Abstract
Optogenetics comprise a promising alternative to electrical stimulation for characterization of neural circuits and for the next generation of neural prostheses. Optogenetic stimulation relies on expression of photosensitive microbial proteins in animal cells to initiate a flow of ions into the cells in response to visible light. Here, we generated a novel transgenic mouse model in which we studied the optogenetic activation of spiral ganglion neurons, the primary afferent neurons of the auditory system, and showed a strong optogenetic response, with a similar amplitude as the acoustically evoked response. A twofold increase in the level of channelrhodopsin expression significantly increased the photosensitivity at both the single cell and organismal levels but also partially compromised the native electrophysiological properties of the neurons. The importance of channelrhodopsin expression level to optogenetic stimulation, revealed by these quantitative measurements, will be significant for the characterization of neural circuitry and for the use of optogenetics in neural prostheses.
NEW & NOTEWORTHY This study reveals a dose-response relationship between channelrhodopsin expression and optogenetic excitation. Both single cell and organismal responses depend on the expression level of the heterologous protein. Expression level of the opsin is thus an important variable in determining the outcome of an optogenetic experiment. These results are key to the implementation of neural prostheses based on optogenetics, such as next generation cochlear implants, which would use light to elicit a neural response to sound.
Keywords: auditory brainstem response, ChR2 expression level, optogenetic stimulation, spiral ganglion neuron
INTRODUCTION
Optogenetics has revolutionized the capacity to probe neural circuits and has led to fundamental advances in neuroscience. The specificity that can be obtained in modulating neuronal activity (Deisseroth and Hegemann 2017; Williams and Denison 2013) makes optogenetic stimulation at the brain-machine interface a powerful candidate for the therapy of neurologic and psychiatric disorders.
The use of channelrhodopsin for activation of neurons (Ayling et al. 2009; Boyden et al. 2005; Huff et al. 2013; Rolls et al. 2011) has potential application in sensory biology including the auditory system (Hernandez et al. 2014; Shimano et al. 2013). Recently, in pursuit of precise and effective activation of the auditory nerve in cochlear implant users, the potential for a multichannel cochlear implant based on optogenetics has been explored (Jeschke and Moser 2015). Shimano et al. introduced channelrhodopsin-2 (ChR2) into the cochlear nucleus (CN) and demonstrated light-evoked increases in auditory neural activity. Building on the results of Shimano et al. we showed that optogenetic stimulation of the CN resulted in activation of downstream auditory pathways, including the inferior colliculus and auditory cortex (Darrow et al. 2015; Hight et al. 2015). Hernandez et al. (2014) elicited light-evoked responses from the peripheral auditory system in Thy1.2-ChR2-YFP transgenic mice expressing ChR2 in spiral ganglion neurons (SGNs) of the cochlea.
Overall, these studies demonstrate potential for optogenetic activation of the auditory system from the periphery, where SGNs comprise the first order afferent neurons that transmit the signal from hair cells to the brainstem. Hernandez et al. (2014) have shown that considerable optogenetic excitation of the auditory nerve can be achieved, but several hurdles remain (Weiss et al. 2016). Better understanding of the parameters governing light-evoked activity in the cochlea is needed to improve the fidelity of optogenetic activity. Providing proper illumination is crucial for effective excitation and to avoid direct tissue damage (Cardin et al. 2010). A neuron’s optogenetic sensitivity can be modulated by using different types of opsins and by regulating the level of opsin expression. A series of opsins, including ChR2 (Boyden et al. 2005), Chronos (Klapoetke et al. 2014), and ReaChR (Lin et al. 2013), with varying degrees of photoreactivity have been developed (Berndt et al. 2011; Dawydow et al. 2014). Among them, ChR2R, which is sensitive to ~473-nm blue light with an effective power density for 50% activation (EPD50) near 0.7 mW/mm2 (Mattis et al. 2012), is the most widely studied opsin. The excitation generated by individual channels, as well as the expression level of those channels, could determine the responsiveness of individual neurons to optical stimulation. Expression could affect sensitivity, which is crucial to the use of opsins as an experimental tool and for a prosthetic device, but overexpression of opsins can have deleterious effects (Miyashita et al. 2013). However, the relationship between expression level and responsiveness has not been addressed in the auditory system or elsewhere in the brain (Allen et al. 2015).
The magnitude and latency of the response may be important in the fast-adapting neurons of the peripheral auditory system. Here, we develop a transgenic mouse model that provides a controllable differential expression of ChR2 in SGNs. The model is useful for studying the firing properties and characteristics of the peripheral neurons and tracing the auditory signal through the brainstem to the auditory cortex. We further examined the effect of expression level on the photoresponse and found that higher expression levels increased photosensitivity but also had an effect on the intrinsic properties of the neurons. The response we observe could have a practical application in the development of an auditory prosthesis (Williams and Denison 2013).
MATERIALS AND METHODS
Generation of Bhlhb5-ChR2R mice.
Bhlhb5-Cre mice (generously provided by Sarah Ross and Michael Greenberg) (Ross et al. 2010) were mated with a floxed ChR2 line, Ai32 (Madisen et al. 2012), obtained from the Jackson Laboratory (stock no. 012569). Complete loss of the Bhlhb5 gene can cause severe self-inflicted skin lesion (Ross et al. 2010). We therefore excluded Bhlhb5Cre/Cre mice. ChR2 expression in the transgenic is driven by the CAG promoter, which is a combination of the cytomegalovirus (CMV) early enhancer element and chicken beta-actin promoter. Genotyping was done in all offspring, and carried out by PCR using KAPA Express Extract and Mouse Genotyping Kits (KAPA BIOSYSTEMS). The primers for Bhlhb5-Cre mutants were as follows: 5′-CCTGACTCTCCAGCCCAGGTG-3′ (forward), 5′-ATCAGCGGGCTCGAAACAGC-3′ (Wt-reverse), 5′-GGCAACACCATTTTTTCTGACC-3′ (Mut-reverse) to detect Bhlhb5+/+ (241 bp), Bhlhb5+/Cre (241 and 280 bp), and Bhlhb5Cre/Cre (280 bp). The primers for Ai32 mutants were 5′-AAGGGAGCTGCAGTGGAGTA-3′ (Wt-forward), 5′-CCGAAAATCTGTGGGAAGTC-3′ (Wt-reverse), 5′-ACATGGTCCTGCTGGAGTTC-3′ (Mut-forward), 5′-GGCATTAAAGCAGCGTATCC-3′ (Mut-reverse) to detect ChR2R-eYFP+/+(297 bp), ChR2R-eYFP+/f (212 and 297 bp), and ChR2R-eYFP fl/fl (212 bp). All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals; study protocols were approved by Massachusetts Eye and Ear IACUC.
ABR recordings.
For acoustically evoked auditory brainstem response (ABR; aABR) recordings, clicks and tone pips were presented to the ears as described previously (Kujawa and Liberman 2009). Adult mice (more than 2 mo old) were anesthetized with ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip). A third of the initial dose was given as a booster when needed. The recording electrodes comprised three subdermal needles placed in the back near the tail (ground), in the scalp between the ears, and behind the left pinna. Sound stimuli were presented to the left ear at a rate of 27/s. Sound level was incremented in 5-dB steps, from 10 dB below threshold to 80 dB (for pure tone) or 90 dB (for click) sound pressure level (SPL). In pure tone threshold tests, seven half-octave-step-sized tone pips (from 5.6 to 45.3 kHz, 5-ms-long pulses with 0.5-ms rise-fall time) were delivered. The aABR waveforms shown in this paper were averaged from 1,024 samples. Threshold for aABR was defined as the lowest stimulus level at which a repeatable morphology could be identified in the response waveform.
Surgical access to the cochlea for optical stimulation.
To access the cochlea for optical stimulation, a postauricular incision was made. Blunt dissection of subcutaneous tissue revealed the sternocleidomastoid muscle, which was retracted to reveal the facial nerve overlying the bulla. After dissection of soft tissue to expose the bony surface of the bulla, a 0.5-mm burr (Meisinger) was used to perforate the bulla and extend the opening to gain access to the cochlea, round window, and overlying stapedial artery. A hand drill (OmniDrill 35, WPI) was then used to perform a cochleostomy (diameter ~1.0 mm) at the second turn of the cochlea, rostral to the stapedial artery. To do this, first the most superficial protrusion of the second turn was scored and the drill was slowly rotated to remove bone, taking care to enter the scala tympani and avoid penetration of the basilar membrane, osseous spiral lamina, or overlying stapedial artery. The facial nerve was then transected.
Optical stimulation and oABR recording.
Optically evoked ABRs (oABRs) were recorded similarly to the acoustically evoked ABR (aABR), with three subdermal electrodes placed in the same positions, but using blue light pulses rather than sound for the stimulus. Optical stimuli produced by a laser (BL473T-100FC, Shanghai Laser & Optics Century) were delivered via an optical fiber (400 µm diameter) placed directly into the cochleostomy (Fig. 6A). Blue light (473 nm) pulses of 1-ms duration were presented at 28 pulses/s. A total of 100 pulses for each light power level, ranging from 0.7 to 13.4 mW, were applied. To calibrate the light power level delivered by the fiber with respect to the voltage delivered to the laser, we positioned the optical fiber 2 mm from a high-sensitivity thermopile sensor (Coherent PS19Q) connected to a power meter (Coherent LabMax-TOP). The voltage command parameters were systematically varied based on the range of known pulse rates, durations, and amplitudes used for optical stimulation. The resulting laser power levels (radiant exposure, mW) were calibrated to the respective voltage input.
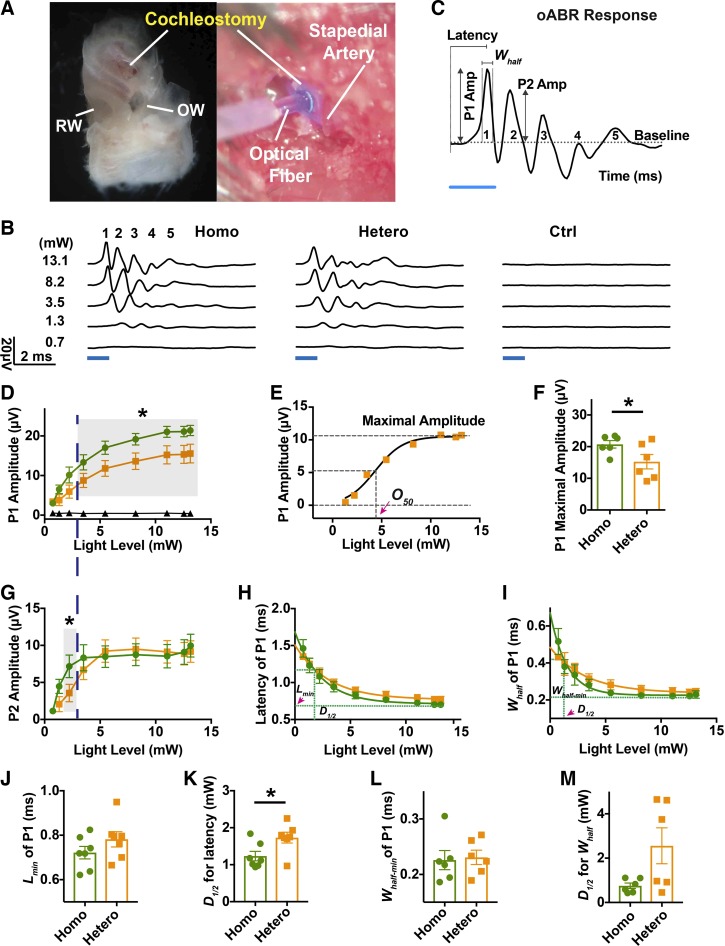
Fig. 6.
ChR2-level-dependent optically evoked auditory brainstem responses (oABRs) from adult mice. A: the cochleostomy made in the lateral wall of the left cochlea for optic fiber placement to deliver light to the inner ear. The round window (RW) and oval window (OW) are marked for orientation. B: oABRs in response to increasing light power levels in homozygous (Homo), heterozygous (Hetero), and control (Ctrl) adult (3–5 mo old) littermates. Each trace is an average of 100 trials. Blue bar represents the light pulse applied. The criterion to recognize peak 1 of the oABR was the amplitude no less than 1 µV. C: illustration to show parameters measured from oABR waves. P1 and P2 Amp represent the amplitudes of oABR peaks 1 and 2. Latencies were measured from the onset of light pulses. Whalf represents width at half-height of maximal oABR peak 1 amplitude. D: peak 1 amplitude of oABR as a function of light power level from homozygous (green, n = 6), heterozygous (orange, n = 6) and control (black, n = 3) ears. Gray shaded area indicates significance (paired t test, P < 0.05) between homozygous and heterozygous oABR peak 1 amplitudes. E: Boltzmann sigmoid fitting of peak 1 amplitudes of a heterozygous ear (orange squares). The baseline was constrained to zero. Predicted maximal amplitudes are illustrated by dashed lines and pink arrowhead. F: comparisons of maximum response magnitudes between homozygous and heterozygous littermates. G: amplitudes of oABR peak 2 as a function of light power level. Gray shaded area indicates significance (paired t test, P < 0.05). H: latencies of oABR peak 1 as a function of light power level. Lmin (pink arrowhead) indicates predicted minimal latency of peak 1 from heterozygous group (green). I: width at half-height (Whalf) of oABR peak 1 as a function of light power level. Horizontal dashed line (green) illustrates the predicted minimum of Whalf (Whalf-min). Vertical dashed lines in H and I, indicated by pink arrowhead in I, illustrate the light level decay to achieve half change (D1/2) of latency or Whalf toward their minimums predicted by one-phase exponential decay equation. J–M: comparisons of Lmin, D1/2 for latency, Whalf-min and D1/2 for Whalf between homozygous and heterozygous ears. *Significance (paired t test). Green represents data from the homozygous group; orange represents the data from the heterozygous group.
Western blot to identify expression level of ChR2-eYFP.
In these experiments, both cochleae were dissected out from ~3- to 5-mo-old Bhlhb5- ChR2R-eYFP mice, including control, heterozygous, and homozygous littermates. The cochlear lateral wall was chipping away with a fine forceps (FST). The spiral ligament and stria vascularis were removed carefully from the organ of Corti and modiolus. The cochlear explants were frozen in a −80°C freezer before homogenization in RIPA buffer (Sigma, no. R0278). Extracted proteins were separated on 4–12% NuPAGE Bis-Tri gels (Invitrogen) and transferred to 0.2-μm PVDF membranes (Bio-Rad). The membranes were probed with mouse anti-YFP (Santa Cruz Biotechnology, sc-9996, 1:200) and mouse anti-HSC70 (Santa Cruz Biotechnology, 1:10,000), followed by horseradish peroxidase-conjugated anti-mouse secondary antibody (Jackson Immunoresearch Laboratories). The blots were processed with ECL Western Blot Substrates (Thermo). Band intensity was quantified by densitometry using Quantity One software (Bio-Rad); each band was normalized with HSC70 and expressed as a ratio to the control. The mean and standard error of the mean were calculated and analyzed for significance. An unpaired two-tailed Student’s t test was used to calculate significance with Prism 6 software.
Histology and Immunohistochemistry.
Mice were euthanized with an overdose of ketamine and perfused with normal saline followed by 4% paraformaldehyde in phosphate-buffered saline (PBS) at pH 7.4. Cochleae were dissected and postfixed in the same solution for 2 h at room temperature. Some cochleae were decalcified (0.1 M EDTA), and embedded in OCT for frozen sectioning and staining. Others were dissected into half-turns for whole-mount staining (Fig. 2B). Immunostaining began with a blocking buffer [PBS with 1% bovine serum albumin (BSA, Sigma), and 1% Triton X-100] for 1 h at room temperature and was followed by incubation with a combination of the following primary antibodies: 1) chicken anti-GFP (AbCam) at 1:1,000; 2) mouse anti-TuJ (Covance) at 1:200; 3) rabbit anti-Myo7a (Proteus) at 1:400; 4) goat anti-ChAT (Chemicon) at 1:200; and 5) rabbit anti-peripherin (AbCam) at 1:500, diluted in blocking buffer with 10% goat serum. Primary incubations were carried out at 4°C overnight, followed by 60-min incubation at room temperature in species-appropriate secondary antibodies (coupled to Alexa Fluor dyes) with 0.1% Triton X-100 and 10% goat serum in PBS. Nuclear staining was performed with DAPI. The immunostaining was analyzed by confocal microscopy.
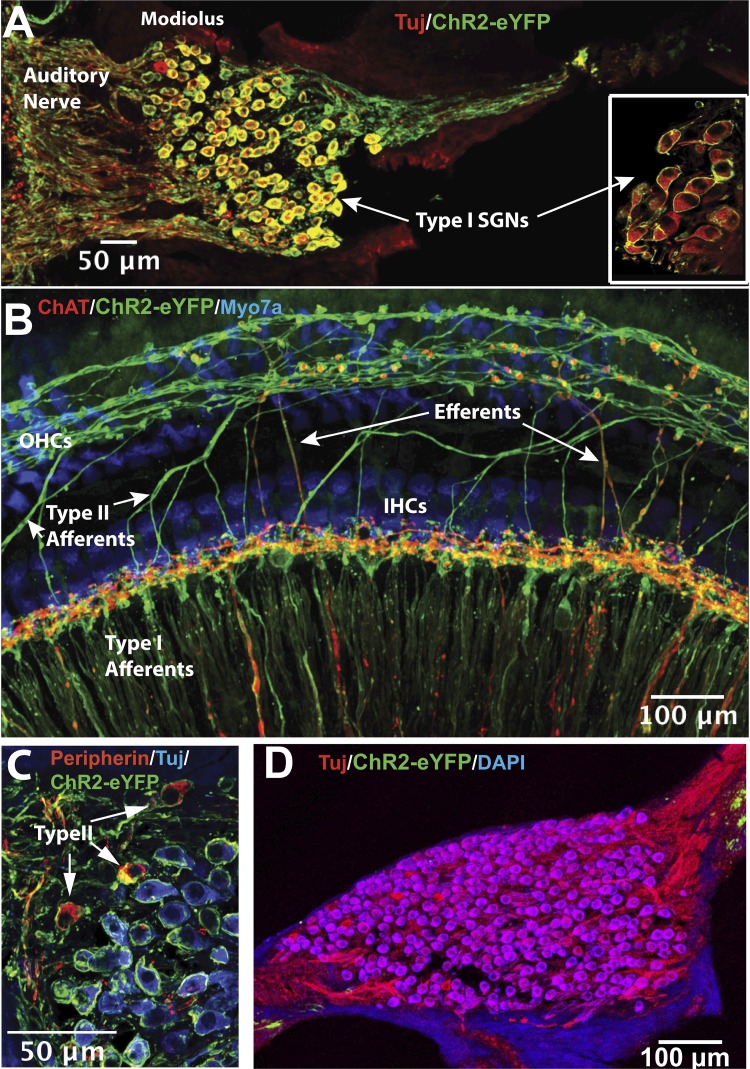
Fig. 2.
Expression pattern of ChR2R-eYFP in the cochlea. A: cross section through the modiolus from a Bhlhb5-ChR2R heterozygous mouse cochlea showing ChR2R-eYFP (anti-GFP in green) expressed in almost all type I spiral ganglion neurons (SGNs; labeled by class III β tubulin antibody, TuJ in red). Yellow shows double labeling. Inset, with similar immunostaining, shows a high-power view of type I SGN cell bodies from a whole-mount of a Bhlhb5-ChR2R homozygous cochlea. B: whole-mount of a portion of the organ of Corti. ChR2R-eYFP (in green) is strongly expressed in radially oriented type I afferent fibers and spirally oriented type II afferent fibers. Expression was also observed in olivocochlear fibers (“Efferents”) near inner hair cells (IHCs), which were labeled by anti-choline acetyltransferase (ChAT, in red). Inner and outer (OHC) hair cells did not show expression (labeled blue by Myo7a antibody). C: cross section of a modiolus from a Bhlhb5-ChR2R heterozygous cochlea showing ChR2R-eYFP (in green) expressed in type I and type II SGNs. Type II SGNs were identified by anti-peripherin staining (red). D: cross section through the modiolus from a control mouse (ChR2R-eYFP+/fl). No ChR2R-eYFP (green) was found in SGNs (TuJ antibody, in red) in these control mice.
Neonatal SGN preparation and whole cell recording.
SGNs were acutely dissected from neonatal mice, ranging in age from postnatal day (P) 2 to P4. After rapid decapitation, cochleae were extracted from the temporal bone and bathed in sterile MEM with GlutaMAX (GIBCO), supplemented with 10 mM HEPES (Sigma-Aldrich) and 25 mg ampicillin (Sigma-Aldrich), pH 7.4. The bony labyrinth surrounding the cochlea was removed to gain access to the whole cochlear turn, and the spiral ligament and stria vascularis were peeled off. The central fiber tracts connecting the SGNs to the cochlear nucleus were carefully severed. No enzymatic treatments were used. The SGN peripheral fibers and the organ of Corti were left relatively intact. SGN explants were then bisected into base and apex in relation to the cochlear tonotopic axis and mounted on glass coverslips (Thermo Fisher Scientific), with the organ of Corti facing up. The sections were pinned under two thin glass fibers glued to the coverslip with Sylgard 184 (Dow Corning). SGN organotypic explants were incubated at 37°C in a humidified incubator (5% CO2) for 1–2 h before use in acute electrophysiological experiments and used within 6 h of dissection.
Recordings were made from SGN cell bodies in voltage- and current-clamp modes. The whole cell, tight-seal patch-clamp configuration was used in all recordings. All electrophysiological recordings were performed at room temperature (22–24°C). A custom-made recording chamber was used and SGN explants were viewed under an Axioskop FS upright microscope (Carl Zeiss) equipped with ×63 water-immersion lens and differential interface contrast optics. A standard external solution was used to bathe the tissue containing (in mM) 137 NaCl, 0.7 NaH2PO4, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 5.6 d-glucose, 10 HEPES, amino acids (1:50; GIBCO), and vitamins (1:100; GIBCO). The pH was adjusted to 7.4 with NaOH and the measured osmolality was 303 mosmol/kg. Recording pipettes (2–4 MΩ) were pulled from R-6 soda lime capillaries (King Precision Glass) using a two-stage vertical pipette puller (PC-10; Narishige) and the tips were coated with ski wax to minimize pipette capacitance. A standard internal solution was used to fill recording pipettes containing (in mM) 135 KCl, 2.5 MgCl2, 2.5 K2-ATP, 5.0 HEPES, 5.0 EGTA, and 0.1 CaCl2, pH 7.4 (KOH), 283 mosmol/kg. All reagents for electrophysiology solution were purchased from Sigma-Aldrich, unless otherwise noted. The hyperpolarization-activated current (Ih) was recorded immediately after the cell membrane was broken through at gigaohm (GΩ) seal and stable membrane currents were reached. Series resistance (Rs) and membrane capacitance (Cm) were corrected. Both parameters were continuously monitored to ensure stable recording. Compensated residual Rs was <7 MΩ on average. The transmembrane currents were recorded using a series of 100-ms voltage steps after the GΩ seal formed.
To examine light-evoked optogenetic responses, a series of 10-ms-long ~473 nm light pulses were presented by a laser (DL473-050-O, CrystaLaser) through an optic fiber (FT200EMT, Thorlabs) coupled with a collimator (CFC-11X-A, Thorlabs). The corrected parallel blue light beam (Ø = ~2.1 mm) was guided to the target cells at the center of the optical field of the microscope. The distance between the end of the collimator output and the center of the optical field of the microscope was fixed and maintained in all the experiments. The laser was calibrated by positioning the collimator end ~2.5 cm from a photodiode sensor (S121C, Thorlabs) connected to a power and energy meter interface (PM100USB, Thorlabs). The photocurrents were acquired with the cells clamped at −80 mV.
Electrophysiological data from SGNs was recorded using an Axopatch200B (Molecular Devices) amplifier in a dark room. Signals were filtered at 1 kHz with a low-pass Bessel filter and digitized at ≥20 kHz using 12-bit acquisition system, Digidata 1332 (Axon Instruments), and pClamp 9.0 (Molecular Devices). All stimulus protocols were generated using pClamp 9.0, and data were stored on a PC. Data were analyzed offline with Clampfit 10.2 and Prism 7 (GraphPad Software).
Lidocaine application and VIIIth cranial nerve ablation.
Two (lidocaine application) and three (cranial nerve VIII ablation) adult homozygous and/or heterozygous mice were anesthetized with ketamine and xylazine and held in a Kopf small-animal stereotaxic apparatus (Tujunga, CA) before surgery (with anesthesia and maintenance, as well as oABR recording performed as described above). After the first oABR recording 20 μL of lidocaine (Hospira, 20 mg/mL) was slowly applied through the cochleostomy in the test ear. The oABRs were monitored continuously from the time of application. For cranial nerve ablation, a craniotomy (diameter ~2.0 mm) was carried out on the posterior skull surface close to the test ear. Partial cerebellar aspiration exposed the dorsal surface of the brainstem with the ampulla of the semicircular canal as a landmark (Darrow et al. 2015). Following the exposure of the auditory meatus, a needle blade was used to cut cranial nerve VIII. A second oABR test was performed after the ablation.
Data analysis.
In whole cell recording analysis, each cell had to pass two criteria to be included: 1) genotyping and 2) occurrence of evoked action potentials by electrical current injection. For photocurrent and photodepolarization analysis, cells were excluded unless the photocurrents evoked by maximum light level (413 μW) were no less than 10 pA. The photocurrent amplitude (IO) was measured from baseline to the extreme of the negative valley on the voltage clamp traces 50 ms after onset of the light pulse (Fig. 3A). Photocurrent density (IO-density) was derived from IO divided by cell capacitance (IO-density = IO/Cm). The photodepolarization (∆VO) was calculated as the magnitude of depolarization of the membrane potential under subthreshold stimulation in 80 ms from baseline to the maximum on the current clamp traces following the light pulse onset (Fig. 3B). The resting membrane potentials (RMPs) were obtained from the first trace when the recording mode was switched to current clamp, usually calculated by the average of voltage in the first 50 ms on the traces. Threshold voltages (Vθ) for firing were defined as the maximal membrane potential excited by a minimal level of current to generate action potentials (Liu and Davis 2007).
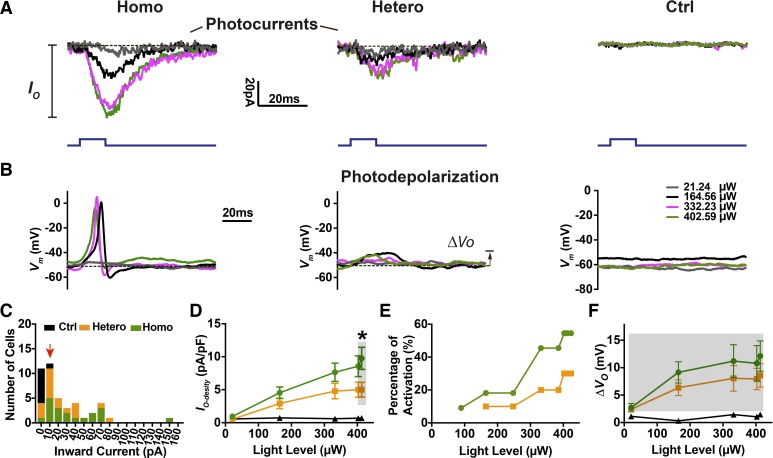
Fig. 3.
Expression-level dependent optogenetic excitation of spiral ganglion neurons (SGNs) in whole cell recordings. Photocurrents (IO; A) and photodepolarization (∆VO; B) were observed in SGNs from homozygous (Homo), heterozygous (Hetero), and control (Ctrl) littermates at postnatal day (P)2 to P4. Light pulses (10 ms; blue traces) were presented to cells at a series of power levels. Dashed lines are representative baselines at the maximum light power level used (413 µW). Vm, membrane potential. C: histogram of photocurrent magnitude distribution at maximum light stimulation. Bin size = 10 pA. The cells included here met the criterion (red arrowhead) of IO ≥ 10 pA. D–F: averaged photocurrent density (IO-density), percentage of SGNs with optically evoked action potentials, and subthreshold photodepolarization (∆VO) as a function of light power level are derived from 15 homozygous, 12 heterozygous, and 8 control cells. Significance was examined by the Mann–Whitney t test. *P < 0.05.
For oABR and cochlear function analysis, mice were selected by the following criteria: 1) normal pure tone ABRs and 2) genotyping. In oABR analysis, amplitudes of oABR peaks were measured from the baseline (Fig. 6C), and the criterion to recognize oABR peak 1 was an amplitude no less than 1 µV (response peaks greater than 1 µV were not seen in the control group) (Fig. 6D). Peak 1 latency was the time difference between light pulse onset and the first response peak. To quantify photosensitivity, and maximal peak 1 amplitudes, the oABR peak 1 amplitude was first plotted against light power levels, and then fitted to the Boltzmann sigmoid equation (Ramekers et al. 2014): Y = Bottom+(Top−Bottom)/{1+exp[(O50−X)/slope)]}, where Y is the oABR peak 1 amplitude, X is the light power level, and Bottom is the background discharge and was constrained to 0. Top is the predicted maximal oABR peak 1 amplitude; O50 represents the light power level to achieve 50% of maximal peak 1 amplitude and is considered an indicator of photosensitivity of the ear.
Latencies (L) and width at half-height of the maximal ABR peak 1 (Whalf) under tested light power levels were assessed and the relevant growth functions were generated by fitting a one phase exponential decay equation, Y = Span*e−K*X+Plateau, where Span represents the initial value, K represents decay rate, and the half-life of the decay (D1/2) was calculated by 0.6932/K, representing light power used to achieve half decay toward the minimum (Plateau).
A custom MATLAB program was used to extract peak amplitudes, latencies, and Whalf from ABR waves. Nonlinear regression and statistical tests were performed in Prism 7. The best-fit values were used in analysis. Data were tested for normality before analysis by paired t test (oABRs) or unpaired t test (whole cell recording data) examining the statistical significance between homozygous and heterozygous groups. In one-way ANOVA analysis, a post hoc two-sample t test was performed using different correction methods (specified in results) for pairwise multiple comparisons when statistically significant interactions were identified. Data are presented as means ± SD unless otherwise noted. In graphs, error bars indicate ±SE.
RESULTS
Bhlhb5-ChR2R-eYFP mice and the expression level of ChR2 in SGNs.
To investigate the optogenetic excitation of SGNs, we developed a novel mouse model by crossing a Bhlhb5-Cre line (Appler et al. 2013; Druckenbrod and Goodrich 2015) with a Cre-dependent, floxed ChR2 line (Ai32; Fig. 1A) (Madisen et al. 2012). We chose the Bhlhb5-Cre line because recombinase activity was SGN specific in the cochlea (Appler et al. 2013), although there was also expression in central nervous system neurons (Cai et al. 2016). The mice were divided into control, ChR2 heterozygous, and ChR2 homozygous groups for all experiments (Fig. 1A). Bhlhb5-Cre mice were kept as heterozygotes because complete loss of the Bhlhb5 gene can cause severe self-inflicted skin lesions (Ross et al. 2010).
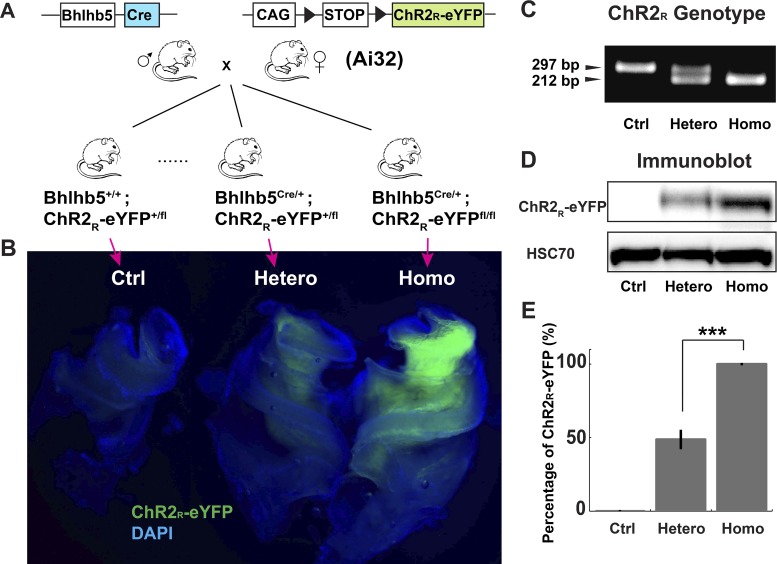
Fig. 1.
Expression levels of ChR2R-eYFP in adult Bhlhb5-ChR2R-eYFP mice. A: conditional expression of ChR2R-eYFP in spiral ganglion neurons (SGNs) is achieved by crossing Bhlhb5Cre/+ mice with ChR2R-eYFP mice. Open reading frames are indicated by the blue box (the recombinase, Cre) and the green box (ChR2R fusion with enhanced yellow fluorescent protein, ChR2R-eYFP). LoxP sites are indicated by the black triangles. B: central cochlear explants from littermates visualized by fluorescence. The control (Ctrl) is the ChR2R-eYFP+/fl mouse, and heterozygous (Hetero) and homozygous (Homo) represent Bhlhb5+/Cre; ChR2R-eYFP +/fl and Bhlhb5+/Cre; ChR2R-eYFPfl/fl, respectively. C: an example of a genotyping gel. D: immunoblot analysis of ChR2R-eYFP from control, heterozygous, and homozygous cochlear explants. Tissue analyzed was from the modiolus (cochlear nerve trunk and spiral ganglion). Blots were also probed with an HSC70-specific antibody as a loading reference. E: quantification of the immunoblots. Error bars represent standard error of the mean. ***P < 0.005. The number of cochlear explants is 5 in each group. The y-axis is the percentage of expression relative to the homozygote.
Differential fluorescence intensities were identified in cochlear explants from control, ChR2 heterozygous, and ChR2 homozygous littermates (Fig. 1B) and were apparently caused by variable expression level of ChR2R-eYFP in the ChR2 genotypes (Fig. 1C). To test this hypothesis, we collected cochlear explants from littermates and examined the ChR2R-eYFP level by Western blot. Significantly higher expression of ChR2R-eYFP was detected from homozygous (n = 5) as compared with heterozygous explants (Fig. 1, D and E, P = 0.0009, n = 5). This was also confirmed in newborn mice (Supplemental Fig. S1; https://zenodo.org/record/3359275#.XUSztJNKgc0). There was an almost twofold greater expression in the homozygous compared with the heterozygous group (Fig. 1E). In summary, the histology and expression results indicate that the Bhlhb5-ChR2R-eYFP mice provide neuron-specific expression of ChR2 across the lifespan and that the genotype correlates with differential expression levels.
ChR2R-eYFP expression was detected in SGNs in the cochlea from heterozygous and homozygous mice. ChR2R-eYFP was seen in SGNs, including both type I (identified by TuJ, Fig. 2A) and type II (identified by peripherin, Fig. 2A), localized in the cell membrane of cell bodies and the central and peripheral processes (Fig. 2A, inset, and Fig. 2C). Expression was also observed in olivocochlear fibers (identified by ChAT immunostaining; Darrow et al. 2006; Guinan 2006), but not in hair cells or other cochlear cell types. ChR2R-eYFP was found throughout apical, middle, and basal regions of the cochlea. Expression was stable from P1 to P5, and at 1, 3, and 7 mo (Supplemental Fig. S2; https://zenodo.org/record/3359275#.XUSztJNKgc0). ChR2R-eYFP was completely absent in the cochlea from control animals (Fig. 2D).
Optogenetic responses from SGNs in Bhlhb5-ChR2R-eYFP mice.
To characterize the optogenetic excitation of SGNs at the cellular level, we tested SGN responses to light using whole cell recording. Significant photocurrents and photodepolarization were observed in most of the homozygous (17 of 21) and heterozygous (13 of 18) SGNs. However, optically evoked action potentials were apparent in only 41.2% (7 of 17) of homozygous SGNs, and 30.8% (4 of 13) of heterozygous SGNs, which may be related to the immature stage of the neonatal mice used. Figure 3, A and B illustrate a typical set of photocurrents and photodepolarizations recorded from SGNs of homozygous, heterozygous, and control littermates. Light pulses induced photocurrents and evoked action potentials at higher rates in homozygous compared with heterozygous neurons; optically evoked action potentials were of similar duration as electrically evoked responses from these immature neurons (see Fig. 4C). Neither photocurrents nor photodepolarization were present in SGNs from control animals. A 10-pA threshold was set as the criterion for identification of genuine SGN photocurrents for inclusion in the following analysis (Fig. 3C). Greater photocurrents did not always evoke more action potentials or photodepolarization in homozygous or heterozygous SGNs (Supplemental Fig. S3; https://zenodo.org/record/3359275#.XUSztJNKgc0). Since the size of SGN cell bodies varies and since larger cell membrane areas can accommodate more transmembrane proteins, we used the photocurrent density and degree of depolarization in our characterization of the optogenetic responses of ChR2R-eYFP SGNs. Photocurrent density and photodepolarization (including fully activated neurons with action potentials) from both groups were light intensity dependent (Fig. 3, D–F). At saturation, homozygous cells showed greater photocurrent density (9.76 ± 6.05 pA/pF at the 413.2 µW level, Mann–Whitney test, P = 0.016) than heterozygous neurons (5.0 ± 3.89 pA/pF), suggesting a significant contribution from the higher level of ChR2 expression. Depolarization via ChR2 (Nagel et al. 2003) induced neuronal excitation in two stages: subthreshold photodepolarization and suprathreshold action potentials. Although there were no significant differences in subthreshold photodepolarization of SGNs between homozygous (12.11 ± 5.53 mV, 413.2 µW light level) and heterozygous (8.59 ± 5.25 mV, Mann–Whitney test, P = 0.476) cells (Fig. 3F), we found a higher probability of optically evoked action potentials in homozygous SGNs at all tested light levels, consistent with the data on photocurrent density.
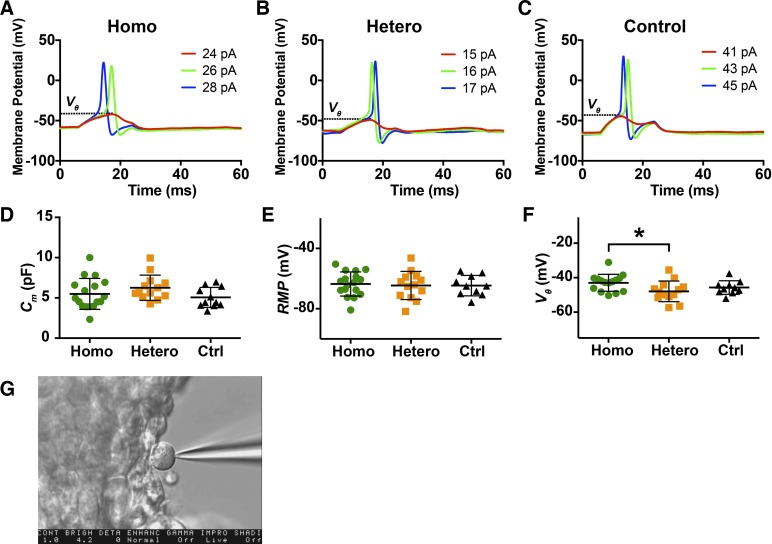
Fig. 4.
Impact of ChR2R expression on electrically evoked action potentials in spiral ganglion neurons (SGNs). A–C: a series of electrical currents were injected to examine the voltage threshold of each cell. The membrane potential traces from homozygous (Homo), heterozygous (Hetero), and control (Ctrl) cells from P2–P4 mice are illustrated. Dashed lines indicate the voltage thresholds (Vθ). D–F: cell capacitances (Cm), resting membrane potentials (RMP), and Vθ recorded from SGNs. Significance was tested by one-way ANOVA. *Significant differences (adjusted P < 0.05). G: illustration of whole cell recording in a single cell.
Influence of ChR2R-eYFP expression on intrinsic properties of SGNs.
To explore the influence of opsin expression level on the intrinsic properties and electrical excitability of SGNs, we examined the Cm, RMP, and threshold voltage (Vθ, Fig. 4, A–C) of newborn SGNs. We found no significant differences in Cm (one-way ANOVA, P = 0.206, Fig. 4D), and RMPs (one-way ANOVA, P = 0.926, Fig. 4E) and a small but statistically significant difference in threshold voltages (one-way ANOVA, P = 0.047, Fig. 4F) among homozygous, heterozygous, and control SGNs. The pairwise comparison after Bonferroni correction showed more depolarized thresholds in homozygous (−43.0 ± 4.92 mV) than in heterozygous cells (−47.95 ± 5.98 mV, adjusted P = 0.0375). The criteria described in methods were applied to all cells to be included in the analysis,
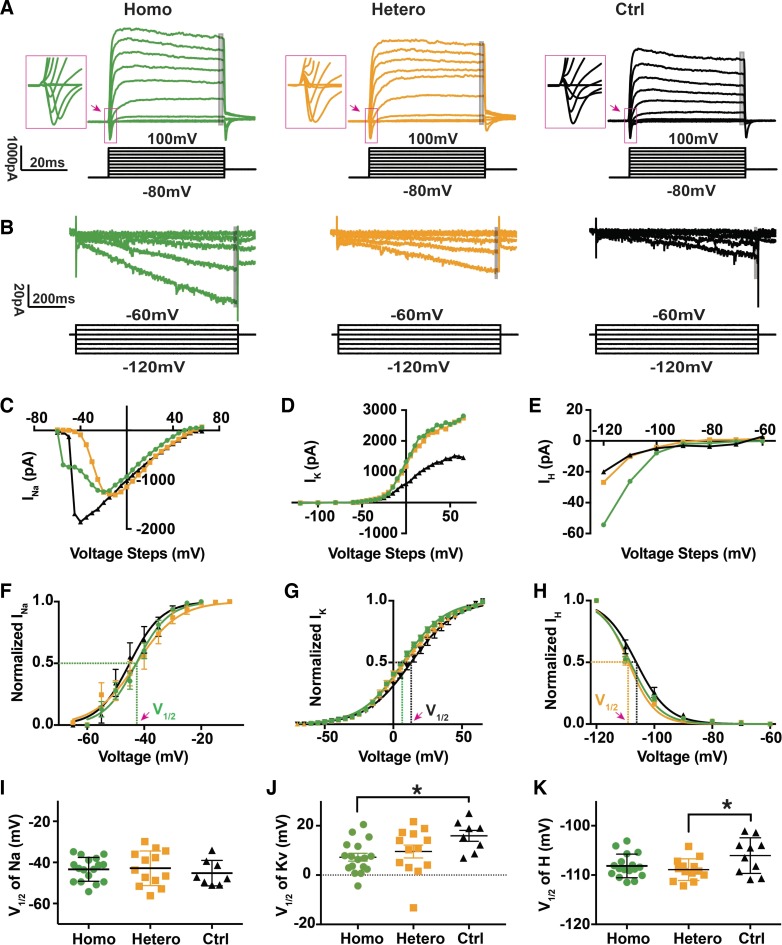
Since threshold voltage is determined by multiple ion channels, mainly voltage-gated potassium (KV) and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels on the SGN membrane (Kim and Holt 2013; Kim and Rutherford 2016; Liu et al. 2014), we examined the function of voltage-gated sodium (NaV), Kv, and HCN channels in all three groups of SGNs. In Fig. 5, we illustrate two sets of representative whole cell currents recorded from homozygous, heterozygous, and control SGNs. In voltage-clamp mode, we observed fast inward Na+ currents and slowly developing K+ currents (Browne et al. 2017; Santos-Sacchi 1993) in all SGNs when holding potentials were varied from −80 to 100 mV (Fig. 5, A, C, and D). Sodium current amplitudes were reduced in the ChR2 expressing neurons. We found that half-activation voltages of Kv were slightly hyperpolarized in homozygous (7.23 ± 6.53 mV, Fig. 5J) compared with control (15.9 ± 6.28 mV, Tukey’s test, adjusted P = 0.031) cells, and intermediate activation voltages in heterozygous cells (9.54 ± 9.42 mV). No significant differences were detected in activation voltages of NaV among homozygous, heterozygous, and control SGNs (Fig. 5I). When we stepped membrane potential to −120 mV, hyperpolarization-activated inward currents appeared in all three types of cells (Fig. 6, B and E). As reported previously, hyperpolarization-activated inward currents are carried by HCN channels (32). Therefore, we calculated the activation range of HCN channels, through normalized current-voltage functions for hyperpolarization applied to all three types of SGNs (Fig. 5H). We found slightly hyperpolarized HCN half-activation voltages in homozygous (−108.2 ± 2.39 mV) and heterozygous (−108.9 ± 2.2 mV, Fig. 5K) cells compared with control (−106.1 ± 3.61 mV, Tukey’s test, adjusted P = 0.042). In summary, we found that while insertion of a Cre sequence in the Bhlhb5 gene open reading frame and ChR2 expression may have caused minor changes in activation range of Kv and HCN channels, whole cell ion channel and action potential firing properties were largely unaffected by ChR2 expression level in SGNs.
Fig. 5.
Effects of differential expression of ChR2R on voltage-activated currents. A: whole cell voltage-clamp recordings of inward and outward currents activated in homozygous, heterozygous, and control spiral ganglion neurons (SGNs) at postnatal day (P)2 to P4 in response to 20-mV voltage steps up to 100 mV from a holding potential of −80 mV. Insets illustrate the relevant inward currents (in pink squares) in high power. Gray area indicates the 2-ms trace where we calculated averaged potassium current. B: whole cell voltage-clamp recordings of inward currents activated in response to 10 mV voltage steps down to −120 mV from a holding potential of −60 mV from the same neurons as in A. Gray area indicates the 30-ms trace where we calculated averaged hyperpolarized inward current. C–E: sodium, potassium, and hyperpolarized inward current magnitudes (INa, IK, and IH, respectively) plotted as a function of voltage levels for individual recordings in the same 3 cells. In a few cases, the fast activating Na currents were too large and evaded voltage control, as apparent in the control current-voltage relation (C). The plateau in K current seen at higher voltages (D) may be influenced by internal Mg ions. F–H: normalized sodium, potassium, and HCN activation curves for the average of all recordings in each group. I–K: averaged half-activation voltage (V1/2) for voltage-gated sodium (NaV, left), potassium (KV, middle), and hyperpolarization-activated cyclic nucleotide-gated (HCN, right) channels from all 3 groups were plotted together. NS, no significant difference. *Significant differences (P < 0.05).
Differential optogenetic auditory brainstem responses in Bhlhb5-ChR2R-eYFP mice.
The differential expression level of ChR2R-eYFP in homozygous versus heterozygous mice permitted us to ask whether the optogenetic response was dependent on expression level. To test this, we examined the oABR from adult mice in response to pulsed blue light provided by an optical fiber guided through a cochleostomy (Fig. 6A). Robust oABRs were elicited in homozygous and heterozygous mice (Fig. 6B). A typical oABR waveform comprised five peaks in the first 8 ms (Fig. 6C). In other experiments, oABR peaks were inhibited or eliminated by either lidocaine application to the inner ear or intracranial ablation of the cranial nerve VIII (Supplemental Fig. S4; https://zenodo.org/record/3359275#.XUSztJNKgc0). This indicated that the oABR peak 1 responses were from the ear and not from other cranial neurons.
In aABRs, peak 1 is generated from the summed activity of the SGNs, and peak 2 results largely from the activity of the ventral cochlear nucleus (Melcher et al. 1996), which represents the postsynaptic response to the auditory nerve. To assess the impact of expression level of ChR2 on optogenetic excitation of the auditory nerve, oABR peaks 1 and 2 were examined across littermates. With increasing light intensity, the amplitudes of all oABR peaks increased, with peak 1 showing sloping saturation (Fig. 6D) and peak 2 showing complete saturation (Fig. 6G). The differences in peak 1 amplitudes between homozygous and heterozygous littermates became significant when light levels exceeded 3.5 mW. Maximal peak 1 amplitudes predicted by Boltzman sigmoid fitting (Fig. 6E) (20.73 ± 2.89 μV, n = 6) in homozygous ears were greater than those (15.24 ± 5.59 μV, n = 6, paired t test, P = 0.047) in heterozygous ears (Fig. 6F). Maximal peak 2 amplitudes (9.97 ± 3.78 µV, n = 6) in homozygous ears were about the same as those (9.16 ± 3.57 µV, n = 6, paired t test, P = 0.706) in heterozygous ears at the maximal tested level (13.1 mW, Fig. 6G) but were significantly higher in homozygous ears for lower light levels (Fig. 6G, at 2.2 mW level, paired t test, P = 0.034). No significant responses were evoked during blue light stimulation in control animals.
We next examined the temporal parameters of peak 1 in the optogenetic excitation of the cochlear nerve in littermates with different ChR2 expression level. We measured the latency (L), the time between the stimulus and the response, and the width at half-height (Whalf). Both parameters of oABR peak 1 decreased exponentially as the light intensity rose, with no significant differences detected in the minimal latencies (Lmin, Fig. 6H and J; in homozygous ears, Lmin = 0.72 ± 0.074 ms, n = 6; in heterozygous ears, Lmin = 0.78 ± 0.091 ms, n = 6; paired t test, P = 0.175) and the minimal Whalf (Whalf-min in Fig. 6I and L; in homozygous ears, Whalf-min = 0.23 ± 0.042 ms, n = 6; in heterozygous ears, Whalf-min = 0.23 ± 0.032 ms, n = 6; paired t test, P = 0.746). The temporal parameters are therefore determined by the intrinsic properties of the opsins, and not the expression level, when the cochlear nerve is fully excited. They were also comparable to the minimal widths at half-height of the acoustic ABR peak 1. However, the light levels at which the nerve reached the half-minimal latencies (D1/2) in homozygous ears (D1/2 = 1.23 ± 0.34 mW, n = 6) were significantly less than those in heterozygous ears (D1/2 = 1.73 ± 0.39 mW, n = 6, paired t test, P = 0.033, Fig. 6K), suggesting stronger excitation of SGNs in ears with greater ChR2 expression when the cochlear nerve was not maximally excited. The light levels that elicited half-minimal Whalf did not differ significantly between homozygous (D1/2 = 0.75 ± 0.31 mW, n = 6) and heterozygous (D1/2 = 2.56 ± 1.98 mW, n = 6, paired t test, P = 0.0897, Fig. 6M) ears, indicating the synchronization of these neurons was not affected by the expression level of ChR2.
Impact of ChR2 expression on cochlear function.
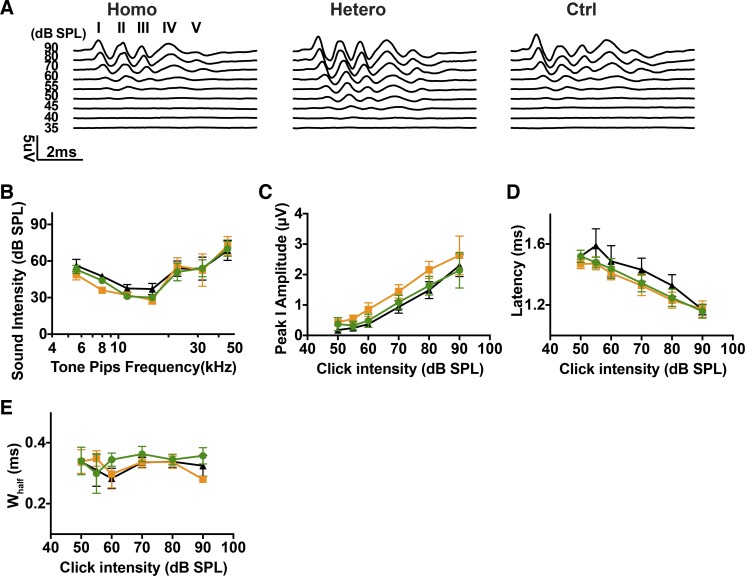
Since heterozygous and homozygous mice have different expression levels of ChR2R-eYFP, we assessed the influence of expression level on auditory nerve physiology. Adult (more than 5 wk old) Bhlhb5-ChR2R-eYFP mice showed normal ABRs, in all but one of six homozygous mice and one of six control mice that was found to have fluid in the middle ear. We tested the response to clicks (Fig. 7A) and tone bursts (Fig. 7B) in homozygous (n = 5), heterozygous (n = 6), and control (n = 5) Bhlhb5-ChR2R-eYFP mice. We observed similar pure tone ABR thresholds among all groups (one-way ANOVA, P = 0.2943, at 16 kHz). Following the pure tone ABR threshold testing, clicks were presented at variable intensity levels and ABRs were measured for all ears. The most prominent peaks in the waveforms were peaks 1 and 2 (Fig. 7A). No significant differences were found in the average ABR peak 1 amplitudes, latencies and width at half-height among all ears at all tested levels (Fig. 7, C–E). Average ABR widths at half-height remained stable throughout the click intensity scan range (Fig. 7E), which averaged ~0.3 ms. These results suggest that ChR2R-eYFP expression has little deleterious effect on the normal ABRs.
Fig. 7.
Acoustic responses in Bhlhb5-ChR2R mice. A: click-evoked auditory brainstem response (ABR) waveforms (I–V) from homozygous (Homo; green), heterozygous (Hetero; orange), and control (Ctrl; black) littermates. B: ABR thresholds for pure tone test from homozygous (n = 5), heterozygous (n = 6), and control (n = 5) mice. C–E: amplitudes, latencies, and widths at half-height (Whalf) of acoustically evoked ABR peak 1 as a function of click intensity. The number of ears from each group was the same as in B.
DISCUSSION
The use of optogenetic excitation for research and clinical application requires precise control of neuronal activity by exogenous opsins. In this study, we successfully generated a mouse line that expressed ChR2R in SGNs and showed that the opsin expression level had a strong influence on in vivo physiology as well as the firing properties of individual SGNs. Specific SGN stimulation was achieved by targeting ChR2R-eYFP expression to Bhlhb5-positive SGNs. Differential expression levels of ChR2R-eYFP were associated with an increase in SGN firing in higher expressing (homozygous) compared with the lower expressing (heterozygous) ears, which translated into increased responsiveness of cochlear nucleus neurons as measured by ABR. A similar increase in action potentials was observed in whole cell patch-clamp recordings but also revealed an influence of ChR2R-eYFP expression on voltage-gated ion channels, indicating a potential effect of high expression levels on sound-evoked cochlear function. In previous studies, optogenetic photosensitivity was surmised from in vitro experiments (Mattis et al. 2012). Here, we provide data from SGNs in vitro and in vivo.
Optogenetic responses depended on expression level.
Bhlhb5 is expressed in a group of neurons within the dorsal horn (Ross et al. 2010) and retina (Feng et al. 2006; Huang et al. 2014), as well as the otic placode from embryonic day 10.5 (Brunelli et al. 2003). We chose the Bhlhb5-Cre because of its stable and cell type-specific expression in the inner ear (Appler et al. 2013). We crossed it with the Ai32 line containing a floxed “STOP” cassette upstream of ChR2R in frame with an eYFP gene. These sequences are targeted to the Rosa26 locus and driven by a strong and ubiquitous CAG promoter (Madisen et al. 2012). The Cre recombinase deleted the floxed “STOP” DNA sequence upstream of ChR2R-eYFP specifically and permanently in SGNs and the CAG promoter resulted in robust expression of ChR2R-eYFP in SGNs of the cochlea as well as efferent neurons that send fibers into the cochlea from the brainstem. ChR2 expression was maintained in SGNs up to at least 7 mo (Supplemental Fig. S2; https://zenodo.org/record/3359275#.XUSztJNKgc0).
Our knock-in Bhlhb5-Cre-LoxP system has the advantage that opsins are expressed from one or both alleles in a consistent genomic environment, allowing reliable comparisons of in vivo performance to be made between the two genotypes. Recently, Hernandez et al. (2014) reported on ChR2 transgenic mice with ChR2 expression in SGNs driven by the Thy1.2 promoter. Compared with Thy1.2-ChR2-YFP mice and viral models, the knock-in Bhlhb5-Cre and flox-STOP-ChR2R-eYFP lines showed stable and specific expression of ChR2R-eYFP in SGNs in both heterozygous and homozygous mice. These responses are mediated by ChR2R, a mutant of wild-type ChR2 with an H134R amino acid substitution (Nagel et al. 2005), which allows cations to flow into SGNs and eventually depolarizes or fires the neuron.
In our Bhlhb5-ChR2R-eYFP model, we observed robust light-evoked inward currents and action potentials as well as subthreshold depolarization in single SGNs, and these translated into measurable oABRs in adult mice. Compared with sound-evoked ABRs, oABRs had greater amplitudes, shorter latencies, and narrower peaks, suggesting greater synchronization to the optical stimulus. Although clicks are temporally brief, the SGN synchrony may be less because apical neurons are activated later due to the traveling wave delay. Although ChR2R induces a larger stationary photocurrent compared with wild-type ChR2 (Nagel et al. 2005), the maximum predicted saturation amplitude of peak 1 in the oABR was 20.7 µV (Fig. 4F), which is consistent with the magnitude of sound-evoked responses without optogenetics and is much smaller than the N1 peaks reported by Hernandez et al. (2014).
Optogenetic responses originating in SGNs highly correlated to expression levels of ChR2R-eYF. Higher expression levels of ChR2R led to greater photocurrent density and a higher probability of firing (Fig. 3, D and E). The effect of ChR2R level on optogenetic excitation was consistent with the increased excitation in cochleae with more ChR2R (homozygous ears) and the greater excitation in SGNs and postsynaptic (cochlear nucleus) neurons (Fig. 4G) at lower light intensity levels. The higher amplitudes in oABR peak 1 from homozygous ears was ascribed to the higher level of ChR2, based on our Western blot data showing that homozygous cochleae expressed more ChR2R-eYFP compared with their littermate counterparts and the increased size of photocurrents per unit size of the cell membrane from homozygous SGNs (Fig. 3D). The expression level will need to be considered in applications where viruses are used to deliver ChR2. Viral delivery is of course highly relevant to the clinical application of channelrhodopsins and has been successfully applied in an animal model of auditory neuropathy (Wrobel et al. 2018), where opsins were expressed in the auditory nerve. However, these studies have not determined the effect of the level of channelrhodopsin, nor have they considered the potential effects of opsin expression on endogenous ion channels. These considerations will be important in applications of channelrhodopsins throughout the nervous system.
Intrinsic properties of auditory neurons.
We saw a difference in the voltage threshold for action potentials between homozygous and heterozygous SGNs. This can be partially explained by the difference in ion channel expression. We also saw a difference in the amplitude of the voltage-gated sodium current but found no difference in the level of activation needed to initiate an action potential, and we observed differences in hyperpolarization-activated and potassium currents (which can modulate the voltage threshold and firing rate). Without pharmacological, immunohistochemical, or mRNA expression characterization, it is not possible to ascertain which specific ion channel activity is altered in the homozygous SGN. One possible explanation for ion channel differences is that higher ChR2 expression in the homozygous SGNs could disrupt expression, trafficking, or insertion of other ion channels into the membrane (Coetzee et al. 1999; Leão et al. 2005; Lin 2011; Trimmer 2015). Even if the expression of pore-forming subunits of the ion channels were not affected, auxiliary subunits, which modulate channel properties, could be altered.
Implications for experimental and clinical use of optogenetics.
Our findings could be useful in the application of optogenetic stimulation for research and for an eventual auditory prosthesis. Current auditory prostheses suffer from spread of electric current (Friesen et al. 2001; Padilla and Landsberger 2016). Ideally, a single electrode should stimulate a small, unique region of the cochlea but, in reality, electric fields from neighboring electrodes overlap and can interfere with one another. For eventual clinical use, several advantages are apparent. The focused beam of the laser might stimulate more narrowly, allowing better presentation of specific frequencies of sound to particular regions of the cochlea and increasing discrimination of speech sounds (Shannon 2012; Srinivasan et al. 2013). Our results show that increasing opsin gene expression increases photocurrents and evoked action potentials of SGNs, enhancing photosensitivity. The excitation level needed is likely to be higher as neurons mature, due to increased K currents and decreased input resistance. Modulating the level of opsins becomes an important variable that must be considered to fine tune an optogenetic response, in attempts to manipulate neural activity via optogenetics.
GRANTS
This project was supported by NIH R21 (DC-012422-02) and NIH R01 (DC-007174).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.M., S.M., M.C.B., J.R.H., D.J.L., and A.S.B.E. conceived and designed research; X.M., S.M., Y.C., J.L., A.E.H., and V.V.K. performed experiments; X.M., S.M., M.C.B., J.R.H., D.J.L., and A.S.B.E. analyzed data; X.M. and A.S.B.E. interpreted results of experiments; X.M. prepared figures; X.M., S.M., and A.S.B.E. drafted manuscript; X.M., M.C.B., J.R.H., D.J.L., and A.S.B.E. edited and revised manuscript; X.M., S.M., Y.C., J.L., A.E.H., V.V.K., M.C.B., J.R.H., D.J.L., and A.S.B.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Leslie Liberman for assistance in cochlear immunostaining and Kenneth Hancock for help with cochlear function tests and optics calibration.
REFERENCES
- Allen BD, Singer AC, Boyden ES. Principles of designing interpretable optogenetic behavior experiments. Learn Mem 22: 232–238, 2015. doi: 10.1101/lm.038026.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appler JM, Lu CC, Druckenbrod NR, Yu WM, Koundakjian EJ, Goodrich LV. Gata3 is a critical regulator of cochlear wiring. J Neurosci 33: 3679–3691, 2013. doi: 10.1523/JNEUROSCI.4703-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayling OG, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods 6: 219–224, 2009. doi: 10.1038/nmeth.1303. [DOI] [PubMed] [Google Scholar]
- Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc Natl Acad Sci USA 108: 7595–7600, 2011. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Browne L, Smith KE, Jagger DJ. Identification of persistent and resurgent sodium currents in spiral ganglion neurons cultured from the mouse cochlea. eneuro 4: ENEURO.0303-17.2017, 2017. doi: 10.1523/eneuro.0303-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S, Innocenzi A, Cossu G. Bhlhb5 is expressed in the CNS and sensory organs during mouse embryonic development. Gene Expr Patterns 3: 755–759, 2003. doi: 10.1016/S1567-133X(03)00135-2. [DOI] [PubMed] [Google Scholar]
- Cai X, Kardon AP, Snyder LM, Kuzirian MS, Minestro S, de Souza L, Rubio ME, Maricich SM, Ross SE. Bhlhb5:flpo allele uncovers a requirement for Bhlhb5 for the development of the dorsal cochlear nucleus. Dev Biol 414: 149–160, 2016. doi: 10.1016/j.ydbio.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc 5: 247–254, 2010. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Morena H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci 868: 233–255, 1999. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback balances interaural sensitivity. Nat Neurosci 9: 1474–1476, 2006. doi: 10.1038/nn1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Slama MC, Kozin ED, Owoc M, Hancock K, Kempfle J, Edge A, Lacour S, Boyden E, Polley D, Brown MC, Lee DJ. Optogenetic stimulation of the cochlear nucleus using channelrhodopsin-2 evokes activity in the central auditory pathways. Brain Res 1599: 44–56, 2015. doi: 10.1016/j.brainres.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawydow A, Gueta R, Ljaschenko D, Ullrich S, Hermann M, Ehmann N, Gao S, Fiala A, Langenhan T, Nagel G, Kittel RJ. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc Natl Acad Sci USA 111: 13972–13977, 2014. doi: 10.1073/pnas.1408269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Hegemann P. The form and function of channelrhodopsin. Science 357: eaan5544, 2017. doi: 10.1126/science.aan5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckenbrod NR, Goodrich LV. Sequential retraction segregates SGN Processes during target selection in the cochlea. J Neurosci 35: 16221–16235, 2015. doi: 10.1523/JNEUROSCI.2236-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development 133: 4815–4825, 2006. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am 110: 1150–1163, 2001. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27: 589–607, 2006. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Hernandez VH, Gehrt A, Reuter K, Jing Z, Jeschke M, Mendoza Schulz A, Hoch G, Bartels M, Vogt G, Garnham CW, Yawo H, Fukazawa Y, Augustine GJ, Bamberg E, Kügler S, Salditt T, de Hoz L, Strenzke N, Moser T. Optogenetic stimulation of the auditory pathway. J Clin Invest 124: 1114–1129, 2014. doi: 10.1172/JCI69050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hight AE, Kozin ED, Darrow K, Lehmann A, Boyden E, Brown MC, Lee DJ. Superior temporal resolution of Chronos versus channelrhodopsin-2 in an optogenetic model of the auditory brainstem implant. Hear Res 322: 235–241, 2015. doi: 10.1016/j.heares.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Hu F, Feng L, Luo XJ, Liang G, Zeng XY, Yi JL, Gan L. Bhlhb5 is required for the subtype development of retinal amacrine and bipolar cells in mice. Dev Dyn 243: 279–289, 2014. doi: 10.1002/dvdy.24067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc Natl Acad Sci USA 110: 3597–3602, 2013. doi: 10.1073/pnas.1219593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke M, Moser T. Considering optogenetic stimulation for cochlear implants. Hear Res 322: 224–234, 2015. doi: 10.1016/j.heares.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Kim KX, Rutherford MA. Maturation of NaV and KV channel topographies in the auditory nerve spike initiator before and after developmental onset of hearing function. J Neurosci 36: 2111–2118, 2016. doi: 10.1523/JNEUROSCI.3437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Holt JR. Functional contributions of HCN channels in the primary auditory neurons of the mouse inner ear. J Gen Physiol 142: 207–223, 2013. doi: 10.1085/jgp.201311019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods 11: 338–346, 2014. [Erratum in Nat Methods 11: 971, 2014.] doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29: 14077–14085, 2009. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von Gersdorff H. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J Neurosci 25: 3724–3738, 2005. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY. A user’s guide to channelrhodopsin variants: features, limitations and future developments. Exp Physiol 96: 19–25, 2011. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci 16: 1499–1508, 2013. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Davis RL. Regional specification of threshold sensitivity and response time in CBA/CaJ mouse spiral ganglion neurons. J Neurophysiol 98: 2215–2222, 2007. doi: 10.1152/jn.00284.2007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lee E, Davis RL. Heterogeneous intrinsic excitability of murine spiral ganglion neurons is determined by Kv1 and HCN channels. Neuroscience 257: 96–110, 2014. doi: 10.1016/j.neuroscience.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ III, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15: 793–802, 2012. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, Yizhar O, Deisseroth K. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods 9: 159–172, 2012. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Guinan JJ Jr, Knudson IM, Kiang NY. Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hear Res 93: 28–51, 1996. doi: 10.1016/0378-5955(95)00179-4. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Shao YR, Chung J, Pourzia O, Feldman DE. Long-term channelrhodopsin-2 (ChR2) expression can induce abnormal axonal morphology and targeting in cerebral cortex. Front Neural Circuits 7: 8, 2013. doi: 10.3389/fncir.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284, 2005. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 100: 13940–13945, 2003. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla M, Landsberger DM. Reduction in spread of excitation from current focusing at multiple cochlear locations in cochlear implant users. Hear Res 333: 98–107, 2016. doi: 10.1016/j.heares.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SF, Grolman W. Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol 15: 187–202, 2014. doi: 10.1007/s10162-013-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA 108: 13305–13310, 2011. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65: 886–898, 2010. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Voltage-dependent ionic conductances of type I spiral ganglion cells from the guinea pig inner ear. J Neurosci 13: 3599–3611, 1993. doi: 10.1523/JNEUROSCI.13-08-03599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV. Advances in auditory prostheses. Curr Opin Neurol 25: 61–66, 2012. doi: 10.1097/WCO.0b013e32834ef878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano T, Fyk-Kolodziej B, Mirza N, Asako M, Tomoda K, Bledsoe S, Pan ZH, Molitor S, Holt AG. Assessment of the AAV-mediated expression of channelrhodopsin-2 and halorhodopsin in brainstem neurons mediating auditory signaling. Brain Res 1511: 138–152, 2013. doi: 10.1016/j.brainres.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan AG, Padilla M, Shannon RV, Landsberger DM. Improving speech perception in noise with current focusing in cochlear implant users. Hear Res 299: 29–36, 2013. doi: 10.1016/j.heares.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS. Subcellular localization of K+ channels in mammalian brain neurons: remarkable precision in the midst of extraordinary complexity. Neuron 85: 238–256, 2015. doi: 10.1016/j.neuron.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, Voss A, Hemmert W. Optogenetic stimulation of the cochlea: a review of mechanisms, measurements, and first models. Network 27: 212–236, 2016. doi: 10.1080/0954898X.2016.1224944. [DOI] [PubMed] [Google Scholar]
- Williams JC, Denison T. From optogenetic technologies to neuromodulation therapies. Sci Transl Med 5: 177ps6, 2013. doi: 10.1126/scitranslmed.3003100. [DOI] [PubMed] [Google Scholar]
- Wrobel C, Dieter A, Huet A, Keppeler D, Duque-Afonso CJ, Vogl C, Hoch G, Jeschke M, Moser T. Optogenetic stimulation of cochlear neurons activates the auditory pathway and restores auditory-driven behavior in deaf adult gerbils. Sci Transl Med 10: eaao0540, 2018. doi: 10.1126/scitranslmed.aao0540. [DOI] [PubMed] [Google Scholar]