N, Satoh T, Kurosawa R, Nogi M, Otsuki T, Kozu K, Numano K, Suzuki K, Sunamura S, Tatebe S, Aoki T, Sugimura K, Miyata S, Hoshikawa Y, Okada Y, Shimokawa H. Protective roles of endothelial AMP-activated protein kinase against hypoxia-induced pulmonary hypertension in mice. Circ Res 119: 197–209, 2016. doi: 10.1161/CIRCRESAHA.115.308178. [DOI] [PubMed] [Google Scholar]
- 55.Ormiston ML, Chang C, Long LL, Soon E, Jones D, Machado R, Treacy C, Toshner MR, Campbell K, Riding A, Southwood M, Pepke-Zaba J, Exley A, Trembath RC, Colucci F, Wills M, Trowsdale J, Morrell NW. Impaired natural killer cell phenotype and function in idiopathic and heritable pulmonary arterial hypertension. Circulation 126: 1099–1109, 2012. doi: 10.1161/CIRCULATIONAHA.112.110619. [DOI] [PubMed] [Google Scholar]
- 56.Païssé S, Valle C, Servant F, Courtney M, Burcelin R, Amar J, Lelouvier B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 56: 1138–1147, 2016. doi: 10.1111/trf.13477. [DOI] [PubMed] [Google Scholar]
- 57.Perlemuter G. Circulating bugs in blood in alcoholic liver disease! Hepatology 67: 1207–1209, 2018. doi: 10.1002/hep.29624. [DOI] [PubMed] [Google Scholar]
- 58.Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care 22: 152–160, 2016. doi: 10.1097/MCC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 59.Potgieter M, Bester J, Kell DB, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev 39: 567–591, 2015. doi: 10.1093/femsre/fuv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puri P, Liangpunsakul S, Christensen JE, Shah VH, Kamath PS, Gores GJ, Walker S, Comerford M, Katz B, Borst A, Yu Q, Kumar DP, Mirshahi F, Radaeva S, Chalasani NP, Crabb DW, Sanyal AJ; TREAT Consortium . The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology 67: 1284–1302, 2018. doi: 10.1002/hep.29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu J, Zhou H, Jing Y, Dong C. Association between blood microbiome and type 2 diabetes mellitus: a nested case-control study. J Clin Lab Anal 33: e22842, 2019. doi: 10.1002/jcla.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabiller A, Nunes H, Lebrec D, Tazi KA, Wartski M, Dulmet E, Libert JM, Mougeot C, Moreau R, Mazmanian M, Humbert M, Hervé P. Prevention of gram-negative translocation reduces the severity of hepatopulmonary syndrome. Am J Respir Crit Care Med 166: 514–517, 2002. doi: 10.1164/rccm.200201-027OC. [DOI] [PubMed] [Google Scholar]
- 63.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rafikova O, Al Ghouleh I, Rafikov R. Focus on early events: pathogenesis of pulmonary arterial hypertension development. Antioxid Redox Signal ars.2018.7673, 2019. doi: 10.1089/ars.2018.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranchoux B, Bigorgne A, Hautefort A, Girerd B, Sitbon O, Montani D, Humbert M, Tcherakian C, Perros F. Gut-lung connection in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 56: 402–405, 2017. doi: 10.1165/rcmb.2015-0404LE. [DOI] [PubMed] [Google Scholar]
- 66.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy P, Reid L, Vreim C, Williams G. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 107: 216–223, 1987. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 67.Roberts JD, Suckling CA, Peedle GY, Murphy JA, Dawkins TG, Roberts MG. An exploratory investigation of endotoxin levels in novice long distance triathletes, and the effects of a multi-strain probiotic/prebiotic, antioxidant intervention. Nutrients 8: 733, 2016. doi: 10.3390/nu8110733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, Metzger D, Wahli W, Desvergne B, Naccari GC, Chavatte P, Farce A, Bulois P, Cortot A, Colombel JF, Desreumaux P. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-γ. J Exp Med 201: 1205–1215, 2005. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 50: 1561–1569, 2007. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 70.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, Kalra PR, Buhner S, Herrmann R, Springer J, Doehner W, von Haehling S, Anker SD, Rauchhaus M. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol 157: 80–85, 2012. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 71.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AA, Jonkers DM, Oosting M, Joosten LA, Netea MG, Franke L, Zhernakova A, Fu J, Wijmenga C, McCarthy MI. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet 51: 600–605, 2019. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, Wibowo MC, Wurth RC, Punthambaker S, Tierney BT, Yang Z, Hattab MW, Avila-Pacheco J, Clish CB, Lessard S, Church GM, Kostic AD. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 25: 1104–1109, 2019. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah NB, Allegretti AS, Nigwekar SU, Kalim S, Zhao S, Lelouvier B, Servant F, Serena G, Thadhani RI, Raj DS, Fasano A. Blood microbiome profile in CKD: a pilot study. Clin J Am Soc Nephrol 14: 692–701, 2019. doi: 10.2215/CJN.12161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microbes 8: 607–615, 2017. doi: 10.1080/19490976.2017.1349041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma S, Bobhate PR, Sable S, Kumar S, Yadav K, Maheshwari S, Amin S, Chauhan A, Varma V, Kapoor S, Kumaran V. Abernethy malformation: single-center experience from India with review of literature. Indian J Gastroenterol 37: 359–364, 2018. doi: 10.1007/s12664-018-0884-3. [DOI] [PubMed] [Google Scholar]
- 76.Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One 8: e80604, 2013. doi: 10.1371/journal.pone.0080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33: 496–503, 2015. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 78.Sikora M, Chrabąszcz M, Maciejewski C, Zaremba M, Waśkiel A, Olszewska M, Rudnicka L. Intestinal barrier integrity in patients with plaque psoriasis. J Dermatol 45: 1468–1470, 2018. doi: 10.1111/1346-8138.14647. [DOI] [PubMed] [Google Scholar]
- 79.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 80.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W. Influence of diet on the gut microbiome and implications for human health. J Transl Med 15: 73, 2017. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, Appleby S, Shanahan CM, Bloch KD, Pepke-Zaba J, Upton P, Morrell NW. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 859–872, 2015. doi: 10.1164/rccm.201408-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122: 920–927, 2010. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 84.Spruijt OA, Bogaard HJ, Vonk-Noordegraaf A. Pulmonary arterial hypertension combined with a high cardiac output state: three remarkable cases. Pulm Circ 3: 440–443, 2013. doi: 10.4103/2045-8932.113185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 2009. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, Raizada MK. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 67: 1555–1557, 2018. doi: 10.1136/gutjnl-2017-314759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 32: 403–432, 2014. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 89.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 109: 867–879, 2011. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91–119, 2014. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 91.Tan Y, Sheng Z, Zhou P, Liu C, Zhao H, Song L, Li J, Zhou J, Chen Y, Wang L, Qian H, Sun Z, Qiao S, Xu B, Gao R, Yan H. Plasma trimethylamine N-oxide as a novel biomarker for plaque rupture in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 12: e007281, 2019. doi: 10.1161/CIRCINTERVENTIONS.118.007281. [DOI] [PubMed] [Google Scholar]
- 92.Thenappan T, Chan SY, Weir EK. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 315: H1322–H1331, 2018. doi: 10.1152/ajpheart.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thenappan T, Goel A, Marsboom G, Fang YH, Toth PT, Zhang HJ, Kajimoto H, Hong Z, Paul J, Wietholt C, Pogoriler J, Piao L, Rehman J, Archer SL. A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med 183: 1080–1091, 2011. doi: 10.1164/rccm.201008-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 360: j5492, 2018. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc 13: 276–284, 2016. doi: 10.1513/AnnalsATS.201509-599FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Traykova D, Schneider B, Chojkier M, Buck M. Blood microbiome quantity and the hyperdynamic circulation in decompensated cirrhotic patients. PLoS One 12: e0169310, 2017. doi: 10.1371/journal.pone.0169310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41: 296–310, 2014. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H, Hou L, Kwak D, Fassett J, Xu X, Chen A, Chen W, Blazar BR, Xu Y, Hall JL, Ge JB, Bache RJ, Chen Y. Increasing regulatory T cells with interleukin-2 and interleukin-2 antibody complexes attenuates lung inflammation and heart failure progression. Hypertension 68: 114–122, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang W, Wang YL, Chen XY, Li YT, Hao W, Jin YP, Han B. Dexamethasone attenuates development of monocrotaline-induced pulmonary arterial hypertension. Mol Biol Rep 38: 3277–3284, 2011. doi: 10.1007/s11033-010-0390-x. [DOI] [PubMed] [Google Scholar]
- 100.Whittle E, Leonard MO, Harrison R, Gant TW, Tonge DP. Multi-method characterization of the human circulating microbiome. Front Microbiol 9: 3266, 2019. doi: 10.3389/fmicb.2018.03266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23: 850–858, 2017. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 102.Wu Q, Turturice B, Wagner S, Huang Y, Gupta PK, Schott C, Metwally A, Ranjan R, Perkins D, Alegre ML, Finn P, Budinger GR, Shilling R, Bharat A. Gut microbiota can impact chronic murine lung allograft rejection. Am J Respir Cell Mol Biol 60: 131–134, 2019. doi: 10.1165/rcmb.2018-0139LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuille S, Reichardt N, Panda S, Dunbar H, Mulder IE. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS One 13: e0201073, 2018. doi: 10.1371/journal.pone.0201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Zhao R, Shi D, Sun S, Ren H, Zhao H, Wu W, Jin L, Sheng J, Shi Y. Characterization of the circulating microbiome in acute-on-chronic liver failure associated with hepatitis B. Liver Int 39: 1207–1216, 2019. doi: 10.1111/liv.14097. [DOI] [PubMed] [Google Scholar]
- 105.Zheng L, Zheng J, Xie Y, Li Z, Guo X, Sun G, Sun Z, Xing F, Sun Y. Serum gut microbe-dependent trimethylamine N-oxide improves the prediction of future cardiovascular disease in a community-based general population. Atherosclerosis 280: 126–131, 2019. doi: 10.1016/j.atherosclerosis.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 106.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB, Federici S, Cohen Y, Linevsky R, Rothschild D, Moor AE, Ben-Moshe S, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Shapiro H, Pevsner-Fischer M, Sharon I, Halpern Z, Segal E, Elinav E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174: 1388–1405.e21, 2018. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]