Abstract
Introduction
Diabetes Mellitus (DM) increases worldwide, mostly in low- and middle-income countries. In Mali, the prevalence in the adult population is estimated at 1.8%, but tuberculosis (TB) patients are not systematically screened. The goal of our study was to determine the prevalence of DM among newly diagnosed TB patients.
Methods
We conducted a cross sectional study and a pilot prospective cohort study in four health centers in Bamako. All patients underwent fasting capillary-blood glucose (FCBG) test at Day 0, and repeated after one-week of TB treatment. Venous FBG test was performed for discrepancies between the two FCBG results. Thereafter, FCBG was performed for pilot study at month-2 (M2) and M5 of TB treatment.
Results
Two hundred and one patients were enrolled in this study. Impaired fasting blood glucose was identified in 17 (8.5%), of whom 11 (5.5%) had DM (VFBG >7 mmol/L). Among patients with DM, seven (63.6%) had successful TB treatment outcome, versus 142 (74.7%) of those without DM (p = 0.64), and (OR: 1.69, 95%CI 0.47–6.02).
Conclusion
The prevalence of DM among TB patients in Bamako exceeds that of the general population and screening at TB diagnosis suffices to identify those with DM. Systematic screening of both diseases will allow better treatment.
Keywords: Diabetes Mellitus, Tuberculosis, Prevalence, Bamako, Mali
1. Introduction
The World Health Organization (WHO) has identified Diabetes Mellitus (DM) as a global epidemic, mostly affecting low- and middle-income countries (LMIC) where 80% of all deaths from DM occur [1]. This obesity related disease has reached populations where tuberculosis (TB) continues to be a major public health problem [2]. Both incidence and prevalence of DM are increasing worldwide, with one third of the estimated 425 million people affected aged 65 or older [3]. Another 352 million people worldwide have glucose intolerance, which places them at high risk of developing overt diabetes [3]. According to the International Diabetes Federation (IDF), Africa region had 16 million people with diabetes in 2017 and this prevalence is estimated to rise to 41 million people by 2045 [3]. Mali, located in West Africa, reported a prevalence of DM of 1.8% in the adult population [1].
Diabetes Mellitus, a non-communicable disease (NCD) associated with obesity, weakens the immune system and increases the risk of several infectious diseases, such as tuberculosis [2]. In 2014 alone, Diabetes was responsible for 1.5 million deaths worldwide [2]. With an estimated 10.4 incidence cases and 1.7 million deaths worldwide, TB remains one of the leading causes of death from an infectious disease. According to the International Union Against Tuberculosis and Lung Diseases (IUTLD), 16–46% of people infected with TB have diabetes, and many are still unaware of it [3]. The association of TB and diabetes has been recognized well before chemotherapy for TB was developed, with an increased incidence in Diabetes patients [4], [5], [6], [7]. As a key TB associated factor in addition to Human Immunodeficiency Virus (HIV), this increase in DM prevalence in LMIC is worrisome for the disease eradication. Moreover, while DM was recognized as a high risk of death in TB patients in Maryland (aOR= 6.5 (95%CI 1.1–38.0)), a recent meta-analysis reported that the relative risk of TB in diabetic patients in Taiwan was 3.11 (95% CI 2.27–4.26) as compared with individuals without DM in cohort studies [8,9].
To overcome this dual burden of disease, prevention, bi-directional screening of TB and DM, and treatment of both diseases together as a model similar to the TB-HIV program may be the most effective approach as recommended by WHO, IUTLD and national TB and Diabetes programs [10].
While waiting for full implementation of this approach in Mali, the goal of our study was to determine the prevalence of DM and the outcome of TB treatment in newly infected TB patients with and without DM in four health centers in the Bamako region. Moreover, of interest was the association of DM with specific lineages within the M. tuberculosis complex.
2. Materials and methods
2.1. Setting, tuberculosis and diabetes programs
The Mali National TB Program (NTP) is based in Bamako and has 77 different centers for diagnosis and treatment through the country [11]. Currently, TB patients are not systematically screened for DM, but the NTP is planning to implement this screening strategy.
As for DM management through the National Diabetes program, it is usually done in public and private hospitals or by non-governmental organizations (NGOs) involved in health care in the country. In Bamako, DM patients are mainly offered self-pay care by specialists in the referral and teaching hospital and in an NGO Centre, or by family medical doctors. Patients with DM are not systematically screened either for TB at the time of DM diagnosis or at their regular follow-up visits. However, those with symptoms suggestive of TB are tested according to the NTP guidelines.
2.2. Study design
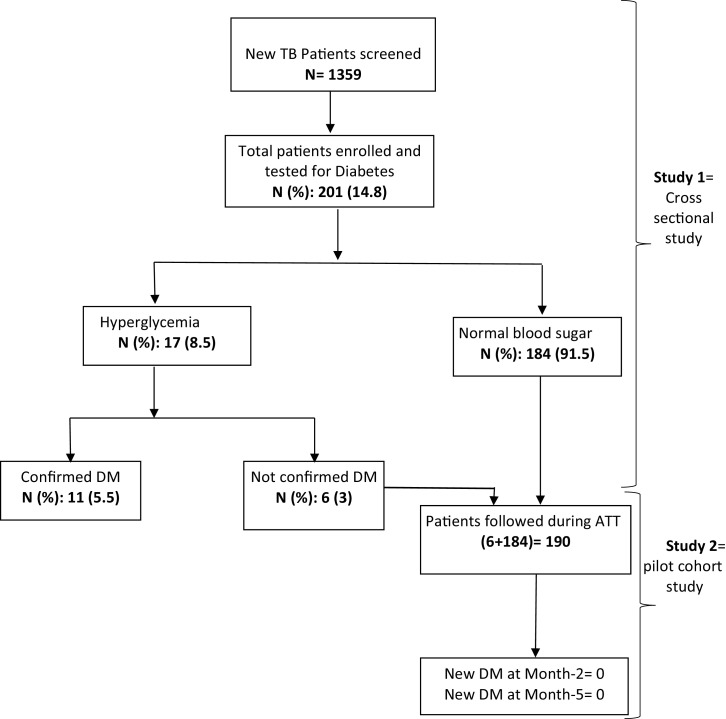
Between January and July 2018, we conducted a cross sectional study, and a pilot prospective cohort study (Fig. 1), by enrolling consecutive new adult (18 years and above), smear positive pulmonary tuberculosis patients (PTB) from four local TB diagnostic and treatment centers, in Bamako. New tuberculosis-infected patients were approached by the study team for study explanation. Thereafter, and at voluntary basis, patients were enrolled after providing written informed consent. The patients not having DM during the enrollment were followed in a pilot prospective study to see if they will develop DM during TB treatment. Bamako, the capital city, has a population of approximately two million people, which is about 14% of the country population. The city is divided in six urban municipalities, with each having a referral health center, where TB diagnostic and treatment services are available. In 2015 alone, more than one third of the total TB patients in Mali (7015 patients) were diagnosed and managed in Bamako [12].
Fig. 1.
Flow Chart of the study Design.
DM: Diabetes Mellitus; TB: Tuberculosis; ATT: Anti tuberculosis treatment; month-2 and month-5 of category 1 tuberculosis treatment.
The study protocol was approved by the Ethics Committee of the University of Sciences, Techniques, and Technologies of Bamako (USTTB) (2014/104/CE/FMPOS). Permission was also obtained from the administration of the health centers where data were collected. Patient's information was anonymized and de-identified before analysis.
2.3. Study population
Convenient sampling strategy was used to screen and enroll consecutive patients with presumptive pulmonary TB had sputum screened at the study sites by either Ziehl Neelsen (ZN) or Auramine/Rhodamine (AR) staining. Adult (age ≥ 18) smear positive consented participants were enrolled into the study after providing writing informed consent after study explanation. Only new patients were enrolled in this study. Patients were treated in accordance with the national guidelines of the Mali TB program [11], which recommends new TB patients to receive a fixed dose combination of two months of rifampin (R), isoniazid (H) pyrazinamide (Z) and ethambutol (E) and four months of RH (2RHZE/4RH). After baseline screening (D0) just before starting TB treatment, and at month-2 (M2) and M5 fresh sputum samples were collected for microscopy. For DM testing, all enrolled TB patients underwent capillary fasting blood glucose test (CFBG) at Day 0, and repeated at one-week of TB treatment. Venous FBG test was performed for those with discrepancies between the two CFGB results. Thereafter, CFBG was performed at M2 and M5 of TB treatment for patients included in the pilot study. The pilot study included those patients who didn't have DM at the baseline screening, and they were followed prospectively to see if they will develop DM during TB treatment (Fig. 1). Patients with confirmed DM were referred for diabetes care. In general newly diagnosed DM/TB patients usually started directly with insulin because of the TB infection, and thereafter were putted on oral anti-diabetics (OAD).
Body mass index (BMI) was calculated by dividing the weight in kilogram (Kg) at each visit by the square of the height in centimeters (cm2) measured at baseline. Programmatic outcome was recorded at M6, including ‘cure’ and ‘treatment completed’ considered as ‘good outcome’ and ‘lost to follow-up’, ‘transferred out’, ‘death’, ‘failure’ considered ‘poor outcome’.
The sample size was calculated based on the assumption in the literature that the prevalence of DM in TB population ranged from 16% to 46% [3]. Even though our first study showed a prevalence of 5.7% of DM patients who developed TB [13]. Considering that we expected to have around 7000 TB cases yearly in Bamako city [14]. At least 159 TB cases will be enrolled in order to have 80% as study power and an error margin of 5%.
2.4. Laboratory tests
Pre-enrollment sputum smear microscopy by Ziehl Neelsen (ZN) or direct fluorescent microscopy (FM) using AR (BBL™ Becton Dickinson, Sparks MD, USA) at local reference centers was followed by fluorescein diacetate vital (FDA) staining and culture at the University Clinical Research Center (UCRC) BSL-3 laboratory, which is certified by the college of American pathologists (CAP), including external quality controls.
2.5. TB culture and strain identification
In the UCRC laboratory, initial strain isolation is done in both liquid (manual reading of Mycobacterium Growth Incubator Tubes (BBL™ MGIT™ Becton Dickinson, Sparks MD, USA)), and solid (Middlebrook 7H11 Agar and Selective 7H11 Agar) media, following standard protocols (10, 12). Speciation of positive mycobacterial cultures was based on Acid-Fast Bacilli-positivity on smear microscopy and colony morphologies on solid medium, with was confirmation by Capilia TB Test (TAUNS Laboratories, Numazu, Japan), or by nucleic acid probes (AccuProbe® GenProbe, San Diego, CA, USA).
2.6. Molecular strain typing
Spoligotyping was performed on boiled bacterial lysates using a commercially available kit (Ocimum) [15] or by in-house prepared membrane. Data entry from the film was verified by a second assessor. Lineage classification was based on the SPOTCLUST (SpolDB3-based, http://tbinsight.cs.rpi.edu/run_spotclust.html) database, TB Miner http://info-demo.lirmm.fr/tbminer/index.php.
2.7. HIV testing
The human immunodeficiency virus (HIV) serology status was determined using previously described methods [12,16].
2.8. Data & statistical analysis
We used the WHO and/or IDF definitions as follow ([10,17]):
-
ü
DM: venous FBG ≥7 mmol/l or if patients reported a known history of DM
-
ü
Impaired fasting glucose (IFG): FBG between 6.1 and 6.9 mmol/l
-
ü
Hypertension: blood pressure >140/90 mmHg
-
ü
Obesity: body mass index ≥ 30 kg/m2.
-
ü
Good Outcome: Tuberculosis patients who are cured and those who have completed treatment
-
ü
Poor Outcome: Tuberculosis patients who are defaulter, died, transferred out, and patients with treatment failure.
Structured questionnaire data were first recorded in a notebook at health centers, and were double entered in Excel, and discordant results were verified and modified accordingly. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC) and Epi info version 7(CDC, Atlanta, USA). Chi-square test was used to compare the frequencies, and results were considered significant when p-values were less than 0.05. Univariate logistic regression model was done to evaluate the risk of TB treatment outcome in TB and TB/DM study group.
3. Results
Among the 201 new TB patients enrolled between January and July 2018, at D0, 17 (8.5%) had hyperglycemia and 11(5.5%) had DM. Most patients (67.2%) were between 18 and 35 years old, and 147 (73.1%) of all patients were male. Median age was 31 with interquartile range (IQR: 25–40), and the mean age was 32.96 ± 11.79 years old. The prevalence of HIV coinfection was 6.5%, and none of the patients having HIV/TB are diagnose with DM.
Among the 11 patients with DM, five were newly diagnosed while six were already known to have the disease and were on DM treatment, either oral or with insulin. No one of the 11 patients was also coinfected with HIV. Moreover, while no one was obese, three were more than 45 years old (Table 1).
Table 1.
Characteristics of patients.
| TB & DM n (%) 11(100%) | TB alone n (%) 190 (100%) | p (value) | |
|---|---|---|---|
| Gender | |||
| Male | 6 (54.5) | 141 (74.2) | 0.279 |
| Female | 5 (45.5) | 49 (25.8) | 0.279 |
| HIV Status | |||
| Positive | – | 13(6.8) | |
| Negative | 11(100) | 177(93.2) | |
| Age | |||
| ≤45 | 8(72.7) | 164(86.3) | 0.420 |
| ˃45 | 3(27.3) | 26(13.7) | 0.420 |
| BMI median at D0 | |||
| <18.5 | 2(18.2) | 98(51.6) | |
| 18.5–24.9 | 8(72.7) | 88(46.3) | – |
| 25–29.9 | 1(9.1) | 4(2.1) | – |
| ≥30 | – | - | - |
| Tuberculosis Treatment Outcome | |||
| Cured | 7(63.6) | 142(74.7) | 0.643 |
| Failed | 1(9.1) | 23(12.1) | 0.858 |
| Death | 1 (9.1) | 7 (3.7) | – |
| Loss to Follow Up/Transferred | 2(18.2) | 18(9.5) | 0.674 |
| Spoligotyping Results | |||
| Lineage 4 | 4(36.3) | 108(56.8) | 0.309 |
| Lineage 6 | 3(27.3) | 50(26.3) | 0.778 |
| Family 33 (Unclassified) | 3(27.3) | 23(12.1) | 0.319 |
| Lineage 2 | 1(9.1) | 2 ( 1.1) | – |
| Others Lineages (L1, L3) and M. bovis | – | 7(3.7) | |
January 2018- July 2018, Bamako, Mali.
TB= Tuberculosis; DM= Diabetes Mellitus; L= Lineage; BMI= Body Mass Index.
Due to logistically conditions, 70 (34%) patients included the 17 with hyperglycemia were followed for the pilot study. While some of those patients tested had impaired fasting glucose (IFG) range during TB treatment (M2 and/or M5), all with DM were identified at D0. Thus, during M2 and M5 follow up, we didn't found any additional diabetes patient.
For TB treatment outcome, 63.6% of patient's experienced good outcome versus 36.4% had poor outcome, including one case of failure without any resistance to anti-tuberculosis drug, and one death (p = 0.64) (Table 1), and (OR: 1.69, 95%CI 0.47 – 6.02) (Table 2).
Table 2.
Tuberculosis treatment outcome by the Diabetes status.
| Good outcome | Poor outcome | Total | |
|---|---|---|---|
| No diabetes | 142 | 48 | 190 |
| Diabetes | 7 | 4 | 11 |
| Total | 149 | 52 | 201 |
Univariate logistic regression model was used to evaluation the association of unfavorable TB treatment outcome and the TB/DM (TB only group as reference) status and resulted to an OR: 1.69, 95%CI 0.47 – 6.02).
Of the 11 DM, 8 (4 under insulin, and 4 under oral drugs) experienced combine dual treatment, and there was no worsening of diabetes during patients.
We didn't identified a patient with hypertension at the screening, and also during the follow up of the pilot study. All the patients had normal and/or low blood pressure (Values not shown).
We found that, approximatively three out of four patients (72.7%) in the dual burden group (TB and DM) had normal BMI at baseline, whereas 51.6% in the TB alone group had normal BMI (Table 1).
By comparing the phylogenetic lineages within the MTBc in TB patients with and without DM, we did not identify an overt association of DM with specific lineages within the M. tuberculosis complex, with modern lineage 4 (in four DM patients), ancestral lineage 6 (in three DM patients), lineage 2 (in one DM patient), and three patients with Family 33 (all spoligotype spacers present) (Table 1). In non-DM TB patients, modern L4 accounts for about 70% in the Bamako region [18].
4. Discussion
Our study aimed to estimate the prevalence of DM in consecutive newly diagnosed adult TB patients and TB treatment outcome with and without DM in Bamako region. Our prevalence estimates of the DM-TB association at 5.5% is similar to our previous study conducted in DM patients in 2011 which was 5.7% [19]. This prevalence is similar to the 5% seen in Zambia in 2018, but higher than the 1.9% observed in Benin in 2015, and lower than in Pakistan (8.8%, and 18%) and in USA (14%) [9,[20], [21], [22], [23]]. These differences in numbers could be due to different sample size, or explained by population dietary, lifestyles and daily behaviors in different countries.
We found that the prevalence of DM in TB patients is three-fold higher than that observed in the general adult population in Mali, which is at 1.8% [1]. As described in the literature years ago, the relative risk of TB in a diabetic patient is in the order of 2–3 [24]. Of the patients burdened by DM and TB in our study population, eight were aged ≤ 45 years old, which was not different from the age distribution of patients with TB alone.
In TB and DM patients, 63.6% had good TB treatment outcome versus 74.7% of those with only TB, in line with Mali global treatment outcome of 77% in 2016, which is far below the WHO/Directly observed treatment short course (DOTS) strategy target of 85% [25]. While our sample size precludes conclusions on treatment outcome, previous studies identified an increased risk of poor outcome of TB treatment in TB and DM patients [26], [27], [28]. Patients with DM should be trained on self-management of the DM for better control [29], which may also improve the outcome of TB treatment [2]. In the pilot study, and during follow-up visits, we did not identify incident DM during TB treatment, confirming earlier observations that TB and its treatment may not be a risk factor for developing DM [7]. Screening new TB patients at diagnosis will thus suffice to identify those with DM.
We found a normal BMI in 72.7% of TB and DM patients despite the associated burden at the screening, compare to 51.6% with normal BMI in the TB alone group. This was no statistically significant, and also BMI was not associated with unfavorable outcome. This is different from the study of Mukhtar et al. who found an association between BMI less than 18.5 and unfavorable outcome [23]. This difference could be probably explained by the high prevalence of TB/DM in Pakistan compared to Mali ie. 18% vs. 5.5%,% respectively.
Our study compared phylogenetic lineages within the MTBc in TB patients with and without DM, we did not identify an overt association of DM with specific lineages within the M. tuberculosis complex. In non-DM TB patients, modern L4 accounts for about 70% in the Bamako region [18]. Ancestral L6 is usually associated with a weakened immune system due to HIV or factors [30]. We expected to see high prevalence of L6 in DM population. However, larger studies are needed to test whether L6 is also more prone to cause TB in patients with uncontrolled-DM.
In the best of our knowledge, this is one of the first study in Mali investigating the association of TB and DM with a pilot prospective study design in a TB population, along with repeated testing of patients for hyperglycemia.
Our study has some limitations as well, including a relatively small number of patients diagnose with DM. Our diagnosis of DM was based on blood glucose levels, which are known to fluctuate, rather than the HbA1c, glycated hemoglobin test. Lastly, we were unable to assess the uptake of DM care referral and the quality of DM care, including whether glucose control was achieved.
In summary, the prevalence of DM among TB patients in Bamako appears to be three-fold greater than that of the general adult population. We didn't found an association between unfavorable treatment outcome and the dual disease burden. Given that the patients can be referred for free diabetes care, the National TB program should make available FBG tests in all TB centers, and diabetes clinics can implement systematic screening for TB to improve the diagnosis and management of dually affected patients. Thus, we recommend increased sensitization of the population about symptoms of TB such as cough and/or weight loss that should prompt seeking care for timely diagnosis of TB, and also symptoms of DM such as frequent urinary for timely diagnosis of DM. Also health care workers may benefit from retraining on early recognition of both TB and DM symptoms. In addition, the diagnosis of TB and/or DM should be completely free of charge for the patient, including the consultation, chest X-ray, and other diagnostic means. Currently only sputum smear examination is free of cost for the patients, who pay on average 2.3 EUR for the consultation and 11.5 EUR in case an X-ray is indicated, whereas for DM, both consultation and blood tests would cost at least 5 EUR.
Whether DM associated TB is caused by different mycobacterial lineages will require a larger study.
As a perspective of this study, we plan to perform a large cohort study to measure glucose levels in non-TB, and TB patients. In addition we will follow patients for relapses, in order to find out the potential implication of DM in post-treatment relapses.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgment
We express our special thanks to all the health centers in Bamako district for their assistance in the recruitment of patients, and also thank all the study volunteers for participating in this study. We expressed our special thanks to Dr Boubacar Coulibaly, Dr Bakary Diarra, M. Mamby Diabaté at health centers, and Dr. Drissa Goita, Ms. Bintou Fané, Ms. Oumou Niare, Miss Fanta Sanogo, Ms. Mariam Goumané, and Miss Hawa B. Dramé all at SEREFO/UCRC for their assistance in collecting/testing the samples.
Funding
This work was done at the University Clinical Research Center (UCRC) of the University of Sciences, Techniques and Technologies of Bamako (USTTB), Mali, and was partially funded by the USTTB through NIH/ R01 grant R01AI110386 and NIH/FIC D71 TWO10428-01A1, the Northwestern University (Chicago, IL, USA) through NIH/FIC D43 TW10350. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. B.D. was supported by a TDR fellowship, TIMS ID B40072, the special programme for research and training in tropical diseases, co-sponsored by UNICEF, UNDP, World Bank and WHO.
Footnotes
Ethical Statement
The study protocol was approved by the ethic committee of the faculty of medicine, pharmacy and dentistry (FMOS/FAPH) of the University of Sciences, Techniques and Technologies of Bamako (USTTB).
References
- 1.Federation I.D., IDF africa members. 2018 (https://www.idf.org/our-network/regions-members/africa/members/17-mali.html). 2018.
- 2.TB alert fafwt, TB and diabetes. 2018; https://www.tbalert.org/about-tb/global-tb-challenges/tb-and-diabetes, 2018.
- 3.Whiting D.R. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Kim S.J. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76(6):529–533. doi: 10.1016/0962-8479(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 5.Shah B.R., Hux J.E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 6.Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raw N. The influence of diabetes and gout on the tuberculous infections of the human body. Tuberculosis (Berlin) 1911;10(5):169–174. [Google Scholar]
- 8.Chiang C.Y. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley K.E. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80(4):634–639. [PMC free article] [PubMed] [Google Scholar]
- 10.Jali M.V., Mahishale V.K., Hiremath M.B. Bidirectional screening of tuberculosis patients for diabetes mellitus and diabetes patients for tuberculosis. Diabetes Metab J. 2013;37(4):291–295. doi: 10.4093/dmj.2013.37.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministère de la Santé . Programme National de lutte contre la Tuberculose: Rapport annuel des activités de lutte contre la Tuberculose, 2009. Mali; 2010. Direction Nationale de la Santé (DNS). Division Prévention et Lutte contre la maladie. [Google Scholar]
- 12.Diarra B. Tuberculosis drug resistance in bamako, mali, from 2006 to 2014. BMC Infect Dis. 2016;16(1):714. doi: 10.1186/s12879-016-2060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diarra B., D.A. Maiga M, Sanogo M., Diallo M.H., Baya B. Tuberculose et diabète à bamako, mali: prévalence et caractéristiques épidémiocliniques de l'association. Revue Malienne d'Infectiologie et de Microbiologie. 2014;2 [Google Scholar]
- 14.Ministère de la Santé . Programme National de lutte contre la Tuberculose: Rapport annuel des activités de lutte contre la Tuberculose, 2010. Mali; 2010. Direction Nationale de la Santé (DNS). Division Prévention et Lutte contre la maladie. [Google Scholar]
- 15.Kamerbeek J. Simultaneous detection and strain differentiation of mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35(4):907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traore B. Molecular strain typing of mycobacterium tuberculosis complex in bamako, mali. Int J Tuberc Lung Dis. 2012;16(7):911–916. doi: 10.5588/ijtld.11.0397. [DOI] [PubMed] [Google Scholar]
- 17.Wang H.T. Frequency of tuberculosis among diabetic patients in the people's republic of china. Ther Clin Risk Manag. 2014;10:45–49. doi: 10.2147/TCRM.S38872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Togo A.C.G. The most frequent mycobacterium tuberculosis complex families in mali (2006-2016) based on spoligotyping. Int J Mycobacteriol. 2017;6(4):379–386. doi: 10.4103/ijmy.ijmy_140_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diarra B., D.A., Maiga M., Sanogo M., Diallo M.H., Baya B. Tuberculose et diabète à bamako, mali: prévalence et caractéristiques épidémiocliniques de l'association. Revue Malienne d'Infectiologie et de Microbiologie. 2014 [Google Scholar]
- 20.Ade S. Low prevalence of diabetes mellitus in patients with tuberculosis in cotonou, benin. Public Health Action. 2015;5(2):147–149. doi: 10.5588/pha.14.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latif A. Did diabetes mellitus affect treatment outcome in drug-resistant tuberculosis patients in pakistan from 2010 to 2014? Public Health Action. 2018;8(1):14–19. doi: 10.5588/pha.17.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fwoloshi S. Screening for diabetes mellitus among tuberculosis patients: findings from a study at a tertiary hospital in lusaka, zambia. Can J Infect Dis Med Microbiol. 2018;2018 doi: 10.1155/2018/3524926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhtar F., Butt Z.A. Risk of adverse treatment outcomes among new pulmonary tb patients co-infected with diabetes in pakistan: a prospective cohort study. PLoS ONE. 2018;13(11) doi: 10.1371/journal.pone.0207148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.H.F. R. The association of diabetes and tuberculosis* epidemiology, pathology, treatment and prognosis. N. Engl. J. Med. 1934;210(1):1–13. [Google Scholar]
- 25.World Health Organization. Global Tuberculosis Reports 2018. Who.int/global-tuberculosis-report-2018-who-2018.
- 26.Garcia-Basteiro A.L. Poor tuberculosis treatment outcomes in southern mozambique (2011-2012) BMC Infect Dis. 2016;16:214. doi: 10.1186/s12879-016-1534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshi D.C. Profile, outcomes, and determinants of unsuccessful tuberculosis treatment outcomes among HIV-Infected tuberculosis patients in a nigerian state. Tuberc Res Treat. 2014;2014 doi: 10.1155/2014/202983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker M.A. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debussche X. Structured peer-led diabetes self-management and support in a low-income country: the ST2EP randomised controlled trial in mali. PLoS ONE. 2018;13(1) doi: 10.1371/journal.pone.0191262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jong B.C., Antonio M., Gagneux S. Mycobacterium africanum–review of an important cause of human tuberculosis in west africa. PLoS Negl Trop Dis. 2010;4(9):e744. doi: 10.1371/journal.pntd.0000744. [DOI] [PMC free article] [PubMed] [Google Scholar]