Abstract
The leishmaniases are multifactorial zoonotic diseases requiring a multidisciplinary One Health approach for diagnosis and control. For leishmaniasis diagnosis, here we describe production of a new recombinant protein based on a kinesin-related gene of Leishmania braziliensis (Lbk39), which shows 59% amino acid identity to the L. infantum homologue. The Lbk39 gene was synthesized, inserted into the pLEXSY-sat2 vector and transfected into L. tarentolae cells by electroporation. Culturing was carried out, and the secreted recombinant protein with a C-terminal histidine tag purified using nickel affinity chromatography on the culture supernatant, yielding a final product at 0.4 mg/mL. An indirect enzyme linked immunosorbent assay (ELISA) was standardised using sera from 74 Brazilian patients with cutaneous leishmaniasis and 11 with visceral leishmaniasis. Optimal ELISA conditions were established for the Lbk39 antigen in comparison with a crude extract from L. braziliensis. The sensitivity, specificity analysis and receiver operating characteristic (ROC) curve were determined with a significance level of 5%. The ROC curve showed a good accuracy with an area under curve (AUC) = 0.967, p < 0.001 (0.941–0.993) for CL patients and an AUC = 100 (100-100) for VL patients. The values of sensitivity and specificity were 88 and 98% for CL and 100 and 100% for VL, respectively. The study showed good production and expression of the target protein and has generated a potential new antigen for the diagnosis of leishmaniasis.
Keywords: Diagnosis, Cutaneous leishmaniasis, Visceral leishmaniasis, Recombinant protein, Kinesin, rK39, Leishmania braziliensis
1. Introduction
The leishmaniases are a group of largely zoonotic, vector-borne diseases transmitted by sand flies and caused by heteroxenous parasites of the genus Leishmania Ross, 1903. The complexity of their epidemiology means they require a One Health approach for their diagnosis and control, and they present a significant ongoing global public health challenge [3,42]. Three varieties of epidemiological cycles are known: a sylvatic cycle in which human infection is accidental and transmission occurs in wild foci; a peridomestic cycle in which the reservoirs of infection are domestic animals and transmission to humans occurs around or within human dwellings; and an anthroponotic cycle in which transmission is human to human via anthropophilic sand flies [11]. Depending on the parasite species and disease focus various combinations of these cycles may co-exist. Another layer of complexity is provided by the environmental factors that influence mammalian reservoir and sand fly vector distributions. For example, the emergence of new foci or re-emergence of leishmaniases has occurred due to invasion of the sand flies into urban areas [41].
Upon infection these parasites interact with the host immune system in a variety of ways to enhance their survival [19]. A common feature of infection is that the ability of the host to control the parasite depends on the production of cell-mediated immune responses, which in turn are able to activate macrophages to eliminate the intracellular parasites [40]. Although the resolution of the infection is largely mediated by Th1 cells secreting IFN-γ in response to an increase of IL-12, there is also development of a Th2 cell response. The resulting increase of IL-4 can result in progression of lesions and lead to systemic disease, but in addition, some Leishmania antigens can drive the differentiation of T-cells that can activate B-lymphocytes to produce immunoglobulins. It is also known that antibodies are produced through neutrophil stimulation at the very beginning of the infection. Although various studies suggest that such antibodies play no role in host protection, they can be useful in diagnosis for determining the presence of the parasite [1,26].
The correct diagnosis of the leishmaniases is performed through a combination of clinical, epidemiological and laboratory findings [37]. A range of diagnostic tools is available, but none of them are perfect and new more reliable diagnostic tests must still be developed, which should be easy to handle, cheap to produce, and perform with high sensitivity and specificity [25]. Some purified recombinant antigens of various Leishmania species have been produced and used in serological assays, such as the rK39 antigen for the serodiagnosis of visceral leishmaniasis (VL) [4,15]. The rK39 antigen is a recombinant protein derived from Leishmania infantum that contains 6.5 tandem copies of a B-cell antigenic epitope composed of 39 amino acids. This antigen is related to a kinesin motor protein, which is well conserved between L. infantum and L. donovani, and the corresponding gene reveals a single open-reading frame that encodes a total of 298 amino acids with a predicted molecular mass of 32.7 kDa [10]. The Leishmania motor protein is involved in various intracellular processes and is present in the amastigote forms of many species.
Production of an antigenic protein of Leishmania, by heterologous expression of its specific epitopes in a prokaryotic system such as Escherichia coli, is a relatively straightforward technique that is both inexpensive for culturing and quick for processing the target recombinant protein. However, such systems lack eukaryotic post-translational activity, which is a significant disadvantage in producing many eukaryotic proteins. Further, high concentrations of the unfolded protein can occur, leading to a decline in effective yield, and culturing at a temperature optimal for E. coli can also reduce yields of recombinant protein and increase protein degradation [20]. The protozoan Leishmania tarentolae, which is not pathogenic to mammals, has been explored as a general eukaryotic host to develop a platform that allows complex eukaryotic protein expression at high levels, and which also has the ability to produce proteins with appropriate post-translational processing [5]. Moreover, the host is easy to manipulate and can be cultivated on a cheap medium with a 6 to 8 h doubling time. The maintenance of a transfected culture of L. tarentolae is performed under specific antibiotic selection and maintains the same level of protein expression after several months of culturing [9,21,24]. Finally, specifically with respect to this study, when the desired recombinant antigen itself is derived from a species of Leishmania use of this system maximises the probability of successful expression.
Based on what has been described above, the aim of this study was to explore the use of L. tarentolae as a host for the expression and secretion of a L. braziliensis kinesin-related recombinant protein, which was identified based on the reference kinesin-related rK39 gene of L. infantum. The diagnostic efficiency of this new antigen was evaluated by developing an indirect ELISA for leishmaniasis detection. Until now, no studies have reported on the levels of antibodies against L. braziliensis kinesin in cutaneous leishmaniasis (CL) patients.
2. Materials and methods
2.1. Serum sample collection
The patients enrolled in the study were divided into four groups, according to clinical classification (Table 1). In Group 0, 50 healthy individuals from a non-endemic area and medically examined to eliminate any previous CL infection, were used to determine the cut off for the ELISA test and the specificity. Patients with L. braziliensis, diagnosed with infection by parasite isolation and clinical examination, were classified in Group 1 (n = 74). Patients with L. infantum diagnosed by serology and PCR were classified in the Group 2 (n = 11). Patients with a positive leishmaniasis diagnosis were treated, by local service staff, in accordance with the guidelines of the Brazilian Ministry of Health, as described in the Manual of surveillance and control of American Integumentary Leishmaniasis [7,8]. Patients with Chagas disease (n = 13), confirmed by serology, were also studied to assess the possibility of cross-reactivity. Patient serum samples were stored frozen (−20 °C) before use.
Table 1.
Identification of samples used in this study.
| Group | N | Description |
|---|---|---|
| 0 | 50 | Healthy individuals from non-endemic areas - Curitiba |
| 1 | 74 | L. braziliensis CL patients with active lesions and no treatment |
| 2 | 11 | Positive patient for visceral leishmaniasis (VL) |
| 3 | 13 | Patients with Chagas disease (CD) |
N: number of patients in each group.
This study was conducted in accordance with the International Ethical Guidelines for Biomedical Research in Human Beings. In addition, ethical approval was obtained from the Universidade Federal do Paraná Ethical Committee under number 684.244, and in accordance with the law of the Southern Common Market Treaty (Mercosur), Resolution No. 129/96.
2.2. Lbk39 plasmid construction, cloning, and propagation in Escherichia coli
A homology search was performed by means of BLAST similarity [2] in the TritrypDB database website. Sequences derived from a kinesin-related gene of L. braziliensis, henceforth called Lbk39, and comprised of 828 nucleotides (nt) were used for initial plasmid construction (Suppl. Fig. 1A). These were identified by homology with the kinesin-related gene of L. infantum - Genebank: L07879, described by Burns et al. [10], and containing 39 amino acid repeats. Lbk39 also contains a related 39 amino acid sequence (Suppl. Fig. 1B) and is also predicted to comprise immunologically dominant B-cell epitopes (BepiPred; http://www.cbs.dtu.dk/services/BepiPred/).
For expression of the target recombinant protein, the synthetic gene Lbk39 was assembled from synthetic oligonucleotides by Invitrogen (Germany), and the fragment was inserted into the pLEXSY-sat2 recombinant vector, developed by Jena Bioscience (Germany), and cloned with a 6 × His-tag into the corresponding site of the above-mentioned recombinant vector. The expression vector was designed for integration into the chromosomal 18SrRNA (ssu) locus of the parasite [9], allowing for the true expression of the eukaryotic protein; also, for this specific study, the target protein was selected to be secreted into the culture medium.
Following the construction of the Lbk39 plasmid, the One Shot™TOP10 Chemically Competent Escherichia coli strain (Invitrogen) was chosen for the plasmid cloning and propagation, and the procedure for culturing was followed according to the manufacturer's instructions, except for the incubation temperature, which was 30 °C for plasmid stability reasons. After that, the plasmid was purified from the E. coli strain using the Geneflow Q-Spin Plasmid DNA Purification Kit and was sent for sequencing. The forward P1442 (5′-CCGACTGCAACAAGGTGTAG-3′) and reverse A264 (5′-CATCTATAGAGAAGTACACGTAAAAG-3′) sequencing primers, included in the LEXSY kit, were used to confirm the plasmid identity and sequence.
2.3. Lkb39 plasmid transfection into LEXSY culture and Lbk39 LEXSY culturing
The propagated and purified Lbk39 plasmid from the E. coli strain was linearised through digestion with the SwaI (SmiI) enzyme, from Streptococcus milleri S - 10 U/μL (Thermo Fischer Scientific), to prepare for plasmid transfection into the LEXSY host L. tarentolae, according to the manufacturer's protocol. To confirm the correct procedure for linearization and to isolate the fragment corresponding to the plasmid, 1% agarose gel-isolation of the expression cassette with an Agarose Gel Extraction Kit (Jena Bioscience) was performed according to the manufacturer's instruction. The LEXSY strain was previously prepared for transfection according to the LEXSYcon2 Expression Kit manual (for detail see https://www.jenabioscience.com/images/ae3a4f50f1/EGE-1310.pdf). When ready for transfection through electroporation, the cultured cells were handled according to the same manual mentioned above. Other aliquots of LEXSY cells were electroporated without DNA under the same conditions as a negative control. Then the electroporated cells were transferred to tissue culture flasks containing 10-mL Brain Heart Infusion (BHI) medium supplemented with porcine hemin (Jena Bisocience) and penicillin and streptomycin (Pen-Strep, Jena Bioscience) at 26 °C in the dark under aerated conditions. As soon as the cultures became slightly turbid (24 h after electroporation), the specific Streptothricin-class of aminoglycoside antibiotic Nourseothricin (LEXSY NTC, Jena Bioscience) for the pLEXSY-sat2 vector was added, and the culture maintained under the same conditions by subpassage every four days.
The genomic integration of the Lbk39 plasmid into the chromosomal 18SrRNA (ssu) locus of L. tarentolae strain was confirmed by PCR. Genomic DNA was extracted from 2 mL of a dense Lbk39 LEXSY culture by means of the DNeasy Blood and Tissue Quick-start kit (Qiagen) according to the manufacturer's recommendation. After that, 200 ng of genomic DNA was added to two 0.2-mL microtubes (100 ng in each), containing the mixed solution of ultra-pure RNAse-free water, 5xHotStar HiFidelity PCR Buffer (including dNTPs), HotStar HiFidelity DNA Polymerase from HotStar HiFidelity Polymerase Kit (Qiagen) and into the first tube the specific primers (Jena Bioscience) for the genomic integration diagnostic: the F3001 forward primer (5′-GATCTGGTTGATTCTGCCAGTAG-3′), responsible for the integration of all ssu expression vectors; and the A1715 reverse primer (5′-TATTCGTTGTCAGATGGCGCAC-3′), responsible for the integration of all “AP” expression vectors with 5′UTR aprt. The second tube was prepared identically except for the primers (Jena Bioscience): the F2999 forward primer (5′-CCTAGTATGAAGATTTCGGTGATC-3′), responsible for the integration diagnostics of all sat expression vectors; and the F3002 reverse primer (5′-CTGCAGGTTCACCTACAGCTAC-3′), responsible for the integration diagnostics of all ssu integration vectors. The PCR conditions for the F3001/A1715 pair of primers were as follows: 1 cycle at 95 °C for 5 min for the initial denaturation, followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 1 min, and 1 cycle at 72 °C for 10 min for final extension; whereas the PCR conditions for the F2999/F3002 pair of primers were: 1 cycle of 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 53 °C for 30 s and 72 °C for 1 min, and 1 cycle at 72 °C for 10 min.
Lbk39 recombinant protein purification was carried out using a HisTrap HP 1-mL column (GE HealthCare) by loading the culture media onto the column according to the manufacturer's instructions. Afterwards, salts and imidazole were removed by dialysis in a PBS buffer at 4 °C, twice for 2 h and once overnight. Then, lyophilisation was performed to concentrate the purified recombinant protein. To analyse whether the purification process had been successful, the purified and dialysed recombinant protein was concentrated with trichloroacetic acid (TCA), as indicated in the LEXSYcon2 Expression kit manual, loaded on a 15% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and stained with silver nitrate. The protein determination was performed using the Micro BCA™ Protein Assay Kit (Thermo Fischer Scientific) following the manufacturer's procedure.
2.4. Enzyme-linked immunosorbent assay (ELISA)
To determine whether levels of anti-Leishmania antibodies in human serum from uninfected individuals and those infected with CL and other diseases can be detected using Lbk39 epitopes, optimal ELISA conditions were established [14]. A range of serum dilutions (1:100, 1:200, 1:400 and 1:800), antibody-conjugate dilutions (1:5000, 1:10,000 and 1:20,000) and antigen dilutions (0.1 μg, 0.5 μg and 0.85 μg/100 μL/well) were tested in various combinations.
High-binding polystyrene microtiter plates (96 well EIA/RIA 1 × 8 Stripwell Plate, Costar, USA) were coated overnight at 4 °C with 100 μL/well solution of antigen diluted in a carbonate–bicarbonate buffer (pH 9.6). On the following day, the plates were washed twice with 200 μL/well of a washing solution (0.9% w/v NaCl, 0.05% v/v Tween 20), and then the wells were blocked with 120 μL of a blocking solution (PBS + 0.1% w/v casein) for 1 h at 37 °C. Afterwards, they were washed twice again with 200 μL/well of the washing solution. Following the washing step, serum samples were diluted in an incubation solution (PBS + 0.25% w/v casein) and were added in their respective wells and incubated at 37 °C for 1 h. Then the plates were washed four times with 200 μL/well of the washing solution, and a polyclonal goat anti-human IgG HRP conjugate (2 mg/mL, SanBio Científica) was diluted and was added to each well for 1 h at 37 °C. Finally, the reaction was developed by adding 100 μL of a 10.5-mL citrate buffer (4.5% w/v Na2PO4, 3.25% w/v citric acid, pH 5.0), with 2 mg of o-Phenylenediamine dihydrochloride (2 mg/tablet, Sigma, USA) and 2 μL of 30% (w/w) H2O2 to each well at room temperature for 15 min, avoiding light, and then 20 μL of a solution 1:20 of H2SO4 was added to stop the reaction. Plates were read in a Powerwave HT reader (BioTek) at 492 nm, and values were expressed in absorbance.
Soluble proteins from the crude extract of L. braziliensis promastigotes (strain MHOM/BR/84/LTB300) were included in the study as a positive control. As additional controls, the pooled positive and negative serums were included in each plate when testing individual sera; each sample was measured in triplicate, and the whole assay described above was performed in duplicate.
2.5. Statistical analysis
The receiver operating characteristic (ROC) curve was derived based on the logistic regression model, considering the classification of the samples (presence or absence of the disease) as a dependent variable and each antigen as an independent variable. Logistic regression model, ROC curve and sensitivity and specificity analyses were performed using R software [35] with an auxiliary pROC system [36]. We used analysis of variance (ANOVA) and Tukey's test to compare the differences in absorbance between the groups. A significance level of p < 0.05 was adopted.
3. Results
3.1. Lbk39 plasmid construction
Gene synthesis using pLEXSY E.coli/L.tarentolae shuttle vectors was used to construct the Lbk39 plasmid, encoding L. braziliensis sequences homologous to the L. infantum rK39 antigen (Suppl. Fig. 1A). BLAST similarity sequence analysis of the cloned sequence confirmed it comprised a 843-bp product (828 plus 15 bp vector flanking sequences) homologous to the locus LBRM_14_1110 of L. braziliensis (strain MHOM/BR/75/M2904), and which exhibited 84% nucleotide sequence identity and 59% amino acid identity with the equivalent kinesin-related gene of L. infantum (Suppl. Fig. 1C). The predicted protein encoded a protein of 281 amino acids with a predicted molecular mass of 30 kDa, with six copies of 39 AA repeats. The cloned sequence was then inserted into the pLEXSY-sat2 vector for secretion and addition of a C-terminal 6 × His-tag (Suppl. Fig. 1B). The purification of the Lbk39 plasmid yielded 1.5 μg/μL of DNA, and plasmid identity was confirmed by sequencing the purified product, with 100% identity for both forward P1442 and reverse A264 sequencing primers.
3.2. Lbk39 plasmid transfection into Leishmania tarentolae
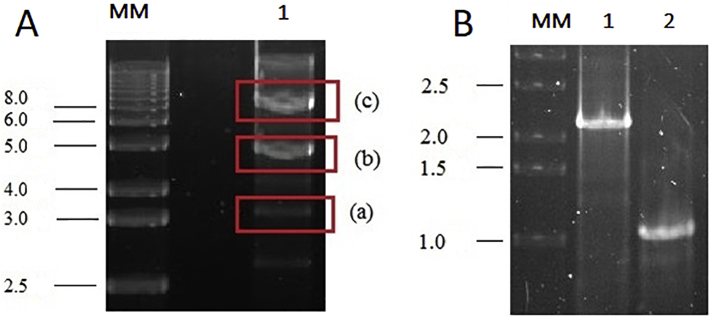
Linearization of the plasmid through digestion with the Swal (Smil) enzyme generated a 2.9-kbp fragment related to the E. coli part and a larger fragment (approximately 5 kbp) related to the Lbk39 plasmid (Fig. 1A). The linearized plasmid was used to transfect L. tarentolae under antibiotic selection, and after approximately 10 days of Lbk39 LEXSY culturing, the cultures became turbid (107 cells/mL), and there was no noticeable growth of the parasites in the negative control flasks. On the 12th day of culturing, another passage was made, and 2 mL of that dense culture were withdrawn to perform the confirmation of Lbk39 genomic integration through PCR. For this objective, two pairs of primers were used: one of them amplifying from within the expression cassette and the other amplifying to a chromosomal ssu-flanking sequence that was not present on the plasmid. The PCR reactions resulted in two DNA fragments of different sizes, one for each pair of primers, as expected and indicated by the manufacturer: a 1.1 kbp fragment size for F3001/A1715 primers and a 2.3 kbp fragment size for F2999/F3002 (Fig. 1B).
Fig. 1.
A. Linearization and partial digestion of the Lbk39 plasmid with the SwaI (SmiI) enzyme. MM: molecular mass markers (1 kb DNA ladder); Lane 1 (a): 2.9 kbp fragment related to the E coli part; Lane 1 (b): a larger fragment (approximately 5 kbp) related to the Lbk39 plasmid; Lane 1 (c): the entire Lbk39 plasmid, linearised, with 8486 bp. B. PCR for confirmation of the Lbk39 genomic integration into the chromosomal 18SrRNA (ssu) locus of L. tarentolae resulted in two DNA fragments of different sizes: 2.3 kbp fragment size for F2999/F3002 pair of primers (lane 1) and 1.1 kbp fragment size for F3001/A1715 pairs of primers (lane 2) MW: molecular weight markers.
3.3. Purification of Lbk39 protein
The Lbk39 protein was expressed as a 6xHis-tagged recombinant protein in the pLEXSY-sat2 vector, inserted into the chromosomal 18SrRNA (ssu) locus of L. tarentolae and designed to be secreted into the culturing media with predicted molecular mass of 31.2 kDa (Suppl. Fig. 1B). The purification procedure and the protein expression were confirmed by SDS-PAGE, with the purified recombinant protein exhibiting a molecular mass of approximately 35 kDa (Fig. 2). The purified Lbk39 recombinant protein was obtained at a final concentration of 0.4 mg/mL.
Fig. 2.
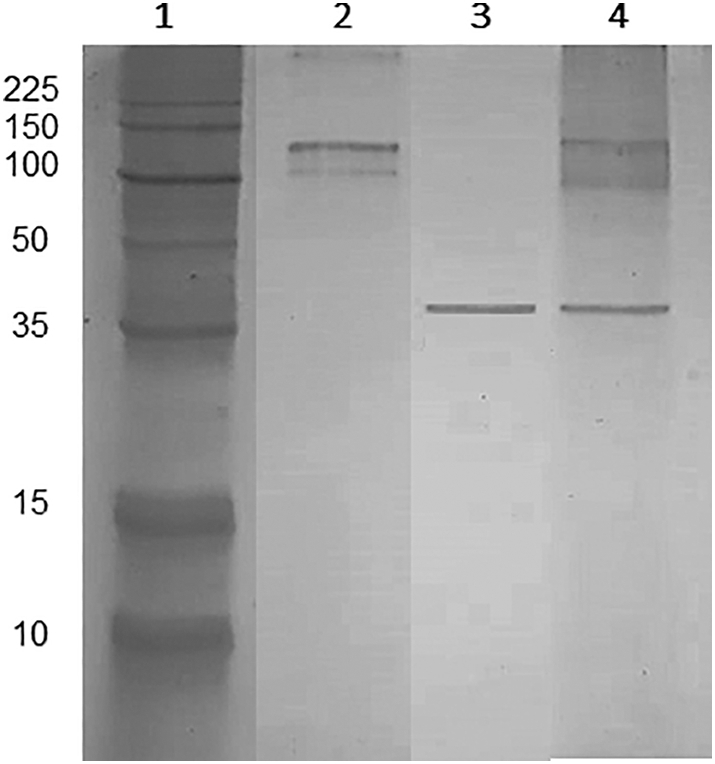
The purified Lbk39 recombinant protein expression confirmed by SDS-PAGE. Lane 1: molecular mass markers (10–225 kDa); Lane 2: crude extract of L. tarentolae non-transfected; Lane 3: purified Lbk39 recombinant protein; Lane 4: crude extract of L. tarentolae transfected.
3.4. Enzyme-linked immunosorbent assay (ELISA)
An indirect ELISA was developed and standardised using the recombinant protein as an antigen for detection of specific anti-Leishmania antibodies in the sera of leishmaniasis patients. The optimum combination of conditions were found to be as follows: antigen concentration of 0.1 μg/100 μL/well; serum samples diluted to 1:200 in incubation solution (PBS + 0.25% w/v casein); and polyclonal goat anti-human IgG HRP conjugate (2 mg/mL) diluted to 1:10,000.
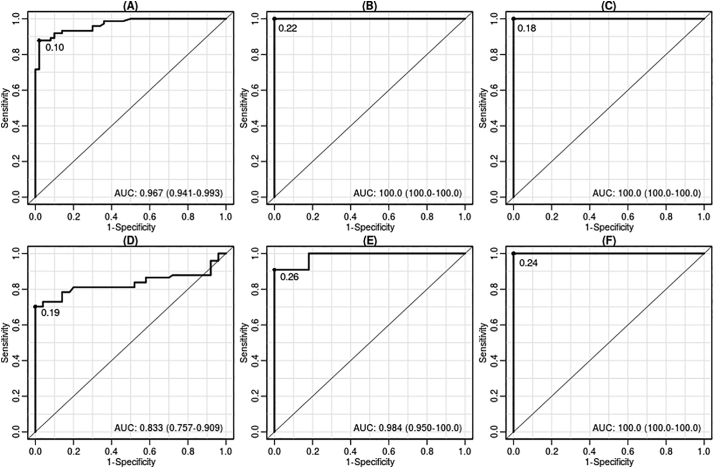
The presence of anti-Leishmania antibodies was determined by comparing antibody levels in patients infected with CL and VL with healthy individuals living in the same endemic area, as well as individuals with Chagas disease, using the Lbk39 recombinant protein as the antigen. The results obtained were compared using the same parameters and the same sample groups using as a positive control antigen a crude extract of L. braziliensis promastigotes. Comparing the two types of Leishmania antigen for patients known to have leishmaniasis, the Lbk39 antigen showed sensitivity of 88% for CL and of 100% for VL patients. The specificity was 98% and 100%, respectively (Table 2 and Fig. 3). Based on the percentage positivity for all serum samples and sensitivity and specificity values, the Lbk39 antigen was able to detect antibodies from both CL and VL patients. The results obtained with the Lbk39 antigen were equivalent to or better than those using crude promastigote extract, except for a small reduction in specificity for CL patients (98% versus 100%).
Table 2.
ROC curve analysis for Lbk39 antigen compared to the ROC curve analysis for the positive control (L. braziliensis) performed using MedCalc software.
| CL |
VL |
|||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| L. braziliensis | 70 | 100 | 90 | 100 |
| Lbk39 | 88 | 98 | 100 | 100 |
L. braziliensis: soluble proteins from crude extract from Leishmania (Viannia) braziliensis culture (strain MHOM/BR/84/LTB300.
Fig. 3.
Receiver operating characteristic (ROC) curve analysis for Lbk39 antigen compared to the positive control L. braziliensis crude extract of Leishmania (Viannia) braziliensis (strain MHOM/BR/84/LTB300). A to C = Lbk39 antigen ROC curve comparing results of the group healthy non-endemic individuals vs. the group with active skin lesions (A); visceral leishmaniasis (B) and Chagas disease (C). D to F = ROC curve results from crude extract of the L. (V.) braziliensis in healthy non-endemic individuals vs. group with active skin lesions (D); visceral leishmaniasis (E) and Chagas disease (F). AUC = area under curve.
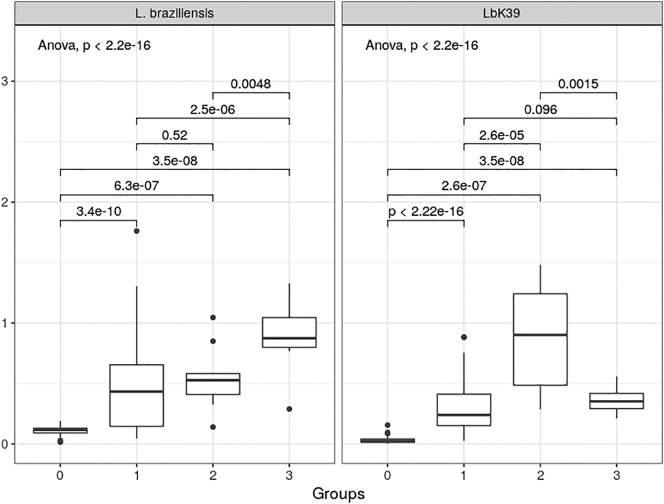
The range of absorbance readings obtained in these assays are shown in Fig. 4. Using the crude antigen, the range of readings obtained was high with the CL patients (Group 1), otherwise the readings were well grouped. However, the crude antigen also gave high readings with patients diagnosed with Chagas disease (Group 3). With the recombinant Lbk39 antigen the cross-reactivity with Chagas patient sera was much reduced, and the readings with the CL patient group were more tightly grouped. Even so the median value for the Chagas patients (Group 3) was still higher than that for CL patients. However, a wider range was found in the VL group using the LbK39 antigen. ANOVA indicated significant differences between the means of the various groups for both antigens. Pairwise comparisons using Tukey's test showed these differences were significant (p < 0.05) in all cases except between Groups 1 and 2 (CL and VL patients) with L. braziliensis crude antigen, and between Groups 1 and 3 (CL and Chagas patients) with LbK39. In all cases the control patients (Group 0) were significantly different to all infection groups.
Fig. 4.
Levels of anti-Lbk39 antibodies detected by means of indirect ELISA in individuals from CL endemic and non-endemic areas in Brazil compared to levels of anti-L. braziliensis antibodies detected by the same technique. Group 0: healthy individuals from non-endemic areas; Group 1: CL patients with active lesion and culture positives; Group 2: VL: positive patients for VL; Group 3: serum from patients with Chagas disease. ANOVA p values are shown for each antigen, together with pairwise Tukey's tests for comparisons between groups for the same antigen.
4. Discussion
Several recombinant proteins have been investigated for their anti-Leishmania antibody responses in patients in attempts to develop the most suitable antigens for diagnostic purposes, of which the best so far is the rK39 antigen for VL [4]. However, although recombinant proteins have been extensively used for specific antibody detection, their use in diagnostic tests has revealed some problems. For example, they can be less immunoreactive than the corresponding purified antigen due to the absence of post-translational modifications, depending on the protein expression system. Further, their production in high quality and quantity is almost always laborious and they can be expensive to produce. Here we explored the use of the pLEXSY/Leishmania tarentolae system for cloning, transfection and recombinant protein production for leishmaniasis immunodiagnosis, in particular for the diagnosis of L. braziliensis infection.
We designed a sequence based on L. braziliensis to generate a product that we named Lbk39, which exhibited 59% amino acid identity to the kinesin-related gene of L. infantum (rK39) that is already used in the serodiagnosis of VL. The Lbk39 sequence was synthesized, inserted into pLEXSY-sat2 recombinant vector and cloned with a 6×His-tag, then inserted into the chromosomal 18SrRNA (ssu) locus of L. tarentolae and selected to be secreted into the culturing media. Successful expression of a ~35 kDa protein was achieved, correlating with the predicted molecular mass of the 289 amino acid 31.2 kDa recombinant protein (Suppl. Fig. 1B). Therefore, we can now add Lbk39 to the wide range of proteins that can be expressed in the L. tarentolae system [24]. Recombinant protein production via large-scale fermentation of L. tarentolae is not expensive and allows yields of 0.1 to 5 mg/L to be achieved [5,22,[23], [30]]. As used in our study, improved expression is also obtained if the target gene is followed by the 3’-UTR (intergenic untranslated regions) from a highly expressed gene, because in trypanosomatids the regulation of protein expression generally occurs by a post-transcriptional process involving the UTRs as in present work [16,31,39]. Expression and purification of the target antigen in the current study yielded recombinant Lbk39 antigen at 0.400 mg/mL.
Lbk39 was evaluated for the serodiagnosis of CL due to L. braziliensis and showed 88% sensitivity and 98% specificity, compared to 98% sensitivity and 100% specificity in VL patients. Absorbance values ranged between 0.46 and 0.016 for CL and between 0.92 and 0.03 for VL patients. The difference between positive and negative sera was similar (30 and 28.4 times for VL and CL, respectively). These findings show that, whilst the target protein was produced from a kinesin-related gene of L. braziliensis and performed well with L. braziliensis patients, antibodies from patients with VL (L. infantum) were also able to recognize Lbk39. The recombinant protein was also recognised by sera from Chagas patients. These are interesting results, however, they do not compromise the use of Lbk39 for serodiagnosis of CL as the other clinical features of infection in VL or Chagas disease are quite different. In fact, given the difficulty in making a positive diagnosis for CL, it may be an advantage and facilitate the usage of Lbk39 as a general immunodiagnostic antigen for both VL and CL, since the main issue is a lack of existing tools for the latter. A greater sampling of patients with cutaneous leishmaniases coming from different regions of Latin America will be needed to confirm if this is a useful approach. The cross-reactivity between the CL and VL patients likely arises from two factors, the first of which is the conservation between Lbk39 and the homologue from L. infantum (Suppl. Fig. 1), which, while only 59% at the amino-acid level, is concentrated in several immunogenic repeat motifs. The second factor is the very high antibody response stimulated by VL infection compared to CL, which also explains the relatively high values seen in the ELISA results for VL sera with Lbk39, and the high sensitivity.
The sensitivity and specificity of a leishmaniasis immunodiagnostic test is influenced by various factors such as antigenic and structural properties of the antigen itself, the duration of the infection, number of lesions, and variation in the parasite and host population. Regarding properties of the antigen, although no previous studies have investigated the levels of antibodies against L. braziliensis kinesin-related proteins in CL and VL patients until this study, a few have investigated the reaction of antibodies from CL patients against L. infantum kinesin-related recombinant proteins. For example, Molinet et al. [28] found that all of 272 serum samples from patients with CL in Brazil were negative when using one commercially available rK39 rapid test. Hartzell et al. [18] observed that both the rK39 rapid test and ELISA using the rK39 antigen demonstrated a positivity of only 10.2 and 28.8%, respectively, in United States soldiers stationed in Afghanistan and Iraq who had contracted CL (mostly due to L. major). Likewise, Oliveira et al. [32] evaluated several recombinant antigens that demonstrated the ability to identify Leishmania infantum-infected patients and found that CL patients were generally less well identified. Interestingly, only 3 out of 26 CL patients showed a positive result using an antigen that encoded a C-terminal fragment of an L. infantum kinesin. One potentially important difference to the current study is that the recombinant antigens described by Oliveira et al. were expressed in E. coli, perhaps compromising their sensitivity for CL diagnosis. According to Moreno et al. [29], the high titres of the anti-rK39 antibody in patients with acute VL are explained by expression in the high number of amastigotes present, compared to asymptomatic patients that have lower numbers of amastigotes. However, here we show that Lbk39 is capable of detecting antibodies in CL patients, which also have low numbers of amastigotes. Another factor to consider is the potential diversity of the diagnostic antigen. In that regard, Bhattacharyya et al. [6] showed that there is diversity in rK39 sequences between L. infantum and L. donovani, which may explain the poorer performance of rK39 in diagnosis of East African VL due to L. donovani. Therefore, potential diversity in Lbk39 should also be investigated in further studies [13]. Finally, the reasons why kinesin-related cytoskeletal proteins have been found to be good antigens for serodiagnosis is not fully understood but presumably is related to their structure, as they contain repetitive amino acid sequences thus presumably providing multiple stimulation of antibody responses.
Regarding other factors that affect sensitivity and specificity in the current context, duration of the infection is significant. In patients with recent lesions (1 to 6 months of progression), serological negativity is higher, and parasitological tests are more sensitive and specific [12]. Also, in the case of positive serology, the mean titres are significantly higher in patients with multiple lesions, reflecting the higher antigenicity induced by a larger number of parasites. Even within the same species, genetic variability of responses can be high and the antigen used for serological testing can give different results. For example, the rK39 antigen used to detect VL antibodies in several regions of the world, shows variable sensitivity and specificity according to geographical region and ethnic groups [38,27]. Different host immune responses may also be responsible for the variability of serological test results. Goto et al. [17] analysed antibody responses to the rK39 antigen in humans and dogs with VL, and they noticed that humans showed much stronger immune responses to the rK39 antigen than dogs, concluding that the rK39 recombinant antigen is very specific towards detecting VL in humans only. However, Porrozzi et al. [33] revealed that the IgG response to the rK39 antigen was variable in asymptomatic dogs (sensitivity of 66%) and significantly higher in symptomatic dogs (sensitivity of 100%). Likewise, 33% of L. braziliensis-infected dogs were positive for the rK39, and 11% of dogs with leptospirosis were also positive showing cross reactivity. For intervention programmes of leishmaniases in humans and dogs, an ideal serodiagnostic tests must be able to identify infected and non-infected reservoirs, specifically in dogs [34], thus providing the possibility of guided control and treatment.
In summary, this study showed that the recombinant Lbk39 protein produced was able to recognize Leishmania infection in the serum of humans with cutaneous or visceral leishmaniases in Brazil. This is a particularly important result for the L. braziliensis CL patients, where there is a lack of serological tests. Further work is required to investigate the potential use of this antigen in different population sera, as well as in different geographical regions in order to determine the specific ability to detect anti-Leishmania antibody levels in patients with CL or VL. In addition, analysis of the response to Lbk39 by sera of dogs infected with L. infantum should be considered in order to determine how the antigen behaves in different hosts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank both Universidade Federal do Paraná (UFPR), Brazil, and Lancaster University (LU), UK, for the approved use of the Molecular Biology Laboratory of the Bioprocess, as well as the Biotechnology Engineering Department of UFPR facilities and the Division of Biomedical and Life Sciences laboratories. This study was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de nível Superior (CAPES, Brazil - Process number 99999.008072/2014-00) and Lancaster University, UK.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2019.100111.
Appendix A. Supplementary data
Supplementary material: Nucleotide and amino acid sequences of Lbk39.
References
- 1.Al-Qadhi B.N., Musa I.S., Hummadi Y.M.K.A. Comparative immune study on cutaneous leishmaniasis patients with single and multiple sores. J. Parasit. Dis. 2015;39:361–370. doi: 10.1007/s12639-013-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped Blast and Psi-Blast: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badaró R., Benson D., Eulalio M.C., Freire M., Cunha S., Netto E.M., Pedral-Sampaio D., Madureira C., Burns J.M., Houghton R.L., David J.R., Reed S.G. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J. Infect. Dis. 1996;173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 5.Basile G., Peticca M. Recombinant protein expression in Leishmania tarentolae. Mol. Biotechnol. 2009;43:273–278. doi: 10.1007/s12033-009-9213-5. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya T., Boelaert M., Miles M.A. Comparison of visceral leishmaniasis diagnostic antigens in African and Asian Leishmania donovani reveals extensive diversity and region-specific polymorphisms. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasil . 1ed. Ministério da Saúde; Brasília: 2010. Manual de Vigilância da Leishmaniose Tegumentar Americana/Ministério da Saúde, Secretaria de Vigilância em Saúde. [in Portuguese] [Google Scholar]
- 8.Brasil . 2ed. Ministério da Saúde; Brasília: 2014. Manual de vigilância e controle da leishmaniose visceral/Ministério da Saúde, Secretaria de Vigilância em Saúde. [in Portuguese] [Google Scholar]
- 9.Breitling R., Klingner S., Callewaert N., Pietrucha R., Geyer A., Ehrlich G., Hartung R., Muller A., Contreras R., Beverley S.M., Alexandrov K. Non-pathogenic trypanosomatid protozoa as a platform for protein research and production. Protein Expr. Pur. 2002;25:209–218. doi: 10.1016/s1046-5928(02)00001-3. [DOI] [PubMed] [Google Scholar]
- 10.Burns J.M., Shreffler W.G., Benson D.R., Ghalib H.W., Badaró R., Reed S.G. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. U. S. A. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burza S., Croft S.L., Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 12.Costa C.A., Toledo V.P.C.P., Genaro O., Williams P., Mayrink W. Montenegro skin test. Evaluation of the composition and stability of the antigen preparation. Mem. Inst. Oswaldo Cruz. 1996;91:193–194. doi: 10.1590/s0074-02761996000200013. [DOI] [PubMed] [Google Scholar]
- 13.Cupolillo E., Brahim L.R., Toaldo C.B., Oliveira-Neto M.P., Brito M.E.F., Falqueto A., de Farias-Naiff M., Grimaldi G., Jr. Genetic polymorphism and molecular epidemiology of Leishmania (Viannia) braziliensis from different hosts and geographic areas in Brazil. J. Clin. Microbiol. 2003;41:3126–3132. doi: 10.1128/JCM.41.7.3126-3132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Souza L.M.B., Thomaz-Soccol V., Petterle R.R., Bates M.D., Bates P.A. Analysis of Leishmania mimetic neoglycoproteins for the cutaneous leishmaniasis diagnosis. Parasitol. 2018;145:1938–1948. doi: 10.1017/S0031182018000720. [DOI] [PubMed] [Google Scholar]
- 15.De Vries H.J.C., Reedijk S.H., Schallig H.D. Cutaneous Leishmaniasis: recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015;16:99–109. doi: 10.1007/s40257-015-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández F.J., Veja M.C. Technologies to keep an eye on: alternative hosts for protein production in structural biology. Curr. Opin. Struct. Biol. 2013;23:365–373. doi: 10.1016/j.sbi.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Goto Y., Howard R.F., Bhatia A., Trigo J., Nakatani M., Netto E.M., Reed S.G. Distinct antigen recognition pattern during zoonotic visceral leishmaniasis in humans and dogs. Vet. Parasitol. 2009;23:215–220. doi: 10.1016/j.vetpar.2008.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartzell J.D., Aronson N.E., Weina P.J., Howard R.S., Yadava A., Wortmann G.W. Positive rK39 serologic assay results in US servicemen with cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2008;79:843–846. [PubMed] [Google Scholar]
- 19.Kaye P., Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 20.Khow O., Suntrarachun S. Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac. J. Trop. Biomed. 2012;2:159–162. doi: 10.1016/S2221-1691(11)60213-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klatt S., Konthur Z. Secretory signal peptide modification for optimized antibody-fragment expression-secretion in Leishmania tarentolae. Microbial Cell Fact. 2012;11:97. doi: 10.1186/1475-2859-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovtun O., Mureev S., Jung W., Kubala M.H., Johnston W., Alexandrov K. Leishmania cell-free protein expression system. Methods. 2011;55:58–64. doi: 10.1016/j.ymeth.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Kushnir S., Gase K., Breitling R., Alexandrov K. Development of an inducible protein expression system based on the protozoan host Leishmania tarentolae. Protein Expr. Pur. 2005;42:37–46. doi: 10.1016/j.pep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Kushnir S., Cirstea I.C., Basiliya L., Lupilova N., Breitling R., Alexandrov K. Artificial linear episome-based protein expression system for protozoon Leishmania tarentolae. Mol. Biochem. Parasitol. 2011;176:69–79. doi: 10.1016/j.molbiopara.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Maia Z., Lirio M., Mistro S., Mendes C.M.C., Mehta S.R., Badaró R. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: systematic review with meta-analysis. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins V.T., Lage D.P., Duarte M.C., Costa L.E., Chávez-Fumagalli M.A., Roatt B.M., Menezes-Souza D., Tavares C.A.P., Coelho E.A.F. Cross-protective efficacy from an immunogen firstly identified in Leishmania infantum against tegumentary leishmaniasis. Parasite Immunol. 2016;38:108–117. doi: 10.1111/pim.12304. [DOI] [PubMed] [Google Scholar]
- 27.Mohapatra T.M., Singh D.P., Sem M.R., Bharti K., Sundar S. Comparative evaluation of rK9, rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. J. Infect. Dev. Ctries. 2010;4:114–117. doi: 10.3855/jidc.544. [DOI] [PubMed] [Google Scholar]
- 28.Molinet F.J.L., Ampuero J.S., Costa R.D., Noronha E.F., Romero G.A.S. Specificity of the rapid rK39 antigen-based immunochromatographic test Kalazar Detect® in patients with cutaneous leishmaniasis in Brazil. Mem. Inst. Oswaldo Cruz. 2013;108:293–296. doi: 10.1590/S0074-02762013000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno E.C., Gonçalves A.V., Chaves A.V., Melo M.N., Lambertucci J.R., Andrade A.S.R., Negrão-Corrêa D., Antunes C.M.F., Carneiro M. Inaccuracy of enzyme-linked immunosorbent assay using soluble and recombinant antigens to detect asymptomatic infection by Leishmania infantum. PLoS Negl. Trop. Dis. 2009;3:e536. doi: 10.1371/journal.pntd.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mureev S., Kovtun O., Nguyen U.T.T., Alexandrov K. Species-independent translational leaders facilitate cell-free expression. Nature Biotechnol. 2009;27:747–752. doi: 10.1038/nbt.1556. [DOI] [PubMed] [Google Scholar]
- 31.Niimi T. Recombinant protein production in the eukaryotic protozoan parasite Leishmania tarentolae: A review. In: Lorence A., editor. Recombinant Gene Expression: Reviews and Protocols. 3rd ed. Vol. 824. Springer Science and Business Media; 2012. pp. 307–315. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira G.G.S., Magalhães F.B., Teixeira M.A., Pereira A.M., Pinheiro C.M., Santos L.R., Nascimento M.B., Bedor C.N.G., Albuquerque A.L., Dos-Santos W.L.C., Gomes Y.M., Moreira-Jr E.D., Brito M.E.F., Carvalho L.C.P., Melo-Neto O.P. Characterization of novel Leishmania infantum recombinant proteins encoded by genes from five families with distinct capacities for serodiagnosis of canine and human visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2011;85:1025–1034. doi: 10.4269/ajtmh.2011.11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porrozzi R., Costa M.V.S., Teva A., Falqueto A., Ferreira A.L., Santos C.D., Fernandes A.P., Gazzinelli R.T., Campos-Neto A., Grimaldi-Jr G. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin. Vaccine Immunol. 2007;14:544–548. doi: 10.1128/CVI.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinnell R.J., Carson C., Reithinger R., Garcez L.M., Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team R: A language and environment for statistical computing R Foundation for Statistical Computing. 2018. https://wwwR-projectorg/ Vienna, Austria; URL.
- 36.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.C., Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szargiki R., Castro E.A., Luz E., Kowalthuk W., Machado A.M., Thomaz-Soccol V. Comparison of serological and parasitological methods for cutaneous leishmaniasis diagnosis in the state of Paraná, Brazil. Braz. J. Infect. Dis. 2009;13:47–52. doi: 10.1590/s1413-86702009000100011. [DOI] [PubMed] [Google Scholar]
- 38.Singh D.P., Sundar S., Mohapatra T.M. The rK39 strip test is non-predictor of clinical status for kala-azar. BMC Res. Notes. 2009;2:187. doi: 10.1186/1756-0500-2-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugino M., Niimi T. Lorence, A Recombinant Gene Expression: Reviews and Protocols. 3rd ed. Vol. 824. Springer Science and Business Media; 2012. Expression of multisubunit proteins in Leishmania tarentolae; pp. 317–325. [DOI] [PubMed] [Google Scholar]
- 40.Taslimi Y., Zahedifard F., Rafati S. Leishmaniasis and various immunotherapeutic approaches. Parasitol. 2018;145:497–507. doi: 10.1017/S003118201600216X. [DOI] [PubMed] [Google Scholar]
- 41.Thomaz-Soccol V., Gonçalves A.L., Piecknick C.A., Baggio R.A., Boeger W.A., Buchman T.L., Michaliszyn M., Dos Santos D., Celestino A., Aquino J., Jr., Leandro A.S., Paz O.L.S.D., Limont M., Bisetto A., Jr., Shaw J.J., Yadon Z.E., Salomon O.D. Hidden danger: unexpected scenario in the vector-parasite dynamics of leishmanioses in the Brazil side of triple border (Argentina, Brazil and Paraguay) PLoS Negl. Trop. Dis. 2018;2018:12(4). doi: 10.1371/journal.pntd.0006336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilas V.J.D.R., Maia-Elkhoury A.N.S., Yadon Z.E., Cosivi O., Sanchez-Vazquez M.J. Visceral leishmaniasis: a One Health approach. Vet. Rec. 2014;175:42–44. doi: 10.1136/vr.g4378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Nucleotide and amino acid sequences of Lbk39.