Abstract
Diabetic kidney disease (DKD) affects ∼40% of patients with diabetes and is associated with high mortality rates. Among different cellular targets in DKD, podocytes, highly specialized epithelial cells of the glomerular filtration barrier, are injured in the early stages of DKD. Both clinical and experimental data support the role of preserved insulin signaling as a major contributor to podocyte function and survival. However, little is known about the key modulators of podocyte insulin signaling. This review summarizes the novel knowledge that intracellular lipids such as cholesterol and sphingolipids are major determinants of podocyte insulin signaling. In particular, the implications of these lipids on DKD development, progression, and treatment will be addressed.
Keywords: ceramide-1-phosphate, cholesterol, diabetic kidney disease, insulin signaling, podocyte, sphingolipids, sphingosine-1-phosphate
INTRODUCTION
The increasing prevalence of type 2 diabetes mellitus worldwide has resulted in a rise in diabetic kidney disease (DKD). DKD that is due to either type 1 (T1D) or type 2 diabetes mellitus (T2D) is the most common cause of end-stage kidney disease (ESKD) in the world (165). The natural history of DKD is characterized by progressive albuminuria and/or gradual decline in estimated glomerular filtration rate (eGFR) even in the absence of albuminuria.
Multifactorial intervention trials targeting glycemic control, blood pressure, and lifestyle interventions have demonstrated that current treatments slow but do not halt the progression of DKD in both T1D (73) and T2D (55). One of the major obstacles for a definitive cure for DKD resides in the fact that the pathogenesis of DKD remains largely unknown. In fact, key glomerular cell constituents of the renal glomerular filtration barrier are affected in diabetes not only by high glucose and altered hemodynamics but also by a large variety of endocrine and autocrine factors (42, 67).
Insulin resistance is a condition in which cells fail to respond to the normal action of insulin. Insulin resistance can occur as a consequence of a mutation of the insulin receptor (IR) and downstream mediators or it can be caused by a multiplicity of factors affecting proper phosphoinositide 3-kinase (PI3K)/Akt pathway signaling responsible for glucose transporters trafficking among other metabolic pathways. Although the glomerular filtration barrier consists of three cellular components and of a glomerular basement membrane, the focus of this review will be on podocytes, which are terminally differentiated cells that have been shown to express functional IRs (38). Podocytes can become insulin resistant before the development of DKD (145, 160). Podocytes can also take up glucose in response to insulin via glucose transporter (GLUT)1 (185) and GLUT4 (62), both being major contributors to podocyte function. We will review both the clinical and experimental evidence supporting the importance of podocyte insulin signaling in the preservation of the glomerular filtration barrier. We will also review the contribution to insulin signaling of two insulin receptor (IR) subtypes (A and B) with specific roles in the modulating of cell growth, differentiation, apoptosis, and metabolism. A major focus of our review will be on lipid mediators of insulin signaling in podocytes, as we have reported a link between podocyte cholesterol content and insulin signaling (111). Furthermore, the contribution of certain sphingolipid species to insulin signaling via different IR isoforms will be discussed.
INSULIN RESISTANCE CONTRIBUTES TO DKD: CLINICAL EVIDENCE
Insulin resistance leads to important hemodynamic changes in the kidney, including increased sympathetic nervous tone, hypertension, and atherosclerosis of the renal microvasculature. Insulin resistance is a risk factor for DKD and may directly contribute to the development of the disease (175). Insulin resistance is frequently observed in patients with mild, advanced, or end-stage chronic kidney disease (for a review, see Ref. 163), and many studies have demonstrated a correlation between the presence of insulin resistance and albuminuria. A recent study on adult patients with newly diagnosed diabetes (with no differentiation between T1D and T2D) revealed that a higher risk of DKD is associated with insulin resistance and poor metabolic control (high HbA1c) (3). Few cross-sectional studies of patients with T2D have demonstrated a positive correlation between measured or calculated insulin resistance and levels of albuminuria (16, 134, 135). Longitudinal studies have demonstrated that even in normalbuminuric patients with T2D or nondiabetic siblings of patients with T2D, insulin resistance predicts the development of albuminuria development (52, 126).
Moreover, similar findings have been reported for patients with T1D (46, 182). The EURODIAB study demonstrated that other conditions, such as diabetes duration, glycemic control, or waist-to-hip ratio, predict microalbuminuria prevalence in patients with T1D (25). In a study on 652 patients with T1D (including 6.2 yr of followup), insulin resistance predicted the development of albuminuria (17, 44). Smaller studies with euglycemic clamps confirmed these findings in patients with T1D as well as in their family members (181, 182).
The Study of Glucose and Insulin in Renal Disease showed that participants with moderate to severe CKD have lower insulin sensitivity and impaired glucose tolerance (39). Hyperinsulinemia and abnormal insulin resistance were associated with kidney failure in nondiabetic adults with CKD from the United States (29). Interventional studies have suggested that attenuating of insulin resistance (for example, using thiazolidinediones) may have renoprotective effects. In randomized trials, normalization of insulin sensitivity reduced the incidence and severity of albuminuria in patients with T2D (117, 149). Another recent trial demonstrated that use of metformin for 36 mo resulted in a preservation of eGFR (141). The evidence that insulin resistance may contribute to chronic liver disease also supports a potential causative role in other organs (21). Whether insulin resistance is the cause or the consequence of CKD remains to be established, but several experimental studies have suggested that insulin resistance contributes to DKD development and progression, as reviewed in more detail below.
INSULIN RESISTANCE AND THE KIDNEY: EXPERIMENTAL EVIDENCE
The development of insulin resistance does not include defects in the binding of insulin to its receptor on cell membranes. Instead, insulin resistance results in “postreceptor defects” that interfere with intracellular signaling processes. The insulin signaling cascade is divided into major pathways such as the PI3K/Akt pathway and MAPK/MEK pathway; in insulin resistance, these pathways are not equally impaired. Recently, it has been suggested that impairment of insulin signaling also occurs in the kidney (113). The kidney is unique in that insulin can access its target cells both from the lumen (in the case of podocytes) and from the basolateral side (in the case of tubular cells) (76). Despite compelling evidence that the kidney is an insulin-responsive organ, whether the kidney in general or in part is affected by insulin resistance similarly to the classical target organs, such as muscles, the liver, and adipose tissue, is not clear. While in adipose tissue both insulin receptor substrate 1 (IRS)1- and IRS2-dependent signals are impaired in the setting of insulin resistance, renal proximal tubule insulin signaling is impaired via IRS1 but not via IRS2 (113, 123). The human kidney abundantly expresses IR isoform B similarly to the classical target organs; glucose uptake is insulin dependent in podocytes only (38, 62, 101, 150) but not in tubules or mesangial cells (7, 170). In diabetic rats, only the PI3K/Akt pathway, but not the MAPK/ERK pathway, was affected in isolated glomeruli (113). Moreover, protein expression of IRS1 in glomeruli was reduced but could be restored by inhibition of PKC-β (113). The impairment of IRS2 signaling in podocyte in the onset of diabetic nephropathy has recently been suggested (147). Together, these data indicate that glomeruli, but not tubuli, can develop insulin resistance and that insulin resistance is selective (for a review, see Ref. 72).
Overall, insulin resistance has a lot of implications for podocyte biology, and all factors associated with systemic insulin resistance have been shown to disrupt podocyte insulin signaling, as has been recently reviewed (97).
INSULIN SIGNALING IN PODOCYTES
Insulin exerts control over cell metabolism by binding to its cell surface receptor, which has been characterized in great detail (34, 98, 128, 129, 176). The occupied receptor is autophosphorylated on tyrosine residues and can thereby tyrosine phosphorylate other cellular proteins, such as the IRS family of proteins, to transduce the insulin signal into the signal network of a cell. The further downstream events involve the generation of second messengers. Among them, PI3K has a major role in insulin function, mainly via phosphorylation of Akt/PKB and PKC (PKC-ζ) cascades. Activated Akt induces glycogen synthesis through inhibition of glycogen synthase kinase 3 (GSK-3), protein synthesis via mammalian target of rapamycin (mTOR), and downstream elements and cell survival through inhibition of several proapoptotic agents (Bad, FoxO transcription factors, GSK3, and macrophage stimulating 1). Insulin signaling also has growth and mitogenic effects (Akt cascade and the Ras/MAPK pathway), inhibits autophagy (via Unc-51-like autophagy activating kinase; see Ref. 119), stimulates glucose uptake (for a review, see Ref. 174), inhibits gluconeogenesis in the liver [through disruption of cAMP response element-binding protein (CREB)/CREB-binding protein (CBP)/mTOR complex (mTORC)2 binding; see Ref. 172], induces fatty acid and cholesterol synthesis (via the regulation of sterol regulatory element-binding protein transcription factors; see Refs. 102, 132, 140, and 180), and promotes fatty acid synthesis (through activation of upstream transcription factor 1 and liver X receptor; see Refs. 142, 156, 173, and 177).
The glomerulus is composed of three cell types: podocytes, endothelial glomerular cells, and mesangial cells. All of these cells have been shown to respond to insulin stimulation. However, podocytes have the highest IR and IRS1 expression levels compared with endothelial and mesangial cells (113). Podocytes constantly express all components of insulin signaling (38, 160) and modulate glucose uptake via GLUT1 (185) and GLUT4 (62), both of which have been shown to be involved in the pathogenesis of DKD (for a review, see Ref. 37). Loss of insulin-stimulated Akt phosphorylation has been reported in podocytes of both T1D (43) and T2D (160) mouse models. The inability to signal through Akt2 was associated with increased podocyte susceptibility to cell death (23). More evidence for the critical role of insulin in podocyte function came from mice with podocyte-specific deletion of the IR (175). In these animals, albuminuria developed, along with effacement of the podocyte foot process, apoptosis, thickening of the glomerular basement membrane, and increased glomerulosclerosis. Additionally, studies have reported that expression levels of the IR and IRS1 were decreased in the glomeruli of insulin-resistant and diabetic rats (113, 164). IRS2 has also been reported to affect podocyte insulin sensitivity. Podocytes depleted of IRS2 were unable to phosphorylate Akt and failed in GLUT4-mediated glucose uptake in association with marked reduction of cortical F-actin remodeling and increased cell motility (147). In addition to these functions, insulin signaling may also regulate podocyte function by modulation of VEGF-A production (65), and it can modify podocyte contractility by regulating Ca2+ influx (via transient receptor potential C6 and calcineurin-dependent pathways) (178). Finally, insulin signaling in podocytes can regulate the activity of equilibrative nucleoside transporters responsible for controlling extracellular levels of adenosine (5) and the adaptive endoplasmic reticulum stress response (via p85-X box-binding protein 1) (108).
In a recent study (23), a link between activation of Akt and mTOR in podocytes has been suggested. Activation of mTORC1 downstream of Akt has been shown to induce podocyte injury in DKD (58, 77), whereas inhibition of mTORC2 has been shown to promote insulin resistance in Drosophila (148).
IR ISOFORMS IN HEALTH AND DISEASE
Alternative splicing of the IR adds additional complexity to the understanding of insulin signaling and may have implications in the development of DKD. The IR exists in two isoforms, which are formed due to the absence (isoform A) or presence (isoform B) of exon 11 of the IR gene. The expression of IR isoforms is tissue specific. IRA is predominantly expressed in fetal and cancer tissues (54), whereas IRB is predominantly expressed in insulin target organs such as the liver, muscles, adipose tissue, and, interestingly, kidney (see Refs. 120 and 169; for a review, see Ref. 12). IR splicing is a conserved mechanism in mammals responsible for the specificity in insulin signaling. A predominant expression of IRA is associated with a decrease in metabolic signaling of insulin and in an increase in the signaling of IGFs (i.e., in developmental and fetal growth). In contrast, increased expression of IRB is associated with increased metabolic actions of insulin. Additionally, the use of IR isoform-selective insulin analogs has demonstrated that IRA expression more than IRB is relevant to efficient glycogen synthesis, whereas IRB more than IRA exerted a stronger effect on glycogen accumulation and lipogenesis (169). Dysregulation of the balance between expression of IRA and IRB in adult life plays an important role in different pathological processes (12). The first evidence that IR isoforms are differentially expressed in disease was obtained in cancer fibroblasts and breast cancer cells (54, 153) and later was shown in a wide variety of cancers (86, 154, 166). In murine hepatocytes, it has been shown that IRA has a stronger effect than IRB in favoring basal glucose uptake by binding GLUT1/2 and inducing GSK-3α/β phosphorylation (41). It also been shown that patients with diabetes have an increased IRA-to-IRB ratio in skeletal muscle, which is linked to insulin resistance (139). While the importance of podocyte IR signaling in DKD has been elegantly described (175), the specific contribution of different IR isoforms in the pathogenesis of DKD has not yet been established.
CAVEOLAE AND INSULIN SIGNALING IN PODOCYTES
Caveolin-1, a critical regulator of insulin receptor expression, is highly expressed in podocytes, where it binds the key slit diaphragm proteins nephrin and CD2-associated protein (157). Cholesterol and sphingolipids together with caveolin are required for caveolae to form and for the different components of the slit diaphragm to function (50, 151, 167). Caveolae are involved in numerous cellular processes such as receptor-mediated uptake, receptor-mediated signaling, and vesicular trafficking (see Refs. 66 and 104; for a review, see Ref. 22). Interestingly, binding of IRs to caveolin-1 can be dissociated by plasma membrane molecules such as monosialodihexosylganglioside (GM3) (85) as well as by circulating TNF-α (155). GM3 can also interfere with raft clustering in podocytes (81). In a recent study (171) on zebrafish podocytes, it was shown that overexpression of caveolin-1 causes significant proteinuria and podocyte injury, suggesting that caveolin-1 could be another therapeutic target in nephrotic syndrome and podocyte injury. Whether and how this would interfere with insulin signaling remains to be established. In fact, some recent studies have demonstrated that IR signaling occurs in a particular subset of membrane microdomains and requires caveolin-2 (93, 94), and thus further studies are needed to elucidate the link between caveolin-2, insulin signaling, and podocyte function.
CHOLESTEROL AND INSULIN SIGNALING
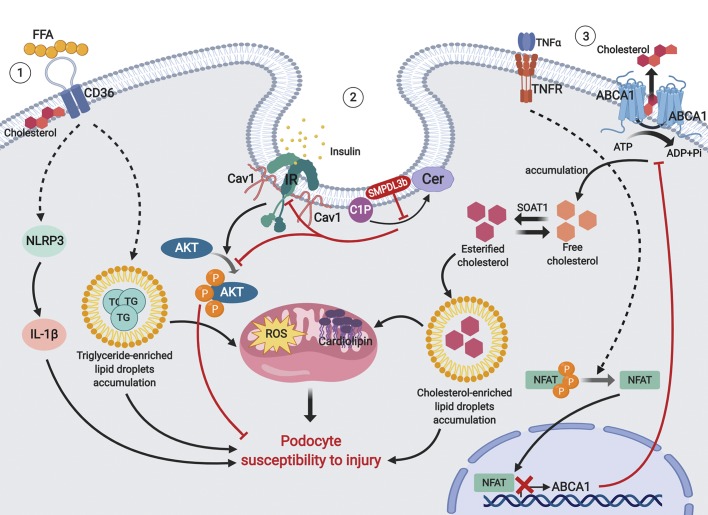
Among several lipids involved in the modulation of insulin signaling, cholesterol has been extensively studied as a key modulator of skeletal muscle cell function (10). We have recently identified cholesterol as a major mediator of podocyte function (111, 115, 137). In fact, sequestration of cholesterol with cyclodextrin was found to protect podocytes in DKD and to restore proper insulin signaling (111). In rats fed a high-cholesterol diet, activity of IRS1 and Akt was impaired, and tyrosine phosphorylation of caveolin-1 failed in response to insulin stimulation in the liver (63). In rat adipocytes, inhibition of downstream insulin signaling via IRS1 has been shown to be in a state of cholesterol depletion (133), suggesting an important role of cholesterol in the metabolic control of insulin signaling. A further link between intracellular cholesterol and insulin signaling is suggested in mice with hepatocyte-specific knockout of ATP-binding cassette transporter A1 (ABCA1), where impairment of ABCA1-dependent cholesterol efflux was associated with reduced hepatic insulin-stimulated Akt phosphorylation (87). More recently, treatment with small-molecule ABCA1 inducers was found to be sufficient to protect from experimental DKD (45). Interestingly, a 40% decrease in insulin-stimulated glucose uptake was reported in skeletal muscle of mice on a high-fat diet (106), suggesting that cholesterol may be a novel regulator of GLUT4 trafficking to the plasma membrane (for a review, see Ref. 10). Our own findings also suggested that accumulation of triglyceride-enriched lipid droplets via increased uptake of free fatty acids by CD36 (82) or esterified cholesterol-enriched lipid droplets via suppressed activity of ABCA1 (137) may both contribute to podocytes injury (Fig. 1).
Fig. 1.
Proposed mechanisms of podocyte susceptibility to lipid-mediated injury. 1) Upregulation of free fatty acids (FFA). The CD36 pathway leads to the accumulation of triglyceride (TG)-enriched lipid droplets and activation of inflammasome pathways, which causes mitochondrial damage and podocyte injury. 2) Overexpression of sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b) leads to the accumulation of ceramide (Cer)-1-phosphate (C1P) and disruption of insulin receptor (IR)/caveolin-1 (Cav1) interactions, which results in decreased phosphorylation of PKB (Akt) and increased podocyte injury. 3) Local TNF-α causes activation of nuclear factor of activated T cells (NFAT) and its translocation to the nucleus, where it blocks transcription of ATP-binding cassette transporter A1 (ABCA1), resulting in the accumulation of cholesterol and esterified cholesterol-enriched lipid droplets, leading to mitochondrial damage and podocyte injury. NLRP3, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing 3; ROS, reactive oxygen species; TNFR, TNF receptor; SOAT1, sterol O-acyltransferase 1.
SPHINGOLIPIDS AND INSULIN SIGNALING
Bioactive sphingolipids such as ceramide, ceramide-1-phosphate (C1P), and sphingosine-1-phosphate (S1P) play numerous roles in cell survival, proliferation, differentiation, and multiple aspects of stress responses. They also interact with intracellular signaling pathways (6), bind transmembrane domains of signaling proteins within the lipid bilayer (36), or even create membrane pores in mitochondria (35). Moreover, sphingolipids play a pivotal role in glomerular disorders of genetic and nongenetic origins (for a review, see Ref. 112).
Ceramide has attracted the most attention due to its roles not only in cell death and senescence but also in cell differentiation, membrane fluidity, protein anchoring, immune activation, and insulin resistance. Plasma ceramide levels are elevated in patients with T2D (18, 68) and are inversely associated with insulin sensitivity (68). Thus, both the liver and skeletal muscle of mice lacking dihydroceramide desaturase 1, which converts metabolically inactive dihydroceramide into active ceramide, have been shown as a target of ceramide-induced insulin resistance (70). Ceramide inhibits activation of Akt/PKB (27) but does not affect early signaling events such as IRS1 phosphorylation or PI3K activity (159). Moreover, patients with Gaucher’s disease, a lysosomal storage disease caused by failure to degrade glycosylated ceramides, are insulin resistant (95). Inhibition of glucosylceramide synthase improves insulin sensitivity in obese rodents. It has been shown that mice lacking GM3 synthase, which causes production of glycosphingolipids, showed improved insulin sensitivity in adipocytes (179), whereas in polycystic kidney disease loss of GM3 synthase has protective effects against cyst formation (125). However, ceramide and GM3 seems to act at different loci in different tissues, as glucosylceramide synthase inhibitors increased ceramide levels and antagonized signaling to Akt/PKB in myotubes (28). In addition, a recent study (90) showed that accumulation of ceramide in sphingomyelin synthase 2 knockout mouse embryonic fibroblasts fails to impair insulin signaling.
Both sphingosine and ceramide can be phosphorylated into S1P and C1P, respectively. The prosurvival activity of S1P highlights its role as a potential therapeutic target in neurodegenerative disorders (40, 83, 121), rheumatoid arthritis (32), cancer (15, 47, 122, 186), lung disorders (for a review, see Ref. 118), heart disorders (30, 79), renal oncology (4), acute kidney injury (9, 138), and nephrotic syndrome (143). Interestingly, the plasma S1P level is increased in the genetically obese ob/ob mouse model (92, 146) and in rodent models of T1D (53). In murine adipocytes, deficiency of serine palmitoyltransferase C2, which catalyzes the first step of de novo sphingolipid synthesis, causes markedly reduced adipose tissue mass and systemic insulin resistance and hyperglycemia (100). In vitro studies using C2C12 myoblasts have shown that S1P can stimulate basal glucose uptake by transphosphorylation of the IR (144), and in rat primary adipocytes, S1P has been shown to stimulate lipolysis (84). In vivo studies on transgenic mice overexpressing sphingosine kinase 1 showed improved insulin sensitivity compared with wild-type mice after 6 wk of a high-fat diet (19) and glucose tolerance, insulin uptake, and Akt phosphorylation through ceramide reduction in muscles (20). In primary hepatocytes, activated expression of sphingosine kinase 2 was associated with elevated expression of Akt, with no alteration of IRS phosphorylation, thus ameliorating glucose intolerance and insulin resistance (99). It has been suggested that S1P contributes to hepatic insulin resistance in primary rat and human hepatocytes as well as in the liver of high-fat diet-fed New Zealand obese mice (48). Overall, high-fat diet-fed mice demonstrated increased amounts of ceramide species, sphingomyelin species, sphingosine, and S1P in the liver, skeletal muscles, adipose tissue, heart, and plasma (91).
The role of sphingolipids in the kidney has remained largely unknown. It has been demonstrated that renal levels of S1P were increased in the streptozotocin mouse model, a phenomenon that was prevented by intraperitoneal insulin injections (127). Other studies in humans revealed that polymorphism in the SGPL1 gene, which encodes S1P lyase 1, is associated with reduced enzymatic activity of S1P lyase 1 and with the development of nephrotic syndrome (103, 107). In mice, lack of the Sgpl1 gene causes progression of foot process effacement, resulting in severe proteinuria (107). The use of unselective S1P receptor agonist FTY720, acting on S1P receptors 1, 3, 4, and 5 but not on S1P receptor 2, showed nephroprotection in streptozotocin-induced diabetic nephropathy in rats (8), whereas berberine improved renal injury in DKD via downregulation of S1P receptor 2 (74).
In contrast, much less known about C1P, as its pro- or anti-inflammatory role is still a matter of debate (24, 59, 61, 64). C1P and its precursor ceramide have opposite effects on cell survival, and a correct balance between the concentrations of C1P and ceramide is essential for cell and tissue homeostasis. In contrast to S1P, C1P is most likely not secreted by intact cells but is released by leaky or damaged cells (88). Although C1P has been claimed to stimulate cell proliferation in C2C12 myoblasts (13, 56) and macrophages (130, 131), not all studies have proven this concept. Thus, in leukemic cells, C1P did not affect cell proliferation, but it was described as an important factor mediating cell migration (1). C1P has been shown to modulate Akt phosphorylation in skin fibroblasts, hematopoietic cells (88), macrophages (57, 60), and adipocytes (26), consistent with the observation that bioactive sphingolipids are major modulators of insulin signaling (80, 110, 158). In renal mesangial cells, it has been shown that knockout of ceramide kinase, an enzyme that generates C1P from ceramide, causes proliferation of PGE2 and is efficient for the treatment of mesangioproliferative glomerular diseases (136). Taken together, although the role of S1P and C1P on insulin resistance and PI3K/Akt signaling in the liver, muscles, and pancreatic β-cells is not fully understood, data on kidneys are mostly unavailable.
We have recently reported that the expression of sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b), a lipid-raft associated protein (51) that regulates plasma membrane fluidity (69), is increased in glomeruli from patients with DKD as well as in glomeruli of db/db diabetic mice (183). Upre gulation of SMPDL3b was also found in normal human podocytes exposed to the sera of patients with DKD (183) and was found to be associated with an inability of DKD sera-treated human podocytes to phosphorylate Akt in response to insulin (111). Because SMPDL3b is a protein with homology to acid sphingomyelinase (31% amino acid identity and 48% overall amino acid similarity), we hypothesized that SMPDL3b may activate the sphingomyelin metabolic pathway, leading to the accumulation of sphingolipids other than sphingomyelin. We demonstrated that high SMPDL3b expression negatively affects the availability of C1P and demonstrated that exogenous administration of C1P in SMPDL3b-overexpressing podocytes is sufficient to restore insulin signaling and protects podocytes from injury (114). In the same study, we showed that human podocytes express both IR isoforms, IRA and IRB, and that SMPDL3b may facilitate IRB subtype signaling, thus favoring Akt phosphorylation in response to insulin by modulating IRA/IRB binding to caveolin. In additional in vivo experiments, we demonstrated that diabetic mice with podocyte-specific Smpdl3b deficiency are protected from the development of DKD, along with reduced albuminuria and preservation of podocyte numbers. This occurred in association with the restoration of the C1P content in kidney cortexes and, most importantly, with the restoration of podocyte-specific Akt phosphorylation (114). Overall, these in vitro and in vivo data reveal SMPDL3b as a master modulator of insulin signaling in podocytes (Fig. 1).
IR SIGNALING AS A THERAPEUTIC TARGET FOR DKD
Current DKD therapies are focused mainly on achievement of specific targets for glycemia and for blood pressure. Among the emerging new treatment strategies, the use of oral hyperglycemic agents [dipeptidyl peptidase-4 and Na+-glucose cotransporter (SGLT)2 inhibitors], mineralocorticoid receptor and endothelin receptor antagonists, selective NADPH oxidase 1/4 inhibitors, PKC inhibitors, advanced glycation end-product inhibitors, and agents that interfere with the VEGF pathway and with inflammatory mediators have shown promising results (for a review, see Ref. 89).
Studies with older insulin sensitizers of the class of thiazolidinediones have demonstrated that insulin sensitizers may further reduce albuminuria when achieving the same HbA1c targets as standard of care (149). In addition, the REMOVAL trial demonstrated that use of metformin is associated with significant preservation of eGFR at 36 mo (141). The question of how insulin sensitizers may protect the kidneys has been of interest during the past 15 yr. Interestingly, metformin has been described as an inhibitor of the respiratory chain complex 1 in the mitochondria via AMP-activated kinase, leading to reduced reactive oxygen species production (49, 124). It would be very interesting to know whether improvement of renal insulin sensitivity and improved mitochondrial respiration also contribute to the very prominent renoprotective effect observed with SGLT2 inhibitors (31, 109, 168).
Many ways to selectively regulate IR activity and downstream signaling have been discovered. With regard to targeting specific IR isoforms, it has been shown that proliferative effects of long-acting insulin analogs may occur predominantly via IRA (152), whereas mitogenic responses occur preferentially via IGF-1 receptors (161, 162). The use of IR ligands that might be able to separate metabolic from mitogenic IR actions has been actively studied in the past years. Based on studies of autoantibodies to the IR in patients with rare disease and severe insulin resistance, novel anti-IR monoclonal antibodies were designed to induce distinct structural states of the receptor and, therefore, different signals (11, 78). These antibodies demonstrated the possibility of differently stimulating the pleiotropic biological signals of IR activation and the advantage being specific to IR isoforms and not to the IGF-1 receptor (14). In addition to antibodies, aptamers (184) and small synthetic peptides (96) were obtained to modify IR activity.
Our most recent study supports the possibility of using active sphingolipids for the cure of DKD (Fig. 1). Indeed, kidneys are among the most sensitive organs to sphingolipid alterations (for a review, see Ref. 2). For instance, rapamycin treatment of rats with streptozotocin-induced DKD significantly decreased formation of many sphingolipids species, which have been shown to be elevated in DKD (105). Our group previously showed that increased expression of SMPDL3b was associated with increased RhoA activity and apoptosis in db/db mice (183). Because ceramide, sphingosine, and S1P are known to accumulate in apoptotic cells, we determined the ceramide content in kidney cortexes of db/db mice and found it to be decreased (114). On the other hand, SMPDL3b expression is increased in DKD, and targeting of SMPDL3b would indeed protect the podocytes from damage (51, 183). Another study has revealed that specific sphingomyelin species (SM d18:1/16:0) are accumulated in the glomeruli of diabetic or high-fat diet-fed mice, which leads to suppressed AMP-activated protein kinase, to an elevated ATP-to-AMP ratio and, as a result, to reduced mitochondrial activity and biogenesis (116). Inhibition of sphingomyelin synthases using siRNA reverses these effects. It has been shown that adiponectin, which is known to take part in the regulation of energy metabolism, stimulates ceramidase activity (via its two receptors, AdipoR1 and AdipoR2) and enhances ceramide catabolism and formation of its antiapoptotic metabolite, S1P (71). AdipoRon, a synthetic adiponectin agonist, may ameliorate ceramide-induced lipotoxicity in DKD by decreasing ceramide, oxidative stress, and apoptosis in glomerular endothelial cells and podocytes (33) and might be considered a potential treatment therapy of DKD. More recently, we have reported that exogenous C1P administration was associated with increased Akt phosphorylation in vitro and with protection from albuminuria and mesangial expansion in db/db mice in vivo (114), suggesting exogenous C1P as a new therapeutic strategy to treat diabetic complications such as DKD. Although we have demonstrated that rituximab may target sphingolipid-associated disorders (51), novel therapeutic strategies specifically targeting proteins such as SMPDL3b remain to be developed. Therefore, it is becoming clear that the strategies of manipulating acid sphingomyelinases, SMPDL3b, sphingosine kinases, S1P lyase, glucosylceramide synthase, and GM3 synthase (as reviewed in Ref. 75) may translate into novel sphingolipid therapeutics for DKD and require further investigation.
CONCLUDING REMARKS
Here, we summarized the clinical and experimental evidence supporting a role of lipids in the modulation of renal cell insulin signaling and development of DKD. We also provide evidence of the complexity of IR signaling and of how specific isoform targeting may be needed in future studies. The complex role of IR isoforms A and B in insulin target organs is only partially understood. It is clear that IR isoforms are responsible for the complexity of insulin signaling diversification, which involves different ligand binding affinities, different membrane partitioning, different trafficking, and different abilities to interact with various molecular partners and to preferentially modify selected downstream signaling pathways. More evidence has appeared that dysregulation of the podocyte insulin signaling and perturbations in free fatty acids, cholesterol, and sphingolipid-related pathways play an important role in renal disorders and may contribute to the development and progression of DKD. Because of the knowledge that podocyte insulin signaling and lipid metabolism are connected, novel attractive therapeutic strategies can be developed. Our present understanding of these signaling cascades is incomplete, and future work in this area will be a step forward toward precision medicine in DKD.
GRANTS
A. Fornoni is supported by National Institutes of Health Grants DK-090316, DK-104753, U24-DK-076169, U54-DK-083912, UM1-DK-100846, and 1-UL1-TR-000460.
DISCLOSURES
A. Fornoni is an inventor on pending or issued patents (US10,183,038 and US10,052,345) aimed to diagnose or treat proteinuric renal diseases and stands to gain royalties from their future commercialization. A. Fornoni is also Chief Scientific Officer of L&F Health LLC and is a consultant for ZyVersa Therapeutics. ZyVersa Therapeutics has licensed worldwide rights to develop and commercialize hydroxypropyl-β-cyclodextrin for treatment of kidney disease from L&F Research. The patent associated with the use of hydroxypropyl-β-cyclodextrin is published under US10,195,227. A. Fornoni is Chief Medical Officer of LipoNexT, LLC. A. Mitrofanova and M. A. Sosa have no competing interests.
AUTHOR CONTRIBUTIONS
A.M. prepared figures; A.M. drafted manuscript; M.A.S. and A.F. edited and revised manuscript; A.F. approved final version of manuscript.
REFERENCES
- 1.Abdelbaset-Ismail A, Cymer M, Borkowska-Rzeszotek S, Brzeźniakiewicz-Janus K, Rameshwar P, Kakar SS, Ratajczak J, Ratajczak MZ. Bioactive phospholipids enhance migration and adhesion of human leukemic cells by inhibiting heme oxygenase 1 (HO-1) and inducible nitric oxygenase synthase (iNOS) in a p38 MAPK-dependent manner. Stem Cell Rev Rep 15: 139–154, 2019. doi: 10.1007/s12015-018-9853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou Daher A, El Jalkh T, Eid AA, Fornoni A, Marples B, Zeidan YH. Translational aspects of sphingolipid metabolism in renal disorders. Int J Mol Sci 18: 2528, 2017. doi: 10.3390/ijms18122528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB, Aly DM, Almgren P, Wessman Y, Shaat N, Spégel P, Mulder H, Lindholm E, Melander O, Hansson O, Malmqvist U, Lernmark Å, Lahti K, Forsén T, Tuomi T, Rosengren AH, Groop L. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 6: 361–369, 2018. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad A, Mitrofanova A, Bielawski J, Yang Y, Marples B, Fornoni A, Zeidan YH. Sphingomyelinase-like phosphodiesterase 3b mediates radiation-induced damage of renal podocytes. FASEB J 31: 771–780, 2017. doi: 10.1096/fj.201600618R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alarcón S, Garrido W, Cappelli C, Suárez R, Oyarzún C, Quezada C, San Martín R. Deficient insulin-mediated upregulation of the equilibrative nucleoside transporter 2 contributes to chronically increased adenosine in diabetic glomerulopathy. Sci Rep 7: 9439, 2017. doi: 10.1038/s41598-017-09783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465: 1084–1088, 2010. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnoni CP, Lima C, Cristovam PC, Maquigussa E, Vidotti DB, Boim MA. Regulation of glucose uptake in mesangial cells stimulated by high glucose: role of angiotensin II and insulin. Exp Biol Med (Maywood) 234: 1095–1101, 2009. doi: 10.3181/0902-RM-50. [DOI] [PubMed] [Google Scholar]
- 8.Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, Lynch KR, Okusa MD. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int 79: 1090–1098, 2011. doi: 10.1038/ki.2010.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajwa A, Huang L, Kurmaeva E, Ye H, Dondeti KR, Chroscicki P, Foley LS, Balogun ZA, Alexander KJ, Park H, Lynch KR, Rosin DL, Okusa MD. Sphingosine kinase 2 deficiency attenuates kidney fibrosis via IFN-γ. J Am Soc Nephrol 28: 1145–1161, 2017. doi: 10.1681/ASN.2016030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrientos G, Sánchez-Aguilera P, Jaimovich E, Hidalgo C, Llanos P. Membrane cholesterol in skeletal muscle: a novel player in excitation-contraction coupling and insulin resistance. J Diabetes Res 2017: 1–8, 2017. doi: 10.1155/2017/3941898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedinger DH, Goldfine ID, Corbin JA, Roell MK, Adams SH. Differential pathway coupling of the activated insulin receptor drives signaling selectivity by XMetA, an allosteric partial agonist antibody. J Pharmacol Exp Ther 353: 35–43, 2015. doi: 10.1124/jpet.114.221309. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, Morrione A, Vigneri R. Insulin receptor isoforms in physiology and disease: an updated view. Endocr Rev 38: 379–431, 2017. doi: 10.1210/er.2017-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernacchioni C, Cencetti F, Ouro A, Bruno M, Gomez-Muñoz A, Donati C, Bruni P. Lysophosphatidic acid signaling axis mediates ceramide 1-phosphate-induced proliferation of C2C12 myoblasts. Int J Mol Sci 19: E139, 2018. doi: 10.3390/ijms19010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezwada P, Zhao J, Der K, Shimizu B, Cao L, Ahene A, Rubin P, Johnson K. A novel allosteric insulin receptor-activating antibody reduces hyperglycemia without hypoglycemia in diabetic cynomolgus monkeys. J Pharmacol Exp Ther 356: 466–473, 2016. doi: 10.1124/jpet.115.229690. [DOI] [PubMed] [Google Scholar]
- 15.Bhat VK, Bernhart E, Plastira I, Fan K, Ghaffari-Tabrizi-Wizsy N, Wadsack C, Rechberger G, Eichmann T, Asslaber M, Spassova I, Verhaegen ME, Malle E, Becker JC, Sattler W. Pharmacological inhibition of serine palmitoyl transferase and sphingosine kinase-1/-2 inhibits Merkel Cell Carcinoma cell proliferation. J Invest Dermatol 139: 807–817, 2019. doi: 10.1016/j.jid.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, Nadeau KJ. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care 37: 3033–3039, 2014. doi: 10.2337/dc14-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, Snell-Bergeon JK. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complications 30: 586–590, 2016. doi: 10.1016/j.jdiacomp.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62: 401–410, 2013. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K, Takuwa Y, Meikle PJ, Pitson SM, Febbraio MA. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes 61: 3148–3155, 2012. doi: 10.2337/db12-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce CR, Risis S, Babb JR, Yang C, Lee-Young RS, Henstridge DC, Febbraio MA. The sphingosine-1-phosphate analog FTY720 reduces muscle ceramide content and improves glucose tolerance in high fat-fed male mice. Endocrinology 154: 65–76, 2013. doi: 10.1210/en.2012-1847. [DOI] [PubMed] [Google Scholar]
- 21.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 42: 987–1000, 2005. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 22.Busija AR, Patel HH, Insel PA. Caveolins and cavins in the trafficking, maturation, and degradation of caveolae: implications for cell physiology. Am J Physiol Cell Physiol 312: C459–C477, 2017. doi: 10.1152/ajpcell.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19: 1288–1296, 2013. doi: 10.1038/nm.3313. [DOI] [PubMed] [Google Scholar]
- 24.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci 118: 4605–4612, 2005. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 25.Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int 60: 219–227, 2001. doi: 10.1046/j.1523-1755.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 26.Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, Galam DL, Gopalan V, Prakash KN, Velan SS, Bulchand S, Tsong TJ, Wang M, Siddique MM, Yuguang G, Sigmundsson K, Mellet NA, Weir JM, Meikle PJ, Bin M Yassin MS, Shabbir A, Shayman JA, Hirabayashi Y, Shiow ST, Sugii S, Summers SA. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab 24: 820–834, 2016. doi: 10.1016/j.cmet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Chavez JA, Holland WL, Bär J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280: 20148–20153, 2005. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 28.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 278: 10297–10303, 2003. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, He J. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 14: 469–477, 2003. doi: 10.1097/01.ASN.0000046029.53933.09. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Cai X, Liu J, Bai B, Li X. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci 215: 31–42, 2018. doi: 10.1016/j.lfs.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Cherney DZI, Odutayo A, Verma S. A big win for diabetic kidney disease: credence. Cell Metab 29: 1024–1027, 2019. doi: 10.1016/j.cmet.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Choi HS, Kim KH, Jin S, Kim J, Yoo I, Pack SP, Ha UH, Park TW, Choi SA, Yuk SH, Kang SW, Jung YW. Decreased expression of sphingosine-1-phosphate receptor 1 in the blood leukocyte of rheumatoid arthritis patients. Immune Netw 18: e39, 2018. doi: 10.4110/in.2018.18.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi SR, Lim JH, Kim MY, Kim EN, Kim Y, Choi BS, Kim YS, Kim HW, Lim KM, Kim MJ, Park CW. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism 85: 348–360, 2018. doi: 10.1016/j.metabol.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Cohen P. The Croonian Lecture 1998. Identification of a protein kinase cascade of major importance in insulin signal transduction. Philos Trans R Soc Lond B Biol Sci 354: 485–495, 1999. doi: 10.1098/rstb.1999.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombini M. Ceramide channels and mitochondrial outer membrane permeability. J Bioenerg Biomembr 49: 57–64, 2017. doi: 10.1007/s10863-016-9646-z. [DOI] [PubMed] [Google Scholar]
- 36.Contreras FX, Ernst AM, Haberkant P, Björkholm P, Lindahl E, Gönen B, Tischer C, Elofsson A, von Heijne G, Thiele C, Pepperkok R, Wieland F, Brügger B. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 481: 525–529, 2012. doi: 10.1038/nature10742. [DOI] [PubMed] [Google Scholar]
- 37.Coward R, Fornoni A. Insulin signaling: implications for podocyte biology in diabetic kidney disease. Curr Opin Nephrol Hypertens 24: 104–110, 2015. doi: 10.1097/MNH.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavaré JM, Mathieson PW, Saleem MA. The human glomerular podocyte is a novel target for insulin action. Diabetes 54: 3095–3102, 2005. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 39.de Boer IH, Zelnick L, Afkarian M, Ayers E, Curtin L, Himmelfarb J, Ikizler TA, Kahn SE, Kestenbaum B, Utzschneider K. Impaired glucose and insulin homeostasis in moderate-severe CKD. J Am Soc Nephrol 27: 2861–2871, 2016. doi: 10.1681/ASN.2015070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Pardo A, Basit A, Armirotti A, Amico E, Castaldo S, Pepe G, Marracino F, Buttari F, Digilio AF, Maglione V. De novo synthesis of sphingolipids is defective in experimental models of Huntington’s Disease. Front Neurosci 11: 698, 2017. doi: 10.3389/fnins.2017.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diaz-Castroverde S, Gómez-Hernández A, Fernández S, García-Gómez G, Di Scala M, González-Aseguinolaza G, Fernández-Millán E, González-Rodríguez Á, García-Bravo M, Chambon P, Álvarez C, Perdomo L, Beneit N, Escribano O, Benito M. Insulin receptor isoform A ameliorates long-term glucose intolerance in diabetic mice. Dis Model Mech 9: 1271–1281, 2016. doi: 10.1242/dmm.025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diez-Sampedro A, Lenz O, Fornoni A. Podocytopathy in diabetes: a metabolic and endocrine disorder. Am J Kidney Dis 58: 637–646, 2011. doi: 10.1053/j.ajkd.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drapeau N, Lizotte F, Denhez B, Guay A, Kennedy CR, Geraldes P. Expression of SHP-1 induced by hyperglycemia prevents insulin actions in podocytes. Am J Physiol Endocrinol Metab 304: E1188–E1198, 2013. doi: 10.1152/ajpendo.00560.2012. [DOI] [PubMed] [Google Scholar]
- 44.Duca LM, Maahs DM, Schauer IE, Bergman BC, Nadeau KJ, Bjornstad P, Rewers M, Snell-Bergeon JK. Development and validation of a method to estimate insulin sensitivity in patients with and without type 1 diabetes. J Clin Endocrinol Metab 101: 686–695, 2016. doi: 10.1210/jc.2015-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducasa GM, Mitrofanova A, Mallela SK, Liu X, Molina J, Sloan A, Pedigo CE, Ge M, Santos JV, Hernandez Y, Kim JJ, Maugeais C, Mendez AJ, Nair V, Kretzler M, Burke GW, Nelson RG, Ishimoto Y, Inagi R, Banerjee S, Liu S, Szeto HH, Merscher S, Fontanesi F, Fornoni A. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J Clin Invest 129: 3387–3400, 2019. doi: 10.1172/JCI125316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekstrand AV, Groop PH, Grönhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant 13: 3079–3083, 1998. doi: 10.1093/ndt/13.12.3079. [DOI] [PubMed] [Google Scholar]
- 47.El Buri A, Adams DR, Smith D, Tate RJ, Mullin M, Pyne S, Pyne NJ. The sphingosine 1-phosphate receptor 2 is shed in exosomes from breast cancer cells and is N-terminally processed to a short constitutively active form that promotes extracellular signal regulated kinase activation and DNA synthesis in fibroblasts. Oncotarget 9: 29453–29467, 2018. doi: 10.18632/oncotarget.25658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fayyaz S, Henkel J, Japtok L, Krämer S, Damm G, Seehofer D, Püschel GP, Kleuser B. Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype. Diabetologia 57: 373–382, 2014. doi: 10.1007/s00125-013-3123-6. [DOI] [PubMed] [Google Scholar]
- 49.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab 20: 953–966, 2014. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Fornoni A, Merscher S, Kopp JB. Lipid biology of the podocyte−new perspectives offer new opportunities. Nat Rev Nephrol 10: 379–388, 2014. doi: 10.1038/nrneph.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW III. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3: 85ra46, 2011. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forsblom CM, Eriksson JG, Ekstrand AV, Teppo AM, Taskinen MR, Groop LC. Insulin resistance and abnormal albumin excretion in non-diabetic first-degree relatives of patients with NIDDM. Diabetologia 38: 363–369, 1995. doi: 10.1007/BF00400643. [DOI] [PubMed] [Google Scholar]
- 53.Fox TE, Bewley MC, Unrath KA, Pedersen MM, Anderson RE, Jung DY, Jefferson LS, Kim JK, Bronson SK, Flanagan JM, Kester M. Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res 52: 509–517, 2011. doi: 10.1194/jlr.M010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 19: 3278–3288, 1999. doi: 10.1128/MCB.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gæde P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 56.Gangoiti P, Bernacchioni C, Donati C, Cencetti F, Ouro A, Gómez-Muñoz A, Bruni P. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie 94: 597–607, 2012. doi: 10.1016/j.biochi.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gómez-Muñoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal 20: 726–736, 2008. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gómez-Muñoz A. Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim Biophys Acta 1758: 2049–2056, 2006. doi: 10.1016/j.bbamem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Gómez-Muñoz A, Kong JY, Parhar K, Wang SW, Gangoiti P, González M, Eivemark S, Salh B, Duronio V, Steinbrecher UP. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett 579: 3744–3750, 2005. doi: 10.1016/j.febslet.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 61.Gómez-Muñoz A, Gangoiti P, Granado MH, Arana L, Ouro A.. Ceramide 1-phosphate in cell survival and inflammatory signaling. In: Shingolipids as Signaling and Regulatory Molecules, edited by Chalfant CE, Poeta M. Austin, TX: Landes Bioscience, 2000. –2013, p. 118–130. [DOI] [PubMed] [Google Scholar]
- 62.Guzman J, Jauregui AN, Merscher-Gomez S, Maiguel D, Muresan C, Mitrofanova A, Diez-Sampedro A, Szust J, Yoo TH, Villarreal R, Pedigo C, Molano RD, Johnson K, Kahn B, Hartleben B, Huber TB, Saha J, Burke GW III, Abel ED, Brosius FC, Fornoni A. Podocyte-specific GLUT4-deficient mice have fewer and larger podocytes and are protected from diabetic nephropathy. Diabetes 63: 701–714, 2014. doi: 10.2337/db13-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hahn-Obercyger M, Graeve L, Madar Z. A high-cholesterol diet increases the association between caveolae and insulin receptors in rat liver. J Lipid Res 50: 98–107, 2009. doi: 10.1194/jlr.M800441-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Hait NC, Maiti A. The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediators Inflamm 2017: 1–17, 2017. doi: 10.1155/2017/4806541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hale LJ, Hurcombe J, Lay A, Santamaría B, Valverde AM, Saleem MA, Mathieson PW, Welsh GI, Coward RJ. Insulin directly stimulates VEGF-A production in the glomerular podocyte. Am J Physiol Renal Physiol 305: F182–F188, 2013. doi: 10.1152/ajprenal.00548.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20: 177–186, 2010. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Harjutsalo V, Groop PH. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis 21: 260–266, 2014. doi: 10.1053/j.ackd.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58: 337–343, 2009. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heinz LX, Baumann CL, Köberlin MS, Snijder B, Gawish R, Shui G, Sharif O, Aspalter IM, Müller AC, Kandasamy RK, Breitwieser FP, Pichlmair A, Bruckner M, Rebsamen M, Blüml S, Karonitsch T, Fauster A, Colinge J, Bennett KL, Knapp S, Wenk MR, Superti-Furga G. The lipid-modifying enzyme SMPDL3B negatively regulates innate immunity. Cell Reports 11: 1919–1928, 2015. doi: 10.1016/j.celrep.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17: 55–63, 2011. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G. Selective insulin resistance in the kidney. BioMed Res Int 2016: 1–8, 2016. doi: 10.1155/2016/5825170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, Binder C, Parving HH. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 26: 1258–1264, 2003. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 74.Huang K, Liu W, Lan T, Xie X, Peng J, Huang J, Wang S, Shen X, Liu P, Huang H. Berberine reduces fibronectin expression by suppressing the S1P-S1P2 receptor pathway in experimental diabetic nephropathy models. PLoS One 7: e43874, 2012. [Erratum in PLoS One 7: 2012.] doi: 10.1371/journal.pone.0043874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huwiler A, Pfeilschifter J. Sphingolipid signaling in renal fibrosis. Matrix Biol 68-69: 230–247, 2018. doi: 10.1016/j.matbio.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Hysing J, Ostensen J, Tolleshaug H, Andersen KJ, Kiil F. Luminal and basolateral uptake and degradation of insulin in the proximal tubules of the dog kidney. Acta Physiol Scand 146: 241–250, 1992. doi: 10.1111/j.1748-1716.1992.tb09413.x. [DOI] [PubMed] [Google Scholar]
- 77.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Issafras H, Bedinger DH, Corbin JA, Goldfine ID, Bhaskar V, White ML, Rubin P, Scannon PJ. Selective allosteric antibodies to the insulin receptor for the treatment of hyperglycemic and hypoglycemic disorders. J Diabetes Sci Technol 8: 865–873, 2014. doi: 10.1177/1932296814529886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jadczyk T, Baranski K, Syzdol M, Nabialek E, Wanha W, Kurzelowski R, Ratajczak MZ, Dolegowska B, Niewczas M, Zejda J. Bioactive sphingolipids, complement cascade, and free hemoglobin levels in stable coronary artery disease and acute myocardial infarction. Mediators Inflamm 2018: 2691934, 2018. doi: 10.1155/2018/2691934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jęśko H, Stępień A, Lukiw WJ, Strosznajder RP. The cross-talk between sphingolipids and insulin-like growth factor signaling: significance for aging and neurodegeneration. Mol Neurobiol 56: 3501–3521, 2019. doi: 10.1007/s12035-018-1286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin J, Sison K, Li C, Tian R, Wnuk M, Sung HK, Jeansson M, Zhang C, Tucholska M, Jones N, Kerjaschki D, Shibuya M, Fantus IG, Nagy A, Gerber HP, Ferrara N, Pawson T, Quaggin SE. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151: 384–399, 2012. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 82.Kim JJ, Molina David JT, Varona Santos JT, Merscher SM, Miner JH, Fornoni A. The role of DDR1 in podocyte lipotoxicity and progression of alport syndrome (Abstract) San Diego, CA: American Society of Nephrology Kidney Week, 2018, p. 323 https://www.asn-online.org/education/kidneyweek/2017/program-abstract.aspx?controlId=2782330. [Google Scholar]
- 83.Joly S, Dalkara D, Pernet V. Sphingosine 1-phosphate receptor 1 modulates CNTF-induced axonal growth and neuroprotection in the mouse visual system. Neural Plast 2017: 1–11, 2017. doi: 10.1155/2017/6818970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jun DJ, Lee JH, Choi BH, Koh TK, Ha DC, Jeong MW, Kim KT. Sphingosine-1-phosphate modulates both lipolysis and leptin production in differentiated rat white adipocytes. Endocrinology 147: 5835–5844, 2006. doi: 10.1210/en.2006-0579. [DOI] [PubMed] [Google Scholar]
- 85.Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA 104: 13678–13683, 2007. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA. Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology 143: 3259–3267, 2002. doi: 10.1210/en.2001-211408. [DOI] [PubMed] [Google Scholar]
- 87.Key CC, Liu M, Kurtz CL, Chung S, Boudyguina E, Dinh TA, Bashore A, Phelan PE, Freedman BI, Osborne TF, Zhu X, Ma L, Sethupathy P, Biddinger SB, Parks JS. Hepatocyte ABCA1 deletion impairs liver insulin signaling and lipogenesis. Cell Reports 19: 2116–2129, 2017. doi: 10.1016/j.celrep.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia 26: 106–116, 2012. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim MK. Treatment of diabetic kidney disease: current and future targets. Korean J Intern Med (Korean Assoc Intern Med) 32: 622–630, 2017. doi: 10.3904/kjim.2016.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim YJ, Greimel P, Hirabayashi Y. GPRC5B-mediated sphingomyelin synthase 2 phosphorylation plays a critical role in insulin resistance. iScience 8: 250–266, 2018. doi: 10.1016/j.isci.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kitada Y, Kajita K, Taguchi K, Mori I, Yamauchi M, Ikeda T, Kawashima M, Asano M, Kajita T, Ishizuka T, Banno Y, Kojima I, Chun J, Kamata S, Ishii I, Morita H. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates high-fat diet-induced adipocyte hypertrophy and systemic glucose intolerance in mice. Endocrinology 157: 1839–1851, 2016. doi: 10.1210/en.2015-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One 8: e72449, 2013. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon H, Lee J, Jeong K, Jang D, Pak Y. Fatty acylated caveolin-2 is a substrate of insulin receptor tyrosine kinase for insulin receptor substrate-1-directed signaling activation. Biochim Biophys Acta 1853: 1022–1034, 2015. doi: 10.1016/j.bbamcr.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Kwon H, Lee J, Jeong K, Jang D, Pak Y. A novel actin cytoskeleton-dependent noncaveolar microdomain composed of homo-oligomeric caveolin-2 for activation of insulin signaling. Biochim Biophys Acta 1833: 2176–2189, 2013. doi: 10.1016/j.bbamcr.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 95.Langeveld M, Ghauharali KJ, Sauerwein HP, Ackermans MT, Groener JE, Hollak CE, Aerts JM, Serlie MJ. Type I Gaucher disease, a glycosphingolipid storage disorder, is associated with insulin resistance. J Clin Endocrinol Metab 93: 845–851, 2008. doi: 10.1210/jc.2007-1702. [DOI] [PubMed] [Google Scholar]
- 96.Lawrence CF, Margetts MB, Menting JG, Smith NA, Smith BJ, Ward CW, Lawrence MC. Insulin mimetic peptide disrupts the primary binding site of the insulin receptor. J Biol Chem 291: 15473–15481, 2016. doi: 10.1074/jbc.M116.732180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lay AC, Coward RJM. The evolving importance of insulin signaling in podocyte health and disease. Front Endocrinol (Lausanne) 9: 693, 2018. doi: 10.3389/fendo.2018.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee J, Pilch PF. The insulin receptor: structure, function, and signaling. Am J Physiol 266: C319–C334, 1994. doi: 10.1152/ajpcell.1994.266.2.C319. [DOI] [PubMed] [Google Scholar]
- 99.Lee SY, Hong IK, Kim BR, Shim SM, Sung Lee J, Lee HY, Soo Choi C, Kim BK, Park TS. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology 62: 135–146, 2015. doi: 10.1002/hep.27804. [DOI] [PubMed] [Google Scholar]
- 100.Lee SY, Lee HY, Song JH, Kim GT, Jeon S, Song YJ, Lee JS, Hur JH, Oh HH, Park SY, Shim SM, Yoo HJ, Lee BC, Jiang XC, Choi CS, Park TS. Adipocyte-specific deficiency of de novo sphingolipid biosynthesis leads to lipodystrophy and insulin resistance. 66: 2596–2609, 2017. doi: 10.2337/db16-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lewko B, Bryl E, Witkowski JM, Latawiec E, Gołos M, Endlich N, Hähnel B, Koksch C, Angielski S, Kriz W, Stepinski J. Characterization of glucose uptake by cultured rat podocytes. Kidney Blood Press Res 28: 1–7, 2005. doi: 10.1159/000080889. [DOI] [PubMed] [Google Scholar]
- 102.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 107: 3441–3446, 2010. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Linhares ND, Arantes RR, Araujo SA, Pena SDJ. Nephrotic syndrome and adrenal insufficiency caused by a variant in SGPL1. Clin Kidney J 11: 462–467, 2018. doi: 10.1093/ckj/sfx130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol 4: 231–235, 1994. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 105.Liu G, Han F, Yang Y, Xie Y, Jiang H, Mao Y, Wang H, Wang M, Chen R, Yang J, Chen J. Evaluation of sphingolipid metabolism in renal cortex of rats with streptozotocin-induced diabetes and the effects of rapamycin. Nephrol Dial Transplant 26: 1493–1502, 2011. doi: 10.1093/ndt/gfq633. [DOI] [PubMed] [Google Scholar]
- 106.Llanos P, Contreras-Ferrat A, Georgiev T, Osorio-Fuentealba C, Espinosa A, Hidalgo J, Hidalgo C, Jaimovich E. The cholesterol-lowering agent methyl-β-cyclodextrin promotes glucose uptake via GLUT4 in adult muscle fibers and reduces insulin resistance in obese mice. Am J Physiol Endocrinol Metab 308: E294–E305, 2015. doi: 10.1152/ajpendo.00189.2014. [DOI] [PubMed] [Google Scholar]
- 107.Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, Shril S, Ashraf S, Tan W, Rao J, Airik M, Schapiro D, Braun DA, Sadowski CE, Widmeier E, Jobst-Schwan T, Schmidt JM, Girik V, Capitani G, Suh JH, Lachaussée N, Arrondel C, Patat J, Gribouval O, Furlano M, Boyer O, Schmitt A, Vuiblet V, Hashmi S, Wilcken R, Bernier FP, Innes AM, Parboosingh JS, Lamont RE, Midgley JP, Wright N, Majewski J, Zenker M, Schaefer F, Kuss N, Greil J, Giese T, Schwarz K, Catheline V, Schanze D, Franke I, Sznajer Y, Truant AS, Adams B, Désir J, Biemann R, Pei Y, Ars E, Lloberas N, Madrid A, Dharnidharka VR, Connolly AM, Willing MC, Cooper MA, Lifton RP, Simons M, Riezman H, Antignac C, Saba JD, Hildebrandt F. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J Clin Invest 127: 912–928, 2017. doi: 10.1172/JCI89626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Madhusudhan T, Wang H, Dong W, Ghosh S, Bock F, Thangapandi VR, Ranjan S, Wolter J, Kohli S, Shahzad K, Heidel F, Krueger M, Schwenger V, Moeller MJ, Kalinski T, Reiser J, Chavakis T, Isermann B. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun 6: 6496, 2015. doi: 10.1038/ncomms7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayer GJ, Wanner C, Weir MR, Inzucchi SE, Koitka-Weber A, Hantel S, von Eynatten M, Zinman B, Cherney DZI. Analysis from the EMPA-REG OUTCOME® trial indicates empagliflozin may assist in preventing the progression of chronic kidney disease in patients with type 2 diabetes irrespective of medications that alter intrarenal hemodynamics. Kidney Int 96: 489–504, 2019. doi: 10.1016/j.kint.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 110.Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol 13: 79–91, 2017. doi: 10.1038/nrendo.2016.169. [DOI] [PubMed] [Google Scholar]
- 111.Merscher-Gomez S, Guzman J, Pedigo CE, Lehto M, Aguillon-Prada R, Mendez A, Lassenius MI, Forsblom C, Yoo T, Villarreal R, Maiguel D, Johnson K, Goldberg R, Nair V, Randolph A, Kretzler M, Nelson RG, Burke GW III, Groop PH, Fornoni A; FinnDiane Study Group . Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes 62: 3817–3827, 2013. doi: 10.2337/db13-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merscher S, Fornoni A. Podocyte pathology and nephropathy−sphingolipids in glomerular diseases. Front Endocrinol (Lausanne) 5: 127, 2014. doi: 10.3389/fendo.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mima A, Ohshiro Y, Kitada M, Matsumoto M, Geraldes P, Li C, Li Q, White GS, Cahill C, Rask-Madsen C, King GL. Glomerular-specific protein kinase C-β-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int 79: 883–896, 2011. doi: 10.1038/ki.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, Molina J, Varona Santos J, Ge M, Sloan A, Kim JJ, Pedigo C, Bryn J, Volosenco I, Faul C, Zeidan YH, Garcia Hernandez C, Mendez AJ, Leibiger I, Burke GW, Futerman AH, Barisoni L, Ishimoto Y, Inagi R, Merscher S, Fornoni A. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun 10: 2692, 2019. doi: 10.1038/s41467-019-10584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mitrofanova A, Molina J, Varona Santos J, Guzman J, Morales XA, Ducasa GM, Bryn J, Sloan A, Volosenco I, Kim JJ, Ge M, Mallela SK, Kretzler M, Eddy S, Martini S, Wahl P, Pastori S, Mendez AJ, Burke GW, Merscher S, Fornoni A. Hydroxypropyl-β-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int 94: 1151–1159, 2018. doi: 10.1016/j.kint.2018.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miyamoto S, Hsu CC, Hamm G, Darshi M, Diamond-Stanic M, Declèves AE, Slater L, Pennathur S, Stauber J, Dorrestein PC, Sharma K. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine 7: 121–134, 2016. doi: 10.1016/j.ebiom.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miyazaki Y, Cersosimo E, Triplitt C, DeFronzo RA. Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int 72: 1367–1373, 2007. doi: 10.1038/sj.ki.5002516. [DOI] [PubMed] [Google Scholar]
- 118.Mohammed S, Harikumar KB. Corrigendum: Sphingosine 1-phosphate: a novel target for lung disorders. Front Immunol 9: 1628, 2018. doi: 10.3389/fimmu.2018.01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Møller AB, Voss TS, Vendelbo MH, Pedersen SB, Møller N, Jessen N. Insulin inhibits autophagy signaling independent of counter-regulatory hormone levels, but does not affect the effects of exercise. J Appl Physiol 125: 1204–1209, 2018. doi: 10.1152/japplphysiol.00490.2018. [DOI] [PubMed] [Google Scholar]
- 120.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J 9: 2409–2413, 1990. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Motyl J, Przykaza Ł, Boguszewski PM, Kosson P, Strosznajder JB. Pramipexole and Fingolimod exert neuroprotection in a mouse model of Parkinson’s disease by activation of sphingosine kinase 1 and Akt kinase. Neuropharmacology 135: 139–150, 2018. doi: 10.1016/j.neuropharm.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 122.Nagahashi M, Abe M, Sakimura K, Takabe K, Wakai T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci 109: 3671–3678, 2018. doi: 10.1111/cas.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakamura M, Yamazaki O, Shirai A, Horita S, Satoh N, Suzuki M, Hamasaki Y, Noiri E, Kume H, Enomoto Y, Homma Y, Seki G. Preserved Na/ cotransporter sensitivity to insulin may promote hypertension in metabolic syndrome. Kidney Int 87: 535–542, 2015. doi: 10.1038/ki.2014.351. [DOI] [PubMed] [Google Scholar]
- 124.Nasri RH. Renoprotective effects of metformin. Daru 21: 36, 2013. doi: 10.1186/2008-2231-21-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Natoli TA, Husson H, Rogers KA, Smith LA, Wang B, Budman Y, Bukanov NO, Ledbetter SR, Klinger KW, Leonard JP, Ibraghimov-Beskrovnaya O. Loss of GM3 synthase gene, but not sphingosine kinase 1, is protective against murine nephronophthisis-related polycystic kidney disease. Hum Mol Genet 21: 3397–3407, 2012. doi: 10.1093/hmg/dds172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niskanen L, Voutilainen R, Teräsvirta M, Lehtinen J, Teppo AM, Groop L, Uusitupa M. A prospective study of clinical and metabolic associates of proteinuria in patients with type 2 diabetes mellitus. Diabet Med 10: 543–549, 1993. doi: 10.1111/j.1464-5491.1993.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 127.Nojiri T, Kurano M, Tokuhara Y, Ohkubo S, Hara M, Ikeda H, Tsukamoto K, Yatomi Y. Modulation of sphingosine-1-phosphate and apolipoprotein M levels in the plasma, liver and kidneys in streptozotocin-induced diabetic mice. J Diabetes Investig 5: 639–648, 2014. doi: 10.1111/jdi.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nystrom FH, Quon MJ. Insulin signalling: metabolic pathways and mechanisms for specificity. Cell Signal 11: 563–574, 1999. doi: 10.1016/S0898-6568(99)00025-X. [DOI] [PubMed] [Google Scholar]
- 129.Olefsky JM. The insulin receptor. A multifunctional protein. Diabetes 39: 1009–1016, 1990. doi: 10.2337/diab.39.9.1009. [DOI] [PubMed] [Google Scholar]
- 130.Ouro A, Arana L, Riazy M, Zhang P, Gomez-Larrauri A, Steinbrecher U, Duronio V, Gomez-Muñoz A. Vascular endothelial growth factor mediates ceramide 1-phosphate-stimulated macrophage proliferation. Exp Cell Res 361: 277–283, 2017. doi: 10.1016/j.yexcr.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 131.Ouro A, Arana L, Rivera IG, Ordoñez M, Gomez-Larrauri A, Presa N, Simón J, Trueba M, Gangoiti P, Bittman R, Gomez-Muñoz A. Phosphatidic acid inhibits ceramide 1-phosphate-stimulated macrophage migration. Biochem Pharmacol 92: 642–650, 2014. doi: 10.1016/j.bcp.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, Goldstein JL, Brown MS. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci USA 109: 16184–16189, 2012. doi: 10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parpal S, Karlsson M, Thorn H, Strålfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem 276: 9670–9678, 2001. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- 134.Parvanova A, Iliev I, Filipponi M, Dimitrov BD, Vedovato M, Tiengo A, Trevisan R, Remuzzi G, Ruggenenti P. Insulin resistance and proliferative retinopathy: a cross-sectional, case-control study in 115 patients with type 2 diabetes. J Clin Endocrinol Metab 89: 4371–4376, 2004. doi: 10.1210/jc.2003-032076. [DOI] [PubMed] [Google Scholar]
- 135.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, Remuzzi G, Ruggenenti P. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 55: 1456–1462, 2006. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 136.Pastukhov O, Schwalm S, Römer I, Zangemeister-Wittke U, Pfeilschifter J, Huwiler A. Ceramide kinase contributes to proliferation but not to prostaglandin E2 formation in renal mesangial cells and fibroblasts. Cell Physiol Biochem 34: 119–133, 2014. doi: 10.1159/000362989. [DOI] [PubMed] [Google Scholar]
- 137.Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, Molina-David J, Ge M, Lassenius MI, Forsblom C, Lehto M, Groop PH, Kretzler M, Eddy S, Martini S, Reich H, Wahl P, Ghiggeri G, Faul C, Burke GW III, Kretz O, Huber TB, Mendez AJ, Merscher S, Fornoni A. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest 126: 3336–3350, 2016. doi: 10.1172/JCI85939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Perry HM, Huang L, Ye H, Liu C, Sung SJ, Lynch KR, Rosin DL, Bajwa A, Okusa MD. Endothelial sphingosine 1–phosphate receptor–1 mediates protection and recovery from acute kidney injury. J Am Soc Nephrol 27: 3383–3393, 2016. doi: 10.1681/ASN.2015080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perseghin G, Caumo A, Arcelloni C, Benedini S, Lanzi R, Pagliato E, Sereni LP, Testolin G, Battezzati A, Comi G, Comola M, Luzi L. Contribution of abnormal insulin secretion and insulin resistance to the pathogenesis of type 2 diabetes in myotonic dystrophy. Diabetes Care 26: 2112–2118, 2003. doi: 10.2337/diacare.26.7.2112. [DOI] [PubMed] [Google Scholar]
- 140.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146: 408–420, 2011. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Petrie JR, Chaturvedi N, Ford I, Brouwers MCGJ, Greenlaw N, Tillin T, Hramiak I, Hughes AD, Jenkins AJ, Klein BEK, Klein R, Ooi TC, Rossing P, Stehouwer CDA, Sattar N, Colhoun HM, Nickerson H, Lou O, Dutta S, Haw J, Anderson C, Kean S, Thomson E, Gillespie L, Gibb J, Greenlaw N, Keech A, Jenkins A, March K, Williams S, Coady E, Bots M, Dreyer J, Jan T, Sheffy K, Lusky R, Peleg S, Shore A, Carty D, Donnan P, Witham M, Adler A, Lonn E, Rauchhaus P, Lindsay R, Brouwers M, Van-Melckebeke J, Gillespie L, Hamill T, Cuthbertson L, Murray A, Jolly L, Miller E, Hair J, Bell A, Carmichael S, Douglas E, Surtees P, Dinnett E, Allan J, Watson C, McLaughlin M, Brindley G, Smillie E, Motherwell D, MacDonald S, Ellis P, Stuart D, Travers M, Brearley S, Greig L, Colman P, Nankervis A, Forulanos S, West D, Vaughan S, Bjorasen M, Donlan J, Vrazas J, O’Neal D, Horsburgh J, Pater H, Kent S, Twigg S, Fulcher G, Denner R, Piotrowicz A, Januszewski A, Coy A, Paul T, McDonald C, Tereschyn S, Schmidt N, Weingert M, Heard H, Burke S, Ooi TC, Lochnan H, Sorisky A, Keely E, Malcolm J, Maranger J, Favreau C, Petherick S, Boles K, Rossing P, Hansen TW, Lund S, Hemmingsen B, Thorogood N, Green K, Robinson T, Abouglilia K, Nayman D, Miller C, Warren R, Aizawa K, Balasubramani M, Toth S, Harvey K, Birch G, Atkin S, Sathyapalan T, James A, Javed Z, Wilding J, Martin B, Birch S, Wilcox A, Watson N, Oliver N, Jugnee N, Rutter M, Turgut T, Shaju A, Yau S, Subin S, Walker M, Wake D, Miller C, Millward A, Chong P, Hibbert M, George J, Schaper N, Pinxt J, op het Roodt J, Phillips S, Murray L, Sleigh L, Collier A, Sit LE, Allan K, Cook J, Campbell K, Hodge L, Leese G, Reekie G, Jaap A, Sudworth A, White A, McKnight J, Steven L, McKay G, Llano A, Currie G, Lennon E, Johnstone J, Shields K; REMOVAL Study Group . Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 5: 597–609, 2017. doi: 10.1016/S2213-8587(17)30194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Popineau L, Morzyglod L, Carré N, Caüzac M, Bossard P, Prip-Buus C, Lenoir V, Ragazzon B, Fauveau V, Robert L, Guilmeau S, Postic C, Komatsu M, Canonne-Hergaux F, Guillou H, Burnol AF. Novel Grb14-mediated cross talk between insulin and p62/Nrf2 pathways regulates liver lipogenesis and selective insulin resistance. Mol Cell Biol 36: 2168–2181, 2016. doi: 10.1128/MCB.00170-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Prasad R, Hadjidemetriou I, Maharaj A, Meimaridou E, Buonocore F, Saleem M, Hurcombe J, Bierzynska A, Barbagelata E, Bergadá I, Cassinelli H, Das U, Krone R, Hacihamdioglu B, Sari E, Yesilkaya E, Storr HL, Clemente M, Fernandez-Cancio M, Camats N, Ram N, Achermann JC, Van Veldhoven PP, Guasti L, Braslavsky D, Guran T, Metherell LA. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J Clin Invest 127: 942–953, 2017. doi: 10.1172/JCI90171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rapizzi E, Taddei ML, Fiaschi T, Donati C, Bruni P, Chiarugi P. Sphingosine 1-phosphate increases glucose uptake through trans-activation of insulin receptor. Cell Mol Life Sci 66: 3207–3218, 2009. doi: 10.1007/s00018-009-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rask-Madsen C, King GL. Diabetes: podocytes lose their footing. Nature 468: 42–44, 2010. doi: 10.1038/468042a. [DOI] [PubMed] [Google Scholar]
- 146.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55: 2579–2587, 2006. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 147.Santamaria B, Marquez E, Lay A, Carew RM, González-Rodríguez Á, Welsh GI, Ni L, Hale LJ, Ortiz A, Saleem MA, Brazil DP, Coward RJ, Valverde AM. IRS2 and PTEN are key molecules in controlling insulin sensitivity in podocytes. Biochim Biophys Acta 1853: 3224–3234, 2015. [Erratum in Biochim Biophys Acta 1863: 786, 2016.] doi: 10.1016/j.bbamcr.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 148.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 149.Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P; Quartet [corrected] Study Group . Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab 89: 6068–6076, 2004. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 150.Schiffer M, Susztak K, Ranalletta M, Raff AC, Böttinger EP, Charron MJ. Localization of the GLUT8 glucose transporter in murine kidney and regulation in vivo in nondiabetic and diabetic conditions. Am J Physiol Renal Physiol 289: F186–F193, 2005. doi: 10.1152/ajprenal.00234.2004. [DOI] [PubMed] [Google Scholar]
- 151.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001. doi: 10.1172/JCI200112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sciacca L, Cassarino MF, Genua M, Pandini G, Le Moli R, Squatrito S, Vigneri R. Insulin analogues differently activate insulin receptor isoforms and post-receptor signalling. Diabetologia 53: 1743–1753, 2010. doi: 10.1007/s00125-010-1760-6. [DOI] [PubMed] [Google Scholar]
- 153.Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene 18: 2471–2479, 1999. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 154.Sciacca L, Mineo R, Pandini G, Murabito A, Vigneri R, Belfiore A. In IGF-I receptor-deficient leiomyosarcoma cells autocrine IGF-II induces cell invasion and protection from apoptosis via the insulin receptor isoform A. Oncogene 21: 8240–8250, 2002. doi: 10.1038/sj.onc.1206058. [DOI] [PubMed] [Google Scholar]
- 155.Sekimoto J, Kabayama K, Gohara K, Inokuchi J. Dissociation of the insulin receptor from caveolae during TNFα-induced insulin resistance and its recovery by D-PDMP. FEBS Lett 586: 191–195, 2012. doi: 10.1016/j.febslet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 156.Shavva VS, Bogomolova AM, Nikitin AA, Dizhe EB, Tanyanskiy DA, Efremov AM, Oleinikova GN, Perevozchikov AP, Orlov SV. Insulin-mediated downregulation of apolipoprotein A-I gene in human hepatoma cell line HepG2: the role of interaction between FOXO1 and LXRβ transcription factors. J Cell Biochem 118: 382–396, 2017. doi: 10.1002/jcb.25651. [DOI] [PubMed] [Google Scholar]
- 157.Sörensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol 13: 2639–2647, 2002. doi: 10.1097/01.ASN.0000033277.32822.23. [DOI] [PubMed] [Google Scholar]
- 158.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol 21: 128–135, 2010. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- 159.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 18: 5457–5464, 1998. doi: 10.1128/MCB.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int 73: 1385–1393, 2008. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 161.ter Braak B, Siezen CL, Kannegieter N, Koedoot E, van de Water B, van der Laan JW. Classifying the adverse mitogenic mode of action of insulin analogues using a novel mechanism-based genetically engineered human breast cancer cell panel. Arch Toxicol 88: 953–966, 2014. doi: 10.1007/s00204-014-1201-2. [DOI] [PubMed] [Google Scholar]
- 162.ter Braak B, Wink S, Koedoot E, Pont C, Siezen C, van der Laan JW, van de Water B. Alternative signaling network activation through different insulin receptor family members caused by pro-mitogenic antidiabetic insulin analogues in human mammary epithelial cells. Breast Cancer Res 17: 97, 2015. doi: 10.1186/s13058-015-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thomas SS, Zhang L, Mitch WE. Molecular mechanisms of insulin resistance in chronic kidney disease. Kidney Int 88: 1233–1239, 2015. doi: 10.1038/ki.2015.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrol 18: 2661–2671, 2007. doi: 10.1681/ASN.2006121410. [DOI] [PubMed] [Google Scholar]
- 165.United States Renal Data System 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016. [Google Scholar]
- 166.Vella V, Pandini G, Sciacca L, Mineo R, Vigneri R, Pezzino V, Belfiore A. A novel autocrine loop involving IGF-II and the insulin receptor isoform-A stimulates growth of thyroid cancer. J Clin Endocrinol Metab 87: 245–254, 2002. doi: 10.1210/jcem.87.1.8142. [DOI] [PubMed] [Google Scholar]
- 167.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 168.Verma S, Bhatt DL. More CREDENCE for SGLT2 inhibition. Circulation. In press. doi: 10.1161/CIRCULATIONAHA.119.041181. [DOI] [PubMed] [Google Scholar]
- 169.Vienberg SG, Bouman SD, Sørensen H, Stidsen CE, Kjeldsen T, Glendorf T, Sørensen AR, Olsen GS, Andersen B, Nishimura E. Receptor-isoform-selective insulin analogues give tissue-preferential effects. Biochem J 440: 301–308, 2011. doi: 10.1042/BJ20110880. [DOI] [PubMed] [Google Scholar]
- 170.Wakisaka M, He Q, Spiro MJ, Spiro RG. Glucose entry into rat mesangial cells is mediated by both Na+-coupled and facilitative transporters. Diabetologia 38: 291–297, 1995. doi: 10.1007/BF00400633. [DOI] [PubMed] [Google Scholar]
- 171.Wan X, Chen Z, Choi WI, Gee HY, Hildebrandt F, Zhou W. Loss of epithelial membrane protein 2 aggravates podocyte injury via upregulation of caveolin-1. J Am Soc Nephrol 27: 1066–1075, 2016. doi: 10.1681/ASN.2014121197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang Y, Inoue H, Ravnskjaer K, Viste K, Miller N, Liu Y, Hedrick S, Vera L, Montminy M. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci USA 107: 3087–3092, 2010. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang Y, Wong RH, Tang T, Hudak CS, Yang D, Duncan RE, Sul HS. Phosphorylation and recruitment of BAF60c in chromatin remodeling for lipogenesis in response to insulin. Mol Cell 49: 283–297, 2013. doi: 10.1016/j.molcel.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wasik AA, Lehtonen S. Glucose transporters in diabetic kidney disease−friends or foes? Front Endocrinol (Lausanne) 9: 155, 2018. doi: 10.3389/fendo.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJM. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.White MF, Kahn CR. The insulin signaling system. J Biol Chem 269: 1–4, 1994. [PubMed] [Google Scholar]
- 177.Wong RH, Chang I, Hudak CS, Hyun S, Kwan HY, Sul HS. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell 136: 1056–1072, 2009. doi: 10.1016/j.cell.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xia S, Liu Y, Li X, Thilo F, Tepel M. Insulin increases expression of trpc6 channels in podocytes by a calcineurin-dependent pathway. Cell Physiol Biochem 38: 659–669, 2016. doi: 10.1159/000438658. [DOI] [PubMed] [Google Scholar]
- 179.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA 100: 3445–3449, 2003. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab 14: 21–32, 2011. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yip J, Mattock M, Sethi M, Morocutti A, Viberti G. Insulin resistance in family members of insulin-dependent diabetic patients with microalbuminuria. Lancet 341: 369–370, 1993. doi: 10.1016/0140-6736(93)90167-F. [DOI] [PubMed] [Google Scholar]
- 182.Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet 342: 883–887, 1993. doi: 10.1016/0140-6736(93)91943-G. [DOI] [PubMed] [Google Scholar]
- 183.Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, Mitrofanova A, Leclercq F, Faul C, Li J, Kretzler M, Nelson RG, Lehto M, Forsblom C, Groop PH, Reiser J, Burke GW, Fornoni A, Merscher S. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol 26: 133–147, 2015. doi: 10.1681/ASN.2013111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yunn NO, Koh A, Han S, Lim JH, Park S, Lee J, Kim E, Jang SK, Berggren PO, Ryu SH. Agonistic aptamer to the insulin receptor leads to biased signaling and functional selectivity through allosteric modulation. Nucleic Acids Res 43: 7688–7701, 2015. doi: 10.1093/nar/gkv767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang H, Schin M, Saha J, Burke K, Holzman LB, Filipiak W, Saunders T, Xiang M, Heilig CW, Brosius FC III. Podocyte-specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. Am J Physiol Renal Physiol 299: F91–F98, 2010. doi: 10.1152/ajprenal.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zheng X, Li W, Ren L, Liu J, Pang X, Chen X, Kang D, Wang J, Du G. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol Ther 195: 85–99, 2019. doi: 10.1016/j.pharmthera.2018.10.011. [DOI] [PubMed] [Google Scholar]