Abstract
Introduction:
Percutaneous collagen induction (PCI) or needling techniques are increasingly popular in the reconstructive and aesthetic arena. The underlying mechanisms of action rest on producing a pattern of non-ablative and non-confluent puncture wound pattern to the dermis with a resulting regenerative effect to the skin.
Methods:
A detailed English literature review was conducted using PubMed Medline, Embase and Web of Science; the manuscripts were appraised and classified according to level of evidence as well risk of bias. Results are presented in descending order of evidence for non-atrophic scars.
Discussion:
On the basis of level 1 evidence currently available, the combination of needling and silicone gel can improve the short-term pliability, height and vascularity of hypertrophic and keloid scars. According to level 2 evidence, needling alongside spray keratinocytes can produce a statistically significant improvement to patient/observer scar ratings and improve pigmentation in hypopigmented burn scars at 12-month follow-up. Results from mixed cohort studies also point towards needling having a beneficial effect on fat graft retention. Level 3 data suggest that needling can render significant resurfacing effects to both mature and actively hypertrophic burn scars at 12-month follow-up based on objective scar scales; furthermore, favourable histological changes are seen, including better collagen alignment in the dermis and increased epidermal thickness.
Conclusion:
Needling techniques are promising adjuncts to non-atrophic scar management. Further research with long-term follow-up and comparative design protocols incorporating other resurfacing modalities is warranted before the exact value of needling is delineated in scar management protocols.
Keywords: Percutaneous, collagen, needling, scar, hypertrophic, keloid, microneedling
Lay Summary


Needling techniques are increasingly popular and involve the use of a device to produce numerous tiny perforations in the skin, stimulate collagen production and resurface the treated area. We undertook this study to find out whether the use of needling can have a beneficial effect on scars that are raised (hypertrophic and keloidal). We conclude that, at present, there is some evidence that needling in combination with silicone gel can improve the appearance of bulky scars in the short term; additionally needling can enhance the appearance of discoloured burn scars if used in combination with spray skin cell preparations and improve the take of fat transferred to the treated area. There are also a number of studies that have confirmed the beneficial effects of needling in the architecture of treated skin, which include improved skin structure and increased collagen production. Further research is eagerly awaited to determine the exact position of needling techniques in scar management protocols.
Introduction
Microneedling (MN) or percutaneous collagen induction (PCI) is an increasingly popular resurfacing procedure. A range of devices are available commercially containing fine needles of variable millimetre lengths with a view to creating a non-ablative and non-confluent puncture wound pattern in the skin.1 The philosophy of causing controlled dermal damage in order to stimulate regeneration of scarred skin has been entertained since 1995 by Orentreich et al,2 when ‘subcision’ was described. The authors demonstrated that depressed scars can be elevated by virtue of a needle inserted and manoeuvred below the scar. In 1997, a technique similar to tattooing but without pigment was introduced, based on a similar underlying theoretical basis.3 Further along the timeline, the technique of PCI was popularised with the use of a 200 drum-shaped needling device, offering a more practical and faster needling modality for large scars.4
The basic principle of needling is the repetitive application of the device on the stretched scar in multiple directions (horizontally, vertically and obliquely) until uniform pinpoint bleeding is reached as an endpoint; the procedure is then repeated as necessary at variable intervals.5,6
MN can be delivered using a range of devices, which can be divided into: manual, motorised as well as radiofrequency coupled. Manual devices include rotary drums as well as static needling devices; the latter allow treating smaller more localised scars.7–10 Motorised devices consist of a powered handpiece and a disposable needle cartridge unit with treatments being delivered by moving the motorised device over the skin in multiple directions.11 Radiofrequency needling equipment works by creating radiofrequency thermal zones, hence imparting combined mechanical and thermal stimuli to the dermis.12
A number of animal and human studies have stressed the importance of skin preparation in order to maximise the results of needling treatments using daily topical vitamin A and C for a period between 3 weeks and 3 months.5,13,14 Vitamin A controls 350–1000 genes responsible for cell proliferation/differentiation, angiogenesis as well as neocollagenesis; vitamin C is similarly important in collagen synthesis and both vitamins act in concordance with a number of growth factors involved in the healing processes inherent to needling action including fibroblast growth factor, platelet-derived growth factor and transforming growth factor (TGF) β.15
The effectiveness of PCI is based on the stimulation of a controlled inflammatory/healing reaction and the remodelling of collagen by virtue of growth factor release (such as vascular endothelial [VEGF]/epidermal/ fibroblast and platelet-derived growth factor, as well as TGF). The latter family of factors has been implicated intensely in the regenerative mechanisms of needling. Specifically, rat animal data suggest a preferential stimulation of the anti-fibrogenic TGF-β3 isoform production (as opposed to the pro-fibrogenic β1 and 2). This preferential stimulation appears to be critical for the regenerative effect of needling and support the formation of a physiological lattice pattern of collagen fibres found in normal skin instead of parallel pattern found in scars.16–19 TGF-β1 and β2 tend to show upregulation after two weeks but only faint expression remains after four weeks, whereas β3 remains upregulated past eight weeks following treatment.13 In addition, VEGF is upregulated after needling and appears to play an important role in angiogenesis as well as keratinocyte function.20 Interestingly, needling modalities have been proposed as techniques able to regulate abnormal pigmentation by altering the secretion pattern of melanocyte-stimulating hormone (MSH) and interleukin (IL)-10. IL-10 is an anti-inflammatory cytokine, which is upregulated at two weeks post-procedurally and becomes undetectable after 4–8 weeks. MSH appears to be downregulated two weeks after needling treatment; this is thought to relate to the action of IL-10.21
Based on animal studies, the beneficial effects of needling on the skin include:
(1) Increase in epidermal thickness by 112% over eight weeks; histological findings include denser and more compact epidermis with more cellular layers and fewer gaps in the stratum corneum;19,20
(2) Upregulation of type 1 collagen expression resulting in a denser network of thicker collagen fibre strands;19,20
(3) Increased expression of stromal glycosaminoglycans within the dermis. All the above three effects in the treated skin have been shown to be enhanced by the eight-week addition of skin preparation with vitamins A and D;20
(4) Increased fibronectin expression, which is important in collagen alignment during healing processes and a marker of fibroblast differentiation.20
PCI is a non-ablative technique owing its success on results that are similar to other invasive and non-invasive modalities such as surgical excision and CO2 laser, but without the adverse side effect of hyperpigmentation,18 which is associated with epidermal ablation. Further advantages of PCI include its versatility and short downtime.5
Needling has been appraised extensively in the atrophic acne scar literature with eight existing randomised controlled trials comparing needling to a number of adjuncts including fractional laser, platelet-rich plasma (PRP) and TCA-CROSS techniques. The summative conclusions from these studies is that needling can produce comparable clinical results to other mainstream adjuncts with explicit advantages relating to the lower risk of side effects.22
Nevertheless, the majority of publications in the scar literature focus on the use of PCI in the context of atrophic scars with no currently available summative reports appraising the pertinent evidence relevant to non-atrophic scars.
Materials and methods
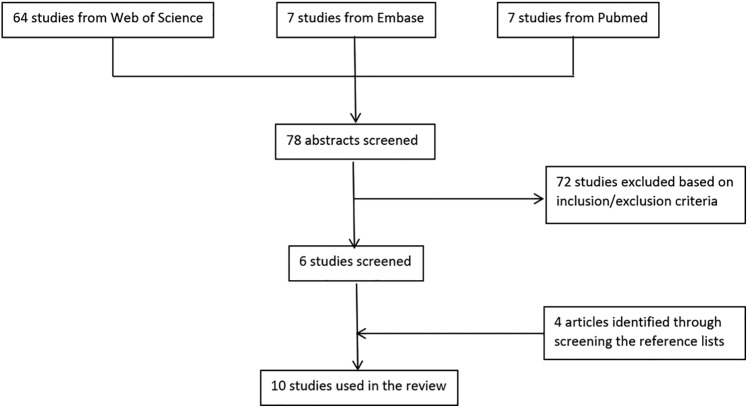
A thorough literature search was conducted using Web of Science, Embase and PubMed Medline databases and the following MESH terms: ‘microneedling’ OR ‘percutaneous collagen induction’ AND ‘scar’ from their individual dates of inception to the present time. Inclusion criteria for the study comprised interventional studies assessing MN effectiveness on human patients with hypertrophic, surgical, keloid, traumatic or burns scars in the English literature. We excluded animal studies and editorials; nevertheless, there were no restrictions on ethnicity, gender, age or severity of scar. A small number of studies had mixed cohorts including some atrophic scars and we decided to include these studies in our work. The study selection was divided into three stages (Figure 1) and the final set of manuscripts were critically appraised by an independent consultant in evidence synthesis for level of evidence (according to the Joanna Briggs Institute Levels of evidence framework) as well as risk of bias (employing the RoB V2.0 and ROBINS-I tools for individually randomised, parallel-group trials and non-randomised studies, respectively).
Figure 1.
Flow chart showing study selection.
Results
Our literature search identified 78 citations relevant to our search terms. Out of these, 72 were excluded based on the inclusion criteria. Of the remaining studies, a thorough search of the reference list identified four more relevant studies making a total number of 10 included studies in our review (Figure 1).
Level 1
Fabbrocini et al. carried out a randomised controlled trial involving 20 patients with previously treated relatively young (age < 5 years) hypertrophic and keloid scars.23 The authors defined both hypertrophic and keloid scars as raised above the skin level, with keloid scars extending beyond the borders of the initial injury. Patients were randomly allocated to groups A or B so that each group had six patients with keloid and four patients with hypertrophic scars. Scars of patients in group A were treated with MN of the total area and application of silicone gel to just half the scar. In group B, scars were treated with topical silicone gel on the total area and MN to one half.
The MN device used was the Dermapen (Dermapen, Salt Lake City, UT, USA) with 1.5–2.5-mm needle lengths (adjusted to scar thickness) and each patient had three treatments at monthly intervals; silicone gel was applied once daily for three months. The authors assessed scar improvement based on photographs taken at baseline and at monthly intervals for the first three months during treatment, and at three-month follow-up after the last treatment session. In addition, ultrasound and the modified Vancouver Scar Scale (VSS) was used to rate improvement three months after the end of treatment compared to baseline.
Overall, the combination of MN and silicone gel rendered a more clinically significant improvement in scar appearance compared to either MN or silicone gel alone (group A: 68% improvement with combined therapy vs. 52% improvement with MN alone [P < 0.01]; group B: 63% improvement with combined therapy vs. 47% improvement with gel alone [P < 0.01]).
VSS results revealed a number of interesting findings. In groups A and B, the part of the scar treated with the combination of needling and silicone gel showed a statistically significant improvement compared to the single modalities in each group (P < 0.01) In terms of vascularity, only group A showed a statistically significant improvement in erythema and pliability at three-month follow-up (1.42 ± 0.55 reduced to 1.12 ± 0.33, P = 0.025 for erythema; 2.87 ± 0.33 reduced to 1.86 ± 0.35, P < 0.001 for pliability). Scar height reduction results also showed the superiority of needling in group A (1.30 ± 0.55 reduced to 1.00 ± 0.29) versus group B (1.38 ± 0.41 reduced to 1.25 ± 0.44); the difference between the two groups was significant (P < 0.05). Ultrasound assessment showed a 60% decrease in keloid thickness in both groups; interestingly, a non-significant improvement in thickness of hypertrophic scars was reported as well. The results regarding pigmentation are difficult to interpret but point towards a reduced index in both groups.
Level 2
Busch et al. carried out an experimental study involving 20 patients, presenting with at least 12 month old hypopigmented scars (from second- and third-degree burns) with an average surface area of 94 cm2 (range = 15–250 cm2).24 The scars had healed with secondary intention and were due to various underlying injury mechanisms including scald, flame, acid accidents, chemical peeling and laser treatment. The treatments were performed as part of a within-subject comparison under general anaesthesia using the following three different modalities: (1) 3-mm MN combined with non-cultured autologous skin cell suspension (NCASCS) using the ReNovaCell (Avita Medical) applied immediately after needling; (2) MN alone (positive control); and (2) no treatment (negative control). Follow-up assessment was conducted using the POSAS tool for the areas treated with needling and keratinocytes at the last visit (6–15 months) and with the Mexameter (Courage + Khazaka electronic GmbH) for melanin quantification at baseline and 12 months after treatment. In terms of patient ratings for the areas treated with needling and NCASCS, these were found to improve by 50% (from 8.0 ± 2.1 SD to 4.0 ± 2.5 SD, P < 0.05) and a similar pattern of improvement by 57.1% (from 7.0 ± 2.6 SD to 3.0 ± 2.3, P < 0.05) was seen in their overall opinion of the scar. Observer ratings showed an improvement of 37.5% (8.0 ± 1.4 SD to 5.0 ± 2.1 SD) for pigmentation and 38.5% (6.5 ± 1.7 SD to 4.0 ± 1.5 SD) for the overall rating (both P < 0.05). Results showed improvement of pigmentation in 17/20 patients. Specifically, the combined group of MN and NCASCS had a median pigmentation change of 29.3% (125.3 ± 31.9 to 162.0 ± 48.2 at follow-up, P < 0.05). In comparison, the MN-only group had an 8.4% decrease in pigmentation (149.5 ± 100.7 at baseline vs. 137.0 ± 77.0 at follow-up), which was not statistically significant. The no treatment group also had a non-significant change in pigmentation of 8.9% (186.0 ± 59.6 at baseline vs. 169.5 ± 90.9 at follow-up).
Another group of 40 patients presenting for facial rejuvenation, asymmetry correction as well as revision of scars from burns and trauma were involved in a quasi-experimental controlled study by Sezgin and Özmen.25 The cohort was divided in two treatment groups: (1) 22 patients in the first group had 1.5-mm needling performed one week before fat transfer under local anaesthetic to pinpoint bleeding and repeated just before the fat grafting procedure; and (2) 18 patients (control group) who had fat grafting alone. In both groups, the fat was harvested with a 3-mm round-tip cannula attached to a 10-mL syringe from the lower abdomen, centrifuged at 3000 rpm for 3 min and injected via 0.9-mm cannula. Patients were followed up at week 1, month 1 and three months postoperatively. Both patients and an independent surgeon scored facial volume and skin quality improvement using the modified Global Aesthetic Improvement Scale (GAIS) and were determined via comparison to baseline photographs. Results suggest that the improvement of facial skin quality was superior in the needling group compared to the fat graft only group in a statistically significant manner for both the aesthetic and reconstructive subgroups (2.27 ± 0.40 vs. 1.33 ± 0.59, P < 0.05). Interestingly, the degree of improvement was significantly higher in the aesthetic indication compared to the reconstructive subgroup undergoing needling and fat grafting (P < 0.05). In terms of volume improvement scores, the pattern was similar with a statistically significant result in favour of the needling and fat graft group for both aesthetic and reconstructive patients (2.32 ± 0.39 vs. 1.56 ± 0.54, P < 0.05) with the aesthetic subgroup gaining a more statistically significant result (P < 0.05). This pattern has been attributed to a greater fibrosis in the reconstructive group, which possibly interferes with the penetrative effect of needling as well as a lower baseline skin quality. A total of 90.9% patients reported they would undergo the procedure in the future if they required it.
Sasaki et al. undertook an experimental study in a cohort of patients with a variety of skin conditions and used different protocols in the two participating facilities recruiting patients.26 Needling was performed using a motorised pen device at depths of 0.25–1 mm (rhytides/skin laxity/acne scars) and 0.5–2.5 mm with PRP (for more severe rhytides/skin laxity and hypertrophic/acne scars and alopecia). Patients’ skin was prepared for at least three months with a topical multivitamin preparation. Results were assessed based on photographic assessment at baseline and 12 months after treatment by patients as well as three independent observers using a visual analogue scale (VAS; 0 = absolutely dissatisfied; 10 = completely satisfied). The study was undertaken in two different locations offering a different treatment protocol; the skin care centre cohort patients (group 1) received an average of eight sessions per year and 53 patients were treated for scarring with an average depth of needle penetration of 0.75 mm (range = 0.25–1.0 mm). At an average follow-up of nine months (range = 6–36 months) after treatment, group 1 POSAS scores for hypertrophic scars improved from 3.2 ± 1.7 to 6.4 ± 1.3 (patient scale) and 4.5 ± 0.1 to 7.0 ± 0.0 (observer scale). The surgical centre cohort (group 2) included 18 patients with scars receiving an average of 1.5 sessions per year at an average depth of 1.25 mm (range = 0.25–2.5 mm) and an average PRP volume of 5 cc (range = 1.5–11.0 cc). POSAS scores at an average follow-up time of 17 months (range = 6–36 months) for hypertrophic scars improved from 6.2 ± 2.3 to 8.6 ± 0.6 (patient scale) and 3.5 ± 2.1 to 6.0 ± 1.2 (observer scale). One of the striking findings presented in this study is the contrasting baseline satisfaction scores in group 1 (more negative) versus their counterparts in group 2 (less negative) compared to observer evaluation scores. Although the overall conclusion by the authors was that MN and PRP resulted in safe and effective treatment for the conditions in the cohort including scars, it is very challenging to draw meaningful studies from this work given the shortcomings including the different protocols between the two groups, the lack of reported statistical analysis for the scar cohort and the fact that a proportion of patients in group 2 did not appear to have provided POSAS scores. The authors commented on the absence of any complications including exacerbation of scarring or hypopigmentation, apart from one patient developing mild hyperpigmentation responding to three months of vitamin A and tyrosinase inhibitors.
Level 3
Aust et al. conducted an observational cohort study of 16 patients, whose burn scars were both mature (at least two years old) as well as actively hypertrophic on various anatomical locations including the extremities and face.27 These scars were caused by open fire (68.8%) or scalds (31.2%), with an average total burn surface area (TBSA) of 20%. 75% of patients had undergone split skin grafting, while 25% had been treated non-operatively. Patients’ skin was prepared with a topical preparation of vitamins A and C for at least one month twice daily (on average applied from 3 ± 1.3 months before treatment) and the medical roll-CIT (Vivida, Cape Town, South Africa) was used for 1–3 sessions under general or local anaesthesia. Results were assessed on the basis of patient-reported VAS (0 = absolutely dissatisfied; 10 = completely satisfied) as well as VSS scores; POSAS assessment at baseline and 12 months postoperatively was performed by two independent observers. Authors additionally obtained 3-mm punch skin biopsies at baseline and 12 months after treatment, which showed normalisation of the collagen/elastin matrix in the reticular dermis and an increase in collagen deposition at 12 months. Additional findings included improved collagen alignment, a normal stratum corneum with thickened stratum spinosum and normal rete ridges. Despite the considerable histological improvements, the authors commented on the lack of complete restoration of the skin architecture to normality by virtue of an overall decreased epidermal and dermal cell density and a remaining partial irregular fibre structure in the dermis. VAS scores showed a statistically significant improvement from 4.5 to 8.5 ± 15.5, P ⩽ 0.005. The VSS scores showed a mean improvement of 2.7 points (P ⩽ 0.005) and POSAS an improvement of 8 (P ⩽ 0.005). Apart from swelling and bruising for 7–10 days, no side effects including scarring, dyspigmentation or photosensitivity were reported.
Level 4
A case series examined the effects of needling in a cohort of 47 paediatric patients with grafted burn scars of a median age of 18 months (range = 4–170 months).14 The cohort consisted of both inactive cicatrices as well as active hypertrophic scars with an interval between injury and first treatment session less than 12 months. Under general anaesthesia, the scars were treated with a 2.5-mm Dermaroller (GmbH, Wolfenbuttel, Germany) and a course of topical vitamin A and C oil preparation (Environ) for four weeks; standard therapy with pressure garments and silicone was re-instituted a few days after the procedure once the oedema and erythema had subsided. Eighteen patients (38.3%) had one session and 29 (61.7%) had two or more treatment sessions with a minimum interval of three months in between. VSS was used in this study to assess the quality of scarring by three independent blinded observers based on photographs at baseline and an average of 14 weeks after the first needling session (range = 4–50 weeks). Scores showed an overall improvement after treatment (9.40 ± 0.265 vs. 7.40 ± 0.284, P < 0.001) with the individual components of vascularity, pliability and height showing a statistically significant change (P < 0.001 for each parameter); nevertheless, there was no statistically significant trend in pigmentation scores. Subjectively, all patients expressed satisfaction with scars being more elastic and homogeneous after treatment. Further analysis of results showed that the time from treatment to follow-up (< 14 weeks vs. > 14 weeks) had no effect on scar improvement pointing towards the longevity/maintenance of the results achieved with needling. Moreover, comparison between mature (> 1 year old) and immature scars also showed no difference in scar improvement. Minor complications were noted in two patients; one relating to severe pruritus and one to prolonged redness and pruritus attributed to the multivitamin oil preparation used.
Schwarz and Laaff published a case series involving 11 patients with traumatic and hypertrophic acne scars undergoing 1.5-mm Dermaroller (Horst Liebl Co., France) needling under local anaesthesia.28 Punch biopsies were taken of the patients’ scars at baseline and 6–8 weeks after treatment for histologic assessment by an independent dermatologist and a pathologist. All patients were reported to be pleased with the results with no side effects noted; 7/11 patients (64%) had a notable increase in elastic fibre content with no statistically relevant increase in epidermal thickness.
Šuca et al. conducted a case series of six patients with mature burn split skin graft scars undergoing three sessions of 2.5-mm dermaroller needling under topical anaesthesia 6–8 weeks apart.29 The scar TBSA varied from 1 to 4.5% and the interval between injury and treatment initiation was in the range of 1–33 years. Assessment was performed using photographs and the VSS, albeit at an unspecified follow-up period. All patients reported subjective improvement in the final scar quality, improved pain profiles and reduced tension; the authors identified an average improvement of 2 points (range = 1–3) on the VSS. Furthermore, the pigment distribution was reported to be more uniform and the hypertrophic and unstable areas were reported to be flatter and more stable.
Aust et al. performed a retrospective analysis of 480 patients undergoing needling for wrinkles, skin laxity, stretch marks and scarring.21 All participants were pre-treated with at least one month of vitamin A and C topical preparation twice daily; most patients had one needling treatment with some receiving up to a maximum of four sessions. Seventy-two patients were treated for scarring from either acne or burns and had a consultation to treatment time of 3.1 ± 1.4 months. Histology at six months showed considerable increase in collagen deposition in a normal lattice pattern as opposed to parallel bundles conventionally seen in scar tissue; other findings included an increase in the elastin content as well as a 40% thickening of the epidermis. VAS ratings at 12 months showed a statistically significant improvement in 50 patients in the scar/wrinkle/stretch mark mixed subgroup from 3.0 to 7.5 (P ⩽ 0.005). In the subgroup of 15 patients with scars and stretch marks, the VSS and POSAS scores (performed by two independent observers at 12 months) showed a statistically significant improvement from 7.5 to 4.8 and 27 to 19, respectively (P ⩽ 0.005). No patients experienced any photosensitivity, hyper- or hypo-pigmentation; two patients developed herpes simplex infection after full-face needling and were treated successfully with acyclovir.
Cho et al. reported on a 49-year-old patient with a longstanding tight facial burn scar treated with five sessions of combined laser (pinhole method at 5-mm intervals) and CIT dermaroller (Horst Liebl, Germany) at four-week intervals. The authors reported favourable subjective observer and patient satisfaction results in terms of texture and colour improvement.30
Discussion
MN or PCI is an increasingly popular resurfacing technique, which rests on the creation of a non-confluent, non-ablative pattern of dermal injury. The majority of reports in the scar literature focus so far on atrophic acne scars and this work aims to appraise the current evidence behind the use of needling techniques in non-atrophic scars. Out of the 10 included studies, only three have a comparative design to allow formal risk of bias assessment; this revealed a high/serious risk of bias for two of these; hence, the conclusions drawn based on the level of evidence need to be interpreted with caution.
There is level 1 evidence to suggest that the combination of needling and topical silicone gel can improve the vascularity, pliability and scar height in hypertrophic and keloid scars at three-month follow-up.23 Level 2 evidence suggests that needling and autologous spray keratinocyte treatment of mature hypopigmented scars can result in statistically significant improvements in both patient and observer POSAS ratings as well as significant change in objective pigmentation scores at 12-month follow-up.24 One further study in this category points towards a beneficial effect of needling in enhancing the aesthetic outcomes of fat grafting including improved fat volume retention at three-months follow-up.25
Level 3 study results confirm the beneficial effects of needling in the histology of both mature and actively hypertrophic burn scars (including a thickened epidermis and normalisation of the dermal collagen/elastin matrix) as well as statistically significant improvements in reported VAS, VSS and POSAS scale ratings at 12-month follow-up.27
Level 4 evidence in paediatric mature and actively hypertrophic scars supports a statistically significant improvement in VSS scores for vascularity, pliability and height at a median follow-up of 20 months.14 A further mixed cohort study reported a significant improvement of scars at 12 months using VAS and POSAS ratings as well as favourable histological findings including epidermal thickening, enriched elastin and collagen content.5,21
One of the advantageous features of PCI is the ability to maintain the integrity of the epidermal layer, which confers a significant advantage in comparison to ablative laser techniques. Interestingly, only one case of post-inflammatory hyperpigmentation and one herpes simplex re-activation have been identified in our literature review.21,26 No reports on scar exacerbation were found and the most frequent side effects of needling include transient swelling and bruising.27
There are a number of limitations of the current literature in appraising needling modalities in non-atrophic scar management; the first relates to the heterogenous mix of scar types within the studies identified as well as the different treatment protocols used.23–25 This is important to recognise because the effect of MN on scar quality is expected to vary depending on the characteristics of the scar being treated and the exact treatment parameters. The second limitation identified in our work relates to the methodology employed in individual studies including the small cohort sizes, limited randomisation/blinding and short follow-up periods. These shortcomings, as confirmed by virtue of the risk of bias assessment, introduce a certain element of bias in the conclusions; this needs to be considered in any initiatives to translate the evidence presented into clinical recommendations.
Conclusion
This work appraised the literature concerning the evidence behind needling techniques for non-atrophic scars. PCI has several advantages compared to other resurfacing techniques, including low risk of side effects, need for non-specialised equipment and versatility. Important limitations of the current literature include the limited number of high-quality studies, small cohort sizes as well as the heterogeneity of outcome measures employed. Further high-quality studies with comparative design protocols are awaited in order to further delineate the role of needling techniques in scar management protocols.
Table 1.
Non-atrophic scars.
| Author | Level of evidence | Patient clinical criteria | Risk of bias (RoB V2.0/ROBINS-I) | Study design | Follow-up | Outcomes |
|---|---|---|---|---|---|---|
| Fabbrocini et al., 2016 (Italy) | RCT (1c) | n = 20 Patients with hypertrophic and keloid scars divided into two groups each with 6 keloid and 4 hypertrophic scars. Inclusion criteria: aged 18–45 years; scar present < 5 years and previously treated. |
High risk of bias (RoBv2.0) | Patients randomised into groups A and B. Group A treatment involved MN to the whole scar and silicone gel to half of the scar already been treated with MN. Group B treatment involved silicone gel to the whole scar and MN used for half of the scar already treated with the gel. MN was carried out with Dermapen at 1.5–2.5-mm needle lengths. |
Follow-up performed with photographic assessment and a modified
VSS on days 0, 30, 60, 90, and at 3 months after the last
session. Scar quality was also assessed with ultrasound imaging before and 3 months after the last session. |
VSS results revealed that the combination of MN and silicone gel produced a statistically significant improvement compared to the single modalities in each group (P < 0.01) Group A only showed a statistically significant improvement in erythema and pliability at 3-month follow-up, (1.42 ± 0.55–1.12 ± 0.33, P = 0.025 for erythema and 2.87 ± 0.33–1.86 ± 0.35, P < 0.001 for pliability). Scar height reduction results also showed the superiority of needling in group A (1.30 ± 0.55 reduced to 1.00 ± 0.29) vs. group B (1.38 ± 0.41 reduced to 1.25 ± 0.44, P < 0.05). Ultrasound assessment showed a 60% decreased keloid thickness in both groups albeit a non-significant improvement in thickness of hypertrophic scars. Trends of reduced pigmentation levels were observed in both treatment groups. |
| Busch et al., 2016 (Germany) | Experimental study with randomisation (2c) |
n = 20 Patients with hypopigmented burn scars; mean age 33 years (range = 6–60). Inclusion criteria: healing by secondary intention, at least 10 cm2 size and 1 year since time of injury; underlying aetiology: scald, fire, acid, chemical peel, laser). |
Serious risk of bias (ROBINS-I) | Patients’ scars were divided into 3 sub-areas, treated with: (1) 3-mm needling and NCASCS; (2) 3-mm needling alone; and (3) no treatment. | Patients were followed up at 3, 6, 9 and 12 months and
pigmentation changes were measured using Mexameter. POSAS at day 0 and in the last visit (6–15 months after treatment). |
An improvement in pigmentation was noted in 17/20 patients in
the combined group (as measured with the Mexameter) of 29.3%,
(P<0.05). The needling only and untreated scar groups had an 8.4% and 8.9% decrease (both not statistically significant). In the combined group, the patient ratings for colour of the scars showed a 50% improvement (P < 0.05) and for the patient’s overall opinion rating a 57.1% improvement (P < 0.05). Observer ratings for pigmentation improved by 37.5% and by 38.5% for overall opinion (p<0.05 for both). |
| Sezgin and Özmen, 2018 (Turkey) | Quasi-experimental prospectively controlled study (2c) | n = 40 Patients needing rejuvenation, asymmetry correction including traumatic and burn scars. Exclusion criteria: chronic illness, e.g. diabetes mellitus; smoking; previous fat grafting procedures. |
Low risk of bias (ROBINS-I) | Patients were divided into 2 groups. Group 1 had fat grafting on the face combined with MN. Group 2 had fat grafting on the face alone (control). A 1.5-mm needle-length MN device, Deeproller, was used. | Follow-up with photographic assessment at day 0 and after 3 months. A modified version of GAIS was used by the patient and one independent surgeon to measure efficacy using 2 variables; facial volume and facial skin quality; these were assessed on a scale of 3 to −1 (exceptionally improved, much improved, improved, same, worse). | The improvement of facial skin quality was better in the MN
group compared to the fat graft group in a statistically
significant manner for both the aesthetic and reconstructive
subgroups (2.27 ± 0.40 vs. 1.33 ± 0.59, P <
0.05). In terms of volume improvement scores, the pattern was
similar with a statistically significant result in favour of the
MN and fat graft group for both aesthetic and reconstructive
patients (2.32 ± 0.39 vs. 1.56 ± 0.54, P <
0.05). 90.9% patients reported they would undergo the procedure in the future if they required it. No complications noted. |
| Sasaki, 2016 (USA) | Experimental study (pre-test – post-test) (2d) | n = 12 Patients with hypertrophic scars. Exclusion criteria: active systemic/local disease; keloidal history; skin cancer; anticoagulant treatment; pregnancy; ablative treatment in previous 6 months. |
N/A | Needling was performed using Dermapen at depths of 0.25–1 mm (rhytides/skin laxity/acne scars) and 0.5–2.5 mm with PRP (for more severe rhytides/skin laxity and hypertrophic/acne scars and alopecia). Patients’ skin was prepared for at least 3 months with a topical multivitamin preparation. | Results were assessed based on photographic assessment at baseline and 12 months after treatment by the patients and three independent observers using a VAS (0 = absolutely dissatisfied; 10 = completely satisfied). | At an average follow-up of 9 months (range = 6–36) after
treatment, group 1 POSAS scores for hypertrophic scars improved
from 3.2 ± 1.7 to 6.4 ± 1.3 (patient scale) and 4.5 ± 0.1 to 7.0
± 0.0 (observer scale). The surgical centre cohort (group 2)
showed an improvement in POSAS scores for hypertrophic scars
from 6.2 ± 2.3 to 8.6 ± 0.6 (patient scale) and 3.5 ± 2.1 to 6.0
± 1.2 (observer scale) at an average follow-up of 17 months
(range 6-36). One patient from the MN alone group had mild hyperpigmentation that responded well to 3 months of treatment with vitamin A and tyrosine inhibitors. |
| Aust et al., 2010 (Germany) | Observational study without a control group (3e) | n = 16 Patients with burn and hypertrophic burn scars. |
N/A | Patients were treated with the medical roll CIT for 1–3 sessions. Pre- and post- treatment preparation with vitamins A and C was used for 3 ± 1.3 months before MN. | Follow-up assessment was performed by patients and two independent observers using the VAS, VSS and POSAS scores 12 months after treatment. Punch biopsies were also taken to assess collagen and elastin quality and quantity. | A mean VAS improvement from 4.5 to 8.5 ± 15.5
(P ⩽ 0.005) was noted, whereas the VSS
showed an improvement of 2.7 points and POSAS of 8 points (both results significant with P ⩽ 0.005). The epidermis showed 45% thickening of the stratum spinosum, normal rete ridges and normalisation of the normal lattice architecture collagen/elastin matrix in the reticular dermis. |
| Kubiak and Lange, 2017 (Germany) | Case series (4c) | n = 47 Patients with burn scars (inactive as well as active hypertrophic) in various bodily locations. Median age at treatment = 3.3 years (range = 0.3–12.4); median time from injury to needling = 1.5 years (range = 0.3–14.2). |
N/A | Patients were treated under general anaesthetic with 2.5-mm Dermaroller; postoperative vitamin A and C oil (Environ) was used for 4 weeks. Standard therapy with pressure garments and silicone was re-instituted a few days after the procedure. Repeat MN sessions performed at least at 3-month intervals. 18 (38.35%) had one treatment and 29 (61.7%) had two or more. | Follow-up assessment using the VSS by patients and three independent observers at day 0 and at least 4 weeks (median = 14 weeks) after the first session. | All patients were satisfied with the treatment, reporting more
elastic and homogeneous scars. VSS individual scores for vascularity, pliability, height and overall scores were statistically significant (P < 0.001) apart from pigmentation (P = 0.063). The magnitude of VSS improvement noted did not vary according to the duration of follow-up (< 14 weeks vs. > 14 weeks). Moreover, comparison of results between immature scars (< 1 year old) and mature scars showed no statistical significance. Two patients developed pruritus; one case was self-limiting and the other responded to discontinuation of the multivitamin oil. |
| Schwarz and Laaff, 2011 (Germany) | Case series (4c) | n = 11 Patients with traumatic and hypertrophic acne scars. |
N/A | Single session with 1.5-mm Dermaroller. | Patient satisfaction and histological assessment at baseline and at 6–8 weeks after MN. | All patients reported to be satisfied with the results and no side effects were reported. 7/11 patients (64%) had a notable increase in elastic fibre content with no statistically relevant increase in epidermal thickness. |
| Šuca et al., 2017 (Czech Republic) | Case series (4c) | n = 6 Patients with mature split skin graft burn scars; time between injury and treatment 1–33 years. |
N/A | Patients were treated with the 2.5-mm Dermaroller device (3 sessions performed 6–8 weeks apart). | Follow-up assessment was performed using VSS and patient rating. | VSS showed an average improvement of 2 points (range = 1–3).
Moreover, there was a more even distribution of pigment,
improvement of hypertrophic/unstable areas as well as the texture of meshed grafts. All patients were satisfied with the result with reduced tension and pain in the treated areas. |
| Aust et al., 2008 (Germany) | Case series (4c) | n = 72 scar (acne and burn) patients with scars – part of a
bigger cohort of 480 with skin laxity/wrinkles Age: 41.7 ± 10.3 years. Consultation to treatment time = 3.1 ± 1.4 months. |
N/A | Most patients underwent one MN treatment but some had up to 4 sessions of Medical-Roll-CIT (1–3 mm); all patients had topical vitamin A and C skin preparation for at least 4 weeks preoperatively. | Histology at 6 months, VAS and POSAS scores at 12 months (two independent observers). |
Histology: considerable increase in collagen and elastin deposition and 40% thickening of the epidermis. VAS ratings at 12 months showed a statistically significant improvement in the scar/wrinkle/stretch mark mixed subgroup (50 patients) from 3.0 to 7.5 (P ⩽ 0.005). In the subgroup of 15 patients with scars and stretch marks, VSS and POSAS scores showed a statistically significant improvement from 7.5 to 4.8 and 27 to 19, respectively (P ⩽ 0.005). No patients experienced photosensitivity, PIH or hypopigmentation; two patients developed herpes simplex infection after full-face needling and were treated successfully with acyclovir. |
| Cho et al., 2008 (South Korea) | Case study (4d) | n = 1 Patient with a longstanding and tight facial burn scar. |
N/A | Combination of CO2 laser and Dermaroller (5 sessions at 4-week intervals). | Physician and patient subjective report. Exact time to follow-up was not stated. | Authors reported relaxation of the contracture, and improvement in texture and colour; patient was satisfied with the treatment. |
MN, microneedling; VAS, visual analogue scale; VSS, Vancouver Scar Scale; POSAS, Patient and Observer Scar Assessment Scale; GAIS, Global Aesthetic Improvement Scale.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: C Iosifidis  https://orcid.org/0000-0002-0148-5674
https://orcid.org/0000-0002-0148-5674
References
- 1. Fernandes D. Percutaneous collagen induction: An alternative to laser resurfacing. Aesthetic Surg J 2002; 22: 307–309. [DOI] [PubMed] [Google Scholar]
- 2. Orentreich D, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg 1995; 21: 543–549. [DOI] [PubMed] [Google Scholar]
- 3. Camirand A, Jocelyne D. Needle dermabrasion. Aesthetic Plast Surg 2013; 21: 48–51. [DOI] [PubMed] [Google Scholar]
- 4. Liebl H. Device and method for applying an active ingredient to the skin. German; WO2001EP14747 20011214, 2002. [Google Scholar]
- 5. Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol 2008; 26: 192–199. [DOI] [PubMed] [Google Scholar]
- 6. Alster T, Graham P. Microneedling: a review and practical guide. Dermatol Surg 2017; 44: 397–404. [DOI] [PubMed] [Google Scholar]
- 7. Jaishree S, Vani Y, Sitara G. Microneedling. In: Mysore V. (ed.) ACS(I) Textbook on Cutaneous & Aesthetic Surgery. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd., 2017, pp. 885–891. [Google Scholar]
- 8. Yadav S, Singh A. Microneedling: Advances and widening horizons. Indian Dermatol Online J 2016; 7: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anastassakis K. The dermaroller series. 2014. Available at: http://www.mtoimportadora.com.br/site_novo/wp-content/uploads/2014/04/Dr.-Anastassakis-Kostas.pdf (accessed 28 Oct 2018).
- 10. Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg 2009; 2: 110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arora S, Gupta P. Automated microneedling device - A new tool in dermatologist’s kit - A review. Journal of Pakistan Association of Dermatologists 2012; 22: 354–357. [Google Scholar]
- 12. Chandrashekar B, Sriram R, Mysore R, et al. Evaluation of microneedling fractional radiofrequency device for treatment of acne scars. J Cutan Aesthet Surg 2014; 7: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aust M, Reimers K, Gohritz A, et al. Percutaneous collagen induction. Scarless skin rejuvenation: fact or fiction? Clin Exp Dermatol 2010; 35: 437–439. [DOI] [PubMed] [Google Scholar]
- 14. Kubiak R, Lange B. Percutaneous collagen induction as an additive treatment for scar formation following thermal injuries: Preliminary experience in 47 children. Burns 2017; 43: 1097–1102. [DOI] [PubMed] [Google Scholar]
- 15. Fernandes D. Minimally Invasive Percutaneous Collagen Induction. Oral Maxillofac Surg Clin North Am 2005; 17: 51–63. [DOI] [PubMed] [Google Scholar]
- 16. Bandyopadhyay B, Fan J, Guan S, et al. A “traffic control” role for TGFβ3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol 2006; 172: 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aust M, Reimers K, Vogt P. Medical needling: improving the appearance of hypertrophic burn-scars. GMS Verbrennungsmedizin 2009;3:Doc03. [Google Scholar]
- 18. Liebl H, Kloth L. Skin cell proliferation stimulated by microneedles. J Am Coll Clin Wound Spec 2012; 4: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeitter S, Sikora Z, Jahn S, et al. Microneedling: Matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration. Burns 2014; 40: 966–973. [DOI] [PubMed] [Google Scholar]
- 20. Aust M, Reimers K, Kaplan H, et al. Percutaneous collagen induction–regeneration in place of cicatrisation. J Plast Reconstr Aesthet Surg 2011; 64: 97–107. [DOI] [PubMed] [Google Scholar]
- 21. Aust M, Reimers K, Repenning C, et al. Percutaneous collagen induction: minimally invasive skin rejuvenation without risk of hyperpigmentation—fact or fiction? Plast Reconstr Surg 2008; 122: 1553–1563. [DOI] [PubMed] [Google Scholar]
- 22. Ramaut L, Hoeksema H, Pirayesh A, et al. Microneedling: Where do we stand now? A systematic review of the literature. J Plast Reconstr Aesthet Surg 2018; 71: 1–14. [DOI] [PubMed] [Google Scholar]
- 23. Fabbrocini G, Marasca C, Ammad S, et al. Assessment of the combined efficacy of needling and the use of silicone gel in the treatment of C-section and other surgical hypertrophic scars and keloids. Adv Skin Wound Care 2016; 29: 408–411. [DOI] [PubMed] [Google Scholar]
- 24. Busch K, Bender R, Walezko N, et al. Combination of medical needling and non-cultured autologous skin cell transplantation (ReNovaCell) for repigmentation of hypopigmented burn scars. Burns 2016; 42: 1556–1566. [DOI] [PubMed] [Google Scholar]
- 25. Sezgin B, Özmen S. Fat grafting to the face with adjunctive microneedling: a simple technique with high patient satisfaction. Turk J Med Sci 2018; 48: 592–601. [DOI] [PubMed] [Google Scholar]
- 26. Sasaki G. Micro-needling depth penetration, presence of pigment particles, and fluorescein-stained platelets: clinical usage for aesthetic concerns. Aesthet Surg J 2016; 37: 71–83. [DOI] [PubMed] [Google Scholar]
- 27. Aust M, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: An alternative treatment for burn scars. Burns 2010; 36: 836–843. [DOI] [PubMed] [Google Scholar]
- 28. Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the dermaroller device. Plast Reconstr Surg 2011; 127: 146e–148e. [DOI] [PubMed] [Google Scholar]
- 29. Šuca H, Zajíček R, Vodsloň Z. Microneedling - a form of collagen induction therapy - our first experiences. Acta Chir Plast 2017; 59: 33–36. [PubMed] [Google Scholar]
- 30. Cho S, Lee S, Kang J, et al. The treatment of burn scar–induced contracture with the pinhole method and collagen induction therapy: a case report. J Eur Acad Dermatol Venereol 2008; 22: 513–514. [DOI] [PubMed] [Google Scholar]
How to cite this article
- Iosifidis C, Goutos I. Percutaneous collagen induction (microneedling) for the management of non-atrophic scars: literature review. Scars, Burns & Healing, Volume 5, 2019. DOI: 10.1177/2059513118880301. [DOI] [PMC free article] [PubMed] [Google Scholar]