INTRODUCTION
In an artice recently published in the American Journal of Physiology-Renal Physiology, Repetti et al. (5) elegantly detailed how consuming foods rich in cholesterol led to cholesterol incorporation into the distal nephrons of kidneys to repress fluid shear stress-induced gene expression. This result was also supported by their in vitro experiments using collecting duct-derived cells. When cholesterol is incorporated into these cells, the expression of natriuresis-associated genes can no longer be induced by fluid shear stress. These natriuresis-associated tubular genes (heme oxygenase-1, cyclooxygenase-2, and nitric oxide synthase 2) are generally known to regulate epithelial Na+ channel (ENaC) activity. Cholesterol modestly but significantly increases Na+ retention and water reabsorption in vivo. Volume expansion by Na+ and water retention can raise blood pressure. The question then becomes: Is this the mechanism by which cholesterol affects blood pressure?
Although the present study did not address this question directly (5), others have shown that a high-fat diet is associated with higher blood pressure (2). Of note, high-cholesterol diet is correlated with hypertension, and it increases ENaC expression, generating an antinatriuretic characteristic (1). Furthermore, lowering cholesterol with lovastatin sufficiently decreases blood pressure in rats (3). Together, these results suggest that cholesterol may play a role in the development of hypertension.
HOW DOES A HIGH-CHOLESTEROL DIET RESULT IN HYPERTENSION?
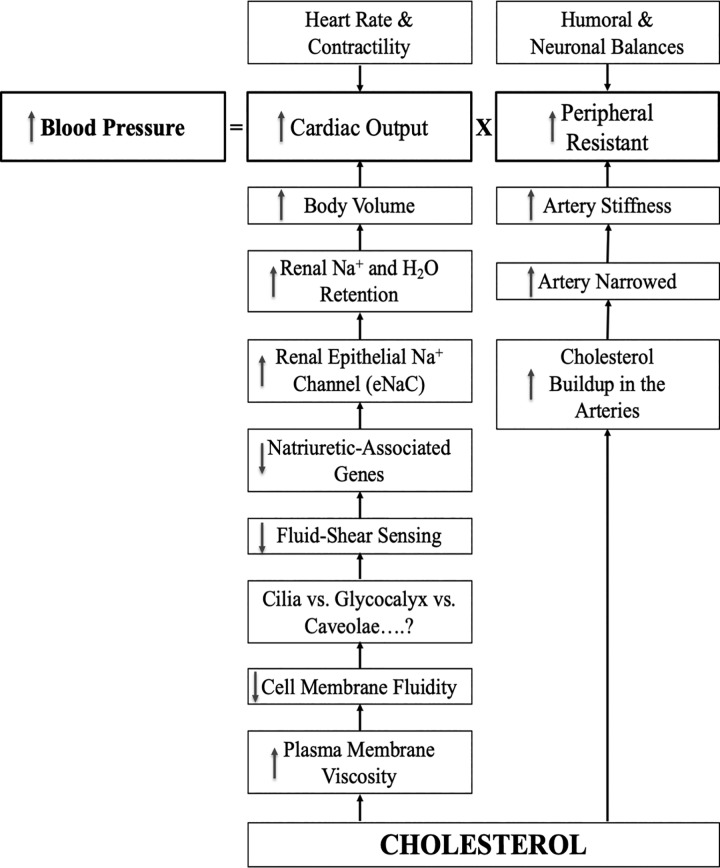
While the exact mechanism for high-cholesterol diet-induced hypertension is still not clear, many studies have eluded to the hypothesis that cholesterol can alter kidney function, resulting in the development of high blood pressure (Fig. 1).
Fig. 1.
Potential mechanisms of high cholesterol leading to high blood pressure. The hypothetical model depicts that cholesterol could induce hypertension via kidney and/or arterial functions. Cholesterol is integrated into the cell membrane of the renal epithelia or arterial lumen within vascular endothelia, resulting in maladaptation of renal and vascular functions.
Because of its rigid ring structure, cholesterol is an important component that dictates the fluidity of the cell membrane. As cholesterol content in the cell membrane increases, the biophysical properties of the plasma membrane change; the viscosity and flexibility of the plasma membrane increases and decreases, respectively. These changes are sufficient to alter how a cell could respond to extracellular biomechanical signals, such as fluid shear stress. Among the cellular sensory components, however, it is currently not known if membrane fluidity primarily alters the mechanically sensitive cilia, glycocalyx, or caveolae.
However, the recent study by Repetti et al. (5) showed that fluid shear stress induces expression of natriuresis-associated genes and, ultimately, kidney ENaCs. This axis is interrupted with increased cholesterol, which is integrated in the cell membrane, resulting in Na+ and water retention. The volume expansion by Na+ and water retention is generally known to induce higher blood pressure.
ROLES OF THE HIGH-CHOLESTEROL DIET IN VASCULAR FUNCTIONS
Aside of kidney function, a high-cholesterol diet has also been proposed to cause arteries to stiffen and narrow, primarily due to a high cholesterol buildup (Fig. 1). Cholesterol-induced artery occlusion is generally known as atherosclerosis. While atherosclerosis is a distinct cardiovascular pathology that is best explained separately, the narrowed and stiffened arteries will impede blood flow. This mechanism is sufficient to increase vascular pressure. Furthermore, obstruction of blood perfusion may trigger a greater force for the heart to pump blood through occluded or stiff arteries, resulting in increased cardiac output and higher blood pressure.
FUTURE STUDIES
The study by Repetti et al. (5) offers several new exciting questions. For example, if high cholesterol incorporation in the cell membrane is sufficient to block mechanosensing in renal epithelia, would the same mechanism be applicable in vascular endothelia? There have been some studies that have eluded to such possibility (4, 6). In particular, vascular endothelia are known to sense blood flow within the lumen of the arteries, resulting in the release of vascular relaxing substances, such as nitric oxide. Another important task is to elucidate the cellular sensing of cilia, glycocalyx, and caveolae with regard to the effect of cholesterol on the cell membrane. The molecular mechanism by which cholesterol is incorporated and may cause modified plasma membrane function is another area in which we still know very little.
Importantly, we need a better understanding on the physiological impact of cholesterol on renal, vascular, and cardiac functions. These renocardiac vascular systems directly regulate blood pressure. As such, we need good in vivo physiological models to dissect the effects of high-cholesterol diets on these organ systems. Without doubt, continuous efforts should be made to integrate the physiological impact of cholesterol into translational and clinical implications toward disease progression.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.N. prepared figures; S.N. drafted manuscript; S.N. edited and revised manuscript; S.N. approved final version of manuscript.
REFERENCES
- 1.Awayda MS, Awayda KL, Pochynyuk O, Bugaj V, Stockand JD, Ortiz RM. Acute cholesterol-induced anti-natriuretic effects: role of epithelial Na+ channel activity, protein levels, and processing. J Biol Chem 286: 1683–1695, 2011. doi: 10.1074/jbc.M110.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mente A, Dehghan M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, Lear S, Li W, Chen H, Yi S, et al.; Prospective Urban Rural Epidemiology (PURE) study investigators Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol 5: 774–787, 2017. doi: 10.1016/S2213-8587(17)30283-8. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MP, Kasiske BL, Katz SA, Schmitz PG, Keane WF. Lovastatin but not enalapril reduces glomerular injury in Dahl salt-sensitive rats. Hypertension 20: 651–658, 1992. doi: 10.1161/01.HYP.20.5.651. [DOI] [PubMed] [Google Scholar]
- 4.Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem 273: 32304–32311, 1998. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- 5.Repetti RL, Meth J, Sonubi O, Flores D, Satlin LM, Rohatgi R. Cellular cholesterol modifies flow-mediated gene expression. Am J Physiol Renal Physiol 317: F815–F824, 2019. doi: 10.1152/ajprenal.00196.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto K, Ando J. Endothelial cell and model membranes respond to shear stress by rapidly decreasing the order of their lipid phases. J Cell Sci 126: 1227–1234, 2013. doi: 10.1242/jcs.119628. [DOI] [PubMed] [Google Scholar]