Abstract
Cystathionine γ lyase (CSE) is the major source of hydrogen sulfide-derived species (H2Sn) in endothelial cells and plays an important role in protecting against atherosclerosis. Here we investigated the molecular mechanisms underlying the regulation of CSE expression in endothelial cells by fluid shear stress/flow. Fluid shear stress decreased CSE expression in human and murine endothelial cells and was negatively correlated with the transcription factor Krüppel-like factor (KLF) 2. CSE was identified as a direct target of the KLF2-regulated microRNA, miR-27b and high expression of CSE in native human plaque-derived endothelial cells, was also inversely correlated with KLF2 and miR-27b levels. One consequence of decreased CSE expression was the loss of Prx6 sulfhydration (on Cys47), which resulted in Prx6 hyperoxidation, decamerization and inhibition, as well as a concomitant increase in endothelial cell reactive oxygen species and lipid membrane peroxidation. H2Sn supplementation in vitro was able to reverse the redox state of Prx6. Statin therapy, which is known to activate KLF2, also decreased CSE expression but increased CSE activity by preventing its phosphorylation on Ser377. As a result, the sulfhydration of Prx6 was partially restored in samples from plaque containing arteries from statin-treated donors. Taken together, the regulation of CSE expression by shear stress/disturbed flow is dependent on KLF2 and miR-27b. Moreover, in murine and human arteries CSE acts to maintain endothelial redox balance at least partly by targeting Prx6 to prevent its decamerization and inhibition of its peroxidase activity.
Abbreviations: (3′UTR), 3′Untranslated region; (CSE), Cystathionine γ lyase; (DHE), Dihydroethidium; (DPPP), Diphenyl-1-pyrenylphosphine; (eNOS), Endothelial nitric oxide synthase; (H2Sn), H2S-related sulfane sulfur compounds; (H2S), Hydrogen sulfide; (H2O2), Hydrogen peroxide; (IL-1β), Interleukin-1β; (KLF2), Krüppel-like factor 2; (LC-MS/MS), Liquid chromatography - tandem mass spectrometry; (NO), Nitric oxide; (Prx), Peroxiredoxin; (ROS), Reactive oxygen species; (siRNA), Small interfering RNA; (O2•-), Superoxide anion; (TBARS), Thiobarbituric acid reactive substances
Graphical abstract
Highlights
-
•
Endothelial expression of CSE is regulated by KLF2 and miRNA-27b in mice and humans.
-
•
CSE derived H2Sn regulate endothelial reactive oxygen species and membrane lipid peroxidation.
-
•
Sulfhydration protects peroxiredoxin 6 from hyperoxidation and preserves its activity.
1. Introduction
The gaseous signaling molecules nitric oxide (NO) and hydrogen sulfide (H2S) are recognized as important regulators of cardiovascular physiology and pathophysiology. Both mediators have been attributed anti-inflammatory, anti-oxidant, anti-apoptotic actions and consequently roles in vasodilation, atherogenesis, endothelial activation and angiogenesis [1]. While the production of NO is determined by the expression and activity of the endothelial nitric oxide synthase (eNOS) [2], vascular generation of H2S-related sulfane sulfur compounds (referred to throughout as H2Sn), in the vascular endothelium is largely attributed to the desulflhydration of cysteine and cystathionine, by cystathionine γ lyase (CSE) [[3], [4], [5]].
eNOS expression in cultured and native endothelial cells has been widely studied and eNOS levels are positively regulated by mechanical stimuli, such as the shear stress exerted on the endothelial cell surface by the blood flowing over it (for review see Ref. [6]). At the molecular level this effect has been attributed to activation of the transcription factor Krüppel-like factor (KLF) 2 [7,8]. However, while eNOS levels are upregulated by exposure to fluid shear stress, the expression of CSE in endothelial cells is decreased [9]. Mapping the expression of the enzymes throughout the aortic tree revealed that eNOS is most highly expressed in atheroprotected areas that experience laminar flow (high shear stress) [6], while CSE expression is highest in atheroprone areas exposed to disturbed flow (low shear stress) [9,10]. This does not mean that CSE activity is itself detrimental to vascular homeostasis, as when it is fully functional, CSE seems to act as a reserve anti-atherosclerotic mechanism, to decrease adhesion molecule expression and monocyte attachment [11]. Indeed, manifest atherosclerosis has been linked with vascular inflammation; at least with increased interleukin (IL)-1β levels, and the phosphorylation and inactivation of CSE. Also, CSE deletion markedly accelerates plaque growth, at least in a mouse model of atherosclerosis [9].
The aim of this study was to assess the molecular mechanisms underlying the regulation of endothelial cell CSE expression by blood flow. Moreover, given that H2Sn elicits its effects by the posttranslational modification of nucleophilic cysteines in a process termed persulfidation or sulfhydration [12], and that it displays anti-oxidant activity [11,13,14], we also studied potential sulfhydration targets that can affect endothelial cell redox balance.
2. Materials and methods
2.1. Materials
Cell culture media were purchased from Gibco (Invitrogen; Darmstadt, Germany), Sulfane Sulfur Probe 4 (SSP4) was from Dojindo (GERBU Biotechnik GmbH, Heidelberg, Germany) and DAPI was from Molecular Probes (Thermo Scientific, Dreieich, Germany). Sodium polythiosulfonate (SG1002) was from Sulfagenix Inc. (New Orleans, Louisiana, USA. The polyclonal anti-CSE antibody (1:1000, rabbit) was from Proteintech (Cat. No. 12217-1-AP, Manchester, UK), the antibody against CD144 (1:100, APC labelled) was from Biolegend (Cat. No. 138011, Koblenz, Germany), the KLF2 (1:1000, goat Cat. No. ab17008) and the non muscle myosin (NMM) antibody (1:5000, rabbit, Cat.No. ab24762) were from Abcam (Cambridge, UK), anti-CBS (1:1000, rabbit, Cat. No. H00000875M01) was from Abnova (Biozol, Echnig, Germany), and anti-MPST (1:2000, rabbit) was from Atlas (Cat. No. HPA001240, Bromma, Sweden). The eNOS (1:1000, mouse) antibody was from BD Biosiences (Cat. No. 610297, Heidelberg, Germany) and anti-peroxiredoxin (Prx) 6 (1:1000, rabbit) was from Cell Signaling Technologies (Cat. No. 64329, Frankfurt, Germany). The phospho-Ser377 CSE antibody was generated as described [9]. Secondary antibodies were from Calbiochem (Darmstadt, Germany). The Alexa-Fluor antibody against rabbit was from Invitrogen (Darmstadt, Germany). The β-actin antibody and all other chemicals (unless otherwise specified), were from Merck (Darmstadt, Germany).
2.2. Human samples
Healthy plaque-free mesenteric arteries were collected from 8 volunteers and carotid plaques were prospectively collected from 45 patients who underwent carotid endarterectomy and had internal carotid artery stenosis of 75–85% (Table S1). Arteriographic evaluation of the carotid bifurcation was performed and the degree of lumen narrowing was determined according to North American Symptomatic Carotid Endarterectomy Trial criteria [15]. Samples were subdivided into those from patients receiving no statins versus simvastatin therapy. All of patients gave their informed consent. The study followed the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the study protocol was approved by the Institutional Ethics Committee (PN1459/02–2017).
2.3. Isolation of endothelial cells from the human arteries
Plaque-free or plaque-containing arteries were isolated and stored in endothelial growth medium (EGM)-2 media (Lonza, Cologne, Germany) for 15–30 min. Thereafter, endothelial cells were isolated using dispase (5 Units/ml, 20 min) in Ham's medium in the absence of serum, recovered by centrifugation and labelled with anti-CD144 antibodies (1:100, Cat. Nr. 348505, Biolegend, Lab supplies, Greece). Cells were subjected to FACS sorting with BD FACSAria III (BD Biosciences, Germany) and CD144 positive cells were collected in EGM-2 media containing 5% orthologous human serum. After 15 min in a humidified chamber with 21% O2, the cells were centrifuged and snap frozen in liquid nitrogen. Samples from 2 to 5 individuals were pooled before being used in subsequent experiments.
2.4. Animals
Floxed CSE (CSEfl/fl) mice were generated as described [16], and crossed with tamoxifen-inducible Cdh5-CreERT2 mice to generate animals specifically lacking CSE in endothelial cells (CSEiEC mice) [17]. Mice were housed in conditions that conform to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH publication no. 85-23). Animals received the usual laboratory diet and all studies were approved by the animal research ethics committees in Athens (790/13-02-2014) and Darmstadt (FU1177, FU1189 and FU1250). To induce robust Cre activity, animals were treated with tamoxifen (75 mg/kg i.p., Sigma‐Aldrich) diluted in corn oil once a day for 5 days. Significant knock down of CSE was observed 7 days post-injection.
2.5. Cell isolation and culture
Human umbilical vein endothelial cells were isolated and cultured as described [18], and confluent cells up to passage 2 were used throughout. The use of human material in this study conforms to the principles outlined in the Declaration of Helsinki and the isolation of endothelial cells was approved in written form by the ethics committee of the Goethe University Hospital. Murine lung endothelial cells were isolated from either wild-type or CSEiEC mice, cultured as described [19], and used up to passage 7. To induce CSE deletion in vitro, cells (passage 5–6) were treated with 10 μmol/L 4-OH-tamoxifen for 7 days. Tamoxifen was removed and the cells passaged a further 2–3 times before experiments were performed. Cells isolated from wild-type mice were treated identically. To decrease lymphatic endothelial cell contamination, vascular endothelial cells were positively selected using CD31-coated magnetic beads prior to experiments. In some experiments, cells were pre-treated with the H2Sn donor SG1002 (10 μmol/L, 15 min).
2.6. Shear stress
Human endothelial cells (1st passage) or murine lung endothelial cells (passages 5–7) were transferred to culture medium containing 2% fetal calf serum and either maintained under static conditions or exposed to shear stress (12 dyn cm−2) in a cone-plate viscosimeter, as described [20]. Undirectional (12 dyn cm−2) or disturbed (0.5 dyn cm−2, 1 Hz) flow was also applied to murine and human endothelial cells in the parallel plate chambers (0.6 μm, ibidi GmBH, Gräfelfing, Germany). Thereafter, the cells were either lysed for biochemical studies or fixed with 4% paraformaldehyde for immunohistochemistry.
2.7. Cell transfection
Endothelial cells were transfected with small interfering RNAs (siRNA) directed against KLF2 (Sigma), or Prx6 (Invitrogen) or a scrambled negative control (Eurogentec), using Lipofectamine RNAiMAX (Invitrogen, Karlsruhe, Germany). Cells were then kept in culture for a further 48 h. In experiments studying microRNA-mediated effects, cells were transfected with a control pre-microRNA or pre-miR27b (Ambion, Köln, Germany) 48 h prior to experiments.
2.8. Immunohistochemistry
Slides were incubated in a blocking buffer consisting of Triton X-100 (0.3%), donkey serum (5%) and BSA (0.5%) in PBS for 2 h at room temperature (RT). Samples were washed with PBS, prior to the addition of primary antibodies and overnight incubation at 4 °C. Subsequently, samples were incubated with anti-CSE at 1:500 in Triton X-100 (0.2% in PBS) and APC-CD144 antibody at a dilution of 1:100, overnight at 4 °C. Thereafter, anti-rabbit secondary antibodies (1:200 in PBS) supplemented with DAPI (10 ng/ml), were added. After washing, samples were mounted with Dako fluorescent mounting medium (Dako, Glostrup, Denmark). Images were taken using a confocal microscope (LSM-780; Zeiss, Jena, Germany) and ZEN software (Zeiss).
2.9. Immunoblotting
Samples (cells) were lysed in ice-cold RIPA buffer (50 mmol/L Tris HCl pH 7.5, 150 mmol/L NaCl, 25 mmol/L NaF, 10 mmol/L Na4P2O7,1% Triton X-100 and 0.5% sodium deoxycholate) supplemented with 0.1% SDS and protease/phosphatase inhibitors. Protein concentrations were determined using the Bradford assay and detergent-soluble proteins were solubilized in SDS-PAGE sample buffer, separated by SDS-PAGE and subjected to Western blotting as described [19]. Proteins were visualized by enhanced chemiluminescence using a commercially available kit (Amersham, Freiburg, Germany) and normalized with β-actin or non muscle myosin (NMM). To evaluate the decameric and dimeric form of Prx6, samples were lysed in non-reducing non-denaturating conditions using SDS-free RIPA buffer and boiled in a DTT-free Laemmli lysis buffer. Western blotting was performed in the absence of SDS at 4 °C. For the detection of the monomeric form of Prx6, the same samples were solubilized in SDS-PAGE sample buffer and subjected to Western blotting.
2.10. Quantification of free amino acids in plasma and plaque material
Cell lysates (100 μl) in lysis buffer were used. Sample preparation was performed using the EZ:faast LC-MS free amino acid analysis kit (Phenomenex, Aschaffenburg, Germany) according to the manufacturer's instructions, with minor modifications. Internal standards (10 μl) were applied to all samples and to the standard curve. The internal standards included homoarginine, methionine-D3 and homophenylalanine. Analysis of metabolites was performed by LC-MS/MS using the EZ:faast AAA-MS HPLC column at 35 °C on an Agilent 1290 Infinity LC system (Agilent, Waldbronn, Germany) coupled to a QTrap 5500 mass spectrometer (Sciex, Darmstadt, Germany). Electro spray ionization in positive mode was employed. The ion source parameter were as follows, Current (Cur) 25 psi, ion spray voltage IS 4000 °C, temperature TEM 425 °C, Gas GS1 40 psi, GS2 40 psi. The intensity of the measured metabolite was normalized to internal standards and protein content of plaque. Analyst 1.6.2 and MultiQuant 3.0 (Sciex, Darmstadt, Germany), were used for data acquisition and analysis, respectively.
2.11. Sulfhydration
Sulfhydration was detected using a modified biotin switch assay, as described [9]. In brief, samples (endothelial cells, or blood cells) were precipitated with 20% trichloroacetic acid (TCA) and stored at −80 °C. TCA precipitates were washed with 10% and then 5% TCA and then centrifuged (16.000 g, 30 min, 4 °C) before being suspended in HENs buffer (250 mmol/L HEPES-NaOH, 1 mmol/L EDTA, 0.1 mmol/L neocuproine, 100 μmol/L deferoxamine, 2,5% SDS) containing 20 mmol/L methanethiosulfonate to block free thiols and protease as well as phosphatase inhibitors. Acetone precipitation was performed and pellets were re-suspended in 300 μL qPerS-SID lysis buffer (6 mol/L urea, 100 mmol/L NaCl, 2% SDS, 5 mmol/L EDTA, 200 mmol/L Tris pH 8.2; 50 mmol/L iodoacetyl-PEG2-biotin, 2.5 mmol/L dimedone), sonicated and incubated for 2 h at room temperature in the dark. Lysates (500 μg) were precipitated with acetone and protein pellets were re-suspended in 50 μl Tris/HCl (50 mmol/L, pH 8.5) containing guanidinium chloride (GdmCl 6 mmol/L), and incubated at 95 °C for 5 min. A negative control was generated for each sample by adding DTT (1 mmol/L) during biotin cross-linking. Biotin was then immunoprecipitated using a high capacity streptavidin resin (Thermo Scientific, Heidelberg, Germany) overnight at 4 °C. Elution was performed by addition of 3% SDS, 1% β-mercaptoethanol, 8 mol/L urea and 0.005% bromophenol blue in PBS for 15 min at room temperature followed by 15 min at 95 °C. Sulfhydrated proteins were detected following SDS-PAGE by Western blotting.
Liquid chromatography/tandem mass spectrometry (LC-MS/MS) was performed using a Thermo Scientific Q Exactive Plus equipped with an ultra-high performance liquid chromatography unit (Thermo Scientific Dionex Ultimate 3000) and a Nanospray Flex Ion-Source (Thermo Scientific). Peptides were loaded on a C18 reversed-phase precolumn (Thermo Scientific) followed by separation on a with 2.4 μm Reprosil C18 resin (Dr. Maisch GmbH) in-house packed picotip emitter tip (diameter 100 μm, 15 cm long from New Objectives) using a gradient from eluent A (4% acetonitrile, 0.1% formic acid) to 50% eluent B (99% acetonitrile, 0.1% formic acid) for 30 min (for experiments with the persulfide donor) or 90 min (for shear stress experiments). MS data were recorded by data dependent acquisition Top10 method selecting the most abundant precursor ions in positive mode for HCD fragmentation. Lock mass option [21] was enabled to ensure high mass accuracy between multiple runs. The full MS scan range was 300–2000 m/z with resolution of 70000, and an automatic gain control (AGC) value of 3E6 total ion counts with a maximal ion injection time of 160 ms. Only higher charged ions (2+) were selected for MS/MS scans with a resolution of 17500, an isolation window of 2 m/z and an automatic gain control value set to 1E5 ions with a maximal ion injection time of 150 ms. Selected ions were excluded in a time frame of 30 s following fragmentation event. Fullscan data were acquired in profile and fragments in centroid mode by Xcalibur software.
MS data analysis: For data analysis MaxQuant 1.6.1.0 [22], N-terminal acetylation (+42.01), oxidation of methionine (+15.99), carbamidomethylation (+57.02) and biotinylation (Peo-biotin, +414.19) were selected as variable on cysteines. The human reference proteome set (Uniprot, February 2018, 71785 entries) was used to identify peptides with a false discovery rate less than 1%. Match between run options were enabled to match features in a time window of 60 s.
2.12. H2Sn measurements
Intracellular levels of H2S were measured by monitoring the selective reaction of SSP4 with H2Sn. In brief, cells were seeded in 12 or 48 well plates and cultured to confluency. The culture medium was replaced with phenol red-free EGM (PeloBiotech, Martinsried, Germany) supplemented with 0.1% BSA. After 2 h, SSP4 (10 μmol/L), l-cysteine (100 μmol/L) and pyridoxal phosphate (10 μmol/L) were added for 60 min. Thereafter, the cell supernatant was collected and floating cells were removed by centrifugation (16.000 g, 10 min, 4 °C). The specific products of the reaction of H2S with SPP4 were quantified by LC-MS/MS as described [4].
2.13. 3′Untranslated region (UTR) assays
The 3′UTR of CSE was subcloned into a pLightswitch-3′UTR plasmid (Active Motif, La Hulpe, Belgium) that contains the luciferase coding sequence. The mutation of the CSE 3′UTR was achieved using the QuickChange kit (Stratagene, Waldbronn, Germany) with a miR27b seeding sequence mutation primer (CSE 5‘-CTATTAGAAGCTGCTTCCTGTGAA, TACTTGAAAAGTTTACTGTGAA-3’ and CSEmut 5‘-CTATTAGAAGCTGCTTCCTGAGTT and TACTTGAAAAGTTTACTGAGTT-3’). HEK-293 cells were transfected with pLightswitch-CSE 3′UTR or the mutated CSE 3′UTR, and the effects of a control precursor (pre-)miR or pre-miR27b on reporter gene activity was determined after 48 h. A construct lacking the CSE 3′UTR was used as a negative control. Luciferase activity was measured with LightSwitch luciferase reporter assay system according to the manufacturers' protocol (Active Motif, La Hulpe, Germany).
2.14. RT-qPCR
Total RNA was extracted using an RNeasy kit (QIAGEN, Hilden, Germany), and equal amounts (1 μg) of total RNA were reverse transcribed (Superscript III; Invitrogen). Gene expression levels were detected using SYBR Green (Absolute QPCR SYBR Green Mix; Thermo Fisher Scientifc).The relative expression levels of the different genes studied was calculated using formula 2−ΔCt (ΔCt = Ct (gene) – Ct (housekeeping gene)) with the 18S RNA as a reference. The primer sequences used were as follows:
| 18s | forward 5′-CTTTGGTCGCTCGCTCCTC-3′ |
| reverse 5′-CTGACCGGGTTGGTTTTGAT-3′ | |
| hCSE | forward 5′- ACTTCAGGCAAGTGGCATCTG-3′ |
| reverse 5′- GCCAAAGGGCGCTTGGTTT-3′ | |
| mCSE | forward 5′- AGGGTGGCATCTGAATTTGG-3′ |
| reverse 5′- GTTGGGTTTGTGGGTGTTTC-3′ | |
| miR-27b | UPL 5′-GTCTCTGCCTGTGCAGGGTCCGAGGTATTCGCACAGG CAGAGACGCAGGAA-3′ |
| reverse 5′- TTCACAGTGGCTAAG-3′ | |
| eNOS | forward 5′- GCTGTTCCAGATTCG-3′ |
| reverse 5′- GCTGCAGGTGTTCGATG-3′ | |
| KLF2 | forward 5′- ATTCCAGTGCCATCTGTGCGAT-3′ |
| reverse 5′- AAACAAAACTCGTCAAGGAGGA-3′ |
2.15. Dihydroethidium measurements
Endothelial cells in culture or from the lesser curvature of aortas from wild type and CSEiEC mice were incubated with dihydroethidium (DHE, 10 μmol/L) for 20 min in the dark, before being snap frozen in liquid nitrogen. LC-MS/MS of DHE derivatives was performed on a 1290 Infinity UHPLC system (Agilent, Waldbronn, Germany) coupled to a 5500 QTrap triple quadrupole mass spectrometer with a TurboV electro spray ionization source (Sciex Deutschland GmbH, Darmstadt, Germany). DHE and its oxidation products were separated on a C18 Phenomenex Kinetex column (150 × 2.1 mm, 2.6 μm), protected by a Phenomenex C18 guard cartridge, using an acetonitrile/water gradient with 0.1% formic acid. The ion source parameters were set as follows: x-axis and y-axis of the source were set to 5.0 mm, TEM = 300 °C, IS = 3500 V, GS1 = 50 p.s.i., GS2 = 40 p.s.i., CUR = 30 p.s.i., collisionally activated dissociation gas (CAD) = medium. Detection of DHE oxidation products (O2•- and ethidium) was achieved by multiple reaction monitoring (MRM) in positive ion mode. The method allowed the detection of DHE degradation product ethidium and the O2•--specific product. System control and analytical data analysis were processed by Analyst software 1.6.2 and MultiQuant 3.0, respectively.
2.16. H2O2 assay
The H2O2-dependent conversion of Amplex Red (50 μmol/L; Invitrogen) to resorufin catalyzed by horseradish peroxidase (2 U/ml) was carried out in 50 mmol/L sodium phosphate buffer, pH 7.4 buffer. Fluorescence (λ excitation 540 nm, λ emission 580 nm) was determined in a fluorimeter (Envision, PerkinElmer Inc., MA, USA).
2.17. Peroxiredoxin activity assay
Peroxiredoxin activity in cell lysates was evaluated based on GSH reductase/GSH/NADPH-coupled assay as described [23]. A buffer containing 50 mmol/L Tris-HCl, 2 mmol/L NaN3, 0.1 mmol/L EDTA (pH 8.0), 0.3 mmol/L NADPH, 0.36 mmol/L GSH, and 0.2 units/ml GSH reductase was used and the absorbance was recorded at λ340 nm. Absorbance was monitored until a steady reading (∼1 min). Subsequently, samples (10 μl, adjusted to 5 μg/ml protein concentration in 1% NP40 lysis buffer) were added and the absorbance at 340 nm was re measured after 5 min. Prx activity was assessed as the change in absorbance at 340 nm (NADPH oxidation) after 5 min. Enzymatic activity was expressed as nmol of NADPH oxidized/min/mg of protein using NADPH standards.
2.18. Lipid peroxidation assays
Lipid peroxidation was assayed with two different methods i.e. using thiobarbituric acid reactive substances (TBARS) and diphenyl-1-pyrenylphosphine (DPPP). TBARS reacts at a 1:2 ratio with malondialdehyde (MDA) and reflects the production of lipid hydroperoxides. TBARS was evaluated fluorimetrically with a commercially available kit (OxiSelect TBARS Assay Kit, New England Biolabs GmbH, Frankfurt, Germany) as described [24]. A DPPP assay was also performed to monitor lipid peroxidation in cell membranes. Cells were incubated with DPPP (10 μmol/L, 4 °C, 30 min) in the dark and cell fluorescence (representing the oxidation product of DPPP) was measured using a microplate reader (λ excitation 352 nm, λ emission 380 nm). Measurements were made before and for up to 6 h after the removal of the H2O2 (100 μmol/L, 2 h) to evaluate recovery.
2.19. Interleukin 1β ELISA
The levels of interleukin 1β were determined in 100 μl of human plasma by ELISA (Invitrogen, eBioscience, Darmstadt, Germany) according to the manufacturer's protocol.
2.20. Statistics
Data are expressed as mean ± SEM. Statistical evaluation was performed using Student's t-test for unpaired data or one-way ANOVA with Bonferroni post hoc analysis where appropriate. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. Regulation of endothelial CSE expression by KLF2 and miR-27b
Consistent with a previous report [9], CSE was expressed in human endothelial cells and its expression was decreased by exposure to shear stress for 24 h (Fig. 1A). KLF2 is known to be induced by shear stress [7,8], and the siRNA-mediated downregulation of KLF2, increased CSE expression in cells maintained under static conditions (Fig. 1B). KLF2 downregulation also attenuated the shear stress-induced decrease in CSE expression. In the same cells, the downregulation of KLF2 prevented the eNOS expression but had no effect on levels of two additional H2S–generating enzymes i.e. cystathionine-β-synthase (CBS) or 3-mercaptopyruvate sulfurtransferase (3MST). CSE expression was mirrored in H2Sn bioavailability, and KLF2 silencing increased intra-endothelial H2Sn both in static and shear stress conditions (Fig. 1C).
Fig. 1.
Relationship between blood flow-KLF2-miR-27b and CSE levels in human endothelial cells. Human endothelial cells (passage 1) were treated with a control siRNA (siCTL), a siRNA directed against KLF2 (siKLF2), a control lentivirus (lv-CTL), and KLF2 lentiviruses (lv-KLF2) or pre-miR-27b (pre-miR). (A) CSE (red) expression in endothelial cells maintained under static conditions or exposed to shear stress (12 dyn cm−2) for 24 h; bar = 50 μm. The results are representative of 5 additional cell batches. (B) Consequences of a control siRNA (siCTL) versus KLF2 downregulation (siKLF2) on the expression of CSE, KLF2 and eNOS in endothelial cells under static conditions or after exposure to shear stress; n = 6 independent cell batches (ANOVA, Newman-Keuls). (C) Fold change of polysulfide (H2Sn) levels in endothelial cells as in B; n = 6 independent cell batches (ANOVA, Newman-Keuls). (D-E) Effect of a control lentivirus (lvCTL) versus KLF2 overexpression (lvKLF2) on the (D) RNA levels of KLF2, miR-27b and CSE and (E) protein levels of KLF2, eNOS, CSE, CBS, 3MST and NMM in endothelial cells under static conditions; n = 6–15 independent cell batches (ANOVA, Newman-Keuls). (F) Effect of pre-miR-27b overexpression (48 h) on CSE protein levels; n = 6 experiments using 6 different cell batches of endothelial cells (Student's t-test). (G) Effects of a negative control pre-miRNA (CTL-miR) and pre-miR-27b on the activity of the wild-type (CSEWT) versus mutated CSE (CSEmut) 3′UTR reporter construct in HEK cells. A construct lacking the CSE 3′UTR was used as a negative control (NC); n = 9 independent experiments (ANOVA, Newman-Keuls). **P < 0.01, ***P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In silico analysis (JASPAR, miRWalk, miRanda and others) failed to identify a KLF2 binding site in the CSE promoter region but did identify a number of microRNA seeding sequences in the 3′ untranslated region (UTR) of CSE. The microRNAs identified included miR-27b, miR-27a, miR-150, miR-513 and miR-510 (Table S2). Of these, miR-27b, was of interest given that it is expressed in endothelial cells and it is regulated by KLF2 [25]. It was possible to confirm a previous report [26] that shear stress elicited a time-dependent increase in miR-27b expression (Fig. S1A), as well as the regulation of miR-27b by KLF2 (Fig. S1B). The lentiviral-mediated overexpression of KLF2, on the other hand increased miR-27b levels (Fig. 1D) and decreased CSE mRNA (Fig. 1D) and protein (Fig. 1E) levels, without affecting either CBS or 3MST (Fig. 1E). The overexpression of precursor (pre)-miR-27b decreased CSE protein levels (Fig. 1F). Moreover, using a CSE 3′UTR construct it was possible to demonstrate that CSE is a bona fide target of miR-27b as its overexpression increased luciferase activity while mutation of the putative miR-27b seeding sequence (Fig. S1C) abolished the effect (Fig. 1G).
In endothelial cells from murine lungs, shear stress induced an increase in eNOS expression and a decrease in CSE expression (Fig. S2A) that correlated temporally with a decrease in H2Sn levels (Fig. S2B). Similarly, the down regulation of KLF2 prevented the shear stress induced upregulation of eNOS and the downregulation of CSE (Fig. S2C). The miR-27b sequence is highly conserved between humans and mice (http://www.mirbase.org/), and shear stress also increased the expression of miR-27b in murine cells with a time course that paralleled the increase in eNOS and KLF2 and the decrease in CSE mRNA (Fig. S2D). Moreover, the overexpression of miR-27b decreased CSE expression (Fig. S2E).
3.2. Regulation of endothelial CSE expression in human atherosclerosis
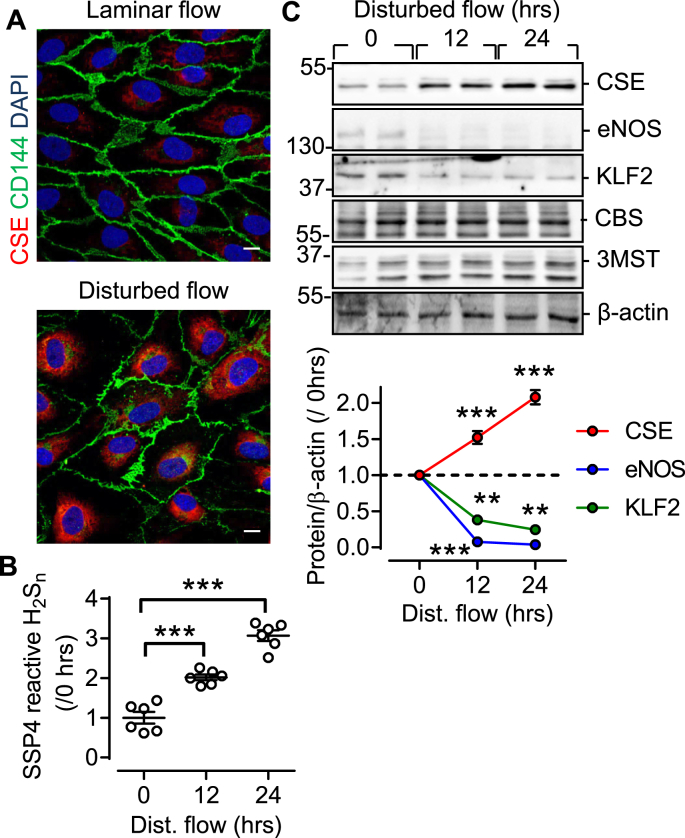
While our in vitro studies focused on the comparison of static conditions versus shear stress, this is not a physiological situation. Rather endothelial cells in vivo are exposed to athero-protective (laminar) flow and athero-prone (oscillatory or disturbed) flow [10]. In cultured human endothelial cells, the application of disturbed flow effectively increased CSE expression (Fig. 2A) and H2Sn generation (Fig. 2B), at the same time as decreasing both KLF2 and eNOS levels (Fig. 2C).
Fig. 2.
Relationship between disturbed flow and CSE expression in human endothelial cells. (A) CSE (red) expression in human endothelial cells exposed to laminar or disturbed flow. CD144 = green, DAPI = grey, bar = 50 μm. Similar findings were observed in 5 additional experiments. (B) H2Sn levels in human endothelial cells exposed to disturbed flow for up to 24 h; n = 6 independent cell batches (ANOVA, Newman-Keuls). (C) CSE, KLF2 and eNOS expression in human endothelial cells exposed to disturbed flow for up to 24 h; n = 6 independent cell batches (ANOVA, Newman-Keuls). **P < 0.01, ***P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
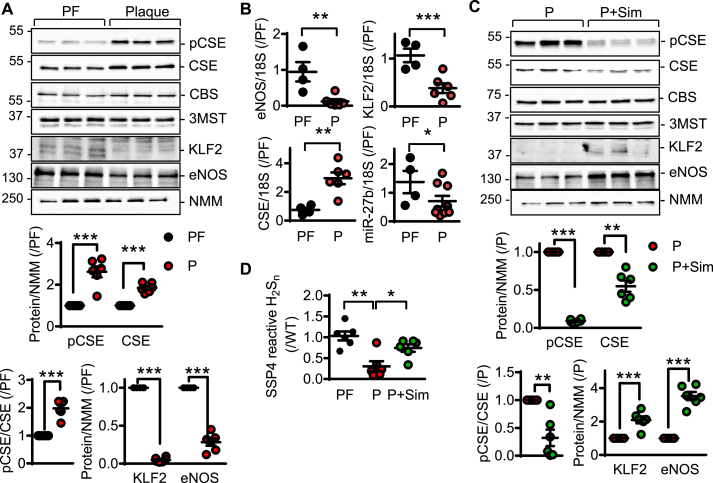
Next, endothelial cells were isolated from 8 plaque-free human mesenteric arteries, or from carotid atherosclerotic plaques derived from 45 patients with internal carotid artery disease (Table S1). Fitting with the assumption that endothelial cells from arteries with atherosclerosis were exposed to disturbed flow, and in line with the effect of disturbed flow on CSE expression in the in vitro studies, the expression of CSE was higher in plaque-containing than in plaque-free samples (Fig. 3A and B). The increase in CSE was paralleled by a decrease in eNOS, KLF2 and miR-27b levels. Many patients with atherosclerosis are treated with HMG CoA inhibitors or “statins”, which in addition to their lipid lowering effects, have also been reported to elicit the activation of KLF2 and to increase eNOS expression [27,28]. Patient samples were therefore separated into those that received simvastatin versus those on non-statin therapy. This revealed a significant increase in KLF2 expression in statin-treated individuals, that was accompanied by an increase in eNOS expression, a decrease in CSE protein levels and a marked decrease in CSE phosphorylation (Fig. 3C). Surprisingly, despite the increase in CSE expression in the intra-plaque endothelium of patients on non-statin therapy, CSE activity was reduced as evidenced by decreased H2Sn levels (Fig. 3D), and increased intra-endothelial levels of the CSE substrate, cystathionine (Fig. S3A). The latter phenomenon is most likely attributable to the inactivation of the enzyme as a consequence of vascular inflammation. Indeed, phosphorylation of CSE on Ser377, which is known to inhibit enzyme activity [9], was elevated in endothelial cells isolated from plaque-containing versus plaque free samples (Fig. 3A). In endothelial cells from statin treated patients, CSE activity was largely normalized (Fig. 3D, Fig. S3A), a phenomenon that was linked with a decrease in circulating levels of IL-1β (Fig. S3B).
Fig. 3.
CSE regulation in human native endothelial cells from healthy and atherosclerotic arteries. (A) Phospho-Ser377 CSE (pCSE), CSE, CBS, 3MST, KLF2, eNOS and NMM protein levels in endothelial cells isolated from plaque-free (PF) or atherosclerotic (P) arteries; n = 4–9 per group (Student's t-test). (B) CSE, KLF2, eNOS and miR-27b RNA levels in samples as in A; n = 4–9 per group (Student's t-test). (C) Phospho-Ser377 CSE, CSE, CBS, 3MST, KLF2, eNOS and NMM protein levels in endothelial cells isolated from patients with atherosclerosis, without (P) and with simvastatin treatment for more than 3 years (P + Sim); n = 6 per group (Student's t-test). (D) H2Sn levels in samples as in A and C. n = 4–6 per group (Student's t-test). *P < 0.05, **P < 0.01, ***P < 0.001.
3.3. CSE and the sulfhydration of peroxiredoxin 6
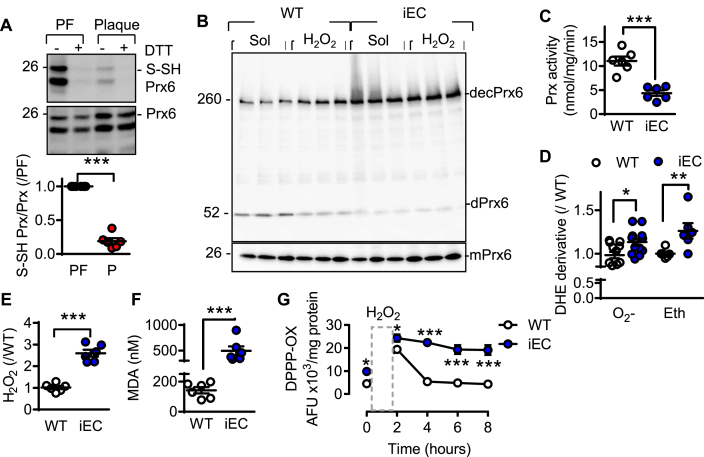
Endogenously generated H2Sn have been linked with processes involved in endothelial cell redox balance [11,29,30]. Given that H2Sn elicit their biological actions via protein sulfhydration [12], sulfhydromic analyses was performed using endothelial cells from plaque-free versus plaque-containing arteries. This process identified only eight sulfhydrated proteins involved in the cell redox homeostasis (GO: 0045454) (Table S3). The most interesting target was peroxiredoxin (Prx) 6, in which the reactive cysteine Cys47, was sulfhydrated in the plaque-free (H2Shigh) endothelial cells. The sulfhydration of Prx6 in endothelial cells from plaque–free vessels was confirmed using the standard biotin switch assay (Fig. 4A) as well as in endothelial cells from wild-type mice (Fig. S4A). No sulfhydrated Prx6 was detected in endothelial cells from CSEiEC mice. Functionally, the loss of sulfhydration in CSEiEC endothelial cells resulted in an increase in the decameric form of Prx6 (Fig. 4B), which is reported to be hyperoxidized [31]. To determine whether or not this was the case, cells were incubated with H2O2 (100 μmol/L), which increased levels of decameric Prx6 in cells from wild-type mice but was without effect in CSE-deficient cells. This indicated that the non sulfhydrated Prx6 was already oxidized. Consistent with the latter observations, Prx activity was significantly lower in cells from CSEiEC mice than from their wild-type littermates (Fig. 4C).
Fig. 4.
Consequence of CSE deletion on redox status and Prx activity in murine endothelial cells. (A) Prx6 sulfhydration (S-SHPrx6). DTT was included to demonstrate the specificity of the signal in endothelial cells isolated from plaque-free (PF) or plaque containing (P) arteries. n = 6–9 per group (Students t-test). (B) Prx6 expression in endothelial cells from wild-type and CSEiEC mice; mPrx6 = monomer Prx6, dPrx6 = dimer Prx6 and decPrx6 = decamer Prx6. Experiments were performed in the absence and presence of H2O2 (100 μmol/L, 10 min). The blot represents the results of 3 independent cell batches and similar results were obtained in 3 additional cell batches. (C) Prdx activity (nmol peroxide/min/mg protein) in endothelial cells from wild-type and CSEiEC mice; n = 6 cell batches. (D) Derivatives of dihydroethidium reaction with reactive oxygen species (O2− as the superoxide-specific products and ethidium/Eth as an unspecific ROS product) in endothelial cells from WT and CSEiEC (iEC) mice; n = 6 cell batches. (E) Fold change of H2O2 levels in endothelial cells from wild-type and CSEiEC mice; n = 6 cell batches. (F) Malondiadehyde levels in endothelial cells from wild-type and CSEiEC mice; n = 6 cell batches. (G) Changes in lipid peroxidation in endothelial cells from wild-type and CSEiEC mice; n = 6 independent cell batches (ANOVA, Newman-Keuls). **P < 0.01, ***P < 0.001.
The antioxidant actions of Prx6 are dependent on the same cysteine i.e Cys47 [32], that was sulfhydrated. Given that Prx6-deficiency in endothelial cells increases their sensitivity to oxidative stress as well as lipid peroxidation [33], we evaluated the consequences of Prx6 sulfhydration on levels of reactive oxygen species (ROS) as well as on lipid peroxidation in murine endothelial cells. Compared with endothelial cells from wild-type mice, CSE deficiency increased superoxide anion (O2•-) generation, detected as adducts of ethidium (Fig. 4D), as well as that of H2O2 (Amplex red) (Fig. 4E). Increased ROS generation was also detected in the lesser curvature of aortae from CSEiEC mice versus their wild-type littermates (Fig. S4B). These observations correlated with increased membrane lipid oxidation (Fig. 4F), as well as a delayed repair of the lipid peroxidation, assessed by determining changes in DPPP oxidation (Fig. 4G).
3.4. Effects of a H2Sn donor
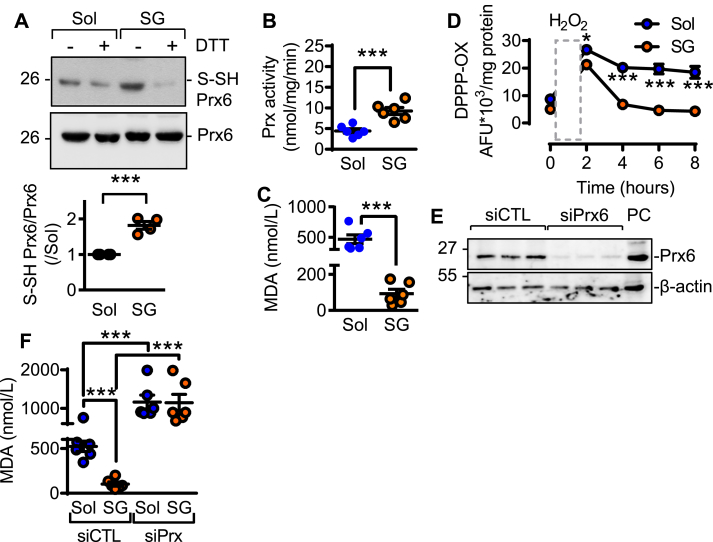
To demonstrate that the effects described were attributable to H2Sn, endothelial cells from CSEiEC mice were treated with the polysulfide donor; SG1002. This resulted in the re-sulfhydration of Prx6 (Fig. 5A), an increase in Prx activity (Fig. 5B), and reduced membrane lipid peroxidation (Fig. 5C&D). The siRNA-mediated downregulation of Prx6 in the CSE-deficient cells (Fig. 5E), potentiated lipid peroxidation and abolished the anti-oxidant effect of SG1002 (Fig. 5F). The in vitro findings were confirmed in vivo inasmuch as ROS levels (Fig. 6A) and lipid peroxidation (Fig. 6B), were increased in endothelial cells from plaque-containing arteries that contained no detectable sulfhydrated Prx6 (Fig. 6C). In samples from statin-treated individuals, CSE activity was increased to restore Prx6 sulfhydration, as well as to decrease H2O2 generation and lipid peroxidation.
Fig. 5.
Effects of H2S supplementation on Prx6 in endothelial cells from CSEiECmice. Endothelial cells from CSEiEC mice were treated with solvent (Sol) or the H2S donor; SG1002 (SG, 10 μmol/L, 15 min). (A) Sulfhydration (S-SH) of Prx6, DTT was included to demonstrate the specificity of the signal; n = 4 different cell batches (Student's t-test). (B) Prdx activity (nmol peroxide/min/mg protein); n = 6 cell batches (Student's t-test). (C) Malondiadehyde levels; n = 6 cell batches (Student's t-test). (D) H2O2-induced changes in lipid peroxidation in endothelial cells from CSEiEC mice treated with solvent or SG1002 (SG, 10 μmol/L, 15 min); n = 6 cell batches (ANOVA repeated measures, Bonferroni). (E) Representative Western blot showing the efficiency of siRNA-mediated Prx6 knockdown; n = 3 independent cell batches. (F) Malondiadehyde levels in cells treated with a control siRNA (siCTL) or siRNA directed against Prx6 (siPrx) before being incubated with SG1002; n = 6 independent cell batches (ANOVA, Newman-Keuls). **P < 0.01, ***P < 0.001.
Fig. 6.
Effect of statins on intra-plaque endothelial redox balance and Prdx sulfhydration. Endothelial cells were isolated from plaque-free (PF) or atherosclerotic (P) arteries of patients without statin treatment or treated with simvastatin for more than 3 years (+Sim). (A) Cellular H2O2 levels; n = 4–6. (B) Cellular malondiadehyde (MDA) levels; n = 4–6. (C) Prx6 sulfhydration (S-SH-Prx6). DTT was included to demonstrate the specificity of the signal. n = 4–9 per group. **P < 0.01, ***P < 0.001 (ANOVA, Newman-Keuls).
4. Discussion
The results of the current investigation revealed that the regulation of miR-27b by KLF2 underlies the downregulation of CSE by shear stress, as well as its upregulation by disturbed flow. While these events were elucidated in cultured cells, the high expression of CSE in native human plaque-derived endothelial cells, was also negatively correlated with KLF2 and miR-27b levels. One consequence of the decrease in H2Sn was the lack of Prx6 sulfhydration (on Cys47), which resulted in its hyperoxidation, decamerization and inhibition, as well as concomitant increase in endothelial cell ROS generation and lipid membrane peroxidation. Importantly statins, which are known to activate KLF2, decreased CSE expression and activity in human plaque-derived endothelial cells. Despite this, statin therapy also decreased circulating levels of the inflammatory mediator IL-1β, to prevent the phosphorylation and inhibition of CSE. The consequence of the latter being the recovery of Prx6 sulfhydration, as well as decreased endothelial cell ROS levels and lipid peroxidation.
H2S has been implicated in vascular homeostasis ever since the first report that it is endogenously generated by vascular cells [34]. Although CSE is the main source of H2S in the endothelium [[3], [4], [5]], only a limited number of studies have evaluated the molecular mechanisms involved in the regulation of its expression. To-date endothelial cell CSE has been reported to be regulated by redox-i.e., Nox4- [35], nutrient i.e. ATF4- [36,37], and NFAT/Ca2+- [38] dependent mechanisms. To assess the mechanism responsible for the regulation of CSE by shear stress, we focused initially on KLF2 which regulates the expression of eNOS [8,39]. The reason for this decision was the fact that the time course of the shear stress-induced upregulation of eNOS mirrored that of the downregulation in CSE. Indeed, the siRNA-mediated downregulation of KLF2 increased CSE expression. The latter relationship was not direct but rather relied on miR-27b which was regulated by shear stress in a KLF2-dependent manner and which directly targeted the 3′UTR of CSE. MiR-27b is interesting as it has been characterized as a serum biomarker for atherosclerosis [40] and asymptomatic carotid artery stenosis [41]. The fact that the expression of eNOS is positively regulated by KLF2 while that of CSE is negatively regulated by the same transcription factor, albeit indirectly, implies that eNOS and CSE are unlikely to be highly expressed in the same cells. This makes it unlikely that the biological actions of CSE-derived H2Sn can be solely attributed to crosstalk with the NO pathway.
To evaluate the pathophysiological relevance of the findings, the expression of CSE was assessed in human endothelial cells from plaque-free and plaque-containing arteries. This comparison required a compromise in the cells studied as plaque free-endothelial cells were isolated from mesenteric arteries while carotid arteries (75–85% stenosis) were used a source of endothelial cells from plaque-containing arteries. We assumed that the endothelial cells from mesenteric arteries were exposed to laminar flow in vivo and those from patients with stenosis were exposed to disturbed flow. This assumption fit well with our previous observations on the changes in CSE expression and activity in mice [9], as well as the fact that KLF2 levels were clearly lower in the endothelial cells from plaque-containing versus the plaque-free arteries. Importantly, the alterations in KLF2 were reflected in endothelial eNOS expression and intra-plaque miR-27b levels, and by a decrease in CSE. Although, CSE levels were high in cells from plaque-containing arteries its activity was reduced, a phenomenon that was attributable to the phosphorylation of the enzyme on Ser377 [42], which inhibits its activity [9]. Of the 45 patients with internal carotid artery disease studied, 15 received statin therapy, and as statins have been reported to activate KLF2, and increase eNOS expression [28] we paid closer attention to this subgroup. Indeed statin therapy was associated with lower CSE levels than in samples from patients receiving non statin therapy, an observation that is consistent with a previous report [43]. Despite this, the Ser377 phosphorylation of CSE was lower in cells from individuals that received statins, which would imply alleviation of inhibition and an increase in CSE-derived H2Sn generation. This fit with the fact that, simvastatin therapy was associated with a reduction in circulating IL-1β levels, an observation that is also consistent with other reports [44,45]. Plasma samples from the same subjects showed reduced cystathionine levels and increased H2Sn levels indicating increased CSE activity. Indeed, an increase in CSE activity was confirmed by the observation that the sulfhydration of one H2Sn target i.e. Prx6, was higher in samples from the statin-treated group.
Endothelial dysfunction and vascular disease are generally preceded by a decrease in NO generation and an increase in ROS production. Indeed, the term “oxidative stress” refers to the state within in the vascular wall when the cellular anti-oxidative defense become overwhelmed. CSE activity has been reported to protect blood vessels against oxidative stress [11,29,30], effects probably linked to the reported effects of H2S donors on a series of antioxidant enzymes including; superoxide dismutases, catalase, glutathione peroxidase, glutathione-S-transferase, xanthine oxidase and the p67phox subunit of the NADPH oxidase complex [46,47]. One transcription factor targeted by H2Sn is Keap1, which regulates the antioxidant response by inhibiting Nrf2 activity [48]. However, no differences in the sulfhydration of previously reported antioxidant enzymes or Keap1 were detected (Bibli. Unpublished Observation 2019). Instead, analysis of endothelial cells from plaque-free versus plaque-containing arteries revealed that only eight proteins involved in the cell redox homeostasis were sulfhydrated in the healthy-CSE enriched conditions. Of these proteins; Prx6, was found to be sulfhydrated on 2 cysteine residues i.e., Cys47 and Cys91. This protein was of particular interest given its role in the repair of peroxidized cell membranes [49], and the fact that dysfunctional Prx6 has been linked with an increased endothelial cell sensitivity to oxidative stress [50] and enhanced lipid peroxidation [51]. While there had been no previous report of a link between Prx6 and H2Sn, during the preparation of this manuscript other members of this protein family, i.e. Prx1‐4 were reported to be sulfhydrated in A549 cell lysates treated with a high concentration (0.5 mM) of exogenous NaHS [52]. However, endogenously generated H2Sn did not result in detectable sulfhydration of Prx1-4 in endothelial cells. Of the cysteine residues within Prx6 targeted by H2Sn, Cys47 seems to be the more crucial because of its high conservation between species that reflects its crucial role in the antioxidant peroxidase activity of Prx6 [53]. In contrast, Cys91 is unique to the human orthologue and its mutation to serine does not affect peroxidase activity. Because of its high affinity for H2O2, Cys47 is extremely susceptible to hyperoxidation, forming peroxidase inactive sulfinic acid and sulfonic acid derivatives and switching the activity of Prx6 to a Ca2+-independent phospholipase A2 [31]. Indeed, hyperoxidation of Prx6 results in the irreversible formation of higher oligomers of the enzyme [54], a state that could be induced by H2O2 in cultured endothelial cells from wild-type mice but that was evident in CSE-deficient endothelial cells even under basal conditions. Given that Prx6 decamerization coincided with a decrease in peroxidase activity as well as an increase in cellular O2•- and H2O2 levels as well as lipid peroxidation, it seems that the sulfhydration of Prx6 on Cys47 protects against its hyperoxidation. Certainly the beneficial effects of the H2Sn donor; SG1002, on lipid peroxidation were dependent on the expression of Prx6.
Taken together, our data show that the regulation of CSE by altered shear stress/flow can be attributed to alterations in KLF2 and miR-27b levels, and that statin treatment can restore CSE activity in endothelial cells from plaque containing arteries. Shear stress has frequently been linked with a decrease in oxidative stress and protection against atherosclerosis [6], via its actions on eNOS expression and NO generation. However, in areas of the vasculature exposed to disturbed flow, this NO-mediated protection is inactive. Our data imply that CSE can partly compensate for the lack of eNOS, to preserve oxidative balance and prevent and lipid peroxidation via the H2Sn–dependent sulfhydration of the antioxidant enzyme Prx6. The sulfhydration of Prx6 prevents its decamerization. This is important as the decameric/hyperoxidized Prdx6 switches from being a peroxidase to become a phospholipase A2. This switch has a negative effect on vascular homeostasis as it contributes to the activation of endothelial cell NADPH oxidases [55,56], thus aggravating endothelial cell ROS production. CSE-derived H2Sn are only able to compensate for the lack of eNOS-derived NO in the absence of vascular inflammation as CSE is exquisitely sensitive to inflammatory cytokines such as IL-1β, which result in the phosphorylation and inhibition of the enzyme. The ability of SG1002 to restore Prx6 sulfhydration indicates that H2Sn donors may be of benefit as a therapeutic approach to attenuate atherosclerosis development in humans.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (CRC1366/1 B1 Project ID: 39404578 to I.F. and S.-I.B.; The Cardio-Pulmonary Institute, EXC 2026, Project ID: 390649896), Förderung FFF Nachwuchsforscher Goethe University Hospital (2018, to S.-I.B.) and Hellenic Research and Innovation (Project # 886, to A.P.)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are indebted to Isabel Winter, Katharina Bruch, Katharina Herbig and Cindy F. Höper for expert technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101379.
Contributor Information
Fragiska Sigala, Email: fsigala@med.uoa.gr.
Ingrid Fleming, Email: fleming@em.uni-frankfurt.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wu D., Hu Q., Zhu D. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid. Med. Cell Longev. 2018;(2018) doi: 10.1155/2018/4579140. 4579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming I., Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 3.Yuan S., Pardue S., Shen X., Alexander J.S., Orr A.W., Kevil C.G. Hydrogen sulfide metabolism regulates endothelial solute barrier function. Redox Biol. 2016;9:157–166. doi: 10.1016/j.redox.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibli S.-I., Luck B., Zukunft S., Wittig J., Chen W., Xian M., Papapetropoulos A., Hu J., Fleming I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 2018;18:295–304. doi: 10.1016/j.redox.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., Snyder S.H., Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine g-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies P.F., Civelek M., Fang Y., Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc. Res. 2013;99:315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekker R.J., van Soest S., Fontijn R.D., Salamanca S., de Groot P.G., VanBavel E., Pannekoek H., Horrevoets A.J.G. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 8.van Thienen J.V., Fledderus J.O., Dekker R.J., Rohlena J., van Ijzendoorn G.A., Kootstra N.A., Pannekoek H., Horrevoets A.J.G. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc. Res. 2006;72:231–240. doi: 10.1016/j.cardiores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Bibli S.-I., Hu J., Sigala F., Wittig I., Heidler J., Zukunft S., Tsilimigras D.I., Randriamboavonjy V., Wittig J., Kojonazarov B., Schürmann C., Siragusa M., Siuda D., Luck B., Abdel Malik R., Filis K.A., Zografos G., Chen C., Wang D.W., Pfeilschifter J., Brandes R.P., Szabo C., Papapetropoulos A., Fleming I. Cystathionine γ lyase sulfhydrates the RNA binding protein Human Antigen R to preserve endothelial cell function and delay atherogenesis. Circulation. 2019;139:101–114. doi: 10.1161/CIRCULATIONAHA.118.034757. [DOI] [PubMed] [Google Scholar]
- 10.Yuan S., Yurdagul A., Peretik J.M., Alfaidi M., Al Yafeai Z., Pardue S., Kevil C.G., Orr A.W. Cystathionine γ-lyase modulates flow-dependent vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2018;38:2126–2136. doi: 10.1161/ATVBAHA.118.311402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mani S., Li H., Untereiner A., Wu L., Yang G., Austin R.C., Dickhout J.G., Lhoták Š., Meng Q.H., Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 12.Meng G., Zhao S., Xie L., Han Y., Ji Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br. J. Pharmacol. 2018;175:1146–1156. doi: 10.1111/bph.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali M.Y., Ping C.Y., Mok Y.-Y., Ling L., Whiteman M., Bhatia M., Moore P.K. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altaany Z., Moccia F., Munaron L., Mancardi D., Wang R. Hydrogen sulfide and endothelial dysfunction: relationship with nitric oxide. Curr. Med. Chem. 2014;21:3646–3661. doi: 10.2174/0929867321666140706142930. [DOI] [PubMed] [Google Scholar]
- 15.North American Symptomatic Carotid Endarterectomy Trial Methods, patient characteristics, and progress. Stroke. 1991;22:711–720. doi: 10.1161/01.str.22.6.711. [DOI] [PubMed] [Google Scholar]
- 16.Syhr K.M.J., Boosen M., Hohmann S.W., Longen S., Köhler Y., Pfeilschifter J., Beck K.-F., Geisslinger G., Schmidtko A., Kallenborn-Gerhardt W. The H2S-producing enzyme CSE is dispensable for the processing of inflammatory and neuropathic pain. Brain Res. 2015;1624:380–389. doi: 10.1016/j.brainres.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 17.Monvoisin A., Alva J.A., Hofmann J.J., Zovein A.C., Lane T.F., Iruela-Arispe M.L. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev. Dynam. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- 18.Busse R., Lamontagne D. Endothelium-derived bradykinin is responsible for the increase in calcium produced by angiotensin-converting enzyme inhibitors in human endothelial cells. Naunyn Schmiedeberg's Arch. Pharmacol. 1991;344:126–129. doi: 10.1007/BF00167392. [DOI] [PubMed] [Google Scholar]
- 19.Fleming I., Fisslthaler B., Dixit M., Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 20.Fisslthaler B., Loot A.E., Mohamed A., Busse R., Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ. Res. 2008;102:1520–1528. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- 21.Olsen J.V., de Godoy L.M.F., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteom. 2005;4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 23.Nelson K.J., Parsonage D. Measurement of peroxiredoxin activity. Curr Protoc Toxicol Chapter. 2011;7 doi: 10.1002/0471140856.tx0710s49. Unit7.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bibli S.-I., Andreadou I., Glynos C., Chatzianastasiou A., Toumpanakis D., Zakynthinos S., Vasilakopoulos T., Iliodromitis E.K., Papapetropoulos A. Exposure to cigarette smoke abrogates the beneficial effect of ischemic postconditioning. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H1321–H1332. doi: 10.1152/ajpheart.00925.2015. [DOI] [PubMed] [Google Scholar]
- 25.Demolli S., Doddaballapur A., Devraj K., Stark K., Manavski Y., Eckart A., Zehendner C.M., Lucas T., Korff T., Hecker M., Massberg S., Liebner S., Kaluza D., Boon R.A., Dimmeler S. Shear stress-regulated miR-27b controls pericyte recruitment by repressing SEMA6A and SEMA6D. Cardiovasc. Res. 2017;113:681–691. doi: 10.1093/cvr/cvx032. [DOI] [PubMed] [Google Scholar]
- 26.Boon R.A., Hergenreider E., Dimmeler S. Atheroprotective mechanisms of shear stress-regulated microRNAs. Thromb. Haemost. 2012;108:616–620. doi: 10.1160/TH12-07-0491. [DOI] [PubMed] [Google Scholar]
- 27.Parmar K.M., Nambudiri V., Dai G., Larman H.B., Gimbrone M.A., García-Cardeña G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 28.Sen-Banerjee S., Mir S., Lin Z., Hamik A., Atkins G.B., Das H., Banerjee P., Kumar A., Jain M.K. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 29.Yan S.-K., Chang T., Wang H., Wu L., Wang R., Meng Q.H. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006;351:485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 30.Bos E.M., Wang R., Snijder P.M., Boersema M., Damman J., Fu M., Moser J., Hillebrands J.-L., Ploeg R.J., Yang G., Leuvenink H.G.D., van Goor H. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 2013;24:759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S.Y., Jo H.-Y., Kim M.H., Cha Y.-y., Choi S.W., Shim J.-H., Kim T.J., Lee K.-Y. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J. Biol. Chem. 2008;283:33563–33568. doi: 10.1074/jbc.M806578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J.W., Dodia C., Feinstein S.I., Jain M.K., Fisher A.B. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J. Biol. Chem. 2000;275:28421–28427. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- 33.Kümin A., Schäfer M., Epp N., Bugnon P., Born-Berclaz C., Oxenius A., Klippel A., Bloch W., Werner S. Peroxiredoxin 6 is required for blood vessel integrity in wounded skin. J. Cell Biol. 2007;179:747–760. doi: 10.1083/jcb.200706090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mistry R.K., Murray T.V.A., Prysyazhna O., Martin D., Burgoyne J.R., Santos C., Eaton P., Shah A.M., Brewer A.C. Transcriptional regulation of cystathionine-γ-lyase in endothelial cells by NADPH oxidase 4-dependent signaling. J. Biol. Chem. 2016;291:1774–1788. doi: 10.1074/jbc.M115.685578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longchamp A., Mirabella T., Arduini A., MacArthur M.R., Das A., Treviño-Villarreal J.H., Hine C., Ben-Sahra I., Knudsen N.H., Brace L.E., Reynolds J., Mejia P., Tao M., Sharma G., Wang R., Corpataux J.-M., Haefliger J.-A., Ahn K.H., Lee C.-H., Manning B.D., Sinclair D.A., Chen C.S., Ozaki C.K., Mitchell J.R. Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell. 2018;173:117–129. doi: 10.1016/j.cell.2018.03.001. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C., Brace L., Longchamp A., Treviño-Villarreal J.H., Mejia P., Ozaki C.K., Wang R., Gladyshev V.N., Madeo F., Mair W.B., Mitchell J.R. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez Bosc L.V., Osmond J.M., Giermakowska W.K., Pace C.E., Riggs J.L., Jackson-Weaver O., Kanagy N.L. NFAT regulation of cystathionine γ-lyase expression in endothelial cells is impaired in rats exposed to intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H791–H799. doi: 10.1152/ajpheart.00952.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Z., Kumar A., SenBanerjee S., Staniszewski K., Parmar K., Vaughan D.E., Gimbrone M.A., Balasubramanian V., García-Cardeña G., Jain M.K. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 2005;96:e48–57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 40.Li T., Cao H., Zhuang J., Wan J., Guan M., Yu B., Li X., Zhang W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin. Chim. Acta. 2011;412:66–70. doi: 10.1016/j.cca.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Dolz S., Górriz D., Tembl J.I., Sánchez D., Fortea G., Parkhutik V., Lago A. Circulating microRNAs as novel biomarkers of stenosis progression in asymptomatic carotid stenosis. Stroke. 2017;48:10–16. doi: 10.1161/STROKEAHA.116.013650. [DOI] [PubMed] [Google Scholar]
- 42.Renga B., Bucci M., Cipriani S., Carino A., Monti M.C., Zampella A., Gargiulo A., Di d'Emmanuele Villa Bianca R., Distrutti E., Fiorucci S. Cystathionine γ-lyase, a H2S-generating enzyme, is a GPBAR1-regulated gene and contributes to vasodilation caused by secondary bile acids. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H114–H126. doi: 10.1152/ajpheart.00087.2015. [DOI] [PubMed] [Google Scholar]
- 43.Sigala F., Efentakis P., Karageorgiadi D., Filis K., Zampas P., Iliodromitis E.K., Zografos G., Papapetropoulos A., Andreadou I. Reciprocal regulation of eNOS, H2S and CO-synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol. 2017;12:70–81. doi: 10.1016/j.redox.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao S., Li Q., Liu L., Xu Z., Xiao J. Simvastatin reduces interleukin-1beta secretion by peripheral blood mononuclear cells in patients with essential hypertension. Clin Chim Acat. 2004;344:195–200. doi: 10.1016/j.cccn.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Boland A.J., Gangadharan N., Kavanagh P., Hemeryck L., Kieran J., Barry M., Walsh P.T., Lucitt M. Simvastatin suppresses interleukin Iβ release in human peripheral blood mononuclear cells stimulated with cholesterol crystals. J. Cardiovasc. Pharmacol. Ther. 2018;23:509–517. doi: 10.1177/1074248418776261. [DOI] [PubMed] [Google Scholar]
- 46.Wen Y.-D., Wang H., Kho S.-H., Rinkiko S., Sheng X., Shen H.-M., Zhu Y.-Z. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suo R., Zhao Z.-Z., Tang Z.-H., Ren Z., Liu X., Liu L.-S., Wang Z., Tang C.-K., Wei D.-H., Jiang Z.-S. Hydrogen sulfide prevents H₂O₂-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol. Med. Rep. 2013;7:1865–1870. doi: 10.3892/mmr.2013.1417. [DOI] [PubMed] [Google Scholar]
- 48.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxidants Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 49.Patel P., Chatterjee S. Peroxiredoxin6 in endothelial signaling. Antioxidants. 2019;8 doi: 10.3390/antiox8030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher A.B., Vasquez-Medina J.P., Dodia C., Sorokina E.M., Tao J.-Q., Feinstein S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018;14:41–46. doi: 10.1016/j.redox.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017;617:68–83. doi: 10.1016/j.abb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu L., Liu K., He J., Tian C., Yu X., Yang J. Direct proteomic mapping of cysteine persulfidation. Antioxidants Redox Signal. 2019 doi: 10.1089/ars.2019.7777. [DOI] [PubMed] [Google Scholar]
- 53.Kang S.W., Baines I.C., Rhee S.G. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J. Biol. Chem. 1998;273:6303–6311. doi: 10.1074/jbc.273.11.6303. [DOI] [PubMed] [Google Scholar]
- 54.Shahnaj S., Chowhan R.K., Meetei P.A., Kakchingtabam P., Herojit Singh K., Rajendrakumar Singh L., Nongdam P., Fisher A.B., Rahaman H. Hyperoxidation of peroxiredoxin 6 induces alteration from dimeric to oligomeric state. Antioxidants. 2019;8 doi: 10.3390/antiox8020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher A.B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res. 2018;59:1132–1147. doi: 10.1194/jlr.R082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chatterjee S., Feinstein S.I., Dodia C., Sorokina E., Lien Y.-C., Nguyen S., Debolt K., Speicher D., Fisher A.B. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 2011;286:11696–11706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.