Abstract
An epidemic of chronic kidney disease of unknown etiology (Mesoamerican nephropathy) has emerged in hot regions of Central America. We have demonstrated that dehydration associated with recurrent heat exposure causes chronic kidney disease in animal models. However, the independent influence of core body temperature on kidney injury has not been explored. In the present study, we tested the hypothesis that kidney injury could be accelerated by increasing body temperature independent of external temperature. Wild-type mice were exposed to heat (39.5°C, 30 min, 2 times daily) with or without the mitochondrial uncoupling agent 2,4-dinitrophenol (DNP) for 10 days. Core temperature, renal function, proteinuria, and renal histological and biochemical analyses were performed. Isolated mitochondria markers of oxidative stress were evaluated from kidney tissue. DNP increased body core temperature in response to heat by 1°C (42 vs. 41°C), which was transient. The mild increase in temperature correlated with worsening albuminuria (R = 0.715, P < 001), renal tubular injury, and interstitial infiltration of monocytes/macrophages. Tubular injury was marked in the outer medulla. This was associated with a reduction in kidney tissue ATP levels (nonheated control: 16.71 ± 1.33 nmol/mg and DNP + heat: 13.08 ± 1.12 nmol/mg, P < 0.01), reduced mitochondria, and evidence for mitochondrial oxidative stress. The results of the present study suggest that kidney injury in heat stress is markedly worsened by increasing core temperature. This is consistent with the hypothesis that clinical and subclinical heat stroke may play a role in Mesoamerican nephropathy.
Keywords: chronic kidney disease of unknown etiology, core temperature, heat shock, heat stress, hyperthermia, Mesoamerican nephropathy
INTRODUCTION
Epidemics of chronic kidney disease (CKD) have recently been documented in agricultural communities in Central America, Mexico, India, and Sri Lanka (23, 32). The disease does not appear to be mediated by common causes, such as glomerulonephritis, hypertension, and diabetes, and is characterized histologically by a dominant pattern of chronic interstitial injury and inflammation, often with some wrinkling of glomerular basement membranes and glomerulosclerosis (45). Clinical and field investigation in communities and workplaces point to a role for heat exposure in pathogenesis. Contributing roles of toxins or infectious agents are more limited. The cause remains unknown (23).
One dominant feature is that the individuals developing CKD are usually working manually in very hot environments where heat stress and recurrent dehydration are frequent. Indeed, it is common to observe acute declines in kidney function across a work shift that are worse in those less hydrated (17, 34, 40, 43, 44). Likewise, recurrent exposure of laboratory animals to heat stress and water restriction can also lead to acute and chronic kidney injury characterized by tubulointerstitial injury and inflammation (15, 16, 35, 36). This has led to the hypothesis that the kidney damage may reflect a type of “heat stress” nephropathy, which is of great concern to worker health and global food security given current projections of global climate change (19).
Heat stress can induce kidney disease in a variety of ways, including by acute tubular injury from heat- and exercise-induced rhabdomyolysis, acute increases in serum and urine uric acid with crystalluria, extracellular volume depletion and resultant renal hypoperfusion, hypokalemia, and hyperosmolarity-mediated mechanisms involving activation of the the intrarenal polyol-fructokinase pathway and effects of vasopressin (23).
Another potential mechanism could be by an increase in core temperature. If the dissipation of heat is unsuccessful, the rise in body temperature can result in heat stroke that can cause acute kidney injury (AKI), which may progress to CKD (24, 25). Heat stroke is increasingly common with rising world temperatures and is a serious concern for the military (8, 20) as well as for individuals living and working in hot climates (9, 26). Although severe heat stroke usually results in obtundation and multiorgan failure (21), it is possible that there may be a continuum of injury from mild to severe related to the degree of elevation in core temperature. Indeed, heat exhaustion is a precursor stage of heat stroke (The National Institute for Occupational Safety and Health Criteria for a Recommended Standard: Occupational Exposure to Heat and Hot Environments, 2016) that is part of a continuum to heat stroke. For example, a recent study (41) reported that wet bulb globe temperature, which is an index of heat stress based on ambient (dry) temperature, solar radiation, and wind speed, has been correlated with AKI in sugarcane workers. Although no study has yet evaluated the relationship of core body temperature of sugarcane workers with AKI in Central America, elevations in core body temperature have been associated with AKI in migrant farm workers in the Central Valley of California (33) and in firefighters exposed to heat and exercise (39)
Heat-related illness is an attractive mechanism to account for the epidemic of CKD. One reason is because there is a classical report of heat stroke demonstrating that people with AKI subsequently developed chronic, progressive interstitial nephritis with impaired renal function (25). Another reason is because some workers in the sugarcane fields develop symptoms strongly suggestive of heat exhaustion, with fever, muscle aches, and fatigue, laboratory findings documenting AKI, and kidney biopsies showing interstitial inflammation and tubular injury (2, 12, 14); some of these workers go on to develop CKD (13). Heat stroke/exhaustion, which is the state of increased body temperature, can be accompanied by AKI, but direct influence of core temperature to cause AKI or CKD has not been proved.
Major regulators of core body temperature are mitochondrial function and metabolism, and nearly 50% of energy expenditure is aimed at maintaining core body temperature (28). When mitochondria make ATP, they also generate heat; these processes occur in opposition such that the more effective the oxidative phosphorylation, the more ATP and less heat generated (5). However, if the proton gradient generated by the mitochondrial electron transport chain is leaky, the dissipation of the gradient results in less ATP and more heat (termed “uncoupling”). There is evidence that Native Americans who entered into the Americas via the Bering Strait in general have higher resting metabolic rates (29) due to mitochondrial mutations that increased mitochondrial uncoupling, which likely provided a survival advantage by increasing their core temperature in the cold Arctic environment (37). Today, most Hispanic Americans in Central America have some Native American genes, which might be associated with higher core temperatures. In contrast, Africans tend to have highly coupled mitochondria, with lower resting metabolic rates and lower core temperatures (1, 30, 31). Such data might provide an explanation for the conundrum that the epidemic may be greater among Hispanic Americans in Central America working the sugarcane fields as opposed to Hispanic Americans from Africa or the Caribbean, the latter having individuals with significant African ancestry (22).
These studies led to the hypothesis that an agent that might increase mitochondrial uncoupling would increase core body temperature in response to heat and that this would be associated with greater kidney injury. To test this hypothesis, we used 2,4-dinitrophenol (DNP), a well-known mitochondrial uncoupling agent (7). We also explored potential mechanisms by which such intervention might contribute to kidney damage.
MATERIALS AND METHODS
Study design.
Twelve-week-old male wild-type mice (C57BL/6J, Jackson Laboratory, Bar Harbor, ME) were used. Mice were kept under temperature- and humidity-controlled conditions within a thermoneutral zone; namely, core temperature of the mice was kept constant. They were also kept in specific pathogen-free conditions and maintained on a 14:10-h dark-light cycle. Mice were allowed ad libitum access to normal laboratory chow (2920X, Harlan Teklad, Madison, WI) and water until the start of the experiment. Two or three mice were housed per cage, and all were treated simultaneously under identical conditions. The experimental protocol was approved by the University of Colorado Animal Care and Use Committee.
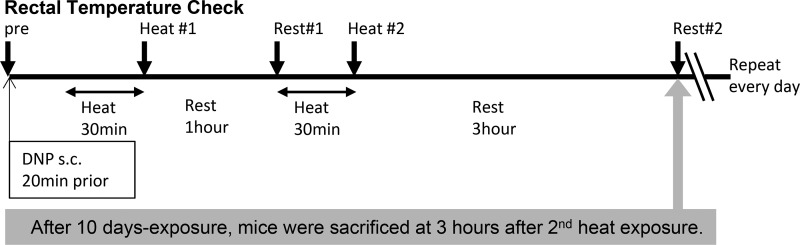
Mice were divided into the following four groups: water with no heat exposure [nontreated (NT) control group], DNP with no heat exposure (DNP group), water with heat exposure (heat group), and DNP with heat exposure (DNP + heat group). DNP (20 mg/kg) was given subcutaneously 20 min before heat exposure as a mitochondrial uncoupling agent to increase body temperature. Mice in the heat-exposed groups were exposed to a hot environment at an ambient temperature of 39.5°C in a heat chamber (Isotemp, Fisher Scientific Incubator, Dubuque, IA) for 30 min. After 30 min of exposure to heat, mice were returned to their cages at normal vivarium room temperature and provided access to water and food for 1 h. This cycle of hot exposure and then rest at room temperature was repeated twice a day for 10 days except during the weekend (Fig. 1). During the experimental period, a few mice of the DNP + heat group showed signs of heat stroke, e.g., muscle cramps, abnormal breathing, and behavioral changes such as hyperreactivity or lack of spontaneous activity and seizures, and were removed from the study. The final number of mice (n) allocated in each group was n = 5 for the control group, n = 7 for the DNP group, n = 5 for the heat group, and n = 5 for the DNP + heat group. To clarify the mechanisms, we evaluated the early stage of renal injury in this model, whereas our previous studies involved longer experimental durations (35, 36).
Fig. 1.
The experimental protocol, in which 0.10–0.15 ml of 5 mg/ml 2,4-dinitrophenol (DNP, 20 mg/kg) or saline were administered subcutaneously 20 min before heat exposure. Mice in the heat-exposed groups were placed in a heat chamber at 39.5°C for 30 min. After 30 min of exposure to heat, mice were returned to their cages at normal vivarium room temperature and provided access to water and food for 1 h. The heat exposure was repeated once again. Body temperature was measured at the time points as shown in the arrows (pre, Heat #1, Rest #1, Heat #2, and Rest #2). DNP administration and heat exposure were performed every day for 10 days except during the weekend.
Body temperature was evaluated with a rectal probe in conscious animals (Ret3, Physitemp Instruments, Clifton, NJ) as follows: we let mice hang on wire mesh lids of cages and held a tail up to be able to see each mouse’s anus. The rectal probe was inserted and held a few seconds until a stable value was displayed on the thermometer. To get accurate and stable temperature, we avoided exciting the mice. The procedures for measurements described above were performed gently and quickly in a quiet room. Mice were acclimated to the measuring procedure for 1 wk before the experiment. The basal temperature was the average of 3 days after acclimation, which was taken in the morning when mice were usually asleep and less likely excitable. Heat exposure was started at the same time every morning to evaluate the increase of body temperature compared with basal measurements. Body temperature was measured immediately after the first cycle of heat exposure (Fig. 1, Heat #1), at the 1-h rest after the first heat exposure (Fig. 1, Rest #1), immediately after the second cycle of heat exposure (Fig. 1, Heat #2), and at 3 h after the second heat exposure (Fig. 1, Rest #2). Body weight, water intake, and food intake were checked every morning and evening for 10 days. On the last day of the experiment, mice were euthanized 3 h after the second cycle of heat exposure to allow for some recovery from dehydration.
Blood testing.
Blood was taken by cardiac puncture before euthanasia under anesthesia with isoflurane. Blood samples were collected in serum separator tubes with clot activator and separation gel (Becton Dickinson, Franklin Lakes, NJ) and placed for 45 min at room temperature. Serum was removed after centrifugation at 3,500 revolutions/min for 10 min at room temperature and kept at −80°C until analysis. Serum creatinine was evaluated with HPLC-MS (27, 42). Blood urea nitrogen (BUN), serum uric acid, and creatinine kinase were measured with a colorimetric detection assay kit (Bio Assay Systems, Hayward, CA). Serum osmolality was measured using an Advance Micro Osmometer (model 3300, Advanced Instruments, Norwood, MA). Serum copeptin (a stable biomarker of vasopressin) was measured with ELISA (Cloud-Clone, Houston, TX)
Urine testing.
Urine was collected before euthanasia by gently holding the mice over a collection plate to stimulate excretion, and it was kept at −80°C until analysis. Urinary albumin was measured by ELISA (Exocell, Philadelphia, PA) and corrected by creatinine (Pointe Scientific, Canton, MI).
Renal histology.
Sliced kidneys were fixed in 10% formalin or methyl Carnoy’s and embedded in paraffin. Sections were cut at 2 µm thickness. General histological examination was done with periodic acid-Schiff’s reagent staining. Immunostaining was performed using classical immunoperoxidase techniques with 3,3-diaminobenzidine for color enhancement. Tubular cell proliferation, macrophage infiltration, and mitochondrial protein [cytochrome c oxidase complex IV (COX IV)] expression in the kidney was determined with anti-proliferating cell nuclear antigen (PCNA) antibody (Sigma, St. Louis, MO), anti-F4/80 antibody (Bio-Rad, Hercules, CA), and anti-COX IV antibody (Abcam, Cambridge, MA). The amount of cell death was assessed by TUNEL staining with 6-µm-thick sections according to the manufacturer’s protocol (Trevigen, Gaithersburg, MD).
The number of positive cells was counted using an Aperio scanner (Aperio Technologies, Vista, CA). The software allows color recognition, and positive cells were identified as the percentage of positive color saturation at ×200 magnification in a blinded manner using at least 10 fields for each biopsy sample (27).
Renal ATP levels.
ATP concentration was analyzed with an ATP assay kit (BioVision, Milpitas, CA). Kidneys (15 mg) were homogenized in 100 μl assay buffer provided in the kit. The amount of ATP was standardized by protein with a BCA assay.
For the one-time exposure experiment, 12-wk-old male C57BL/6J mice were divided into the following two groups: nontreated control (NT control group; n = 6) and heat exposure (heat group; n = 7). Mice in the heat group were put in the heat chamber at 39.5°C for 30 min. Mice were euthanized soon after the one-time heat exposure, and kidneys were removed.
Western blot analysis.
Whole kidney tissues were homogenized in MAPK lysis buffer. Protein concentration was determined by a BCA protein assay (Pierce, ThermoFisher Scientific, Rockford, IL). Each sample equivalent to 30 mg protein was loaded in each lane of a gel. Samples were subjected to SDS-PAGE and then transferred to PVDF membranes. Membranes were incubated with the following primary antibodies: heat shock protein (HSP)70 (Stressgen, San Diego, CA), COX IV (Abcam), succinate dehydrogenase subunit A (SDHA; Proteintech, Rosemont, IL), aconitase-2 (Sigma), NADPH oxidase 4 (NOX4; Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (Cell Signaling Technology, Danvers MA) and visualized using horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) and enhanced chemiluminescence agent (ThermoFisher Scientific). The intensity and area of bands were quantified by ImageJ and standardized by β-actin.
Isolation of mitochondria.
Whole kidney mitochondria were isolated with a Mitochondria Isolation Kit (ThermoFisher Scientific). One-fourth of the hemi-kidney was homogenized in PBS as described in the manufacturer’s protocol. Isolated mitochondrial samples were lysed in lysis buffer for Western blot analysis, and the protein concentration was evaluated by a BCA assay. Each sample equivalent to 5 mg protein was loaded in each lane for Western blots. The intensity and area of bands were quantified by ImageJ and standardized by COX IV.
Statistical analysis.
All values are expressed as means ± SE. Independent replicates for each data point (n) are shown in the figures. Data graphics and statistical analysis were performed using Prism (Graphpad). Analysis for multiple comparisons was performed with a Tukey-Kramer test. Comparison of two groups (NT control and heat groups) was performed with an unpaired two-tailed t-test. Correlation analyses were performed by Pearson's product-moment correlation. Significance was defined as P < 0.05.
RESULTS
Body temperature increased with DNP and heat exposure.
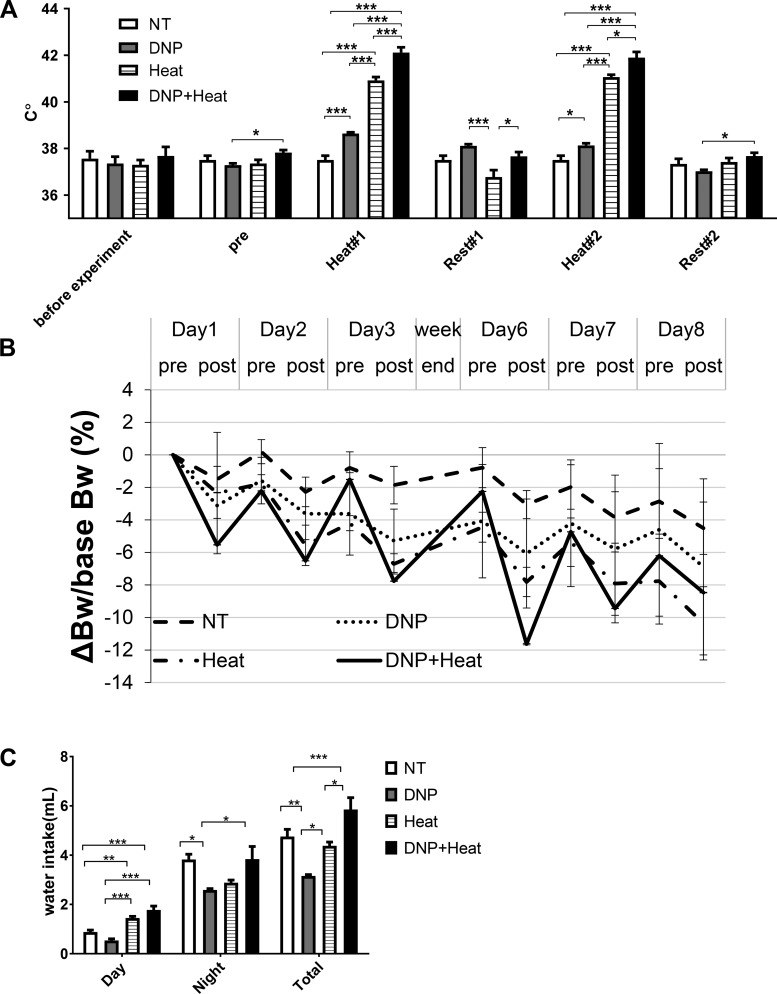
Basal core body temperature before DNP administration and heat exposure was similar between groups (Fig. 2A, before experiment). Although temperature did not change in the control group not exposed to heat, core body temperature increased from 37.50°C (NT control group) to 38.64°C with DNP administration in the absence of heat exposure (Fig. 2A, Heat #1). In contrast, heat-exposed mice had a core body temperature of 40.92°C immediately after heat exposure, whereas core body temperature increased to 42.11°C in the DNP + Heat group (Fig. 2A, Heat #1). No mice in the heat group showed signs of heat stroke, and a few mice in the DNP + heat group with suspected heat stroke were removed, either due to possible signs of convulsion or being less alert or displaying less movement. Core temperature was decreased rapidly after mice were removed from the heat chamber. Core body temperature after the second cycle of heat exposure remained significantly higher in the DNP, heat, and DNP + heat groups, respectively (Fig. 2A, Heat #2). Core body temperature at the 1-h rest period after heat exposure (Fig. 2A, Rest #1) and core body temperature at the 3-h rest period after the second heat exposure (6 h after the start of experiment) (Fig. 2A, Rest #2) returned to baseline core body temperature levels.
Fig. 2.
A: body temperature. Basal body temperature (core body temperature; before experiment) was the average of 3 days before the experiment, and there was no difference among groups. Average core body temperature on each day before 2,4-dinitrophenol (DNP) administration (pre) showed a small but statistically significant difference between the DNP and DNP + heat groups. This difference was not scientifically significant. Core body temperature after the first exposure (Heat #1) and second exposure (Heat #2) was increased by heat and DNP, respectively. Core body temperature at the 1-h rest period after the first heat exposure (Rest #1) and the 3-h rest period after the second heat exposure (Rest #2) showed no difference between the nontreated (NT) control group and each group. B: trend of the body weight (BW) change corrected by basal BW. Calculation of ΔBW/base BW of each mouse was performed with the following formula: (BW at each point – BW before the experiment)/BW before the experiment. The BW change at day 8 after heat and DNP exposure was not different among groups. C: water intake. Water intake during daytime was increased in the heat and DNP + heat groups compared with the NT control and DNP groups, respectively. Water intake during night was decreased in the DNP group compared with the NT control group. Total water intake was not changed between the NT control and heat groups or NT control and DNP + heat groups. n = 5 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001.
Basal body weight was not different among groups (Table 1). After 10 days of experiments, body weight was decreased, but no difference existed among groups (Table 1). The mean decrease of body weight after heat exposure each day (the change in body weight between morning and evening) was more severe in the DNP + heat group compared with the NT control and DNP groups (Table 1 and Fig. 2B). However, because body weight recovered (increased) during night, the decrease of body weight during the entire experimental period was not different among groups (Table 1 and Fig. 2B).
Table 1.
Body weight, food intake, creatinine, blood urea nitroben, osmolality, and copeptin
| NT Control Group | DNP Group | Heat Group | DNP + Heat Group | |
|---|---|---|---|---|
| Body weight before the experiment, g | 27.77 ± 0.68 | 29.88 ± 2.05 | 29.34 ± 1.21 | 27.99 ± 0.93 |
| Body weight after 10 days of the experiment, g | 26.50 ± 0.55 | 27.57 ± 1.22 | 26.24 ± 0.80 | 25.63 ± 0.99 |
| ΔBody weight after 10 days of the experiment, g | −1.27 ± 0.30 | −2.31 ± 0.86 | −3.09 ± 0.55 | −2.35 ± 0.20 |
| Average Δbody weight after heat exposure/day, g | −0.50 ± 0.19 | −0.64 ± 0.07 | −1.00 ± 0.19 | −1.52 ± 0.12a |
| Food intake, g·mouse−1·day−1 | 3.22 ± 0.16 | 2.28 ± 0.12 | 2.19 ± 0.40 | 2.48 ± 0.30 |
| Serum creatinine, mg/dl | 0.79 ± 0.08 | 1.00 ± 0.04b | 0.62 ± 0.05 | 0.96 ± 0.04c |
| Blood urea nitrogen, mg/dl | 25.25 ± 1.38 | 25.57 ± 1.48 | 23.65 ± 0.80 | 36.79 ± 0.84d |
| Serum osmolality, mosm | 323.2 ± 3.9 | 345.1 ± 3.5 | 329.6 ± 3.5 | 362.4 ± 13.7e |
| Serum copeptin, pg/ml | 112.9 ± 11.2 | 115.2 ± 4.0 | 127.2 ± 5.4 | 158.7 ± 7.0f |
Values are means ± SE. NT, nontreated; DNP, 2,4-dinitrophenol.
P < 0.001 vs. the NT control group and P < 0.01 vs. the DNP group;
P < 0.05 vs. the NT control group and P < 0.001 vs. the heat group;
P < 0.01 vs. the heat group;
P < 0.001 vs. the NT control, DNP, and heat groups;
P < 0.01 vs. the NT control group and P < 0.05 vs. the heat group;
P < 0.01 vs. the NT control and DNP groups and P < 0.05 between the DNP + heat group and the other groups.
Water intake during daytime was increased in the heat and DNP + heat groups compared with the NT control and DNP groups (Fig. 2C). Water intake during night was decreased in the DNP group compared with the NT control and DNP + heat groups (Fig. 2C). This latter finding may be due to metabolic water generated from lipid or muscle degradation by DNP. Total water intake per day was not increased in the heat and DNP + heat groups compared with the NT control group but was increased compared with the DNP group (Fig. 2C). Food intake was not different between groups (Table 1).
Proteinuria, tubular injury, and interstitial inflammation are associated with higher core temperatures.
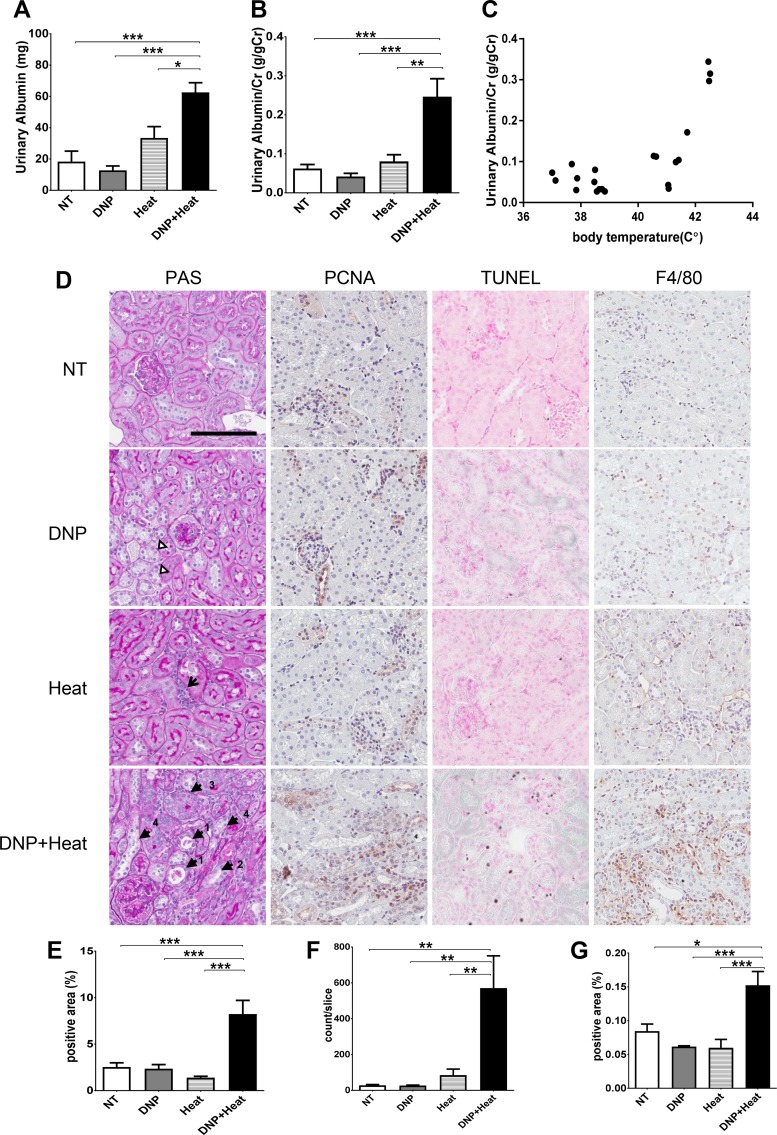
Albuminuria was increased significantly in the DNP + heat group (Fig. 3A). Albuminuria corrected by urinary creatinine was also increased in the DNP + heat group (Fig. 3B). The level of albuminuria correlated with core body temperatures (R = 0.5112, P < 0.01; Fig. 3C).
Fig. 3.
A: albuminuria. Urinary albumin (uAlb; spot urine at the 3-h rest period after the second heat exposure) was increased in the 2,4-dinitrophenol (DNP) + Heat group. B: uAlb corrected by urinary creatinine (uAlb/uCr) was also increased in the DNP + heat group. C: correlation between core body temperature and uAlb/uCr. Core body temperature after the first heat exposure (Heat #1) was correlated to uAlb/uCr (r = 0.715, P < 0.001). D: periodic acid-Schiff (PAS) reagent, TUNEL, and immunohistochemistry with anti-proliferating cell nuclear antigen (PCNA) and F4/80. Magnification: ×400. Scale bar = 100 µm. Renal pathology was observed with PAS staining. PAS showed core body temperature tubular injury in DNP (brightness of the cytosol; white triangles), interstitial cell infiltration in the heat group (arrow), massive tubular injury in the DNP + heat group, tubular dilatation (arrowheads labeled “1”), debris of tubular lumens (arrowheads labeled “1”), loss of the brush border (arrowheads labeled “2”), tubular cell proliferation (arrowheads labeled “3”), and tubular cell swelling and loss of nuclei (arrowheads labeled “4”). PCNA showed the increase of tubular cell proliferation as nuclear and cytosolic stains of brown. TUNEL showed the increase of cell death as dark blue stains of nuclei and normal cells as pink (counterstain with nuclear fast red). F4/80 showed the increase of macrophage as stains of brown. E–G: quantification of the number of positive cells [PCNA (E), TUNEL (F), and F4/80 (G)] was analyzed by an Aperio scanner. n = 5 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001.
A statistically significant increase of serum creatinine was also detected in the DNP and DNP + heat groups, but the increase was minor (Table 1). BUN was increased in the DNP + heat group. Serum osmolality and copeptin (a stable biomarker of vasopressin) were also increased in the DNP + heat group (Table 1). The DNP + heat group drank more water than the other groups, and the decrease of body weight each day was compensated enough such that the groups showed the same body weight by the next morning. Thus, the relative increase in BUN, osmolality, and copeptin suggested that the DNP + heat group had a greater physiological response to a given state of dehydration (Fig. 2C).
Renal histology showed acute tubular injury in the DNP group (brightness of the cytosol; Fig. 3D, white triangles), whereas the heat group showed mild interstitial cell infiltration (Fig. 3D, arrow). However, the DNP + heat group showed focal tubular injury with tubular dilatation (Fig. 3D, arrowheads labeled “1”), debris present in the tubular lumen (Fig. 3D, arrowheads labeled “1”), loss of the brush border (Fig. 3D, arrowhead labeled “2”), tubular cell proliferation (Fig. 3D, arrowhead labeled “3”), tubular cell swelling and loss of nuclei (Fig. 3D, arrowhead labeled “4”), and interstitial cellular infiltration. PCNA (Fig. 3, D and E) and TUNEL (Fig. 3, D and F) staining documented a significant increase of tubular cell proliferation and tubular cell death in the DNP + heat group. There was also a significant increase of F4/80-positive macrophages in the interstititum of DNP + heat-treated mice (Fig. 3, D and G).
A decrease in mitochondrial mass in the outer medulla is associated with transient hyperthermia.
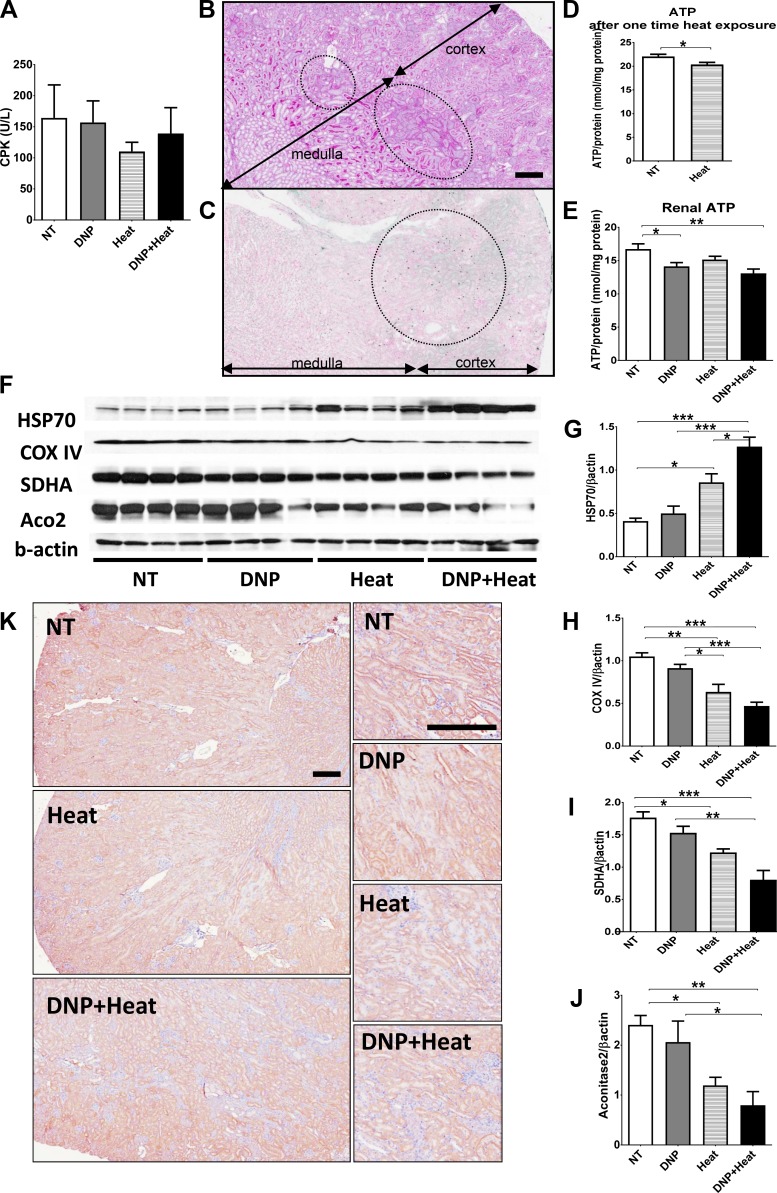
Serum creatinine kinase was not increased by DNP administration and heat exposure (Fig. 4A) and was even slightly lower in the heat and DNP + heat groups. Thus, we concluded that rhabdomyolysis did not contribute to kidney injury in this model. Because tubular injury was severe in the outer medulla and distal tubules, we hypothesized that the tubular cell injury caused by heat stress is related to intracellular hypoxia, as the outer medulla and distal tubules are susceptible to ischemia (Fig. 4, B and C) (11). Consistent with this hypothesis, ATP content of the whole kidney was decreased after mice were exposed to a single 30-min exposure to heat at 39.5°C (Fig. 4D). Similarly, renal ATP was decreased in the DNP + heat group at 10 days, although the decrease in the heat group was not significant (P = 0.13, NT control vs. heat groups; Fig. 4E).
Fig. 4.
A: serum creatinine kinase (CPK) was not increased by 2,4-dinitrophenol (DNP) and heat exposure. B: periodic acid-Schiff stain with low magnification (×80) showed that tubular injury was evident in the outer medulla (inside the circles). C: TUNEL with low magnification (×80) showed that positive cells were observed mainly in the outer medulla (inside the circle). D: renal ATP after one-time heat exposure. ATP in the kidney harvested soon after a one-time, 30-min heat exposure was decreased. E: renal ATP at euthanization. ATP in the kidney harvested after the 10-day experiment (at 3-h rest period after the second heat exposure) was decreased in the DNP + heat group compared with the nontreated (NT) control group. F: Western blot (WB) analysis of heat shock protein (HSP)70 and mitochondrial protein. HSP70 was increased in the heat and DNP + heat groups. Mitochondrial proteins [cytochrome c oxidase complex IV (COX IV), succinate dehydrogenase subunit A (SDHA), and aconitase-2 (Aco2)] were decreased in the heat and DNP + heat groups. β-Actin was used as an internal control. G–J: quantification of the WB analysis [HSP70 (G), COX IV (H), SDHA (I), and Aco2 (J)]. The intensity and area of bands were analyzed with ImageJ and standardized by β-actin. K: immunohistochemistry with COX IV are presented at low magnification (×80) (left) and high magnification of the outer medulla area (×200) (right). COX IV was less expressed in tubular cells of the outer medulla in the heat and DNP + heat groups. Scale bar = 200 μm. n = 5 mice for A, D, and E and 4 mice for G–J. *P < 0.05; **P < 0.01; ***P < 0.001.
HSP70 expression in the kidney was increased in the heat and DNP + heat groups compared with the NT control group (Fig. 4, F and G) even though core body temperature returned to normal when mice were euthanized (Fig. 2A, Rest #2). COX IV, which is a stable marker of mitochondria in complex IV of the mitochondrial electron transfer chain, was decreased in the heat and DNP + heat groups by Western blot analysis (Fig. 4, F and H). Aconitase-2 and SDHA (an enzyme used in the mitochondrial respiratory chain) were also decreased in the heat and DNP + heat groups (Fig. 4, I and J). Immunohistochemistry demonstrated decreased COX IV expression in tubular cells of the outer medulla in the heat group (Fig. 4K). In the DNP + heat group, we also observed focal decreases of COX IV expression in the outer medulla (Fig. 4K) coinciding with areas of tubular injury.
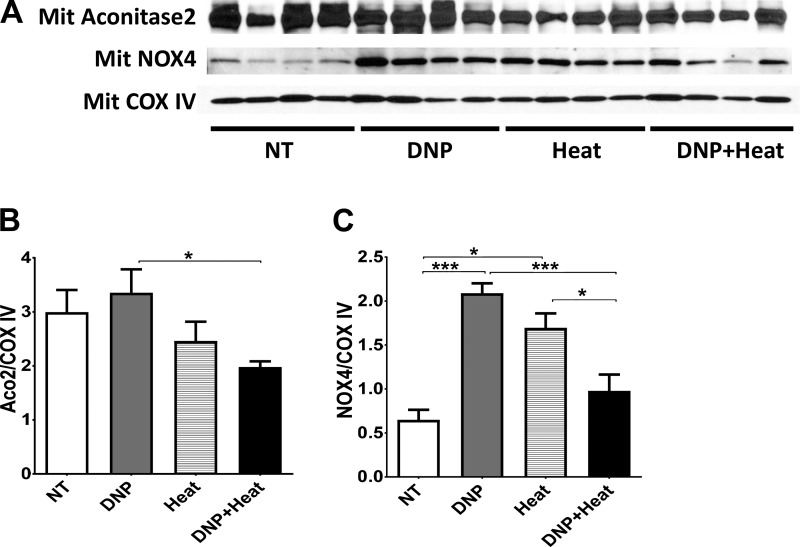
Experiments in isolated mitochondria.
Isolated mitochondria from whole kidney tissue were examined for intramitochondrial expression of enzymes involved in the electron transport chain as well as NOX4 (an enzyme involved in oxidative stress) factored for COX IV, which was used as a general mitochondrial housekeeping gene. Intramitochondrial aconitase-2 tended to be reduced in the Heat group (P = 0.076, NT control vs. heat groups) and was significantly lower in the DNP + Heat group compared with the NT control group (Fig. 5, A and B). NOX4 in mitochondria was also increased in the heat and DNP groups (Fig. 5, A and C). Taken together, increasing body temperature led to loss of mitochondria and greater oxidative stress.
Fig. 5.
A: Western blots of isolated mitochondria from kidney tissue. Mitochondrial aconitase-2, which represents mitochondrial function, was decreased in the 2,4-dinitrophenol (DNP) + heat group. Mitochondrial NADPH oxidase 4 (NOX4), which represents intramitochondrial oxidative stress, was increased in the DNP and heat groups. Cytochrome c oxidase complex IV (COX IV) was used as an internal control. B and C: quantification of intramitochondrial protein expression [aconitase-2 (B) and NOX4 (C)]. The intensity and area of bands were analyzed by ImageJ and standardized by COX IV. n = 4 mice. *P < 0.05; ***P < 0.001.
DISCUSSION
In the present study, we tested the hypothesis that increasing core temperature might accelerate kidney disease in a model of heat stress in mice. Mice were exposed to heat daily (except for the weekend) in the presence or absence of DNP, a mitochondrial uncoupling agent. As expected, we found that DNP resulted in a higher core temperature, ~1°C in the DNP group compared with the NT control group and in the DNP + heat group compared with the heat group; this higher core temperature was transient, being maximal around the time of heat exposure. Mice with the higher temperature drank more fluid but still showed signs of dehydration with greater serum osmolality and vasopressin (copeptin) levels. Our key finding was that administration of DNP with heat resulted in greater kidney injury, with marked albuminuria, tubular injury and proliferation, tubular apoptosis, and interstitial inflammation. Additional experiments showed that kidney injury was associated with a reduction in ATP levels in the kidney, evidence of mitochondrial oxidative stress, and an absolute loss in mitochondrial mass. Thus, these are the first experimental results to suggest that in the setting of hot external temperature, an increased core temperature response specifically induced by mitochondrial uncoupling appears to be a risk factor for heat stress-associated kidney disease.
The first major finding was that raising core body temperature only 1°C was sufficient to induce significant kidney damage, based on the result that only the DNP + heat group showed renal damage and the heat group did not. Exposure to heat commonly raises body temperature, but usually heat stroke relates to disturbance of the central nervous system and multiorgan dysfunction associated with persistent hyperthermia of >40°C (4). The body temperature that results in heat stroke may be higher in rodents compared with humans. Previous studies have suggested that there are critical thermal maximums that result in tissue injury, consisting of 41.6–42°C for 45 min to 8 h in humans, with higher temperatures (44°C) required in mice (4, 6, 46). In our study, exposure to 39.5°C of ambient temperature increased the core temperature of mice up to 41.0°C and up to 42.0°C with an uncoupling agent. In prior studies (4, 6, 46) as well as the present study, no mice in the heat group, whose body temperature was 41.0°C, showed signs of heat stroke. A few mice in the DNP + heat group with suspected heat stroke were removed. The thermoregulatory failure was not coincident because core temperature decreased rapidly after mice were removed from the heat chamber. Thus, the mice that were used in our study did not display any overt signs of heat stroke; nevertheless, they showed moderate kidney damage. Furthermore, kidney damage, as manifested by albuminuria, correlated with the core temperature (Fig. 3C), and there was minimal injury in mice given DNP in the absence of heat. Finally, the rise in temperature was transient in these mice, and temperatures rapidly returned to normal levels within a few hours. Thus, the results of the present study are consistent with a direct relationship between kidney damage and core temperature and suggest that even transient increases in core temperature can lead to significant injury.
The second major finding was that the kidney injury involved more than tubular death; it had a prominent inflammatory component. The injury was not a simple acute tubular necrosis but had components suggestive of acute interstitial nephritis. This is important because an inflammatory renal injury is typical in heat-related induced kidney injury, as reported in classical articles of heatstroke (24, 25), and a similar acute presentation can also occur in sugarcane workers, some of whom go on to develop CKD (12–14).
We also performed experiments to better characterize potential mechanisms for causing the kidney damage. A key finding was the loss of mitochondria mass, as noted by a decrease in the mitochondrial housekeeping protein COX4. Isolated mitochondria demonstrated a loss of aconitase relative to COX4 as well as an increase in NOX4. NOX4 is a type of NADPH oxidase that has been previously reported to localize to mitochondria in conditions such as hyperuricemia and diabetes (3, 38). Inhibition of aconitase and succinate dehydrogenase can affect ATP generation by oxidative phosphorylation and the electron transport pathway, and these effects could contribute to decreased ATP levels. Consistent with this finding, ATP levels were low in the kidneys of mice administered heat and DNP. These results suggest that alterations in energy metabolism might underlie the pathogenesis of kidney injury in this model.
One interesting finding in our study was the observation of high expression of HSP, notably HSP70. HSP70 is a very sensitive marker for heat stress and may have a role in both innate and adaptive immunity. Additionally, serum or urinary HSP70 might be a good marker for detecting workers at high risk of developing CKD because the increase of HSP70 was persistent even after several hours of evacuation from the hot environment, and serum HSP70 was correlated to its local expression (10, 18).
A limitation to this study is that the full mechanism of kidney injury was not elucidated. It also remains possible that the effects of renal injury may relate more to mitochondrial dysfunction induced by DNP as opposed to the effects on temperature per se. Nevertheless, the results presented suggest that future clinical studies might focus on characteristics such as core temperature, serum HSP70 levels, mitochondrial function, and the frequency of single-nucleotide polymorphisms that affect mitochondrial function and heat production.
In conclusion, we present evidence for a key role for core body temperature in driving kidney damage from heat stress. The administration of a mitochondrial uncoupling agent led to hyperthermia, renal injury, mitochondrial loss, and tissue ATP depletion that resulted in acute tubulointerstitial injury. We hypothesize that the core temperature that occurs in workers in the sugarcane fields or other outside environments may have a key role in predicting who is most susceptible to kidney damage. A better understanding of the mechanisms may allow for early detection and intervention that may help reduce or reverse the current epidemic of kidney disease affecting these hot regions of the world.
GRANTS
This work was supported by a grant from Dutch National Postcode Lottery to the Solidaridad Network and La Isla Foundation and by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-108408-01A1. T. Jensen was funded by NIDDK Training Grant 5-T32-DK-007446-34. This paper is considered a contribution by the University of Colorado Climate Change and Health consortium.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.S., C.A.R.-J., and R.J.J. conceived and designed research; Y.S., C.A.R.-J., and M.M. performed experiments; Y.S. analyzed data; Y.S. and R.J.J. interpreted results of experiments; Y.S. prepared figures; Y.S. and R.J.J. drafted manuscript; Y.S., L.G.S.-L., L.S.N., J.G., and R.J.J. edited and revised manuscript; Y.S., A.A.-H., T.J., D.R.T., L.G.S.-L., L.S.N., J.B.-D., C.S., J.G., H.F.D., T.I., T.K., S.M., G.E.G., M.A.L., and R.J.J. approved final version of manuscript.
REFERENCES
- 1.Adzika Nsatimba PA, Pathak K, Soares MJ. Ethnic differences in resting metabolic rate, respiratory quotient and body temperature: a comparison of Africans and European Australians. Eur J Nutr 55: 1831–1838, 2016. doi: 10.1007/s00394-015-1000-4. [DOI] [PubMed] [Google Scholar]
- 2.Badurdeen Z, Nanayakkara N, Ratnatunga NV, Wazil AW, Abeysekera TD, Rajakrishna PN, Thinnarachchi JP, Kumarasiri R, Welagedera DD, Rajapaksha N, Alwis AP. Chronic kidney disease of uncertain etiology in Sri Lanka is a possible sequel of interstitial nephritis! Clin Nephrol 86, Suppl 1: 106–109, 2016. doi: 10.5414/CNP86S115. [DOI] [PubMed] [Google Scholar]
- 3.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA 106: 14385–14390, 2009. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchama A, Knochel JP. Heat stroke. N Engl J Med 346: 1978–1988, 2002. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 5.Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol 6: 36, 2015. doi: 10.3389/fphys.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bynum GD, Pandolf KB, Schuette WH, Goldman RF, Lees DE, Whang-Peng J, Atkinson ER, Bull JM. Induced hyperthermia in sedated humans and the concept of critical thermal maximum. Am J Physiol Regul Integr Comp Physiol 235: R228–R236, 1978. doi: 10.1152/ajpregu.1978.235.5.R228. [DOI] [PubMed] [Google Scholar]
- 7.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 7: 552–560, 2008. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 8.Carter R III, Cheuvront SN, Williams JO, Kolka MA, Stephenson LA, Sawka MN, Amoroso PJ. Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med Sci Sports Exerc 37: 1338–1344, 2005. doi: 10.1249/01.mss.0000174895.19639.ed. [DOI] [PubMed] [Google Scholar]
- 9.Dearden L. Karachi Heat Wave: Death Toll Tops 1,000 as Government and Electricity Company Trade Blame (Online). https://www.independent.co.uk/news/world/asia/pakistan-heatwave-death-toll-tops-1000-as-government-and-electricity-company-trade-blame-10344719.html.
- 10.Dutta SK, Girotra M, Singla M, Dutta A, Otis Stephen F, Nair PP, Merchant NB. Serum HSP70: a novel biomarker for early detection of pancreatic cancer. Pancreas 41: 530–534, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein FH, Agmon Y, Brezis M. Physiology of renal hypoxia. Ann N Y Acad Sci 718: 72–82, 1994. doi: 10.1111/j.1749-6632.1994.tb55706.x. [DOI] [PubMed] [Google Scholar]
- 12.Fischer RS, Mandayam S, Chavarria D, Vangala C, Nolan MS, Garcia LL, Palma L, Garcia F, García-Trabanino R, Murray KO. Clinical evidence of acute Mesoamerican nephropathy. Am J Trop Med Hyg 97: 1247–1256, 2017. doi: 10.4269/ajtmh.17-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer RS, Vangala C, Mandayam S, Chavarria D, García-Trabanino R, Garcia F, Garcia LL, Murray KO. Clinical markers to predict progression from acute to chronic kidney disease in Mesoamerican nephropathy. Kidney Int 94: 1205–1216, 2018. doi: 10.1016/j.kint.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer RS, Vangala C, Truong L, Mandayam S, Chavarria D, Granera Llanes OM, Fonseca Laguna MU, Guerra Baez A, Garcia F, García-Trabanino R, Murray KO. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int 93: 681–690, 2018. doi: 10.1016/j.kint.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 15.García-Arroyo FE, Cristóbal M, Arellano-Buendía AS, Osorio H, Tapia E, Soto V, Madero M, Lanaspa MA, Roncal-Jiménez C, Bankir L, Johnson RJ, Sánchez-Lozada LG. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol 311: R57–R65, 2016. doi: 10.1152/ajpregu.00354.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Arroyo FE, Tapia E, Blas-Marron MG, Gonzaga G, Silverio O, Cristóbal M, Osorio H, Arellano-Buendía AS, Zazueta C, Aparicio-Trejo OE, Reyes-García JG, Pedraza-Chaverri J, Soto V, Roncal-Jiménez C, Johnson RJ, Sánchez-Lozada LG. Vasopressin mediates the renal damage induced by limited fructose rehydration in recurrently dehydrated rats. Int J Biol Sci 13: 961–975, 2017. doi: 10.7150/ijbs.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Trabanino R, Jarquín E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, Glaser J, José Vindell J, Stockfelt L, Roncal C, Harra T, Barregard L. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador—a cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res 142: 746–755, 2015. doi: 10.1016/j.envres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Gelain DP, de Bittencourt Pasquali MA, M Comim C, Grunwald MS, Ritter C, Tomasi CD, Alves SC, Quevedo J, Dal-Pizzol F, Moreira JC. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock 35: 466–470, 2011. doi: 10.1097/SHK.0b013e31820fe704. [DOI] [PubMed] [Google Scholar]
- 19.Glaser J, Lemery J, Rajagopalan B, Diaz HF, García-Trabanino R, Taduri G, Madero M, Amarasinghe M, Abraham G, Anutrakulchai S, Jha V, Stenvinkel P, Roncal-Jimenez C, Lanaspa MA, Correa-Rotter R, Sheikh-Hamad D, Burdmann EA, Andres-Hernando A, Milagres T, Weiss I, Kanbay M, Wesseling C, Sánchez-Lozada LG, Johnson RJ. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin J Am Soc Nephrol 11: 1472–1483, 2016. doi: 10.2215/CJN.13841215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goforth CW, Kazman JB. Exertional heat stroke in navy and marine personnel: a hot topic. Crit Care Nurse 35: 52–59, 2015. doi: 10.4037/ccn2015257. [DOI] [PubMed] [Google Scholar]
- 21.Hart GR, Anderson RJ, Crumpler CP, Shulkin A, Reed G, Knochel JP. Epidemic classical heat stroke: clinical characteristics and course of 28 patients. Medicine (Baltimore) 61: 189–197, 1982. doi: 10.1097/00005792-198205000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RJ. Pro: heat stress as a potential etiology of Mesoamerican and Sri Lankan nephropathy: a late night consult with Sherlock Holmes. Nephrol Dial Transplant 32: 598–602, 2017. doi: 10.1093/ndt/gfx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RJ, Wesseling C, Newman LS. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med 380: 1843–1852, 2019. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 24.Kew MC, Abrahams C, Levin NW, Seftel HC, Rubenstein AH, Bersohn I. The effects of heatstroke on the function and structure of the kidney. Q J Med 36: 277–300, 1967. [PubMed] [Google Scholar]
- 25.Kew MC, Abrahams C, Seftel HC. Chronic interstitial nephritis as a consequence of heatstroke. Q J Med 39: 189–199, 1970. [PubMed] [Google Scholar]
- 26.Kovats RS, Kristie LE. Heatwaves and public health in Europe. Eur J Public Health 16: 592–599, 2006. doi: 10.1093/eurpub/ckl049. [DOI] [PubMed] [Google Scholar]
- 27.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, Inaba S, Li N, Rivard CJ, Kosugi T, Sanchez-Lozada LG, Petrash JM, Sautin YY, Ejaz AA, Kitagawa W, Garcia GE, Bonthron DT, Asipu A, Diggle CP, Rodriguez-Iturbe B, Nakagawa T, Johnson RJ. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 25: 2526–2538, 2014. doi: 10.1681/ASN.2013080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW. Do the obese have lower body temperatures? A new look at a forgotten variable in energy balance. Trans Am Clin Climatol Assoc 120: 287–295, 2009. [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard WR, Sorensen MV, Galloway VA, Spencer GJ, Mosher MJ, Osipova L, Spitsyn VA. Climatic influences on basal metabolic rates among circumpolar populations. Am J Hum Biol 14: 609–620, 2002. doi: 10.1002/ajhb.10072. [DOI] [PubMed] [Google Scholar]
- 30.Manini TM, Patel KV, Bauer DC, Ziv E, Schoeller DA, Mackey DC, Li R, Newman AB, Nalls M, Zmuda JM, Harris TB; Health, Aging and Body Composition Study . European ancestry and resting metabolic rate in older African Americans. Eur J Clin Nutr 65: 663–667, 2011. doi: 10.1038/ejcn.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marino FE, Lambert MI, Noakes TD. Superior performance of African runners in warm humid but not in cool environmental conditions. J Appl Physiol (1985) 96: 124–130, 2004. doi: 10.1152/japplphysiol.00582.2003. [DOI] [PubMed] [Google Scholar]
- 32.Martín-Cleary C, Ortiz A. CKD hotspots around the world: where, why and what the lessons are. A CKJ review series. Clin Kidney J 7: 519–523, 2014. doi: 10.1093/ckj/sfu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyce S, Mitchell D, Armitage T, Tancredi D, Joseph J, Schenker M. Heat strain, volume depletion and kidney function in California agricultural workers. Occup Environ Med 74: 402–409, 2017. doi: 10.1136/oemed-2016-103848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paula Santos U, Zanetta DM, Terra-Filho M, Burdmann EA. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int 87: 792–799, 2015. doi: 10.1038/ki.2014.306. [DOI] [PubMed] [Google Scholar]
- 35.Roncal-Jimenez CA, Sato Y, Milagres T, Andres Hernando A, García G, Bjornstad P, Dawson JB, Sorensen C, Newman L, Krisher L, Madero M, Glaser J, Gárcía-Trabanino R, Romero EJ, Song Z, Jensen T, Kuwabara M, Rodriguez-Iturbe B, Sanchez-Lozada LG, Lanaspa MA, Johnson RJ. Experimental heat stress nephropathy and liver injury are improved by allopurinol. Am J Physiol Renal Physiol 315: F726–F733, 2018. doi: 10.1152/ajprenal.00543.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, González MA, Aragón A, Wesseling C, Sánchez-Lozada LG, Johnson RJ. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 86: 294–302, 2014. doi: 10.1038/ki.2013.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303: 223–226, 2004. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron, Exp Nephrol 121: e71–e78, 2012. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlader ZJ, Chapman CL, Sarker S, Russo L, Rideout TC, Parker MD, Johnson BD, Hostler D. Firefighter work duration influences the extent of acute kidney injury. Med Sci Sports Exerc 49: 1745–1753, 2017. doi: 10.1249/MSS.0000000000001254. [DOI] [PubMed] [Google Scholar]
- 40.Solis Zepeda GA. Impacto de las medidas preventivas para evitar el deterioro de la función renal por el Síndrome de Golpe por Calor en trabajadores agrícolas del Ingenio San Antonio del Occidente de Nicaragua, Ciclo Agrícola 2005–2006 (Thesis). León: Universidad Nacional Autonoma de Nicaragua, 2007. [Google Scholar]
- 41.Sorensen CJ, Butler-Dawson J, Dally M, Krisher L, Griffin BR, Johnson RJ, Lemery J, Asensio C, Tenney L, Newman LS. Risk factors and mechanisms underlying cross-shift decline in kidney function in Guatemalan sugarcane workers. J Occup Environ Med 61: 239–250, 2019. doi: 10.1097/JOM.0000000000001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int 71: 266–271, 2007. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 43.Wegman DH, Apelqvist J, Bottai M, Ekström U, García-Trabanino R, Glaser J, Hogstedt C, Jakobsson K, Jarquín E, Lucas RA, Weiss I, Wesseling C, Bodin T; Work Health and Efficiency (WE) Program Working Group . Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health 44: 16–24, 2018. doi: 10.5271/sjweh.3659. [DOI] [PubMed] [Google Scholar]
- 44.Wesseling C, Aragón A, González M, Weiss I, Glaser J, Bobadilla NA, Roncal-Jiménez C, Correa-Rotter R, Johnson RJ, Barregard L. Kidney function in sugarcane cutters in Nicaragua—a longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res 147: 125–132, 2016. doi: 10.1016/j.envres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Wijkström J, Leiva R, Elinder CG, Leiva S, Trujillo Z, Trujillo L, Söderberg M, Hultenby K, Wernerson A. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis 62: 908–918, 2013. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Wright GL. Critical thermal maximum in mice. J Appl Physiol 40: 683–687, 1976. doi: 10.1152/jappl.1976.40.5.683. [DOI] [PubMed] [Google Scholar]