Short abstract
Background
The antiseizure racetams may provide novel molecular insights into neuropathic pain due to their unique mechanism involving synaptic vesicle glycoprotein 2A. Anti-allodynic effects of levetiracetam have been shown in animal models of neuropathic pain. Here, we studied the effect of brivaracetam, which binds to synaptic vesicle glycoprotein 2A with 20-fold greater affinity, and has fewer off-target effects.
Methods
Mice underwent unilateral sciatic nerve cuffing and were evaluated for mechanical sensitivity using von Frey filaments. Pain behaviors were assessed with prophylactic treatment using levetiracetam (100 or 10 mg/kg) or brivaracetam (10 or 1 mg/kg) beginning after surgery and continuing for 21 days, or with therapeutic treatment using brivaracetam (10 or 1 mg/kg) beginning on day 14, after allodynia was established, and continuing for 28 or 63 days. Spinal cord tissues from the prophylaxis experiment with10 mg/kg brivaracetam were examined for neuroinflammation (Iba1 and tumor necrosis factor), T-lymphocyte (CD3) infiltration, and synaptic vesicle glycoprotein 2A expression.
Results
When used prophylactically, levetiracetam, 100 mg/kg, and brivaracetam, 10 mg/kg, prevented the development of allodynia, with lower doses of each being less effective. When used therapeutically, brivaracetam extinguished allodynia, requiring 10 days with 10 mg/kg, and six weeks with 1 mg/kg. Brivaracetam was associated with reduced neuroinflammation and reduced T-lymphocyte infiltration in the dorsal horn. After sciatic nerve cuffing, synaptic vesicle glycoprotein 2A expression was identified in neurons, activated astrocytes, microglia/macrophages, and T lymphocytes in the dorsal horn.
Conclusion
Synaptic vesicle glycoprotein 2A may represent a novel target for neuropathic pain. Brivaracetam may warrant study in humans with neuropathic pain due to peripheral nerve injury.
Keywords: Brivaracetam, levetiracetam, neuropathic pain, astrocyte, microglia, T lymphocyte, sciatic nerve cuff model, mouse
Introduction
Neuropathic pain, which is characterized by dysesthesia, hyperalgesia, and allodynia, encompasses a wide range of heterogeneous conditions caused by lesions or diseases of the somatosensory nervous system, either at the periphery or centrally.1 Neuropathic pain that occurs after peripheral nerve injury arguably may be the most troubling type, since it is often refractory to treatment and thus is especially burdensome clinically.2 Numerous classes of drugs have been utilized for the treatment of neuropathic pain, including many anticonvulsants.3 Among anticonvulsants, the racetams used for seizure disorders may be of particular interest, due to their unique mechanism of action involving synaptic vesicle glycoprotein 2A (SV2A), a membrane glycoprotein localized to secretory vesicles in neurons, endocrine, and other cells.4–6
Antihyperalgesic and anti-allodynic effects of the antiseizure racetam, levetiracetam (LEV), have been demonstrated in two animal models of chronic neuropathic pain: sciatic nerve (n.) constriction7 and streptozotocin-induced diabetes.7–10 Notably, LEV was more potent in the diabetic pain model (first active dose was 17 mg/kg) than in the sciatic n. constriction model (first active dose was 540 mg/kg).7 LEV has also been found to exert analgesic effects in nonneuropathic pain models such as postsurgical pain11 and inflammatory pain.12–14 In humans, LEV was found to have analgesic effects in the electrical sural n. stimulation pain model15 and has shown efficacy in treating patients with trigeminal neuralgia,16 multiple sclerosis,17,18 and migraine.19 Notably, a recent Cochrane review failed to find the evidence of clinical efficacy for LEV based on six studies of 344 patients with six different types of neuropathic pain.20 However, the failures in these trials may have been due to the fact that numerous patients treated with LEV experienced adverse events that caused them to withdraw from the studies, precluding proper assessment of LEV’s effects on neuropathic pain.
Another anticonvulsant of the racetam group, brivaracetam (BRV; UCB 34714), has gained recognition due to its greater antiseizure potency and reduced off-target effects, compared to LEV.21–23 BRV was first reported in 2004 as a structural derivative of LEV, differing from the parent compound by a single propyl group (Figure 1).24 BRV was identified during a drug-discovery program based on SV2A binding.25,26 Like LEV, BRV binds to SV2A but with 20-fold greater affinity.27,28 BRV, similar to LEV, enters into recycling synaptic vesicles and produces a frequency-dependent decrement in synaptic transmission at 100-fold lower concentrations than LEV.29 BRV is reported to be 10 times more potent as an antiseizure medication compared to LEV.24
Figure 1.
The chemical structures of five racetam drugs found to be effective in rodent neuropathic pain models. The chemical structures shown are based on data from the National Center for Biotechnology Information; https://pubchem.ncbi.nlm.nih.gov (accessed 30 December 2018).
The effects of BRV have been reported in two rat models of neuropathic pain, albeit only as a meeting abstract.30 In both the streptozotocin-induced diabetes model and the sciatic n. constriction model, BRV (21 mg/kg) significantly increased the vocalization thresholds and completely reversed hyperalgesia. BRV (200 and 400 mg/day) was also tested in a clinical trial involving 152 subjects with postherpetic neuropathic pain, with the negative outcome of this study recently being made public.31
Here, we sought to expand on the available literature regarding BRV in neuropathic pain, focusing on pain induced by peripheral nerve injury, because this type is relatively refractory to treatment compared to diabetic pain.2,7 We used a murine model of sustained neuropathic pain induced by sciatic n. cuffing32,33 to examine the effects of BRV when given either prophylactically or therapeutically, and we evaluated not only pain behaviors but also neuroinflammation in the spinal cord, an important factor in the initiation and maintenance of pain hypersensitivity.34–37
Methods
Ethics statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research. Animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore and in accordance with the relevant guidelines and regulations as stipulated in the National Research Council Publication, Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Subjects and surgical procedure
Adult male C57BL/6 mice, approximately 22 to 25 g were obtained from Charles River (Frederick, MD, USA). Mice were given free access to food and water, except during behavioral testing. They were housed in plastic cages in specially constructed rooms with controlled humidity, exchange of air, and controlled lighting (12/12 h light/dark cycle).
For surgery, mice were anesthetized (100 mg/kg ketamine plus 10 mg/kg xylazine, intraperitoneal (IP)) and breathed room air spontaneously. Core temperature was maintained at 37°C using a heating pad (Deltaphase® Isothermal Pad, Braintree Scientific, Braintree, MA, USA). Hair was clipped from the right proximal lateral thigh, and the surgical site was prepared using iodine and alcohol. A sterile environment was maintained throughout the procedure. Lidocaine solution (2%) was injected prior to making an incision.
The procedure for sciatic n. cuffing was as previously described,32,33 with only minor modification. Using a surgical microscope, the common branch of the right sciatic n. was exposed by separating the muscles and the nerve by blunt dissection. After isolation, the nerve was gently stretched for 15 min by placing a 5-mm diameter plastic rod beneath it, which caused the nerve to blanch (see Figure 1 of Benbouzid et al.32). A 2-mm long section of PE20 tubing, presplit and gas sterilized, was placed around the nerve. After the surgical procedure, mice were nursed on a heating pad to maintain temperature approximately 37°C until they emerged from anesthesia.
Exclusions
No mice became infected, required early euthanasia or died. No mice in the vehicle-treated groups failed to develop stable mechanical allodynia. There were no exclusions.
Treatments
LEV was obtained from West-Ward Pharmaceutical Corp. (now Hikma Pharmaceuticals USA Inc., Eatontown, NJ, USA). For doses of 100 and 10 mg/kg LEV, solutions of 500 or 50 mg, respectively, in 5 mL normal saline (NS) were administered via IP injection in a volume of 25 µL once daily.9 BRV was obtained from Toronto Research Chemicals (Ontario, Canada). For doses of 10 and 1 mg/kg BRV, solutions of 25 mg in 10 or 100 mL NS were administered via IP injection in a volume of 100 µL once daily.38 IP injections were performed using a 27-gauge needle with the depth of the injection limited to 3 mm by a sleeve of PE20 tubing placed over the needle.
Sample size calculation
For mechanical allodynia, we based our sample size calculation on a previous study that used the same model of neuropathic pain but tested a different drug. Values derived from Figure 2 of Yalcin et al.33 suggested an effect size (Cohen’s d) of ≈2, where d = (M1−M2)/SDpooled, M1 and M2 are the means, and SDpooled = [(SD1+SD2)/2]½. Using the following assumptions: two-tailed hypothesis, α, 0.05; desired power, 80%; and d, 2.1, sample size calculation indicated a minimum sample size of five mice per group. This group size is similar to other reports using this model.32
Figure 2.
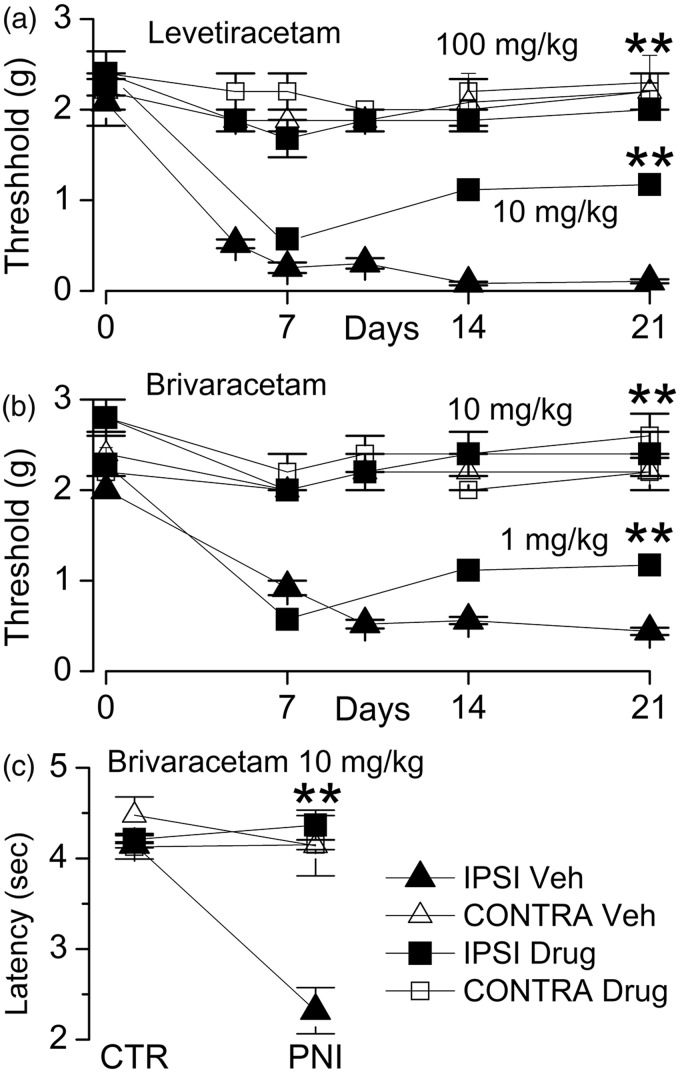
Prophylactic treatment with LEV and BRV prevents the development of mechanical allodynia and thermal hyperalgesia in the murine sciatic n. cuff model. After unilateral sciatic n. cuffing, mice were randomly assigned to receive vehicle (Veh) or LEV (100 or 10 mg/kg) or BRV (10 or 1 mg/kg) starting on the day of surgery and continuing daily until day 21; von Frey filaments were used to assess ipsilateral and contralateral hindpaw withdrawal thresholds; withdrawal thresholds (mean ± standard error) are plotted as a function of time for ipsilateral (filled symbols) and contralateral (empty symbols) hindpaws of mice receiving vehicle versus LEV (a), or vehicle versus BRV (b); five mice per group; **P < 0.01 with respect to vehicle treatment. (c) Thermal sensitivity was assessed on day 14 after sciatic n. cuffing using Hargreaves test in mice-administered vehicle versus BRV (10 mg/kg). CTR: control; PNI: peripheral n. injury; IPSI: ipsilateral; CONTRA: contralateral.
Experimental series
In series 1, 15 mice were randomly divided into three groups and were administered LEV (100 or 10 mg/kg) or vehicle (NS), with treatments beginning shortly after surgery, on postop day 0 (pod-0) and continuing daily until pod-21. In series 2, 15 mice were randomly divided into three groups and were administered BRV (10 or 1 mg/kg) or NS, with treatments beginning shortly after surgery, on postop day 0 (pod-0), and continuing daily until pod-21; tissues from these mice (BRV, 10 mg/kg and NS) were used for immunohistochemistry. In series 3, 10 mice were randomly divided into two groups and were administered BRV (10 mg/kg) or NS, with treatments beginning on pod-14 and continuing daily until pod-28. In series 4, 12 mice were randomly divided into two groups and were administered BRV (1 mg/kg) or NS, with treatments beginning on pod-14 and continuing daily until pod-63. In all cases, the noninjured hind paw served as a control.
Mechanical allodynia
Outcomes were assessed by investigators blinded to treatment group. Mice were handled two to three times weekly for acclimatization to handlers. Sensory testing was performed before surgery and at three- to five-day intervals thereafter. Mice were placed in elevated Perspex cages with a wire mesh floor (15 × 10 × 10 cm) (ITCC Life Science, Woodland Hill, CA, USA) and were acclimatized for 30 min prior to testing. The paw withdrawal threshold to mechanical stimulation of both the ipsilateral and contralateral hind paws was measured using a series of von Frey filaments, which exerted forces ranging from 0.16 to 4 g (North Coast Medical, San Jose, CA, USA). The von Frey filaments were pressed perpendicularly onto the plantar surface of the hind paw for 2 s (four times for each filament), and a positive response was noted if there was a sharp flinching of the hind paw. The “up-down method”39 was used to determine the withdrawal threshold.
Thermal hyperalgesia
Outcomes were assessed by investigators blinded to treatment group. Thermal sensitivity was assessed in mice from series 2 at 14 days after sciatic n. cuffing using the Hargreaves method and a Hargreaves-type apparatus (Plantar Test Analgesia Meter, ITCC Life Science). An unrestrained mouse was placed in a Perspex enclosure on the top of a glass pane. An infrared generator placed below glass pane was aimed at the plantar surface of the hind paw, and the time to withdrawal was recorded automatically via an optical sensor. Paw withdrawal latency was calculated as the mean of three to five different measurements taken at 15-min intervals.
Immunoblot for validation of anti-SV2A antibody
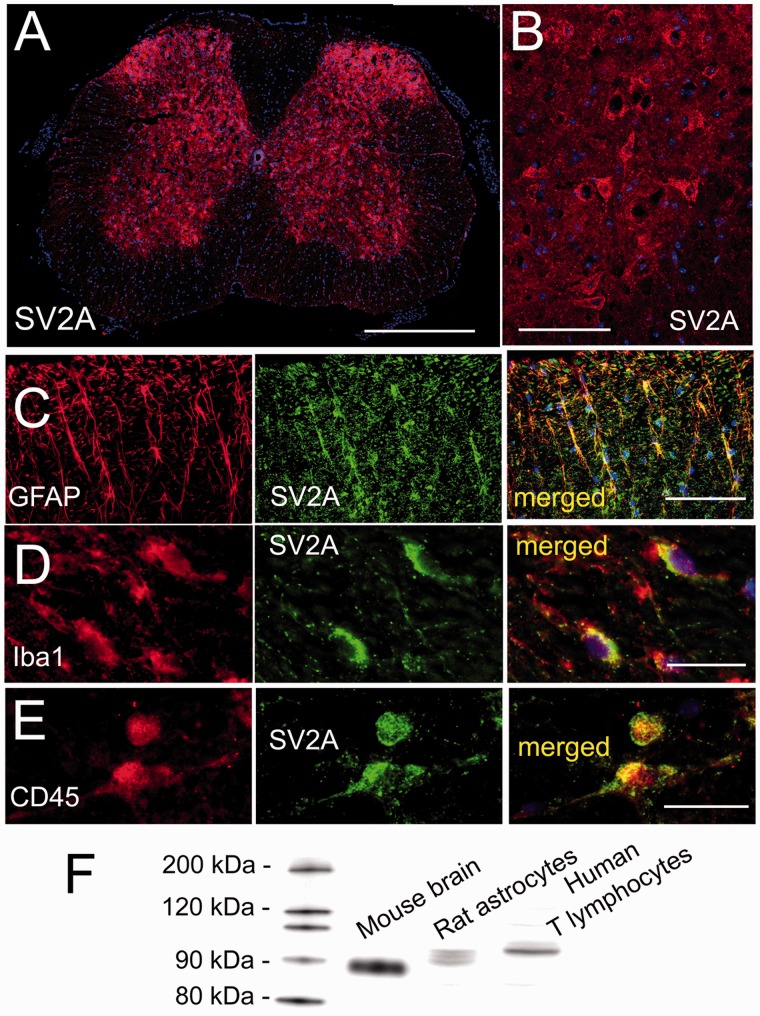
To validate the rabbit anti-SV2A antibody used for immunohistochemistry (cat#TA322365; Origene, Rockville, MD, USA), we performed an immunoblot of lysate from mouse brain, from an immortalized rat astrocyte cell line (DI TNC1; catalogue #CRL-2005; ATCC, Gaithersburg, MD, USA) and from an immortalized human T-lymphocyte cell line (TIB-152; ATCC), using standard methods as we described.40
Immunohistochemistry and quantification of specific labeling
Under deep anesthesia, mice were euthanized, underwent trans-cardiac perfusion with NS (15 mL) followed by 10% neutral buffered formalin (15 mL). Spinal cord tissues at spine segments L1 to L5 were harvested and postfixed. Tissues were cryoprotected with 30% sucrose, frozen in optimal cutting temperature (OCT), and cryosectioned (10 µm).
Immunohistochemistry was performed as described.41 Sections were incubated at 4°C overnight with primary antibodies, including rabbit anti-Iba1 (1:200; cat#019–19741; Wako, Osaka, Japan), goat antitumor necrosis factor (TNF) (1:200; cat#sc1350 (N-19); Santa Cruz Biotechnology, Sant Cruz, CA, USA), mouse antiglial fibrillary acidic protein (GFAP) (1:300; cat#C9205; Sigma, St. Louis, MO, USA), rabbit anti-CD3 (1:100; cat# AB5690; Abcam, Cambridge, UK), mouse anti-CD45 (1:50; cat# 05–1410; EMD Millipore, Temecula, CA, USA), and rabbit anti-SV2A (1:50; cat#TA322365; Origene).
After several rinses in phosphate-buffered saline, sections were incubated with species-appropriate fluorescent secondary antibodies (Alexa Fluor 488 and 555, Molecular Probes; Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. Controls included the omission of primary antibodies.
Unbiased measurements of specific labeling within regions of interest (ROI) were obtained using NIS-Elements AR software (Nikon Instruments, Melville, NY, USA) from sections immunolabeled as a single batch. All images for a given signal were captured using uniform parameters of magnification, area, exposure, and gain. Segmentation analysis was performed by computing a histogram of pixel intensity for a particular ROI, and pixels were classified as having specific labeling based on signal intensity greater than two times that of background. The area occupied by pixels with specific labeling was used to determine the percentage area in the ROI with specific labeling (% ROI). For Iba1 and TNF, the ROI was a rectangle, 500 × 400 µm, positioned at the dorsal edge of the dorsal horn. For CD3, individual CD3+ cells were counted manually as described42 in two distinct areas: the dorsal horn and the remainder of the gray matter, both ipsilateral and contralateral.
Statistics
Nominal data are presented as mean ± standard error. Nominal data were analyzed using a t test or analysis of variance with post hoc Bonferroni correction, as appropriate. Statistical tests were performed using Origin Pro (V8; OriginLab, North Hampton, MA, USA). Significance was assumed if P < 0.05.
Results
LEV and BRV prophylaxis
LEV (540 mg/kg) was tested previously in a rat model with chronic constriction of the sciatic n.7 Here, we tested LEV (100 or 10 mg/kg) in a mouse model with sciatic n. cuffing. LEV or vehicle was administered beginning shortly after sciatic n. cuffing and daily thereafter. Von Frey filaments were used to assess ipsilateral and contralateral hindpaw withdrawal thresholds at several day intervals up to 21 days. In mice that were administered vehicle, sciatic n. cuffing gave rise to mechanical allodynia involving the ipsilateral hindpaw that developed over the course of 7 days and that persisted for the remainder of the 21-day duration of the experiment (Figure 2(a)). Mice that were administered LEV, 10 mg/kg, exhibit allodynia that was significantly less severe than vehicle-treated animals. However, in mice that were administered LEV, 100 mg/kg, hypersensitivity of the ipsilateral hindpaw to mechanical stimuli failed to develop, and withdrawal thresholds were not different from those of the contralateral (uninjured) hindpaw (Figure 2(a)).
We repeated this experiment, except that LEV was replaced by BRV (10 or 1 mg/kg daily). Again, in control mice that were administered vehicle, sciatic n. cuffing gave rise to mechanical allodynia involving the ipsilateral hindpaw that persisted (Figure 2(b)). Mice that were administered BRV, 1 mg/kg, exhibit allodynia that was significantly less severe than vehicle-treated animals. However, in mice that were administered BRV (10 mg/kg daily) beginning at the time of sciatic n. cuffing, hypersensitivity of the ipsilateral hindpaw to mechanical stimuli failed to develop, and withdrawal thresholds were not different from the contralateral (uninjured) hindpaw (Figure 2(b)).
We also tested thermal sensitivity in the mice that had received BRV (10 mg/kg daily) versus vehicle. Thermal sensitivity was assessed on day 14 using Hargreaves test. Compared to the contralateral hindpaw, the ipsilateral hindpaw of vehicle-treated mice exhibited significant thermal hyperalgesia (Figure 2(c)). By contrast, BRV-treated mice exhibited ipsilateral thermal sensitivity that was not different from the contralateral (uninjured) hindpaw (Figure 2(c)).
Neuroinflammation and T-cell infiltration
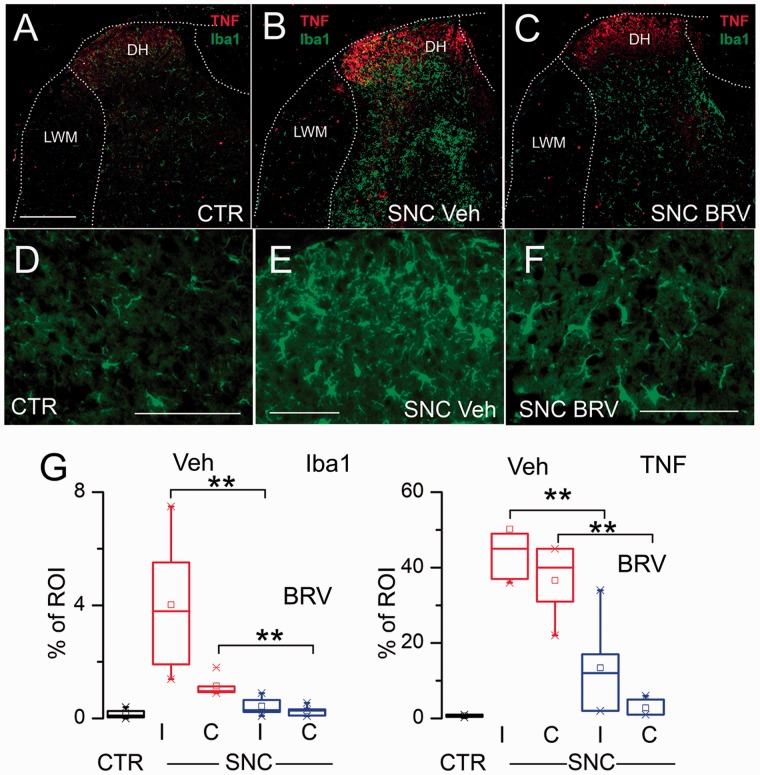
Neuroinflammation with microglial activation is a key component of neuropathic pain37,43 and is reportedly ameliorated by LEV.10 We studied tissues from mice in the foregoing experiment administered BRV (10 mg/kg daily) versus vehicle. The involved spinal cord segment was immunolabeled for Iba1 and colabeled for TNF. In the dorsal horn of uninjured controls, Iba1+ microglia were sparsely distributed and exhibited a ramified, nonreactive morphology, consistent with a quiescent phenotype; minimal immunoreactivity for TNF was evident (Figure 3(a) and (d)). After sciatic n. cuffing, numerous Iba1+ cells with a plump, activated morphology were evident in the ipsilateral dorsal horn and to a lesser extent in the contralateral dorsal horn, and immunoreactivity for TNF was greatly increased (Figure 3(b) and (e)). In mice with sciatic n. cuffing treated with BRV, Iba1+ cells were less prominent, and these cells exhibited less-plump morphologies; TNF immunoreactivity was also less prominent (Figure 3(c) and (f)). Quantification of Iba1 and TNF immunoreactivity confirmed a significant increases in vehicle-treated mice with sciatic n. cuffing compared to uninjured controls and significant decreases in mice with sciatic n. cuffing treated with BRV, both ipsilateral and contralateral (Figure 3(g)).
Figure 3.
Treatment with BRV attenuates DH neuroinflammation in the murine SNC model. Double immunolabeling for Iba1 (green) and TNF (red) of the involved segment from an uninjured control mouse (a), a mouse with SNC-administered vehicle (Veh) (b), and a mouse with SNC-administered BRV (10 mg/kg) (c). High-magnification views of Iba1+ cells from an uninjured control mouse (d), a mouse with SNC-administered vehicle (Veh) (e), and a mouse with SNC-administered BRV (f); bars: 250 μm in (a) to (c), and 100 µm in (d) to (f). (g) Quantification of Iba1 and TNF immunoreactivity in ipsilateral (I) and contralateral (C) DH of uninjured controls, mice with SNC-administered vehicle (Veh), and mice with SNC-administered BRV (10 mg/kg); five to six mice per group; **P < 0.01. CTR: control; BRV: brivaracetam; SNC: sciatic n. cuffing; DH: dorsal horn; TNF: tumor necrosis factor; LWM: lateral white matter; ROI: regions of interest.
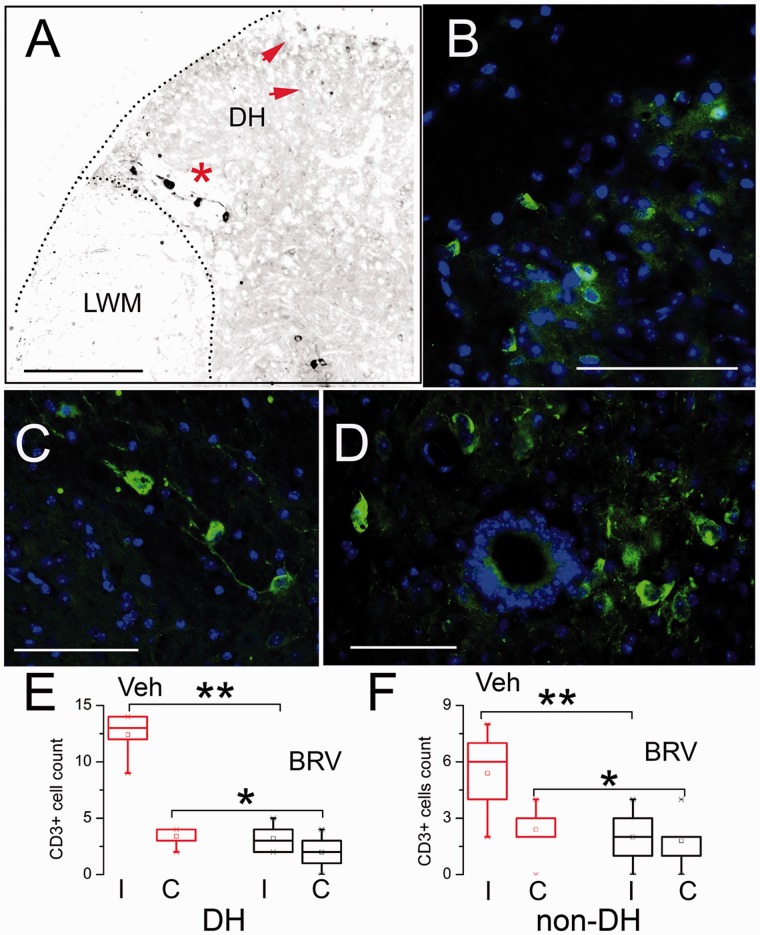
T lymphocytes (T cells) comprise an important component of the inflammatory response to nerve injury.42,44–46 Immunolabeling for CD3 showed small clusters of cells at the involved segment, located predominantly in the ipsilateral dorsal horn, often at the lateral gray-white junction (DH-LGWJ) (Figure 4(a) to (c)), as well as near the central canal (Figure 4(d)).42 Occasionally, a few CD3+ cells could also be identified in the mirror location contralaterally. Counts of CD3+ cells showed a significant increase in the dorsal horn as well as outside the dorsal horn of mice with sciatic n. cuffing treated with vehicle, compared to uninjured controls (Figure 4(e) and (f)). Treatment with BRV resulted in significant reductions in CD3+ cells in all regions (Figure 4(e) and (f)).
Figure 4.
Treatment with BRV reduces spinal cord infiltration of CD3+ cells in the murine sciatic n. cuff model. Low-magnification (a) and high-magnification (b and c) views of the ipsilateral dorsal horn of two mice with sciatic n. cuffing-administered vehicle, immunolabeled for CD3; note several small CD3+ cells in the dorsal most part of the dorsal horn (red arrows), and several larger CD3+ cells at the dorsal horn lateral gray-white junction (red asterisk), the latter shown at high magnification in (c). (d) High-magnification view of the central canal, identified by the circle of 4′,6-diamidino-2-phenylindole (blue) positive ependymal cells, with nearby clusters of CD3+ cells (green); bars: 250 μm in (a), and 100 μm in (b) to (d). (e and f) Quantification of CD3+ cells in ipsilateral (I) and contralateral (C) areas, the DH and the remainder of the gray matter (non-DH), of mice with sciatic n. cuffing-administered vehicle (Veh) or BRV (10 mg/kg); LWM: lateral white matter; five mice per group; *P < 0.05; **P < 0.01. BRV: brivaracetam; DH: dorsal horn.
SV2A expression in the affected spinal cord
A likely site of action of LEV and BRV in neuropathic pain due to sciatic n. cuffing is the spinal cord, where SV2A expression was reported previously using autoradiography and immunoblot.27 Here, we sought to determine which cell type(s) in the spinal cord express SV2A. Spinal cord tissues were immunolabeled using an anti-SV2A antibody that we independently validated (Figure 5). In uninjured controls, SV2A was identified almost exclusively in gray matter neuropil and neurons (not shown), as reported.27 In mice with sciatic n. cuffing, coronal sections of the involved segment showed robust labeling in gray matter (Figure 5(a) and (b)), with occasional, sporadic immunoreactivity in white matter subpial astrocytes. Most of the SV2A immunoreactivity in gray matter was in neuropil and neurons, the latter easily identified in the ventral horn by their large distinct perikarya (Figure 5(a) and (b)). Double immunolabeling for SV2A and GFAP showed that activated astrocytes in the ipsilateral dorsal horn also expressed SV2A (Figure 5(c)). In addition, double immunolabeling for SV2A and Iba1 or CD45 showed that small numbers of microglia/macrophages as well as leukocytes located in the ipsilateral dorsal horn, including the DH-LGWJ, also expressed SV2A (Figure 5(d) and (e)). The CD45+ leukocytes that expressed SV2A were the same cells that were CD3+, consistent with previous reports of SV2A expression by T lymphocytes.47
Figure 5.
SV2A is expressed by numerous cell types in the spinal cord in the murine sciatic n. cuff model. Low-magnification (a) and high-magnification (b) views of spinal cord sections at the involved level immunolabeled for SV2A; note the relative abundance of SV2A expression in gray matter versus white matter (a), with ventral horn motor neurons showing robust expression (b). (c) Double immunolabeling of the dorsal horn for GFAP and SV2A, shown individually and merged, showing expression of SV2A in reactive astrocytes at the involved segment. (d) Double immunolabeling of the dorsal horn for Iba1 and SV2A, shown individually and merged, showing expression of SV2A in microglia/macrophages at the involved segment. (e) Double immunolabeling of the dorsal horn for CD45 and SV2A, shown individually and merged, showing expression of SV2A in peripheral immune cells at the involved segment. All images are representative of findings in five mice per labeling; bars: 500 μm in (a), 100 μm in (b) and (c), and 25 μm in (d) and (e). (f) Immunoblot showing that anti-SV2A antibody used for immunohistochemistry detects proteins at approximately 90 kDa in lysate from mouse brain gray matter, a rat astrocyte line, and a human T lymphocyte line, with slightly different molecular masses attributable to different species and different glycosylation states, as reported;48–50 representative of three replicates. SV2A: synaptic vesicle glycoprotein 2A; GFAP: glial fibrillary acidic protein.
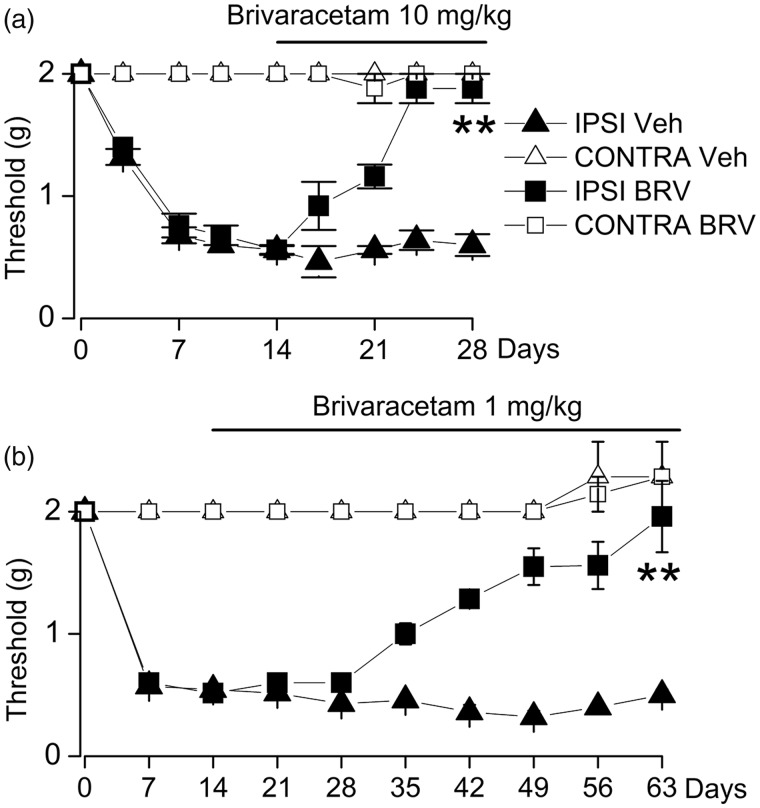
BRV treatment
To examine a clinically relevant scenario, we studied the effect of BRV when administered beginning after allodynia was fully established. Mice underwent sciatic n. cuffing and were allowed to develop mechanical allodynia over the course of two weeks. They then were randomly assigned to one of the four groups: two vehicle control groups, or BRV at two doses, 10 mg/kg or 1 mg/kg daily. Over the course of the next 10 days, mice receiving 10 mg/kg daily gradually reverted to mechanical sensitivities equivalent to the contralateral hindpaw, whereas those receiving vehicle exhibited persistent allodynia (Figure 6(a)). Similarly, mice receiving 1 mg/kg exhibited a slow recovery to normal sensitivities, although at this dose, the recovery required six weeks (Figure 6(b)). Note that vehicle-treated mice in the last experiment showed no extinction of symptoms, continuing to exhibit mechanical allodynia for the full nine weeks of the experiment.
Figure 6.
Therapeutic treatment with BRV reverses mechanical allodynia in the murine sciatic n. cuff model. (a and b) After unilateral sciatic n. cuffing, mice were randomly assigned to receive vehicle (Veh) or BRV (10 mg/kg) or BRV (1 mg/kg) starting on day 14 and continuing daily until day 28 or day 63, as indicated; von Frey filaments were used to assess ipsilateral and contralateral hindpaw withdrawal thresholds; withdrawal thresholds (mean ± standard error) are plotted as a function of time for IPSI (filled symbols) and CONTRA (empty symbols) hindpaws of mice receiving vehicle or BRV, 10 mg/kg (a) or BRV, 1 mg/kg (b); five to six mice per group; **P < 0.01 with respect to vehicle treatment. IPSI: ipsilateral; CONTRA: contralateral.
Discussion
The principal findings of the present study are that (i) both BRV and LEV are effective in reducing neuropathic pain behaviors in the murine sciatic n. cuff model; (ii) the salutary effects of BRV are observed with a dose that is 10× less than that of LEV, similar to findings in seizure models,24 and consistent with a mechanism of action involving SV2A; (iii) the salutary effects of BRV on neuropathic pain behaviors correlate with reduced neuroinflammation in the spinal cord; (iv) BRV exhibits beneficial effects when administered both prophylactically, at the time of sciatic n. cuffing, before the onset of neuropathic pain, and later, after symptoms have fully developed, underscoring its potential for translation to the pain clinic. An interesting, but unexplained observation is the length of time required for BRV to reverse allodynia after it is established—two and six weeks with 10 and 1 mg/kg, respectively.
Neuronal hyperexcitability is a critical element in neuropathic pain, and for this reason, most current drug treatments for neuropathic pain are directed toward decreasing neuronal excitability. However, neuroinflammation linked to glial cell activation is also recognized to play a prominent role in the initiation and maintenance of pain hypersensitivity.34–37 Nonneuronal cells such as immune cells (macrophages and lymphocytes) and glial cells (Schwann cells and satellite cells in the peripheral nervous system (PNS) and astrocytes and microglia in the central nervous system (CNS)) play an important role in the induction and maintenance of neuropathic pain. Injury-induced inflammation at the site of the damaged or affected nerve(s) precedes microglial activation in the dorsal horns of the spinal cord. Under chronic pain conditions, neuroinflammation is characterized by infiltration of immune cells in the dorsal root ganglion and sequential activation of microglia and astrocytes in the spinal cord and brain, with subsequent release of numerous pro-inflammatory cytokines and chemokines.
Our data showing a reduced neuroinflammatory response within the dorsal horn with BRV is in keeping with the concept of a key role for neuroinflammation in neuropathic pain. We showed that BRV significantly reduced microglial activation, TNF expression, and leukocyte infiltration into the dorsal horn, in conjunction with reduced neuropathic pain behaviors. Our observations here with BRV accord with a previous report on LEV, which is also effective in preclinical models of neuropathic pain and also reduces neuroinflammation within the spinal cord.10
Considerable work indicates that LEV and BRV act, at least in part, via a specific mechanism involving SV2A.51 However, apart from SV2A, LEV has additional mechanisms of action, including inhibition of voltage-gated K+ and Ca2+ channels, inhibition of 1,4,5-trisphosphate-mediated release of intracellular Ca2+, and interactions with multiple excitatory and inhibitory ligand-gated ion channels.52–58 By contrast, BRV may be somewhat more selective. A single report suggests that BRV inhibits Na+ current in cultured rat cortical neurons,59 but other studies indicate that BRV does not modulate high- and low-voltage-activated Ca2+ currents, voltage-gated delayed rectifier K+ currents, and persistent voltage-gated Na+ currents, and that BRV is devoid of any direct effect on currents gated by γ-aminobutyric acidergic type A, glycine, kainate, N-methyl-d-aspartate, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.60–62
Given that LEV and BRV overlap strongly in terms of SV2A binding, but not in terms of actions at ion channels or receptors, it seems plausible that the mechanism for the similar salutary effects of LEV and BRV on pain behaviors involves SV2A. The 10-fold higher potency that we observed here with respect to anti-allodynic activity, which is similar to its relative efficacy with respect to antiseizure activity,24 is in keeping with 20-fold greater binding affinity for SV2A by BRV28 and is in keeping with the novel hypothesis that SV2A is the relevant target of the anticonvulsant racetams in neuropathic pain.
Although SV2A may be a target of the drug, the specific role of SV2A in neuropathic pain is enigmatic. SV2A is an integral constituent of synaptic vesicle membranes in presynaptic terminals and has been implicated in mechanisms that control the efficiency of vesicle release as well as upstream vesicle-trafficking mechanisms, accounting for the ability of the anticonvulsant racetams to reduce neuronal excitation.5,6 SV2A is an essential protein for maintaining Ca2+-regulated exocytosis not only at the synapse but in neuroendocrine and other cells as well.
Of the three known SV2 paralogs, SV2A is the only member ubiquitously expressed in the adult brain.5,6,63 SV2A is also expressed in spinal cord, predominantly in gray matter, as previously reported27 and as shown here. SV2A is also found in neuroendocrine cells and at neuromuscular junctions.48,64,65 However, the expression of SV2A by other CNS or PNS cells has not been well studied. In culture, astrocytes reportedly upregulate SV2,66 and in cultured astrocytes, LEV inhibits the release of glutamate67 and exhibits anti-inflammatory properties.68,69 Reports indicate that acutely isolated astrocytes as well as astrocytes in situ do not express SV2,66 whereas here, we found that activated astrocytes in the involved dorsal horn, as well as an astrocyte cell line, express SV2A. Our immunolabeling experiments also revealed the expression of SV2A by Iba1+ microglia/macrophages. Thus, regarding involvement of CNS cell types, neurons, astrocytes, and microglia in the spinal cord may be plausible targets of the racetams in neuropathic pain.
Apart from the nervous system, SV2A mRNA and protein have been identified in T lymphocytes47,70 and in antral mucosal cells,64 and in these cells, like in neurons, Ca2+-regulated exocytosis (in this case, degranulation) is inhibited by LEV.47,71 Here, we found that CD3+ T lymphocytes in situ, as well as a human T-lymphocyte cell line, express SV2A, with counts of T lymphocytes in situ significantly reduced by BRV. T lymphocytes are known to contribute to neuropathic pain induced by peripheral nerve injury. CD4+ and CD8+ T lymphocytes infiltrate the injured sciatic n. after both a local inflammatory insult72 and chronic constriction injury (CCI),44,46 the latter similar to the sciatic n. cuff model used here. Following CCI of the sciatic n., congenitally athymic nude rats,44 as well as RAG-1 knockout mice,46 both of which lack functional T lymphocytes, develop reduced mechanical allodynia and thermal hyperalgesia. In murine nerve transection-induced neuropathic pain models, T lymphocytes infiltrate into the dorsal horn of the spinal cord, and in these models as well, T lymphocyte deficiency (RAG-1 or CD4 knockout) is partially protective.42,73 Thus, both peripheral and central T lymphocyte may be plausible targets of the racetams in neuropathic pain.
A possible peripheral action of the racetams is further supported by other lines of evidence. Ipsilateral but not contralateral intraplantar injection of LEV in a model of localized inflammation (intraplantar carrageenan) produces local peripheral antihyperalgesic and anti-edematous effects.13 Botulinum neurotoxins, which enter neurons by binding to SV2A,65 are injected peripherally as a third-line treatment for neuropathic pain in humans.74 In a model of diabetic neuropathy, untreated mice with allodynia showed degeneration and vacuolization in the sciatic n. along with central neuroinflammation, whereas in mice treated with LEV, allodynia was reduced, nerves showed minimal histopathological changes, and spinal cord microgliosis and astrocytosis were reduced.10 Thus, the anticonvulsant racetams, LEV and BRV, may be acting synergistically via SV2A both centrally in neurons, astrocytes, microglia, and T lymphocytes and peripherally in T lymphocytes and sensory neurons.
Finally, the mechanism for the salutary effects of LEV and BRV on neuropathic pain may extend beyond SV2A. The racetams are a broad class of drugs that share a 2-pyrrolidinone nucleus but may otherwise differ greatly in structure and biological actions.75 Notably, other racetams, including piracetam,76 nefiracetam,77 and dimiracetam,78 have been found to be effective in neuropathic pain models, generally at doses comparable to those used with LEV. In some cases (excluding piracetam), their chemical structures are quite different from those of LEV and BRV (Figure 1). LEV, BRV, and piracetam are known to bind to SV2A,27,28,79 but to our knowledge, SV2A binding has not been demonstrated for dimiracetam and nefiracetam,80 although the latter is reported to have antiseizure properties.81 At present, a unifying molecular mechanism is lacking to explain how such structurally diverse molecules as the racetams depicted in Figure 1 can be effective in neuropathic pain.
This study has limitations. Among them, we studied only a single model of pain in one sex of a single species, and most of our efficacy evaluations focused on mechanical allodynia. Future studies will be needed to broaden our experimental approach, to include other models of neuropathic pain, nonreflexive pain behaviors, both sexes, and other species. Foremost, we implicated SV2A in the neurobiology of neuropathic pain by relying on pharmacological rather than molecular experiments. Although BRV is highly selective for SV2A, molecular approaches may now be within reach to address this shortcoming.82
Conclusion
Neuropathic pain remains a major public health problem that is magnified by the prevalence of opioid use disorder. Its pathogenesis remains incompletely understood, and major challenges remain to discover novel drugs with therapeutic efficacy that will be well tolerated, have minimal side effects, and be devoid of addictive potential. Here, we report that BRV is highly effective in reducing neuropathic pain behaviors in a murine sciatic n. injury model when administered both prophylactically, before the onset of neuropathic pain, and later, after symptoms have fully developed. Attenuation of pain behaviors by BRV was found to correlate with reduced neuroinflammation in the spinal cord. When used therapeutically, especially at low doses, daily administration of BRV for several weeks was required to achieve an optimal benefit. Compared to LEV, BRV’s greater potency, fewer off-target effects, and more benign side-effect profile suggest that it may be an attractive candidate drug for the treatment of some forms neuropathic pain due to peripheral nerve injury.
Author’s Contributions
Study conceptualization, ST; study design and oversight, OT, VG, and JMS; surgeries, OT; data collection for neurofunctional tests, ST, MS, CG, and OT; immunohistochemistry, SI and VG; funding procurement, CS and JMS; first draft of the manuscript, JMS; critical contributions to the manuscript, ST, CS, VG, and JMS. All authors have read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant to CS from The Kahlert Foundation, Sykesville, MD, and by a grant to JMS from the National Institute of Neurological Disorders and Stroke (R01NS105633). ST and MS were supported in part by stipends from the Program for Research Initiated by Students and Mentors, University of Maryland School of Medicine Office of Student Research.
References
- 1.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014; 13: 924–935. [DOI] [PubMed] [Google Scholar]
- 2.Fornasari D. Pharmacotherapy for neuropathic pain: a review. Pain Ther 2017; 6: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waszkielewicz AM, Gunia A, Sloczynska K, Marona H. Evaluation of anticonvulsants for possible use in neuropathic pain. Curr Med Chem 2011; 18: 4344–4358. [DOI] [PubMed] [Google Scholar]
- 4.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA 2004; 101: 9861–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendoza-Torreblanca JG, Vanoye-Carlo A, Phillips-Farfán BV, Carmona-Aparicio L, Gómez-Lira G. Synaptic vesicle protein 2A: basic facts and role in synaptic function. Eur J Neurosci 2013; 38: 3529–3539. [DOI] [PubMed] [Google Scholar]
- 6.Bartholome O, Van den Ackerveken P, Sanchez Gil J, de la Brassinne Bonardeaux O, Leprince P, Franzen R, Rogister B. Puzzling out synaptic vesicle 2 family members functions. Front Mol Neurosci 2017; 10: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardid D, Lamberty Y, Alloui A, Coudore-Civiale MA, Klitgaard H, Eschalier A. Antihyperalgesic effect of levetiracetam in neuropathic pain models in rats. Eur J Pharmacol. 2003; 473: 27–33. [DOI] [PubMed] [Google Scholar]
- 8.Beyreuther B, Callizot N, Stohr T. Antinociceptive efficacy of lacosamide in a rat model for painful diabetic neuropathy. Eur J Pharmacol 2006; 539: 64–70. [DOI] [PubMed] [Google Scholar]
- 9.Ozcan M, Ayar A, Canpolat S, Kutlu S. Antinociceptive efficacy of levetiracetam in a mice model for painful diabetic neuropathy. Acta Anaesthesiol Scand 2008; 52: 926–930. [DOI] [PubMed] [Google Scholar]
- 10.Reda HM, Zaitone SA, Moustafa YM. Effect of levetiracetam versus gabapentin on peripheral neuropathy and sciatic degeneration in streptozotocin-diabetic mice: influence on spinal microglia and astrocytes. Eur J Pharmacol 2016; 771: 162–172. [DOI] [PubMed] [Google Scholar]
- 11.Sliva J, Dolezal T, Prochazkova M, Votava M, Krsiak M. Preemptive levetiracetam decreases postoperative pain in rats. Neuro Endocrinol Lett 2008; 29: 953–957. [PubMed] [Google Scholar]
- 12.Micov A, Tomić M, Popović B, Stepanović-Petrović R. The antihyperalgesic effect of levetiracetam in an inflammatory model of pain in rats: mechanism of action. Br J Pharmacol 2010; 161: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepanovic-Petrovic RM, Micov AM, Tomic MA, Ugresic ND. The local peripheral antihyperalgesic effect of levetiracetam and its mechanism of action in an inflammatory pain model. Anesth Analg 2012; 115: 1457–1466. [DOI] [PubMed] [Google Scholar]
- 14.Smith MD, Woodhead JH, Handy LJ, Pruess TH, Vanegas F, Grussendorf E, Grussendorf J, White K, Bulaj KK, Krumin RK, Hunt M, Wilcox KS. Preclinical comparison of mechanistically different antiseizure, antinociceptive, and/or antidepressant drugs in a battery of rodent models of nociceptive and neuropathic pain. Neurochem Res 2017; 42: 1995–2010. [DOI] [PubMed] [Google Scholar]
- 15.Enggaard TP, Klitgaard NA, Sindrup SH. Specific effect of levetiracetam in experimental human pain models. Eur J Pain 2006; 10: 193–198. [DOI] [PubMed] [Google Scholar]
- 16.Jorns TP, Johnston A, Zakrzewska JM. Pilot study to evaluate the efficacy and tolerability of levetiracetam (Keppra) in treatment of patients with trigeminal neuralgia. Eur J Neurol 2009; 16: 740–744. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S, Mataluni G, Codeca C, Fiore S, Buttari F, Musella A, Castelli M, Bernardi G, Centonze D. Effects of levetiracetam on chronic pain in multiple sclerosis: results of a pilot, randomized, placebo-controlled study. Eur J Neurol 2009; 16: 360–366. [DOI] [PubMed] [Google Scholar]
- 18.Falah M, Madsen C, Holbech JV, Sindrup SH. A randomized, placebo-controlled trial of levetiracetam in central pain in multiple sclerosis. Eur J Pain 2012; 16: 860–869. [DOI] [PubMed] [Google Scholar]
- 19.Kashipazha D, Ghadikolaei HS, Siavashi M. Levetiracetam in compare to sodium valproate for prophylaxis in chronic migraine headache: a randomized double-blind clinical trial. Curr Clin Pharmacol 2017; 12: 55–59. [DOI] [PubMed] [Google Scholar]
- 20.Wiffen PJ, Derry S, Moore RA, Lunn MP. Levetiracetam for neuropathic pain in adults. Cochrane Database Syst Rev 2014: 7: CD010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenstiel P. Brivaracetam (UCB 34714). Neurotherapeutics 2007; 4: 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanon NT, Gagne J, Wolf DC, Aboulamer S, Bosoi CM, Simard A, Messiet E, Desgent S, Carmant L. Favorable adverse effect profile of brivaracetam vs levetiracetam in a preclinical model. Epilepsy Behav 2017; 79: 117–125. [DOI] [PubMed] [Google Scholar]
- 23.Zhu LN, Chen D, Chen T, Xu D, Chen SH, Liu L. The adverse event profile of brivaracetam: a meta-analysis of randomized controlled trials. Seizure 2017; 45: 7–16. [DOI] [PubMed] [Google Scholar]
- 24.Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, Frycia AM, Moureau FG, Klitgaard HV, Gillard MR, Fuks B, Michel P. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem 2004; 47: 530–549. [DOI] [PubMed] [Google Scholar]
- 25.Russo E, Citraro R, Mula M. The preclinical discovery and development of brivaracetam for the treatment of focal epilepsy. Expert Opin Drug Discov 2017; 12: 1169–1178. [DOI] [PubMed] [Google Scholar]
- 26.Klitgaard H, Matagne A, Nicolas JM, Gillard M, Lamberty Y, De Ryck M, Kaminski RM, Leclercq K, Niespodziany I, Wolff C, Wood M, Hannestad J, Kervyn S, Kenda B. Brivaracetam: rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia 2016; 57: 538–548. [DOI] [PubMed] [Google Scholar]
- 27.Lambeng N, Gillard M, Vertongen P, Fuks B, Chatelain P. Characterization of [(3)H]ucb 30889 binding to synaptic vesicle protein 2A in the rat spinal cord. Eur J Pharmacol 2005; 520: 70–76. [DOI] [PubMed] [Google Scholar]
- 28.Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol 2011; 664: 36–44. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Bognar J, Jr., He T, Mohammed M, Niespodziany I, Wolff C, Esguerra M, Rothman SM, Dubinsky JM. Brivaracetam augments short-term depression and slows vesicle recycling. Epilepsia 2015; 56: 1899–1909. [DOI] [PubMed] [Google Scholar]
- 30.Lamberty Y, Ardid D, Eschalier A, Alloui A, Matagne A, Kenda B, Michel P, Klitgaard H. A new pyrrolidone derivative ucb 34714 is effective in neuropathic pain models in rats: comparison with gabapentin. J Pain 2003; 4: 53. [Google Scholar]
- 31.Clinical Trials.gov. A study assessing efficacy of brivaracetam in subjects with persistent pain after shingles (post-herpetic neuralgia), https://clinicaltrials.gov/ct2/show/results/NCT00160667 (2019, accessed 19 October 2019).
- 32.Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, Freund-Mercier MJ, Barrot M. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain 2008; 12: 591–599. [DOI] [PubMed] [Google Scholar]
- 33.Yalcin I, Megat S, Barthas F, Waltisperger E, Kremer M, Salvat E, Barrot M. The sciatic nerve cuffing model of neuropathic pain in mice. J Vis Exp 2014; 89: e51608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 2010; 10: 167–184. [DOI] [PubMed] [Google Scholar]
- 35.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016; 354: 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 2017; 74: 3275–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018; 19: 138–152. [DOI] [PubMed] [Google Scholar]
- 38.Nygaard HB, Kaufman AC, Sekine-Konno T, Huh LL, Going H, Feldman SJ, Kostylev MA, Strittmatter SM. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer’s disease mouse model. Alz Res Therapy 2015; 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 40.Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem 2013; 288: 3655–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol 2010; 69: 1177–1190. [DOI] [PubMed] [Google Scholar]
- 42.Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci 2009; 29: 14415–14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue K. A state-of-the-art perspective on microgliopathic pain. Open Biol 2018; 8: 180154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004; 129: 767–777. [DOI] [PubMed] [Google Scholar]
- 45.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun 2007; 21: 599–616. [DOI] [PubMed] [Google Scholar]
- 46.Kleinschnitz C, Hofstetter HH, Meuth SG, Braeuninger S, Sommer C, Stoll G. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol 2006; 200: 480–485. [DOI] [PubMed] [Google Scholar]
- 47.Li G, Nowak M, Bauer S, Schlegel K, Stei S, Allenhofer L, Waschbisch A, Tackenberg B, Hollerhage M, Hoglinger GU, Wegner S, Wang X, Oertel WH, Rosenow F, Hamer HM. Levetiracetam but not valproate inhibits function of CD8+ T lymphocytes. Seizure 2013; 22: 462–466. [DOI] [PubMed] [Google Scholar]
- 48.Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol 1985; 100: 1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H. Synaptic vesicle glycoprotein 2A ligands in the treatment of epilepsy and beyond. CNS Drugs 2016; 30: 1055–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janz R, Sudhof TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 1999; 94: 1279–1290. [DOI] [PubMed] [Google Scholar]
- 51.Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol Biol Cell 2008; 19: 5226–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukyanetz EA, Shkryl VM, Kostyuk PG. Selective blockade of N-type calcium channels by levetiracetam. Epilepsia 2002; 43: 9–18. [DOI] [PubMed] [Google Scholar]
- 53.Angehagen M, Margineanu DG, Ben-Menachem E, Ronnback L, Hansson E, Klitgaard H. Levetiracetam reduces caffeine-induced Ca2+ transients and epileptiform potentials in hippocampal neurons. Neuroreport 2003; 14: 471–475. [DOI] [PubMed] [Google Scholar]
- 54.Madeja M, Margineanu DG, Gorji A, Siep E, Boerrigter P, Klitgaard H, Speckmann EJ. Reduction of voltage-operated potassium currents by levetiracetam: a novel antiepileptic mechanism of action? Neuropharmacology 2003; 45: 661–671. [DOI] [PubMed] [Google Scholar]
- 55.Pisani A, Bonsi P, Martella G, De Persis C, Costa C, Pisani F, Bernardi G, Calabresi P. Intracellular calcium increase in epileptiform activity: modulation by levetiracetam and lamotrigine. Epilepsia 2004; 45: 719–728. [DOI] [PubMed] [Google Scholar]
- 56.Carunchio I, Pieri M, Ciotti MT, Albo F, Zona C. Modulation of AMPA receptors in cultured cortical neurons induced by the antiepileptic drug levetiracetam. Epilepsia 2007; 48: 654–662. [DOI] [PubMed] [Google Scholar]
- 57.Fukuyama K, Tanahashi S, Nakagawa M, Yamamura S, Motomura E, Shiroyama T, Tanii H, Okada M. Levetiracetam inhibits neurotransmitter release associated with CICR. Neurosci Lett 2012; 518: 69–74. [DOI] [PubMed] [Google Scholar]
- 58.Vogl C, Mochida S, Wolff C, Whalley BJ, Stephens GJ. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol Pharmacol 2012; 82: 199–208. [DOI] [PubMed] [Google Scholar]
- 59.Zona C, Pieri M, Carunchio I, Curcio L, Klitgaard H, Margineanu DG. Brivaracetam (ucb 34714) inhibits Na(+) current in rat cortical neurons in culture. Epilepsy Res 2010; 88: 46–54. [DOI] [PubMed] [Google Scholar]
- 60.Niespodziany I, Andre VM, Leclere N, Hanon E, Ghisdal P, Wolff C. Brivaracetam differentially affects voltage-gated sodium currents without impairing sustained repetitive firing in neurons. CNS Neurosci Ther 2015; 21: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niespodziany I, Lukyanetz EA, Matagne A, Klitgaard H, Wolff C. Brivaracetam does not modulate the major ionic conductances in neurons. Epilepsia 2015; 56: 192–193. [Google Scholar]
- 62.Niespodziany I, Rigo JM, Moonen G, Matagne A, Klitgaard H, Wolff C. Brivaracetam does not modulate ionotropic channels activated by glutamate, gamma-aminobutyric acid, and glycine in hippocampal neurons. Epilepsia 2017; 58: e157–e161. [DOI] [PubMed] [Google Scholar]
- 63.Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci 1994; 14: 5223–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Portela-Gomes GM, Lukinius A, Grimelius L. Synaptic vesicle protein 2, a new neuroendocrine cell marker. Am J Pathol 2000; 157: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006; 312: 592–596. [DOI] [PubMed] [Google Scholar]
- 66.Wilhelm A, Volknandt W, Langer D, Nolte C, Kettenmann H, Zimmermann H. Localization of SNARE proteins and secretory organelle proteins in astrocytes in vitro and in situ. Neurosci Res 2004; 48: 249–257. [DOI] [PubMed] [Google Scholar]
- 67.Sanz-Blasco S, Pina-Crespo JC, Zhang X, McKercher SR, Lipton SA. Levetiracetam inhibits oligomeric Aβ-induced glutamate release from human astrocytes. Neuroreport 2016; 27: 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haghikia A, Ladage K, Hinkerohe D, Vollmar P, Heupel K, Dermietzel R, Faustmann PM. Implications of antiinflammatory properties of the anticonvulsant drug levetiracetam in astrocytes. J Neurosci Res 2008; 86: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 69.Stienen MN, Haghikia A, Dambach H, Thone J, Wiemann M, Gold R, Chan A, Dermietzel R, Faustmann PM, Hinkerohe D, Prochnow N. Anti-inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGFβ1 regulation. Br J Pharmacol 2011; 162: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Expression Atlas. Gene expression across species and biological conditions, www.ebi.ac.uk/gxa/experiments/E-MTAB-3079/Results?geneQuery=%5B%7B%22value%22%3A%22ensmusg00000038486%22%7D%5D (2019, accessed 19 October 2019).
- 71.Harada S, Tanaka S, Takahashi Y, Matsumura H, Shimamoto C, Nakano T, Kuwabara H, Sawabe Y, Nakahari T. Inhibition of Ca(2+)-regulated exocytosis by levetiracetam, a ligand for SV2A, in antral mucous cells of guinea pigs. Eur J Pharmacol 2013; 721: 185–192. [DOI] [PubMed] [Google Scholar]
- 72.Eliav E, Herzberg U, Ruda MA, Bennett GJ. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain 1999; 83: 169–182. 1999/10/27. [DOI] [PubMed] [Google Scholar]
- 73.Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol 2008; 38: 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park J, Park HJ. Botulinum toxin for the treatment of neuropathic pain. Toxins 2017; 9: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malykh AG, Sadaie MR. Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs 2010; 70: 287–312. [DOI] [PubMed] [Google Scholar]
- 76.Mehta AK, Bhati Y, Tripathi CD, Sharma KK. Analgesic effect of piracetam on peripheral neuropathic pain induced by chronic constriction injury of sciatic nerve in rats. Neurochem Res 2014; 39: 1433–1439. [DOI] [PubMed] [Google Scholar]
- 77.Rashid MH, Ueda H. Nonopioid and neuropathy-specific analgesic action of the nootropic drug nefiracetam in mice. J Pharmacol Exp Ther 2002; 303: 226–231. [DOI] [PubMed] [Google Scholar]
- 78.Fariello RG, Ghelardini C, Di Cesare Mannelli L, Bonanno G, Pittaluga A, Milanese M, Misiano P, Farina C. Broad spectrum and prolonged efficacy of dimiracetam in models of neuropathic pain. Neuropharmacology 2014; 81: 85–94. [DOI] [PubMed] [Google Scholar]
- 79.Noyer M, Gillard M, Matagne A, Henichart JP, Wulfert E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur J Pharmacol 1995; 286: 137–146. [DOI] [PubMed] [Google Scholar]
- 80.Danish A, Namasivayam V, Schiedel AC, Muller CE. Interaction of approved drugs with synaptic vesicle protein 2A. Arch Pharm Chem Life Sci 2017; 350: 1700003. [DOI] [PubMed] [Google Scholar]
- 81.Kitano Y, Komiyama C, Makino M, Kasai Y, Takasuna K, Kinoshita M, Yamazaki O, Takazawa A, Yamauchi T, Sakurada S. Effects of nefiracetam, a novel pyrrolidone-type nootropic agent, on the amygdala-kindled seizures in rats. Epilepsia 2005; 46: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 82.Menten-Dedoyart C, Serrano Navacerrada ME, Bartholome O, Sanchez Gil J, Neirinckx V, Wislet S, Becker G, Plenevaux A, Van den Ackerveken P, Rogister B. Development and validation of a new mouse model to investigate the role of SV2A in epilepsy. PLoS One 2016; 11: e0166525. [DOI] [PMC free article] [PubMed] [Google Scholar]