Abstract
The function of actin is regulated by various posttranslational modifications. We have previously shown that in the kidneys of nonobese type 2 diabetes model Goto-Kakizaki rats, increased O-GlcNAcylation of β-actin protein is observed. It has also been reported that both O-GlcNAcylation and phosphorylation occur on Ser199 of β-actin. However, their roles are not known. To elucidate their roles in diabetic nephropathy, we examined the rat kidney for changes in O-GlcNAcylation of Ser199 (gS199)-actin and in the phosphorylation of Ser199 (pS199)-actin. Both gS199- and pS199-actin molecules had an apparent molecular weight of 40 kDa and were localized as nonfilamentous actin in both the cytoplasm and nucleus. Compared with the normal kidney, the immunostaining intensity of gS199-actin increased in podocytes of the glomeruli and in proximal tubules of the diabetic kidney, whereas that of pS199-actin did not change in podocytes but decreased in proximal tubules. We confirmed that the same results could be observed in the glomeruli of the human diabetic kidney. In podocytes of glomeruli cultured in the presence of the O-GlcNAcase inhibitor Thiamet G, increased O-GlcNAcylation was accompanied by a concomitant decrease in the amount of filamentous actin and in morphological changes. Our present results demonstrate that dysregulation of O-GlcNAcylation and phosphorylation of Ser199 occurred in diabetes, which may contribute partially to the causes of the morphological changes in the glomeruli and tubules. gS199- and pS199-actin will thus be useful for the pathological evaluation of diabetic nephropathy.
Keywords: β-actin, diabetic nephropathy, Goto-Kakizaki rat, O-GlcNAcylation, phosphorylation and dephosphorylation
INTRODUCTION
Actin is an important cytoskeletal protein for maintaining the structure and motility of cells (9). Not only is actin a cytoskeletal protein but also, in recent years, it has been revealed that actin participates in the regulation of transcription and chromatin remodeling in the nucleus (8, 24, 33). Functions of actin are regulated by various posttranslational modifications, such as acetylation, arginylation, ADP-ribosylation, methylation, ubiquitination, oxidation, phosphorylation, and O-GlcNAcylation (45). O-GlcNAc modification (O-GlcNAcylation) of proteins occurs on serine or threonine residues of many cytoplasmic and nuclear proteins (19, 48). O-GlcNAcylation often occurs on the same or proximal serine or threonine residues where phosphorylation occurs (7, 19). In diabetes, hyperglycemia increases the flux into the hexosamine biosynthesis pathway (6, 31). The end point of the hexosamine biosynthetic pathway is UDP-GlcNAc, the donor substrate for O-GlcNAc transferase. O-GlcNAcylation of many cytoplasmic and nuclear proteins is increased in diabetes (1, 3, 4, 16, 37, 46). Aberrant O-GlcNAcylation and phosphorylation of proteins causes diabetic nephropathy (2, 11, 15, 26, 36, 47). We have previously shown that in the kidneys of nonobese type 2 diabetes model Goto-Kakizaki (GK) rats, the expression of O-GlcNAc transferase protein increases and is accompanied by a twofold or more increase in O-GlcNAcylation of cytoskeletal proteins, including β-actin, along with morphological changes in glomeruli and proximal tubules (2, 3). However, the role of O-GlcNAcylation of β-actin has not been studied yet, whereas that of phosphorylation of β-actin has been previously examined.
The ultrastructure of the foot process of the podocyte is maintained by a cytoskeleton composed mainly of actin filaments (22). In diabetic nephropathy, morphological changes occur in the glomerulus, e.g., thickening of the basement membrane, fusion of foot processes with each other, disappearance of the slit membrane, and closure of pores in the endothelium (3).
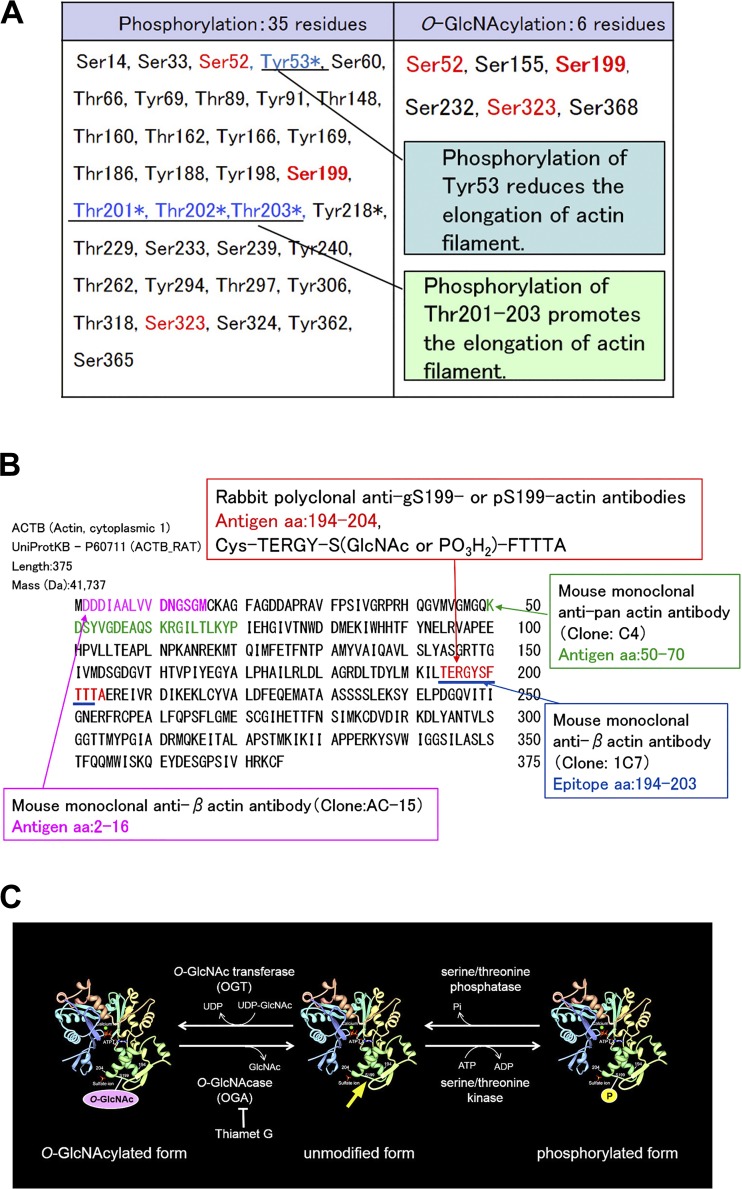
β-Actin has six serine amino acid residues (Ser52, Ser155, Ser199, Ser232, Ser323, and Ser368), each of which is O-GlcNAcylated (Fig. 1) (20, 38). Of these six residues, three of them (Ser52, Ser199, and Ser323) are not only O-GlcNAcylated but also phosphorylated (Fig. 1A) (20, 38). Phosphorylation of Thr201–203 adjacent to Ser199 increases actin polymerization, whereas phosphorylation of Tyr53 next to Ser52 decreases it (12, 14, 30). Ser199, Thr201, Thr202, and Thr203 amino acids are conserved well in most species (41). However, the role of β-actin Ser199 phosphorylation (pS199) and Ser199 O-GlcNAcylation (gS199) in the kidney and the relation between changes in their localization and diabetic nephropathy are not known.
Fig. 1.
A: phosphorylation sites and O-GlcNAcylation sites in β-actin. In the amino acid sequence of β-actin, six serine residues are O-GlcNAcylated. Of them, three serine residues (Ser52, Ser199, and Ser323) are also phosphorylated. Tyr53, Thr201, Thr202, and Thr203 are important amino acid residues for actin polymerization, which is conserved in all species. Phosphorylation of Thr201–203, which neighbor Ser199, promotes the elongation of actin filaments (12, 14), whereas phosphorylation of Tyr53, next to Ser52, reduces the elongation of actin filaments (30). *Known functionally important modification. B: amino acid sequences of polypeptides used as the immunogen to raise the antibodies. C: molecular images of β-actin. The schematic indicates the relationship between O-GlcNAcylation of Ser199-actin, unmodified β-actin, and phosphorylation of Ser199-actin. The yellow arrow indicates the site where the amino acid sequence used as the immunogen is located in the molecule.
To study the role of O-GlcNAcylation and phosphorylation of β-actin Ser199 in diabetic nephropathy, we raised rabbit polyclonal antibodies against gS199-actin and pS199-actin using synthesized oligomeric peptides [Cys-TERGY-S(GlcNAc)-FTTA and Cys-TERGY-S(phospho)-FTTA] as antigens. We examined where gS199-actin and pS199-actin were localized in the normal glomerulus and proximal tubules and whether changes in their localization occurred in the diabetic kidney. To elucidate the role of gS199-actin, we examined the effect of the O-GlcNAcase (OGA) inhibitor Thiamet G on the cellular ultrastructure of cultured podocytes.
MATERIALS AND METHODS
Animal kidney specimens.
Kidney tissues were obtained by dissecting 15-wk-old male (n = 6) Wistar rats (as controls) and GK rats, the latter of which are a nonobese model of noninsulin-dependent diabetes mellitus and had been developed by the selective breading of glucose-intolerant Wistar rats. Both rat types were obtained from CLEA (Tokyo, Japan). All experimental procedures using laboratory animals were approved by the Animal Care and Use Committee of Kyorin University School of Medicine (approval no. 07).
Human kidney specimens.
We collected cryopreserved autopsy samples of kidney from postmortem patients in the Tokyo Metropolitan Geriatric Hospital. Kidney tissues were obtained from eight patients with diabetes with glomerular lesions, from seven patients with diabetes without glomerular lesions, and from four patients who did not have diabetes without glomerular lesions as normal tissue (11 men and 8 women, average age: 82 yr). Histopathological findings of diabetic nephropathy and normal glomerulus are shown in Table 1. Bereaved family members or related persons gave written informed consent for research use of the tissues. The protocols were approved by the Ethics Committee of the Kyorin University (approval no. 626-01) and Tokyo Metropolitan Geriatric Hospital (approval no. R19-16). All experiments were performed in accordance with the relevant guidelines and regulations set out in the Declaration of Helsinki.
Table 1.
Histopathological findings on diabetic nephropathy and normal glomerulus
| Glomerular Lesion |
||||||
|---|---|---|---|---|---|---|
| Age | Sex | Glomerulosclerosis | Nodular lesion | Arteriosclerosis | Arteriosclerosis | |
| DM with glomerular lesion | ||||||
| Patient no. | ||||||
| 1 | 85 | M | + + + | − ~+ ~+ + + | + | +++ |
| 2 | 82 | M | + (1/3–1/4) | + | − | + |
| 3 | 93 | F | + | − | + + | ++ |
| 4 | 78 | F | + | + | − | + |
| 5 | 78 | F | + | ++ | + | + |
| 6 | 75 | M | − | + | − | + |
| 7 | 77 | M | − | + | − | + |
| 8 | 73 | M | − | − | + | ± |
| DM without glomerular lesion | ||||||
| Patient no. | ||||||
| 9 | 90 | M | − | − | − | + |
| 10 | 74 | F | − | − | − | + |
| 11 | 87 | F | − | − | − | + |
| 12 | 81 | M | − | − | − | + |
| 13 | 94 | M | − | − | − | ± |
| 14 | 87 | F | − | − | − | ++ |
| 15 | 72 | M | − | − | − | ± |
| Normal without DM | ||||||
| Patient no. | ||||||
| 16 | 77 | F | − | − | − | − |
| 17 | 79 | M | − | − | − | − |
| 18 | 85 | M | − | − | − | − |
| 19 | 90 | F | − | − | − | − |
DM, diabetes mellitus; F, female; M, male.
Antibodies.
The following antibodies were commercially obtained: mouse monoclonal anti-β-actin antibody (clone, AC15) and anti-vimentin antibody (clone, V9) from Sigma-Aldrich (St. Louis, MO), mouse monoclonal anti-nonfilamentous β-actin antibody (clone, 1C7) from Progen Biotechnik (Heidelberg, Germany) and Abbkine (Wuhan, China), mouse monoclonal anti-pan-actin antibody (clone, C4) from Millipore, and mouse monoclonal anti-O-GlcNAc antibodies (clone, RL2), Alexa 568-labeled secondary antibody, and Alexa 488-phalloidin from ThermoFisher Scientific (Rockford, IL). Mouse monoclonal anti-GAPDH antibody (clone, GT239) was purchased from GeneTex (Irvine, CA). Colloidal gold-conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA).
Generating specific pS199-actin and gS199-actin antibodies.
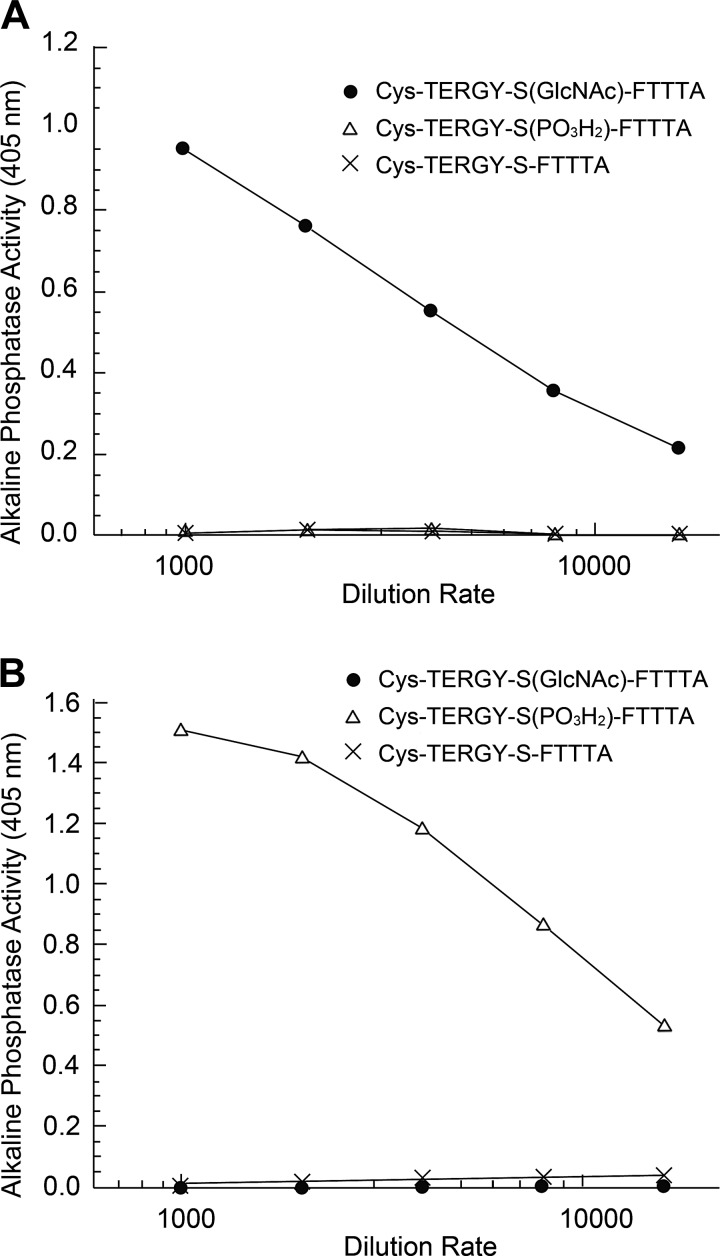
Anti-gS199-actin and anti-pS199-actin polyclonal antiserums were generated against synthetic peptides [TERGY-S(GlcNAc)-FTTTA and TERGY-S(PO3H2)-FTTTA, respectively], corresponding to amino acid sequence 194–204 of rat β-actin, which shares high homology with the human, rat, and mouse proteins (Fig. 1B). Two rabbits were immunized with each antigen, and IgG fractions of the obtained antisera were purified by Peptide Institute (Osaka, Japan). The specificity of the antibodies against the antigens was analyzed by ELISA (Fig. 2).
Fig. 2.
ELISA analysis. Flat-bottomed 96-well plates were coated with synthesized peptides [Cys-TERGY-S(GlcNAc)-FTTTA (●), Cys-TERGY-S(PO3H2)-FTTTA (△), or and Cys-TERGY-S-FTTTA (×)]. Peptide-coated plates were blocked by incubation with casein blocking buffer and then incubated either with affinity-purified anti-O-GlcNAcylation of Ser199 (gS199)-actin peptide antibody or with anti-phosphorylation of Ser199 (pS199)-actin peptide antibody. Antibody binding was detected by incubation with alkaline phosphatase-conjugated goat anti-rabbit IgG. The chromogen development was mediated by the addition of substrate solution (p-nitrophenyl phosphate). Optical density was measured at 405 nm. The results of the ELISA assay showed that gS199-actin antibody reacted with neither unmodified Ser199-actin peptide nor pS199-actin peptide but did react specifically with gS199-actin peptide (A) and that pS199-actin antibody reacted with neither unmodified Ser199-actin peptide nor gS199-actin peptide but reacted specifically with pS199-actin peptide (B).
Immunoprecipitation and immunoblot analysis.
Immunoprecipitation and immunoblot analysis were carried out as previously described (2). The isolation of cytoplasmic and nuclear proteins of cultured podocyte cells was carried out using an SF PTS kit (GL Sciences, Tokyo, Japan) according to the manufacturer’s instructions.
Mass spectrometry.
Bands were excised from a Coomassie blue-stained SDS-PAGE gel and treated with trypsin for 16 h at 37°C. The digested proteins were then desalted and concentrated with StageTips. Analysis of tryptic peptides was performed using two systems of mass spectrometry: LTQ-Orbitrap Velos (ThermoFisher Scientific) for the identification of immunoprecipitated 40-kDa protein and Q-Exactive (ThermoFisher Scientific) for the comparison of sequence coverage between 40- and 42-kDa β-actin. LTQ-Orbitrap Velos was coupled with a direct nano-liquid chromatography system (DiNA, KYA Technologies, Tokyo, Japan). The MASCOT 2.4 (Matrix Science) search engine was used to access the UniProtKB/Swiss-Prot database. In Q-Exactive analyses, tryptic peptides were desalted by using GL-Tip SDB (GL Sciences) followed by LC-MS/MS analysis. Proteome Discoverer 2.2 was used for protein identification to access the database for Rattus norvegicus (SwissProt TaxID no. 10116_and_subtaxionomies).
Immunofluorescence and confocal microscopy.
The immunohistochemical study was carried out as previously described (23). For immunostaining of gS199-actin and pS199-actin, immortalized cultured podocytes were fixed in 4% formaldehyde for 1 h, subjected to autoclave heating using Target Retrieval Solution (Dako) at 120°C for 10 min, and rendered permeable with 0.05% Tween 20 in PBS for 10 min. After incubation with 5% normal donkey serum for 30 min, cells were reacted with primary antibodies for gS199-actin and pS199-actin or normal rabbit IgG at 4°C overnight and then incubated with Alexa Fluor 568-conjugated donkey anti-rabbit IgG and Alexa Fluor 488-conjugated phalloidin. For immunostaining of kidney tissue, rat kidneys were fixed with 4% paraformaldehyde for 1 h. Cryostat sections of the kidney were subjected to autoclave heating using Target Retrieval Solution (Dako) at 120°C for 10 min and incubated with 5% normal donkey serum for 60 min. Sections were reacted with primary antibodies or normal rabbit IgG and secondary antibodies as described above. Nuclei were stained with TO-PRO-3 Iodide (ThermoFisher Scientific), and signals were examined under a confocal laser scanning microscope (LSM-510 META, Carl Zeiss Microscopy). For human kidney specimens, fresh renal tissues were embedded in OCT compound (Sakura Fine Technical, Tokyo, Japan). The embedded tissues were frozen, cut to a thickness of 5 μm using a cryostat, and fixed in 4% paraformaldehyde in PBS for 2 h. The sectioned specimens were used for immunofluorescent labeling experiments as described above.
Immunoelectron microscopic analysis.
Immunoelectron microscopy was performed as previously described (42). Briefly, samples of kidney were fixed in 4% paraformaldehyde and embedded in LR White resin (Polysciences, Warrington, PA). Ultrathin sections were picked up on nickel grids. Sections were then incubated with 5% normal donkey serum in PBS for 10 min. Next, the grids were incubated at 4°C overnight with anti-gS199-actin antibody, anti-pS199-actin antibody, or normal rabbit IgG (5 μg/ml each) diluted with TrueVision Reagent (Vicgene Biotechnology, Mountain View, CA), rinsed with PBS, and reacted with colloidal gold-conjugated (10 nm in diameter) anti-rabbit IgG (1:50) at room temperature for 1 h. Finally, sections were stained with uranyl acetate for 30 s and then examined under an electron microscope (JEM-1010C; JEOL, Tokyo, Japan).
Culture of immortalized podocytes.
A conditionally immortalized mouse podocyte cell line was maintained as previously described (40). Cells were cultured at 37 °C in RPMI-1640 medium that contained 100 U/ml penicillin-streptomycin supplemented with 5% FBS. For the O-GlcNAcase inhibition experiment, cells were cultured in the presence or absence of the OGA inhibitor Thiamet G at a concentration of 100 nM.
Statistics.
A Student’s or Welch’s t-test with equal or unequal variances, respectively, was conducted to detect the difference between mean scores in two groups, based on the equality test of two variances. P values of <0.05 were considered statistically significant in all cases.
RESULTS
Characterization of antibodies against gS199-actin and pS199-actin.
To determine whether β-actin in the kidney was both O-GlcNAcylated and phosphorylated at its Ser199 and to examine possible changes in these posttranslational modifications in the diabetic kidney, we produced antibodies specific for pS199-actin and gS199-actin. Focusing on Ser199, located near the important amino acid sequence Thr201, Thr202, Thr203 for actin polymerization, antibodies were generated against an 11-amino acid-long peptide containing pS199 or gS199 and adjacent amino acids from rat β-actin (Fig. 1B).
ELISA analysis showed that anti-gS199-actin antibody bound specifically to the synthetic gS199-actin oligopeptide but reacted with neither pS199-actin oligopeptides nor unmodified oligopeptides. Also, anti-pS199-actin antibody bound specifically to the synthetic pS199-actin oligopeptide but was reactive with neither gS199-actin oligopeptides nor unmodified oligopeptides (Fig. 2).
Immunoblot and immunoprecipitation analyses of gS199-actin and pS199-actin from normal Wistar and diabetic GK rat kidneys.
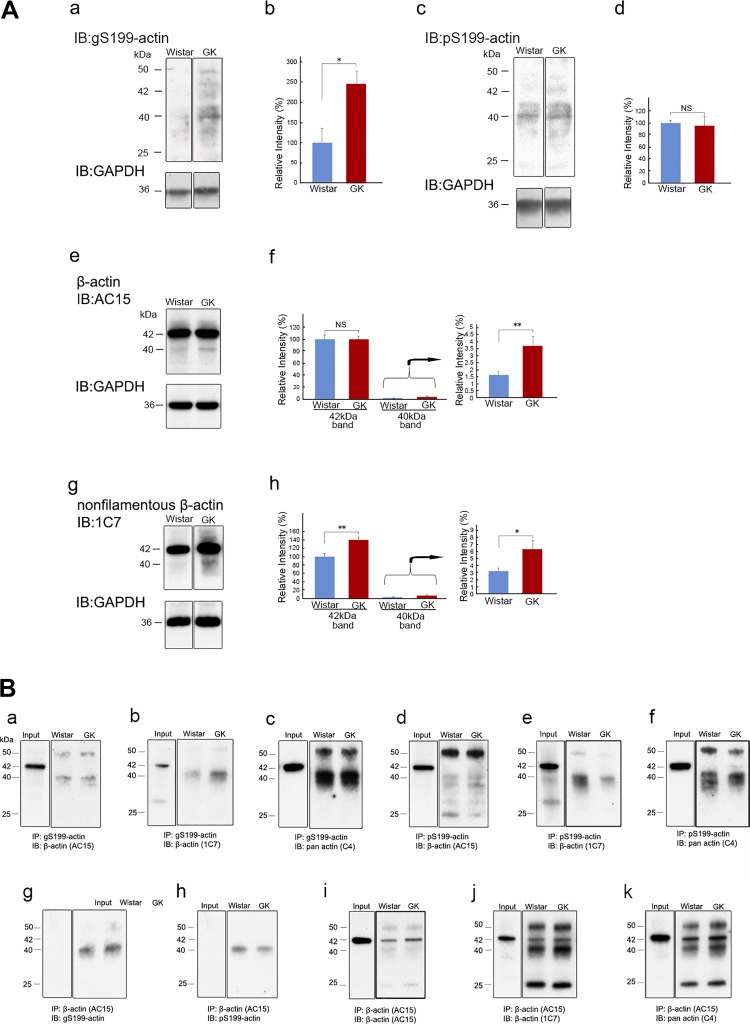
Immunoblot analysis of β-actin from total lysates of normal Wistar and diabetic GK rat kidneys performed with anti-gS199-actin antibody and pS199-actin antibody showed that both antibodies bound weakly to the 42-kDa band of β-actin and strongly to the 40-kDa band (Fig. 3A, a and c). Quantification of the bands revealed that the staining intensity of the 40-kDa band of gS199-actin for the diabetic GK rat kidney was significantly greater than that for the Wistar kidney but that that of the pS199-actin band was the same (Fig. 3A, b and d). Immunoblot analysis using AC15 anti-β-actin antibody, which is one of most used antibodies as a loading control for Western blot analysis and whose epitope (15 amino acids) is located in the NH2-terminal part of β-actin (Fig. 1B), showed that AC15 antibody bound to both 42- and 40-kDa bands and that there was no significant difference between Wistar and GK rats as to the density of the 42-kDa band. However, for the 40-kDa band, there was a significant increase in the diabetic kidney; that is, the ratio of the 40-kDa band density to that of the 42-kDa band density was 1.65 ± 0.23% in the normal kidney, whereas it increased significantly to 3.69 ± 0.69% in the diabetic kidney (Fig. 3A, e and f). On the other hand, clone 1C7 β-actin antibody, which was produced by using a chemically cross-linked actin dimer as the immunogen, recognized nonfilamentous β-actin. Its epitope was amino acids 194–203 of β-actin, almost the same as that of the antibodies used in the present study (Fig. 1B), and it detected a 42-kDa major band and a 40-kDa minor band (Fig. 3A, g and h). The density of both 42- and 40-kDa bands was significantly higher in the diabetic kidney (Fig. 3A, g and h).
Fig. 3.
A: immunoblot (IB) analysis of β-actin from normal Wistar and diabetic Goto-Kakizaki (GK) rat kidneys. Total kidney proteins were immunoblotted with anti-O-GlcNAcylation of Ser199(gS199)-actin antibody (a), anti-phosphorylated Ser199 (pS199)-actin antibody (c), AC15 anti-β-actin antibody (e), or 1C7 anti-nonfilamentous β-actin antibody (g). PVDF membranes in a, c, e, and g (top) were stripped and reprobed with the loading control, anti-GAPDH antibody (a, c, e, and g, bottom). Quantification of 40- and 42-kDa band densities was determined by the ratio of band density to that of the loading control (b, d, f, and h). Relative intensities of bands versus control 40-kDa band for Wistar rats were determined. Data are means ± SE from 5 (b and d) or 6 (f and h) different rats. The bands were cut from the same gel films. *P < 0.05 and **P < 0.01 vs. control Wistar rat. B: immunoprecipitation (IP) analysis of gS199-actin and pS199-actin from normal Wistar and diabetic GK rat kidneys. Total kidney proteins were immunoprecipitated with anti-gS199-actin antibody (a–c), anti-pS199-actin antibody (d–f), or AC15 anti-β-actin antibody (g–k). IB analysis using AC15 anti-β-actin antibody (a, d, and i), 1C7 anti-nonfilamentous β-actin antibody (b, e, and j), C4 anti-pan-actin antibody (c, f, and k), anti-gS199-actin antibody (g), and anti-pS199-actin antibody (h) were performed. The 40-kDa band was detected in all cases, whereas the 42-kDa band was detected in the input lane using AC15 (a, d, and i), 1C7 (b, e, and j), or C4 (c, f, and k) and in the lanes immunoprecipitated with AC15 (i–k). However, the 42-kDa band was not detected, and only the 40-kDa band was detected in the lanes using AC15 for IP and anti-gS199-actin antibody or anti-pS199-actin antibody for IB analysis (g, h). The 25-kDa band was IgG light chain, and the 50-kDa band was IgG heavy chain. The bands in the input were cut from the same gel films.
To confirm whether the different anti-β-actin antibodies also reacted with the 40-kDa protein bound with anti-gS199-actin and pS199-actin antibodies, we carried out immunoprecipitation and immunoblot analysis using other anti-β-actin antibodies. When immunoprecipitates obtained from normal Wistar and diabetic GK rat kidneys by reaction with anti-gS199-actin antibody or pS199-actin antibody were examined for reactivity with the three different anti-β-actin antibodies, the 40-kDa band but not the 42-kDa band was detected: AC15 (Fig. 3B, a and d), 1C7 (Fig. 3B, b and e), and C4 (Fig. 3B, c and f). However, when immunoprecipitates obtained with AC15 were examined using AC15, both 42- and 40-kDa bands were detected by anti-β-actin antibodies (Fig. 3B, i–k). On the other hand, when immunoprecipitates made with AC15 were examined for reactivity with anti-gS199-actin antibody or anti-pS199-actin antibody, only the 40-kDa band was observed (Fig. 3B, g and h). These results indicated that anti-gS199-actin antibody reacted specifically with 40-kDa gS199-actin and that anti-pS199-actin antibody did so specifically with 40-kDa pS199-actin.
Furthermore, we identified 40-kDa proteins immunoprecipitated with anti-gS199-actin, anti-pS199-actin antibody, or AC15 β-actin antibody using mass spectrometry. As a result, these immunoprecipitated proteins were identified as β-actin with gS199 antibody (coverage rate: 45.87%, number of peptides: 6, score: 686.29), pS199 antibody (coverage rate: 22.67%, number of peptides: 7, score: 344.77), and AC15 (coverage rate: 90%, number of peptides: 1, score: 587.84) (Supplemental Table S1; all Supplemental Data are available online at https://doi.org/10.6084/m9.figshare.9784754.v1). We presumed that 40-kDa β-actin was a truncated form of 42-kDa β-actin. However, the detected peptide sequences of 40-kDa β-actin spanned from the NH2-terminal to the COOH-terminal of β-actin, indicating that it was not a degradation product of 42-kDa β-actin (Fig. 4).
Fig. 4.
Comparison of amino acid sequence coverage between 42- and 40-kDa β-actin. The sequence of β-actin is shown (https://www.uniprot.org/uniprot/P60711). Blue underlined and yellow highlighted characters represent the identified peptide sequences from the respective 42- and 40-kDa bands, which were excised from SDS-PAGE gels of the immunoprecipitate obtained with AC15. The false discovery rate confidence was <0.01. Coverage rates of 42- and 40-kDa β-actin were 92.80% and 89.87%, respectively.
Immunofluorescence microscopy, immunoprecipitation, and immunoblot analysis of cultured podocytes.
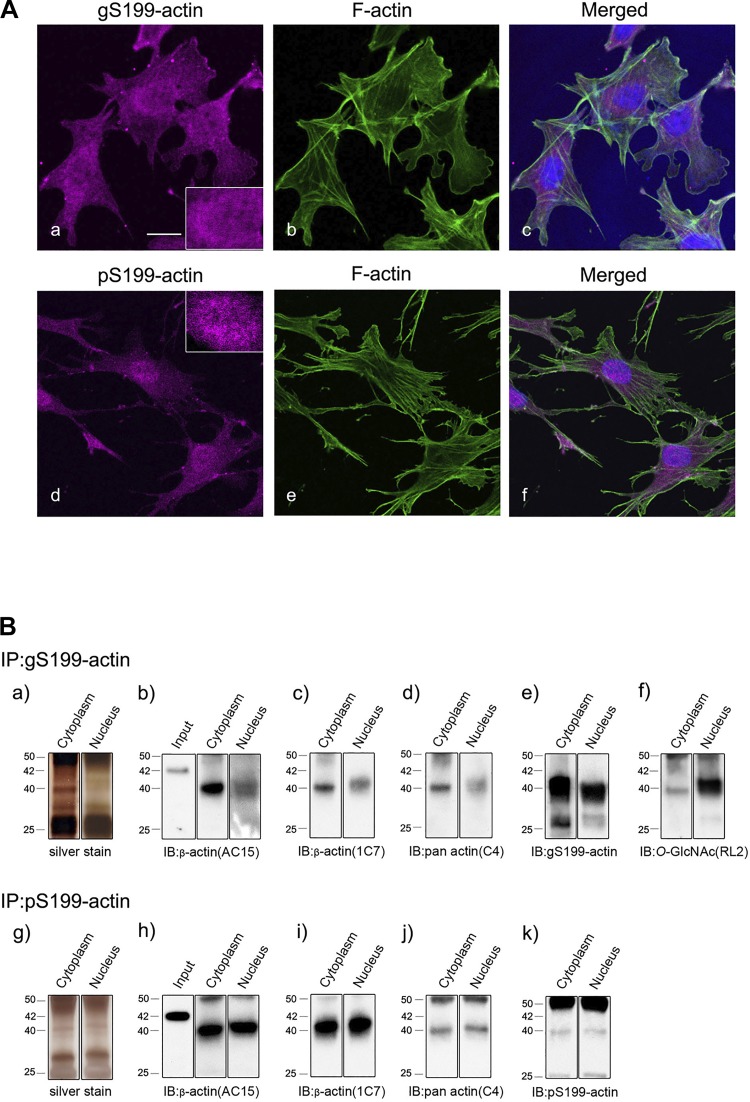
The subcellular localization of gS199-actin and pS199-actin was examined immunohistochemically in cultures of immortalized glomerular epithelial cell podocytes. Immunofluorescence analysis of the images obtained with gS199-actin and pS199-actin antibodies demonstrated that gS199-actin immunostaining (magenta color in Fig. 5Aa) was diffuse in both the nucleus (inset in Fig. 5Aa) and cytoplasm of the cultured podocytes, with filamentous actin (stress fiber, green color in Fig. 5Ab) being observed in the cytoplasm. pS199-actin immunostaining (magenta color in Fig. 5Ad) was intense and punctate in the nucleus (inset in Fig. 5Ad) but weak and diffuse in the cytoplasm of the cultured podocytes. Neither anti-gS199-actin nor anti-pS199-actin antibodies reacted with stress fibers, which consisted of actin filaments (Fig. 5A, a and d). To further show the specificity of the antibodies for their antigen, we carried out immunohistochemical absorption experiments using synthetic antigen oligopeptides. Coincubation of anti-gS199-actin antibody with synthetic gS199-actin oligopeptide inhibited the immunostaining with anti-gS199-actin antibody, whereas coincubation of the antibody with the synthetic pS199-actin oligopeptides did not do so (data not shown). Similarly, coincubation of anti-pS199-actin antibody with synthetic pS199-actin oligopeptide inhibited the immunostaining with anti-pS199-actin antibody, whereas coincubation of the antibody with gS199-actin-peptides did not inhibit it (data not shown). These results show that the anti-gS199-actin and anti-pS199-actin antibodies specifically reacted with gS199-actin and pS199-actin, respectively.
Fig. 5.
A: immunofluorescent images of O-GlcNAcylation of Ser199(gS199)-actin (a) and phosphorylated Ser199 (pS199)-actin (d) in cultured immortalized glomerular epithelial cell podocytes. gS199-actin immunostaining (magenta color) was diffuse in both the nucleus and cytoplasm of cultured podocytes (a). pS199-actin immunostaining (magenta color) was observed to be intense in the nucleus and weak in the cytoplasm of cultured podocytes (d). Filamentous actin (stress fiber, green color) stained with Alexa 488-phalloidin was observed in the cytoplasm (b and e). Insets in a and d show enlarged nuclei. gS199-actin localization was diffuse in the nucleus, whereas that of pS199-actin was punctate there. Merged images of magenta, green, and blue color are shown in c and f. Blue color indicates nuclei stained with TO-PRO3. Scale bar = 10 μm. B: immunoprecipitation (IP) analysis of gS199-actin and pS199-actin from cultured podocytes. Cytoplasmic and nuclear proteins were immunoprecipitated with anti-gS199-actin antibody (a–f) or anti-pS199-actin antibody (g–k). Silver-stained SDS-PAGE gel images are shown in a and g. Immunoblot (IB) analysis using AC15 anti-β-actin antibody (b and h), 1C7 anti-nonfilamentous β-actin antibody (c and i), C4 anti-pan-actin antibody (d and j), anti-gS199-actin antibody (e), anti-pS199-actin antibody (k), and RL2 anti-O-GlcNAc antibody (f) was performed. The 40-kDa band was detected in both the cytoplasm and nucleus in all cases (b–f and h–k), whereas the 42-kDa band was scarcely detected except in the input lanes using AC15 (b and h). For the input lanes, Wistar rat samples were loaded.
To further confirm the existence of gS199-actin and pS199-actin in both the cytoplasm and nucleus of podocytes, we conducted immunoprecipitation and immunoblot analyses using isolated cytoplasmic and nuclear lysates. After immunoprecipitation with gS199-actin or pS199-actin antibodies, immunoblot analysis was carried out with AC15 β-actin antibody, 1C7 nonfilamentous β-actin antibody, C4 pan-actin antibody, and RL2 O-GlcNAc antibody (Fig. 5B). The findings confirmed that gS199-actin and pS199-actin existed in both the cytoplasm and nucleus and that the molecular weights of gS199-actin and pS199-actin were both around 40 kDa but not 42 kDa (Fig. 5B). Furthermore, the 40-kDa protein immunoprecipitated with anti-gS199-actin antibody was O-GlcNAcylated, especially heavily in the nucleus (Fig. 5Bf).
Immunofluorescence and immunoelectron microscopy for the localization of gS199-actin and pS199-actin in the glomerulus of normal and diabetic kidneys.
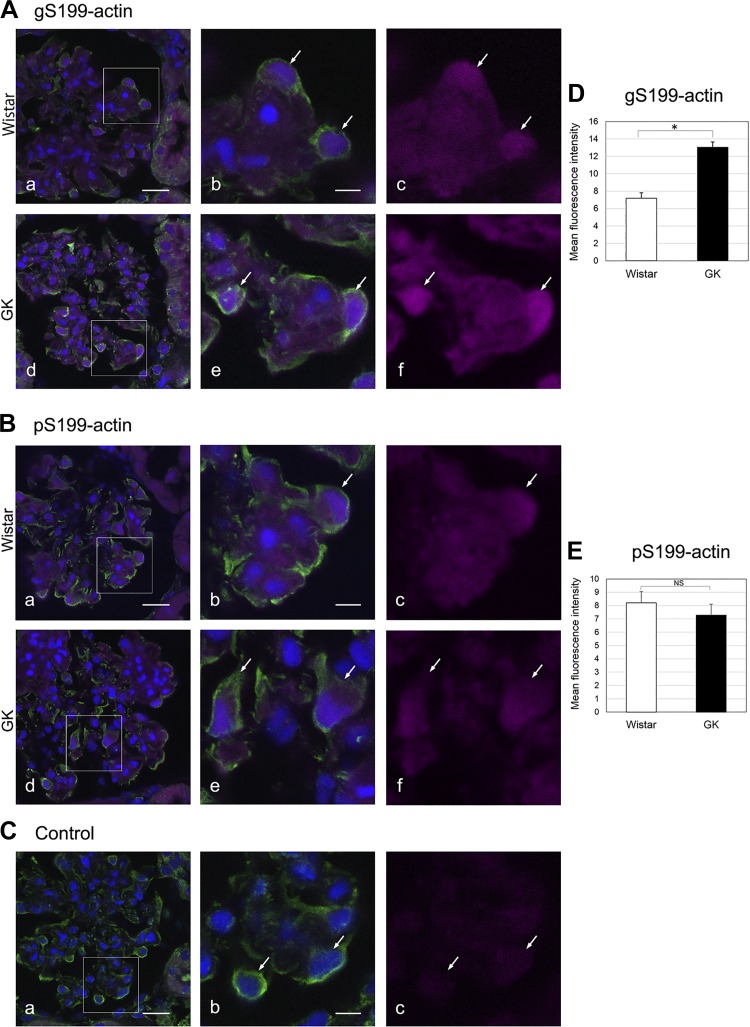
To observe a possible change in the localization of gS199-actin and pS199-actin in the diabetic kidney, we examined the localization of gS199-actin antibody using a confocal laser scanning microscope. In the glomerulus of the diabetic kidney, the immunostaining intensity of gS199-actin was significantly stronger in podocytes compared with other cells (Fig. 6, A, d–f, and D), whereas in the glomerulus of the normal kidney the staining intensity in podocytes was almost the same as that in the other cells (Fig. 6A, a–c). On the other hand, the immunostaining intensity of pS199-actin did not change in podocytes of the diabetic kidney compared with that for the normal kidney (Fig. 6, B and E).
Fig. 6.
Immunofluorescent images of O-GlcNAcylation of Ser199(gS199)-actin (A) and phosphorylated Ser199 (pS199)-actin (B) in the glomerulus of Wistar and Goto-Kakizaki (GK) rat kidneys. The rectangular areas in a and d are enlarged in b and e, respectively. c and f: Immunostained gS199-actin and pS199-actin (magenta color) were found to be diffuse in the glomerulus of the normal Wistar kidney and diabetic GK kidney. Vimentin (green color) was stained as a marker of podocytes (arrows) with Alexa 488-secondary antibody. C: cytochemical control images. c: Normal rabbit IgG showed no reactivity toward kidney sections. D and E: graphical quantification of gS199-actin (D) and pS199-actin (E) immunoreactivities in podocytes of Wistar and GK rat kidneys. Nuclei (blue color) were stained with TO-PRO3. Data are means ± SE from 5 different rats. *P < 0.05. Scale bars = 20 μm in a and 10 μm in b. NS, nonsignificant.
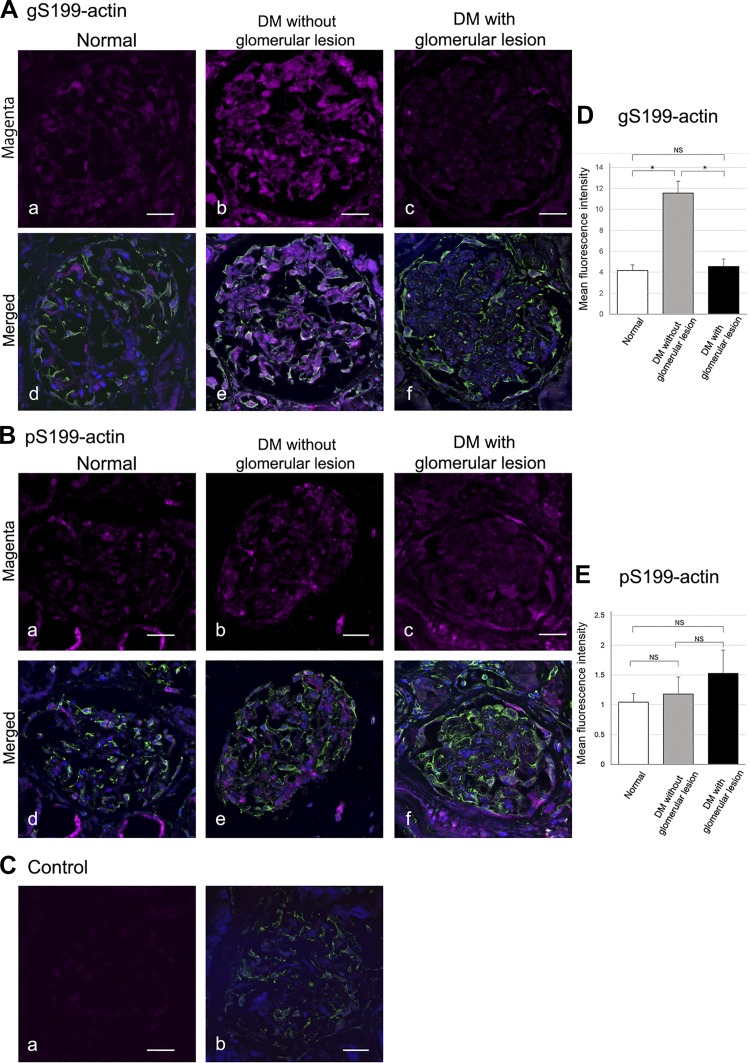
To confirm whether the same changes could be observed in the human diabetic kidney, we carried out immunofluorescent labeling experiments using human specimens. Immunostained gS199-actin (magenta color) was seen to be diffuse and weak in the normal glomerulus (Fig. 7A, a and d), whereas in the diabetic glomerulus without lesions, the immunostaining was significantly increased in intensity, especially in podocytes (Fig. 7, A, b and e, and D). However, in the diabetic glomerulus with lesions, the immunostaining intensity showed almost the same level as that for the normal glomerulus (Fig. 7, A, c and f, and D). As to the immunostaining intensity of pS199-actin, no significant difference was observed between the normal glomerulus and diabetic glomerulus without or with lesions (Fig. 7, B, a–f, and E). It was thus confirmed that the same changes of O-GlcNAcylation and phosphorylation of Ser199-actin occur in the glomerulus of the human diabetic kidney without lesions.
Fig. 7.
A and B: immunofluorescent images of O-GlcNAcylation of Ser199(gS199)-actin and phosphorylated Ser199 (pS199)-actin in the glomerulus of a normal human kidney (a and d), diabetes mellitus (DM) without glomerular lesions (b and e), and DM with glomerular lesions (c and f). Vimentin (green color) was stained as a marker of podocytes (arrows) with Alexa 488-secondary antibody. Immunostained gS199-actin (magenta color) was found to be diffuse and weak in the normal glomerulus (A, a and d), whereas in the diabetic glomerulus without lesions the immunostaining was significantly increased in intensity (A, b and e). The immunostaining intensity of pS199-actin in the diabetic glomerulus was observed to be at almost the same level as that found for the normal glomerulus (B, a–f). C: cytochemical control images. a: Normal rabbit IgG showed no reactivity toward kidney sections. b: Merged image of normal rabbit IgG (magenta color) and vimentin (green color). D and E: graphical quantification of gS199-actin and pS199-actin immunoreactivities in the podocytes of the glomerulus of normal (white bar), DM without glomerular lesions (gray bar), and DM with glomerular lesions (black bar). Nuclei (blue color) were stained with TO-PRO3. Data are means ± SE from 12 normal control glomeruli, 16 diabetic glomeruli without lesions, and 14 diabetic glomeruli with lesions. *P < 0.05. Scale bars = 20 μm. NS, nonsignificant.
As we have previously reported, morphological changes in the ultrastructure occur in the glomerulus of the diabetic GK rat kidney (3). Scanning electron microscopy showed a disordered arrangement of the podocyte foot processes in the glomerulus of the diabetic rat kidney (Fig. 8A). Transmission electron microscopy of the normal Wistar rat kidney and diabetic GK kidney showed fusion of foot processes of podocytes and thickening of the basement membrane (Fig. 9, A and B). To examine the detailed localization of gS199-actin, pS199-actin, and total β-actin, we performed immunoelectron microscopy using the colloidal gold labeling postembedding method. Figure 9, C and D, shows the localization of gS199-actin in the glomerular capillary wall. Labeling of gS199-actin was scarcely observed in the foot processes of podocytes and endothelial cells (Fig. 9C) but was moderate in the cell body of the podocyte (Fig. 9E), whereas intense labeling with AC15 anti-β-actin antibody was localized in both the foot processes of podocytes and endothelial cells (Fig. 8B, a and b). The density of colloidal gold labeling of gS199-actin was significantly greater in the foot processes, cytoplasm, and nucleus of podocytes of the diabetic kidney (Fig. 9, D, F, G, and H).
Fig. 8.
A: ultrastructural changes in the glomerulus of the diabetic rat kidney. a−d: Scanning electron microscopy of the glomerulus of the normal Wistar rat kidney (a and c) and diabetic Goto-Kakizaki (GK) rat kidney (b and d). c and d are enlargements of the rectangular areas in a and b, respectively. Foot processes (FP) of podocytes are regularly arranged in the normal kidney (c) but irregularly arranged in the diabetic kidney (d). Furthermore, many long and thin filopodia of podocytes were found in the diabetic kidney (d), whereas few filopodia were found in the normal kidney (c). Scale bars = 20 μm in a and b and 1 μm in c and d. B: immunoelectron microscopy of β-actin in the glomerulus and proximal tubule of normal (a and c) and diabetic (b and d) kidneys performed with AC15 anti-β-actin antibody by the 10-nm colloidal gold immunolabelling postembedding method. In the glomerular capillary wall, colloidal gold particles are localized in both FPs of podocytes and in endothelial cells (En) (a and b). In the proximal tubule, the particles were found in both the microvilli (MV) and terminal web (TW) (c and d). There was no difference in their distribution between normal and diabetic kidneys. Scale bars = 200 nm. GBM, glomerular basement membrane.
Fig. 9.
A and B: ultrastructural changes in the capillary wall of the glomerulus in the diabetic kidney (B) compared with that in the normal kidney (A). Immunoelectron microscopy of glomerular O-GlcNAcylation of Ser199(gS199)-actin using 10-nm colloidal gold labeling is shown. C and D: localization of gS199-actin in the capillary wall of the glomerulus from the normal kidney (C) and diabetic kidney (D). G: colloidal gold immunolabelling density of gS199-actin was significantly increased in the foot process (FP) of the diabetic podocyte. E and F: localization of gS199-actin in the cell body of a podocyte from a normal kidney (E) and diabetic kidney (F). Dotted lines indicate the boundary between the cytoplasm (Cy) and nucleus (Nu). Colloidal gold labeling was seen in both the cytoplasm and nucleus. H: colloidal gold immunolabelling density of gS199-actin was significantly increased in both the cytoplasm and nucleus of podocytes in the diabetic kidney. Scale bars = 500 nm in A and B and 200 nm in C–F. En, endothelial cells; GBM, glomerular basement membrane; GK, Goto-Kakizaki.
On the other hand, pS199-actin was scarcely detectable by the colloidal gold labeling postembedding method.
Immunofluorescence and immunoelectron microscopy for the localization of gS199-actin and pS199-actin in proximal tubules of normal and diabetic kidneys.
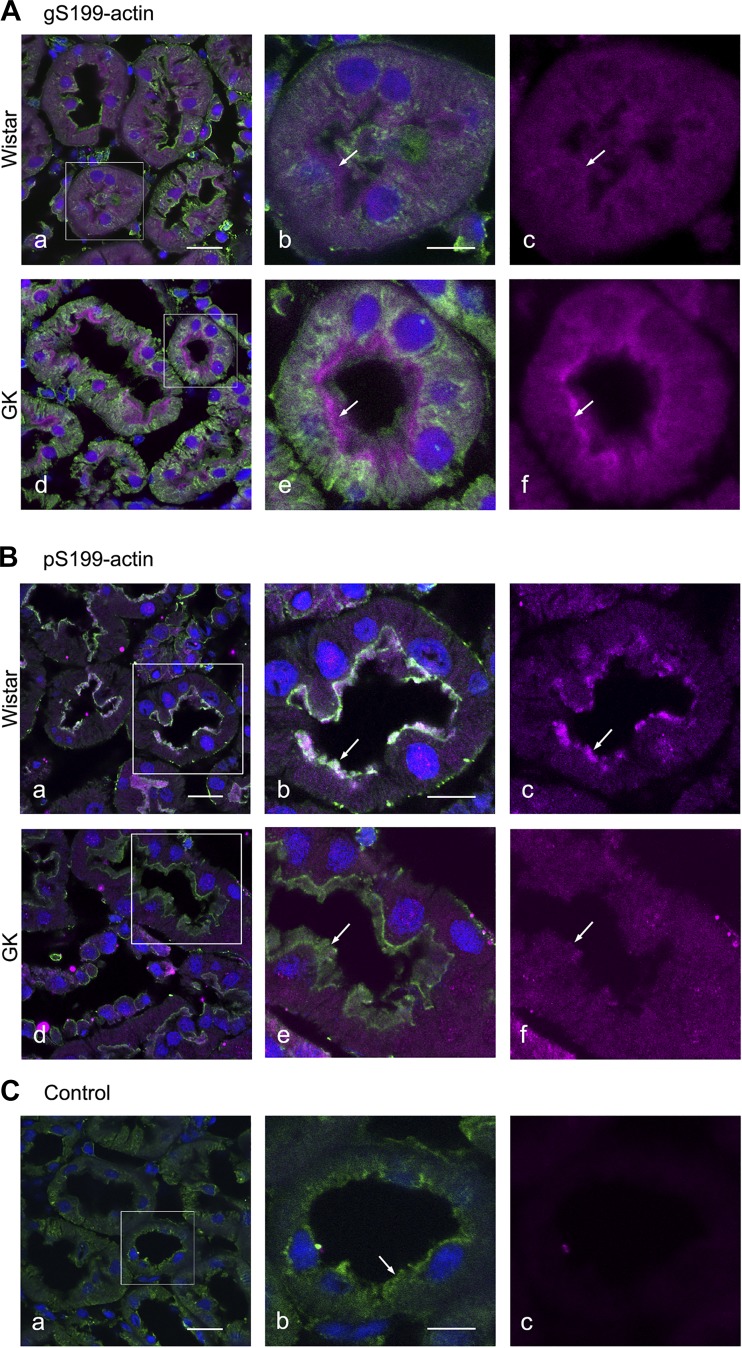
In proximal tubules of normal and diabetic kidneys, positive immunostaining for both gS199-actin and pS199-actin was observed in the brush border, cytoplasm, and nucleus (Fig. 10). The staining intensity of gS199-actin in the brush border was stronger in the diabetic kidneys than in the normal kidneys, whereas that of pS199-actin was weaker in the diabetic specimens (Fig. 10).
Fig. 10.
Immunofluorescent images of O-GlcNAcylation of Ser199(gS199)-actin (A) and phosphorylated Ser199 (pS199)-actin (B) in the proximal tubule of normal Wistar (a–c) and diabetic Goto-Kakizaki (GK) (d–f) kidneys. Immunostaining of gS199-actin and pS199-actin (magenta color) was intense in the brush border but diffuse in the cytoplasm and nucleus. Filamentous actin (green color) was stained with Alexa 488-phalloidin. The rectangular areas in a and d are enlarged in b and e, respectively. Nuclei (blue color) were stained with TO-PRO3. C: cytochemical control images. The rectangular area in a is enlarged in b. c: Normal rabbit IgG showed no reactivity toward kidney sections. Scale bars = 20 μm in a and 10 μm in b.
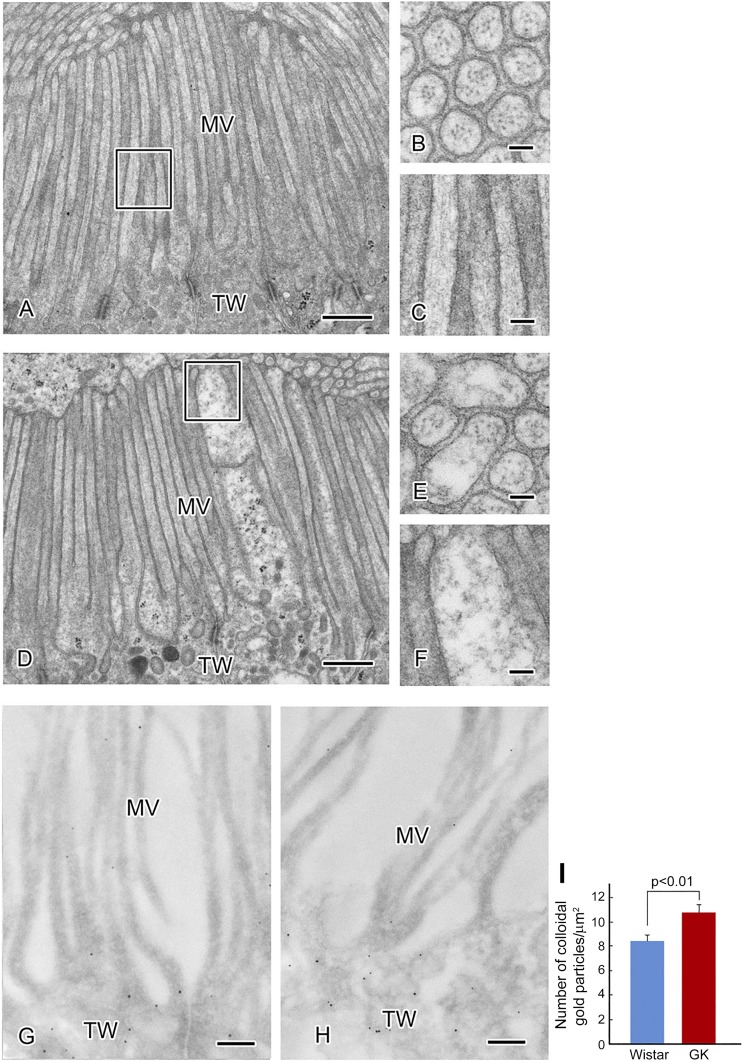
At the electron microscopic level in the diabetic proximal tubules, swollen microvilli and disarranged actin filaments were observed (Fig. 11, A–F). Immunoelectron microscopy with colloidal gold labeling of gS199-actin revealed scarce labels in the microvilli, whereas the gold label was observed at the terminal web in the bottom of the microvilli (Fig. 11, G and H). In the case of colloidal gold labeling with AC15 anti-β-actin antibody, reactivity was observed along the actin filaments in the microvilli in both normal and diabetic proximal tubules (Fig. 8B, c and d). The labeling intensity was significantly stronger in the terminal web of the diabetic microvilli (Fig. 11I).
Fig. 11.
A−F: ultrastructural changes in the microvilli (MV) of the proximal tubule in the diabetic kidney (D–F) compared with the normal kidney (A–C). In the brush border, the structure of the microvilli is maintained by actin filaments. Cross sections (B and E) and longitudinal sections (C and F) of microvilli are shown. A regular arrangement of actin filaments along microvilli was seen in the normal proximal tubule. Swollen microvilli and irregularly arranged actin filaments were observed in the diabetic tubule. Immunoelectron microscopy of O-GlcNAcylation of Ser199(gS199)-actin in the proximal tubule is shown. gS199-actin immunolabelling was moderate in the terminal web (TW) and faint in the microvilli of the proximal tubule in both normal (G) and diabetic (H) kidneys. Colloidal gold immunolabelling density of gS199-actin was significantly increased in the terminal web of the diabetic proximal tubule (I). Scale bars = 500 nm in A and D, 50 nm in B and E, 100 nm in C and F, and 200 nm in G and H. GK, Goto-Kakizaki..
OGA inhibitor Thiamet G induced a change in the immunofluorescence localization of gS199-actin and pS199-actin in cultured podocytes.
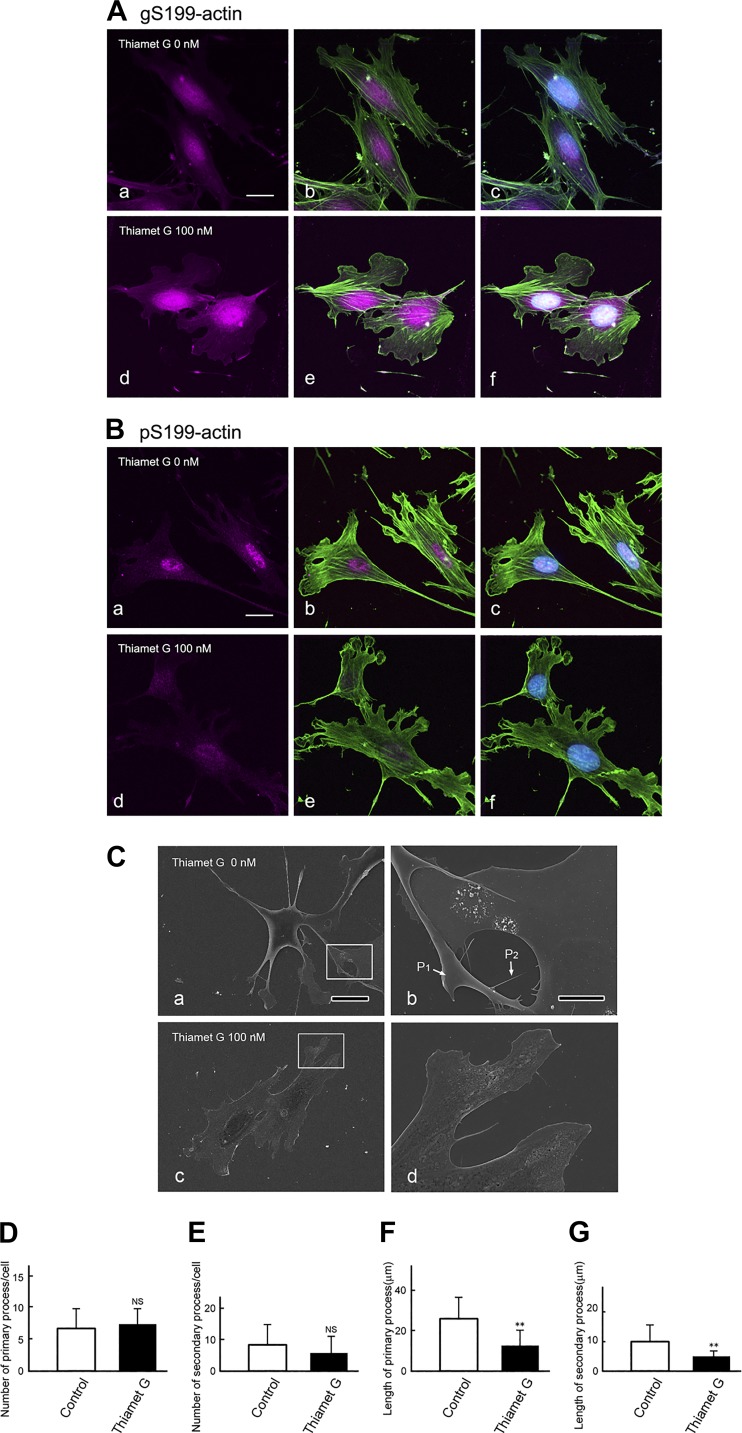
To further examine whether the elevated amount of O-GlcNAcylated β-actin in the diabetic kidney caused a change in gS199-actin and pS199-actin localization and/or cell shape, we examined their localization and cell shape in podocytes cultured with or without Thiamet G, whose inhibitor elevates the O-GlcNAcylation level of proteins. In cells cultured without Thiamet G, gS199-actin immunostaining was observed to be diffuse in both the nucleus and cytoplasm (Fig. 12A, a–c), whereas pS199-actin immunostaining was intense in the nucleus but weak and diffuse in the cytoplasm (Fig. 12B, a–c). In podocytes cultured with Thiamet G, the intensity of immunostaining for gS199-actin was increased, especially in the nucleus (Fig. 12A, d–f), whereas that for pS199-actin was decreased in both the nucleus and cytoplasm (Fig. 12B, d–f). The localization of filamentous actin changed, and its staining intensity was decreased in the Thiamet G-treated cells (Fig. 12, Ae and Be). Scanning electron microscopy revealed that podocytes cultured without Thiamet G displayed long primary processes and short secondary processes (Fig. 12C). Podocytes cultured with Thiamet G showed a significant decrease in the length of both primary and secondary processes (Fig. 12, F and G), although they had almost the same number of these processes as control cells cultured without Thiamet G (Fig. 12, D and E). These results indicate that O-GlcNAcylation and phosphorylation of β-actin Ser199 may be involved in the formation of the foot process of the podocyte.
Fig. 12.
A and B: immunofluorescent images of O-GlcNAcylation of Ser199(gS199)-actin and phosphorylated Ser199 (pS199)-actin in immortalized podocytes cultured without (a–c) or with (d–f) the O-GlcNAcase (OGA) inhibitor Thiamet G used at 100 nM. A and B, a and d: single exposure images (magenta color) of gS199-actin (A, a and d) and pS199-actin (B, a and d), respectively. A and B, b and e: double exposure images of a and d with F-actin (green color). A and B, c and f: triple exposure images of b and e with nuclei (blue color). The fluorescence intensity of gS199-actin was increased in cells in the presence of Thiamet G, whereas that of pS199-actin was decreased in cells treated with Thiamet G. Nuclei (blue color) were stained with TO-PRO3. Scale bars = 10 μm. C: scanning electron microscopic images of immortalized podocytes cultured with (c and d) or without (a and b) the OGA inhibitor Thiamet G used at 100 nM. Rectangular areas in a and c are enlarged in b and d, respectively. In podocytes cultured without Thiamet G, long primary processes (P1) and secondary processes (P2) were seen (a and b), whereas in those cultured with Thiamet G, the secondary processes were poorly developed (c and d). D and E: numbers of primary and secondary processes per cell cultured with (black bars) or without (control; white bars) Thiamet G were counted. F and G: length of primary and secondary processes cultured with (black bars) or without (control; white bars) Thiamet G were measured on ×1,000 single exposure images. Graphs show that podocytes cultured with Thiamet G showed a significant decrease in the length of both primary and secondary processes (F and G), whereas they had almost the same number of these processes as the cells cultured without Thiamet G (D and E). Data are means ± SD. **P < 0.01. Scale bars = 20 μm in A and 5 μm in B. NS, nonsignificant.
DISCUSSION
Several recent studies have shown that posttranslational modifications play an important role in the morphogenesis of cell ultrastructure. For example, phosphorylation of Tyr53 β-actin affects the morphogenesis of dendritic spines in rat hippocampal and cortical neurons (5). O-GlcNAcylation of proteins plays an important role for foot process maturation and survival of podocytes in the kidney (35). In skeletal and cardiac muscle, O-GlcNAcylation of α-actin plays a regulatory role in muscle contraction (21, 29, 38).
In the present study, we showed that β-actin was also Ser199 O-GlcNAcylated and Ser199 phosphorylated in the rat kidney. Both anti-gS199-actin antibody and anti-pS199-actin antibody reacted with nonfilamentous actin but not with filamentous actin. The peptide sequence that contains Ser199 is located in the pointed (minus) end of the actin molecule where actin monomers bind with each other for polymerization (Fig. 1C) (28, 39). We postulate that once actin polymerization occurs, the epitope is masked because of three-dimensional steric hindrance.
Immunoprecipitation and immunoblot analysis showed that both antibodies reacted very weakly with the 42-kDa band but reacted intensely with the 40-kDa band upon SDS-PAGE. β-actin at 40 kDa has been previously reported, and it is considered to be a truncated form in its NH2-terminal portion (49). However, as peptides containing the amino acid sequence of the NH2-terminus were detected by mass spectrometry analysis (Fig. 4), we propose that the difference in the molecular weight between 40 and 42 kDa may be ascribable to the posttranslational modification of β-actin. We are now examining the posttranslational modifications of this 40-kDa protein. The present results demonstrated that the amount of nonfilamentous gS199-actin increased in the diabetic kidney. These results are consistent with those previously reported showing that high glucose levels alter β-actin assembly in glomerular mesangial and epithelial cells (50).
The immunofluorescence experiments using 1C7 β-actin antibody with epitope amino acids 194–203 showed diffuse staining in both the nucleus and cytoplasm of the glomerulus and proximal tubule (data not shown). However, no difference in staining intensity or localization of gS199-actin with 1C7 actin antibody was observed between normal and diabetic kidneys (data not shown). The nuclear diffuse localization of gS199-actin was consistent with the immunohistochemical localization found with 1C7 β-actin antibody (39). 2G2 β-actin antibody with epitopes of amino acids 131–139, 155–169, and 176–187 also reacted only with nonfilamentous β-actin and revealed a distinct pattern of nuclear dots in differentiated myogenic cells (17). AC15 β-actin antibody visualized gS199-actin localized in the foot processes of podocytes and in capillary endothelial cells of the glomerulus (Fig. 8B), as previously reported (22). There was no significant difference in the colloidal gold labeling density of AC15-detected β-actin between normal and diabetic kidneys. The localization pattern of gS199-actin was a little different from that of pS199-actin. gS199-actin was localized diffusely in both the cytoplasm and nucleus of cultured podocytes, whereas pS199-actin was localized densely and in punctate form in the nucleus and weakly and diffusely in the cytoplasm. In kidney tissue, the amount of gS199-actin was increased in podocytes of the diabetic kidney, whereas that of pS199-actin was decreased in these podocytes compared with the normal amount. These results indicate that gS199-actin and pS199-actin were localized in a different pattern and may have different functions in the cytoplasm and in the nucleus of the podocyte and are involved in the morphological changes in podocytes in diabetes.
In the nucleus, β-actin does not form filaments but plays important roles in the transcription and chromatin remodeling as a β-actin monomer or oligomer (8, 13, 18, 24, 25, 27). In the nucleus, there are many O-GlcNAcylated proteins such as RNA polymerase II, Fox01, NF-κB, and histone, all of which are involved in transcription and chromatin formation (19, 48). O-GlcNAc regulates the expression of many transcription factors that regulate gluconeogenic and lipogenic genes (32). It has been previously reported that under the condition of certain diseases, the amount of nuclear β-actin increases, with the appearance of β-actin filaments (44). In the present study, we did not observe β-actin filaments in the nucleus of the diabetic kidney but did find that the amount of gS199-actin increased and that of pS199-actin decreased in the nucleus of podocytes in the diabetic kidney. From these results, we propose the hypothesis that GlcNAcylation of β-actin Ser199 in the nucleus may suppress actin filament formation by inhibiting phosphorylation-mediated binding between actin molecules. The roles of gS199-actin and pS199-actin in the nucleus remain to be elucidated.
Present immunofluorescence and immunoelectron microscopy results for the proximal tubule indicate that in the diabetic kidney, aberrant O-GlcNAcylation and phosphorylation of β-actin Ser199 may cause the disarray of actin filaments in the microvilli of the proximal tubule. As these microvilli are very important for reabsorption of proteins including albumin, increased O-GlcNAcylation of β-actin may cause diabetes-associated proteinuria. Recently, it has been reported that spontaneously hypertensive rats show increased O-GlcNAcylation and O-GlcNAc transferase expression and that this increase in O-GlcNAcylation reduces albumin endocytosis and cell surface expression of megalin in the proximal tubule, thus suggesting the involvement of increased O-GlcNAcylation in hypertension-associated proteinuria (43).
Ca2+-dependent actin-fragmin kinase phosphorylates the threonine residues (Thr201–203) of β-actin, inducing actin filament elongation (10, 14). This is caused by a decrease in the interaction between actin and fragmin, which is a gelsolin-related protein that controls filament length (10, 14). It has been reported that α-helix 191–199 is involved in the binding between strands (34). The peptide used as the immunogen in the present study included Tyr198, Ser199, and Thr201–203 on which phosphorylation occurs, and this sequence is located at the pointed end of the actin tertiary structure and is involved in the binding between actin monomers. From these facts, we suggest that the O-GlcNAcylation of β-actin Ser199 may regulate the elongation of β-actin filaments by inhibiting phosphorylation of β-actin Tyr198, Ser199 and Thr201–203 and that in diabetic nephropathy the elevation of O-GlcNAcylation of β-actin Ser199 may induce a decrease in the phosphorylation of β-actin and cause aberration of β-actin filament elongation.
In conclusion, the present results indicate that O-GlcNAcylation and phosphorylation of β-actin Ser199 in the diabetic kidney differ from those in the normal kidney and that these changes may contribute partially to the causes of the morphological changes in the glomerulus and tubules. In the future, it will be important to explore the role of gS199-actin and pS199-actin in the nucleus of normal and diabetic kidney cells. gS199-actin and pS199-actin will be useful for the pathological short-term evaluation of diabetic nephropathy, as O-GlcNAcylation and phosphorylation are often reciprocal and dynamic posttranslational modifications.
GRANTS
This work was supported in part by Grants-In-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (C-20590198 and 18K06840 to Y. Akimoto), from the Japan Diabetes Foundation (to Y. Akimoto), from the Kazato Research Foundation (to Y. Akimoto), and from Kyorin University School of Medicine, Kyorin Medical Research Award 2017 (to Y. Akimoto) and by National Institutes of Health Grants R01-GM-116891 and R01-DK-61671 (to G. W. Hart).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.A. conceived and designed research; Y.A., Y.M., H.T., T.F., T.A., and Y.C. performed experiments; Y.A., K.Y., Y.M., H.T., T.F., A.K., T.A., Y.C., and S.K. analyzed data; Y.A., K.Y., Y.M., H.T., T.F., D.S., A.K., T.A., S.K., G.W.H., T.E., and H.K. interpreted results of experiments; Y.A., Y.M., and A.K. prepared figures; Y.A., Y.M., and G.W.H. drafted manuscript; Y.A., K.Y., Y.M., H.T., T.T., D.S., A.K., T.A., Y.C., S.K., G.W.H., T.E., and H.K. edited and revised manuscript; Y.A., K.Y., Y.M., H.T., T.T., T.F., D.S., A.K., T.A., Y.C., S.K., G.W.H., T.E., and H.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Sachie Matsubara, Tomoko Miura, and Junri Hayakawa (Laboratory for Electron Microscopy and Department of Anatomy, Kyorin University School of Medicine) and Yasuko Hasegawa (Research Team for Geriatric Pathology, Tokyo Metropolitan Institute of Gerontology) for technical assistance.
REFERENCES
- 1.Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, Kawakami H. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology 17: 127–140, 2007. doi: 10.1093/glycob/cwl067. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto Y, Miura Y, Toda T, Wolfert MA, Wells L, Boons G-J, Hart GW, Endo T, Kawakami H. Morphological changes in diabetic kidney are associated with increased O-GlcNAcylation of cytoskeletal proteins including α-actinin 4. Clin Proteomics 8: 15, 2011. doi: 10.1186/1559-0275-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akimoto Y, Yamamoto K, Munetomo E, Wells L, Vosseller K, Hart GW, Kawakami H, Hirano H. Elevated post-translational modification of proteins by O-linked N-acetylglucosamine in various tissues of diabetic Goto-Kakizaki rats accompanied by diabetic complications. Acta Histochem Cytochem 38: 131–142, 2005. doi: 10.1267/ahc.38.131. [DOI] [Google Scholar]
- 4.Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Mol Aspects Med 51: 1–15, 2016. doi: 10.1016/j.mam.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Bertling E, Englund J, Minkeviciene R, Koskinen M, Segerstråle M, Castrén E, Taira T, Hotulainen P. Actin tyrosine-53-phosphorylation in neuronal maturation and synaptic plasticity. J Neurosci 36: 5299–5313, 2016. doi: 10.1523/JNEUROSCI.2649-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev Nutr 33: 205–229, 2013. doi: 10.1146/annurev-nutr-071812-161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butkinaree C, Park K, Hart GW. O-linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 1800: 96–106, 2010. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caridi CP, D’Agostino C, Ryu T, Zapotoczny G, Delabaere L, Li X, Khodaverdian VY, Amaral N, Lin E, Rau AR, Chiolo I. Nuclear F-actin and myosins drive relocalization of heterochromatic breaks. Nature 559: 54–60, 2018. doi: 10.1038/s41586-018-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlier MF, Pernier J, Montaville P, Shekhar S, Kühn S; Cytoskeleton Dynamics and Motility group . Control of polarized assembly of actin filaments in cell motility. Cell Mol Life Sci 72: 3051–3067, 2015. doi: 10.1007/s00018-015-1914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantin B, Meerschaert K, Vandekerckhove J, Gettemans J. Disruption of the actin cytoskeleton of mammalian cells by the capping complex actin-fragmin is inhibited by actin phosphorylation and regulated by Ca2+ ions. J Cell Sci 111: 1695–1706, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Degrell P, Cseh J, Mohás M, Molnár GA, Pajor L, Chatham JC, Fülöp N, Wittmann I. Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci 84: 389–393, 2009. doi: 10.1016/j.lfs.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Eichinger L, Bomblies L, Vandekerckhove J, Schleicher M, Gettemans J. A novel type of protein kinase phosphorylates actin in the actin-fragmin complex. EMBO J 15: 5547–5556, 1996. doi: 10.1002/j.1460-2075.1996.tb00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falahzadeh K, Banaei-Esfahani A, Shahhoseini M. The potential roles of actin in the nucleus. Cell J 17: 7–14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuhashi K, Hatano S, Ando S, Nishizawa K, Inagaki M. Phosphorylation by actin kinase of the pointed end domain on the actin molecule. J Biol Chem 267: 9326–9330, 1992. [PubMed] [Google Scholar]
- 15.Gellai R, Hodrea J, Lenart L, Hosszu A, Koszegi S, Balogh D, Ver A, Banki NF, Fulop N, Molnar A, Wagner L, Vannay A, Szabo AJ, Fekete A. Role of O-linked N-acetylglucosamine modification in diabetic nephropathy. Am J Physiol Renal Physiol 311: F1172–F1181, 2016. doi: 10.1152/ajprenal.00545.2015. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg HJ, Scholey J, Fantus IG. Glucosamine activates the plasminogen activator inhibitor 1 gene promoter through Sp1 DNA binding sites in glomerular mesangial cells. Diabetes 49: 863–871, 2000. doi: 10.2337/diabetes.49.5.863. [DOI] [PubMed] [Google Scholar]
- 17.Gonsior SM, Platz S, Buchmeier S, Scheer U, Jockusch BM, Hinssen H. Conformational difference between nuclear and cytoplasmic actin as detected by a monoclonal antibody. J Cell Sci 112: 797–809, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Grosse R, Vartiainen MK. To be or not to be assembled: progressing into nuclear actin filaments. Nat Rev Mol Cell Biol 14: 693–697, 2013. doi: 10.1038/nrm3681. [DOI] [PubMed] [Google Scholar]
- 19.Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022, 2007. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 20.Hédou J, Bastide B, Page A, Michalski JC, Morelle W. Mapping of O-linked β-N-acetylglucosamine modification sites in key contractile proteins of rat skeletal muscle. Proteomics 9: 2139–2148, 2009. doi: 10.1002/pmic.200800617. [DOI] [PubMed] [Google Scholar]
- 21.Hédou J, Cieniewski-Bernard C, Leroy Y, Michalski JC, Mounier Y, Bastide B. O-linked N-acetylglucosaminylation is involved in the Ca2+ activation properties of rat skeletal muscle. J Biol Chem 282: 10360–10369, 2007. doi: 10.1074/jbc.M606787200. [DOI] [PubMed] [Google Scholar]
- 22.Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in rat podocytes. J Histochem Cytochem 51: 1589–1600, 2003. doi: 10.1177/002215540305101203. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Katayama K, Nishibori Y, Akimoto Y, Kudo A, Kurayama R, Hada I, Takahashi S, Kimura T, Fukutomi T, Katada T, Suehiro J, Beltcheva O, Tryggvason K, Yan K. Wolf-Hirschhorn syndrome candidate 1-like 1 epigenetically regulates nephrin gene expression. Am J Physiol Renal Physiol 312: F1184–F1199, 2017. doi: 10.1152/ajprenal.00305.2016. [DOI] [PubMed] [Google Scholar]
- 24.Jockusch BM, Schoenenberger CA, Stetefeld J, Aebi U. Tracking down the different forms of nuclear actin. Trends Cell Biol 16: 391–396, 2006. doi: 10.1016/j.tcb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor P, Chen M, Winkler DD, Luger K, Shen X. Evidence for monomeric actin function in INO80 chromatin remodeling. Nat Struct Mol Biol 20: 426–432, 2013. doi: 10.1038/nsmb.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor β1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest 101: 160–169, 1998. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristó I, Bajusz I, Bajusz C, Borkúti P, Vilmos P. Actin, actin-binding proteins, and actin-related proteins in the nucleus. Histochem Cell Biol 145: 373–388, 2016. doi: 10.1007/s00418-015-1400-9. [DOI] [PubMed] [Google Scholar]
- 28.Kudryashov DS, Sawaya MR, Adisetiyo H, Norcross T, Hegyi G, Reisler E, Yeates TO. The crystal structure of a cross-linked actin dimer suggests a detailed molecular interface in F-actin. Proc Natl Acad Sci USA 102: 13105–13110, 2005. doi: 10.1073/pnas.0506429102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert M, Richard E, Duban-Deweer S, Krzewinski F, Deracinois B, Dupont E, Bastide B, Cieniewski-Bernard C. O-GlcNAcylation is a key modulator of skeletal muscle sarcomeric morphometry associated to modulation of protein-protein interactions. Biochim Biophys Acta 1860: 2017–2030, 2016. doi: 10.1016/j.bbagen.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Shu S, Hong MS, Levine RL, Korn ED. Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments. Proc Natl Acad Sci USA 103: 13694–13699, 2006. doi: 10.1073/pnas.0606321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266: 4706–4712, 1991. [PubMed] [Google Scholar]
- 32.McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA 99: 10695–10699, 2002. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obrdlik A, Kukalev A, Louvet E, Farrants AK, Caputo L, Percipalle P. The histone acetyltransferase PCAF associates with actin and hnRNP U for RNA polymerase II transcription. Mol Cell Biol 28: 6342–6357, 2008. doi: 10.1128/MCB.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oda T, Iwasa M, Aihara T, Maéda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature 457: 441–445, 2009. [Erratum in Nature 461: 500, 2009.] doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- 35.Ono S, Kume S, Yasuda-Yamahara M, Yamahara K, Takeda N, Chin-Kanasaki M, Araki H, Sekine O, Yokoi H, Mukoyama M, Uzu T, Araki SI, Maegawa H. O-linked β-N-acetylglucosamine modification of proteins is essential for foot process maturation and survival in podocytes. Nephrol Dial Transplant 32: 1477–1487, 2017. doi: 10.1093/ndt/gfw463. [DOI] [PubMed] [Google Scholar]
- 36.Osicka TM, Russo LM, Qiu ML, Brammar GC, Thallas V, Forbes JM, Comper WD, Jerums G. Additive effects of hypertension and diabetes on renal cortical expression of PKC-α and -ε and α-tubulin but not PKC-β1 and -β2. J Hypertens 21: 2399–2407, 2003. doi: 10.1097/00004872-200312000-00029. [DOI] [PubMed] [Google Scholar]
- 37.Peterson SB, Hart GW. New insights: a role for O-GlcNAcylation in diabetic complications. Crit Rev Biochem Mol Biol 51: 150–161, 2016. doi: 10.3109/10409238.2015.1135102. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, Dias WB, Vecoli C, Hart GW, Murphy AM. O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ Res 103: 1354–1358, 2008. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenenberger C-A, Buchmeier S, Boerries M, Sütterlin R, Aebi U, Jockusch BM. Conformation-specific antibodies reveal distinct actin structures in the nucleus and the cytoplasm. J Struct Biol 152: 157–168, 2005. doi: 10.1016/j.jsb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Sekine Y, Nishibori Y, Akimoto Y, Kudo A, Ito N, Fukuhara D, Kurayama R, Higashihara E, Babu E, Kanai Y, Asanuma K, Nagata M, Majumdar A, Tryggvason K, Yan K. Amino acid transporter LAT3 is required for podocyte development and function. J Am Soc Nephrol 20: 1586–1596, 2009. doi: 10.1681/ASN.2008070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheterline P, Clayton J, Sparrow J. Actin. Protein Profile 2: 1–103, 1996. [PubMed] [Google Scholar]
- 42.Shimizu M, Khoshnoodi J, Akimoto Y, Kawakami H, Hirano H, Higashihara E, Hosoyamada M, Sekine Y, Kurayama R, Kurayama H, Joh K, Hirabayashi J, Kasai K, Tryggvason K, Ito N, Yan K. Expression of galectin-1, a new component of slit diaphragm, is altered in minimal change nephrotic syndrome. Lab Invest 89: 178–195, 2009. doi: 10.1038/labinvest.2008.125. [DOI] [PubMed] [Google Scholar]
- 43.Silva-Aguiar RP, Bezerra NCF, Lucena MC, Sirtoli GM, Sudo RT, Zapata-Sudo G, Takiya CM, Pinheiro AAS, Dias WB, Caruso-Neves C. O-GlcNAcylation reduces proximal tubule protein reabsorption and promotes proteinuria in spontaneously hypertensive rats. J Biol Chem 293: 12749–12758, 2018. doi: 10.1074/jbc.RA118.001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stenzel W, Preuße C, Allenbach Y, Pehl D, Junckerstorff R, Heppner FL, Nolte K, Aronica E, Kana V, Rushing E, Schneider U, Claeys KG, Benveniste O, Weis J, Goebel HH. Nuclear actin aggregation is a hallmark of anti-synthetase syndrome-induced dysimmune myopathy. Neurology 84: 1346–1354, 2015. doi: 10.1212/WNL.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 45.Terman JR, Kashina A. Post-translational modification and regulation of actin. Curr Opin Cell Biol 25: 30–38, 2013. doi: 10.1016/j.ceb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walgren JLE, Vincent TS, Schey KL, Buse MG. High glucose and insulin promote O-GlcNAc modification of proteins, including α-tubulin. Am J Physiol Endocrinol Metab 284: E424–E434, 2003. doi: 10.1152/ajpendo.00382.2002. [DOI] [PubMed] [Google Scholar]
- 47.Weigert C, Brodbeck K, Sawadogo M, Häring HU, Schleicher ED. Upstream stimulatory factor (USF) proteins induce human TGF-β1 gene activation via the glucose-response element-1013/-1002 in mesangial cells: up-regulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem 279: 15908–15915, 2004. doi: 10.1074/jbc.M313524200. [DOI] [PubMed] [Google Scholar]
- 48.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291: 2376–2378, 2001. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita S, Suzuki A, Kamada M, Yanagita T, Hirohata S, Toyoshima S. Possible physiological roles of proteolytic products of actin in neutrophils of patients with Behçet’s disease. Biol Pharm Bull 24: 733–737, 2001. doi: 10.1248/bpb.24.733. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, Hurst RD, Templeton D, Whiteside CI. High glucose alters actin assembly in glomerular mesangial and epithelial cells. Lab Invest 73: 372–383, 1995. [PubMed] [Google Scholar]