Abstract
The emergence, spread, and persistence of antimicrobial resistance (AMR) remains a pressing global concern. Increased promotion of commercial small-scale agriculture within low-resource settings has facilitated an increased use in antimicrobials as growth promoters globally, creating antimicrobial-resistant animal reservoirs. We conducted a longitudinal field study in rural Ecuador to monitor the AMR of Escherichia coli populations from backyard chickens and children at three sample periods with approximately 2-month intervals (February, April, and June 2017). We assessed AMR to 12 antibiotics using generalized linear mixed effects models (GLMM). We also sampled and assessed AMR to the same 12 antibiotics in one-day-old broiler chickens purchased from local venders. One-day-old broiler chickens showed lower AMR at sample period 1 compared to sample period 2 (for 9 of the 12 antibiotics tested); increases in AMR between sample periods 2 and 3 were minimal. Two months prior to the first sample period (December 2016) there was no broiler farming activity due to a regional collapse followed by a peak in annual farming in February 2017. Between sample periods 1 and 2, we observed significant increases in AMR to 6 of the 12 antibiotics in children and to 4 of the 12 antibiotics in backyard chickens. These findings suggest that the recent increase in farming, and the observed increase of AMR in the one-day old broilers, may have caused the increase in AMR in backyard chickens and children. Small-scale farming dynamics could play an important role in the spread of AMR in low- and middle-income countries.
Keywords: Repeated samples, Global health, Antimicrobial resistance, Agriculture, Small-scale agriculture
1. Introduction
Small-scale commercial animal farming remains a growing practice throughout the world [1,2]. Poultry is one of the fastest growing meats produced on the planet [3], especially among low- and middle-income countries (LMICs), due to its cost effectiveness and high protein content [4]. Often, small-scale agricultural development programs initiate interventions through the introduction of broiler meat chickens [5,6]. These broilers are typically reared in intensive agricultural farming systems where the chickens harbor high levels of antimicrobial resistance (AMR) and zoonotic pathogens [1,7,8]. There is a current lack of understanding of public health outcomes related to small-scale agriculture since the majority of research focuses on industrial large-scale conventional animal farming systems [7,9]. Industrial large-scale farming is characterized by raising animals at high densities and using large quantities of chemotherapeutic agents [9]. AMR transmission to human populations from large-scale farming occur primarily through food chain consumption [10], environmental contamination [11], or occupational handling of animals [12]. In contrast, small-scale animal husbandry typically occurs at lower densities within the household setting. These family-operated micro enterprises could potentially promote risk of AMR and zoonosis exposure to community members due to the close proximity of production animals and surrounding human populations [13].
Our study in northwestern coastal Ecuador is an observational investigation, monitoring small-scale broiler farming activity in parallel with human and backyard chicken susceptibility to antimicrobials. In our study communities, small-scale poultry farming of broiler meat chickens co-occurs with farming of local backyard chicken. Typically, broiler chickens are reared within a large-scale farming and purchased as one-day-old chickens by farmers whose operations are based out of either a single household or multiple households within a collective hatchery. Our previous analyses suggested that selection for drug resistance in broilers originates in the large-scale farming setting driven by high amounts of antibiotics delivered in the feed [14]. Villagers administer antibiotics supplemented in chicken water source and indirectly through the purchase of commercial chicken feed laced with antibiotics [14].
Intensive farming can have impacts on the emergence of AMR at the farm-level and surrounding community. Our previous work within this study system has demonstrated that compared to backyard chickens, broiler chickens exhibited greater E. coli phenotypic antibiotic resistance, greater richness of antimicrobial resistant genes, and lower microbial community diversity [6,14,15]. Elsewhere, conventional farming of poultry compared to antimicrobial use free has been associated with higher AMR prevalence in Campylobacter spp. [17]. Multiple studies have noted that vicinity to conventional farming presents increased risk of AMR exposure to human populations [2,16,[18], [19], [20]].
Despite our foundational work, we still have limited understanding of AMR carriages over time in relation to small-scale chicken farming activity. We predict that the introduction of broiler farming activity can function as a driver for changes in AMR profiles over time. Here, we analyzed the variation over time in phenotypic antimicrobial-resistant profiles of backyard chickens and children within rural poultry farming communities in Ecuador.
2. Material and methods
2.1. Field study design
We conducted our study in three villages within the province of Esmeraldas, Ecuador: Borbón (962 households), Colon Eloy (235 households), and Timbiré (166 households). Between February and May 2017, we collected fecal samples from backyard chickens and children during three observation periods [sample period one (S1): February 2 – February 6, sample period two (S2): March 29 – April 1, and sample period three (S3): May 24 – May 27). The inclusion criteria for enrollment was that a household had at least one child (age: 5–18) or at least one backyard chicken present. Child samples were provided by parents. Backyard chicken samples were collected by the field team via cloaca chicken swabs.
During each of the three sampling periods, we visited each enrolled household. If at least one child was present, we collected all child fecal samples provided by the household guardians and we administered a survey on antibiotic use of the children living in enrolled households. If a household had backyard chickens, each received a uniquely colored and numbered identification band (National Band & Tag Company) that provided a unique identification number for every backyard chicken enrolled in the study. The field team collected one fecal sample from a maximum of four chickens.
Concurrently, during each observational period we sampled 30 one-day-old broiler chickens from regional vendors as a baseline for understanding the magnitude of potential antibiotic-resistant E. coli entering the study villages.
All avian and children samples were placed in Cary Blair medium (Thermo Scientific™) [21] on ice and transported to Quito for analysis within 48 h.
Consent to participate was obtained from all households. The head of a household provided consent on the behalf of the child participants, and provided consent to collect fecal samples from birds. All study protocols were reviewed and approved by the University of Michigan Institutional Review Board (HUM00121496), University of Michigan Institutional Animal Use & Care Committee (PRO00008191), and the Universidad San Francisco de Quito Bioethics Committee (MSP-SDM-10-2013-1019-O).
2.2. Antimicrobial susceptibility testing
We collected field samples and immediately placed them in Cary Blair Transport Medium and cultured within 48 h. In Quito, we plated samples on MacConkey lactose (MKL) agar and then selected up to three lactose positive colonies, which we transferred to a nutrient agar to allow them to grow. Each purified colony was tested for β-glucoronidase activity to identify E. coli using Chromocult agar (Merck, Darmstadt, Germany) After confirmation, we again cultured each colony on a nutrient agar for additional growth prior to testing each colony for phenotypic resistance. We used the Kirby-Bauer disc diffusion method [22] for 12 antibiotics: amoxicillin-clavulanate (AMC; 10 μg per antibiogram unit), ampicillin (AMP; 10), ciprofloxacin (CIP; 5), cefotaxime (CTX; 30), cephalothin (CF; 30), chloramphenicol (C; 30), enrofloxacin (ENO; 5), gentamicin (GM; 10), streptomycin (S; 10), sulfisoxazole (G; 1 mg), tetracycline (TE; 30), and trimethoprim/sulfamethoxazole (SXT; 25). Zones of inhibition were measured after a 24-h incubation period using digital calipers. Antibiotic sensitivity was recorded for a given child or chicken if at least one of the three isolates was resistant to the antibiotic tested according to Clinical and Laboratory Standards Institute (CLSI) [23,24]. We used reference strains (E. coli ATCC 25922, Staphylococcus aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 27853) as controls for each batch of disc diffusion test. We classified phenotypic resistance as resistant or sensitive (intermediate isolates were categorized as sensitive). Analyses were conducted at the sample level. Multidrug resistance (MDR) was categorized as being resistant to two or more antibiotic classes.
2.3. AMR E. coli profile turnover
For each sampled child or backyard chicken, we define an AMR profile as the binary categorization of presence or absence of resistance to each of the 12 antibiotics, where we define sample-level resistance as above. For each observational sample period, we estimated the number of profiles by quantifying the number of unique binary categorizations. Profile turnover is defined as the number of unique profiles when comparing one sample period to the next.
2.4. Statistical analyses
We used generalized linear mixed effects models (GLMM) to examine changes in phenotypic AMR for individual chickens and children over time. We used sample period as the time variable and included it in the model as a fixed effect. On the other hand, community and individual IDs were included as random effects to account for both community-level clustering and intra-subject correlation, respectively. Conversely, analyses of one-day-old broiler chickens did not include an individual ID. Since multiple chickens might have been acquired from the same vendor, vendor source was included as a random effect to account for vendor-level clustering. Odds ratios and 95% confidence intervals were calculated from GLMM estimates. All analyses were performed using R Statistical Software version 3.5.2 (2019).
3. Results
3.1. Chicken-farming activities before and during study period
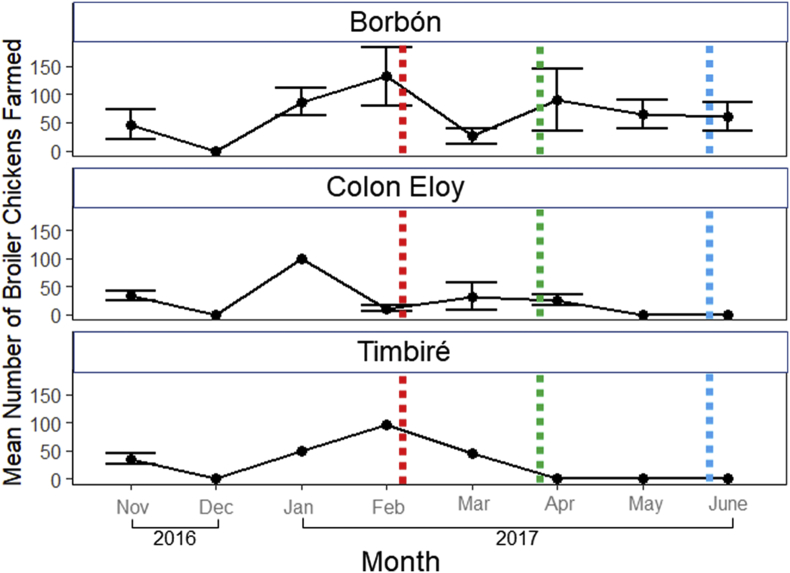
During our three sampling periods, the mean number of households raising broiler chickens ranged from 2 to 7 in Borbón, 1 to 3 in Colon Eloy and 0 to 1 in Timbiré (Fig. 1). Overall, the mean number of broilers chickens reared per farm was 58 (min-max: 0–132). There was no farming activity in December 2016. By February 2017 there was a mean of 114 chickens per farming household. Anecdotally, our field supervisor reported that the collapse in December 2016 in broiler farming was due to an earthquake in the region leading to high broiler mortality. We enrolled 70 households (34 from Borbón, 25 from Colon Eloy and 11 from Timbiré). Of these households, 57 had backyard chickens and at least one child, 13 had only backyard chickens.
Fig. 1.
2016–2017 monthly mean (± SE) number of broiler chicken farmed in three villages within the Esmeraldas Province, Ecuador. Standard error was calculated by the square root of the number of houses actively farming (points without SE bars indicate n households ≤1). Colors correspond to dates of sample periods (red: sample period one, green: sample period two, blue sample period three). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. AMR in one-day-old broiler chicken from vender sources
Multiple drug resistance (MDR) of one-day-old broiler chickens (resistance to two or more unique antibiotic classes) was 68.4%, 100% and 100% for each observational period S1, S2, and S3 respectively (Table 1). Across the three observational periods, the mean number of antibiotics that a given isolate was resistant to was 7, with 64% of the isolates resistant to 7 or more antibiotics. We recorded the resistance to “AMP-G-AMC-CTX-CF-C-CIP-SXT-ENO-TE-S" (n = 13) as the most frequently observed resistance profile for one-day-old broiler chickens also present in one child and two backyard chickens. Comparing S1 and S2, we detected an increase in AMR for 10 of the 12 antibiotics tested (Table 1). The mean number of antibiotics an isolate was resistant to, increased from 3 (S1) to 9 (S2). In general, resistance levels for S3 was similar to that of S2. Resistance prevalence to 5 of the 12 antibiotics tested were all above 90% during S2 [cephalothin (CF), sulfisoxazole (G), tetracycline (TE), streptomycin (S), and trimethoprim/sulfamethoxazole (SXT)] and S3 [ampicillin (AMP), cephalothin (CF), sulfisoxazole (G), tetracycline (TE), and trimethoprim/sulfamethoxazole (SXT)]. Comparing S2 and S3, we observed a statistically significant decline in resistance to only amoxicillin-clavulanate (AMC).
Table 1.
Phenotypic resistance profiles of E. coli isolates collected from one-day-old vendor source broiler chickens from three regional poultry vendors located in Borbón, San Lorenzo, and Esmeraldas City in Esmeraldas Province, Ecuador (Borbón, San Lorenzo during sample periods one (S1), two (S2), and three (S3). A GLMM was used to compare S1 vs. S2 and S2 vs. S3. A significant difference between S2 and S1 (P-value <.05) is represented by the symbol * in column 2. For the comparison between S2 and S3, the symbol is located in column 3. Each cell contains the number of antibiotic-resistant E. coli isolates and the percentage resistant of those tested, n. MDR, multiple drug resistance, is define as resistance to two or more unique antibiotic classes.
| Antibiotic | S1 |
S2 |
S3 |
|---|---|---|---|
| (n = 76) | (n = 87) | (n = 90) | |
| MDR | 52 (68.4) | 87 (100) | 90 (100) |
| Amoxicillin-clavulanate | 22 (28.9) | 45 (51.7)* | 15 (16.7)* |
| Ampicillin | 18 (23.7) | 69 (79.3) | 83 (92.2) |
| Cefotaxime | 2 (2.6) | 60 (69.0)* | 65 (72.2) |
| Cephalothin | 54 (71.1) | 79 (90.8)* | 82 (91.1) |
| Chloramphenicol | 13 (17.1) | 76 (87.4)* | 63 (70.0) |
| Ciprofloxacin | 2 (2.6) | 57 (65.5)* | 42 (46.7) |
| Enrofloxacin | 3 (3.9) | 56 (64.4)* | 60 (66.7) |
| Gentamicin | 1 (3.7) | 10 (11.5)* | 17 (18.9) |
| Streptomycin | 37 (46.7) | 82 (94.3)* | 75 (83.3) |
| Sulfisoxazole | 23 (30.3) | 79 (90.8)* | 82 (91.1) |
| Tetracycline | 47 (61.8) | 87 (100) | 90 (100) |
| Trimethoprim/ | 23 (30.3) | 79 (90.8)* | 82 (91.1) |
| Sulfamethoxazole |
3.3. Change in AMR in children and backyard chickens over time
We detected high E. coli phenotypic resistance. For example, MDR ranged from 76.1 to 88.3% in children and 81.1 to 83.3% in backyard chickens (Table 2). The gain in the proportion of children isolates with AMR between sample S1 and S2 varied from 13.7% (GM) to 45.1% (SXT) (Supplementary Materials; Table 1), while gains in backyard chickens isolates varied from 8.7% (S) to 52.2% (SXT) (Supplementary Materials; Table 2). Specifically, we detected increased AMR in children samples (n = 220) for GM, S, AMP, CTX, G, and SXT comparing S1 to S2 (Table 3; GLMM, p < .05). Among samples from backyard chickens (n = 96), we detected increased AMR levels from S1 to S2 for GM, CIP, ENO, and SXT (Table 3, Table 4; GLMM, p < .05). From S2 to S3, we detected decreased AMR levels in children for AMC, C, and MDR, and in backyard chicken for AMC, CF and MDR. This result remained constant when including additional covariates including flock size, binary categorization of broiler chickens farmed, and total number of farming durations during the study period.
Table 2.
Phenotypic resistance prevalence of E. coli isolates collected from backyard chickens and children during sample periods one (S1), two (S2), and three (S3). Each cell contains the number of antibiotic-resistant E. coli isolates and the percentage resistant of those tested, n. MDR, multiple drug resistance is defined as to two or more unique antibiotic classes.
| Antibiotic | Child |
Backyard chicken |
||||
|---|---|---|---|---|---|---|
| S1 |
S2 |
S3 |
S1 |
S2 |
S3 |
|
| (n = 72) | (n = 77) | (n = 71) | (n = 30) | (n = 37) | (n = 29) | |
| MDR | 56 (77.8) | 68 (88.3) | 54 (76.1) | 25 (83.3) | 30 (81.1) | 21 (81.1) |
| Amoxicillin-clavulanate | 72 (31.9) | 24 (31.1) | 12 (16.9) | 18 (60.0) | 13 (35.1) | 2 (6.9) |
| Ampicillin | 72 (51.4) | 51 (66.2) | 41 (57.7) | 13 (43.3) | 19 (51.4) | 16 (55.2) |
| Cefotaxime | 8 (11.1) | 19 (24.7) | 17 (23.9) | 7 (23.3) | 10 (27.0) | 3 (10.3) |
| Cephalothin | 52 (72.2) | 66 (85.7) | 57 (80.3) | 25 (83.3) | 25 (67.6) | 17 (58.6) |
| Chloramphenicol | 17 (23.6) | 15 (19.5) | 12 (16.9) | 6 (20.0) | 12 (32.4) | 6 (20.7) |
| Ciprofloxacin | 8 (11.1) | 18 (23.4) | 8 (11.3) | 1 (3.3) | 5 (13.5) | 2 (6.9) |
| Enrofloxacin | 11 (15.3) | 19 (24.7) | 14 (19.7) | 3 (10.0) | 8 (21.6) | 5 (17.2) |
| Gentamicin | 6 (8.3) | 62 (19.5) | 9 (12.7) | 2 (6.7) | 10 (27.0) | 6 (20.7) |
| Streptomycin | 72 (84.7) | 74 (96.1) | 59 (83.1) | 27 (90.0) | 33 (89.2) | 26 (89.7) |
| Sulfisoxazole | 26 (36.1) | 45 (58.4) | 36 (50.7) | 10 (33.3) | 20 (54.1) | 14 (48.3) |
| Tetracycline | 49 (68.1) | 58 (75.3) | 49 (69.0) | 19 (63.3) | 25 (67.6) | 20 (69.0) |
| Trimethoprim/ | 18 (25.0) | 42 (54.5) | 35 (49.3) | 7 (23.3) | 20 (54.1) | 12 (41.4) |
| Sulfamethoxazole | ||||||
Table 3.
Odds ratio and 95% CI comparing E. coli sample phenotypic resistance to 12 antibiotics among children samples comparing sample period one (S1) to two (S2) and S2 and three (S3).
| Antibiotic |
S1 vs S2 |
S2 vs S3 |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Amoxicillin-clavulanate | 0.7 | 0.4 — 1.2 | 0.7 | 0.2 — 1.6 |
| Ampicillin | 2.3 | 1.4 — 4.0 | 0.5 | 0.3 — 2.0 |
| Cefotaxime | 2.9 | 1.4 — 5.9 | 0.5 | 0.2 — 3.6 |
| Cephalothin | 2.1 | 1.0 — 4.2 | 0.4 | 0.2 — 2.2 |
| Chloramphenicol | 1.3 | 1.3 — 1.3 | 0.5 | 0.2 — 1.7 |
| Ciprofloxacin | 2.1 | 1.0 — 4.3 | 0.2 | 0.1 — 1.8 |
| Enrofloxacin | 1.9 | 0.9 — 3.7 | 0.4 | 0.1 — 2.2 |
| Gentamicin | 2.7 | 1.2 — 6.0 | 1.9 | 0.1 — 3.6 |
| Streptomycin | 2.9 | 1.1 — 7.7 | 0.1 | 0.1 — 1.5 |
| Sulfisoxazole | 2.2 | 1.3 — 3.8 | 0.5 | 0.2 — 2.0 |
| Tetracycline | 1.1 | 0.6 — 1.9 | 0.7 | 0.2 — 1.9 |
| Trimethoprim/ | 2.7 | 1.5 — 4.7 | 0.4 | 0.2 — 2.0 |
| Sulfamethoxazole | ||||
Table 4.
Odds ratio and 95% CI comparing E. coli sample phenotypic resistance to 12 antibiotics among backyard chicken samples comparing sample period one (S1) to two (S2) and sample period two (S2) to three (S3).
| Antibiotic |
S1 vs S2 |
S2 vs S3 |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Amoxicillin-clavulanate | 0.7 | 0.4 — 1.2 | 0.6 | 0.1 — 2.8 |
| Ampicillin | 1.0 | 1.0 — 1.5 | 1.0 | 0.4 — 2.5 |
| Cefotaxime | 1.8 | 1.0 — 1.5 | 0.3 | 0.1 — 1.4 |
| Cephalothin | 1.2 | 0.7 — 2.1 | 0.5 | 0.1 — 1.2 |
| Chloramphenicol | 2.3 | 1.0 — 3.2 | 0.3 | 0.1 — 1.5 |
| Ciprofloxacin | 231.1 | 6.3 — 784.7 | 0.0 | 0.0 — 57.9 |
| Enrofloxacin | 7.3 | 1.4 — 36.9 | 0.0 | 0.0 — 6.6 |
| Gentamicin | 2.7 | 1.5 — 5.4 | 0.5 | 0.2 — 2.8 |
| Streptomycin | 1.5 | 0.8 — 3.1 | 0.6 | 0.2 — 2.8 |
| Sulfisoxazole | 1.3 | 0.8 — 2.0 | 0.9 | 0.4 — 2.3 |
| Tetracycline | 1.1 | 0.7 — 1.8 | 1.0 | 0.4 — 2.8 |
| Trimethoprim/ | 1.8 | 1.1 — 2.8 | 0.6 | 0.3 — 2.0 |
| Sulfamethoxazole | ||||
Among children and backyard chickens there were 103 AMR profiles identified in S1, 114 in S2, and 100 in S3 (Table 5). We observed a high amount of unique E. coli AMR profiles in each sample period: 69 for S1, 86 for S2, and 69 for S3. At the individual level, we collected as many as 3 isolates. Comparing S1 to S2, we observed that 77% of the samples had an AMR profile in all 3 isolates, 18.9% of the samples had an AMR profile turnover in 2 of the 3 isolates, and 4.1% of the samples had an AMR profile turnover in 1 of the 3 isolates. We did not observe any samples that retained the same profile when comparing S1 and S2. In sum, 92% and 68% of the isolates in S2 were new profiles in backyard chickens and children respectively. We observed new profiles when comparing S2 to S3 in both backyard chickens (96%) and children (64%) (Table 5).
Table 5.
Summary of AMR E. coli profiles (binary categorizations, either resistance or susceptible, of the 12 antibiotics tested) detected among children and backyard chicken isolates.
| S1 | S2 | S3 | |
|---|---|---|---|
| No. of isolates tested | 103 | 114 | 100 |
| Backyard Chickens | 31 | 37 | 29 |
| Children | 72 | 77 | 71 |
| No. of unique profiles identified (%) | 69 (67) | 86 (75) | 69 (69) |
| Backyard Chickens | 23 (74) | 34 (92) | 24 (83) |
| Children | 46 (64) | 52 (68) | 45 (63) |
| No. of new profiles relative to prior sample period (%) | |||
| Backyard Chickens | – | 28 (82) | 23 (96) |
| Children | – | 38 (73) | 29 (64) |
4. Discussion
The emergence, spread, and persistence of antibiotic resistance remains a pressing concern especially among LMICs where the practice of small-scale animal husbandry is common [6,[25], [26], [27]]. Our observed increase in E. coli resistance among backyard chickens and children could have been in response to a resurgence in farming activity in our Ecuadorian study system. Increases in the resistance levels of one-day-old broiler chickens originating from the large-scale farm could have also contributed to the observed resistant patterns. Our additional observation of a high rate of profile turnover when comparing isolates between S1 and S2, suggests either the introduction of new E coli strains and/or high levels of horizontal gene transfer. Together, these results suggest that AMR can readily spillover from farmed chickens to backyard chickens and ultimately to children.
In December 2016, we observed a collapse in small-scale broiler farming activity followed by a large peak in February 2017. A earthquake within the region. This magnitude of mortality is not surprising, since meat broiler chickens are vulnerable to various environmental pressures, including natural disasters [28], infectious diseases [29], and temperature change [30]. All of these drivers of avian mortality are likely intensified within tropical environments [31,32].
We suspect that any observed increase in phenotypic resistance levels in backyard chickens due to the increase in farming intensity is likely delayed given that it takes time for the drug-resistant microbes and genes to spread into the environment. To ensure we captured the response to the increase in farming we set our sampling period to 60 days. Previous work has recorded AMR bacteria and genes persisting in animal feces and soil for up to 60 days [33,34], suggesting that 60 days was a reasonable choice.
We speculate that antibiotic use in broiler farming is an important driver for AMR increases in children and backyard chickens. Our previous studies have all reported limited to no antibiotic use in backyard chickens and significantly higher levels of AMR in broiler chickens [6,14,15]. In addition, while antibiotic use in humans within our study system is significant [35], based on our data on child antibiotic use prior to each sampling period, there was not a significant increase in use patterns that would explain the increases we observed in AMR.
Previous longitudinal studies within industrial food animal agricultural systems have demonstrated that decreases in antibiotic use in farming practices can decrease AMR levels. An 18-month study documented a decline in swine MRSA carriage following a 44% decline in antimicrobial use [36]. From 2011 to 2013, loss in extended-spectrum beta-lactamase-producing E. coli was associated with the absence of cephalosporin use in swine farming [37]. The impact of the European Union ban on antibiotic use as growth promotors in farming, however, has had mixed results. In Demark, resistance to vancomycin among certain bacterial species appeared to persist following the ban on growth promoters [38].
Our study is distinct in showing the impact of a recent resurgence of farming intensity on AMR. Interestingly, only for trimethoprim-sulfamethoxazole did we detect a gain in resistance to both children and backyard chickens. We reported a similar finding in our prior work, which is likely due the physical link between a mobile genetic element (int1) and trimethoprim-sulfamethoxazole and the observation that int1 is strongly associated with farming activity [15]. It is surprising that we observed gains in ciprofloxacin resistance from backyard chicken samples because ciprofloxacin is a wide spectrum clinically relevant quinolone that is seldom used in humans within our study site [35].
The other main source of antibiotics are the one-day-old chickens coming from the large-scale farms, which constantly change the antibiotics applied to the chickens prior to being sold to farmers. As a result, the resistant profiles of the one-day-old broiler chickens also varied over time. The high turnover rate of resistance profiles in E. coli may reflect the frequent exposure to novel E. coli strains in the environment driven by broiler farming and/or may reflect a high rate of horizontal gene transfer. Other studies have documented how resistant strains of commensal bacteria can outcompete non-resistant species leading to overall lower microbiota diversity [17,39]. Our previous studies have detected decreases in broiler E. coli phenotypic resistance levels over time, largely due to the absence of selection pressure from the large-scale farm setting [6]. Analogously, within the same studied villages, we reported greater microbial diversity and lower abundance in antimicrobial-resistant genes sampled from backyard chickens compared to broiler chickens [15].
5. Conclusion
Small-scale chicken farming is an important economic activity in our study communities. It is also an important source of antibiotic resistance. The specific implications of this source of AMR is complex. Future studies should focus on disentangling these ecological complexities. In this regard, longitudinal studies can be useful. Following newborns to monitor long-term patterns of AMR in humans, chickens and the environment can provide important data on determinants of the spread of AMR. Other important data include knowledge of antimicrobials and concentrations delivered in feed to understand the dynamic selection pressures within this system, and bacterial strain sequencing to measure the rate of genetic exchange between animals and humans [40]. These focused inquires can help us more deeply understanding the dynamics of antibiotic susceptibility in animal and human populations.
Declaration of Competing Interest
The authors do not have any conflict of interest regarding this research.
Acknowledgements
We are grateful for the invaluable contributions of the field coordinator Jorge Mejía Zamora and the entire “Resistencia Zoonotica” field team members: Eduardo Zamora Castillo, Mauricio Ayovi, Leonar Hurtado, Evelin Melisa Valdéz, Sulay Borja Perlaza, and Eduardo Zamora Castillo. We thank Sonia Zapata Mena for access to laboratory work environments at Universidad San Francisco de Quito, Ecuador. We thank Sanchitha Meda for laboratory assistance at Michigan State University. This project was funded through the Dow Sustainability Fellowship and the Integrated Training in Microbial Systems program at the University of Michigan. We are grateful for statistical analysis assistance from Michael Clark. We are grateful for the careful review of Tom Duda and Carl Marrs. Procedures contributing to this work comply with the ethical standards were reviewed and approved by the University of Michigan Institutional Review Board and the Universidad San Francisco de Quito Bioethics Committee (Protocol: HUM00121496). Animal use protocol was approved by the Institutional Animal Care & Use Committee at the University of Michigan (Protocol:PRO00006200).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2019.100112.
Contributor Information
H.D. Hedman, Email: hedmanh@umich.edu.
J.N.S. Eisenberg, Email: jnse@umich.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.Cuong N.V. Antimicrobial usage in animal production: a review of the literature with a focus on low- and middle-income countries. Antibiotics. 2018;7(3):1–20. doi: 10.3390/antibiotics7030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrique-Mas J. Mortality, disease and associated antimicrobial use in commercial smallscale chicken flocks in the Mekong Delta of Vietnam. Prev. Vet. Med. 2019;165:15–22. doi: 10.1016/j.prevetmed.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84(4):634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 4.Tilman D. Global food demand and the sustainable intensification of agriculture. Proc. Nat. Acad. Sci. 2011;108(50):20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert M. Income disparities and the global distribution of intensively farmed chicken and pigs. PLoS One. 2015;10(7):1–14. doi: 10.1371/journal.pone.0133381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedman H.D. High prevalence of extended-spectrum beta-lactamase CTX-M-producing Escherichia coli in small-scale poultry farming in rural Ecuador. Amer. J. Trop. Med. Hyg. 2019;100(2):374–376. doi: 10.4269/ajtmh.18-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham J.P. Hyperendemic Campylobacter jejuni in Guinea pigs (Cavia porcellus) raised for food in a semi-rural community of Quito, Ecuador. Environ. Microb. Report. 2016;8(3):382–387. doi: 10.1111/1758-2229.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasco K. Detection of zoonotic enteropathogens in children and domestic animals in a semirural community in Ecuador. Appl. Environ. Microb. 2016;82(14):4218–4224. doi: 10.1128/AEM.00795-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilchrist M.J. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ. Health Persp. 2007;115(2):313–316. doi: 10.1289/ehp.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verraes C. Antimicrobial resistance in the food chain: a review. Int. J. Environ. Res. Pub. Health. 2013;10(7):2643–2669. doi: 10.3390/ijerph10072643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie W.Y. Antibiotics and antibiotic resistance from animal manures to soil: a review, euro. J. Soil Sci. 2017;69(7):181–195. [Google Scholar]
- 12.Trung N. Prevalence and risk factors for carriage of antimicrobial-resistant Escherichia coli on household and small-scale chicken farms in the Mekong Delta of Vietnam. J. Antimicrob. Chemother. 2015;70(7):2144–2152. doi: 10.1093/jac/dkv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham J.P. Small-scale food animal production and antimicrobial resistance: mountain, molehill, or something in-between? Environ. Health Perspect. 2017;125(10) doi: 10.1289/EHP2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braykov N.P. Antibiotic resistance in animal and environmental samples associated with small-scale poultry farming in northwestern Ecuador. mSphere. 2016;1(1):1–15. doi: 10.1128/mSphere.00021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X. Antibiotic resistome associated with small-scale poultry production in rural Ecuador. Environ.Sci.Tech. 2018;52(15):8165–8172. doi: 10.1021/acs.est.8b01667. [DOI] [PubMed] [Google Scholar]
- 16.Moser K.A. The role of mobile genetic elements in the spread of antimicrobial-resistant Escherichia coli from chickens to humans in small-scale production poultry operations in rural Ecuador. Amer. J. Epid. 2017;187(3):558–567. doi: 10.1093/aje/kwx286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luangtongkum T. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. App. Environ. Microb. 2006;72(5):3600–3607. doi: 10.1128/AEM.72.5.3600-3607.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jernberg C. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1(1):56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 19.Trung N. Zoonotic transmission of mcr-1 colistin resistance gene from small-scale poultry farms, Vietnam. Emerg. Infect. Dis. 2017;23(3):529–532. doi: 10.3201/eid2303.161553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Y., Yamamoto Colistin-resistant Escherichia coli with mcr genes in the livestock of rural small-scale farms in Ecuador. BMC Res. Notes. 2019;12:121. doi: 10.1186/s13104-019-4144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann D.A. Cary-Blair, a transport medium for Vibrio parahemolyticus. Am. J. Clin. Pathol. 1972;57:33–34. doi: 10.1093/ajcp/57.1.33. [DOI] [PubMed] [Google Scholar]
- 22.Biemer J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Institute Clin. Sci. 1973;74(2):511–519. [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute . Approved Standard M31-A4. Vol. 28. Clinical and Laboratory Standards Institute; Wayne, PA: 2009. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; p. 47. [Google Scholar]
- 24.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, PA: 2012. Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement. CLSI; pp. M100–S22. [Google Scholar]
- 25.Andoh L.A. Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry in Ghana. Epidemiol. Infect. 2016;144(15):3288–3299. doi: 10.1017/S0950268816001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.E.O., Ngbebe Antimicrobial resistance and virulence profile of enterococci isolated from poultry and cattle sources in Nigeria. Trop. Anim. Health Prod. 2017;49(3):451–458. doi: 10.1007/s11250-016-1212-5. [DOI] [PubMed] [Google Scholar]
- 27.Bui T.K.N. Potential transmission opportunity of CTX-M-producing Escherichia coli on a large-scale chicken farm in Vietnam. J. Global Antimicrob. Res. 2018;13:1–6. doi: 10.1016/j.jgar.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Glassey S., Wilson T. Animal welfare impact following the 4 September 2010 Canterbury (Darfield) earthquake. Australas. J.Disaster & Trauma Stud. 2011:49–59. [Google Scholar]
- 29.Agunos A. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can. Vet. J. 2012;53(12):1289–1300. [PMC free article] [PubMed] [Google Scholar]
- 30.Ritz C.W. Evaluation of hot weather thermal environment and incidence of mortality associated with broiler live haul. Poult. Sci Assoc. 2005;14(3):594–602. [Google Scholar]
- 31.Henry I. Prevalence and risk factors for campylobacter spp. in chicken broiler flocks in Reunion Island (Indian Ocean) Prev. Vet. Med. 2011;100(1):64–70. doi: 10.1016/j.prevetmed.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Tirawattanawanich C. The effects of tropical environmental conditions on the stress and immune responses of commercial broilers, Thai indigenous chickens, and crossbred chickens. Appl. Poult. Res. 2011;20(4):409–420. [Google Scholar]
- 33.Heuer H., Smalla K. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol. 2007;9(3):657–666. doi: 10.1111/j.1462-2920.2006.01185.x. [DOI] [PubMed] [Google Scholar]
- 34.Scott A. Enrichment of antibiotic resistance genes in soil receiving composts derived from swine manure, yard wastes, or food wastes, and evidence for multiyear persistence of swine Clostridium spp. Can. J. Microbiol. 2018;64(3):201–208. doi: 10.1139/cjm-2017-0642. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg J.N.S. In-roads to the spread of antibiotic resistance: regional patterns of microbial transmission in northern coastal Ecuador. J. R. Soc. Interface. 2012;45(7):1029–1039. doi: 10.1098/rsif.2011.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorado-García A. Dose-response relationship between antimicrobial drugs and livestock-associated MRSA in pig farming. Emerg. Infect. Dis. 2015;21(6):950–959. doi: 10.3201/eid2106.140706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dohmen W. Risk factors for ESBL-producing Escherichia coli on pig farms: a longitudinal study in the context of reduced use of antimicrobials. PLoS One. 2017;12(3):1–14. doi: 10.1371/journal.pone.0174094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aarestrup F.M. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agen. Chemoth. 2001;45(7):2054–2059. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J. Response of intestinal microbiota to antibiotic growth promoters in chickens. Food Pathogens. Dis. 2013;10(4):331–337. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- 40.Armand-Lefevre L. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg. Infect. Dis. 2005;11(5):711–714. doi: 10.3201/eid1105.040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material