Graphical abstract
Keywords: Toilet tissue paper, PAHs, Phthalates, 8270 SVOCs, SVCOCs, Risk assessment, PCA-MLR source apportionment
Abstract
The presence of phthalates, polycyclic aromatic hydrocarbons (PAHs) and semi-volatile chlorinated organic compounds (SVCOC) in toilet tissue papers may be detrimental to the health of consumers upon exposure. This study therefore, sought to investigate the levels of these toxicants in toilet tissue papers on the Ghanaian market and the associated risk of exposure. The study also sought to conduct source apportionments for analytes.
A total of 32 composite toilet tissue samples from 8 different brands were analysed in replicates for PAHs, phthalates and SVCOCs. Analysis was conducted using Shimadzu GCMS QP 2020 with the MS operated in SIM mode.
The results showed elevated levels of PAHs, phthalates, and appreciable levels of SVCOCs in the toilets tissue papers. The risk assessment conducted, showed an associated elevated cancer risk >10−4 for PAHs in all samples and DEHP in samples NN, BB and SF. The risk associated with the levels of carcinogenic SVCOCs were found to be > 10-5 but < 10−4.The hazard indices (HI) calculated for non-cancer effects, showed risk levels < 1.0 for phthalates in most toilet paper samples except for samples BB and SF. The HI recorded for chlorophenols were all <1.
Cumulatively, these values suggested elevated cancer and non-cancer risk associated with the dermal use of the toilet tissue papers on the Ghanaian market. The PCA-MLR source apportionment suggested two significant sources of SVOCs in the toilet tissue papers. PAHs, phthalates and 2-chloronaphthalene were of one source (oil base source) whereas SVCOCs were of another source (bleaching process).
1. Introduction
Toilet tissue paper finds extensive use in many households, for example, for bathroom hygiene, nose care, wiping up spills, removing makeup and sometime for bathroom cleaning chores. In Ghana, toilet tissue papers are also used by some women as a sanitary towel or its support. These tissue papers, classified as personal care products, suggest toilet tissue papers need to be clean and hygienic [1].
Usually, toilet tissue papers are made from various proportions of bleached Kraft pulps with relatively little refining of the stock, rendering them soft, bulky, and good absorbent. They are classified into two namely; virgin paper products formed from chipped wood and recycled paper products [1].
The international production laws for toilet tissues papers, regulate compounds such as polychlorinated biphenyls (PCBs) and pentachlorophenols (PCP) that could result from the bleaching process in the case of recycled paper products [[1], [2], [3], [4]]. Notwithstanding, in Ghana and most developing countries, tissue papers are mostly produced from recycled waste papers and are poorly regulated. Unfortunately, raw papers used in the production are usually soiled with coal tar, bitumen, printer’s inks, paints, oils and other substances that contain toxic semi-volatile organic compounds (SVOCs) such as polycyclic aromatic hydrocarbons (PAHs), phthalate plasticizers etc and or their precursors, which are not captured in the regulations. Even those captured, seem not be strictly adhered to by most manufacturers because of poor regulatory institutions.
The presence of PAHs in personal care products such as, cosmetics, disinfectants and washing others from Nigeria was recently reported by Adekunle et al. [5], but literature is sparse on their presence in the highly patronized personal care product like toilet tissue paper. Reported health risk associated with exposure to PAHs include growth retardation, low birth weight, teratogenicity, low IQ [[6], [7], [8]], and skin allergies [9] as well as endocrine disruption effects with reproductive related effects in both male and female [10,11]. According to the International Agency for Research on Cancer (IARC) monograph, most PAHs studied in this work are carcinogenic [12]. IARC reported benzo[a]pyrene as a definite carcinogen (group 1), whereas the rest of the EPA prioritied 16-PAHs were classified under probable (group 2A) and possible human carcinogens (group 2B) [12].
Phthalates and other plasticizers may be found in plastic products, adhesives, inks and lubricating oil, as well as solvents in paints, insecticides and personal care products [[13], [14], [15]]. The six most commonly used phthalate plasticizers in consumer products are Bis (2-ethylhexyl) phthalate (DEHP), di-n-octyl phthalate (DnOP) and di-n-butyl phthalate (DBP), as well as diisononyl phthalate (DINP), diisodecyl phthalate (DIDP) and benzyl butyl phthalate (BBP), whereas others are used only for selective applications [16]. The widely used and the ubiquitous phthalates have been detected in various environmental samples [[17], [18], [19]], foodstuff [20,21], indoor dust [[22], [23], [24]], air inside vehicles [25] and even in human breast milks [[26], [27], [28], [29]]. But literature has reported no study on the possible presence of phthalates and other plasticizers in tissue papers, especially those from recycled papers.
Phthalates have both reproductive toxicity and endocrine-disrupting properties [20,[30], [31], [32]]. The IARC [12] monograph has classified Bis(2-ethylhexyl) phthalate (DEHP) as a possible human carcinogen (Class 2B). Phthalates are also reported to be teratogen [33,34], with adverse effects on child behaviour, intellectual and motor development as well as anogenital distance complications [[35], [36], [37], [38], [39], [40], [41], [42]].
Semivolatile chlorinated organic compounds (SVCOCs) such as chlorophenols, may be produced at elevated levels, when very contaminated paper products are being bleached for recycled toilet tissue papers. Chlorinated organic compounds such as polychlorophenols (including 2,4,6-trichlorophenol and tetrachlorophenols), 4-chloroaniline, and hexachloroethane, as well as chlorobenzens (including 1,4-dichlorobenzene, and hexachlorobenzene) are classified by IARC [43] as possible human carcinogen (class 2B).
The WHO, recently reported an upsurge in cancer incidences, where colorectal cancer (1.80 million cases) and skin cancer (non-melanoma) (1.04 million cases) were implicated as among the most commonly diagnosed cancers [43,44]. The report, also cited colorectal cancer as second most common cause of cancer death (862 000 deaths) in the world.
With respect to the aforementioned issues and the extensive usage of toilet tissue papers, it was imperative for a study to be conducted to investigate residual levels of SVOCs in toilet tissue papers that have direct contacts to vital and cancer susceptible part of the human body. These toxicants, may seep easily into the lymph upon dermal exposure and get circulated to other organs. Here for example, carcinogenic PAHs in toilet tissue paper used privately to clean the anus, may seep into the lymph or contact the rectum, get circulated and initiate colorectal cancer. A study in this regard, would help raise awareness of the issues at stake in the production of toilet tissue papers and cause the regulatory bodies to increase surveillance in this regard to correct the menace.
This study therefore sought to analyse the residual levels of selected 8270 SVOCs (compounds identified by the U.S. EPA Method 8270D/E) including PAHs, phthalate plasticizers and other semi-volatile chlorinated organic compounds (SVCOCs) in toilet tissue papers on the Ghanaian market. The study also sought to conduct health risk assessment on the levels of SVOCs in toilet tissue papers to estimate their contribution to the upsurge in cancer and other cancer related incidences in Ghana and the world. The study further sought to conduct source apportionment of the SVOCs in the toilet papers using principal component analysis (PCA) with multiple linear regression analysis (MLR).
2. Materials and methods
2.1. Reagents and standards
All reagents and standards used were of high purity. The 8270 Mega mix standards (#31850), SV internal standards (6 components; 31206), B/N Surrogates mix (4/89 SOW, #31062) and GCMS tuning mixture (benzidine; DFTPP; 4,4′-DDT and pentachlorophenol, #31615) used were all purchased from Restek. GC grade Hexane (≥ 99.8 %) and dichloromethane (≥ 99.8 %, # K4799165633) solvents were purchased from Millipore Corporation, Germany; Silica gel (60−120 mesh) was from BDH Chemicals Limited Poole, England and anhydrous Na2SO4 (99.0 %, #7630-4405)were purchased from DAEJUNG chemical & metal Co. Ltd.
2.2. Sampling
Toilet tissue papers on the Ghanaian market were purchased from various shops in Greater Accra and Central region of Ghana. Ten (10) single rolls of toilet tissue papers belonging to a brand, were randomly purchased from different shops to make a composite for each brand from each region. Sampling was done within a week each, for two different months (i.e. First week of February, 2019 and First week of April, 2019). The samples were kept in their wrappers until further preparation for analysis. A total of 32 composite samples from 8 different brands mostly patronized by the average Ghanaian, were used for the study. The samples were labelled with abbreviations of the brand names. According to the manufacturers’ descriptions, six samples, labelled as SS, SL, NN, BB, NRP, and SF were produced from recycled paper products and two, labelled as PTN and F were products of virgin papers.
2.3. Sample preparations and extractions
About 100 g of each composited toilet tissue paper brand was homogenized and blended to fine size forms. Ten gram (10 g) each, of the blended samples, were then transferred into a porcelain mortar and further homogenized with anhydride Na2SO4. The homogenate, was transferred into a thimble and put in a 200 mL Soxhlet extraction chamber. A Soxhlet apparatus consisting of 250 mL round bottom flask, an extraction chamber, condenser and water circulator were mounted on a temperature controlled heating mantle for the extractions. Extractions were done for 16 h with 250 mL 2:3 DCM/hexane solvent mixture.
The extracts were concentrated using Rotavapor R-114 at a temperature of 45 °C to about 5 mL and further concentrated to about 1.0 mL using a stream of an inert nitrogen gas (EPA Method 3540) and allowed to concentrate further to about 0.5 mL in a desiccator.
2.4. Post-extraction clean-up
The 0.5 mL concentrated extract was loaded onto a packed silica gel column. The column used was prepared by packing 2.0 g of activated silica gel into a chromatographic column (0.5 mL ID). About 0.5 g of anhydrous Na2SO4 was added to the top of the column. Both ends of the packed column were plugged with glass wools. The packed column was then preconditioned with 1.0 mL DCM followed by 1.0 mL Hexane. The 0.5 mL concentrated extract was then applied on top of the column and eluted with 2.0 mL hexane followed 3 mL DCM/hexane (2:3 v/v) two successive elution. About 5.0 mL of eluent collected was concentrated to 1.0 mL using a stream of nitrogen and left to left to concentrate to almost dryness in a desiccator. Ten microliters of 5.0 mg/L internal standard and surrogates were added and reconstituted hexane to a volume of 0.3 mL prior to GC/MS analysis (EPA Method 3630; 8270D).
2.5. GC/MS analysis of samples
The EPA method 8270 (SIM) with slight modification to improve selectivity and sensitivity was employed for the GC/MS analysis.
A Shimadzu GCMS QP2020 system, equipped with AOC 20i auto-injector was used for the analysis. The dimension of capillary column used was 30.0 m (length) × 0.25 mm (ID) × 0.25 μm (thickness) Rtx-5 ms fused capillary column. Helium (purity: 99.9995) gas was used as the carrier gas.
2.5.1. GC operation conditions
The injection port temperature was set at 265.0 °C and the column oven temperature was initially set at 70.0 °C. Temperature programming was used for GC operations. Here the temperature was initially set at 70 °C and held for 2.0 min. it was then ramp at a rate of 20 °C/min to 90 °C and ramp again at 10 °C/min to 250 °C. The temperature was further ramped at 5.0 °C/min to 300 °C and held for 3.0 min. A total program time of 32.00 min was used. The injection volume was 1.0 μL. The linear velocity flow control mode was used: the linear velocity was 42.3 cm/sec for a column flow of 1.33 mL/min, and a total flow of 8.7 mL/min.
2.5.2. MS operation conditions
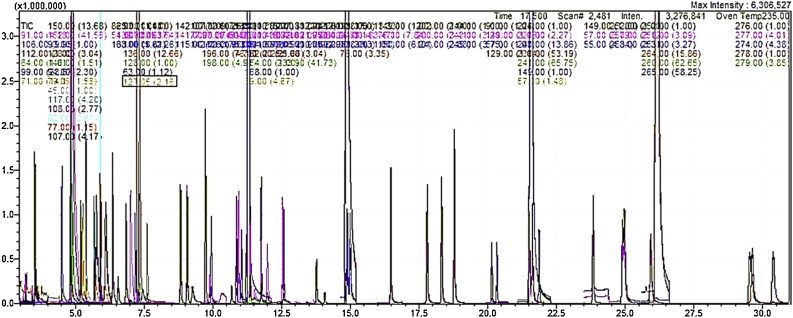
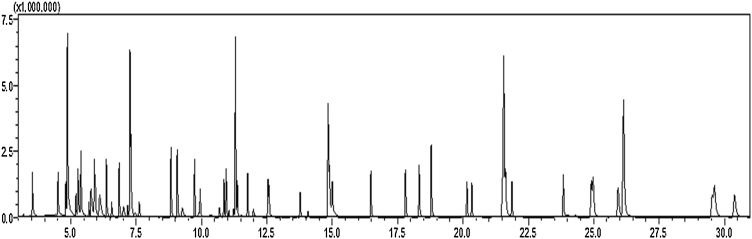
The electron impact ionization source was used and quantitative data were collected using Selected Ion Monitoring (SIM) mode with ≥ 2 ions monitored for each compound (Table 1 and Fig. 1). The temperatures of the ion source and the interface were set at 230 °C and 280 °C respectively. Table 1 and Fig. 1 show the selected 8270 compounds analysed, their respective target and reference ions and retention times. A typical real sample chromatogram of the selected 8270 SVOCs is also shown in Fig. 2.
Table 1.
Calibration curves parameters for target compounds, internal standards (ISTD & Ref) and surrogates (S) used.
| ID# | Retention Time | Name | Type | ISTD Group# | Target ion, m/z | R2 | Reference ions, m/z | Equation for Calibration Curve, Y | RF % RSD | Detection limitation, μg/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.548 | 2-fluorophenol (S) | Target | 1 | 112 | 0.998 | 64 - 92, | 0.4324X + 0.0020 | 7.8522 | 39.74 |
| 2 | 4.537 | Bis(2-chloroethyl) ether | Target | 1 | 93 | 0.9996 | 63 - 95 | 6.6218X- 0.0138 | 17.7054 | 0.89 |
| 3 | 4.819 | Benzene, 1,2-dichloro- | Target | 1 | 146 | 0.9999 | 111 - 148 | 2.1660X- 0.0016 | 10.8499 | 0.75 |
| 4 | 4.823 | Phenol, 2-chloro- | Target | 1 | 128 | 0.9997 | 64 - 63 | 2.1192X- 0.0039 | 16.8385 | 0.85 |
| 5 | 4.875 | 1,4-Dichlorobenzene-d4 | ISTD& Ref | 1 | 150 | 152 | ||||
| 6 | 4.902 | Benzene, 1,3-dichloro- | Target | 1 | 146 | 0.9999 | 111 - 148 | 2.8364X- 0.0021 | 10.5885 | 0.77 |
| 7 | 5.213 | Benzene, 1,4-dichloro- | Target | 1 | 146 | 0.9999 | 148 - 111 | 2.2248X- 0.0008 | 10.3473 | 0.73 |
| 8 | 5.423 | Bis(2-chloroisopropyl) ether | Target | 1 | 45 | 0.9957 | 121 - 77 | 8.0417X- 0.0098 | 12.2907 | 0.78 |
| 9 | 5.7 | Ethane, hexachloro- | Target | 1 | 117 | 0.9963 | 119 - 201 | 0.8573X- 0.0019 | 15.0917 | 0.73 |
| 10 | 5.918 | Nitrobenzene-D5 (S) | Target | 2 | 82 | 0.9996 | 54-128 | 0.3971X+ 0.0016 | 4.8743 | |
| 11 | 6.402 | Isophorone | Target | 2 | 82 | 0.9995 | 54 - 138 | 2.3984X- 0.0028 | 7.8019 | 0.26 |
| 12 | 6.873 | Methane, bis(2-chloroethoxy)- | Target | 2 | 93 | 0.9994 | 63 - 95 | 1.7325X- 0.0023 | 9.4991 | 0.29 |
| 13 | 7.187 | Benzene, 1,2,4-trichloro- | Target | 2 | 180 | 0.9998 | 182 - 74 | 0.7183X- 0.0005 | 5.1811 | 0.23 |
| 14 | 7.276 | Naphthalene-D8 | ISTD & Ref | 2 | 136 | 54 - 108 | ||||
| 15 | 7.338 | Naphthalene | Target | 2 | 128 | 0.9998 | 51 - 127 | 2.8436X- 0.0019 | 4.7522 | 0.17 |
| 16 | 7.471 | Phenol, 2,4-dichloro- | Target | 2 | 63 | 1.0 | 63 - 164 | Quadratic | 33.2198 | 0.65 |
| 17 | 7.637 | 1,3-Butadiene, 1,1,2,3,4,4-hexachloro- | Target | 2 | 127 | 0.9988 | 227 - 223 | 0.8612X- 0.0014 | 8.9866 | 0.43 |
| 18 | 7.637 | 4-Chloroaniline | Target | 2 | 127 | 0.9988 | 65 - 129 | 0.8612X- 0.0014 | 8.9866 | 0.43 |
| 19 | 8.86 | Naphthalene, 1-Methyl- | Target | 2 | 142 | 0.9994 | 141 - 115 | 1.9541X- 0.0027 | 9.2094 | 0.30 |
| 20 | 9.1 | Naphthalene, 2-Methyl- | Target | 2 | 142 | 0.9993 | 141 - 115 | 1.8462X- 0.0025 | 9.0410 | 0.32 |
| 21 | 9.294 | 4-chloro-3-methylphenol | Target | 2 | 107 | 0.9993 | 77 -142 | 0.6058X- 0.0011 | 14.7357 | 0.33 |
| 22 | 9.752 | 2-fluorobiphenyl (S) | Target | 3 | 172 | 0.9996 | 171 - 170 | 1.1003X+ 0.0111 | 6.0411 | 0.13 |
| 23 | 9.901 | Phenol, 2,4,6-trichloro- | Target | 3 | 97 | 0.9967 | 198 - 196 | 0.4297X- 0.0019 | 36.6714 | 0.91 |
| 24 | 9.974 | Naphthalene, 2-chloro- | Target | 3 | 162 | 0.9995 | 127 - 164 | 3.3579X- 0.0039 | 8.0727 | 0.33 |
| 25 | 10.304 | Phenol, 2,4,5-trichloro- | Target | 3 | 196 | 0.9996 | 196 - 97 | 0.1499X- 0.00009 | 24.9247 | 4.0 |
| 26 | 10.898 | Dimethyl phthalate | Target | 3 | 163 | 0.9991 | 77 - 76 | 3.6343X- 0.0054 | 9.0912 | 0.39 |
| 27 | 10.977 | Acenaphthylene | Target | 3 | 152 | 0.9991 | 76 - 151 | 4.0016X- 0.0063 | 10.0823 | 0.41 |
| 28 | 11.315 | Acenaphthene-d10 | ISTD & Ref | 3 | 164 | 162 - 160 | ||||
| 29 | 11.398 | Acenaphthene | Target | 3 | 154 | 0.9994 | 153 - 152 | 3.2506X- 0.0037 | 6.4589 | 0.35 |
| 30 | 12.449 | Phenol, 2,3,5,6-tetrachloro- | Target | 3 | 65 | 0.9980 | 230 - 234 | 0.6514X+ 0.012 | 41.1876 | 0.76 |
| 31 | 12.54 | Phenol, 2,3,4,5-tetrachloro- | Target | 3 | 232 | 0.9988 | 230 - 131 | 0.0207X- 0.00006 | 27.3923 | 0.93 |
| 32 | 12.577 | Diethyl Phthalate | Target | 3 | 149 | 0.9986 | 160 - 165 | 3.5046X+ 0.0064 | 18.9221 | 0.14 |
| 33 | 12.618 | 9H-Fluorene | Target | 3 | 166 | 0.9988 | 165 - 82 | 3.5534X - 0.0062 | 11.1171 | 0.45 |
| 34 | 13.445 | 2,4,6-tribromophenol (S) | Target | 3 | 332 | 0.9983 | 62 - 330 | 0.0235X - 0.0008 | 15.8856 | 18.3 |
| 35 | 13.793 | 4-bromophenyl phenyl ether | Target | 4 | 248 | 0.9991 | 250 - 77 | 0.7581X - 0.0014 | 13.7724 | 0.41 |
| 36 | 14.097 | Benzene, hexachloro- | Target | 4 | 286 | 0.9997 | 284-282 | 0.1665X+ 0.00009 | 15.9099 | 0.84 |
| 37 | 14.865 | Phenanthrene-D10 | ISTD & Ref | 4 | 188 | 80 - 94 | ||||
| 38 | 14.938 | Anthracene | Target | 4 | 178 | 0.9993 | 76 - 89 | 2.6414X- 0.0036 | 7.7782 | 0.33 |
| 39 | 15.042 | Phenanthrene | Target | 4 | 178 | 0.9995 | 76 - 89 | 3.0189X- 0.0036 | 8.4975 | 0.36 |
| 40 | 16.517 | Dibutyl phthalate | Target | 4 | 149 | 0.9995 | 150 - 57 | 3.3163X- 0.0027 | 8.453233 | 0.38 |
| 41 | 17.848 | Fluoranthene | Target | 4 | 202 | 0.9996 | 101 -200 | 3.3491X- 0.0035 | 6.2156 | 0.32 |
| 42 | 18.372 | Pyrene | Target | 5 | 202 | 0.9995 | 101 -100 | 2.9473X- 0.0032 | 5.6275 | 0.35 |
| 43 | 18.813 | p-Terphenyl-d14 (S) | Target | 6 | 244 | 0.9994 | 243 - 245 | 0.6542X+ 0.0017 | 5.1000 | 12.3 |
| 44 | 20.188 | Benzyl butyl phthalate | Target | 5 | 149 | 0.9986 | 91 - 65 | 1.1980X+ 0.0002 | 12.0877 | 0.49 |
| 45 | 20.37 | Hexanedioic acid, dioctyl ester | Target | 5 | 129 | 0.9986 | 57 - 71 | 1.0651X+ 0.0000 | 13.6516 | 0.50 |
| 46 | 21.567 | Benz[a]Anthracene | Target | 5 | 228 | 0.9996 | 226 - 114 | 2.9580X - 0.0010 | 11.0788 | 0.34 |
| 47 | 21.591 | Chrysene-D12 | ISTD & Ref | 5 | 240 | 236 - 241 | ||||
| 48 | 21.688 | Chrysene | Target | 5 | 228 | 0.9998 | 226 - 113 | 3.0885X- 0.0018 | 3.4619 | 0.32 |
| 49 | 21.91 | Bis(2-ethylhexyl) phthalate | Target | 5 | 149 | 0.9995 | 57 - 71 | 1.5115X- 0.0015 | 5.5767 | 0.36 |
| 50 | 23.892 | Di-n-octyl phthalate | Target | 5 | 149 | 0.9996 | 57 - 71 | 2.5745X - 0.0033 | 10.0511 | 0.35 |
| 51 | 25.055 | Benzo[b]fluoranthene | Target | 6 | 252 | 0.9987 | 250 - 253 | 2.6753X - 0.0049 | 11.6721 | 0.52 |
| 52 | 25.055 | Benzo[k]fluoranthene | Target | 6 | 252 | 0.9996 | 250 - 253 | 3.8256X - 0.0035 | 4.9722 | 0.36 |
| 53 | 26.008 | Benzo[a]pyrene | Target | 6 | 252 | 0.9989 | 250 - 253 | 3.0565X - 0.0052 | 10.5567 | 0.48 |
| 54 | 26.184 | Perylene-D12 | ISTD & Ref | 6 | 264 | 260 - 265 | ||||
| 55 | 29.622 | Indeno[1,2,3-cd]pyrene | Target | 6 | 276 | 0.9992 | 277 - 274 | 3.6582X - 0.0054 | 9.5846 | 0.43 |
| 56 | 29.708 | Dibenz[a,h]anthracene | Target | 6 | 278 | 0.9990 | 276 - 279 | 3.1282X - 0.0053 | 11.7075 | 0.39 |
| 57 | 30.488 | Benzo[ghi]perylene | Target | 6 | 276 | 0.9993 | 277 - 274 | 3.1291X - 0.0043 | 8.2272 | 0.33 |
Fig. 1.
A SIM chromatogram of 200 ppb Phthalates, PAHs, SVCOCs and the internal standard analysed.
Fig. 2.
A real sample chromatogram of selected 8270 SVOCs analysed.
2.6. Analytical quality control
Internal standard quantitative method was employed in this study. A five point calibration curve for 8270 standards ranging from 0.01 to 0.5 mg/L, for which 50.0 μL of 5.0 mg/L internal standard (ISTD) has been added to each, was used for quantification. Surrogates standards (S) were also added to each standard (0.30–3.0 mg/L of S) and sample to check for method recovery (EPA method 8270). Initial calibration standards (ICVs) at 0.2 mg/L was ran and also CCVs at 0.5 mg/L were also ran to validate the GCMS method for each 10 continuous sample runs. Method reagent black spiked with ISTD and surrogates were first analysed for each batch of sample analysis. Using the GCMS tuning mixture, manual tuning was conducted for every 12 h in conformity to criteria for method 8270 E/D.
2.7. Carcinogenic risk assessment using TEF
Toxicity equivalency factors (TEFs) risk assessment protocol employed by Essumang et al. [6], were employed for the risk assessment
That is;
| (1) |
Where is the measured individual PAHs concentrations for the ‘ith’ compound with the assigned .
This approach, has also been adopted because PAHs usually exist as a mixture of compounds [45,46] that can exert synergistic effect on human health.
The calculated for the seven USEPA classified carcinogens (mutagens) were used to estimate carcinogenic risk involved in the use of toilet tissue papers for an adult’s life time of 70.0 years [47]. The total risk due to exposure to mixtures of carcinogenic PAHs is:
| (2) |
Where is the dermal carcinogenic slope factor for benzo[a]pyrene (25 per mg/kg/day).
The B[a]P equivalent daily dose for dermal exposure to mixtures of carcinogenic PAHs is given as;
| (3) |
Where DADB[a]P is the B[a]P equivalent daily dose for dermal exposure (mg kg−1 day−1), SA is the surface area of the body part exposed to the toxicant. Here for lack of data on the surface area of the private area, where toilet papers are used and also considering the resemblance of that surface to human hands, EPA default SA for hands (904 cm2) was used. Again, considering the delicate nature of the part of application and the extent of direct application on the body part considered, the EPA default 95th percentile AF (dermal adherence factor) for utility worker 0.9 mg/cm2 was used. EF is the exposure frequency (350 day year−1), ED is the exposure duration (30 years), and ABS is the dermal absorption factor, i.e. 0.13 for B[a]P and other PAHs [48]; 0.1 for other semivolatile organic compounds [47,49]. Adults average body weight (BW) of 70 kg and a life time (AT) of 70 years were used. Dermal cancer slope factor SFd (mg/kg-day)−1 for a specific chemical was also accordingly used. These exposure assumptions were made to be consistent with EPA guidance on risk assessment for Superfund [47].
The risk involved upon dermal contacts to the rest of chemicals, were also calculated using Eqs. (2) and (3), as well as the same exposure assumptions with slight modification for the respective toxicants. Hazard quotients were calculated using the following formula:
| (4) |
Where RfDABS is the absorbed reference dose value for the contaminant understudy. Using EPA default RfDABS values of 0.1, 0.2, 0.8, 0.1 and 0.02 mg/kg bw/day for DBP, BBP, DEP, DMP and DEHP respectively, a target hazard quotients HQ were calculated. The hazard index (HI) was calculated as the sum of these five HQ for each sample. Also the HI of the chlorophenols were calculated using the respective RfDABS values of 0.005, 0.003, 0.1 and 0.03 mg/kg bw/day for 2-chlorophenol (2-CP), 2,4-dichlorophenol and 2,4,5-trichlorophenol (2,4,5-TCP) as well as 2,3,4,6-Tetrachhlorophenol (2,3,4,6-TeCP) [71].
The cancer risk of DEHP is prioritized over the rest of the plasticizers, thus, carcinogenic risk was calculated for only DEHP and hazard quotient was calculated for all the plasticizers.
For the carcinogenic risk assessment, due to the lack of dermal slope factor for DEHP in literature, the slope factor SFd was assumed to be equal to:
| (5) |
Where SFo [0.014 (mg/kg-day)−1] is the oral slope factor for DEHP, and ABSGI is the fraction of DEHP absorbed in gastrointestinal tract in the critical toxicity study [47]. Considering the critical nature of the point of applications of toilet tissue paper, the ABSGI was chosen to be 100 % as recommended by [50,49]).
2.8. Statistical analysis
Analysis of variance (ANOVA) and %RSD statistics were conducted using Microsoft Excel Toolpak. Factor analysis, that is principal component analysis (PCA) and multiple linear regression (MLR) analysis were conducted with IBM SPSS statistics version 22 software.
3. Results and discussion
3.1. Quality control results
The calibration curves obtained for the analysis showed a good linear range of calibration (R2 < 0.995) over the five-points internal standards (Table 1). Good Response factors (RF) percent relative standard deviations (% RSD < 15) were obtained for 84 % of the compounds analysed and the rest mostly the plasticizers, fell within EPA method 8000 acceptance criteria of RF % RSD < 20. Only five of the analytes (<10 %), all belonging to chlorinated phenols, had RF % RSD > 20 % (Table 1), which were accepted using the calibration curves R2 > 0.99, in compliance to EPA method 8000 acceptance criteria as referenced in method 8270E. The percent recoveries for ICVs and CCVs ran with mid standards levels ranged from 98 to 105% and 84–111.6 % respectively. The mean recoveries of the surrogates standards (S) used ranged between 99.5–126 %. The mean recoveries of the spiked samples used for extraction method validation ranged between 78–109 %. The phthalates, had the highest recovery in all samples.
3.2. Levels of PAHs in tissue papers
From the results in Table 2, significant levels of PAHs were recorded for all the tissue papers analysed. The total mean PAHs recorded ranged between 174.53–1664.55 μg/kg. PTN and F tissue papers that recorded the least were analysed as controls to the toilet tissue papers since they are virgin tissue papers. Among the toilet tissue papers, “NN” toilet paper recorded the least level of mean total PAHs (392.82 μg/kg) and “BB” toilet paper recorded the highest (4450.39 μg/kg) (Table 2). The mean total PAH levels in this study are comparable to levels of 840–12300 μg/kg with an average of 4800 μg/kg obtained in contaminated dust by Wang et al. [51], though levels in this study are relatively significantly smaller than the maximum of 12,300 μg/kg.
Table 2.
Mean Concentrations in μg/kg of PAHs (n = 4) in Toilet-tissue papers on the Ghanaian Market.
| PAH | SS | SL | NN | BB | NRP | SF | F | PTN |
|---|---|---|---|---|---|---|---|---|
| Naphthalene | 128.62 | 263.01 | 42.19 | 33.32 | 15.86 | 9.26 | 89.53 | 5.99 |
| 1-Methyl-Naphthalene | 190.17 | 247.74 | 60.72 | 34.62 | 40.94 | 21.35 | ND | ND |
| 2-Methyl- Naphthalene | 116.80 | 150.59 | 35.65 | 34.05 | 40.29 | 11.23 | ND | ND |
| Acenaphthylene | 3.54 | 5.03 | 2.26 | 11.67 | 7.05 | 1.96 | 0.17 | BLD |
| Acenaphthene | 10.18 | 18.08 | 11.88 | 28.29 | 16.06 | 22.79 | 11.81 | 9.97 |
| Fluorene | 80.28 | 94.92 | 17.16 | 333.34 | 38.50 | 10.24 | 2.07 | 2.35 |
| Anthracene | 188.03 | 349.59 | 49.21 | 1785.79 | 257.16 | 52.57 | 1.68 | 2.00 |
| Phenanthrene | 22.65 | 305.88 | 14.01 | 982.88 | 52.15 | 10.95 | BLD | BLD |
| Fluoranthene | 61.87 | 70.57 | 36.56 | 359.59 | 109.49 | 26.81 | BLD | BLD |
| Pyrene | 79.77 | 84.22 | 56.19 | 238.03 | 173.59 | 23.12 | 0.35 | 0.32 |
| Benz[A]Anthracene | 6.57 | 29.09 | 14.83 | 75.33 | 270.69 | 59.62 | 29.07 | 30.05 |
| Chrysene | 16.51 | 14.83 | 16.38 | 342.81 | 872.60 | 57.84 | 25.36 | 26.29 |
| Benzo[B]Fluoranthene | 4.34 | 9.05 | 7.09 | 48.25 | 154.42 | 47.48 | 24.79 | 25.19 |
| Benzo[K]Fluoranthene | 2.11 | 4.22 | 6.95 | 12.79 | 79.38 | 34.28 | 21.10 | 21.31 |
| Benzo[A]Pyrene | 5.49 | 1.94 | 6.28 | 17.37 | 108.71 | 26.55 | 25.47 | 24.90 |
| Indeno[1,2,3-CD]Pyrene | 1.97 | 6.44 | 3.82 | 61.48 | 14.46 | 5.67 | 12.95 | 14.73 |
| Dibenz[A,H]Anthracene | 3.67 | 4.69 | 7.73 | 29.88 | 56.11 | 14.53 | 11.17 | 9.82 |
| Benzo[GHI]Perylene | 3.83 | 4.67 | 3.93 | 20.89 | 5.77 | 6.86 | 0.66 | 1.62 |
| Mean Total PAHs | 926.39 | 1664.55 | 392.82 | 4450.39 | 2313.24 | 443.12 | 256.2 | 174.53 |
ND means not detected, whereas BLD means below method?"s detection limit.
These levels recorded, may have dire consequences on the health of consumers upon dermal contacts to these products. The elevated levels of PAHs, in almost all the samples taken, may be attributed to the extent of contamination and poor pre-treatment of recycled waste papers or paper products used for the production of these toilet tissue papers. The presence and appreciable levels of alkylated PAHs, 1-methylnaphthalene (21.35–247.74 μg/kg) and 2-methylnaphthalene (11.23–116.80 μg/kg) in the recycled paper toilet tissue products (Table 2) is an indication of petroleum contamination [[52], [53], [54]].The levels of benzo[a]pyrene, B[a]P a definite carcinogen ranged between 1.94–108.71 μg/kg (Table 2). PAHs such as pyrene and benzo[a]pyrene have been linked to certain cancers in human due to their elevated levels in rectal cancer, liver cancer, gastric cancer and lung cancer tissues [[55], [56], [57]]. The elevated levels of PAHs in the toilet tissue papers may have contributed significantly to the upsurge in cancer and non-cancer incidences as reported by Bray et al. [44] and WHO (2018). There is thus, a cause for concern on the unwholesomeness of toilet tissue papers on the Ghanaian market.
Statistical analysis of variance (ANOVA) conducted at the 95 % CL showed no significant difference (p > 0.05) in individual and total PAHs levels for replicate samples (n = 4). Two-way ANOVA conducted at 95 % CL showed significant difference between individual PAH levels (p = 0.03) and also between different toilet tissue paper samples with respect to their PAH levels (p = 0.0074). This is an indication of the fact that, the levels of PAHs in samples, is brand dependant suggesting differences in treatments processes of the recycled papers.
3.3. Plasticizers in toilet tissue paper
From the results (Table 3), the mean total plasticizers in toilet tissue papers ranged between 27055.16 μg/kg (0.003 %)–181306.62 μg/kg (0.02 %). Among the individual phthalates analysed, Bis(2-Ethylhexyl) Phthalate (DEHP) recorded the highest levels in most samples [12885.62 (0.0.1 %) - 58640.82 μg/kg (0.006 %)] followed by Hexanedioic acid, dioctyl ester [2978.92 (0.003 %) - 95473.99 μg/kg (0.01 %)]. Considering the extent and point of applications of these toilet papers, the elevated levels of plasticizers in some toilet tissue paper samples, especially in “BB” and “NRP” may have dire health consequences on consumers though levels obtained were below the EC and US Consumer Product Safety Improvement Act limits of 0.1 % [32,58]. The appreciable level of plasticizers may be attributed to the use of poor recycle papers and or contaminations of the products during processing due to poor manufacturing practices. This therefore require of the regulatory bodies to increase surveillance to curb the menace. The levels of the plasticizers obtained in this study, are comparable with levels obtained by Koniecki et al. [59] in cosmetics and some personal care products, though levels obtained in their study was quite higher. This meant that, these toilet tissue papers are not that worst though improvement is required to reduce the risk associated with the presence of these phthalates especially DEHP levels.
Table 3.
Mean concentrations in μg/kg of plasticizers (n = 4) in toilet tissue papers on the Ghanaian Market.
| Compound | SS | SL | NN | BB | NRP | SF |
|---|---|---|---|---|---|---|
| Dimethyl phthalate | 39.06 | 30.63 | 9.95 | 78.02 | 68.91 | 51.56 |
| Diethyl Phthalate | 966.56 | 660.50 | 337.14 | 847.03 | 1241.58 | 2236.95 |
| Dibutyl phthalate | 8777.74 | 8167.83 | 9447.35 | 18537.50 | 9760.68 | 8269.71 |
| Benzyl butyl phthalate | 654.06 | 1086.15 | 732.49 | 3595.43 | 724.62 | 296.90 |
| Hexanedioic acid, dioctyl ester | 2978.92 | 5202.58 | 5438.84 | 95473.99 | 46720.28 | 4967.97 |
| Bis(2-Ethylhexyl) Phthalate | 12885.62 | 11630.44 | 24583.90 | 58640.82 | 41950.39 | 13923.25 |
| Di-n-octyl phthalate | 753.19 | 1262.23 | 1940.60 | 4133.83 | 1253.28 | 618.53 |
| Mean total | 27055.16 (0.003 %) | 28040.36 (0.003%) | 42490.27 (0.004 %) | 181306.62 (0.02 %) | 101719.75 (0.01 %) | 30364.88 (0.003 %) |
Two-way ANOVA at 95 % CL, showed statistical significant difference (p = 0.048) between the total mean levels of plasticizers in the various toilet tissue paper sample studied, and also significant difference (p = 0.0002) between the levels of individual plasticizers studied. These indicate that the levels of plasticizers in samples differ from brands implying difference in the pre-treatment procedures used.
From Table 4, the mean total concentrations of semi-volatile chlorinated organic compounds found in the toilet tissue paper samples ranged from 2216.27 μg/kg (in NN) to 14626.04 μg/kg (in “SL”). The elevated levels of SVCOC recorded in samples analysed especially in “SL” and “SS” are suggestive of the extent of chlorine bleaching the recycled papers were subjected to during the production of the tissue papers. This may be attributed to the fact that the raw materials used were highly contaminated and as such compelled the producers to apply a copious amount of bleach to produce relatively cleaner products. Unfortunately these elevated levels may have a dire consequences on the health of consumers.
Table 4.
Mean total concentrations (μg/kg) of Semi-volatile chlorinated organic compounds (n = 4) in toilet tissue paper samples from the Ghana Market.
| Compound | SS | SL | NN | BB | NRP | SF |
|---|---|---|---|---|---|---|
| Bis (2-chloroethyl) ether | 26.14 | 20.15 | 6.93 | 9.72 | 81.85 | 32.76 |
| Bis(2-chloroisopropyl) ether | 114.78 | 206.60 | 187.09 | 74.97 | 101.28 | 14.74 |
| Methane, bis(2-chloroethoxy)- | 9.27 | 10.00 | 3.53 | 5.91 | 5.63 | 7.90 |
| Ethane, hexachloro- | 26.27 | 16.19 | 5.43 | 66.20 | 14.37 | 13.03 |
| 1,3-Butadiene, 1,1,2,3,4,4-hexachloro- | 2.60 | 35.32 | 18.17 | 19.67 | 4.04 | 32.16 |
| Benzene, 1,2-Dichloro- | 11.09 | 23.31 | 3.16 | 1.97 | 1.09 | 7.36 |
| Benzene, 1,3-dichloro- | 1.88 | 12.13 | 1.66 | 4.34 | 16.45 | 13.18 |
| Benzene, 1,4-Dichloro- | 2.39 | 6.19 | 1.09 | 5.30 | 25.99 | 2.78 |
| Benzene, 1,2,4-trichloro- | 1.80 | 1.90 | 1.28 | 2.59 | 2.00 | 3.63 |
| Benzene, Hexachloro- | 2.27 | 1.61 | 31.58 | 3.21 | 1.28 | 2.13 |
| 4-Chloroaniline | 21.98 | 5.05 | 9.47 | 4.63 | 4.38 | 10.67 |
| Naphthalene, 2-chloro- | 1.12 | 0.87 | 2.49 | 5.96 | 1.80 | 4.07 |
| Phenol, 2-chloro- | 3.56 | 1.62 | 2.14 | 8.22 | 3.11 | 10.15 |
| Phenol, 2,4-dichloro- | 114.32 | 47.35 | 121.12 | 94.77 | 81.96 | 20.51 |
| 4-Chloro-3-Methylphenol | 19.76 | 43.56 | 13.88 | 29.92 | 10.31 | 6.26 |
| Phenol, 2,4,6-Trichloro- | 5617.79 | 12414.31 | 1080.78 | 1209.17 | 1500.72 | 545.11 |
| Phenol, 2,4,5-Trichloro- | 160.53 | 233.92 | 104.59 | 55.13 | 48.58 | 16.18 |
| Phenol, 2,3,5,6-Tetrachloro- | 1789.31 | 1497.40 | 589.45 | 205.41 | 53.95 | 2681.63 |
| Phenol, 2,3,4,5-Tetrachloro- | 76.92 | 47.86 | 23.85 | 2766.04 | 434.79 | 33.83 |
| 4-Bromophenyl Phenyl Ether | 1.29 | 0.71 | 8.61 | 12.95 | 2.87 | 6.59 |
| Mean total | 8005.08 | 14626.04 | 2216.27 | 4586.06 | 2396.45 | 3464.68 |
Among the classes of SVCOC investigated, the chlorophenols recorded the highest levels in all samples, with 2,4,6-Trichlorophenol recording the highest levels (545.11–12414.31 μg/kg) in almost all samples followed by 2,3,5,6-Tetrachlorophenol (Table 4). Chlorinated ethers, were second in levels with elevated maximum of 206.60 μg/kg bis(2-chloroisopropyl) ether. The carcinogenic Bis(2-chloroethyl) ether in the latter class recorded values that ranged from 9.72 μg/kg (in BB) to 49.84 μg/kg (NRP) (Table 4). The class of SVCOC with the third highest levels were the polychlorinated aliphatics including hexachloroethane, (5.43–66.20 μg/kg) and 1,1,2,3,4,4-hexachloro-1,3-Butadiene, (2.60–35.32 μg/kg). The SVCOC class with the least levels in samples analysed were the chlorobenzenes (Table 4). The elevated levels of polychlorophenols, Bis(2-chloroethyl) ether and other class B carcinogens in the toilet tissue papers analysed may have contributed to the upsurge in cancer incidence in Ghana and the world as a whole as reported by Ghana health service and thus Bray et al. [44].
Two-way ANOVA at 95 CL showed no statistical significant difference (p = 0.46) in the mean total levels of SVCOC between toilet tissue paper samples investigated. This may suggest that the problem cuts across the spheres of tissue papers on the Ghanaian market.
3.4. Risk assessment
3.4.1. PAHs cancer risks
The carcinogenic risk involved upon exposure to PAHs were computed using the proposed values in Table 5. From Table 6, the ∑B[a]P-TEQ calculated for toilet tissue samples ranged from 10.48 to 210.44 μg/kg and the respective DADB[a]P also ranged from 6.5 × 10−6 to 1.3 × 10-4 mg/kg-day in samples SS and NRP. The cancer risk associated with these exposures also ranged from1.6 × 10-4 and 3.3 × 10-3 respectively for adults’ life time of 70 years (Table 6). These values were above the USEPA acceptable cancer risk of 1.0 × 10-6 to 1.0 × 10-4 [47].
Table 5.
Proposed benzo[a]pyrene toxicity equivalent factors (TEF) for carcinogenic PAH.
| PAH | TEF [60] |
|---|---|
| Chrysene | 0.001 |
| Benz[a]anthracene | 0.100 |
| Benzo[b]fluoranthene | 0.100 |
| Benzo[k]fluoranthene | 0.010 |
| Benzo[a]pyrene | 1.000 |
| Indeno[1,2,3-Cd]pyrene | 0.100 |
| Dibenz[a,h]anthracene | 1.000 |
Table 6.
Cancer risk assessment for dermal exposure to chemicals in toilet tissue papers.
| Risk Parameter | SS | SL | NN | BB | SF | NRP | FP | PTN |
|---|---|---|---|---|---|---|---|---|
| For PAHs | ||||||||
| ∑ BaP-TEQ | 10.48 | 11.14 | 16.66 | 66.22 | 52.76 | 210.44 | 43.56 | 41.96 |
| DADBaP, (mg/kg-day) | 6.5E-06 | 6.9E-06 | 1.0E-05 | 4.1E-05 | 3.3E-05 | 1.3E-04 | 2.7E-05 | 2.6E-05 |
| Cancer Risk | 1.6E-04 | 1.7E-04 | 2.6E-04 | 1.0E-03 | 8.2E-04 | 3.3E-03 | 6.8E-04 | 6.5E-04 |
| For Phthalates | ||||||||
| DAD (mg/kg-day)for DEHP | 6.2E-03 | 5.6E-03 | 1.2E-02 | 2.8E-02 | 2.0E-02 | 6.6E-03 | ||
| Cancer RISK for DEHP | 8.6E-05 | 7.8E-05 | 1.6E-04 | 3.9E-04 | 2.8E-04 | 9.3E-05 | ||
| Hazard Index (HI) | 3.5E-01 | 3.2E-01 | 6.3E-01 | 1.5 | 1.1 | 3.7E-01 | ||
| SVCOC | ||||||||
| Bis (2-chloroethyl) ether | 1.4E-05 | 1.1E-05 | 3.6E-06 | 5.1E-06 | 4.3E-05 | 1.7E-05 | ||
| 1,4-dichllorobenzene | 2.7E-08 | 7.1E-08 | 1.2E-08 | 6.1E-08 | 3.0E-07 | 3.2E-08 | ||
| Hexachloroethane | 1.8E-07 | 1.1E-07 | 3.6E-08 | 4.4E-07 | 9.6E-08 | 8.7E-08 | ||
| 2,4,6-Trichlorophenol | 2.9E-05 | 6.5E-05 | 5.7E-06 | 6.4E-06 | 7.9E-06 | 2.9E-06 | ||
| 4-chloroaniline | 2.1E-06 | 4.8E-07 | 9.1E-07 | 4.4E-07 | 4.2E-07 | 1.0E-06 | ||
| Cumulative cancer Risk | 4.6E-05 | 7.6E-05 | 1.0E-05 | 1.2E-05 | 5.2E-05 | 2.1E-05 | ||
| Chlorophenols | ||||||||
| Hazard Index, HI | 4.8E-02 | 3.3E-02 | 2.9E-02 | 1.9E-02 | 1.4E-02 | 4.7E-02 | ||
Elevated risk of colorectal cancer mortality was found among PAH-exposed gas furnace workers in an occupational cohort study in Germany [61]. Similar studies have reported an associations between PAHs exposure, particularly B(a)P exposure and risk of colorectal adenoma [[62], [63], [64], [65]]. The risk outcome suggested that, the presence of PAHs in the toilet tissue papers may have contributed significantly to the upsurge in global cancer burden, especially to colorectal cancer and adenoma as reported by [43].
Surprisingly, even the controls used had appreciable cancer risk associated with their use, implying some level of contaminations with PAHs which may stem from the fact that these hand tissue papers were not virgin tissue papers but rather recycled tissue papers.
3.4.2. Risk assessment for phthalate plasticizers in Toilet tissue paper
The DAD for DEHP in the toilet tissue paper samples were found to range from 5.6 × 10−3 to 2.8 × 10-2 mg/kg-day (samples SL to BB respectively) and the respective cancer risk for adult’s life time of 70 years were also found to range from 7.8 × 10-5 to 3.9 × 10-4 (Table 6). Samples NN, BB and SF had risk values above the USEPA acceptable Upper bound value 10-4 implying that consumers are at high risk of cancer upon use such unwholesome products. These elevated risk observed may be contributing to the upsurge in cancer incidences in Ghana and the world as a whole and cause for concern.
Hazard index (HI) for the phthalates were calculated to explore the non-cancer health effects [47] associated upon exposure to these contaminants in toilet tissue paper. An HI < 1.0 indicate(s) may pose no significant non-cancer adverse health effect to human.
The hazard Index (HI) calculated for dermal exposure to phthalates in the samples ranged from 3.2 × 10−1 to 1.5 in samples SL to BB (Table 6). With the exception of sample BB and NRP that recorded HI values greater than maximum acceptable value of 1.0, the rest of the samples recorded values <1. The elevated HI (>1) values recorded for BB and NRP may be contributing to the upsurge in non-cancer health effects such as reproductive disorders and birth defect among Ghanaians.
3.4.3. Risk assessment of SVCOC in toilet tissue papers on the Ghanaian market
Using Eq. (5) with the follow respective slope factors 1.10, 0.024, 0.014, 0.011 and 0.2 (mg/kg-day)−1 and the calculated DADABS, the carcinogenic risk assessment conducted for the classified carcinogenic SVCOCs, recorded values ranging from 3.6 × 10-6 – 4.3 × 10-5; 1.2 × 10-8 – 2.9 × 10-7; 3.6 × 10-8 - 4.4 × 10-7; 2.9 × 10-6 – 6.5 × 10-5; and 4.2 × 10-7 – 2.1 × 10-6 for Bis (2-chloroethyl) ether, 1,4-dichlorobenzene, hexachloroethane, 2,4,6-Trichlorophenol and 4-chloroaniline respectively (Table 6). The cumulative cancer risk for these SVCOC in the toilet tissue papers ranged from 1.0 × 10-5 – 7.7 × 10-5 (Table 6). The cumulative cancer risk recorded is relatively just above the moderate cancer risk threshold of 10-5. This is suggestive of little cancer risk in this regard, but not withstanding these may added unto the earlier calculated risk from the other toxicants present and thus may increase significantly the cumulative cancer risk in the use of toilet tissues on the Ghanaian market.
Using Eq. (4), the calculated hazard index for chlorophenols including 2-CP, 2,4-DCP, 2,4,5-TCP and 2,3,4,6-TeCP ranged from 1.4 × 10−2 to 4.8 × 10-2 (Table 6). This indicates low or no non- cancer risk associated with chlorophenols in the toilet tissue paper samples, thus low contribution to the non-cancer risk levels associated with the use of toilet tissue paper samples analysed.
3.5. Source apportionment using principal component analysis and multiple linear regression (PCA-MLR)
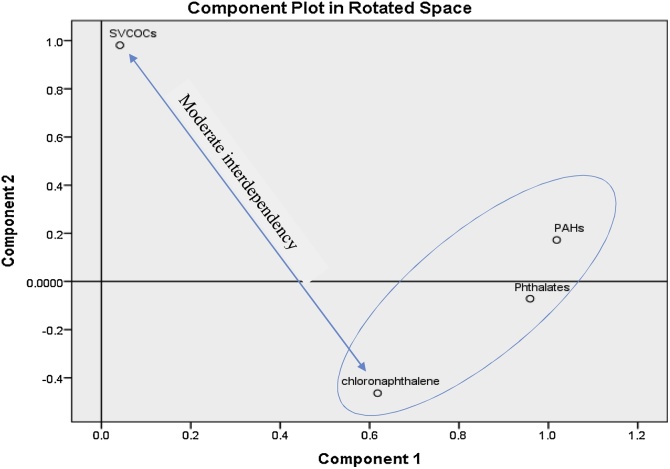
Principal component analysis with multiple linear regression (PCA-MLR) have been successfully employed for source apportionment in environmental studies [[66], [67], [68]]. The PCA-MLR were conducted to help apportion likely sources for PAHs, phthalates, 2-chloronaphthalene and SVCOCs in the toilet tissue papers.
The PCA extraction results (Fig. 3), showed two significant factor components (KMO = 0.54) after Oblimin with Kaiser Normalization rotation (with Eigenvalues ≥ 1.0). The two factor components (FC1 and FC2) contributed about 90.96 % of the total percent variance. Factor component 1 (FC1) with 67.15 % of variance, had high loading (> 0.85) for PAHs, phthalates and 2-chloronapthalenes, whereas Factor component 2 (FC2) with 23.81 % of the variance, loaded high for SVCOCs. The reproduced correlation from the factor analysis suggested, a moderately strong negative correlation (−0.61) between SVCOCs and 2-Chloronaphthalene, whereas a weak negative correlation was found between SVCOCs and PAHs (−0.09), as well as between SVCOCs and phthalates (−0.32). A strong positive correlation (0.94) was found between PAHs and phthalates, whereas 2-chloronaphthalene also correlated moderately strong with PAHs (r = 0.66) and phthalates (r = 0.78). These factor analysis results, suggested two major sources, likely to be of oil based substances (FC1) and bleaching source (FC2).
Fig. 3.
A PCA component plot showing the source apportionment of Phthalates, PAH, SVCOCs and 2-choronapthalene in toilet tissue papers.
To complete the source apportionment, FCs (sources) contributions to PAHs, phthalates, SVCOCs and 2-chloronaphthalene were further determined with a combined PCA-MLR analysis. Here, the levels of PAHs, phthalates, SVCOCs and 2-chloronaphthalene were used as the dependant variables whereas the factor components (the sources) were used as the independent variables for the PCA-MLR model [69,70].
The results from the model summary (Table 7), showed that, the two components in the four models estimated accounted for 96.1 % (R2 = 0.961), 96.7 % (R2 = 0.967), 89.9 % (R2 = 0.899) and 77.1 % (R2 = 0.771) of the variation in the PAHs, Phthalates, SVCOCs and 2-chloronaphthalene respectively. Factor component “1” contributed significantly (β > 1.0; p < 0.05) to PAHs, and Phthalates but not SVCOCs (β = 0.041; p > 0.05). Factor component “2”, also contributed significantly to SVCOCs (β = 0.98; p = 0.007) in the toilet tissue papers. However, the standardized coefficients of FC2 in predicting PAHs and Phthalates were statistically not significant (p > 0.05). The PCA-MLR model results (Table 7) further showed that, both FC1 and FC2 contributed to the variance in 2-chloronaphthalene. But FC1 contributed greater and positively (β = 0.62) than FC2 (−0.46) to 2-chloronaphthalene.
Table 7.
Model summary for Multiple Linear regression Analysis for source apportionment.
| Significant Predictor | Dependent variable | R2 | Standardized coefficients, β | Significance, p-value |
|---|---|---|---|---|
| REGR factor score 1 | PAHs | 0.961 | 1.02 | 0.003 |
| REGR factor score 1 | Phthalates | 0.967 | 1.01 | 0.003 |
| REGR factor score 2 | SVCOCs | 0.899 | 0.98 | 0.007 |
| REGR FC1 > FC2 | 2-Chloronaphthalene | 0.771 | 0.62 (FC1) and -0.46 (FC2) | > 0.05 |
Where FC means factor component.
The PCA-MLR analysis results suggested that, PAHs, phthalates and 2-chloronaphthalene in the toilet tissue paper may have come from one common source. The possible source could be oil based substances such as coal t ar, bitumen, printer’s inks, paints, petroleum oil etc. or mixtures of two or more of these on a soiled recycled paper used for the production of the tissue paper. The results also suggested SVCOCs to come from a significantly different source altogether, which may possibly come from the bleaching processes employed for the production of toilet tissue paper. The model also predicted 2-chloronaphthalene to have its major source from oil base substances, but bleaching source also contributed negatively to the source of 2-choronaphthalene as predicted by the factor analysis.
4. Conclusion
The study found elevated levels of PAHs, phthalates and SVCOCs in toilet tissue papers on the Ghanaian market. These levels suggested elevated cancer and non-cancer risk associated with the dermal use of the toilet tissue papers by an adult Ghanaian for a life time of 70 years. The cumulative effect of the risk of exposure to carcinogenic SVOCs in toilet tissue papers, may have contributed significantly to the upsurge in cancer incidence in Ghana and the world as a whole. It is therefore, recommended that FDA and other regulatory bodies in Ghana increase surveillance to help curb the menace in the toilet tissue paper production industries. This may help reduce the significant contributions of these consumer products and their likes to the cancer incidences. The source apportionment conducted with PCA-MLR, revealed two major possible sources, which indicated phthalates, PAHs and 2-chhloronaphthalene to have come from one common source, possibly an oil base source whereas SVCOCs came from another source, possibly a bleaching source.
CRediT authorship contribution statement
Joseph Kweku Adjei: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Supervision, Visualization, Writing - original draft, Writing - review & editing. David Kofi Essumang: Validation, Resources, Supervision, Writing - review & editing. Evelyn Twumasi: Conceptualization, Writing-original draft, Formal analysis, Visualization, Resources. Eric Nyame: Writing - original draft, Investigation. Ishmael Muah: Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to express our profound gratitude to the School of Physical Sciences, University of Cape Coast for making available their instrument for the sample analysis. Our final thanks go to the Government of Ghana for their financial support.
References
- 1.Nordic Ecolabelling . 2016. Nordic Ecolabelling of Tissue Paper. Version 5.3. [Google Scholar]
- 2.EU regulation; 2001/95/EC. Directive 2001/95/EC of the European Parliament and of the Council of 3 December 2001 on General Product Safety.
- 3.EC 850/2004. Corrigendum to Regulation (EC) No 850/2004 of the European Parliament and of the Council of 29 April 2004 on persistent organic pollutants and amending Directive 79/117/EEC. Off. J. Eur. Union L 158 of 30 April 2004.
- 4.CNS . Chinese National Standards; Taiwan: 2016. Toilet Tissue Paper- CNS 1091:2016. 2016-3-1, pg. 7. [Google Scholar]
- 5.Adekunle A.S., Oyekunle J.A.O., Ola I.J., Obisesan O.R., Maxakato N.W. Determination of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in some personal care products in Nigeria. Toxicol. Rep. 2018;5:994–1001. doi: 10.1016/j.toxrep.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essumang D.K., Dodoo D.K., Adjei J.K. Effect of smoke generation sources and smoke curing duration on the levels of polycyclic aromatic hydrocarbon (PAH) in different suites of fish. Food Chem. Toxicol. 2013;58:86–94. doi: 10.1016/j.fct.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Perera F., Wang S., Vishnevetsky J., Zhang B., Cole K.J., Tang D. Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behaviour scores in New York City children. Environ. Health Perspect. 2011;119(8):1176–1181. doi: 10.1289/ehp.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards S.C., Jedrychowski W., Butscher M., Camann D., Agnieszka K.A. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and childrens intelligence at 5 years of age in a prospective cohort study in Poland. Environ. Health Perspect. 2010;118(9):1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IPCS . International Programme On Chemical Safety; 2010. Polycyclic Aromatic Hydrocarbons, Selected Non-Heterocyclic.http://www.inchem.org/documents/ehc/ehc/ehc202.htm [Google Scholar]
- 10.Luderer U., Myers M.B., Banda M., Mckim K.L. Ovarian effects of prenatal exposure to benzo[a]pyrene: roles of embryonic and maternal glutathione status. Reprod. Toxicol. 2017;69:187–195. doi: 10.1016/j.reprotox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essumang D.K., Kowalski K., Sogaard E.G. Levels, distribution and source characterization of polycyclic aromatic hydrocarbons (PAHs) in topsoils and roadside soils in Esbjerg, Denmark. Bull. Environ. Contam. Toxicol. 2011;86:438. doi: 10.1007/s00128-011-0232-0. [DOI] [PubMed] [Google Scholar]
- 12.IARC . Vols. 1–103. 2012. http://monographs.iarc.fr/ENG/Classification/ClassificationsAlphaOrder.pdf&updated (Agents Classified by the IARC Monographs). Last updated 30 July 2018. [Google Scholar]
- 13.Schettler T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 14.Ling W., Gui-Bin C., Ya-Qi H. Cloud point extraction coupled with HPLC-UV for the determination of phthalate esters in environmental water samples. J. Environ. Sci. 2007;19:874–878. doi: 10.1016/s1001-0742(07)60145-4. [DOI] [PubMed] [Google Scholar]
- 15.Huang P.-C., Tien C.-J., Sun Y.-M., Hsieh C.-Y., Lee C.-C. Occurrence of phthalates in sediment and biota: Relationship to aquatic factors and the biots-sediment accumulation factor. Chemosphere. 2008;73:539–544. doi: 10.1016/j.chemosphere.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 16.ATSDR . Department of Health and Human Services; Atlanta, Georgia: 2006. Toxicological Profile for Di-(2-Ethylhexyl) Phthalate (DEHP). Agency for Toxic Substances and Disease Registry, Atlanta. [PubMed] [Google Scholar]
- 17.Wormuth M., Scheringer M., Vollenweider M., Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26(3):803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 18.Wittassek M., Wiesmuller G.A., Koch H.M., Eckard R., Dobler L., Muller J. Internal phthalate exposure over the last two decades—a retrospective human biomonitoring study. Int. J. Hyg. Environ. Health. 2007;210:319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Wittassek M., Angerer J. Phthalates: metabolism and exposure. Int. J. Androl. 2008;31 doi: 10.1111/j.1365-2605.2007.00837.x. 131–1. [DOI] [PubMed] [Google Scholar]
- 20.Benson Hazard to the developing male reproductive system from cumulative exposure to phthalate esters--dibutyl phthalate, diisobutyl phthalate, butylbenzyl phthalate, diethylhexyl phthalate, dipentyl phthalate, and diisononyl phthalate. Regul. Toxicol. Pharmacol. 2009;53(2):90–101. doi: 10.1016/j.yrtph.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 21.De Toni L., Tisato F., Seraglia R., Roverso M. Phthalates and heavy metals as endocrine disruptors in food: a study on pre-packed coffee products. Toxicol. Rep. 2017;4:234–239. doi: 10.1016/j.toxrep.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutschle T., Reiter R., Butte W., Heinzow B., Keck T., Riechelmann H. A controlled challenge study on di(2-ethylhexyl) phthalate (DEHP) in house dust and the immune response in human nasal mucosa of allergic subjects. Environ. Health Perspect. 2008;116(11):1487–1493. doi: 10.1289/ehp.11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolarik B., Naydenov K., Larsson M., Bornehag C.G., Sundell J. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ. Health Perspect. 2008;116:98–103. doi: 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schripp T., Fauck C., Salthammer T. Chamber studies on mass-transfer of di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DnBP) from emission sources into house dust. Atmos. Environ. 2010;44(24):2840–2845. [Google Scholar]
- 25.Geiss O., Salvatore T., Barrero-Moreno J., Kotzias D. Investigation of volatile organic compounds and phthalates present in the cabin air of used private cars. Environ. Int. 2009;35(8):1188–1195. doi: 10.1016/j.envint.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Main K.M., Mortensen G.K., Kaleva M.M., Boisen K.A., Damgaard Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 2006;114(2):270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takatori S., Akutsu K., Kondo F., Izumi S., Makino T., Nakazawa H. Determination of phthalate monoester levels in human breast milk by high pressure liquid chromatography/tandem mass spectrometry [in Japanese] Bunseki Kagaku. 2007;56(12):225–231. [Google Scholar]
- 28.Hines E.P., Calafat A.M., Silva M.J., Mendola P., Fenton S.E. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ. Health Perspect. 2009;117(1):86–92. doi: 10.1289/ehp.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latini G., Wittassek M., Del Vecchio A., Presta G., De Felice C., Angerer J. Lactational exposure to phthalates in Southern Italy. Environ. Int. 2009;35:236–239. doi: 10.1016/j.envint.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Foster P.M. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006;29(1):140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 31.Howdeshell K.L., Wilson V.S., Furr J., Lambright C.R., Rider C.V. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 2008;105(1):153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 32.EC Communication from the commission: on the finalisation of the restriction process on the four phthalates (DEHP, DBP, BBP and DIBP) under Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). (2014/C 260/01) Off. J. Eur. Union. 2014 C 260/1. [Google Scholar]
- 33.Ferguson K.K., McElrath T.F., Ko Y.A., Mukherjee B., Meeker J.D. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int. 2014;70C:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson K.K., McElrath T.F., Meeker J.D. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel S.M., Zhu C., Berkowitz G.S., Calafat A.M., Silva M.J. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522–528. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y., Ha E.H., Kim E.J., Park H., Ha M. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ. Health Perspect. 2011;119(10):1495–1500. doi: 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki Y., Yoshinaga J., Mizumoto Y., Serizawa S., Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int. J. Androl. 2012;35(3):236–244. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 38.Whyatt R.M., Liu X., Rauh V.A., Calafat A.M., Just A.C. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ. Health Perspect. 2012;120(2):290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bustamante-Montes L., Hernández-Valero M., Flores-Pimentel D., García-Fábila M., Amaya-Chávez A., Barr D., Borja-Aburto V. Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. J. Dev. Orig. Health Dis. 2013;4(4) doi: 10.1017/S2040174413000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Factor-Litvak P., Insel B., Calafat A.M., Liu X., Perera F., Rauh V.A., Whyatt R.M. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One. 2014;9(12):e114003. doi: 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bornehag C.G., Carlstedt F., Jönsson B.A., Lindh C.H. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ. Health Perspect. 2015;123(1):101–107. doi: 10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swan S.H., Sathyanarayana S., Barrett E.S., Janssen S. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 2015;30(4):963–972. doi: 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.IARC . WHO; Geneva, Switzerland: 2018. IARC Report-Latest Global Cancer Data: Cancer burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018.https://www.who.int/cancer/PRGlobocanFinal.pdf [Google Scholar]
- 44.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018 doi: 10.3322/caac.21492. in press. The online GLOBOCAN 2018 database is accessible at http://gco.iarc.fr/, as part of IARC’s Global Cancer Observatory. [DOI] [PubMed] [Google Scholar]
- 45.Engraff M., Solere C., Smith K., Mayer P., Dahllof I. Aquatic toxicity of PAHs and PAH mixtures at saturation to benthic amphipods: linking toxic effects to chemical activity. Aquat. Toxicol. 2011;102:142–149. doi: 10.1016/j.aquatox.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Fisher T.T., Law R.J., Rumney H.S., Kirby M.F., Kelly C. Towards a scheme of toxic equivalency factors (TEFs) for the acute toxicity of PAHs in sediment. Ecotoxicol. Environ. Saf. 2011;74:2245–2251. doi: 10.1016/j.ecoenv.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 47.EPA . 2004. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment). EPA/540/R/99/005, OSWER 9285.7-02EP, PB99-9633. 12 July 2004. [Google Scholar]
- 48.Wester R.C., Maibach H.I., Bucks D.A.W., Sedik L., Melendres J. Percutaneous absorption of [14C]DDT and [14C]benzo[a]pyrene from soil. Fundam. Appl. Toxicol. 1990;15:510–516. [PubMed] [Google Scholar]
- 49.EPA . National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency; Washington, DC: 2007. Dermal exposure assessment: a summary of EPA approaches; p. 20460. EPA/600/R-07/040F. [Google Scholar]
- 50.EPA . United States Environmental Protection Agency; Washington DC, United States: 2005. Guidelines for Carcinogenic Risk Assessment. 70FR17765-1717. EPA/630/P-03/001F. [Google Scholar]
- 51.Wang W., Huang M.-J., Kang Y. Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: status, sources and human health risk assessment. Sci. Total Environ. 2011;409:4519–4527. doi: 10.1016/j.scitotenv.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 52.Brandt C.A., Becker M., Porta A. Distribution of polycyclic aromatic hydrocarbons in soils and terrestrial biota after a spill of crude oil in Trecate, Italy. Environ. Toxicol. Chem. 2002;21:1638–1643. [PubMed] [Google Scholar]
- 53.Zakaria M.P., Takada H., Tsutsumi S. Distribution of polycyclic aromatic hydrocarbon (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environ. Sci. Technol. 2002;36:1907–1918. doi: 10.1021/es011278+. [DOI] [PubMed] [Google Scholar]
- 54.Ou S., Zheng J., Zheng J. Petroleum hydrocarbons and polycyclic aromatic hydrocarbons in the surficial sediments of Xiamen Harbour and Yuan Dan Lake, China. Chemosphere. 2004;56:107–112. doi: 10.1016/j.chemosphere.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Jiang G., Lun L., Cong L. Association between polycyclic aromatic hydrocarbons and human rectal tumor or liver cancer. Chin.-German J. Clin. Oncol. 2012;11(7):391–394. [Google Scholar]
- 56.Wang J., Lun L.M. Association between polycyclic aromatic hydrocarbons and human gastric tumor. Shandong Med. J. (Chinese) 2008;48:20–21. [Google Scholar]
- 57.Zhang T., Lun L.M. The detection of polycyclic aromatic hydrocarbons in human lung cancer. Med. J. Qilu (Chinese) 2009;2009(24):202–204. [Google Scholar]
- 58.U.S. Consumer Product Safety Commission . 2010. Review of Exposure and Toxicity Data for Phthalate Substitutes: Di-isononyl-cyclohexane-1, 2-dicarboxylate (DINCH)www.cpsc.gov/about/cpsia/phthalsub.pdf Available at. [Google Scholar]
- 59.Koniecki D., Wang R., Moody R.P., Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ. Res. 2011;111:329–336. doi: 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 60.EPA . 1993. Carcinogenic Polycyclic Aromatic Hydrocarbons. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons. p. 28. [Google Scholar]
- 61.Berger J., Manz A. Cancer of the stomach and the colon-rectum among workers in a coke gas plant. Am. J. Ind. Med. 1992;22:825–834. doi: 10.1002/ajim.4700220605. [DOI] [PubMed] [Google Scholar]
- 62.Botteri E., Iodice S., Bagnardi V. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 63.Cross A.J., Ferrucci L.M., Risch A. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Z., Shrubsole M.J., Smalley W.E. Association of meat intake and meat-derived mutagen exposure with the risk of colorectal polyps by histologic type. Cancer Prev. Res. (Phila.) 2011;2011(4):1686–1697. doi: 10.1158/1940-6207.CAPR-11-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrucci L.M., Sinha R., Huang W.Y. Meat consumption and the risk of incident distal colon and rectal adenoma. Br. J. Cancer. 2012;106:608–616. doi: 10.1038/bjc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang B., Zhou L., Xue N., Li F., Li Y., Vogt R.D. Source apportionment of polycyclic aromatic hydrocarbons in soils of Huanghuai Plain, China: comparison of three receptor models. Sci. Total Environ. 2013;443:31–39. doi: 10.1016/j.scitotenv.2012.10.094. [DOI] [PubMed] [Google Scholar]
- 67.Pedersen K.B., Lejon T., Jensen P.E., Ottosen L.M. Chemometric analysis for pollution source assessment of harbour sediments in arctic locations. Water Air Soil Pollut. 2015;226(5):1–15. [Google Scholar]
- 68.Jiang J.J., Lee C.L., Fang M.D., Boyd K.G., Gibb S.W. Source apportionment and risk assessment of emerging contaminants: an approach of pharmaco-signature in water systems. PLoS One. 2015;10(4):e0122813. doi: 10.1371/journal.pone.0122813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morandi M.T., Daisey J.M., Lio P.J. Development of a modified factor analysis/multiple regression model to apportion suspended particulate matter in a complex urban airshed. Atmos. Environ. 1987;21(8):1821–1831. (1967) [Google Scholar]
- 70.Nasir M.F.M., Samsudin M.S., Mohamad I., Awaluddin M.R.A. River water quality modeling using combined principle component analysis (PCA) and multiple linear regressions (MLR): a case study at Klang River, Malaysia. World Appl. Sci. J. 2011;14:73–82. (Exploring Pathways to Sustainable Living in Malaysia: Solving the Current Environmental Issues) [Google Scholar]
- 71.IRIS . U.S. Environmental Protection Agency; Washington, DC: 1998. Integrated Risk Information System. [Google Scholar]