Abstract
cAMP is a critical second messenger of numerous endocrine signals affecting water-electrolyte transport in the renal tubule. Exchange protein directly activated by cAMP (Epac) is a relatively recently discovered downstream effector of cAMP, having the same affinity to the second messenger as protein kinase A, the classical cAMP target. Two Epac isoforms, Epac1 and Epac2, are abundantly expressed in the renal epithelium, where they are thought to regulate water and electrolyte transport, particularly in the proximal tubule and collecting duct. Recent characterization of renal phenotype in mice lacking Epac1 and Epac2 revealed a critical role of the Epac signaling cascade in urinary concentration as well as in Na+ and urea excretion. In this review, we aim to critically summarize current knowledge of Epac relevance for renal function and to discuss the applicability of Epac-based strategies in the regulation of systemic water-electrolyte homeostasis.
Keywords: cAMP, collecting duct, proximal tubule, renal transport
MOLECULAR ASPECTS OF cAMP-EPAC SIGNALING
Exchange protein directly activated by cAMP (Epac) was discovered in 1998 by two independent research groups in a quest of finding new cAMP-interacting partners exhibiting both cAMP-binding and guanine nucleotide exchange factor properties to mediate activation of small G proteins from the Ras family, specifically Rap1, in response to elevated cAMP levels independently from the classical protein kinase A (PKA) pathway (9, 16). Importantly, cAMP binds and activates Epac with a similar affinity as PKA (14). Due to the broad distribution in multiple tissues and organs, the Epac signaling cascade governs a novel and much less explored dimension of cAMP-dependent signaling mechanisms, which are often independent of PKA. Indeed, Epac signaling has been implicated in a wide spectrum of cellular processes, including regulation of insulin secretion, vascular permeability, regulation of intracellular Ca2+ concentration ([Ca2+]i) homeostasis, generation and shaping the action potentials in neurons and muscle cells, and regulation of intracellular pH, to name a few (25). Inappropriately activated Epac-dependent pathways have been found in malignant tumors, such as metastatic melanoma and gastric, breast, and pancreatic cancers (for a review, see Ref. 25). Moreover, several Epac1 and Epac2 polymorphisms have been linked to anxiety, depression, and autism disorders (1, 22).
In the absence of cAMP, both known Epac isoforms, Epac1 and Epac2, exist in a tight inactive configuration, where the COOH-terminal catalytic region containing the CDC25 homology domain is hindered due to a close spatial association with the cyclic nucleotide-binding motif (for a review, see Ref. 25) (see Fig. 1, A and B). Increases in intracellular cAMP levels, for instance after stimulation of Gs-coupled heterotrimeric G protein-coupled receptors by a hormone, result in direct cAMP binding to the cyclic nucleotide-binding motif. This induces conformational changes in Epac and lifts CDC25 inhibition, thereby enabling activation of Epac downstream effectors, most notably monomeric small G proteins Rap1 and Rap2 (8). While Epac1 and Epac2 share virtually identical molecular mechanisms of activation and signal transduction, there seems to be very little redundancy and compensation between them (25). This could be, in part, attributed to isoform-specific distribution in different tissues. Thus, whereas Epac1 expression is notably higher in the skeletal muscle, thyroid, ovary, adipose tissue, and kidney, Epac2 levels are more prominent in the central nervous system, pancreas, and adrenal gland (13). Epac1 and Epac2 localization within the same cell is also genuine with both isoforms existing in distinct macromolecular signaling complexes (i.e., signalomes) (21, 23, 24). This, in turn, allows isoform-specific control of discrete signaling pathways or different stages of the same pathway with minimal redundancy and compensation.
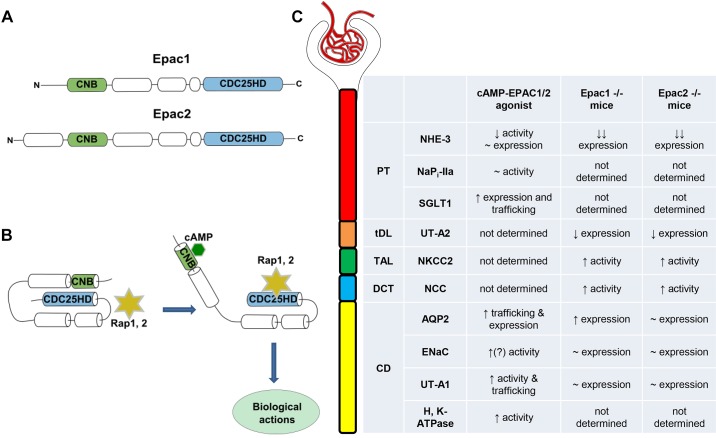
Fig. 1.
Exchange protein directly activated by cAMP (Epac) signaling in the regulation of renal tubular transport. A: an outline of major domain organization of Epac1 and Epac2 isoforms. Both isoforms contain one (for Epac1) or two (for Epac2) cyclic nucleotide binding (CNB) motif(s) at their regulatory domain at the NH2-terminus. CDC25 homology domain (CDC25-HD) is essential for interaction with Epac downstream effectors, mainly Rap1 and Rap2. B: schematic representation of Epac activation by cAMP. Binding of cAMP to the CNB motif promotes an open active Epac configuration, allowing enzymatically active CDC25-HD to interact with Rap1 and Rap2. C: summary table comparing the reported effects of pharmacological Epac stimulation and genetic deletion of Epac1 and Epac2 isoforms on the transporting systems in different segments of the renal tubule. AQP2, aquaporin 2; CD, collecting duct; DCT, distal convoluted tubule; ENaC, epithelial Na+ channel; NaPi-IIa, Na+-phosphate cotransporter type IIa; NCC, Na+-Cl− cotransporter; NHE3, Na+/H+ exchanger type 3; NKCC2, Na+-K+-2Cl− cotransporter; PT, proximal tubule; SGLT1, Na+-glucose cotransporter type 1; TAL, thick ascending limb; tDL, thin descending limb; UT-A2, urea transporter type A2; UT-A1, urea transporter type A1.
REGULATION OF RENAL TUBULE TRANSPORT BY THE EPAC SIGNALING CASCADE
A little over a decade ago, Li and associates (20) detected Epac1 and Epac2 expression with immunohistochemistry in almost all segments of the renal tubule, but not in the thin descending limb, of the rat and human kidney. Since both isoforms can be found at the apical and basolateral regions of renal epithelial cells, this indicates that the Epac signaling cascade might play a role in the regulation of water and solute transport. In the proximal tubule (PT), Epac1/2 expression was largely restricted to the brush border, which coincides with the place of Na+/H+ exchanger type 3 (NHE3) residency in these cells (20). Stimulation of cultured PT cells and kidney sections with an Epac-selective cAMP analog (8-pCPT-2′-O-Me-cAMP) inhibited NHE3-dependent H+ extrusion without changes in NHE3 expression or its inactive phosphorylated Ser552 NHE3 form (15). In contrast, stimulation of Epac did not affect cAMP-sensitive Na+-coupled inorganic phosphate cotransporter IIa (NaPi-IIa), also expressed at the brush border of PT cells. Moreover, Epac signaling had a major role in the stimulation of Na+-glucose cotransporter type 1 expression and trafficking via a mechanism involving caveolin-1 and F-actin in PT cells (19). Epac1 and Epac2 are also prominently expressed in the thick ascending limb and distal convoluted tubule (20). However, there is no currently available evidence in support of Epac regulation of electrolyte transport at these sites. In the collecting duct (CD), Epac2 was found in both aquaporin 2 (AQP2)-positive principal and AQP2-negative intercalated cells, whereas Epac1 expression was found rather in AQP2-negative intercalated cells (20). Stimulation of the cAMP-Epac pathway with 8-pCPT-2′-O-Me-cAMP recapitulated arginine vasopressin (AVP)-induced [Ca2+]i responses in inner medullary CDs and promoted apical AQP2 translocation independently of PKA (2, 29, 30). Furthermore, long-term stimulation of AQP2 expression by AVP might involve Epac signaling in cultured cortical CDs, with AVP increasing Epac1 but decreasing Epac2 expression (17). Consistent with its putative antidiuretic actions, the Epac activator 8-pCPT-2′-O-Me-cAMPS significantly augmented urea permeability in perfused rat inner medullary CDs by increasing phosphorylation and plasma membrane levels of urea transporter UT-A1 (27). Epithelial Na+ channel activity can also be regulated by cAMP-dependent signaling in response to AVP in split-opened CDs (3). Thus, it is quite possible that the Epac cascade contributes to this regulation, while direct evidence is currently missing. Finally, Epac can be also involved in the regulation of acid-base balance in the CD by stimulating activity of H+-K+-ATPase (18). A summary of the major Epac actions on transporting systems in different tubular segments is shown in Fig. 1C.
CONTRIBUTION OF EPAC ISOFORMS IN URINE PRODUCTION
The aforementioned studies have implicated Epac signaling in the regulation of the major transporting systems predominantly in the PT and CD at baseline and in response to hormonal inputs. At the same time, it is hard to grasp how the Epac-mediated regulation of renal transport systems can be extrapolated into changes in renal function from these important but focused studies. To remedy this, we recently explored how deletion of individual Epac isoforms affects urinary production in mice. Compared with Epac wild-type mice, both Epac1−/− and Epac2−/− mice exhibited polyuria and polydipsia in the presence of elevated AVP, suggesting deficient renal water conservation (4). Furthermore, the defect persisted after 24-h water deprivation, pointing to resistance to AVP actions by the renal tubule. However, Epac1 and Epac2 deletion had no effect on AVP-induced [Ca2+]i elevations in split-opened CDs (4). The most unexpected result was that ablation of individual Epac isoforms did not reduce AQP2 levels or compromise AQP2 trafficking (4). In fact, AQP2 levels were increased in Epac1−/− mice, and the AQP2 reporting signal was enriched in the apical region of CDs from both Epac1−/− and Epac2−/− mice (4). This suggests that deletion of individual Epac isoforms is not sufficient to disrupt AVP-dependent [Ca2+]i signaling and AQP2 trafficking, as was proposed from experiments using cAMP-Epac activators to concomitantly activate Epac1 and Epac2 (5, 30, 31). Thus, investigation of water balance and renal water handling on simultaneous deletion of both isoforms would be decisive in solving this conundrum.
Deletion of Epac1 and Epac2 also resulted in urea wasting on water deprivation (4), which is in agreement with a proposed role of the Epac cascade in stimulation of urea permeability by AVP (27). Since UT-A1 levels were unchanged in Epac1−/− and Epac2−/− mice, this effect is likely associated with reduced UT-A1 phosphorylation and/or defective translocation to the apical membrane in CD cells. Future studies are necessary to directly test this prediction. Moreover, both knockouts have significantly decreased UT-A2 levels, also indicating an impaired mechanism of urea recycling (4).
Since deletion of individual Epac isoforms did not compromise AVP-dependent AQP2 expression and localization in the CD, a defect in upstream segments of the renal tubule has to be responsible for the urinary concentrating defect in knockouts. In agreement with this notion, deletion of Epac1 and Epac2 caused a drastic (over 75%) reduction of NHE3 expression, without apparent changes in its subcellular localization in PT cells (4). Interestingly, the overall renal phenotype of Epac1−/− and Epac2−/− mice is very similar to that recently observed in mice with selective NHE3 deletion in the PT (12). This is quite unexpected, since NHE3 has been shown to be inhibited by cAMP as a result of dopamine and parathyroid hormone actions in the PT (6, 28). It is possible that Epac signaling is necessary to maintain NHE3 expression by stabilizing its interaction with the cytoskeleton at the brush-border membrane via a mechanism likely involving NHE3 regulator factor 1 (7, 11). Consistently, NHE3 regulator factor 1 renal levels were altered in Epac1−/− and Epac2−/− mice (4). Further studies are needed to explore this possibility.
Mice lacking Epac isoforms also have increased active (phosphorylated) levels of Na+-K+-2Cl− cotransporter type 2 and Na+-Cl− cotransporter, pointing to the augmented Na+ reabsorption in the thick ascending limb and distal convoluted tubule, respectively (4). While the direct effect of Epac deletion on the transporters cannot be excluded, this most likely represents a compensatory response to the diminished NHE3 expression in the PT. Of interest, epithelial Na+ channel levels in the CD were not upregulated, despite the increased aldosterone levels in Epac knockouts (4) (V. N. Tomilin, unpublished observations). This argues that the Epac cascade might also contribute to fine-tuning Na+ reabsorption in the aldosterone-sensitive distal nephron during variations in dietary Na+. Thus, inhibition of Epac would favor natriuresis and might be of clinical relevance for treatment of hypertension associated with high circulating aldosterone. Again, careful future investigations are required to tackle complex contribution of individual Epac isoforms and their synergy in control of renal salt handling. A summary of changes in transporting systems in Epac1−/− and Epac2−/− mice is shown in Fig. 1C.
CONCLUSIONS AND OTHER THOUGHTS
With the development of pharmacological tools and genetic animal models, we now begin to appreciate the significance of previously underappreciated cAMP-Epac1/2 pathways in controlling renal water-electrolyte handling and tubular transport. Epac1 and Epac2 play a notable role in the regulation of urinary production by affecting multiple transporting systems in the PT and CD. The seeming discordance between some of the observed effects induced by Epac stimulation and deletion of Epac isoforms simply indicates that we are just peeking into the complex and multifactorial relevance of Epac signaling in the regulation of urinary production and water-solute balance. In addition, the cAMP-Epac cascade has also been proposed to play a role in reducing oxidative stress after ischemia-reperfusion renal injury and decreasing renal fibrosis (10, 26). There is no doubt, though, that future advancements in understanding Epac significance for kidney function will be of high clinical relevance.
GRANTS
The research program in O. Pochynyuk’s laboratory is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-095029 and DK-117865 (to O. Pochynyuk) and American Heart Association Grant AHA-19CDA34660148 (to V. N. Tomilin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.N.T. and O.M.P. conceived and designed research; V.N.T. prepared figures; O.M.P. drafted manuscript; V.N.T. and O.M.P. edited and revised manuscript; V.N.T. and O.M.P. approved final version of manuscript.
REFERENCES
- 1.Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G, Parr J, Beyer KS, Klauck SM, Poustka A, Bailey AJ, Monaco AP, Maestrini E; International Molecular Genetic Study of Autism Consortium (IMGSAC) . Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry 8: 916–924, 2003. doi: 10.1038/sj.mp.4001340. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian L, Sham JS, Yip KP. Calcium signaling in vasopressin-induced aquaporin-2 trafficking. Pflugers Arch 456: 747–754, 2008. doi: 10.1007/s00424-007-0371-7. [DOI] [PubMed] [Google Scholar]
- 3.Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol 297: F1411–F1418, 2009. doi: 10.1152/ajprenal.00371.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherezova A, Tomilin V, Buncha V, Zaika O, Ortiz PA, Mei F, Cheng X, Mamenko M, Pochynyuk O. Urinary concentrating defect in mice lacking Epac1 or Epac2. FASEB J 33: 2156–2170, 2019. doi: 10.1096/fj.201800435R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- 6.Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem 275: 31601–31608, 2000. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham R, Steplock D, Wang F, Huang H, E X, Shenolikar S, Weinman EJ. Defective parathyroid hormone regulation of NHE3 activity and phosphate adaptation in cultured NHERF-1−/− renal proximal tubule cells. J Biol Chem 279: 37815–37821, 2004. doi: 10.1074/jbc.M405893200. [DOI] [PubMed] [Google Scholar]
- 8.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 275: 20829–20836, 2000. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 9.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 10.Ding H, Bai F, Cao H, Xu J, Fang L, Wu J, Yuan Q, Zhou Y, Sun Q, He W, Dai C, Zen K, Jiang L, Yang J. PDE/cAMP/Epac/C/EBP-β signaling cascade regulates mitochondria biogenesis of tubular epithelial cells in renal fibrosis. Antioxid Redox Signal 29: 637–652, 2018. doi: 10.1089/ars.2017.7041. [DOI] [PubMed] [Google Scholar]
- 11.Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. NHE3 regulatory complexes. J Exp Biol 212: 1638–1646, 2009. doi: 10.1242/jeb.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int 92: 397–414, 2017. doi: 10.1016/j.kint.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355–375, 2010. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 14.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol 577: 5–15, 2006. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honegger KJ, Capuano P, Winter C, Bacic D, Stange G, Wagner CA, Biber J, Murer H, Hernando N. Regulation of sodium-proton exchanger isoform 3 (NHE3) by PKA and exchange protein directly activated by cAMP (EPAC). Proc Natl Acad Sci USA 103: 803–808, 2006. [Erratum in Proc Natl Acad Sci USA 103: 4328, 2006.] doi: 10.1073/pnas.0503562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279, 1998. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 17.Kortenoeven ML, Trimpert C, van den Brand M, Li Y, Wetzels JF, Deen PM. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am J Physiol Renal Physiol 302: F1395–F1401, 2012. doi: 10.1152/ajprenal.00376.2011. [DOI] [PubMed] [Google Scholar]
- 18.Laroche-Joubert N, Marsy S, Luriau S, Imbert-Teboul M, Doucet A. Mechanism of activation of ERK and H-K-ATPase by isoproterenol in rat cortical collecting duct. Am J Physiol Renal Physiol 284: F948–F954, 2003. doi: 10.1152/ajprenal.00394.2002. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Kim MO, Ryu JM, Han HJ. Regulation of SGLT expression and localization through Epac/PKA-dependent caveolin-1 and F-actin activation in renal proximal tubule cells. Biochim Biophys Acta 1823: 971–982, 2012. doi: 10.1016/j.bbamcr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Konings IB, Zhao J, Price LS, de Heer E, Deen PM. Renal expression of exchange protein directly activated by cAMP (Epac) 1 and 2. Am J Physiol Renal Physiol 295: F525–F533, 2008. doi: 10.1152/ajprenal.00448.2007. [DOI] [PubMed] [Google Scholar]
- 21.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 22.Middeldorp CM, Vink JM, Hettema JM, de Geus EJ, Kendler KS, Willemsen G, Neale MC, Boomsma DI, Chen X. An association between Epac-1 gene variants and anxiety and depression in two independent samples. Am J Med Genet B Neuropsychiatr Genet 153B: 214–219, 2010. doi: 10.1002/ajmg.b.30976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T, Seino S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol 219: 652–658, 2009. doi: 10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- 24.Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem 277: 26581–26586, 2002. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- 25.Robichaux WG III, Cheng X. Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development. Physiol Rev 98: 919–1053, 2018. doi: 10.1152/physrev.00025.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stokman G, Qin Y, Booij TH, Ramaiahgari S, Lacombe M, Dolman ME, van Dorenmalen KM, Teske GJ, Florquin S, Schwede F, van de Water B, Kok RJ, Price LS. Epac-Rap signaling reduces oxidative stress in the tubular epithelium. J Am Soc Nephrol 25: 1474–1485, 2014. doi: 10.1681/ASN.2013070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Klein JD, Blount MA, Martin CF, Kent KJ, Pech V, Wall SM, Sands JM. Epac regulates UT-A1 to increase urea transport in inner medullary collecting ducts. J Am Soc Nephrol 20: 2018–2024, 2009. doi: 10.1681/ASN.2008121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiederkehr MR, Di Sole F, Collazo R, Quiñones H, Fan L, Murer H, Helmle-Kolb C, Moe OW. Characterization of acute inhibition of Na/H exchanger NHE-3 by dopamine in opossum kidney cells. Kidney Int 59: 197–209, 2001. doi: 10.1046/j.1523-1755.2001.00480.x. [DOI] [PubMed] [Google Scholar]
- 29.Yip KP. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538: 891–899, 2002. doi: 10.1113/jphysiol.2001.012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yip KP. Epac-mediated Ca2+ mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol 291: F882–F890, 2006. doi: 10.1152/ajprenal.00411.2005. [DOI] [PubMed] [Google Scholar]
- 31.Yip KP, Sham JS. Mechanisms of vasopressin-induced intracellular Ca2+ oscillations in rat inner medullary collecting duct. Am J Physiol Renal Physiol 300: F540–F548, 2011. doi: 10.1152/ajprenal.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]