Abstract
Tuberculosis diagnosis and treatment currently revolves around clinical features and microbiology. The disease however adversely affects patients’ psychological, economic, and social well-being as well, and therefore our focus also additionally needs to shift towards quality of life (QOL). The disease influences all QOL domains and substantially adds to patient morbidity, and these complex and multidimensional interactions pose challenges in accurately quantifying impairment in QOL. For this review, PubMed database was queried using keywords like quality of life, health status and tuberculosis, and additional publications identified by a bibliographic review of shortlisted articles. Both generic and specific QOL scales show a wide variety of derangements in scores, and results vary across countries and patient groups. In particular, diminished capacity to work, social stigmatization, and psychological issues worsen QOL in patients with tuberculosis. Although QOL has been consistently shown to improve during standard anti-tubercular therapy, many patients continue to show residual impairment. It is also not clear if specific situations like presence of comorbid illnesses, drug resistance, or co-infection with human immunodeficiency virus additionally worsen QOL in these patients. There is a definite need to incorporate QOL assessment as adjunct outcome measures in tuberculosis control programs. Governments and program managers need to step up socio-cultural reforms and health education, and provide additional incentives to patients, to counter impairment in QOL.
Keywords: Quality of life, Questionnaire, Stigma, Tuberculosis
1. Background
Worldwide, tuberculosis (TB) continues to be an important public health issue, and a major cause of morbidity and mortality. Despite advances in diagnosis and therapy nearly ten million incident TB cases were reported, and an estimated 1.6 million deaths occurred due to TB, globally in 2017 [1]. Almost a quarter of the world's population is latently infected with TB, and therefore at risk of progressing to active disease sometime during their lifetime [1].
According to the World Health Organization, health is defined as a state of complete physical, mental, and social well-being and not a mere absence of disease or infirmity. The impact of any disease, especially a chronic illness like tuberculosis, on an individual patient is therefore often all-encompassing, affecting not only his physical health but also his psychological, economic, and social well-being.
At present, the TB control services are geared towards optimizing microbiological cure, and using this parameter as an indicator for successful treatment. Although this is extremely important from a public health perspective, such an approach does not adequately address the physical, mental and social suffering of patients due to TB [2]. Patients suffer not only because of the symptoms of the disease, but also because of the resultant general deterioration in their quality of life (QOL). Despite this, patient perceptions about disease and their health have remained largely unknown.
QOL is a broad and complex multidimensional concept that incorporates physical, social, psychological, economic, spiritual and other domains. It is therefore difficult to define and measure, but may be broadly described as individuals’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns [3]. QOL therefore is an expression of patient preferences and values rather than clinician's assessment. For the latter, one simply needs to ask the patient “How high is your fever?”, while for the former, patient response to the question “How much are you bothered by your fever?” or “To what extent do you feel that fever prevents you from doing what you need to do?” can be recorded. Self-reported health-related QOL is therefore an important adjunct measure in understanding and quantifying the actual impact of TB on patients.
This review was conducted to summarize the various issues related to QOL among patients with all forms of TB. A broad search was conducted through the PubMed platform using keywords like quality of life, health status and tuberculosis. Relevant publications for detailed evaluation were identified through an abstract review of the search results. Additional key references were identified from bibliography of shortlisted publications during their full-text review. Data from large and well-conducted studies was preferentially used to summarize and tabulate important findings.
2. Instruments for describing and quantifying QOL in TB
An objective assessment of patient's QOL attempts to quantify the functional effects of an illness and its consequent therapy on a patient, as perceived by that patient. A wide variety of questionnaires and scales have been employed to evaluate self-rated QOL in patients with active TB [4], [5], [6], [7]. Some of these evaluate QOL holistically, whereas others focus on specific domains like physical health or psychological morbidity.
The simplest approach to QOL assessment is using only one summary item as a global descriptor of QOL. This can take the form of a single question, a visual analogue scale (VAS), or a standard gamble approach [8], [9], [10], [11], [12], [13]. However, this is likely to miss important information on several important facets of QOL that may be important to TB patients. More and more investigators therefore rely on standardized multidimensional scales to obtain a more comprehensive picture of the relevant facets and domains. Several of these instruments are generic, which means that they can be used across a wide spectrum of disorders (and even among healthy individuals). A commonly used scale in TB research is Short Form 36 (SF-36) [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. This gives scaled scores across eight domains – Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health, and two summary scores – Physical Component Score and Mental Component Score. The EQ-5D, developed by the European QOL Group, is another commonly used instrument [10,[30], [31], [32], [33], [34]]. It has two components – health state description and evaluation. In the description part, health status is measured through five dimensions (5D) – mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. VAS is used to assess overall health status in the evaluation part. The abbreviated World Health Organization Quality of Life scale (WHOQOL-Bref) is another popular generic instrument [35], [36], [37], [38], [39], [40], [41], [42]. This 26-item instrument evaluates QOL across four domains – Physical, Psychological, Social relationships, and Environment. Some other generic scales used include Medical Outcome Survey (MOS) [9] Social Functioning 12 (SF-12) [32,43], variants of the WHOQOL scale family [19,44,45], and other uncommon or in-house instruments [46], [47], [48].
Although generic measures permit comparisons across interventions and diagnostic categories, they fail to adequately capture facets particularly important to a particular disease. More specific instruments may prove better in this regard, and these can be either system-specific or disease-specific. Since lungs are the predominant organ involved in TB, it is intuitive that respiratory-system specific questionnaires may be appropriate in pulmonary TB. The St. George's Respiratory questionnaire (SGRQ) has been used in some studies [32,49,50]. It has 76 items, whose responses can be aggregated into an overall score and three domain scores for Symptoms, Activity and Impact. Another approach is to use disease specific instruments. Unfortunately, TB-specific QOL instruments have not been widely used. Dhingra and Rajpal proposed a disease-specific QOL instrument (DR-12) from data on TB patients treated under programmatic conditions in India [51]. This scale has 12 items over two domains – Symptoms, and Sociopsychological/exercise adaptation. However, scale development was not scientifically rigorous, and phrasing of items suggests it to be more of a health status rather than QOL instrument. It has been sparsely used [52,53]. Another disease specific instrument FACIT-TB (Functional Assessment of Chronic Illness Therapy – Tuberculosis) has been developed, and psychometrically validated in Arabic, for quantifying QOL in TB patients in Iraq [54,55]. This questionnaire, which is a part of the FACIT measurement system, consists of 45 items across five domains – Physical well-being, Social and economic well-being, Emotional well-being/Stigma of having TB, Functional well-being, and Spiritual well-being. More recently, the generic module of the Chinese Quality of Life Instruments for Chronic Diseases (QLICD) has been modified by addition of a pulmonary TB scale. The resultant QLICD-PT instrument has three domains (with 28 items) for general QOL and one pulmonary TB specific domain (with 12 items) [56]. The scale has been shown to have acceptable degree of validity, reliability and responsiveness. To the best of our knowledge, both these new QOL instruments have not been used by other independent researchers.
In addition, more specific instruments have been used to explore individual QOL domains in TB. This is most evident in evaluation of psychological morbidity, where several tools such as General Health Questionnaire 12 (GHQ12) [57], Patient Health Questionnaire (PHQ-9) [12,[58], [59], [60]], Centre for Epidemiologic Studies Depression Scale (CES-D) [31], State-trait Anxiety Short-Form (STAI-6) [31], Kessler-10 item scale (K-10) [45,61], Hospital Anxiety and Depression Scale (HADS) [25,32], and others have been used.
3. QOL evaluation in TB patients
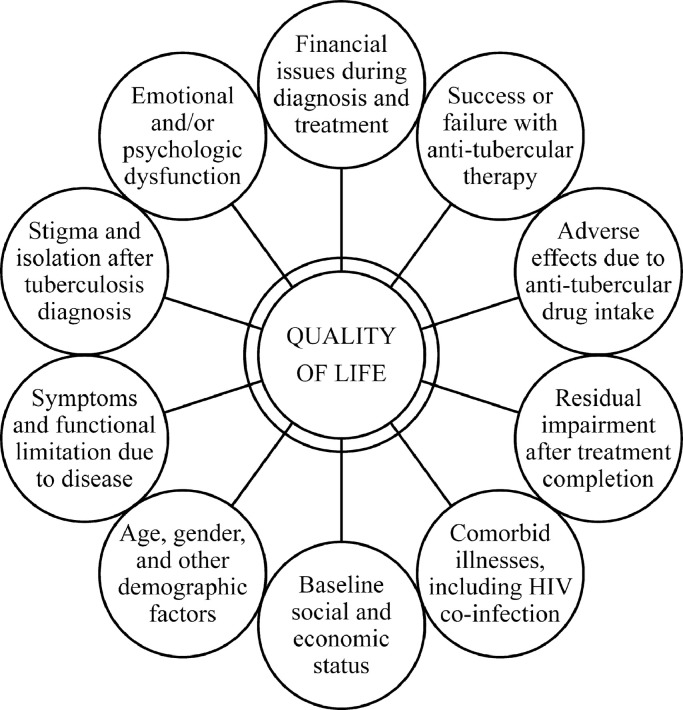
QOL can be influenced by several patient, disease, and treatment-related factors in TB patients (Fig. 1). Few investigators have evaluated QOL in adult patients through cross-sectional studies (Table 1). In general, QOL of TB patients is poorer as compared to healthy individuals across most domains, with physical functioning domain affected more severely than others [5,14,19,25,31,[35], [36], [37], [38],62]. Patients with active TB generally also perceive their health status to be worse as compared to people with latent TB or previously cured TB [5,8,13,16,17,21]. However, the relative contribution of TB towards impairment of QOL can sometimes be problematic as several patients have other comorbid illnesses or socio-economic problems [63]. Overall, QOL seems largely independent of age and gender [48,64]. However, some studies report advancing age to be negatively correlated with QOL [10,13,38]. Others report worse QOL among women [17,36,38]. One Indian study has shown higher QOL scores for physical and psychological domains among women, probably suggestive of better coping strength [36]. Also, lower education level and poor socio-economic status may be associated with greater impairment of QOL [17,48]. Patients with relapse or retreatment tend to show the greatest impairment in QOL [36].
Fig. 1.
Important determinants of quality of life with tuberculosis.
Table 1.
Characteristics of selected recent studies in tuberculosis patients reporting cross-sectional data from multi-dimensional quality of life scales.
| Investigator | Study setting | Study subjects | HIV+ | Comorbid disorders | Non-TB comparator groups | QOL measures | Timing of QOL assessment |
|---|---|---|---|---|---|---|---|
| Dion et al. [30] | Public hospital in Canada | 17 culture confirmed pulmonary TB patients | None | Excluded | 25 latent TB, 8 previously treated TB | SF-36, EQ-5D, VAS, Standard gamble | Before or during treatment |
| Duyan et al. [48] | Hospital in Turkey | 120 inpatients with pulmonary TB | None | Excluded | None | In-house instrument | At least one month after hospitalization |
| Dhuria et al. [35] | DOT centres in India | 90 patients with pulmonary TB (20% retreatment cases) | NR | Excluded | 90 age and gender matched healthy persons | WHOQOL-Bref | Within 3 days of initiating TB treatment |
| Guo et al. [13] | TB clinic in Canada | 84 patients on TB treatment | NR | 45% | 78 persons on treatment for latent TB | SF-36, VAS, Health Utilities Index | Within 2 months of diagnosis of active/latent TB |
| Unalan et al. [17] | TB dispensary in Turkey | 196 patients with TB | NR | 26.5% | 196 healthy persons, and 108 with latent/healed TB | SF-36 | During treatment |
| Deribew et al. [44] | Hospitals in Ethiopia | 124TB/HIV co-infected patients | 100% | Excluded | 467 HIV+ patients | WHOQOL-HIV | During intensive phase of TB treatment |
| Babikako et al. [9] | TB clinics in Uganda | 133TB patients | 50% | NR | None | Medical Outcome Survey, VAS | Variable – before, during, or end of treatment |
| Chung et al. [37] | Hospitals in Taiwan | 140 patients with pulmonary TB | NR | NR | 130 age and gender matched healthy persons | WHOQOL-Bref | Within 2 weeks of initiating treatment |
| Kittikraisak et al. [10] | Hospitals in Thailand | 92TB patients | 53.3% | NR | 49 HIV+ patients, 81 patients with treated TB | EQ-5D | Variable |
| Louw et al. [46] | Primary care clinics in South Africa | 4900TB patients | 59.9% | NR | None | Social functioning 12 | Within one month of treatment initiation |
| Unalan et al. [19] | TB sanitorium in Turkey | 92TB patients | NR | NR | None | SF-36, WHOQOL-100 | Early during treatment |
| Sharma et al. [39] | TB clinic in India | 60 MDR TB and 60 retreatment TB patients | None | Excluded | 60 persons (details NR) | WHOQOL-Bref | Variable during treatment |
| Kisaka et al. [24] | Hospital in Uganda | 210 smear positive pulmonary TB patients (one third each at diagnosis, end of intensive phase, and treatment completion) | 59% | NR | None | SF-36 | Variable during treatment |
| Dos Santos et al. [25] | Hospital in Brazil | 86 inpatients with pulmonary TB | 37.2% | 3.5% | None | SF-36 | NR |
| Roba et al. [27] | Hospitals, health centres in Ethiopia | 300TB, and 100 MDR TB, patients | 13.8% | NR | None | SF-36 | At least one month after treatment initiation |
| Shahdadi et al. [28] | Diabetes clinic in Iran | 62 diabetic patients with pulmonary TB | NR | 100% | None | SF-36 | NR |
| Laxmeshwar et al. [42] | TB clinics in India | 95 MDR TB patients | 4.2% | NR | None | WHOQOL-Bref | Variable during treatment |
| Sineke et al. [29] | TB treatment site in South Africa | 149 patients with drug-resistant TB | 77.9% | 4.4% | None | SF-36 | NR |
DOT Directly observed treatment, EQ European Quality of Life, HIV Human immunodeficiency virus, MDR Multi-drug resistant, NR Not reported, QOL Quality of life, SF-36 Short Form 36, TB Tuberculosis, VAS Visual analogue scale, WHOQOL World Health Organization Quality of Life.
4. Physical functioning and role limitation
Physical functioning is a reflection of an individual patient's capacity to carry out basic day-to-day activities, and role functioning encompasses a person's ability to function in designated roles at work, society, and home. The physical effects of TB are highly variable, and depend on patient's premorbid health status, severity of symptoms, and duration of illness. Debilitating somatic symptoms are often the hallmark of active TB, and patients are often specially concerned about generalized weakness and weight loss [63]. Poor performance status has been shown to be a strong predictor of mortality in Japanese patients with active pulmonary TB [65]. The diagnosis of tuberculosis in the family increases the workload on the family primary caregivers (wives and mothers), and diminishes the caregiver's ability to generate income and care for the remainder of the family [66].
5. Social functioning
One of the most important facets affecting QOL is the stigma associated with TB, both at the family and the community level [63,67]. In a study from urban Zambia, 82% TB patients reported stigma [68]. In another study from southern India, 51.2% TB patients felt stigmatized, and stigma was greater among sputum smear positive patients [69]. In a study using an improvised scale to quantify stigma, mean TB-related stigma score in Chinese patients was 9.33 (maximum scale score of 27) [70].
TB is most commonly stigmatized due to the perceived risk of transmission from patients to other susceptible community members [71]. In other instances, the reasons could relate to the association of TB with HIV infection or low socio-economic status, and traditional myths about TB [68]. Patients often report issues such as loss of friends, lack of respect among colleagues, and social isolation at workplace [72,73]. The stigma associated with disease may be greater among women and inability to get married, and divorce, have both been commonly reported in developing countries [74], [75], [76]. Contrary to popular belief, stigmatization of TB patients is not just confined to developing countries, but may be also be widely prevalent in low-TB burden countries as well [77].
6. Emotional and psychological health
A wide range of psychological reactions are observed once TB is diagnosed. Worry is a common feeling after disclosure of diagnosis [15,78]. The diagnosis may come as a shock to the patient, and there are instances of denial of diagnosis [63,78,79]. Another common feeling at diagnosis is fear of seclusion and social boycott, and sometimes even death [63,75]. In particular, hospitalization and isolation of patients (a common practice in several low-burden countries) can have important emotional and psychological ramifications [63,80].
Depressive symptoms such as low mood, tiredness, reduced sexual desire, sleep disturbances, anorexia, loss of weight, etc. are commonly seen [57]. Cross-sectional, community-based data from the World Health Survey on nearly 250,000 adults from low- and middle-income countries has shown a much higher prevalence of depressive episodes in patients with TB (23.7% vs. 6.8% among those without TB) [81]. The odds for subsyndromal depression and brief depressive episodes were also higher among TB patients. Interaction analysis showed that depression amplified difficulties in self-care in TB patients but did not affect other health status domains. Using PHQ as a screening tool, a Nigerian study identified 27.7% patients with depression [58]. In another study on patients attending public primary care clinics in South Africa, 32.9% showed psychological distress and 8.3% were receiving anti-depressant therapy [61]. On multivariable analysis older age, lower formal education, and poverty were independently associated with psychological distress. A cross-sectional study in Brazil on hospitalized TB patients found that 31.4% had depression, 38.4% had anxiety, and 23.3% suffered from low self-esteem [25]. Patients with depression or anxiety also had lower overall QOL scores as compared to patients without. In a study from Ethiopia, 53.9% patients were categorized as having probable depression at start of treatment, and QOL impairment, loss to follow-up, and mortality were significantly higher among this subset [12]. A study from southern India reported depression in 40.8% TB patients receiving anti-tubercular therapy (ATT) [60]. Most patients had mild or moderate depression, with a higher prevalence in pulmonary as compared to extrapulmonary disease (80.4% vs. 19.6%).
Adequate treatment can ameliorate some of these psychological issues. A South African study using HADS showed that both anxiety and depression domains changed by +95% from a state of ‘moderate problems’ to a state of reporting ‘no problems’ [32].
7. Economic well-being
Patients of TB are most commonly in the economically productive age group, and hence the resultant economic cost is rather substantial. Several patients and families feel the financial burden of disease, resulting both from cost of treatment as well as indirectly from loss of wages [79,82]. A study in Thailand noted that adult TB patients spent more than 15% of their income on out-of-pocket expenses for diagnosis and therapy of TB, and often needed to take loans or sell property [66]. Another study on southern India reported expenditure up to 40% of patients’ income, with non-medical expenses (such as travel costs), and diagnosis/treatment in the private sector, also imposing a disproportionate burden on poor households [82].
8. Effect of treatment
Few investigators have longitudinally evaluated QOL in cohorts of adult patients on ATT, mostly from endemic or high-burden countries (Table 2). The greatest improvement in QOL seems to occur within the initial 2–3 months of therapy [5]. A study from South India reported improvement in patient perceptions about physical and mental well-being after treatment [15]. In a study from northern India, QOL improved significantly at end of intensive phase, and further at end of treatment [41]. Similar results were reported from another north Indian study, where overall QOL, and all domains except social, improved after treatment for three months, and all domains improved further at treatment completion at six months [36]. Another study from north India showed that QOL improved across all domains among patients showing microbiological conversion on sputum examination, but not among those with persistent sputum positivity at end of intensive phase of treatment [38]. In a study from Pakistan, mean QOL scores more than doubled in TB patients after completing ATT [34]. A study from China reported gradual improvement in QOL with TB treatment, with physical function, role-motional, bodily pain, and general health domain scores comparable to healthy individuals after treatment [14]. In one study from Iraq that longitudinally used a TB-specific QOL questionnaire, physical well-being, functional well-being, and the total QOL scores were significantly increased after two months of ATT [55]. All QOL subscales, except social and economic well-being and spiritual well-being, improved at end of treatment, and the total QOL score had a statistically significant contribution towards predicting likelihood of favourable response to ATT. In a study from Yemen, both physical and mental summary scores improved at end of intensive phase of treatment [23]. While the former improved further at treatment completion, the latter remained largely static, with mean scores still below population norms. In a study from Indonesia, 94% patients showed a clinically significant improvement in SGRQ scores after two months of treatment, and 80% achieved additional significant improvement by end of treatment at six months [50]. Progressive improvement across all QOL domains was also reported among Malaysian patients receiving ATT [20]. This suggests that QOL correlates with other objectives measures of response to therapy. In a study from Uganda, QOL progressively improved as the patients’ duration of TB treatment increased [9]. In a study from Uganda, both physical and mental component summary scores significantly improved at end of intensive phase, and further by end of treatment completion [24]. In two studies from South Africa, QOL improved significantly during treatment and at treatment completion, with biggest gains in the physical health scores [32,43]. No socio-demographic traits were significantly associated with this improvement, suggesting that TB treatment was the principal determinant of change in QOL. Maximum improvements were seen in physical, followed by psychological domain.
Table 2.
Characteristics of selected recent longitudinal studies reporting data from multi-dimensional quality of life scales among patients receiving treatment for tuberculosis.
| Investigator | Study setting | Study subjects | HIV+ | Comorbid disorders | Non-TB comparator groups | QOL measures | Timing of serial QOL assessment |
|---|---|---|---|---|---|---|---|
| Chamla, 2004 [14] | TB center in China | 102 patients with pulmonary TB | NR | NR | 103 age and gender matched healthy persons | SF-36 | ST, EIP, ET |
| Rajeswari et al. [15] | TB units in India | 610 patients with TB | NR | NR | None | SF-36 | ST, EIP, ET |
| Marra et al. [16] | TB clinic in Canada | 7.7% | 10.6% | 102 persons with latent TB | SF-36 | Baseline, 3 months, 6 months | |
| Dhuria et al. [36] | DOT centres in India | 90 patients with pulmonary TB | NR | Excluded | 90 persons (details NR) | WHOQOL-Bref | Baseline, 3 months, 6 months |
| Maguire et al. [50] | TB clinic in Indonesia | 115 patients with pulmonary TB | 4.5% | NR | None | SGRQ | Baseline, 2 months, 6 months |
| Guo et al. [18] | TB control clinics in Canada | 89 patients with TB | NR | 46% | None | SF-36 | Baseline, 3 months, 6 months |
| Kruijshaar et al. [31] | Clinics in UK | 61 patients with TB (20 had extrapulmonary disease) | NR | NR | None | SF-36, EQ-5D | Baseline, 2 months |
| Aggarwal et al. [38] | DOT centres in India | 1034 patients with pulmonary TB | NR | NR | None | WHOQOL-Bref | ST, EIP, ET |
| Deribew et al. [45] | Hospitals in Ethiopia | 124TB/HIV coinfected patients | 100% | Excluded | 465 HIV+ patients | WHOQOL HIV-Bref | During intensive phase, 6 months later |
| Atif et al. [20] | Chest clinic in Malaysia | 216 patients with pulmonary TB | None | Excluded | None | SF-36 | ST, EIP, ET |
| Bauer et al. [21] | Hospitals in Canada | 48 patients with pulmonary TB (8 had extrapulmonary disease) | NR | Excluded | 105 persons with latent TB, 110 healthy persons | SF-36 | 1, 2, 4, 6, 9 and 12 months |
| Dujaili et al. [55] | Specialist Respiratory Centre in Iraq | 305 patients with pulmonary TB | None | Excluded | None | FACIT-TB | ST, EIP, ET |
| Ahmad et al. [22] | Hospital in Pakistan | 81 patients with MDR TB | NR | 12.3% | None | SF-36 | Baseline, 12 months, ET (>20 months) |
| Jaber et al. [23] | TB centres in Yemen | 243 patients with TB | NR | 16.5% | None | SF-36 | ST, EIP, ET |
| Louw et al. [43] | Primary care clinics in South Africa | 1196 patients with TB | NR | 36.8% | None | SF-12 | Baseline, 6 months |
| Mthiyane et al. [47] | Hospitals in South Africa | 62TB/HIV coinfected patients | 100% | Excluded | 20 HIV+ patients | FAHI | Baseline, 3 months, 6 months, 12 months |
| Kastien-Hilka et al. [32] | Primary care clinics in South Africa | 131 patients with pulmonary TB | None | 20.6% | None | SF-12, EQ-5D, SGRQ | ST, 4, 8, 16 weeks, ET |
| Ramkumar et al. [26] | DOT centres in India | 92 patients with TB | NR | NR | 83 age and gender matched healthy persons | SF-36 | ST, 3 months, ET |
| Siddiqui et al. [53] | DOT centres in India | 316 patients with TB (50 had diabetes) | NR | 15.8% | None | DR..−12 | ST, EIP, ET |
| Singh et al. [40] | Hospital in India | 50 patients with pulmonary TB | NR | NR | 50 age and gender matched healthy persons | WHOQOL-Bref | Baseline, 2 months, 6 months |
| Jorstad et al. [33] | Hospital in Tanzania | 69 patients with extrapulmonary TB | 23.2% | NR | 63 patients without TB | EQ-5D | ST, 2–3 months, ET |
| Saleem et al. [34] | TB clinic in Pakistan | 226 patients with pulmonary TB | NR | Excluded | None | EQ-5D | ST, EIP, ET |
| Dar et al. [41] | Hospital in India | 198 patients with pulmonary TB | NR | NR | None | WHOQOL-Bref | ST, EIP |
| Jaber and Ibrahim [89] | TB centres in Yemen | 80 patients with MDR TB | NR | 28.8% | None | SF-36 | Baseline, ET, 12 months after ET |
DOT Directly observed treatment, DR−12 Dhingra and Rajpal scale, EIP End of intensive phase, EQ European Quality of Life, ET End of treatment, FACIT Functional Assessment of Chronic Illness Therapy, FAHI Functional Assessment of HIV Infection, HIV Human immunodeficiency virus, MDR Multi-drug resistant, NR Not reported, QOL Quality of life, SF-12 Social Functioning 12, SF-36 Short Form 36, SGRQ St George's Respiratory Questionnaire, St Start of treatment, TB Tuberculosis, WHOQOL World Health Organization Quality of Life.
Relatively little information is available from low TB burden countries. In a study from Canada, QOL was better in most domains after TB treatment, with most significant improvements observed in vitality, physical functioning, role physical, social functioning, and role emotional domains [16]. In contrast, another study on Canadian patients showed that while mental component summary scores improved throughout treatment, the physical component summary score improved only slightly during the 2–4 month period and then slightly declined again [21].
On the other hand, adverse effects from ATT may sometimes paradoxically worsen QOL. For instance, gastrointestinal disturbances, visual impairment or peripheral neuropathy may hamper physical functioning [63]. A Canadian study reported that major, but not minor, adverse drug reactions were associated with significant reductions in a few mental and physical subscales of SF-36 [18]. This study also showed that patients with low pre-treatment QOL scores were more likely to experience adverse drug reactions. A study from UK suggested that while the psychological burden from depression improved with treatment, that with anxiety did not [31].
Although most patients report normal or near-normal QOL after successful TB treatment, a small proportion can still show residual impairment of QOL [15,16,20,23,31,38,49,83]. In particular, a recent systematic review suggests that psychological well-being and social functioning continue to remained impaired even after successful microbiological cure with treatment [62]. In addition to persistent physical changes, patients also report continued emotional distress or impaired mental health even after completion of ATT [20,84].
The long term impact of successful TB treatment on QOL is not clear. Few studies show that the overall QOL in patients previously treated 1–2 years back was largely similar to that in the general population [21,30,83,85]. Other investigators report substantial impairment in QOL, even several years after completing treatment, although it was still better when compared to other chronic respiratory disorders [86]. Apart from the global assessment, individual QOL facets may be important for patients. For instance, overcoming stigma and resuming normal social life (including joining work, resuming interactions with friends and colleagues, etc.) may be difficult for some patients. Others may have significant organ damage (such as extensive lung fibrosis or destruction) that can result in persistent symptoms and inability to resume normal daily activities. Other events (such as loss of job or divorce) due to TB diagnosis may also have long-lasting social, psychological and financial implications.
9. Extrapulmonary disease
There is only sparse data on how extrapulmonary TB (EPTB) influences QOL. A study from China reported similar QOL scores between pulmonary and extrapulmonary TB, though site distribution or numbers for the latter were not provided [14]. In general, QOL is likely to be related to the anatomic location of disease, and some forms are more likely to be associated with substantial morbidity and long-term disability. Therefore, the impact of skeletal tuberculosis or tuberculous meningitis is likely to be much different in comparison to tuberculous lymphadenitis or pleural tuberculosis. A study from UK found that patients with lymph node disease appeared to report better QOL than patients with pulmonary TB at time of diagnosis [31]. Another prospective study in Zanzibar followed up patients with presumptive EPTB, and reduction in working capacity was reported in a lower proportion of patients with lymphadenitis as compared to other patients [33]. These patients had better self-rated QOL at baseline as compared to EPTB at other sites. Overall, QOL improved in all patients with adequate treatment, but residual impairment was not reported for any site.
10. Drug resistance
In general, patients with multi-drug resistant (MDR) TB have endured disease and treatment in the past as well, and hence face additional difficulties related to family life, social stigmatization, and financial hardships. Treatment for MDR TB is also longer, more complex, associated with frequent adverse effects, and associated with suboptimal outcomes. It is therefore not hard to imagine that QOL among patients with MDR TB is likely to be much more impaired [87,88]. A study from north India showed that patients with MDR TB had worse QOL as compared to drug-susceptible patients receiving retreatment with ATT [39]. In contrast, an Ethiopian study found that QOL was similarly reduced among MDR and gender-matched drug-susceptible patients with TB [27]. However, MDR patients reported worse general health scores and extensive stigmatization. In a retrospective study on 61 HIV/MDR-TB patients in India, 16% had depression at baseline, and all except one improved with ATT and psychological support [59]. In a follow-up study of MDR TB patients programmatically managed in Pakistan, QOL was severely impaired across all domains before starting treatment [22]. At one year of treatment, there was minimal and clinically insignificant improvement in QOL scores. At completion of treatment, there was significant improvement in QOL domain scores and summary component measures, but the scores still remained below standard population norms, suggesting significant residual impairment of QOL. A study from western India found that psychological and physical health domains were the most affected among patients receiving treatment for MDR TB, and that loss of work adversely affected the social relationships and environmental domains [42]. However, QOL in this study was not as low as reported in some other studies, and was not influenced by drug-resistance pattern. Qualitatively, pill burden significantly affected QOL. A study from Yemen showed clinically important improvement in QOL scores at end of treatment for MDR TB, but there was no further improvement over next one year. Duration of illness before diagnosis of MDR TB was an important predictor of improvement in both physical and mental domain scores [89].
A cross-sectional study in Namibia attempted to correlate adverse drug events with QOL around the time of completion of MDR TB treatment [90]. QOL ratings were moderately low in these patients and were not correlated with adverse reactions (which were most commonly mild). In another study from South Africa, patients on drug-resistant TB treatment who reported an adverse event had poorer QOL (principally mental health and well-being) as compared to patients who did not, especially those on intensive phase treatment for six months or less [29]. However, in both studies, most adverse events had already occurred much before quantification of QOL, while some were persistent for variable length of time.
A recent systematic review and meta-analysis obtained a summary prevalence of 25% for depression across 15 studies, and 24% for anxiety across three studies [87].
11. TB and human immunodeficiency virus (HIV) co-infection
Nearly 9% of TB patients are co-infected with HIV, and TB/HIV co-infection seems to be driving the resurgence of TB in the developed world [1]. In a study from Ethiopia, TB/HIV co-infected patients were documented to have poorer QOL across all domains when compared to HIV seronegative TB patients, even after adjusting for potential confounders like age, gender, occupation, social support, WHO staging, and CD4 lymphocyte count [44]. Similarly, another study from Ethiopia showed that QOL in TB/HIV co-infected patients was more impaired as compared to that in HIV seropositive patients without TB, and treatment led to greater improvement in QOL in the former group [45]. Similar observations were also reported from a cross-sectional study from India [91]. In contrast, a study from Brazil found that QOL was similarly impaired among patients receiving treatment for HIV infection, active TB, and TB/HIV con-infection, with the maximal decrease being observed in the physical domain in the last group [11]. Substantial impairment of physical and mental health was documented in a study on HIV-infected TB patients treated in Thailand [92]. Physical symptoms were largely relieved with treatment, but mental health remained unchanged or worsened in nearly two-third patients. In contrast, a study from South Africa reported greater impairment in physical functioning, but better mental health, among TB/HIV co-infected patients [46]. Another study from South Africa showed overall improvement in QOL with therapy in TB/HIV coinfected patients [47]. This improvement appeared similar among those receiving, and not receiving concomitant anti-retroviral therapy. However, patients with CD4 counts below 200/µL had a poorer QOL, both pre-treatment and during and after completion of treatment.
12. Impact of other comorbidities
Several TB patients have other concurrent comorbid illnesses that can themselves influence QOL. In particular, diabetes is a common association [1]. It is possible that QOL in such patients may be worse. However, most QOL studies conducted in specific disease states tend to either exclude patients with comorbid illnesses that can confound quantification of QOL, or ignore the associated clinical conditions while describing QOL (Tables 1 and 2). Hence data in this area is rather sparse. In one study from northern India, TB patients having diabetes shower poorer QOL at start of treatment, as compared to patients without diabetes [53]. In another study on diabetic patients in Iran, a significant inverse association was noted between QOL and hemoglobin A1c levels, suggesting that poor glycemic control may worsen QOL in TB patients [28].
13. Quality of TB care
As per the International Standards of Tuberculosis Care, a patient-centered approach to therapy needs to be developed for all patients in order to promote adherence, improve QOL, and relieve suffering [93]. Unfortunately, quality of TB care is still far from optimal, especially in high burden countries [94]. There are still considerable delays in TB diagnosis and, and several patients are lost even before treatment can be initiated; this contributes to prolonged patient suffering [95,96]. Several patients find the current mechanisms of directly observed ATT to be inflexible and intrusive, and prefer the less effective unsupervised treatment [97]. The quality of drugs supplied through TB programs, and their guaranteed availability, are also important issues. Most TB programs still do not address the non-medical aspects of tuberculosis with enough seriousness. All these factors directly influence QOL in TB patients. These issues may adversely affect speed of recovery as well as treatment outcomes, and thereby indirectly contribute to impairment of QOL.
14. What can be done to improve QOL in TB patients
From a programmatic perspective, one must deviate from the traditional indicators of disease severity and treatment response to capture the overall health status, with a greater emphasis on patient's, rather than clinician's, perspective of disease [2]. TB control programs need to look beyond clinical and microbiological aspects, and try and include socio-cultural and psychological dimensions that impact the disease and its treatment as part of evaluation and monitoring tools. QOL and related measures can therefore be used more frequently as an adjunct to routine disease outcome indices, and perhaps included into forthcoming guidelines. This can assist health care providers to target specific mental and physical health components that are adversely affected by the disease or treatment [98]. For this, there is also a need for developing psychometrically robust and ethnically appropriate TB-specific QOL measures in different countries. We were unable to locate any quality data that described QOL in children with TB, and this is one area where information needs to be generated. Given that the interaction between healthcare providers and patients, as well as the services rendered by the clinical team, can heavily impact a patient's QOL, there is urgent need improve the overall quality of TB care [99]. TB control programs should also try and implement patient friendly regimens that reduce pill burden, and keep hospitalization and isolation at a minimum. Metrics such as quality-adjusted life years (QALYs) can be used for economic assessment of interventions, and choosing those that provide the greatest health effects [100].
The other major target should be to promote awareness and try and bring about relevant social reforms. There is a need to understand the roots of misconceptions about TB and to address the lack of knowledge about disease. Good communication, especially at time of diagnosis and initiating treatment, is necessary, and psychological counseling should be an integral part of TB management [101]. TB patients receiving adequate social support from family, friends and community are likely to have better QOL [102]. Hence, there may be a case of implementing wellness interventions at family and/or community level to improve QOL. There is also a potential role for targeted, culturally relevant psychosocial support interventions for persons treated for TB disease, especially during the early months of treatment that integrate patients back into their communities as quickly as possible. At a higher level, policymakers should promote social protection and livelihood-strengthening interventions, such as poverty alleviation, food security, cash transfers, etc. [103]. Another major set of interventions are required to reduce TB stigma. Education and support programs aimed at healthcare providers, TB patients, and at-risk community members may prove useful [71]. Other important measures to combat stigmatization include advocacy, communication, social mobilization, and personal empowerment of marginalized groups and women who disproportionally bear the burden of TB stigma. TB clubs or social networks could be created for patients to improve patient interaction with other stakeholders [104].
Funding
None.
Declaration of Competing Interest
None.
References
- 1.World Health Organization . World Health Organization; Geneva: 2018. Global tuberculosis report 2018. [Google Scholar]
- 2.Aggarwal A.N. Health-related quality of life: a neglected aspect of pulmonary tuberculosis. Lung India. 2010;27(1):1–3. doi: 10.4103/0970-2113.59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The world health organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41(10):1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 4.Guo N., Marra F., Marra C.A. Measuring health-related quality of life in tuberculosis: a systematic review. Health Qual Life Outcomes. 2009;7:14. doi: 10.1186/1477-7525-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer M., Leavens A., Schwartzman K. A systematic review and meta-analysis of the impact of tuberculosis on health-related quality of life. Qual Life Res. 2013;22(8):2213–2235. doi: 10.1007/s11136-012-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J., Capocci S., Smith C., Morris S., Abubakar I., Lipman M. Health status and quality of life in tuberculosis. Int J Infect Dis. 2015;32:68–75. doi: 10.1016/j.ijid.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 7.Khan S., Tangiisuran B., Imtiaz A., Zainal H. Health status and quality of life in tuberculosis: systematic review of study design, instruments, measuring properties and outcomes. Health Sci J. 2017;11(1):1–10. [Google Scholar]
- 8.Dion M.J., Tousignant P., Bourbeau J., Menzies D., Schwartzman K. Measurement of health preferences among patients with tuberculous infection and disease. Med Decis Making. 2002;22(5 Suppl):S102–S114. doi: 10.1177/027298902237706. [DOI] [PubMed] [Google Scholar]
- 9.Babikako H.M., Neuhauser D., Katamba A., Mupere E. Feasibility, reliability and validity of health-related quality of life questionnaire among adult pulmonary tuberculosis patients in urban Uganda: cross-sectional study. Health Qual Life Outcomes. 2010;8:93. doi: 10.1186/1477-7525-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kittikraisak W., Kingkaew P., Teerawattananon Y., Yothasamut J., Natesuwan S., Manosuthi W. Health related quality of life among patients with tuberculosis and HIV in Thailand. PLoS One. 2012;7(1):e29775. doi: 10.1371/journal.pone.0029775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowdy D.W., Israel G., Vellozo V., Saraceni V., Cohn S., Cavalcante S. Quality of life among people treated for tuberculosis and human immunodeficiency virus in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2013;17(3):345–347. doi: 10.5588/ijtld.12.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambaw F., Mayston R., Hanlon C., Medhin G., Alem A. Untreated depression and tuberculosis treatment outcomes, quality of life and disability, Ethiopia. Bull World Health Organ. 2018;96(4):243–255. doi: 10.2471/BLT.17.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo N., Marra C.A., Marra F., Moadebi S., Elwood R.K., Fitzgerald J.M. Health state utilities in latent and active tuberculosis. Value Health. 2008;11(7):1154–1161. doi: 10.1111/j.1524-4733.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 14.Chamla D. The assessment of patients' health-related quality of life during tuberculosis treatment in Wuhan, China. Int J Tuberc Lung Dis. 2004;8(9):1100–1106. [PubMed] [Google Scholar]
- 15.Rajeswari R., Muniyandi M., Balasubramanian R., Narayanan P.R. Perceptions of tuberculosis patients about their physical, mental and social well-being: a field report from south India. Soc Sci Med. 2005;60(8):1845–1853. doi: 10.1016/j.socscimed.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Marra C.A., Marra F., Colley L., Moadebi S., Elwood R.K., Fitzgerald J.M. Health-related quality of life trajectories among adults with tuberculosis: differences between latent and active infection. Chest. 2008;133(2):396–403. doi: 10.1378/chest.07-1494. [DOI] [PubMed] [Google Scholar]
- 17.Unalan D., Soyuer F., Ceyhan O., Basturk M., Ozturk A. Is the quality of life different in patients with active and inactive tuberculosis? Indian J Tuberc. 2008;55(3):127–137. [PubMed] [Google Scholar]
- 18.Guo N., Marra F., Fitzgerald J.M., Elwood R.K., Marra C.A. Impact of adverse drug reaction and predictivity of quality of life status in tuberculosis. Eur Respir J. 2010;36(1):206–208. doi: 10.1183/09031936.00159409. [DOI] [PubMed] [Google Scholar]
- 19.Unalan D., Soyuer F., Ozturk A. Comparison of SF-36 and WHOQOL-100 life quality scales in early period tuberculosis subjects. J Pak Med Assoc. 2012;62(11):1161–1167. [PubMed] [Google Scholar]
- 20.Atif M., Sulaiman S.A., Shafie A.A., Asif M., Sarfraz M.K., Low H.C. Impact of tuberculosis treatment on health-related quality of life of pulmonary tuberculosis patients: a follow-up study. Health Qual Life Outcomes. 2014;12:19. doi: 10.1186/1477-7525-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer M., Ahmed S., Benedetti A., Greenaway C., Lalli M., Leavens A. Health-related quality of life and tuberculosis: a longitudinal cohort study. Health Qual Life Outcomes. 2015;13:65. doi: 10.1186/s12955-015-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad N., Javaid A., Syed Sulaiman S.A., Basit A., Afridi A.K., Jaber A.A. Effects of multidrug resistant tuberculosis treatment on patients' health related quality of life: results from a follow up study. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaber A.A., Khan A.H., Syed Sulaiman S.A., Ahmad N., Anaam M.S. Evaluation of health-related quality of life among tuberculosis patients in two cities in yemen. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kisaka S.M., Rutebemberwa E., Kasasa S., Ocen F., Nankya-Mutyoba J. Does health-related quality of life among adults with pulmonary tuberculosis improve across the treatment period? a hospital-based cross sectional study in Mbale Region, Eastern Uganda. BMC Res Notes. 2016;9(1):467. doi: 10.1186/s13104-016-2277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dos Santos A.P., Lazzari T.K., Silva D.R. Health-Related quality of life, depression and anxiety in hospitalized patients with tuberculosis. Tuberc Respir Dis (Seoul) 2017;80(1):69–76. doi: 10.4046/trd.2017.80.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramkumar S., Vijayalakshmi S., Seetharaman N., Pajanivel R., Lokeshmaran A. Health-related quality of life among tuberculosis patients under revised National Tuberculosis Control Programme in rural and urban Puducherry. Indian J Tuberc. 2017;64(1):14–19. doi: 10.1016/j.ijtb.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Roba A.A., Dasa T.T., Weldegebreal F., Asfaw A., Mitiku H., Teklemariam Z. Tuberculosis patients are physically challenged and socially isolated: a mixed methods case-control study of health related quality of life in Eastern Ethiopia. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0204697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahdadi H., Salarzaee M., Balouchi A. Quality of life of diabetic patients with smear positive PTB in southeastern Iran: a cross-sectional study in a poor region of Iran. Indian J Tuberc. 2018;65(2):159–163. doi: 10.1016/j.ijtb.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Sineke T., Evans D., Schnippel K., van Aswegen H., Berhanu R., Musakwa N. The impact of adverse events on health-related quality of life among patients receiving treatment for drug-resistant tuberculosis in Johannesburg, South Africa. Health Qual Life Outcomes. 2019;17(1):94. doi: 10.1186/s12955-019-1155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dion M.J., Tousignant P., Bourbeau J., Menzies D., Schwartzman K. Feasibility and reliability of health-related quality of life measurements among tuberculosis patients. Qual Life Res. 2004;13(3):653–665. doi: 10.1023/B:QURE.0000021320.89524.64. [DOI] [PubMed] [Google Scholar]
- 31.Kruijshaar M.E., Lipman M., Essink-Bot M.L., Lozewicz S., Creer D., Dart S. Health status of UK patients with active tuberculosis. Int J Tuberc Lung Dis. 2010;14(3):296–302. [PubMed] [Google Scholar]
- 32.Kastien-Hilka T., Rosenkranz B., Sinanovic E., Bennett B., Schwenkglenks M. Health-related quality of life in South African patients with pulmonary tuberculosis. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorstad M.D., Amus J., Marijani M., Sviland L., Mustafa T. Diagnostic delay in extrapulmonary tuberculosis and impact on patient morbidity: a study from Zanzibar. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0203593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem S., Malik A., Ghulam A., Ahmed J., Hussain H. Health-related quality of life among pulmonary tuberculosis patients in Pakistan. Qual Life Res. 2018;27(12):3137–3143. doi: 10.1007/s11136-018-1954-9. [DOI] [PubMed] [Google Scholar]
- 35.Dhuria M., Sharma N., Ingle G. Impact of tuberculosis on the quality of life. Indian J Community Med. 2008;33(1):58–59. doi: 10.4103/0970-0218.39249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhuria M., Sharma N., Narender Pal S., Ram Chander J., Saha R., Gopal Krishan I. A study of the impact of tuberculosis on the quality of life and the effect after treatment with dots. Asia Pac J Public Health. 2009;21(3):312–320. doi: 10.1177/1010539509336242. [DOI] [PubMed] [Google Scholar]
- 37.Chung W.S., Lan Y.L., Yang M.C. Psychometric testing of the short version of the world health organization quality of life (WHOQOL-BREF) questionnaire among pulmonary tuberculosis patients in Taiwan. BMC Public Health. 2012;12:630. doi: 10.1186/1471-2458-12-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aggarwal A.N., Gupta D., Janmeja A.K., Jindal S.K. Assessment of health-related quality of life in patients with pulmonary tuberculosis under programme conditions. Int J Tuberc Lung Dis. 2013;17(7):947–953. doi: 10.5588/ijtld.12.0299. [DOI] [PubMed] [Google Scholar]
- 39.Sharma R., Yadav R., Sharma M., Saini V., Koushal V. Quality of life of multi drug resistant tuberculosis patients: a study of north India. Acta Med Iran. 2014;52(6):448–453. [PubMed] [Google Scholar]
- 40.Singh S.K., Agrawal A., Tiwari K.K. Improvement in quality of life in pulmonary tuberculosis patients: a prospective study. Trop Doct. 2017;47(2):97–100. doi: 10.1177/0049475516643256. [DOI] [PubMed] [Google Scholar]
- 41.Dar S.A., Shah N.N., Wani Z.A., Nazir D. A prospective study on quality of life in patients with pulmonary tuberculosis at a tertiary care hospital in Kashmir, Northern India. Indian J Tuberc. 2019;66(1):118–122. doi: 10.1016/j.ijtb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Laxmeshwar C., Stewart A.G., Dalal A., Kumar A.M.V., Kalaiselvi S., Das M. Beyond 'cure' and 'treatment success': quality of life of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2019;23(1):73–81. doi: 10.5588/ijtld.18.0149. [DOI] [PubMed] [Google Scholar]
- 43.Louw J.S., Mabaso M., Peltzer K. Change in health-related quality of life among pulmonary tuberculosis patients at primary health care settings in south africa: a prospective cohort study. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0151892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deribew A., Tesfaye M., Hailmichael Y., Negussu N., Daba S., Wogi A. Tuberculosis and HIV co-infection: its impact on quality of life. Health Qual Life Outcomes. 2009;7:105. doi: 10.1186/1477-7525-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deribew A., Deribe K., Reda A.A., Tesfaye M., Hailmichael Y., Maja T. Change in quality of life: a follow up study among patients with HIV infection with and without TB in Ethiopia. BMC Public Health. 2013;13:408. doi: 10.1186/1471-2458-13-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louw J., Peltzer K., Naidoo P., Matseke G., McHunu G., Tutshana B. Quality of life among tuberculosis (TB), TB retreatment and/or TB-HIV co-infected primary public health care patients in three districts in South Africa. Health Qual Life Outcomes. 2012;10:77. doi: 10.1186/1477-7525-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mthiyane T., Pym A., Dheda K., Rustomjee R., Reddy T., Manie S. Longitudinal assessment of health related quality of life of HIV infected patients treated for tuberculosis and HIV in a high burden setting. Qual Life Res. 2016;25(12):3067–3076. doi: 10.1007/s11136-016-1332-4. [DOI] [PubMed] [Google Scholar]
- 48.Duyan V., Kurt B., Aktas Z., Duyan G.C., Kulkul D.O. Relationship between quality of life and characteristics of patients hospitalised with tuberculosis. Int J Tuberc Lung Dis. 2005;9(12):1361–1366. [PubMed] [Google Scholar]
- 49.Pasipanodya J.G., Miller T.L., Vecino M., Munguia G., Bae S., Drewyer G. Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest. 2007;132(5):1591–1598. doi: 10.1378/chest.07-0755. [DOI] [PubMed] [Google Scholar]
- 50.Maguire G.P., Anstey N.M., Ardian M., Waramori G., Tjitra E., Kenangalem E. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tuberc Lung Dis. 2009;13(12):1500–1506. [PubMed] [Google Scholar]
- 51.Dhingra V.K., Rajpal S. Health related quality of life (HRQL) scoring in tuberculosis. Indian J Tuberc. 2003;50:99–104. [Google Scholar]
- 52.Dhingra V.K., Rajpal S. Health related quality of life (HRQL) scoring (DR-12 score) in tuberculosis – additional evaluative tool under dots. J Commun Dis. 2005;37(4):261–268. [PubMed] [Google Scholar]
- 53.Siddiqui A.N., Khayyam K.U., Siddiqui N., Sarin R., Sharma M. Diabetes prevalence and its impact on health-related quality of life in tuberculosis patients. Trop Med Int Health. 2017;22(11):1394–1404. doi: 10.1111/tmi.12968. [DOI] [PubMed] [Google Scholar]
- 54.Abdulelah J., Sulaiman S.A.S., Hassali M.A., Blebil A.Q., Awaisu A., Bredle J.M. Development and psychometric properties of a tuberculosis-specific multidimensional health-related quality-of-life measure for patients with pulmonary tuberculosis. Value Health Reg Issues. 2015;6:53–59. doi: 10.1016/j.vhri.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Dujaili J.A., Sulaiman S.A., Hassali M.A., Awaisu A., Blebil A.Q., Bredle J.M. Health-related quality of life as a predictor of tuberculosis treatment outcomes in iraq. Int J Infect Dis. 2015;31:4–8. doi: 10.1016/j.ijid.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Sun Y., Yang Z., Wan C., Xu C., Chen L., Xu L. Development and validation of the pulmonary tuberculosis scale of the system of quality of life instruments for chronic diseases (QLICD-PT) Health Qual Life Outcomes. 2018;16(1):137. doi: 10.1186/s12955-018-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aydin I.O., Ulusahin A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: applicability of GHQ-12. Gen Hosp Psychiatry. 2001;23(2):77–83. doi: 10.1016/s0163-8343(01)00116-5. [DOI] [PubMed] [Google Scholar]
- 58.Issa B.A., Yussuf A.D., Kuranga S.I. Depression comorbidity among patients with tuberculosis in a university teaching hospital outpatient clinic in Nigeria. Ment Health Fam Med. 2009;6(3):133–138. [PMC free article] [PubMed] [Google Scholar]
- 59.Das M., Isaakidis P., Van den Bergh R., Kumar A.M., Nagaraja S.B., Valikayath A. HIV, multidrug-resistant TB and depressive symptoms: when three conditions collide. Glob Health Action. 2014;7:24912. doi: 10.3402/gha.v7.24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shyamala K.K., Naveen R.S., Khatri B. Depression: a neglected comorbidity in patients with tuberculosis. J Assoc Physicians India. 2018;66(12):18–21. [PubMed] [Google Scholar]
- 61.Peltzer K., Naidoo P., Matseke G., Louw J., McHunu G., Tutshana B. Prevalence of psychological distress and associated factors in tuberculosis patients in public primary care clinics in South Africa. BMC Psychiatry. 2012;12:89. doi: 10.1186/1471-244X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kastien-Hilka T., Abulfathi A., Rosenkranz B., Bennett B., Schwenkglenks M., Sinanovic E. Health-related quality of life and its association with medication adherence in active pulmonary tuberculosis- a systematic review of global literature with focus on South Africa. Health Qual Life Outcomes. 2016;14:42. doi: 10.1186/s12955-016-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansel N.N., Wu A.W., Chang B., Diette G.B. Quality of life in tuberculosis: patient and provider perspectives. Qual Life Res. 2004;13(3):639–652. doi: 10.1023/B:QURE.0000021317.12945.f0. [DOI] [PubMed] [Google Scholar]
- 64.Jankowska-Polanska B.K., Kaminska M., Uchmanowicz I., Rycombel A. Quality of life and health behaviours of patients with tuberculosis - sex differences. Pneumonol Alergol Pol. 2015;83(4):256–265. doi: 10.5603/PiAP.2015.0046. [DOI] [PubMed] [Google Scholar]
- 65.Horita N., Miyazawa N., Yoshiyama T., Kojima R., Omori N., Kaneko T. Poor performance status is a strong predictor for death in patients with smear-positive pulmonary TB admitted to two Japanese hospitals. Trans R Soc Trop Med Hyg. 2013;107(7):451–456. doi: 10.1093/trstmh/trt037. [DOI] [PubMed] [Google Scholar]
- 66.Kamolratanakul P., Sawert H., Kongsin S., Lertmaharit S., Sriwongsa J., Na-Songkhla S. Economic impact of tuberculosis at the household level. Int J Tuberc Lung Dis. 1999;3(7):596–602. [PubMed] [Google Scholar]
- 67.Kelly P. Isolation and stigma: the experience of patients with active tuberculosis. J Community Health Nurs. 1999;16(4):233–241. doi: 10.1207/S15327655JCHN1604_3. [DOI] [PubMed] [Google Scholar]
- 68.Cremers A.L., de Laat M.M., Kapata N., Gerrets R., Klipstein-Grobusch K., Grobusch M.P. Assessing the consequences of stigma for tuberculosis patients in urban Zambia. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shivapujimath R., Rao A.P., Nilima A.R., Shilpa D.M. A cross-sectional study to assess the stigma associated with tuberculosis among tuberculosis patients in Udupi district, Karnataka. Indian J Tuberc. 2017;64(4):323–326. doi: 10.1016/j.ijtb.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Yin X., Yan S., Tong Y., Peng X., Yang T., Lu Z. Status of tuberculosis-related stigma and associated factors: a cross-sectional study in central China. Trop Med Int Health. 2018;23(2):199–205. doi: 10.1111/tmi.13017. [DOI] [PubMed] [Google Scholar]
- 71.Courtwright A., Turner A.N. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep. 2010;125(Suppl 4):34–42. doi: 10.1177/00333549101250S407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johansson E., Diwan V.K., Huong N.D., Ahlberg B.M. Staff and patient attitudes to tuberculosis and compliance with treatment: an exploratory study in a district in Vietnam. Tuber Lung Dis. 1996;77(2):178–183. doi: 10.1016/s0962-8479(96)90035-0. [DOI] [PubMed] [Google Scholar]
- 73.Johansson E., Long N.H., Diwan V.K., Winkvist A. Attitudes to compliance with tuberculosis treatment among women and men in Vietnam. Int J Tuberc Lung Dis. 1999;3(10):862–868. [PubMed] [Google Scholar]
- 74.Hudelson P. Gender differentials in tuberculosis: the role of socio-economic and cultural factors. Tuber Lung Dis. 1996;77(5):391–400. doi: 10.1016/s0962-8479(96)90110-0. [DOI] [PubMed] [Google Scholar]
- 75.Khan A., Walley J., Newell J., Imdad N. Tuberculosis in Pakistan: socio-cultural constraints and opportunities in treatment. Soc Sci Med. 2000;50(2):247–254. doi: 10.1016/s0277-9536(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 76.Long N.H., Johansson E., Diwan V.K., Winkvist A. Fear and social isolation as consequences of tuberculosis in Vietnam: a gender analysis. Health Policy (New York) 2001;58(1):69–81. doi: 10.1016/s0168-8510(01)00143-9. [DOI] [PubMed] [Google Scholar]
- 77.Craig G.M., Daftary A., Engel N., O'Driscoll S., Ioannaki A. Tuberculosis stigma as a social determinant of health: a systematic mapping review of research in low incidence countries. Int J Infect Dis. 2017;56:90–100. doi: 10.1016/j.ijid.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Venkatraju B., Prasad S. Psychosocial trauma of diagnosis: a qualitative study on rural TB patients' experiences in Nalgonda district, Andhra Pradesh. Indian J Tuberc. 2013;60(3):162–167. [PubMed] [Google Scholar]
- 79.Liefooghe R., Michiels N., Habib S., Moran M.B., De Muynck A. Perception and social consequences of tuberculosis: a focus group study of tuberculosis patients in Sialkot, Pakistan. Soc Sci Med. 1995;41(12):1685–1692. doi: 10.1016/0277-9536(95)00129-u. [DOI] [PubMed] [Google Scholar]
- 80.Marra C.A., Marra F., Cox V.C., Palepu A., Fitzgerald J.M. Factors influencing quality of life in patients with active tuberculosis. Health Qual Life Outcomes. 2004;2:58. doi: 10.1186/1477-7525-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koyanagi A., Vancampfort D., Carvalho A.F., DeVylder J.E., Haro J.M., Pizzol D. Depression comorbid with tuberculosis and its impact on health status: cross-sectional analysis of community-based data from 48 low- and middle-income countries. BMC Med. 2017;15(1):209. doi: 10.1186/s12916-017-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajeswari R., Balasubramanian R., Muniyandi M., Geetharamani S., Thresa X., Venkatesan P. Socio-economic impact of tuberculosis on patients and family in India. Int J Tuberc Lung Dis. 1999;3(10):869–877. [PubMed] [Google Scholar]
- 83.Muniyandi M., Rajeswari R., Balasubramanian R., Nirupa C., Gopi P.G., Jaggarajamma K. Evaluation of post-treatment health-related quality of life (HRQoL) among tuberculosis patients. Int J Tuberc Lung Dis. 2007;11(8):887–892. [PubMed] [Google Scholar]
- 84.Dias A.A., de Oliveira D.M., Turato E.R., de Figueiredo R.M. Life experiences of patients who have completed tuberculosis treatment: a qualitative investigation in southeast Brazil. BMC Public Health. 2013;13:595. doi: 10.1186/1471-2458-13-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C.T., Chu K.H., Reiher B., Kienene T., Chien L.Y. Evaluation of health-related quality of life in patients with tuberculosis who completed treatment in Kiribati. J Int Med Res. 2017;45(2):610–620. doi: 10.1177/0300060517694491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Banu Rekha V.V., Ramachandran R., Kuppu Rao K.V., Rahman F., Adhilakshmi A.R., Kalaiselvi D. Assessment of long term status of sputum positive pulmonary TB patients successfully treated with short course chemotherapy. Indian J Tuberc. 2009;56(3):132–140. [PubMed] [Google Scholar]
- 87.Alene K.A., Clements A.C.A., McBryde E.S., Jaramillo E., Lonnroth K., Shaweno D. Mental health disorders, social stressors, and health-related quality of life in patients with multidrug-resistant tuberculosis: a systematic review and meta-analysis. J Infect. 2018;77(5):357–367. doi: 10.1016/j.jinf.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Vo N.X., Xuan Doan T.B., Kha Vo D.N., Tran T.K., Vo T.Q. Assessing quality of life for multidrug-resistant and extensively drug-resistant tuberculosis patients. J Pak Med Assoc. 2019;69:S137–Ss57. (Suppl 2)(6) [PubMed] [Google Scholar]
- 89.Jaber A.A.S., Ibrahim B. Health-related quality of life of patients with multidrug-resistant tuberculosis in Yemen: prospective study. Health Qual Life Outcomes. 2019;17(1):142. doi: 10.1186/s12955-019-1211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sagwa E.L., Ruswa N., Mavhunga F., Rennie T., Leufkens H.G., Mantel-Teeuwisse A.K. Adverse events and patients' perceived health-related quality of life at the end of multidrug-resistant tuberculosis treatment in Namibia. Patient Prefer Adherence. 2016;10:2369–2377. doi: 10.2147/PPA.S116860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jha D.K., Jha J., Jha A.K., Achappa B., Holla R. Quality of life among HIV-tuberculosis co-infected patients. Perspect Clin Res. 2019;10(3):125–129. doi: 10.4103/picr.PICR_99_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kittikraisak W., Burapat C., Nateniyom S., Akksilp S., Mankatittham W., Sirinak C. Improvements in physical and mental health among HIV-infected patients treated for TB in Thailand. Southeast Asian J Trop Med Public Health. 2008;39(6):1061–1071. [PubMed] [Google Scholar]
- 93.TB CARE I . 3rd ed. TB CARE I; The Hague: 2014. International standards for tuberculosis care. [Google Scholar]
- 94.Daniels B., Kwan A., Pai M., Das J. Lessons on the quality of tuberculosis diagnosis from standardized patients in China, India, Kenya, and South Africa. J Clin Tuberc Other Mycobacterial Dis. 2019;16 doi: 10.1016/j.jctube.2019.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naidoo P., Theron G., Rangaka M.X., Chihota V.N., Vaughan L., Brey Z.O. The south african tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis. 2017;216(suppl_7):S702–Ss13. doi: 10.1093/infdis/jix335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Subbaraman R., Nathavitharana R.R., Satyanarayana S., Pai M., Thomas B.E., Chadha V.K. The tuberculosis cascade of care in india's public sector: a systematic review and meta-analysis. PLoS Med. 2016;13(10) doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinto L.M., Udwadia Z.F. Private patient perceptions about a public programme; what do private indian tuberculosis patients really feel about directly observed treatment? BMC Public Health. 2010;10:357. doi: 10.1186/1471-2458-10-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atif M., Sulaiman S.A., Shafie A.A., Ali I., Hassali M.A., Saleem F. WHO guidelines for treatment of tuberculosis: the missing links. Int J Clin Pharm. 2012;34(4):506–509. doi: 10.1007/s11096-012-9657-8. [DOI] [PubMed] [Google Scholar]
- 99.Reid M.J.A., Arinaminpathy N., Bloom A., Bloom B.R., Boehme C., Chaisson R. Building a tuberculosis-free world: the lancet commission on tuberculosis. Lancet. 2019;393(10178):1331–1384. doi: 10.1016/S0140-6736(19)30024-8. [DOI] [PubMed] [Google Scholar]
- 100.Miller T.L., McNabb S.J., Hilsenrath P., Pasipanodya J., Weis S.E. Personal and societal health quality lost to tuberculosis. PLoS One. 2009;4(4):e5080. doi: 10.1371/journal.pone.0005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peddireddy V. Quality of life, psychological interventions and treatment outcome in tuberculosis patients: the Indian scenario. Front Psychol. 2016;7:1664. doi: 10.3389/fpsyg.2016.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zarova C., Chiwaridzo M., Tadyanemhandu C., Machando D., Dambi J.M. The impact of social support on the health-related quality of life of adult patients with tuberculosis in Harare, Zimbabwe: a cross-sectional survey. BMC Res Notes. 2018;11(1):795. doi: 10.1186/s13104-018-3904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hargreaves J.R., Boccia D., Evans C.A., Adato M., Petticrew M., Porter J.D. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654–662. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Macq J., Solis A., Martinez G., Martiny P., Dujardin B. An exploration of the social stigma of tuberculosis in five "municipios" of Nicaragua to reflect on local interventions. Health Policy (New York) 2005;74(2):205–217. doi: 10.1016/j.healthpol.2005.01.003. [DOI] [PubMed] [Google Scholar]