Graphical abstract
Abbreviations: TAM, tamoxifen; SIM, simvastatin; ER+, estrogen receptor-positive; TNF-α, tumor necrosis factor α; NF-KB, nuclear factor kappa-B; NOx, nitric oxide; GSH, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; VEGF, vascular endothelial growth factor; MMP, 2&9 metalloproteinases-2and9; Bax/Bcl-2, ratio Bcl-2-AssociatedXprotein/B-cell lymphoma 2 ratio; EAC, ehrlich ascites carcinoma
Keywords: Tamoxifen, Simvastatin, Cytotoxicity, Apoptosis, Vascular endothelial growth factor, Oxidative stress
Highlights
-
•
The combination of TAM and SIM exerted synergistic effects in T47D cells.
-
•
The combination increased the apoptotic and necrotic cell death in T47 cells.
-
•
VEGF and MMP 2&9 were decreased after using the combination regimen.
-
•
In vivo, non-significant decrease in tumor volume in combination regimen.
Abstract
Tamoxifen (TAM) is a nonsteroidal antiestrogen drug, used in the prevention and treatment of all stages of hormone-responsive breast cancer. Simvastatin (SIM), a lipid-lowering agent, has been shown to inhibit cancer cell growth. The study aimed at investigating the impact of using SIM with TAM in estrogen receptor-positive (ER+) breast cancer cell line, T47D, as well as in mice-bearing Ehrlich solid tumor. The cell line was treated with different concentrations of TAM or/and SIM for 72 h. The effects of treatment on cytotoxicity, oxidative stress markers, apoptosis, angiogenesis, and metastasis were investigated. Our results showed that the combination treatment decreased the oxidative stress markers, glucose uptake, VEGF, and MMP 2 &9 in the cell line compared to TAM- treated cells. Drug interaction of TAM and SIM was synergistic in T47D by increasing the apoptotic makers Bax/BCL-2 ratio and caspase 3 activity. Additionally, in vivo, the combination regimen resulted in a non-significant decrease in the tumor volume compared to TAM treated group. Moreover, the combined treatment decreased the protein expression of TNF-α, NF-kB compared to control. In conclusion, our results suggest that SIM may serve as a promising treatment with TAM for improving the efficacy against estrogen receptor-positive (ER+) breast cancer.
1. Introduction
Breast cancer is the most common female cancer worldwide [1]. Estrogen receptor (ER+) positive breast cancer represents more than 70% of all breast cancer patients. Tamoxifen (TAM) is the mainstay in the treatment and prevention of ER + breast cancer in both pre and postmenopausal females [2]. It reduces breast cancer recurrence by 50% and the annual mortality rate by 31%. Despite this success, 20–30% of tumors develop resistant to TAM therapy after 3–5 years of its intake, in addition to its side effects [3].
Obesity is a risk factor for (ER+) postmenopausal breast cancer patients, attributed to increases in circulating insulin, insulin-like growth factors, estrogen and inflammatory cytokines [4,5]. Hypercholesterolemia, comorbidity of obesity, has been identified as an independent risk factor for breast cancer [6,7]. Statins, the 3-hydroxy-3- methylglutaryl (HMG) – CoA reductase inhibitors, beyond their cardiovascular effects, they have been reported to have possible benefits as anticancer, and inhibition of cancer progression [8,9]. Besides, a potential role for statins as a radiosensitizer for aggressive breast cancer has been suggested [10]. Despite the convincing preclinical evidence for anticancer effects of statin, their role in breast cancer recurrence and mortality are still not conclusive [[11], [12], [13], [14], [15]]. Some data support a beneficial role for their use in breast cancer management [12,13], other studies are less promising and argue against their prescription in cancer treatment [14,15]. However, the results differ depending on the type of statin used, when it is used, duration of treatment, follow-up time, and patient characteristics [16]. Moreover, all these studies were carried out using statins alone, its effectiveness in combination with TAM as adjuvant therapy in ER + breast cancer has not yet been explored. Therefore, it is worthwhile examining whether SIM can potentiate the tumor response of TAM or not, the conventional breast cancer therapy. The importance of this interaction is intensified as TAM is a pioneering medicine for the treatment and prevention of breast cancer, and confers dramatic reductions in breast cancer recurrence and mortality. Also, SIM may be prescribed with TAM for breast cancer patients because of hypercholesterolemia. Therefore, the current study was designed to investigate the combined antitumor effect of TAM and SIM in the ER + breast cancer cell line, T47D as well as in mice bearing Ehrlich solid tumor as a model of mammary carcinoma established in studying the effect of chemotherapy in vivo.
2. Material and methods
2.1. Drugs
Tamoxifen (TAM citrate), and Simvastatin (SIM) were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). It was dissolved in dimethyl sulfoxide (DMSO) to yield stock solution1mM and serially diluted in RPMI-1640 supplemented medium immediately before use to yield a concentration range of 2.5–40 μM for TAM and 1–16 μM for SIM, the final concentration of DMSO never exceeded 0.1% (v/v) in both control and treated samples
2.2. Chemicals
Dimethyl sulfoxide DMSO), RPMI-1640 medium, fetal bovine serum (FBS), Penicillin/ Streptomycin antibiotic, trypsin-EDTA, Ellman's reagent [5,5-Dithio-bis-(2-nitrobenzoic acid)], reduced glutathione,1,1.3,3-tetramethoxypropane, trichloroacetic acid (TCA), thiobarbituric acid, β-mercaptoethanol, sodium dodecyl sulfate (SDS),sodium bicarbonate and methanol were all purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Acetonitrile was obtained from (Alliance Bio Co., USA). All other chemicals and solvents used were of the highest purity grade available.
2.3. Human cancer cell line
Human breast carcinoma cell line T47D was obtained frozen in liquid nitrogen (-180 °C) from American Type Culture Collection (ATCC; Washington, DC, USA). The tumor cell line was maintained as monolayer cultures in RPMI-1640 supplemented with 10% FBS and 1% penicillin-streptomycin.
2.4. Animals
Female Swiss albino mice weighing 20–25 g were obtained from the animal facility, Pharmacology Unit, National Cancer Institute (NCI), Cairo University, Egypt. The treatment protocol was approved by the research ethics committee of the Faculty of Pharmacy, Cairo University, Cairo, Egypt (Permit Number: PT 1567)
In-vitro parameters
2.5. Cytotoxicity assay
Cytotoxicity was determined using the sulforhodamine-B (SRB) method according to that of Skehan et al., [17]. Cells were seeded in 96-well microtiter plates at a concentration of 3 × 103 cells/well. They were left to attach for 24 h before incubation with drugs. The cells were treated for 72 h with different concentrations (2.5–40 μM) of TAM, SIM (1–16 μM), and combination of 5 μM of TAM (half of IC50) and different concentrations of SIM (0.5, 1, 1.5, 2, 2.5 μM).The optical density (O. D) of each well was measured spectrophotometrically at 570 nm using an ELISA microplate reader (TECAN Sunrise™, Germany). The mean values were estimated as the percentage of cell viability as follows: O.D (treated cells) / O.D (control cells) × 100. The IC50 value (the concentration that produces 50% inhibition of cell growth) of each drug was calculated using dose-response curve-fitting models (Graph-Pad Prism software, version 5).
2.6. Evaluation of drug interaction
For designing an effective combination regimen, we used a fixed concentration of TAM, 5 μM (half IC50), with different concentrations (0.5–2.5 μM) of SIM (Fig. 1C).To assess the modulatory effect of SIM on the cytotoxicity of TAM, the degree of interaction between the two drugs was calculated using the combination index according to the isobologram equation according to [18]: the combination index (CI) = d1/D1 + d2/D2. d1 and d2 signify the respective concentrations of TAM and SIM used in combination to produce a fixed level of inhibition, while D1 and D2 represent their concentrations that are alone able to produce the same magnitude of the effect. If "CI" is less than 1, the effect of the combination is synergistic, whereas if CI = 1 or >1, the effect is additive or antagonistic, respectively.
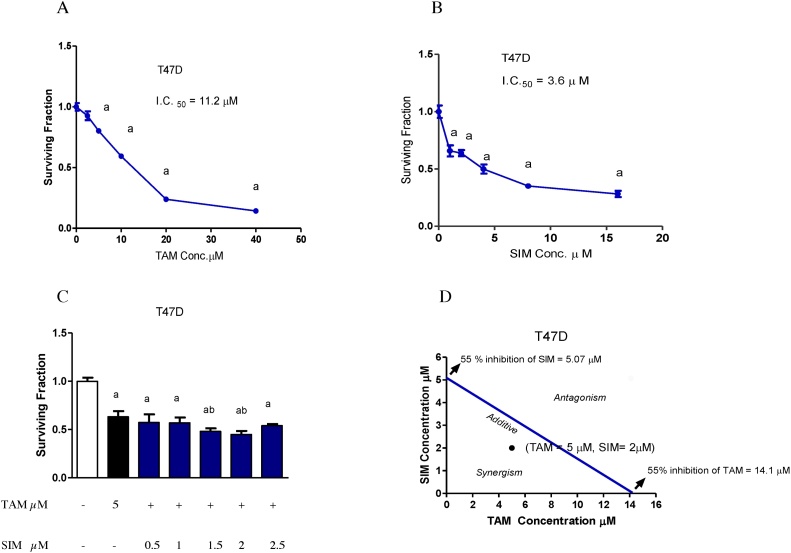
Fig. 1.
Cytotoxicity of TAM, SIM and their combinations in T47D breast cancer cell line after 72 h. Surviving fraction of T47D treated with different concentrations of TAM (A). Surviving fractions of T47D treated with different concentrations of SIM (B). Combined cytotoxicity effect of 5 μM TAM and different concentrations of SIM (0.5–2.5 μM) in T47D cells (C). Isobologram analysis of combination of TAM and SIM in T47D cell line (D). Values are the means ± SD of three independent experiments performed in triplicates. a: significantly different from the control group and b: significantly different from TAM-treated group at P value < 0.05.
2.7. For the preparation of cell-free media and cell lysate
Cells were cultured in T75 flasks, left for 24 h, and then treated with TAM or/and SIM for 72 h. The medium was collected and used for the determination of LDH leakage, glucose uptake, and NOx level. Cell pellets were prepared by removing the cells from the flasks by trypsinization. The treated and control cell pellet were collected, washed, and suspended in cold lysis buffer, then sonicated and centrifuged, and the clear supernatant was taken into another Eppendorf.
2.8. Determination of lipid peroxidation
Lipid peroxidation products were determined by measuring malondialdehyde (MDA) level in cell lysate using the method of Buege and Aust [19]. The principle mainly depends on the reaction of malondialdehyde with thiobarbituric acid to form thiobarbituric acid reactive substances, which has a pink color with absorption in spectrophotometry at 535 nm wavelength. The results were expressed as nmol/mg protein.
2.9. Determination of non-protein reduced thiols content (glutathione content)
Reduced glutathione (GSH) in cell lysate was determined according to the method of Ellman [20], it is based on the reduction of Ellman's reagent [5,5′-dithio-bis- (2- nitrobenzoic acid)] by SH groups to form 1 mol of 2-nitro-5- mercaptobenzoic acid per mole of SH. The optical density was measured at 412 nm against a reagent blank and the results were expressed as μmol/mg protein.
2.10. Determination of superoxide dismutase (SOD)
Superoxide dismutase (SOD) activity was assayed using a commercial Assay Kit-WST (Sigma- Aldrich, St. Louis, MO, USA). SOD assay was carried out using WST-1 (2-(4-Iodophenyl)- 3-(4-nitrophenyl)-5-(2,4-disulfophenyl)- 2Htetrazolium, monosodium salt) that produces a water-soluble formazan dye upon reduction with a superoxide anion. The rate of the reduction can be determined by a calorimetrically at 440 nm. Enzymatic activity was expressed in the form of U/ml.
2.11. Determination of total nitrate/nitrite (NOx)
Total nitrate/nitrite (NOx) was measured in cell culture media as a stable end product, nitrite, according to the method of Miranda [21]. The assay is based on the reduction of nitrate by vanadium trichloride combined with detection by the acidic Griess reaction. The diazotization of sulfanilic acid with nitrite at acidic pH is subsequent coupling with N-(10-naphthyl) ethylenediamine to an intensely colored product that is determined spectrophotometrically at 540 nm and expressed as nmol/mg protein
2.12. Determination of protein concentration
Protein concentration was assessed in the medium and cell lysate by using the Bradford method [22]. The method based on the binding of Coomassie brilliant blue G-250 dye with protein and forming a complex which can be detected spectrophotometrically at 595 nm then the concentration was determined using a standard calibration curve.
2.13. Determination of glucose uptake
Glucose in media was determined using a colorimetric assay kit (Randox, County Antrim, UK) according to the manufacturer's instructions following the method of Trinder and Ann [23] using glucose oxidase with an oxygen acceptor. The glucose in the medium was determined spectrophotometrically at 546 nm. The results were expressed as mg/dl.
2.14. Determination of LDH level
LDH level was determined in the cell culture supernatant using a colorimetric assay kit (Randox, County Antrim, UK) according to the manufacturer's instructions. The O.D was read at 500 nm. The results were expressed as U/L.
2.15. Determination of the VEGF level
The VEGF level in the medium was determined using an ELISA kit provided by Biosciences (San Diego, CA, USA) following the manufacturer's protocol and the method described by Kim et al. [24]. The O.D of each well was measured at 450 nm and the results were expressed as pg/ml.
2.16. Determination of the enzymatic activity of caspase 3
Caspase 3 activity was measured colorimetrically using a caspase 3 assay kit (Sigma-Aldrich Chemical Co., USA). Assay buffer was added in a 96 well plate to cell lysates of different treated groups. The reaction was then started by adding a caspase-3 substrate to each well and gently mixed by shaking. After incubating at 37 °C overnight, the absorbance was read at 405 nm. The results were calculated using a calibration curve prepared using a stock solution at the concentration range of 10–200 μM p-nitroaniline.
2.17. Determination of TAM uptake using liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis
To study whether the addition of SIM can affect the level of TAM, the level of TAM alone and in its combination with SIM were determined using LC/MS/MS. Cells were seeded at a density of 2 × 104 cells/well in 24-well plate and left for 24 h. Cells were incubated with TAM alone and with SIM, the medium was then aspirated at 0, 2, 4, 6, 24 and 48 h, centrifuged and the supernatants were used for the assay. Two- hundred microliters of the supernatant were mixed thoroughly with 200 μL acetonitrile (Alliance Bio, USA) and centrifuged at 1400 rpm for 15 min at 4∘ C. The clear supernatant was injected into AB SCIEX LC/MS/MS system (AB SCIEX 3200 QTRAP, Germany) adapting the method of Gjerde et al. [25] for TAM determination. The system is equipped with an electrospray ionization (ESI) source and an Agilent 1260 affinity HPLC system, consisting of a vacuum degasser, a binary pump, and an autosampler to determine the concentration of TAM. Analyst 1.5.2 software was used for data acquisition and processing. The analytical column used was Agilent Poroshell 120-C18 (50 mm × 3 mm ×2.7 μm, Agilent, Germany) at 25∘ C. The mobile phase consists of 0.1% formic acid/water (solvent A) and 0.1% formic acid/acetonitrile (solvent B), delivered at a flow rate of 0.5 mL/min and the analysis was performed using the positive ion mode.
2.18. Determination MMP-2 and 9 activities by gelatin zymography
Briefly, MMP-2 and MMP-9 enzymatic activities in the collected medium were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE) gelatin zymography [26]. The gel was incubated for 15 min in renaturation buffer containing 2.5% Triton X-100 at room temperature then it incubated for 1 h. The gel was washed twice with water, then incubated overnight at 37 °C in developing buffer (50 mMTris–HCl, pH 7.5, 0.2 M NaCl, Triton-X5 ml and 5 mM CaCl2. The gel was stained with Coomassie brilliant blue R1 250 for 1 h and de-stained in a 10% methanol and 5% acetic acid solution. Enzyme digested regions were observed as clear bands against a dark blue background. The gel was scanned using image Scanner III LabScan6.0. To determine the mean intensity of each band (mean pixel), the band densities were measured with Scion Image Beta 4.0.2 (Scion Co., Frederick, MD, U.S.A.) software.
2.19. Immunohistochemical staining of NF-KB, Bax, and BCL-2
For the detection of immunoreactive proteins, treated cells were treated with 5μM of TAM, 2μM of SIM and their combination for 72 h. Cells were trypsinized, centrifuged, and resuspended in 500 μl medium. Slides were washed and sterilized with ethanol, coated with cells and incubated overnight at 37°. Cells were fixed in ethanol/methanol 1:1 for 5 min, washed in 0.1 M PBS, and nonspecific antigens were blocked with normal goat serum for 20 min at room temperature. After blocking, slides were incubated 1 h with the primary monoclonal antibodies specific to NF-KB, Bax, and BCL-2 (Abcam, Cambridge, UK) at a concentration of 1 μg/ml. Thereafter, samples were incubated at room temperature for 1 h with goat anti-rabbit secondary antibody, sections were then washed with TBS and incubated in diaminobenzidine (DAB) solution containing H2O2 and Counterstain was performed using hematoxylin, and the slides were visualized using a digital camera installed on a Leica DMLB2 light microscope (Leica Microsystems, Germany).
In-vivo study
2.20. Determination of Tumor volume and tumor mass
The in vitro results stimulated us to test the combination in vivo using the available murine cell line (EAC). Animals were group housed and kept under controlled environmental conditions and were allowed free access to water and pelleted standard rat chow diet. Animals were kept under a controlled lighting condition (light: dark, 13 h: 11 h). Ehrlich carcinomas (EAC)-cells (2 × 106) were transplanted subcutaneously in the right thigh of the lower limb of mice. Twenty-four mice with a palpable tumor mass (100 mm3) that developed within 7 days after implantation were divided into 4 groups each 6 animals. The number of animals chosen according to the requirement of the statistician's opinion of the ethical committee and also guided by previous colleagues' studies conducted in our lab & used a similar group size [27] Group I, mice were served as a control group. Group II animals received TAM (2.5 mg/kg) [27] by oral gavage. Group III, mice were given SIM (2 mg/kg) [28] by oral gavage. Group IV, mice were treated by the combination of both TAM and SIM at the pre-mentioned doses for a period of 8 days. The change in tumor volume was measured every other day, using a Vernier caliper using the following formula. Mice were sacrificed on day 9, and tumors were dissected and weighed.
| Tumor volume (mm3)=4(A/2)2 X (B/2)/ 3 |
Where A and B denote the minor and the major tumor axis, respectively.
2.21. Determination of MDA, GSH, SOD activity and NOx level in solid tumor tissue
Animals were anesthetized by ketamine, (100 mg/kg) (EIMC Pharmaceuticals Co., Cairo, Egypt), then sacrificed by decapitation and the tumor was excised, weighed. The tumor was dissected for histopathology and biochemical parameters. Ten % tumor homogenate in phosphate buffer saline was prepared using a Branson sonifier (250, VWR Scientific, Danbury, CT, USA). The homogenates were centrifuged at 800 g for 5 min at 4 C° to separate the nuclear debris and then the supernatant was centrifuged again at 10,500 g for 20 min at 4 C°. Levels of MDA, GSH, SOD, and NOx were determined as previously described.
2.22. Histopathology and immunohistochemical detection of TNF-α and NF-KB
The samples were fixed in 10% neutral buffered formalin, dehydrated through alcohols, cleared in xylene and then embedded in paraffin wax. Sections (5 mm thick) were stained with hematoxylin and eosin. Paraffin-embedded EAC sections were first rehydrated in xylene and then in graded ethanol solutions. Primary antibodies specific to NF-kB and TNF-α (Abcam, Cambridge, UK) at a concentration of 1 μg/ml antibodies were used and thereafter processed as detailed above.
2.23. Statistical analysis
Differences between obtained values (mean ± SD) were carried out by one-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test. ANOVA with repeated and mixed model followed by the Bonferroni test for adjustment for multiple comparisons was used for comparison between two groups in TAM uptake. A value of 0.05 or less was taken as a criterion for a statistically significant difference.
3. Results
3.1. TAM, SIM and their combination inhibited cellular proliferation of T47D cells
Treatment of T47D cell line with different concentrations of TAM for 72 h produced a decrease in cell survival with IC 50 of 11.2 μM (Fig. 1A). On the other hand, the IC50 values of SIM were 3.6 μM (Fig. 1B). Our results showed that the most effective concentration of SIM that impeded the cell viability in the cell line was 2 μM. Therefore, the following regimens (5 μM TAM with 2 μM SIM) for T47D were used for all the following studies (Fig. 1C).
3.2. The combination regimen of TAM and SIM was synergistic in T47D cells
To verify whether SIM modulation of TAM cytotoxicity in a human cell line (T47D) was due to an additive, antagonistic or synergistic effect, evaluation of drug interaction was performed. Using the isobologram equation, the combination index (CI) of the regimen in which 2 μM SIM combined with 5 μM of TAM for 72 h of treatment was applied. Synergistic drug interaction of 5 μM TAM and 2μM SIM in T47D with combination index, CI = 0.75. (Fig. 1D).
3.3. The combination regimen significantly increased GSH and SOD in T47D cells
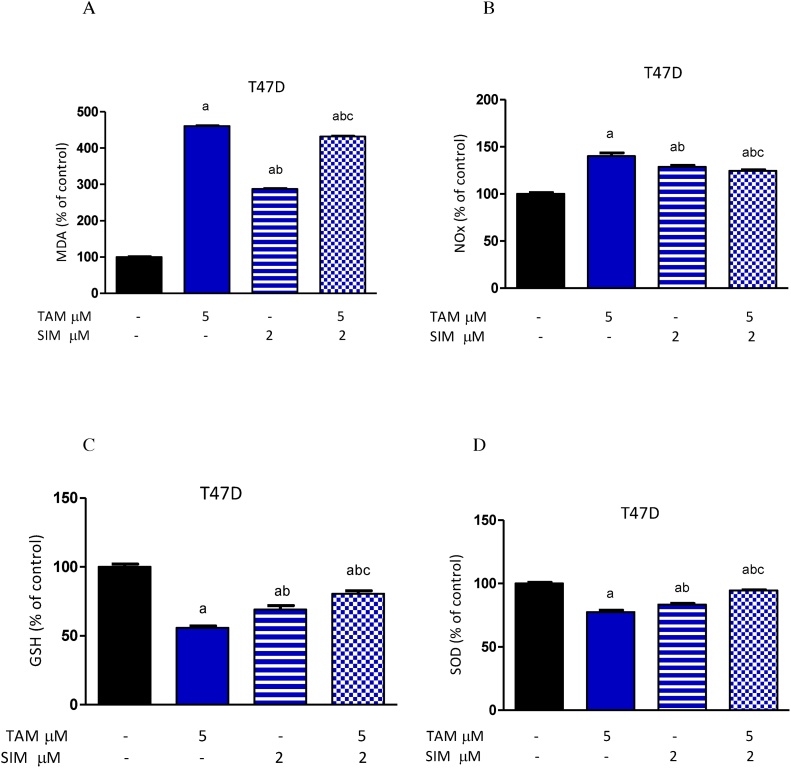
Treatment of either TAM or SIM alone significantly increased the MDA level and NOx level (Fig. 2A and B) accompanied by a significant reduction in the GSH content and SOD in T47D cells (Fig. 2C and D) compared to normal untreated cells. The combination of SIM and TAM produced a significant inhibition in the MDA and NOx level by 6.25% and 11.2% respectively compared to TAM-treated cells, while it is still significantly higher than the normal untreated cells (Fig. 2A and B). Besides, SIM and TAM increased significantly the GSH content by 44.6% and SOD activity by 22.2% compared to TAM treated cells (Fig. 2C and D). However, the antioxidants substances GSH and SOD were still significantly lower than normal control.
Fig. 2.
Effect of treatment of TAM, SIM and their combinations on oxidative stress and antioxidants in T47Dcell line. MDA (A), NO (B), GSH level (C), SOD (D). Data were expressed as means ± SD of three independent experiments. a: significantly different from the control group, b: significantly different from TAM and c: significantly different from SIM at P value <0.05.
3.4. The combination treatment decreased significantly glucose uptake in T47D cells
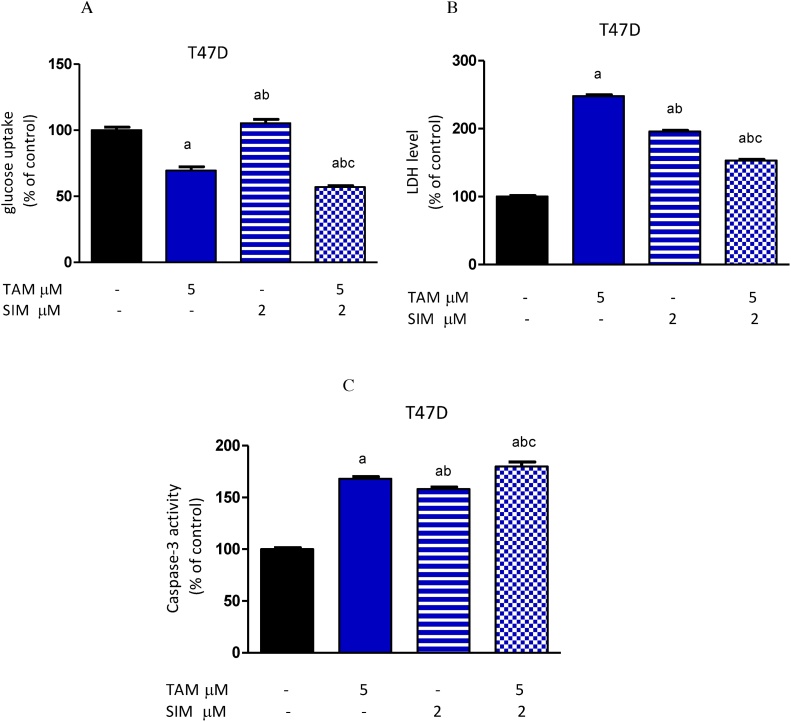
Compared to the control, TAM alone decreased the glucose consumption of T47D cells. On the other hand, SIM significantly increased glucose uptake compared to normal non-treated cells. Moreover, the combination of SIM and TAM produced a significant decrease in glucose uptake compared to the untreated control and TAM treated group (Fig. 3A).
Fig. 3.
Effect of TAM, SIM and their combinations on glucose uptake, lactate dehydrogenase (LDH) and caspase-3 activity in media of T47D cell line after 72 h. Glucose uptake (A), levels of LDH (B) and caspase-3 activity (C). Results were expressed as means ± SD of three independent experiments performed in triplicates. a: significantly different from the control group, b: significantly different from the TAM and c: significantly different from SIM at P value < 0.05.
3.5. The combination regimen decreased LDH leakage in the media
Treatment with SIM or TAM alone significantly increased the released LDH level in media of breast cancer cells compared to vehicle-treated cells. The combination of TAM and SIM significantly decreased the LDH level by 38.3% in T47D cells when compared to the TAM-treated group (Fig. 3B). Compared to non-treated cells, TAM and SIM combination regimen significantly increased LDH leakage in media.
3.6. The combination regimen significantly increased caspase-3 activity
Treatment of T47D cells with TAM produced a significant increase in caspase-3 activity by 68% compared to control. Furthermore, SIM significantly increased caspase-3 activity by 58% compared to non-treated cells. The combination regimen induced a significant increase in caspase-3 activity reaching 7.1% as compared to TAM treated cell and 79.8% as compared to non-treated cells (Fig. 3C).
3.7. The combination regimen inhibited MMP-2 and 9 in the T47D cell line
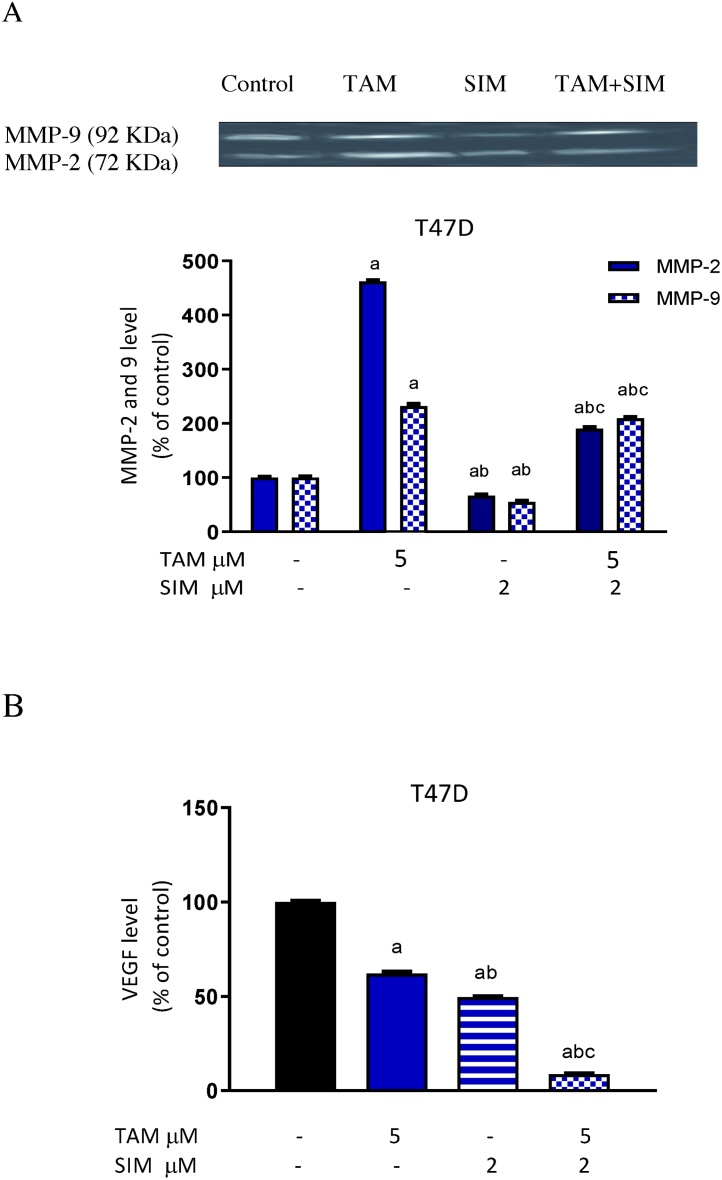
TAM treatment increased the activity of MMP 2 and 9 in T47 cells. On the other hand, SIM alone decreased the expression activity of both 2 and 9 MMPs enzymes in the breast cells. The combination of SIM and TAM inhibited the activity of MMPs compared to TAM- treated cells (Fig. 4A).
Fig. 4.
Effect of treatment with TAM, SIM and their combinations on extracellular level of MMP-2 and 9 using gelatin zymography and VEGF level in T47D cell line. Level of MMP-2 and 9 (A)and level of VEGF (B). Results are expressed as means ± SD of 2 independent experiments performed in duplicates. a: significantly different from the control group, b: significantly different from the TAM and c: significantly different from SIM at P value < 0.05. negative staining (0–25%) consider negative; (25–50%) mild; (50–75%) moderate; (75–100%) severe.
3.8. The combination regimen inhibited VEGF in the breast cancer cell line
Incubation of T47D cells with TAM significantly decreased in the level of VEGF by 37.9% compared to the control-treated group. SIM alone caused a significant decrease in VEGF by 50.4%, compared to non-treated cells. The combination of SIM with TAM produced a significant decrease compared to the TAM treated group and non-treated cells (Fig. 4B).
3.9. Expression of NF-kB, BCL- 2 and bax immunohistochemically
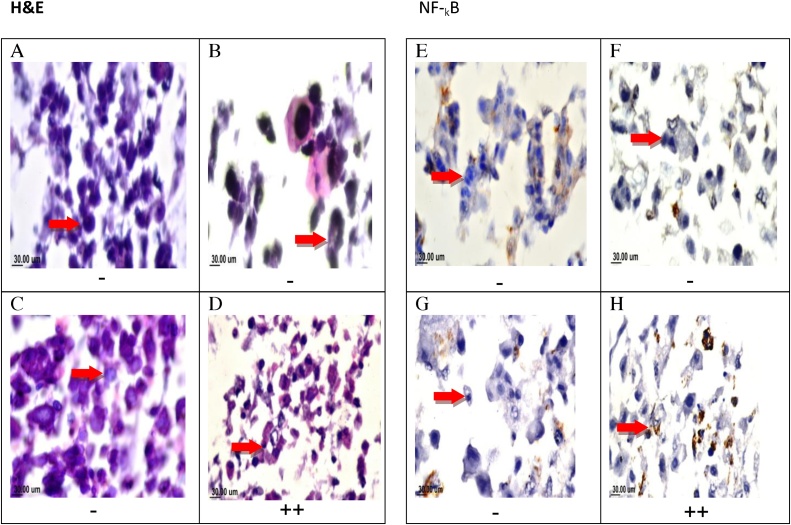
Fig. 5(A–D) display H &E stain examination of T47D cells, TAM-treated, SIM-treated and the combination showing nuclear pyknosis with basophilic smaller size cells. Immune reactivity localized intracellularly with brown color at different density according to the severity was seen in different treatments of T47cells. T47D cells showed weak staining for NF-kB in either TAM or SIM treated cells (0–25%), while, the combination regimen presented with moderate (++) staining (50–75%) Fig. 5(E–G).
Fig. 5.
Photomicrographs and NF-??B immunohistochemistry of T47D cells treated with TAM, SIM, and their combinations. Photomicrograph T47D cells sections stained by H&E(X160). control cells (A), TAM-treated (B), SIM treated (C) combination-treated (D). Immunohistochemical staining ofNF-??B of control non-treated cells (E), TAM treated(F), SIM treated (G), and combination treated(H) showing moderate positive staining (++) NF-??B. (X160).
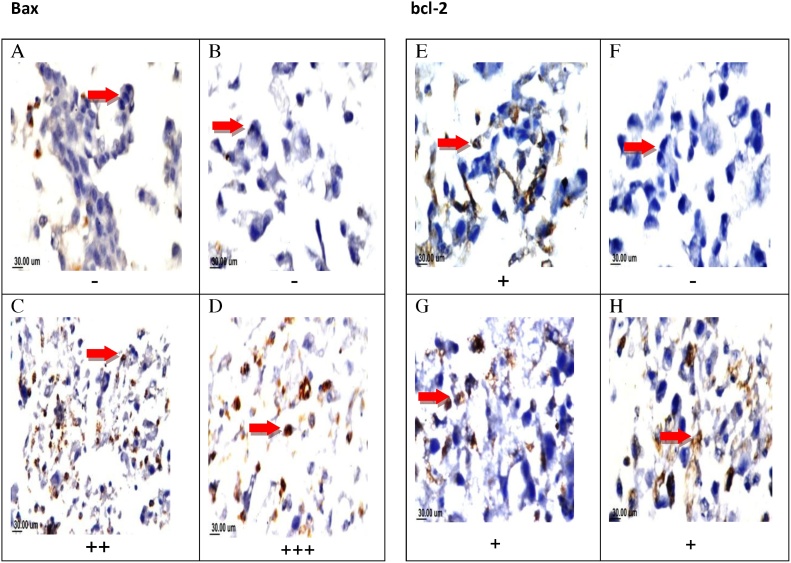
Both treatedT47 D cells with SIM and the combined regimen showed mild positive staining for BCL-2 by (+) (25–50%), while TAM decreases its expression (0–25%). Cells treated with SIM and combination showed positive staining in Bax by (++) and (+++) respectively (Fig. 6).
Fig. 6.
Effect of TAM, SIM, and their combinations on the expression levels of Bax and Bcl-2 in T47D cell line. Immunohistochemical staining of Bax for control (A) (-), TAM treated group(-) (B), SIM treated (++) moderate positive staining (C) and combination (+++) severe positive staining(D). Immunohistochemical staining of bcl-2for control (+) mild positive staining (E), TAM treated (-)(F), SIM treated (+) (G) and combination (+)mild positive staining (H). (X160).
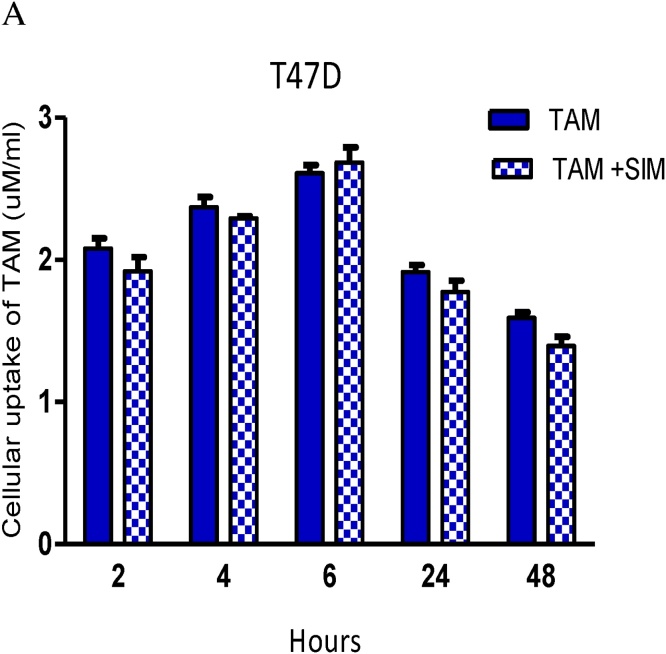
3.10. SIM did not affect cellular uptake of TAM in the breast cancer cell line
By studying the effect of time, a significant increase in TAM uptake was shown in both TAM & TAM + SIM groups up to 6 h followed by a significant drop at 24 h (p = 0.043).
The addition of SIM did not significantly affect the concentration of TAM at all the studied times. Studying group interaction, although T47D showed a higher level in TAM group from 2 h up to 48 h, (significant only at 2 (p = 0.041), 24 (p = 0.025), 48 h (p = 0.013), but p-value for interaction was non-significant (p = 0.563) (Fig. 7).
Fig. 7.
Effect of SIM on cellular uptake of TAM at different time intervals inT47D cell line. The results are expressed as the mean ± SD of 2 separate experiments performed in duplicates.
In vivo result:
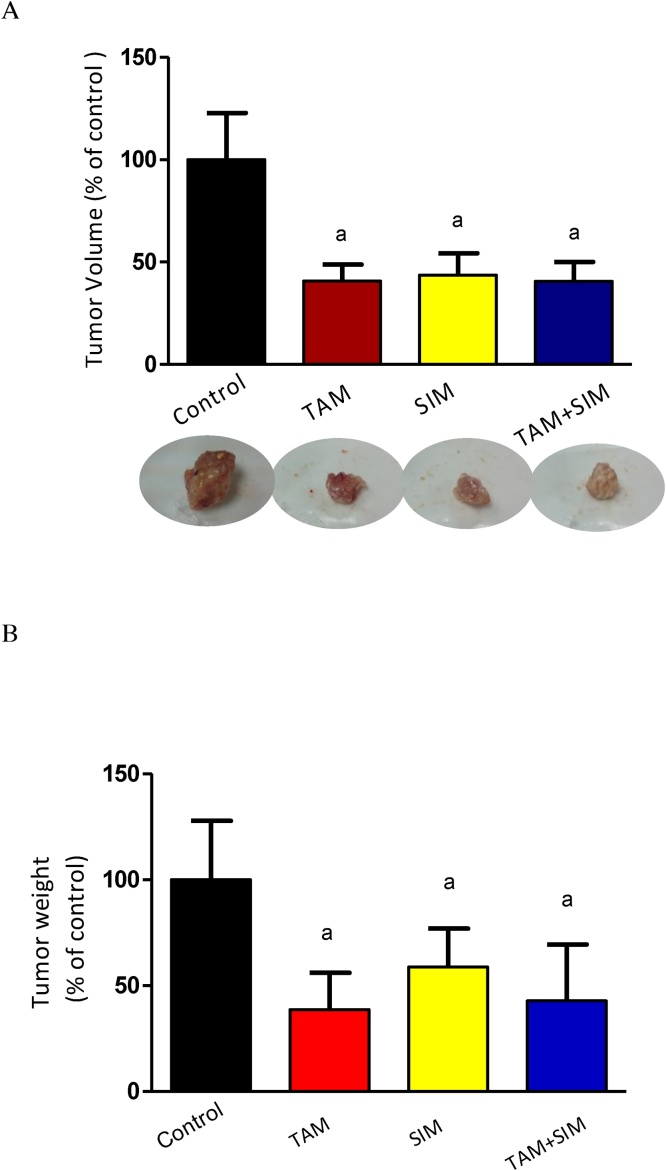
3.11. In vivo, SIM did not significantly reduce tumor volume or weight induced by TAM
The volume of the solid tumor was decreased by 59% in TAM treated group compared to the control group; however, SIM treated group & the combination regimen treated group did not significantly different from the effect of the TAM treated group (Fig. 8).
Fig. 8.
Effect of TAM, SIM and their combinations on tumor volume (A) and tumor weight (B) of solid EAC of mice. Effect of TAM (2.5 mg/kg), SIM (2 mg/kg) and their combination on tumor volume of EAC-bearing mice (A) and tumor weight (B). Values were given as mean ± SD (n = 6). a Significantly different from the control group at P < 0.05.
3.12. In vivo, SIM significantly decreased the oxidative stress of TAM
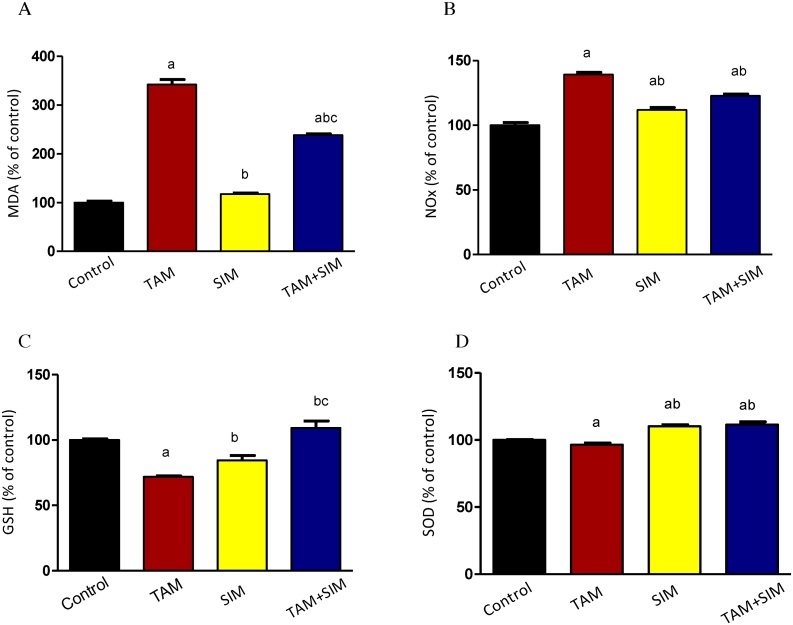
TAM increased significantly MDA and NO accompanied by a significant decrease in GSH and SOD activity. Compared to the TAM treated group, the combination treatment caused a significant decrease in the level of MDA by 30%, and NOx by 11.9% while, an increase in SOD activity by15.6% (Fig. 9)
Fig. 9.
Effect of TAM, SIM and their combinations on oxidative stress markers of solid EAC of mice. MDA (A), NOx (B), GSH (C) and SOD (D). Results are expressed as means ± SD of tumor volume from 6 mice. a Significantly different from the control group, b significantly different from the TAM and c from SIM at P value < 0.05.
3.13. Immunohistochemical results
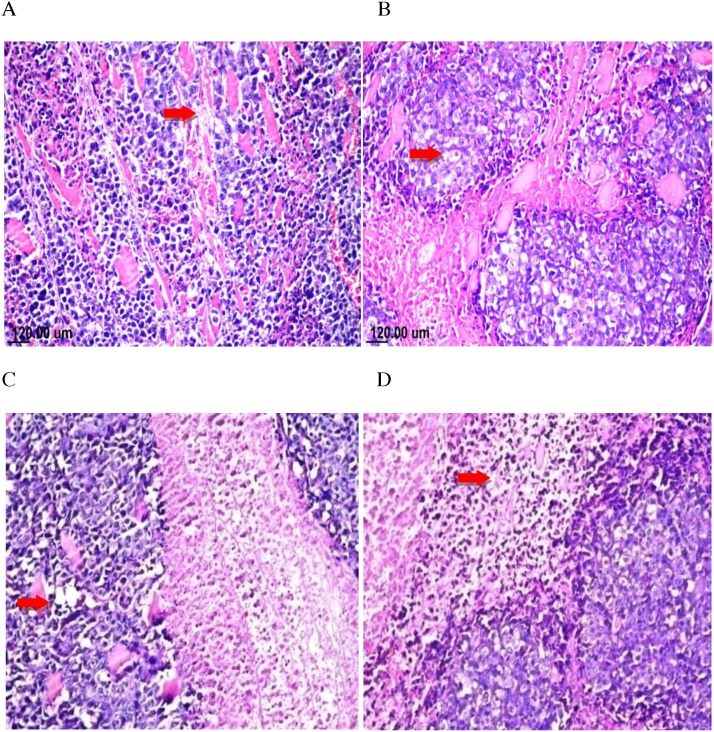
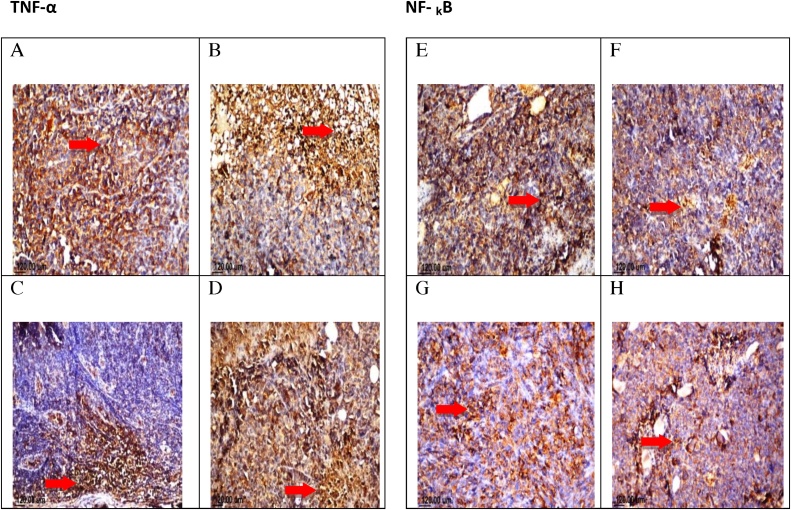
Histopathological examination of mouse solid tumor in all the studied groups showed necrosis and apoptosis as presented in solid EAC tumor of control mice (A). EAC tumor-bearing mice treated with TAM (2.5 mg/kg) showing severe necrosis and apoptosis (B), SIM (2 mg/kg) (C), and combinations treated group (TAM + SIM) showing moderate necrosis and apoptosis (D) (Fig. 10). The untreated tumor showed severe immunostaining(75–100%) of TNF-α and NF-kB expression, while the TAM-treated, SIM-treated and the combination regimen had lower expression compared to the control group, moderate staining level, (50–75%) expression of TNF-α and NF-kB was detected (Fig. 11).
Fig. 10.
Photomicrographs of solid tumor of EAC. Sections stained by H&E(X40). Sections taken from solid EAC tumor of control mice showing mild necrosis and apoptosis(A), sections taken from solid EAC tumor-bearing mice treated with TAM (2.5 mg/kg) showing sever necrosis and apoptosis (B), SIM (2 mg/kg) (C) and combinations (TAM + SIM) showing moderate necrosis and apoptosis (D).
Fig. 11.
Photomicrographs of EAC tumorsections stained by H&E(X160)treated with TAM, SIM, and their combinations.Effect of TAM, SIM and their combinations on TNF-α and NF-??B expression in EAC solid tumor. Immunohistochemical staining of TNF-α in EAC solid tumor sections (X40) control (A), TAM (2.5 mg/kg) (B), SIM (2 mg/kg) (C) and combination(D). Immunohistochemical staining of NF-??B in EAC solid tumor sections(X40).Control (E), TAM (2.5 mg/kg) (F), SIM (2 mg/kg) (G)and combination (H).
4. Discussion
Results of the present study showed that either TAM or SIM treatment inhibited cell proliferation of breast cancer cells by significantly increased ROS, LDH leakage, Bax/BCL-2 expression ratio, and caspase 3 activity. TAM has an anti-proliferative effect mediated by estrogen-dependent and/or estrogen-independent manners [29]. TAM exerts its antiproliferative effect via binding competitively to the estrogen receptor, thereby blocking the mitogenic effect of estrogen [30]. In addition, it induces apoptosis of cancer cells through several distinct mechanisms including the modulation of signaling proteins, such as protein kinase C, transforming growth factor-β (TGF-β), and the upregulation of p53 [31]. In addition, the increase in oxidative stress by TAM was suggested as a prerequisite leading to cell death [32] and the reduction in antioxidant activity sensitizes the cells to ROS –induced cell death [33]. Moreover, the leakage of LDH is a well-known indicator of necrotic cell death or damage to the cell membrane of breast cancer cells treated with TAM [34]. Like our data, a significant increase in caspase-3 activity by TAM as well as ceramide accumulation was detected [35]. Data from the present study depicted that TAM decreased glucose consumption and VEGF, with an increase in MMP2 & 9. VEGF and MMPs are critical markers in malignant tumors for angiogenesis and metastases [36] Similarly, Hesselbarth et al. [37], reported the treatment of breast cancer cells with TAM decreased glucose uptake and caused a significant increase in glycated hemoglobin HbA1c in C57BL/6NTac mice. TAM was shown to decrease the angiogenic and metastatic potential by diminished VEGF release [34,38]. However, TAM significantly increased MMP-2/MMP-9 activity and endostatin levels in human breast cancer, suggesting a possible role of MMP modulation associated with a generation of anti-angiogenic fragments in the therapeutic effect of TAM in breast cancer [39,40]. It was suggested that activation of NF-kB via may be a significant mechanism for the development of resistance in breast cancer, and that inhibition of NF-kB may be an effective treatment strategy to limit the progression of this disease [41].
Apart from their cardiovascular effects, statins have demonstrated significant although heterogeneous, anti-tumor activities in preventing breast cancer progression [9,42]. Our data showed that SIM increased significantly oxidative stress markers, LDH release, caspase 3 activity, Bax/ BCL2 ratio, and increased glucose uptake. SIM was found to have anticancer effects on many types of cancer cell lines including; melanoma [43] leukemia [44]and endometrial cancer [45]. Similarly, Sanchez et al. [46] reported that statin-induced growth inhibition of breast cancer due to apoptotic and necrotic cell death by an increase in the formation of superoxide and oxidative stress in cytotoxic activity [47]. SIM reduces not only serum cholesterol levels but also mevalonate synthesis, a precursor of several major products, regulating the cell cycle, and signal transduction involved in cell proliferation, differentiation, and apoptosis [9,42]. Moreover, a significant increase in LDH release in C2C12 cells exposed to SIM was reported [48]. SIM was found to increase the apoptotic markers by blocking cell proliferation in the G0/G1 phase, decrease the expression of Ki 67 and an increase in the protein tyrosine phosphorylation leading to apoptosis [47,49]. SIM increased glucose uptake and decrease MMP2,9 and VEGF. Like our results SIM was found to decrease breast cancer cell proliferation by deactivating NF-kB, reducing expression of the anti-apoptotic protein BCL-xl, especially in cell lines with constitutively active RAS or overexpressed HER2 [50,51]. Similar findings by Spampanato et al. [51] who documented up-regulation of Bax proteins and downregulation of Bcl-2 after statin treatment.
Results of the present study revealed that the combination of SIM and TAM resulted in significant growth inhibition with synergistic drug interaction via an increase in apoptotic cell death, caspase-3 activity, and overexpression of Bax/Bcl-2 ratio. However, the combination regimen resulted in a significant decrease in ROS levels and LDH leakage compared to TAM, while they are still significantly higher compared to untreated cells. This decrease in necrotic cell death may be a defense mechanism of the combined treatment due to the increase in antioxidant substances (GSH and SOD). Similarly, Domoki et al. [52] and Wang et al. [43] documented that SIM increases the cellular defense against ROS and reduces ischemia–reperfusion-evoked LDH release through its antioxidant effect [53]. In addition, statin treatment-induced SOD and catalase activities in triple-negative breast cancer (TNBC) cells [54]. Moreover, Sohn et al. [53] illustrated that SIM had an antioxidant effect; it decreased free radical production of TAM. Furthermore, SIM increased the level of glutathione content [52] and reducing LDH release through its antioxidant effects [53]. The synergistic drug interaction may be due to the apoptotic and to lesser extent of necrotic cell deaths in addition to other mechanisms. Liang et al. [29] found that the combination of SIM and TAM suppressed the growth, induced apoptosis and subsequently inducing DNA damage in TAM-resistant breast cancer cells. Moreover, it was reported that statins have an impact on the outcomes of patients with aggressive triple-negative breast cancer [55]. Furthermore, SIM suppressed senescence-associated growth factors and cytokines activation in breast cancer cells that might confer endocrine resistance to breast cancer cells [56]. Our data illustrated decreases in glucose uptake, NF-κB protein expression, and VEGF by SIM. It was observed that statin induces hyperglycemia in clinical trials that may be due to impair of cellular glucose uptake by inducing cholesterol-dependent conformational changes in glucose transporter [57]. In addition, the study of Malenda et al. [58] indicated that statins could effectively inhibit glucose uptake by tumor cells thereby impairing the adaptation of tumor cells to micro-environmental conditions associated with tumor progression. The current study showed that SIM has a potential role in decreasing, NF-κB, and VEGF, and metastatic potential. It was suggested that the statin inhibited invasion and metastasis in the aggressive breast cancer cell line MDA-MB 231 via blockade of the mevalonate pathway [59] and inhibition of both MMP-2 and MMP-9. The matrix metalloproteinases (MMPs) play significant roles in tumor growth, angiogenesis, and metastasis by the degradation of collagen and other extracellular matrix components [60].
The present data showed a significant decrease in VEGF upon exposure of the cells to either TAM or/and SIM. An effect may be due to the effect of SIM. In agreement with our results, Coimbra et al. [59] and Wang et al. [61] found that statins inhibited angiogenesis. SIM was found to have a direct effect on tumor AMP kinase signaling, which impedes downstream hypoxia-inducible factor-1α (HIF-1α)-induced angiogenesis [61]. Results of the current study showed that SIM inhibited the increase of MMPs induced by TAM in the breast cancer cells. An effect that may be due to statin and was supported by the study of Thunya- kitpisal and Chaisuparat, [62] who reported that SIM inhibited MMP-9 expression in osteoblastic cells and HT1080 fibrosarcoma cells. Additionally, SIM treatment also suppressed MMP-9 but not MMP-2 expression in human leukemia U937 and KU812 cells [44]. Moreover, SIM down-regulates H-Ras-induced MMP-9 expression [63].
Data of thein Vivo study displayed that the administration of either TAM or SIM significantly reduced the tumor volume, weight, TNF-α, NF-κB of mice bearing EAC tumor. Previous studies in vivo documented a significant reduction in the growth of the tumor by TAM [27,35]. Similarly, SIM alone impairs the growth of human breast tumor xenografts in mice; [50,51]. However, the combination of TAM and SIM resulted in a non-significant decrease in the tumor volume or weight compared to the TAM-treatment group. The insignificant change observed in a combination treatment may be related to the differences in the biological type of the tumor, as EAC is a murine tumor and doses of the drugs used in vivo.
In conclusion, this study illustrates that SIM enhanced the apoptotic, anti-metastatic potential of TAM and may be used in combination with TAM for a subset of breast cancer patients, depending on their biological features, anti-apoptosis mechanisms, and the proteins involved in cell growth stimulation. No doubts that further studies are required to confirm these results in a preclinical and clinical setting.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgment
The authors of the manuscript acknowledge the National Cancer Institute, Cairo University for providing facility and supporting this work.
References
- 1.Siegel R., Miller K., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Subramani T., Yeap S., Ho W., Ho C., Omar A., Aziz S., Rahman M.Abd., Alitheen N. Vitamin C suppresses cell death in MCF-7 human breast cancer cells induced by tamoxifen. J. Cell. Mol. Med. 2014;18:305–313. doi: 10.1111/jcmm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H., Man H.T., Zhao X.D., Dong N., Ma S. Estrogen receptor-positive breast cancer molecular signatures and therapeutic potentials. Biomed. Rep. 2013;2:41–52. doi: 10.3892/br.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capasso I., Esposito E., Pentimalli F., Crispo A., Montella M., Grimaldi M., De Marco M., Cavalcanti E., D’Aiuto M., Fucito A., Frasci G., Maurea N., Esposito G., Pedicini T., Vecchione A., D’Aiuto A., Gand Giordano A. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol. Ther. 2011;10:1240–1243. doi: 10.4161/cbt.10.12.13473. [DOI] [PubMed] [Google Scholar]
- 5.Gérard C., Brown K.A. Obesity and breast cancer - role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell. Endocrinol. 2018;466:15–30. doi: 10.1016/j.mce.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Ferraroni M., Gerber M., Decarli A., Richardson S., Marubini E., Crastes de Paulet P., Crastes de Paulet A., Pujol H. HDL-cholesterol and breast cancer: a joint study in northern Italy and southern France. Int. J. Epidemiol. 1993;22:772–780. doi: 10.1093/ije/22.5.772. [DOI] [PubMed] [Google Scholar]
- 7.Kitahara C.M., Berrington de González A., Freedman N.D., Huxley R., Mok Y., Jee S.H., Samet J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanan B., Venkatesan N., Subburaju T., Fathah A. Chemopreventive role of combination of Etoricoxib and Atorvastatin on colon cancer induced by 1, 2-dimethylhydrazine on rats. Int. J. Pharm. Pharm. Sci. 2015;7:299–303. [Google Scholar]
- 9.Sripriyalakshmi S., Anjali C., George Priya Doss C., Rajith B., Aswathy R. BSA Nanoparticle loaded atorvastatin calcium—a new facet for an old drug. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacerda L., Reddy J.P., Liu D., Larson R., Li L., Masuda H., Brewer T., Debeb B.G., Xu W., Hortobágyi G.N., Buchholz T.A., Ueno N.T., Woodward W.A. Simvastatin radiosensitizes differentiated and stem-like breast cancer cell lines and is associated with improved local control in inflammatory breast cancer patients treated with postmastectomy radiation. Stem Cells Transl. Med. 2014;3:849–856. doi: 10.5966/sctm.2013-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undela K., Srikanth V., Bansal D. Statin use and risk of breast cancer: a meta- analysis of observational studies. Breast Cancer Res. Treat. 2012;135:261–269. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 12.Ahern T.P., Pedersen L., Tarp M., Cronin-Fenton D.P., Garne J.P., Silliman R.A., Sorensen H.T., Lash T.L. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J. Natl. Cancer Inst. 2011;103:1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen S.F., Nordestgaard B.G., Bojesen S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 14.Cote-Daigneault J., Mehandru S., Ungaro R., Atreja A., Colombel J.F. Potential immunomodulatory effects of statins in inflammatory bowel disease. Inflamm. Bowel Dis. 2016;22:724–732. doi: 10.1097/MIB.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 15.Kavalipati N., Shah J., Ramakrishan A., Vasnawala H. Pleiotropic effects of statins. Ind. J. Endocrinol. Metabol. 2015;19:554–562. doi: 10.4103/2230-8210.163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Wyhe R.D., Rahal O.M., Woodward W.A. Effect of statins on breast cancer recurrence and mortality: a review. Breast Cancer Targets Ther. 2017;9:559–565. doi: 10.2147/BCTT.S148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 18.Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drugcombination studies. Pharmacol. Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 19.Buege A.J., Aust S.D. Microsomal lipid peroxidation. Methods. Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 20.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Miranda K.M., Espey M.G., Wink D.A. A rapid, simple Spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 22.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Trinder P., Ann Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann. Clin. Biochem. 1969 [Google Scholar]
- 24.Kim K.J., Li B., Houck K., Winer J., Ferrara N. The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7:53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- 25.Gjerde J., Kisanga E.R., Hauglid M., Holm P.I., Mellgren G., Lien E.A. Identification and quantification of tamoxifen and four metabolites in serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2005;1082:6–14. doi: 10.1016/j.chroma.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Snoek-van Beurden P.A., Von den Hoff J.W. Zymographic techniques for analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- 27.Motawia T.K., Abdelazima S.A., Darwisha H.A., Elbaza E.M., Shouman S.A. Could caffeicacidphenethylester expand the antitumor effect of tamoxifen in breast carcinoma? Nutr. Cancer. 2016;68:435–445. doi: 10.1080/01635581.2016.1153669. [DOI] [PubMed] [Google Scholar]
- 28.Kochuparambil S.T., Al-Husein B., Goc A., Soliman S., Somanath P.R. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J. Pharmacol. Exp. Ther. 2011;336:496–505. doi: 10.1124/jpet.110.174870. [DOI] [PubMed] [Google Scholar]
- 29.Liang Z., Li W., Liu J., Li J., He F., Jiang Y., Yang L., Li P., Wang B., Wang Y., Ren Y., Yang J., Luo Z., Vaziri C., Peijun Liu P. Simvastatin suppresses the DNA replication licensing factor MCM7 and inhibits the growth of tamoxifen-resistant breast cancer cells. Sci. Rep. 2017;7:41776–41786. doi: 10.1038/srep41776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aesoy R., Sanchez B.C., Norum J.H., Lewensohn R., Viktorsson K., Linderholm B. An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4- hydroxytamoxifen in MCF-7 breast cancer cells. Mol. Cancer Res. 2008;6:1630–1638. doi: 10.1158/1541-7786.MCR-07-2172. [DOI] [PubMed] [Google Scholar]
- 31.Lagadec C., Adriaenssens E., Toillon R.A., Chopin V., Romon R., Van Coppenolle F., Hondermarck H., Le Bourhis X. Tamoxifen and Trail synergistically induce apoptosis in breast cancer cells. Oncogene. 2008;27:1472–1477. doi: 10.1038/sj.onc.1210749. [DOI] [PubMed] [Google Scholar]
- 32.Hwang J.J., Kim H.N., Kim J., Cho D.H., Kim M.J., Kim Y.S., Kim Y., Park S.J., Koh J.Y. Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals. 2010;23:997–1013. doi: 10.1007/s10534-010-9346-9. [DOI] [PubMed] [Google Scholar]
- 33.Bruning A., Friese K., Burges A., Mylonas I. Tamoxifen enhances the cytotoxic effect of nelfinavir in breast cancer cells. Breast Cancer Res. 2010;12:45–56. doi: 10.1186/bcr2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darakhshan S., Bidmeshkipour A., Khazaei M., Rabzia A., Ghanbari A. Synergistic effects of tamoxifen and tranilast on VEGF and MMP-9 regulation in cultured human breast cancer cells. Asian Pac. J. Cancer Prev. 2013;14:6869–6874. doi: 10.7314/apjcp.2013.14.11.6869. [DOI] [PubMed] [Google Scholar]
- 35.Bekele R.T., Venkatraman G., Liu R.Z., Tang X., Mi S., Benesch M.G.K., Mackey J.R., Godbout R., Curtis J.M., McMullen T.P.W., David N., Brindley D.N. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: implications for tamoxifen therapy and resistance. Sci. Rep. 2016;6:21164. doi: 10.1038/srep21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmeliet p. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 37.Hesselbarth N., Pettinelli C., Gericke M., Berger C., Kunath A., Stumvoll M., Blüher M., Kloting N. Tamoxifen affects glucose and lipid metabolism parameters causes browning of subcutaneous adipose tissue and transient body composition changes in C57BL /6NTac mice. Biochem. Biophys. Res. Commun. 2015;464:724–729. doi: 10.1016/j.bbrc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Johnson K.E., Forward J.A., Tippy M.D., Ceglowski J.R., El-Husayni S., Kulenthirarajan R., Machlus K.R., Mayer E.L., Italiano J.E., Battinelli E.M. Tamoxifen directly inhibits platelet angiogenic potential and platelet-mediated metastasis. Arterioscler. Thromb.Vasc. Biol. 2017;37:664–674. doi: 10.1161/ATVBAHA.116.308791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garvin S., Nilsson U.W., Huss F.R., Kratz G., Dabrosin C. Estradiol increases VEGF in human breast studied by whole tissue culture. Cell Tissue Res. 2006;325:245–251. doi: 10.1007/s00441-006-0159-7. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson U.W., Garvin S., Dabrosin C. MMP-2 and MMP-9 activity is regulated by estradiol and tamoxifen in cultured human breast cancer cells. Breast Cancer Res. Treat. 2007;102:253–261. doi: 10.1007/s10549-006-9335-4. [DOI] [PubMed] [Google Scholar]
- 41.Park M.H., Hong J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5:1–15. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopalan A., Yu W., Sanders B.G., Kline K. Simvastatin inhibition of mevalonate pathway induces apoptosis in human breast cancer cells via activation of JNK/CHOP/DR5 signaling pathway. Cancer Lett. 2013;329:9–16. doi: 10.1016/j.canlet.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Xu S.L., Wu Y.Z., Zhao M.S., Xu W.J., Yang H.Y., Li Y.X. Simvastatin induces caspase-dependent apoptosis and activates P53 in OCM-1 cells. Exp. Eye Res. 2013;113:128–134. doi: 10.1016/j.exer.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y.J., Chang L.S. Simvastatin induces NFkB/p65 down-regulation and JNK1/c-Jun/ATF- 2 activation leading to matrix metalloproteinase-9 (MMP-9) but not MMP-2 down-regulation in human leukemia cells. Biochem. Pharmacol. 2014;92:530–543. doi: 10.1016/j.bcp.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Schointuch M.N., Gilliam T.P., Stine J.E., Han X., Zhou C., Gehrig P.A., Kim K., Bae-Jump V.L. Simvastatin, an HMG-CoA reductase inhibitor exhibits anti-metastatic and anti-tumorigenic effects in endometrial cancer. Gynecol. Oncol. 2014;134:346–355. doi: 10.1016/j.ygyno.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez C.A., Rodriguez E., Varela E., Zapata E., Paez A., Masso F.A., Montano L.F., Loopez-Marure R. Statin induced inhibition of MCF-7 breast cancer cell proliferation is related to cell cycle arrest and apoptotic and necrotic cell death mediated by an enhanced oxidative stress. Clin. Cancer Investig. J. 2008;26:698–707. doi: 10.1080/07357900701874658. [DOI] [PubMed] [Google Scholar]
- 47.Buranrat B., Suwannaloet W., Naowaboot J. Simvastatin potentiates doxorubicin activity against MCF-7 breast cancer cells. Oncol. Lett. 2017;14:6243–6250. doi: 10.3892/ol.2017.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taha D.A., De Moor C.H., Barrett D.A., Lee J.B., Gandhi R.D., Hoo C.W., Gershkovich P. The role of acid-base imbalance in statin-induced myotoxicity. Transl. Res. 2016;174:140–160. doi: 10.1016/j.trsl.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keyomarsi K., Sandoval L., Band V., Pardee A.B. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 50.Ghosh-Choudhury N., Mandal C.C., Ghosh-Choudhury N., Ghosh Choudhury G. Simvastatin induces derepression of PTEN expression via NFκB to inhibit breast cancer cell growth. Cell. Signal. 2010;22:749–758. doi: 10.1016/j.cellsig.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spampanato C., De Maria S., Sarnataro M., Giordano E., Zanfardino M., Baianoet S., Carteni M., Morelli F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int. J. Oncol. 2012;40:935–941. doi: 10.3892/ijo.2011.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Domoki F., Kis B., Gaspar T., Snipes J.A., Parks J.S., Bari F., Busija D.W. Rosuvastatin induces delayed preconditioning against oxygen-glucose deprivation in cultured cortical neurons. Am. J. Physiol. Cell Physiol. 2009;296:C97–C105. doi: 10.1152/ajpcell.00366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sohn H.M., Hwang J.Y., Ryu J.H., Kim J., Park S., Park J.W., Han S.H. Simvastatin protects ischemic spinal cord injury from cell death and cytotoxicity through decreasing oxidative stress: in vitro primary cultured rat spinal cord model under oxygen and glucose deprivation reoxygenation conditions. J. Orthop. Surg. Res. 2017;12:36–44. doi: 10.1186/s13018-017-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanugula A.K., Kotamraju S. Statin-induced molecular mechanisms of breast cancer cell death: recent developments. Cancer Cell Microenviron. 2014;1:e390. [Google Scholar]
- 55.Shaitelman S.F., Stauder M.C., Allen P., Reddy S., Lakoski S., Atkinson B., Reddy J., Amaya D., Guerra W., Ueno N., Caudle A., Tereffe W., Woodward W.A. Impact of statin use on outcomes in triple negative breast cancer. J. Cancer. 2017;8:2026–2032. doi: 10.7150/jca.18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S., Uppal H., Demaria M., Desprez P.Y., Campisi J., Kapahi P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci. Rep. 2015;5:17895. doi: 10.1038/srep17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nowis D., Malenda A., Furs K., Oleszczak B., Sadowski R., Chlebowska J., Firczuk M., Bujnicki J.M., Staruch A., Zagozdzon R., Glodkowska-Mrowka E., Szablewski L., Golab J. Statins impair glucose uptake in human cells. BMJ Open Diabetes Res. Care. 2014;2 doi: 10.1136/bmjdrc-2014-000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malenda A., Skrobanska A., Issat T., Winiarska M., Bil J., Oleszczak B., Sinski M., Firczuk M., Bujnicki J.M., Chlebowska J., Staruch A.D., Glodkowska- Mrowka E., Kunikowska J., Krolicki L., Szablewski L., Gaciong Z., Koziak K., Jakobisiak M., Golab J., Nowis D.A. Statins impair glucose uptake in tumor cells. Neoplasia. 2012;14:311–323. doi: 10.1593/neo.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coimbra M., Banciu M., Fens M.H., De Smet L., Cabaj M., Metselaar J.M., Storm G., Schiffelers R.M. Liposomal pravastatin inhibits tumor growth by targeting cancer-related inflammation. J. Control Release. 2010;148:303–310. doi: 10.1016/j.jconrel.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Klein G., Vellenga E., Fraaije M.W., Kamps W.A., Bont E.S. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit. Rev. Oncol. Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Wang G., Cao R., Wang Y. Simvastatin induces cell cycle arrest and inhibits proliferation of bladder cancer cells via PPARγ signalling pathway. Sci. Rep. 2016;6:35783. doi: 10.1038/srep35783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thunyakitpisal P.D., Chaisuparat R. Simvastatin, an HMG-CoA reductase inhibitor, reduced the expression of matrix metalloproteinase-9 (Gelatinase B) in osteoblastic cells and HT1080 fibrosarcoma cells. J. Pharmacol. Sci. 2004;94:403–409. doi: 10.1254/jphs.94.403. [DOI] [PubMed] [Google Scholar]
- 63.Kang S., Kim E.S., Moon A. Simvastatin and lovastatin inhibit breast cell invasion induced by H-Ras. Oncol. Rep. 2009;21:1317–1322. doi: 10.3892/or_00000357. [DOI] [PubMed] [Google Scholar]