Abstract
Natriuretic peptides (NPs) are well known to promote renal Na+ excretion, counteracting the effects of the renin-angiotensin-aldosterone system. Thus, NPs serve as a key component in the maintenance of blood pressure, influencing fluid retention capabilities via osmoregulation. Recently, NPs have been shown to affect lipolysis and enhance lipid oxidation and mitochondrial respiration. Here, we provide an overview of current knowledge about the relationship between NPs and mitochondria-mediated processes such as reactive oxygen species production, Ca2+ signaling, and apoptosis. Establishing a clear physiological and mechanistic connection between NPs and mitochondria in the cardiovascular system will open new avenues of research aimed at understanding and potentially using it as a therapeutic target from a completely new angle.
Keywords: atrial natriuretic peptide, cGMP, guanylate cyclase, mitochondria, natriuretic peptides
NATRIURETIC PEPTIDES AND THEIR FUNCTIONS
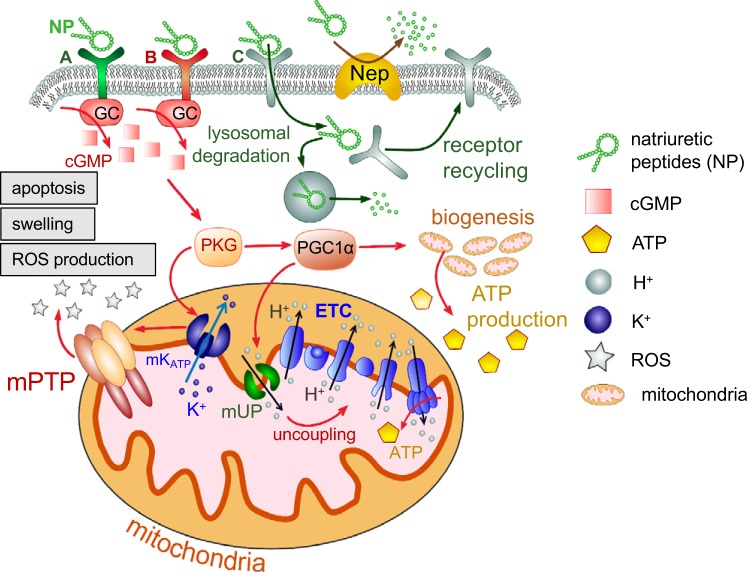
Natriuretic peptides (NPs) are hormones secreted from the heart to promote Na+ excretion by the kidneys. Currently, there are three known peptides in the NP family: atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP). Binding of these NPs to their respective guanylate cyclase (GC)-coupled receptors results in the conversion of GTP to the secondary messenger cGMP. cGMP activates a plethora of downstream signaling cascades, which leads to various biological responses (40). So far three natriuretic peptide receptors have been discovered: natriuretic peptide receptor A (NPRA; GC coupled), natriuretic peptide receptor B (NPRB; GC coupled), and natriuretic peptide receptor C (NPRC). ANP and BNP have high affinity to NPRA, whereas CNP has a higher affinity to NPRB. NPRC serves as a “clearance receptor” for all three natriuretic peptides (25). Upon binding to NPRC, the NP is internalized and degraded by lysosomal ligand hydrolysis, and NPRC is recycled back in the plasma membrane (38). Degradation of NPs is also modulated by proteolytic enzymes such as neprilysin or the insulin-degrading enzyme in the extracellular space (38). A simplified schematic illustration of the NP receptor binding, downstream signaling, and degradation is shown in Fig. 1.
Fig. 1.
Schematic overview of natriuretic peptide (NP)-mediated regulation of mitochondria function in various tissues. Upon binding to natriuretic peptide receptor A (NPRA) and natriuretic peptide receptor B (NPRB), NPs trigger the receptors’ guanylyl cyclase (GC) activity and production of cGMP. cGMP activates PKG, which can increase the peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α level and therefore stimulate mitochondria biogenesis and ATP synthesis. PGC-1α is also capable of activating mitochondrial uncoupling protein (mUP), which uncouples the electron transport chain (ETC). In addition, PKG is known to activate the mitochondrial ATP-sensitive K+ (mKATP) channel, which attenuates mitochondrial permeability transition pore (mPTP) formation, mitochondria swelling, and eventually apoptosis. Binding of NPs to natriuretic peptide receptor C (NPRC) leads to internalization of the NP-NPRC complex, lysosomal degradation of NPs, and receptor recycling back to the plasma membrane. Circulating levels of NPs are regulated by neprilysin (Nep), a membrane-bound metalloproteinase capable of degrading NPs.
Well-established physiological effects of NPs include a decrease in blood pressure via vasorelaxation, antagonism of the renin-angiotensin-aldosterone system, and inhibition of cardiac hypertrophy (3). Much of the natriuretic activity of NPs is mainly attributed to an inhibitory effect on various Na+-reabsorbing channels and transporters along the nephron. For example, NPs have been shown to inhibit tubular-basolateral Na+-K+-ATPase, Na+-K+-Cl− cotransporter, and epithelial Na+ channel activity as well as vasopressin-induced renal water reabsorption (50). The cumulative inhibitory effect on these channels and transporters leads to diminished renal Na+ reabsorption and thus decreased water retention and blood pressure.
Recently, it has been discovered that NPs are involved in lipolysis, lipid oxidation, and mitochondrial respiration (40). Evidence of a correlation between the activity of the NP system and mitochondrial respiration and biogenesis has been explored to some extent; however, little is known about these pathways in the kidney. Because mitochondria play a major role in programmed cell death and ATP provision for general cell metabolic needs, further exploration of their relationship with the NP system may lead to a better understanding of how NPs affect various cardiorenal and metabolic functions in health and disease and to the discovery of novel pharmacological targets. This review will focus on the effects of NPs on mitochondria metabolism and biogenesis and the potential importance of this interaction for cardiorenal disease.
MITOCHONDRIA IN THE CARDIORENAL SYSTEM
Mitochondria play key roles in a plethora of physiological and pathophysiological processes. First and foremost, they are the source of energy (ATP) in most tissues and organs (8). Additionally, mitochondria mediate Ca2+ signaling, reactive oxygen species (ROS) and nitrogen species production, as well as metabolism of endogenous and exogenous compounds. In the cardiovascular system in particular, mitochondria produce nitric oxide, which affects blood pressure via smooth muscle relaxation (43). Muscle cells are rich with mitochondria and rely on them to provide ATP for contraction, and the role of mitochondria as regulators of Ca2+ is very important in this case (16). Another critical function of mitochondria is the initiation of cell death (apoptosis and necrosis), which is an essential physiological response that is tightly controlled but, under certain conditions, may cause damage. For example, during ischemia-reperfusion (I/R) injury of the heart, mitochondria are involved in the processes that injure the cardiomyocytes and cardiac vasculature (through activation of mitochondria membrane protein complexes referred to as “death channels,” impairment of the oxidative phosphorylation pathway, and decreased antioxidant defenses; see Refs. 9, 27, and 49).
The structure, density, and function of the mitochondria vary greatly depending on the needs of different tissues and organs. For instance, some cells are more dependent on glycolysis and either do not contain a large number of mitochondria or do not have them at all, like red blood cells (5). In other cell types, with considerable energy needs, mitochondria density is very high, and cell function is therefore directly dependent on mitochondria health (41). For instance, kidney cells have the second highest mitochondrial content and oxygen consumption rate after the heart (35, 37), with considerable energy needs (42). Disruption of mitochondrial homeostasis in the early stages of acute kidney injury (AKI) is an important factor that has been shown to drive tubular injury and renal dysfunction. Hyperglycemia-induced ATP depletion triggers changes in renal mitochondrial morphology that lead to the onset of diabetic nephropathy in diabetes mellitus (2). A recent study by Kruger et al. (22) revealed that normalizing tubular cell mitochondrial function and energy balance could be an important preventative strategy in kidney disease. Therefore, correcting abnormal electron transport chain function directly, and/or by targeting the pathways that regulate mitochondrial biogenesis, may be used to improve renal disease outcomes by restoring mitochondrial function and thus assisting with organ repair (46).
THE NP-MITOCHONDRIA SIGNALING AXIS IN PHYSIOLOGY AND PATHOPHYSIOLOGY
NPs have been shown to have diverse effects on mitochondria in various tissues. It has been established that NPs can promote mitochondrial biogenesis and fat oxidation (28), and ANP has been reported to upregulate mitochondrial fat oxidative capacity and respiration in mouse skeletal muscle (33). In addition, a positive correlation was established in the muscles between gene expression of Npra and Ppargc1a, the master regulator for mitochondrial biogenesis, which encodes for peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α protein (17). In the same cells, BNP has been demonstrated to decrease mitochondrial ROS production and lead to the opening of mitochondrial ATP-sensitive (mKATP) K+ channels (48). A study (19) involving adipocytes revealed increased intracellular temperature and increased mRNA levels of uncoupling protein-1 after 1 h of incubation with ANP, whereas the intracellular ATP concentration was not changed, implying the presence of uncoupled mitochondrial respiration.
One of the major downstream NP-related cellular events is an increase in cGMP levels that results from activation of the NP receptors’ GC activity (4). cGMP is a common regulator of ion channel conductance, glycogenolysis, and cellular apoptosis (1). In addition, it is a known regulator of smooth muscle cell contractility and, therefore, vascular blood flow (39). The effects of cGMP on mitochondria have been extensively described in the literature; cGMP induces mitochondrial biogenesis in vitro and in vivo and therefore might have beneficial effects in AKI and other pathologies characterized by mitochondrial dysfunction and suppressed mitochondrial biogenesis (51). cGMP has been shown to inhibit the formation of mitochondrial permeability transition pore (mPTP) and reduce cell death from I/R injury in the heart and other tissues (11). Furthermore, it is believed that PGC-1α gene expression, along with the expression of several oxidative phosphorylation pathway genes, is partially induced in a cGMP-dependent manner (17). CNP, acting through NPRB, can stimulate the production of cGMP in neurons (53). Some studies have suggested that NPs, specifically via the GC-cGMP and cGMP-dependent protein kinase pathway, can promote skeletal muscle mitochondrial biogenesis and fat oxidation to prevent obesity and glucose intolerance (28); NPs were shown to induce lipolysis and acutely increase free fatty acid availability in humans (3). At the same time, other publications revealed that cGMP, on the other hand, can decrease the efficiency of mitochondrial ATP synthesis (without impacting mitochondrial content or ultrastructure; see Ref. 29). Despite the abundant evidence of the importance of NP-mediated cGMP effects in various tissues and organs, further research is required on the specific mechanisms mediating these events, especially in disease states.
The known effects of NPs on Ca2+ signaling are of particular interest, especially taking into consideration the importance of mitochondria in Ca2+ handling; however, the data are limited. BNP exerted protective effects in I/R injury by blocking the mitochondrial Ca2+ uniporter (44). In addition, BNP has been shown to modulate overall Ca2+ levels in airway smooth muscle cells (36). In addition, in failing cardiomyocytes, ANP was able to normalize aberrant diastolic Ca2+ sparks, through suppression of mitochondrial ROS generation, which might be a contributing factor in cardiomyocyte survival during heart failure (34). An increase in plasma NP levels is typically used as a clinical marker of cardiac dysfunction and heart failure (13). Several studies have shown that, in I/R injury, NPs may exhibit protective effects through mitochondria-mediated mechanisms. For instance, intravenous administration of ANP (30 min) inhibited I/R-induced mitochondrial damage in the pig myocardium. Further examination of myocardial tissue using electron microscopy revealed swollen mitochondria with sparse cristae in the control group, as opposed to normal mitochondrial morphology in the ANP-treated group. BNP showed similar protection against I/R in the skeletal muscle of Wistar rats (48). It has also been reported that BNP did not exhibit such protective functions when mitochondrial mKATP channels were blocked, suggesting that opening of this channel can contribute to the observed beneficial effects (48). Another study that investigated into mKATP channels in rat myocardial mitochondria indicated that cGMP mediates the opening of this channel (11) through the activation of PKG that leads to mitochondrial ROS production (12). In this case, ROS production would serve as a secondary messenger, resulting in the activation of downstream kinases, including PKC, which then blocks the mPTP. In support of this, BNP prevented I/R-induced mPTP opening in cultured cardiomyocytes and exerted protective effects by suppressing the mitochondrial death pathway in a phosphatidylinositol 3-kinase/Akt-dependent manner (45).
ANP is an intracrine signaling molecule, and adrenal cortical cells are among the target cells that have been shown to internalize it. High-affinity binding sites for ANP have been identified on adrenal mitochondrial membranes, and intracellular ANP has been found in association with mitochondria (18, 30, 31). Interestingly, the published data are very sparse as to the regulation of mitochondria function by NPs in the kidney. A study in the early 1980s showed that direct application of recombinant ANP to renal mitochondria inhibited step 3 mitochondrial respiration and decreased the ADP-to-oxygen ratio; ANP also induced dramatic mitochondrial swelling (20). A 2016 study by Moriyama et al. (32) pointed out that oxygen consumption in renal mitochondria was attenuated by ANP in Sprague-Dawley rats. A possibility of a link between NPs and mitochondria in renal tissues has not been explored in enough detail; however, plenty of studies have shown that cGMP (produced because of GC activity of NPRA and NPRB) can play an important role in the kidney (10, 21). Based on the literature, we can hypothesize that the effects of ANP on mitochondria function can be cGMP mediated, since cGMP is known to prevent mitochondria permeability transition, decrease mitochondrial depolarization, and reduce cell death (6, 10, 11, 21, 24, 47). In renal tissue, activation of particulate GC A (pGC-A) in the kidney yields increased Na+ and water excretion (7, 14). There are several mechanisms that contribute to this effect, including reduced Na+ reabsorption, an increased glomerular membrane ultrafiltration coefficient, and elevated glomerular hydrostatic pressure (10). pGC-A/cGMP activity is also involved in the modulation of renal vascular function and glomerular filtration rate. Specifically, it was found that cGMP induces efferent arteriole constriction and afferent arteriole dilation, thus enhancing the glomerular filtration rate (15, 26). Renal dysfunction is observed in rodents with pGC-A gene knockout, which also exhibit augmented fibrosis, indicative of a cGMP-dependent protective effect against fibrosis (23, 52). Despite these physiologically relevant findings, data linking NPs and their receptor activity via cGMP to mitochondria function or associated cascades are essentially lacking.
CONCLUSIONS
It is becoming clear that NPs play a role in mitochondria functionality, especially in the heart and skeletal muscles. However, the overall effect of NP on mitochondria is not well defined, and further studies are warranted before NPs can be targeted in mitochondria-related therapeutic pathways. Although the effects of NPs on renal function are known, there is little knowledge on how NPs affect renal mitochondria functionality and biogenesis. Elucidation of the precise mechanisms of this NP-mitochondria interaction could potentially lead to novel therapeutic mechanisms for mitochondria-affecting diseases, such as AKI and chronic kidney disease.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R00-DK-105160, Polycystic Kidney Disease Foundation Grant 221G18a, and American Physiological Society Research Career Enhancement and Lazaro J. Mandel Young Investigator awards (to D. V. Ilatovskaya) and by NIH Grant U54-DA-016511 and Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award IK2BX003922 (to K. Y. DeLeon-Pennell). This work was also financially supported, in part, by the 2019 American Physiological Society S&R Foundation Ryuji Ueno Award (to K. Y. DeLeon-Pennell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D., A.B.N., and D.V.I. prepared figures; M.D., A.B.N., K.Y.D.-P., and D.V.I. drafted manuscript; M.D., A.B.N., K.Y.D.-P., and D.V.I. edited and revised manuscript; M.D., A.B.N., K.Y.D.-P., and D.V.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mikhail Fomin, Andrey Ilatovskiy, Tengis Pavlov, and Yuliia Kashyrina for critical reading of this review and helpful edits.
REFERENCES
- 1.Berisha F, Nikolaev VO. Cyclic nucleotide imaging and cardiovascular disease. Pharmacol Ther 175: 107–115, 2017. doi: 10.1016/j.pharmthera.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkenfeld AL, Adams F, Schroeder C, Engeli S, Jordan J. Metabolic actions could confound advantageous effects of combined angiotensin II receptor and neprilysin inhibition. Hypertension 57: e4–e5, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165159. [DOI] [PubMed] [Google Scholar]
- 4.Borán MS, Baltrons MA, García A. The ANP-cGMP-protein kinase G pathway induces a phagocytic phenotype but decreases inflammatory gene expression in microglial cells. Glia 56: 394–411, 2008. doi: 10.1002/glia.20618. [DOI] [PubMed] [Google Scholar]
- 5.Brinkkoetter PT, Bork T, Salou S, Liang W, Mizi A, Ozel C, Koehler S, Hagmann HH, Ising C, Kuczkowski A, Schnyder S, Abed A, Schermer B, Benzing T, Kretz O, Puelles VG, Lagies S, Schlimpert M, Kammerer B, Handschin C, Schell C, Huber TB. Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep 27: 1551–1566.e5, 2019. doi: 10.1016/j.celrep.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown GC. Nitric oxide and mitochondria. Front Biosci 12: 1024–1033, 2007. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 7.Burnett JC Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol Renal Fluid Electrolyte Physiol 247: F863–F866, 1984. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 8.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17: 65–134, 1956. [DOI] [PubMed] [Google Scholar]
- 9.Charles AL, Guilbert AS, Bouitbir J, Goette-Di Marco P, Enache I, Zoll J, Piquard F, Geny B. Effect of postconditioning on mitochondrial dysfunction in experimental aortic cross-clamping. Br J Surg 98: 511–516, 2011. doi: 10.1002/bjs.7384. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Burnett JC. Particulate guanylyl cyclase A/cGMP signaling pathway in the kidney: physiologic and therapeutic indications. Int J Mol Sci 19: 1006, 2018. doi: 10.3390/ijms19041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res 77: 344–352, 2007. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 12.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jabůrek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol 290: H406–H415, 2006. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 13.Coué M, Moro C. Natriuretic peptide control of energy balance and glucose homeostasis. Biochimie 124: 84–91, 2016. doi: 10.1016/j.biochi.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 14.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94, 1981. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 15.Dunn BR, Ichikawa I, Pfeffer JM, Troy JL, Brenner BM. Renal and systemic hemodynamic effects of synthetic atrial natriuretic peptide in the anesthetized rat. Circ Res 59: 237–246, 1986. doi: 10.1161/01.RES.59.3.237. [DOI] [PubMed] [Google Scholar]
- 16.Eisner V, Csordás G, Hajnóczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle−pivotal roles in Ca2+ and reactive oxygen species signaling. J Cell Sci 126: 2965–2978, 2013. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest 122: 4675–4679, 2012. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heisler S. Direct binding of atrial natriuretic factor to adrenocortical mitochondria. Eur J Pharmacol 162: 281–288, 1989. doi: 10.1016/0014-2999(89)90291-4. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Nagoshi T, Yoshii A, Kashiwagi Y, Tanaka Y, Ito K, Yoshino T, Tanaka TD, Yoshimura M. The thermogenic actions of natriuretic peptide in brown adipocytes: the direct measurement of the intracellular temperature using a fluorescent thermoprobe. Sci Rep 7: 12978, 2017. doi: 10.1038/s41598-017-13563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohashi N, Trippodo NC, MacPhee AA, Frohlich ED, Cole FE. Rat atrial natriuretic peptides inhibit oxygen consumption by rat kidney. Hypertension 7: 491–498, 1985. doi: 10.1161/01.HYP.7.4.491. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan SM, Kraehling JR, Eitner F, Bénardeau A, Sandner P. The impact of the nitric oxide (NO)/soluble guanylyl cyclase (sGC) signaling cascade on kidney health and disease: a preclinical perspective. Int J Mol Sci 19: 1712, 2018. doi: 10.3390/ijms19061712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruger C, Nguyen TT, Breaux C, Guillory A, Mangelli M, Fridianto KT, Kovalik JP, Burk DH, Noland RC, Mynatt R, Stadler K. Proximal tubular cell-specific ablation of carnitine acetyltransferase causes tubular disease and secondary glomerulosclerosis. Diabetes 68: 819–831, 2019. doi: 10.2337/db18-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Gogulamudi VR, Periasamy R, Raghavaraju G, Subramanian U, Pandey KN. Inhibition of HDAC enhances STAT acetylation, blocks NF-κB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice. Am J Physiol Renal Physiol 313: F781–F795, 2017. doi: 10.1152/ajprenal.00166.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Rehman H, Krishnasamy Y, Lemasters JJ, Zhong Z. 8-pCPT-cGMP prevents mitochondrial depolarization and improves the outcome of steatotic partial liver transplantation. Int J Physiol Pathophysiol Pharmacol 9: 69–83, 2017. [PMC free article] [PubMed] [Google Scholar]
- 25.Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science 238: 675–678, 1987. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- 26.Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 324: 473–476, 1986. doi: 10.1038/324473a0. [DOI] [PubMed] [Google Scholar]
- 27.Maximilian Buja L. Mitochondria in ischemic heart disease. Adv Exp Med Biol 982: 127–140, 2017. doi: 10.1007/978-3-319-55330-6_7. [DOI] [PubMed] [Google Scholar]
- 28.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 58: 2880–2892, 2009. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon Y, Balke JE, Madorma D, Siegel MP, Knowels G, Brouckaert P, Buys ES, Marcinek DJ, Percival JM. Nitric oxide regulates skeletal muscle fatigue, fiber type, microtubule organization, and mitochondrial ATP synthesis efficiency through cGMP-dependent mechanisms. Antioxid Redox Signal 26: 966–985, 2017. doi: 10.1089/ars.2016.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel G, Chabot JG, Garcia-Caballero T, Gossard F, Dihl F, Belles-Isles M, Heisler S. Synthesis, internalization, and localization of atrial natriuretic peptide in rat adrenal medulla. Endocrinology 123: 149–158, 1988. doi: 10.1210/endo-123-1-149. [DOI] [PubMed] [Google Scholar]
- 31.Morel G, Mesguich P, Chabot JG, Belles-Isles M, Jeandel L, Heisler S. Internalization of atrial natriuretic peptide by adrenal glomerulosa cells. Biol Cell 65: 181–188, 1989. doi: 10.1111/j.1768-322X.1989.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 32.Moriyama T, Kanmura Y, Lindahl SG. Atrial natriuretic peptide attenuation of renal ischemia-reperfusion injury after major surgery. J Surg Res 201: 213–218, 2016. doi: 10.1016/j.jss.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 33.Moro C. Natriuretic peptides and fat metabolism. Curr Opin Clin Nutr Metab Care 16: 645–649, 2013. doi: 10.1097/MCO.0b013e32836510ed. [DOI] [PubMed] [Google Scholar]
- 34.Murakami W, Kobayashi S, Susa T, Nanno T, Ishiguchi H, Myoren T, Nishimura S, Kato T, Hino A, Oda T, Okuda S, Yamamoto T, Yano M. Recombinant atrial natriuretic peptide prevents aberrant Ca2+ leakage through the ryanodine receptor by suppressing mitochondrial reactive oxygen species production induced by isoproterenol in failing cardiomyocytes. PLoS One 11: e0163250, 2016. doi: 10.1371/journal.pone.0163250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor PM. Renal oxygen delivery: matching delivery to metabolic demand. Clin Exp Pharmacol Physiol 33: 961–967, 2006. doi: 10.1111/j.1440-1681.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 36.Orlandi A, Calzetta L, Doldo E, Tarquini C, Matera MG, Passeri D. Brain natriuretic peptide modulates calcium homeostasis and epidermal growth factor receptor gene signalling in asthmatic airways smooth muscle cells. Pulm Pharmacol Ther 31: 51–54, 2015. doi: 10.1016/j.pupt.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123, 2008. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J 278: 1808–1817, 2011. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem 277: 3310–3317, 2002. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- 40.Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther 144: 12–27, 2014. doi: 10.1016/j.pharmthera.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Schuster VL, Bonsib SM, Jennings ML. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol 251: C347–C355, 1986. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- 42.Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol 48: 9–31, 1986. doi: 10.1146/annurev.ph.48.030186.000301. [DOI] [PubMed] [Google Scholar]
- 43.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491: 473–477, 2012. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Deng T, Lu N, Yan M, Zheng X. B-type natriuretic peptide protects cardiomyocytes at reperfusion via mitochondrial calcium uniporter. Biomed Pharmacother 64: 170–176, 2010. doi: 10.1016/j.biopha.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Zhang Y, Yan M, Wu Y, Zheng X. B-type natriuretic peptide-induced cardioprotection against reperfusion is associated with attenuation of mitochondrial permeability transition. Biol Pharm Bull 32: 1545–1551, 2009. doi: 10.1248/bpb.32.1545. [DOI] [PubMed] [Google Scholar]
- 46.Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int 90: 997–1011, 2016. doi: 10.1016/j.kint.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Takuma K, Phuagphong P, Lee E, Mori K, Baba A, Matsuda T. Anti-apoptotic effect of cGMP in cultured astrocytes: inhibition by cGMP-dependent protein kinase of mitochondrial permeable transition pore. J Biol Chem 276: 48093–48099, 2001. doi: 10.1074/jbc.M108622200. [DOI] [PubMed] [Google Scholar]
- 48.Talha S, Bouitbir J, Charles AL, Zoll J, Goette-Di Marco P, Meziani F, Piquard F, Geny B. Pretreatment with brain natriuretic peptide reduces skeletal muscle mitochondrial dysfunction and oxidative stress after ischemia-reperfusion. J Appl Physiol (1985) 114: 172–179, 2013. doi: 10.1152/japplphysiol.00239.2012. [DOI] [PubMed] [Google Scholar]
- 49.Thaveau F, Zoll J, Rouyer O, Chafke N, Kretz JG, Piquard F, Geny B. Ischemic preconditioning specifically restores complexes I and II activities of the mitochondrial respiratory chain in ischemic skeletal muscle. J Vasc Surg 46: 541–547, 2007. doi: 10.1016/j.jvs.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 50.Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Renal Physiol 308: F1047–F1055, 2015. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitaker RM, Wills LP, Stallons LJ, Schnellmann RG. cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J Pharmacol Exp Ther 347: 626–634, 2013. doi: 10.1124/jpet.113.208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshihara F, Tokudome T, Kishimoto I, Otani K, Kuwabara A, Horio T, Kawano Y, Kangawa K. Aggravated renal tubular damage and interstitial fibrosis in mice lacking guanylyl cyclase-A (GC-A), a receptor for atrial and B-type natriuretic peptides. Clin Exp Nephrol 19: 197–207, 2015. doi: 10.1007/s10157-014-0982-1. [DOI] [PubMed] [Google Scholar]
- 53.Zielińska M, Fresko I, Konopacka A, Felipo V, Albrecht J. Hyperammonemia inhibits the natriuretic peptide receptor 2 (NPR-2)-mediated cyclic GMP synthesis in the astrocytic compartment of rat cerebral cortex slices. Neurotoxicology 28: 1260–1263, 2007. doi: 10.1016/j.neuro.2007.05.012. [DOI] [PubMed] [Google Scholar]