Highlights
-
•
Decreased meta-memory processing is related to tauopathy.
-
•
No association was found with amyloid pathology.
-
•
Decreased meta-memory may be a sensitive clinical indicator of AD pathophysiology in the pre-symptomatic phase.
Keywords: Alzheimer's disease, Amyloid, Feeling-of-knowing, Meta-memory, Tau
Abstract
The ability to accurately judge memory efficiency (meta-memory monitoring) for newly learned (episodic) information, is decreased in older adults and even worse in Alzheimer's disease (AD), whereas no differences have been found for semantic meta-memory. The pathological substrates of this phenomenon are poorly understood. Here, we examine the association between meta-memory monitoring for episodic and semantic information to the two major proteinopathies in AD: amyloid (Aβ) and tau pathology in a group of cognitively unimpaired older adults. All participants underwent multi-tracer PET and meta-memory monitoring was assessed using a feeling-of-knowing (FOK) task for non-famous (episodic) and famous (semantic) face-name pairs. Whole brain voxel-wise correlations between meta-memory and PET data were conducted (controlling for memory), as well as confirmatory region-of-interest analyses. Participants had reduced episodic FOK compared to semantic FOK. Decreased episodic FOK was related to tauopathy in the medial temporal lobe regions, including the entorhinal cortex and temporal pole, whereas decreased semantic FOK was related to increased tau in regions associated with the semantic knowledge network. No association was found with Aβ-pathology. Alterations in the ability to accurately judge memory efficiency (in the absence of memory decline) may be a sensitive clinical indicator of AD pathophysiology in the pre-symptomatic phase.
1. Introduction
The ability to accurately monitor and control one's cognitive abilities, known as meta-cognition (Nelson and Narens, 1990), guides much of human behavior. Meta-cognition plays an important role throughout life: in early childhood when we learn new skills, or in old age when cognitive abilities decline. In the early stages of Alzheimer's disease (AD), a time when many individuals begin to experience difficulties with memory, ongoing judgments (self-monitoring) of material in memory can help individuals change and adapt their behavior. This may involve engaging in compensatory or mnemonic strategies (control) that can help them avoid forgetting. Past research suggests that our meta-memory abilities (i.e. the processes involved in memory self-monitoring) are affected by the aging process (Souchay et al., 2007; Morson et al., 2015) and deteriorate further along the prodromal (Perrotin et al., 2007) and AD dementia stages (Souchay et al., 2002a,b; Rosen et al., 2014). In AD, poor meta-cognition also facilitates anosognosia (i.e. impaired awareness of their disease) (Babinski, 1914), a condition with far-reaching clinical implications for the individual. For example, anosognosia is associated with diminished treatment adherence, increased engagement in high-risk situations, and increased caregiver burden (Turro-Garriga et al., 2013; Turro-Garriga et al., 2016). Although there has been extensive work on the behavioral characterization of meta-memory, our knowledge of the pathological substrates underlying meta-memory disturbance in aging and AD remains very limited.
Studies of meta-memory typically ask the person to predict their memory performance on a given task. Their predictions are then compared to their actual memory performance, thus forming an estimate of how well the person is aware of their own memory. One of the most studied meta-memory processes in aging and AD is the feeling-of-knowing, FOK (Hart, 1965; Nelson and Narens, 1990) in which predictions are made about the likelihood of subsequent recognition of currently non-recallable information. FOK judgments can be assessed using either semantic (Hart, 1965; Nelson and Narens, 1990) or recently learned episodic information (Schacter, 1983; Souchay et al., 2000). A large number of studies have confirmed that subjective confidence is predictive of objective memory performance (e.g. Hart, 1965; Freedman and Landauer, 1966; Hart, 1967; Gardiner et al., 1973; Gruneberg and Monks, 1974; Schacter, 1983; Nelson, 1984; Leonesio and Nelson, 1990; Kelemen et al., 2000; Nelson and Narens, 1990). For instance, in two subsequent studies that tested FOK using face-name association tasks, one study by Chua and colleagues demonstrated that young individuals’ FOK predictions on episodic or non-famous faces and names were accurate (Chua et al., 2009), and the other by Hosey and colleagues further demonstrated that FOK predictions on semantic or famous faces and names are accurate in young individuals (Hosey et al., 2009). However, these two meta-memory processes seem to be differentially affected in age and disease. While semantic FOK is preserved in aging (e.g. Bäckman and Karlsson, 1985; Backman and Lipinska, 1993; Lipinska and Bäckman, 1996; Allen-Burge and Storandt, 2000; Marquie and Huet, 2000; Souchay et al., 2007), episodic FOK has been shown to decline with old age (Souchay et al., 2007; Morson et al., 2015) and further decrease in mild cognitive impairment (prodromal stage of AD) (Perrotin et al., 2007; Anderson and Scmitter-Edgecomb, 2010) and AD dementia (Souchay et al., 2003; Souchay and Moulin, 2013; Rosen et al., 2014), although one study showed decreased semantic FOK but not episodic FOK in mild AD (Pappas et al., 1992).
While the pathological basis of altered meta-memory processes remains elusive, recent neuroimaging findings reveal associations with brain regions implicated in internally directed cognition or self-referential processing (Northoff et al., 2006; van der Meer et al., 2010) and self-awareness (Zamboni and Wilcock, 2011), including frontal, lateral temporal, and medial parietal regions. Studies investigating FOK found that less accurate meta-memory in a group of cognitively diverse older adults was associated with reduced cortical thickness in the posterior cingulate and medial prefrontal cortex (Bertrand et al., 2018) as well as in the right insula (Cosentino et al., 2015). Interestingly, these brain regions are vulnerable to the hallmark pathologies of AD, including extracellular amyloid-beta (Aβ) in the association cortex of temporal, parietal, and frontal lobes (Klunk et al., 2004; Engler et al., 2006; Kemppainen et al., 2006; Mintun et al., 2006; Edison et al., 2007; Forsberg et al., 2008). Further, in initial stages of the AD pathophysiologic process (Braak I-III), intraneuronal neurofibrillary tangles (tau) are found concentrated in medial temporal lobe structures (Braak and Braak, 1991, 1995). An important question that remains unsolved is whether early evidence of tau and/or Aβ pathology may be related to changes in meta-memory monitoring in older individuals. Moreover, as previous FOK studies primarily utilized episodic information to investigate FOK, it is unknown whether the pathological substrates underlying deficits in FOK are the same or different for episodic and semantic information.
The present study aimed to answer these questions and extend previous findings by investigating whether there is a behavioral difference in FOK for episodic and semantic information in a group of cognitively unimpaired older adults, as well as by investigating the association of FOK to the two major proteinopathies in AD (Aβ and tau) using multi-tracer PET.
2. Methods and materials
2.1. Participants
A total of 102 cognitively unimpaired older adults from the Harvard Aging Brain Study (HABS) (Dagley et al., 2015) underwent 18F Flortaucipir imaging (FTP-PET) for tau and C11 Pittsburg Compound-B (PiB) PET imaging for Aβ, as well as a functional magnetic resonance imaging (fMRI) task to investigate meta-memory at the Massachusetts General Hospital (MGH) and Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA. Both PET procedures were performed within 6 months of the FOK MRI task [PiB-PET: 0.2 years (S.D. = 0.29), FTP-PET: 0.31 years (S.D. = 0.62)]. Participants completed an extensive neurocognitive testing battery (see (Dagley et al., 2015) for full list of tests), including the selective reminding test (SRT) (Grober et al., 2000) to test episodic memory, and the category fluency test (CAT) (Benton, 1968) to test semantic fluency. Inclusion criteria to participate in the study were: a Mini-Mental State Examination (MMSE) (Folstein et al., 1975) score of 27–30 (inclusive with educational adjustment); and a global Clinical Dementia Rating (CDR) (Morris, 1993) score of 0. Exclusionary criteria were: history of neurologic or major psychiatric disorder, contraindications for MRI; severe cardiovascular disease; alcohol or substance abuse; or known cerebrovascular disease [as determined by a Hachinski Ischemic Score (Rosen et al., 1980) higher than 4 and/or presence of cortical infarct; multiple lacunar strokes; or extensive white matter hyperintensities on structural MRI]. Two participants were removed from the analysis due to having no errors of omission, meaning that no meta-memory data could be analyzed for these participants. Demographic information for remaining individuals is detailed in Table 1. This study was approved by and conducted under the auspices of the Partners Human Research Committee at the BWH and MGH (Boston, MA). Every participant provided informed written consent prior to experimental procedures.
Table 1.
Demographic, clinical and cognitive variables.
| Mean and S.D. | Range | |
|---|---|---|
| Age (years) | 76.1 (6.1) | 64.8–92.3 |
| Gender (%) = F | 63 | NA |
| Education (years) | 16.2 (3.2) | 6–20 |
| CDR Sum-of-Boxes | 0.2 (0.4) | 0–2 |
| MMSE (unadjusted score) | 29.3 (1.1) | 25–30 |
| Depression (GDS score) | 3.8 (3.9) | 0–21 |
| Episodic memory (SRT delayed recall) | 6.7 (3.4) | 0–12 |
| Semantic memory (CAT (n = 97)) | 43.9 (11.6) | 21–80 |
| APOE status (%) (n = 93) = e4+ | 31 | NA |
| Amyloid status (%) = positive | 33 | NA |
| FR (%) Episodic | 0.6 (0.3) | 0–1.0 |
| Semantic | 0.5 (0.3) | 0–1.0 |
| FCR (%) Episodic | 0.5 (0.2) | 0–1.0 |
| Semantic | 0.4 (0.2) | 0.1–0.8 |
Mean and standard deviation (S.D.). CDR = clinical dementia rating; GDS = Geriatric depression scale; MMSE = Mini-Mental State Examination. FR = Free recall, FCR = forced choice recognition; SRT = selective reminding test; CAT = category fluency test.
2.2. Feeling-of-knowing task
The meta-memory task consisted of a modified version of the Recall-Judgment-recognition (RJR) paradigm, originally proposed by Dr. Joseph Hart (Hart, 1965). The task was created as an event-related fMRI task. Given that this study focused on the FOK behavioral responses in relation to PET data, only details of the FOK task itself will be presented here. The RJR paradigm was a face–name association task executed in consecutive phases: encoding (for episodic stimuli) and recall (both episodic and semantic stimuli) was performed in the scanner, and judgment and recognition (both episodic and semantic stimuli) was performed outside the scanner.
Episodic stimuli consisted of 75 face photographs of non-famous individuals, with a fictional first name printed in white Times New Roman 36-point font underneath. During the encoding phase, participants were explicitly told to try and remember the name associated with each face and indicate with a button press whether they thought the name was a good fit, not a good fit, or if they were uncertain. This was a purely subjective decision. The rating of how well a name fits to a face has been shown to enhance encoding processes (Sperling et al., 2003). The experiment consisted of three encoding runs. In each run, participants viewed 25 face-name pair stimuli, each shown for 2.75 s , and which were presented in a pseudorandom order in groups of five face-name pairs. Each of the 25 face-name pairs were repeated twice (with a mean delay of 15 s between first and second presentations) during the encoding task. Face-name pairs were randomly intermixed with fixation trials of a white crosshair (+) centered on a black background. Participants were told to focus their attention on the fixation cross during presentation of the crosshair. Each encoding run lasted for 5.7 min.
After each encoding run, participants were asked to recall the names for these previously (n = 25) encoded items as well as recall the names of (n = 25) famous people (recall phase). In total, there were 3 recall phases, and the total amount of semantic stimuli consisted of 75 pictures of face photographs of famous people. Each face was presented for 4 s and the participant was instructed to try and recall the first name of the person within the presentation time, and press a button as quickly as possible to indicate whether they remembered or did not remember each stimuli.
Outside the scanner, all the non-remembered face stimuli (both famous and non-famous) were presented again to the participants, and they were then asked to judge the likelihood of recognizing the name (feeling-of-knowing, FOK) on an upcoming test (judgment phase). They ranked their degree of FOK using one of 3 levels from 1 (maximum degree of FOK) to 3 (minimum degree of FOK). We tested the accuracy of participant's FOK using a forced-choice-recognition (FCR) test with three-alternatives as well as a ‘don't know’ option for all the FOK trials (recognition phase). For the recalled trials, the participants had a free recall (FR) test of each face image. The behavioral outcome (FR and FCR) of the task are presented in Table 1.
The paradigm was designed and generated on an external (Macbook pro®) computer using JAVA software. Responses were collected using an MR compatible fiber-optical key press device with 3 buttons held in the right hand, and responses were recorded by a computer interfaced with the optical switch outside the scanner room. Participants received detailed oral instructions prior to each run and completed a practice session both inside and outside of the MR-scanner before the experiment began.
2.3. Relative accuracy of judgments
To investigate whether participants could discriminate between their FOK responses, a Goodman–Kruskal gamma was calculated for each participant, which indicates the strength of association between ordinal variables by examining item-by-item associations between (FOK) predictions and memory (FCR) performance. A score approaching −1 indicates discordance between prediction and outcome, whereas scores approaching +1 indicate concordance.
2.4. PET acquisition
All PET imaging took place at the PET facility at MGH and acquired as previously described (Johnson et al., 2007; Gomperts et al., 2008; Rentz et al., 2010) with a Siemens/CTI ECAT HR scanner. Synthesis, preparation and administration of the PET tracers were done according to MGH Radioactive Drug Research Committee-approved protocols (Shoup et al., 2013).
For measuring Aβ deposition, acquisition of Pittsburgh Compund-B (PiB)-PET imaging data was done following a transmission scan, where a 10–15 mCi 11C-PiB bolus was injected intravenously, immediately followed by a 60-min dynamic PET scan in 3-D mode (63 image planes, 15.2 cm axial field of view, 5.6 mm transaxial resolution and 2.4 mm slice interval; 69 frames: 12 × 15 s, 57 × 60 s).
For measuring tau, acquisition of Flortaucipir (FTP)-PET (also known as 18 F-labeled AV1451 or T807) was done using a 3D list mode dynamic protocol on the PET camera described above. Images were acquired from 80 to 100 min in 4 × 5-min frames after a mean (SD) bolus injection of 10.0 (1.0) mCi.
All PET data were reconstructed, attenuation-corrected, evaluated for head motion, and co-registered to the corresponding T1 image for each participant using 6 df rigid body registration. For PiB-PET and FTP-PET, cerebellar gray matter was used as the reference region from the FreeSurfer Desikan-Killiany atlas as previously described (Becker et al., 2011; Johnson et al., 2016). FTP-PET measures were computed as the standardized uptake value ratios (SUVRs) and for PiB-PET, a summary distribution volume ratio (DVR) was used. For PiB-PET and FTP-PET surface analyses, we performed a partial volume correction by using the Müller-Gartner correction, estimating the signal in each grey matter voxel, as implemented in FreeSurfer software (version 6.0; https://surfer.nmr.mgh.harvard.edu/). For confirmatory Regions-of-Interest (ROI) analyses, we performed partial volume correction on PET data by using the geometric transform matrix method (Rousset et al., 1998) as implemented in FreeSurfer software (version 6.0; https://surfer.nmr.mgh.harvard.edu/) and described in (Greve et al., 2016), providing one mean binding value per region. Partial volume correction processing was performed assuming a uniform 6-mm point spread function.
Neocortical PiB retention was assessed using a large cortical ROI aggregate that included frontal, lateral temporal and retrosplenial cortices (FLR) and used as a variable when investigating the contribution of amyloid to the association between meta-memory and tau pathology.
2.5. Statistical analyses
One sample t-tests were used to test whether the mean for episodic respectively semantic FOK were significantly different from zero, indicating whether meta-memory monitoring was above chance accuracy or not. A paired sample t-tests was performed to test whether there was a behavioral difference for episodic and semantic FOK in the individuals. Pearson correlation was used to investigate the relationship between episodic and semantic FOK, as well as the association between FOK with demographic and clinical variables.
Whole-brain vertex-vise correlations were used to determine meta-memory-associated tau and Aβ deposition separately for episodic or semantic FOK. The maps were created by taking the participants’ image-mapping native PET images to the fs average surface in FreeSurfer and smoothing with the equivalent of an 8 mm Gaussian kernel. An uncorrected threshold (p < 0.01) was applied to the maps, and maps were first unadjusted, and then adjusted for age, education, and memory performance. Additional analyses were also performed adjusting for either meta-memory or amyloid pathology in the maps.
Analyses were conducted using MATLAB (generalized linear model scripts can be accessed at http://mrtools.mgh.harvard.edu/).
To confirm the results found in the surface based analyses we performed sensitivity analyses using an ROI approach: using the average FTP SUVR within individual corresponding Desikan–Killiany atlas regions (using FreeSurfer) that overlapped with regions identified in the previous exploratory whole-brain map approach. A series of linear regressions were then conducted to determine the influence of tau on meta-memory using these independent Desikan–Killiany regions. These models were both unadjusted and adjusted for age, education, and memory performance. Multiple comparisons for these models were accounted for using a Bonferroni correction method.
3. Results
3.1. Relative accuracy of judgments
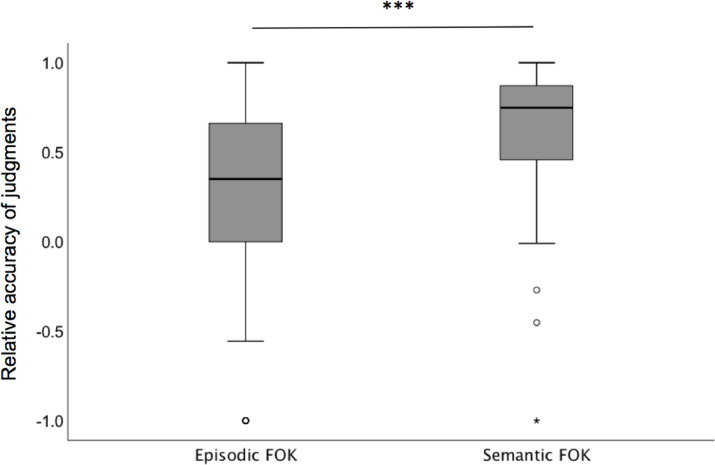
We found that the mean for both episodic and semantic FOK showed overall concordance (Fig. 1). Both were significantly greater than zero (episodic FOK, t99 = −4.7, p < 0.001 and semantic FOK t99 = −16.1, p < 0.001) indicating that meta-memory monitoring was above chance accuracy. However, a paired sample t-test demonstrated that participants were significantly better at meta-memory monitoring for semantic relative to episodic information (t99 = 6.8, p < 0.001).
Fig. 1.
Relative accuracy of FOK judgements for episodic and semantic information
A significant association was found between episodic and semantic FOK (r = 0.38, p < 0.001), indicating that decreased resolution of the episodic FOK was related to decreased resolution of the semantic FOK.
The mean of both episodic and semantic FOK showed overall concordance. A one sample t-test demonstrated that both meta-memory processes were above chance. A paired sample t-test however demonstrated that meta-memory was better for semantic as compared to episodic information, *** = p < 0.001.
3.2. Association of FOK with demographics and clinical variables
Meta-memory monitoring for episodic information was not significantly associated with education (r = 0.12, p = 0.12), episodic memory (using the SRT delayed recall) (r = 0.16, p = 0.12) or semantic memory (using CAT fluency task) (r = 0.15, p = 0.13). There was no sex difference in meta-memory for episodic information (t98 = 0.35, p = 0.73). However, we did find a significant association between meta-memory for episodic information and age (r = -0.23, p = 0.03) as well as MMSE score (r = 0.28, p = 0.004), such that older age as well as lower MMSE scores were related to lower resolution of the episodic gamma score. Similarly, meta-memory monitoring for semantic information was not significantly associated with semantic memory (using the CAT fluency task) (r = 0.05, p = 0.61), episodic memory (using SRT delayed recall) (r = 0.01, p = 0.92), age (r = −0.02, p = 0.82), or MMSE scores (r = 0.19, p = 0.06). There was no sex difference in meta-memory monitoring for semantic information (t98 = −1.51, p = 0.14). We found a significant association with education (r = 0.25, p = 0.01), such that individuals with higher education had better resolution of the semantic gamma score.
3.3. Association between meta-memory and amyloid pathology
Using a whole brain voxel-vise analysis we did not observe any significant association for either episodic or semantic FOK and PiB-PET binding.
3.4. Association between episodic meta-memory and tau pathology
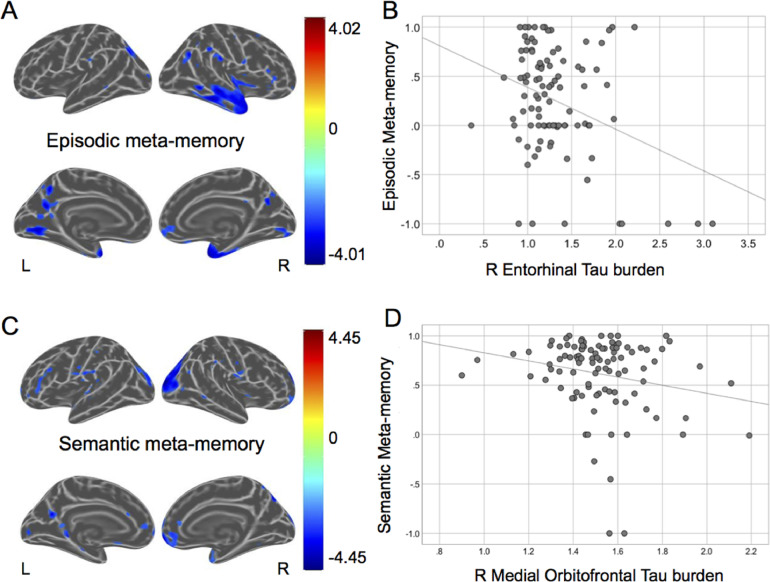
We found that lower meta-memory monitoring for episodic information was related to greater FTP binding in medial temporal lobe regions including the temporal pole and entorhinal cortex, in addition to the superior temporal cortex (Fig. 2A and B). These effects remained significant after controlling for age, education, accuracy on the FCR task, and accuracy on an episodic memory task (SRT delayed recall; Supplemental Figure 1 A). No significant positive associations between meta-memory monitoring for episodic information and FTP-PET binding were found.
Fig. 2.
The association between tau pathology and meta-memory accuracy.
Whole brain vertex-wise analyses visualizing the association between greater FTP-PET binding and lower meta-memory for episodic (a) and semantic (c) information. Maps are unadjusted and corrected at p = 0.01. The color bar represents t-values. Scatterplots showing the association between greater FTP-PET binding and lower meta-memory for episodic (b) and semantic (d) information in representative regions-of-interests.
3.5. Controlling for semantic meta-memory
To investigate whether association between episodic meta-memory and tau was independent of semantic meta-memory, we ran additional whole-brain vertex-wise analyses controlling for semantic meta-memory. Our findings remained the same (see Supplemental Fig. 1 B) such that lower meta-memory monitoring for episodic information was related to greater FTP binding in the medial temporal lobe regions, the temporal pole and superior temporal cortex.
3.6. Investigating the contribution of amyloid pathology on the association between episodic meta-memory and tau pathology
To further investigate the contribution of amyloid pathology on our findings, we ran a whole-brain vertex-wise analyses controlling for amyloid pathology from an aggregate region (see methods). Our findings remained the same (see Supplemental Fig. 1 C) such that lower meta-memory monitoring for episodic information was related to greater FTP binding in the medial temporal lobe regions, including the temporal pole and superior temporal cortex.
3.7. Association between semantic meta-memory and tau pathology
We found that lower semantic FOK was related to greater FTP binding in several neocortical brain regions including the superior parietal lobe and inferior parietal lobe such as the angular gyrus (Fig. 2C and D). The temporal and medial orbitofrontal cortex was also significantly associated with greater FTP-PET binding. These effects remained significant after controlling for age, education, accuracy on the FCR task, and accuracy on an semantic memory task (category fluency; Supplemental Fig. 1 A. No significant positive associations were found between meta-memory monitoring for semantic information and FTP-PET binding.
3.8. Controlling for episodic meta-memory
To investigate whether the association between semantic meta-memory and tau was independent of episodic meta-memory, we ran additional whole-brain vertex-wise analyses controlling for semantic meta-memory. Our findings remained largely the same (see Supplemental Fig. 1 B) such that lower meta-memory monitoring for semantic information was related to greater FTP binding in the superior parietal lobe and inferior parietal lobe. The findings in the temporal and medial orbitofrontal cortex demonstrated reduced spatial extent as compared to our original maps.
3.9. Investigating the contribution of amyloid pathology on the association between semantic meta-memory and tau pathology
To further investigate the contribution of amyloid pathology on our findings, we ran a whole-brain vertex-wise analyses controlling for amyloid pathology from an aggregate region (see Methods). Again, our findings remained largely the same (see Supplemental Fig. 1 C). In particular, lower meta-memory monitoring for episodic information was related to greater FTP binding in the superior parietal lobe and inferior parietal lobe.
3.10. Regions-of-interest analysis
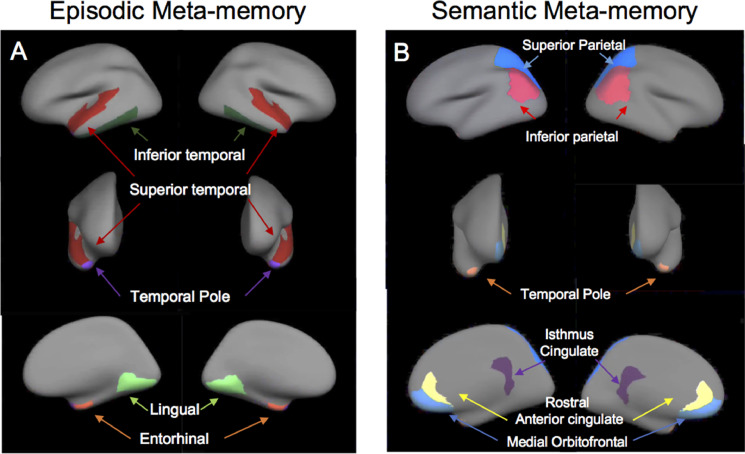
Confirmatory ROI analyses were performed by extracting the tau signal within individual corresponding Desikan–Killiany atlas regions (using FreeSurfer) that overlapped with regions identified in the whole-brain map approach (see Fig. 3A and B).
Fig. 3.
Regions-of-interests.
We could confirm the negative association found between meta-memory for episodic information and FTP binding in regions in the right hemisphere including the temporal pole, right entorhinal cortex, and right superior temporal cortex (see example in Fig. 2B). An association between FTP-PET binding in the right inferior temporal cortex and meta-memory for episodic information was just above the corrected p-value threshold (Table 2).
Table 2.
Association between FOK and tau pathology in regions-of-interest.
| Region-of-interest | B | SE B | ß | t | p |
|---|---|---|---|---|---|
| Episodic FOK | |||||
| Temporal pole BH | −0.83 | 0.25 | −0.33 | −3.28 | 0.001 |
| RH | −0.54 | 0.17 | −0.32 | −3.15 | 0.002 |
| LH | −0.59 | 0.26 | −0.23 | −2.27 | 0.025 |
| Entorhinal BH | −0.37 | 0.17 | −0.24 | −2.14 | 0.035 |
| RH | −0.40 | 0.13 | −0.32 | −2.99 | 0.004 |
| LH | −0.16 | 0.18 | −0.12 | −0.87 | 0.39 |
| Superior temporal BH | −0.71 | 0.43 | −0.18 | −1.65 | 0.10 |
| RH | −0.97 | 0.37 | −0.27 | −2.67 | 0.009 |
| LH | −0.16 | 0.38 | −0.05 | −0.43 | 0.67 |
| Inferior temporal BH | −0.39 | 0.30 | −0.15 | −1.29 | 0.20 |
| RH | −0.61 | 0.24 | −0.28 | −2.52 | 0.013 |
| LH | 0.05 | 0.29 | 0.02 | 0.19 | 0.85 |
| Lingual | −0.64 | 0.42 | −0.17 | −1.52 | 0.13 |
| RH | −0.25 | 0.39 | −0.07 | −0.65 | 0.52 |
| LH | −0.73 | 0.35 | −0.22 | −2.1 | 0.04 |
| Semantic FOK | |||||
| Temporal pole BH | −0.40 | 0.18 | −0.23 | −2.23 | 0.025 |
| RH | −0.31 | 0.12 | −0.26 | −2.67 | 0.009 |
| LH | −0.15 | 0.19 | −0.08 | −0.78 | 0.44 |
| Inferior parietal BH | −0.43 | 0.27 | −0.17 | −1.58 | 0.12 |
| RH | −0.34 | 0.25 | −0.15 | −1.36 | 0.18 |
| LH | −0.38 | 0.26 | −0.16 | −1.47 | 0.14 |
| Superior parietal BH | −0.33 | 0.23 | −0.15 | −1.41 | 0.16 |
| RH | −0.52 | 0.19 | −0.26 | −2.59 | 0.01 |
| LH | −0.01 | 0.22 | −0.01 | −0.06 | 0.95 |
| Isthmus cingulate BH | −0.45 | 0.27 | −0.18 | −1.65 | 0.10 |
| RH | −0.13 | 0.24 | −0.06 | −0.53 | 0.59 |
| LH | −0.59 | 0.25 | −0.25 | −2.40 | 0.018 |
| Rostral anterior cingulate BH | −0.43 | 0.19 | −0.22 | −2.16 | 0.033 |
| RH | −0.39 | 0.19 | −0.21 | −2.11 | 0.037 |
| LH | −0.38 | 0.19 | −0.21 | −2.03 | 0.045 |
| Medial orbitofrontal BH | −0.45 | 0.22 | −0.21 | −2.02 | 0.046 |
| RH | −0.49 | 0.20 | −0.24 | −2.43 | 0.017 |
| −0.29 | 0.21 | −0.14 | −1.37 | 0.17 |
Linear regression models are adjusted for age, education, and memory performance. Multiple comparisons were accounted for using Bonferroni corrected a of 0.01 (for episodic FOK) respectively 0.0083 (for semantic FOK). BH =Bilateral hemispheres, RH = Right hemisphere, LH = Left hemisphere.
Maps showing the Desikan–Killiany atlas regions (using FreeSurfer) used in the regions-of-interests analyses for episodic (A) and semantic (B) FOK.
The ROI analyses for meta-memory for semantic information revealed weak relationships between FOK and FTP-PET binding across 6 ROIs. While none survived adjusting for multiple comparisons, an association with FTP-PET binding in the right temporal pole was just above the significance threshold (Table 2).
When we also conducted the episodic FOK models while correcting for semantic FOK, the association with FTP binding in the temporal pole remained significant (beta = -0.25, p =0.01. No semantic FOK model remained significant after adjusting for episodic FOK.
4. Discussion
This work investigated the association between meta-memory monitoring for episodic and semantic information to the two major proteinopathies in AD: amyloid (Aβ) and tau pathology in a group of cognitively unimpaired older adults. We found that while older adults were overall concordant in their FOK accuracy, they displayed reduced accuracy of FOK when predictions were made on an episodic task as compared to when predictions were made on a semantic task. Furthermore, lower feeling-of-knowing for both episodic and semantic information were related to tauopathy, but in different brain regions. The different spatial patterns in the association between episodic and semantic FOK with tau pathology may highlight the relative contributions of episodic and semantic processes to the genesis of FOK. We found no associations with Aβ pathology.
Overall, these findings may help to better understand the mechanisms underlying meta-memory deficits in aging and AD.
Consistent with previous findings we found that older adults have preserved overall accuracy of FOK, but that older adults have reduced FOK accuracy when predictions are made on an episodic task as compared to FOK accuracy when predictions are made on a semantic task (Souchay et al., 2007). This dissociation has been proposed to be a result from the fact that episodic and semantic FOK draw upon different aspects of inferential processing (Souchay et al., 2007). That is, according to Koriat (1993), when a participant is unable to answer a question, the FOK judgment will be based on partial information accessed during the search for the right answer (Koriat, 1993). As a consequence, the accuracy of the FOK depends on the overall accuracy of the partial information (Koriat, 1993), providing a feeling of recollection. Previous studies investigating episodic memory have found that older adults fail to retrieve contextual information (Craik and Salthouse, 1992), and in an elegant study by Souchay and colleagues (2007), these findings were extended to include episodic FOK (Souchay et al., 2007). Specifically, using a task design where they compared FOK in tasks which did (episodic FOK) and did not (semantic FOK) rely on proficiency of recollection, they only found an age effect on FOK accuracy when predictions were made on an episodic memory task but not on a semantic task (Souchay et al., 2007). In a second experiment, they examined the relationship between FOK accuracy and the availability of contextual cues and partial information available during examination as indexed by ‘remembering’(Souchay et al., 2007). They found that participants who made more accurate FOK predictions prior to the test phase made more recognition judgments on the basis of remembering. The authors concluded that this demonstrates ‘a failure of metacognitive processes at retrieval, not encoding, that characterizes the aging deficits in meta-cognition.’
Our findings demonstrate that meta-memory for episodic respectively semantic information were related to tauopathy, but not Aβ-pathology, in different brain regions. Specifically, we found that deficits in metacognitive episodic knowledge were related to increased tau burden in episodic memory-related brain regions, brain regions vulnerable to pathological changes in early AD. Results remained whilst controlling for age, memory performance (overall accuracy on the task & SRT dr), and even semantic FOK. In contrast, we found that decreased semantic meta-memory was related to increased tau burden in the temporal pole (similar as the episodic FOK) but also tau in regions associated with the semantic knowledge network. This later finding, in contrast to episodic meta-memory, suggests that the association between tau and meta-memory for semantic information is expanding beyond Braak stages I-II. However, the association between semantic FOK and tau burden in these regions did not survive in the ROI analyses after correction for multiple comparisons. These findings can be interpreted in regard to Koriat's (1993) accessibility model, in which FOK judgments are based on the amount of information produced during retrieval. In this view, awareness of memory requires the retrieval of memory either fully or partially, which gives rise to a subjective state of consciousness such as recalling. Moreover, retrieval of episodic information has been associated with increased activation in the MTL regions, including the hippocampus e.g., (Yonelinas et al., 2005). Our findings may indicate that tau pathology in the MTL regions, especially in the entorhinal cortex, might influence an individual's judgment and reduce their accuracy for episodic FOK. That is, we speculate that older adults harboring MTL tau pathology may have particular difficulties in accessing or utilizing cues from retrieval attempts to accurately predict future recognition. Furthermore, the magnitude of tau-pathology maybe also be an important contributor to the observed behavioral deficit, as increased tau pathology was observed to be related to decreased meta-memory for episodic information (see Fig. 2B). In addition, the differential age effects between episodic and semantic FOK reported previously and also observed in the current study, might actually reflect differential amounts of underlying tau pathology. The fact that this effect remained significant even after adjusting for episodic memory performance (overall accuracy on the task & SRT dr) suggests that this ‘state of consciousness’ is separate from the process of recalling. The fact that we did not see the same pattern for semantic FOK supports the idea that FOK for semantic and episodic memory relies on different partial information (Yonelinas and Jacoby, 1995; Souchay et al., 2002b). An alternative interpretation of these findings is that it is conceivable that the functionality of the memory systems is differentially impaired, such that neurodegenerative process have already affected the different memory system.
5. Limitations
We acknowledge that there are several limitations to this study. The focus of this study was to investigate the pathological correlates of FOK. However, future research is needed to investigate whether different measurements of meta-cognition e.g. judgment-of-learning, show different underlying cognitive processes and consequently different brain correlates. In addition, the current study only investigated cognitively unimpaired older adults. Future studies should investigate whether these results are similar or change in individuals with mild cognitively impairment or AD, as meta-cognitive deficits may increase in magnitude.
In conclusion, we found that older adults had reduced accuracy of FOK when predictions were made on episodic information as compared to when FOK predictions were made on semantic information. Our findings may help define and distinguish pathological substrates underlying metacognitive monitoring of episodic and semantic memory. Overall, these findings may help to better understand the mechanism underlying meta-memory deficits in aging and ultimately unawareness (anosognosia) in Alzheimer's disease.
Financial disclosures
Dr. Rentz has served as a consultant for Eli Lilly, Biogen, and Janssen Pharmaceutical, and sits on the Scientific Advisory Board for Neurotrack.
Dr Johnson has served as paid consultant for Bayer, GE Healthcare, Janssen Alzheimer's Immunotherapy, Siemens Medical Solutions, Genzyme, Novartis, Biogen, Roche, ISIS Pharma, AZTherapies, GEHC, Lundberg, and AbbVie. He is a site co-investigator for Lilly/Avid, Pfizer, Janssen, and Navidea.
Dr. Sperling has served as a paid consultant for AbbVie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. She has served as a co-investigator for Avid, Eli Lilly, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen. She receives research support from Janssen and Eli Lilly.
CRediT authorship contribution statement
Patrizia Vannini: Conceptualization, Formal analysis, Writing - original draft. Federico d'Oleire Uquillas: Conceptualization, Formal analysis, Writing - original draft. Heidi I.L. Jacobs: Conceptualization, Formal analysis, Writing - original draft. Jorge Sepulcre: Writing - review & editing. Jennifer Gatchel: Writing - review & editing. Rebecca E. Amariglio: Writing - review & editing. Bernard Hanseeuw: Writing - review & editing. Kathryn V. Papp: Writing - review & editing. Trey Hedden: Writing - review & editing. Dorene M. Rentz: Writing - review & editing. Alvaro Pascual-Leone: Writing - review & editing. Keith A. Johnson: Writing - review & editing. Reisa. A. Sperling: Writing - review & editing.
Declaration of competing interest
None.
Acknowledgements
This research was carried out in whole or in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Regional Resource supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. Dr. P. Vannini received funding from NIH-NIA grant K01AG048287. Additional funding came from NIA P01 AG036694 to R.A.S. and K.A.J., K24 AG035007 to R.A.S as well as the Alzheimer's Association (IIRG-06-27374 to R.A.S.). H.I.L.J. received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie Grant agreement (IF-2015-GF, 706714). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary material associated with this article can be found, in the online version, at 10.1016/j.nicl.2019.102097.
Appendix. Supplementary Materials
References
- Allen-Burge R., Storandt M. Age equivalence in feeling-of-knowing experiences. J. Gerontol. B Psychol. Sci. Soc. Sci. 2000;55:214–223. doi: 10.1093/geronb/55.4.p214. [DOI] [PubMed] [Google Scholar]
- Anderson J.W., Edgecomb S. Mild cognitive impairment and feeling-of-knowing in episodic memory. J. Clin. Exp. Neuropsychol. 2010;32:505–514. doi: 10.1080/13803390903224944. [DOI] [PubMed] [Google Scholar]
- Babinski M.J. Contibution a l’etudedes troubles mantaux dans l’hemiplegie organique cerebrale (Anasognosie) Rev. Neurol. 1914;12:845–848. [Google Scholar]
- Bäckman L., Karlsson T. The relation between level of general knowledge and feeling-of-knowing: an adult age study. Scand. J. Psychol. 1985;26:249–258. doi: 10.1111/j.1467-9450.1985.tb01162.x. [DOI] [PubMed] [Google Scholar]
- Backman L., Lipinska L. Monitoring of general knowledge: evidence for preservation in early Alzheimer's disease. Neuropsychologia. 1993;31:335–345. doi: 10.1016/0028-3932(93)90157-u. [DOI] [PubMed] [Google Scholar]
- Becker J.A., Hedden T., Carmasin J., Maye J., Rentz D.M., Putcha D., Fischl B., Greve D.N., Marshall G.A., Salloway S., Marks D., Buckner R.L., Sperling R.A., Johnson K.A. Amyloid-β associated cortical thinning in clinically normal elderly. Ann. Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A.L. Differential behavioural effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Bertrand E., Azar M., Rizvi B., Brickman A.M., Huey E.D., Habeck C., Landeira-Fernandez J., Mograbi D.C., Cosentino S. Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology. 2018;32:700–710. doi: 10.1037/neu0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Staging of Alzheimer´s disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Chua E.F., Schacter D.L., Sperling R.A. Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. J. Cognit. Neurosci. 2009;21:1751–1765. doi: 10.1162/jocn.2009.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S., Brickman A.M., Griffith E., Habeck C., Cines S., Farrell M., Shaked D., Huey E.D., Briner T., Stern Y. The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia. 2015;75:163–169. doi: 10.1016/j.neuropsychologia.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F.I.M., Salthouse T.A. Hillsdale; Erlbaum: 1992. Handbook of Aging and cognition. [Google Scholar]
- Dagley A., LaPoint M., Huijbers W., Hedden T., McLaren D.G., Chatwal J.P., Papp K.V., Amariglio R.E., Blacker D., Rentz D.M., Johnson K.A., Sperling R.A., Schultz A.P. Harvard aging brain study: dataset and accessibility. NeuroImage. 2015;144:255–258. doi: 10.1016/j.neuroimage.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P.M., Archer H.A.M., Hinz R.P., Hammers A.P., Pavese N.M., Tai Y.F.M., Hotton G.M., Cutler D.B., Fox N.P., Kennedy A.M., Rossor M.M.D.D., Brooks D.J.M.D.D. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- Engler H., Forsberg A., Almkvist O., Blomquist G., Larsson E., Savitcheva I., Wall A., Ringheim A., Langstrom B., Nordberg A. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHough P.R. “Mini-Mental State”: a practical method for grading cognit9ive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Engler H., Almkvist O., Blomquist G., Hagman G., Wall A., Ringheim A., Långström B., Nordberg A. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol. Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Freedman J.L., Landauer T.K. Retrieval of long-term memory: “tip-of-the-tongue” phenomenon. Psychon. Sci. 1966;4:309–310. [Google Scholar]
- Gardiner F.M., Craik F.I., Bleasdale F.A. Retrieval difficulty and subsequent recall. Mem. Cognit. 1973;1:213–216. doi: 10.3758/BF03198098. [DOI] [PubMed] [Google Scholar]
- Gomperts S.N.M.D.P., Rentz D.M.P., Moran E.B., Becker J.A.P., Locascio J.J.P., Klunk W.E.M.D.P., Mathis C.A.P., Elmaleh D.R.P., Shoup T.P., Fischman A.J.M., Hyman B.T.M.D.P., Growdon J.H.M., Johnson K.A.M. Imaging amyloid deposition in Lewy body diseases SYMBOL. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Salat D.H., Bowen S.L., Izquierdo-Garcia D., Schultz A.P., Catana C., Becker J.A., Svarer C., Knudsen G.M., Sperling R.A., Johnson K.A. Different partial volume correction methods lead to different conclusions: an (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334–343. doi: 10.1016/j.neuroimage.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E., Lipton R.B., Hall C., Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Gruneberg M.M., Monks J. “Feeling of knowing” and cued recall. Acta Psychol. 1974;38:257–265. [Google Scholar]
- Hart J. Memory and the feeling-of-knowing experience. J. Educ. Psychol. 1965;56:208–216. doi: 10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Hart J. Second-try recall, recognition, and the memory-monitoring process. J. Educ. Psychol. 1967;58:193–197. doi: 10.1037/h0024908. [DOI] [PubMed] [Google Scholar]
- Hosey L.A., Peynircioğlu Z.F., Rabinovitz B.E. Feeling of knowing for names in response to faces. Acta Psychol. 2009;130:214–224. doi: 10.1016/j.actpsy.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Johnson K.A., Gregas M., Becker J.A., Kinnecom C., Salat D.H., Moran E.K., Smith E.E., Rosand J., Rentz D.M., Klunk W.E., Mathis C.A., Price J.C., DeKosky S.T., Fischman A.J., Greenberg S.M. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann. Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Johnson K.A., Schultz A., Betensky R.A., Becker J.A., Sepulcre J., Rentz D., Mormino E., Chhatwal J., Amariglio R., Papp K., Marshall G., Albers M., Mauro S., Pepin L., Alverio J., Judge K., Philiossaint M., Shoup T., Yokell D., Dickerson B., Gomez-Isla T., Hyman B., Vasdev N., Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen W.L., Frost P.J., CAr W. Individual differences in metacognition: evidence against a general metacognitive ability. Mem. Cognit. 2000;28:92–107. doi: 10.3758/bf03211579. [DOI] [PubMed] [Google Scholar]
- Kemppainen N.M.M.D.P., Aalto S.M., Wilson I.A.P., Nagren K.P., Helin S.M., Bruck A.M.D.P., Oikonen V.M., Kailajarvi M.M.D.P., Scheinin M.M.D.P., Viitanen M.M.D.P., Parkkola R.M.D.P., Rinne J.O.M.D.P. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006;67:1575–1580. doi: 10.1212/01.wnl.0000240117.55680.0a. [DOI] [PubMed] [Google Scholar]
- Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P., Bergström M., Savitcheva I., Huang G.-F., Estrada S., Ausén B., Debnath M.L., Barletta J., Price J.C., Sandell J., Lopresti B.J., Wall A., Koivisto P., Antoni G., Mathis C.A., Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Koriat A. How do we know that we know? The accessibility model of the feeling of knowing. Psychol. Rev. 1993;100:609–639. doi: 10.1037/0033-295x.100.4.609. [DOI] [PubMed] [Google Scholar]
- Leonesio R.J., Nelson T.O. Do different metamemory judgments tap the same underlying aspects of memory? J. Exp. Psychol. Learn. Mem. Cognit. 1990;16:464–467. doi: 10.1037//0278-7393.16.3.464. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Bäckman L. Feeling-of-knowing in fact retrieval: further evidence for preservation in early Alzheimer’s disease. J. Int. Neuropsychol. Soc. 1996;2:350–358. doi: 10.1017/s1355617700001375. [DOI] [PubMed] [Google Scholar]
- Marquie J.C., Huet N. Age differences in feeling-of-knowing and confidence judgements as a function of knowledge domain. Psychol. Aging. 2000;15:451–460. doi: 10.1037//0882-7974.15.3.451. [DOI] [PubMed] [Google Scholar]
- Mintun M.A., LaRossa G.N., Sheline Y.I., Dence C.S., Lee S.Y., Mach R.H., Klunk W.E., Mathis C.A., DeKosky S.T., Morris J.C. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morson S.M., Moulin C.J.A., Souchay C. Ageing: A Novel Use of the General Knowledge Task. Vol. 15. 2015. Selective deficits in episodic feeling of knowing; pp. 85–92. [DOI] [PubMed] [Google Scholar]
- Nelson T.O. A comparison of current measures of the accuracy of feeling-of-knowing predictions. Psychol. Bull. 1984;95:109–113. [PubMed] [Google Scholar]
- Nelson T.O., Narens L. Metamemory: A theoretical framework and new findings. Psychol. Learn. Motiv. 1990;26:125–173. [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. Neuroimage. 2006;15:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pappas B.A., Sunderland T., Weingartner H.M., Vitiello B., Martinson H., Putnam K. Alzheimer’s disease and feeling-of-knowing for knowledge and episodic memory. J. Gerontol. 1992;47:159–164. doi: 10.1093/geronj/47.3.p159. [DOI] [PubMed] [Google Scholar]
- Perrotin A., Belleville S., Isingrini M. Metamemory monitoring in mild cognitive impairment: evidence of a less accurate episodic feeling-of-knowing. Neuropsychologia. 2007;45:2811–2826. doi: 10.1016/j.neuropsychologia.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rentz D.M., Locascio J.J., Becker J.A., Moran E.K., Eng E., Buckner R.L., Sperling R.A., Johnson K.A. Cognition, reserve, and amyloid deposition in normal aging. Ann. Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Alcantar O., Zakrzewski J., Shimamura A.P., Neuhaus J., Miller B.L. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2014;28:436–447. doi: 10.1037/neu0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen W.G., Terry R.D., Fuld P.A., Katzman R., Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann. Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Rousset O.G., Ma Y., Evans A.C. Correction for partial volume effects in PET: principle and validation. J. Nucl. Med. 1998;39:904–911. [PubMed] [Google Scholar]
- Schacter D.L. Feeling-of-Knowing in episodic memory. J. Exp. Psychol. 1983;9:39–54. [Google Scholar]
- Shoup T.M., Yokell D.L., Rice P.A., Jackson R.N., Livni E., Johnson K.A., Brady T.J., Vasdev N. A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807. J. Label. Comp. Radiopharm. 2013;56:736–740. doi: 10.1002/jlcr.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchay C., Insigrini M., Gil R. Alzheimer's disease and feeling-of-knowing in episodic memory. Neuropsychologia. 2002;40:2386–2396. doi: 10.1016/s0028-3932(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Souchay C., Insigrini M., Roger G. Alzheimer’s disease and feeling-of-knowing. Episodic Mem. Neuropsychol. 2002;40:2386–2396. doi: 10.1016/s0028-3932(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Souchay C., Isingrini M., Espagnet L. Aging, episodic memory feeling-of-knowing, and frontal functionning. Neuropsychology. 2000;2:299–309. doi: 10.1037//0894-4105.14.2.299. [DOI] [PubMed] [Google Scholar]
- Souchay C., Isingrini M., Pillon B., Gil R. Metamemory accuracy in Alzheimer's disease and frontotemporal lobe dementia. Neurocase. 2003;9:482–492. doi: 10.1076/neur.9.6.482.29376. [DOI] [PubMed] [Google Scholar]
- Souchay C., Moulin C.J.A. Eliciting the implicit: metacognition in Alzheimer’s disease. Cognit. Neurosci. 2013 doi: 10.1080/17588928.2013.853657. [DOI] [PubMed] [Google Scholar]
- Souchay C., Moulin C.J.A., Clarys D., Taconnat L., Isingrini M. Diminished episodic memory awareness in older adults: evidence from feeling-of-knowing and recollection. Conscious. Cognit. 2007;16:769–784. doi: 10.1016/j.concog.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Sperling R.A., Bates J.F., Chua E.F., Cocchiarella A.J., Rentz D.M., Rosen B.R., Schacter D.L., Albert M.S. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turro-Garriga O., Garre-Olmo J., Vilalta-Franch J., Conde-Sala J.L., de Gracia Blanco M., Lopez P. Burden associated with the presence of anosognosia in Alzheimer’s disease. Int. J. Geriatr. Psychatr. 2013;28(S I):291–297. doi: 10.1002/gps.3824. [DOI] [PubMed] [Google Scholar]
- Turro-Garriga O., Garre-Olmoa J., Rene-Ramırez R., Laia Calvo-Perxasa L., Gascon-Bayarri J., Conde-Sala J.-L. Consequences of anosognosia on the cost of caregivers’ care in Alzheimer’s disease. J. Alzheimer’s Dis. 2016;54:1551–1560. doi: 10.3233/JAD-160419. [DOI] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Yonelinas A.P., Jacoby L.L. Dissociating automatic and controlled processes in a memory-search task: beyond implicit memory. Psychol. Res. 1995;57:156–165. doi: 10.1007/BF00431277. [DOI] [PubMed] [Google Scholar]
- Yonelinas A.P., Otten L.J., Shaw K.N., Rugg M.D. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 2005;16:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G., Wilcock G. Lack of awareness of symptoms in people with dementia: the structural and functional basis. Int. J. Geriatr. Psychiatry. 2011;26:783–792. doi: 10.1002/gps.2620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.