Abstract
Among mitochondrial diseases, isolated complex V (CV) deficiency represents a rare cause of respiratory chain (RC) dysfunction. In mammalian mitochondrial DNA (mtDNA), MT-ATP6 partly overlaps with MT-ATP8 making double mutations possible, yet extremely rarely reported principally in patients with cardiomyopathy. Here, we report a novel m.8561 C>T substitution in the overlapping region of MT-ATP6 and MT-ATP8 in a child with early-onset ataxia, psychomotor delay and microcephaly, enlarging the clinical manifestations spectrum associated with CV deficiency.
Keywords: Mitochondrial disorders, ATP synthase, Ataxia, Psychomotor delay, Microcephaly
Abbreviations: ADP, adenosine triphosphate; ATP, adenosine triphosphate; BN-PAGE, Blue Native-PolyAcrylamide Gel Electrophoresis; CV, complex V; MRI, Magnetic resonance imaging; mtDNA, mitochondrial DNA; NARP, Neuropathy, Ataxia, Retinitis Pigmentosa; NGS, Next-generation sequencing; OXPHOS, oxidative phosphorylation; PCR, polymerase chain reaction; PVDF, PolyVinyliDene Fluoride; RC, respiratory chain; RFLP, Restriction Fragment Length Polymorphism; WT, wild-type
1. Introduction
The mitochondrial oxidative phosphorylation (OXPHOS) system, involved in cellular adenosine triphosphate (ATP) production, is composed of five complexes (complexes I-V). ATP synthase or complex V (CV) synthesizes ATP from adenosine diphosphate (ADP) in the mitochondrial matrix using the energy provided by the proton electrochemical gradient. Among mitochondrial diseases, isolated CV deficiency represents a rare cause of respiratory chain (RC) dysfunction [1,2]. The majority of CV mutations described to date is located in MT-ATP6 and causes different clinical phenotypes including NARP (Neuropathy, Ataxia, Retinitis Pigmentosa) and Leigh syndromes (www.mitomap.org). Mutations in MT-ATP8 have been reported more rarely [3]. Last, the 5′ part of MT-ATP6 partly overlaps with MT-ATP8 in human mitochondrial DNA (mtDNA) making double mutations possible that have been reported in a very few number of patients with cardiomyopathy [4,5]. In 2016, Laura Kytövuori et al. described a novel heteroplasmic mutation m.8561C>G in the overlapping region of MT-ATP6 and MT-ATP8 in two adults siblings presenting with cerebellar ataxia, peripheral neuropathy, diabetes mellitus and hypergonadotropic hypogonadism [6]. Here, we report a novel C>T substitution at the same position (m.8561) in a child with early-onset severe neurological signs.
2. Methods
All biological samples were obtained after the obtention of informed consents. The biopsies were taken from the quadriceps femoris muscle. Spectrophotometric analysis of the individual RC complexes in muscle was performed at 37° on crude homogenates as previously described [7], and proteins were measured by Bradford microassay [8]. For Blue Native-PolyAcrylamide Gel Electrophoresis (BN-PAGE) analysis in muscle, equal amounts (15 μg) of mitochondrial proteins were subjected to blue native-PAGE, blotted onto a PVDF membrane and then incubated with specific antibodies as previously described [9]. Next-generation sequencing (NGS) of mtDNA was performed as previously described [10]. The relative proportion of the m.8561C>T was determined in different tissues by PCR-RFLP analysis. The forward primer was 5′-atg gcc cac cat aat tac cc-3′ and the reverse primer containing mismatches was 5′-cgg gta ggc cta gga tca tg-3′ (mismatches shown in bold). These mismatches create a BspHI restriction site in the mutant PCR product which cuts the 193 bp amplicon into two fragments of 20 bp and 173 bp. Digested and undigested PCR products were separated through a 8% acrylamide gel. In order to analyze the conservation of mutated amino acids, we used the Web interface for EMBOSS Stretcher (https://www.ebi.ac.uk/Tools/psa/emboss_stretcher/) to calculate an optimal global alignment of human and S. cerevisiae MT-ATP6 and MT-ATP8 sequences. In order to predict putative impact of these substitutions on ATP synthase structure and function, we looked at their positioning on the crystallographic structure of S. cerevisiae mitochondrial ATP synthase [11,12] using the interface Swiss-Pdb Viewer (https://spdbv.vital-it.ch/, PDB code: 6B8H).
3. Results
3.1. Case report
The patient is a boy, born of non-consanguinous parents with no family history. He has a healthy younger sister. He presented hypotonia and microcephaly (-2DS) since age 4 months. He started associating words at age 2 years, but with dysarthria needing therapy. Walking was acquired at age 29 months and became stable only by age 6 years. Property was also acquired at 6 years. He gradually developed ataxia. Today, at age 13 years he presents ataxia increasing with effort, dysarthria, gait disorders, slowness, learning difficulties, exercise intolerance and fatigue needing to rest. Ophthalmological examination shows bilateral retinal hypoplasia. Cardiac, pulmonary and abdominal examinations are normal. Brain MRI showed bilaterally symmetric lesions in the basal ganglia, that correspond to a typical aspect of Leigh syndrome (data not shown).
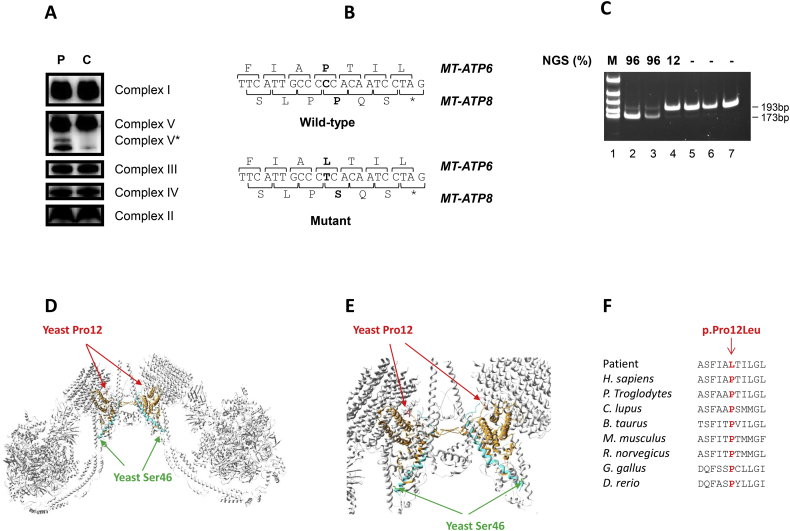
Blood lactate/pyruvate ratio was slightly elevated (17; normal <10). Histological examination of muscle was normal. Spectrophotometric analysis of the individual RC complexes in muscle revealed an isolated decrease of the CV specific activity (Supplementary Table 1). BN-PAGE analysis in patient's muscle revealed an isolated CV defect with a pattern similar to the one observed in other patients with MT-ATP6 or MT-ATP8 mutations (Fig. 1a) [3,6,[13], [14], [15]]. Amounts of the fully assembled ATP synthase were similar between patient and control. However, as previously described by Kytövuori et al., an increased amount of subcomplex F1 was observed in patient muscle with an additional assembly intermediate of complex V, referred as V*, detected in patient but not in control sample. By mtDNA NGS analysis, we identified a novel heteroplasmic substitution (m.8561C>T) in the overlapping region of MT-ATP6 and MT-ATP8 (Fig. 1b). This variant leads to a p.Pro12Leu substitution in MT-ATP6 depending subunit a and, a p.Pro66Ser substitution in MT-ATP8 depending subunit A6L of CV. PCR-RFLP analysis showed a high mutant load (96%) in patient's muscle and blood. The patient's mother was asymptomatic. Mutant load was of 12% in urines with comparable values in blood and buccal swab (Fig. 1c).
Fig. 1.
A novel m.8561C>T variant associated with early-onset neurological phenotype. A. BN-PAGE in muscles of control subject (C) and patient (P). The amount of subcomplex F1 is increased in the patient and V* corresponds to the supplementary band detected by the anti-complex V antibody. (C) is matched for age and gender to the patient. B: WT and mutated mtDNA sequences obtained by NGS. C: PCR-RFLP analysis of m.8561C>T in different tissues from the patient (lane 2: muscle, lane 3 blood), his mother (lane 4: urinary epithelial cells, lane 5: buccal swab, lane 6: blood) and a negative control (lane 7). M: molecular weight marker. Mutant loads detected by NGS are indicated (top): 96% in patient's muscle and blood, 12% in patient's asymptomatic mother urines and approximatelly the same load in her blood and buccal swab. D–E: Localization of the concerned amino acids on the crystallographic structure of S. cerevisiae mitochondrial ATP synthase (e: higher magnification). The MT-ATP6 and MT-ATP8 subunits are in orange and blue, respectively. The Pro12 (in human and S.cerevisiae) is highlighted in red, and the yeast Ser46 (Pro66 in human) is in green. F: Cross-species protein conservation of MT-ATP6, flanking the altered proline amino acid. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The human MT-ATP6 protein and the corresponding yeast protein have 31.7% identity and 51% amino acid similarity and Pro12 in human corresponds to Pro12 in yeast, indicating the conservation of this amino acid between these two organisms. With regard to the alignment of the human MT-ATP8 and S. cerevisiae sequences, apart from the first four N-terminal amino acids, the sequences are poorly conserved (19.1% identity and 32.4% similarity). In addition, the amino acid sequence in yeast is shorter (20 amino acids less than in humans). According to the alignment of these two sequences, Pro66 in humans is not preserved in yeast and would correspond to Ser46.
The Pro12 (corresponding to Pro12 in humans) is located on the N-terminal loop of the MT-ATP6 subunit and probably has a structural role in ensuring the proper positioning of the N-terminal end for the stabilization of this region of the ATPase complex. The Ser46 (corresponding to Pro66 in humans) is located at the end of an helix (Fig. 1d–e). The human Pro12 is also highly conserved through several other species which suggests a deleterious effect of the mutant (Fig. 1f).
4. Discussion
The m.8561C>G mutation, reported by Kytövuori's et al., leads to p.Pro12Arg substitution in MT-ATP6 predicted to be pathogenic and p.Pro66Ala substitution in MT-ATP8 predicted to be neutral [6]. Muscle heteroplasmy was very high (99%) with decreased ATP production and impaired assembly of complex V similar to what we found in our patient's muscle. Patients described by Kytövuori's et al. and the one we report have an ataxia. The phenotype is more severe in our case with psychomotor delay and microcephaly appearing during first months of life. However, we have no objective argument to explain this phenotypic variability, frequently found in mitochondrial diseases.
5. Conclusion
In conclusion, our results confirm the importance of proline residues at position 12/66 in MT-ATP6/8 and enlarge the spectrum of clinical manifestations associated with CV deficiency.
Spectrophotometric analysis of RC enzymatic activities in patient's muscle showing isolated CV activity decrease.
Funding
We received no specific funding for this work.
Author's contributions
KF performed the biochemical examinations, analyzed and interpreted of the patient's data and wrote the manuscript with VP-F supervision. CA, SB and CR performed the molecular examinations, analyzed and interpreted of the patient's data. AC and BC expertized clinical data. VS performed the crystallographic studies. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content, given final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We thank Gaëlle Auge, Mathieu Berthet, Christelle Camuso, Bernadette Chafino, Charlotte Cochaud, and Sandra Foustoul for technical help.
References
- 1.Jonckheere A.I., Smeitink J.A., Rodenburg R.J. Mitochondrial ATP synthase: architecture, function and pathology. J. Inherit. Metab. Dis. 2012;35(2):211–225. doi: 10.1007/s10545-011-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu T., Pagadala V., Mueller D.M. Understanding structure, function, and mutations in the mitochondrial ATP synthase. Microb. Cell. 2015;2(4):105–125. doi: 10.15698/mic2015.04.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonckheere A.I., Hogeveen M., Nijtmans L., van den Brand M., Janssen A., Diepstra H., van den Brandt F., van den Heuvel B., Hol F., Hofste T., Kapusta L., Dillmann U., Shamdeen M., Smeitink J., Smeitink J., Rodenburg R. A novel mitochondrial ATP8 gene mutation in a patient with apical hypertrophic cardiomyopathy and neuropathy. BMJ Case Rep. 2009 doi: 10.1136/bcr.07.2008.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware S.M., El-Hassan N., Kahler S.G., Zhang Q., Ma Y.W., Miller E., Wong B., Spicer R.L., Craigen W.J., Kozel B.A., Grange D.K., Wong L.J. Infantile cardiomyopathy caused by a mutation in the overlapping region of mitochondrial ATPase 6 and 8 genes. J. Med. Genet. 2009;46(5):308–314. doi: 10.1136/jmg.2008.063149. [DOI] [PubMed] [Google Scholar]
- 5.Imai A., Fujita S., Kishita Y., Kohda M., Tokuzawa Y., Hirata T., Mizuno Y., Harashima H., Nakaya A., Sakata Y., Takeda A., Mori M., Murayama K., Ohtake A., Okazaki Y. Rapidly progressive infantile cardiomyopathy with mitochondrial respiratory chain complex V deficiency due to loss of ATPase 6 and 8 protein. Int. J. Cardiol. 2016;207:203–205. doi: 10.1016/j.ijcard.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Kytövuori L., Lipponen J., Rusanen H., Komulainen T., Martikainen M.H., Majamaa K. A novel mutation m.8561C>G in MT-ATP6/8 causing a mitochondrial syndrome with ataxia, peripheral neuropathy, diabetes mellitus, and hypergonadotropic hypogonadism. J. Neurol. 2016;263(11):2188–2195. doi: 10.1007/s00415-016-8249-2. [DOI] [PubMed] [Google Scholar]
- 7.Rustin P., Chretien D., Bourgeron T., Gérard B., Rötig A., Saudubray J.M., Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta. 1994;228(1):35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 9.Fragaki K., Chaussenot A., Boutron A., Bannwarth S., Rouzier C., Chabrol B., Paquis-Flucklinger V. Assembly defects of multiple respiratory chain complexes in a child with cardiac hypertrophy associated with a novel ACAD9 mutation. Mol. Genet. Metab. 2017;121(3):224–226. doi: 10.1016/j.ymgme.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Plutino M., Chaussenot A., Rouzier C., Ait-El-Mkadem S., Fragaki K., Paquis-Flucklinger V., Bannwarth S. Targeted next generation sequencing with an extended gene panel does not impact variant detection in mitochondrial diseases. BMC Med. Genet. 2018;19(1) doi: 10.1186/s12881-018-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo H., Bueler S.A., Rubinstein J.L. Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science. 2017;358(6365):936–940. doi: 10.1126/science.aao4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dautant A., Meier T., Hahn A., Tribouillard-Tanvier D., di Rago J.P., Kucharczyk R. ATP synthase diseases of mitochondrial genetic origin. Front. Physiol. 2018;9:329. doi: 10.3389/fphys.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houstĕk J., Klement P., Hermanská J., Houstková H., Hansíková H., Van den Bogert C., Zeman J. Altered properties of mitochondrial ATP-synthase in patients with a T—>G mutation in the ATPase 6 (subunit a) gene at position 8993 of mtDNA. Biochim. Biophys. Acta. 1995;1271(2–3):349–357. doi: 10.1016/0925-4439(95)00063-a. [DOI] [PubMed] [Google Scholar]
- 14.Nijtmans L.G., Henderson N.S., Attardi G., Holt I.J. Impaired ATP synthase assembly associated with a mutation in the human ATP synthase subunit 6 gene. J. Biol. Chem. 2001;276(9):6755–6762. doi: 10.1074/jbc.M008114200. [DOI] [PubMed] [Google Scholar]
- 15.Wittig I., Meyer B., Heide H., Steger M., Bleier L., Wumaier Z., Karas M., Schägger H. Assembly and oligomerization of human ATP synthase lacking mitochondrial subunits a and A6L. Biochim. Biophys. Acta. 2010;1797(6–7):1004–1011. doi: 10.1016/j.bbabio.2010.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectrophotometric analysis of RC enzymatic activities in patient's muscle showing isolated CV activity decrease.