Abstract
Background:
The graft bending angle (GBA), the angle between the femoral bone tunnel and the line connecting the femoral and tibial tunnel apertures, has been proven to influence stress within the graft and could be an important factor in graft healing within the joint and bone tunnel. However, the influence of the GBA on functional outcomes, particularly on return to sports (RTS), is rarely reported.
Purpose/Hypothesis:
The purpose of this study was to investigate the influence of the GBA on graft maturation, the femoral tunnel, and functional outcomes at 12 months after anterior cruciate ligament reconstruction (ACLR). We hypothesized that a greater GBA might be related to bone tunnel widening, poor graft healing, and inferior functional outcomes after ACLR.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A total of 43 consecutive patients who underwent unilateral ACLR with hamstring tendon autografts participated in this study. Their knees were evaluated using functional scores (International Knee Documentation Committee [IKDC] score, Lysholm knee activity score, Tegner activity scale, RTS) and the anterior tibial translation side-to-side difference (ATTD), as measured using a KT-1000 arthrometer and 3.0-T magnetic resonance imaging (MRI), at 12 months after surgery. Based on MRI, the signal/noise quotient (SNQ) of the graft, the GBA, and the femoral tunnel diameter were measured.
Results:
The mean GBA was 56° (range, 41°-69°). The GBA had a significant positive correlation with the SNQ (rho, 0.45; P = .003) and bone tunnel diameter (rho, 0.35; P = .02), but it had no significant correlation with any functional scores. Patients were divided into 3 groups based on GBA values: low GBA (LGBA; 40° < GBA ≤ 50°), middle GBA (MGBA; 50° < GBA ≤ 60°), and high GBA (HGBA; 60° < GBA ≤ 70°). The HGBA group had a significantly higher mean SNQ than both the LGBA (P = .01) and MGBA groups (P = .02). It also had a greater mean tunnel diameter than the LGBA group (P = .04). There was no significant difference in IKDC scores, Lysholm scores, ATTD, Tegner scores, or rates of RTS among groups.
Conclusion:
The GBA did not affect functional outcomes at 12 months after ACLR, although it affected the SNQ of the graft and the femoral tunnel diameter.
Keywords: MRI, anterior cruciate ligament, functional outcomes, signal/noise quotient (SNQ), GBA
Most anterior cruciate ligament (ACL) injuries require surgical reconstruction to return to sports.3,26 Postoperatively, the new reconstructed ACL graft will experience healing, which influences patients’ rehabilitation process and time to return to sports (RTS).31,35 Poor healing could result in graft failure.12,14,32
The graft bending angle (GBA), the angle between the femoral bone tunnel and the line connecting the femoral and tibial tunnel apertures,20,24 has been proven to influence stress within the graft, and it might be an important factor in graft healing within the joint and bone tunnel.18,28,33 A higher GBA could lead to tunnel widening.21 It has also been found to correlate significantly with a higher graft signal intensity (SI) on magnetic resonance imaging (MRI), which might indicate poorer ACL graft healing.2,4,27 Meanwhile, the influence of the GBA on functional outcomes, particularly RTS, is rarely reported.
The purpose of this study was to investigate the influence of the GBA on the femoral bone tunnel, graft healing, and functional outcomes at 12 months after ACL reconstruction (ACLR). We hypothesized that a high GBA might be related to bone tunnel widening, poor graft healing, and inferior functional outcomes after ACLR.
Methods
Participants
The Health Sciences Institutional Review Board of our hospital approved this study. From January 2014 to September 2016, patients undergoing ACLR with hamstring tendon autografts were invited to participate in this study. The inclusion criteria were as follows: (1) unilateral ACLR, (2) age between 18 and 50 years, (3) hamstring tendon autograft, (4) transportal (medial portal) technique for the femoral tunnel, (5) 8 mm diameter of the femoral and tibial bone tunnels, (6) femoral fixation with Endobutton CL (Smith & Nephew), and (7) tibial fixation with Bio-Intrafix (Mitek). The exclusion criteria were as follows: (1) fixation with other implants, (2) bone tunnels with other diameters, (3) combined with other ligament reconstruction, (4) infection after surgery, (5) revision surgery, and (6) osteoarthritis.
Surgical Technique
One senior surgeon (J.C.) performed arthroscopic single-bundle ACLR with hamstring tendon autografts in all cases. The semitendinosus and gracilis tendons were harvested and prepared as a 4-strand double-looped autograft with a diameter of 8 mm. Then, the femoral tunnel (8 mm in diameter) was produced through a medial portal and located at the center of the ACL femoral footprint, and the tibial tunnel (8 mm in diameter) was created using a tibial guide. The autograft was pulled into the tunnels and fixed with Endobutton CL at the femoral side. The tibial side was fixed with Bio-Intrafix in 20° of knee flexion with 20 N of tension.
Clinical Evaluation
Both the clinical evaluation and MRI were performed at 12 months after surgery. An experienced orthopaedic surgeon (J.C.) performed the clinical evaluation, including the subjective functional examinations and the knee stability examinations. Subjective functional examinations included the International Knee Documentation Committee (IKDC) score, Lysholm knee activity score, and Tegner activity scale. The anterior drawer test and Lachman test were used to evaluate knee stability. In addition, anterior tibial translation was measured with the KT-1000 knee arthrometer (MEDmetric), when maximum manual posterior-anterior external force applied at the tibia set off the alarm at 30 lb. Both knees were examined, and the anterior tibial translation side-to-side difference (ATTD) was recorded. The rate of RTS was also investigated.
MRI and Image Analysis
A 3.0-T MRI scanner (MAGNETOM Verio; Siemens) was used to scan the knees. Sagittal images were obtained with oblique proton density–fat saturation imaging. Also, 3-dimensional (3D) dual-echo steady-state imaging or 3D proton density imaging was performed on each patient. All of these images were imported into RadiAnt DICOM viewer 4.0.3 software (Medixant) for analysis. The MRI evaluation focused on 3 measurements:
The signal/noise quotient (SNQ) of the graft: The SI was measured in 3 regions of interest (proximal, midsubstance, and distal sites) on proton density–fat saturation images according to a previous study.27 To quantify the normalized SI of the ACL graft, the SNQ of the graft was calculated using the equation shown in Figure 1.
The GBA: The femoral and tibial tunnel apertures were identified through a 3D multiplanar reconstruction model. The plane containing the femoral and tibial tunnel aperture centers and the proximal femoral tunnel exit was chosen to evaluate the GBA according to a previous study (Figure 2).4 The GBA was calculated as the angle between the long axis of the femoral bone tunnel and the line connecting the femoral and tibial tunnel apertures.
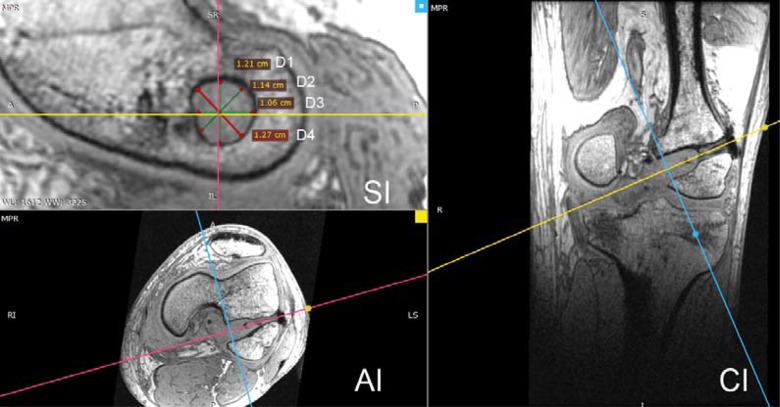
The femoral bone tunnel diameter: To reduce the bias, 3 consecutive oblique sagittal images perpendicular to the long axis of the femoral tunnel at the femoral aperture were chosen to measure the diameter. The 3 consecutive oblique sagittal images were 0, 0.8, and 1.6 mm away from the tunnel aperture, respectively. Within each sagittal image, the diameter measurements from 4 directions was averaged, then the average measurement from the 3 consecutive sagittal images was calculated for the overall femoral tunnel diameter (Figure 3).
All of the measurements were performed by 2 investigators (H.L., S.L.), and repeated measurements were made on 2 days at least 1 week apart.
Figure 1.
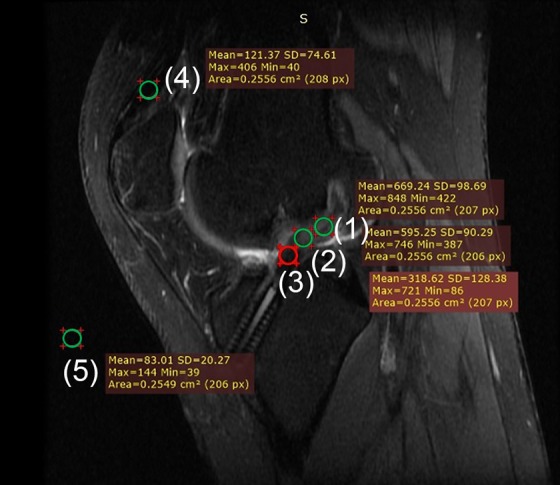
Sagittal magnetic resonance imaging of the knee shows the positions of the 5 regions of interest (area of the circle = 0.25 cm2), which included the (1) proximal site, (2) midsubstance site, (3) distal site, (4) quadriceps tendon, and (5) background (∼2 cm anterior to the patellar tendon). The signal/noise quotient (SNQ) of the graft was calculated using the following equation: {[(SI proximal + SI midsubstance + SI distal) / 3] − SI quadriceps} / SI background, where SI = signal intensity.
Figure 2.
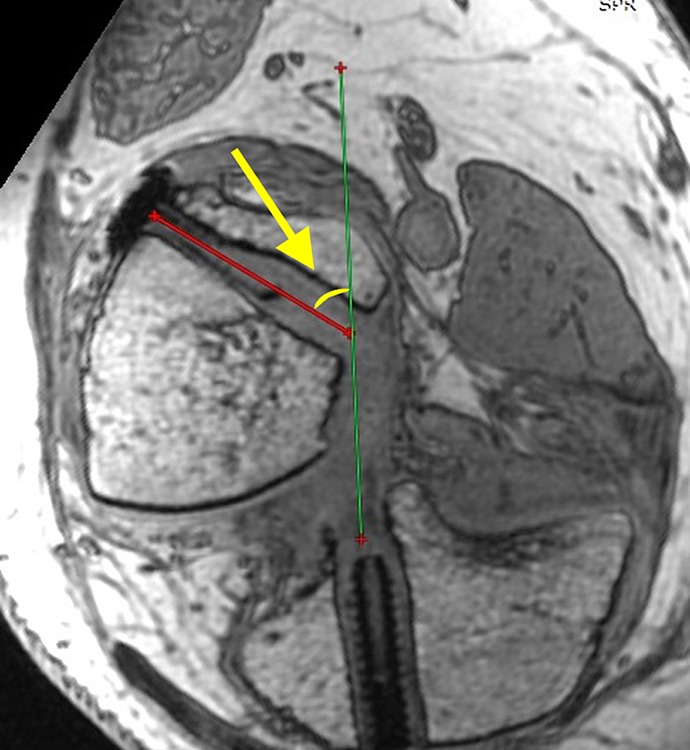
The graft bending angle (arrow) is the angle between the femoral bone tunnel and the line connecting the femoral and tibial tunnel apertures.
Figure 3.
Measurement of the femoral bone tunnel diameter at the aperture. On the sagittal image (SI), the bone tunnel circular plane is perpendicular to the long axis of the femoral tunnel. The bone tunnel diameter was calculated using the following equation: (D1 + D2 + D3 + D4) / 4; where D = distance. On the axial image (AI), the purple line is the long axis of the femoral tunnel, and the blue line is perpendicular to the long axis of the femoral tunnel. On the coronal image (CI), the yellow line is the long axis of the femoral tunnel, and the blue line is perpendicular to the long axis of the femoral tunnel.
Statistical Analysis
Data were analyzed with Stata 10.0 software (StataCorp) and are reported as means and standard deviations. To quantify the proportion of the variance for all measurements, the intraclass correlation coefficient (ICC) was assessed by intraobserver and interobserver reliabilities. The ICC was interpreted as poor (ICC < 0.40), marginal (0.40 ≤ ICC ≥ 0.75), or good (ICC > 0.75). A chi-square test was used to compare the categorical variables. Also, 1-way analysis of variance was used to compare the continuous variables between groups. Spearman correlation coefficients were calculated between the GBA and demographic data, graft SNQ, femoral tunnel diameter, and functional scores. The significance level was set at .05.
Results
At 12 months after surgery, 43 patients participated in this study (Table 1). None of the patients had reinjured the operated knee, and all knees were confirmed stable by the anterior drawer test and Lachman test. In the cohort, the mean GBA was 56° (range, 41°-69°). The respective ICCs of interobserver and intraobserver reliabilities were 0.79 and 0.80 for the SNQ, 0.75 and 0.77 for the GBA, and 0.75 and 0.74 for the tunnel diameter.
TABLE 1.
Participant Demographic Data a
| Age, y | 30 (18-40) |
| Body mass index, kg/m2 | 24 (21-26) |
| Sex, male/female, n | 37/6 |
| Operative side, left/right, n | 23/20 |
| GBA, deg | 55.8 (40.6-68.7) |
| SNQ of the graft | 19.4 (2.0-46.0) |
| Tunnel diameter, mm | 9.3 (7.4-12.1) |
aData are reported as mean (range) unless otherwise specified. GBA, graft bending angle; SNQ, signal/noise quotient.
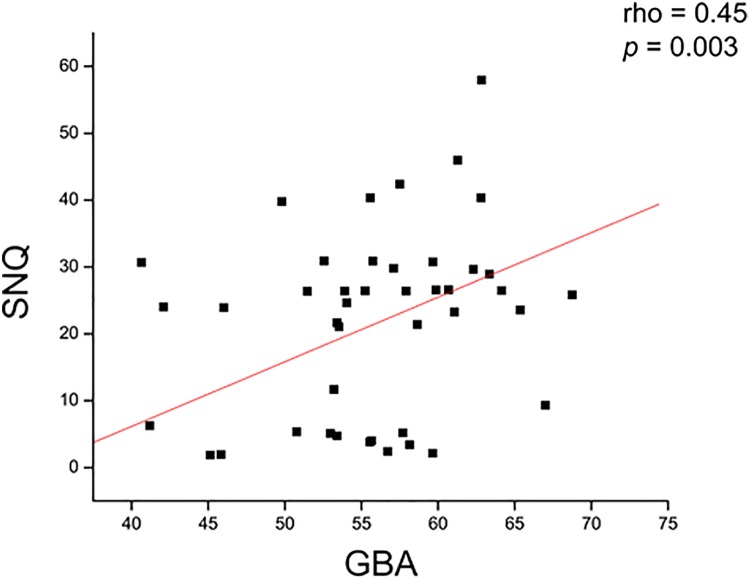
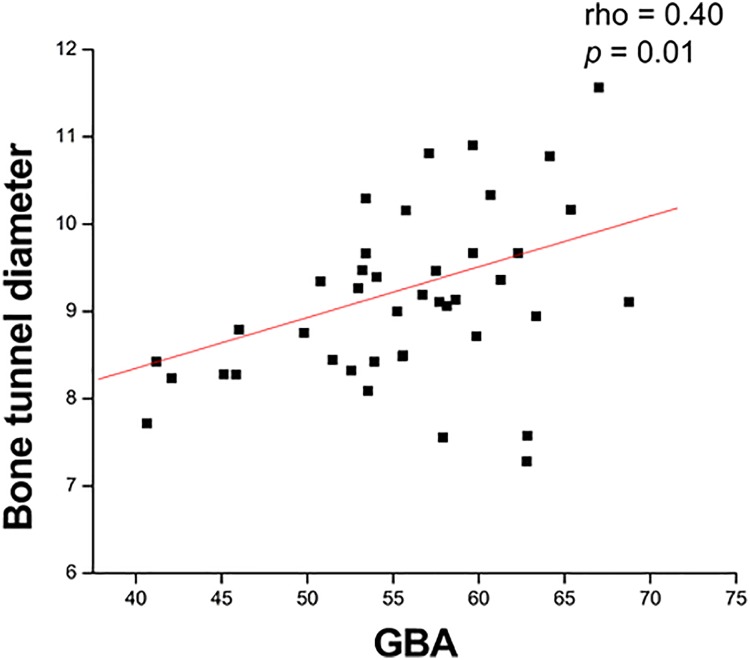
The possible correlations between the GBA and various factors are shown in Table 2. At 12 months after surgery, the GBA had a significant positive correlation with the SNQ (rho = 0.45; P = .003) (Figure 4) and femoral bone tunnel diameter (rho = 0.40; P = .01) (Figure 5); however, it had no significant correlation with any functional scores.
TABLE 2.
Possible Correlations Between GBA and Various Factorsa
| Rho | P Value | |
|---|---|---|
| SNQ of the graft | 0.45 | .003b |
| Tunnel diameter | 0.35 | .02b |
| IKDC score | –0.21 | .19 |
| Lysholm score | –0.25 | .11 |
| Tegner score | –0.05 | .75 |
| ATTD | 0.07 | .67 |
aATTD, anterior tibial translation side-to-side difference; GBA, graft bending angle; IKDC, International Knee Documentation Committee; SNQ, signal/noise quotient.
bStatistically significant (P < .05).
Figure 4.
The graft bending angle (GBA) had a significant positive correlation with the graft’s signal/noise quotient (SNQ).
Figure 5.
The graft bending angle (GBA) had a significant positive correlation with the femoral tunnel diameter.
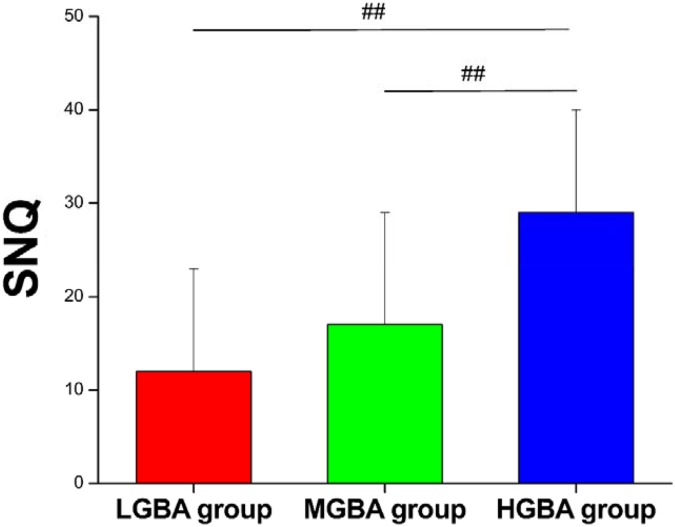
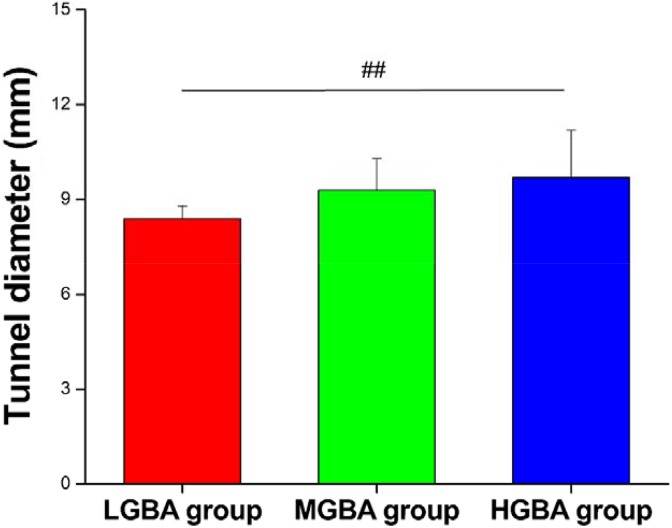
Given that the observed GBA ranged from 41° to 69° in this study, we divided the patients into 3 groups for further analysis: low GBA (LGBA; 40° < GBA ≤ 50°), middle GBA (MGBA; 50° < GBA ≤ 60°), and high GBA (HGBA; 60° < GBA ≤ 70°). There were 7, 25, and 11 patients enrolled in the LGBA, MGBA, and HGBA groups, respectively (Table 3). No significant differences in age, body mass index, or sex were found among the groups. Interestingly, the SNQ of group HGBA (29 ± 11) was significantly higher than that of group LGBA (12 ± 11) (P = .01) and group MGBA (17 ± 12) (P = .02) (Figure 6). Moreover, group HGBA had a wider tunnel diameter than group LGBA (9.7 ± 1.5 vs 8.4 ± 0.4 mm, respectively; P = .04) (Figure 7). There were no significant differences in the IKDC scores, Lysholm scores, ATTD measured by the KT-1000 arthrometer, Tegner scores, or RTS rates among the groups.
TABLE 3.
Participant Demographic Data and Functional Outcomes by Groupa
| LGBA (n = 7) | MGBA (n = 25) | HGBA (n = 11) | |
|---|---|---|---|
| Age, y | 28 ± 5 | 29 ± 5 | 33 ± 4b |
| Body mass index, kg/m2 | 24 ± 1 | 24 ± 1 | 23 ± 1b |
| Sex, male/female, n | 5/2 | 23/2 | 9/2b |
| IKDC score | 76 ± 10 | 68 ± 13 | 64 ± 10b |
| Lysholm score | 67 ± 25 | 59 ± 26 | 45 ± 11b |
| Tegner score | 5 ± 1 | 5 ± 2 | 5 ± 1b |
| ATTD | 0.8 ± 0.7 | 0.9 ± 0.8 | 1.2 ± 1.0b |
| RTS rate, % | 71 | 64 | 55b |
| GBA, deg | 44 ± 3 | 56 ± 3c | 64 ± 3d,e |
| SNQ of the graft | 12 ± 11 | 17 ± 12 | 29 ± 11d,e |
| Tunnel diameter, mm | 8.4 ± 0.4 | 9.3 ± 1.0 | 9.7 ± 1.5d |
aData are reported as mean ± SD unless otherwise specified. ATTD, anterior tibial translation side-to-side difference; GBA, graft bending angle; HGBA, high graft bending angle; IKDC, International Knee Documentation Committee; LGBA, low graft bending angle; MGBA, middle graft bending angle; RTS, return to sports; SNQ, signal/noise quotient.
bNo significant difference between groups.
cSignificant difference between MGBA and LGBA groups.
dSignificant difference between HGBA and LGBA groups.
eSignificant difference between HGBA and MGBA groups.
Figure 6.
Comparison of signal/noise quotients (SNQs) between groups. ##Significant difference between groups. HGBA, high graft bending angle; LGBA, low graft bending angle; MGBA, middle graft bending angle.
Figure 7.
Comparison of femoral tunnel diameters between groups. ##Significant difference between groups. HGBA, high graft bending angle; LGBA, low graft bending angle; MGBA, middle graft bending angle.
Discussion
The most important finding in this study was that the GBA had no significant influence on the IKDC score, Lysholm score, Tegner score, ATTD, or RTS at 12 months after ACLR. Yet, a higher GBA significantly correlated with a higher SNQ of the graft and a wider femoral tunnel. These findings indicated that a high GBA does not influence functional outcomes at 12 months after ACLR, but it could be associated with slower graft healing and femoral bone tunnel widening.
GBA values with different evaluation methods range from 50° to 90° (some studies used the adjacent supplementary angle as the GBA).8,9,18,24,33 In this cohort, the GBA was defined as the angle between the femoral bone tunnel and the intra-articular part of the graft (the line connecting the femoral tunnel aperture and the tibial tunnel aperture). The mean GBA of 56° was in the lower range compared with previous studies, probably because of the surgical technique and evaluation method used in this study. First, the femoral tunnel was drilled with the knee at maximal flexion in our cohort, which might have resulted in a more posterior femoral tunnel exit.28,29 Because it has been reported that a more posterior femoral tunnel exit correlates with a smaller GBA, our surgical technique might have contributed to low GBA values.29 Second, the GBA and bone tunnel were measured on 3D MRI based on the graft axis in this study. Traditionally, the GBA has been measured on 3D computed tomography based on the femoral and tibial tunnel aperture centers and the proximal femoral tunnel exit.8,9,24 Considering the potential tunnel enlargement, our evaluation method might have influenced the GBA calculation.
In this study, there were no significant differences in the IKDC scores, Lysholm scores, ATTD, Tegner scores, or RTS rates among the LGBA, MGBA, and HGBA groups. Previously, Sim et al25 reported that the outside-in technique showed a significantly more acute graft tunnel angle than the transportal technique, but there were no statistically significant differences in the IKDC or Tegner scores between the 2 groups. Niki et al17 also reported that the outside-in technique provided a more acute GBA, but no significant differences were observed in the Lysholm score, pivot-shift test, or anteroposterior laxity at >2.5-year follow-up between the outside-in and transportal techniques. Moreover, Lee et al10 demonstrated that the modified transtibial group had a lower femoral GBA and higher graft maturity, but there were no significant differences in functional outcomes. Our study supported these findings that although the GBA correlated positively with the SI, it did not influence functional outcomes.
Femoral bone tunnel widening, which usually occurs within the first year after ACLR,1,23 might be influenced by a high GBA value. Severe femoral tunnel widening may result in impairment of graft incorporation, joint laxity, and complications in revision surgery.1,23 Tashiro et al28 used a quadriceps tendon autograft with a bone block in ACLR, and they demonstrated that a higher GBA moderately correlated with greater tunnel widening. Segawa et al22 reported that a higher GBA may increase mechanical stress on the anterior margin of the femoral tunnel. In the present study, it was also found that the GBA had a significantly positive correlation with the femoral bone tunnel diameter. Group HGBA had greater tunnel enlargement than group LGBA at 12 months after surgery.
It has been proved that a high GBA might be a risk factor for delayed graft healing.2 The SI12,13,34 and SNQ11,15 are MRI parameters for evaluating the integrity and maturation of the ACL graft. As the more precise method, the SNQ has been widely used to evaluate the integrity and maturation of the graft after ACLR,12,13,34 and higher SNQ values might indicate poorer graft healing.5,6,12 In the present study, it was found that the GBA had a significantly positive correlation with the SNQ, and group HGBA had a significantly higher SNQ compared with group LGBA. These results indicate that a higher GBA leads to poorer graft healing. This could be explained by increased abrasive force on the graft by a higher GBA, which might even result in graft failure.16,30 It has been reported that a steep graft with a high GBA might cause high stress on the graft and bone interface at full extension after anatomic ACLR. Nohmi et al19 observed that the ACL graft was deformed at the corner of the femoral tunnel aperture after cycling anterior tibial loads in a biomechanical study. In a study investigating the most common rupture patterns of ACLR, van Eck et al32 found that the graft tunnel angle was one of the factors that influenced the rupture pattern. Thus, although we found no evidence of a correlation between GBA and functional scores after ACLR, it may be worthwhile to consider postponing the time of RTS for patients with a high GBA to avoid graft failure and ACL reinjuries.
There are several limitations in this study. First, a 10° difference in the GBA was applied to divide the cohort into the LGBA, MGBA, and HGBA groups. Yet, there was a lack of criteria of the GBA difference to indicate graft failure.7 Further biomechanical studies are needed to learn about the correlation between GBA and graft failure. Second, the GBA ranged from 41° to 69° in this study, and it is still unclear if a lower GBA (<40°) will have poorer or better clinical outcomes. Third, the sample size of patients included and analyzed was small. Because this was a prospective study, the number of patients recruited was limited, which might have introduced bias and inaccuracy. Moreover, the cohort excluded ACLR with allografts, but it was unclear whether the graft source could influence the results. Furthermore, the GBA was measured on 3D MRI based on the graft axis, whereas it has traditionally been measured on 3D computed tomography based on the femoral and tibial tunnel aperture centers and the proximal femoral tunnel exit. This may have contributed to measurement errors of the GBA. However, the ICCs of interobserver and intraobserver reliabilities for the GBA were 0.75 and 0.77, respectively. A previous study also revealed that it is proper to measure the GBA based on 3D MRI.4 In addition, this study only investigated ACLR with hamstring tendon autografts with suspensory fixation. It is still unknown if there is a same result with aperture fixation. Finally, this study documented only 12-month outcomes, and more research is necessary to determine long-term outcomes in this patient population.
Conclusion
Collectively, there was no significant difference in the IKDC scores, Lysholm scores, ATTD, Tegner scores, or RTS rates among the groups. The GBA did not influence functional outcomes at 12 months after ACLR, although it had a positive correlation with the SNQ of the graft and the femoral tunnel diameter.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by the Shanghai Sports Science and Technology “Comprehensive Plan” Project (18Z004). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Health Sciences Institutional Review Board of Huashan Hospital.
References
- 1. Aga C, Wilson KJ, Johansen S, et al. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn JH, Jeong HJ, Lee YS, et al. Graft bending angle is correlated with femoral intraosseous graft signal intensity in anterior cruciate ligament reconstruction using the outside-in technique. Knee. 2016;23(4):666–673. [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Taylor NF, Feller JA, Webster KE. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2012;40(1):41–48. [DOI] [PubMed] [Google Scholar]
- 4. Chen L, Wu Y, Lin G, et al. Graft bending angle affects allograft tendon maturity early after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(10):3048–3054. [DOI] [PubMed] [Google Scholar]
- 5. Fukuda H, Asai S, Kanisawa I, et al. Inferior graft maturity in the PL bundle after autograft hamstring double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):491–497. [DOI] [PubMed] [Google Scholar]
- 6. Hofbauer M, Soldati F, Szomolanyi P, et al. Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jang KM, Park SC, Lee DH. Graft bending angle at the intra-articular femoral tunnel aperture after single-bundle posterior cruciate ligament reconstruction: inside-out versus outside-in techniques. Am J Sports Med. 2016;44(5):1269–1275. [DOI] [PubMed] [Google Scholar]
- 8. Kim JG, Chang MH, Lim HC, et al. An in vivo 3D computed tomographic analysis of femoral tunnel geometry and aperture morphology between rigid and flexible systems in double-bundle anterior cruciate ligament reconstruction using the transportal technique. Arthroscopy. 2015;31(7):1318–1329. [DOI] [PubMed] [Google Scholar]
- 9. Kim JG, Wang JH, Lim HC, Ahn JH. Femoral graft bending angle and femoral tunnel geometry of transportal and outside-in techniques in anterior cruciate ligament reconstruction: an in vivo 3-dimensional computed tomography analysis. Arthroscopy. 2012;28(11):1682–1694. [DOI] [PubMed] [Google Scholar]
- 10. Lee DW, Kim JG, Lee JH, Park JH, Kim DH. Comparison of modified transtibial and outside-in techniques in anatomic single-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2018;34(10):2857–2870. [DOI] [PubMed] [Google Scholar]
- 11. Li H, Chen J, Li H, Wu Z, Chen S. MRI-based ACL graft maturity does not predict clinical and functional outcomes during the first year after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3171–3178. [DOI] [PubMed] [Google Scholar]
- 12. Li H, Tao H, Cho S, Chen S, Yao Z. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40(7):1519–1526. [DOI] [PubMed] [Google Scholar]
- 13. Liu S, Li H, Tao H, et al. A randomized clinical trial to evaluate attached hamstring anterior cruciate ligament graft maturity with magnetic resonance imaging. Am J Sports Med. 2018;46(5):1143–1149. [DOI] [PubMed] [Google Scholar]
- 14. Muller B, Bowman KF, Jr, Bedi A. ACL graft healing and biologics. Clin Sports Med. 2013;32(1):93–109. [DOI] [PubMed] [Google Scholar]
- 15. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038–1044. [DOI] [PubMed] [Google Scholar]
- 16. Natsu-ume T, Shino K, Nakata K, et al. Endoscopic reconstruction of the anterior cruciate ligament with quadrupled hamstring tendons: a correlation between MRI changes and restored stability of the knee. J Bone Joint Surg Br. 2001;83(6):834–837. [DOI] [PubMed] [Google Scholar]
- 17. Niki Y, Nagai K, Harato K, et al. Effects of femoral bone tunnel characteristics on graft-bending angle in double-bundle anterior cruciate ligament reconstruction: a comparison of the outside-in and transportal techniques. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1191–1198. [DOI] [PubMed] [Google Scholar]
- 18. Nishimoto K, Kuroda R, Mizuno K, et al. Analysis of the graft bending angle at the femoral tunnel aperture in anatomic double bundle anterior cruciate ligament reconstruction: a comparison of the transtibial and the far anteromedial portal technique. Knee Surg Sports Traumatol Arthrosc. 2009;17(3):270–276. [DOI] [PubMed] [Google Scholar]
- 19. Nohmi S, Ishibashi Y, Tsuda E, et al. Biomechanical comparison between single-bundle and double-bundle anterior cruciate ligament reconstruction with hamstring tendon under cyclic loading condition. Sports Med Arthrosc Rehabil Ther Technol. 2012;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park JS, Park JH, Wang JH, et al. Comparison of femoral tunnel geometry, using in vivo 3-dimensional computed tomography, during transportal and outside-in single-bundle anterior cruciate ligament reconstruction techniques. Arthroscopy. 2015;31(1):83–91. [DOI] [PubMed] [Google Scholar]
- 21. Ra HJ, Celik H, Kim HJ, Lee DH. Femoral tunnel widening is similar between anteromedial portal and transtibial techniques following single-bundle anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):626–635. [DOI] [PubMed] [Google Scholar]
- 22. Segawa H, Omori G, Tomita S, Koga Y. Bone tunnel enlargement after anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):206–210. [DOI] [PubMed] [Google Scholar]
- 23. Siebold R, Cafaltzis K. Differentiation between intraoperative and postoperative bone tunnel widening and communication in double-bundle anterior cruciate ligament reconstruction: a prospective study. Arthroscopy. 2010;26(8):1066–1073. [DOI] [PubMed] [Google Scholar]
- 24. Sim JA, Kim JM, Lee S, Bae JY, Seon JK. Comparison of tunnel variability between trans-portal and outside-in techniques in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1227–1233. [DOI] [PubMed] [Google Scholar]
- 25. Sim JA, Kim JM, Lee S, Song EK, Seon JK. No difference in graft healing or clinical outcome between trans-portal and outside-in techniques after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(8):2338–2344. [DOI] [PubMed] [Google Scholar]
- 26. Spindler KP, Wright RW. Clinical practice: anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tashiro Y, Gale T, Sundaram V, et al. The graft bending angle can affect early graft healing after anterior cruciate ligament reconstruction: in vivo analysis with 2 years’ follow-up. Am J Sports Med. 2017;45(8):1829–1836. [DOI] [PubMed] [Google Scholar]
- 28. Tashiro Y, Sundaram V, Thorhauer E, et al. In vivo analysis of dynamic graft bending angle in anterior cruciate ligament-reconstructed knees during downward running and level walking: comparison of flexible and rigid drills for transportal technique. Arthroscopy. 2017;33(7):1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomihara T, Hashimoto Y, Taniuchi M, Shimada N. Relationship between femoral tunnel location and graft bending angle in outside-in and transportal technique for ACL double bundle reconstruction in 3D-CT study. Arch Orthop Trauma Surg. 2015;135(6):839–846. [DOI] [PubMed] [Google Scholar]
- 30. Tomihara T, Hashimoto Y, Taniuchi M, et al. Shallow knee flexion angle during femoral tunnel creation using modified transtibial technique can reduce femoral graft bending angle in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):618–625. [DOI] [PubMed] [Google Scholar]
- 31. Unterhauser FN, Bail HJ, Hoher J, Haas NP, Weiler A. Endoligamentous revascularization of an anterior cruciate ligament graft. Clin Orthop Relat Res. 2003;414:276–288. [DOI] [PubMed] [Google Scholar]
- 32. van Eck CF, Kropf EJ, Romanowski JR, et al. ACL graft re-rupture after double-bundle reconstruction: factors that influence the intra-articular pattern of injury. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang JH, Kim JG, Lee DK, Lim HC, Ahn JH. Comparison of femoral graft bending angle and tunnel length between transtibial technique and transportal technique in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1584–1593. [DOI] [PubMed] [Google Scholar]
- 34. Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging: a two-year study in sheep. Am J Sports Med. 2001;29(6):751–761. [DOI] [PubMed] [Google Scholar]
- 35. Yoshikawa T, Tohyama H, Enomoto H, et al. Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):804–810. [DOI] [PubMed] [Google Scholar]