Highlights
-
•
Oral exposure to BPA relevant to human exposure aggravated allergic asthma.
-
•
Low dose BPA with allergen reduced lung mRNA levels of hormone receptors.
-
•
Low dose BPA with allergen altered lymph node and bone marrow microenvironments.
Abbreviations: Ar, androgen receptor; AhR, aryl hydrocarbon receptor; BPA, bisphenol a; BM, bone marrow; ER, estrogen receptor; FACS, fluorescence-activated cell-sorting; GR, glucocorticoid receptor; Gr-1, granulocyte-differentiation antigen; Hprt1, hypoxanthine phosphoribosyltransferase 1; Ig, immunoglobulin; IFN-γ, interferon-gamma; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein 1-alpha; MLN, mediastinal lymph node; OVA, ovalbumin; RANTES, normal T cell expressed and secreted; SDF-1α, stromal cell derived factor 1 alpha; Th, T helper
Keywords: Bisphenol A, Allergic asthma, Endocrine disruptor, Low dose effects, Th2 response, Hormone receptor
Abstract
Bisphenol A (BPA) is widely used in many consumer products and has adverse effects on human health including allergic diseases. We investigated the effects of low dose BPA, comparable to actual human oral exposure, on allergic asthma in mice. C3H/HeJ male mice were fed a chow diet containing BPA (equivalent to 0.09, 0.90, or 9.01 μg/kg/day) and were intratracheally administered ovalbumin (OVA, 1 μg/animal) every two weeks from 5–11 weeks of age. All doses of BPA plus OVA enhanced pulmonary inflammation and airway hyperresponsiveness, and increased lung mRNA levels of Th2 cytokine/chemokine, and serum OVA-specific IgE and IgG1 compared to OVA alone, with greater effects observed in the middle- and high-dose BPA plus OVA groups. Furthermore, high-dose BPA with OVA decreased lung mRNA levels of ERβ and AR compared with OVA. Furthermore, BPA enhanced OVA-restimulated cell proliferation and protein levels of IL-4 and IL-5 in mediastinal lymph node (MLN) cells in OVA-sensitized mice. In bone marrow (BM) cells, middle-dose BPA with OVA increased Gr-1 expression. In conclusion, oral exposure to low-dose BPA at levels equivalent to human exposure can aggravate allergic asthmatic responses through enhancement of Th2-skewed responses, lung hormone receptor downregulation, and MLN and BM microenvironment change.
1. Introduction
Bisphenol A (BPA), a widely used environmental chemical in polycarbonate plastics and epoxy resins, has been detected in children’s toys, dental sealants, medical devices, internal coating of cans, and food and beverage containers [[1], [2], [3]]. Therefore, BPA is globally ubiquitous [4] and can be detected in blood and urine samples in 95 % of the U.S. population according to biomonitoring surveys by the U.S. Centers for Disease Control and Prevention [5]. The main source of BPA exposure in humans is due to food and beverages, while the exposure via house dust ingestion, dental surgery, skin absorption from thermal paper has been estimated at 5 % or less [6]. The Food and Drug Administration (FDA) estimated that the daily intake of BPA for adults and infants from food additive uses is 0.185 μg kg − 1 day−1 and 0.7 μg kg−1 day−1, respectively [7]. In Japan, the predicted maximum oral exposure has been estimated to be 0.09 μg kg − 1 day−1 [8].
BPA is well known to show endocrine disrupting effects by binding to various receptors, such as estrogen receptors, androgen receptors, thyroid hormone receptors, aryl hydrocarbon receptors, and toll-like receptors [9,10]. Previous studies mentioned that BPA could disrupt developmental, reproductive, cardiovascular, nervous, and metabolic systems, resulting in adverse effects on human health [[11], [12], [13]]. Furthermore, several reports suggested that BPA has potential mutagenicity [14] and is associated with the development of tumors, such as prostate, breast, and lung cancers [[15], [16], [17]]. Regarding to the impact of BPA on immune/allergic system, an epidemiological study showed that prenatal exposure to BPA was associated with pediatric respiratory outcomes among boys, but not girls [18]. The urine concentration of BPA is related to atopic dermatitis symptom aggravation in children [19]. In animal studies, Bauer et al. reported that prenatal and postnatal exposure to BPA (0, 0.5, 5, 50 or 500 μg kg − 1 day−1) enhanced allergic lung inflammation in female but not male offspring of mice [20]. These studies have only focused on the indirect effects such as prenatal exposure, however, the impact of direct exposure to BPA during the postnatal period or throughout life on allergic responses is poorly understood. He et al. reported that repetitive oral exposure to BPA (1 mg per mouse, four times) enhanced lung eosinophilia by promoting Th2-type immune responses in allergen-sensitized male mice [21]. A recent study was demonstrated that oral administration of BPA (0.06, 0.2 mg/kg, single injection) enhanced toluene-2, 4-diisocyanate (Th2 type hapten)-induced airway allergic inflammation, but not atopic dermatitis [22]. These doses in previous studies are approximately 2000 to 40000-fold of the predicted maximum exposure dose in Japan. Thus, the effects of low dose BPA comparable to the level of human exposure and the underlying mechanisms are poorly understood. Our recent study indicated that intratracheal exposure to low dose BPA (0.0015 μg/kg/day; equivalent to 5 times of the predicted maximum exposure dose from the general atmosphere in Japan of 0.0003 μg/kg/day) aggravates allergic airway inflammation and disrupts the immune systems in mice [23]. However, the effects of oral BPA, which is the main route of BPA exposure, remain unclear, particularly at levels equivalent to human exposure.
In the current study, we aimed to evaluate the effects of oral exposure to low doses of BPA relevant to human exposure in a murine model of allergic asthma. We also investigated the relationship between dietary exposure to BPA and the changes in lymph node and bone marrow microenvironments on allergic asthma.
2. Materials and methods
2.1. Animals and experimental design
Four-week-old male C3H/HeJSlc mice were purchased from Japan SLC, Inc. (Shizuoka, Japan) and used for experiments. Five-week-old mice were randomly divided into eight groups: 1) Vehicle, 2) 0.09 μg/kg/day BPA (BPA-L), 3) 0.9 μg/kg/day BPA (BPA-M), 4) 9 μg/kg/day BPA (BPA-H), 5) ovalbumin (OVA), 6) OVA + BPA-L, 7) OVA + BPA-M, and 8) OVA + BPA-H. We selected dietary exposure to BPA, and took into account actual human exposure levels, as serum levels of BPA are different between dietary exposure and forced administration in an animal model [24]. From 5–11 weeks of age, mice were fed a chow diet mixed with 0, 7.5, 75, or 750 μg/10 kg of BPA based on soy-free AIN-76A to avoid phytoestrogenic effects that might mask possible estrogenic effects of BPA (Japan Clea Co., Tokyo, Japan). Since the daily food consumption in mice was approximately 3 g, the exposure amount of BPA was estimated to be 0.09 μg/kg/day in the BPA-L group. The BPA-L dose is equivalent to the estimated peak oral exposure dose of BPA identified by the Ministry of the Environment in Japan [8]. Food and water were provided ad libitum. Mice were housed in an animal facility maintained at 22 °C – 26 °C and 40 %–69 % humidity under a 12 h light/dark cycle. To minimize background BPA exposure, we used animal cages made of polymethylpentene and water bottles made of polypropylene. BPA concentration in the drinking water was analyzed using HPLC and was less than 1 ng/mL. Mice were anesthetized with isoflurane (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and intratracheally instilled with 50 μL aqueous suspension. Mice received phosphate-buffered saline (PBS; pH7.4; Thermo Fisher Scientific, Inc., IL, USA) in the vehicle and BPA-treated groups or 1 μg of OVA (20 μg/mL; Sigma-Aldrich Co., St Louis, MO, USA) dissolved in PBS in the OVA-treated groups every 2 weeks from 5–11 weeks of age. Forty-eight hours after the final OVA instillation, all mice were euthanized with an intraperitoneal injection of sodium pentobarbital (150 mg/kg) at 11 weeks of age. All procedures were approved by the Animal Care and Use Committee of National Institute for Environmental Studies (approval number; AE-16-01 and AE-17-10), which was conducted in accordance with the guideline for the Care and Use of Laboratory Animals of the National Institute for Environmental Studies. Animals were humanely treated and were alleviated of suffering.
2.2. Retrieval of serum and bronchoalveolar lavage (BAL)
All mice were euthanized under anesthesia 48 h after the final intratracheal instillation (5–6 animals per group). The chest and abdominal walls were opened, and blood was retrieved by cardiac puncture. The BAL fluid was aspirated twice with 0.8 mL of sterile saline at 37 °C by syringe. The average volume was 90 % of the amount instilled. The BAL fluid was centrifuged at 300 g for 10 min at 4 °C to recover alveolar free cells. The total cell count was determined on a fresh fluid specimen using a hemocytometer. Differential cell counts were prepared using Autosmear (Sakura Seiki Co., Tokyo, Japan) and stained with Diff-Quik (International Reagents Co., Kobe, Japan). A total of 500 cells were counted under a microscope (AX80; Olympus, Tokyo, Japan). The lung tissue was immediately extirpated after BAL retrieval. The serum and lungs were stored at −80 °C until use.
2.3. Determination of pulmonary function
Airway responsiveness in the OVA-treated mice was measured 24 h after the last OVA intratracheal instillation using whole body plethysmography (WBP) in a noninvasive fashion (Buxco FinePointe System, Buxco, Wilmington, USA) according to the manufacturer’s instructions (5–6 animals per group). Mice were given 5 min for acclimation and then exposed to nebulized PBS to set a baseline value, followed by increasing concentrations of 50 μL nebulized methacholine chloride (Sigma-Aldrich, German; 3.125, 6.25, 12.5, 25, and 50 mg/mL in PBS) for 2 min. Airway responsiveness to methacholine was monitored continuously for 3 min following methacholine inhalation and then evaluated using the enhanced pause (Penh) values and respiratory frequency (f).
2.4. Quantification of antigen-specific immunoglobulin in serum
Blood was sampled by cardiac puncture (5–8 animals per group). Serum was collected and stored at −80 °C until use. OVA-specific IgE and IgG1 in serum were measured using mouse anti-OVA IgE (DS Pharma Biomedical Co., Ltd, Tokyo, Japan) and IgG1 ELISA Kit (Shibayagi Co., Gunma, Japan) according to the manufacturers’ instructions.
2.5. Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA from lungs was extracted using RNAiso Plus (Takara Bio Inc., Shiga, Japan) and then treated with DNase I and purified using RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions (3–7 animals/group). The total RNA concentration was assessed spectrophotometrically with a NanoDrop spectrometer (Thermo Fisher Scientific). Total RNA was reverse transcribed to cDNA using a High-Capacity RNA-to-cDNA™ Kit (Thermo Fisher Scientific). mRNA expressions of interleukin-4 (Il4), Il5, Il13, Il33, monocyte chemoattractant protein-1 (Mcp1), macrophage inflammatory protein 1-alpha (Mip1a), eotaxin, regulated on activation, normal T cell expressed and secreted (Rantes), estrogen receptor alpha (Era), estrogen receptor beta (Erb), androgen receptor (Ar), and Muc5ac were quantified using the StepOne Plus™ Real-time PCR System (Thermo Fisher Scientific). RT PCR was then performed at 50 °C for 2 min, 95 °C for 10 min, 95 °C for 15 s, and 60 °C for 1 min, with the last two steps repeated for 40 cycles. Data were analyzed by the critical threshold (ΔCT) and the comparative critical threshold (ΔΔCT) methods using StepOne Plus™ Software version 2.2.2. The relative intensity was normalized to an endogenous control gene (hypoxanthine phosphoribosyltransferase 1; Hprt1). TaqMan probes and pairs for target genes were designed and purchased from Thermo Fisher Scientific and these sequences were not disclosed.
2.6. Preparation of mediastinal lymph node cells
Mediastinal lymph nodes (MLNs) (6 animals per group) were pushed through a sterile stainless wire mesh in PBS (pH7.4; Takara Bio Inc., Shiga, Japan). MLN cells were collected by centrifugation at 400 g for 5 min at 20 °C and red blood cells were lysed with ammonium chloride. After washing with PBS, cells were resuspended in culture medium R10, consisting of Gibco RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 10 % heat inactivated fetal bovine serum (MP Biomedicals Inc., Eschwege, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin (Sigma-Aldrich), and 50 μM 2-mercaptoethanol (Thermo Fisher Scientific). Total cell numbers and viability were determined by trypan blue staining (Thermo Fisher Scientific).
2.7. Proliferation and cytokine secretion of mediastinal lymph node cells
MLN cells (1 × 106/mL) were cultured with or without OVA (100 μg/mL) in 200 μL of R10 medium in 96-well flat-bottom plates. These cultures were performed in triplicate at 37 °C in a 5 % CO2/95 % air atmosphere. After 67 h, culture supernatant was collected and stored at −80 °C until use. Cell proliferation was measured by adding 5-bromo-2′-deoxyuridine to each well 20 h before the measurement using an ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany), according to the manufacturer’s instructions. Levels of IL-4, IL-5, interferon-gamma (IFN-γ) (Thermo Fisher Scientific) in MLN cell culture supernatant were measured using ELISA kits according to the manufacturer's instructions.
2.8. Preparation of bone marrow (BM) cells and BM lavage fluid
For preparation of BM cells, BM was flushed with 2 mL of PBS from the right femur (6 animals per group) and centrifuged at 400 g for 5 min at 4 °C. Red blood cells were lysed with ammonium chloride. After washing the cells with PBS, the total cell numbers and viability were determined by the trypan blue exclusion method. For preparation of BM lavage fluid, BM was flushed with 200 μL of PBS from the left femur (6 animals per group). The suspension was centrifuged at 5000 g for 10 min at 4 °C and the supernatant was collected as BM lavage fluid and stored at −80 °C until use.
2.9. Flow cytometry analysis
The expression of granulocyte-differentiation antigen (Gr-1) in BM cells were examined using fluorescence-activated cell-sorting (FACS) analysis. We used the monoclonal antibody for Gr-1 (RB6-8C5, Rat IgG2b κ PE-conjugated; BD Biosciences, San Diego, CA, USA). The cells were incubated with each antibody for 30 min on ice, and fluorescence was measured on a FACSCalibur (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) as previously reported [25,26].
2.10. Statistical analysis
We tested the significance of differences between the groups using two-way analysis of variance followed by a Tukey or Scheffe test. We also performed non parametric Kruskal-Wallis test followed by Steel test. All statistical analyses were carried out using Ekuseru-Toukei 2010 statistical software (Social Survey Research Information Co., Ltd., Tokyo, Japan). Statistical significance was defined as a P value of < 0.05.
3. Results
3.1. BPA has no effects body weight and food intake with or without allergen
Mice were weighed biweekly with monitoring of daily food intake in all groups at 6, 8, and 10 weeks of age and there were no significant changes in each group (data not shown).
3.2. BPA aggravates allergen-induced pulmonary inflammation and goblet cell hyperplasia
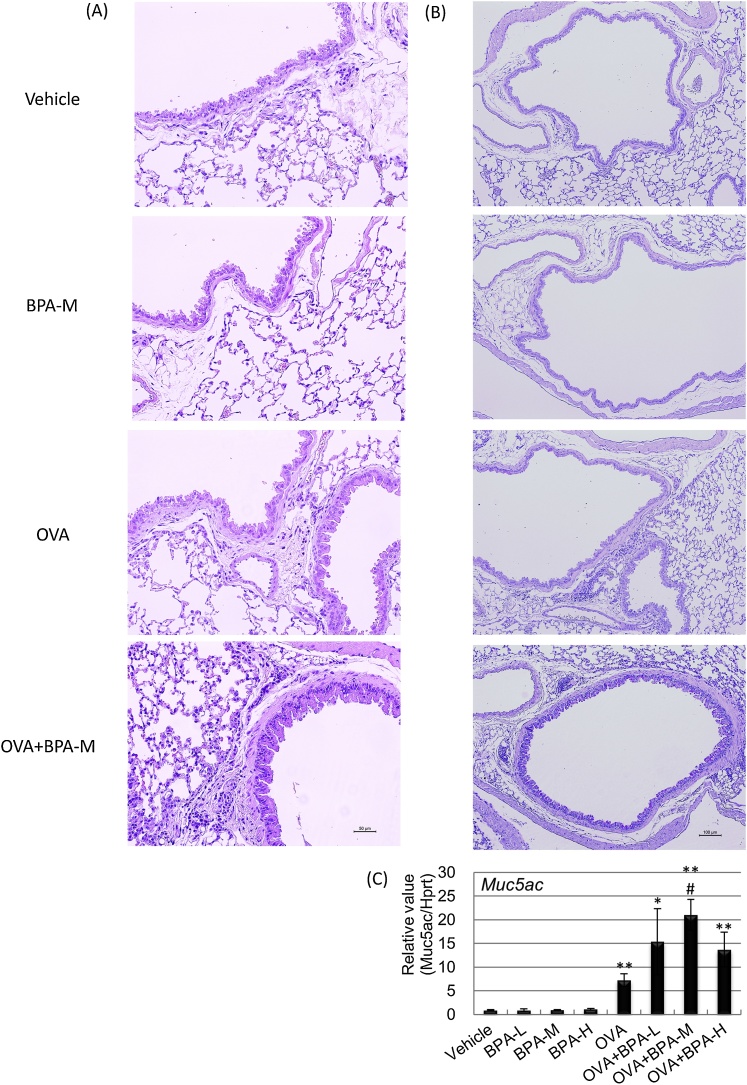
We investigated the cellular profile in BAL fluid 48 h after the last intratracheal instillation to evaluate whether oral exposure to BPA affects allergen-induced pulmonary inflammation. Lung infiltration of eosinophils and lymphocytes was significantly increased in the OVA + BPA groups compared with the Vehicle group (Table 1). Lymphocytes were significantly increased in the OVA + BPA-M and OVA + BPA-H groups compared with the OVA group (P < 0.05). Additionally, eosinophil accumulation was greater in the OVA+BPA-H group than in the OVA group (P < 0.05). Furthermore, we examined H&E- and PAS-stained lung sections. The combined administration of BPA and OVA enhanced the increase in eosinophil and lymphocyte accumulation in the peribronchial and perivascular regions (Fig. 1A) and goblet cell hyperplasia in the bronchial epithelium (Fig. 1B) compared with OVA administration alone, which was more prominent in the OVA + BPA-M and OVA + BPA-M groups. Furthermore, mRNA levels of Muc5ac were higher in the OVA + BPA-M group than in the OVA group (P < 0.05, Fig. 1C).
Table 1.
Cell number in the BAL fluid.
| Group | Total cells | Macrophages | Neutrophils | Eosinophils | Lymphocytes |
|---|---|---|---|---|---|
| Vehicle | 13.75 ± 1.24 | 13.75 ± 1.36 | 0.004 ± 0.005 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| BPA-L | 18.00 ± 2.61 | 17.86 ± 2.89 | 0.132 ± 0.105 | 0.005 ± 0.006 | 0.000 ± 0.000 |
| BPA-M | 19.08 ± 2.03 | 19.05 ± 2.23 | 0.029 ± 0.024 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| BPA-H | 20.42 ± 2.46 | 20.31 ± 2.63 | 0.110 ± 0.120 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| OVA | 20.30 ± 2.40 | 20.19 ± 2.38 | 0.026 ± 0.011 | 0.065 ± 0.041 | 0.020 ± 0.012 |
| OVA + BPA-L | 18.10 ± 3.54 | 17.27 ± 3.07 | 0.019 ± 0.014 | 0.764 ± 0.483** | 0.050 ± 0.013* |
| OVA + BPA-M | 17.42 ± 1.53 | 16.57 ± 1.36 | 0.103 ± 0.059 | 0.568 ± 0.179** | 0.181 ± 0.069**,# |
| OVA + BPA-H | 22.92 ± 2.32 | 21.86 ± 2.29 | 0.069 ± 0.030 | 0.770 ± 0.104**,# | 0.221 ± 0.086**,# |
The total cell and differential cell in the BAL fluid were evaluated 48 h after the final intratracheal instillation. Data were expressed as means ± SE for 5–6 animals per group. * P < 0.05 versus vehicle group, ** P < 0.01 versus vehicle group, # P < 0.05 versus OVA group.
Fig. 1.
Histological findings and mRNA Muc5ac levels in the lung. Histological changes using H&E- and PAS-staining and gene expression using RT-PCR analysis in the lung were examined 48 h after the final intratracheal administration. (A) H&E staining. (B) PAS staining. (C) Muc5ac mRNA level. Data were expressed as mean ± SE for 3 animals per group for histological findings and for 3–7 animals per group for RT-PCR analysis, respectively. * P < 0.05 versus vehicle group, ** P < 0.01 versus vehicle group, # P < 0.05 versus OVA group.
3.3. BPA enhances allergen-induced airway hyperresponsiveness
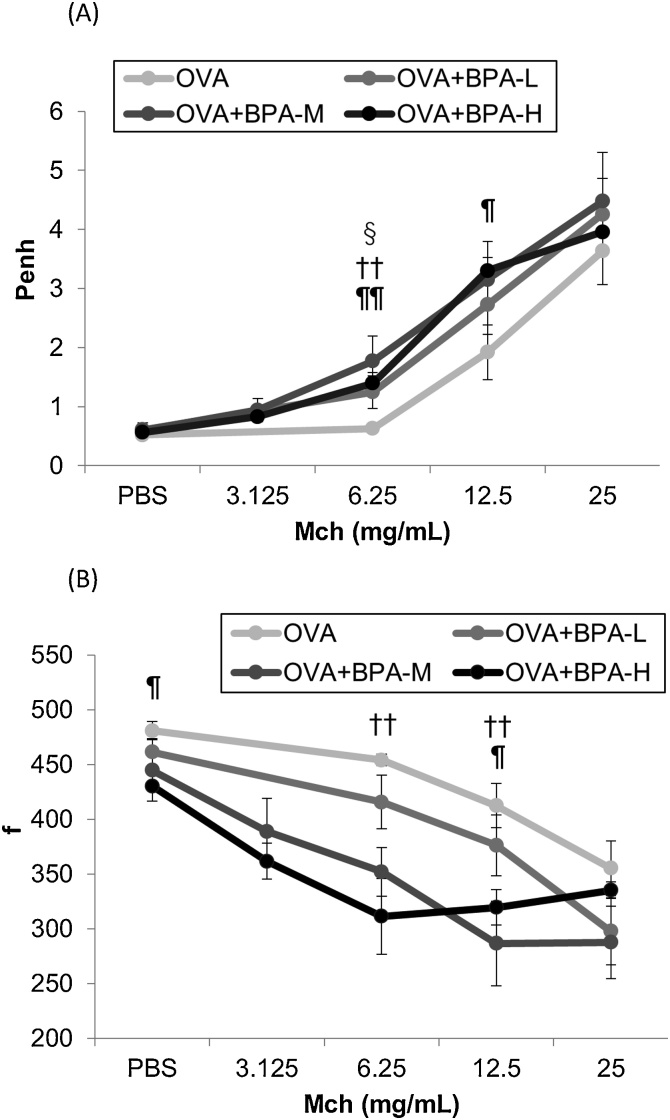
To evaluate the effects of BPA exposure on noninvasive airway responsiveness in allergic asthma, enhanced pause (Penh) and respiratory frequency (f) to nebulized methacholine were analyzed 24 h after the last OVA instillation. As shown in Fig. 2, oral exposure to BPA increased Penh values compared to PBS exposure in OVA-sensitized mice. This result was more prominent in the OVA + BPA-M and OVA + BPA-H groups. In addition, the OVA + BPA-M and OVA + BPA-H groups showed significantly reduced respiratory frequency compared to the OVA group (Fig. 2B).
Fig. 2.
Changes in airway responsiveness to methacholine in OVA-sensitized mice. They were performed 24 h after the final OVA intratracheal administration using whole body plethysmography in a noninvasive fashion. (A) Enhanced pause values (Penh). (B) Airway frequency (f). § P < 0.05 OVA+BPA-L group versus OVA group, †† P < 0.01 OVA+BPA-M group versus OVA group, ¶ P < 0.05 OVA+BPA-H group versus OVA group, ¶¶ P < 0.01 OVA+BPA-H group versus OVA group.
3.4. BPA elevates serum OVA-specific Ig antibody production
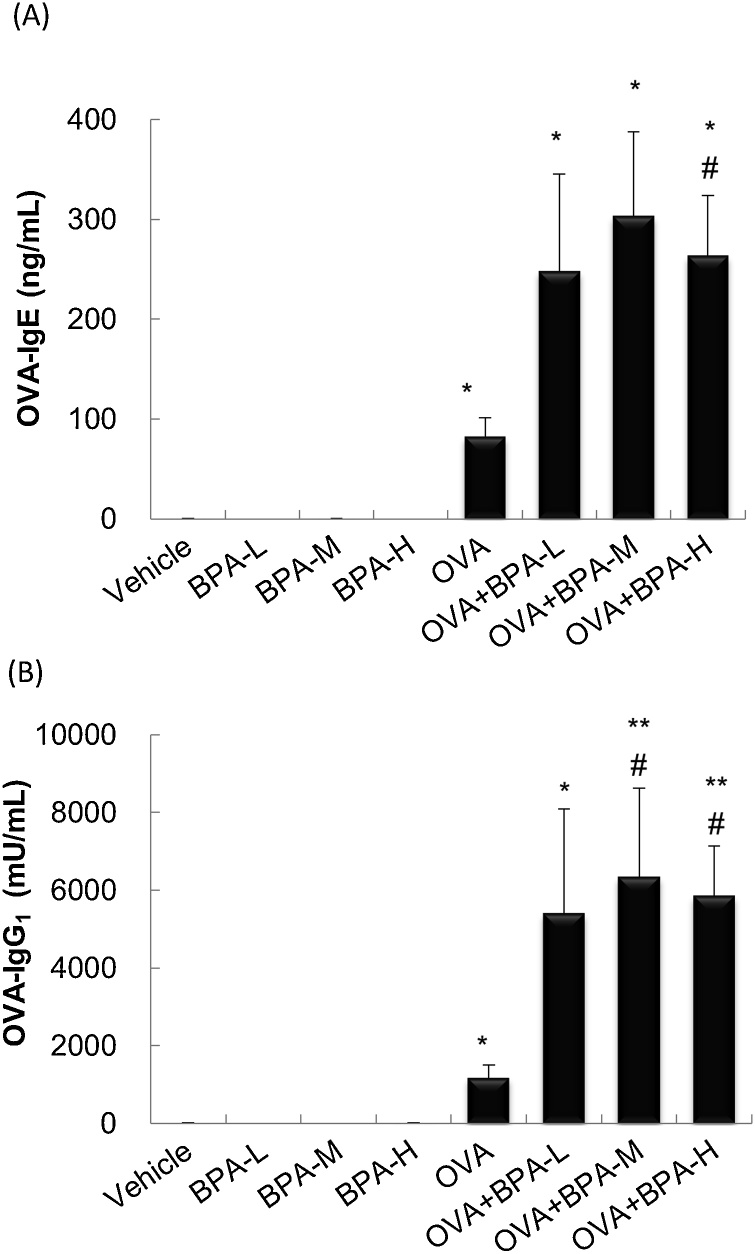
To examine the adjuvant activity of BPA, the levels of serum OVA-specific IgE and IgG1 48 h after the last intratracheal administration were measured. OVA-treated groups had significantly increased levels of OVA-specific IgE (P < 0.05, Fig. 3A) and IgG1 (P < 0.05, Fig. 3B) compared with Vehicle group. OVA-IgE in the OVA + BPA-H group and OVA-IgG1 in the OVA + BPA-M and OVA + BPA-H groups were greater than in the OVA group (P < 0.05). Co-exposure to OVA and BPA-L also tended to increase the OVA-specific Ig antibodies compared with OVA exposure alone although this did not reach statistical significant difference (P < 0.10 for IgE, P < 0.22 for IgG1).
Fig. 3.
Serum levels of OVA-specific Ig antibodies. OVA-specific IgE and IgG1 in serum were measured 48 h after the last intratracheal administration by ELISA. (A) OVA-IgE. (B) OVA-IgG1. Data were expressed as mean ± SE for 5–6 animals per group. ** P < 0.01 versus vehicle group. * P < 0.05 versus vehicle group, ** P < 0.01 versus vehicle group, # P < 0.05 versus OVA group, ## P < 0.01 versus OVA group.
3.5. BPA elevates mRNA levels of cytokines/chemokines in the lungs
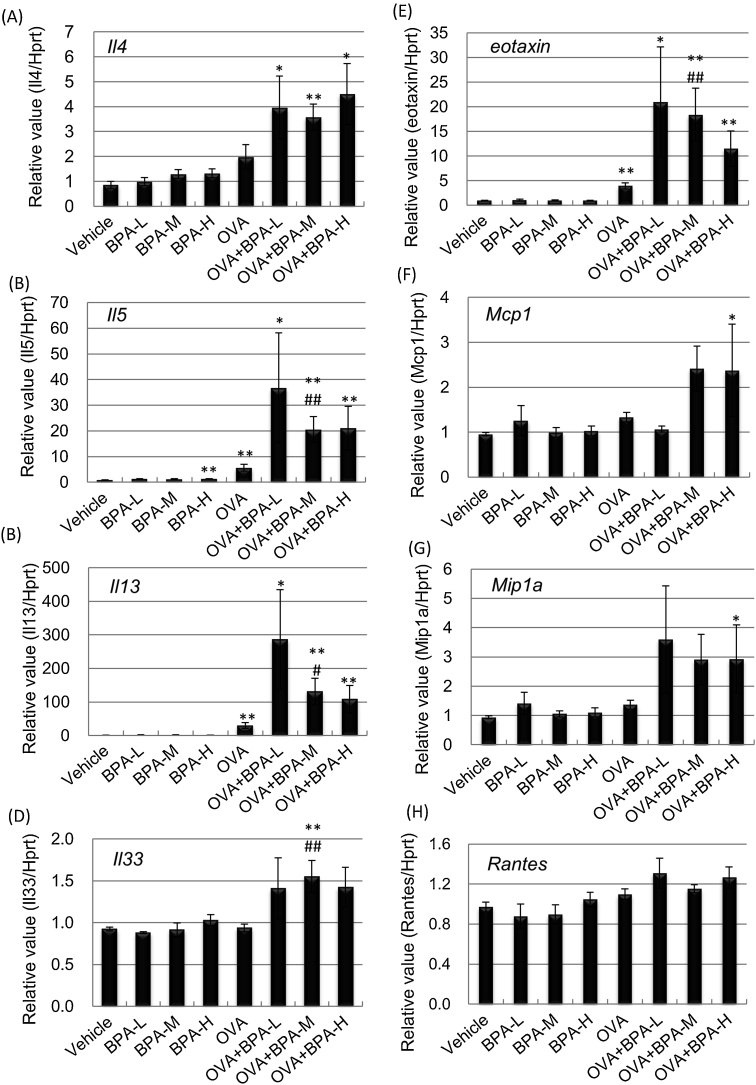
We investigated whether oral exposure of BPA affects the gene expression of Th1/Th2 cytokines and chemokines in the lungs 48 h after the last intratracheal instillation. OVA administration increased lung gene expression of Il5, Il13 and eotaxin compared with vehicle administration (P < 0.01, Fig. 4). Overall, co-exposure to OVA and BPA tended to increase Th2 cytokine/chemokine levels compared with OVA exposure alone. In particular, mRNA levels of Il5, Il13, Il33 and eotaxin in the OVA + BPA-M group were significantly higher than in the OVA group (Il13; P < 0.05, Il5, Il33 and ccl11; P < 0.01). The gene expression of Mcp1 and Mip1a was higher in the OVA + BPA-H group than in the Vehicle group (P < 0.05). BPA plus OVA tended to increase mRNA levels of Rantes compared to OVA alone, although there was no significant change (Fig. 4H). For Th1 cytokine expression in the lung, mRNA levels of IL-12 β was greater in OVA-treated mice than in PBS-treated mice, and BPA exposure had no effect (P < 0.05, data not shown). Administration of BPA-M with OVA showed a tendency to decrease IL-12 α mRNA compared to OVA administration alone (P < 0.085, data not shown). No statistically significant change was observed in IFN-γ (data not shown).
Fig. 4.
Cytokine and chemokine mRNA levels in the lung. Gene expression in the lung was examined 48 h after the last intratracheal administration by RT-PCR analysis. (A) Il4. (B) Il5. (C) Il13. (D) Il33. (E) eotaxin. (F) Mcp1, (G) Mip1a. (H) Rantes. The relative intensity was normalized to Hprt1. Data were expressed as mean ± SE for 3–7 animals per group. ** P < 0.01 versus vehicle group. * P < 0.05 versus vehicle group, ** P < 0.01 versus vehicle group, # P < 0.05 versus OVA group, ## P < 0.01 versus OVA group.
3.6. BPA disrupts lung hormone receptor mRNA levels
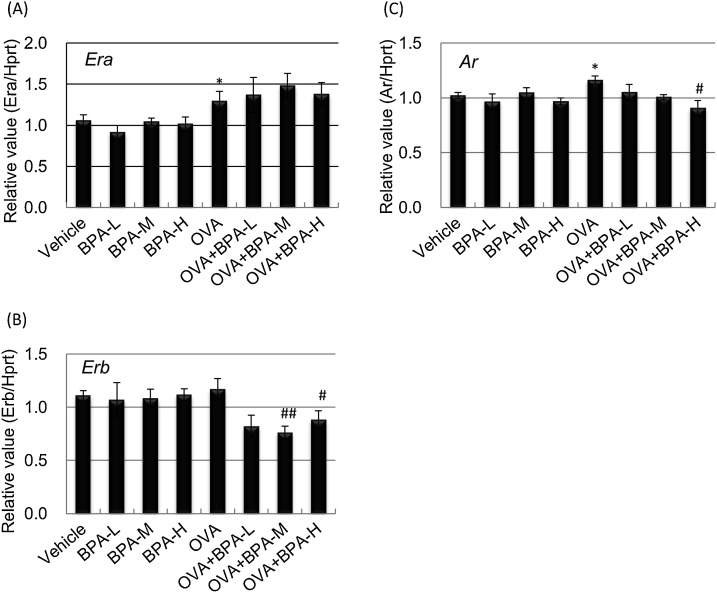
BPA is a major endocrine disrupting chemical and can modulate immune responses via binding to various receptors such as estrogen receptors, estrogen-related receptors, androgen receptor (AR), glucocorticoid receptor (GR), and aryl hydrocarbon receptor (AhR) [27]. We performed RT-PCR analysis to investigate whether oral exposure to BPA affects allergic responses through changes in receptor gene expression in the lung. OVA treatment induced Era expression compared to PBS treatment (Fig. 5A), but BPA exposure had no significant effect. In contrast, Erb mRNA levels was lower in the OVA groups than in the PBS groups (Fig. 5B). Furthermore, exposure to OVA with BPA-M or BPA-H significantly reduced Erb mRNA compared with OVA exposure alone (P < 0.05; OVA+BPA-H, P < 0.01; OVA+BPA-M). The expression of Ar was lower in OVA + BPA-H-treated mice than in OVA-treated mice (P < 0.05, Fig. 5C). There was no significant change in GR, AhR, and estrogen receptor-related gamma levels in all groups (data not shown).
Fig. 5.
Hormone receptor mRNA levels in the lung. Gene expression in the lung was examined 48 h after the last intratracheal administration by RT-PCR analysis. (A) Era. (B) Erb. (C) Ar. Data were expressed as mean ± SE for 3–7 animals per group. The relative intensity was normalized to Hprt1. ** P < 0.01 versus vehicle group. * P < 0.05 versus vehicle group, ** P < 0.01 versus vehicle group, # P < 0.05 versus OVA group, ## P < 0.01 versus OVA group.
3.7. BPA activates mediastinal lymph node microenvironment
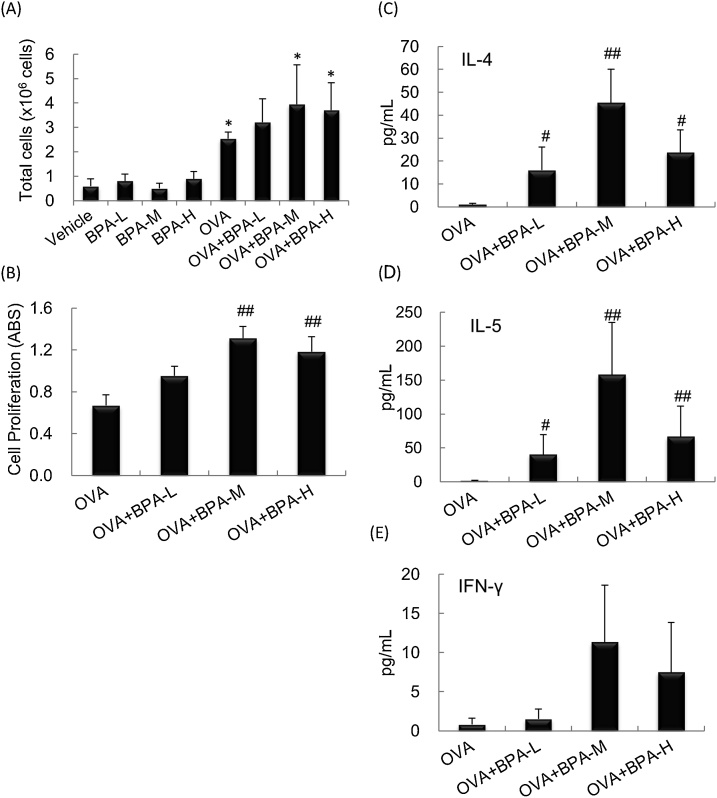
To evaluate whether BPA exposure enhance OVA-induced activation of MLN cells, total cell numbers and cell proliferation and cytokine secretion of OVA-restimulated MLN cells 48 h after the last intratracheal instillation were examined. Total number of MLN cells was significantly increased in the OVA-treated mice compared with the PBS-treated mice (P < 0.01, Fig. 6A). Next, cell proliferation and cytokine levels in the culture supernatant after OVA restimulation for 67 h in OVA-administered mice were analyzed. The OVA + BPA-M and OVA + BPA-H groups showed significantly enhanced cell proliferation compared to the OVA group (P < 0.01, Fig. 6B). The protein levels of IL-4 and IL-5 were higher in the OVA + BPA groups than in the OVA group, which was more prominent in the OVA + BPA-M group (P < 0.01, Fig. 6C and 6D). IFN-γ and SDF-1α (data not shown) levels showed a similar trend, but changes were not statistically significant.
Fig. 6.
Changes in cell number and activation in MLN cells. MLN cells were prepared 48 h after the final OVA intratracheal administration and the total cell number was counted. Cell proliferation and cytokine expression in culture supernatants were examined after 67 h of culture in the presence of OVA. (A) Total cell number. (B) Cell proliferation (Abs). (C) IL-4. (D) IL-5. (E) IFN-γ. Data were expressed as mean ± SEM of 6 animals per group. *; P < 0.05 versus Vehicle group, # P < 0.05 versus OVA group, ## P < 0.01 versus OVA group.
3.8. BPA activates bone marrow microenvironment
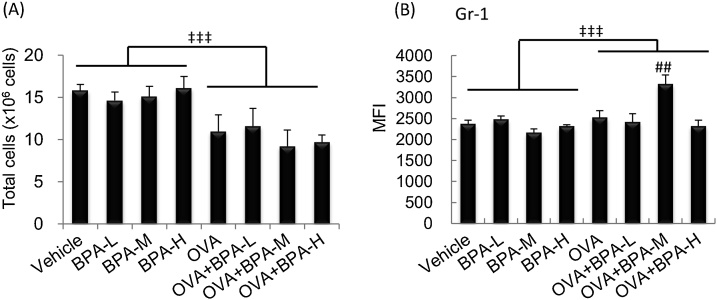
Next, the activation of bone marrow (BM) cells following exposure to BPA with allergen was analyzed. OVA treatment significantly decreased the total number of cells compared to PBS treatment. There was a tendency to decrease in the OVA + BPA-M or OVA + BPA-H groups compared to the OVA group (Fig. 7A). Gr-1 expression, which directly correlates with granulocyte differentiation and maturation, was significantly elevated in the OVA + BPA-M group compared to the OVA group (Fig. 7B).
Fig. 7.
Changes in cell number and Gr-1 expression in BM cells. BM cells were prepared 48 h after the final intratracheal administration and the total cell number was counted. Cell surface molecule expression was determined by FACS analysis. (A) Total cell number. (B) MFI of Gr-1+ cells. Data were expressed as mean ± SE for 6 animals per group. ## P < 0.01 versus OVA group, ‡‡‡ P < 0.001 OVA groups versus PBS groups.
4. Discussion
We showed that oral exposure to low dose BPA (equivalent to a dose of 0.09 μg/kg/day) promotes airway hyperresponsiveness and MLN activation in OVA-sensitized mice. In addition, higher doses of BPA (0.9 and 9 μg/kg/day) significantly enhanced allergic pulmonary inflammation, airway hyperresponsiveness, Th2-polarized immune responses, serum OVA-specific Ig production, and hormone receptor downregulation. These results were accompanied with enhanced cell proliferation and T helper 2 (Th2)-cytokine production in OVA-restimulated MLN cells. In bone marrow, there was a decrease in cell number and an increase in Gr expression in the OVA + BPA-M group. In contrast, these changes were not observed with BPA exposure alone. Previous reports have been limited to the effects of high doses BPA, however, this is the first report showing that oral exposure to low dose BPA, equivalent to actual human oral exposure levels, enhances allergic responses.
Several studies have suggested that prenatal and/or perinatal exposure to BPA have slight or remarkable effects on allergic asthma in offspring [[28], [29], [30]]. O'Brien et al. also reported that maternal exposure to BPA aggravates allergic responses in female but not male pups [29]. In contrast, Petzold et al. showed that exposure to BPA from infancy to adulthood aggravates allergic airway inflammation in pups, although prenatal and perinatal exposure had no effects [31]. In addition, BPA exposure in adult mice attenuated allergic immune responses. We also found that intratracheal exposure to low doses BPA (equivalent to 0.0015 μg/kg/day; 5 times that of the predicted maximum exposure dose from the general atmosphere in Japan of 0.0003 μg/kg/day) aggravates allergic airway inflammation during the juvenile period of development in mice [23]. Furthermore, even lower doses of BPA (0.000075 μg/kg/day) significantly enhanced immunocompetent cell function. These results suggest that direct (intratracheal) exposure to BPA may be more effective at lower doses than indirect (oral) exposure on allergic asthma. These inconsistent results may be due to differences in exposure concentrations, exposure periods, exposure route, gender, and strain. Although the reasons underlying this discrepancy is still unclear, BPA may disrupt immune responses through binding to hormone receptors. We found that exposure to moderate or high doses of BPA caused a decrease in ER β and AR mRNA levels in allergic asthmatic lungs. BPA is known to affect the immune system as well as the endocrine systems via binding to various receptors, such as ERs and AR. In particular, BPA is a well-known ER modulator. ERs are expressed in immunocompetent cells such as T- and B-lymphocytes and macrophages. Salem reviewed that ER could induce anti-inflammatory cytokines and inhibit APC activation, T cell proliferation, and cytokine production [32]. Our recent study showed that intratracheal exposure to BPA increases the gene expression of ERβ in the lungs, resulting in the attenuation of allergic airway inflammation in mice [23]. BPA can also act as an AR antagonist [33]. Cephus et al. reported that androgen reduced Th2-related allergic responses such as allergic inflammation and type 2 innate immune responses through AR [34]. AR is expressed in non-reproductive tissues as well as reproductive tissues. In the murine lung, AR is mainly expressed in the bronchial epithelium and type II pneumocytes and its expression is increased by androgen administration [35]. Furthermore, AR deficiency in monocytes/macrophages attenuated allergic pulmonary inflammation in mice [36]. Taken together, BPA may contribute to aggravate allergic pulmonary inflammation via the downregulation of hormone receptors such as ER β and AR in the lung.
Allergic asthma has been considered to be a Th2 cell–mediated immune response and is characterized by airway inflammation with pulmonary eosinophilia, airway hyperreactivity, and increased serum allergen-specific immunoglobulin levels [37]. Th2 cytokines and chemokines play a crucial role in allergic diseases [38]. IL-5 is an important regulator of eosinophil growth, differentiation, and activation, and is essential for eosinophil migration [39,40]. IL-13 has several actions similar to those of IL-4 and induces IgE secretion and mucus hypersecretion and goblet cell hyperplasia [41]. Mucus production and hypersecretion are important pathophysiological features of asthma. MUC5AC is a major gel-forming respiratory mucin and is overexpressed in asthmatic subjects. IL-33, a member of the IL-1 cytokine family, promotes secretion of Th2 cytokines such as IL-5 and IL-13 as well as IgE secretion [42,43]. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, and inflamed tissues.
Members of the C—C branch of the chemokine family such as MCP-1, eotaxin, MIP-1α, and RANTES play a key role in the recruitment of inflammatory cells to the lung, resulting in allergic inflammation, and are expressed in lung tissue and bronchial lavage fluid of asthmatic subjects [44,45]. Eotaxin, MIP-1α, and RANTES are chemotactic for lymphocytes, eosinophils, and monocytes [46,47], while the targets of MCP-1 are limited to monocytes/macrophages, lymphocytes [48,49], and basophils [50]. We showed that a moderate dose of BPA elevates the gene expression of IL-5, IL-13, IL-33, and eotaxin in OVA-treated lungs. MCP-1 and MIP-1α levels were greater in the OVA + BPA-H group than in the Vehicle group. In contrast, no significant changes were observed in Th1 cytokines such as IFN-γ and IL-12. Previous reports and the present findings suggest that oral exposure to BPA may enhance Th2-polarization resulting in aggravated allergic pulmonary inflammation.
Next, we examined MLN activation. BPA exposure slightly increased total cell number and enhances APC activation (the percentage of MHC class II + CD86+ cells and mean fluorescence intensity (MFI) of MHC class II + cells) in allergic asthmatic mice, although there was no statistically significant difference (data not shown). Co-exposure to BPA with OVA increased OVA-restimulated cell proliferation and Th2 cytokine production in MLN cells. Our previous study showed that exposure to intratracheal exposure to BPA increases MHC class II and CD86 expression in MLN cells in OVA-sensitized mice [23]. BPA exposure may enhance cell proliferation and cytokine expression after allergen restimulation and result in the subsequent migration of immune cells to the lung, resulting in aggravated allergic pulmonary inflammation.
To further elucidate the underlying mechanism by which oral exposure to BPA exacerbates allergic responses, we focused on changes in the bone marrow microenvironment. All immune cells originate from hematopoietic stem cells located in the bone marrow in the adult animal. Hematopoietic stem and progenitor cells contribute to allergic inflammation. Allergen-induced proinflammatory cytokines can impact the differentiation of hematopoietic progenitor cells resulting in increased production of effector cells such as eosinophils and basophils. In this study, OVA + BPA-M significantly enhanced Gr-1 expression in BM cells. Gr-1 in bone marrow is related to granulocyte differentiation and maturation. Hematopoietic stem and progenitor cells leave the bone marrow and are recruited to the inflamed site via the activation of stromal cell derived factor 1 alpha (SDF-1α) [51]. SDF-1, a major leukocyte chemoattractant factor, is decreased in bone marrow and increased in the airway mucosa of asthmatic subjects [52]. Our data showed that SDF-1α was slightly decreased in BM lavage fluid and increased in OVA-restimulated MLN cell culture supernatant in the OVA + BPA-M group (data not shown). In addition, the proportion of granulocyte/monocyte subsets was higher in the OVA + BPA-M group than in the OVA group (44 % vs. 36 %), and this was associated with an increase in cell size and intracellular granule formation/density (data not shown). These findings suggest that oral exposure to BPA together with an allergen may partly modulate the bone marrow microenvironment, which consequently affects the proliferation and recruitment of immune cells to local inflammatory sites.
5. Conclusions
The current study showed that oral exposure to low doses of BPA that were equivalent to actual human exposure levels can aggravate allergic asthmatic responses through the enhancement of Th2-skewed responses in the lung and changes in the lymph node and bone marrow microenvironments. Furthermore, these adverse effects may be due to the disruption of lung hormone receptor expression.
Declaration of Competing Interest
All authors have no competing financial interests.
Funding
This work was supported in part by a grant from the Research project on health impacts of chemicals on children and future generations (National Institute for Environmental Studies, Japan: 1620AA041) and by JSPS KAKENHI Grant-in-Aid for Scientific Research (S) (Grant Number JP16H06308).
Acknowledgements
The authors thank Satomi Abe for technical assistance. The authors would like to thank Enago (http://www.enago.jp) and GeniusPlus (https://genius.jp.net/) for the English language review.
References
- 1.Konieczna A., Rutkowska A., Rachon D. Health risk of exposure to Bisphenol A (BPA) Rocz. Panstw. Zakl. Hig. 2015;66(1):5–11. [PubMed] [Google Scholar]
- 2.Tzatzarakis M.N., Karzi V., Vakonaki E., Goumenou M., Kavvalakis M., Stivaktakis P., Tsitsimpikou C., Tsakiris I., Rizos A.K., Tsatsakis A.M. Bisphenol A in soft drinks and canned foods and data evaluation. Food Addit. Contam. Part B Surveill. 2017;10(2):85–90. doi: 10.1080/19393210.2016.1266522. [DOI] [PubMed] [Google Scholar]
- 3.Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Kang J.H., Kondo F., Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226(2-3):79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Calafat A.M., Kuklenyik Z., Reidy J.A., Caudill S.P., Ekong J., Needham L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005;113(4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geens T., Aerts D., Berthot C., Bourguignon J.P., Goeyens L., Lecomte P., Maghuin-Rogister G., Pironnet A.M., Pussemier L., Scippo M.L., Van Loco J., Covaci A. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50(10):3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food, Drug Administration . 2008. Draft Assessment of Bisphenol A for Use in Food Contact Applications.http://www.fda.gov/about-fda/website-policies/website-disclaimer (Accessed 11 July 2019) [Google Scholar]
- 8.2004. Ministry of the Environment of Japan, Bisphenol A.http://www.env.go.jp/chemi/report/h16-01/pdf/chap01/02_2_15.pdf (in Japanese) (Accessed 22 April 2019) [Google Scholar]
- 9.Liao S.L., Tsai M.H., Lai S.H., Yao T.C., Hua M.C., Yeh K.W., Chiang C.H., Huang S.Y., Huang J.L. Prenatal exposure to bisphenol-A is associated with Toll-like receptor-induced cytokine suppression in neonates. Pediatr. Res. 2016;79(3):438–444. doi: 10.1038/pr.2015.234. [DOI] [PubMed] [Google Scholar]
- 10.Rogers J.A., Metz L., Yong V.W. Review: Endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013;53(4):421–430. doi: 10.1016/j.molimm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Patel B.B., Di Iorio M., Chalifour L.E. Metabolic response to chronic bisphenol A exposure in C57bl/6n mice. Toxicol. Rep. 2014;1:522–532. doi: 10.1016/j.toxrep.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moustafa G.G., Ahmed A.A.M. Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: genotoxic and immunohistochemical implications. Toxicol. Rep. 2016;3:685–695. doi: 10.1016/j.toxrep.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y., Liu H., Wu J., Yuan L., Wang Y., Du X., Wang R., Marwa P.W., Petlulu P., Chen X., Zhang H. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019;176 doi: 10.1016/j.envres.2019.108575. [DOI] [PubMed] [Google Scholar]
- 14.Jalal N., Surendranath A.R., Pathak J.L., Yu S., Chung C.Y. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 2018;5:76–84. doi: 10.1016/j.toxrep.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K.S., Chen H.Q., Chen Y.S., Qiu K.F., Zheng X.B., Li G.C., Yang H.D., Wen C.J. Bisphenol A stimulates human lung cancer cell migration via upregulation of matrix metalloproteinases by GPER/EGFR/ERK1/2 signal pathway. Biomed. Pharmacother. 2014;68(8):1037–1043. doi: 10.1016/j.biopha.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Tse L.A., Lee P.M.Y., Ho W.M., Lam A.T., Lee M.K., Ng S.S.M., He Y., Leung K.S., Hartle J.C., Hu H., Kan H., Wang F., Ng C.F. Bisphenol A and other environmental risk factors for prostate cancer in Hong Kong. Environ. Int. 2017;107:1–7. doi: 10.1016/j.envint.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Leung Y.K., Govindarajah V., Cheong A., Veevers J., Song D., Gear R., Zhu X., Ying J., Kendler A., Medvedovic M., Belcher S., Ho S.M. Gestational high-fat diet and bisphenol A exposure heightens mammary cancer risk. Endocr. Relat. Cancer. 2017;24(7):365–378. doi: 10.1530/ERC-17-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckley J.P., Quiros-Alcala L., Teitelbaum S.L., Calafat A.M., Wolff M.S., Engel S.M. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7years. Environ. Int. 2018;115:79–88. doi: 10.1016/j.envint.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim E.H., Jeon B.H., Kim J., Kim Y.M., Han Y., Ahn K., Cheong H.K. Exposure to phthalates and bisphenol A are associated with atopic dermatitis symptoms in children: a time-series analysis. Environ. Health. 2017;16(1):24. doi: 10.1186/s12940-017-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer S.M., Roy A., Emo J., Chapman T.J., Georas S.N., Lawrence B.P. The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood. Toxicol. Sci. 2012;130(1):82–93. doi: 10.1093/toxsci/kfs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He M., Ichinose T., Yoshida S., Takano H., Nishikawa M., Shibamoto T., Sun G. Exposure to bisphenol A enhanced lung eosinophilia in adult male mice. Allergy Asthma Clin. Immunol. 2016;12:16. doi: 10.1186/s13223-016-0122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajiki-Nishino R., Makino E., Watanabe Y., Tajima H., Ishimota M., Fukuyama T. Oral administration of bisphenol a directly exacerbates allergic airway inflammation but not allergic skin inflammation in mice. Toxicol. Sci. 2018;165(2):314–321. doi: 10.1093/toxsci/kfy132. [DOI] [PubMed] [Google Scholar]
- 23.Koike E., Yanagisawa R., Win-Shwe T.T., Takano H. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice. Int. J. Immunopathol. Pharmacol. 2018;32 doi: 10.1177/2058738418774897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieli P.T., Jasarevic E., Warzak D.A., Mao J., Ellersieck M.R., Liao C., Kannan K., Collet S.H., Toutain P.L., Vom Saal F.S., Rosenfeld C.S. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ. Health Perspect. 2011;119(9):1260–1265. doi: 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koike E., Inoue K., Yanagisawa R., Takano H. Di-(2-ethylhexyl) phthalate affects immune cells from atopic prone mice in vitro. Toxicology. 2009;259(1-2):54–60. doi: 10.1016/j.tox.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Koike E., Yanagisawa R., Sadakane K., Inoue K., Ichinose T., Takano H. Effects of diisononyl phthalate on atopic dermatitis in vivo and immunologic responses in vitro. Environ. Health Perspect. 2010;118(4):472–478. doi: 10.1289/ehp.0901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J., Huang G., Guo T.L. Developmental bisphenol a exposure modulates immune-related diseases. Toxics. 2016;4(4) doi: 10.3390/toxics4040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midoro-Horiuti T., Tiwari R., Watson C.S., Goldblum R.M. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ. Health Perspect. 2010;118(2):273–277. doi: 10.1289/ehp.0901259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien E., Bergin I.L., Dolinoy D.C., Zaslona Z., Little R.J., Tao Y., Peters-Golden M., Mancuso P. Perinatal bisphenol A exposure beginning before gestation enhances allergen sensitization, but not pulmonary inflammation, in adult mice. J. Dev. Orig. Health Dis. 2014;5(2):121–131. doi: 10.1017/S204017441400004X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima Y., Goldblum R.M., Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ. Health. 2012;11:8. doi: 10.1186/1476-069X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petzold S., Averbeck M., Simon J.C., Lehmann I., Polte T. Lifetime-dependent effects of bisphenol A on asthma development in an experimental mouse model. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salem M.L. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr. Drug Targets Inflamm. Allergy. 2004;3(1):97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Ding Z., Shi Q.M., Ge X., Wang H.X., Li M.X., Chen G., Wang Q., Ju Q., Zhang J.P., Zhang M.R., Xu L.C. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology. 2017;387:10–16. doi: 10.1016/j.tox.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Cephus J.Y., Stier M.T., Fuseini H., Yung J.A., Toki S., Bloodworth M.H., Zhou W., Goleniewska K., Zhang J., Garon S.L., Hamilton R.G., Poloshukin V.V., Boyd K.L., Peebles R.S., Jr., Newcomb D.C. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21(9):2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.P., Janne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010;317(1-2):14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Bielecki B., Mattern C., Ghoumari A.M., Javaid S., Smietanka K., Abi Ghanem C., Mhaouty-Kodja S., Ghandour M.S., Baulieu E.E., Franklin R.J., Schumacher M., Traiffort E. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc. Natl. Acad. Sci. U. S. A. 2016;113(51):14829–14834. doi: 10.1073/pnas.1614826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umetsu D.T., McIntire J.J., Akbari O., Macaubas C., DeKruyff R.H. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 2002;3(8):715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 38.Galli S.J., Tsai M., Piliponsky A.M. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clutterbuck E.J., Sanderson C.J. Human eosinophil hematopoiesis studied in vitro by means of murine eosinophil differentiation factor (IL5): production of functionally active eosinophils from normal human bone marrow. Blood. 1988;71(3):646–651. [PubMed] [Google Scholar]
- 40.Faccioli L.H., Mokwa V.F., Silva C.L., Rocha G.M., Araujo J.I., Nahori M.A., Vargaftig B.B. IL-5 drives eosinophils from bone marrow to blood and tissues in a guinea-pig model of visceral larva migrans syndrome. Med. Inflamm. 1996;5(1):24–31. doi: 10.1155/S096293519600004X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingram J.L., Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol. 2012;130(4):829–842. doi: 10.1016/j.jaci.2012.06.034. quiz 843-4. [DOI] [PubMed] [Google Scholar]
- 42.Liew F.Y., Pitman N.I., McInnes I.B. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 43.Oboki K., Ohno T., Kajiwara N., Saito H., Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol. Int. 2010;59(2):143–160. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 44.Teran L.M., Noso N., Carroll M., Davies D.E., Holgate S., Schroder J.M. Eosinophil recruitment following allergen challenge is associated with the release of the chemokine RANTES into asthmatic airways. J. Immunol. 1996;157(4):1806–1812. [PubMed] [Google Scholar]
- 45.Alam R., York J., Boyars M., Stafford S., Grant J.A., Lee J., Forsythe P., Sim T., Ida N. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am. J. Respir. Crit. Care Med. 1996;153(4 Pt 1):1398–1404. doi: 10.1164/ajrccm.153.4.8616572. [DOI] [PubMed] [Google Scholar]
- 46.Kuna P., Reddigari S.R., Schall T.J., Rucinski D., Sadick M., Kaplan A.P. Characterization of the human basophil response to cytokines, growth factors, and histamine releasing factors of the intercrine/chemokine family. J. Immunol. 1993;150(5):1932–1943. [PubMed] [Google Scholar]
- 47.Carr M.W., Roth S.J., Luther E., Rose S.S., Springer T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. U. S. A. 1994;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushima K., Larsen C.G., DuBois G.C., Oppenheim J.J. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J. Exp. Med. 1989;169(4):1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kameyoshi Y., Dorschner A., Mallet A.I., Christophers E., Schroder J.M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J. Exp. Med. 1992;176(2):587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schall T.J., Bacon K., Toy K.J., Goeddel D.V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 51.Fischer K.D., Agrawal D.K. Hematopoietic stem and progenitor cells in inflammation and allergy. Front. Immunol. 2013;4:428. doi: 10.3389/fimmu.2013.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoshino M., Aoike N., Takahashi M., Nakamura Y., Nakagawa T. Increased immunoreactivity of stromal cell-derived factor-1 and angiogenesis in asthma. Eur. Respir. J. 2003;21(5):804–809. doi: 10.1183/09031936.03.00082002. [DOI] [PubMed] [Google Scholar]