Short abstract
Neuropeptide Y signaling plays an important role in inhibiting chronic pain in the spinal cord of mice. However, little is known about the respective roles of two major neuropeptide Y receptors, Y1R and Y2R, in evoked and spontaneous pain behavior under normal physiological condition. Using intrathecal administration approach, we found that pharmacological inhibition of Y2R, unexpectedly, gave rise to spontaneous pain behavior. In addition, Y2R antagonism also resulted in long-lasting mechanical but not thermal hypersensitivity. By contrast, neither overt spontaneous pain behavior nor mechanical and thermal hypersensitivity were detected after pharmacological inhibition of Y1R. Remarkably, the activation of Y1R produced powerful analgesic effect: blocking both evoked and spontaneous pain behavior resulted from Y2R antagonism. These findings highlight the pivotal role of endogenous Y2R in gating mechanical and spontaneous pain transmission. Importantly, our results suggest that Y1R could be a therapeutic target that may be exploited for alleviating spontaneous pain without affecting acute pain transmission.
Keywords: NPY, Y2R, Y1R, pain, spinal cord
Introduction
Spinal nociceptive transmission involves a wide variety of neuropeptides and transmitters. Neuropeptide Y (NPY), a 36-amino acid peptide, is a major signaling peptide that inhibits nociceptive and itch transmission.1–4 NPY receptor family comprises five members in mammals with relative low sequence similarity.4 NPY is barely detectable in sensory neurons, but present in GABAergic neurons of the spinal cord.5–9 In the dorsal root ganglion (DRG) neurons and the spinal cord, major NPY receptors identified are Y1R and Y2R, two Gi/o protein-coupled receptors (GPCRs) whose activation results in inhibition of chronic pain transmission.3,10 Y2R is expressed in a subset of medium- and large-size peptidergic A-fiber nociceptors, which peripherally innervate hairy and glabrous skin and centrally lamina II of the spinal cord.10,11 In contrast, Y1R is expressed in small-diameter nociceptors in DRGs,11 and in somatostatin (SOM)-positive excitatory interneurons in laminae I–II of the spinal cord.6,12,13 Y1R mRNA or Y1R signaling is upregulated in DRG neurons and the spinal cord under inflammatory and neuropathic pain conditions in rodents.5,14,15 Intrathecal (i.t.) activation of Y1R has been shown to inhibit both chemical and mechanical itch16,17 as well as mechanical and cold allodynia in neuropathic pain models.18 The NPY receptor system is important for tonic inhibition of inflammatory and neuropathic pain.19
Although numerous studies using i.t. administration of NPY have suggested an important role of NPY signaling in pain transmission, the respective role of Y1R or Y2R is unclear. Moreover, prior research on Y2R signaling has relied on mechanical and thermal stimuli-evoked reflex measurement in the setting of chronic pain conditions, leaving its function in gating pain transmission under normal physiological condition undetermined. Using highly selective Y2R and Y1R antagonists, the present study aims to determine the function of Y1R and Y2R in nociceptive transmission under normal physiological condition.
Materials and methods
Animals
Experiments were carried out on 7- to 12-week-old male C57BL/6J mice (Jackson Labs, Sacramento, CA). All mice were housed in clear plastic cages with no more than five mice per cage in a controlled environment at a constant temperature of ∼23°C and humidity of 50 ± 10% with a light–dark cycle of 12 h–12 h. The animals had food and water available ad libitum. All experiments conform to guidelines set by the National Institutes of Health and the International Association for the Study of Pain and were reviewed and approved by the Institutional Animal Care and Use Committee at Washington University School of Medicine. All the efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs
Y2R antagonist BIIE0246, Y1R antagonist BIBO3304, and Y1R agonist LP-NPY were purchased from Tocris (MN, USA). GRP18-27 was from Bachem (CA, USA). Morphine was from Hospira (IL, USA). BIIE0246 and BIBO3304 were first dissolved in dimethylsulfoxide and then diluted in sterile saline for injections. LP-NPY and gastrin-releasing peptide (GRP) were dissolved in sterile saline.
Acute scratching behavior
I.t. injections and acute scratching behaviors were performed as previously described.20,21 Briefly, the caudal paralumbar region of the mice was shaved at least three days before experiments. Mice were placed in a plastic box (10 × 11 × 15 cm) for 30 min per day to acclimate for three days. On the testing day, mice were given 15 min to acclimate in the box prior to i.t. injections using a 30-gauge needle inserting into the fifth intervertebral space. The injection volume was 10 μl. Mice were returned to the box and the behaviors were videotaped from a side angle. An observer blinded to the treatments of mice quantified the number of scratching behaviors. One scratch is defined as a lifting of the hind limb toward the body and then a shifting of the limb to the mouth or back to the floor, regardless of how many scratching strokes take place between those two movements.
Rotarod test
A rotarod system of accelerating treadmills was used to assess coordinate motor activity and general motor disability as described.22 Mice were trained to maintain its belaying walking on a rotarod apparatus at five revolution per minute (r/min) for 5 min on the first two days. On the third day, mice received i.t. injections of BIIE0246 or LP-NPY. Two hours after BIIE0246 injection or 15 min after LP-NPY injection, the mice were tested for three trials at accelerating speed (5–40 r/min) with 15-min intervals. The latencies of mice to fall off were recorded for analysis. The cut-off time was 300 s.
Acute pain behavior tests
Mice were habituated to the wire mash 1 h per day for three days before the test. Mechanical sensitivity was assessed using a set of calibrated von Frey filaments (North Coast, CA, USA). Each filament was applied five consecutive times with 10-s intervals and the smallest filament that evoked reflexive flinches of the hindpaw on three of five trials was taken as paw withdrawal threshold.
Thermal sensitivity was determined using a Hargreaves apparatus. The hindpaw withdrawal latency to a beam of radiant heat was measured three times with 10-min intervals and averaged for analysis.
Statistical analysis
All the data were presented as means ± standard error of the mean. Statistical tests are indicated in figure legends. Two-tailed, unpaired Student’s t test was used for two-group comparisons. One-way analysis of variance (ANOVA) with Tukey's post hoc was used for the comparisons of more than two groups. Two-way ANOVA with Tukey's post hoc was used for multiple elements comparisons. Statistical analyses were performed using Prism 7 (v7.0c, GraphPad, San Diego, CA). Normality and equal variance tests were performed for all statistical analyses. A value of p < 0.05 was considered statistically significant.
Results
Pharmacological inhibition of Y2R in the spinal cord induces spontaneous pain behavior
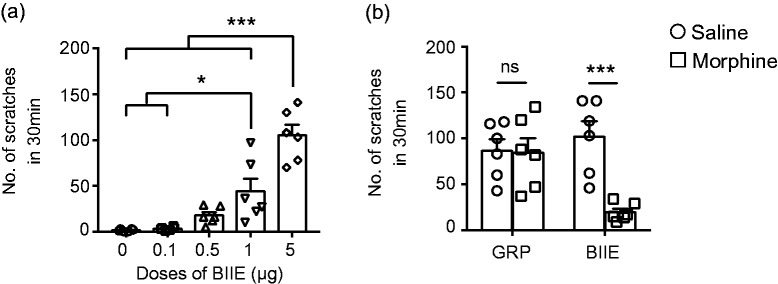
To examine the role of Y2R in nociceptive transmission under normal physiological condition, we tested the effect of a highly selective and potent Y2R antagonist, BIIE0246 (thereafter referred to as BIIE),23 by i.t. injection of BIIE into wild-type C57 mice. At 0.1 μg, no overt behavior was observed within 30 min (Figure 1(a)). At 0.5 μg, mice exhibited slightly increased scratching behavior, but not statistically significant as compared to the saline control (Figure 1(a)). However, significant increase of scratching behavior was detected at 1 μg and 5 μg, (Figure 1(a)). To ascertain whether inhibition of Y2R-induced spontaneous scratching behavior reflects itch or pain, we tested the effect of i.t. morphine (0.3 nmol) on BIIE-ineduced scratching behavior. Gastrin-releasing peptide receptor (GRPR) is a Gq protein-coupled receptor expressed in the spinal cord and is important for mediating nonhistaminergic itch transmission.20,21,24 I.t. GRP-induced scratching behavior and morphine-mediated analgesia are mediated through distinct molecular and neural pathways in the spinal cord.25,26 While morphine failed to inhibit GRP-induced scratching behavior, it significantly attenuated spontaneous scratching behavior induced by BIIE (Figure 1(b)). These data suggested that BIIE treatment resulted in spontaneous pain behavior, manifested in pain-related scratching behavior, and the effect was mediated by Y2R because it was dose dependent.
Figure 1.
Inhibition of Y2R in the spinal cord induced pain-related scratching behaviors. (a) I.t. injections of Y2R antagonist, BIIE (0.1 μg, 0.5 μg, 1 μg, and 5 μg), induced dose-dependent scratching behaviors. n = 6 mice per group. *p < 0.05, ***p < 0.001, one-way ANOVA with Tukey’s post hoc tests. (b) Preinjection of morphine (0.3 nmol, i.t.) for 30 min inhibited the scratching behaviors induced by i.t. injection of BIIE (5 μg) but not GRP (0.1 nmol). n = 6 mice per group. ns: not significant, ***p < 0.001, two-way ANOVA with Tukey’s post hoc tests. GRP: gastrin-releasing peptide.
Pharmacological inhibition of Y2R in the spinal cord induces mechanical allodynia which could be inhibited by morphine
To examine whether BIIE treatment may affect the motor function of mice, we examined mouse behaviors using the rotarod test after i.t. injections of a series of dose of BIIE. For the mice treated with BIIE up to 1 μg, no major difference in the fall off latencies was observed (Figure 2). However, BIIE at 5 μg severely blunted the rotarod test (Figure 2). To test the effect of BIIE on acute mechanical sensitivity, we examined the threshold of evoked hindpaw withdrawal responses using von Frey test. Remarkably, BIIE at 0.1 μg showed significantly reduced withdrawal threshold, which lasted for at least 6 h, as compared to the saline control (Figure 3(a)). Interestingly, there was no major difference in the threshold of evoked paw withdrawal responses between 0.1, 0.5, and 1 μg (Figure 3(a)), indicating that BIIE has reached the ceiling effect at 0.1 μg. Next, we examined the thermal pain behavior of these mice treated with BIIE using Hargreaves test and found no significant difference in the thermal sensitivity compared to the saline control (Figure 3(b)). We also examined whether acute mechanical pain could be reversed by morphine and found that coinjection of morphine significantly attenuated BIIE-induced mechanical hypersensitivities at the first 2 h (Figure 3(c)). The lack of analgesia effect of morphine at late phase could be attributable to wear off the drug.
Figure 2.
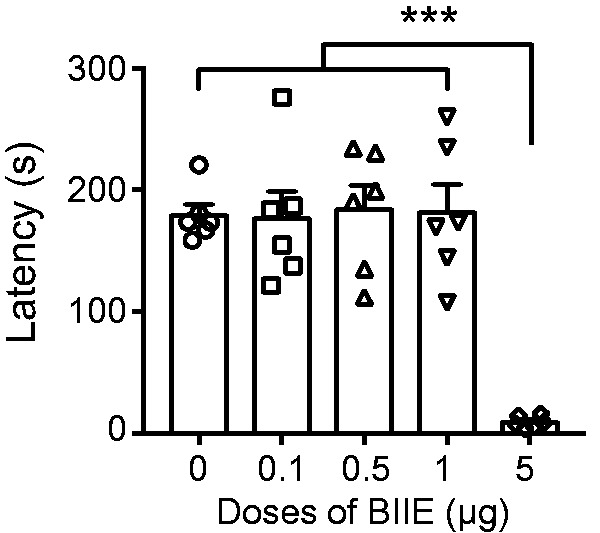
High dose of Y2R antagonist impaired motor function. I.t. injection of BIIE at 5 μg significantly decreased the fall off latencies in rotarod test compared with saline and low doses of BIIE (0.1 μg, 0.5 μg and 1 μg). n = 6 mice per group. ***p < 0.001, one-way ANOVA with Tukey’s post hoc test.
Figure 3.
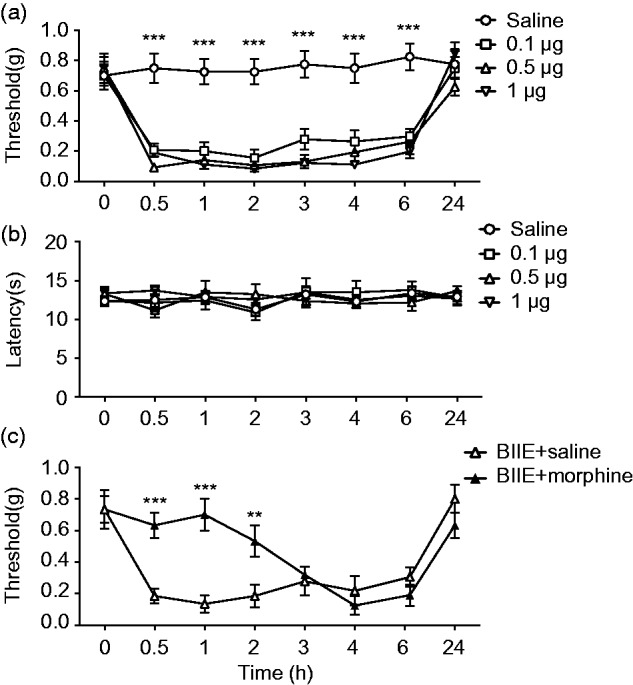
Inhibition of Y2R in the spinal cord induced mechanical allodynia. Von Frey test (a) and Hargraves test (b) showed that i.t. injections of BIIE at 0.1 μg, 0.5 μg, or 1 μg significantly decreased the mechanical pain threshold for at least 6 h (a), while thermal pain sensitivity was not affected (b). n = 6–8 mice per group, ***p < 0.001, two-way ANOVA with Tukey’s post hoc test. (c) Coinjection of morphine (0.3 nmol) significantly blocked 0.1 μg BIIE-induced mechanical hypersensitivity for the first 2 h. n = 6–8 mice per group. **p < 0.01, ***p < 0.001, two-way ANOVA with Tukey’s post hoc test.
Pharmacological activation of Y1R in the spinal cord reverses mechanical allodynia induced by BIIE
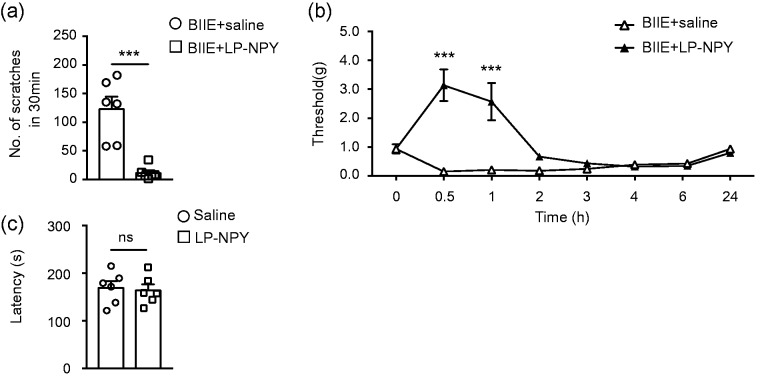
To examine the relationship between the Y1R and Y2R, we first tested whether the activation of Y1R could inhibit spontaneous pain behavior unmasked by Y2R antagonism. Strikingly, following i.t. administration of a highly selective Y1R agonist, LP-NPY (1 nmol),27 no spontaneous pain behavior was detected as a result of BIIE administration (Figure 4(a)). These results suggest that Y1R activation, or inhibition of Y1R neurons, precludes spontaneous pain transmission as a result from Y2R antagonism. Next, we tested whether the activation of Y1R could reverse mechanical allodynia following BIIE administration or Y2R antagonism. Remarkably, i.t. LP-NPY treatment not only block the effect of BIIE but further increased the mechanical threshold up to around 3 g and 2.5 g after injection 30 min and 1 h, respectively (Figure 4(b)). Importantly, mice received LP-NPY injections were apparently normal and the fall off latencies on rotarod were comparable between LP-NPY group and saline group (Figure 4(c)). Thus, pharmacological activation of Y1R, or inhibition of Y1R neurons, could result in potent analgesic effect stronger than either i.t. morphine (Figure 3(c)) or ablation of spinal SOM neurons.28
Figure 4.
The activation of Y1R in the spinal cord alleviated BIIE-induced pain behaviors. (a) Coinjection of Y1R agonist, LP-NPY (1 nmol) significantly decreased scratching behavior induced by BIIE (5 μg) treatment. n = 6 mice per group. ***p < 0.001, unpaired t test. (b) Coinjection of LP-NPY (1 nmol) reversed mechanical hypersensitivity induced by BIIE (0.1 μg) treatment for 1 h. n = 6 mice per group. ***p < 0.001, two-way ANOVA with Tukey’s post hoc test. (c) I.t. injection of LP-NPY (1 nmol) did not affect the fall off latencies in rotarod test compared with saline group. n = 6 mice per group. ns: not significant, unpaired t test.
Pharmacological inhibition of Y1R in the spinal cord does not induce spontaneous, tactile, or thermal pain behavior
Lastly, we investigated whether inactivation of Y1R may alter nociceptive transmission by i.t. administration of BIBO 330429 (thereafter referred to as BIBO), a highly selective Y1R antagonist that can significantly reinstate mechanical and thermal hypersensitivity of mice with chronic pain.19 In contrast to BIIE, BIBO had no significant effect on spontaneous scratching behavior compared to the control (Figure 5(a)). However, pretreatment of BIBO (1 µg) increased the spontaneous pain behavior inhibited by LP-NPY in BIIE-treated mice (Figure 5(b)). I.t. BIBO (1 µg) also failed to alter mechanical and thermal sensitivities under normal physiological condition (Figure 5(c) and (d)), a finding consistent with the previous study.19
Figure 5.
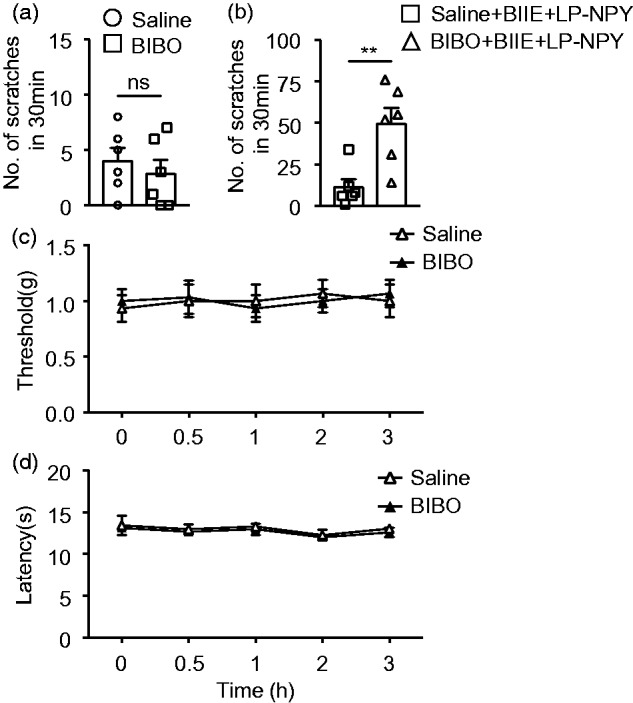
Inhibition of Y1R in the spinal cord did not affect mechanical or thermal sensitivities. (a) I.t. injection of Y1R antagonist BIBO (1 μg) did not induce scratching behavior. n = 6 mice per group, ns: not significant, unpaired t test. (b) Preinjection of BIBO (1 μg) for 15 min blocked the inhibiting effect of LP-NPY (1 nmol) on BIIE-induced scratching behaviors. n = 6 mice per group. **p < 0.01, unpaired t test. I.t. injections of BIBO (1 μg) had no effect on the mechanical sensitivity as tested by von Frey test (c) or thermal sensitivity as tested by Hargreaves test (d). n = 6 mice per group, two-way ANOVA with Tukey’s post hoc test.
Discussion
The present study shows that Y2R is a pivotal inhibitory GPCR that gates the nociceptive transmission under normal physiological condition. There are two distinct aspects of Y2R function in gating nociceptive transmission. First, pharmacological inhibition of Y2R results in disinhibition of nociceptive transmission, which manifests in spontaneous pain behaviors. This indicates that the endogenous NPY-Y2R signaling pathway exerts powerful tonic inhibition of nociceptive circuitry at the spinal level under normal physiological condition. To the best of our knowledge, this is the first description of spontaneous pain behavior after inhibition of a GPCR in the spinal cord under normal physiological condition. Second, the finding that pharmacological inhibition of Y2R activity causes mechanical but not thermal hypersensitivity uncovers a unique role of Y2R signaling in gating mechanical pain, under normal physiological condition. Prior studies have shown that the delta opioid receptor (DOR) and mu opioid receptor (MOR) regulate mechanical and thermal hypersensitivity in DRGs and spinal cord, respectively.30,31 DOR is expressed in myelinated nonpeptidergic fibers, whereas MOR in small peptidergic pain fibers.31 Our observation of the selective involvement of Y2R in mechanical but not thermal pain is reminiscent of the role of DOR. Interestingly, DOR is expressed in spinal SOM+ neurons that gate mechanical but not thermal pain.28,30 Therefore, it will be of interest to determine whether Y2R is coexpressed with DOR or marks distinct subset of dorsal horn neurons in future studies. It is possible that Y2R neurons are integral part of the microcircuits that gate mechanical pain. Combined with the important role of Y2R in the development and maintenance of inflammatory and neuropathic pain,19 Y2R has emerged as a key player in regulating spontaneous, acute mechanical, and chronic pain transmission.
Because Y2R is expressed in both DRGs and the spinal cord,10,11 the action site of BIIE remains unclear. However, given NPY is not detectable in DRGs,32,33 endogenous spinal NPY is likely to be a major source for targeting spinal Y2R rather than presynaptic Y2R. By contrast, Y2R in DRGs may function peripherally rather than centrally.10 Recently, Arcourt et al. showed that the activation of peripheral Y2R fibers induces mechanical pain,10 raising the possibility that Y2R may have similar function both peripherally and centrally. It will be of interest to determine whether peripheral Y2R may be dedicated to gating nociceptive transmission.
Our finding reveals distinct roles of Y2R and Y1R in nociceptive transmission. Unlike Y2R, Y1R is dispensable for gating spontaneous pain and mechanical pain. On the other hand, the observation that the activation of Y1R neurons could block spontaneous and mechanical pain unmasked by disinhibition of Y2R neurons implies that Y1R neurons nevertheless function downstream of Y2R neurons. This suggests that additional inhibitory signaling mechanisms are likely to work in concert in Y1R neurons to gate nociceptive transmission. Indeed, that inhibition of Y1R neurons by pharmacological activation of Y1R resulted in analgesic effect more potent than either i.t. morphine or ablation of spinal SOM neurons28 is in support of this notion. Nevertheless, one should be cautious when inferring the endogenous role of a GPCR from behavior resulted from pharmacological activation.
One interesting observation is that depending on the behavioral tests, the same dose of BIIE may have discrete effects. For example, while i.t. 5 μg is too high for mice to endure rotarod test, it does not preclude mice from performing hindlimb-directed pain-related scratching behavior. It is also interesting that while the ceiling effect on acute mechanical allodynia was observed at 0.1 μg, this dose is insufficient to cause spontaneous pain behavior. Nevertheless, the data point to a much more potent inhibition of Y2R for breaking the gate to enable spontaneous than mechanical pain transmission.
Elucidation of spinal inhibitory neuropeptide receptor signaling mechanisms by which nociceptive transmission is gated in the spinal cord has been technically challenging for several reasons. First, a conventional or even conditional knockout of a peptide or receptor approach always risks developmental compensatory effect when no overt phenotype is detected. Second, pharmacological manipulation of GPCR is of limited use because highly selective antagonists/agonists are often not available for many GPCRs. By taking advantage of highly specific antagonists for Y1/2R and agonist for Y1R, we are able to dissect the respective roles of Y2R and Y1R in gating nociceptive transmission under physiological condition. While Y2R is a pivotal receptor that gates spontaneous and mechanical pain, Y1R may be a preferred therapeutic target that could be harnessed for alleviating spontaneous and mechanical pain without impacting acute pain transmission.
Author Contributions
S Chen and X Liu designed experiments and analyzed data; Y Jiao provided advices on the project; S Chen performed experiments; Z Chen and W Yu conceived, supervised the project, and wrote the manuscript. All authors read and approved the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project was financially supported by the NIH grants (1R01AR056318-06, R01NS094344, and R01 DA037261-01A1 to Z Chen) and National Natural Science Foundation of China (Grant No. 81571048 to W Yu).
References
- 1.Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides 2007; 28: 464–474. [DOI] [PubMed] [Google Scholar]
- 2.Duan B, Cheng LZ, Ma QF. Spinal circuits transmitting mechanical pain and itch. Neurosci Bull 2018; 34: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-delCastillo M, Woldbye DP, Heegaard AM. Neuropeptide Y and its involvement in chronic pain. Neuroscience 2018; 387: 162–169. [DOI] [PubMed] [Google Scholar]
- 4.Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. Embo Mol Med 2010; 2: 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J Neurosci 1994; 14: 6423–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Tong Y-G, Bao L, Hokfelt T. The neuropeptide Y Y1 receptor is a somatic receptor on dorsal root ganglion neurons and a postsynaptic receptor on somatostatin dorsal horn neurons. Eur J Neurosci 1999; 11: 2211–2225. [DOI] [PubMed] [Google Scholar]
- 7.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 2015; 350: 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laing I, Todd AJ, Heizmann CW, Schmidt HHHW. Subpopulations of GABAergic neurons in laminae I-III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience 1994; 61: 123–132. [DOI] [PubMed] [Google Scholar]
- 9.Boyle KA, Gutierrez-Mecinas M, Polgár E, Mooney N, O’Connor E, Furuta T, Watanabe M, Todd AJ. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 2017; 363: 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, Lechner SG. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron 2017; 93: 179–193. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Shi T, Holmberg K, Landry M, Huang W, Xiao H, Ju G, Hokfelt T. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proc Natl Acad Sci USA 1997; 94: 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamessian A, Young M, Qadri Y. Transcriptional profiling of somatostatin interneurons in the spinal dorsal horn. Sci Rep 2018; 8: 6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson TS, Fu W, Donahue RR, Corder GF, Hökfelt T, Wiley RG, Taylor BK. Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci Rep 2019; 9: 7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor BK, Fu W, Kuphal KE, Stiller C-O, Winter MK, Chen W, Corder GF, Urban JH, McCarson KE, Marvizon JC. Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons. Neuroscience 2014; 256: 178–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marvizon JC, Chen W, Fu W, Taylor BK. Neuropeptide Y release in the rat spinal cord measured with Y1 receptor internalization is increased after nerve injury. Neuropharmacology 2019; 158: 107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao T, Ma H, Xu B, Bergman J, Larhammar D, Lagerström MC. The neuropeptide Y system regulates both mechanical and histaminergic itch. J Invest Dermatol 2018; 138: 2405–2411. [DOI] [PubMed] [Google Scholar]
- 17.Acton D, Ren X, Di Costanzo S, Dalet A, Bourane S, Bertocchi I, Eva C, Goulding M. Spinal neuropeptide Y1 receptor-expressing neurons form an essential excitatory pathway for mechanical itch. Cell Rep 2019; 28: 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malet M, Leiguarda C, Gastón G, McCarthy C, Brumovsky P. Spinal activation of the NPY Y1 receptor reduces mechanical and cold allodynia in rats with chronic constriction injury. Peptides 2017; 92: 38–45. [DOI] [PubMed] [Google Scholar]
- 19.Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A 2011; 108: 7224–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007; 448: 700–703. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y-G, Zhao Z-Q, Meng X-L, Yin J, Liu X-Y, Chen Z-F. Cellular basis of itch sensation. Science 2009; 325: 1531–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malmberg AB, Gilbert H, McCabe TR, Basbaum AI. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain 2003; 101: 109–116. [DOI] [PubMed] [Google Scholar]
- 23.Dumont Y, Cadieux A, Doods H, Pheng LH, Abounader R, Hamel E, Jacques D, Regoli D, Quirion R. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y(2) receptor antagonist. Br J Pharmacol 2000; 129: 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan L, Jin H, Liu X-Y, Jeffry J, Barry DM, Shen K-F, Peng J-H, Liu X-T, Jin J-H, Sun Y, Kim R, Meng Q-T, Mo P, Yin J, Tao A, Bardoni R, Chen Z-F. Distinct roles of NMB and GRP in itch transmission. Sci Rep 2017; 7: 15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X-Y, Ginosar Y, Yazdi J, Hincker A, Chen Z-F. Cross-talk between human spinal cord mu-opioid receptor 1Y isoform and gastrin-releasing peptide receptor mediates opioid-induced scratching behavior. Anesthesiology 2019; 131: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X-Y, Liu Z-C, Sun Y-G, Ross M, Kim S, Tsai F-F, Li Q-F, Jeffry J, Kim J-Y, Loh HH, Chen Z-F. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 2011; 147: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuhlendorff J, Gether U, Aakerlund L, Langeland-Johansen N, Thogersen H, Melberg SG, Olsen UB, Thastrup O, Schwartz TW. [Leu31, Pro34]neuropeptide Y: a specific Y1 receptor agonist. Proc Natl Acad Sci U S A 1990; 87: 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014; 159: 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol 1998; 125: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Tawfik VL, Corder G, Low SA, François A, Basbaum AI, Scherrer G. Functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron 2018; 98: 90–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherrer G, Imamachi N, Cao Y-Q, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009; 137: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett 1991; 124: 200–203. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Nicholas AP, Hökfelt T. Ultrastructural studies on peptides in the dorsal horn of the spinal cord–I. Co-existence of galanin with other peptides in primary afferents in normal rats. Neuroscience 1993; 57: 365–384. [DOI] [PubMed] [Google Scholar]